Diagnostic Cytology of the Gastrointestinal Tract
Helen H. Wang
Gamze Ayata
Introduction
The popularity of gastrointestinal (GI) cytology for the diagnosis of infection and malignancy has waxed and waned during the past few decades. The ability to distinguish between high-grade dysplasia or carcinoma in situ and invasive carcinoma in biopsy specimens and the more prevalent expertise of surgical pathology cause some to consider cytology an unnecessary duplication of GI mucosal biopsies.1,2 However, the combined use of endoscopy, ultrasound guidance, and fine-needle aspiration (FNA) has expanded the horizons of GI cytology.3
Specimen Types
Types of GI tract specimens commonly received in the cytology laboratory include those obtained by endoscopic brushings and ultrasound-guided endoscopic FNA. Endoscopic FNA has enabled endoscopists to reach farther than they can with biopsy forceps to sample mural and extramural lesions, including lesions adjacent to the GI tract. The nonendoscopic specimens obtained with balloon- or mesh-type samplers have been evaluated in the research setting to ascertain their usefulness in the surveillance of populations at high risk for esophageal carcinoma.4–6
Specimen Preparations
Direct smears can be made from materials collected on the endoscopic brush, in the needle, or on the balloon and mesh samplers; these can then be either fixed immediately in 95% ethanol and stained with the Papanicolaou method or left to air-dry and stained with Diff-Quik (Dade-Behring, Inc., Deerfield, IL) or Wright-Giemsa stain. Alternatively, the material can be rinsed into a medium such as CytoLyt, CytoRich, or 50% ethanol for liquid-based preparations. The specimen can then be processed by a concentration method, such as ThinPrep Processor (Hologic, Marlborough, MA) or Cytospin (ThermoFisher Scientific, Waltham, MA),7,8 to make slides that are then stained with the Papanicolaou method. According to a College of American Pathologists Interlaboratory Comparison Program in Nongynecologic Cytology, ThinPrep preparations performed better than non-ThinPrep preparations.7 However, liquid-based preparations, including ThinPrep, involve altered morphology and artifacts that require adjustment by cytopathologists, such as cleaner background with altered or reduced background and extracellular elements, architectural changes (smaller cell clusters and sheets and more three-dimensional clusters), altered cell distribution (more dyshesion—dissociation of cells at the periphery of cell clusters or as single cells), and changes in cytologic morphology (enhanced nuclear features and smaller cell size).9 Residual material from liquid-based preparations lends itself to cell block making with thromboplastin-plasma cell block technique,10 the Cellient Automated Cell Block System (Hologic), or other techniques for ancillary studies.
Value and Accuracy of Specimens
Cytology specimens have some advantages over specimens obtained by endoscopic biopsy. The brush can sample a wider area, and the fine needle can reach deeper lesions than can be reached by biopsy forceps. Also, both the brush and the fine needle are less invasive than biopsy forceps and less likely to cause bleeding. In addition, cytology has a shorter turnaround time than histology. Direct smears can be ready for review within minutes with no compromise of the quality of the preparation (unlike frozen sections of biopsy specimens, which compromise the quality of the final or permanent preparation). However, as mentioned, cytology is limited in its ability to distinguish between high-grade dysplasia or carcinoma in situ and invasive carcinoma.
Despite the potential duplication of cytology and biopsy, the literature has consistently shown that the highest diagnostic yield is obtained with the combined use of these specimens.11–13 The yield of cytology is significantly higher when the brushing is performed before rather than after the biopsy.14
Normal Morphology
Esophagus
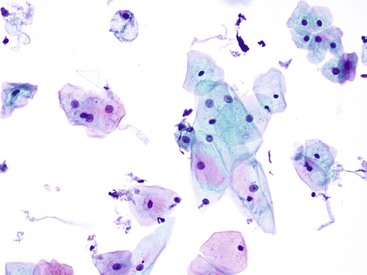
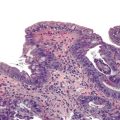
Intermediate-type squamous cells with abundant cytoplasm and vesicular nuclei are seen in the normal esophagus (Fig. 3.1). Superficial-type squamous cells with abundant cytoplasm and small pyknotic nuclei can also be seen in small numbers. Single cells and clusters of ciliated columnar cells from the respiratory tract with no clinical significance may be seen rarely.
