Chapter 38 Diagnostic Pancreatography
Indications
Despite the reduction in use of ERP and ERCP, indications have remained essentially unchanged over the past decade. As for all endoscopic procedures, ERP is indicated only when the potential findings would alter the management of the patient’s condition in a meaningful way.1,2 Appropriate indications have been published by national societies and interest groups2 and revised for application in individual practice settings. Our standard indications for ERCP include investigation of symptoms strongly suspected to relate to the pancreas on the basis of associated abnormal laboratory or imaging tests, investigation of specific abnormalities on prior imaging or laboratory testing, and investigation of known pancreatic abnormalities for which intervention is needed or anticipated (Box 38.1). Diagnostic ERCP alone is not indicated to confirm findings clearly shown on other testing if therapy would not be changed by its performance. ERCP alone also is not indicated for investigation of isolated abdominal pain when other tests are normal. A National Institutes of Health consensus conference addressing the clinical applications of ERCP emphasized that it is indicated for investigation of isolated abdominal pain only when equipment and skills are available for concurrent therapy and for manometric investigation for potential sphincter of Oddi dysfunction.3
Preparation
Pancreatography may be performed alone or in concert with diagnostic or therapeutic endoscopic cholangiography (ERCP). Patient preparation, sedation, and positioning for pancreatography are the same as for endoscopic cholangiography. Recommendations for fasting intervals before sedation and intubation vary among centers.4 Most patients are asked to fast overnight; however, patients scheduled for afternoon procedures generally can be allowed a small clear liquid breakfast early in the day. Administration of antibiotics before the procedure is indicated in any patient with known or suspected parenchymal necrosis, duct obstruction, duct leak, or potential filling of poorly drained fluid collections or spaces such as peripancreatic fluid collections and pseudocysts.
Sedation for ERCP is usually accomplished by titrated parenteral administration of a narcotic and a benzodiazepine. Fentanyl is the narcotic of choice for most gastrointestinal (GI) endoscopy; however, compared with meperidine (Demerol), it has a shorter half-life and a greater stimulatory effect on the sphincter of Oddi.5,6 Fentanyl is less advantageous for performance of ERCP, and meperidine tends to be the narcotic of choice. Midazolam (Versed) is the usual benzodiazepine for all GI endoscopy, including performance of ERCP. Midazolam has been shown to reduce sphincter of Oddi pressures7,8 and is therefore not recommended during sphincter of Oddi manometry. Diazepam (Valium) does not influence the normotensive sphincter of Oddi, but little is known regarding its effects on the hypertensive sphincter. It is the preferred agent during manometric procedures.9 Propofol has become a popular agent for use during endoscopy by virtue of its extremely rapid induction and reversal and the deep sedation it provides. For ERCP, propofol is typically administered by an anesthesia specialist. Propofol does not seem to influence sphincter pressures at commonly used doses and can be used for all aspects of ERCP practice.10
Technique
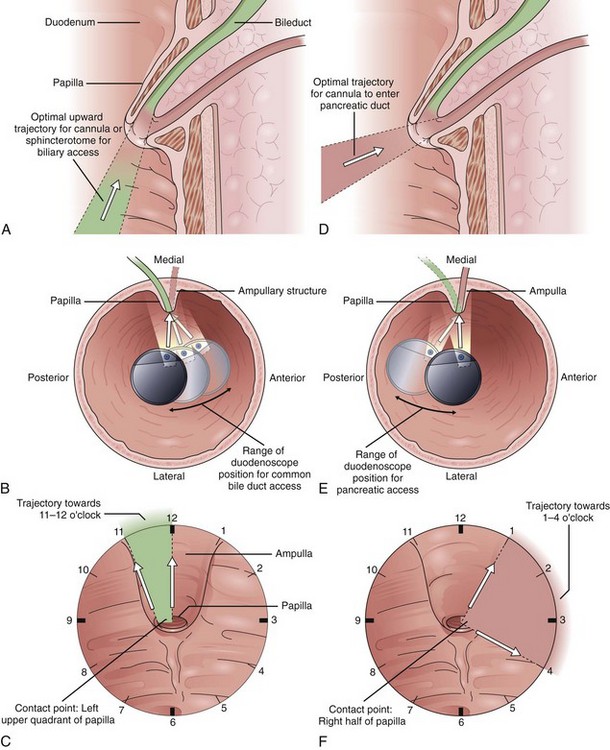
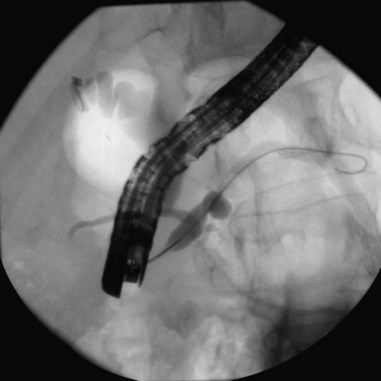
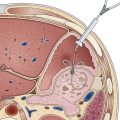
Endoscope positioning within the duodenum is crucial for efficient performance of cholangiography or pancreatography. For cholangiography, the optimal lens position is below the papilla looking upward or backward toward the proximal second portion of the duodenum. The cannula follows an upward and slightly posterior trajectory to access the bile duct located in the 10 o’clock to 12 o’clock position relative to the papillary os. In contrast, for pancreatography via the major papilla, the optimal lens position is relatively en face to the papilla with a slightly anterior directed view. The cannula is directed in a slightly forward and upward trajectory to access the pancreatic duct, which lies roughly between 1 o’clock and 3 o’clock relative to the papillary os (Fig. 38.1) (see Videos![]() ).11
).11
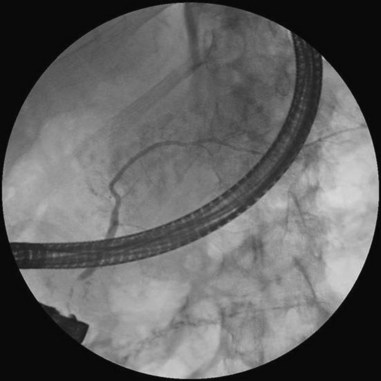
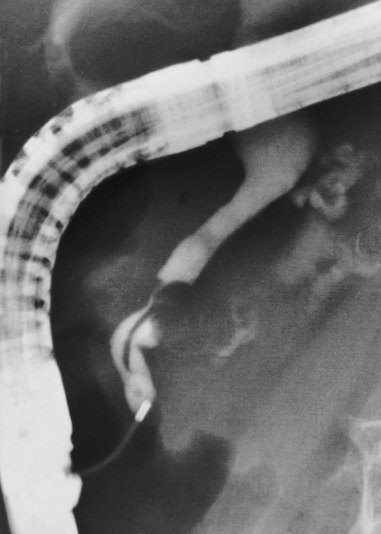
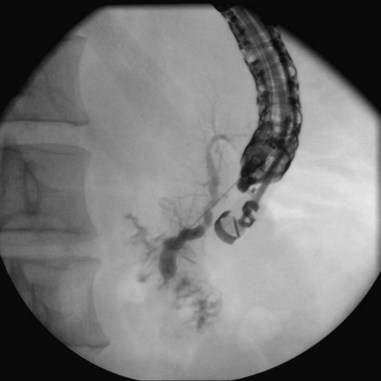
