CHAPTER 43 DIAPHRAGMATIC INJURY
ANATOMY AND PHYSIOLOGY
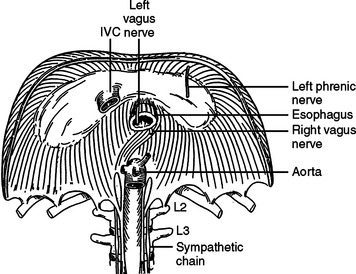
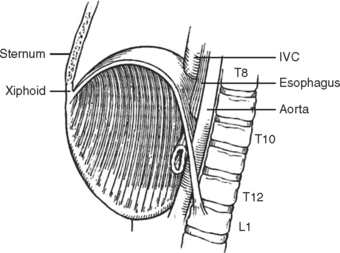
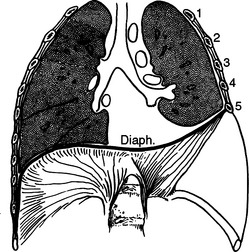
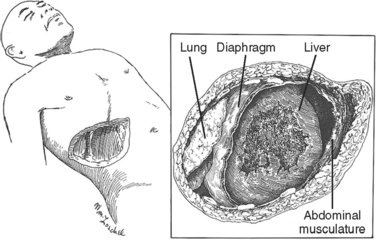
The diaphragm is a musculotendinous organ that separates the thoracic cavity from the abdominal cavity.1,2 This important organ arises from the confluence of the abdominal peritoneum and the parietal pleural during the first trimester of pregnancy. The muscular ingrowth represents an extension from the circumference of the thoracic inlet, specifically the posterior sternal border, the inner surfaces of the lower six costal cartilages, and the posterior lumbocostal arches. These three muscular groups join together as a central tendon, which has three leaflets, namely, left, right, and central leaflets (Figures 1 and 2). The most medial posterior margins are formed by the crura. The central leaflet is located posterior to the sternum and inferior to the heart; it contributes to the pericardial fibers. The right and left leaflets are located posterolaterally in each hemithorax. Incomplete closure of this posterior lateral leaflet results in herniation in the posterior lateral foramen of Bochdalek. Partial closure may result in pleural and peritoneal apposition without union as a central tendon.3 This results in an eventration that may be an important consideration in the differential diagnosis of blunt diaphragmatic injury (Figures 3 and 4).3
The diaphragm, as a muscular organ, varies widely in contour during the ventilatory cycle.1 During full expiration, the diaphragmatic dome extends high into the thoracic cavity. When the diaphragm contracts during forced inspiration, the central tendon is pulled inferiorly and becomes more plate-like.1 This reduces the intrathoracic pressure and raises the intra-abdominal pressure. The excursion of the diaphragm during forced expiration is great; the dome of the diaphragm elevates to the level of the fourth intercostal space at the sternal junction on the right side and to about the level of the fifth intercostal space on the left side.1,2 The diaphragm may extend inferiorly at least three intercostal spaces during full inspiration. The most inferior extension posteriorly occurs at the crura. The right crus arises from the bodies and fibrocartilages of the lumbar vertebrae one through three, whereas the smaller left crus arises from the first and second lumbar vertebrae. They join superiorly to encompass the esophagus at the esophageal foramen. The posterior lateral sulcus or recess of the diaphragm is directly posterior to many of the intra-abdominal organs. Consequently, the posterior sulcus extends inferiorly to the midportion of the kidneys. This inferior extension is often not appreciated when patients with upper abdominal gunshot wounds or stab wounds are treated for intra-abdominal injuries; thus, perforation of the diaphragm in this area is easily overlooked. The resultant hemothorax is diagnosed postoperatively. Careful inspection followed by repair and tube thoracostomy precludes the development of this complication.
The diaphragm has three major foramina (see Figure 1). The aortic hiatus is the most posterior, located between the diaphragmatic muscle and the 12th vertebra. The esophageal hiatus is bordered by the strong muscular crura; the left crus encircles the esophagus and forms an important part of the lower esophageal sphincter. Immediately posterior to the esophagus, sinuous fibers from each hemidiaphragm join as the median arcuate ligament, which is anterior to the aorta and superior to the celiac axis. Immediately posterior to the esophageal foramen on the right side is the foramen for the inferior vena cava (see Figure 1). There are also lesser diaphragmatic apertures. The anterior foramen of Morgagni is in the retroxiphoid space; the internal mammary arteries pass through this foramen while coursing from the mediastinum before dividing into the superior epigastric arteries and the subcostal arteries.4 The accompanying veins follow the same course.
The median plane of the diaphragm extending from the foramen of Morgagni to the esophageal foramen, the so-called median raphe, is less vascular than the more lateral muscular portions of the diaphragm (see Figure 2). This plane can be divided to provide better exposure to the inferior mediastinum. Care must be taken, however, to prevent injury to the phrenic vein, which may cross this median plane about 1 cm above the esophageal hiatus. This vein, if not controlled while dividing the median raphe between the foramen of Morgagni and esophageal hiatus, can lead to major hemorrhage.
The diaphragm is innervated by the phrenic nerve with arises from the cervical portion of the spinal cord, namely, C3, C4, and C5 (see Figure 1).1 After these nerves pass inferior through the mediastinum to the medial portion of the diaphragm, they curve laterally, like a fan, from the central medial portion of the diaphragm to the anterior, lateral, and posterior attachments (see Figure 1). In theory, one should protect these nerves while performing surgery on the diaphragm; this is accomplished by detachment of the diaphragm from the peripheral thorax. This is seldom necessary for most patients with diaphragmatic injury. When the origins of the diaphragm are to be relocated for treatment of unusual injuries, however, care should be taken to protect the neural innovation as much as is practical.
