Diagnostic Cytology of the Biliary Tract and Pancreas
Barbara A. Centeno
Introduction
Endobiliary brushing is currently the preferred method to sample the pancreatobiliary system in patients with a stricture or obstruction without an associated mass. It is also used to screen patients with primary sclerosing cholangitis (PSC). Guidance methods include endoscopy, endoscopic retrograde cholangiopancreatography (ERCP), and percutaneous transhepatic cholangiopancreatography (PTC). Fine-needle aspiration biopsy (FNAB) is the most effective procedure to sample solid and cystic masses of the pancreatobiliary tract. Guidance techniques include intraoperative palpation and direct visualization, transabdominal ultrasound (TUS), intraoperative ultrasound, computed tomography (CT), and endoscopic ultrasound (EUS).
EUS is the preferred imaging technique at many academic centers and large community hospitals. Briefly, a biopsy needle is passed through the biopsy chamber of an echoendoscope, which consists of a linear array transducer mounted distal to the end of the viewing optical component of the endoscope. The needle is passed through either the duodenum or the stomach into the pancreatobiliary mass in real-time imaging.
Three manufacturers construct the needles used for EUS. They are made in 25-, 22-, and 19-gauge diameters and have a standard tip design. New sampling devices have been developed to improve the cellular collection from pancreatic masses. A through-the-needle cytologic brush system, the EchoBrush Endoscopic Ultrasound Cytology Brush (Cook Medical, Bloomington, Ind.), samples the interior lining of pancreatic cysts. Initial studies show that diagnostic yield and cellularity are better with the EchoBrush than with the standard EUS needle.1–3
The EchoTip ProCore High-Definition Ultrasound Biopsy Needle (Cook Medical) is a single-use, disposable needle for EUS-FNAB. It is designed as a regular needle but has an added reverse bevel, which increases tissue collection. The larger-gauge needles obtain corelike samples, but the 25-gauge needle obtains fragmented, fluid-like samples, similar to those obtained with a regular EUS needle.
Cytologic Sampling of the Pancreatobiliary Tract
Indications
Indications for sampling of the biliary tree and pancreatic ducts using brushing cytology include the presence of a stricture or obstruction. Brushing cytology is also used to monitor patients with PSC4,5 who are at risk for cholangiocarcinoma. FNAB is indicated for sampling either solid or cystic masses of the pancreatobiliary tract.
Contraindications
Contraindications to brushing of the biliary tract or pancreatic ducts include contraindications to the techniques used to guide the procedures for ERCP and PTC. Absolute contraindications to ERCP include patient refusal to undergo the procedure; unstable cardiopulmonary, neurologic, or cardiovascular status; and existing bowel perforation. Relative contraindications include structural abnormalities of the esophagus, stomach, or small intestine. PTC is contraindicated in patients with bleeding diatheses or significant ascites.
Contraindications to EUS-guided FNAB include the presence of an uncorrectable bleeding disorder and lack of a safe needle access route.6 Gastrointestinal obstruction is an absolute contraindication for EUS-FNAB because of the risk of intestinal perforation.7
Rapid On-Site Evaluation
The role of rapid on-site evaluation (ROSE) is not clearly established for brushing cytology. However, if smears are prepared on site, then it is preferable that they be prepared by an experienced cytotechnologist or pathologist.
ROSE, performed by a cytotechnologist or a pathologist, is probably the single greatest factor responsible for improvement in the diagnostic accuracy of FNAB for solid masses of the pancreatobiliary tract. ROSE of selected smears ensures adequacy of the specimen and reduces the number of inadequate specimens caused by poor localization of the lesion, scant cellularity, poor sample preparation, poor sample preservation, or obscuring blood. Thus, ROSE produces significant cost savings and may spare the patient from the need for another procedure.8–12 ROSE also allows the interpreter to determine the need for adjunctive studies such as flow cytometry, immunohistochemistry, or microbiology and prepare the sample accordingly. ROSE is not indicated for purely cystic masses but can be performed on the solid component of a cystic mass.
Sensitivity and Specificity
Biliary Brushing Cytology
Prospective and retrospective studies have documented a higher level of sensitivity for biliary brushings compared with exfoliative cytology in the detection of biliary carcinomas.13 A review of the literature shows that duct brushing cytology has an overall sensitivity of 26% to 88.9%, a specificity of 80% to 100%, and an overall accuracy of 48.1% to 96%. In PSC, the sensitivity was reported to be 60%, with a specificity of 89%. The sensitivity of bile duct brushings has been shown to increase after repeated attempts.14,15 In fact, after three negative brushings, the probability that a patient has a carcinoma is less than 6%.16 Predictors of positive yield include older patient age, mass size greater than 1 cm, and stricture length greater than 1 cm17,18 or the presence of a true stricture. The presence of stones correlates with benign cytology.19
Most false-negative results are caused by sampling error,14,15 which can occur if the tumor does not invade biliary mucosa. In addition, tumors with sclerotic desmoplastic stroma do not exfoliate cells as readily as tumors without desmoplasia and therefore may not be detected with brushings. Poor visualization of the area by the endoscopist may negatively impact the sampling. Interpretation errors (17%) and technical errors (17%) are the second most frequent causes of false-negative results. Most interpretation errors result from underinterpretation of adenocarcinoma caused by the difficulty of distinguishing adenocarcinoma from reactive changes.20 The converse is also true: Reactive changes can mimic adenocarcinoma and lead to false-positive findings. Degeneration of malignant cells is another source of false-negative results.
False positive findings commonly result from overinterpretation of reactive and degenerative changes21 or of adenomatous epithelium from villous tumors of the ampulla22 or the bile duct. Intraductal neoplasms and dysplasia within the biliary ductal system may also lead to false-positive results.
FNAB Cytology
The reported sensitivity for percutaneous pancreatic FNAB, with the use of a variety of guidance techniques, ranges from 45% to 97%. The specificity is almost 100%. Diagnostic accuracy ranges from 75% to 100%.23 The low sensitivity and low negative predictive values are cited as major limitations of pancreatic FNAB.23 However, in our experience, the sensitivity for a diagnosis of adenocarcinoma is greater than 90% when a cytotechnologist is on site to ensure specimen adequacy.12 The sensitivity for EUS-FNAB has ranged from 60% to 96% in large series, with a specificity of almost 100%.24–28
Factors that affect the accuracy of the procedure include accurate localization of the mass by the operator, adequate sampling of the mass, correct sample preparation, and correct interpretation by the pathologist. Accurate localization and adequate sampling are highly dependent on the skill of the operator. The sample should be representative of the intended target and should contain a sufficient number of cells for proper interpretation. Certain lesions may not yield diagnostic material regardless of the skill of the operator. These include most cystic lesions and sclerotic, vascular, or necrotic tumors. Aspiration of the periphery of necrotic tumors may help obtain better-preserved material. Poor sample preparation also hinders accurate interpretation by the pathologist. Factors that affect interpretation include blood, air-drying artifact on smears intended for alcohol fixation, crush artifact, and smears that are spread too thickly.
Specimen Preparation
Preparation of Pancreatobiliary Tract Brushing Specimens
The traditional method of specimen preparation is to prepare smears from the brushing—both air-dried smears for on site interpretation and alcohol-fixed smears for Papanicolaou stains. The brush may also be collected in Cytolyt solution (Hologic, Marlborough, Mass.) and the sample prepared as a ThinPrep (Hologic). The latter technique shows greater sensitivity.19 In another method, the brush is vortexed and then the sheath and guide wire are cut into 5-cm pieces and vortexed separately. The specimen is prepared as a cytospin. This procedure significantly increases the cellularity.29 Another published procedure entails processing the entire brush as an actual surgical pathology specimen.30
Preparation of FNAB Specimens
Preparation methods used for FNAB samples include direct smears, cytospins (Shandon CytoSpin, Thermo Fisher Scientific, Waltham, Mass.), liquid-based preparations such as ThinPrep or SurePath Prep (TriPath, Burlington, N.C.), and cellblocks. Guidelines for preparation of the various sample types follow.
Samples from Cystic Lesions.
An aliquot of cyst fluid obtained by FNAB should be submitted for measurement of carcinoembryonic antigen (CEA) and amylase. It may be possible to send a sample of the supernatant after the fluid has been spun down. If the sample is insufficient, it may be diluted and then the final CEA and amylase values corrected by the dilution ratio.31 A sample of the fluid may also be sent for molecular analyses (e.g., KRAS mutational analysis). It is recommended that the remainder of the fluid be prepared by using cytospin and cell block techniques rather than smears.
The EchoBrush is used to sample the interior wall of cystic masses. The EchoBrush is passed through the biopsy chamber and used to brush the cyst wall. The endoscopy staff cuts off the brush tip and put it in saline. The brush tip is spun down with the saline. The sample is then used to make cytospins and cell blocks.
Samples from Solid Masses.
At the Moffitt Cancer Center, a cytotechnologist is present on site for adequacy interpretations of solid masses. Two smears are prepared from an aliquot of each pass; one is used to make an air-dried Diff-Quik–stained smear and the other to make an alcohol-fixed Papanicolaou-stained smear. The remainder of the sample is rinsed in saline for processing as a cell block. If lymphoma is suspected, the sample is rinsed in Roswell Park Memorial Institute (RPMI) medium.
The ProCore needle obtains a sample similar to a core biopsy when the larger-gauge needles are used. Touch imprints may be made from the larger-gauge needles for on-site evaluation. The 25-gauge needle most often yields fragmented, liquid-like material that can be smeared. The cores are placed in saline and prepared with the use of a cell block technique.
Contaminants
Contaminants from the duodenum may occasionally be seen with ERCP-guided brushing cytology of the pancreatobiliary tree, and contaminants from other sites may be obtained with FNAB of either the biliary tract or pancreas (Box 35.1).
Mesothelial Cells.