Cannulation of the major papilla is usually performed with the same equipment used in the biliary tree. Following apparent cannulation, fluoroscopically guided contrast agent injection should be more judicious and gradual than in the biliary tree. The normal pancreatic duct has a much smaller volume than the bile duct, and overfilling can occur after relatively small volumes of contrast agent have been injected; this may be recognized as acinarization, or filling of acini in a fluffy, more dense pattern throughout the distribution of the ducts (Fig. 38.2). Cannulation of normal side branches or of anomalous ductal systems can lead to overfilling of small segments almost immediately after injection is begun. Similarly, wire-guided cannulation must be more circumspect than in the bile duct because wire-induced perforation of a side branch can occur early, before contrast agent injection. When the appropriate position is confirmed within the desired system, more complete filling can proceed. Full-strength contrast agent is usually employed for pancreatography to optimize visualization of narrow ducts and fine detail.
In the setting of pancreatic fluid collections, for which formal drainage or surgery is not planned, caution should be employed to avoid excessive contamination of cysts that may not drain spontaneously. When difficult anatomy, pathology, or pancreas divisum prevents performance of pancreatography from the major papilla, it can be accomplished via the minor papilla approximately 90% of the time.12 The minor papilla is usually located 2 to 3 cm proximal and slightly anterior to the major papilla. It is usually less apparent than the major papilla and may not have an obvious opening. If identification of either the papilla or the os is difficult, secretin can be administered (0.2 mcg/kg body weight by intravenous injection over one minute) to stimulate the flow of pancreatic juice into the duodenum, which occurs within minutes of administration.13 Methylene blue can be sprayed on the duodenal wall to facilitate visualization of the focal source of drainage of clear pancreatic juice.14 Once identified, the minor papilla can be approached with a nearly en face view using either a semi–long scope position or an extremely short scope position with slightly posterior orientation. Cannulation usually follows a slightly posterior and horizontal to mildly cephalad path.
Complications of Pancreatography
The complications of diagnostic pancreatography are similar to the complications seen with therapeutic pancreatography and ERCP in general; a major exception is the rarity of perforation and bleeding. Complications related to sedation and intubation are similar. Pancreatitis is the predominant concern. The performance of pancreatography during ERCP is one of the most consistently positive risk factors identified in studies of procedural pancreatitis.15 Some studies note an even higher incidence with overfilling to the point of acinarization. Investigation of patients with past episodes of pancreatitis, particularly procedure-related pancreatitis, and investigation of patients with suspected sphincter of Oddi dysfunction are also risk factors for post-ERCP pancreatitis.
Numerous studies have investigated medications and interventions intended to reduce the incidence of post-ERCP pancreatitis. The details are beyond the scope of this chapter; however, on rigorous study, most interventions have not proven useful.15 Temporary prophylactic placement of a small-caliber pancreatic stent has been shown to reduce ERCP-related pancreatitis, or the severity of pancreatitis, in various patients,16 including patients in whom needle-knife sphincterotomy is used for access to the bile duct,15 patients in whom sphincterotomy is performed for treatment of sphincter of Oddi dysfunction,17 patients in whom sphincter dilation is performed for biliary stone removal,18 and patients with other high-risk indicators including “difficult cannulation.”19
Infection is a risk of pancreatography in predictable subgroups of patients, particularly patients with necrosis, duct leaks, and communicating cystic spaces such as pseudocysts. Although minimal data exist to guide the use of antibiotics in these settings, risk-to-benefit considerations suggest potential benefit of prophylactic antibiotics in the peri-ERCP period. In the past, pancreatic pseudocysts were considered a relative contraindication to ERCP, unless surgical drainage was planned within 24 hours. Experience has not supported this concern, however.20 Nonetheless, prudent practice might employ antibiotic prophylaxis and limited filling of chambers that would not effectively drain.
