Diagnostic Techniques in Ocular Surface Disease
Slit Lamp Examination
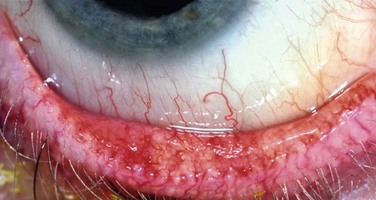
The slit lamp examination is a crucial part of the process when evaluating any ophthalmologic patient, and is no different for the individual with ocular surface disease. Careful, systematic examination from the outside to the inside of the eye should be performed each and every time. Care should be taken to specifically evaluate the condition of the meibomian glands (Fig. 7.1) and the entire conjunctival surface, including the palpebral areas, looking for inflammation and scarring. Once this is all performed, without anesthesia and stains, the examination can proceed to the next steps, including Schirmer testing and then ocular surface staining.
Schirmer Testing
The Schirmer test is a simple test that was first described in 19031 and it is still commonly performed in the office to assess aqueous tear production. There are three variations of this test, but the most popular is the Schirmer I test which measures both basal and reflex tear production. In this test, a strip of filter paper is placed on the lower eyelid margin without anesthesia, after 5 minutes, the strip is removed, and the amount of wetting is measured in millimeters (Fig. 7.2). Although this test is used frequently in the office, it has been found to lack accuracy and reproducibility: the same person’s test results taken at the same time each day for several days can fluctuate widely, and the mean Schirmer I test results for normal individuals have been reported to range from 8.1 mm to 33.1 mm.2 As such, many ophthalmologists do not even use this test anymore, but for those who do, in general, any value below 10 mm is considered abnormal. Many other ophthalmologists consider this test as a reasonable diagnostic tool only for severe dry eyes, where there is moderate reproducibility,3 with many practitioners only considering values of less than 5 mm to be significant.
Ocular Surface Staining
Fluorescein sodium is one the most frequently used methods for evaluating corneal staining, and it has been used since the end of the nineteenth century. Fluorescein penetrates poorly into the lipid layer of the corneal epithelium, and therefore, it does not stain normal cornea. Instead, the surface is stained whenever there is disruption of the cell-to-cell junctions.4 Although fluorescein is a very effective stain for the diseased cornea, it is more difficult to detect fluorescein staining of the conjunctiva because of the poor scleral contrast. However, this staining can be more readily viewed if a yellow (blue-free) filter is used.
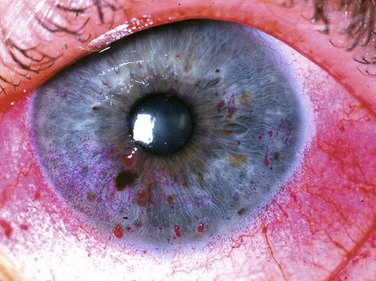
Rose bengal stain has also been used for a very long time: in this case for nearly a century. It is a derivative of fluorescein and is used to detect damage on the ocular surface, especially on the conjunctiva (Fig. 7.3). Although originally thought to stain dead or devitalized cells, rose bengal is currently believed to stain any part of the ocular surface that is not adequately protected by the tear film,4,5 specifically, in areas lacking membrane-associated mucins.6 Though an excellent diagnostic tool, rose bengal has been shown to be toxic to epithelial cells, and patients often complain about the burning and stinging upon instillation.
Lissamine green is a synthetic organic acid dye that stains in a similar fashion to rose bengal, but without causing stinging and without affecting the viability of the cells. For this reason, it has gained popularity in its use. However, staining with lissamine green is dose-dependent and an inadequate volume results in weak staining that can be overlooked. A minimal dosage of 10–20 µL is recommended for accurate diagnostic ability (Fig. 7.4).
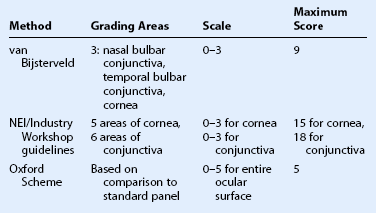
There are three commonly used methods to grade ocular surface staining: the van Bijsterveld system,7 the NEI/Industry Workshop guidelines,8 and the Oxford Scheme.9 At the present time, there is no evidence that any one method is superior to another for grading the ocular surface staining patterns (Table 7.1).