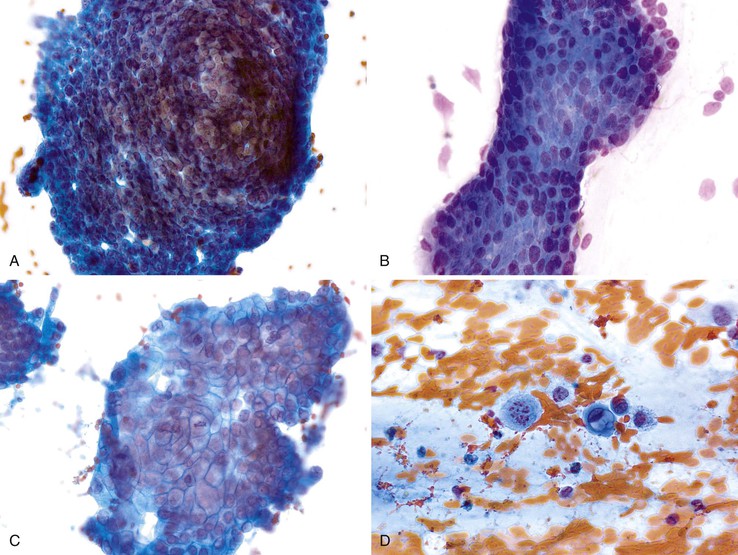
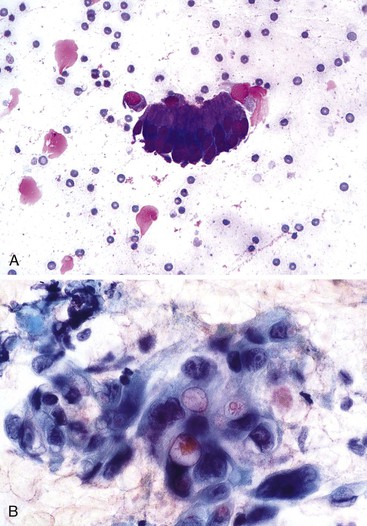
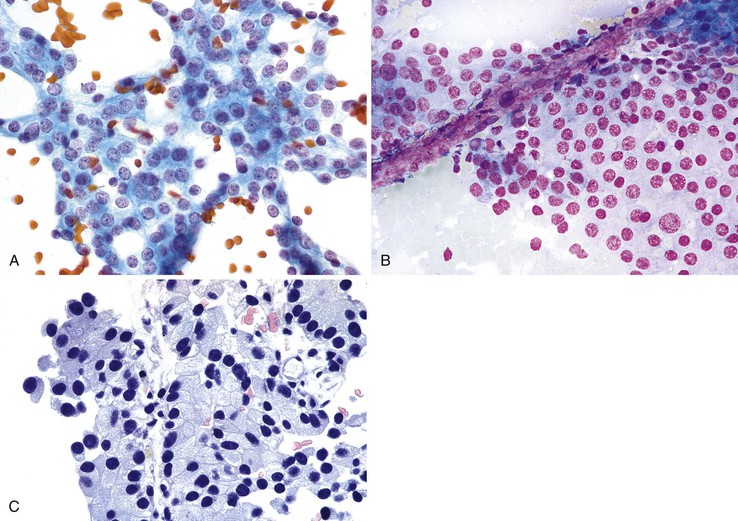
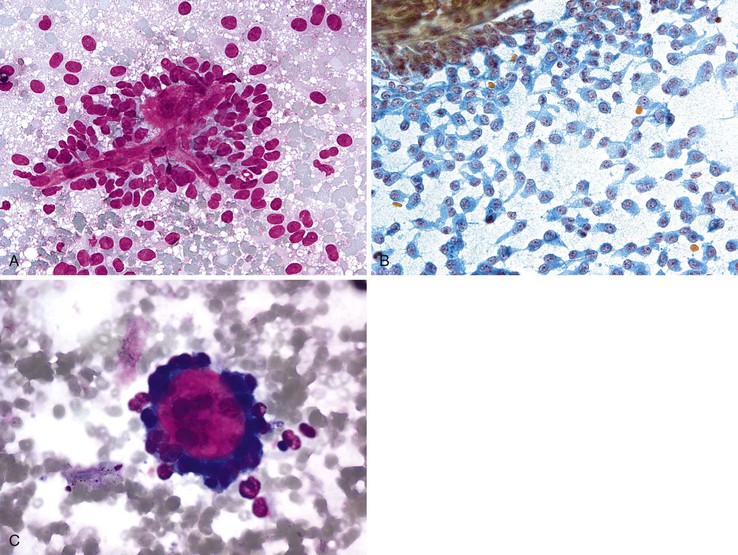
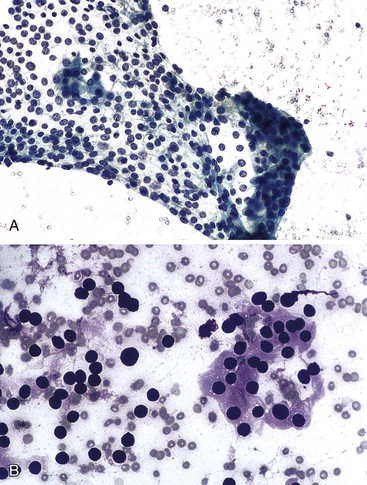
Mesothelial cells are polygonal cells arranged in flat sheets that show round to oval nuclei and intercytoplasmic “windows” (Fig. 35.1). They may be mistaken for benign ductal cells or possibly squamous cells.
Hepatocytes.
Normal hepatocytes are large polygonal cells with dense, sharply defined cytoplasm and round, central or eccentric nuclei with prominent nucleoli (Fig. 35.2).
Gastrointestinal Epithelium.
Gastrointestinal epithelium is a frequent contaminant in EUS-FNAB samples.
Gastric Epithelium.
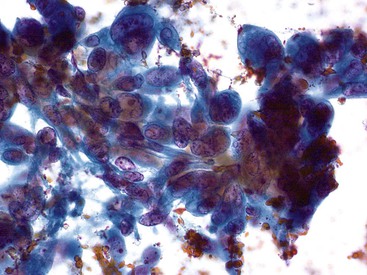
The surface mucus cells of the gastric epithelium are columnar cells arranged in large folded sheets, palisaded rows, or single cells, usually associated with background mucin. Typically, a luminal border may be seen along one edge of the cellular aggregates. The sheets are typically monolayered but occasionally may be folded or thick (Fig. 35.3). The gastric pits may appear in some groups of cells as rosettes present in the center of a cellular sheet. The mucus glands of the cardia or pylorus are indistinguishable from the foveolar surface mucus cells. The cells derived from the foveolae and mucus glands display mucinous cytoplasm, often contained in the upper third of the cytoplasmic compartment (Fig. 35.4), although the mucin may extend to the nucleus.
Gastric epithelium may also appear as stripped, bland, slightly elongated nuclei within a background of mucin (Fig. 35.5).32,33 Chief and parietal cells may be observed if the needle has traversed the fundic or body region of the stomach (Fig. 35.6). Intact fragments may show attachment of surface epithelium to the lamina propria. Gastric epithelium is associated with abundant mucus that may contain degenerated cells if there is an inflammatory process.
Small-Intestinal Epithelium.
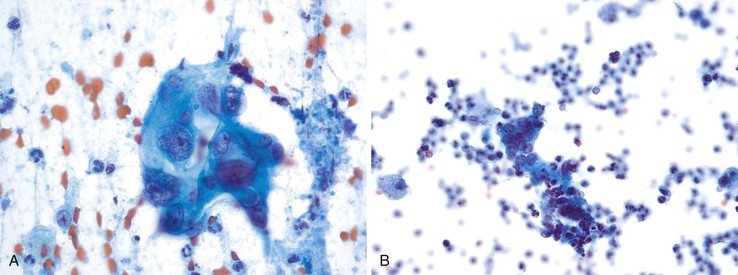
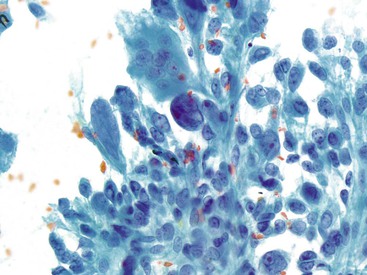
Small-intestinal epithelium has an architectural appearance similar to that of the surface mucus epithelium of the stomach, but the epithelial component is composed of absorptive enterocytes with interspersed goblet cells and is also associated with background mucin (Fig. 35.7, A). The brush border is visible when the cells are seen on edge (see Fig. 35.7, B). The epithelium may contain lymphocytes, which appear as darker, small cells within the epithelium.32,33 Paneth cells are occasionally seen and are identified by the presence of coarse eosinophilic granules in the apical cytoplasm.
Both small-intestinal epithelium and gastric epithelium may produce abundant degenerated material that should not be confused with neoplastic cells. Both may also be associated with lamina propria that may resemble a stromal neoplasm.
Cytology of the Biliary Tract
Because brushing cytology is the technique most frequently used to sample the biliary tract, the cytologic descriptions in this section pertain to brushing cytology. The cytologic criteria for the FNAB diagnosis of benign and malignant entities in the biliary tract are similar to those in the pancreas.
Normal Biliary Tract
Normal bile duct epithelial cells are tall and columnar or cuboidal in appearance The cells are typically arranged in flat monolayered sheets (Fig. 35.8, A) or in a picket-fence arrangement with basally located nuclei (see Fig. 35.8, B). The nuclei are round to oval, and the cytoplasmic borders are distinct (Box 35.2).
Reactive Changes
Inflammation of the biliary system results from choledocholithiasis, sclerosing cholangitis, acute cholangitis, infections, calculi, stents, or instrumentation. The changes induced by inflammation include loss of surface structures, vacuolization of the cytoplasm, nuclear enlargement, coarse hyperchromasia, and multinucleolation34 (Fig. 35.9). The epithelium remains monolayered and lacks pseudoacinar structures. In situations of inflammation or injury, the epithelial lining may develop mucinous metaplasia or squamous metaplasia (Box 35.3).
Dysplasia
Premalignant lesions of the bile ducts have historically been referred to as biliary dysplasia or atypical biliary epithelium. A new consensus classification of biliary intraepithelial neoplasia (BilIN) was published in 2007.35 This proposal classified BilIN into three grades, similar to the scheme used for other organs such as the pancreas and prostate. The histopathologic criteria are similar to those for other intraepithelial lesions. The cytopathologic criteria have not been defined but can be assumed to be similar to what has been described as dysplasia in the biliary tract, with grade 1 lesions causing atypia on bile duct brushings previously referred to as low-grade dysplasia5 and grade 3 lesions causing atypia previously referred to as high-grade dysplasia.
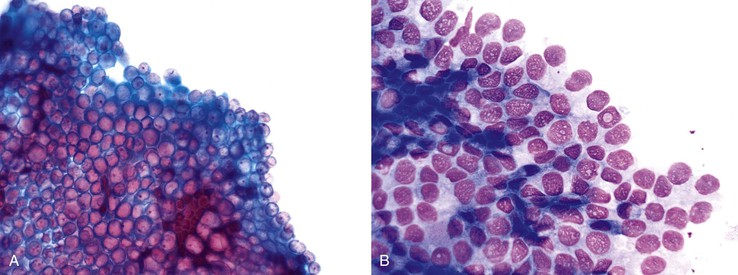
Lesions with grade 2 dysplasia may fall into either category on brushings. Low-grade dysplasia is characterized by sheets and clusters of cells that show nuclear crowding and overlapping, smooth nuclear membranes, and a moderate nucleus–to-cytoplasm (N : C) ratio (Fig. 35.10, A). The chromatin is characteristically clear in appearance and granular, with mild clumping. Low-grade dysplastic cells may have one or two distinct nucleoli. More pronounced nuclear crowding and overlapping occurs in BilIN grade 3, or high-grade dysplasia. In these cases, nuclear membranes are irregular and the N : C ratio is significantly increased; chromatin is coarse; and nucleoli are distinct and prominent (Box 35.4; see Fig. 35.10, B).
Adenoma of the Biliary Tract and Ampulla
Adenomatous epithelium appears as slender, elongated, columnar cells arranged singly or in small sheets and clusters. The long, thin, basally oriented nuclei occupy approximately one third of the cell. The chromatin is typically fine and granular with one or more nucleoli (Fig. 35.11).36 It may be difficult to separate these lesions from adenocarcinoma or dysplasia based on brushings (Box 35.5).