INCIDENCE OF DIAPHRAGMATIC INJURIES
The reported incidence of diaphragmatic injury varies widely. Asensio et al.,2 in a multicenter review, identified a 3% incidence of diaphragmatic injuries for all patients sustaining torso trauma with a range of 0.8%–5.8%; this wide range reflects the different types of injuries treated at each institution and the diligence one places on confirming a diaphragmatic perforation. Rural trauma centers are more likely to treat patients with blunt diaphragmatic injury, whereas penetrating diaphragmatic injury predominates in patients presenting to inner city trauma centers.5
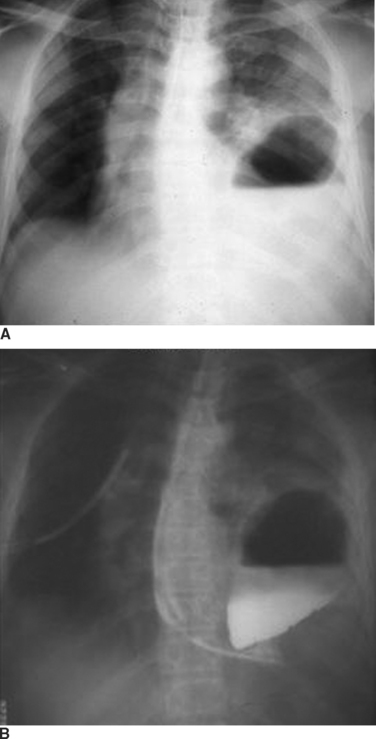
The reported incidence of diaphragmatic rupture may also reflect the different therapeutic approaches to patients with both blunt and penetrating abdominal wounds.6 Most patients with penetrating stab wounds to the abdomen are now being treated nonoperatively when the patient exhibits no signs of peritonitis or hemoperitoneum.7,8 This is also true for patients with lower thoracic stab wounds causing hemopneumothorax which, based on prior experiences with a more liberal approach to exploratory laparotomy, would be associated with a high incidence of confirmed diaphragmatic penetration.8 This includes some patients who have hemothorax treated by tube thoracostomy; many of these patients have unrecognized diaphragmatic perforation (Figure 5). Moreover, patients with through-and-through anterior to posterior right upper quadrant gunshot wounds near the liver and associated right-sided hemopneumothorax are being treated by tube thoracostomy alone, even though there are at least two diaphragmatic perforations. The missile in this setting would pass through the lower rib cage anteriorly, enter the anterior hemithorax, pass through the anterior portion of the diaphragm, transit the liver, re-enter the chest through the posterior portion of the diaphragm, and exit through the posterior chest wall.9 When treated only with right tube thoracostomy for hemothorax, the diaphragmatic perforations are not diagnosed, and therefore, not coded in the trauma registry.8 When diagnostic peritoneal lavage (DPL) was performed routinely for patients with penetrating wounds of the lower rib cage, the effluent would be pink with a red cell count less 100,000/cm3 in patients with isolated diaphragmatic perforation.8 Laparotomy in these patients would confirm a diaphragmatic perforation that was sutured.10 The current trend for routine ultrasonography (US) in patients with abdominal injury has replaced DPL; US is less sensitive for identifying small amounts of blood. Consequently, clinically insignificant diaphragmatic perforations are not being recognized and are not coded in the trauma registry. Penetrating diaphragmatic perforation after upper abdominal wounds may also go unrecognized despite laparotomy for repair of other intra-abdominal visceral injuries. The perforation may be missed because the missile is thought to have penetrated only the transversalis abdominus muscle. When a subsequent hemothorax appears postoperatively, the treating physician, hopefully, will recognize that there was a missed diaphragmatic injury. Patients with blunt injury are less likely to have a diaphragmatic injury that is not recognized during the same hospitalization (Figure 6). There are, however, a number of patients who present with a diaphragmatic hernia years after major blunt torso trauma when diaphragmatic injury was not recognized initially.
During the 1970s, when routine laparotomy was performed for all penetrating abdominal wounds and careful examination of the inferior diaphragmatic extensions was routine, the incidence of diaphragmatic perforation was approximately 19% for upper abdominal gunshot wounds and 11% for upper abdominal stab wounds.8 Now that laparotomy for penetrating abdominal wounds is performed more selectively, the incidence of confirmed diaphragmatic perforation is about 8% for gunshot wounds and 2% for stab wounds. In contrast, the recent incidence of diaphragmatic injury in patients admitted after blunt torso injury is less than 1%; most patients with blunt rupture of the liver or spleen are treated nonoperatively. Patients requiring laparotomy for blunt abdominal injury have about a 3% incidence of diaphragmatic rupture.5,8
MECHANISM AND LOCATION OF INJURY
The location of diaphragmatic perforation varies with mechanism of injury.2,8 The classic scenario for blunt diaphragmatic injury is a head-on motor vehicle collision or a T-bone impact causing a marked increase in the intra-abdominal pressure, thereby stretching the diaphragm to the point of rupture. The rupture typically occurs in the posterior lateral segment in the central tendon of the left diaphragm, often with extension into the muscular portion of the diaphragm. Blunt rupture can also occur after assaults, stompings, falls from a height, and explosions.11 Although the posterior lateral location is the most common site for injury, rupture may occur adjacent to the esophageal hiatus, near the bare area of the liver on the right side, and in a subxyphoid location on either side.