Tear Break-up Time
The tear break-up time (TBUT) is defined as the time interval between a complete blink and the first appearance of a dry spot in the tear film after fluorescein administration.10,11 It is believed that this represents an unstable tear film, whereby the mucous layer may rupture, allowing the aqueous to come in contact with exposed epithelium,12 but the exact mechanism is poorly understood. Like the Schirmer test, the TBUT test has been criticized as being unreliable and not reproducible. Many factors may lead to its non-reproducibility, including the volume of fluorescein administered, as well as the presence of preservatives, such as benzalkonium chloride, which may shorten TBUT. Despite this unreliability, it is generally agreed that a TBUT of less than 10 seconds suggests tear film instability, and less than 5 seconds suggests definite dry eye.13
Patient Questionnaire
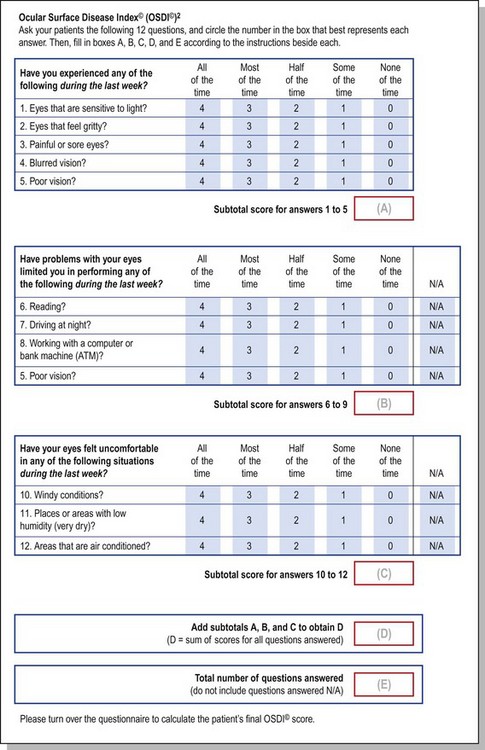
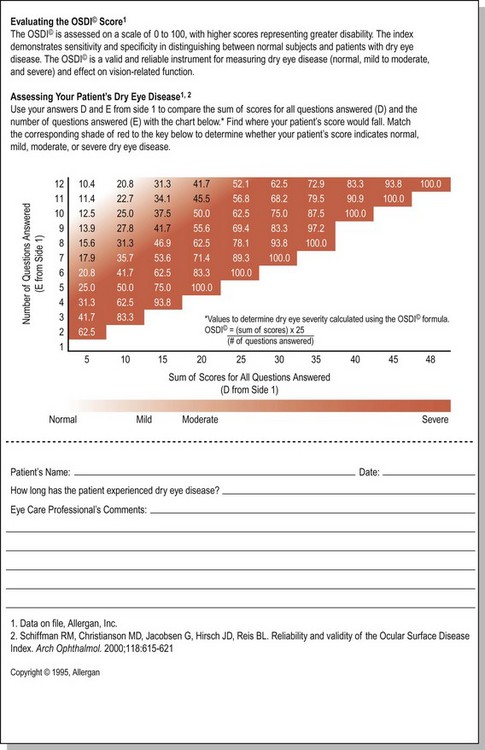
The Ocular Surface Disease Index (OSDI) is a questionnaire that has been validated to discriminate between normal, mild to moderate, and severe dry eye disease as defined by the physician’s assessment and a composite disease severity score (Fig. 7.5). The OSDI has also been correlated significantly with the McMonnies Dry Eye Questionnaire, the National Eye Institute Visual Functioning Questionnaire, the physical component summary score of the Short Form-12 Health Status Questionnaire, patient perception of symptoms, and artificial tear usage.14 It has been demonstrated to have the necessary psychometric properties to be used as an end point in clinical trials, and as such, it could be an important tool for in-office support for the diagnosis of ocular surface disease that is easy to administer.15
Impression Cytology
Impression cytology is a powerful tool for the diagnosis of ocular surface disease. This minimally invasive procedure involves applying nitrocellulose filter paper to the area of interest on the ocular surface to remove the superficial 2–3 layers of cells.16 As first described by Egbert and colleagues,17 the cells are air dried and stained with periodic acid – Schiff and hematoxylin. This test has been modified several times, and now these cells can then be subject to histological, immunohistochemical, and molecular testing to help diagnose the ocular surface disease. Electron microscopy of the cells can even be done.
