CHAPTER 35 DIAGNOSTIC AND THERAPEUTIC ROLES OF BRONCHOSCOPY AND VIDEO-ASSISTED THORACOSCOPY IN THE MANAGEMENT OF THORACIC TRAUMA
Direct injury to the chest and pulmonary complications after any major trauma account for a significant proportion of trauma-related morbidity and mortality. In the past the role of thoracic endoscopy was limited to bronchoscopic diagnosis of major airway injury and assistance with pulmonary toilet. Major injuries to the tracheobronchial tree, significant hemorrhage within the chest, failure of nonoperative management of chest injuries, and major pulmonary complications invariably required thoracotomy with its high morbidity and mortality. Technical advances in fiber optics and videoscopic imaging have led to rapid advances in the field of minimally invasive surgery. In the thoracic region, this has led to the advent of videoassisted thoracoscopic surgery (VATS) and the broadening of the role of bronchoscopy, both in terms of diagnosis and therapy. These minimally invasive endoscopic techniques have significantly lower morbidity and mortality. The current chapter focuses on the evolving role of thoracic endoscopy (thoracoscopy and bronchoscopy) following chest trauma and major pulmonary complications after any trauma.
INCIDENCE
Thoracic trauma accounts for a significant burden of disease in terms of morbidity and mortality. Twenty percent of trauma-associated deaths involve chest injury, and chest trauma is second only to head and spinal cord injuries as a cause of death following trauma.1 Death as a result of chest trauma in the acute setting is related to either airway injury, direct trauma to the heart, or massive bleeding in the chest cavity. In addition to the acute deaths that are a direct result of chest trauma, pulmonary complications following chest trauma or any trauma add to the mortality and morbidity attributable to the chest.2
Most chest trauma comprises of abrasions, rib fractures, and simple pneumothoraces that are easily diagnosed with chest x-rays and/or computed tomography (CT) and can be treated with simple measures—pulmonary toilet, pain management, and tube thoracostomy. The indications for open thoracotomy in the acute setting are principally major airway disruption and massive bleeding in the chest or airway. Approximately 1% of all trauma admissions require open thoracotomy in the acute setting.3 Open thoracotomy in this setting is associated with a very high morbidity and mortality.3 Bronchoscopic control of bleeding within the airway and stenting of major airway injury can offer a lower-risk alternative to open surgery in selected patients.4,5 Similarly, VATS offers a lower-risk alternative for managing hemorrhage within the chest cavity.6 In the nonacute setting, trauma patients with or without direct injury to the chest have a high incidence of pulmonary complications, including pneumonia, retained hemothorax, fibrothorax, empyema, and acute respiratory distress syndrome (ARDS), posing diagnostic and therapeutic challenges.1,2 Thoracic endoscopy in this setting is playing an increasingly important role in early diagnosis and therapy for these complications, and possibly improving outcome.
DIAGNOSTIC AND THERAPEUTIC ROLES OF VATS IN CHEST TRAUMA
Indications and Patient Selection
The first diagnostic and therapeutic application of thoracoscopy for the management of thoracic pathologies such as pleural effusions, pleural adhesions, empyemas, and thoracic malignancy, was recorded by Jacobeaus at the University of Stockholm in 1922.7 Thoracoscopy for the treatment of traumatic injuries was first described in 1946 by Branco for the management of hemothorax in penetrating chest injuries.8 In the last decade, the advent of minimally invasive access to the thoracic cavity combined with video-assisted technology and selective lung ventilation has revolutionized the diagnosis and the treatment of thoracic injuries with improved use and outcomes.9,10
Patient selection is very important for the application of VATS in thoracoabdominal trauma. The current indications are both diagnostic and therapeutic and include mainly the evaluation of a structural injury (the diaphragm, the pericardium, lung parenchyma, the thoracic duct, etc.) or the drainage of a pleural collection and repair of any structural damage. Aside from the usual bleeding disorders, the main contraindications of VATS include an unstable patient, or a patient with underlying lung and cardiac pathologies that preclude the use of single lung ventilation. Table 1 lists the current indications and contraindications for the use of VATS.
| Indications | Contraindications |
|---|---|
| Persistent pneumothorax | Hemodynamic instability |
Diaphragmatic Injuries
The incidence of blunt diaphragmatic injuries have been reported as low as 0.8% and as high as 7%.11,12 Blunt trauma accounts for 10%–30% of traumatic diaphragmatic ruptures (TDR) in North American series from urban trauma centers.13 The incidence of diaphragmatic injuries has been reported to be as high as 67% in penetrating thoracoabdominal trauma.14
Diaphragmatic injuries are particularly difficult to diagnose with the use of radiographic imaging such as chest x-ray or CT and can be missed in up to 30% of patients.14,15 Together with laparoscopy, thoracoscopy has become the diagnostic tool of choice for diaphragmatic injuries when compared with other nonoperative modalities.16,17 Thoracoscopy is particularly useful when laparoscopy may not be optimal or feasible. It is useful for evaluation of right-sided diaphragmatic injuries and of posterior wounds from the posterior axillary line to the spine.18 It is also useful for avoidance of abdominal procedures, particularly in patients with previous laparotomies and expected presence of extensive adhesions.