Intraductal Neoplasms of the Biliary Tract
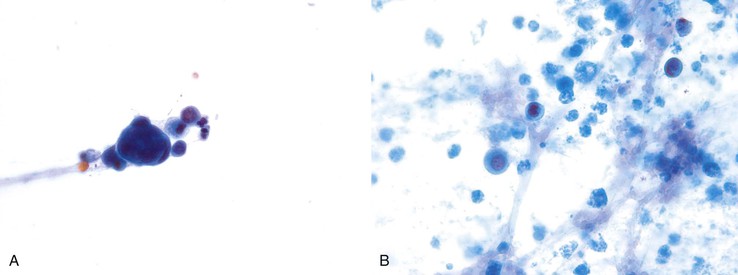
Intraductal papillary mucinous neoplasms of the bile ducts (IPMN-B) correspond to lesions previously referred to as biliary papillomatosis or as cystic or mucinous lesions of the biliary tract,37 similar to intraductal papillary mucinous neoplasms of the pancreas (IPMN-P).38 The role of cytology in diagnosing these lesions is not established. What have been previously described as atypical papillary or mucinous cells indicative of well-differentiated adenocarcinoma are probably derived from these lesions.17 Cytologic features of papillary differentiation include groups with crowding and overlapping, papillary formations, and elongated nuclei. Features of mucinous differentiation are groups with mucinous cytoplasm (Fig. 35.12).
Adenocarcinoma
Cytology preparations of adenocarcinoma tend to be cellular and contain both cohesive and single cells. Groups of cells are usually arranged in sheets with nuclear overlapping and crowding and loss of polarity. A very useful finding is the presence of two-cell populations (Fig. 35.13). The nuclei lose their normal round shape and become rectangular, pointed, or angulated with convolutions and notches, particularly in high-grade carcinomas (Fig. 35.14, A). The N : C ratio is altered. The chromatin is typically coarse but may be pale in well-differentiated adenocarcinomas. Carcinomas with more abundant mucin in their cytoplasm show both nuclear and cellular enlargement and appear in an exaggerated honeycomb pattern. Pseudoacinar formations are features of adenocarcinoma (Box 35.6; see Fig. 35.14, A).
Mucinous and papillary differentiation have been described as subtle findings that need to be recognized because they may represent well-differentiated adenocarcinoma.17 The recommendation was to report any mucinous change as possibly representative of a mucinous neoplasm and any papillary change as suggestive of a papillary neoplasm. It now seems that these morphologic patterns are derived from IPMN-B if identified on a brushing (see earlier discussion). These findings identified on an FNAB of a solid mass are more likely to correlate with an invasive process.
A pitfall occurs with the papillary pattern because normal bile epithelium may form pseudopapillary clusters. Papillary clusters in normal duct cells are two dimensional. The cell clusters in true papillary lesions are crowded, and nuclei are usually arranged haphazardly. The nuclei of true papillary lesions are pointy and elongated, in contrast to their normal round to oval shape. Occasionally, the nuclei of benign epithelium may appear elongated, but again, the architectural arrangement is key. Carcinomas show a greater degree of architectural abnormalities and the presence of hypochromatic, transparent nuclei.
Differential Diagnosis
Reactive Processes versus Adenocarcinoma in the Biliary Tract
The key problem when assessing pancreatobiliary brushing cytology specimens is differentiating a reactive process from adenocarcinoma. A number of studies have focused on identifying sets of criteria that are most specific for adenocarcinoma. Initial studies assessing cytologic criteria on smears found architectural abnormalities, nuclear enlargement, “cell-in-cell” arrangement, loss of polarity, a bloody background, the presence of flat nuclei, nuclear molding, and chromatin clumping39–41 to be indicative of the presence of adenocarcinoma, although a cytologist’s overall gestalt assessment appeared to be just as good.41 Another report described adenocarcinoma as showing architectural features of high-grade dysplasia but characterized by the presence of a greater number of single cells.34
In ThinPrep-prepared specimens, features typical of carcinomas were three-dimensional micropapillae, anisonucleosis, high N : C ratio, nuclear contour irregularity, and prominent nucleoli. Cytomorphologic features that were not helpful in distinguishing positive and negative cases were single naked nuclei, chromatin granularity, and necrosis.42
A prospective study of brush smears obtained during ERCP found tightly cohesive, small, three-dimensional cell clusters that formed cell balls as the most consistent feature of malignancy. The cells in the clusters displayed features of malignancy. The authors concluded that the presence of single cells, cytoplasmic vacuoles, and prominent nucleoli are not essential for a diagnosis of malignancy.43
In summary, cytologic features that are indicative of malignancy include loss of normal honeycomb pattern, loss of polarity, nuclear enlargement, anisonucleosis, nuclear membrane irregularities, and chromatin abnormalities. Identification of a two-cell population is also a very helpful feature. Single cells are not always identified in malignancy, and macronucleoli and necrosis may be seen in inflammatory processes (Table 35.1).
Table 35.1
Biliary Tract: Reactive Processes versus Adenocarcinoma
| Criterion | Reactive Processes | Adenocarcinoma |
| Architecture | Retained honeycomb pattern, 2-dimensionality | Loss of honeycomb pattern, 3-dimensionality |
| Loss of polarity | Absent | Present |
| Nuclear molding | Absent | Present |
| Nucleus–to-cytoplasm ratio | Low | Increased |
| Nuclear membrane irregularities | Minimal | Loss of roundness, nuclei become angulated |
| Anisonucleosis | Minimal | >4 : 1 |
| Nuclear enlargement | Absent to minimal | Present |
| Chromatin | Finely dispersed | Coarse or hypochromatic |
| Cell population | Range of atypia | Two-cell population |
| Single, malignant cells | Absent | Present |
Ancillary Studies
Molecular Investigations
One study that assessed a panel of 12 polymorphic microsatellite markers linked to six tumor suppressor genes and KRAS codon 12 mutation detection showed that this panel was effective.44 A subsequent study assessing 20 bile duct brushing specimens for mutations in TP53, KRAS, BRAF, and EGFR found these mutations too infrequent in biliary tract adenocarcinomas to be of added diagnostic benefit.45
Aneuploidy and Chromosomal Analyses
Two advanced cytologic techniques for detecting aneuploidy—digital image analysis (DIA) and fluorescence in situ hybridization (FISH)—are reported to improve the sensitivity of brushing cytology to diagnose adenocarcinoma in pancreatobiliary strictures. In one study, the authors confirmed that conventional cytology has a low sensitivity (4% to 20%) but 100% specificity. When conventional cytology results were negative, FISH had an increased sensitivity (35% to 60%) in assessing for chromosomal gains (polysomy) while preserving the specificity of cytology. The sensitivity and specificity of DIA was intermediate compared with routine cytology and FISH but was additive to FISH values, demonstrating only trisomy of chromosome 7 or chromosome 3.46 When the panel developed to detect urothelial carcinoma (UroVysion panel) was used, FISH alone increased the sensitivity of routine cytology.47,48 In another study, FISH had a sensitivity of 94%, compared with 81% for routine cytomorphology.49 A technique of intraductal ultrasound combined with standard cytology, DIA, and FISH enhances the accuracy of standard techniques in the evaluation of indeterminate bile duct strictures, allowing diagnosis of malignancy in a substantial number of patients with false-negative cytology.50
The combination of KRAS mutation and FISH analyses appears to increase the cancer detection rate in patients with pancreatobiliary strictures51 beyond that of cytology and FISH alone. KRAS mutation analysis was performed on residual cytology specimens by using the quantitative, polymerase chain reaction (PCR)-based DxS KRAS Mutation Test Kit (DxS Ltd., Manchester, U.K.) in patients who also had cytologic evaluation and FISH analysis of their brushing specimens. KRAS mutations were identified in 24 (69%) of pancreatic adenocarcinoma specimens, and FISH results were positive in 22 (63%), with a combined sensitivity of 86% (30/35). KRAS mutations and polysomic FISH results were identified in fewer cholangiocarcinoma patients—in 12 (29%) and 17 (41%) of cholangiocarcinoma specimens, respectively—with a combined sensitivity of 54% (22/41).
Protein Analysis
Some proteins reportedly increase the sensitivity of brushing cytology for detection of adenocarcinoma. Insulin-like growth factor-II mRNA-binding protein 3 (IMP3 cytology), when used alone, showed a sensitivity of 64.1% for diagnosis of adenocarcinoma; the sensitivity rose to 74.1% when combined with routine cytology. Cytology alone showed a sensitivity of 33.3%.52 S100 protein P has been reported to be upregulated in cholangiocarcinoma.53 Analysis of S100P expression by reverse transcriptase (RT)-PCR showed that this protein was frequently expressed in adenocarcinomas of the biliary tract but not in normal tissues. K-homology domain containing protein overexpressed in cancer (KOC) and S100A4 are two additional proteins evaluated in biliary brushing specimens. The authors of this study assessed protein expression on alcohol-fixed smears and included cases that were negative, indeterminate, and diagnostic for malignancy. In this study, the sensitivity and specificity were, respectively, 83% and 95% for cytology, 92% and 95% for KOC, and 79% and 95% for S100A4 protein. The combined use of KOC and S100A4 showed a sensitivity of 100% and a specificity of 95%.54
Cytology of the Pancreas
Assessment of the pancreatic FNAB sample needs to begin before the slides are reviewed. An integrative approach to the evaluation of pancreatic aspirates incorporating the clinical history, radiologic findings, cytologic findings, and ancillary studies yields the most clinically relevant interpretation of the aspirate material (Box 35.7).
Age and gender are important because some neoplasms show specific gender and age predilections. Because metastases to the pancreas from known primaries may occur, it is important to obtain a history of any previous malignancies. A prolonged history of pancreatitis without an underlying cause may suggest IPMN. A cyst associated with a history of alcohol-induced pancreatitis may suggest pseudocyst.
Most crucial are the imaging findings indicating whether the mass is solid or cystic; this information will determine the cytopathologic algorithm. Different entities are considered, depending on whether the imaging studies show a mass that is solid, solid and cystic, purely cystic, or cystic with a connection to the ductal system (intraductal lesion). Chronic pancreatitis, lobular atrophy, ductal adenocarcinoma, pancreatic neuroendocrine tumor (PanNET), acinar cell carcinoma (ACC), and metastases are among the entities that are considered if the lesion is solid. Solid and cystic lesions include any solid tumor that has undergone cystic degeneration, such as PanNET, and also solid-pseudopapillary neoplasm (SPN). Purely cystic-appearing lesions include mucinous cystic neoplasms (MCN), serous cystadenoma, side branch IPMNs, and pseudocysts. More specific information, such as an impression of serous cystadenoma, may be provided by the person reporting the imaging findings.
A stepwise analysis of the aspirate sample begins with evaluation of the gross sample. This evaluation is particularly informative for cystic lesions. At low power, assessment of cellularity, architectural features, and background yields a great deal of information, which may in itself be diagnostic. At intermediate power, architectural details can be assessed in greater detail. At high power, the nuclear, cytoplasmic, and mitotic features are appreciated.
Ancillary studies include immunohistochemistry, flow cytometry for suspected lymphoma, cyst fluid analysis for CEA and amylase, and mutational analyses. Ancillary studies are most routinely used for the differential diagnosis of nonductal neoplasms and metastases and in the work-up of pancreatic cyst fluids.
Normal Pancreas and Contaminants
Table 35.2 summarizes the cytologic features of normal pancreatic epithelia.