Confocal Microscopy
In vivo confocal microscopy has evolved into a popular method of imaging the anterior segment at a cellular level because the images obtained are comparable to ex vivo histochemical methods.18 Not only has this technology been used to evaluate corneal nerves and to aid in the diagnosis of corneal infections, such as Acanthamoeba keratitis, but it has also garnered interest for its use in the conjunctiva and the eyelids: studies have demonstrated that confocal microscopy can aid in the diagnosis of dry eye or superior limbic keratoconjunctivitis by its ability to evaluate the conjunctival epithelium for squamous metaplasia.19 Further, confocal scanning laser microscopy has been found to be an efficient and noninvasive tool for the quantitative assessment of conjunctival inflammation, as well as epithelial cell densities and conjunctival morphologic alterations, such as microcysts in patients with Sjögren’s and non-Sjögren’s syndrome dry eye disease.20 The ability to assess for conjunctival inflammation also allows for this technology to help diagnose atopic keratoconjunctivitis. Further, confocal microscopy has also been shown to have high potential in the diagnosis of meibomian gland dysfunction.21 Although in vivo confocal microscopy technology is still evolving, it has already been demonstrated to have significant value in aiding in the evaluation of patients with ocular surface disease. At the present time, this technology may not be available in a widespread fashion, but its further development in the future will hopefully result in decreasing costs so that it will be available to more practitioners.
Tear Film Interferometry
Additional aqueous tear deficiency assessment includes measuring the thickness of the precorneal tear film. Tear film interferometry can achieve this by using wavelength-dependent fringes: the optical path difference from the reflection at the surface of the tear film and at the interface of the tear film and the cornea results in an interference wave, which is calculated to be the precorneal tear film thickness. Normal precorneal tear thicknesses vary by study from 2.7 to 11.0 µm, but studies that have compared the thicknesses of individuals with dry eyes versus controls demonstrate that the controls have a much thicker tear film.22–25 Furthermore, this technology can also be used to evaluate specifically for the thickness of the lipid layer of the tear film.26 Along with a careful evaluation of the meibomian gland status, this technique helps to assess for the mechanism for ocular surface dryness.
The technology of interferometry has also been applied in a kinetic fashion: evaluating the spread of lipids through the tear film with blinking. It has been found that in dry eye disease, due to lipid deficiency from meibomian gland dysfunction, lipids are seen to spread slowly with vertical streaking patterns compared to normal subjects without dry eye who have rapid spreading of the lipids in a horizontal pattern.27 This technology has significant promise as a powerful diagnostic technique, especially in assessing for changes after institution of therapy, but at the present time it is also not widely available, and it still needs to undergo validation.