After blunt injury, most ruptures occur in the left hemidiaphragm.12 A review of 32 published series with 1589 patients by Asensio et al.2 showed a distribution of left-sided rupture in 1187 patients (75%), right-sided rupture in 363 patients (23%), and bilateral rupture in 39 patients (2%). This observation parallels the authors’ experience. The relative protection of the right hemidiaphragm, historically, has been attributed to the protective effects of the liver, which blunts the rapid rise in the intraabdominal against the right hemidiaphragm.13 A less common explanation may reflect a weaker left hemidiaphragm that undergoes a later intrauterine closure14; an inherent tendinous or muscular weakness, however, has not been demonstrated with carefully constructed bursting tests.15 The site of injury with penetrating wounds may be anywhere. Bilateral perforations are more likely with missiles. Stab wounds are more commonly located along the periphery of the diaphragm.8
SEVERITY OF INJURY
The severity of diaphragmatic rupture or injury is best judged by the organ injury scale for diaphragmatic injury developed by the American Association for the Surgery of Trauma.16 This scale identifies five grades of injury. Grade I injury is a contusion or hematoma without rupture. Grade II injury is a laceration less than 2 cm in diameter. Grade III rupture is a laceration greater than 2 cm but less that 10 cm in magnitude. Grade IV rupture is greater than 10 cm or has loss of diaphragmatic tissue, which is less than 25 cm2. Grade V injury is an extensive tear with tissue loss greater than 25 cm2.
Patients with penetrating stab wounds and gunshot wounds likely have minor ruptures (grades I and II); some patients with low-velocity missile perforations will have grade III perforations. Patients with blunt injury are more likely to have grade III or grade IV lacerations. The massive injuries with extensive tissue loss (grade V) are more likely to be caused by a close-range shotgun blast, high-velocity rifle perforation, or explosions.17,18
DIAGNOSIS OF DIAPHRAGMATIC INJURY
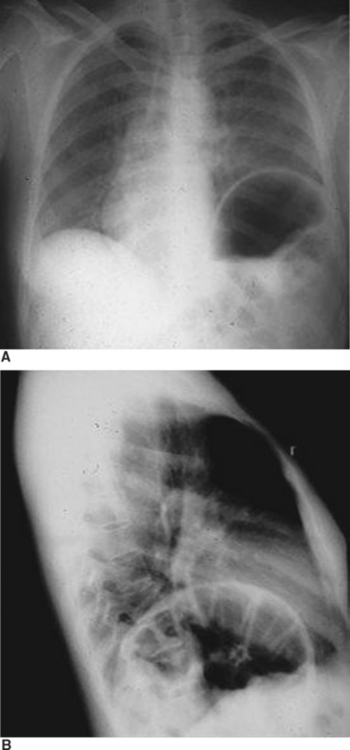
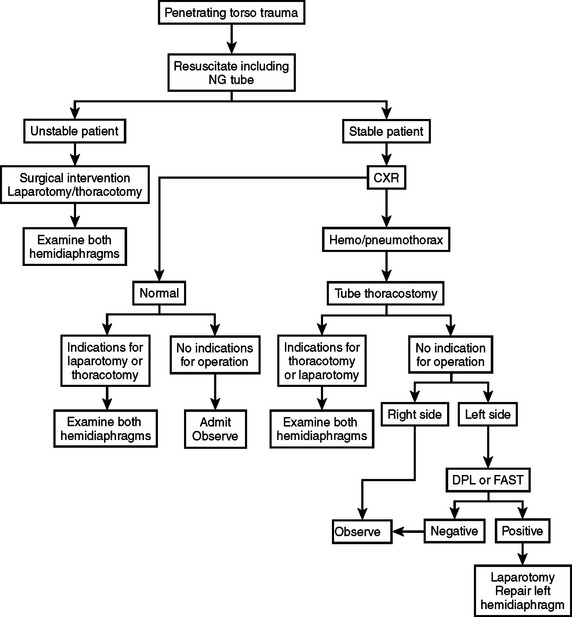
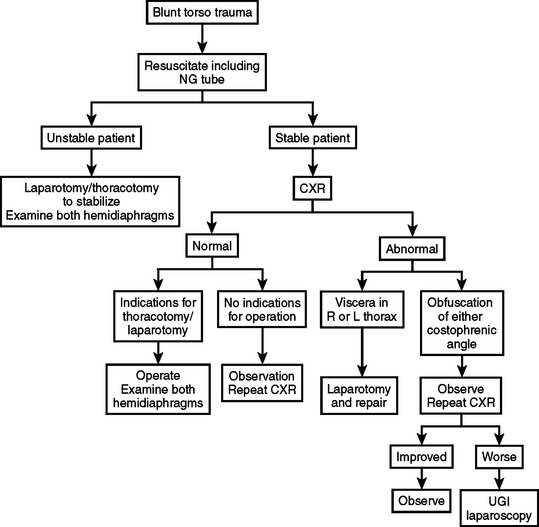
The diagnosis of diaphragmatic rupture after blunt injury is usually made on the basis of a chest x-ray that demonstrates the gastric bubble in the left hemithorax (see Figure 6). This can easily be confirmed to be the gastric bubble by showing that the nasogastric tube will pass up into the thorax and contrast agent injected through this tube will fill the gastric bubble. Because a routine chest x-ray is obtained in all patients with significant blunt injury, this provides the simplest and quickest method of diagnosis.2,7,19 Patients with multisystem injuries involving the chest and pelvis in the presence of hypotension, are more likely to have an associated diaphragmatic injury.20 When the diaphragmatic perforation is not appreciated on the initial chest x-rays, a subsequent computed tomography (CT) scan of the trunk may show the injury; the diagnosis of diaphragmatic rupture by CT, however, is not very sensitive.21 The features which lead to the diagnosis being made or suspected by chest x-ray include obfuscation of the costal phrenic angle, apparent elevation of the hemidiaphragm, and the presence of the gastric bubble in a location that is too superior to be intra-abdominal (see Figure 6). Although not readily apparent, the spleen and splenic flexure of the colon, with or without omentum, are also commonly relocated into the left thorax after blunt diaphragmatic rupture.2,8
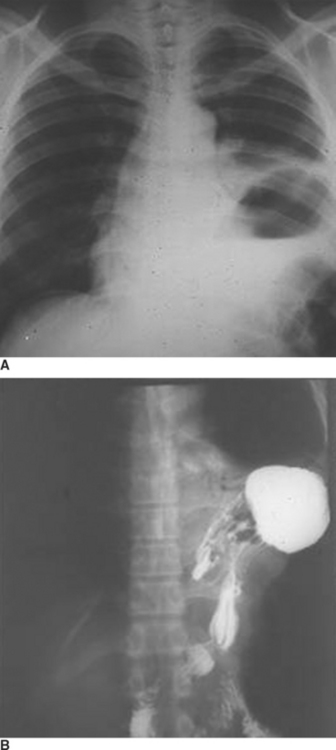
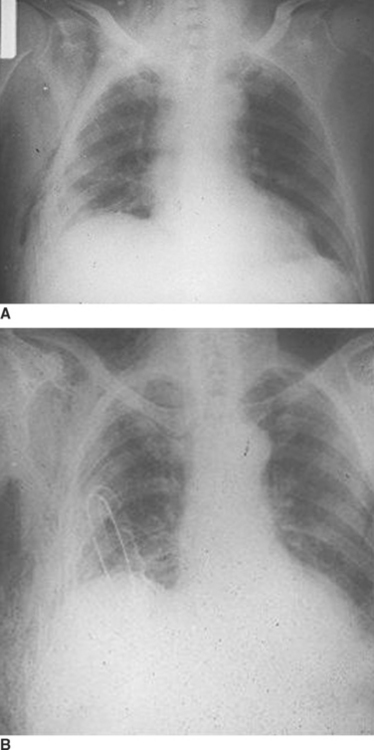
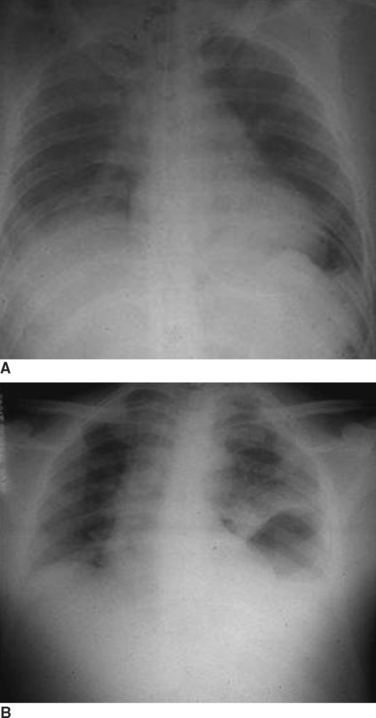