Table 35.2
Cytologic Characteristics of Pancreatic Epithelia
| Epithelium | Cytoplasm | Nuclei | Architectural Features |
| Ductal epithelium | Tall columnar or cuboidal, with mucin | Round to oval | Flat, honeycomb sheets or palisade columns of cells |
| Acinar epithelium | Pyramidal, abundant, well defined, contains zymogen granules | Round, with prominent nucleoli | Acinar groups of cells or single cells |
| Islet cells | Scant and variable, amphophilic, contains neuroendocrine granules | Round to oval with salt-and-pepper chromatin | Loosely cohesive groups of cells |

Ductal Cells
The lining of the large pancreatic ducts is composed of columnar epithelium arranged in flat, honeycombed, two-dimensional sheets of cells with centrally located nuclei (Fig. 35.15). The ductal cells may also be present in a palisaded, “picket-fence” arrangement in which the nuclei are basally located. In contrast to the epithelium of the large ducts, intralobular duct epithelium is composed of cuboidal-shaped cells with scant basophilic cytoplasm and are usually present as flat sheets, small clusters, or tubular structures.
Acinar Cells
Aspirates of the normal pancreas consist predominantly of acinar-type epithelium, which is typically arranged either singly or in small acinar structures (Fig. 35.16). The cells are pyramidal or triangular in shape and contain abundant, granular cytoplasm with numerous intracytoplasmic zymogen granules. The nuclei are round, have a granular chromatin pattern, contain prominent nucleoli, and are either central or eccentrically located.
Islet Cells
Islet cells are only rarely detected in aspirates of the normal pancreas. More often, they are seen in aspirates from patients with chronic pancreatitis with islet cell hyperplasia. When present, they occur as loose aggregates of cells that contain wispy, ill-defined amphophilic cytoplasm and oval nuclei with a stippled chromatin pattern (Fig. 35.17).
Solid Masses
Cytologic Approach to Aspirates of Solid Masses: Ductal Pattern versus Solid Cellular Pattern
Primary neoplasms of the pancreas manifesting as solid masses have predominantly two distinct morphologic patterns. The first is the typical histopathologic pattern of ductal neoplasms, which shows ductal-type groups with significant pleomorphism associated with desmoplastic stroma. Aspiration may be difficult because of the fibrous stroma, and sometimes the stroma results in samples that are scantly cellular. The differential diagnosis is usually with pancreatitis. The other pattern is that of the solid, cellular neoplasm. PanNET, ACC, SPN, and pancreatoblastoma (PB) fall in this category (see later discussion). Histopathology shows a neoplastic process growing as solid cell sheets, without a desmoplastic stroma. The cytology smears are diffusely cellular with numerous single cells and loose groups. These neoplasms may also be vascular.
Reactive Processes
Reactive processes of the pancreas that may be sampled by FNAB include acute and chronic pancreatitis and autoimmune pancreatitis. The role of FNAB is to rule out adenocarcinoma or other neoplasms. Occasionally, the FNAB may sample a reactive process adjacent to a neoplasm; clinical follow-up and further evaluation is always indicated for patients with clinical and imaging findings suspicious for adenocarcinoma or neoplasia (Box 35.8).
Acute Pancreatitis
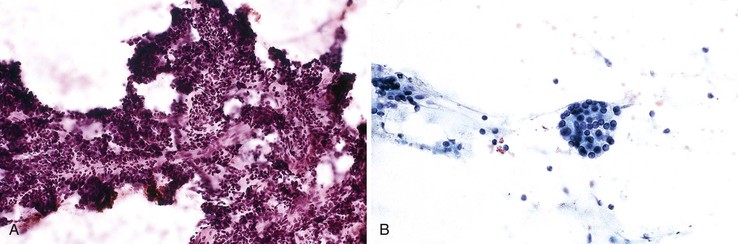
Typical smears from patients with acute pancreatitis show dirty, necrotic background cells, cellular debris, and fat necrosis with saponification. Acute inflammation may be prominent. The normal pancreatic elements, when present, may show evidence of necrosis and degeneration (Fig. 35.18). Pancreatic ductal epithelium may show various degrees of reactive atypia as well (see Box 35.8).
Chronic Pancreatitis
Aspirates of chronic pancreatitis are not typically very cellular; they consist of varying amounts of inflammatory cells, ductal cells, acinar cells, calcifications, fibrous tissue fragments (Fig. 35.19), and debris.55 Islet cells are sparse or absent. The inflammatory component usually consists of mononuclear cells and histiocytes. Acinar cells may be associated with inflammation and fibrosis. The ductal cells show mild atypia but remain monolayered and organized (see Box 35.8).
Autoimmune Pancreatitis
FNAB smears show a paucity of ductal epithelium and are composed of inflammatory cells and stromal fragments. The key feature that helps distinguish this process from others is the presence of cellular stromal fragments with embedded lymphocytes.56 A dense plasmacytic infiltrate and eosinophils are also common components of this process, but a lymphocytic background is rare.
Reactive Atypia
Reactive atypia induced by pancreatitis or other processes shows a spectrum of changes (Fig. 35.20). When severe, the atypia may mimic adenocarcinoma. A key feature is that the atypical groups are fewer in number than the number of groups associated with ductal adenocarcinoma. Another feature is that there is usually a range of atypia in the ductal groups, from benign to malignant. The differentiation of benign and reactive processes from adenocarcinoma is discussed later (see Differential Diagnosis and Pitfalls).
Ductal Adenocarcinoma
Diagnosis of pancreatic ductal adenocarcinoma (PDA), not otherwise specified (NOS), based on FNAB samples is usually straightforward. Occasionally, it is difficult to distinguish between benign lesions and adenocarcinoma or between reactive lesions and adenocarcinoma.
Diagnostic Criteria
Several studies have evaluated a number of cytomorphologic criteria in pancreatic FNAB specimens to identify a set of minimal cytologic criteria that can reliably separate benign processes from adenocarcinoma.57–59 Although the criteria identified as most accurate vary and their accuracy has not been proven in a prospective study, it is clear that a systematic approach incorporating these criteria leads to improved diagnostic accuracy. The criteria can be categorized into cellular composition and cellularity, architectural features, background, cytoplasmic features, and nuclear features and mitotic activity (Box 35.9).
Cellular Composition
Benign pancreas is composed of a mixture of ductal cells and acinar cells, whereas PDA is usually composed of a relatively pure population of ductal-type cells (Fig. 35.21). This fact is most useful in the examination of aspirates obtained at laparotomy, when it is presumed that the surgeon has inserted the needle directly into the mass. Care must be taken in EUS-FNAB not to misinterpret gastrointestinal contamination as lesional.
Cellularity
Adenocarcinoma tends to produce cellular smears (see Fig. 35.21). However, tumors with a sclerotic stroma or necrosis may yield paucicellular samples. In this case, close attention to the atypia of particular groups is needed. Preparation of a cellblock from the collected material is helpful because it may contain diagnostic tissue, or even tissue fragments with invasive cells, when the smears are scant and indeterminate.
Architectural Features
Architectural features are the most helpful aspect. Malignant groups lose the normal, honeycomb, two-dimensional patterns of ductal epithelium. Architectural atypia is manifested as three-dimensionality, crowding and overlapping of the nuclei (Fig. 35.22, A and B), or an exaggerated (drunken) honeycomb sheet arrangement (see Fig. 35.22, C). Cribriforming is also a feature of malignancy. Cells may be arranged in sheets or in small clusters or groups. Other helpful features include loss of polarity and nuclear molding (see Fig. 35.22, A and B). Loss of cohesion and single cells (see Fig. 35.22, D) are a feature of malignancy, but well-differentiated carcinomas may retain their cohesion and not have any malignant single cells.
Background
Coagulative necrosis is typical of malignancy but is more frequent in higher-grade carcinomas (Fig. 35.23, A). Well-differentiated carcinomas may have a clean background (see Fig. 35.22, A through C). Other features that can be assessed as part of the background are inflammation (see Fig. 35.23, B), secretory products, and cellular dyshesion.
Cytoplasmic Features
Because normal pancreatic ductal cells do not display visible cytoplasmic mucin, the presence of abundant cytoplasmic mucin (as in Fig. 35.22, C) is a feature of a neoplastic process. In the setting of a radiologically detected mass lesion, it is a feature that supports a malignant interpretation. The cells may appear as columnar cells seen on edge with columnar, mucin-containing cytoplasm (Fig. 35.24, A), or the cells may show abnormal cytoplasmic mucin vacuoles (see Fig. 35.24, B).
Nuclear Features
Nuclear features include changes in nuclear size, membrane profile, and distribution of the chromatin. Normal nuclei are round and uniform (see Fig. 35.15, A). Malignant nuclei lose that roundness and develop irregular nuclear membranes with angulated, sharp edges or rectangular, flattened sides, or they become more elongated and carrot shaped (see Fig. 35.22, B). The nuclei may show noses or blebs and nuclear membrane folding (see Fig. 35.22, C). Notches and convolutions become more apparent in higher-grade carcinomas (see Fig. 35.23, A). Hypochromasia with parachromatin clearing is more often a feature of well-differentiated adenocarcinoma (see Fig. 35.22, C), whereas hyperchromasia is more apparent in higher-grade carcinomas.
Nuclear Enlargement.
Carcinoma nuclei are approximately 1.5 times the size of a red blood cell on Romanowsky stain or twice the size of the nuclei of normal ductal cells (see Fig. 35.24, A).
Anisonucleosis.
Variation in nuclear size of greater than 4 : 1 is a feature of malignancy (see Fig. 35.23, B).
Mitoses.
Abnormal mitotic figures are diagnostic when identified.
A comparison of the cytologic criteria distinguishing well-differentiated and high-grade PDA is presented in Table 35.3.