The therapeutic role of VATS in the treatment of diaphragmatic injuries is well documented.14,19 In a report of 24 patients who underwent VATS for thoracic injuries, 9 of 10 patients were successfully diagnosed with diaphragmatic injuries. VATS was used for repair of the diaphragm in four patients.19 Martinez et al.14 evaluated 52 patients with penetrating thoracoabdominal trauma admitted to the General Hospital for Accidents in Guatemala City. VATS was used to diagnose 35 patients with diaphragmatic injuries. All 35 diaphragmatic injuries were successfully repaired thoracoscopically. Even though successful thoracoscopic repair of diaphragm injuries is reported as feasible, safe, and expeditious, currently no long-term outcome results are available.
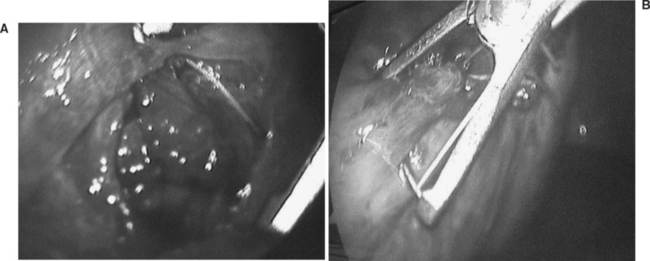
In areas where other abdominal injuries are suspected, a laparoscopic or open surgical approach is preferable depending on the available surgical expertise. A combined thoracic and abdominal cavitary endoscopy can also be useful. Figure 1 shows the thoracoscopic evaluation of a right diaphragm injury from an impaled object in the chest of a patient evaluated in our trauma center. This was followed by the thoracoscopic repair of the diaphragm after the laparoscopic confirmation of a nonbleeding liver laceration, and no other associated abdominal injuries. In all cases where a diaphragm injury is found, an exploratory laparoscopy or laparotomy is mandatory to rule out associated intra-abdominal injuries.
Retained Thoracic Collection
The evacuation of a retained hemothorax is one of the main indications for VATS. Inadequate evacuation of blood from the pleural space and prolonged thoracostomy tube drainage put the patient at risk for developing empyema and fibrothorax with prolonged hospital stays and increasing costs.9,20 The incidence of a retained hemothorax and empyema post–tube thoracostomy placement ranges from 4% to 20% and from 4% to 10%, respectively.8,9,19,21–23 A prospective randomized study of 39 patients with thoracic trauma and retained hemothoraces from Parkland Memorial Hospital showed that early evacuation with VATS compared with conventional therapy of a secondary chest tube placement lead to a significantly shorter duration of tube drainage (2.5 days), shorter hospital stay (2.7 days), and reduced hospital costs ($6000).9 These advantages of VATS were attributed to rapid and complete evacuation of the pleural space, optimal video-assisted positioning of the thoracostomy tubes, and identification and treatment of the sources of the bleeding and of other associated intrathoracic injuries.
It is important to note that these advantages rest on the early use (day 4–7 postinjury) of VATS for the evacuation of the hemothorax. In a study by Heniford et al.,20 19 of 25 patients (76%) with retained hemothorax were successfully treated with VATS. Failure of VATS correlated with time interval from injury to operation, and with the type of fluid collection (hemothorax vs. empyema). The mean time between admission and operation for the successful versus unsuccessful thoracoscopic drainage was 4.5 days and 14.5 days, respectively.20
The application of VATS for traumatic empyema is dependent on the phase of the empyema. For the acute/exudative empyema occurring between 1 and 5 days, VATS is uniformly effective. The success rate for the transitional/fibrinopurulent stage (day 6–14) is around 75%–85%, with a sharp drop to around 50% in the organized/chronic phase (>2 weeks).24,25 The presence of a thick fibrin peel with entrapped lung requires mature surgical judgment for early conversion to open thoracotomy and avoidance of the risk of further pulmonary parenchymal injury or the creation of a bronchopleural fistula.
Persistent Hemorrhage
Video-assisted thoracoscopic surgery is also useful for patients with persistent but slow hemorrhage and no hemodynamic instability. Unstable patients with suspected thoracic bleed require open thoracotomy. Smith et al.26 performed VATS on five hemodynamically stable patients for persistent hemorrhage from intercostal vessels. In three patients, the bleeding was successfully controlled with diathermy. Other techniques for hemorrhage control, including endoclips or argon beam coagulators, can be used.15 Intracorporeal suture placement around the rib was used successfully in our center for control of a persistent intercostal bleed not amenable to endoclip placement. The success rate for the thoracoscopic control of a nonhemodynamically compromising hemorrhage is around 80% with a thoracotomy conversion rate of 15%–20%.24,27
Persistent Pneumothorax
The incidence of persistent air leak and lung re-expansion 72 hours post–thoracostomy tube placement ranges from 4% to 23%.27,28 Conservative management with continuous pleural suction leads to prolonged chest tube drainage, prolonged hospital length of stay (LOS), and increased hospital costs.28 VATS has been shown to be safe and effective in the treatment of persistent pneumothorax with decreased number of chest tube days, hospital LOS, and cost.9,28,29 In our trauma center, endo-GIA staplers are routinely employed to staple off the affected lung parenchyma. Recently the use of a topical synthetic nonreactive surgical sealant (Coseal by Baxter, Freemont, CA) for the creation of an elastic watertight seal has also been reported.17 Chemical pleurodesis or pleural scarification by electrocoagulation remains a viable option especially in recurrences post-VATS. Carillo et al.28 reported the successful use of VATS in 10 of 11 patients with persistent pneumothorax post-traumatic injuries. The 11th patient was successfully treated with chemical pleurodesis. The inflammatory reaction that occurs with chemical pleurodesis, however, is often associated with increased pleural edema, drainage, and postoperative pain.15 Prior to committing a patient to VATS, it is important to aggressively evaluate the cause of the air leak and rule out a malfunctioning or a malpositioned chest tube, the presence of a foreign body, or a deeply penetrating rib fragment. The patient should be evaluated thoroughly with chest CT and bronchoscopy to evaluate the tracheobronchial tree, the distal parenchyma, and the pleural cavity.
