PREVENTION AND ERADICATION OF POLIOVIRUS
![]() (See also Chap. 148) After a peak of 57,879 cases of poliomyelitis in the United States in 1952, the introduction of IPV in 1955 and of OPV in 1961 ultimately eradicated disease due to wild-type poliovirus in the Western Hemisphere. Such disease has not been documented in the United States since 1979, when cases occurred among religious groups who had declined immunization. In the Western Hemisphere, paralysis due to wild-type poliovirus was last documented in 1991.
(See also Chap. 148) After a peak of 57,879 cases of poliomyelitis in the United States in 1952, the introduction of IPV in 1955 and of OPV in 1961 ultimately eradicated disease due to wild-type poliovirus in the Western Hemisphere. Such disease has not been documented in the United States since 1979, when cases occurred among religious groups who had declined immunization. In the Western Hemisphere, paralysis due to wild-type poliovirus was last documented in 1991.
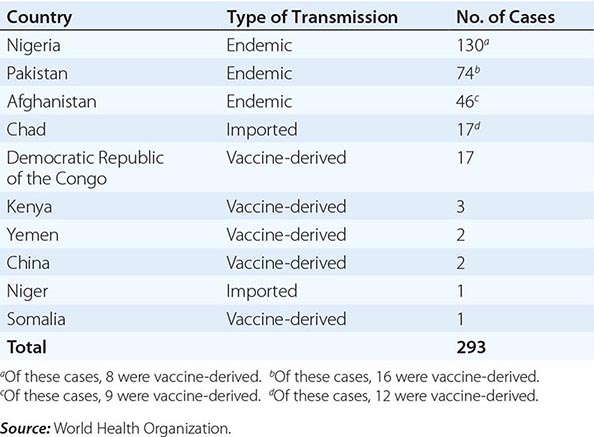
In 1988, the World Health Organization adopted a resolution to eradicate poliomyelitis by the year 2000. From 1988 to 2001, the number of cases worldwide decreased by >99%, with only 496 confirmed cases reported in 2001. Wild-type poliovirus type 2 has not been detected in the world since 1999. The Americas were certified free of indigenous wild-type poliovirus transmission in 1994, the Western Pacific Region in 2000, and the European Region in 2002. However, in 2002, there were 1922 cases of polio, with 1600 cases reported in India. In fact, after the nadir of 496 cases in 2001, 21 countries that had previously been free of polio reported cases imported from 6 polio-endemic countries in 2002–2005. By 2006, polio transmission had been reduced in most of these 21 countries. In 2012, 293 cases of polio were reported (the lowest number ever in a 1-year period); 85% were from Nigeria, Pakistan, and Afghanistan, the only countries where polio remains endemic (Table 228-2). As of November 2013, there had been 390 cases of polio in 2013 compared with 293 cases in 2012. The increase was associated with a marked rise in imported cases, including more than 180 cases in Somalia, more than 10 cases each in Kenya and Syria, and cases in Cameroon and Ethiopia. Also in 2013, wild-type poliovirus was detected in sewage in Israel, prompting a massive vaccination campaign with OPV. As of November 2013, India had not reported a case of polio since January 2011. Polio is a source of concern for unimmunized or partially immunized travelers. Importation of poliovirus accounted for ~50% of cases in 2013. Clearly, global eradication of polio is necessary to eliminate the risk of importation of wild-type virus. Outbreaks are thought to have been facilitated by suboptimal rates of vaccination, isolated pockets of unvaccinated children, poor sanitation and crowding, improper vaccine-storage conditions, and a reduced level of response to one of the serotypes in the vaccine. While the global eradication campaign has markedly reduced the number of cases of endemic polio, doubts have been raised as to whether eradication is a realistic goal, given the large number of asymptomatic infections and the political instability in developing countries.
|
LABORATORY-CONFIRMED CASES OF POLIOMYELITIS IN 2012 |
The occurrence of outbreaks of poliomyelitis due to circulating vaccine-derived poliovirus of all three types has been increasing, especially in areas with low vaccination rates. In Egypt, 32 cases of vaccine-derived polio occurred in 1983–1993; in the Dominican Republic and Haiti, 21 cases occurred in 2000–2001; in Indonesia, 46 cases were reported in 2005; in Nigeria, 385 cases occurred in 2005–2012; in the Democratic Republic of the Congo, 64 cases were reported in 2008–2012; in Pakistan, 16 cases occurred in 2012, and at least 30 cases occurred in 2013. These OPV-derived viruses reverted to a more neurovirulent phenotype after undetected circulation (probably for >2 years). The epidemic in Hispaniola was rapidly terminated after intensive vaccination with OPV. In 2005, a case of vaccine-derived polio occurred in an unvaccinated U.S. woman returning from a visit to Central and South America. In the same year, an unvaccinated immunocompromised infant in Minnesota was found to be shedding vaccine-derived poliovirus; further investigation identified 4 of 22 infants in the same community who were shedding the virus. All 5 infants were asymptomatic. These outbreaks emphasize the need for maintaining high levels of vaccine coverage and continued surveillance for circulating virus.
IPV is used in most industrialized countries and OPV in most developing countries, including those in which polio still is or recently was endemic. While IM injections of other vaccines (live or attenuated) can be given concurrently with OPV, unnecessary IM injections should be avoided during the first month after OPV vaccination because they increase the risk of vaccine-associated paralysis. Since 1988, an enhanced-potency inactivated poliovirus vaccine has been available in the United States.
After several doses of OPV alone, the seropositivity rate for individual poliovirus serotypes may still be suboptimal for children in developing countries; one or more supplemental doses of IPV can increase the rate of seropositivity for these serotypes. Against a given serotype, monovalent OPV containing only that serotype is more immunogenic than trivalent vaccine because of a lack of interference from other serotypes. With eradication of wild-type poliovirus type 2, bivalent OPV (types 1 and 3), which was shown to be superior to trivalent OPV, has been the vaccine of choice to eliminate polio and has markedly reduced rates of polio in Nigeria. As the frequency of wild-type polio declines and reports of polio associated with circulating vaccine-derived viruses increase, the World Health Organization is investigating whether IPV can be produced from OPV strains that require less biocontainment, ultimately replacing OPV.
OPV and IPV induce antibodies that persist for at least 5 years. Both vaccines induce IgG and IgA antibodies. Compared with recipients of IPV, recipients of OPV shed less virus and less frequently develop reinfection with wild-type virus after exposure to poliovirus. Although IPV is safe and efficacious, OPV offers the advantages of ease of administration, lower cost, and induction of intestinal immunity resulting in a reduction in the risk of community transmission of wild-type virus. Because of progress toward global eradication of polio and the continued occurrence of cases of vaccine-associated polio, an all-IPV regimen was recommended in 2000 for childhood poliovirus vaccination in the United States, with vaccine administration at 2, 4, and 6–18 months and 4–6 years of age. The risk of vaccine-associated polio should be discussed before OPV is administered. Recommendations for vaccination of adults are listed in Table 228-3.
|
RECOMMENDATIONS FOR POLIOVIRUS VACCINATION OF ADULTS |
Abbreviation: IPV, inactivated poliovirus vaccine.
Source: Modified from Pickering LK, ed. Red Book 2012: Committee on Infectious Diseases, 29th ed.
There are concerns about discontinuing vaccination in the event that endemic spread of poliovirus is eliminated. Among the reasons for these concerns are that poliovirus is shed from some immunocompromised persons for >10 years, that vaccine-derived poliovirus can circulate and cause disease, and that wild-type poliovirus is present in research laboratories.
PARECHOVIRUSES
Human parechoviruses (HPeVs), like enteroviruses, are members of the family Picornaviridae. The 16 serotypes of HPeV commonly cause infections in early childhood. HPeV-1 infections occur throughout the year, while other parechovirus infections occur more commonly in summer and fall. Infections with HPeVs present similarly to those due to enteroviruses and may cause generalized disease of the newborn, aseptic meningitis, encephalitis, transient paralysis, exanthems, respiratory tract disease, and gastroenteritis. While HPeV-1 is the most common serotype and generally causes mild disease, deaths of infants in the United States have been associated with HPeV-1, HPeV-3, and HPeV-6. HPeVs can be isolated from the same sites as enteroviruses, including the nasopharynx, stool, and respiratory tract secretions. PCR using pan-enterovirus primers does not detect HPeVs, and while PCR assays are performed by the CDC and research laboratories, many commercial laboratories do not perform the test.
REOVIRUSES
Reoviruses are double-stranded RNA viruses encompassing three serotypes. Serologic studies indicate that most humans are infected with reoviruses during childhood. Most infections either are asymptomatic or cause mild upper respiratory tract symptoms. Reovirus is considered a rare cause of mild gastroenteritis or meningitis in infants and children. Speculation regarding an association of reovirus type 3 with idiopathic neonatal hepatitis and extrahepatic biliary atresia is based on an elevated prevalence of antibody to reovirus in some affected patients and the detection of viral RNA by PCR in hepatobiliary tissues in some studies. New orthoreoviruses have been associated with human disease—e.g., Melaka and Kampar viruses with fever and acute respiratory disease in Malaysia, and Nelson Bay virus with acute respiratory disease in a traveler from Bali.
229 |
Measles (Rubeola) |
DEFINITION
Measles is a highly contagious viral disease that is characterized by a prodromal illness of fever, cough, coryza, and conjunctivitis followed by the appearance of a generalized maculopapular rash. Before the widespread use of measles vaccines, it was estimated that measles caused between 5 million and 8 million deaths worldwide each year.
GLOBAL CONSIDERATIONS
![]() Remarkable progress has been made in reducing global measles incidence and mortality rates through measles vaccination. In the Americas, intensive vaccination and surveillance efforts—based in part on the successful Pan American Health Organization strategy of periodic nationwide measles vaccination campaigns (supplementary immunization activities, or SIAs)—and high levels of routine measles vaccine coverage interrupted endemic transmission of measles virus. In the United States, high-level coverage with two doses of measles vaccine eliminated endemic measles virus transmission in 2000. More recently, progress has been made in reducing measles incidence and mortality rates in sub-Saharan Africa and Asia as a consequence of increasing routine measles vaccine coverage and provision of a second dose of measles vaccine through mass measles vaccination campaigns and childhood immunization programs.
Remarkable progress has been made in reducing global measles incidence and mortality rates through measles vaccination. In the Americas, intensive vaccination and surveillance efforts—based in part on the successful Pan American Health Organization strategy of periodic nationwide measles vaccination campaigns (supplementary immunization activities, or SIAs)—and high levels of routine measles vaccine coverage interrupted endemic transmission of measles virus. In the United States, high-level coverage with two doses of measles vaccine eliminated endemic measles virus transmission in 2000. More recently, progress has been made in reducing measles incidence and mortality rates in sub-Saharan Africa and Asia as a consequence of increasing routine measles vaccine coverage and provision of a second dose of measles vaccine through mass measles vaccination campaigns and childhood immunization programs.
In 2003, the World Health Assembly endorsed a resolution urging member countries to reduce the number of deaths attributed to measles by 50% (compared with 1999 estimates) by the end of 2005. This target was met. Global measles mortality rates were further reduced in 2008; during that year, there were an estimated 164,000 deaths due to measles (uncertainty bounds: 115,000 and 222,000 deaths). These achievements attest to the enormous public-health significance of measles vaccination. However, recent large outbreaks of measles in Europe and Africa illustrate the challenges faced in sustaining measles control: in these outbreaks, measles was imported into countries that had eliminated indigenous transmission of measles virus.
The Measles and Rubella Initiative, a partnership led by the American Red Cross, the United Nations Foundation, UNICEF, the U.S. Centers for Disease Control and Prevention (CDC), and the World Health Organization (WHO), is playing an important role in reducing global measles incidence and mortality rates. Since its inception in 2001, the Initiative has provided governments and communities in more than 80 countries with technical and financial support for routine immunization activities, mass vaccination campaigns, and disease surveillance systems. Through its 2012–2020 Global Measles and Rubella Strategic Plan, the Initiative aims to reduce measles deaths by 95% (compared with year 2000 estimates) by 2015 and to eliminate measles from at least five of the six WHO regions by 2020. As regional goals for measles elimination are set, global measles eradication is likely to become a public health goal in the near future.
ETIOLOGY
Measles virus is a spherical, nonsegmented, single-stranded, negative-sense RNA virus and a member of the Morbillivirus genus in the family Paramyxoviridae. Measles was originally a zoonotic infection, arising from animal-to-human transmission of an ancestral morbillivirus ~10,000 years ago, when human populations had attained sufficient size to sustain virus transmission. Although RNA viruses typically have high mutation rates, measles virus is considered to be an antigenically monotypic virus; i.e., the surface proteins responsible for inducing protective immunity have retained their antigenic structure across time and distance. The public health significance of this stability is that measles vaccines developed decades ago from a single strain of measles virus remain protective worldwide. Measles virus is killed by ultraviolet light and heat, and attenuated measles vaccine viruses retain these characteristics, necessitating a cold chain for vaccine transport and storage.
EPIDEMIOLOGY
Measles virus is one of the most highly contagious directly transmitted pathogens. Outbreaks can occur in populations in which <10% of persons are susceptible. Chains of transmission are common among household contacts, school-age children, and health care workers. There are no latent or persistent measles virus infections that result in prolonged contagiousness, nor are there animal reservoirs for the virus. Thus, measles virus can be maintained in human populations only by an unbroken chain of acute infections, which requires a continuous supply of susceptible individuals. Newborns become susceptible to measles virus infection when passively acquired maternal antibody is lost; when not vaccinated, these infants account for the bulk of new susceptible individuals.
![]() Endemic measles has a typical temporal pattern characterized by yearly seasonal epidemics superimposed on longer epidemic cycles of 2–5 years or more. In temperate climates, annual measles outbreaks typically occur in the late winter and early spring. These annual outbreaks are probably attributable to social networks facilitating transmission (e.g., congregation of children at school) and environmental factors favoring the viability and transmission of measles virus. Measles cases continue to occur during interepidemic periods in large populations, but at low incidence. The longer epidemic cycles occurring every several years result from the accumulation of susceptible persons over successive birth cohorts and the subsequent decline in the number of susceptibles following an outbreak.
Endemic measles has a typical temporal pattern characterized by yearly seasonal epidemics superimposed on longer epidemic cycles of 2–5 years or more. In temperate climates, annual measles outbreaks typically occur in the late winter and early spring. These annual outbreaks are probably attributable to social networks facilitating transmission (e.g., congregation of children at school) and environmental factors favoring the viability and transmission of measles virus. Measles cases continue to occur during interepidemic periods in large populations, but at low incidence. The longer epidemic cycles occurring every several years result from the accumulation of susceptible persons over successive birth cohorts and the subsequent decline in the number of susceptibles following an outbreak.
Secondary attack rates among susceptible household and institutional contacts generally exceed 90%. The average age at which measles occurs depends on rates of contact with infected persons, protective maternal antibody decline, and vaccine coverage. In densely populated urban settings with low-level vaccination coverage, measles is a disease of infants and young children. The cumulative distribution can reach 50% by 1 year of age, with a significant proportion of children acquiring measles before 9 months—the age of routine vaccination in many countries, in line with the schedule recommended by the WHO’s Expanded Programme on Immunization. As measles vaccine coverage increases or population density decreases, the age distribution shifts toward older children. In such situations, measles cases predominate in school-age children. Infants and young children, although susceptible if not protected by vaccination, are not exposed to measles virus at a rate sufficient to cause a large disease burden in this age group. As vaccination coverage increases further, the age distribution of cases may be shifted into adolescence and adulthood; this distribution is seen in measles outbreaks in the United States and necessitates targeted measles vaccination programs for these older age groups.
Persons with measles are infectious for several days before and after the onset of rash, when levels of measles virus in blood and body fluids are highest and when cough, coryza, and sneezing, which facilitate virus spread, are most severe. The contagiousness of measles before the onset of recognizable disease hinders the effectiveness of quarantine measures. Viral shedding by children with impaired cell-mediated immunity can be prolonged.
Medical settings are well-recognized sites of measles virus transmission. Children may present to health care facilities during the prodrome, when the diagnosis is not obvious although the child is infectious and is likely to infect susceptible contacts. Health care workers can acquire measles from infected children and transmit measles virus to others. Nosocomial transmission can be reduced by maintenance of a high index of clinical suspicion, use of appropriate isolation precautions when measles is suspected, administration of measles vaccine to susceptible children and health care workers, and documentation of health care workers’ immunity to measles (i.e., proof of receipt of two doses of measles vaccine or detection of antibodies to measles virus).
As efforts at measles control are increasingly successful, public perceptions of the risk of measles as a disease diminish and are replaced by concerns about possible adverse events associated with measles vaccine. As a consequence, numerous measles outbreaks have occurred because of opposition to vaccination on religious or philosophical grounds or unfounded fears of serious adverse events (see “Active Immunization,” below).
PATHOGENESIS
Measles virus is transmitted primarily by respiratory droplets over short distances and, less commonly, by small-particle aerosols that remain suspended in the air for long periods. Airborne transmission appears to be important in certain settings, including schools, physicians’ offices, hospitals, and enclosed public places. The virus can be transmitted by direct contact with infected secretions but does not survive for long on fomites.
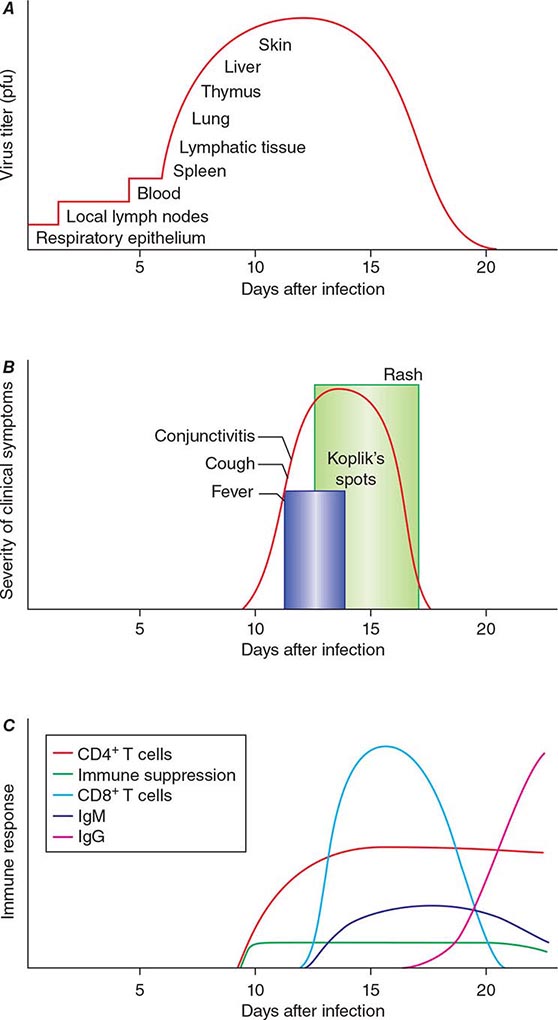
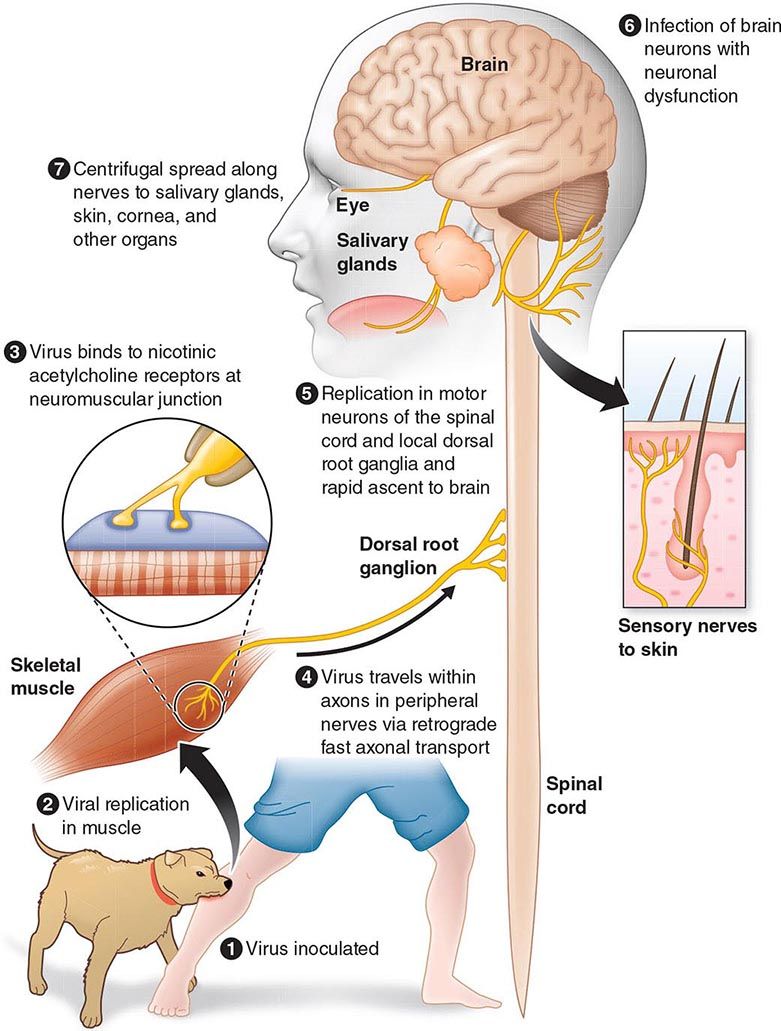
The incubation period for measles is ~10 days to fever onset and 14 days to rash onset. This period may be shorter in infants and longer (up to 3 weeks) in adults. Infection is initiated when measles virus is deposited on epithelial cells in the respiratory tract, oropharynx, or conjunctivae (Fig. 229-1A). During the first 2–4 days after infection, measles virus proliferates locally in the respiratory mucosa and spreads to draining lymph nodes. Virus then enters the bloodstream in infected leukocytes (primarily monocytes), producing the primary viremia that disseminates infection throughout the reticuloendothelial system. Further replication results in secondary viremia that begins 5–7 days after infection and disseminates measles virus throughout the body. Replication of measles virus in these target organs, together with the host’s immune response, is responsible for the signs and symptoms of measles that occur 8–12 days after infection and mark the end of the incubation period (Fig. 229-1B).