Tear Meniscus Measurement
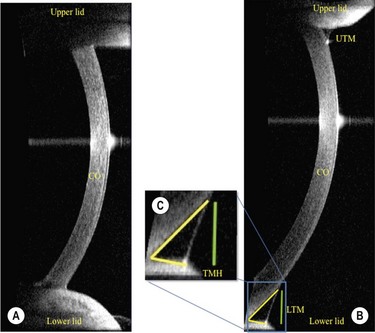
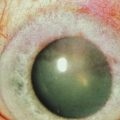
In addition to precorneal tear film thickness, the measurement of tear meniscus dimensions has also been shown to be of great value in the assessment of the patient with ocular surface disease.28 In the past, the meniscus variables, such as height, width, cross-sectional area, and meniscus curvature, have all been reported to be useful in the diagnosis of dry eye, but with limitations in the measurement techniques, due to their invasive nature causing stimulation of the reflex tearing. Recently, however, the Visante Anterior Segment Optical Coherence Tomography (OCT; Carl Zeiss Meditec, International, Dublin, CA, USA), has been shown to produce accurate measurements of tear height in a noninvasion fashion with acceptable sensitivity, specificity, and reproducibility when compared with slit lamp tear meniscus height measurements, tear function vital staining scores, and Schirmer testing29 (Fig. 7.6). The Spectral OCT (also known as Fourier domain, high-speed, or three-dimensional OCT) has also been shown to be well correlated with Schirmer testing, TBUT, and subjective symptoms. The advantage of the Spectral OCT is that it has improved sensitivity and a short acquisition time, which improves the quality of the two-dimensional images and thereby enables accurate three-dimensional modeling. The high acquisition speed also allows for the evaluation of tear meniscus changes in real time.30 As with the other previously described technologies, OCT imaging of the tear meniscus has proven to be a powerful diagnostic tool, but at the present time it is not widely available to all practitioners.
Esthesiometry
Because corneal epithelial disturbances are frequently due to decreased corneal sensation, esthesiometry is an important adjunctive technique for diagnosing ocular surface disease. The classic technique for performing esthesiometry is with the Cochet – Bonnet esthesiometer,31 which consists of a fine nylon filament, the length of which can be adjusted to apply different intensities of stimuli. While seemingly an objective measurement, this test is fraught with limitations including difficulties with alignment, placement, and replication of the force applied with the nylon filament. In addition, use of this test causes disruption of the epithelial surface by the filament. Rather than use this instrument, some practitioners simply use a cotton swab with the cotton pulled into a wisp, and then used with a subjective semiquantitative grading scale. Recently, a non-contact air jet esthesiometer has been introduced and tested.32 This instrument, the CRCERT – Belmonte esthesiometer, allows for better stimulus reproducibility and better control over stimulus characteristics. Since this technique relies on having the patient in their baseline sensitivity state, this technique must be employed prior to anesthetic instillation in the eye.
Osmolarity
The Dry Eye Workshop Report introduced the concept that an increase in tear osmolarity is a hallmark of dry eye disease, and it is now thought to be the central mechanism in the cause of ocular surface damage in dry eyes.33 From this report, tear osmolarity has been reported to be the single best objective marker for dry eye disease. Unfortunately, at that time, measurements were limited to laboratory instruments which required very large volumes of tears. Recently, with the introduction of the TearLab Test (TearLab Corp, San Diego, CA, USA), the clinician can easily collect and measure osmolarity in a 50 µL tear sample with minimal disturbance to the tear film.34 This microfluidic technology produces a reading within seconds before evaporation can influence the concentration of solutes in the tear sample.
In a prospective, observational case series to determine the clinical usefulness of tear osmolarity compared to commonly used objective tests to diagnose dry eye, tear osmolarity was found to be the best single metric to diagnose and classify dry eye disease. In this study, it was found that a cutoff of more than 308 mOsms/L achieved a 90.7% rate of a correct diagnosis of severe dry eye patients, and had a true negative rate of 81.3%.35
Rapid Testing For Inflammatory Markers
Matrix metalloproteinase 9 (MMP-9) plays a critical role in wound healing and inflammation, and is primarily responsible for the pathologic alterations to the ocular surface in various conditions.36 MMP-9 has been demonstrated to be significantly elevated in the tears of patients with blepharitis, allergic eye disease, dry eye disease, and conjunctivochalasis.37,38 The ability to test for MMP-9 in the tear film may prove to be an important tool to help in the diagnosis of ocular surface disease. Recently, a commercially available point-of-care test, RPS InflammaDry Detector (RPS, Inc, Sarasota, FL, USA) offers an easy-to-administer and rapid turn-around test (10 minutes) for measuring MMP-9 levels in the tear film.