Normal Pancreatic Ductal Anatomy
Terminology for the linear extremes of the pancreatic duct is sometimes confusing because proximal and distal sometimes correlate with direction of flow (distal bile ducts are at the major papilla), but in the pancreas, proximal and distal usually refer to relative distance from a point of reference at the duodenal wall (proximal pancreatic duct relative to or near the major papilla). In line with this classic use, surgical terminology uses distal pancreatectomy to refer to resections beginning with the tail. To avoid confusion, especially as may occur among three specialties providing procedural, radiographic, and surgical interpretations, it is clarifying to speak of upstream segments or locations as being toward the tail relative to another location and downstream segments or locations as being toward the duodenum.21
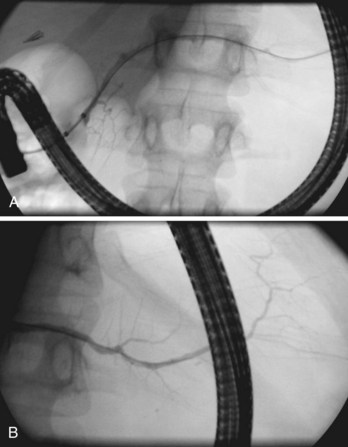
Globally, in the anteroposterior projection, the pancreatogram extends in an oblique fashion from the tail, located left of the spine at the T12 level, to the major papilla located right of the spine at the L2 level (Fig. 38.3). There is significant variation, however, in both the overall extent and the course of individual portions of the main duct. Within the head, the duct runs cephalad about 15 degrees from parallel to the spine; in the body, it runs horizontally or perpendicular to the spine; and in the tail, it usually rises further at a gentle angle but may even descend.22 The tail segment tends to be the most variable in course and shape. Occasionally, it is bifid, or split, left of the spine. In the anteroposterior projection, the retroperitoneal main pancreatic duct appears two-dimensional. However, oblique or lateral views show that it begins posteriorly in the tail, extends anteriorly around the spine, then extends back to a relatively posterior position at the genu and at the junction with the second portion of the duodenum. This excursion is highly variable and not useful for interpretation of displacement by adjacent space-occupying lesions.23
The main pancreatic duct is smooth with minor undulations and a general decline in caliber from the head to the tail. Focal nonpathologic indentations are sometimes noted at the genu, near the approximate junction with the accessory duct, and in the body, where it is in close proximity to the superior mesenteric vasculature.24 The former is well described but uncommon, and the latter is infrequently reported. Numerous studies have reported the length and caliber of the pancreatic duct as obtained from postmortem and endoscopic studies.25 Postmortem values tend to be slightly higher. With age, the duct caliber appears to increase slightly, while the length is stable. The length in normal glands averages about 16 to 17 cm, but it may range from 9 to 24 cm. When the main duct is less than 9 cm in length, obstruction should be suspected. The caliber of the pancreatic duct is most variable within the head of the gland, where the normal diameter is 3 to 4 mm but may range up to 6 mm, after correction for radiographic magnification. Accepted corrected diameters for the body and tail are 2 to 3 mm (up to 5 mm) and 1 to 2 mm (up to 3 mm).26
Visualization of side branches during pancreatography depends largely on technique and adequacy of contrast agent injection. Their visualization may be immaterial to the clinical question being addressed, or visualization may be crucial and of primary importance for resolution of clinical issues. Depending on the indication, lack of side-branch filling may not be indicative of a suboptimal or inadequate study. Side branches are highly variable and asymmetric in the pancreatic head but quite regular and symmetric, with alternating junctions along the main duct throughout the body and tail. A postmortem study reported a mean of 56 first-order branch ducts (range 52 to 66).27 Far fewer branch ducts are usually seen during even forceful pancreatography. A single large, inferiorly directed “uncinate” branch is seen in 55% to 62% of pancreatograms.24,28 The accessory pancreatic duct is shown in only 14% to 62% of endoscopic pancreatograms, even though postmortem studies can show its presence in 100% of autopsy specimens.25 It communicates with the main pancreatic duct in approximately 90% of specimens. Patency of the accessory duct and minor papilla together is highly variable. It is seen in more than 60% of general ERCP studies but in only 17% of studies in patients being evaluated for biliary pancreatitis, implying that when patent, the accessory duct serves to decompress the briefly obstructed main pancreatic duct.
Pancreatic Duct Variants
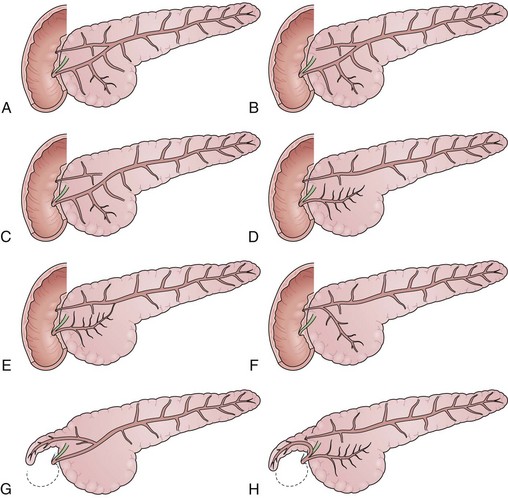
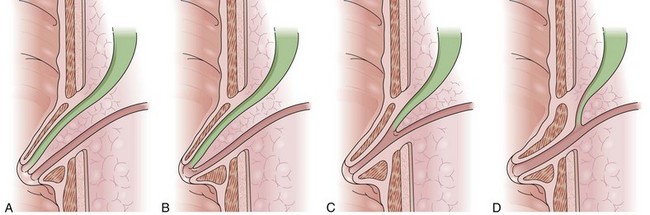
The pancreatic ducts are highly subject to abnormal formation during embryogenesis (Fig. 38.4). Most variants are of no clinical consequence, other than recognition when identified during diagnostic studies. Several risk the occurrence of pain or pancreatitis, however, on the basis of partial obstruction to flow of pancreatic juice after major stimulation. The most important and common variants occur as a result of either malrotation or malfusion of the ventral or dorsal embryonic pancreatic anlage. Normally, during the 6th to 8th week of embryogenesis, the smaller ventral pancreas rotates posteromedially as the duodenum rotates and migrates laterally. Once in a medial position neighboring the larger dorsal pancreas, the parenchyma and ducts fuse, yielding a main pancreatic duct comprising both dorsal and ventral segments. Failure of normal rotation and migration can yield an annular pancreas, in which some portion of the ventral pancreas remains behind in the path of migration (Fig. 38.5). The band of incompletely migrated duct and associated parenchyma typically form a partial to complete ring around the second portion of the duodenum, just proximal to the major papilla.
Many cases of annular pancreas remain asymptomatic, but more extreme variants can cause partial duodenal obstruction in infancy, childhood, or adulthood.29 The preferred treatment for intestinal obstruction is duodenal bypass, rather than excision of the offending segment. Annular pancreas may also cause episodic pancreatitis, but its association with other abnormalities makes interpretation of the exact etiology uncertain. Approximately one-third of cases are associated with pancreas divisum.