Stomach
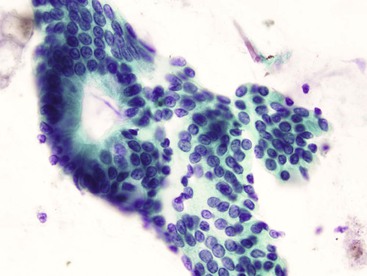
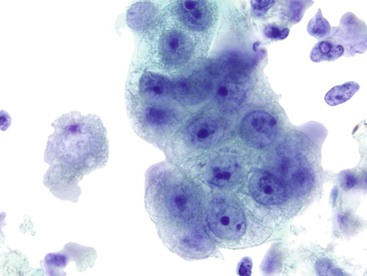
Gastric surface foveolar cells can shed as single cells or in sheets. When in sheets, the columnar cells exhibit abundant cytoplasm, regularly spaced nuclei, and open chromatin arranged in a honeycomb or palisaded pattern (Fig. 3.2), depending on the orientation. When they are shed as single cells, they often lose their cytoplasm to become naked nuclei. In endoscopic FNA specimens, the sheets of foveolar cells can mimic cells from a mucinous neoplasm, and the single naked nuclei, because of their small, monomorphic appearance, can mimic cells from a pancreatic endocrine tumor.
Small Intestine
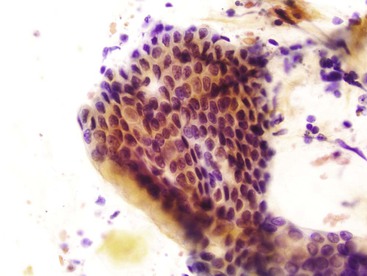
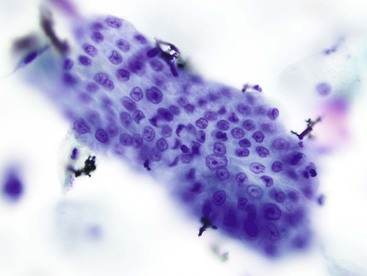
The lining cells of the small intestine can be easily distinguished from gastric foveolar cells by the presence of goblet cells. On low magnification, the specimen typically has a Swiss cheese appearance, with the “holes” representing either goblet cells or gland openings of the crypts (Fig. 3.3). On high magnification, the absorptive cells have either finely granular or vacuolated cytoplasm, and the goblet cells have single large mucin vacuoles and crescent-shaped nuclei with rounded contours. The striated border of the absorptive cells may be seen at the periphery of the sheets.
Large Intestine
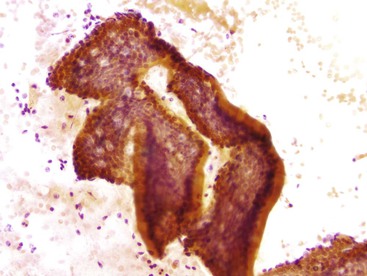
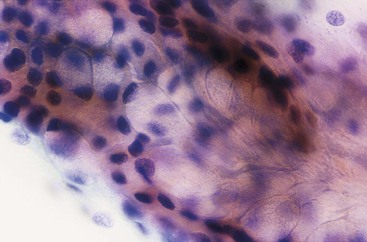
Normal epithelium is characterized on cytology by sheets or strips of tall columnar cells with abundant cytoplasm and basal nuclei. Partial or complete openings of the colonic crypts may be seen (Fig. 3.4).
Infections
Most infectious agents that affect human hosts can infect the GI tract of immunocompetent and immunocompromised patients.15 Some infectious agents have a predilection for the GI tract. The more common ones are discussed in this section.
