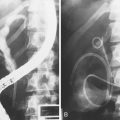
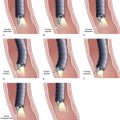
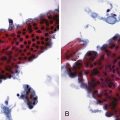
Chapter 37 Diagnostic Cholangiography
Introduction
Endoscopic cannulation of the major papilla with imaging of the biliary tree and the pancreatic ductal system (endoscopic retrograde cholangiopancreatography [ERCP]) was first successfully accomplished with an end-viewing duodenoscope and reported in 1968.1 Subsequent development of side-viewing endoscopes with a catheter-deflecting elevator greatly facilitated the technique. Diagnostic studies were supplemented by the first endoscopic sphincterotomies in the early 1970s.2,3 These developments permitted less invasive diagnostic and therapeutic maneuvers in the bile duct previously limited to open surgical and percutaneous techniques. Although these procedures are more technically demanding than most other gastrointestinal (GI) endoscopic techniques, they are now being widely used and are the method of choice for many clinical problems involving the pancreatic ductal and hepatobiliary systems.
This chapter focuses on diagnostic endoscopic retrograde cholangiography (ERC). Radiographic visualization of the biliary tree is often key to establishing a clinical diagnosis and formulating a therapeutic plan.4,5 With the aid of noninvasive imaging via transcutaneous ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI),6–13 thorough ductal filling at ERC is needed less frequently and is contraindicated in certain cases. Diagnostic ERC is just one portion of commonly combined ERCP and associated therapeutic maneuvers.
Endoscopic Retrograde Cholangiopancreatography
Indications and Contraindications
The role for diagnostic ERC alone has nearly disappeared as other, less invasive and noninvasive imaging techniques (e.g., CT scans, endoscopic ultrasound [EUS], magnetic resonance cholangiopancreatography [MRCP]) have become more widely used. Imaging of the biliary ductal system without anticipated therapy is clinically helpful in only a few clinical settings, such as cholestasis without dilated ducts. In certain settings, such as inflammatory bowel disease, patients with early sclerosing cholangitis may have ductal changes visible only via invasive cholangiography such as ERC (i.e., missed by noninvasive imaging). ERC is mainly indicated in clinical settings in which there is significant suspicion of obstructing, inflammatory, or neoplastic pancreatobiliary lesions that, if detected or ruled out, would alter clinical management. A general classification of indications is listed in Box 37.1.
Most contraindications of ERC are relative, and the degree of risk must be balanced against the potential benefit.14–16 In certain settings, even very ill and unstable patients, such as with acute cholangitis with shock or sepsis from bile duct stones or biliary strictures, diagnostic (followed by therapeutic) ERCP may be lifesaving. ERC in patients with necrotizing acute pancreatitis and low clinical suspicion for ductal stones is considered relatively contraindicated because pancreatography may result in bacterial contamination of the pancreatic bed. Other relative contraindications include unstable cardiopulmonary disease or severe coagulopathy. Patients with comorbid life-threatening conditions can often have ERC performed in the intensive care unit (with or without fluoroscopy) if deemed medically necessary. ERC is generally not indicated in type III suspected sphincter of Oddi dysfunction (unless manometry is included).
Preparation for Endoscopic Retrograde Cholangiography
Assembling the Team
We recommend that ERCP be done independently only by physicians with prior formal training in ERCP. An adequate number of examinations during training varies greatly with the trainee but should include at least 200 examinations.17 This number includes at least 100 therapeutic examinations. Successful biliary cannulation rates should be at least 85% and preferably 90%. We recommend that nurses have experience with at least 1000 upper GI and colonoscopy examinations before “graduating up” to the ERCP suite. Nurses should train alongside experienced ERCP nurses for 100 to 200 examinations before independent guidewire and accessory management is undertaken. Two nurses are needed per examination: one for sedation and analgesia administration and one for accessories management. Radiology technicians working in ERCP should maintain longevity and be team members rather than rotate frequently. In nearly all centers, a radiologist no longer assists in fluoroscopy or image acquisition except in the most difficult cases. Collaborative reading of final images may aid in the accuracy of final interpretation. Final reading by general radiologists with little pancreatobiliary training, experience, or interest may be counterproductive.
Patient Preparation
Risk factors such as anticoagulant therapy, prosthetic heart valves, and allergies must be addressed.18 Patients with iodine allergy are at very low risk of allergic reaction19; nevertheless, some centers continue to use prednisone, 30 to 40 mg orally, 15 hours and 3 hours before the examination. Diphenhydramine (Benadryl), 25 mg intravenously, may be added if serious past reactions have occurred. Iodine allergy is not a reason to omit a needed examination, but limiting the volume of contrast medium used is logical. Air20 can be successfully used for cholangiography if needed. If possible, aspirin and nonsteroidal antiinflammatory drugs should be avoided for 7 days before the procedure.
Informed Consent
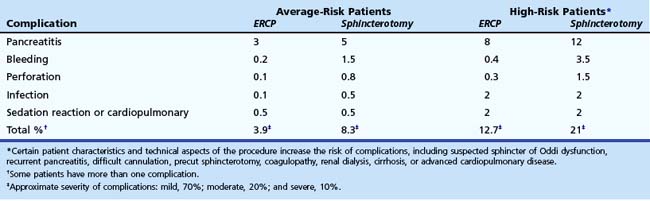
Informed consent for ERCP must be obtained. It is both legally and ethically necessary to apprise the patient (and family members if applicable) of the risks, benefits, and alternatives of the anticipated procedure. Table 37.1 lists potential complications of diagnostic and therapeutic ERCP and their relative frequency. Although legal standards continue to evolve, we recommend that patients be informed of the potential complications and the relative frequencies of complications. In addition, we recommend that patients be told that a severe complication possibly may result in a prolonged hospital stay, intensive care unit monitoring, or open surgery and very rarely may result in permanent disability or death. Complication rates vary according to patient and procedure risk factors and the disease process being evaluated and treated. Patients with uncomplicated biliary stones, malignancy, or chronic pancreatitis have lower complication rates, whereas patients with acute recurrent pancreatitis and suspected sphincter of Oddi dysfunction have twofold to fourfold higher complication rates. Procedure techniques associated with higher complication rates include repeated cannulation attempts, repeated pancreatic duct injections, pancreatic parenchymal acinarization, and precut sphincterotomy (without associated protective pancreatic stent). Attention to details of the technique and patient selection can minimize, but not eliminate, complications. Morbidity can be limited by early recognition and treatment of complications.