Other Indications/Application
Other indications for use of VATS in chest trauma include diagnosis of bronchopleural fistulas, removal of retained foreign bodies, ligation of injured thoracic duct, drainage of chylothorax, and assessment of cardiac and mediastinal structure.10,15 Although pericardioscopy for suspected penetrating cardiac injury has been reported as feasible and safe in the hemodynamically stable patient, it remains very controversial, with potential for iatrogenic life-threatening injuries.30 In a stable patient, the gold standard approach for suspected cardiac injury remains the use of echocardiography or subxyphoid pericardial window followed by immediate sternotomy or left thoracotomy for evacuation of hemopericardium and repair of cardiac injury.
Morbidity and Complication Management
The reported complication rates for thoracoscopy are less than 10% and the missed injury rates are less than 1%.24,31 The perioperative complications include intrathoracic bleed (parietal, intercostal, or parenchymal), recurrent pneumothorax, and hemothorax. Other complications include intercostal neuritis and iatrogenic lung laceration. Conversion to open thoracotomy is reported to be less than 8% and usually results from pleural adhesions or uncontrollable bleed.31 This underscores the importance of the timing of the procedures, within 5–7 days—early enough to avoid pleural adhesions and fibrosis, and late enough to ensure adequate hemostasis. Persistent air leak in the postoperative period is attributed to underlying lung pathology such as emphysema or apical bleb disease. Late complications are rare and include the development of pneumonia, pleural edema, and empyema.9,31 Airway complications from malpositioned dual-lumen endotracheal tubes or the development of tension pneumothorax during one lung ventilation have also been reported.32
DIAGNOSTIC AND THERAPEUTIC ROLE OF BRONCHOSCOPY
Attempts to directly visualize the interior of the airway date as far back as the time of Hippocrates. However, the first recorded bronchoscopy was performed by Gustav Killian of Frieburg, Germany in 1887. The only available instrument at that time was a rigid bronchoscope and the principal indications were therapeutic, the commonest being removal of inhaled foreign objects. The field was advanced by Chevalier Jackson, the father of American bronchoesophagology, who designed modern rigid bronchoscopes. In 1963, Shigeto Ikeda introduced the flexible fibroptic bronchoscope, primarily as a diagnostic instrument.33 Flexible bronchoscopes were much easier to use, and flexible bronchoscopy became a diagnostic tool with wide application. The only therapeutic indication that persisted was removal of foreign bodies from within the tracheobronchial tree. Recent technical advances in the instrument itself and in the availability of other therapeutic tools such as stents, electrocautery, lasers, and so on, are allowing bronchoscopy to regain a role in therapy and also broadening its well established diagnostic role. While in some very limited situations rigid bronchoscopy may offer some advantages, the ease of use and greater experience in the use of flexible bronchoscopes have made rigid bronchoscopy rare in trauma settings.
Basic Technique of Flexible Fibroptic Bronchoscopy
Preparation
The patient should be placed on 100% oxygen, and the ventilator rate set at 10–12 breaths per minute. Adequate sedation is essential to avoid inducing stress. This is especially important in head injured patients as an acute rise in intracranial pressure (ICP) is well documented during bronchoscopy.34 We use a benzodiazepine (Ativan or Versed 2–4 mg intravenously) and a narcotic analgesic (morphine 4–8 mg intravenously). While bronchoscopy can be performed without paralysis, we have found that, to be able to perform it comfortably, temporary paralysis using vecuronium (10 mg intravenously) is very helpful. Adequate time should be given to allow the patient to get preoxygenated and the medications to take effect before starting the procedure. Recently, bispectral EEG monitoring (BIS) is being used to determine optimal sedation. Extra medications should be available to be given whenever needed to avoid stress to the body.