FIGURE 229-1 Measles virus infection: pathogenesis, clinical features, and immune responses. A. Spread of measles virus, from initial infection of the respiratory tract through dissemination to the skin. B. Appearance of clinical signs and symptoms, including Koplik’s spots and rash. C. Antibody and T cell responses to measles virus. The signs and symptoms of measles arise coincident with the host immune response. (Source: Modified from WJ Moss, DE Griffin: Nat Rev Microbiol 4:900, 2006.)
IMMUNE RESPONSES
Host immune responses to measles virus are essential for viral clearance, clinical recovery, and the establishment of long-term immunity (Fig. 229-1C). Early nonspecific (innate) immune responses during the prodromal phase include activation of natural killer cells and increased production of antiviral proteins. The adaptive immune responses consist of measles virus–specific antibody and cellular responses. The protective efficacy of antibodies to measles virus is illustrated by the immunity conferred to infants from passively acquired maternal antibodies and the protection of exposed, susceptible individuals after administration of anti–measles virus immunoglobulin. The first measles virus–specific antibodies produced after infection are of the IgM subtype, with a subsequent switch to predominantly IgG1 and IgG4 isotypes. The IgM antibody response is typically absent following reexposure or revaccination and serves as a marker of primary infection.
The importance of cellular immunity to measles virus is demonstrated by the ability of children with agammaglobulinemia (congenital inability to produce antibodies) to recover fully from measles and the contrasting picture for children with severe defects in T lymphocyte function, who often develop severe or fatal disease (Chap. 374). The initial predominant TH1 response (characterized by interferon γ) is essential for viral clearance, and the later TH2 response (characterized by interleukin 4) promotes the development of measles virus–specific antibodies that are critical for protection against reinfection.
The duration of protective immunity following wild-type measles virus infection is generally thought to be lifelong. Immunologic memory to measles virus includes both continued production of measles virus–specific antibodies and circulation of measles virus–specific CD4+ and CD8+ T lymphocytes.
However, the intense immune responses induced by measles virus infection are paradoxically associated with depressed responses to unrelated (non–measles virus) antigens, which persist for several weeks to months beyond resolution of the acute illness. This state of immune suppression enhances susceptibility to secondary infections with bacteria and viruses that cause pneumonia and diarrhea and is responsible for a substantial proportion of measles-related morbidity and deaths. Delayed-type hypersensitivity responses to recall antigens, such as tuberculin, are suppressed, and cellular and humoral responses to new antigens are impaired. Reactivation of tuberculosis and remission of autoimmune diseases after measles have been described and are attributed to this period of immune suppression.
CLINICAL MANIFESTATIONS
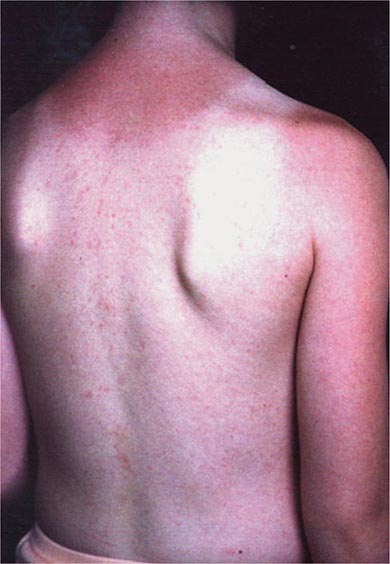
In most persons, the signs and symptoms of measles are highly characteristic (Fig. 229-1B). Fever and malaise beginning ~10 days after exposure are followed by cough, coryza, and conjunctivitis. These signs and symptoms increase in severity over 4 days. Koplik’s spots (see Fig. 25e-2) develop on the buccal mucosa ~2 days before the rash appears. The characteristic rash of measles (see Fig. 25e-3) begins 2 weeks after infection, when the clinical manifestations are most severe, and signal the host’s immune response to the replicating virus. Headache, abdominal pain, vomiting, diarrhea, and myalgia may be present.
Koplik’s spots (see Fig. 25e-2) are pathognomonic of measles and consist of bluish white dots ~1 mm in diameter surrounded by erythema. The lesions appear first on the buccal mucosa opposite the lower molars but rapidly increase in number to involve the entire buccal mucosa. They fade with the onset of rash.
The rash of measles begins as erythematous macules behind the ears and on the neck and hairline. The rash progresses to involve the face, trunk, and arms (see Fig. 25e-3), with involvement of the legs and feet by the end of the second day. Areas of confluent rash appear on the trunk and extremities, and petechiae may be present. The rash fades slowly in the same order of progression as it appeared, usually beginning on the third or fourth day after onset. Resolution of the rash may be followed by desquamation, particularly in undernourished children.
Because the characteristic rash of measles is a consequence of the cellular immune response, it may not develop in persons with impaired cellular immunity (e.g., those with AIDS; Chap. 226). These persons have a high case-fatality rate and frequently develop giant-cell pneumonitis caused by measles virus. T lymphocyte defects due to causes other than HIV-1 infection (e.g., cancer chemotherapy) also are associated with increased severity of measles.
A severe atypical measles syndrome was observed in recipients of a formalin-inactivated measles vaccine (used in the United States from 1963 to 1967 and in Canada until 1970) who were subsequently exposed to wild-type measles virus. The atypical rash began on the palms and soles and spread centripetally to the proximal extremities and trunk, sparing the face. The rash was initially erythematous and maculopapular but frequently progressed to vesicular, petechial, or purpuric lesions (see Fig. 25e-22).
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of measles includes other causes of fever, rash, and conjunctivitis, including rubella, Kawasaki disease, infectious mononucleosis, roseola, scarlet fever, Rocky Mountain spotted fever, enterovirus or adenovirus infection, and drug sensitivity. Rubella is a milder illness without cough and with distinctive lymphadenopathy. The rash of roseola (exanthem subitum) (see Fig. 25e-5) appears after fever has subsided. The atypical lymphocytosis in infectious mononucleosis contrasts with the leukopenia commonly observed in children with measles.
DIAGNOSIS
Measles is readily diagnosed on clinical grounds by clinicians familiar with the disease, particularly during outbreaks. Koplik’s spots (see Fig. 25e-2) are especially helpful because they appear early and are pathognomonic. Clinical diagnosis is more difficult (1) during the prodromal illness; (2) when the rash is attenuated by passively acquired antibodies or prior immunization; (3) when the rash is absent or delayed in immunocompromised children or severely undernourished children with impaired cellular immunity; and (4) in regions where the incidence of measles is low and other pathogens are responsible for the majority of illnesses with fever and rash. The CDC case definition for measles requires (1) a generalized maculopapular rash of at least 3 days’ duration; (2) fever of at least 38.3°C (101°F); and (3) cough, coryza, or conjunctivitis.
Serology is the most common method of laboratory diagnosis. The detection of measles virus–specific IgM in a single specimen of serum or oral fluid is considered diagnostic of acute infection, as is a fourfold or greater increase in measles virus–specific IgG antibody levels between acute- and convalescent-phase serum specimens. Primary infection in the immunocompetent host results in antibodies that are detectable within 1–3 days of rash onset and reach peak levels in 2–4 weeks. Measles virus–specific IgM antibodies may not be detectable until 4–5 days or more after rash onset and usually fall to undetectable levels within 4–8 weeks of rash onset.
Several methods for measurement of antibodies to measles virus are available. Neutralization tests are sensitive and specific, and the results are highly correlated with protective immunity; however, these tests require propagation of measles virus in cell culture and thus are expensive and laborious. Commercially available enzyme immunoassays are most frequently used. Measles can also be diagnosed by isolation of the virus in cell culture from respiratory secretions, nasopharyngeal or conjunctival swabs, blood, or urine. Direct detection of giant cells in respiratory secretions, urine, or tissue obtained by biopsy provides another method of diagnosis.
For detection of measles virus RNA by reverse-transcriptase polymerase chain reaction amplification of RNA extracted from clinical specimens, primers targeted to highly conserved regions of measles virus genes are used. Extremely sensitive and specific, this assay may also permit identification and characterization of measles virus genotypes for molecular epidemiologic studies and can distinguish wild-type from vaccine virus strains.
COMPLICATIONS
Most complications of measles involve the respiratory tract and include the effects of measles virus replication itself and secondary bacterial infections. Acute laryngotracheobronchitis (croup) can occur during measles and may result in airway obstruction, particularly in young children. Giant-cell pneumonitis due to replication of measles virus in the lungs can develop in immunocompromised children, including those with HIV-1 infection. Many children with measles develop diarrhea, which contributes to undernutrition.
Most complications of measles result from secondary bacterial infections of the respiratory tract that are attributable to a state of immune suppression lasting for several weeks to months after acute measles. Otitis media and bronchopneumonia are most common and may be caused by S. pneumoniae, H. influenzae type b, or staphylococci. Recurrence of fever or failure of fever to subside with the rash suggests secondary bacterial infection.
Rare but serious complications of measles involve the central nervous system (CNS). Postmeasles encephalomyelitis complicates ~1 in 1000 cases, affecting mainly older children and adults. Encephalomyelitis occurs within 2 weeks of rash onset and is characterized by fever, seizures, and a variety of neurologic abnormalities. The finding of periventricular demyelination, the induction of immune responses to myelin basic protein, and the absence of measles virus in the brain suggest that postmeasles encephalomyelitis is an autoimmune disorder triggered by measles virus infection. Other CNS complications that occur months to years after acute infection are measles inclusion body encephalitis (MIBE) and subacute sclerosing panencephalitis (SSPE). In contrast to postmeasles encephalomyelitis, MIBE and SSPE are caused by persistent measles virus infection. MIBE is a rare but fatal complication that affects individuals with defective cellular immunity and typically occurs months after infection. SSPE is a slowly progressive disease characterized by seizures and progressive deterioration of cognitive and motor functions, with death occurring 5–15 years after measles virus infection. SSPE most often develops in persons infected with measles virus at <2 years of age.
PROGNOSIS
![]() Most persons with measles recover and develop long-term protective immunity to reinfection. Measles case-fatality proportions vary with the average age of infection, the nutritional and immunologic status of the population, measles vaccine coverage, and access to health care. Among previously vaccinated persons who do become infected, disease is less severe and mortality rates are significantly lower. In developed countries, <1 in 1000 children with measles die. In endemic areas of sub-Saharan Africa, the measles case-fatality proportion may be 5–10% or even higher. Measles is a major cause of childhood deaths in refugee camps and in internally displaced populations, where case-fatality proportions have been as high as 20–30%.
Most persons with measles recover and develop long-term protective immunity to reinfection. Measles case-fatality proportions vary with the average age of infection, the nutritional and immunologic status of the population, measles vaccine coverage, and access to health care. Among previously vaccinated persons who do become infected, disease is less severe and mortality rates are significantly lower. In developed countries, <1 in 1000 children with measles die. In endemic areas of sub-Saharan Africa, the measles case-fatality proportion may be 5–10% or even higher. Measles is a major cause of childhood deaths in refugee camps and in internally displaced populations, where case-fatality proportions have been as high as 20–30%.
PREVENTION
Passive Immunization Human immunoglobulin given shortly after exposure can attenuate the clinical course of measles. In immunocompetent persons, administration of immunoglobulin within 72 h of exposure usually prevents measles virus infection and almost always prevents clinical measles. Administered up to 6 days after exposure, immunoglobulin will still prevent or modify the disease. Prophylaxis with immunoglobulin is recommended for susceptible household and nosocomial contacts who are at risk of developing severe measles, particularly children <1 year of age, immunocompromised persons (including HIV-infected persons previously immunized with live attenuated measles vaccine), and pregnant women. Except for premature infants, children <6 months of age usually will be partially or completely protected by passively acquired maternal antibody. If measles is diagnosed in a household member, all unimmunized children in the household should receive immunoglobulin. The recommended dose is 0.25 mL/kg given intramuscularly. Immunocompromised persons should receive 0.5 mL/kg. The maximum total dose is 15 mL. IV immunoglobulin contains antibodies to measles virus; the usual dose of 100–400 mg/kg generally provides adequate prophylaxis for measles exposures occurring as long as 3 weeks or more after IV immunoglobulin administration.
Active Immunization The first live attenuated measles vaccine was developed by passage of the Edmonston strain in chick embryo fibroblasts to produce the Edmonston B virus, which was licensed in 1963 in the United States. Further passage of Edmonston B virus produced the more attenuated Schwarz vaccine that currently serves as the standard in much of the world. The Moraten (“more attenuated Enders”) strain, which was licensed in 1968 and is used in the United States, is genetically closely related to the Schwarz strain.
Lyophilized measles vaccines are relatively stable, but reconstituted vaccine rapidly loses potency. Live attenuated measles vaccines are inactivated by light and heat and lose about half their potency at 20°C and almost all their potency at 37°C within 1 h after reconstitution. Therefore, a cold chain must be maintained before and after reconstitution. Antibodies first appear 12–15 days after vaccination, and titers peak at 1–3 months. Measles vaccines are often combined with other live attenuated virus vaccines, such as those for mumps and rubella (MMR) and for mumps, rubella, and varicella (MMR-V).
The recommended age of first vaccination varies from 6 to 15 months and represents a balance between the optimal age for seroconversion and the probability of acquiring measles before that age. The proportions of children who develop protective levels of antibody after measles vaccination approximate 85% at 9 months of age and 95% at 12 months. Common childhood illnesses concomitant with vaccination may reduce the level of immune response, but such illness is not a valid reason to withhold vaccination. Measles vaccines have been well tolerated and immunogenic in HIV-1-infected children and adults, although antibody levels may wane. Because of the potential severity of wild-type measles virus infection in HIV-1-infected children, routine measles vaccination is recommended except for those who are severely immunocompromised. Measles vaccination is contraindicated in individuals with other severe deficiencies of cellular immunity because of the possibility of disease due to progressive pulmonary or CNS infection with the vaccine virus.
The duration of vaccine-induced immunity is at least several decades if not longer. Rates of secondary vaccine failure 10–15 years after immunization have been estimated at ~5% but are probably lower when vaccination takes place after 12 months of age. Decreasing antibody concentrations do not necessarily imply a complete loss of protective immunity: a secondary immune response usually develops after reexposure to measles virus, with a rapid rise in antibody titers in the absence of overt clinical disease.
Standard doses of currently licensed measles vaccines are safe for immunocompetent children and adults. Fever to 39.4°C (103°F) occurs in ~5% of seronegative vaccine recipients, and 2% of vaccine recipients develop a transient rash. Mild transient thrombocytopenia has been reported, with an incidence of ~1 case per 40,000 doses of MMR vaccine.
Since the publication of a report in 1998 hypothesizing that MMR vaccine may cause a syndrome of autism and intestinal inflammation, much public attention has focused on this purported association. The events that followed publication of this report led to diminished vaccine coverage in the United Kingdom and provide important lessons in the misinterpretation of epidemiologic evidence and the communication of scientific results to the public. The publication that incited the concern was a case series describing 12 children with a regressive developmental disorder and chronic enterocolitis; 9 of these children had autism. In 8 of the 12 cases, the parents associated onset of the developmental delay with MMR vaccination. This simple temporal association was misinterpreted and misrepresented as a possible causal relationship, first by the lead author of the study and then by elements of the media and the public. Subsequently, several comprehensive reviews and additional epidemiologic studies refuted evidence of a causal relationship between MMR vaccination and autism.
PROSPECTS FOR MEASLES ERADICATION
Progress in global measles control has renewed discussion of measles eradication. In contrast to poliovirus eradication, the eradication of measles virus will not entail challenges posed by prolonged shedding of potentially virulent vaccine viruses and environmental viral reservoirs. However, in comparison with smallpox eradication, higher levels of population immunity will be necessary to interrupt measles virus transmission, more highly skilled health care workers will be required to administer measles vaccines, and containment through case detection and ring vaccination will be more difficult for measles virus because of infectivity before rash onset. New tools, such as aerosol administration of measles vaccines, will facilitate mass vaccination campaigns. Despite enormous progress, measles remains a leading vaccine-preventable cause of childhood mortality worldwide and continues to cause outbreaks in communities with low vaccination coverage rates in industrialized nations.
230e |
Rubella (German Measles) |
Rubella was historically viewed as a variant of measles or scarlet fever. Not until 1962 was a separate viral agent for rubella isolated. After an epidemic of rubella in Australia in the early 1940s, the ophthalmologist Norman Gregg noticed the occurrence of congenital cataracts among infants whose mothers had reported rubella infection during early pregnancy, and congenital rubella syndrome (CRS; see “Clinical Manifestations,” below) was first described.
ETIOLOGY
Rubella virus is a member of the Togaviridae family and the only member of the genus Rubivirus. This single-stranded RNA enveloped virus measures 50–70 nm in diameter. Its core protein is surrounded by a single-layer lipoprotein envelope with spike-like projections containing two glycoproteins, E1 and E2. There is only one antigenic type of rubella virus, and humans are its only known reservoir.
PATHOGENESIS AND PATHOLOGY
Although the pathogenesis of postnatal (acquired) rubella has been well documented, data on pathology are limited because of the mildness of the disease. Rubella virus is spread from person to person via respiratory droplets. Primary implantation and replication in the nasopharynx are followed by spread to the lymph nodes. Subsequent viremia occurs, which in pregnant women often results in infection of the placenta. Placental virus replication may lead to infection of fetal organs. The pathology of CRS in the infected fetus is well defined, with almost all organs found to be infected; however, the pathogenesis of CRS is only poorly delineated. In tissue, infections with rubella virus have diverse effects, ranging from no obvious impact to cell destruction. The hallmark of fetal infection is chronicity, with persistence throughout fetal development in utero and for up to 1 year after birth.
Individuals with acquired rubella may shed virus from 7 days before rash onset to ~5–7 days thereafter. Both clinical and subclinical infections are considered contagious. Infants with CRS may shed large quantities of virus from bodily secretions, particularly from the throat and in the urine, up to 1 year of age. Outbreaks of rubella, including some in nosocomial settings, have originated with index cases of CRS. Thus only individuals immune to rubella should have contact with infants who have CRS or who are congenitally infected with rubella virus but are not showing signs of CRS.
EPIDEMIOLOGY
The largest recent rubella epidemic in the United States took place in 1964–1965, when an estimated 12.5 million cases occurred, resulting in ~20,000 cases of CRS. Since the introduction of the routine rubella vaccination program in the United States in 1969, the number of rubella cases reported each year has dropped by >99%; the rate of vaccination coverage with rubella-containing vaccine has been >90% among children 19–35 months old since 1995 and >95% for kindergarten and first-grade entrants since 1980. In 1989 a goal for the elimination of rubella and CRS in the United States was set, and in 2004 a panel of experts agreed unanimously that rubella was no longer an endemic disease in this country. The criteria used to document lack of endemic transmission included low disease incidence, high nationwide rubella antibody seroprevalence, outbreaks that were few and contained (i.e., small numbers of cases), and lack of endemic virus transmission (as assessed by genetic sequencing). In the United States, interruption of endemic transmission of rubella virus has been sustained since 2001; in 2012, however, three cases of CRS were reported in infants whose mothers had acquired rubella infection abroad. Thus health care providers should remain vigilant, considering the possibility of rubella infection in patients emigrating or returning from countries without rubella control programs and the accompanying potential for CRS among their infants.
![]() Although rubella and CRS are no longer endemic in the United States, they remain important public health problems globally. The number of rubella cases reported worldwide in 1999 was ~900,000; this figure declined steadily to 94,030 in 2012. However, numbers of rubella cases are substantially underestimated because cases in many countries are identified through measles surveillance systems that are not specific for rubella. In 2010, it was estimated that 103,000 cases of CRS occur globally.
Although rubella and CRS are no longer endemic in the United States, they remain important public health problems globally. The number of rubella cases reported worldwide in 1999 was ~900,000; this figure declined steadily to 94,030 in 2012. However, numbers of rubella cases are substantially underestimated because cases in many countries are identified through measles surveillance systems that are not specific for rubella. In 2010, it was estimated that 103,000 cases of CRS occur globally.
CLINICAL FEATURES
Acquired Rubella Acquired rubella commonly presents with a generalized maculopapular rash that usually lasts for up to 3 days (Fig. 230e-1), although as many as 50% of cases may be subclinical or without rash. When it occurs, the rash is usually mild and may be difficult to detect in persons with darker skin. In children, rash is usually the first sign of illness. However, in older children and adults, a 1- to 5-day prodrome often precedes the rash and may include low-grade fever, malaise, and upper respiratory symptoms. The incubation period is 14 days (range, 12–23 days).
FIGURE 230e-1 Mild maculopapular rash of rubella in a child.
Lymphadenopathy, particularly occipital and postauricular, may be noted during the second week after exposure. Although acquired rubella is usually thought of as a benign disease, arthralgia and arthritis are common in infected adults, particularly women. Thrombocytopenia and encephalitis are less common complications.
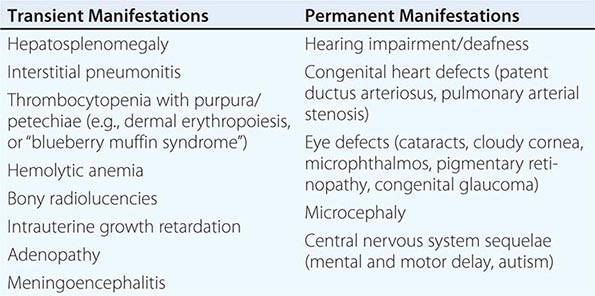
Congenital Rubella Syndrome The most serious consequence of rubella virus infection can develop when a woman becomes infected during pregnancy, particularly during the first trimester. The resulting complications may include miscarriage, fetal death, premature delivery, or live birth with congenital defects. Infants infected with rubella virus in utero may have myriad physical defects (Table 230e-1), which most commonly relate to the eyes, ears, and heart. This constellation of severe birth defects is known as congenital rubella syndrome. In addition to permanent manifestations, there are a host of transient physical manifestations, including thrombocytopenia with purpura/petechiae (e.g., dermal erythropoiesis, “blueberry muffin syndrome”). Some infants may be born with congenital rubella virus infection but have no apparent signs or symptoms of CRS and are referred to as “infants with congenital rubella infection only.”
|
COMMON TRANSIENT AND PERMANENT MANIFESTATIONS IN INFANTS WITH CONGENITAL RUBELLA SYNDROME |
DIAGNOSIS
Acquired Rubella Clinical diagnosis of acquired rubella is difficult because of the mimicry of many illnesses with rashes, the varied clinical presentations, and the high rates of subclinical and mild disease. Illnesses that may be similar to rubella in presentation include scarlet fever, roseola, toxoplasmosis, fifth disease, measles, and illnesses with suboccipital and postauricular lymphadenopathy. Thus laboratory documentation of rubella virus infection is considered the only reliable way to confirm acute disease.