When the blunt injury occurs on the right side, the liver may prevent abdominal viscera from entering the right hemithorax with the result that the injury may be overlooked.7 Patients presenting with blunt trauma and significant bleeding from a right-sided pneumothorax, however, should be suspected of having a right-sided hemidiaphragmatic tear in the bare area of the liver, which is also injured and is the source of bleeding (Figure 7). Bilateral diaphragmatic rupture is much less frequent but, when present, may lead to a delay in diagnosis resulting from the apparent lack of diaphragmatic elevation on either side. Both costophrenic angles, however, show lack of clarity (Figure 8). Repeat chest x-rays in such patients will typically show further elevation of one or both hemidiaphragms (see Figure 8). The elevation on the left side would be more likely to show an intrathoracic gastric bubble even in patients after a delay in diagnosis. When the chest x-ray shows what appears to be a large rupture of the left hemidiaphragm in a patient who has minimal symptoms, one should obtain a good history to rule out previous anatomic changes of the left hemidiaphragm and, if possible, obtain old chest x-rays that might identify the presence of an eventration (see Figure 8).
When the chest x-ray does not confirm a diaphragmatic rupture, a number of other studies may be obtained. As indicated, CT is not highly accurate in patients with diaphragmatic injury.21 Laparoscopy has become more popular for identifying diaphragmatic injury but this has been used primarily in patients with penetrating wounds.22,23 Likewise, technetium scanning has been employed but has not been that helpful.24 DPL may lead to suspicion of diaphragmatic injury in patients with associated penetrating wounds to the lower thorax, but has not been too reliable in patients with blunt torso injuries.8,25 Thorascopy has also been used in patients with late manifestations of a previously missed diaphragmatic injury.26 Finally, magnetic resonance imaging has been attempted but is of limited value.27
The diagnosis of a diaphragmatic injury after a penetrating wound is usually made at the time of laparotomy performed for the treatment of other organ injuries.2,8 As indicated previously, many diaphragmatic perforations are not currently being diagnosed because of the nonoperative treatment of highly selected patients with torso stab wounds and torso gunshot wounds. When a patient has a stab wound to the lower chest associated with hemothorax, however, a DPL yielding a small amount of hemoperitoneum strongly suggests diaphragmatic perforation.8,25 This can be confirmed by means of laparoscopy, which will also allow for the small grade II perforation to be repaired.22,23 A missed diaphragmatic injury is more likely to occur now that patients with these asymptomatic penetrating wounds associated with hemothorax no longer undergo laparotomy (see Figure 5). These missed injuries are more complicated to repair because of associated adhesions; this repair, however, can usually be accomplished through a transabdominal approach.8,28 When the perforation occurs along the medial portion of the diaphragm, near the esophageal foramen, the herniated viscus may also extend into the pericardium, which makes the diagnosis somewhat more difficult.29
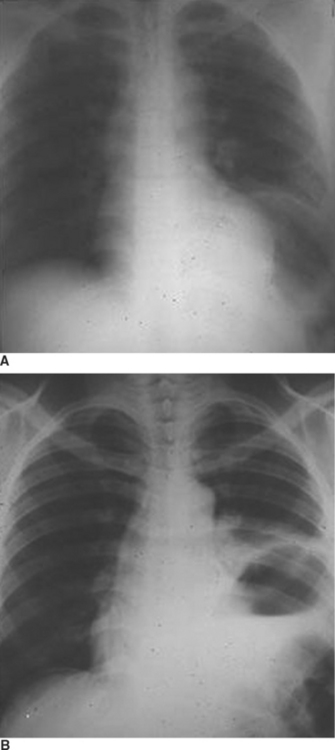
The location of diaphragmatic rupture along the more medial portion of the diaphragm increases the likelihood that the rupture will be missed when laparotomy is performed for repair of other injuries (Figure 9). After completing therapy for associated intra-abdominal injuries, the surgeon should carefully examine all of the diaphragm, including the posterior portions of the diaphragm behind the triangular ligaments, especially on the left side.