Complications of Bronchoscopy
A large number of complications have been described after flexible fibroptic bronchoscopy (Table 2). The incidence of complications is higher the longer the procedure, the sicker the patient, and when the bronchoscopy is performed for therapeutic indications rather than diagnostic. However, in experienced hands and when the above precautions of technique and monitoring are observed, fibroptic flexible bronchoscopy is a fairly safe procedure.
| Hypoxemia | Hypercapnia |
| Barotrauma | Hypotension |
| Hypertension | Hemorrhage |
| Aspiration | Intracranial hypertension |
| Infection | Laryngospasm |
| Damage to scope | Cardiac arrhythmias |
Diagnostic Role of Flexible Fibroptic Bronchoscopy
Acute Trauma
Tracheobronchial injury
Tracheobronchial injury following trauma is very rare; however, the consequences of missed injuries can be significant. While some injuries can be managed nonoperatively and will heal, if an injury does require repair, delaying the repair significantly increases the chances of failure. Fibroptic bronchoscopy should be performed early in any patient who may potentially have a tracheobronchial injury.35 In some instances, if the patient is not acutely hypoxic and tracheobronchial injury is suspected, in addition to its diagnostic role, bronchoscopy can be an invaluable aid to intubation and correct tube placement. In such situations, bronchoscopy should be performed by an experienced bronchoscopist with the endotracheal tube prepositioned over the scope.36 There is a role for rigid bronchoscopy in patients with injury to the cervical trachea where the rigid scope can identify the distal ruptured end and align it with the proximal end allowing the patient to be intubated and the balloon of the tube passed beyond the site of injury. Once an injury has been diagnosed, the bronchoscopic findings are useful in planning appropriate therapy—nonoperative management, open surgery, or bronchoscopic placement of stent. In a report by Lin et al.,37 bronchoscopy was useful in management decisions in one-third of the instances.
Acute or late onset bleeding in the tracheobronchial tree
Bleeding within the tracheobronchial tree following trauma is usually caused by pulmonary contusion and rarely by injury to the tree. In later stages, hemoptysis may be caused by pulmonary embolism, infections, tracheobronchial erosions, or tracheoinnominate fistula. Fibroptic bronchoscopy is useful for diagnosing the cause of the bleeding and to localize the site. It may help temporarily control the hemorrhage, isolate the site to avoid flooding the nonbleeding areas of the lung, and, in selected cases, provide definitive control of the bleeding2 (see following section).
Inhalational injury
Fibroptic bronchoscopy plays an invaluable role in the diagnosis and management of patients suspected of inhalational injury. Any patient suspected of having suffered inhalational burns to the tracheobronchial tree should undergo early diagnostic evaluation. If signs of impeding loss of airway are present and the patient does not have an endotracheal tube, the bronchoscope may be used as a guide for safe intubation. The bronchoscopic signs of inhalational injury are observed within a few hours of the injury and may be classified as acute, subacute, and chronic.38 In the acute stage, the most prominent finding is airway edema with soot deposition within the mucosa. As the injury progresses to the subacute phase, necrosis of the lining mucosa, and hemorrhagic tracheobronchitis are prominent. The subacute phase may last from several hours to days, and in this stage the patient may demonstrate massive bronchorrhea. Repeated bronchoscopic toileting may be necessary to maintain airway patency. Finally, in the chronic phase formation of granulation tissue with stenosis, scarring, and obliterative bronchiolitis are observed. The initial bronchoscopic appearance is poorly correlated with the need and duration of mechanical ventilatory requirements and also the final outcome. Hence, repeated examinations may be required to accurately plan therapy.39
Ventilator-Associated Pneumonia
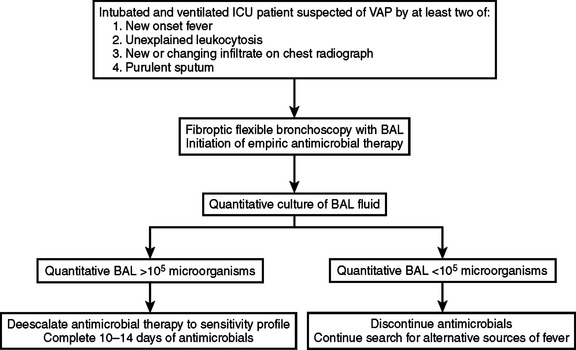
Ventilator-associated pneumonia (VAP) is one of the most common nosocomial infections in the modern intensive care unit (ICU). It is associated with high morbidity and mortality, and each incidence of VAP significantly increases the cost of care.40 Early appropriate antimicrobial therapy has been shown to improve outcomes.41 Despite its relative frequency, the diagnosis of VAP can be challenging, especially in the trauma patient. The reasons for this are primarily that the diagnostic criteria for pneumonia (fever with productive cough and leukocytosis, new or changing infiltrate on chest radiograph, and sputum culture demonstrating predominant growth of a single organism) are either nonspecific or falsely positive because of tracheobronchial colonization in the ICU. Quantitative examination of a lower respiratory specimen (lavage or brush) has been suggested as one method of accurately differentiating between nonpathogenic tracheobronchial colonization and VAP. Bronchoalveolar lavage (BAL) specimens from the lower respiratory tree can be easily obtained through the bronchoscope. This method has been proven to be safe and accurate in not only diagnosing VAP, but in also ruling it out so that patients are spared unnecessary antimicrobial therapy.42 A simple scheme practiced at the authors’ institution is outlined in Figure 2. Institution of this scheme, in which bronchoscopy plays a central role has significantly reduced the use of antimicrobials, and related to that, reduced microbial resistance within our ICU.