Laboratory assessment of rubella infection is conducted by serologic and virologic methods. For acquired rubella, serologic diagnosis is most common and depends on the demonstration of IgM antibodies in an acute-phase serum specimen or a fourfold rise in IgG antibody titer between acute- and convalescent-phase specimens. The enzyme-linked immunosorbent assay IgM capture technique is considered most accurate for serologic diagnosis, but the indirect IgM assay also is acceptable. After rubella virus infection, IgM antibody may be detectable for up to 6 weeks. In case of a negative result for IgM in specimens taken earlier than day 5 after rash onset, serologic testing should be repeated. Although uncommon, reinfection with rubella virus is possible, and IgM antibodies may be present. To detect a rise in IgG antibody titer indicative of acute disease, the acute-phase serum specimen should be collected within 7–10 days after onset of illness and the convalescent-phase specimen ~14–21 days after the first specimen.
IgG avidity testing is used in conjunction with IgG testing. Low-avidity antibodies indicate recent infection. Mature (high-avidity) IgG antibodies most likely indicate an infection occurring at least 2 months previously. This test helps distinguish primary infection from reinfection. Avidity testing may be particularly useful in diagnosing rubella in pregnant women and assessing the risk of CRS.
Rubella virus can be isolated from the blood and nasopharynx during the prodromal period and for as long as 2 weeks after rash onset. However, as the secretion of virus in individuals with acquired rubella is maximal just before or up to 4 days after rash onset, this is the optimal time frame for collecting specimens for viral cultures. Rubella can also be diagnosed by viral RNA detection in a reverse-transcriptase polymerase chain reaction (RT-PCR) assay.
Congenital Rubella Syndrome A clinical diagnosis of CRS is reasonable when an infant presents with a combination of cataracts, hearing impairment, and heart defects; this pattern is seen in ~10% of infants with CRS. Infants may present with different combinations of defects depending on when infection occurs during gestation. Hearing impairment is the most common single defect of CRS. However, as with acquired rubella, laboratory diagnosis of congenital infection is highly recommended, particularly because most features of the clinical presentation are nonspecific and may be associated with other intrauterine infections. Early diagnosis of CRS facilitates appropriate medical intervention for specific disabilities and prompts implementation of infection control measures.
Diagnostic tests used to confirm CRS include serologic assays and virus detection. In an infant with congenital infection, serum IgM antibodies are normally present for up to 6 months but may be detectable for up to 1 year after birth. In some instances, IgM may not be detectable until 1 month of age; thus infants who have symptoms consistent with CRS but who test negative shortly after birth should be retested at 1 month. A rubella serum IgG titer persisting beyond the time expected after passive transfer of maternal IgG antibody (i.e., a rubella titer that does not decline at the expected rate of a twofold dilution per month) is another serologic criterion used to confirm CRS.
In congenital infection, rubella virus is isolated most commonly from throat swabs and less commonly from urine and cerebrospinal fluid. Infants with congenital rubella may excrete virus for up to 1 year, but specimens for virus isolation are most likely to be positive if obtained within the first 6 months after birth. Rubella virus in infants with CRS can also be detected by RT-PCR.
Rubella Diagnosis in Pregnant Women In the United States, screening for rubella IgG antibodies is recommended as part of routine prenatal care. Pregnant women with a positive IgG antibody serologic test are considered immune. Susceptible pregnant women should be vaccinated postpartum.
A susceptible pregnant woman exposed to rubella virus should be tested for IgM antibodies and/or a fourfold rise in IgG antibody titer between acute- and convalescent-phase serum specimens to determine whether she was infected during pregnancy. Pregnant women with evidence of acute infection must be clinically monitored, and gestational age at the time of maternal infection must be determined to assess the possibility of risk to the fetus. Of women infected with rubella virus during the first 11 weeks of gestation, up to 90% deliver an infant with CRS; for maternal infection during the first 20 weeks of pregnancy, the CRS rate is 20%.
PREVENTION
![]() After the isolation of rubella virus in the early 1960s and the occurrence of a devastating pandemic, a vaccine for rubella was developed and licensed in 1969. Currently, the majority of rubella-containing vaccines (RCVs) used worldwide are combined measles and rubella (MR) or measles, mumps, and rubella (MMR) formulations. A tetravalent measles, mumps, rubella, and varicella (MMRV) vaccine is available but is not widely used.
After the isolation of rubella virus in the early 1960s and the occurrence of a devastating pandemic, a vaccine for rubella was developed and licensed in 1969. Currently, the majority of rubella-containing vaccines (RCVs) used worldwide are combined measles and rubella (MR) or measles, mumps, and rubella (MMR) formulations. A tetravalent measles, mumps, rubella, and varicella (MMRV) vaccine is available but is not widely used.
The public health burden of rubella infection is measured primarily through the resulting CRS cases. The 1964–1965 rubella epidemic in the United States encompassed >30,000 infections during pregnancy. CRS occurred in ~20,000 infants born alive, including >11,000 infants who were deaf, >3500 infants who were blind, and almost 2000 infants who were mentally retarded. The cost of this epidemic exceeded $1.5 billion. In 1983, the cost per child with CRS was estimated at $200,000.
In most countries, there is little documented evidence to illuminate the epidemiology of CRS. Clusters of CRS cases have been reported in developing countries. Before the introduction of an immunization program, the incidence of CRS is 0.1–0.2 per 1000 live births during endemic periods and 1–4 per 1000 live births during epidemic periods. Where rubella virus is circulating and women of childbearing age are susceptible, CRS cases will continue to occur.
The most effective method of preventing acquired rubella and CRS is through vaccination with an RCV. One dose induces seroconversion in ≥95% of persons ≥1 year of age. Immunity is considered long-term and is probably lifelong. The most commonly used vaccine globally is the RA27/3 virus strain. The current recommendation for routine rubella vaccination in the United States is a first dose of MMR vaccine at 12–15 months of age and a second dose at 4–6 years. Target groups for rubella vaccine include children ≥1 year of age, adolescents and adults without documented evidence of immunity, individuals in congregate settings (e.g., college students, military personnel, child care and health care workers), and susceptible women before and after pregnancy.
Because of the theoretical risk of transmission of live attenuated rubella vaccine virus to the developing fetus, women known to be pregnant should not receive an RCV. In addition, pregnancy should be avoided for 28 days after receipt of an RCV. In follow-up studies of 680 unknowingly pregnant women who received rubella vaccine, no infant was born with CRS. Receipt of an RCV during pregnancy is not ordinarily a reason to consider termination of the pregnancy.
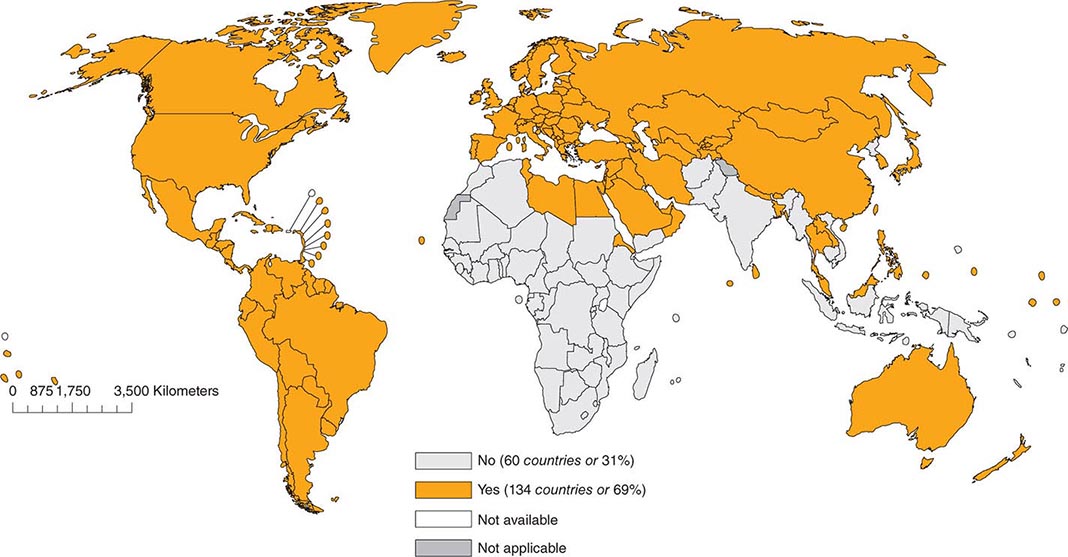
As of 2012, 134 (69%) of the 194 member countries of the World Health Organization recommended inclusion of an RCV in the routine childhood vaccination schedule (Fig. 230e-2). Goals for control or elimination of rubella and CRS have been established in the American Region, the European Region, the South-East Asia Region, and the Western Pacific Region. The other two regions (Eastern Mediterranean and African) have not yet set such goals.
FIGURE 230e-2 Countries using rubella vaccine in their national immunization schedule, 2012. (From the World Health Organization.)
231e |
Mumps |
DEFINITION
Mumps is an illness characterized by acute-onset unilateral or bilateral tender, self-limited swelling of the parotid or other salivary gland(s) that lasts at least 2 days and has no other apparent cause.
ETIOLOGIC AGENT
![]() Mumps is caused by a paramyxovirus with a negative-strand, nonsegmented RNA genome of 15,384 bases encoding at least 8 proteins: the nucleo- (N), phospho- (P), V, matrix (M), fusion (F), small hydrophobic (SH), hemagglutinin-neuraminidase (HN), and large (L) proteins. The N, P, and L proteins together provide the polymerase activity responsible for genome transcription and replication. The viral genome is surrounded by a host cell–derived lipid bilayer envelope containing the M, F, SH, and HN proteins. The M protein is involved in viral assembly, whereas the HN and F proteins are responsible for cell attachment and entry and are the major targets of virus-neutralizing antibody. The V and SH proteins are accessory proteins, acting as antagonists of the host antiviral response; the former interferes with the interferon response and the latter with the tumor necrosis factor α (TNF-α)–mediated apoptotic signaling pathway. Because of the hypervariability of the SH gene, its nucleotide sequence is used to “genotype” the virus for molecular epidemiologic purposes. Thus far, 12 mumps virus genotypes have been assigned by SH gene sequence and are designated A–N (with the exclusion of E and M, which have been merged with genotypes C and K, respectively).
Mumps is caused by a paramyxovirus with a negative-strand, nonsegmented RNA genome of 15,384 bases encoding at least 8 proteins: the nucleo- (N), phospho- (P), V, matrix (M), fusion (F), small hydrophobic (SH), hemagglutinin-neuraminidase (HN), and large (L) proteins. The N, P, and L proteins together provide the polymerase activity responsible for genome transcription and replication. The viral genome is surrounded by a host cell–derived lipid bilayer envelope containing the M, F, SH, and HN proteins. The M protein is involved in viral assembly, whereas the HN and F proteins are responsible for cell attachment and entry and are the major targets of virus-neutralizing antibody. The V and SH proteins are accessory proteins, acting as antagonists of the host antiviral response; the former interferes with the interferon response and the latter with the tumor necrosis factor α (TNF-α)–mediated apoptotic signaling pathway. Because of the hypervariability of the SH gene, its nucleotide sequence is used to “genotype” the virus for molecular epidemiologic purposes. Thus far, 12 mumps virus genotypes have been assigned by SH gene sequence and are designated A–N (with the exclusion of E and M, which have been merged with genotypes C and K, respectively).
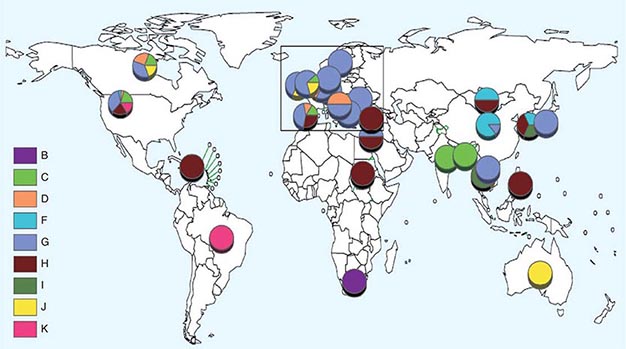
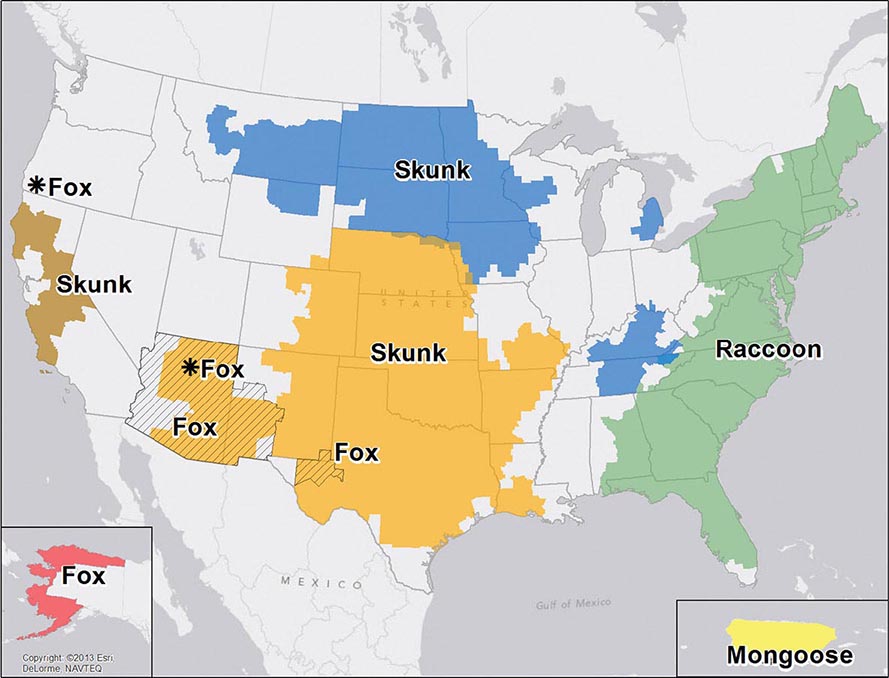
![]() Nucleotide sequencing of clinical isolates shows that virus genotypes D and G circulate predominantly in the Western Hemisphere; genotypes F, C, and I in the Asia–Pacific region; and genotypes B, H, J, and K in the Southern Hemisphere (Fig. 231e-1). Although numerous mumps virus genotypes have been identified and some vary antigenically from others, only one serotype exists, and there is no evidence to suggest that certain circulating virus strains are more virulent or contagious than others.
Nucleotide sequencing of clinical isolates shows that virus genotypes D and G circulate predominantly in the Western Hemisphere; genotypes F, C, and I in the Asia–Pacific region; and genotypes B, H, J, and K in the Southern Hemisphere (Fig. 231e-1). Although numerous mumps virus genotypes have been identified and some vary antigenically from others, only one serotype exists, and there is no evidence to suggest that certain circulating virus strains are more virulent or contagious than others.
FIGURE 231e-1 Distribution of reported mumps genotypes, 2005–2011 (data as of April 20, 2012). Pie-slice size is proportional to the number of years each genotype was reported. (Figure courtesy of WHO, with permission; http://www.who.int/immunization_monitoring/diseases/mumps/en/index.html; accessed September 11, 2012.)
EPIDEMIOLOGY
![]() Mumps is endemic worldwide, with epidemics every 3–5 years in unvaccinated populations. These epidemics typically occur in locations where children and young adults congregate, such as schools, military barracks, and other institutions. In countries without national mumps vaccination programs, the estimated annual global incidence is 100–1000 cases per 100,000 population. After the introduction of mumps vaccine in the United States in 1967, the number of reported cases declined dramatically. By 2001, fewer than 300 cases were reported, representing a 99.8% reduction from prevaccine-era levels. Mumps incidence remained at historic lows in the United States until 2006, when 6584 cases were reported—the largest outbreak since 1987. At the time of the 2006 outbreak, the disease was resurging globally, even in populations with high-level vaccination coverage. The number of reported U.S. cases declined precipitously in the 2 years that followed but then spiked in 2009–2010, with focal outbreaks in New York and New Jersey, and again in 2011, with a focal outbreak in California. A recent study by the Centers for Disease Control and Prevention (CDC) showed that two-dose coverage with measles–mumps–rubella (MMR) vaccine in major U.S. cities (94.8%) remains at or very near the level needed to contain these childhood infections; however, focal areas with inadequate vaccination coverage still leave some children at risk. Sporadic, large-scale mumps outbreaks continue to be reported worldwide, sometimes in countries where the disease was once under control.
Mumps is endemic worldwide, with epidemics every 3–5 years in unvaccinated populations. These epidemics typically occur in locations where children and young adults congregate, such as schools, military barracks, and other institutions. In countries without national mumps vaccination programs, the estimated annual global incidence is 100–1000 cases per 100,000 population. After the introduction of mumps vaccine in the United States in 1967, the number of reported cases declined dramatically. By 2001, fewer than 300 cases were reported, representing a 99.8% reduction from prevaccine-era levels. Mumps incidence remained at historic lows in the United States until 2006, when 6584 cases were reported—the largest outbreak since 1987. At the time of the 2006 outbreak, the disease was resurging globally, even in populations with high-level vaccination coverage. The number of reported U.S. cases declined precipitously in the 2 years that followed but then spiked in 2009–2010, with focal outbreaks in New York and New Jersey, and again in 2011, with a focal outbreak in California. A recent study by the Centers for Disease Control and Prevention (CDC) showed that two-dose coverage with measles–mumps–rubella (MMR) vaccine in major U.S. cities (94.8%) remains at or very near the level needed to contain these childhood infections; however, focal areas with inadequate vaccination coverage still leave some children at risk. Sporadic, large-scale mumps outbreaks continue to be reported worldwide, sometimes in countries where the disease was once under control.
Although historically a disease of unvaccinated children, with the largest proportion of cases occurring in children 5–9 years of age during the prevaccine era, mumps now frequently occurs in older age groups—primarily college students, most of whom were vaccinated in early childhood. This shift in age distribution and the occurrence of mumps in vaccinated populations are probably the result of several coincident circumstances, including (1) situations promoting the spread of respiratory viruses among young adults (e.g., residence in college dormitories), (2) waning of vaccine immunity with time, (3) lack of endemically circulating wild-type virus to periodically boost vaccine-induced immune responses, and (4) continuing global epidemics (due to either lack of mumps vaccination programs or, where such programs do exist, low rates of mumps vaccination). The notable decline in mumps vaccination–induced immunity with time may be due to both declining titers and decreasing avidity of antibodies. The waning of mumps immunity over time is supported by studies suggesting that a third dose of MMR vaccine significantly reduces the mumps attack rate; however, these studies were not adequately controlled to rule out the possibility that the observed declines in mumps incidence were unrelated to the intervention. Therefore, the effectiveness of a third dose of MMR vaccine remains to be demonstrated.
PATHOGENESIS
Humans are the only natural hosts for mumps virus infection. The incubation period of mumps is ~19 days (range, 7–23 days). The virus is transmitted by the respiratory route via droplets, saliva, and fomites. Mumps virus is typically shed from 1 week before to 1 week after symptom onset, although this window appears to be narrower in vaccinated individuals. Persons are most contagious 1–2 days before onset of clinical symptoms. Inference from related respiratory diseases and animal studies indicates that primary replication likely occurs in the nasal mucosa or upper respiratory mucosal epithelium. Mononuclear cells and cells within regional lymph nodes can become infected; such infection facilitates the development of viremia and poses a risk for a wide array of acute inflammatory reactions. Classic sites of mumps virus replication include the salivary glands, testes, pancreas, ovaries, mammary glands, and central nervous system (CNS).
Little is known about the pathology of mumps since the disease is rarely fatal. The virus replicates well in glandular epithelium, but classic parotitis is not a necessary component of mumps infection. Affected glands contain perivascular and interstitial mononuclear cell infiltrates and exhibit hemorrhage with prominent edema. Necrosis of acinar and epithelial duct cells is evident in the salivary glands and in the germinal epithelium of the seminiferous tubules of the testes. The virus probably enters cerebrospinal fluid (CSF) through the choroid plexus or via transiting mononuclear cells during plasma viremia. Although relevant data are limited, typical mumps encephalitis appears to be secondary to respiratory spread and is probably a parainfectious process, as suggested by perivenous demyelination, perivascular mononuclear cell inflammation, and relative sparing of neurons. Although rare, presumed primary encephalitis has been associated with mumps virus isolation from brain tissue. Evidence of placental and intrauterine spread in pregnancy has been found in both early and late gestation.
CLINICAL MANIFESTATIONS
Up to half of mumps virus infections are asymptomatic or lead to nonspecific respiratory symptoms. Inapparent infections are more common in adults than in children. The prodrome of mumps consists of low-grade fever, malaise, myalgia, headache, and anorexia. Mumps parotitis—acute-onset unilateral or bilateral swelling of the parotid or other salivary glands that lasts >2 days and has no other apparent cause—develops in 70–90% of symptomatic infections, usually within 24 h of prodromal symptoms but sometimes as long as 1 week thereafter. Parotitis is generally bilateral, although the two sides may not be involved synchronously. Unilateral involvement is documented in about one-third of cases. Swelling of the parotid is accompanied by tenderness and obliteration of the space between the earlobe and the angle of the mandible (Figs. 231e-2 and 231e-3). The patient frequently reports an earache and finds it difficult to eat, swallow, or talk. The orifice of the parotid duct is commonly red and swollen. The submaxillary and sublingual glands are involved less often than the parotid gland and are almost never involved alone. Glandular swelling increases for a few days and then gradually subsides, disappearing within 1 week. Recurrent sialadenitis is a rare sequela of mumps parotitis. In ~6% of mumps cases, obstruction of lymphatic drainage secondary to bilateral salivary gland swelling may lead to presternal pitting edema, associated often with submandibular adenitis and rarely with the more life-threatening supraglottic edema.