Candida
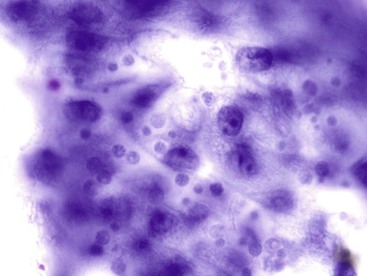
Candida almost exclusively involves the esophageal portion of the GI tract. Brushings are more sensitive than biopsy specimens in the detection of esophageal candidiasis.13 Contamination by oral Candida is usually not a problem because the brush is contained within a sheath when it is passed into and out of the endoscope and is expelled from the sheath only to sample the lesion. The organisms appear as pink to purple pseudohyphae and yeast formations on Papanicolaou stain (Fig. 3.5). Reactive squamous cells and inflammatory cells are often observed in the background.
Herpes Simplex Virus
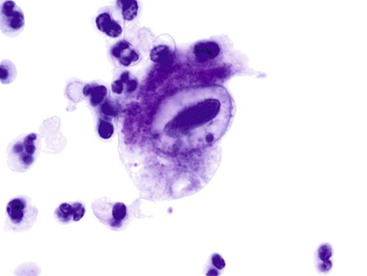
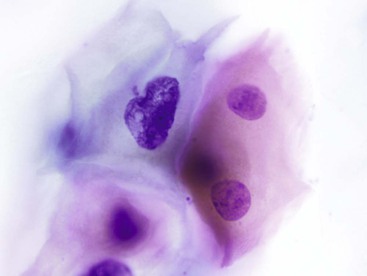
Herpes simplex virus infection can theoretically affect epithelial cells anywhere along the GI tract, but it is most commonly seen in the esophagus. Multinucleation, nuclear molding, ground-glass chromatin, and eosinophilic intranuclear inclusions are the characteristic features of infected cells (Fig. 3.6).
Cytomegalovirus
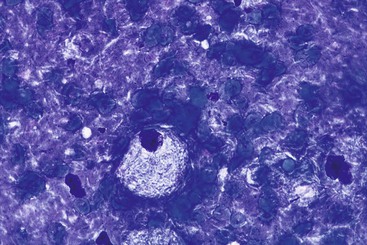
Cytomegalovirus infection affects epithelial, stromal, and endothelial cells along the GI tract and is characterized by large cells with a single large basophilic intranuclear inclusion with a perinuclear halo (Fig. 3.7). Intracytoplasmic textured inclusions can occasionally be seen in the affected cells.
Helicobacter pylori
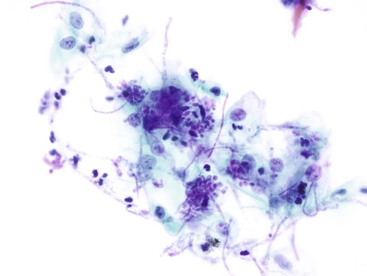
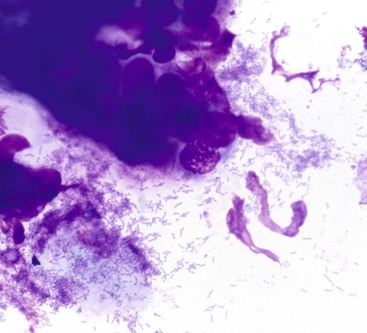
Helicobacter pylori infection occurs exclusively in the stomach and is perhaps the most common infection of the GI tract. These organisms can be demonstrated on imprint smears of gastric biopsies or on brush cytology specimens.16,17 Examination of imprint and brushing cytology specimens is comparable, if not superior, in sensitivity (88%) and specificity (61%) to histologic examination of sections stained with hematoxylin and eosin (H&E) and modified Giemsa stain.16,17 The benefits of imprint and brushing cytology are rapid results, high specificity, and low cost. However, the efficacy of cytologic detection depends on the extent of colonization by these organisms. When present in large quantity, they are evident even at low magnification, but they can be difficult to identify when present in small numbers. On Papanicolaou stain, H. pylori organisms appear as faintly basophilic, S-shaped rods admixed with mucus in the vicinity of glandular cell clusters (Fig. 3.8). Special stains, such as a triple stain combining silver, H&E, and Alcian blue at pH 2.5, can enhance their detection by cytology.18
Giardia
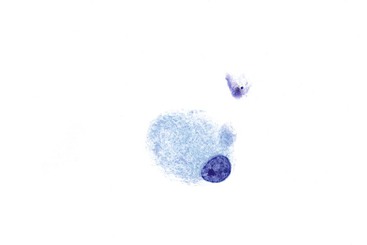
Giardia affects the duodenum of both immunocompetent and immunocompromised hosts. Brush cytology is a useful method for detecting Giardia because the organisms are on the luminal surfaces of the intestinal epithelial cells. They are flat, gray, pear-shaped, and binucleate, with four pairs of flagella (Fig. 3.9).19 Giardia lamblia trophozoites have been found to be immunoreactive for the protooncogene KIT (C-kit, CD117), which may help to identify the organisms.20