The technique of obtaining BAL specimen through a flexible bronchoscope is simple. The scope is passed into a tertiary level bronchiole and wedged in it. Suction is avoided during insertion to maintain sterility of the working channel of the scope. The site is selected by using one or more of the following criteria: (1) site of new infiltrate on chest radiograph, (2) site of maximum purulence as observed through the scope, and (3) most common site of VAP, the right lower lobe. Once the scope has been wedged in the selected bronchiole, five aliquots of 20-ml, sterile nonbacteriostatic saline are instilled through the working port and immediately aspirated. A good specimen is indicated by aspirating more than 50% of the instilled saline and observing floating froth (evidence of surfactant) in the aspirate. This specimen is then sent to the laboratory for quantitative culture and empiric antimicrobials are initiated based on the prevailing flora in the ICU. When the final quantitative culture results are available (usually in 48–72 hours), the antimicrobial therapy is tailored to the culture and sensitivity profile. In our ICU, a threshold of more than 105 colony-forming units per milliliter (CFU/ml) of bronchoalveolar lavage alteration (BAL) is used for the diagnosis of VAP. Other ICUs use a smaller number. An alternative to lavage is the protected specimen brush where a special brush-tipped catheter is passed through the scope and used to scrape the lining of the bronchiole. A diagnostic threshold of 103 CFU/ml is often adopted when the brush is used. Although nonbronchoscopic methods of obtaining the lower respiratory specimen are available, no comparative trials have been performed to compare the bronchoscopic and nonbronchoscopic methods.40
Stricture
Most causes of stricture within the tracheobronchial tree are related to neoplasia. In the trauma setting, strictures may be caused by prolonged intubation, scarring at the site of previous injury, or following inhalational injury. Bronchoscopy has both a diagnostic and therapeutic role in the management of strictures. Initially, bronchoscopy can confirm the presence of the stricture and localize its site. In addition, the bronchoscopic features in terms of site, length, and character of the tissue can help with the planning of appropriate treatment. Also bronchoscopy can help with management of the atelectasis of the pulmonary segments beyond the stricture and to treat the infections in these atelectatic segments.
Therapeutic Role of Flexible Fibroptic Bronchoscopy
Control of Acute or Late Onset Hemoptysis
Massive hemoptysis is defined as when the volume of blood in the tracheobronchial tree leads to a life-threatening situation by causing airway obstruction. Bronchoscopy has been used to not only diagnose the source of hemorrhage, but also to remove the blood, thereby overcoming the airway obstruction. In addition, in some selected cases, bronchoscopic techniques can provide either temporary control of hemorrhage until preparations for definitive control, by surgery or bronchial artery embolization, are made. In selected patients, bronchoscopic techniques can even provide definitive therapy. The therapeutic tools to control hemorrhage include ice-cold saline lavage, injection of 1:20,000 epinephrine, instillation of fibrin glue or other topical hemostatics, balloon tamponade, electrocautery, and laser coagulation. While most reports about the use of such techniques are from the medical literature, the same techniques are being used for acute hemorrhage following trauma or late onset hemoptysis in the surgical ICU patient.4
Stent Repair of Acute Airway Trauma
Major disruptions of tracheobronchial tree are rare, but life threatening. In the past the only available treatment was major surgery. Recently, covered expandable metallic stents have been developed that can be deployed under bronchoscopic control. This offers a low risk alternative of repairing these potentially devastating injuries. When injury to the tracheobronchial tree is suspected early evaluation by bronchoscopy is very helpful in diagnosing or ruling out the injury and then planning therapy. Bronchoscopy can define the site of injury, and help with safe placement of the endotracheal tube. Once the patient’s airway is secured, a careful assessment should be made of the characteristics of the injury especially the site in relation to branches, and the size of the injury. While open repair has been the traditional method of therapy, consideration should be given for stent repair if the injury is so amenable and especially if the patient’s other injuries make him/her a poor surgical candidate. If none of the shelf stent is available, customized stents can be ordered to suit the anatomy of the specific injury. A recent case at the authors’ institution exemplified this point. An 18-year-old man presented after a high speed motor vehicle collision with the chest CT demonstrating aortic transection. In addition, because of his massive pneumothorax and persistent high volume air leak through the chest tube, airway injury was suspected and confirmed at the distal trachea by bronchoscopy. A custom-made stent was placed via the bronchoscope, and the air leak stopped. A few days later the patient underwent successful repair of his aortic injury via a thoracotomy. Subsequent bronchoscopies revealed that the tracheal injury had healed with formation of granulation tissue around the stent. The stent was removed intact after 8 weeks, and 2 weeks following that mucosa was found to be covering the injury site.43
Removal of Foreign Body
Before the availability of bronchoscopy, foreign body removal from the tracheobronchial tree carried high morbidity and mortality. Availability of bronchoscopy revolutionized the care of such patients as it offered a very low-risk alternative to major surgery. Although the first removals were performed using a rigid scope, currently the large majority of such cases are performed with flexible scopes that can be inserted into more distal airways and that have a working channel through which instruments can be passed.44,45 When inhalation of a foreign body is suspected and the patient has survived the acute obstruction, careful planning should go into any further intervention as poor planning can lead to airway obstruction and death. The procedure should be performed by an experienced endoscopist with the availability and facility with both rigid and flexible scopes.44 While the procedure can be performed with the patient awake, often general anesthesia is required. Careful consultation between the endoscopist and the anesthesiologist as to how the airway shall be safely managed is essential. Once the airway plan is safely in place, endoscopy is carried out. Besides the availability of the two types of scopes, accessory instruments are very helpful in safely removing various bodies that may have become embedded into the mucosa.45 These instruments include balloon catheters, special grasping forceps, and wire baskets. In addition, other adjunctive techniques have been developed to safely remove the foreign body. These include the cryoprobe that can cause the body to adhere to the end of the instrument or neodymium-ytterium-aluminum-garnet (Nd:YAG) laser to break up the foreign body, vaporize the surrounding granulation tissue to dislodge it, and blunting the sharp edges for safe removal.44,45
Toilet for Pulmonary Collapse, Massive Secretions, and Aspiration