Endoscopic Equipment
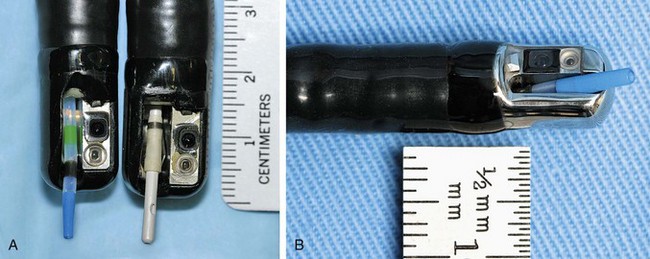
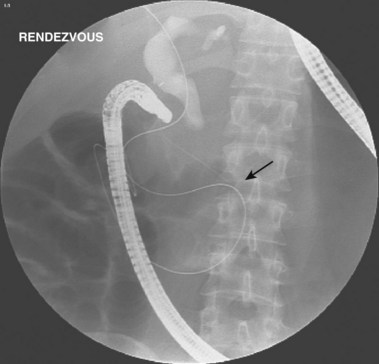
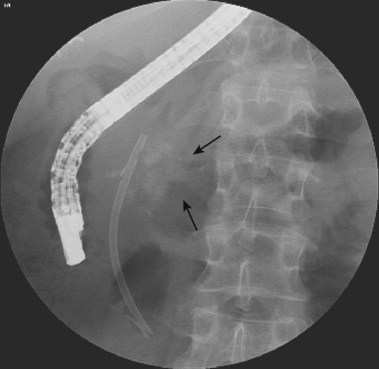
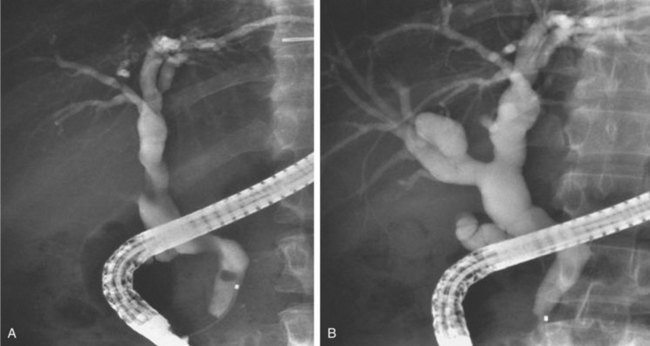
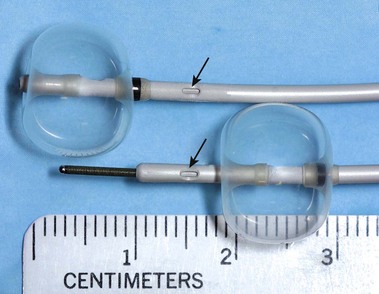
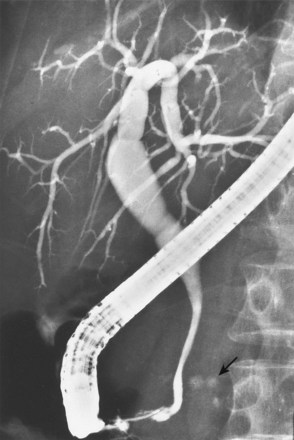
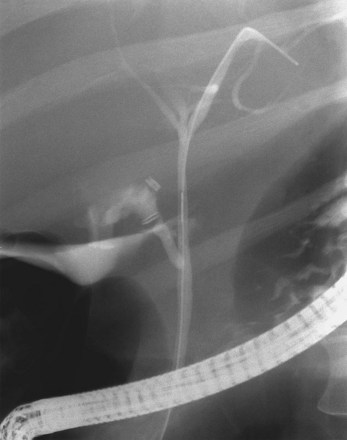
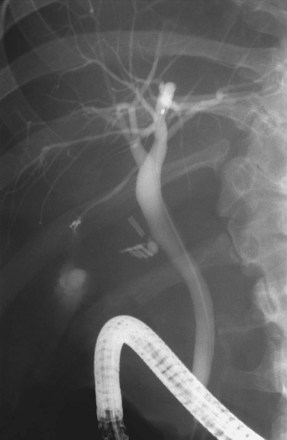
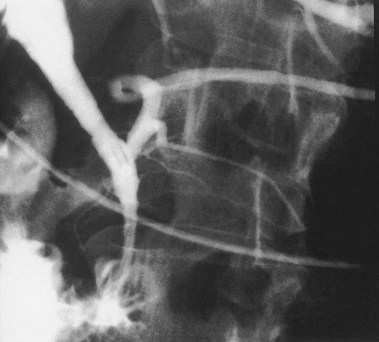
ERC can be performed with fiberoptic or video chip, side-viewing instruments. Endoscopes are 120-cm working length and generally categorized as diagnostic (approximately 10-mm diameter) or therapeutic (12- to 13-mm diameter). Video systems offer the advantage of television monitor viewing by all persons in the endoscopy suite; this offers better teaching capabilities and allows better coordination between the endoscopist and nursing assistants. Some newer generation endoscopes combine a large working channel diameter up to 4 mm with a standard 10- to 11-mm outer diameter (Fig. 37.1). A newer generation pediatric videoendoscope with outer diameter less than 7 mm is now available from Olympus America Inc. Most ERCP examinations are done with air insufflation. Limited data show that carbon dioxide inflation reduces postprocedure abdominal distention.21 For patients undergoing Billroth II procedures, we generally start with a standard side-viewing duodenoscope, but an end-viewing endoscope is occasionally needed. In patients with a long Roux-en-Y gastroenterostomy or choledochojejunostomy, a 160-cm pediatric colonoscope or a 220-cm enteroscope can reach the bile duct in greater than half of patients.22 Double-balloon enteroscopy has facilitated Roux-en-Y limb traversal.23,24 The lack of a catheter-deflecting elevator and limited compatibility accessories make end-viewing endoscopy difficult in these settings. Rendezvous with transhepatic wire passage is occasionally helpful (Fig. 37.2).
Current-generation endoscopes are capable of undergoing submersion disinfection. After cleaning, endoscopes should be hung in vertical position to facilitate drying. In the past, Pseudomonas infections were directly linked to inadequate ERCP scope disinfection. Ideally, endoscopes should be cultured periodically. Patients developing infections after undergoing ERCP should be cultured for the presence of Pseudomonas species (see Chapter 4).
Radiology Suite
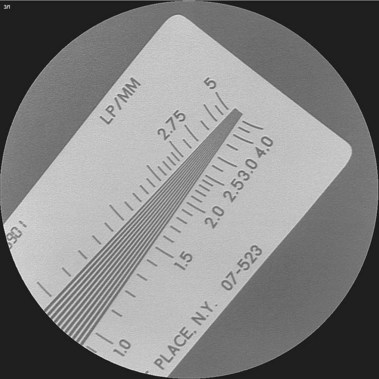
Few endoscopists have a dedicated suite for ERCP. We are aware of no manufacturer who markets a fluoroscopy unit specifically for ERCP. Most endoscopists schedule time in the radiology department and use general purpose or angiographic units. Film documentation is likely to disappear in the next decade, with digital formats viewed only on high-resolution monitors, which are becoming the new standard. Quality digital images now rival film quality. Flat tables with fixed overhead carriage have limited versatility. The preferred x-ray table includes the capability to tilt the patient’s head up and down 30 degrees and has C-arm carriage, which allows axial, cranial, caudal, vertical, and horizontal movements, allowing viewing at multiple angles (Fig. 37.3). Because the patient is usually positioned prone with the head at the “foot” of the table, ability to reverse the viewing image in both the vertical and the horizontal axes is helpful. In the past, endoscopists used older generation x-ray units, including portable C-arm units with limited image resolution. This practice is no longer acceptable because fluoroscopy and saved image quality are key to accurate diagnosis and management. High-quality ERCP imaging requires resolution equivalent to that for neuroradiology (brain blood vessels). Resolution of greater than 2.5 line pair per millimeter is strongly recommended for both fluoroscopy and final images (Fig. 37.4). This resolution is best accomplished with smaller diameter image intensifiers of 6 to 9 inches.
Radiation safety standards should be followed.25 Monitoring of personal exposure and review of methods to limit exposure are needed. Attention to coning the field of view to the area of interest is good practice. Lead aprons or shields around the patient limit x-ray beam scatter. Use of newer generation pulse fluoroscopy gives intermittent viewing, which is slightly jerky but often adequate with one-tenth the radiation exposure. Appropriate lead aprons, lead glasses, and thyroid shields are recommended (Fig. 37.5).
Technique
Patient Positioning, Preparation, and Sedation
Most centers prefer to have the patient positioned in a prone or slightly left lateral decubitus position on a fluoroscopic table. Less often, supine position is preferred, such as in patients with recent abdominal incisions, patients with multiple abdominal drain tubes, and patients undergoing Billroth II procedures.26 Intravenous access and monitoring equipment for blood pressure, pulse, and pulse oximetry are needed. Electrocardiogram (ECG) monitoring is desirable for patients with angina or a history of a cardiac arrhythmia and in other less stable patients.
Sedation and analgesia are achieved by slow intravenous administration of diazepam (10 to 40 mg), midazolam (2 to 10 mg), and meperidine (25 to 150 mg) or fentanyl (50 to 150 mg). The general ranges mentioned here are given over a 30- to 60-minute examination. Droperidol (2.5 to 10 mg) is a common supplement or alternative, particularly for alcoholics or persons regularly taking narcotics or benzodiazepines.27 However, more recent concerns about arrhythmias and Q–T interval prolongation have limited the use of droperidol for endoscopy. Our policy is to use droperidol routinely for patients whose baseline ECG has a normal corrected Q–T interval.28 More recently, propofol has been used for deep sedation and may offer better procedure tolerance and a much shorter recovery time than standard sedation. Propofol use, when administered by endoscopists and endoscopy nurses, is apparently safe for standard upper GI endoscopy and colonoscopy but has not been well studied for ERC.29–31
Upper Gastrointestinal Endoscopy
Before attempts at cannulation, fluoroscopic visualization (or still image acquisition) of the field of interest should be performed to look for stents, calcifications, masses, and residual contrast material (Fig. 37.6). The choice of initial cannulation tool is a personal preference (similar to choosing a tennis racket or golf club). One may begin with a simple single-lumen 5-Fr polyethylene catheter, without a guidewire, in many cases. The relative flexibility (not rigid), maneuverability, low cost, and simplicity are attractive features. A manometry catheter is initially used if ERCP findings are likely to be nonspecific or normal and if manometry is likely to be potentially helpful. If a sphincterotomy is almost certainly needed, a sphincterotome is a good starting tool. If the orifice appears small, a more tapered tip catheter or sphincterotome may be chosen. A few centers prefer various shaped metal tip catheters. Two-lumen or three-lumen catheters or sphincterotomes may be preferred because they have a separate lumen for a guidewire and contrast medium. A guidewire may be used at any point to aid cannulation or maintain intraductal stability. For biliary cannulation, 0.025-inch or 0.035-inch diameter wires are preferred. Soft-tipped wires have the advantage of less tissue trauma (e.g., fewer submucosal or other extraductal dissections). The specific devices used are much less important than the skill of the endoscopist (Fig. 37.7).