Table 35.3
Comparison of Well-Differentiated and Moderately or Poorly Differentiated Adenocarcinoma
| Criteria | Well-Differentiated | Moderately or Poorly Differentiated |
| Cellularity | Variable | Variable |
| Background | Clean or bloody | Coagulative necrosis |
| Architecture | Large, folded groups; crowding and nuclear overlapping | More three-dimensional groups, smaller atypical groups |
| Dyshesion | Infrequent; cohesion more typical | Present |
| Anisonucleosis | Present as defined but subtle | More variable |
| Nuclear enlargement | Minimal as defined | Larger and more variable |
| Chromatin appearance | More often hypochromatic | Hyperchromasia and abnormal parachromatin clearing |
| Nuclear membrane abnormalities | Elongations and angulation | More obvious notches and convolutions |
| Mitoses | Infrequent | More apparent |
| Macronuceloli | Absent | More apparent |
Undifferentiated (Anaplastic) Adenocarcinoma
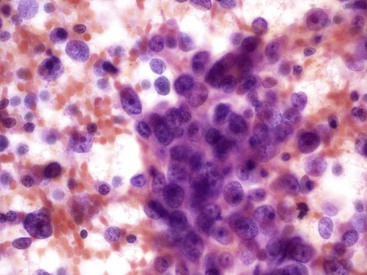
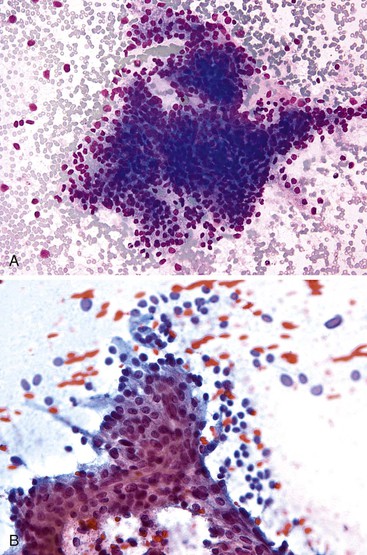
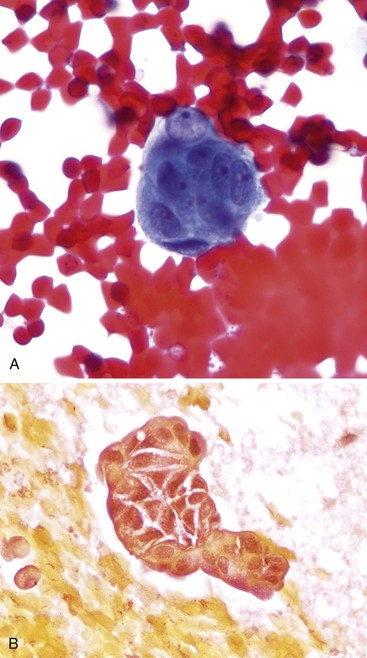
The cytologic features (Box 35.10) of undifferentiated adenocarcinoma have been described. Smears are usually very cellular. The carcinoma is composed of mononucleated and multinucleated cells. The mononuclear cells appear singly or in small clusters and can range from medium-sized, polygonal epithelioid cells with clear cytoplasm to large, bizarre sarcomatoid cells with dense and/or spindled cytoplasm. The multinucleated cells form bizarre giant cells60,61 (Fig. 35.25). Cytophagocytosis, tumor necrosis, and marked inflammation are additional common findings.
Undifferentiated carcinomas are usually easily recognizable as malignant. However, the differential diagnosis includes metastatic undifferentiated carcinomas, sarcomas, and melanomas. Immunohistochemistry will separate these various entities.
Undifferentiated Carcinoma with Osteoclast-Like Giant Cells
Undifferentiated carcinoma with osteoclast-like giant cells, a variant of PDA, is composed of malignant, usually mononuclear, epithelial cells admixed with benign osteoclast-type giant cells. Aspirate smears are typically hypercellular with two cell populations—an obviously malignant mononuclear epithelial proliferation, similar to that as described in undifferentiated carcinoma, and benign-appearing osteoclast-like giant cells (Fig. 35.26; see Box 35.10). The giant cells vary in number and often contain 10 or more bland appearing, centrally clustered and slightly overlapping nuclei with even chromatin and occasionally prominent nucleoli.62–65
Variants of Ductal Adenocarcinoma
Adenosquamous Carcinoma
Adenosquamous carcinoma is the most common variant of PDA. These tumors are typically high grade; aspirate smears typically show moderate to high cellularity and necrosis. The squamous component may predominate, with focal intracellular mucin or glandular groups of cells as evidence of glandular differentiation. Atypical, keratinized squamous cells may represent the only evidence of squamous differentiation in a predominantly glandular tumor66 (Fig. 35.27). Without keratin, the squamous cells show dense cytoplasm. The cell block may show classic components as well (Boxes 35.11 and 35.12). The differential diagnosis includes metastatic squamous cell carcinoma.
Colloid (Mucinous Noncystic) Carcinoma
The classic cytomorphologic appearance of colloid carcinoma is that of malignant glandular cells floating in thick, background mucin (Fig. 35.28; see Box 35.12). It may be difficult to distinguish this carcinoma from metastases of similar tumors from the lung, colon, or breast, particularly in patients with a history of carcinoma at these sites, or from intracystic or intraductal mucinous neoplasms. When the FNAB is obtained from a solid mass, the diagnosis of this invasive process can be made. Uncertainty may occur if a similar pattern is found in material aspirated from what is described as a cyst or dilated duct, in which case the differential includes MCN and IPMN. The background mucin and malignant features of the glandular epithelium distinguish this process from gastrointestinal contamination. In the absence of neoplastic epithelium, the diagnosis cannot be rendered.
Signet Ring Cell Carcinoma
The FNAB cytomorphology of signet ring cell carcinoma in the pancreas is the same as that in other sites. Signet ring cells are single malignant cells with a cytoplasmic mucin vacuole that distends and compresses the nucleus (Box 35.13). These should be recognized as malignant, but the differential diagnosis includes metastatic or secondary involvement by gastric signet ring cell carcinoma.
Medullary Carcinoma
The FNAB features of medullary carcinoma have not been reported. One case encountered at the Moffitt Cancer Center revealed syncytial groups composed of neoplastic cells with rounded to oval nuclei and prominent nucleoli (Fig. 35.29). Lymphocytes and plasma cells were associated with the neoplastic cells (Box 35.14).
Differential Diagnosis and Pitfalls
Key diagnostic problems include differentiating adenocarcinoma from benign or reactive epithelium (Table 35.4) and differentiating primary from metastatic disease. Identification of well-differentiated adenocarcinoma can be particularly problematic because the cytomorphologic features of well-differentiated carcinoma are subtle. Crowded, hypercellular groups are the most frequent finding. The cells show nuclear enlargement and significant loss of polarity. Moderately and poorly differentiated adenocarcinomas are easier to diagnose (see Table 35.4), although on an inflamed smear, the differential may include reactive ductal groups. Variants of PDA are usually recognized as malignant; the differential diagnosis is with metastatic disease from other sites that produce carcinomas with similar features. In this case, immunohistochemistry is indicated.
Table 35.4
Benign Processes vs. Adenocarcinoma with FNAB
| Feature | Benign Epithelium and Reactive Processes | Adenocarcinoma |
| Cellularity | Scant (except GI contamination) | Moderate to high |
| Architecture | Flat and cohesive | Irregular |
| Loss of polarity | Minimal | Prominent |
| Nuclear crowding | Minimal | Prominent |
| Nuclear membrane contour | Round, oval | Angulation, elongation, notches, grooves |
| Chromatin | Fine, granular | Parachromatin clearing |
| Mitoses | Minimal/normal | Present/atypical |
| Single atypical cells | Absent | Present |
| Nuclear enlargement | Minimal | 1.5 × RBC, or 2-3 × neutrophil nucleus |
| Nuclear size variation | Minimal | 4 : 1 |
GI, Gastrointestinal; RBCs, red blood cell.
Ancillary Studies
Indications for the use of ancillary studies when dealing with ductal-type neoplasms are to differentiate benign from malignant disease or to differentiate primary from metastatic disease.
Studies have focused on assessment of samples for molecular alterations identified in PDA. KRAS, the first mutation analyzed in PDA and has been the most frequently assessed mutation in small biopsy samples. In two studies that evaluated fine needle aspiration, the addition of KRAS mutation analysis to standard cytomorphology increased the detection of adenocarcinoma.67,68 KRAS lacks specificity, however, because it is expressed in early lesions before significant morphological alterations occur, leading to false-positive results. Other studies have focused on other proteins altered in pancreatic carcinogenesis. Loss of DPC4 protein expression can serve as a marker of malignancy in FNAB samples, because benign tissues retain expression of DPC4. However, only 52% of PDA cases show loss of expression, so it is not a reliable marker by itself.69 An approach with a panel of markers, such as analysis of KRAS mutations and loss of protein expression for TP53 and DPC4, is more sensitive and more specific.70
In recent years, there has been an explosion in the identification of genes and their related proteins involved in pancreatic carcinogenesis.71 Examples of these markers include prostate stem cell antigen (PSCA) and mesothelin,72 von Hippel-Lindau gene product (pVHL) and S100P,73 KOC,74,75 IMP3,76–78 and the mucins MUC1 and MUC2.79 A recent study analyzed a large number of markers that are reported as useful for diagnosing PDA. This study found that the combination of pVHL (which is not expressed in PDA), mammary serine protease inhibitor (maspin), S100P, and IMP3 was best for diagnosing PDA on samples obtained by fine-needle aspiration.80 Although these results are promising, to date none have been validated for routine use.
Analysis of microRNA profiles is promising because this technology is easily applied to the small samples obtained from FNABs. PDA has been found to have restricted microRNA patterns that can differentiate it from benign conditions of the pancreas.81
Because the pancreas is a site of metastasis, the immunohistochemical work-up may need to be geared toward differentiating primary from metastatic disease. This may be more of an issue with the variants of PDA, although adenocarcinoma from other sites can also metastasize to the pancreas and create a diagnostic dilemma.
Cytologic Diagnosis of Nonductal Pancreatic Neoplasms
Included in the category of nonductal pancreatic neoplasms are PanNET, ACC, SPN, and PB. The histopathologic pattern of these neoplasms is described as the solid, cellular pattern on histopathology. The low-power cytopathologic picture is that of a very cellular, monomorphic-appearing aspirate with numerous single cells, stripped nuclei, and loosely cohesive clusters. These neoplasms are vascular and may demonstrate this vascularity on the cytology samples. The vascularity is a key feature of SPNs.
Well-Differentiated Pancreatic Neuroendocrine Tumors
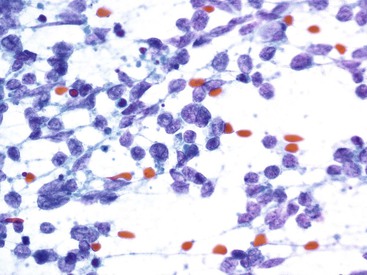
Aspirates of well-differentiated PanNET (WDPanNET) are very cellular. They are composed of a monomorphic, dyshesive population of cells occurring most often as single cells but also in loosely cohesive clusters (Fig. 35.30, A) that may occasionally show pseudorosettes. These patterns are often accompanied by stripped nuclei. The nuclei are round to oval, and the chromatin pattern has a characteristic salt-and-pepper appearance. Nucleoli are variably present but are prominent in some tumors. The cytoplasm varies in quantity and quality. It may be scant and wispy,77,82 plasmacytoid, or vacuolated, as in the clear cell variant.83 Less common variants include oncocytic and rhabdoid variants. The vascularity of WDPanNETs may be seen on the corresponding cytology smears and is characterized by vascular cores with loosely attached neoplastic cells (Box 35.15; see Fig. 35.30, B).
The immunoprofile of WDPanNET is characterized by expression of cytokeratins and markers of neuroendocrine differentiation, including synaptophysin, chromogranin, and neural cell adhesion molecule 1 (CD56). These neoplasms also show expression of a variety of hormones, but immunohistochemical analysis for hormone expression does not add further value to the diagnosis or prognosis.
The key entities in the differential diagnosis of WDPanNET include islet cell hyperplasia and a number of neoplasms, specifically ACC, SPN, adenocarcinoma (i.e., PDA), lymphomas, and metastases to the pancreas (e.g., melanoma, renal cell carcinoma). WDPanNET, ACC, SPN, and PB have many characteristics in common; their differential diagnosis is summarized at the end of this section and in Table 35.5.