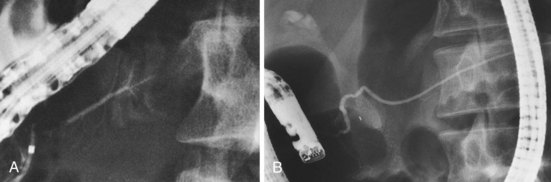
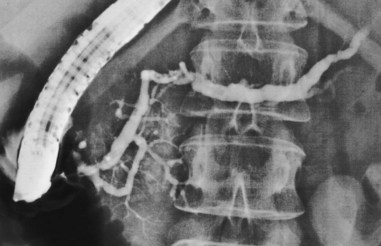
Pancreas divisum is the most common and important of the congenital variants (Fig. 38.6). It occurs as a result of incomplete fusion of the ventral and dorsal ducts after migration of the ventral anlage. Pancreas divisum occurs in approximately 7% (1% to 14%) of autopsy and pancreatography series. Approximately 20% of cases are incompletely divided, with persisting tiny communications via side branches of the dorsal and ventral systems. The technical approach to pancreatography via the minor papillae has been described previously. In pancreas divisum, the ventral duct may be generous, small, or nonexistent in up to 30% of cases. It should appear finely tapering with gradually smaller side branches, suggesting the appearance of a delicate Christmas tree. Abrupt termination of the main duct should prompt concern about a potential obstructing lesion. Dorsal ductography and EUS usually resolve this question.
The junction between the pancreatic duct and the bile duct at the major papilla is also highly variable (Fig. 38.7). Differences in junctional anatomy are primarily important for the challenges they present during selective cannulation of one or the other system during ERCP. A so-called anomalous pancreaticobiliary junction exists when the ducts unite proximal to the pancreatic sphincter and the duodenal wall. In this setting, there is no barrier for prevention of reflux of bile or pancreatic juice into the alternative system. This abnormality has been associated with the development of choledochal cystic dilation and acute idiopathic pancreatitis. Whether endoscopic sphincterotomy is adequately efficacious remains questionable because the junction is often above the boundary of safe incision. Duct duplications, with the resulting appearance of a bifid system, occur most commonly in the body and tail.30 Duplications may be associated with unusual patterns of glandular parenchyma, but they are generally of no clinical consequence.
Periampullary Pathology Related to Pancreatic Disease
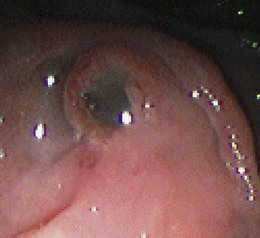
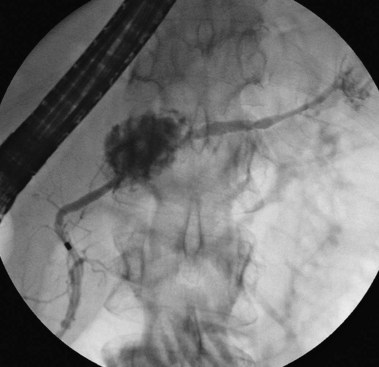
Pathologic appearances of the ampulla, the papilla of Vater, and the medial wall of the duodenum can be due to intrinsic conditions or can reflect underlying pathology within the neighboring pancreas or the pancreatic ducts. The most common extrinsic causes of an abnormal appearance are changes related to underlying malignancy or pancreatitis. Cancer within the head of the pancreas often encroaches on the periampullary region, causing mucosal and ampullary swelling, induration, and inflammation. In the absence of malignant ulceration, the changes are nonspecific and similar to changes seen with underlying severe acute or subacute pancreatitis. Severe pancreatitis often produces more prominent and widespread edema of the duodenal mucosa as well. Changes of the papillary os are uncommon and usually seen after stone passage from the bile duct. Transpapillary passage of white chalky pancreatic stones from the pancreatic duct is rarely seen endoscopically but has been noted during investigation of obstructive cholangitis in patients with chronic calcific pancreatitis and duct stones.31 Observation of thick plugs of mucus extruding from a dilated papillary duct is pathognomonic of intraductal papillary mucin-producing neoplasm (IPMN) of either the bile duct or the pancreatic duct.32 The latter is far more common (Fig. 38.8).
Pathologic Patterns Seen during Pancreatography
During pancreatography, recognition of pathology requires familiarity with normal findings and patterns of abnormalities that might be encountered. Various potential findings include (1) filling defects, (2) abnormalities in duct caliber or contour, (3) duct leaks, and (4) filling into cystic spaces. Each abnormality is associated with a differential diagnosis of possible causes, many of which overlap or contribute to multiple abnormalities (Box 38.2). Filling defects include stones, parasites, and mucus secreted by IPMN. Abnormalities in contour include partial or complete obstruction, duct dilation, and focal or diffuse irregularities secondary to malignancy or benign inflammatory processes. Leaks can be free, contained, or communicating with other organs as fistulas and occur as a result of trauma, surgery, endoscopy, and acute or chronic pancreatitis. Filling of cystic spaces can be within or extrinsic to the pancreas itself and may be associated with pseudocysts, central areas of necrosis within solid lesions, and communicating cystic neoplasms.