Atypical Mycobacteria
Atypical mycobacteria accumulate within macrophages in the lamina propria, and very rigorous brushing is required for the infected macrophages to be included in the cytology sample. The presence of isolated foamy histiocytes on the smear should raise the level of suspicion of an atypical mycobacterial infection (Fig. 3.10). In general, the organisms are present in large numbers. On Diff-Quik–stained smears, the mycobacteria form numerous rod-shaped negative images, either within the histiocytes or in the background (Fig. 3.11).21 Special stains for acid-fast bacilli are necessary to confirm the diagnosis.
Cryptosporidia
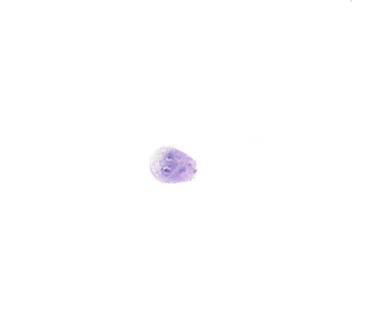
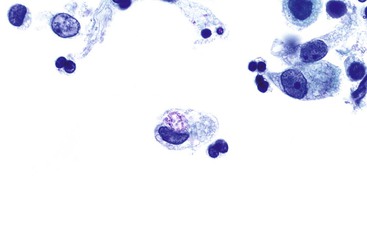
Cryptosporidia can involve any glandular epithelium of the GI tract in patients infected with the human immunodeficiency virus (HIV) and can be detected by examination of stool and cytology specimens.22 Cryptosporidia are 2- to 5-µm, round, basophilic bodies on the luminal surfaces of the epithelial cells. Therefore, they are seen only when the plane of focus is shifted to the surfaces of the cells where the organisms reside (Fig. 3.12). When in doubt, confirmatory Gomori methenamine-silver stain can be applied.
Microsporidia
Microsporidia can be detected on cytologic specimens such as stool, nasal secretions, duodenal aspirates, and bile, as well as on brushing specimens from the duodenum and biliary tract.23–25 On Papanicolaou stain, they appear in aggregates as brightly eosinophilic, rod-shaped or ovoid organisms measuring 1 to 3 µm in diameter (Fig. 3.13). They are present in both epithelial cells and inflammatory cells. In epithelial cells, they are located in the supranuclear portion of the cytoplasm and therefore, like cryptosporidia, are seen at a slightly different plane of focus from that of the epithelial nuclei.
Inflammatory, Reactive, and Metaplastic Changes
Nonspecific Changes
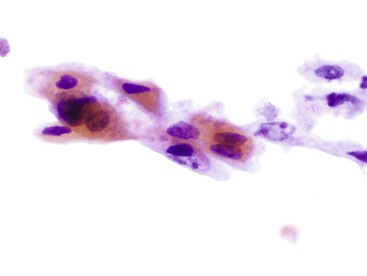
Any injury to the mucosa can evoke a nonspecific inflammatory or reactive epithelial change. When the injury is sufficient to result in ulceration, the change (i.e., the epithelial repair) can become so extreme that it may mimic a malignancy. It is often difficult to determine whether the reparative epithelium is of glandular or squamous origin. Although epithelial repair is characterized by prominent eosinophilic nucleoli, they are usually neither huge nor numerous (i.e., more than three or four) (Fig. 3.14). The appearance of atypical stromal cells or their stripped nuclei from granulation tissue can also be quite alarming (Fig. 3.15). In spite of striking nuclear enlargement of such cells, hyperchromasia is absent. Instead, they have fine, homogeneous chromatin and thin, smooth nuclear membranes.