Table 35.5
Differential Diagnosis of WDPanNET, ACC, SPN, and PB
| Criteria | WDPanNET | ACC | SPN | PB |
| Nuclei | Salt-and-pepper chromatin Round and oval ± Nucleoli |
Round to oval Prominent nucleoli |
Grooves Uniform Small nucleoli |
Variable amounts of acinar, squamous, endocrine, ductal, and primitive component |
| Cytoplasm | Scant and wispy Oncocytic Clear |
Dense, well-defined Zymogen granules Negative image |
Scant Tail like Inclusions |
— |
| Stroma | Vascular | Vascular | Mucoid fibrovascular stroma | Variable, may be cartilage or bone |
| Architecture | Single cells Pseudorosettes Loose clusters |
Single cells Stripped nuclei Grape-like clusters |
Papillary fronds Single cells |
— |
| Immunoprofile | Cytokeratin+ CD56, synaptophysin, and chromogranin+ β-Catenin− E-cadherin+ CD10− |
Cytokeratin+ Trypsin and chymotrypsin+ |
Neuron-specific enolase+ Vimentin+ Cytokeratin± CD10+ E-cadherin− β-Catenin+ |
As for components |
ACC, Acinar cell carcinoma; PB, pancreatoblastoma; SPN, solid-pseudopapillary neoplasm; WDPanNET, well-differentiated pancreatic neuroendocrine tumor.
WDPanNET may occasionally be mistaken for adenocarcinoma, if the nuclei have prominent nucleoli and show significant size variation, and the groups are cohesive with crowding or some pseudorosettes. Features that distinguish WDPanNET from PDA are that the nuclei retain a rounded or oval appearance and lack the nuclear angulations, sharpness, and convolutions seen in PDA. PDA tends to be more cohesive, particularly when well differentiated, and shows evidence of glandular differentiation.
The stripped nuclei and dyshesive pattern of WDPanNET raises the differential diagnosis with non-Hodgkin lymphomas (NHL). Lymphomas are characterized by lymphoglandular bodies in the background. Flow cytometry identifies a monoclonal lymphoid population. Melanoma84 and plasmacytoma85 may also be considered in the differential diagnosis of WDPanNET because of their plasmacytoid morphology. Melanoma is typically much more pleomorphic and has intranculear inclusions and prominent nucleoli. The presence of melanin pigment is diagnostic. Plasmacytomas show a perinuclear hof (Golgi zone) and a clock-face nuclear chromatin pattern, distinguishing these cells from WDPanNET. The pancreas is a common site for metastasis from renal cell carcinoma, which is also a vascular tumor and may have stripped nuclei, similar to WDPanNET. Renal cell carcinoma has much more nuclear pleomorphism and clear cell cytoplasm. There should also be a history of a renal mass or of renal cell carcinoma. Immunohistochemistry is beneficial in sorting out these various neoplasms.
Poorly Differentiated Pancreatic Neuroendocrine Carcinoma
Poorly differentiated PanNETs (PDPanNETs) resemble poorly differentiated neuroendocrine carcinomas from other sites, such as small cell carcinoma (Box 35.16). The cytomorphologic features are those of fusiform, small cells with molding, abundant necrosis, and mitotic activity (Fig. 35.31). These neoplasms are as aggressive as pulmonary small cell carcinomas. Large cell neuroendocrine carcinomas show vesicular nuclei with more abundant cytoplasm and prominent mitotic activity.
Acinar Cell Carcinoma
ACC typically produces moderately cellular smears with cohesive clusters, single cells, and stripped nuclei.86 The background is clean but may contain cytoplasmic granules from the stripped cytoplasm of tumor cells. The tumor cells, when cohesive, have a number of architectural patterns, including clusters and an organoid appearance (Fig. 35.32, A). The cohesive groups lose their normal architectural arrangements and may form crowded, three-dimensional groups. These neoplasms are vascular, and vessels may be prominent on the smears (see Fig. 35.32, B). The individual acinar carcinoma cells are polygonal and uniform in size and shape and have a low N : C ratio. The nuclear chromatin in the tumor cells is generally coarsely clumped, and usually one, or sometimes two, prominent nucleoli are seen. Cytoplasmic granularity resulting from the zymogen granules is seen on Diff-Quik smears as vacuoles or negative images. Poorly differentiated ACC shows more prominent nucleoli and more significant nuclear pleomorphism and nuclear membrane irregularity (Box 35.17). The immunoprofile of ACC is characterized by expression for cytokeratins, trypsin, chymotrypsin, Bcl-10, and CD10 and nuclear Beta-catenin expression due to mutations in the Beta-catenin pathways. They express other pancreatic enzyme markers, but these are not as frequently assessed.
The cytologic differential diagnosis includes PanNET, PB, SPN, and non-neoplastic acinar parenchyma. The differential diagnosis with PanNET, SPN, and PB is discussed at the end of this section. As for PanNET, the differential diagnosis for the stripped nuclei seen in ACC includes NHL, but in ACC the stripped nuclei are the same as the nuclei in the intact tumor cells and not of lymphocytic origin. Furthermore, the smear of the ACC lacks the background lymphoglandular bodies seen in NHL.
FNAB of benign pancreas may yield abundant benign acinar cells, and these may be misinterpreted as ACC. The organoid, cohesive, grapelike pattern of normal acinar cells hints at their benign nature. Normal acinar cells form organized, uniform, grapelike clusters, either singly or attached to a fibrovascular matrix; this pattern provides a more monomorphic appearance than ACC on low power scanning. Benign acinar cells retain a very low N : C ratio, and groups do not show any crowding.
Solid-Pseudopapillary Neoplasm
SPN of the pancreas has a characteristic cytologic picture. Smears are typically quite cellular and contain numerous branching, papillary-like groups of epithelial cells with a central fibrovascular core. The papillary fronds have three layers: a central vessel, a mucoid stromal layer, and an outer neoplastic layer (Fig. 35.33, A). The layer of neoplastic epithelium is composed of monomorphic epithelial cells with round to oval nuclei, finely dispersed chromatin, nuclear indentations or grooves, and small nucleoli. The cytoplasm of these cells is basophilic, varies in shape and quantity, may be vacuolated or may contain periodic acid–Schiff (PAS)-positive granules, and may demonstrate cytoplasmic tails (see Fig. 35.33, B). The pathognomonic feature is “balls” of mucoid stroma that may or may not contain a rim of epithelial cells87 (Box 35.18; see Fig. 35.33, C). The characteristic immunohistochemical profile is strong, and diffuse expression of vimentin, neuron-specific enolase (NSE), α1-antitrypsin, and alpha1-antichymotrypsin chymotrypsin, and CD10 and nuclear expression of β-catenin.88,89 These neoplasms also express progesterone receptors. They are variably positive for cytokeratins and neuroendocrine markers and negative for e-cadherin.90
PanNET, ACC, and PB are the key entities in the differential diagnosis. The differential diagnosis with these entities is discussed at the end of this section.
Pancreatoblastoma
PB produces cellular smears with both cell clusters and single cells. The appearance of the epithelial cells varies depending on the direction of differentiation.91–93 Cells with acinar differentiation have an oval to cuboidal shape, round central to eccentric nuclei, one or more small nucleoli, and a moderate amount of granular cytoplasm closely resembling ACC (Fig. 35.34, A and B). This component cannot be reliably differentiated from ACC. Cells with endocrine differentiation can resemble acinar cells but demonstrate a more cuboidal shape; a higher N : C ratio; denser, less granular cytoplasm; and less conspicuous nucleoli. In general, however, the tumor cell differentiation is difficult to appreciate clearly on smears. The distinguishing feature of these neoplasms is the squamoid nest or corpuscle, which is best recognized in cell block preparations. Stromal fragments may be scant or prominent with traversing capillaries (Box 35.19; see Fig. 35.34, A).
The immunophenotype depends on the components present. These neoplasms may show histochemical and immunohistochemical features of acinar differentiation, neuroendocrine differentiation, ductal differentiation, or squamous differentiation. Markers of acinar and neuroendocrine differentiation have already been discussed. The squamous areas may express markers of squamous differentiation such as cytokeratins CK5/6 and protein p63. The ductal areas show cytoplasmic mucin demonstrated by special stains for mucin, such as mucicarmine.
Differential Diagnosis of PanNET, ACC, SPN, and PB
These nonductal pancreatic neoplasms can be differentiated based on assessment of their cytomorphologic features and immunohistochemical findings (see Table 35.5). The cytomorphologic features to consider are nuclear, cytoplasmic, and stromal. The nuclei of WDPanNET show a salt-and-pepper chromatin pattern; the nuclei of SPN are subtly more irregular and pale, with grooves and micronucleoli; and the nuclei of ACC and PB with acinar differentiation may show prominent nucleoli. The cytoplasm of WDPanNET is variable in amount and quantity; when it is plasmacytoid, this neoplasm may be more readily recognized. The cytoplasm of ACC shows the presence of granularity due to zymogen granules. The cytoplasm of PB may be similar to that of ACC. Only the cytoplasm of PB will show squamous differentiation in the form of squamous corpuscles of nests. The cytoplasm of SPN may show tails, vacuoles, or inclusions, which are unique to this neoplasm.
All of these neoplasms are vascular, and a vascular stromal component will be present on the smears to a variable extent. However, only SPN shows a layer of mucoid stroma surrounding the capillaries in the fibrovascular cores. This mucoid stroma, when identified, is pathognomonic for SPN.
When the cytomorphologic features do not provide a clear diagnosis, immunohistochemical studies are indicated. A panel consisting of cytokeratins, vimentin, neuroendocrine markers, pancreatic enzymes, CD10, progesterone receptor, e-cadherin, and β-catenin can differentiate among these tumors (see Table 35.5).
Pancreatic Cysts
Many pancreatic cysts are discovered incidentally, as part of a work-up for abdominal pain or other reasons. In contrast to other organs, in which an incidentally discovered mass is more likely to be benign, in the pancreas, they are more likely to represent either a preinvasive or an invasive neoplasm. In fact, 78% of all incidentally discovered masses in the pancreas are premalignant or malignant.94 Among incidentally discovered cysts, preinvasive neoplasms account for more than 50% of cases.95
FNAB is used in the preoperative work-up of cystic masses of the pancreas. FNAB is performed to answer two questions: (1) Is the cyst neoplastic or non-neoplastic? and (2) If it is neoplastic, does it contain a neoplasm that is indolent or unlikely to progress, or is there a high potential to progress to invasion?
Cytology has excellent specificity but poor sensitivity for diagnosis of cystic neoplasms of the pancreas. Therefore, an integrative approach that includes correlation of the findings from clinical, radiologic, cytologic, and ancillary studies, is even more necessary for assessing pancreatic cysts (see discussion at the beginning of this chapter).
Analysis of the cytologic specimen needs to begin with assessment of the gross appearance of the fluid aspirated, because this can provide critical information about the nature of the cyst.96 The background, cellular components, architectural appearance of groups, cytoplasm, and nuclear features should then be assessed. Ancillary studies are essential for the work-up of pancreatic cysts.
Ancillary Studies
Mucin Stains
Mucicarmine stains and Alcian blue stain aid in identifying background material as mucinous when its nature is questionable. However, mucin from gastrointestinal contaminants may lead to a false positive interpretation.97 The presence of abundant, thick background mucin or cells containing mucin lead in the direction of an MCN or IPMN and excludes serous cystadenoma and pseudocyst. If the sample is obviously mucinous, mucin stains are not indicated.