Flexible bronchoscopy has become an invaluable tool for managing atelectasis and complete or partial pulmonary collapse resulting from tenacious mucoid secretions. There is an immediate benefit observed in patients with tenacious mucoid secretions obstructing central airways. In such patients, bronchoscopic suctioning for whole lung collapse or lobar atelectasis has been shown to improve oxygenation.46 In other patients, however, the benefits of bronchoscopic clearing of airways over traditional chest percussion therapy are less clear.47 In trauma patients, though, it may not be possible to provide good percussion therapy at times because of other injuries, and bronchoscopy may be the only effective method of clearing secretions. For patients who have suffered large-volume aspiration of gastric contents, early bronchoscopy and lavage of the airways can help clear the airways and possibly limit chemical damage. Although bronchoscopy in such settings is often used for this aim, no studies have conclusively shown its benefits. Based on anecdotal evidence, a reasonable approach may be to perform bronchoscopy and lavage on patients suspected of large volume aspiration if they are already intubated or require intubation immediately after the episode. If the patient does not require intubation, he or she should be carefully monitored and managed with aggressive percussion therapy and other measures to encourage pulmonary toilet. As with all procedures it is necessary to balance potential harm with benefit. Extensive suctioning within the airways during bronchoscopy leads to derecruitment of alveoli that can lead to problems with oxygenation and also extensive washing within the airways can cause diffusion problems further worsening oxygenation (see previous discussion of complications).
Percutaneous Dilatational Tracheostomy
Percutaneous dilatational tracheostomy has gained in popularity as an alternative to open tracheostomy that can be performed in the ICU at lower cost. While it is possible to perform the procedure without bronchoscopy, many believe that the addition of bronchoscopic control adds to the safety of the procedure.48 The procedure consists of placing a needle within the trachea, and passing a guide wire through the needle. Once a guide wire has been placed the tract from the skin to the trachea is sequentially dilated and finally a tracheostomy tube, preloaded over the final dilator, is passed into the trachea via the established tract. When the procedure is performed under bronchoscopic control, it is possible to ensure that the needle, guide wire, and dilators are indeed passing into the trachea, as they are supposed to, and not into a false passage within the soft tissues of the neck.
Indications of percutaneous tracheostomy are the same as for open tracheostomy. Skin infection, unstable cervical spine and elevated ICP are absolute contraindications to the procedure while obesity, high ventilatory requirements, coagulopathy, and any anatomical abnormality in the area are relative contraindications.49 Reported complications include mucosal tears, submucosal placement of tube, perforation of the posterior tracheal wall with formation of a tracheoesophageal fistula, paratracheal placement, barotrauma, and damage to the endotracheal tube and bronchoscope. Like the open procedure there is an incidence of late tracheoinnominate fistula and subglottic stenosis.49 There have been retrospective meta-analyses and prospective studies to compare open and percutaneous tracheostomy. The results suggest that percutaneous tracheostomy has a steep learning curve; however, in experienced hands the percutaneous technique has equivalent perioperative results and possibly improved long-term results as compared with open tracheostomy.50
Management of Bronchopleural Fistula
Bronchopleural fistulae present as a persistent air leak from a thoracostomy tube. When conservative measures, including maintaining continuous negative pleural pressure and chemical pleurodesis, fail to close the fistula by 1–3 weeks, surgical correction may be necessary. Bronchoscopy can be useful in identifying the offending segment from which the fistula emanates. Passing the scope into different bronchopleural segments of the lung with the suspected fistula and observing for telltale granulation tissue through which bubbles are emanating is fairly accurate in diagnosing the site of the fistula. After identification a balloon-tipped catheter can be passed with the help of the bronchoscope and the balloon inflated to occlude the site. If this results in cessation of the air leak, the diagnosis and site are confirmed.51 Once the diagnosis is confirmed and site identified, a number of substances including fibrin glue, gel foam, and lead-shot plugs have been used to temporarily seal the site prior to surgery, and, in selected cases, even offer permanent control obviating the need of major surgery. Fresh autologous blood clot delivered to the site by pasting it onto the balloon of a balloon tipped catheter has also been used. When blood clot is used, an antifibrolytic agent (e.g., epsilon aminocaproic acid), tetracycline, or doxycycline instillation can enhance the success rate.51
Dilatation/Laser Therapy of Tracheobronchial Strictures
Tracheobronchial strictures in trauma patients are usually related to prolonged intubation. However, they can also be caused by granulation tissue resulting from infections and occur after surgical or stent repairs of airway injuries. While surgical excision and repair of the strictured area has a high success rate,52 bronchoscopic dilatation, with or without laser vaporization, of the stricture is a lower risk alternative that can offer temporary relief till surgical repair can be carried out or in some cases lead to permanent cure.53 The technical details of the procedure are beyond the scope of this text, but careful consideration has to be given to plan optimal therapy in each individual situation. The important considerations include the anatomy of the stricture—site, length, and type of tissue—and, if laser therapy is opted for, the type of laser to be used—Nd:YAG, CO2, or potassium-titanil-phosphate (KTP). In addition, local expertise and experience are important considerations. Adjunctive techniques that can be used along with laser vaporization include balloon dilatation, placement of a stent, and infusion of mitomycin C to reduce the recurrence rate following laser therapy. No comparative trials have been performed comparing surgical resection with laser therapy; a multidisciplinary approach to the management of each individual patient is the best way to obtain optimal results.53
Drainage of Lung Abscess
Lung abscess is a serious complication following chest trauma or pneumonia following any trauma, with high morbidity and mortality. Traditional methods of treatment include adequate early antibiotic therapy and postural drainage. Failing this, surgical drainage was the only available option, but it carried high morbidity and mortality. Interventional radiological techniques have allowed drainage of the abscess without high-risk surgery. However, radiologically placing drainage catheters deep within the lung in proximity of major airways runs the risk of developing persistent bronchopleural fistulas. Transbronchial approach via the bronchoscope is an additional technique that can be used for aspirating such abscesses, and by leaving a drainage catheter the technique allows for irrigation of the cavity and continuous drainage. Good results have been reported but care should be exercised to minimize spillage of pus into the airways.54