Pancreatic Neoplasia
Pancreatography is a key means of identifying neoplastic lesions of the pancreas. Pancreatic carcinoma produces characteristic duct abnormalities, most of which are nonspecific and must be differentiated from similar changes caused by chronic pancreatitis. Patterns include complete obstruction, focal stricturing or stenosis, irregular narrowing of moderate degree, distortion or obliteration of side branches, and stenosis with entry into necrotic cystic spaces.33 The two dominant patterns are complete obstruction and focal strictures. Obstruction related to cancer may appear blunt, serrated, or abruptly pointed, whereas obstruction related to chronic pancreatitis may appear smoothly tapered, rounded, or concave from a meniscus sign related to intraductal stones. Malignant strictures tend to be more abrupt, with irregular contours, whereas strictures from chronic pancreatitis are either very short and suggestive of focal weblike scars or lengthy and smoothly tapered.34
In malignant obstruction or stenosis, the side branches are commonly absent near the lesion, dilated upstream from the obstruction, and normal downstream. In chronic pancreatitis, the side branches are intact but perhaps abnormal near the main duct lesion, and the main duct and side branches exhibit either dilation or chronic distortion upstream and changes of chronic pancreatitis downstream from the obstruction. Key findings for differentiating pancreatic carcinoma from autoimmune pancreatitis during pancreatography include the length of main pancreatic duct strictures (autoimmune pancreatitis > 3 mm, pancreatic carcinoma < 3 cm), presence (autoimmune pancreatitis) or absence (pancreatic carcinoma) of skip lesions, maximum caliber of upstream dilation (autoimmune pancreatitis < 3 to 5 mm, pancreatic carcinoma > 5 mm), and presence of demonstrable side branches in strictured segments of autoimmune pancreatitis but not in pancreatic carcinoma.35 Successful interpretation varies among readers but can be learned and appropriately applied.36
The presence of concomitant neighboring abnormalities in both the pancreatic and the bile ducts is highly suggestive of malignancy. Findings may include dual obstruction or stenosis (“double duct” sign), displacement by tethering or compression, and effacement.37 The double duct sign is highly suggestive and should be considered diagnostic of cancer until proven otherwise (Fig. 38.9). Lesions in the head are often first brought to attention by associated biliary obstruction and jaundice or pruritus. In this setting, ERCP often accesses the biliary obstruction for tissue sampling and palliative stent placement. If computed tomography (CT) or ultrasound findings are diagnostic of cancer, pancreatography is usually unnecessary. If no mass has been seen by cross-sectional imaging when ERCP shows a distal biliary stricture, it is very useful to obtain a pancreatogram at least within the pancreatic head, ideally including both uncinate and accessory branches. Cases of pancreatic carcinoma involving a nonfilling accessory duct with an otherwise “normal” pancreatogram have been reported.38
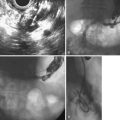
Of all the pancreatic neoplasms, findings with IPMN of the pancreatic ducts are among the most specific.32 The characteristic production of mucus may occur silently, with eventual identification of dilated segments on cross-sectional imaging, or it may manifest clinically with episodes of pain or pancreatitis related to intermittent obstruction.39 When IPMN involves the main pancreatic duct, pancreatography typically shows dilation and soft, bulky filling defects that can be stripped from the lumen with an occlusion balloon. When present only in side branches, the diagnosis is commonly suspected after cross-sectional imaging with CT or ultrasound. Contrast agent filling of segments harboring side-branch IPMN may require forceful injection above an occlusion balloon (see Fig. 38.8). This maneuver may not be justified, given the improving identification of this process by EUS. Gross papillary changes of the duct lining mucosa are infrequently evident radiographically but when present can be sampled with transpapillary biopsy and brushing to diagnose IPMN.
Transpapillary pancreatoscopy and intraductal ultrasound during ERCP have been described as useful in preoperative staging of the longitudinal spread of main duct IPMN.40,41 Mucus markedly distorts the endoscopic view but wire-guided intraductal ultrasound identifies both the mucus and papillary changes if they are accessible. None of these endoscopic tools are highly sensitive for identifying the progression to invasive malignancy.42
Pancreatography in Acute Pancreatitis
Relative consensus now exists regarding the use of endoscopic cholangiography for acute pancreatitis. Despite the known risk of pancreatitis attributable to ERCP, performance of cholangiography in the setting of acute pancreatitis has been shown to be generally safe.43 Cholangiography and sphincterotomy, as indicated by findings, are now accepted in the setting of severe acute pancreatitis with associated biliary obstruction or cholangitis.44 These procedures may also be beneficial for severe biliary pancreatitis without biliary obstruction; however, data are mixed on this point,45,46 and one study suggested detrimental effects in this setting.47 Subsequent to resolution of one or more episodes of acute pancreatitis, pancreatography is useful for identification of potential etiologies, including biliary stone disease, anomalous anatomy (e.g., pancreas divisum, anomalous junction), occult neoplasia, and chronic pancreatitis.48
Venu and colleagues48 reported use of ERCP and sphincter of Oddi manometry in 116 patients with recurrent idiopathic pancreatitis. A treatable cause of pancreatitis was identified in 37%, including a mixture of anatomic abnormalities, stones, and sphincter hypertension. As previously described, evolving guidelines propose that in this elective setting ERCP should be performed only if skills for therapy and performance of sphincter of Oddi manometry are also available. The use of pancreatography during episodes of severe acute pancreatitis is generally not indicated. However, smoldering pancreatitis that is not resolving and late morbidity related to strictures or leaks after improvement in the most severe inflammatory process are both settings in which pancreatography and associated therapies may be beneficial.49–52
Nonspecific findings that may be seen during acute pancreatitis, for which intervention is not indicated, include increased irregularity in contour of the main duct and side branches, perhaps less prompt filling of side branches, and occasional early parenchymal staining even before side-branch filling, suggestive of local necrosis. Duct leaks and strictures resulting from acute pancreatitis occur most frequently near the genu and likely result from focal necrosis at that site. Leaks identified within the first few weeks appear free within the retroperitoneum, whereas leaks identified after longer intervals enter more circumscribed spaces or mature pseudocysts. The timing of onset for duct leaks remains incompletely defined. Data from Neoptolemos and coworkers53 suggested that most leaks occur after the 4th day. These investigators retrospectively stratified patients to modest versus extensive (>25%) glandular necrosis by CT scan then determined the incidence and timing of duct leaks based on pancreatography. No leaks were found among 89 patients with limited necrosis. In contrast, 7 of 16 patients in the extensive necrosis group exhibited leaks, including none of 4 patients studied before day 5 and 7 of 12 studied at day 5 or later.
In a prospective study of pancreatic duct integrity during acute pancreatitis, Uomo and colleagues54 showed a 31% incidence of leaks by pancreatography performed during the 1st week (mean 4.2 days). Leaks did not correlate with need for surgical management. In both acute and chronic pancreatitis, it is useful to clarify whether leaks are being potentiated by downstream obstruction related to strictures or stones because this has implications for endoscopic and surgical management.
The details of therapeutic interventions are beyond the scope of this chapter. There is growing interest in the potential utility of early investigation and prophylactic intervention or early treatment for significant duct injury in severe necrotizing pancreatitis.55 However, only limited data support this approach, and the potential for secondary infection of necrosis and peripancreatic tissues mandates that prospective studies be performed before this approach becomes common practice.