Ocular Surface Scraping
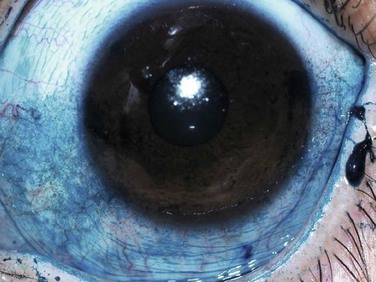
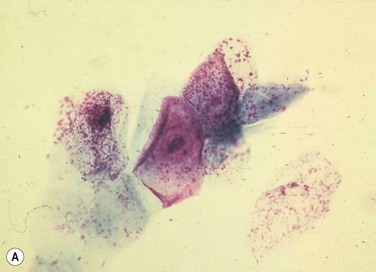
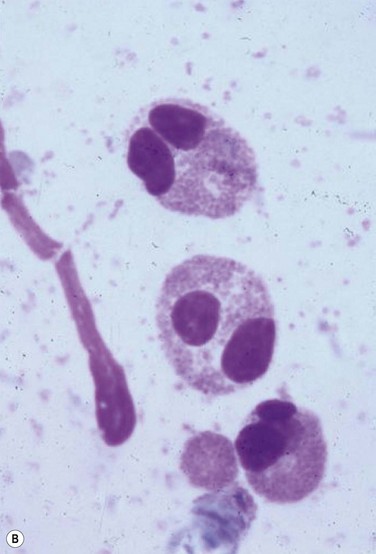
Despite the plethora of novel ideas and tests for evaluating ocular surface disease, sometimes it is necessary to revert to the tried and true method of taking a sample and evaluating it under light microscopy. Conjunctival scrapings (or swabbings) can be performed to obtain a specimen for cytologic examination (Fig. 7.7). The specimens that are collected may reveal cells or microorganisms under light microscopy that may be helpful in the diagnostic process. Although the use of these techniques requires either the ophthalmologist or a microbiologist to evaluate the specimen, it is a technique that can be used widely compared to the expensive new technology that has been described above.
References
1. Schirmer, O. Studien zur physiologie und pathologie der tranenabsonderung und tranenabfuhr. Graefes Arch Clin Exp Ophthalmol. 1903;56:197–291.
2. Savini, G, Prabhawasat, P, Kojima, T, et al. The challenge of dry eye diagnosis. Clin Ophthalmol. 2008;2:31–55.
3. Tsubota, K, Xu, KP, Fujihara, T, et al. Decreased reflex tearing is associated with lymphocytic infiltration in lacrimal glands. J Rheumatol. 1996;23:313–329.
4. Feenstra, RP, Tseng, SC. Comparison of fluorescein and rose bengal staining. Ophthalmology. 1992;99:605–617.
5. Feenstra, RP, Tseng, SC. What is actually stained by rose bengal? Arch Ophthalmol. 1992;110:984–993.
6. Argüeso, P, Tisdale, A, Spurr-Micharud, S, et al. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest Ophthalmol Vis Sci. 2006;47:113–119.
7. van Bijsterveld, OP. Diagnostic tests in the sicca syndrome. Arch Ophthalmol. 1969;82:10–14.
8. Lemp, MA. Report of the National Eye Institute/Industry Workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–232.
9. Bron, AJ, Evans, VE, Smith, JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–650.
10. Norn, MS. Desiccation of the precorneal tear film. I. Corneal wetting-time. Acta Ophthalmol (Copenh). 1969;47:865–880.
11. Lemp, MA. Breakup of the tear film. Int Ophthalmol Clin. 1973;13:97–102.
12. Sharma, A, Ruckenstein, E. Mechanism of tear film rupture and its implications for contact lens tolerance. Am J Optom Physiol Opt. 1985;62:246–253.
13. Shimazaki, J. Definition and criteria of dry eye. Ganka. 1995;37:765–770.
14. Schiffman, RM, Chirstianson, MD, Jacobsen, G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621.
15. Ozcura, F, Aydin, S, Helvaci, MR. Ocular surface disease index for the diagnosis of dry eye syndrome. Ocular Immunol Inflamm. 2007;15:389–393.