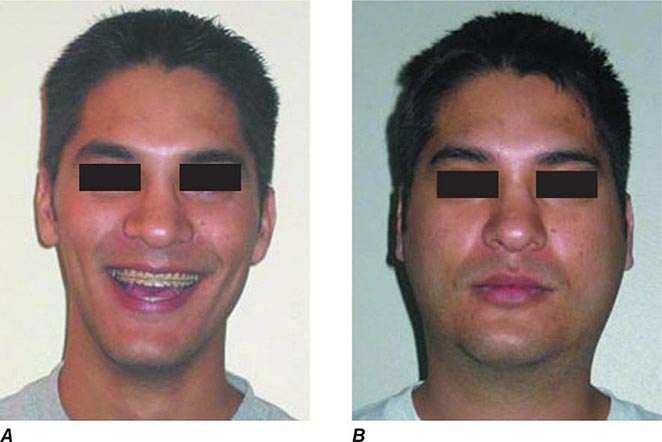
FIGURE 231e-2 A comparison of a person before acquiring mumps (A) and on day 3 (B) of acute bilateral parotitis. (Courtesy of patient C.M. From Shanley JD. The resurgence of mumps in young adults and adolescents. Cleve Clin J Med 2007; 74:42-48. Reprinted with permission. Copyright © 2007 Cleveland Clinic Foundation. All rights reserved.)
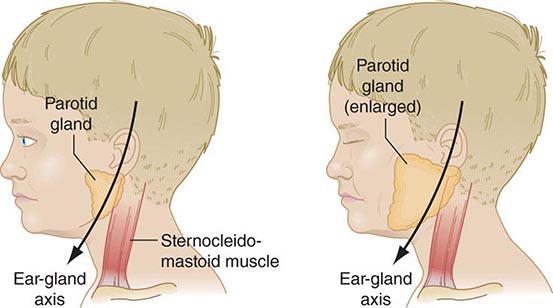
FIGURE 231e-3 Schematic drawing of a parotid gland infected with mumps virus (right) compared with a normal gland (left). An enlarged cervical lymph node is usually posterior to the imaginary line. (Reprinted with permission from Gershon A et al: Mumps, in Krugman’s Infectious Diseases of Children, 11th ed. Philadelphia, Elsevier, 2004, p 392.)
Epididymo-orchitis is the next most common manifestation of mumps, developing in 15–30% of cases in postpubertal males, with bilateral involvement in 10–30% of those cases. Orchitis, accompanied by fever, typically occurs during the first week of parotitis but can develop up to 6 weeks after parotitis or in its absence. The testis is painful and tender and can be enlarged to several times its normal size; this condition usually resolves within 1 week. Testicular atrophy develops in one-half of affected men. Sterility after mumps is rare, although subfertility is estimated to occur in 13% of cases of unilateral orchitis and in 30–87% of cases of bilateral orchitis. Oophoritis occurs in ~5% of women with mumps and may be associated with lower abdominal pain and vomiting but has only rarely been associated with sterility or premature menopause. Mumps infection in postpubertal women may also present with mastitis.
Documented CSF pleocytosis indicates that mumps virus invades the CNS in ~50% of cases; however, symptomatic CNS disease, typically in the form of aseptic meningitis, occurs in <10% of cases, with a male predominance. CNS symptoms of aseptic meningitis (e.g., stiff neck, headache, and drowsiness) appear ~5 days after parotitis and also occur often in the absence of parotid involvement. Within the first 24 h polymorphonuclear leukocytes may predominate in CSF (1000–2000 cells/μL), but by the second day nearly all the cells are lymphocytes. The glucose level in CSF may be low and the protein concentration high, a pattern reminiscent of bacterial meningitis. Mumps meningitis is a self-limited manifestation without significant risk of death or long-term sequelae. Cranial nerve palsies have occasionally led to permanent sequelae, particularly deafness. The reported incidence of mumps-associated hearing loss varies between 1 in 1000 and 1 in 100,000. In ~0.1% of infections, mumps virus may cause encephalitis, which presents as high fever with marked changes in the level of consciousness, seizures, and focal neurologic symptoms. Electroencephalographic abnormalities may be seen. Permanent sequelae are sometimes identified in survivors, and adult infections more commonly have poor outcomes than do pediatric infections. The mortality rate associated with mumps encephalitis is ~1.5%. Other CNS problems occasionally associated with mumps include cerebellar ataxia, facial palsy, transverse myelitis, hydrocephalus, Guillain-Barré syndrome, flaccid paralysis, and behavioral changes.
Mumps pancreatitis, which may present as abdominal pain, occurs in ~4% of infections but is difficult to diagnose because an elevated serum amylase level can be associated with either parotitis or pancreatitis. An etiologic association of mumps virus and juvenile diabetes mellitus remains controversial. Myocarditis and endocardial fibroelastosis are rare and self-limited but may represent severe complications of mumps infection; however, mumps-associated electrocardiographic abnormalities have been reported in up to 15% of cases. Other unusual complications include thyroiditis, nephritis, arthritis, hepatic disease, keratouveitis, and thrombocytopenic purpura. Abnormal renal function is common, but severe, life-threatening nephritis is rare. It remains at issue whether an excessive number of spontaneous abortions are associated with gestational mumps. Mumps in pregnancy does not appear to lead to premature birth, low birth weight, or fetal malformations.
DIFFERENTIAL DIAGNOSIS
During a mumps outbreak, the diagnosis is made easily in patients with parotitis and a history of recent exposure; however, when disease incidence is low, other causes of parotitis should be considered and laboratory testing is required for case confirmation. Infectious causes of parotitis include other viruses (e.g., HIV, coxsackievirus, parainfluenza virus type 3, influenza A virus, Epstein-Barr virus, adenovirus, parvovirus B19, lymphocytic choriomeningitis virus, human herpesvirus 6), gram-positive bacteria, atypical mycobacteria, and Bartonella species. Rarely, other gram-negative or anaerobic bacteria are associated with parotitis. Parotitis can also develop in the setting of sarcoidosis, Sjögren’s syndrome, Mikulicz’s syndrome, Parinaud’s syndrome, uremia, diabetes mellitus, laundry starch ingestion, malnutrition, cirrhosis, and some drug treatments. Unilateral parotitis can be caused by ductal obstruction, cysts, and tumors. In the absence of parotitis or other salivary gland enlargement, symptoms of other visceral organ and/or CNS involvement may predominate, and a laboratory diagnosis is required. Other entities should be considered when manifestations consistent with mumps appear in organs other than the parotid. Testicular torsion may produce a painful scrotal mass resembling that seen in mumps orchitis. Other viruses (e.g., enteroviruses) may cause aseptic meningitis that is clinically indistinguishable from that due to mumps virus.
LABORATORY DIAGNOSIS
Laboratory diagnosis is primarily based on detection of viral RNA by reverse-transcriptase polymerase chain reaction (RT-PCR) or on serology. Detection of viral antigens (e.g., via mumps virus–specific immunofluorescent staining of cultured clinical specimens) is comparatively inefficient and is no longer commonly performed.
For RT-PCR-based testing, viral RNA can be extracted either directly from clinical samples or from cell cultures incubated with clinical samples. Buccal swabs appear to be the best specimens for virus detection, particularly when obtained within 2 days of clinical onset; however, mumps virus can also be detected readily in throat swabs and saliva and, in cases of meningitis, in CSF. Despite the apparent high frequency of viremia during mumps, mumps virus has rarely been detected in blood. The ability to detect viral RNA in clinical samples rapidly diminishes beyond the first week after symptom onset, and in several studies rates of virus detection were substantially lower in recipients of two vaccine doses than in unvaccinated persons or recipients of one dose. The rate of false-negative RT-PCR findings can be quite high, approaching 70% in some studies.
A serologic diagnosis of mumps is typically made by enzyme-linked immunosorbent assay (ELISA). The data must be interpreted with caution. In vaccinated persons with mumps, IgM is typically absent; thus, a negative IgM result in a vaccinated person does not rule out mumps. In addition, regardless of vaccination status, IgM may not be detectable if serum is assayed too early (prior to day 3 of symptom onset) or too late (beyond 6 weeks after symptom onset) in the course of disease. Reliance on a rise in IgG titer in paired acute- and convalescent-phase sera also is problematic: IgG titers in convalescent-phase sera may be only nominally greater than those in acute-phase sera. Thus, at present, the capacity of RNA or viral antigen detection to confirm cases is much greater than that of serologic testing. Traditional and labor-intensive serologic tests such as complement fixation, hemagglutination inhibition, and virus neutralization are now performed only rarely. The main downside to replacement of these functional serologic assays with the more rapid ELISA method is the latter’s detection of all virus-specific antibodies, including those that are nonneutralizing (i.e., nonprotective). Thus, an individual who is seropositive by ELISA may lack protective levels of antibody. While there is a strong association between the presence of mumps virus neutralizing antibody and protection from disease, an absolute antibody titer predictive of serologic protection is lacking; in this respect, mumps differs from other respiratory infections, such as measles.
PREVENTION
![]() Vaccination is the only practical control measure. Nearly all developed countries use mumps-containing vaccines, but in many countries mumps is not a notifiable disease and vaccination is often voluntary. However, where used, mumps vaccination has had a tremendous impact, with reductions in incidence and morbidity typically exceeding 90%. Despite the tremendous success of mumps vaccination programs, large mumps outbreaks continue to occur globally, even in settings of high-level two-dose vaccine coverage. Whereas outbreaks historically involved young (often unvaccinated) children in primary and secondary schools, more recent outbreaks have predominantly involved young adults, particularly on college and university campuses. While primary and secondary (waning-immunity) vaccine failures have been hypothesized to be factors in mumps outbreaks in several countries, in some countries other factors may have played a role, such as lack of compliance with the recommended vaccine schedule, changes to vaccination schedules resulting in missed cohorts, or changes in population demographics, such as large-scale immigration.
Vaccination is the only practical control measure. Nearly all developed countries use mumps-containing vaccines, but in many countries mumps is not a notifiable disease and vaccination is often voluntary. However, where used, mumps vaccination has had a tremendous impact, with reductions in incidence and morbidity typically exceeding 90%. Despite the tremendous success of mumps vaccination programs, large mumps outbreaks continue to occur globally, even in settings of high-level two-dose vaccine coverage. Whereas outbreaks historically involved young (often unvaccinated) children in primary and secondary schools, more recent outbreaks have predominantly involved young adults, particularly on college and university campuses. While primary and secondary (waning-immunity) vaccine failures have been hypothesized to be factors in mumps outbreaks in several countries, in some countries other factors may have played a role, such as lack of compliance with the recommended vaccine schedule, changes to vaccination schedules resulting in missed cohorts, or changes in population demographics, such as large-scale immigration.
In the United States, the benefit-cost ratios for mumps vaccination alone are >13 for direct costs (e.g., medical expenses) and >24 for societal costs (including productivity losses for patients and caregivers). Several mumps virus vaccines are used throughout the world; in the United States, only the live attenuated Jeryl Lynn strain is used. Current recommendations are that mumps vaccine be administered as part of the combined trivalent measles-mumps-rubella vaccine (M-M-R® II) or the quadrivalent measles-mumps-rubella-varicella vaccine (ProQuad®). Monovalent vaccine is no longer produced for the U.S. market but is available in other countries.
Before administering mumps-containing vaccine, physicians should always consult the latest recommendations from the Advisory Committee on Immunization Practices (ACIP). Current recommendations for children specify two doses of mumps-containing vaccine: the first dose given on or after the first birthday and the second dose administered no earlier than 28 days after the first. In the United States, children often receive the second dose between the ages of 4 and 6 years.
In 2009, the ACIP revised its recommendations for evidence of mumps immunity in health care personnel to include (1) documented administration of two doses of a preparation containing live mumps vaccine, (2) laboratory evidence of immunity or laboratory confirmation of disease, or (3) birth date before 1957. For unvaccinated health care personnel born before 1957 who lack laboratory evidence of mumps immunity or laboratory confirmation of mumps, health care facilities should consider two doses of MMR vaccine separated by the appropriate interval; during a mumps outbreak, vaccination of these individuals is recommended.
Mumps vaccine contains live attenuated virus. It is not recommended for pregnant women, for individuals who have had a life-threatening allergic reaction to components of the vaccine, or for people in settings of clinically significant primary or secondary immunosuppression. (For details, see the ACIP guidelines on the CDC’s website [www.cdc.gov/vaccines/pubs/acip-list.htm].) Occasionally, febrile reactions and parotitis have been reported soon after mumps vaccination. Allergic reactions after vaccination (e.g., rash and pruritus) are uncommon and are usually mild and self-limited. More serious complications, such as aseptic meningitis, have been causally associated with certain vaccine strains but not with the Jeryl Lynn strain.
Immunity to mumps is associated with the development of neutralizing antibody, although a specific correlate of protection has not been established. Seroconversion occurs in ~95% of recipients of the Jeryl Lynn strain; however, vaccine efficacy is ~80% for one dose and 90% for two doses. Recent data indicate declining seropositivity rates with time since vaccination. Studies are under way to assess the value of including a third dose in the immunization schedule. Although it is generally accepted that mumps virus is serologically monotypic, antigenic differences between virus isolates have been detected. It is unclear whether such differences can lead to immune escape. The role of the cellular arm of the immune response is unclear, but there is evidence that it may help limit virus spread and complications.
ACKNOWLEDGMENT
The authors thank and acknowledge Dr. Anne Gershon, the author of this chapter in earlier editions.
232 |
Rabies and Other Rhabdovirus Infections |
RABIES
Rabies is a rapidly progressive, acute infectious disease of the central nervous system (CNS) in humans and animals that is caused by infection with rabies virus. The infection is normally transmitted from animal vectors. Rabies has encephalitic and paralytic forms that progress to death.
ETIOLOGIC AGENT
Rabies virus is a member of the family Rhabdoviridae. Two genera in this family, Lyssavirus and Vesiculovirus, contain species that cause human disease. Rabies virus is a lyssavirus that infects a broad range of animals and causes serious neurologic disease when transmitted to humans. This single-strand RNA virus has a nonsegmented, negative-sense (antisense) genome that consists of 11,932 nucleotides and encodes 5 proteins: nucleocapsid protein, phosphoprotein, matrix protein, glycoprotein, and a large polymerase protein. Rabies virus variants, which can be characterized by distinctive nucleotide sequences, are associated with specific animal reservoirs. Six other non–rabies virus species in the Lyssavirus genus have been reported to cause a clinical picture similar to rabies. Vesicular stomatitis virus, a vesiculovirus, causes vesiculation and ulceration in cattle, horses, and other animals and causes a self-limited, mild, systemic illness in humans (see “Other Rhabdoviruses,” below).
EPIDEMIOLOGY
![]() Rabies is a zoonotic infection that occurs in a variety of mammals throughout the world except in Antarctica and on some islands. Rabies virus is usually transmitted to humans by the bite of an infected animal. Worldwide, endemic canine rabies is estimated to cause 55,000 human deaths annually. Most of these deaths occur in Asia and Africa, with rural populations and children most frequently affected. Thus, in many resource-poor and resource-limited countries, canine rabies continues to be a threat to humans. However, in Latin America, rabies control efforts in dogs have been quite successful in recent years. Endemic canine rabies has been eliminated from the United States and most other resource-rich countries. Rabies is endemic in wildlife species, and a variety of animal reservoirs have been identified in different countries. Surveillance data from 2012 identified 6162 confirmed animal cases of rabies in the United States (including Puerto Rico). Only 8% of these cases were in domestic animals, including 257 cases in cats, 84 in dogs, and 115 in cattle. In North American wildlife reservoirs, including bats, raccoons, skunks, and foxes, the infection is endemic, with involvement of one or more rabies virus variants in each reservoir species (Fig. 232-1). “Spillover” of rabies to other wildlife species and to domestic animals occurs. Bat rabies virus variants are present in every state except Hawaii and are responsible for most indigenously acquired human rabies cases in the United States. Raccoon rabies is endemic along the entire eastern coast of the United States. Skunk rabies is present in the midwestern states, with another focus in California. Rabies in foxes occurs in Texas, New Mexico, Arizona, and Alaska.
Rabies is a zoonotic infection that occurs in a variety of mammals throughout the world except in Antarctica and on some islands. Rabies virus is usually transmitted to humans by the bite of an infected animal. Worldwide, endemic canine rabies is estimated to cause 55,000 human deaths annually. Most of these deaths occur in Asia and Africa, with rural populations and children most frequently affected. Thus, in many resource-poor and resource-limited countries, canine rabies continues to be a threat to humans. However, in Latin America, rabies control efforts in dogs have been quite successful in recent years. Endemic canine rabies has been eliminated from the United States and most other resource-rich countries. Rabies is endemic in wildlife species, and a variety of animal reservoirs have been identified in different countries. Surveillance data from 2012 identified 6162 confirmed animal cases of rabies in the United States (including Puerto Rico). Only 8% of these cases were in domestic animals, including 257 cases in cats, 84 in dogs, and 115 in cattle. In North American wildlife reservoirs, including bats, raccoons, skunks, and foxes, the infection is endemic, with involvement of one or more rabies virus variants in each reservoir species (Fig. 232-1). “Spillover” of rabies to other wildlife species and to domestic animals occurs. Bat rabies virus variants are present in every state except Hawaii and are responsible for most indigenously acquired human rabies cases in the United States. Raccoon rabies is endemic along the entire eastern coast of the United States. Skunk rabies is present in the midwestern states, with another focus in California. Rabies in foxes occurs in Texas, New Mexico, Arizona, and Alaska.
FIGURE 232-1 Distribution of the major rabies virus variants among wild terrestrial reservoirs in the United States and Puerto Rico, 2008–2012. *Potential host-shift event. (From JL Dyer et al: J Am Vet Med Assoc 243:805, 2013.)
In Canada and Europe, epizootics of rabies in red foxes have been well controlled with the use of baits containing rabies vaccine. A similar approach is used in Canada to control raccoon rabies.
Rabies virus variants isolated from humans or other mammalian species can be identified by reverse-transcription polymerase chain reaction (RT-PCR) amplification and sequencing or by characterization with monoclonal antibodies. These techniques are helpful in human cases with no known history of exposure. Worldwide, most human rabies is transmitted from dogs in countries with endemic canine rabies and dog-to-dog transmission, and human cases can be imported by travelers returning from these regions. In North America, human disease is usually associated with transmission from bats; there may be no known history of bat bite or other bat exposure in these cases. Most human cases are due to a bat rabies virus variant associated with silver-haired and tricolored bats. These are small bats whose bite may not be recognized, and the virus has adapted for replication at skin temperature and in cell types that are present in the skin.
Transmission from nonbite exposures is relatively uncommon. Aerosols generated in the laboratory or in caves containing millions of Brazilian free-tail bats have rarely caused human rabies. Transmission has resulted from corneal transplantation and also from solid organ transplantation and a vascular conduit (for a liver transplant) from undiagnosed donors with rabies in Texas, Florida, and Germany. Human-to-human transmission is extremely rare, although hypothetical concern about transmission to health care workers has prompted the implementation of barrier techniques to prevent exposures.
PATHOGENESIS
The incubation period of rabies (defined as the interval between exposure and the onset of clinical disease) is usually 20–90 days but in rare cases is as short as a few days or >1 year. During most of the incubation period, rabies virus is thought to be present at or close to the site of inoculation (Fig. 232-2). In muscles, the virus is known to bind to nicotinic acetylcholine receptors on postsynaptic membranes at neuromuscular junctions, but the exact details of viral entry into the skin and SC tissues have not yet been clarified. Rabies virus spreads centripetally along peripheral nerves toward the CNS at a rate of up to ~250 mm/d via retrograde fast axonal transport to the spinal cord or brainstem. Once the virus enters the CNS, it rapidly disseminates to other regions of the CNS via fast axonal transport along neuroanatomic connections. Neurons are prominently infected in rabies; infection of astrocytes is unusual. After CNS infection becomes established, there is centrifugal spread along sensory and autonomic nerves to other tissues, including the salivary glands, heart, adrenal glands, and skin. Rabies virus replicates in acinar cells of the salivary glands and is secreted in the saliva of rabid animals that serve as vectors of the disease. There is no well-documented evidence for hematogenous spread of rabies virus.
FIGURE 232-2 Schematic representation of the pathogenetic events following peripheral inoculation of rabies virus by an animal bite. (Adapted from Jackson AC: Human disease, in Rabies: scientific basis of the disease and its management, 3rd ed., AC Jackson [ed], Oxford, UK, Elsevier Academic Press, 2013, pp 269–298; with permission.)
Pathologic studies show mild inflammatory changes in the CNS in rabies, with mononuclear inflammatory infiltration in the leptomeninges, perivascular regions, and parenchyma, including microglial nodules called Babes nodules. Degenerative neuronal changes usually are not prominent, and there is little evidence of neuronal death; neuronophagia is observed occasionally. The pathologic changes are surprisingly mild in light of the clinical severity and fatal outcome of the disease. The most characteristic pathologic finding in rabies is the Negri body (Fig. 232-3). Negri bodies are eosinophilic cytoplasmic inclusions in brain neurons that are composed of rabies virus proteins and viral RNA. These inclusions occur in a minority of infected neurons, are commonly observed in Purkinje cells of the cerebellum and in pyramidal neurons of the hippocampus, and are less frequently seen in cortical and brainstem neurons. Negri bodies are not observed in all cases of rabies. The lack of prominent degenerative neuronal changes has led to the concept that neuronal dysfunction—rather than neuronal death—is responsible for clinical disease in rabies. The basis for behavioral changes, including the aggressive behavior of rabid animals, is not well understood.
FIGURE 232-3 Three large Negri bodies in the cytoplasm of a cerebellar Purkinje cell from an 8-year-old boy who died of rabies after being bitten by a rabid dog in Mexico. (From AC Jackson, E Lopez-Corella: N Engl J Med 335:568, 1996. © Massachusetts Medical Society.)