Pancreatography in Chronic Pancreatitis
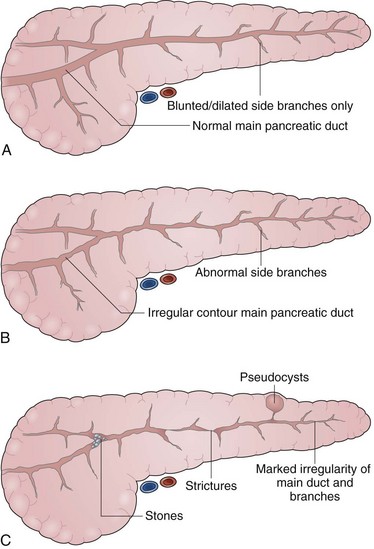
The indications for pancreatography in chronic pancreatitis include (1) to establish a diagnosis, (2) to characterize the anatomy before surgical management, and (3) to characterize the anatomy as a component of endoscopic therapy. Pancreatography has been the “gold standard” for diagnosing chronic pancreatitis for several decades. Several classification systems for chronic pancreatitis have been proposed. Although none are perfect or all-encompassing, the Cambridge Classification of 1983 proposed a sequential gradation of ductal abnormalities21 that remains perhaps the most straightforward and widely used. Pancreatograms are termed normal, equivocal, or indicative of mild, moderate, or marked pancreatitis on the basis of side-branch abnormalities; main duct abnormalities; or additional advanced irregularities including presence of cavities, complete obstruction, filling defects (stones), severe dilation (>1 cm), and segmentation (“chain of lakes”) (Figs. 38.10 and 38.11).
The severity of pancreatographic findings correlates loosely with progression of disease and decline in pancreatic function. The main challenges in the interpretation of pancreatography for the diagnosis of chronic pancreatitis are the differentiation of normal from early disease with minimal changes and the differentiation of pancreatic carcinoma from chronic disease with focal stricturing. Comments on the latter have been made earlier. Mild changes on pancreatography—blunting, dilation, or shortening of the side branches—are the least specific and most subjective because they are highly dependent on technique and adequacy of filling and potentially a result of age-related changes or transient effects of acute injury. ERP is more sensitive for early changes of chronic pancreatitis than CT, transabdominal ultrasound, or magnetic resonance cholangiopancreatography (MRCP). EUS is both highly sensitive for early chronic pancreatitis and safe.56 In centers with adequate EUS experience, it is becoming the procedure of choice for making this diagnosis when CT or MRCP are normal.
Pancreatic duct morphology is best characterized by pancreatography, and this remains the procedure of choice for anatomic characterization before surgery or in concert with other, less invasive therapies for pseudocysts, fistulas, leaks, and pancreatic ascites related to chronic or postnecrotic pancreatitis.57,58 In a prospectively documented series of 41 patients with pseudocysts associated with chronic or resolving acute pancreatitis, Nealon and coworkers59 showed that preoperative ERCP led to significant alterations in the surgical management in 24 patients (59%). In particular, ERCP led to pseudocyst drainage combined with concurrent drainage of the pancreatic duct in 19 patients and of the common bile duct in 11 patients with chronic pancreatitis. In the current era of transmural and transpapillary endoscopic drainage of pseudocysts, the role of endoscopic characterization of the pancreatic duct remains incompletely defined. Its utility is likely to be equivalent or greater than when used in concert with surgery.
Miscellaneous Settings
After pancreatic surgery, questions pertaining to anastomotic leaks and strictures are often best answered by pancreatography (Fig. 38.12). Duct decompression and drainage are feasible at the same procedure, similar to the procedure described for acute and chronic pancreatitis leaks. Resection procedures generally yield leaks at closure lines of main ducts, whereas surgical enucleation or excision procedures are more prone to smaller side-branch or lateral main duct leaks. Pancreatography may also be useful for investigation and therapy of late postoperative anastomotic strictures. Pancreaticojejunal anastomoses fashioned in decompressive procedures, including the Peustow lateral pancreaticojejunostomy and the Frey procedure, can be studied via the pancreatic os at the major papilla. Pancreaticojejunal anastomosis employed in the Whipple procedure and the Beger procedure must be accessed via enteroscopy into an afferent limb or a Roux limb; this is significantly more difficult.
In our experience, ERP is successful in only 10% to 15% of patients after the Whipple procedure.60 In most patients with post-Whipple anatomy, a pancreatogram can be obtained most easily via transgastric EUS-guided duct puncture (Fig. 38.13).61 This maneuver is also used as a prelude to EUS-guided transgastric stent decompression of obstructed ducts resulting from cancer or chronic pancreatitis. These therapies may be accomplished in usual retrograde fashion after rendezvous passage of a guidewire or directly from stomach to pancreas.61 Percutaneous duct access for pancreatography and associated therapies has also been described.62
Pancreatography is also very useful during investigation of blunt midabdominal trauma, from which mid–pancreatic duct leaks can occur owing to rupture of the gland over the spine. Identification and localization of traumatic leaks can guide surgical63,64 or endoscopic management.
Magnetic Resonance Pancreatography
Performance of MRCP is predominantly dependent on the strength of the magnet and the use of sophisticated software algorithms for generating optimal views of the region of interest. Magnetic resonance pancreatography (MRP) has slightly less spatial resolution than ERP, but optimal performance approaches that of endoscopic studies and is generally adequate for clinical decision making.65 MRP is highly accurate for the diagnosis of pancreas divisum.66 In chronic pancreatitis, MRP can show glandular and periglandular atrophy and inflammation, in addition to the status of the ducts. The duct distention and higher spatial resolution of ERCP yield more optimal imaging of fine detail and side branches. Secretin stimulation during MRCP can enhance distention and interpretation of both duct detail and assessment of parenchymal function.67
In a retrospective study of 32 patients who underwent ERCP, CT, and abdominal ultrasound for advanced chronic pancreatitis, Varghese and colleagues68 assessed dual interpretations of MRP. They reported sensitivity, specificity, and diagnostic accuracy for detection of filling defects in the pancreatic duct (56% to 78%, 100%, 87% to 94%), ductal strictures (75% to 88%, 92% to 96%, 88% to 94%), and pseudocysts (100%, 100%, 100%). MRP failed in two patients because of respiratory motion artifacts. Among all others, the duct was completely visualized in 84%, partially visualized in 9%, and not at all visualized in 6%. ERCP failed in two patients, resulting from Billroth II anatomy in one and a duct stricture and pseudocyst in another. ERCP was inadequate in five (6%) patients because of poor opacification of the duct, which was insufficient for diagnosis. There was good correlation between MRCP and ERCP with respect to duct size. Varghese and colleagues68 concluded MRP is poorly sensitive but specific for duct abnormalities and, when combined with CT or transabdominal ultrasound, can be sufficient for planning therapy in most patients with advanced chronic pancreatitis.