MANAGEMENT OF DIAPHRAGMATIC INJURY
The severity of diaphragmatic injury plus the magnitude of the associated injuries determines the optimal approach to surgical treatment.2,8 The decisions regarding resuscitation and prioritization of specific organ injury treatment are discussed elsewhere in this text. Antibiotics should be administered.30 Most patients with grade II injury, contusion or hematoma, are diagnosed at the time of laparotomy or thoracotomy performed for some other reason.2,8 These injuries require no special surgical treatment. The surgeon should make sure that the hematoma or contusion is not hiding a full-thickness perforation. When a full-thickness perforation is seen, it is small and can be treated by simple closure. When diagnosed by laparoscopy, isolated grade I perforation may be repaired through the scope.22,23 The type of suture used for closure of diaphragmatic perforations varies according to surgical preference; the authors prefer 2-0 or 1-0 absorbable polyglycolic sutures,8 while others prefer 2-0 polypropylene sutures, either in an interrupted fashion using Halsted horizontal mattress sutures or in a running fashion.
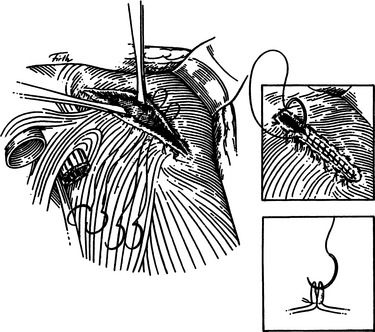
Grade II injuries with full perforation less than 2 cm in size result from stab wounds or small-caliber gunshot wounds. These are treated with primary closure (Figure 10). When the perforation is located posteriorly, suture placement may be difficult. This can be facilitated by hooking the diaphragm through the perforation with a long right-angle clamp. This permits the first suture to be placed and tied. The first suture then becomes the handle to expose the diaphragm for placement of subsequent sutures (Figure 11).
Larger grade III rents of 2–10 cm can be closed primarily using the same techniques described previously when the tear is linear, as might be caused by a large knife or, in some patients, after blunt injury (see Figure 11). The grade III tears that are seen after large high velocity missile impact or with blunt injury, however, are often irregular. Once the perforation is exposed, meticulous debridement of devitalized tissue is in order, followed by initial placement of strategic sutures designed to reapproximate the irregular borders, which helps with the subsequent closure. Minimal debridement of irregular fragments may help with the approximation. The authors prefer to use interrupted 1-0 absorbable suture for approximation of irregular borders, followed by the placement of running 1-0 absorbable sutures for the definitive closure.
The reconstruction of grade IV lacerations greater than 10 cm in size and associated with modest tissue loss is more challenging.2,8 These larger injuries often are associated with herniation of abdominal viscera into the left hemithorax. Classically, the stomach, spleen, and colon are inherniated; omentum and small bowel may also be in the thorax.2,8 Once exposed, these organs need to be relocated within the abdomen. Gentle traction should be tried initially. If the viscera resist gentle traction, the surgeon should place a hand within the thorax, cup the spleen, and gently relocate the viscera into the peritoneal cavity. When the diaphragmatic rent is too tight around the herniated viscera, opening the rent further in a radial direction will allow the surgeon’s hand to get superior to the viscera to successfully restore them into the abdomen. This precaution protects the herniated spleen which, surprisingly, is often not injured in these patients. Most grade IV injuries, however, can be closed primarily without using mesh or fascia lata and without altering diaphragmatic attachments. The diaphragm has significant redundancy in the relaxed state so that this approximation can be achieved without tension. Although there will be decreased excursion of the repaired hemidiaphragm after primary closure in this setting, there is seldom long-term impairment. When approximation of ragged borders is difficult, a modified Z-plasty is helpful. In theory, the incisions made in creating the Z-plasty should be radial, extending from the medial plane to the periphery to maximally preserve the branches of the phrenic nerve; however, the long-term result of successful approximation of a viable diaphragm is almost always excellent, even when branches of the phrenic nerve are severed.2,8 When repairing large defects, one should reapproximate the midportions of the defect so that the lateral portions can be accurately reapposed without creating a dog-ear type of irregular closure.
The grade V diaphragmatic injury with tissue loss exceeding 25 cm2 presents a major surgical challenge. Many of these large defects with major tissue loss, however, can be closed, primarily if the adjacent chest cage and abdominal wall are intact. When the central defect precludes a tension-free approximation, even in the relaxed state, the peripheral diaphragmatic attachments can be severed anteriorly, laterally, and posteriorly from their costal origins so that the defect can be closed and the diaphragmatic edges reattached two or three interspaces more cephalad.17 This permits generous advancement of healthy tissue and allows a tension-free primary closure. The long-term results are good.17
MORTALITY
The reported morbidity and mortality after diaphragmatic injury vary depending on the etiology, severity of injury, and the extent of injury to other organs.2,8 Most complications and deaths are caused by the associated injuries. Patients sustaining blunt diaphragmatic rupture often have associated injury to the liver, spleen, or more importantly, the underlying lung. The association of multiple rib fractures and pulmonary contusion with diaphragmatic rupture often causes severe pulmonary contusion resulting in respiratory compromise and prolonged ventilatory support; a fatal outcome is not unusual. In contrast, patients presenting with penetrating wounds to the diaphragm often have associated injuries to the lung and a number of intra-abdominal viscera. When these injuries involve major vessels, the mortality rate is extraordinarily high.