CLINICAL MANIFESTATIONS
In rabies, the emphasis must be on postexposure prophylaxis (PEP) initiated before any symptoms or signs develop. Rabies should usually be suspected on the basis of the clinical presentation. The disease usually presents as atypical encephalitis with relative preservation of consciousness. Rabies may be difficult to recognize late in the clinical course when progression to coma has occurred. A minority of patients present with acute flaccid paralysis. There are prodromal, acute neurologic, and comatose phases that usually progress to death despite aggressive therapy (Table 232-1).
|
CLINICAL STAGES OF RABIES |
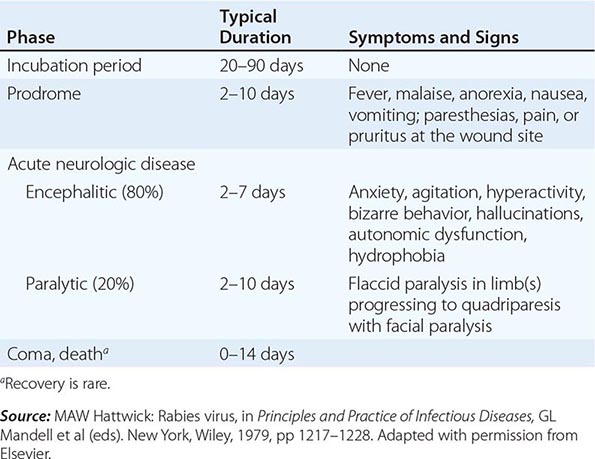
Prodromal Features The clinical features of rabies begin with nonspecific prodromal manifestations, including fever, malaise, headache, nausea, and vomiting. Anxiety or agitation may also occur. The earliest specific neurologic symptoms of rabies include paresthesias, pain, or pruritus near the site of the exposure, one or more of which occur in 50–80% of patients and strongly suggest rabies. The wound has usually healed by this point, and these symptoms probably reflect infection with associated inflammatory changes in local dorsal root or cranial sensory ganglia.
Encephalitic Rabies Two acute neurologic forms of rabies are seen in humans: encephalitic (furious) in 80% and paralytic in 20%. Some of the manifestations of encephalitic rabies, including fever, confusion, hallucinations, combativeness, and seizures, may be seen in other viral encephalitides as well. Autonomic dysfunction is common and may result in hypersalivation, gooseflesh, cardiac arrhythmia, and priapism. In encephalitic rabies, episodes of hyperexcitability are typically followed by periods of complete lucidity that become shorter as the disease progresses. Rabies encephalitis is distinguished by early brainstem involvement, which results in the classic features of hydrophobia (involuntary, painful contraction of the diaphragm and accessory respiratory, laryngeal, and pharyngeal muscles in response to swallowing liquids) and aerophobia (the same features caused by stimulation from a draft of air). These symptoms are probably due to dysfunction of infected brainstem neurons that normally inhibit inspiratory neurons near the nucleus ambiguus, resulting in exaggerated defense reflexes that protect the respiratory tract. The combination of hypersalivation and pharyngeal dysfunction is also responsible for the classic appearance of “foaming at the mouth” (Fig. 232-4). Brainstem dysfunction progresses rapidly, and coma—followed within days by death—is the rule unless the course is prolonged by supportive measures. With such measures, late complications can include cardiac and/or respiratory failure, disturbances of water balance (syndrome of inappropriate antidiuretic hormone secretion or diabetes insipidus), noncardiogenic pulmonary edema, and gastrointestinal hemorrhage. Cardiac arrhythmias may be due to dysfunction affecting vital centers in the brainstem or to myocarditis. Multiple-organ failure is common in patients treated aggressively in critical care units.
FIGURE 232-4 Hydrophobic spasm of inspiratory muscles associated with terror in a patient with encephalitic (furious) rabies who is attempting to swallow water. (Copyright DA Warrell, Oxford, UK; with permission.)
Paralytic Rabies About 20% of patients have paralytic rabies in which muscle weakness predominates and cardinal features of encephalitic rabies (hyperexcitability, hydrophobia, and aerophobia) are lacking. There is early and prominent flaccid muscle weakness, often beginning in the bitten extremity and spreading to produce quadriparesis and facial weakness. Sphincter involvement is common, sensory involvement is usually mild, and these cases are commonly misdiagnosed as Guillain-Barré syndrome. Patients with paralytic rabies generally survive a few days longer than those with encephalitic rabies, but multiple-organ failure nevertheless ensues.
LABORATORY INVESTIGATIONS
Most routine laboratory tests in rabies yield normal results or show nonspecific abnormalities. Complete blood counts are usually normal. Examination of cerebrospinal fluid (CSF) often reveals mild mononuclear cell pleocytosis with a mildly elevated protein level. Severe pleocytosis (>1000 white cells/μL) is unusual and should prompt a search for an alternative diagnosis. CT head scans are usually normal in rabies. MRI brain scans may show signal abnormalities in the brainstem or other gray-matter areas, but these findings are variable and nonspecific. Electroencephalograms show only nonspecific abnormalities. Of course, important tests in suspected cases of rabies include those that may identify an alternative, potentially treatable diagnosis (see “Differential Diagnosis,” below).
DIAGNOSIS
In North America, a diagnosis of rabies often is not considered until relatively late in the clinical course, even with a typical clinical presentation. This diagnosis should be considered in patients presenting with acute atypical encephalitis or acute flaccid paralysis, including those in whom Guillain-Barré syndrome is suspected. The absence of an animal-bite history is common in North America. The lack of hydrophobia is not unusual in rabies. Once rabies is suspected, rabies-specific laboratory tests should be performed to confirm the diagnosis. Diagnostically useful specimens include serum, CSF, fresh saliva, skin biopsy samples from the neck, and brain tissue (rarely obtained before death). Because skin biopsy relies on the demonstration of rabies virus antigen in cutaneous nerves at the base of hair follicles, samples are usually taken from hairy skin at the nape of the neck. Corneal impression smears are of low diagnostic yield and are generally not performed. Negative antemortem rabies-specific laboratory tests never exclude a diagnosis of rabies, and tests may need to be repeated after an interval for diagnostic confirmation.
Rabies Virus–Specific Antibodies In a previously unimmunized patient, serum neutralizing antibodies to rabies virus are diagnostic. However, because rabies virus infects immunologically privileged neuronal tissues, serum antibodies may not develop until late in the disease. Antibodies may be detected within a few days after the onset of symptoms, but some patients die without detectable antibodies. The presence of rabies virus–specific antibodies in the CSF suggests rabies encephalitis, regardless of immunization status. A diagnosis of rabies is questionable in patients who recover from rabies without developing serum neutralizing antibodies to rabies virus.
RT-PCR Amplification Detection of rabies virus RNA by RT-PCR is highly sensitive and specific. This technique can detect virus in fresh saliva samples, skin, CSF, and brain tissues. In addition, RT-PCR with genetic sequencing can distinguish among rabies virus variants, permitting identification of the probable source of an infection.
Direct Fluorescent Antibody Testing Direct fluorescent antibody (DFA) testing with rabies virus antibodies conjugated to fluorescent dyes is highly sensitive and specific and can be performed quickly and applied to skin biopsies and brain tissue. In skin biopsies, rabies virus antigen may be detected in cutaneous nerves at the base of hair follicles.
DIFFERENTIAL DIAGNOSIS
The diagnosis of rabies may be difficult without a history of animal exposure, and no exposure to an animal (e.g., a bat) may be recalled. The presentation of rabies is usually quite different from that of acute viral encephalitis due to most other causes, including herpes simplex encephalitis and arboviral (e.g., West Nile) encephalitis. Early neurologic symptoms may occur at the site of the bite, and there may be early features of brainstem involvement with preservation of consciousness. Anti-N-methyl-D-aspartate receptor (anti-NMDA) encephalitis occurs in young patients (especially females) and is characterized by behavioral changes, autonomic instability, hypoventilation, and seizures. Postinfectious (immune-mediated) encephalomyelitis may follow influenza, measles, mumps, and other infections; it may also occur as a sequela of immunization with rabies vaccine derived from neural tissues, which are used only in resource-limited and resource-poor countries. Rabies may present with unusual neuropsychiatric symptoms and may be misdiagnosed as a psychiatric disorder. Rabies hysteria may occur as a psychological response to the fear of rabies and is often characterized by a shorter incubation period than rabies, aggressive behavior, inability to communicate, and a long course with recovery.
As previously mentioned, paralytic rabies may mimic Guillain-Barré syndrome. In these cases, fever, bladder dysfunction, a normal sensory examination, and CSF pleocytosis favor a diagnosis of rabies. Conversely, Guillain-Barré syndrome may occur as a complication of rabies vaccination with a neural tissue–derived product (e.g., suckling mouse brain vaccine) and may be mistaken for paralytic rabies (i.e., vaccine failure).
PROGNOSIS
Rabies is an almost uniformly fatal disease but is nearly always preventable with appropriate postexposure therapy during the early incubation period (see below). There are seven well-documented cases of survival from rabies. All but one of these patients had received rabies vaccine before disease onset. The single survivor who had not received vaccine had neutralizing antibodies to rabies virus in serum and CSF at clinical presentation. Most patients with rabies die within several days of the onset of illness, despite aggressive care in a critical care unit.
PREVENTION
Postexposure Prophylaxis Since there is no effective therapy for rabies, it is extremely important to prevent the disease after an animal exposure. Figure 232-5 shows the steps involved in making decisions about PEP. On the basis of the history of the exposure and local epidemiologic information, the physician must decide whether initiation of PEP is warranted. Healthy dogs, cats, or ferrets may be confined and observed for 10 days. PEP is not necessary if the animal remains healthy. If the animal develops signs of rabies during the observation period, it should be euthanized immediately; the head should be transported to the laboratory under refrigeration, rabies virus should be sought by DFA testing, and viral isolation should be attempted by cell culture and/or mouse inoculation. Any animal other than a dog, cat, or ferret should be euthanized immediately and the head submitted for laboratory examination. In high-risk exposures and in areas where canine rabies is endemic, rabies prophylaxis should be initiated without waiting for laboratory results. If the laboratory results prove to be negative, it may safely be concluded that the animal’s saliva did not contain rabies virus, and immunization should be discontinued. If an animal escapes after an exposure, it must be considered rabid, and PEP must be initiated unless information from public health officials indicates otherwise (i.e., there is no endemic rabies in the area). Although controversial, the use of PEP may be warranted when a person (e.g., a small child or a sleeping adult) has been present in the same space as a bat and an unrecognized bite cannot be reliably excluded.
FIGURE 232-5 Algorithm for rabies postexposure prophylaxis. RIG, rabies immune globulin. (From L Corey, in Harrison’s Principles of Internal Medicine, 15th ed. E Braunwald et al [eds]: New York, McGraw-Hill, 2001; adapted with permission.)
PEP includes local wound care and both active and passive immunization. Local wound care is essential and may greatly decrease the risk of rabies virus infection. Wound care should not be delayed, even if the initiation of immunization is postponed pending the results of the 10-day observation period. All bite wounds and scratches should be washed thoroughly with soap and water. Devitalized tissues should be debrided, tetanus prophylaxis given, and antibiotic treatment initiated whenever indicated.
All previously unvaccinated persons (but not those who have previously been immunized) should be passively immunized with rabies immune globulin (RIG). If RIG is not immediately available, it should be administered no later than 7 days after the first vaccine dose. After day 7, endogenous antibodies are being produced, and passive immunization may actually be counterproductive. If anatomically feasible, the entire dose of RIG (20 IU/kg) should be infiltrated at the site of the bite; otherwise, any RIG remaining after infiltration of the bite site should be administered IM at a distant site. With multiple or large wounds, the RIG preparation may need to be diluted in order to obtain a sufficient volume for adequate infiltration of all wound sites. If the exposure involves a mucous membrane, the entire dose should be administered IM. Rabies vaccine and RIG should never be administered at the same site or with the same syringe. Commercially available RIG in the United States is purified from the serum of hyperimmunized human donors. These human RIG preparations are much better tolerated than are the equine-derived preparations still in use in some countries (see below). Serious adverse effects of human RIG are uncommon. Local pain and low-grade fever may occur.
Two purified inactivated rabies vaccines are available for rabies PEP in the United States. They are highly immunogenic and remarkably safe compared with earlier vaccines. Four 1-mL doses of rabies vaccine should be given IM in the deltoid area. (The anterolateral aspect of the thigh also is acceptable in children.) Gluteal injections, which may not always reach muscle, should not be given and have been associated with rare vaccine failures. Ideally, the first dose should be given as soon as possible after exposure; failing that, it should be given without further delay. The three additional doses should be given on days 3, 7, and 14; a fifth dose on day 28 is no longer recommended. Pregnancy is not a contraindication for immunization. Glucocorticoids and other immunosuppressive medications may interfere with the development of active immunity and should not be administered during PEP unless they are essential. Routine measurement of serum neutralizing antibody titers is not required, but titers should be measured 2–4 weeks after immunization in immunocompromised persons. Local reactions (pain, erythema, edema, and pruritus) and mild systemic reactions (fever, myalgias, headache, and nausea) are common; anti-inflammatory and antipyretic medications may be used, but immunization should not be discontinued. Systemic allergic reactions are uncommon, but anaphylaxis does occur rarely and can be treated with epinephrine and antihistamines. The risk of rabies development should be carefully considered before the decision is made to discontinue vaccination because of an adverse reaction.
![]() Most of the burden of rabies PEP is borne by persons with the fewest resources. In addition to the rabies vaccines discussed above, vaccines grown in either primary cell lines (hamster or dog kidney) or continuous cell lines (Vero cells) are satisfactory and are available in many countries outside the United States. Less expensive vaccines derived from neural tissues are still used in a diminishing number of developing countries; however, these vaccines are associated with serious neuroparalytic complications, including postinfectious encephalomyelitis and Guillain-Barré syndrome. The use of these vaccines should be discontinued as soon as possible, and progress has been made in this regard. Worldwide, >10 million individuals receive postexposure rabies vaccine each year.
Most of the burden of rabies PEP is borne by persons with the fewest resources. In addition to the rabies vaccines discussed above, vaccines grown in either primary cell lines (hamster or dog kidney) or continuous cell lines (Vero cells) are satisfactory and are available in many countries outside the United States. Less expensive vaccines derived from neural tissues are still used in a diminishing number of developing countries; however, these vaccines are associated with serious neuroparalytic complications, including postinfectious encephalomyelitis and Guillain-Barré syndrome. The use of these vaccines should be discontinued as soon as possible, and progress has been made in this regard. Worldwide, >10 million individuals receive postexposure rabies vaccine each year.
If human RIG is unavailable, purified equine RIG can be used in the same manner at a dose of 40 IU/kg. Before the administration of equine RIG, hypersensitivity should be assessed by intradermal testing with a 1:10 dilution. The incidence of anaphylactic reactions and serum sickness has been low with recent equine RIG products.
Preexposure Rabies Vaccination Preexposure rabies prophylaxis should be considered for people with an occupational or recreational risk of rabies exposures, including certain travelers to rabies-endemic areas. The primary schedule consists of three doses of rabies vaccine given on days 0, 7, and 21 or 28. Serum neutralizing antibody tests help determine the need for subsequent booster doses. When a previously immunized individual is exposed to rabies, two booster doses of vaccine should be administered on days 0 and 3. Wound care remains essential. As stated above, RIG should not be administered to previously vaccinated persons.
OTHER RHABDOVIRUSES
OTHER LYSSAVIRUSES
![]() A growing number of lyssaviruses other than rabies virus have been discovered to infect bat populations in Europe, Africa, Asia, and Australia. Six of these viruses have produced a very small number of cases of a human disease indistinguishable from rabies: European bat lyssaviruses 1 and 2, Australian bat lyssavirus, Irkut virus, and Duvenhage virus. Mokola virus, a lyssavirus that has been isolated from shrews with an unknown reservoir species in Africa, may also produce human disease indistinguishable from rabies.
A growing number of lyssaviruses other than rabies virus have been discovered to infect bat populations in Europe, Africa, Asia, and Australia. Six of these viruses have produced a very small number of cases of a human disease indistinguishable from rabies: European bat lyssaviruses 1 and 2, Australian bat lyssavirus, Irkut virus, and Duvenhage virus. Mokola virus, a lyssavirus that has been isolated from shrews with an unknown reservoir species in Africa, may also produce human disease indistinguishable from rabies.
VESICULAR STOMATITIS VIRUS
Vesicular stomatitis is a viral disease of cattle, horses, pigs, and some wild mammals. Vesicular stomatitis virus is a member of the genus Vesiculovirus in the family Rhabdoviridae. Outbreaks of vesicular stomatitis in horses and cattle occur sporadically in the southwestern United States. The animal infection is associated with severe vesiculation and ulceration of oral tissues, teats, and feet and may be clinically indistinguishable from the more dangerous foot-and-mouth disease. Epidemics are usually seasonal, typically beginning in the late spring, and are probably due to arthropod vectors. Direct animal-to-animal spread can also occur, although the virus cannot penetrate intact skin. Transmission to humans usually results from direct contact with infected animals (particularly cattle) and occasionally follows laboratory exposure. In human disease, early conjunctivitis is followed by an acute influenza-like illness with fever, chills, nausea, vomiting, headache, retrobulbar pain, myalgias, substernal pain, malaise, pharyngitis, and lymphadenitis. Small vesicular lesions may be present on the buccal mucosa or on the fingers. Encephalitis is very rare. The illness usually lasts 3–6 days, with complete recovery. Subclinical infections are common. A serologic diagnosis can be made on the basis of a rise in titer of complement-fixing or neutralizing antibodies. Therapy is symptom-based.
233 |
Arthropod-Borne and Rodent-Borne Virus Infections |
This chapter summarizes the major features of selected arthropod-borne and rodent-borne viruses. Numerous viruses of this category are transmitted in nature among animals without ever infecting humans. Other viruses incidentally infect humans, but only a proportion of these viruses induce human disease. In addition, certain viral agents are regularly introduced into human populations or spread among humans by certain arthropods (specifically, insects and ticks) or by chronically infected rodents. These zoonotic viruses are taxonomically diverse and therefore differ fundamentally from one another in terms of virion morphology, replication strategies, genomic organization, and genome sequence. While a virus’s classification in a taxon is enlightening with regard to natural maintenance strategies, sensitivity to antiviral agents, and particular aspects of pathogenesis, the classification does not necessarily predict which clinical signs and symptoms (if any) the virus will cause in humans. Zoonotic viruses are evolving, and “new” zoonotic viruses are regularly discovered. The epizootiology and epidemiology of zoonotic viruses continue to change as a result of environmental alterations affecting vectors, reservoirs, wildlife, livestock, and humans. Zoonotic viruses are most numerous in the tropics but are also found in temperate and even frigid climates. The distribution and seasonal activity of a zoonotic virus may vary, and the rate at which it changes is likely to depend largely on ecologic conditions (e.g., rainfall and temperature), which can affect the density of virus vectors and reservoirs and the development of infection.
Arthropod-borne viruses (arboviruses) infect their vectors after ingestion of a blood meal from a viremic, usually nonhuman vertebrate; some arthropods may also become infected by saliva-activated transmission. The arthropod vectors then develop chronic, systemic infection as the viruses penetrate the gut and spread throughout the body to the salivary glands; such virus dissemination, referred to as extrinsic incubation, typically lasts 1–3 weeks in mosquitoes. At this point, if the salivary glands become involved, the arthropod vector is competent to continue the chain of transmission by infecting a vertebrate during a subsequent blood meal. An alternative mechanism for virus maintenance in its arthropod vector is transovarial transmission. The arthropod generally is unharmed by the infection, and the natural vertebrate partner usually has only transient viremia with no overt disease.
Rodent-borne viruses are maintained in nature by transmission between rodents, which become chronically infected. Usually a high degree of rodent–virus specificity is observed, and overt disease in the reservoir host is rare.