If the major papilla is not initially evident, gentle lifting of folds, greater air distention, and use of glucagon to inhibit peristalsis would likely expose the structure. If duodenal diverticula are present, the major papilla is most commonly on the diverticular rim, but the papilla is within the diverticulum per se in approximately 5% to 10% of cases (Fig. 37.8). The major papilla is then cannulated. Orientation of the catheter tip toward the 11 o’clock to 12 o’clock position (Fig. 37.9) would more likely enter the bile duct; orientation of the catheter toward the 3 o’clock to 5 o’clock position would more likely enter the pancreatic duct. Biliary orifice location may vary from 10 o’clock to 2 o’clock. Cannulation may be initially done by gentle impaction of the catheter tip in the papillary orifice. Deep cannulation (>1 cm penetration of the catheter into the duct) more securely establishes an intraductal position, which allows contrast agent injection, fluid aspiration, patient position changes, and endoscope position changes without loss of access to the duct.
Selective Deep Biliary Cannulation
Pancreatic cannulation is easier than biliary cannulation. Selective biliary entry is mandatory in most cases of biliary pathology. We often start with a standard 5-Fr catheter and add a guidewire or a sphincterotome for assistance. If the cannulation angle fails to achieve adequate cephalad orientation, a sphincterotome or curved top guidewire generally helps to achieve that angle. There are increasing observations that initial use of a guidewire facilitates cannulation and decreases post-ERCP pancreatitis.32 In patients with a prominent major papilla (protruding well into the duodenal lumen), the path of the biliary lumen is nearly always stair-stepped. Cannulation is initially cephalad, then more perpendicular into the wall, and then more cephalad again. The cannulation is partially accomplished by pulling the endoscope more cephalad, lowering the elevator, and moving the viewing lens very close to the papilla. A sharp guidewire may puncture the ampullary segment roof at the first cephalad-perpendicular junction. Gentle guidewire manipulations and more perpendicular catheter orientation are required.
Limited data indicate that cannulation may be facilitated in difficult cases with use of drugs (e.g., nitroglycerin) to relax the sphincter. If biliary entry is mandatory (obstructive jaundice) and initial attempts fail, precut entry is generally required (see Chapter 42). In this setting, the endoscopist must balance decisions regarding transfer to a more experienced facility33,34 and consider percutaneous or surgical opinions versus proceeding with more aggressive endoscopic techniques.
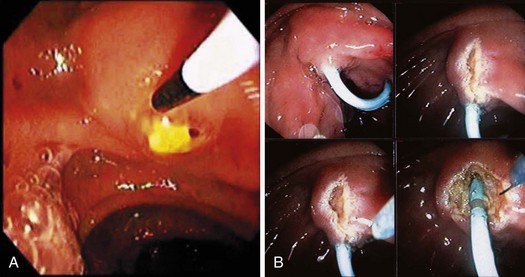
Precut sphincterotomy35–39 involves cutting the papilla to gain deep intraductal access to the biliary tree. This technique should be used by experienced endoscopists only and mostly applied in patients with a high clinical suspicion of obstructive pathology (e.g., impacted stone; Fig. 37.10A) or jaundiced patients with dilated bile ducts on noninvasive imaging after standard techniques fail. Precutting can be achieved by impaction of a short-nosed pull-type sphincterotome into the papillary orifice with sequential shallow cephalad cuts until the biliary orifice is identified. Similar sequential shallow cuts can be made with a needle-knife. We prefer to place a 3-Fr to 4-Fr, 6-cm long, no intraductal flange polyethylene stent into the pancreatic duct first, if possible, and use the stent to guide needle-knife cutting (Fig. 37.10B).
Contrast Medium and Image Acquisition
Standard ionic contrast medium (e.g., meglumine diatrizoate) at a 25% to 30% concentration is often called half-strength and is most commonly used for cholangiography. Half-strength contrast medium permits adequate visualization of small ducts of 2 to 6 mm diameter and allows filling defects (stones) to be seen in more dilated ducts. However, biliary stricture detail and peripheral intrahepatic ducts are better defined with full-strength contrast medium (50% to 60% concentration). Nonionic and lower osmolality contrast agents, which are more expensive, offer no safety advantage.40 Many manufacturers have abandoned marketing of inexpensive ionic agents making use of nonionic agents necessary.
We prefer 20-mL syringes because this avoids the need to exchange syringes as often (and potentially introduce air bubbles). With each syringe exchange, one should aspirate back to remove any air bubbles from the Luer connector and flush to ensure that the contrast medium extends to the catheter tip. Contrast agent injection is done with continuous fluoroscopic monitoring. Contrast medium is more dense than bile and flows along the most dependent route. The left lobe fills quickest (lowest) with the patient prone (Fig. 37.11A), the right lobe anterior segments fill next, and the right lobe posterior segments fill last (and may remain unfilled unless adequate volume and injection force are applied) (Fig. 37.11B). The extent of ductal filling should be correlated with the clinical history and the need to know the ductal anatomy. High-resolution fluoroscopy is required to see fine detail of small ducts.
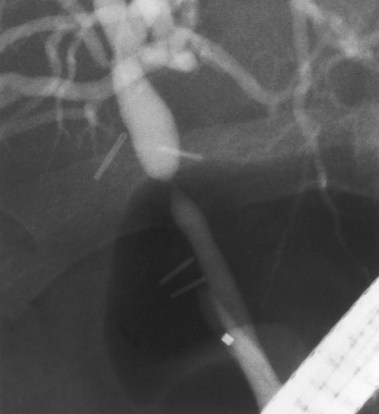
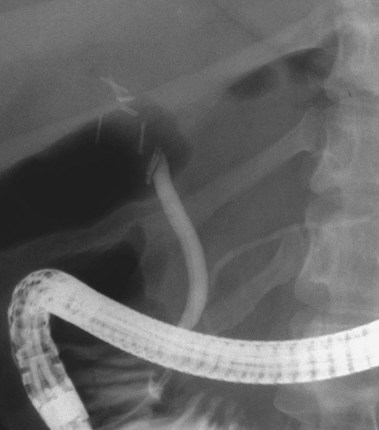
Multiple views of initial distal bile duct filling are recommended to see potential small filling defects (stones) that may be washed upstream (and no longer visible) or masked by more dense contrast concentration in a dilated duct (Figs. 37.12 and 37.13). Complete cholangiography requires filling of the peripheral intrahepatic radicles. The left lobe is more dependent in the prone position and fills preferentially. Right lobe filling may require tilting the patient’s head down 15 to 20 degrees on the fluoroscopy table, more forceful injection (a balloon occlusion catheter is helpful), selective right lobe cannulation, or turning the patient to the supine position. Contrast medium mixes slowly with gallbladder bile. Multiple films during early filling are recommended. Final films may be best taken in the supine position after withdrawal of the endoscope. Occasionally, delaying gallbladder films until 4 to 24 hours after completion of the procedure allows for passage of intraluminal gas, giving better diagnostic film quality. In settings of tight biliary strictures, limited contrast medium filling upstream should be done until catheter access above the stricture is achieved (Fig. 37.14).41–43
Problem Solving
Table 37.2 reviews multiple general problems encountered with ERC and potential ways to solve them.44–46 Biliary manometry usually is performed at the time of ERCP. All drugs that relax (e.g., anticholinergics, nitrates, calcium channel blockers, glucagon) or stimulate (e.g., certain narcotics, cholinergic agents) should be avoided for at least 8 to 12 hours before the study and during the manometry. Manometry is performed using a low-compliance infusion pump system and a 5-Fr catheter (see Chapter 49).