Asensio and colleagues2 collectively reviewed 33 published reports on diaphragmatic injury with an overall mortality rate that ranged from 4.3% to 41%, with the average morality rate being 13.7%. The total number of patients included in this review was 1799. The lower mortality rate was reported by Aronoff et al.,19 who treated patients with mostly penetrating diaphragmatic wounds; the higher mortality rate was reported by Boulanger and colleagues,12 who treated patients, primarily, with blunt rupture. The reason that the patients with blunt diaphragmatic rupture have a higher mortality rate than the patients with penetrating wounds is related to the magnitude of associated injury involving the rib cage, lungs, and brain.8,12
The observed mortality rate will also reflect different therapeutic approaches to treating patients with torso injuries after both penetrating wounds and blunt injury. When all stable patients with penetrating wounds to the abdomen undergo exploratory laparotomy despite the lack of evidence of hypotension or peritoneal irritation, many diaphragmatic perforations will be identified and closed.2,8,19 The vast majority of these patients would have done well without operation. During the period of time when this approach to penetrating abdominal wounds was followed, the authors observed a mortality rate of well under 4% in patients treated with penetrating diaphragmatic wounds.8 This same phenomenon was seen in patients with gunshot wounds who had stable vital signs, no evidence of blood loss, and no clinical evidence of hollow viscus perforation. Over the past generation, there have been major changes in the approach to penetrating wounds to the torso. Despite known penetration, patients who are stable and have no evidence of peritonitis are watched overnight and discharged home the next day. Many of these patients likely have perforated diaphragms that are not diagnosed, not treated, and not included in the statistical review of diaphragmatic injuries. In contrast, those patients who do undergo operation for penetrating wounds of the torso have evidence of hypotension and/or peritonitis; these patients have life-threatening injuries to other organs so the repair of a diaphragmatic perforation represents the minor part of their total treatment. Consequently, the mortality rate now exceeds 30% for penetrating diaphragmatic injuries; the deaths are related to hemorrhage from associated injuries, particularly for those patients sustaining thoracoabdominal injuries.
MORBIDITY
Like mortality, the observed morbidity after diaphragmatic injury results, primarily, from injuries to other organs. Pulmonary insufficiency resulting from atelectasis, pneumonia, and contusion is more likely in patients with multiple rib fractures. An intrapulmonary hematoma that becomes infected and develops into a lung abscess is more commonly seen after a gunshot wound. Occasionally, there will be a surgical disruption of a diaphragmatic repair after one has performed extensive mobilization to achieve primary closure for a grade IV or grade V injury. Breakdown of the diaphragmatic repair, however, is an unusual occurrence. Other complications that occur with diaphragmatic injury include phrenic nerve paralysis, supradiaphragmatic empyema, and subdiaphragmatic abscess. Rodriguez-Morales et al.5 reported a 65% incidence of atelectasis and a 5% incidence of empyema. Wiencek and colleagues32 reported a 31% complication rate primarily caused by atelectasis; most complications were not life threatening.
COMPLICATED DIAPHRAGMATIC REPAIR WITH THORACIC INJURY
The greatest technical challenge with the treatment of grade V diaphragmatic injuries occurs when there are associated injuries to the chest wall and/or abdominal wall.17,18 These massive injuries are usually caused by close-range shotgun blast or high-velocity rifle wounds. There are few descriptions of the technical challenges associated with these rare injuries, probably because the mortality rate is extraordinarily high. Successful care of these huge defects requires the combined reconstruction of the diaphragm and the torso wall; this may take place in multiple phases.
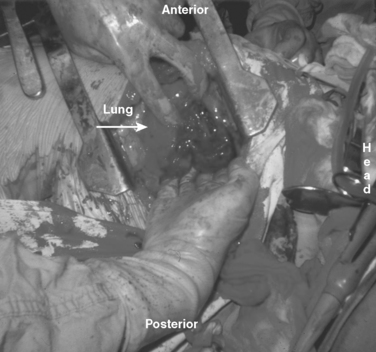
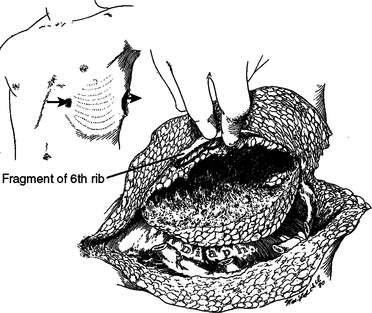
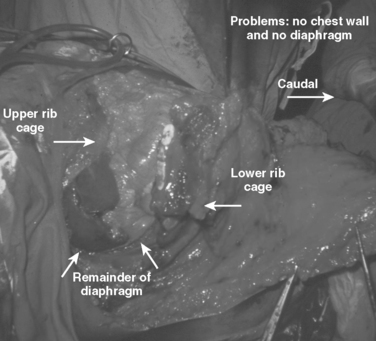
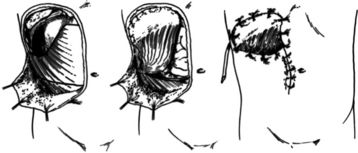
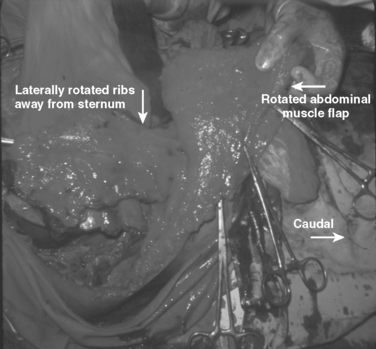
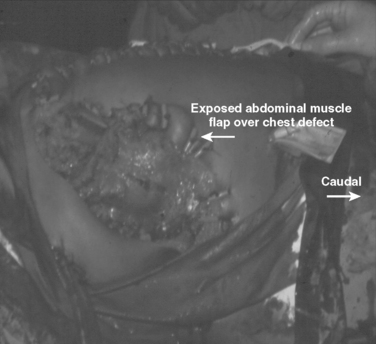