ETIOLOGY
Arthropod-borne and rodent-borne zoonotic viruses belong to at least seven families: Arenaviridae, Bunyaviridae, Flaviviridae, Orthomyxoviridae, Reoviridae, Rhabdoviridae, and Togaviridae (Table 233-1).
|
ZOONOTIC ARTHROPOD- AND RODENT-BORNE VIRUSES THAT INFECT HUMANS |
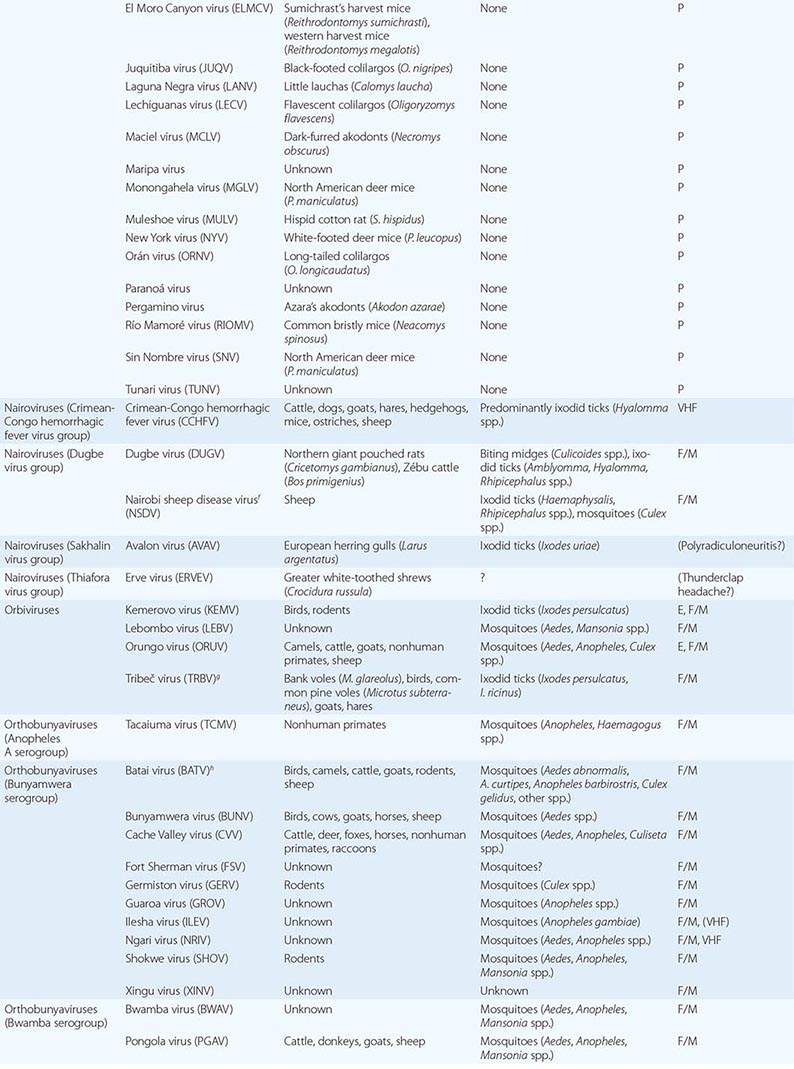
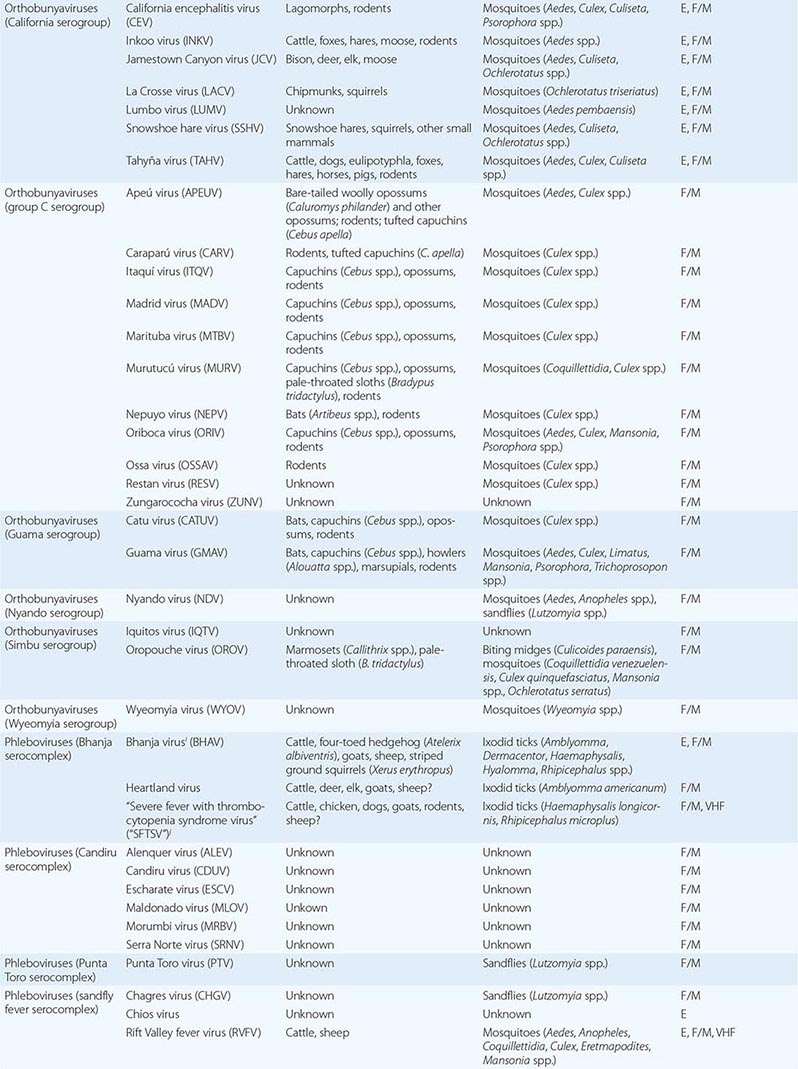
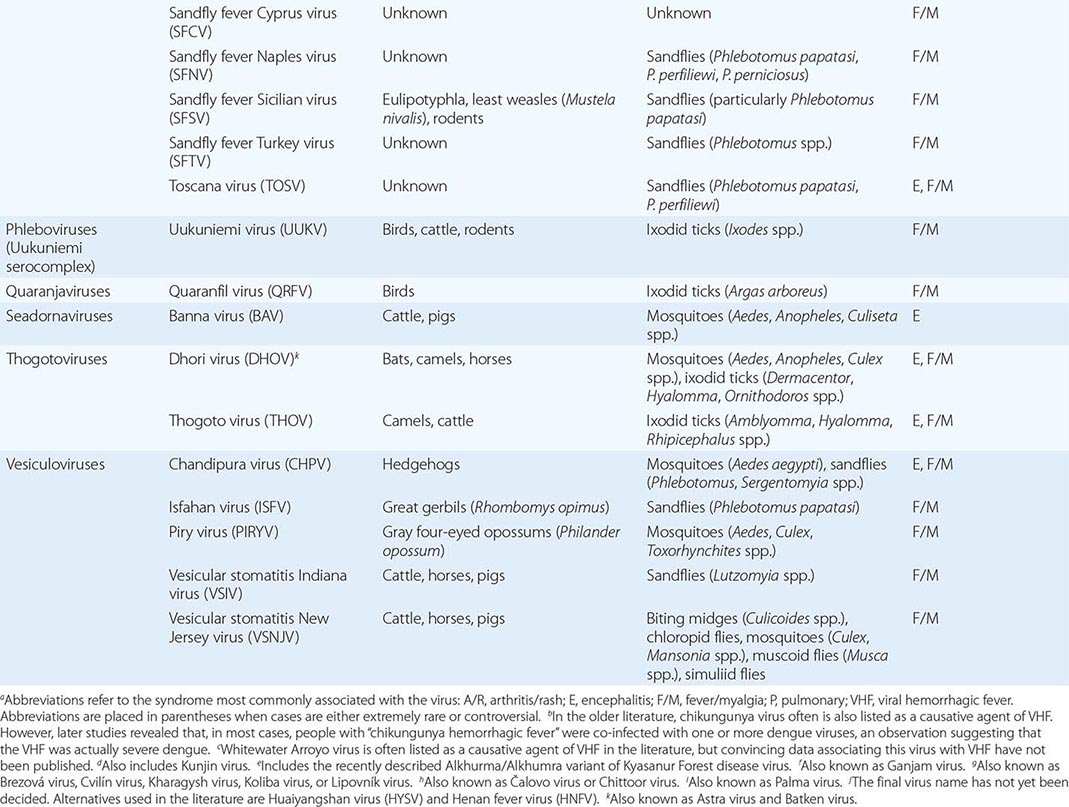
ARENAVIRIDAE
The members of the family Arenaviridae that infect humans are all assigned to the genus Arenavirus. The members of this genus are divided into two main phylogenetic branches: Old World viruses (the Lassa–lymphocytic choriomeningitis serocomplex) and New World viruses (the Tacaribe serocomplex). Human arenaviruses form spherical, oval, or pleomorphic enveloped and spiked virions (~50–300 nm in diameter) that bud from the infected cell’s plasma membrane. The particles contain two genomic single-stranded RNAs (S, ~3.5 kb; and L, ~7.5 kb) encoding structural proteins in an ambisense orientation. Most arenaviruses persist in nature by chronically infecting rodents. The Old World viruses are maintained by murid rodents that often are persistently viremic and commonly transmit viruses vertically and horizontally. New World viruses are found in cricetid rodents; horizontal transmission is typical, vertical infection may occur, and persistent viremia may be observed. Strikingly, each arenavirus is predominantly adapted to one particular type of rodent. Humans usually become infected through inhalation of or direct contact with infected rodent excreta or secreta (e.g., aerosols of rodents in harvesting machines; aerosolized dried rodent urine or feces in barns or houses; direct contact with rodents in traps). Person-to-person transmission of arenaviruses is uncommon.
BUNYAVIRIDAE
The family Bunyaviridae includes four medically significant genera: Hantavirus, Nairovirus, Orthobunyavirus, and Phlebovirus. The members of all these genera form spherical to pleomorphic enveloped virions containing three genomic single-stranded RNAs (S, ~1–2 kb; M, 3.6–5.3 kb; and L, 6.4–12.3 kb) of negative (hantaviruses, nairoviruses, orthobunyaviruses) or ambisense (nairoviruses) polarity. Bunyaviruses mature into particles ~80–120 nm in diameter in the Golgi complex of infected cells and exit these cells by exocytosis.
Hantaviruses are unique among the bunyaviruses in that they are not transmitted by arthropods but instead are maintained in nature by rodents that chronically shed virions. Old World hantaviruses are harbored by murid and cricetid rodents, and New World hantaviruses are maintained by cricetid rodents. As with arenaviruses, individual hantaviruses usually are specifically adapted to a particular type of rodent. However, hantaviruses do not cause chronic viremia in their rodent hosts and are transmitted only horizontally from rodent to rodent. Similar to arenaviruses, hantaviruses infect humans primarily through inhalation of or direct contact with rodent excreta or secreta, and person-to-person transmission is not a common event (with the notable exception of Andes virus). Although there is overlap, the human Old World hantaviruses usually are the etiologic agents of hemorrhagic fever with renal syndrome, whereas the New World viruses usually cause hantavirus pulmonary syndrome.
Nairoviruses are maintained by ixodid ticks, which vertically (transovarially and transstadially) transmit these viruses to progeny tick generations and horizontally spread them through viremic vertebrate hosts. Humans are usually infected via a tick bite or during handling of infected vertebrates.
Orthobunyaviruses are largely mosquito-borne and rarely midge-borne and have viremic vertebrate intermediate hosts. Many orthobunyaviruses are also transovarially transmitted in their mosquito host. Numerous orthobunyaviruses have been associated with human infection and disease. They have been considered to be members of ~19 serogroups based on antigenic cross-reactions, but this grouping is currently undergoing revision with the accumulation of new genomic data and phylogenetic analyses. Human viruses are found in at least nine serogroups.
Phleboviruses are transmitted vertically (transovarially) in their arthropod hosts and horizontally through viremic vertebrate hosts. Phleboviruses are divided into two groups: the phlebotomus group viruses are transmitted by sandflies and the Uukuniemi group viruses by ticks. Phleboviruses are assigned to at least 10 serocomplexes; human pathogens are found in at least four of these serocomplexes.
FLAVIVIRIDAE
The family Flaviviridae currently includes four genera, one of which (Flavivirus) comprises arthropod-borne human viruses. Flaviviruses sensu stricto have single-stranded positive-sense RNA genomes (~11 kb) and form spherical enveloped particles 40–60 nm in diameter. The flaviviruses discussed here belong to two phylogenetically and antigenically distinct groups and are transmitted among vertebrates by mosquitoes and ixodid ticks, respectively. Vectors are usually infected when they feed on viremic hosts; as in the case of most other viruses discussed here, humans are accidental hosts who usually are infected by arthropod bites. Arthropods maintain flavivirus infections horizontally, although transovarial transmission has been documented. Under certain circumstances, flaviviruses can also be transmitted by aerosols or via contaminated food products; in particular, raw milk can transmit tick-borne encephalitis virus.
ORTHOMYXOVIRIDAE
The family Orthomyxoviridae includes two genera of medically relevant arthropod-borne viruses: Quaranjavirus and Thogotovirus. Quaranjaviruses are transmitted among birds by ixodid ticks, whereas thogotoviruses have a predilection for mammalian host reservoirs and can be transmitted by both ixodid ticks and mosquitoes.
REOVIRIDAE
The family Reoviridae contains viruses with linear, multisegmented, double-stranded RNA genomes (~16–29 kb in total). These viruses produce particles that have icosahedral symmetry and are 60–80 nm in diameter. In contrast to all other virions discussed here, reovirions are not enveloped and thus are insensitive to detergent inactivation. Fifteen genera of reoviruses are currently recognized. Human arthropod-borne viruses are found among the genera Coltivirus (subfamily Spinareovirinae), Orbivirus, and Seadornavirus (subfamily Sedoreovirinae). Arthropod-borne coltiviruses possess 12 genome segments. Coltiviruses are transmitted by numerous tick types transstadially but not transovarially. Overall maintenance of the transmission cycle, therefore, involves viremic mammalian hosts infected by tick bites. Arthropod-borne orbiviruses have 10 genome segments and are transmitted by mosquitoes or ixodid ticks, whereas relevant seadornaviruses have 12 genome segments and are transmitted exclusively by mosquitoes.
RHABDOVIRIDAE
The family Rhabdoviridae is included in the order Mononegavirales. Viruses of the nine rhabdovirus genera have linear, nonsegmented, single-stranded RNA genomes of negative polarity (~11–15 kb) and form bullet-shaped to pleomorphic enveloped particles (100–430 nm long and 45–100 nm wide). Only the genus Vesiculovirus includes human arthropod-borne viruses, all of which are transmitted by insects (biting midges, mosquitoes, and sandflies). The general properties of rhabdoviruses are discussed in more detail in Chap. 232.
TOGAVIRIDAE
The members of the family Togaviridae have linear, single- and positive-stranded RNA genomes (~9.7–11.8 kb) and form enveloped icosahedral virions (~60–70 nm in diameter) that bud from the plasma membrane of the infected cell. The togaviruses discussed here are all members of the genus Alphavirus and are transmitted among vertebrates by mosquitoes.
EPIDEMIOLOGY
The distributions of arthropod-borne and rodent-borne viruses are restricted by the areas inhabited by their reservoir hosts and/or vectors. Consequently, a patient’s geographic origin or travel history can provide important clues in the differential diagnosis. Table 233-2 lists the approximate geographic distribution of most arthropod-borne and rodent-borne infections. Many of these diseases can be acquired in either rural or urban settings; these diseases include yellow fever, dengue (previously called dengue fever), severe dengue (previously called dengue hemorrhagic fever and dengue shock syndrome), chikungunya virus disease, hemorrhagic fever with renal syndrome caused by Seoul virus, sandfly fever caused by sandfly fever Naples and Sicilian viruses, and Oropouche virus disease.
|
GEOGRAPHIC DISTRIBUTION (UNITED NATIONS GEOSCHEME) OF ZOONOTIC ARTHROPOD-BORNE OR RODENT-BORNE VIRAL DISEASES |
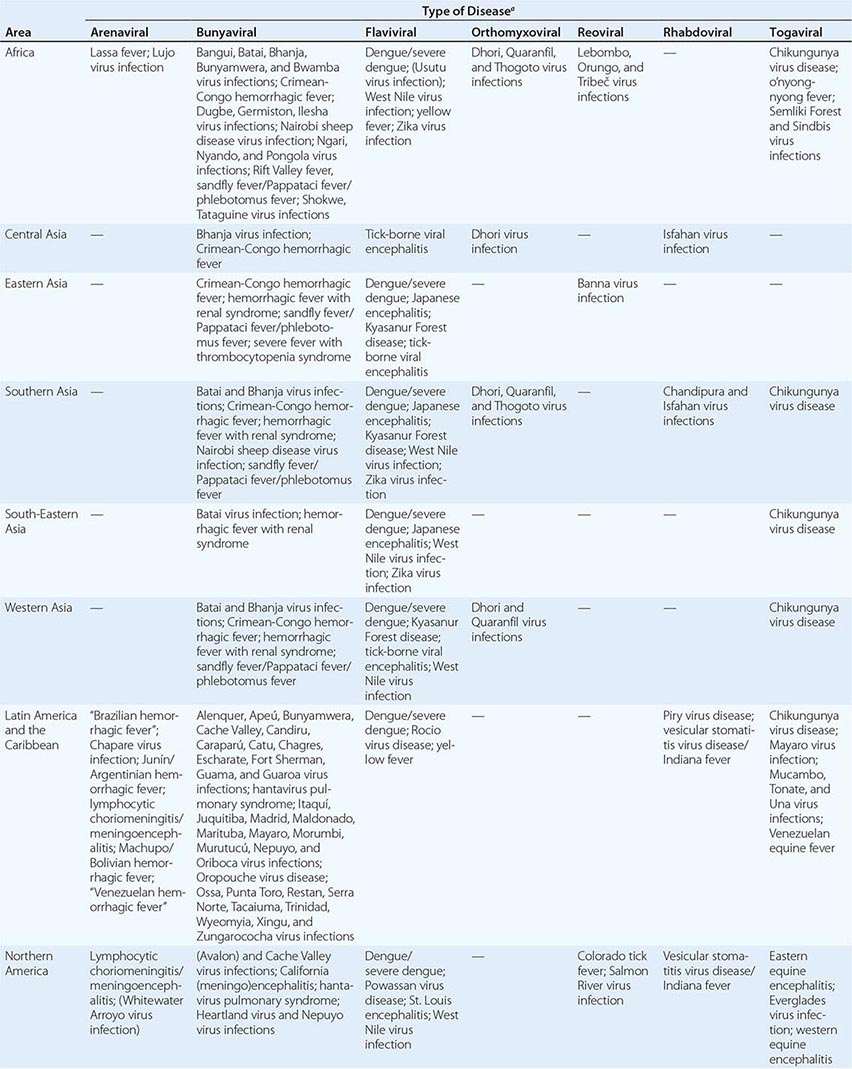
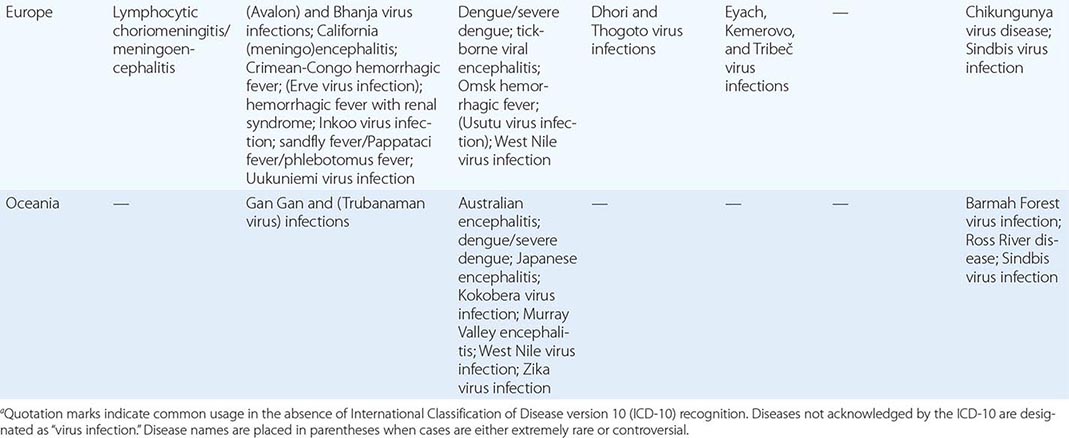
DIAGNOSIS
In patients with suspected viral infection, a recognized history of mosquito bite has little diagnostic significance, but a history of tick bite is more diagnostically useful. Exposure to rodents is sometimes reported by persons infected with arenaviruses or hantaviruses. Laboratory diagnosis is required in all cases, although epidemics occasionally provide enough clinical and epidemiologic clues for a presumptive etiologic diagnosis. For most arthropod-borne and rodent-borne viruses, acute-phase serum samples (collected within 3 or 4 days of onset) have yielded isolates. Paired serum samples have been used to demonstrate rising antibody titers. Intensive efforts to develop rapid tests for viral hemorrhagic fevers have resulted in reliable antigen-detection enzyme-linked immunosorbent assays (ELISAs), IgM-capture ELISAs, and multiplex polymerase chain reaction (PCR) assays. These tests can provide a diagnosis based on a single serum sample within a few hours and are particularly useful in patients with severe disease. More sensitive reverse-transcription PCR (RT-PCR) assays may yield diagnoses based on samples without detectable antigen and may also provide useful genetic information about the etiologic agent.
Hantavirus infections differ from other viral infections discussed here in that severe acute disease is immunopathologic; patients present with serum IgM that serves as the basis for a sensitive and specific test. At diagnosis, patients with encephalitides generally are no longer viremic or antigenemic and usually do not have virions in cerebrospinal fluid (CSF). In this situation, the value of serologic methods for IgM determination and RT-PCR is high. IgM-capture ELISA is increasingly being used for the simultaneous testing of serum and CSF. IgG ELISA or classic serology is useful in the evaluation of past exposure to viruses, many of which circulate in areas with minimal medical infrastructures and sometimes cause only mild or subclinical infection.