Table 37.2 Common General Problems and Challenges Encountered at Endoscopic Retrograde Cholangiography
| Cholangiographic Challenge or Clinical Suspicion | Potential Steps to Solve Problem |
|---|---|
| Obese patient | Increase kilovoltage |
| Take extra exposures (a few are likely to be adequate) | |
| Review still images before deciding on therapy (i.e., do not rely only on fluoroscopy view) | |
| Patient moves frequently | Take multiple exposures (one is likely to be clear) |
| Increase kilovoltage to shorten exposure time | |
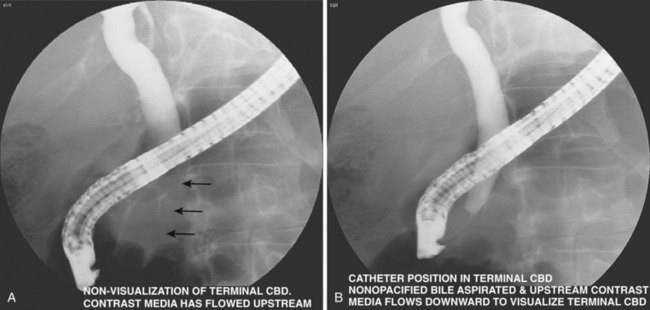
| Terminal (preampullary) CBD not well seen | If patient has cholangitis with risk of sepsis if greater filling done, pass stone retrieval balloon to mid-CBD, inflate balloon, and inject contrast agent downstream to balloon (need appropriate “below the balloon” injection port). Tilt head up 5–20 degrees (Fig. 37.15) |
| If moderate amount of contrast agent already upstream in intrahepatic ducts, place catheter tip 1 cm above sphincter. Aspirate nonopacified bile until upstream contrast agent flows back into terminal CBD (Fig. 37.16) | |
| Patient has typical postcholecystectomy pain, but ERCP (or MRCP) is normal | Perform manometry37–39 Do not initiate ERCP if ducts are not dilated by noninvasive imaging and liver serum chemistries are normal, unless manometry is immediately available |
| Cannot find papilla | Check fluoroscopy to ensure endoscope tip is in descending duodenum. Be sure of surgical anatomy—Roux-en-Y gastrojejunostomy? Bile present—follow trail. Gently lift folds in candidate area. Find minor papilla, and search left and inferior. Give cholecystokinin or secretin to stimulate fluid flow |
| Left and right hepatic ductal systems overlap at hilum, not well defined | Fixed fluoroscopy table, roll patient to slight left posterior oblique position; use C-arm rotation to separate systems |
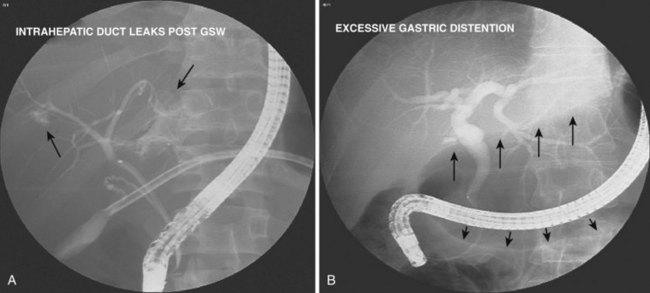
| Bile leak expected | Obtain multiple early images to locate leak site precisely before leak site contrast agent obscures view |
| Limit injection to small amount of spilled contrast agent (Fig. 37.17A) | |
| Air bubbles introduced | Observe where bubbles went and where collected; if distal CBD, tilt head down and aspirate bubbles, bile, and contrast agent from terminal duct |
| Consider tilt head up and observe bubble passage into intrahepatic ducts | |
| Contrast agent or air in duodenum or stomach detracts from image quality | Aspirate all contrast agent and air from duodenum before imaging; do this routinely when injecting contrast agent (Fig. 37.17B) |
| Endoscope repeatedly covers area of interest | Use C-arm (or patient positioning) to change angle Move endoscope from short (lesser curve) to long (greater curve) position |
| Place catheter upstream to hilum, slowly back endoscope into stomach aspirating air and spilled contrast agent as if nasobiliary tube placement were being done | |
| Pyloric or duodenal narrowing precludes endoscope passage | Pass guidewire and 5-Fr to 7-Fr catheter through the narrowing and ahead into transverse duodenum; an extra-stiff guidewire (Amplatz Super Stiff Guidewire; Boston Scientific, Billerica, MA) is especially helpful |
| Pass endoscope over wire, paying attention to fluoroscopic alignment more than the endoscopic view |
CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography; MRCP, magnetic resonance cholangiopancreatography.
Normal Findings
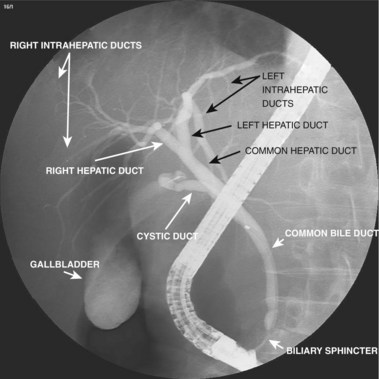
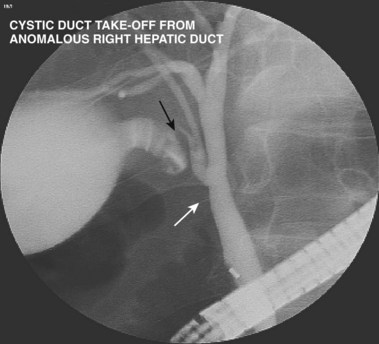
A normal cholangiogram is shown in Fig. 37.18. Although there is controversy, the weight of evidence indicates that the biliary tree does not dilate after cholecystectomy in the absence of obstructing pathology. The common hepatic and common bile duct diameter on ERCP is commonly 2 to 3 mm greater than seen on CT or ultrasound. This difference is accounted for by filling (or overfilling) the ductal system with extra fluid (contrast medium) under greater pressure than physiologic secretory pressure.47 Many centers accept the upper limits of normal diameter for the common bile duct as 10 mm in adults. The cystic duct commonly joins the common duct approximately halfway from the hilum to the papilla, but this junction may be quite variable. The intrahepatic radicles have a leafless tree–like branch pattern with marked variation in distribution. An aberrant, low insertion right hepatic duct, which connects to right posterior hepatic segments, is seen in 5% of patients (Fig. 37.19). These may be transected during laparoscopic cholecystectomy and give rise to problematic bile leaks from the disconnected segmental branch (Fig. 37.20). Because the transected duct does not fill on ERCP, MRCP may be more diagnostic.48,49 Numerous other normal anatomic variants have been reported (Fig. 37.21)49–51 and are beyond the scope of this chapter.
Biliary Stones
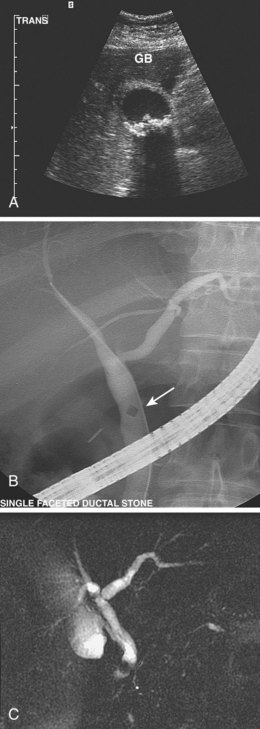
In white and African American patients, most stones are cholesterol or mixed type and occur in the gallbladder. These patients do not require ERC unless stones also occur in or migrate into the bile ducts.52–57 Fig. 37.22A shows a 35-year-old female patient with multiple small stones in the gallbladder (transcutaneous ultrasound). Fig. 37.22B shows a single small ductal stone on ERC. Gallstone pancreatitis is one common outcome of small stone (<5 mm diameter) passage. In this latter setting, more than 80% of patients spontaneously pass the stones from the bile duct into the duodenum. Selective use of ERC is recommended for patients who have persistent (≥12 hours) upper abdominal pain, persistent or worsening cholestasis, cholangitis, or dilated extrahepatic bile ducts.58–66
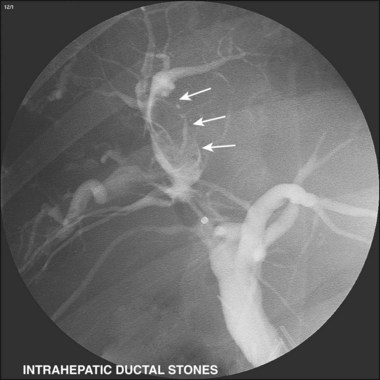
A meta-analysis of prospective randomized trials showed benefit of therapeutic ERCP in the setting of acute pancreatitis of suspected gallstone origin.67 Additional noninvasive imaging is helpful but adds expense and should probably be reserved for higher risk patients (e.g., cardiopulmonary disease) and patients with relatively low probability (10% to 25%) of ductal stones. Patients with higher stone probability should have ERC without preceding MRCP or EUS (Fig. 37.22C).68 A larger ductal stone is seen in Fig. 37.23 in a patient after cholecystectomy. Intrahepatic stones69–72 (with or without extrahepatic stones) are common in Asians. Fig. 37.24 shows right segmental branches with large fusiform stones. A stone impacted in the cystic duct may compress the common hepatic or common bile duct and cause obstructive jaundice (Mirizzi’s syndrome). Table 37.3 reviews problems encountered in biliary stone cases and ways to solve them.