These patients typically present in shock because of associated injuries to major vessels, lung or intraperitoneal viscera, and especially the liver.17,18 During rapid resuscitation, the surgeon must remember that a complicated repair of the torso wall in conjunction with a large diaphragmatic rupture is facilitated by having separate airway control of both mainstem bronchi. Intubation with a double-lumen tube allows for the injured lung to be deflated while the abdominal or thoracic wall reconstruction is performed in conjunction with the diaphragmatic repair.17,18 Likewise, the double-lumen airway tube prevents blood from flowing, by gravity, from the injured side, across the carina into the dependent uninjured side; this results in respiratory insufficiency and is likely to be lethal. The primary intraoperative objective is to get rapid control of hemorrhage, which may require pulmonary lobectomy for a massively injured lung (Figure 12). This is followed by debridement of devitalized tissues of the chest wall and diaphragm (Figure 13). Often the resultant defect after massive injury precludes successful closure. When the wound occurs solely in the abdomen, the abdominal wall pack technique can be used.32 However, when the wound involves the thorax, it is impossible to pack the thorax open, so some type of imaginative reconstruction must take place. Sometimes, it is possible to relocate the diaphragm to a more superior position in the thorax (Figure 14). This is accomplished by detaching the anterior, lateral, and posterior attachments from their peripheral origins and reattaching them, superiorly, to an inner space that is above the destroyed chest wall (Figure 15). This allows for the thoracic cavity to be closed and the defect, which is now in the rib cage that covers only the abdominal cavity, can be treated by the abdominal wall packed technique.31 Continued dressing changes will allow for granulation tissues to develop, after which a split-thickness skin graft can be placed over the granulation tissues (Figure 16). Later construction of the defect can be accomplished by rotating fascial grafts after the patient has recovered from the underlying insult.
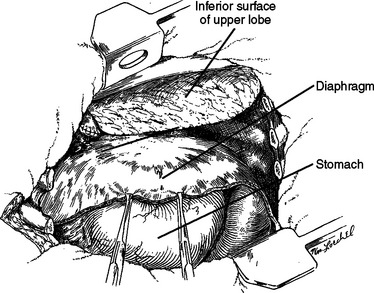
Figure 14 After the extensive debridement shown in Figure 13, the diaphragm was detached anteriorly, laterally, and posteriorly so that it could be reattached three inner spaces higher, thereby converting a chest wall defect into an abdominal defect.
The diaphragm is a large muscular organ with an excellent medially based blood supply, thereby facilitating easy detachment and resuturing. Being native tissue, there is less risk of infection. Even if the diaphragm has been partially destroyed by the blast, the redundancy should allow for relocation. This technique is ideally suited for lower thoracic full-thickness chest wall defects.17 The technique reported herein is not suited for superiorly located chest wall defects. More superiorly located full-thickness defects from shotgun blasts can be managed by upper lobectomy combined with wound coverage by a thoracoplasty or rotation of a latissimus dorsi muscle flap. An anteriorly located wound that dismembers the ipsilateral breast and underlying chest wall can be managed by relocation of the uninjured contralateral breast.
COMBINED CHEST WALL AND ABDOMINAL DEFECT WITH DIAPHRAGMATIC RUPTURE
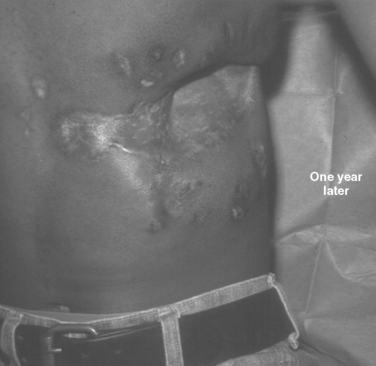
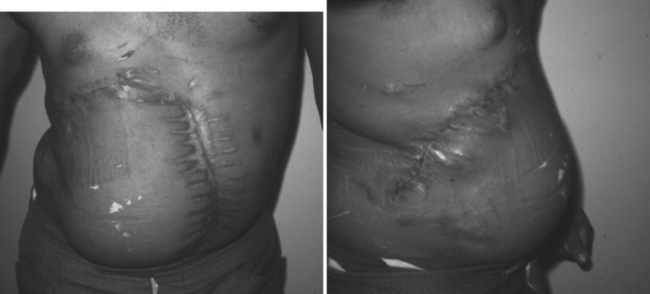
The challenge is greater when both the chest wall and abdominal wall are associated with diaphragmatic rupture. The technical challenges of successful treatment of such an injury are best illustrated by the care provided to a young man who presented minutes after sustaining a close-range shotgun blast of the right inferior and lateral chest wall plus the superior and lateral abdominal wall (Figure 17). During rapid resuscitation, a double-lumen endotracheal tube was inserted to prevent blood aspiration into the left lung. The presence of a large combined injury of the abdominal wall, chest wall, and hemidiaphragm should not influence the surgeon to minimize debridement of devitalized tissue in order to achieve primary closure. The likely result will be ischemia, infection, and an exposed lung; this result is fatal. When mature and proper debridement results in a huge defect of the chest and abdomen walls, unusual techniques must be used to achieve a tension-free closure. Different types of mesh may be tried, but are doomed to failure if there is associated colon spill. Successful closure of this combined defect may be achieved with native tissues by a number of rotation flaps of chest wall with rib fragments and abdominal wall musculature (Figures 18, 19, and 20). The primary concern is coverage; resultant hernias can be repaired later when the patient has recovered. The exposed tissues without skin coverage are treated with frequent dressing changes until later skin graft coverage (Figure 21). After removal of the skin graft and primary closure, the patient shown herein elected not to have his full-thickness abdominal wall defect repaired (Figure 22).
