Chapter 366 Diagnostic Approach to Respiratory Disease
Physical Examination
Respiratory dysfunction usually produces detectable alterations in the pattern of breathing. Values for normal respiratory rates are presented in Table 62-1 and depend on many factors, most importantly, age. Repeated respiratory rate measurements are necessary because respiratory rates, especially in the young, are exquisitely sensitive to extraneous stimuli. Sleeping respiratory rates are more reproducible in infants than those obtained during feeding or activity. These rates vary among infants but average 40-50 breaths/min in the 1st few weeks of life and usually <60 breaths/min in the 1st few days of life.
Respiratory control abnormalities can cause the child to breathe at a low rate or periodically. Mechanical abnormalities produce compensatory changes that are generally directed at altering minute ventilation to maintain alveolar ventilation. Decreases in lung compliance require increases in muscular force and breathing rate, leading to variable increases in chest wall retractions and nasal flaring. The respiratory excursions of children with restrictive disease are shallow. An expiratory grunt is common as the child attempts to raise the functional residual capacity (FRC) by closing the glottis at the end of expiration. Children with obstructive disease might take slower, deeper breaths (Chapter 365). When the obstruction is extrathoracic (from the nose to the mid-trachea), inspiration is more prolonged than expiration, and an inspiratory stridor can usually be heard. When the obstruction is intrathoracic, expiration is more prolonged than inspiration, and the patient often has to make use of accessory expiratory muscles. Intrathoracic obstruction results in air trapping and, therefore, a larger residual volume and, perhaps, greater functional residual capacity.
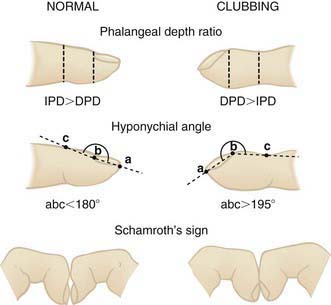
Auscultation confirms the presence of inspiratory or expiratory prolongation and provides information about the symmetry and quality of air movement. In addition, it often detects abnormal or adventitious sounds such as stridor (a predominant inspiratory monophonic noise), crackles (or rales) (high-pitched, interrupted sounds found during inspiration and more rarely during early expiration, which denote opening of previously closed air spaces), or wheezes (musical, continuous sounds usually caused by the development of turbulent flow in narrow airways) (Table 366-1). Digital clubbing is a sign of chronic hypoxia and chronic lung disease (Fig. 366-1) but may be due to nonpulmonary etiologies (Table 366-2).
| TYPE | SOUND |
|---|---|
| DISCONTINUOUS | |
| Fine (high pitch, low amplitude, short duration) | Fine crackles/rales |
| Coarse (low pitch, high amplitude, long duration) | Coarse crackles |
| CONTINUOUS | |
| High pitch | Wheezes |
| Low pitch | Rhonchi |
From Cugell DW: Lung sound nomenclature, Am Rev Respir Dis 136:1016, 1987, with permission; from Chernick V, Boat TF: Kendig’s disorders of the respiratory tract in children, ed 6, Philadelphia, 1998, WB Saunders, p 97.
Table 366-2 NONPULMONARY DISEASES ASSOCIATED WITH CLUBBING
CARDIAC
HEMATOLOGIC
GASTROINTESTINAL
OTHER
UNILATERAL CLUBBING
From Chernick V, Boat TF: Kendig’s disorders of the respiratory tract in children, ed 6, Philadelphia, 1998, WB Saunders, p 102.
Blood Gas Analysis
Blood gas exchange is evaluated most accurately by the direct measurement of arterial PO2, PCO2, and pH (Chapters 95.3 and 365). The blood specimen is best collected anaerobically in a heparinized syringe containing only enough heparin solution to displace the air from the syringe. The syringe should be sealed, placed in ice, and analyzed immediately. Although these measurements have no substitute in many conditions, they require arterial puncture and have been replaced to a great extent by noninvasive monitoring, such as capillary samples and/or oxygen saturation.
The age and clinical condition of the patient need to be taken into account when interpreting blood gas tensions. With the exception of neonates, values of arterial PO2 <85 mm Hg are usually abnormal for a child breathing room air at sea level. Calculation of the alveolar-arterial oxygen gradient is useful in the analysis of arterial oxygenation, particularly when the patient is not breathing room air or in the presence of hypercarbia. Values of arterial PCO2 >45 mm Hg usually indicate hypoventilation or a severe ventilation-perfusion mismatch, unless they reflect respiratory compensation for metabolic alkalosis (Chapter 52).
Radiographic Techniques
Sinus and Nasal Films
The general utility of roentgenographic examination of the sinuses is uncertain because of the large number of films with positive findings (low sensitivity and specificity, Table 366-3). Imaging studies are not necessary to confirm the diagnosis of sinusitis in children <6 yr. CT scans are indicated if surgery is required, in cases of complications due to sinus infection, in immunodeficient patients, and for recurrent infections that are not responsive to medical management.
Table 366-3 FACTORS CONTRIBUTING TO LOW SENSITIVITY AND SPECIFICITY OF IMAGING SINUSITIS IN PEDIATRIC PATIENTS
From Slovis TL, editor: Caffey’s pediatric diagnostic imaging, ed 11, vol 1, Philadelphia, 2008, Mosby, p 571.