| Probable bile duct stones (most are in terminal CBD at beginning of examination) | Inject contrast agent with catheter tip in sphincter segment (not deeply cannulated) Inject contrast agent slowly Take film exposures early after only 1–2 cm of duct filled and again at each 1–2 cm further filling |
| With patient prone, tilt table head up 5–20 degrees to keep contrast agent near papilla | |
| Gallbladder stones | Take multiple early filling gallbladder films |
| If overfilled, advance guidewire and catheter into gallbladder and aspirate excess contrast agent (Fig. 37.25) | |
| Take delayed films supine in 4–24 hr | |
| Probable sludge seen in terminal CBD on early films | Stop contrast agent injection; aspirate bile through “see-through” (nonopaque) catheter and confirm granular material |
| Cholangitis manifesting with purulent bile (with or without sepsis) | Aspirate bile from CBD (send for culture) and replace aspirated bile (e.g., 30 mL with less than  volume—10 mL of contrast media); limit intrahepatic filling; do definitive intrahepatic duct and stone evaluation later when cholangitis resolved volume—10 mL of contrast media); limit intrahepatic filling; do definitive intrahepatic duct and stone evaluation later when cholangitis resolved |
CBD, common bile duct.
Biliary Strictures
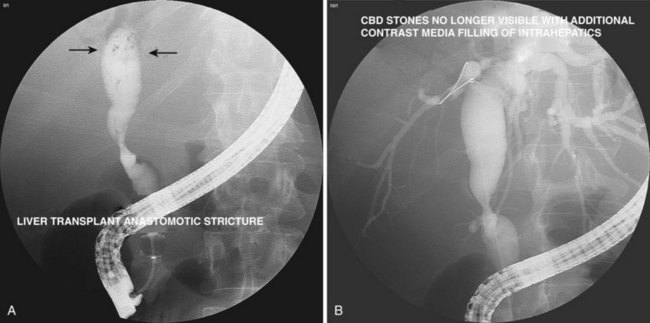
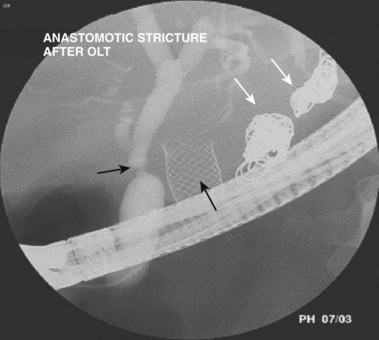
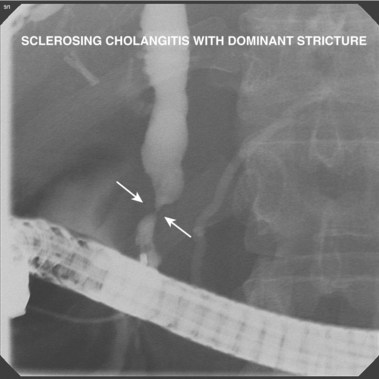
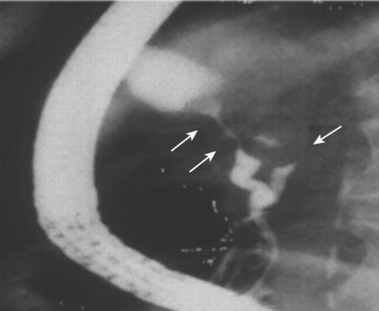
Biliary strictures73–84 are abnormal narrowings of the ductal system resulting from compression (e.g., chronic pancreatitis), scar formation (e.g., postoperative), or neoplasm (e.g., cholangiocarcinoma). These typically manifest clinically with cholestasis, obstructive jaundice, or cholangitis. The etiology of the stricture is usually evident from the history (e.g., recent biliary surgery, ethanol abuse, or elderly patient with weight loss). Fig. 37.26 shows a smooth, tapered long narrowing within the head of the pancreas in a patient with calcific chronic pancreatitis. Laparoscopic cholecystectomy85–87 is associated with thermal or mechanical injury, which results in stricture formation in 0.25% to 0.5% of patients. Fig. 37.27 shows a typical postlaparoscopy stricture. Injuries at open cholecystectomy occur less frequently. Duct transections (Fig. 37.28) and duct resections are the most serious injuries. Orthotopic liver transplant with duct-to-duct anastomosis results in pathologic narrowing at the anastomosis in 15% of patients (Fig. 37.29).88,89 Primary sclerosing cholangitis90–96 is characterized by multifocal extrahepatic or intrahepatic strictures, or both (Fig. 37.30). The gallbladder and cystic duct are spared.
The goals of ERC in suspected primary sclerosing cholangitis are to (1) establish a diagnosis, (2) identify treatable dominant strictures, and (3) identify (or rule out) concomitant cholangiocarcinoma, which occurs in up to 40% of advanced cases going on to transplantation. Pancreatic ductal cell origin adenocarcinoma is the most common cancer encountered on ERC. Pancreatic cancer97,98 of the head has the classic double-duct sign (Fig. 37.31). This sign may also be seen in patients with chronic pancreatitis. Tissue sampling99–101 should be done on strictures that clinically have any suspicion of neoplasm. Brush cytology is easiest to obtain but detects cancer when present in only 30% to 50% of cases. Additional sampling with a second brush, forceps, or endoluminal needles each adds approximately 10% to the diagnostic sensitivity.
Biliary Leaks
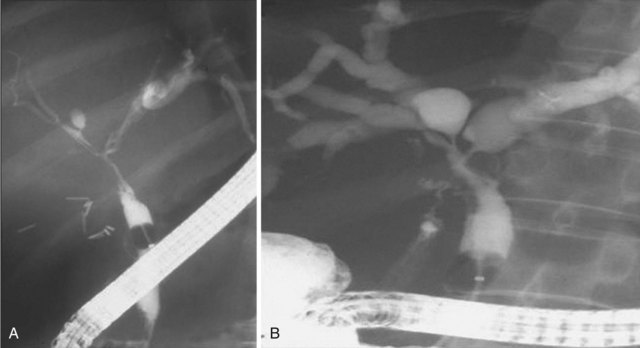
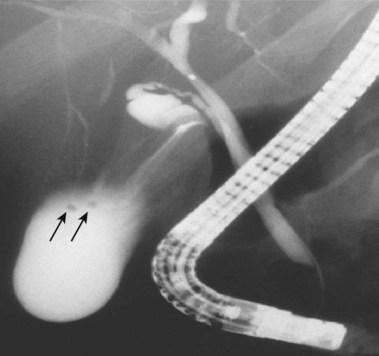
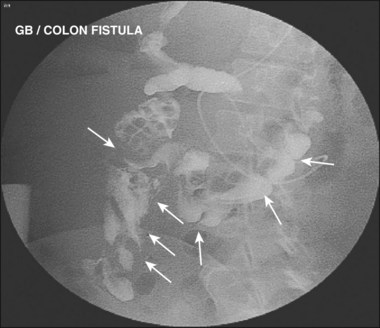
Biliary leaks102–108 result from surgery complications or trauma (penetrating or nonpenetrating). Laparoscopic cholecystectomy is most commonly associated with leaks from the cystic duct or duct of Luschka (Fig. 37.32). Bile leaks typically cause right upper quadrant pain, fever, mildly abnormal serum liver chemistries, and leukocytosis. A typical duct of Luschka leak is seen in Fig. 37.33 with leak occurring from a small intrahepatic duct. A subhepatic contrast collection is seen in Fig. 37.34 from a cystic duct leak occurring after laparoscopic cholecystectomy. Table 37.4 reviews common problems encountered on ERC when dealing with strictures and leaks. Gallbladder disease is commonly found on ERC. Fig. 37.35 shows stones of less than 2 mm diameter in a partially filled gallbladder. Fig. 37.36 shows gallbladder stones and right colon filling via gallbladder fistula to the colon.