Both cellular arrangements and the features of individual cells are useful in distinguishing between severe reactive and neoplastic changes. Cells with reactive or reparative changes are usually arranged in flat sheets without three-dimensionality or prominent cell dyshesion. In contrast, dyshesion, presented either as “feathering” (dissociation of cells) at the periphery of cell clusters or as the dispersion of numerous isolated cells, is usually evident with neoplasms, as is three-dimensionality. In addition, the enlarged nuclei in reactive or reparative changes usually have uniform size and a similar number of small, prominent nucleoli, in contrast to the variation in nuclear and nucleolar size and shape as well as the chromatin pattern in the neoplastic lesions. Specific types of reactive cells may also be seen, such as those with radiation-induced changes (Fig. 3.16). As in other organs, the cells are proportionally enlarged, with metachromatic cytoplasm and nuclear or cytoplasmic vacuoles.
Pemphigus
Rarely, pemphigus vulgaris, an autoimmune disease of the skin and mucous membranes that attacks the intercellular junctions and causes a suprabasilar bleb or blister as well as acantholysis, may affect the esophagus. Numerous acantholytic cells are usually present. The characteristic cells are round to polygonal, uniform, parabasal-sized, and isolated.26,27 The cytoplasm is dense and may have perinuclear eosinophilic staining or a clear halo. The cells appear atypical because of the high nucleus-to-cytoplasm ratio, the enlarged nuclei, and the prominent multiple, even irregular, nucleoli (Fig. 3.17). A bar- or bullet-shaped nucleolus is characteristic.28 However, the cells have smooth nuclear membranes, and the chromatin is pale, fine, and even. Normal mitotic figures can be seen. These atypical cells resemble those in repair except for the increased number of single cells.
Barrett Esophagus
Cytology is not the optimal tool for the diagnosis of Barrett esophagus. When glandular epithelial cells are seen in a cytology specimen, it is difficult to be certain whether they represent cells from the gastric side of the esophagogastric junction or metaplastic glandular cells from the esophagus. It has also been shown that cytology is neither sensitive nor specific for the detection of goblet cells,29,30 a hallmark of this condition, in part because of the absence of a blue hue of acid mucin with the Papanicolaou stain. However, a long segment of Barrett esophagus is more readily appreciated by cytology because of the reduced probability of sampling error.30 Its appearance is similar to that of the lining epithelium of the small intestine, with a Swiss cheese pattern at low magnification and goblet cells with single, large cytoplasmic vacuoles on high magnification (Fig. 3.18). The honeycomb arrangement of the glandular cells in Barrett esophagus usually tends to be slightly more irregular than that of normal small intestinal epithelium.
Neoplastic Lesions
Squamous Dysplasia or Carcinoma
Squamous dysplastic cells of the esophagus have morphology similar to that of the dysplastic cells on cervicovaginal Papanicolaou smears (Box 3.1 and Figs. 3.19 and 3.20).31 The cellular features of squamous cell carcinoma vary with the degree of differentiation (Box 3.2 and Fig. 3.21; Box 3.3 and Fig. 3.22).