1 LoCicero JIII, Mattox KL. Epidemiology of chest trauma. Surg Clin North Am. 1989;69:15-19.
2 Shapiro MB, Anderson HLIII, Bartlett RH. Surg Clin North Am. 2000;80:871-883.
3 Karmy-Jones R, Jurkovich GJ, Shatz DV, et al. Management of traumatic lung injury: a Western Trauma Association multicenter review. J Trauma. 2001;51:1049-1053.
4 Karmy-Jones R, Cuschieri J, Vallieres E. Role of bronchoscopy in massive hemoptysis. Chest Surg Clin North Am. 2001;11:873-906.
5 Allen JN. Self-expanding metallic stents in interventional pulmonary medicine. Lung Biol Health Dis. 2004;189:239-257.
6 Karmy-Jones R, Jurkovich GJ. Blunt chest trauma. Curr Prob Surg. 2004;41:1223-1380.
7 Jacobaues HC. The practical importance of thoracoscopy in surgery of the chest. Surg Gynecol Obstet. 1922;34:289-293.
8 Branco JM C. Thoracoscopy as a method of exploration in penetrating injuries of the chest. Dis Chest. 1946;12:330-335.
9 Meyer DM, Jessen ME, Wait MA, Estrera AS. Early evacuation of traumatic retained hemothoraces using thoracoscopy: a prospective, randomized trial. Ann Thorac Surg. 1997;64:1396-1401.
10 Manlulu AV, Lee TW, Thung KH, Wong R, Yim AP C. Current indications and results of VATS in the evaluation and management of hemodynamically stable thoracic injuries. Eur J Cardiothorac Surg. 2004;25:1048-1053.
11 Rodriguez-Morales G, Rodriguez A, Shatney CH. Acute rupture of the diaphragm in blunt trauma: analysis of 60 patients. J Trauma. 1986;26:438-444.
12 Rosati C. Acute traumatic injury of the diaphragm. Chest Surg Clin North Am. 1998;8:371-379.
13 Johnson CD. Blunt injuries of the diaphragm. Br J Surg. 1988;75:226-230.
14 Martinez M, Britz JE, Carrillo EH. Delayed thoracoscopy facilitates the diagnosis and treatment of diaphragmatic injuries safely and expeditiously. Surg Endosc. 2001;15:28-32.
15 Carrillo EH, Richardson JD. Thoracoscopy for the acutely injured patient. Am J Surg. 2005;190:234-238.
16 Fabian T, Croce M, Stewart B, et al. A prospective analysis of diagnostic laparoscopy in trauma. Ann Surg. 1993;217:557-565.
17 Livingston DH, Tortella B, Blackwood J, et al. The role of laparoscopy in abdominal trauma. J Trauma. 1992;33:471-475.
18 Ivatury RR, Oschner MG, Simon R, et al. Cavitary endoscopy. In: Ivatury RR, Gayten CG, editors. The Textbook of Penetrating Trauma. Media, PA: Williams & Wilkins; 1996:416-428.
19 Eddy AC, Luna GK, Copass M: Empyema thoracis in patients undergoing emergent closed tube thoracostomy for thoracic trauma. Am J Surg 157:494–497.
20 Heniford BT, Carrillo EH, Spain DA, et al. The role of thoracoscopy in the management of retained thoracic collections after trauma. Ann Thorac Surg. 1997;63:940-943.
21 Coselli JS, Mattox KL, Beal AC. Reevaluation of early evacuation of clotted hemothorax. Am J Surg. 1984;148:786-790.
22 Graham JM, Mattox KL, Beall ACJr. Penetrating trauma of the lung. J Trauma. 1979;19:665-669.
23 Helling TS, Gyles NRIII, Eisenstein CL, et al. Complications following blunt and penetrating injuries in 216 victims of chest trauma requiring tube thoracostomy. J Trauma. 1989;29:1367-1370.