Table 37.4 Challenges in Detection and Optimal Viewing of Strictures
| Hilar stricture | Take multiple early filling views with varying degrees of angulation; especially obtain Y confluens view with left and right main hepatic ducts separated |
| Fill only a tiny amount of duct upstream initially, and get guidewire upstream before filling more; avoid thorough upstream filling (rely on MRCP or CT if that information clinically needed) unless guidewire and catheter already passed upstream and full stent drainage is certain | |
| Common bile or common hepatic duct stricture with obviously dilated upstream ducts (per CT or MRI); need upper rim of stricture definition | Avoid thorough filling above stricture; advance large-diameter stone retrieval balloon above stricture, inflate, and fill downstream to balloon to see upper stricture definition better |
| Right lobe not filling—is obstruction present? | Inject more contrast agent with greater force (unless purulent bile) |
| Advance catheter into right duct | |
| Consider aspirating bile from near hilum to “empty” right lobe to make space for contrast agent | |
| If prior sphincterotomy, use balloon occlusion | |
| Tilt head down 5–20 degrees | |
| Probable sclerosing cholangitis setting or other intrahepatic stricturing; contrast agent preferentially enters gallbladder | Limit gallbladder filling in primary sclerosing cholangitis because post-ERCP cholecystitis may occur; inflate balloon catheter above cystic duct takeoff and inject upstream; after more aggressive intrahepatic filling, patient should remain on broad-spectrum antibiotics for 5–7 days |
| Sphincter segment appears narrow | This is usually a normal finding; dilated duct upstream or abnormal liver serum chemistries present suggests pathology |
| Measure length of segment—>12 mm suggests scar or tumor narrowing; correlate with normal or abnormal appearance of papilla; brush cytology, manometry, sphincterotomy with viewing inside ampulla, or endoscopic ultrasound may be needed to clarify | |
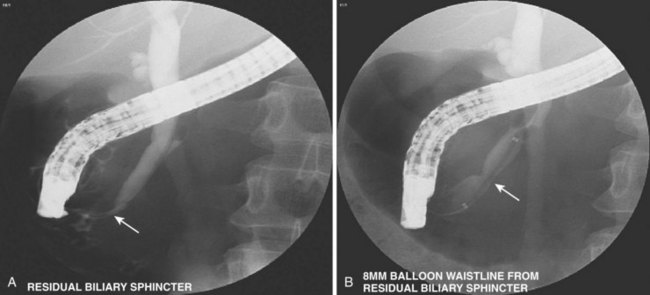
| Is postsphincterotomy biliary orifice adequate? | Do manometry, pull-through stone retrieval balloon; size with hydrostatic balloon (see Fig. 37.34) |
CT, computed tomography; MRCP; magnetic resonance cholangiopancreatography; MRI, magnetic resonance imaging.
Anomalous Pancreatobiliary Ductal Union
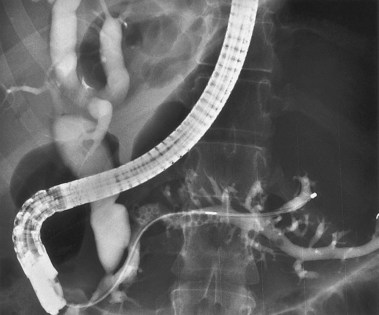
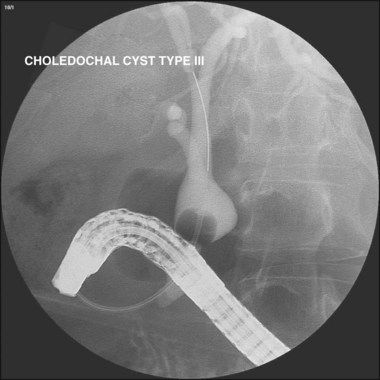
Anomalous pancreatobiliary ductal union109,110 occurs in approximately 2% of Asians but only 0.2% of whites. In this condition, the pancreatobiliary junction occurs outside the duodenal wall, and simultaneous biductal filling occurs from major papilla injection (Figs. 37.37 and 37.38). Approximately one-third of these patients have an associated choledochal cyst (Fig. 37.39). There is also an association with gallbladder cancer.
1 McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of Vater: A preliminary report. Ann Surg. 1968;167:752-756.
2 Classen M, Demling L. [Endoscopic sphincterotomy of the papilla of Vater and extraction of stones from the choledochal duct (author’s transl)]. Dtsch Med Wochenschr. 1974;99:496-497.
3 Kawai K, Akasaka Y, Murakami K, et al. Endoscopic sphincterotomy of the ampulla of Vater. Gastrointest Endosc. 1974;20:148-151.
4 Arguedas MR, Dupont AW, Wilcox CM. Where do ERCP, endoscopic ultrasound, magnetic resonance cholangiopancreatography, and intraoperative cholangiography fit in the management of acute biliary pancreatitis? A decision analysis model. Am J Gastroenterol. 2001;96:2892-2899.
5 Fayad L, Holland GA, Bergin D, et al. Functional magnetic resonance cholangiography (fMRC) of the gallbladder and biliary tree with contrast-enhanced magnetic resonance cholangiography. J Magn Reson Imaging. 2003;18:449-460.
6 Maniatis P, Triantopoulou C, Sofianou E, et al. Virtual CT cholangiography in patients with choledocholithiasis. Abdom Imaging. 2003;28:536-544.
7 Soto JA, Alvarez O, Munera F, et al. Diagnosing bile duct stones: Comparison of unenhanced helical CT, oral contrast-enhanced CT cholangiography, and MR cholangiography. AJR Am J Roentgenol. 2000;175:1127-1134.
8 Stockberger S, Wass JL, Sherman S, et al. Intravenous cholangiography with helical CT: Comparison with endoscopic retrograde cholangiography. Radiology. 1994;192:675-680.
9 Zidi SH, Prat F, Le Guen O, et al. Performance characteristics of magnetic resonance cholangiography in the staging of malignant hilar strictures. Gut. 2000;46:103-106.
10 Urbach D, Khajanchee YS, Jobe BA, et al. Cost-effective management of common bile duct stones: A decision analysis of the use of endoscopic retrograde cholangiopancreatography (ERCP), intraoperative cholangiography, and laparoscopic bile duct exploration. Surg Endosc. 2001;15:4-13.
11 Textor H, Flacke S, Pauleit D, et al. Three-dimensional magnetic resonance cholangiopancreatography with respiratory triggering in the diagnosis of primary sclerosing cholangitis: Comparison with endoscopic retrograde cholangiography. Endoscopy. 2002;34:984-990.
12 Sackmann M, Beuers U, Helmberger T. Biliary imaging: Magnetic resonance cholangiography versus endoscopic retrograde cholangiography. J Hepatol. 1999;30:334-338.
13 Cabada Giadas T, Sarria Octavio de Toledo L, Martinez-Berganza Asensio MT, et al. Helical CT cholangiography in the evaluation of the biliary tract: Application to the diagnosis of choledocholithiasis. Abdom Imaging. 2002;27:61-70.
14 Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918.
15 Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: A prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434.
16 Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest Endosc. 1991;37:383-393.
17 Jowell PS, Baillie J, Branch MS, et al. Quantitative assessment of procedural competence: A prospective study of training in endoscopic retrograde cholangiopancreatography. Ann Intern Med. 1996;125:983-989.
18 ASGE Standards of Practice CommitteeAnderson MA, Ben-Menachem T, Gan SI, et al. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060-1070.
19 Draganov PV, Forsmark CE. Prospective evaluation of adverse reactions to iodine-containing contrast media after ERCP. Gastrointest Endosc. 2008;68:1098-1101.
