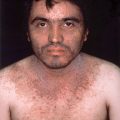
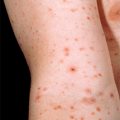
Chapter 3 Diagnostic techniques
Head E: Laboratory diagnosis of the superficial fungal infections, Dermatol Clin 2:93–108, 1984.
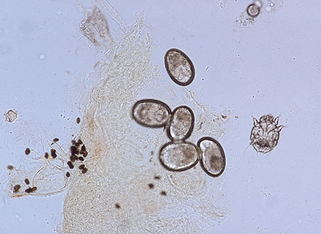
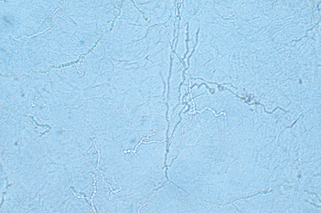
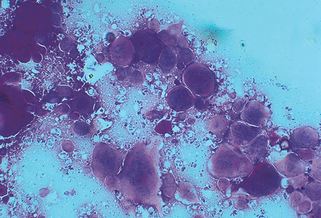
Figure 3-6. A positive scraping for scabies showing an immature mite, eggs, and numerous fecal pellets.
(Courtesy of James E. Fitzpatrick, MD.)
Hay RJ: Scabies and pyodermas—diagnosis and treatment, Dermatol Ther 22:466–464, 2009.
Holder NA: Gonococcal infections, Pediatr Rev 29:228–234, 2008.
Goossens A: Recognizing and testing allergens, Dermatol Clin 27:219–226, 2009.
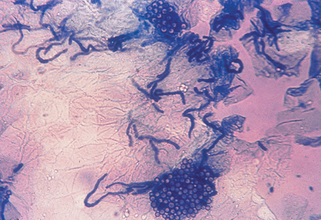
Key Points: Diagnosis of Fungal Infections
Key Points: Biopsy Techniques
Zillikens D: Diagnosis of autoimmune bullous skin disease, Clin Lab 54:491–503, 2008.