Cyst Fluid Viscosity
The original studies published on the topic of cyst fluid viscosity used viscosity measurements as a means of distinguishing between nonmucinous and mucinous lesions. A more recent study emphasized again the importance of assessing the viscosity of the fluid.98
Tumor Markers
A large number of tumor markers have been evaluated and reported on in the literature for the evaluation of pancreatic cysts.31 Currently, CEA levels are the most useful. An elevated CEA level correlates with the presence of a cystic mucinous neoplasm. Serous cystadenomas and pseudocysts have low levels of CEA. A cutoff level of 192 ng/mL was established in a prospective, multiinstitutional series99 as indicative of the presence of a mucinous-type neoplasm, either MCN or IPMN. However, it is recommended that each laboratory establish its own threshold. CEA levels do not correlate with the grade of dysplasia.100
Benign entities such as enteric duplication cysts,101 mesothelial inclusion cysts,102 and lymphoepithelial cyst of the pancreas (LECP) may also produce elevated CEA levels.103,104 Therefore, CEA levels should not be used as the sole criterion for the diagnosis of a cystic mucinous neoplasm.
Enzymes
Elevated amylase levels are associated with a pseudocyst and may also be encountered in IPMN. Typically, serous cystadenomas and MCN do not have elevated amylase levels. LECP may have elevated amylase levels.104
Mutations
KRAS mutations are identified in both MCN and IPMN.105 GNAS mutations are identified in IPMN. Neither of these mutations identifies high-grade dysplasia.106 Cytology still remains the gold standard, because some entities, such as intraductal oncocytic papillary neoplasms (IOPN, discussed later), may have neither mutation.
Non-neoplastic Cysts
Pseudocysts
Pseudocysts are the most common non-neoplastic cyst of the pancreas. Aspiration of a pseudocyst typically produces an abundant amount of turbid, brown fluid. The cytology sample demonstrates hemosiderin-laden macrophages, few acute inflammatory cells or lymphocytes, fibrin, debris, and bile pigment (Fig. 35.35) and lacks neoplastic lining epithelium (Box 35.20).107,108 These cysts should not have background mucin, although mucin may be obtained as a contaminant from the gastrointestinal epithelium.97 Benign cells from adjacent pancreatic parenchyma108 may be aspirated and represent a potential pitfall. The cytologic features of pseudocysts are listed in Box 35.20.
The cyst fluid aspirated from a pseudocyst is thin, not viscid.98 Pseudocyst fluid shows elevated levels of amylase and low levels of CEA.31,99,105,109 KRAS mutations should not be detected105; however, if adjacent pancreatic parenchyma containing pancreatic intraepithelial neoplasia or IPMN is sampled, KRAS mutations may be detected in the fluid (unpublished data).
The differential diagnosis of pseudocysts includes other types of inflammatory or infectious cysts as well as true cysts and cystic neoplasms. Secondary bacterial infection may produce cyst fluid that contains abundant neutrophils and proteinaceous background debris.107 Cultures of the fluid are usually positive. Hydatid cyst is a rare cause of cysts in the pancreas and should be considered a possibility in endemic regions.110–112 Diagnosis is usually suspected preoperatively, based on imaging findings and serologies, and aspiration is contraindicated,113 but some patients do not have positive serology.112 Cytology aspirates show scolices, hooklets, and laminated fragments.111 The presence of a neoplastic lining epithelium or background mucin excludes a diagnosis of pseudocyst, although false-positive mucin stains occur because of gastrointestinal contamination.97
Ciliated Foregut Cyst
Aspirates from ciliated foregut cysts are characterized by ciliated columnar cells and detached ciliary tufts. The background fluid contains amorphous debris and rare macrophages.114 The differential diagnosis includes cystic neoplasms. The presence of ciliated cells is diagnostic of this entity.
Lymphoepithelial Cyst of the Pancreas
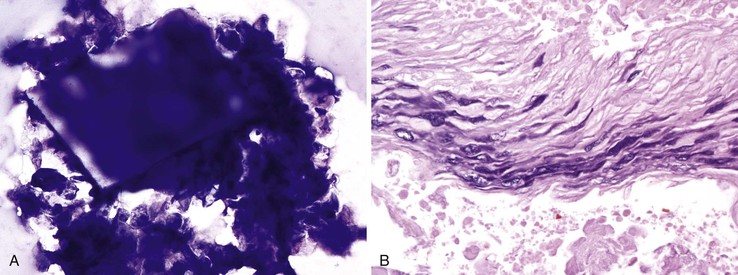
LECP is typically discovered incidentally. These lesions have a very typical appearance on computed tomography. They show a well-circumscribed, loculated or lobulated mass with low attenuation and with a density equal to water. TUS shows an inhomogeneous echogenic mass with posterior enhancement.115 EUS imaging shows a hypoechoic, unilocular or multilocular mass possibly with hyperechoic, sludgelike material in the cyst.116 Aspirates of LECP usually yield a variable amount of pasty, yellow-gray or yellow-white material.117 The smears show abundant anucleated squamous cells, keratinous debris , and few nucleated squamous cells. The background may also contain lymphocytes, histiocytes, background debris, and cholesterol clefts and crystals (Fig. 35.36, A).118,119 The cell block may show squamous cells with a preserved granular layer (see Fig. 35.36, B). This cytologic finding is diagnostic (Box 35.21).
Measurement of cyst fluid levels may lead to misinterpretation of an LECP as a MCN or IPMN, because LECP may produce high levels of cyst fluid CEA, and the cancer antigen, CA 19-9. The reported range of CEA levels is 2700 to 35,028 ng/mL.104,119–121 The cyst fluid amylase levels range from normal to 480 U/L.104,119,122,123 The cyst fluid CA 19-9 level may be elevated,121,122 reportedly as high as 5 × 106 U/mL or greater.104 The wide range of these test results helps to emphasize why cytology is considered the gold standard diagnostic method.
The differential diagnosis of LECP includes pseudocysts and cystic neoplasms. The presence of squamous material within the cytology sample helps exclude these other entities. The morphologic differential diagnosis includes any other entity with a squamous component, such as squamous metaplasia, squamous cyst of the pancreas, dermoid cyst, epidermoid cyst involving intrapancreatic accessory spleen, adenosquamous carcinoma, and metastatic squamous cell carcinoma. Squamous metaplasia occurs mainly in association with chronic pancreatitis and is unlikely to produce a cystic mass lesion. It may be difficult to differentiate dermoid cysts of the pancreas and splenic epidermoid cysts from LECP on the basis of cytologic features alone. Epidermoid cysts arising in intrapancreatic accessory spleen show mostly macrophages and debris.124 Squamous cyst of the pancreatic ducts (SCOPD) is a recently described entity that is characterized by a benign-appearing squamous component and mucinous epithelium. The lack of malignant epithelium helps exclude adenosquamous and squamous cell carcinoma.
Squamoid Cyst of the Pancreatic Ducts
SCOPD is a recently described entity that manifests as a large cystic mass on imaging studies.125 The cysts are lined by a layer of mucinous epithelium in the lumen and a layer of squamous epithelium at the base (Fig. 35.37). I have encountered one case on cytology. The patient had a large, multiloculated mass in the tail of the pancreas. The cytologic cyst fluid sample showed groups of benign-appearing squamous cells with benign mucinous columnar cells either surrounding the squamous epithelium or occurring separately. The background contained degenerated cellular debris. The cytologic features of SCOPD are listed in Box 35.22. The CEA fluid was elevated at more than 400 ng/mL.
The differential diagnosis of SCOPD may include MCN or IPMN, which should lack such a well-formed squamous component. However, in the absence of a squamous component, and because of the elevated CEA level, these cysts present a potential for a false positive diagnosis of a cystic mucinous neoplasm. The combination of mucinous epithelium with squamous epithelium, the lack of abundant anucleated squamous cells, and the location within the pancreas instead of outside the pancreas, should differentiate this entity from LECP.
Cystic Neoplasms
Cystic neoplasms can be either inherently cystic neoplasms, such as serous cystadenomas and MCNs; intraductal neoplasms presenting as cysts, such as IPMNs; or solid neoplasms with cystic degeneration, such as PanNETs or SPNs.
Serous Cystic Neoplasm (Serous Cystadenoma/Serous Cystadenocarcinoma)
Aspiration of a serous cystadenoma is generally nondiagnostic because of scant cellularity.105 Typically, a scant amount of clear, thin fluid is aspirated. The background is usually watery and may contain fibrovascular stromal fragments that are stripped of epithelial cells. The cells are usually arranged in flat, monolayered sheets or as dispersed single cells or stripped nuclei (Fig. 35.38). The nuclei are round and uniform in shape but may vary in size. The nuclei lack nucleoli and mitoses.126 Aspirates may contain only a few cuboidal cells or histiocytes.127 Because the stroma is typically quite vascular, these cysts may hemorrhage, in which case the aspirate may reveal blood and hemosiderin-laden macrophages. The cytologic features of serous cystic neoplasms are listed in Box 35.23.
Cytology lacks sensitivity for serous cystic neoplasms because of the difficulty in obtaining cells from these neoplasms.126 Therefore, knowledge of the radiologic findings is essential. If imaging studies are interpreted as characteristic of serous cystadenoma, then the pathologist should evaluate the sample carefully for diagnostic cells. Smears of serous cystic neoplasms do not contain many diagnostic cells. Oligocystic128 and solid variants129,130 of serous cystic neoplasms occur as well.
A diagnosis of cystadenocarcinoma depends primarily on histologic identification of tissue invasion.131,132 There are no reliable differences in the cytologic appearance between benign and malignant serous neoplasms because there are no reliable differences in histopathologic specimens.133
The PAS stain, with and without diastase digestion, may be performed on cytology samples to demonstrate the presence of cytoplasmic glycogen, a characteristic feature of serous cystadenomas. CEA and amylase levels in the cyst fluid will be low.31,99,105 These neoplasms are negative for mutations in KRAS and GNAS.106
The differential diagnosis includes pseudocyst, MCN, and solid neoplasms manifesting as cysts. Because aspirates of these neoplasms frequently lack neoplastic epithelium, psuedocyst remains a diagnosis of exclusion on cytology alone.
Mucinous Cystic Neoplasm
MCN manifests as a solitary unilocular or multilocuar cyst in the distal pancreas in middle-aged women.134 Histology is characterized by columnar, mucinous cyst-lining cells surrounded by ovarian-type stroma. The fluid is viscous and difficult to aspirate. Aspirates of MCN are characterized by thick background mucin and neoplastic epithelial cells containing cytoplasmic mucin. The cytoplasm and nuclear atypia can be variable in appearance. The cytology of MCN is similar to that of IPMN. Additional details about the cytologic features of both entities are discussed later.
These fluids have a very high viscosity.98 Analysis of pancreatic cyst fluid shows low levels of amylase and elevated levels of CEA.105
Intraductal Papillary Mucinous Neoplasm
The diagnosis of IPMN may be suggested on the imaging studies if they show a cyst connecting to the main pancreatic duct or multiple large cysts. However, a connection to the ductal system is not always identified.
Cytology for Mucinous Cysts
Aspiration of fluid from an MCN/IPMN may be difficult because the fluid is often extremely viscous. Gross examination often reveals highly viscous fluid or clear fluid with strands of mucus plugs. In fact, the material may be expressed from the needle as a “plug” of mucin. This feature is considered diagnostic, regardless of the cellularity of the aspirate.