20 Choudari CP, Fogel E, Kalayci C, et al. Therapeutic biliary endoscopy. Endoscopy. 1999;31:80-87.
21 Maple JT, Keswani RN, Hovis RM, et al. Carbon dioxide insufflation during ERCP for reduction of postprocedure pain: A randomized, double-blind, controlled trial. Gastrointest Endosc. 2009;70:278-283.
22 Wright BE, Cass OW, Freeman ML. ERCP in patients with long-limb Roux-en-Y gastrojejunostomy and intact papilla. Gastrointest Endosc. 2002;56:225-232.
23 Haber GB. Double balloon endoscopy for pancreatic and biliary access in altered anatomy (with videos). Gastrointest Endosc. 2007;66:S47-S50.
24 Koornstra JJ, Fry L, Monkemuller K. ERCP with the balloon-assisted enteroscopy technique: A systematic review. Dig Dis. 2008;26:324-329.
25 Heyd RL, Kopecky KK, Sherman S, et al. Radiation exposure to patients and personnel during interventional ERCP at a teaching institution. Gastrointest Endosc. 1996;44:287-292.
26 Ferreira LE, Baron TH. Comparison of safety and efficacy of ERCP performed with the patient in supine and prone positions. Gastrointest Endosc. 2008;67:1044-1045.
27 Wille RT, Barnett JL, Chey WD, et al. Routine droperidol pre-medication improves sedation for ERCP. Gastrointest Endosc. 2000;52:362-366.
28 Yimcharoen P, Fogel EL, Kovacs RJ, et al. Droperidol, when used for sedation during ERCP, may prolong the QT interval. Gastrointest Endosc. 2006;63:979-985.
29 Krugliak P, Ziff B, Rusabrov Y, et al. Propofol versus midazolam for conscious sedation guided by processed EEG during endoscopic retrograde cholangiopancreatography: A prospective, randomized, double-blind study. Endoscopy. 2000;32:677-682.
30 Gillham MJ, Hutchinson RC, Carter R, et al. Patient-maintained sedation for ERCP with a target-controlled infusion of propofol: A pilot study. Gastrointest Endosc. 2001;54:14-17.
31 Hansen J, Ulmer B, Rex D. Technical performance of colonoscopy in patients sedated with nurse-administered propofol. Am J Gastroenterol. 2004;99:52-56.
32 Cheung J, Tsoi KK, Quan WL, et al. Guidewire versus conventional contrast cannulation of the common bile duct for the prevention of post-ERCP pancreatitis: A systematic review and meta-analysis. Gastrointest Endosc. 2009;70:1211-1219.
33 Kumar S, Sherman S, Hawes RH, et al. Success and yield of second attempt ERCP. Gastrointest Endosc. 1995;41:445-447.
34 Choudari CP, Sherman S, Fogel EL, et al. Success of ERCP at a referral center after a previously unsuccessful attempt. Gastrointest Endosc. 2000;52:478-483.
35 Heiss FW, Cimis RSJr, MacMillan FPJr. Biliary sphincter scissor for pre-cut access: Preliminary experience. Gastrointest Endosc. 2002;55:719-722.
36 Kasmin FE, Cohen D, Batra S, et al. Needle-knife sphincterotomy in a tertiary referral center: Efficacy and complications. Gastrointest Endosc. 1996;44:48-53.
37 Cotton PB. Precut papillotomy—a risky technique for experts only. Gastrointest Endosc. 1989;35:578-579.
38 Binmoeller KF, Seifert H, Gerke H, et al. Papillary roof incision using the Erlangen-type pre-cut papillotome to achieve selective bile duct cannulation. Gastrointest Endosc. 1996;44:689-695.
39 Goff JS. Long-term experience with the transpancreatic sphincter pre-cut approach to biliary sphincterotomy. Gastrointest Endosc. 1999;50:642-645.
40 George S, Kulkarni AA, Stevens G, et al. Role of osmolality of contrast media in the development of post-ERCP pancreatitis: A metaanalysis. Dig Dis Sci. 2004;49:503-508.
41 Hintze RE, Abou-Rebyeh H, Adler A, et al. Magnetic resonance cholangiopancreatography-guided unilateral endoscopic stent placement for Klatskin tumors. Gastrointest Endosc. 2001;53:40-46.
42 De Palma GD, Galloro G, Siciliano S, et al. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: Results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547-553.
43 Freeman ML, Overby C. Selective MRCP and CT-targeted drainage of malignant hilar biliary obstruction with self-expanding metallic stents. Gastrointest Endosc. 2003;58:41-49.
44 Lehman G, Sherman S. Sphincter of Oddi dysfunction. In: Yamada T, Alpers DH, Laine L, et al, editors. Textbook of gastroenterology. Philadelphia: Lippincott Williams & Wilkins; 1999:2343-2354.
45 Hogan WJ, Sherman S, Pasricha P, et al. Sphincter of Oddi manometry. Gastrointest Endosc. 1997;45:342-348.
46 Sherman S, Gottlieb K, Uzer MF, et al. Effects of meperidine on the pancreatic and biliary sphincter. Gastrointest Endosc. 1996;44:239-242.
47 Blaut U, Sherman S, Fogel E, et al. Influence of cholangiography on biliary sphincter of Oddi manometric parameters. Gastrointest Endosc. 2000;52:624-629.
48 Kalayci C, Aisen A, Canal D, et al. Magnetic resonance cholangiopancreatography documents bile leak site after cholecystectomy in patients with aberrant right hepatic duct where ERCP fails. Gastrointest Endosc. 2000;52:277-281.
49 Hand B. Anatomy and embryology of the biliary tract and pancreas. In: Sivak MVJr, editor. Gastroenterologic endoscopy. Philadelphia: Saunders; 1987:863-877.
50 Hand B. Anatomy and function of the extrahepatic biliary system. Rev Gastroenterol Mex. 1973;2:3-29.
51 Gazelle GS, Lee MJ, Mueller PR. Cholangiographic segmental anatomy of the liver. Radiographics. 1994;14:1005-1013.
52 Boraschi P, Gigoni R, Braccini G, et al. Detection of common bile duct stones before laparoscopic cholecystectomy: Evaluation with MR cholangiography. Acta Radiol. 2002;43:593-598.
53 Charfare H, Cheslyn-Curtis S. Selective cholangiography in 600 patients undergoing cholecystectomy with 5-year follow-up for residual bile duct stones. Ann R Coll Surg Engl. 2003;85:167-173.
54 Coppola R, Riccioni ME, Ciletti S, et al. Selective use of endoscopic retrograde cholangiopancreatography to facilitate laparoscopic cholecystectomy without cholangiography: A review of 1139 consecutive cases. Surg Endosc. 2001;15:1213-1216.
55 Sherman S, Hawes RH, Lehman GA. Management of bile duct stones. Semin Liver Dis. 1990;10:205-221.
56 Bergman JJ, Rauws EA, Tijssen JG, et al. Biliary endoprostheses in elderly patients with endoscopically irretrievable common bile duct stones: Report on 117 patients. Gastrointest Endosc. 1995;42:195-201.
57 Prat F, Tennenbaum R, Ponsot P, et al. Endoscopic sphincterotomy in patients with liver cirrhosis. Gastrointest Endosc. 1996;43(2 Pt 1):127-131.
58 Folsch UR, Nitsche R, Ludtke R, et al. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med. 1997;336:237-242.
59 Fan ST, Lai EC, Mok FP, et al. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328:228-232.
60 Neoptolemos JP, Carr-Locke DL, London NJ, et al. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979-983.
61 Fogel E, Sherman S. Acute biliary pancreatitis: When should the endoscopist intervene? Gastroenterology. 2003;125:229-235.
62 Nowak A, Nowakowska-Dulawa E, Marek TA, et al. Final results of the prospective, randomized, controlled study on endoscopic sphincterotomy versus conventional management in acute biliary pancreatitis. Gastroenterology. 1995;108:A380.
63 Connors P, Carr-Locke D. Endoscopic retrograde cholangiopancreatography findings and endoscopic sphincterotomy for cholangitis and pancreatitis. Gastrointest Endosc Clin N Am. 1991;1:27-50.
64 Kozarek RA. Role of ERCP in acute pancreatitis. Gastrointest Endosc. 2002;56:S231-S236.
65 Uomo G, Manes G, Laccetti M. Endoscopic sphincterotomy and recurrence of acute pancreatitis in gallstone patients considered unfit for surgery. Pancreas. 1997;14:28-30.