16. Singh, R, Joseph, A, Umapathy, T, et al. Impression cytology of the ocular surface. Br J Ophthalmol. 2005;89:1655–1659.
17. Egbert, PR, Lauber, S, Maurice, DM. A simple conjunctival biopsy. Am J Ophthalmol. 1977;84:798–801.
18. Crazat, A, Pavan-Langston, D, Hamrah, P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–177.
19. Kojima, T, Matsumoto, Y, Ibrahim, OMA, et al. In vivo evaluation of superior limbic keratoconjunctivitis using laser scanning confocal microscopy and conjunctival impression cytology. Invest Ophthalmol Vis Sci. 2010;51:3986–3992.
20. Wakamatsu, TH, Sato, EA, Matsumoto, Y, et al. Conjunctival in vivo confocal scanning laser microscopy in patients with Sjögren syndrome. Invest Ophthalmol Vis Sci. 2010;51:144–150.
21. Ibrahim, OMA, Matsumoto, Y, Dogru, M, et al. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117:665–672.
22. Danjo, Y, Nakamura, M, Hamano, T. Measurement of the precorneal tear film thickness with a non-contact optical interferometry film thickness measurement system. Jpn J Ophthalmol. 1994;38:260–266.
23. King-Smith, PE, Fink, BA, Fogt, N, et al. The thickness of the human precorneal tear film: evidence from reflection spectra. Invest Ophthalmol Vis Sci. 2001;41:3348–3359.
24. King-Smith, PE, Fink, BA, Hill, RM, et al. The thickness of the tear film. Curr Eye Res. 2004;29:357–368.
25. Hosaka, E, Kawamorita, T, Ogasawara, Y, et al. Interferometry in the evaluation of precorneal tear film thickness in dry eye. Am J Ophthalmol. 2011;151:18–23.
26. Goto, E, Dogru, M, Kojima, T, et al. Computer-synthesis of an interference color chart of human tear lipid layer by a colorimetric approach. Invest Ophthalmol Vis Sci. 2003;44:4693–4697.
27. Goto, E, Tseng, SC. Kinetic analysis of tear interference images in aqueous tear deficiency dry eye before and after punctual occlusion. Invest Ophthalmol Vis Sci. 2003;44:1897–1905.
28. Mainstone, JC, Bruce, AS, Golding, TR. Tear meniscus measurement in the diagnosis of dry eye. Curr Eye Res. 1996;15:653–661.
29. Ibrahim, OMA, Dogru, M, Takano, Y, et al. Application of Visante Optical Coherence Tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology. 2010;117:1923–1929.
30. Czajkowski, G, Kaluzny, BJ, Laudencka, A, et al. Tear meniscus measurement by spectral optical coherence tomography. Optom Vis Sci. 2012;89:1–7.
31. Cochet, P, Bonnet, R. L’Esthesie corneenne. Sa mesure clinique. Ses variations physiologiques et pathologieques. La Clinique Ophtalomologique. 1960;4:3–27.
32. Golebiowshi, B, Papas, E, Stapleton, F. Assessing the sensory function of the ocular surface: implications of use of a non-contact air jet aesthesiometer versus the Cochet–Bonnet aesthesiometer. Exp Eye Res. 2011;92:408–413.
33. International Dry Eye Workshop. The definition and classification of dry eye disease. In: 2007 Report of the International Dry Eye Workshop (DEWS). Ocul Surf. 2007;5:75–92.
34. Sullivan, BD, Whitmer, D, Nichols, KK. An objective approach to severity in dry eye disease. Invest Ophthalmol Vis Sci. 2010;51:6125–6130.
35. Lemp, MA, Bron, AJ, Baudouin, C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151:792–798.
36. Sambursky, R, O’Brien, TP. MMP-9 and the perioperative management of LASIK surgery. Curr Opin Ophthalmol. 2011;22:294–303.
37. Acera, A, Rocha, G, Vecino, E, et al. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res. 2008;40:315–321.
38. Chotikavanich, S, de Paiva, CS, Li de, Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:3203–3209.