24 Villavicencio RT, Aucar JA, Wall MJ. Analysis of thoracoscopy in trauma. SurgEndosc. 1999;13:3-9.
25 Landreneau RJ, Kennan RJ, Hazelrigg SR, et al. Thoracoscopy for empyema and hemothorax. Chest. 1995;109:18-24.
26 Smith RS, Fry WR, Tsoi EK M, et al. Preliminary report on videothoracoscopy in the evaluation and treatment of thoracic injury. Am J Surg. 1993;166:690-695.
27 Kern JA, Tribble CG, Spotnitz WD, et al. Thoracoscopy in the subacute management of patient with thoracoabdominal trauma. Chest. 1993;104:942-945.
28 Carillo EH, Schmacht DC, Gable DR, et al. Thoracoscopy in the management of posttraumatic persistent pneumothorax. J Am Coll Surg. 1998;186:636-640.
29 Shremer CR, Matterson BD, Demarest GBIII, et al. A prospective evaluation of video-assisted thoracic surgery for persistent air leak due to trauma. Am J Surg. 1999;177:480-484.
30 Pons F, Lang-Lazdunski L, Kerangal X, et al. The role of videothoracos-copy in the management of precordial thoracic penetrating injuries. Eur J Cardiothorac Surg. 2002;2:7-12.
31 Krasna MJ, Deshmukh S, Mclaughlin JS. Complications of thoracoscopy. Ann Thorac Surg. 1996;61:1066-1069.
32 Weng W, DeCrosta DJ, Zhang H. Tension pneumothorax during one-lung ventilation: a case report. J Clin Anesth. 2002;14:529-531.
33 Herth FJ F, Ernst A, Beamis JFJr. History of rigid bronchoscopy in interventional pulmonary medicine. Lung Biol Health Dis. 2004;189:1-12.
34 Kerwin AJ, Croce MA, Timmons SD, et al. Effects of fiberoptic bronchoscopy on intracranial pressure in patients with brain injury: a prospective clinical study. J Trauma. 2000;48:876-882.
35 Barmada H, Gibbons JR. Tracheobronchial injury in blunt and penetrating chest trauma. Chest. 1994;106:74-78.
36 Baumgartner F, Sheppard B, De Virgilio C, et al. Tracheal and main bronchial disruptions after blunt chest trauma: presentation and management. Ann Thorac Surg. 1990;50:569-574.
37 Lin MC, Lin HC, Lan RS, et al. Emergent flexible bronchoscopy for the evaluation of acute chest trauma. J Bronchol. 1995;1:188-193.
38 Prakash UB S. Chemical warfare and bronchoscopy. Chest. 1486;100:1991.
39 LIunt JL, Agee RN, Pruitt BAJr. Fibroptic bronchoscopy in acute inhalation injury. J Trauma. 1975;15:641-649.
40 American Thoracic Society, Infectious Diseases Society of America. guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
41 Kollef MH, Sherman G, Ward S, et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital acquired mortality among critically ill patients. Chest. 1999;115:462-474.
42 Croce MA, Fabian TC, Schurr MJ, et al. Using bronchoalveolar lavage to distinguish nosocomial pneumonia from systemic inflammatory response syndrome: a prospective analysis. J Trauma. 1995;39:1134-1140.
43 Chambers AS, Shepherd RW, Moses L, et al. Novel tracheal injury management with aortic transaction. J Bronchol. 2006;13:32-34.
44 Rafanan AL, Mehta AC. Adult airway foreign body removal. Clin Chest Med. 2001;22:319-330.
45 Kelly SM, Marsh BR. Airways foreign bodies. Chest Surg Clin North Am. 1996;6:253-276.
46 Stevens RP, Lillington GA, Parsons GH. Fibroptic bronchoscopy in the intensive care unit. Heart Lung. 1981;10:1037-1045.
47 Marini J, Pierson D, Hudson L. Acute lobar atelectasis: a prospective comparison of bronchoscopy and respiratory therapy. Am Rev Respir Dis. 1979;119:971-978.
48 Lee P, Mehta AC. Therapeutic flexible bronchoscopy: overview in interventional pulmonary medicine. Lung Biol Health Dis. 2004;189:49-87.
49 Noppen N. Percutaneous dilatational tracheostomy. In: Bollinger CT, Mathur PN, editors. Interventional Bronchoscopy. Basel: Karger; 2000:215-225.
50 deBoisblanc BP. Percutaneous dilatational tracheostomy in interventional pulmonary medicine. Lung Biol Health Dis. 2004;189:567-583.
51 McManigle JR E, Fletcher GL, Tenholder ME. Bronchoscopy in the management of bronchopleural fistula. Chest. 1990;97:1235-1238.
52 Rea F, Callegaro D, Toy M, et al. Benign tracheal and laryngotracheal stenosis: surgical treatment and results. J Cardiovasc Surg Eur J Cardiovasc Surg. 2002;22:352-356.
53 Shapshay SM, Valdez TA. Bronchoscopic management of benign stenosis. Chest Surg Clin North Am. 2001;11:749-768.
54 Schmitt GS, Ohar JM, Kanter KR, et al. Indwelling transbronchial catheter drainage of pulmonary abscess. Ann Thorac Surg. 1988;45:43-47.