66 Boerma D, Rauws EA, Keulemans YC, et al. Wait-and-see policy or laparoscopic cholecystectomy after endoscopic sphincterotomy for bile-duct stones: A randomized trial. Lancet. 2002;360:761-765.
67 Sharma VK, Howden CW. Metaanalysis of randomized controlled trials of endoscopic retrograde cholangiography and endoscopic sphincterotomy for the treatment of acute biliary pancreatitis. Am J Gastroenterol. 1999;94:3211-3214.
68 ASGE Standards of Practice CommitteeMaple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc. 2010;71:1-9.
69 Lee SK, Seo DW, Myung SJ, et al. Percutaneous transhepatic cholangioscopic treatment for hepatolithiasis: An evaluation of long-term results and risk factors for recurrence. Gastrointest Endosc. 2001;53:318-323.
70 Kim M, Sekijima J, Lee S. Primary intrahepatic stones. Am J Gastroenterol. 1995;90:540-548.
71 Jeng K. Treatment of intrahepatic biliary strictures associated with hepatolithiasis. Hepatogastroenterology. 1997;44:342-351.
72 Kim M, Lim BC, Myung SJ, et al. Epidemiological study on Korean gallstone disease: A nation-wide cooperative study. Dig Dis Sci. 1999;44:1674-1683.
73 Smith MT, Sherman S, Lehman GA. Endoscopic management of benign strictures of the biliary tree. Endoscopy. 1995;27:253-266.
74 Davids PH, Rauws EA, Coene PP, et al. Endoscopic stenting for post-operative biliary strictures. Gastrointest Endosc. 1992;38:12-18.
75 Berkelhammer C, Kortan P, Haber GB. Endoscopic biliary prostheses as treatment for benign postoperative bile duct strictures. Gastrointest Endosc. 1989;35:95-101.
76 Bergman JJ, Burgemeister L, Bruno MJ, et al. Long-term follow-up after biliary stent placement for postoperative bile duct stenosis. Gastrointest Endosc. 2001;54:154-161.
77 Costamagna G, Pandolfi M, Mutignani M, et al. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001;54:162-168.
78 Davids PH, Tanka AK, Rauws EA, et al. Benign biliary strictures: Surgery or endoscopy? Ann Surg. 1993;217:237-243.
79 Dumonceau JM, Deviere J, Delhaye M, et al. Plastic and metal stents for postoperative benign bile duct strictures: The best and the worst. Gastrointest Endosc. 1998;47:8-17.
80 Smits ME, Rauws EA, van Gulik TM, et al. Long-term results of endoscopic stenting and surgical drainage for biliary stricture due to chronic pancreatitis. Br J Surg. 1996;83:764-768.
81 Barthet M, Bernard JP, Duval JL, et al. Biliary stenting in benign biliary stenosis complicating chronic calcifying pancreatitis. Endoscopy. 1994;26:569-572.
82 Farnbacher MJ, Rabenstein T, Ell C, et al. Is endoscopic drainage of common bile duct stenoses in chronic pancreatitis up-to-date? Am J Gastroenterol. 2000;95:1466-1471.
83 Ludwig K, Bernhardt J, Steffen H, et al. Contribution of intraoperative cholangiography to incidence and outcome of common bile duct injuries during laparoscopic cholecystectomy. Surg Endosc. 2002;16:1098-1104.
84 Hirao K, Miyazaki A, Fujimoto T, et al. Evaluation of aberrant bile ducts before laparoscopic cholecystectomy: Helical CT cholangiography versus MR cholangiography. AJR Am J Roentgenol. 2000;175:713-720.
85 A prospective analysis of 1518 laparoscopic cholecystectomies. The Southern Surgeons Club. N Engl J Med. 1991;324:1073-1078.
86 Gouma D, Go P. Bile duct injury during laparoscopic and conventional cholecystectomy. J Am Coll Surg. 1994;178:229-233.
87 Bergman JJ, van den Brink GR, Rauws EA, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut. 1996;38:141-147.
88 Rerknimitr R, Sherman S, Fogel EL, et al. Biliary tract complications after orthotopic liver transplantation with choledochostomy anastomosis: Endoscopic findings and results of therapy. Gastrointest Endosc. 2002;55:224-231.
89 Morelli J, Mulcahy HE, Willner IR, et al. Long-term outcomes for patients with post-liver transplant anastomotic biliary strictures treated by endoscopic stent placement. Gastrointest Endosc. 2003;58:374-379.
90 Campbell WL, Ferris JV, Holbert BL, et al. Biliary tract carcinoma complicating primary sclerosing cholangitis: Evaluation with CT, cholangiography, US, and MR imaging. Radiology. 1998;207:41-50.
91 Campbell WL, Peterson MS, Federle MP, et al. Using CT and cholangiography to diagnose biliary tract carcinoma complicating primary sclerosing cholangitis. AJR Am J Roentgenol. 2001;177:1095-1100.
92 Ernst O, Asselah T, Sergent G, et al. MR cholangiography in primary sclerosing cholangitis. AJR Am J Roentgenol. 1998;171:1027-1030.
93 Lee S, Kim MH, Lee SK, et al. MR cholangiography versus cholangioscopy for evaluation of longitudinal extension of hilar cholangiocarcinoma. Gastrointest Endosc. 2002;56:25-32.
94 Baluyut AR, Sherman S, Lehman GA, et al. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. Gastrointest Endosc. 2001;53:308-312.
95 Chalasani N, Baluyut A, Ismail A, et al. Cholangiocarcinoma in patients with primary sclerosing cholangitis: A multicenter case-control study. Hepatology. 2000;31:7-11.
96 Tanaka Y, Koshiyama H, Nakao K, et al. Rapid progress of acute suppurative cholangitis to secondary sclerosing cholangitis sequentially followed-up by endoscopic retrograde cholangiography. Endoscopy. 2001;33:633-635.
97 Costamagna G, Pandolfi M, Mutignani M. Carcinoma of the pancreatic head area. Diagnostic imaging. Direct cholangiography: ERCP. Rays. 1995;20:269-279.
98 Kozarek RA. Endoscopy in the management of malignant obstructive jaundice. Gastrointest Endosc Clin N Am. 1996;6:153-176.
99 De Bellis M, Sherman S, Fogel EL, et al. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 1). Gastrointest Endosc. 2002;56:552-561.
100 De Bellis M, Sherman S, Fogel EL, et al. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 2). Gastrointest Endosc. 2002;56:720-730.
101 Jailwala J, Fogel EL, Sherman S, et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000;51(4 Pt 1):383-390.
102 Brooks DC, Becker JM, Connors PJ, et al. Management of bile leaks following laparoscopic cholecystectomy. Surg Endosc. 1993;7:292-295.
103 Frakes JT, Bradley SJ. Endoscopic stent placement for biliary leak from an accessory duct of Luschka after laparoscopic cholecystectomy. Gastrointest Endosc. 1993;39:90-92.
104 Peters J, Ollila D, Nichols K. Diagnosis and management of bile leaks following laparoscopic cholecystectomy. Surg Endosc. 1994;4:163-170.
105 Barkun A, Rezieg M, Mehta SN, et al. Postcholecystectomy biliary leaks in the laparoscopic era: Risk factors, presentation, and management. McGill Gallstone Treatment Group. Gastrointest Endosc. 1997;45:277-282.
106 Bjorkman DJ, Carr-Locke DL, Lichtenstein DR, et al. Postsurgical bile leaks: Endoscopic obliteration of the transpapillary pressure gradient is enough. Am J Gastroenterol. 1995;90:2128-2133.
107 Barton JR, Russell RC, Hatfield AR. Management of bile leaks after laparoscopic cholecystectomy. Br J Surg. 1995;82:980-984.
108 Raijman I, Catalano MF, Hirsch GS, et al. Endoscopic treatment of biliary leakage after laparoscopic cholecystectomy. Endoscopy. 1994;26:741-744.
109 Samavedy R, Sherman S, Lehman GA. Endoscopic therapy in anomalous pancreatobiliary duct junction. Gastrointest Endosc. 1999;50:623-627.
110 Schmidt HG, Bauer J, Wiessner V, et al. Endoscopic aspects of choledochoceles. Hepatogastroenterology. 1996;43:143-146.