SYNDROMES
The spectrum of possible human responses to infection with arthropod- or rodent-borne viruses is wide, and knowledge of the outcome of most of these infections is limited. People infected with these viruses may not develop signs of illness. If viral disease is recognized, it can usually be grouped into one of five broad categories: arthritis and rash, encephalitis, fever and myalgia, pulmonary disease, or viral hemorrhagic fever (VHF) (Table 233-3). These categories often overlap. For example, infections with West Nile and Venezuelan equine encephalitis viruses are discussed here as encephalitides, but during epidemics many patients present with much milder febrile syndromes. Similarly, Rift Valley fever virus is best known as a cause of VHF, but the attack rates for febrile disease are far higher, and encephalitis and blindness occasionally occur as well. Lymphocytic choriomeningitis virus is classified here as a cause of fever and myalgia because this syndrome is the most common disease manifestation; even when central nervous system (CNS) disease evolves during infection with this virus, neural manifestations are usually mild and are preceded by fever and myalgia. Infection with any dengue virus type (1, 2, 3, or 4) is considered as a cause of fever and myalgia because this syndrome is by far the most common manifestation worldwide. However, severe dengue is a VHF with a complicated pathogenesis that is of tremendous importance in pediatric practice in certain areas of the world. Unfortunately, most of the known arthropod- or rodent-borne viral diseases have not been studied in detail with modern medical approaches; thus available data may be incomplete or biased. The reader must be aware that data on geographic distribution are often fuzzy: the literature frequently is not clear as to whether the data pertain to the distribution of a particular virus or the areas where human disease has been observed. In addition, the designations for viruses and viral diseases have changed multiple times over decades. Here, virus and taxon names are in line with the latest reports of the International Committee on Taxonomy of Viruses, and disease names are largely in accordance with the World Health Organization’s International Classification of Disease version 10 (ICD-10) and more recent updates.
|
CLINICAL SYNDROMES CAUSED BY ZOONOTIC ARTHROPOD-BORNE OR RODENT-BORNE VIRUSES |
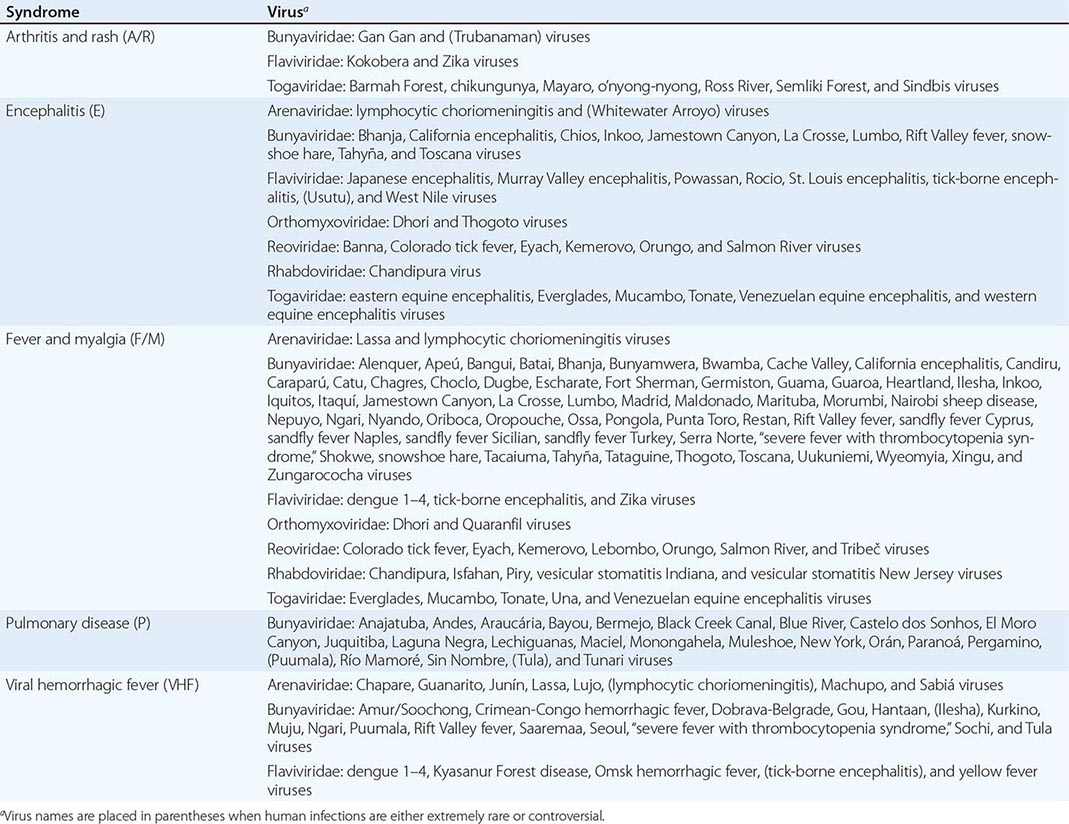
ARTHRITIS AND RASH
Arthritides are common accompaniments of several viral diseases, such as hepatitis B, parvovirus B19 infection, and rubella, and occasionally accompany infection due to adenoviruses, enteroviruses, herpesviruses, and mumps virus. Two ungrouped bunyaviruses, Gan Gan virus and Trubanaman virus, and the flavivirus Kokobera virus have been associated with single cases of polyarthritic disease. Arthropod-borne alphaviruses are also common causes of arthritides—usually acute febrile diseases accompanied by the development of a maculopapular rash. Rheumatic involvement includes arthralgia alone, periarticular swelling, and (less commonly) joint effusions. Most alphavirus infections are less severe and have fewer articular manifestations in children than in adults. In temperate climates, these ailments are summer diseases. No specific therapies or licensed vaccines exist. The most important alphavirus arthritides are Barmah Forest virus infection, chikungunya virus disease, Ross River disease, and Sindbis virus infection. A large (>2 million cases), albeit isolated, epidemic was caused by o’nyong nyong virus in 1959–1961 (o’nyong nyong fever). Mayaro, Semliki Forest, and Una viruses have caused isolated cases or limited and infrequent epidemics (30 to several hundred cases per year) in the past. Signs and symptoms of infections with these viruses often are similar to those observed with chikungunya virus disease.
Chikungunya Virus Disease Disease caused by chikungunya virus is endemic in rural areas of Africa. Intermittent epidemics take place in towns and cities of both Africa and Asia. Aedes aegypti mosquitoes are the usual vectors for the disease in urban areas. In 2004, a massive epidemic began in the Indian Ocean region (in particular on the islands of Réunion and Mauritius) and was most likely spread by travelers; Aedes albopictus was identified as the major vector of chikungunya virus during that epidemic. Between 2013 and 2014, several thousand chikungunya virus infections were reported (and several tens of thousands of cases were suspected) from Caribbean islands. The virus was imported to Italy, France, and the United States by travelers from the Caribbean. Chikungunya virus poses a threat to the continental United States as suitable vector mosquitoes are present in the southern states. The disease is most common among adults, in whom the clinical presentation may be dramatic. The abrupt onset of chikungunya virus disease follows an incubation period of 2–10 days. Fever (often severe) with a saddleback pattern and severe arthralgia are accompanied by chills and constitutional symptoms and signs, such as abdominal pain, anorexia, conjunctival injection, headache, nausea, and photophobia. Migratory polyarthritis mainly affects the small joints of the ankles, feet, hands, and wrists, but the larger joints are not necessarily spared. Rash may appear at the outset or several days into the illness; its development often coincides with defervescence, which occurs around day 2 or 3 of the disease. The rash is most intense on the trunk and limbs and may desquamate. Young children develop less prominent signs and are therefore less frequently hospitalized. Children also often develop a bullous rather than a maculopapular/petechial rash. Maternal–fetal transmission has been reported and in some cases has led to fetal death. Recovery may require weeks, and some elderly patients may continue to experience joint pain, recurrent effusions, or stiffness for several years. This persistence of signs and symptoms may be especially common in HLA-B27-positive patients. In addition to arthritis, petechiae are occasionally seen and epistaxis is not uncommon, but chikungunya virus should not be considered a VHF agent. A few patients develop leukopenia. Elevated concentrations of aspartate aminotransferase (AST) and C-reactive protein have been described, as have mildly decreased platelet counts. Treatment of chikungunya virus disease relies on nonsteroidal anti-inflammatory drugs and sometimes chloroquine for refractory arthritis.
Barmah Forest Virus Infection and Ross River Disease Barmah Forest virus and Ross River virus cause diseases that are indistinguishable on clinical grounds alone (hence the previously common disease designation epidemic polyarthritis for both infections). Ross River virus has caused epidemics in Australia, Papua New Guinea, and the South Pacific since the beginning of the twentieth century and continues to be responsible for ~4800 cases of disease in rural and suburban areas annually. In 1979–1980, the virus swept through the Pacific Islands, causing more than 500,000 infections. Ross River virus is predominantly transmitted by Aedes normanensis, Aedes vigilax, and Culex annulirostris. Wallabies and rodents are probably the main vertebrate hosts. Barmah Forest virus infections have been on the rise in recent years. In 2005–2006, roughly 2000 cases were recorded in Australia. Barmah Forest virus is transmitted by both Aedes and Culex mosquitoes and has been isolated from biting midges. The vertebrate hosts remain to be determined, but serologic studies implicate horses and possums.
Of the human Barmah Forest and Ross River virus infections surveyed, 55–75% were asymptomatic; however, these viral diseases can be debilitating. The incubation period is 7–9 days; the onset of illness is sudden, and disease is usually ushered in by disabling symmetrical joint pain. A nonitchy, diffuse, maculopapular rash (more common in Barmah Forest virus infection) generally develops coincidentally or follows shortly, but in some patients it can precede joint pains by several days. Constitutional symptoms such as low-grade fever, asthenia, headache, myalgia, and nausea are not prominent or are absent in many patients. Most patients are incapacitated for considerable periods (≥6 months) by joint involvement, which interferes with grasping, sleeping, and walking. Ankle, interphalangeal, knee, metacarpophalangeal, and wrist joints are most often involved, although elbows, shoulders, and toes may also be affected. Periarticular swelling and tenosynovitis are common, and one-third of patients have true arthritis (more common in Ross River disease). Myalgia and nuchal stiffness may accompany joint pains. Only half of all patients with arthritis can resume normal activities within 4 weeks, and 10% still must limit their activity after 3 months. Occasional patients are symptomatic for 1–3 years but without progressive arthropathy.
In the diagnosis of either infection, clinical laboratory values are normal or variable. Tests for rheumatoid factor and antinuclear antibodies are negative, and the erythrocyte sedimentation rate is acutely elevated. Joint fluid contains 1000–60,000 mononuclear cells/μL, and viral antigen can usually be detected in macrophages. IgM antibodies are valuable in the diagnosis of this infection, although such antibodies occasionally persist for years. Isolation of the virus from blood after mosquito inoculation or growth of the virus in cell culture is possible early in the illness. Because of the great economic impact of annual epidemics in Australia, an inactivated Ross River virus vaccine is under development. Nonsteroidal anti-inflammatory drugs such as naproxen or acetylsalicylic acid are effective for treatment.
Sindbis Virus Infection Sindbis virus is transmitted among birds by infected mosquitoes. Infections with northern European or southern African variants are particularly likely in rural environments. After an incubation period of <1 week, Sindbis virus infection begins with rash and arthralgia. Constitutional clinical signs are not marked, and fever is modest or lacking altogether. The rash, which lasts ~1 week, begins on the trunk, spreads to the extremities, and evolves from macules to papules that often vesiculate. The arthritis is multiarticular, migratory, and incapacitating, with resolution of the acute phase in a few days; the ankles, elbows, knees, phalangeal joints, wrists, and—to a much lesser extent—proximal and axial joints are involved. Persistence of joint pain and occasionally of arthritis is a major problem and may continue for months or even years despite lack of deformities.
Zika Virus Infection Zika virus is an emerging pathogen that is transmitted among nonhuman primates and humans by Aedes mosquitoes. Human infections are usually benign and are most likely misdiagnosed as dengue or influenza. Zika virus infection is characterized by influenza-like clinical signs, including fever, headaches, and malaise. A maculopapular rash, conjunctivitis, myalgia, and arthralgia usually accompany or follow those manifestations. Zika virus infection was first documented in Africa in 1947 and was later recognized in southeastern and southern Asia. In recent years, the number of Zika virus infections reported from Micronesia and Polynesia has increased steadily.
ENCEPHALITIS
The major encephalitis viruses are found in the families Bunyaviridae, Flaviviridae, Rhabdoviridae, and Togaviridae. However, individual agents of other families, including Dhori virus and thogotovirus (Orthomyxoviridae) as well as Banna virus (Reoviridae), have been known to cause isolated cases of encephalitis as well. Arboviral encephalitides are seasonal diseases, commonly occurring in the warmer months. Their incidence varies markedly with time and place, depending on ecologic factors. The causative viruses differ substantially in terms of case–infection ratio (i.e., the ratio of clinical to subclinical infections), lethality rate, and residual disease. Humans are not important amplifiers of these viruses.
All the viral encephalitides discussed in this section have a similar pathogenesis. An infected arthropod ingests blood from a human and thereby initiates infection. The initial viremia is thought to originate from the lymphoid system. Viremia leads to multifocal entry into the CNS, presumably through infection of olfactory neuroepithelium, with passage through the cribriform plate; “Trojan horse” entry with infected macrophages; or infection of brain capillaries. During the viremic phase, there may be little or no recognizable disease except in tick-borne flavivirus encephalitides, which may manifest with clearly delineated phases of fever and systemic illness.
CNS lesions arise partly from direct neuronal infection and subsequent damage and partly from edema, inflammation, and other indirect effects. The usual pathologic features of arboviral encephalitides are focal necroses of neurons, inflammatory glial nodules, and perivascular lymphoid cuffing. Involved areas display the “luxury perfusion” phenomenon, with normal or increased total blood flow and low oxygen extraction. The typical patient presents with a prodrome of nonspecific constitutional signs and symptoms, including fever, abdominal pain, sore throat, and respiratory signs. Headache, meningeal signs, photophobia, and vomiting follow quickly. The severity of human infection varies from an absence of signs/symptoms to febrile headache, aseptic meningitis, and full-blown encephalitis. The proportions and severity of these manifestations vary with the infecting virus. Involvement of deeper brain structures in less severe cases may be signaled by lethargy, somnolence, and intellectual deficit (as disclosed by the mental status examination). More severely affected patients are obviously disoriented and may become comatose. Tremors, loss of abdominal reflexes, cranial nerve palsies, hemiparesis, monoparesis, difficulty swallowing, limb-girdle syndrome, and frontal lobe signs are all common. Spinal and motor neuron diseases are documented after West Nile and Japanese encephalitis virus infections. Seizures and focal signs may be evident early or may appear during the course of the disease. Some patients present with an abrupt onset of fever, convulsions, and other signs of CNS involvement. The acute encephalitis usually lasts from a few days to as long as 2–3 weeks. The infections may be fatal, or recovery may be slow, with weeks or months required for the return of maximal recoupable function, or incomplete, with persisting long-term deficits. Difficulty concentrating, fatigability, tremors, and personality changes are common during recovery.
The diagnosis of arboviral encephalitides depends on the careful evaluation of a febrile patient with CNS disease and the performance of laboratory studies to determine etiology. Clinicians should (1) consider empirical acyclovir treatment for herpesvirus meningoencephalitis and antibiotic treatment for bacterial meningitis until test results are received; (2) exclude intoxination and metabolic or oncologic causes, including paraneoplastic syndromes, hyperammonemia, liver failure, and anti-NMDA receptor encephalitis; and (3) rule out a brain abscess or a stroke. Leptospirosis, neurosyphilis, Lyme disease, cat-scratch disease, and more recently described viral encephalitides (e.g., Nipah virus infection) should be considered if epidemiologically relevant. CSF examination usually shows a modest increase in leukocyte counts—in the tens or hundreds or perhaps a few thousand. Early in the process, a significant proportion of these leukocytes may be polymorphonuclear, but mononuclear cells are usually predominant later. CSF glucose concentrations are generally normal. There are exceptions to this pattern of findings: in eastern equine encephalitis, for example, polymorphonuclear leukocytes may predominate during the first 72 h of disease and hypoglycorrhachia may be detected. In lymphocytic choriomeningitis/meningoencephalitis, lymphocyte counts may be in the thousands, and the glucose concentration may be diminished. A humoral immune response is usually detectable at or near the onset of disease. Both serum (acute- or convalescent-phase) and CSF should be examined for IgM antibodies and viruses by plaque-reduction neutralization assay and/or (RT)-PCR. Virus generally cannot be isolated from blood or CSF, although Japanese encephalitis virus has been recovered from CSF of patients with severe disease. RT-PCR analysis of CSF may yield positive results. Viral antigen is present in brain tissue, although its distribution may be focal. Electroencephalography usually shows diffuse abnormalities and is not directly helpful.
Experience with medical imaging is still evolving. Both computed tomography (CT) and magnetic resonance imaging (MRI) scans may be normal except for evidence of preexisting conditions or occasional diffuse edema. Imaging is generally nonspecific in that most patients do not present with pathognomonic lesions, but it can be used to rule out other suspected causes of disease. It is important to remember that imaging may yield negative results if done early in the disease course but later may detect lesions. For example, eastern equine encephalitis (focal abnormalities) and severe Japanese encephalitis (hemorrhagic bilateral thalamic lesions) have caused lesions detectable by medical imaging.
Comatose patients may require management of intracranial pressure elevations, inappropriate secretion of antidiuretic hormone, respiratory failure, or seizures. Specific therapies for these viral encephalitides are not available. The only practical preventive measures are vector management and personal protection against the arthropod transmitting the virus. For Japanese encephalitis or tick-borne viral encephalitis, vaccination should be considered in certain circumstances (see relevant sections below).
Bunyaviruses: California (Meningo)encephalitis The isolation of California encephalitis virus established California serogroup orthobunyaviruses as causes of encephalitides. However, California encephalitis virus has been implicated in only a very few cases of encephalitis, whereas its close relative, La Crosse virus, is the major cause of encephalitis in this serogroup (~70 cases per year in the United States). California (meningo)encephalitis due to La Crosse virus infection is most commonly reported from the upper Midwest of the United States but is also found in other areas of the central and eastern parts of the country, most often in West Virginia, Tennessee, North Carolina, and Georgia. The serogroup includes 13 other viruses, some of which (e.g., Inkoo, Jamestown Canyon, Lumbo, snowshoe hare, and Tahyña viruses) also cause human disease. Transovarial transmission is a strong component of transmission of the California serogroup viruses in Aedes and Ochlerotatus mosquitoes. The mosquito vector of La Crosse virus is Ochlerotatus triseriatus. In addition to transovarial transmission, acquisition through feeding on viremic chipmunks and other mammals as well as venereal transmission can result in infection of this mosquito. O. triseriatus breeds in sites such as tree holes and abandoned tires and bites during daylight hours. The habits of this mosquito correlate with the risk factors for human cases: recreation in forested areas, residence at a forest’s edge, and the presence of water-containing abandoned tires around the home. Intensive environmental modification based on these findings has reduced the incidence of disease in a highly endemic area in the U.S. Midwest.
Most humans are infected from July through September. The Asian tiger mosquito (A. albopictus) efficiently transmits La Crosse virus to mice and also transmits the agent transovarially in the laboratory; this aggressive anthropophilic mosquito has the capacity to urbanize, and its possible impact on transmission of virus to humans is of concern. The prevalence of antibody to La Crosse virus in humans is ≥20% in endemic areas, a figure indicating that infection is common but often asymptomatic. CNS disease has been recognized primarily in children <15 years of age.
The illness from La Crosse virus varies from aseptic meningitis accompanied by confusion to severe and occasionally fatal encephalitis (lethality rate, <0.5%). The incubation period is ~3–7 days. Although there may be prodromal symptoms/signs, the onset of CNS disease is sudden, with fever, headache, and lethargy often joined by nausea and vomiting, convulsions (in one-half of patients), and coma (in one-third of patients). Focal seizures, hemiparesis, tremor, aphasia, chorea, Babinski signs, and other evidence of significant neurologic dysfunction are common, but residual disease is not. Approximately 10% of patients have recurrent seizures in the succeeding months. Other serious sequelae of La Crosse virus infection are rare, although a decrease in scholastic standing in children has been reported and mild personality change has occasionally been suggested.
The blood leukocyte count is commonly elevated in patients with La Crosse virus infection, sometimes reaching 20,000/μL, and is usually accompanied by a left shift. CSF leukocyte counts are typically 30–500/μL with a mononuclear cell predominance (although 25–90% of cells are polymorphonuclear in some patients). The blood protein concentration is normal or slightly increased, and the glucose concentration is normal. Specific virologic diagnosis based on IgM-capture assays of serum and CSF is efficient. The only human anatomic site from which virus has been isolated is the brain.
Treatment is supportive over a 1- to 2-week acute phase during which status epilepticus, cerebral edema, and inappropriate secretion of antidiuretic hormone are important concerns. A phase 2B clinical trial of IV ribavirin in children with La Crosse virus infection was discontinued during dose escalation because of adverse effects.
Jamestown Canyon virus has been implicated in several cases of encephalitis in adults, usually with a significant respiratory illness at onset. Human infection with this virus has been documented in New York, Wisconsin, Ohio, Michigan, Ontario, and other areas of North America where the vector mosquito, Aedes stimulans, feeds on its main host, the white-tailed deer. Tahyña virus can be found in central Europe, Russia, China, and Africa. The virus is a prominent cause of febrile disease but can also cause pharyngitis, pulmonary syndromes, aseptic meningitis, or meningoencephalitis.
Flaviviruses The most important flavivirus encephalitides are Japanese encephalitis, St. Louis encephalitis, tick-borne encephalitis, and West Nile virus infection. Australian encephalitis (Murray Valley encephalitis) and Rocio virus infection resemble Japanese encephalitis but are documented only occasionally in Australia and Brazil, respectively. Powassan virus has caused ~50 cases of often-severe disease (lethality rate, ~10%), frequently occurring among children in eastern Canada and the United States. Usutu virus has caused only individual cases of human infection, but such infections may be underdiagnosed.
JAPANESE ENCEPHALITIS Japanese encephalitis is the most important viral encephalitis in Asia. Each year 35,000–50,000 cases and more than 15,000 deaths are reported. Japanese encephalitis virus is found throughout Asia, including far eastern Russia, Japan, China, India, Pakistan, and southeastern Asia, and causes occasional epidemics on western Pacific islands. The virus has been detected in the Torres Strait islands, and five human encephalitis cases have been identified on the nearby Australian mainland. The virus is particularly common in areas where irrigated rice fields attract the natural avian vertebrate hosts and provide abundant breeding sites for mosquitoes such as Culex tritaeniorhynchus, which transmit the virus to humans. Additional amplification by pigs, which suffer abortion, and horses, which develop encephalitis, may be significant as well. Vaccination of these additional amplifying hosts may reduce the transmission of the virus.
Clinical signs of Japanese encephalitis emerge after an incubation period of 5–15 days and range from an unspecific febrile presentation (nausea, vomiting, diarrhea, cough) to aseptic meningitis, meningoencephalitis, acute flaccid paralysis, and severe encephalitis. Common findings are cerebellar signs, cranial nerve palsies, and cognitive and speech impairments. A Parkinsonian presentation and seizures are typical in severe cases. Effective vaccines are available. Vaccination is indicated for summer travelers to rural Asia, where the risk of acquiring Japanese encephalitis is considered to be about 1 per 5000 to 1 per 20,000 travelers per week if travel duration exceeds 3 weeks. Usually two intramuscular doses of the vaccine are given 28 days apart, with the second dose administered at least 1 week prior to travel.
ST. LOUIS ENCEPHALITIS St. Louis encephalitis virus is transmitted between mosquitoes and birds. This virus causes a low-level endemic infection among rural residents of the western and central United States, where Culex tarsalis is the vector (see “Western Equine Encephalitis,” below). The more urbanized mosquitoes Culex pipiens and Culex quinquefasciatus have been responsible for epidemics resulting in hundreds or even thousands of cases in cities of the central and eastern United States. Most cases occur in June through October. The urban mosquitoes breed in accumulations of stagnant water and sewage with high organic content and readily feed on humans in and around houses at dusk. The elimination of open sewers and trash-filled drainage systems is expensive and may not be possible, but screening of houses and implementation of personal protective measures may be an effective approach to the prevention of infection. The rural mosquito vector is most active at dusk and outdoors; its bites can be avoided by modification of activities and use of repellents.