The classic cytomorphologic appearance is the presence of thick background mucin combined with neoplastic mucinous epithelium. Assessment therefore includes a stepwise approach to the background and cellular component.
The key feature is the presence of background mucin, which can vary in quantity. The most diagnostic appearance is when the mucin covers the entire slide and forms a thick film, similar to colloid from thyroid aspirates. On Papanicolaou staining, the mucin has a characteristic pink hue (Fig. 35.39). When not diffuse, it may be clumped. Ferning may be seen. Psammomatous calcifications are a feature of IPMNs. The background may also contain acute inflammation and necrosis. The mucin is usually accompanied by degenerated cells, called “oncotic cells,”138 and macrophages (Fig. 35.41, B). The presence of thick background mucin with these characteristics indicates MCN/IPMN.
The cellularity is variable. The cells may be arranged singly, on edge, in sheets, in papillary clusters, or in small papillary tufts. The sheets or groups do not form a honeycomb pattern; rather, they are subtly hypercellular and crowded (see Fig. 35.40, A and B).
The appearance of the cytoplasm varies. The characteristic cell type is a columnar cell with abundant cytoplasmic mucin and the appearance of mucinous metaplasia (see Fig. 35.39), or the cells may be rounder, with vacuolated cytoplasm.
The nuclei show varying degrees of abnormalities. The subtlest are mild nuclear enlargement and hypochromasia with subtle nuclear membrane foldings. The hypochromatic nuclei may have small, peripherally located nucleoli, similar to those seen in papillary thyroid carcinoma. Nuclear grooves and pseudoinclusions become more evident and are evidence of MCN/IPMN. The chromatin becomes coarser, with abnormal paranuclear clearing and micronucleoli (see Fig. 35.40). Mitotic figures will be evident in high-grade dysplasia. The single dysplastic cell, which is a small cell with a high N : C ratio and nuclear convolutions (see Fig. 35.41), is diagnostic of a neoplastic process; it is typically associated with higher-grade dysplasias. Because these cells may be obscured by the cystic debris, careful examination of the specimen, particularly in the areas of thick mucin, is needed (Box 35.24).
Invasive Carcinoma Associated with MCN or IPMN
Invasion typically cannot be determined on the basis of evaluation of cystic or ductal fluid only. Aspiration of a solid area of an MCN/IPMN, when visualized with radiologic guidance, may prove diagnostic of invasive adenocarcinoma.139
The presence of coagulative tumor necrosis, cells with significant anisonucleosis and mitotic features, and other criteria of adenocarcinoma may indicate the presence of an invasive ductal carcinoma component. The intestinal type of IPMN is associated with noncystic mucinous carcinoma.
Intraductal Oncocytic Papillary Neoplasms and Invasive Oncocytic Carcinomas
IOPN is an intraductal neoplasm that has morphologic and clinical features overlapping those of IPMN.140 Aspirates from these lesions may obtain viscous fluid. Cytology smears show a pattern similar to that of IPMN. The cells are usually arranged in papillary clusters with background mucin that contains macrophages and cell debris (Box 35.25). However, the main distinction is that most of the cells have dense oncocytic cytoplasm (Fig. 35.42). Invasive oncocytic carcinomas develop from IOPNs. Aspirate smears show features of malignancy. The cells have abundant oncocytic cytoplasm; others have glandular-type cytoplasm. The nuclei show grooves and inclusions.
These oncocytic neoplasms lack mutations in KRAS.141 The differential diagnosis includes ACC, PanNET with oncocytic cytoplasm, and hepatoid PDA. Immunohistochemistry will assist with these differential diagnoses. ACC expresses trypsin and Bcl-10.142 PanNET expresses neuroendocrine markers, and hepatoid carcinomas shows expression for HepPar and α-fetoprotein. Oncocytic carcinomas do not express either of these markers. Furthermore, the oncocytic epithelium is intermixed with glandular, mucinous-containing epithelium and shows papillary fronds and background mucin.
Role of Cytology in the Management of Mucinous Cysts
Analysis of cyst fluid for cytology and ancillary studies is accurate at classifying pancreatic cysts as mucinous or nonmucinous.44,143,144 Current management strategies are to resect all mucinous cysts that appear to be MCNs based on correlation with imaging findings. A mucinous cyst is suspected to be an IPMN if it connects to the ductal system or if the disease is multicentric. Preoperative assessment of patients with suspected IPMN now focuses on identifying patients who are at high risk of harboring high-grade dysplasia or invasive carcinoma by using a combination of clinical history, gender, imaging characteristics, cytology, and cyst fluid biochemical and mutational analyses.
The 2012 international consensus guidelines have identified certain features as high-risk stigmata for the presence of malignancy and other features as worrisome for malignancy. If worrisome features are present, the patient is referred for EUS assessment with FNAB.145 It is at this juncture that cytology becomes critical, because the patient will be referred for surgery if the aspirate is suspicious or positive for malignancy. In this context, cytology is considered a screening test for pancreatic cysts,146 and it should be assessed with an eye toward identifying cells that indicate the presence of at least high-grade dysplasia, if not invasive carcinoma.
The cytologic features that are predictive of high-grade dysplasia or adenocarcinoma have been grouped together as “high-grade epithelial atypia,” because outright features of malignancy are infrequently present and there is some overlap in the features of high-grade dysplasia with moderate dysplasia. The specific cytologic features that warrant classifying a cyst as showing high-grade dysplasia are discussed earlier (see Cytology for Mucinous Cysts) (Box 35.26).
Cystic Pancreatic Neuroendocrine Tumors
Cystic PanNETs are uncommon, accounting for 3.4% of all cystic neoplasms and 4.3% of all PanNETs in one series.147 However, cystic degeneration in large, nonfunctional tumors is a relatively common occurrence.148 The cytologic features of these tumors are similar to those of their solid counterpart (see Fig. 35.30). A pitfall is that the aspirates may be scantly cellular. Therefore, a lower threshold is needed to diagnose these tumors. Cyst fluid analysis shows low values for CEA, CA 125, and CA 15.3; low viscosity; and variable amylase content.149
Differential Diagnosis and Pitfalls: Neoplastic Mucinous Epithelium versus Gastrointestinal Contaminant
The main pitfall in the evaluation of pancreatic cysts is distinguishing benign gastric or duodenal epithelium from cystic mucinous neoplasms, MCN, or IPMN. Gastrointestinal epithelium mimics the epithelium derived from these neoplasms because it consists of glandular, mucin-containing epithelium and background mucin.
An approach to differentiating gastrointestinal epithelium from neoplastic mucinous epithelium incorporates assessment of (1) gross appearance of the fluid, (2) appearance of mucin on smears, and (3) cytomorphologic features of the cells.
It is also critical to know where the mass is located because aspirates of the pancreatic head and uncinate process traverse the duodenum, and aspirates of the neck, body, and tail traverse the stomach. Therefore, the location determines what type of contaminant one is trying to identify. In general, duodenal epithelium is easier to recognize because of the goblet cells and enterocytes.
Gastric and duodenal contents are usually watery and thin, in contrast to the mucin from MCN/IPMN, which is viscous. The mucin of gastrointestinal epithelium appears thinner and wispier on smears and is associated with the gastrointestinal epithelium. The mucin of neoplasms contains histiocytes and oncotic cells.138 Mucin from gastrointestinal epithelium may contain degenerated cells.32
Key to the diagnosis of a neoplasm is evaluation of the cytologic features. Gastric or duodenal epithelium retains a two-dimensional architecture in which the nuclei are evenly spaced within the sheets. Epithelium from MCN/IPMN shows crowding and nuclear overlap, which may be subtle. The nuclei are irregularly distributed.
Both gastric and duodenal epithelium may yield single cells. Single columnar cells with abundant columnar cytoplasm containing mucin that extends to the nucleus—metaplastic mucinous cells—are usually neoplastic. The mucin in foveolar cells typically does not fill the entire cytoplasm. Single cells with a brush border are enterocytes and are derived from the duodenum. Goblet cells are seen in IPMN and MCN, but these are less uniform and are unevenly distributed, in contrast to the goblet cells in the duodenum.
The nuclei from gastrointestinal epithelium are round and uniform. Neoplastic nuclei are subtly atypical, with nuclear membrane irregularities. Pseudoinclusions and grooves are typical of neoplastic epithelium, although gastric epithelium may also exhibit grooves and inclusions as a result of degeneration. Gastric epithelium frequently produces stripped nuclei with atypia and grooves in a background of mucin; this pattern should be recognized as part of the spectrum of gastric epithelium and not overinterpreted as being from gastric epithelium.
Even with these criteria, it is difficult to differentiate gastrointestinal epithelium, particularly gastric epithelium, from neoplastic epithelium. However, as discussed earlier (see Role of Cytology in the Management of Mucinous Cysts), it is not as crucial to differentiate gastric epithelium from low-grade, foveolar-type neoplastic epithelium. What is crucial is identifying the features that indicate the presence of high-grade dysplasia or invasive carcinoma.
Rare Pancreatic and Extrapancreatic Cysts and Neoplasms
Endothelial tumors (hemangiomas and lymphangiomas) are quite rare. Peripancreatic or retroperitoneal cysts and cystic neoplasms that appear to be pancreatic in origin on radiologic studies comprise approximately 21% of percutaneously aspirated lesions.102 These lesions may be misdiagnosed if the possibility of an extrapancreatic cyst is not considered. A few examples of these lesions are hydroureter, adrenal cyst, mesothelial cyst, mesenteric lymphangioma, gastric primary tumor, retroperitoneal serous cystadenoma, retroperitoneal leiomyoma, malignant lymphoma in a retroperitoneal lymph node, gastrointestinal stromal tumor, and gastric leiomyosarcoma.102,150,151
Pancreatic Metastases
FNAB is also useful in the evaluation of secondary pancreatic tumors.152
Cancers of the lung, breast, stomach, and colon are the primary adenocarcinomas that most commonly metastasize to the pancreas. Immunoperoxidase studies may provide helpful information in the differential diagnosis of these tumors. These studies include cytokeratin (CK) 7, CK20, CK17, BRST-2 (GCDFP-15), napsin A, and thyroid transcription 1 (TTF-1).153–157 Breast, colon, and gastric adenocarcinomas have cytomorphologic features that suggest their site of origin. Metastatic lung adenocarcinoma is difficult to distinguish from pancreatic carcinoma because of similar morphology. Both tumors may demonstrate squamous differentiation. Immunophenotyping for napsin and TTF-1 assists with the differential diagnosis.
Malignant melanoma is one of the most common types of metastases to the pancreas.152 The presence of melanin pigment is diagnostic but is not always present. In the absence of melanin pigment, the diagnostic ancillary work-up should include markers of melanoma such as S100, human melanoma black 45, MART1 (melan-A), and MITF1.
Renal cell carcinoma is the most common source of metastasis to the pancreas.152,158–161 The characteristic appearance is a uniform population of clear cells with central round nuclei and prominent nucleoli. Stripped nuclei, which occur in renal cell carcinoma, are not characteristic of PDA. The differential diagnosis includes PanNET with clear cell features.162 Both tumors may occur in association with VHL disease.