Helical CT and optimal MRCP yield equivalent results for detection of pancreatic neoplasms, vascular invasion, and neighboring lymphadenopathy.65 Cystic tumors and the contents of tumors and fluid collections are more accurately characterized with MRCP than with CT. Most centers use MRCP (or CT) for diagnosis or characterization of advanced disease and for patients who are not good candidates for sedation and intubation or in whom the clinical suspicion is quite low. ERCP is reserved for patients in whom more subtle duct abnormalities are anticipated or endoscopic therapy is planned. Local preferences dictate which study is used for preoperative anatomic characterization.
1 Lambert R, Rey JF. Appropriateness of diagnostic digestive endoscopy. Dig Dis. 2002;20:236-241.
2 American Society for Gastrointestinal Endoscopy. Appropriate use of gastrointestinal endoscopy. Gastrointest Endosc. 2000;52:831-837.
3 National Institutes of Health State-of-the-Science Conference Statement (Final Statement: June 10, 2002). Endoscopic Retrograde Cholangiopancreatography (ERCP) for Diagnosis and Therapy. January 14–16, 2002. Available at http://consensus.nih.gov/ta/020/020sos_statement.htm
4 Warner MA, Caplan RA, Epstein BS, et al. American Society of Anesthesiology. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures. Available at http://www.asahq.org/publicationsAndServices/NPO.pdf
5 Radnay PA, Brodman E, Mankikar D, et al. The effect of equianalgesic doses of fentanyl, morphine, meperidine, and pentazocine on common bile duct pressure. Anaesthetist. 1980;29:26-29.
6 Thune A, Baker RA, Saccone GT. Differing effects of pethidine and morphine on human sphincter of Oddi motility. Br J Surg. 1990;77:992-995.
7 Fazel A, Burton FR. The effect of midazolam on the normal sphincter of Oddi: A controlled study. Endoscopy. 2002;34:78-81.
8 Fazel A, Burton FR. A controlled study of the effect of midazolam on abnormal sphincter of Oddi motility. Gastrointest Endosc. 2002;55:637-640.
9 Ponce Garcia J, Garrigues V, Sala T, et al. Diazepam does not modify the motility of the sphincter of Oddi. Endoscopy. 1988;20:87.
10 Goff JS. Effect of propofol on human sphincter of Oddi. Dig Dis Sci. 1995;40:2364-2367.
11 Petersen BT. Cannulation techniques: Biliary and pancreatic. Tech Gastrointest Endosc. 2003;5:17-27.
12 Benage D, McHenry R, Hawes RH, et al. Minor papilla cannulation and dorsal ductography in pancreas divisum. Gastrointest Endosc. 1990;36:553-557.
13 O’Connor KW, Lehman GA. An improved technique for accessory papilla cannulation in pancreas divisum. Gastrointest Endosc. 1985;31:13-17.
14 Park SH, de Bellis M, McHenry L, et al. Use of methylene blue to identify the minor papilla or its orifice in patients with pancreas divisum. Gastrointest Endosc. 2003;57:358-363.
15 Freeman ML. Prevention of post-ERCP pancreatitis: Pharmacologic solution or patient selection and pancreatic stents? Gastroenterology. 2003;124:1977-1980.
16 Singh P, Das A, Isenberg G, et al. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60:544-550.
17 Tarnasky P, Palesch YY, Cunningham JT, et al. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518-1524.
18 Aizawa T, Ueno N. Stent placement in the pancreatic duct prevents pancreatitis after endoscopic sphincter dilation for removal of bile duct stones. Gastrointest Endosc. 2001;54:209-213.
19 Fazel A, Quadri A, Catalano M, et al. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc. 2003;57:291-294.
20 Kolars JC, O’Connor M, Ansel H, et al. Pancreatic pseudocysts: Clinical and endoscopic experience. Am J Gastroenterol. 1989;84:259-264.
21 Axon AT, Classen M, Cotton PB, et al. Pancreatography in chronic pancreatitis: International definitions. Gut. 1984;25:1107-1112.
22 Classen M, Hellwig H, Rosch W. Anatomy of the pancreatic duct: A duodenoscopic-radiological study. Endoscopy. 1973;5:14-17.
23 Varley PF, Rohrmann CA, Silvis SE, et al. The normal endoscopic pancreatograms. Radiology. 1976;118:295-300.
24 Sivak MV, Sullivan BH. Endoscopic retrograde pancreatography: Analysis of the normal pancreatogram. Dig Dis. 1976;21:263-269.
25 Sivak MVJr. The normal retrograde pancreatogram and cholangiogram. In: Sivak MVJr, editor. Gastrointestinal endoscopy. ed 2. Philadelphia: Saunders; 2000:878-889.
26 Cotton PB. The normal endoscopic pancreatograms. Endoscopy. 1974;6:65-70.
27 Ishibashi T, Matsubara O. Studies on the retrograde pancreatography in autopsy specimens. Bull Tokyo Med Dent Univ. 1997;24:43-51.
28 Rienhoff WFJr, Pickrell KL. Pancreatitis: An anatomic study of the pancreatic and extrahepatic biliary systems. Arch Surg. 1945;51:205-219.
29 Zyromski NJ, Sandoval JA, Pitt HA, et al. Annular pancreas: Dramatic differences between children and adults. J Am Coll Surg. 2008;206:1019-1027.
30 Halpert RH, Shabot JM, Heare BR, et al. The bifid pancreas: A rare anatomical variation. Gastrointest Endosc. 1990;36:60-61.
31 Little TE, Kozarek RA. Pancreatic stones as a cause of bile duct and ampullary obstruction: Endoscopic treatment approaches. Gastrointest Endosc. 1993;39:709-712.
32 Venu RP, Atia G, Brown RD, et al. Intraductal papillary mucinous tumor of the pancreas: ERCP, EUS, and pancreatoscopy findings. Gastrointest Endosc. 2002;55:82.
33 Fukumoto K, Nakajima M, Murakami K, et al. Diagnosis of pancreatic cancer by endoscopic pancreatocholangiography. Am J Gastroenterol. 1974;62:210-213.
34 Rohrmann CAJr, Silvis SE, Vennes JA. The significance of pancreatic ductal obstruction in differential diagnosis of the abnormal endoscopic retrograde pancreatogram. Radiology. 1976;121:311-314.
35 Kamisawa T, Imai M, Chen PY, et al. Strategy for differentiating autoimmune pancreatitis from pancreatic cancer. Pancreas. 2008;37:e62-e67.