Pulmonary Function Testing
Whether restrictive or obstructive, most forms of respiratory disease cause alterations in lung volume and its subdivisions (Chapter 365). Restrictive diseases typically decrease total lung capacity (TLC). TLC includes residual volume, which is not accessible to direct determinations. It must therefore be measured indirectly by gas dilution methods or, preferably, by plethysmography. Restrictive disease also decreases vital capacity (VC). Obstructive diseases produce gas trapping and thus increase residual volume and FRC, particularly when these measurements are considered with respect to TLC.
Airway obstruction is most commonly evaluated from determinations of gas flow in the course of a forced expiratory maneuver. The peak expiratory flow is reduced in advanced obstructive disease. The wide availability of simple devices that perform this measurement at the bedside makes it useful for assessing children who have airway obstruction. Evaluation of peak flows requires a voluntary effort, and peak flows may not be altered when the obstruction is moderate or mild. Other gas flow measurements require that the child inhale to TLC and then exhale as far and as fast as possible for several seconds. Cooperation and good muscle strength are therefore necessary for the measurements to be reproducible. The forced expiratory volume in 1 sec (FEV1) correlates well with the severity of obstructive diseases. The maximal midexpiratory flow rate, the average flow during the middle 50% of the forced vital capacity (FVC), is a more reliable indicator of mild airway obstruction. Its sensitivity to changes in residual volume and vital capacity, however, limits its use in children with more severe disease. The construction of flow-volume relationships during the FVC maneuvers overcomes some of these limitations by expressing the expiratory flows as a function of lung volume (Chapter 365).
A spirometer is used to measure VC and its subdivisions and expiratory (or inspiratory) flow rates (see Fig. 365-1). A simple manometer can measure the maximal inspiratory and expiratory force a subject generates, normally at least 30 cm H2O, which is useful in evaluating the neuromuscular component of ventilation. Expected normal values for VC, FRC, TLC, and residual volume are obtained from prediction equations based on body height.
Flow rates measured by spirometry usually include the FEV1 and the maximal midexpiratory flow rate. More information results from a maximal expiratory flow-volume curve, in which expiratory flow rate is plotted against expired lung volume (expressed in terms of either VC or TLC). Flow rates at lung volumes <~75% VC are relatively independent of effort. Expiratory flow rates at low lung volumes (<50% VC) are influenced much more by small airways than are flow rates at high lung volumes (FEV1). The flow rate at 25% VC (V25) is a useful index of small airway function. Low flow rates at high lung volumes associated with normal flow at low lung volumes suggest upper airway obstruction (Chapter 365).
Exercise Testing
Exercise testing (Chapter 417.5) is a more-direct approach for detecting diffusion impairment as well as other forms of respiratory disease. Exercise is a strong provocateur of bronchospasm in susceptible patients, so exercise testing can be useful in the diagnosis of patients with asthma that is only apparent with activity. Measurements of heart and respiratory rate, minute ventilation, oxygen consumption, carbon dioxide production, and arterial blood gases during incremental exercise loads often provide invaluable information about the functional nature of the disease. Often a simple assessment of the patient’s exercise tolerance in conjunction with other, more static forms of respiratory function testing can allow a distinction between respiratory and nonrespiratory disease in children.
Sleep Studies
The sleep state has an important influence on respiratory function at all ages. Polysomnographic studies are often helpful for diagnosing airway obstruction during sleep, when abnormalities of central respiratory control, muscular disorders, or respiratory complications from gastroesophageal reflux (GER) are suspected. Polysomnography is now considered the gold standard test for obstructive sleep apnea or hypoventilation during sleep. pH probe studies are indicated and are added to such sleep studies when GER is suspected. In these studies, a pH probe is placed in the esophagus and prolonged (usually over several hours) monitoring is undertaken (Chapter 315). These studies, which usually include the simultaneous assessment of ventilatory effort, airway gas flow, gas exchange, and sleep state, are also useful in the diagnosis and management of airway and respiratory control disorders and nocturnal hypoxemia and hypercapnia in children with chronic respiratory disease (Chapter 365).
Airway Visualization and Lung Specimen–Based Diagnostic Tests
American Academy of Pediatrics, Subcommittee on Management of Sinusitis and Committee on Quality Improvement. Clinical practice guideline: management of sinusitis. Pediatrics. 2001;108:798-808.
Bush A. Update in pediatric lung disease 2008. Am J Respir Crit Care Med. 2009;179:637-649.
Deutsch GH, Young LR, Deterding RR, et aland the Pathology Cooperative Group, on behalf of the ChILD Research Co-operative. Diffuse lung disease in young children. Application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120-1128.
Goodman TR, McHugh K. The role of radiology in the evaluation of stridor. Arch Dis Child. 1999;81:456-459.
Hirsch W, Sorge I, Krohmer S, et al. MRI of the lungs in children. Eur J Radiol. 2008;68(2):278-288.
Margolis P, Ferkol T, Marsocci S, et al. Accuracy of the clinical examination in detecting hypoxemia in infants with respiratory illness. J Pediatr. 1994;124:552-560.
Schechter MS. Snoring: investigations guidelines. Pediatr Pulmonol Suppl. 2004;26:172-174.
Schechter MS, Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:e69.
Welsby PD, Earis JE. Some high pitched thoughts on chest examination. Postgrad Med J. 2001;77:617-620.