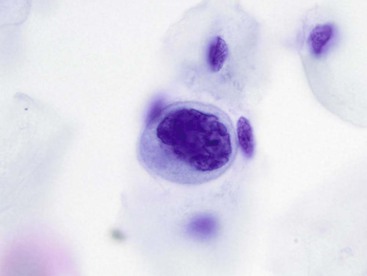
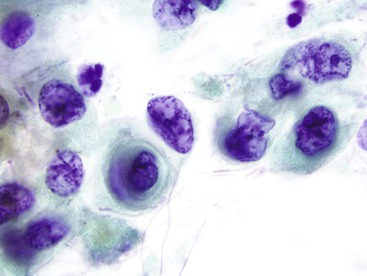
Glandular Dysplasia or Carcinoma
Glandular dysplasia and carcinoma in the esophagus usually arise in the setting of Barrett esophagus. The precursor lesions of adenocarcinoma in the stomach and in the intestine can manifest as either polypoid or flat dysplastic lesions. Adenomas of the stomach and dysplasia of the esophagus or stomach are similar in cytologic appearance. Although the few reported studies on this topic were based on very small numbers of cases29,30,32,33 and were insufficient to provide definitive conclusions on the usefulness of cytologic surveillance,34 the preliminary results appear promising. Low-grade dysplasia may be difficult to distinguish from artifactual crowding, whereas high-grade dysplasia may be confused with either severe reparative change or invasive carcinoma (Box 3.4 and Fig. 3.23; Box 3.5 and Fig. 3.24; Box 3.6 and Fig. 3.25). Ancillary molecular studies may be helpful to identify dysplasia and carcinoma in esophageal brushing specimens from patients with Barrett esophagus.35–37
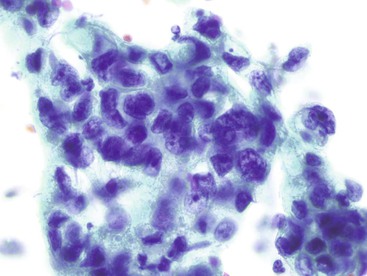
The amount and characteristics of the cytoplasm of the tumor cells depend on the degree of differentiation. Appearance varies from abundant vacuolated or granular cytoplasm to scant dense cytoplasm that is difficult to distinguish from that of a poorly differentiated squamous cell carcinoma.
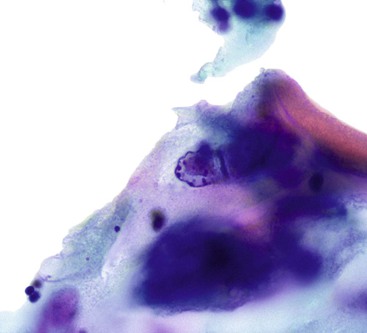
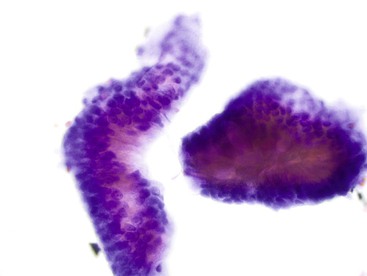
Signet ring cell carcinoma, a type of adenocarcinoma that occurs most commonly in the stomach, is worthy of special consideration because it can be difficult to detect on both cytologic and histologic preparations. Because the malignant cells infiltrate predominantly the lamina propria, they are often not included in the brush cytology sample unless mucosal ulceration is present. The reactive or reparative epithelial changes associated with an ulcer can distract the pathologist from the real lesion. In addition, the numerous inflammatory cells from the ulcer can obscure the scattered, isolated tumor cells (Box 3.7 and Fig. 3.26). Even when detected, some signet ring cells have such bland nuclei that they can be mistaken for histiocytes, which have intracytoplasmic phagocytized material and a very low nucleus-to-cytoplasm ratio. A high degree of suspicion is the best safeguard against failure to detect a signet ring cell carcinoma by cytology. When in doubt, immunocytochemical studies can be applied to the cytologic material to determine whether the phenotype of the cells of interest is epithelial or histiocytic. Carcinoma cells should be positive for epithelial markers such as keratin and epithelial membrane antigen, whereas histiocytes express CD68 (as detected by the KP1 antibody) and CD163.
Endocrine Tumors
GI neuroendocrine neoplasms are classified into two main categories according to the latest classification from the World Health Organization38: (1) neuroendocrine tumor (NET)—including grade 1 (carcinoid) and grade 2; and (2) neuroendocrine carcinoma—both large cell and small cell. In addition to morphology, the distinction between grade 1 and grade 2 NET depends on mitoses and the Ki67 proliferation index,39 which also distinguish NET from neuroendocrine carcinoma.