36 Sugumar A, Levy MJ, Kamisawa T, et al. Endoscopic retrograde pancreatography criteria to diagnose autoimmune pancreatitis: An international multicentre study. Gut. 2011;60:666-670.
37 Freeny PC, Bilbao MK, Katon RM. “Blind” evaluation of endoscopic retrograde cholangiopancreatography (ERCP) in the diagnosis of pancreatic carcinoma: The “double duct” and other signs. Radiology. 1976;119:271-274.
38 Kowdley KV, Variyam EP, Sivak MVJr. Obstructive jaundice caused by pancreatic carcinoma in the setting of a normal pancreatogram. Gastrointest Endosc. 1995;41:158-160.
39 Loftus EVJr, Olivares-Pakzad BA, Batts KP, et al. Intraductal papillary-mucinous tumors of the pancreas: Clinicopathologic features, outcome, and nomenclature. Gastroenterology. 1996;110:1909-1918.
40 Hara T, Yamaguchi T, Ishihara T, et al. Diagnosis and patient management of intraductal papillary-mucinous tumor of the pancreas by using peroral pancreatoscopy and intraductal ultrasonography. Gastroenterology. 2002;122:34-43.
41 Yamao K, Ohashi K, Nakamura T, et al. Evaluation of various imaging methods in the differential diagnosis of intraductal papillary mucinous tumor (IPMT) of the pancreas. Hepatogastroenterology. 2001;48:962-966.
42 Cellier C, Cuillerier E, Palazzo L, et al. Intraductal papillary and mucinous tumors of the pancreas: Accuracy of preoperative computed tomography, endoscopic retrograde pancreatography and endoscopic ultrasonography, and long-term outcome in a large surgical series. Gastrointest Endosc. 1998;47:42-49.
43 Brambs HJ, Scholmerich J, Gross V, et al. Endoscopic retrograde cholangiopancreatography in acute pancreatitis. Dig Surg. 1988;5:156-159.
44 Fan ST, Lai ECS, Mok FPT, et al. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328:228-232.
45 Barkun AN. Early endoscopic management of acute gallstone pancreatitis—an evidence-based review. J Gastrointest Surg. 2001;5:243-250.
46 Neoptolemos JP, Carr-Locke DL, London NJ, et al. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979-983.
47 Foelsch UR, Nitsche R, Luedtke R, et al. German Study Group on Acute Biliary Pancreatitis. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. N Engl J Med. 1997;336:237-242.
48 Venu RP, Geenen JE, Hogan W, et al. Idiopathic recurrent pancreatitis: An approach to diagnosis and treatment. Dig Dis Sci. 1989;34:56-60.
49 Kozarek RA. Endoscopic therapy of complete and partial pancreatic duct disruptions. Gastrointest Endosc Clin North Am. 1998;8:39-53.
50 Varadarajulu S, Noone T, Hawes R, et al. Pancreatic duct stent insertion for functional smoldering pancreatitis. Gastrointest Endosc. 2003;58:438-441.
51 Kozarek RA, Ball TJ, Patterson DJ, et al. Endoscopic transpapillary therapy for disrupted pancreatic duct and peripancreatic fluid collections. Gastroenterology. 1991;100:1362-1370.
52 Levy MJ, Geenen JE, Catalano MF, et al. Pancreatic duct stent therapy for “smoldering” pancreatitis. Gastrointest Endosc. 2000;51:AB203.
53 Neoptolemos JP, London NJ, Carr-Locke DL. Assessment of main pancreatic duct integrity by endoscopic retrograde pancreatography in patients with acute pancreatitis. Br J Surg. 1993;80:94-99.
54 Uomo G, Molino D, Visconti M, et al. The incidence of main pancreatic duct disruption in severe biliary pancreatitis. Am J Surg. 1998;176:49-52.
55 Lau ST, Simchuk EJ, Kozarek RA, et al. A pancreatic ductal leak should be sought to direct treatment in patients with acute pancreatitis. Am J Surg. 2001;181:411-415.
56 Sahai AV. EUS and chronic pancreatitis. Gastrointest Endosc. 2002;56:S76-S81.
57 O’Connor M, Kolars J, Ansel H, et al. Preoperative endoscopic retrograde cholangiopancreatography in the surgical management of pancreatic pseudocysts. Am J Surg. 1986;151:18-24.
58 Kuo Y, Wu C. The role of endoscopic retrograde pancreatography in pancreatic ascites. Dig Dis Sci. 1994;39:1143-1146.
59 Nealon WH, Townsend CM, Thompson JC. Preoperative endoscopic retrograde cholangiopancreatography (ERCP) in patients with pancreatic pseudocyst associated with resolving acute and chronic pancreatitis. Ann Surg. 1989;209:532-540.
60 Chahal P, Baron TH, Topazian MD, et al. Endoscopic retrograde cholangiopancreatography in post-Whipple patients. Endoscopy. 2006;38:1241-1245.
61 Will U, Fueldner F, Thieme AK, et al. Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J Hepatobiliary Pancreat Surg. 2007;14:377-382.
62 Simmons DT, Baron TH, LeRoy A, et al. Percutaneous pancreatography for treatment of complicated pancreatic duct strictures. Pancreatology. 2008;8:194-198.
63 Sugawa C, Lucas CE. The case for preoperative and intraoperative ERCP in pancreatic trauma. Gastrointest Endosc. 1988;34:145-147.
64 Plancq MC, Villamizar J, Ricard J, et al. Management of pancreatic and duodenal injuries in pediatric patients. Pediatr Surg Int. 2000;16:35-39.
65 Reinhold C. Magnetic resonance imaging of the pancreas in 2001. J Gastrointest Surg. 2002;6:133-135.
66 Bret PM, Reinhold C, Taourel P, et al. Pancreas divisum: Evaluation with MR cholangiopancreatography. Radiology. 1996;199:99-103.
67 Manfredi R, Costamagna G, Brizi MG, et al. Severe chronic pancreatitis versus suspected pancreatic disease: Dynamic MR cholangiopancreatography after secretin stimulation. Radiology. 2000;214:849-855.
68 Varghese JC, Masterson A, Lee MJ. Value of MR pancreatography in the evaluation of patients with chronic pancreatitis. Clin Radiol. 2002;57:393-401.