1 Williams PL, Bannister LH, editors. Gray’s Anatomy, 38th ed., Edinburgh, New York: Churchill Livingstone, 1993.
2 Asensio JA, Petrone P, Demetriades D. Injury to the diaphragm. In: Zietlow SP, Feliciano DV, editors. Trauma Surgery. Philadelphia: WB Saunders; 2000:613-634.
3 Michelson E. Eventration of the diaphragm. Surgery. 1961;49:410.
4 Morgagni GB: Seats and Causes of Diseases. Monograph on Hernia of the Diaphragm. London, Zellts 54, 1769.
5 Rodriguez-Morales G, Rodriguez A, Shatney CH. Acute rupture of the diaphragm in blunt trauma: analysis of 60 patients. J Trauma. 1986;26:438.
6 Murray JA, Demetriades D, Asensio JA, et al. Occult injuries to the diaphragm: prospective evaluation of laparoscopy in penetrating injuries to the lower left chest. J Am Coll Surg. 1998;187(6):626-630.
7 Payne JH, Yellin AE. Traumatic diaphragmatic hernia. Arch Surg. 1982;117:18.
8 Lucas CE, Ledgerwood AM. Diaphragmatic injury. In: Cameron JL, editor. Current Surgical Therapy. 6th ed. St. Louis, MO: Mosby; 1998:944-952.
9 Murray JA, Demetriades D, Cornwell EE, et al. Penetrating left thoracoabdominal trauma: the incidence and clinical presentation of diaphragm injuries. J Trauma. 1997;43(8):824.
10 Pagliarello G, Carter J. Traumatic injury to the diaphragm: timely diagnosis and treatment. J Trauma. 1992;33:194.
11 Voeller GR, Reisser JR, Fabian TC, et al. Blunt diaphragm injuries. Am Surg. 1990;56:28.
12 Boulanger BR, Milzman DP, Rosato C, et al. A comparison of right and left blunt traumatic diaphragmatic rupture. J Trauma. 1993;35:255.
13 Somers JM, Gleeson FV, Flower CD R. Rupture of the right hemidiaphragm following blunt trauma: the use of ultrasound in diagnosis. Clin Radiol. 1990;42:97.
14 Andrus CH, Morton JH. Rupture of the diaphragm after blunt trauma. Am J Surg. 1970;119:686.
15 Brown LG, Richardson DJ. Traumatic diaphragmatic hernia: a continuing challenge. Ann Thorac Surg. 1984;39:170.
16 Moore EE, Malangoni MA, Cogbill T, et al. Organ injury scaling. IV: thoracic, vascular, lung, cardiac and diaphragm. J Trauma. 1994;36:299.
17 Bender JS, Lucas CE. Management of close-range shotgun injuries to the chest by diaphragmatic transposition: case reports. J Trauma. 1990;30(12):1581-1584.
18 Carrasquilla C, Watts J, Ledgerwood AM, Lucas CE. Management of massive thoracocoabdominal wall defect from close-range shotgun blast. J Trauma. 1971;11(8):715-717.
19 Aronoff RJ, Reynolds J, Thal ER. Evaluation of diaphragmatic injuries. Am J Surg. 1982;144:671.
20 Beal SL, McKennan M. Blunt diaphragmatic rupture: a morbid injury. Arch Surg. 1988;123:828.
21 Chen JC, Wilson SE. Diaphragmatic injuries: recognition and management in sixty-two patients. Am Surg. 1991;57:810.
22 Ivatury RR, Simon RJ, Stahl WM. A critical evaluation of laparoscopy in penetrating abdominal trauma. J Trauma. 1993;34:822.
23 Salvino CK, Esposito TJ, Marshall WJ, et al. The role of diagnostic laparoscopy in the management of trauma patients: a preliminary assessment. J Trauma. 1993;34:506.
24 Ramirez JS, Moreno AJ, Otero C, et al. Detection of diaphragmatic disruptions by peritoneoscintigraphy using technetium-99M diethylenetriamine pentaacetic acid. J Trauma. 1988;28(6):818-822.
25 Merlotti G, Marcel G, Sheaff EM, et al. Use of peritoneal lavage to evaluate abdominal penetration. J Trauma. 1985;25:228.
26 Ochsner MG, Rozycki GS, Lucente V, et al. Prospective evaluation of thoracoscopy for diagnosing diaphragmatic injury in thoracoabdominal trauma: a preliminary report. J Trauma. 1993;34:704.
27 Boulanger BR, Mirvis SE, Rodriguez A. Magnetic resonance imaging in traumatic diaphragmatic rupture: case reports. J Trauma. 1992;32:89.
28 Feliciano DV, Cruse PA, Matox KL, et al. Delayed diagnosis of injuries to the diaphragm after penetrating wounds. J Trauma. 1988;28:1135.
29 de Rooiwj PD, Haarman HT M. Herniation of the stomach into the pericardial sac combined with cardiac luxation caused by blunt trauma: a case report. J Trauma. 1993;34:453.
30 Jones RJ, Thal ER, Johnson NA, et al. Evaluation of antibiotic therapy following penetrating abdominal trauma. Surgery. 1985;201:576.
31 Lucas CE, Ledgerwood AM. Autologous closure of giant abdominal wall defects. Am. Surg. 1998;64(7):607-610.
32 Wiencek RG, Wilson RF, Steiger Z. Acute injuries of the diaphragm: analysis of 165 cases. J Thorac Cardiovasc Surg. 1986;92:989.