The prognosis of NET depends on the grade and on features that cannot be evaluated on cytologic preparations, including size and site of the lesion, presence of local invasion, angioinvasion, patterns of hormone production, and metastases.40 Cytologic atypia, mitotic index, and the proliferative index obtained by Ki67 immunostaining can be evaluated to some extent on cytologic materials. Along the GI tract, the small intestine is the most common site for such tumors, followed by the rectum and appendix,41 with the stomach a distant fourth. These tumors account for fewer than 1% of all gastric malignancies.42 However, cytologic specimens from the appendix, ileum, and rectum are almost never seen. Our experience with cytology of grade I NET has primarily involved tumors in the stomach and duodenum (Box 3.8 and Fig. 3.27).
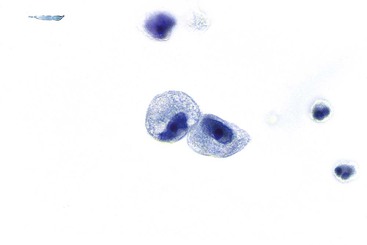
The tendency of NET cells to lose their cytoplasm causes them to mimic lymphomas with small cell morphology because of their small size and characteristic monomorphism. Such stripped nuclei can be distinguished from low-grade small cell lymphoma by their complete absence of cytoplasm and their finely granular (“salt-and-pepper”) chromatin pattern. Of course, one should always find intact cells to confirm the diagnosis. Small cell neuroendocrine carcinomas (small cell carcinomas) of the GI tract are similar to those seen elsewhere and are characterized by small cells with scant cytoplasm, showing nuclear molding and a finely dispersed chromatin pattern. Mitoses and necrosis are also prominent features of these tumors.
Mesenchymal Tumors
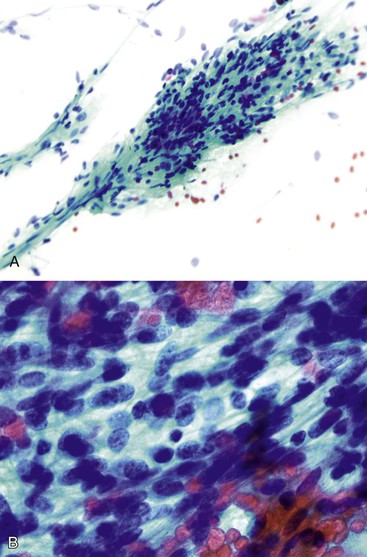
Mesenchymal tumors common in the GI tract include leiomyomas (predominantly of the muscularis mucosae of the esophagus and colorectum), GI stromal tumors, and leiomyosarcomas. Because of their submucosal or mural location, these tumors are not usually accessible by endoscopic brush unless the tumor is ulcerated. Endoscopic FNA with or without ultrasound guidance is the preferred method of sampling. Specimens from leiomyomas usually consist of sparse bland, cohesive spindle cells arranged in parallel lines with evenly spaced nuclei and abundant intercellular fibrillary matrix.43 However, specimens from GI stromal tumors and leiomyosarcomas are usually cellular with loose and crowded fragments and individual spindle or epithelioid cells (Box 3.9 and Fig. 3.28).
The individual cells of GI stromal tumors have a tendency to lose their cytoplasm to become stripped, spindle-shaped, or round to oval nuclei.44,45 Perinuclear or paranuclear vacuoles are present in some cells. Delicate cytoplasm and prominent nuclear palisading have also been noted.46 The tumor cells may appear spindly or epithelioid.47,48 Although leiomyosarcomas tend to show more significant nuclear pleomorphism and atypia as well as a less prominent vascular pattern than GI stromal tumors,49,50 immunocytochemistry, polymerase chain reaction (PCR) analysis of KIT, or both are needed to make the definitive distinction between the two. Most GI stromal tumors show strong diffuse positivity for CD117 and DOG1, whereas leiomyosarcomas are typically positive for desmin and actin50,51 and negative for CD117 and DOG1.
Although immunocytochemical staining for CD117 and DOG1 is useful in confirming a cytologic diagnosis of GI stromal tumor, the diagnosis of malignancy still depends on evaluation of the resected specimen. Detection of a KIT mutation in an FNA specimen was found to be promising in predicting malignant behavior, although absence of mutation does not preclude malignancy.52
Lymphoid Tumors
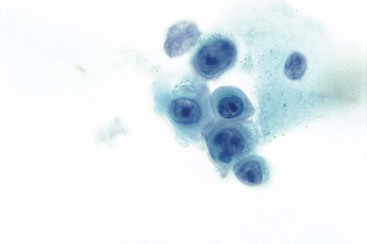
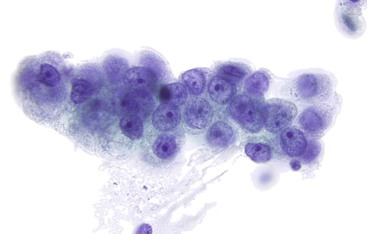
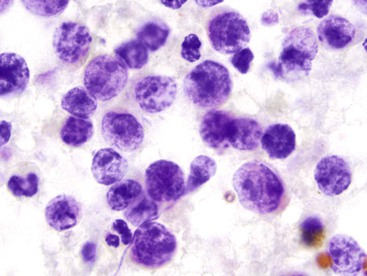
The cytologic appearance of lymphoma of the GI tract depends on its subtype. With adequate material and a combination of morphology and flow cytometry, a diagnosis of lymphoma can be established on the basis of a cytology specimen.53 The large cell type usually does not pose any diagnostic difficulty on morphology, because large malignant lymphoid cells are sufficiently atypical to raise the suspicion of a malignancy (Fig. 3.29). The challenge is to recognize them as being lymphoid and to distinguish them from poorly differentiated epithelial or mesenchymal tumors. Their lymphoid nature may in fact be easier to identify on cytology than in a small biopsy specimen. Large cell lymphoma cells shed as isolated, relatively monomorphic, large atypical cells with scant cytoplasm, vesicular nuclei, and a single large nucleolus or multiple prominent nucleoli.54 The absence of any true cohesion is the principal diagnostic feature of a lymphoma. Although a poorly differentiated carcinoma may shed predominantly as single cells, cell clusters can usually be found after a careful search. In addition, a poorly differentiated carcinoma often has more abundant cytoplasm, which may or may not be vacuolated, and a greater degree of nuclear pleomorphism than a large cell lymphoma. Immunocytochemical staining facilitates the distinction between lymphoma and carcinoma.
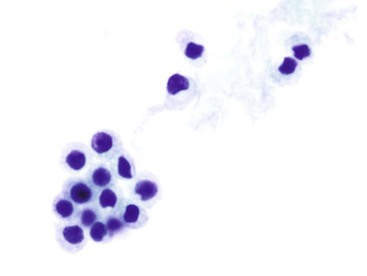
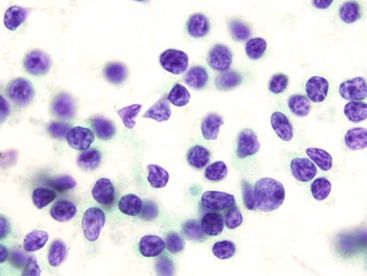
A low-grade small cell lymphoma, such as a lymphoma of the mucosa-associated lymphoid tissue (MALT lymphoma or MALToma), can be difficult to diagnose by cytology. It may be mistaken for an inflammatory process (Box 3.10 and Fig. 3.30), because it may contain a polymorphous population of small, intermediate-sized, and large cells.55–56 The dominant cell population is usually intermediate-sized lymphoid cells that contain a moderate amount of cytoplasm, show slight nuclear membrane irregularities, and have inconspicuous or completely absent nucleoli. These cells may show “plasmacytoid” morphology on air-dried preparations. Diagnosis of MALToma by cytology is challenging. A definitive diagnosis is usually made by cytology in only 50% of the cases,55,57 and reactive follicular hyperplasia is often erroneously diagnosed.56 Whereas brushings may obtain limited materials, endoscopic ultrasound-guided FNA has been shown to yield sufficient materials for immunohistologic, flow cytometric, and cytogenetic assessments for diagnosis of lymphoproliferative disorders.58