Disease severity increases with age. St. Louis encephalitis virus infections that result in aseptic meningitis or mild encephalitis are concentrated among children and young adults, while severe and fatal cases primarily affect the elderly. Infection rates are similar in all age groups; thus the greater susceptibility of older persons to disease is a biologic consequence of aging. St. Louis encephalitis has an abrupt onset after an incubation period of 4–21 days, sometimes following a prodrome, and begins with fever, lethargy, confusion, and headache. In addition, nuchal rigidity, hypotonia, hyperreflexia, myoclonus, and tremors are common. Severe cases can include cranial nerve palsies, hemiparesis, and seizures. Patients often report dysuria and may have viral antigen in urine as well as pyuria. The overall rate of lethality is generally ~7% but may reach 20% among patients >60 years of age. Recovery is slow. Emotional lability, difficulties with concentration and memory, asthenia, and tremors are commonly prolonged in older convalescent patients. The CSF of patients with St. Louis encephalitis usually contains tens to hundreds of leukocytes, with a lymphocytic predominance and a left shift. The CSF glucose concentration is normal in these patients.
TICK-BORNE VIRAL ENCEPHALITIS Tick-borne encephalitis viruses are currently subdivided into four groups: the western/European subtype (previously called central European encephalitis virus), the (Ural-) Siberian subtype (previously called Russian spring-summer encephalitis virus), the Far Eastern subtype, and the louping ill subtype (previously called louping ill virus or, in Japan, Negishi virus). Small mammals and grouse, deer, and sheep are the vertebrate amplifiers for these viruses, which are transmitted by ticks. The risk of infection varies by geographic area and can be highly localized within a given area; human infections usually follow either outdoor activities resulting in tick bites or consumption of raw (unpasteurized) milk from infected goats or, less commonly, from other infected animals (cows, sheep). Milk seems to represent the main transmission route for louping ill viruses, which cause disease only very rarely. The western/European subtype viruses are transmitted mainly by Ixodes ricinus from Scandinavia to the Ural Mountains. (Ural-)Siberian viruses are transmitted predominantly by Ixodes persulcatus from Europe across the Ural Mountains to the Pacific Ocean; louping ill viruses seem to be confined primarily to Great Britain. Several thousand infections with tick-borne encephalitis virus are recorded each year among people of all ages. Human tick-borne viral encephalitis occurs between April and October, with a peak in June and July.
Western/European viruses classically caused bimodal disease. After an incubation period of 7–14 days, the illness begins with a fever–myalgia phase (arthralgia, fever, headaches, myalgia, nausea) that lasts for 2–4 days and is thought to correlate with viremia. A subsequent remission for several days is followed by the recurrence of fever and the onset of meningeal signs. The CNS phase (7–10 days before onset of improvement) varies from mild aseptic meningitis, which is more common among younger patients, to severe (meningo-)encephalitis with coma, seizures, tremors, and motor signs. Spinal and medullary involvement can lead to typical limb-girdle paralysis and respiratory paralysis. Most patients with western/European virus infections recover (lethality rate, 1%), and only a minority of patients have significant deficits. However, the lethality rate from (Ural-)Siberian virus infections reaches 7–8%.
Infections with Far Eastern viruses generally run a more abrupt course. The encephalitic syndrome caused by these viruses sometimes begins without a remission from the fever–myalgia phase and has more severe manifestations than the western/European syndrome. The lethality rate is high (20–40%), and major sequelae—most notably, lower motor neuron paralyses of the proximal muscles of the extremities, trunk, and neck—are common, developing in approximately one-half of patients. Thrombocytopenia sometimes develops during the initial febrile illness, resembling the early hemorrhagic phase of some other tick-borne flavivirus infections, such as Kyasanur Forest disease. In the early stage of the illness, virus may be isolated from the blood. In the CNS phase, IgM antibodies are detectable in serum and/or CSF.
Diagnosis of tick-borne viral encephalitis primarily relies on serology and detection of viral genomes by RT-PCR. There is no specific therapy for infection. However, effective alum-adjuvanted, formalin-inactivated virus vaccines are produced in Austria, Germany, and Russia in chicken embryo cells (FSME-Immun® and Encepur®). Two doses of the Austrian vaccine separated by an interval of 1–3 months appear to be effective in the field, and antibody responses are similar when vaccine is given on days 0 and 14. Because rare cases of postvaccination Guillain-Barré syndrome have been reported, vaccination should be reserved for persons likely to experience rural exposure in an endemic area during the season of transmission. Cross-neutralization for the western/European and Far Eastern variants has been established, but there are no published field studies on cross-protection among formalin-inactivated vaccines.
Because 0.2–4% of ticks in endemic areas may be infected, the use of immunoglobulin prophylaxis of tick-borne viral encephalitis has been raised. Prompt administration of high-titered specific antibody preparations should probably be undertaken, although no controlled data are available to prove the efficacy of this measure. Immunoglobulins should be considered because of the risk of antibody-mediated enhancement of infection or antigen–antibody complex deposition in tissues.
WEST NILE VIRUS INFECTION West Nile virus is now the primary cause of arboviral encephalitis in the United States. In 2012, 2873 cases of neuroinvasive disease (e.g., meningitis, encephalitis, acute flaccid paralysis), with 270 deaths, and 2801 cases of non-neuroinvasive infection were reported. West Nile virus was initially described as being transmitted among wild birds by Culex mosquitoes in Africa, Asia, and southern Europe. In addition, the virus has been implicated in severe and fatal hepatic necrosis in Africa. West Nile virus was introduced into New York City in 1999 and subsequently spread to other areas of the northeastern United States, causing die-offs among crows, exotic zoo birds, and other birds. The virus has continued to spread and is now found in almost all states as well as in Canada, Mexico, South America, and the Caribbean islands. C. pipiens remains the major vector in the northeastern United States, but several other Culex species and A. albopictus are also involved. Jays compete with crows and other corvids as amplifiers and lethal targets in other areas of the country.
West Nile virus is a common cause of febrile disease without CNS involvement (incubation period, 3–14 days), but it occasionally causes aseptic meningitis and severe encephalitis, particularly among the elderly. The fever–myalgia syndrome caused by West Nile virus differs from that caused by other viruses in terms of the frequent—rather than occasional—appearance of a maculopapular rash concentrated on the trunk (especially in children) and the development of lymphadenopathy. Back pain, fatigue, headache, myalgia, retroorbital pain, sore throat, nausea and vomiting, and arthralgia (but not arthritis) are common accompaniments that may persist for several weeks. Encephalitis, sequelae, and death are all more common among elderly, diabetic, and hypertensive patients and among patients with previous CNS insults. In addition to the more severe motor and cognitive sequelae, milder findings may include tremor, slight abnormalities in motor skills, and loss of executive functions. Intense clinical interest and the availability of laboratory diagnostic methods have made it possible to define a number of unusual clinical features. Such features include chorioretinitis, flaccid paralysis with histologic lesions resembling poliomyelitis, and initial presentation with fever and focal neurologic deficits in the absence of diffuse encephalitis. Immunosuppressed patients may have fulminant courses or develop persistent CNS infection. Virus transmission through both transplantation and blood transfusion has necessitated screening of blood and organ donors by nucleic acid–based tests. Occasionally, pregnant women infect their fetuses with West Nile virus.
Rhabdoviruses: Chandipura Virus Infection Chandipura virus seems to be an emerging and increasingly important human virus in India, where it is transmitted among hedgehogs by mosquitoes and sandflies. In humans, the disease begins as an influenza-like illness, with fever, headache, abdominal pain, nausea, and vomiting; these manifestations are followed by neurologic impairment and infection-related or autoimmune-mediated encephalitis. Chandipura virus infection is characterized by high lethality in children. Several hundred cases of infection are recorded in India every year. Infections with other arthropod-borne rhabdoviruses (Isfahan, Piry, vesicular stomatitis Indiana, vesicular stomatitis New Jersey) may imitate the early febrile stage of Chandipura virus infection.
Togaviruses • EASTERN EQUINE ENCEPHALITIS This disease is encountered primarily in swampy foci along the eastern coast of the United States, with a few inland foci as far removed as Michigan. Infected humans present for medical care from June through October. During this period, the bird–Culiseta mosquito cycle spills over into other mosquitoes such as Aedes sollicitans or Aedes vexans, which are more likely to feed on mammals. There is concern over the potential role of the introduced anthropophilic mosquito species A. albopictus, which has been found to be infected with eastern equine encephalitis virus and is an effective experimental vector in the laboratory. Horses are a common target for the virus. Contact with unvaccinated horses may be associated with human disease, but horses probably do not play a significant role in amplification of the virus.
Eastern equine encephalitis is one of the most destructive of the arboviral diseases, with a sudden onset after an incubation period of ~5–10 days, rapid progression, 50–75% lethality, and frequent sequelae in survivors. This severity is reflected in the extensive necrotic lesions and polymorphonuclear infiltrates found at postmortem examination of the brain. Acute polymorphonuclear CSF pleocytosis, often occurring during the first 1–3 days of disease, is another indication of severity. In addition, leukocytosis with a left shift is a common feature. A formalin-inactivated vaccine has been used to protect laboratory workers but is not generally available or applicable.
VENEZUELAN EQUINE ENCEPHALITIS Venezuelan equine encephalitis viruses are separated into epizootic viruses (subtypes IA/B and IC) and enzootic viruses (subtypes ID, IE, and IF). Closely related enzootic viruses are Everglades virus, Mucambo virus, and Tonate virus. Enzootic viruses are found primarily in humid tropical-forest habitats and are maintained between culicoid mosquitoes and rodents. These viruses cause human disease but are not pathogenic for horses and do not cause epizootics. Enzootic viruses are common causes of acute febrile disease. Everglades virus has caused encephalitis in humans in Florida. Extrapolation from the rate of genetic change suggests that Everglades virus may have been introduced into Florida <200 years ago. Everglades virus is most closely related to the ID subtype viruses that appear to have given evolutionary rise to the epizootic variants active in South America.
Epizootic viruses have an unknown natural cycle but periodically cause extensive epizootics/epidemics in equids and humans in the Americas. These epizootics/epidemics are the result of high-level viremia in horses and mules, which transmit the infection to several types of mosquitoes. Infected mosquitoes in turn infect humans and perpetuate virus transmission. Humans also have high-level viremia, but their role in virus transmission is unclear. Epizootics of Venezuelan equine fever occurred repeatedly in South America at intervals of ≤10 years from the 1930s until 1969, when a massive epizootic spread throughout Central America and Mexico, reaching southern Texas in 1971. Genetic sequencing suggested that the virus from that outbreak originated from residual “un-inactivated” IA/B subtype virus in veterinary vaccines. The outbreak was terminated in Texas with a live attenuated vaccine (TC-83) originally developed for human use by the U.S. Army; the epizootic virus was then used for further production of inactivated veterinary vaccines. No further epizootic disease was identified until 1995, when additional epizootics took place in Colombia, Venezuela, and Mexico. The viruses involved in these epizootics as well as previously epizootic IC viruses are close phylogenetic relatives of known enzootic ID viruses. This finding suggests that active evolution and selection of epizootic viruses are under way in South America.
During epizootics, extensive human infection is the rule, with clinical disease in 10–60% of infected individuals. Most infections result in notable acute febrile disease, while relatively few infections (5–15%) result in neurologic disease. A low rate of CNS invasion is supported by the absence of encephalitis among the many infections resulting from exposure to aerosols in the laboratory setting or from vaccination accidents. The most recent large epizootic of Venezuelan equine fever occurred in Colombia and Venezuela in 1995; of the more than 85,000 clinical cases, 4% (with a higher proportion among children than adults) included neurologic symptoms/signs, and 300 cases ended in death.
The prevention of epizootic Venezuelan equine fever depends on vaccination of horses with the attenuated TC-83 vaccine or with an inactivated vaccine prepared from that variant. Enzootic viruses are genetically and antigenically different from epizootic viruses, and protection against the former with vaccines prepared from the latter is relatively ineffective. Humans can be protected by immunization with similar vaccines prepared from Everglades virus, Mucambo virus, and Venezuelan equine encephalitis virus, but the use of the vaccines is restricted to laboratory personnel because of reactogenicity, possible fetal pathogenicity, and limited availability.
WESTERN EQUINE ENCEPHALITIS The primary maintenance cycle of western equine encephalitis virus in the United States is between C. tarsalis and birds, principally sparrows and finches. Equids and humans become infected, and both suffer encephalitis without amplifying the virus in nature. St. Louis encephalitis virus is transmitted in a similar cycle in the same regions harboring western equine encephalitis virus; disease caused by the former occurs about a month earlier than that caused by the latter (July through October). Large epidemics of western equine encephalitis occurred in the western and central United States and Canada during the 1930s through 1950s, but in recent years the disease has been uncommon. From 1964 through 2010, only 640 cases were reported in the United States. This decline in incidence may reflect in part the integrated approach to mosquito management that has been employed in irrigation projects and the increasing use of agricultural pesticides. The decreased incidence of western equine encephalitis almost certainly reflects the increased tendency for humans to be indoors behind closed windows at dusk—the peak biting period by the major vector.
After an incubation period of ~5–10 days, western equine encephalitis virus causes a typical diffuse viral encephalitis, with an increased attack rate and increased morbidity among the young, particularly children <2 years old. In addition, the lethality rate is high among the young and the very elderly (3–7% overall). One-third of individuals who have convulsions during the acute illness have subsequent seizure activity. Infants <1 year old—particularly those in the first months of life—are at serious risk of motor and intellectual damage. Twice as many males as females develop clinical encephalitis after 5–9 years of age. This difference in incidence may be related to greater outdoor exposure of boys to the vector but may also be due in part to biologic differences. A formalin-inactivated vaccine has been used to protect laboratory workers but is not generally available.
FEVER AND MYALGIA
The fever and myalgia syndrome is most commonly associated with zoonotic virus infection. Many of the numerous viruses listed in Table 233-1 probably cause at least a few cases of this syndrome, but only some of these viruses have prominent associations with the syndrome and are of biomedical importance. The fever and myalgia syndrome typically begins with the abrupt onset of fever, chills, intense myalgia, and malaise. Patients may also report joint or muscle pains, but true arthritis is not found. Anorexia is characteristic and may be accompanied by nausea or even vomiting. Headache is common and may be severe, with photophobia and retroorbital pain. Physical findings are minimal and are usually confined to conjunctival injection with pain on palpation of muscles or the epigastrium. The duration of symptoms/signs is quite variable (generally 2–5 days), with a biphasic course in some instances. The spectrum of disease varies from subclinical to temporarily incapacitating. Less constant findings include a nonpruritic maculopapular rash. Epistaxis may occur but does not necessarily indicate a bleeding diathesis. A minority of the patients may develop aseptic meningitis. This diagnosis is difficult to make in remote areas, given patients’ photophobia and myalgia as well as the lack of opportunity to examine the CSF. Although pharyngitis or radiographic evidence of pulmonary infiltrates is found in some patients, the agents causing this syndrome are not primary respiratory pathogens.
The differential diagnosis includes anicteric leptospirosis, rickettsial diseases, and the early stages of other syndromes discussed in this chapter. The fever and myalgia syndrome is often described as “influenza-like,” but the usual absence of cough and coryza makes influenza an unlikely confounder except at the earliest stages. Treatment is supportive, but acetylsalicylic acid is avoided because of the potential for exacerbated bleeding or Reye’s syndrome. Complete recovery is the general outcome for people with this syndrome, although prolonged asthenia and nonspecific symptoms have been described in some patients, particularly after infection with lymphocytic choriomeningitis virus or dengue virus types 1–4.
Efforts at prevention of viral infection are best based on vector control, which, however, may be expensive or impossible. For mosquito control, destruction of breeding sites is generally the most economically and environmentally sound approach. Emerging containment technologies include the release of genetically modified mosquitoes and the spread of Wolbachia bacteria to limit mosquito multiplication rates. Depending on the vector and its habits, other possible approaches include the use of screens or other barriers (e.g., permethrin-impregnated bed nets) to prevent the vector from entering dwellings, judicious application of arthropod repellents such as N,N,-diethyltoluamide (DEET) to the skin, wearing of long-sleeved and ideally permethrin-impregnated clothing, and avoidance of the vectors’ habitats and times of peak activity.
Arenaviruses Lymphocytic choriomeningitis/meningoencephalitis is the only human arenavirus infection resulting predominantly in fever and myalgia. Lymphocytic choriomeningitis virus is transmitted to humans from the common house mouse (Mus musculus) by aerosols of excreta and secreta. The virus is maintained in the mouse mainly by vertical transmission from infected dams. The vertically infected mouse remains viremic and sheds virus for life, with high concentrations of virus in all tissues. Infected colonies of pet hamsters also can serve as a link to humans. Infections among scientists and animal caretakers can occur because the virus is widely used in immunology laboratories as a model of T cell function and can silently infect cell cultures and passaged tumor lines. In addition, patients may have a history of residence in rodent-infested housing or other exposure to rodents. An antibody prevalence of ~5–10% has been reported among adults from Argentina, Germany, and the United States.
Lymphocytic choriomeningitis/meningoencephalitis differs from the general syndrome of fever and myalgia in that the onset is gradual. Conditions occasionally associated with the disease are orchitis, transient alopecia, arthritis, pharyngitis, cough, and maculopapular rash. An estimated one-fourth of patients (or fewer) experience a febrile phase of 3–6 days. After a brief remission, many develop renewed fever accompanied by severe headache, nausea and vomiting, and meningeal signs lasting for ~1 week (the CNS phase). These patients virtually always recover fully, as do the rare patients with clear-cut signs of encephalitis. Recovery may be delayed by transient hydrocephalus. During the initial febrile phase, leukopenia and thrombocytopenia are common, and virus can usually be isolated from blood. During the CNS phase, the virus may be found in the CSF, and antibodies are present in the blood. The pathogenesis of lymphocytic choriomeningitis/meningoencephalitis is thought to resemble that following direct intracranial inoculation of the virus into adult mice. The onset of the immune response leads to T cell–mediated immunopathologic meningitis. During the meningeal phase, CSF mononuclear-cell counts range from the hundreds to the low thousands per microliter, and hypoglycorrhachia is found in one-third of patients.
IgM-capture ELISA, immunochemistry, and RT-PCR are used in the diagnosis of lymphocytic choriomeningitis/meningoencephalitis. IgM-capture ELISA of serum and CSF usually yields positive results; RT-PCR assays have been developed for probing CSF. Because patients who have fulminant infections transmitted by recent organ transplantation do not mount an immune response, immunohistochemistry or RT-PCR is required for diagnosis. Infection should be suspected in acutely ill febrile patients with marked leukopenia and thrombocytopenia. In patients with aseptic meningitis, any of the following suggests lymphocytic choriomeningitis/meningoencephalitis: a well-marked febrile prodrome, adult age, occurrence in the autumn, low CSF glucose levels, or CSF mononuclear-cell counts of >1000/μL. In pregnant women, infection may lead to fetal invasion with consequent congenital hydrocephalus and chorioretinitis. Because the maternal infection may be mild, causing only a short febrile illness, antibodies to the virus should be sought in both the mother and the fetus under suspicious circumstances, particularly in TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes simplex, and HIV)–negative neonatal hydrocephalus.
Bunyaviruses Numerous bunyaviruses cause fever and myalgia. Many of these viruses cause individual infections and usually do not result in epidemics—e.g., the viruses of the orthobunyavirus Anopheles A serogroup (e.g., Tacaiuma virus), Bwamba serogroup (Bwamba virus, Pongola virus), Guama serogroup (Catu virus, Guama virus), Nyando serogroup (Nyando virus), and Wyeomyia serogroup (Wyeomyia virus); the unclassified bunyavirus Tataguine virus; the phlebovirus Bhanja complex (Bhanja virus, Heartland virus) and Candiru complex (Alenquer, Candiru, Escharate, Maldonado, Morumbi, and Serra Norte viruses); the hantavirus Choclo virus; and the Dugbe and Nairobi sheep disease nairoviruses. In the relevant orthobunyaviral Bunyamwera serogroup (Bunyamwera, Batai, Cache Valley, Fort Sherman, Germiston, Guaroa, Ilesha, Ngari, Shokwe, and Xingu viruses), Ngari virus recently has been implicated in a large epidemic in Africa.
ORTHOBUNYAVIRUS GROUP C SEROGROUP Apeú, Caraparú, Itaquí, Madrid, Marituba, Murutucú, Nepuyo, Oriboca, Ossa, Restan, and Zungarococha viruses are among the most common causes of arboviral infection in humans entering South American jungles. These viruses cause acute febrile disease and are transmitted by mosquitoes in neotropical forests.
ORTHOBUNYAVIRUS SIMBU SEROGROUP Oropouche virus is transmitted in Central and South America by a biting midge, Culicoides paraensis, which often breeds to high density in cacao husks and other vegetable detritus found in towns and cities. Explosive epidemics involving thousands of patients have been reported from several towns in Brazil and Peru. Rash and aseptic meningitis have been detected in a number of patients. Iquitos virus, a recently discovered reassortant and close relative of Oropouche virus, causes disease that is easily mistaken for Oropouche virus disease; its overall epidemiologic significance remains to be determined.
PHLEBOVIRUS SANDFLY FEVER SEROGROUP A previous designation for sandfly fever, “3-day fever,” instructively describes the brief debilitating course associated with this essentially benign infection. There is neither a rash nor CNS involvement, and complete recovery is the rule. Sandfly fever is caused by at least six distinct phleboviruses of the phlebovirus sandfly fever serocomplex (Chagres virus, sandfly fever Cyprus virus, sandfly fever Naples virus, sandfly fever Sicilian virus, sandfly fever Turkey virus, and Toscana virus). Sandfly fever Naples virus, sandfly fever Sicilian virus, and Toscana viruses are the most important human pathogens of this group. Phlebotomus sandflies transmit the viruses, probably among small mammals, and infect humans by bites. Female sandflies may be infected by the oral route as they take a blood meal and may transmit the virus to offspring when they lay their eggs after a second blood meal. This prominent transovarial transmission confounds virus control.
Sandfly fever is found in the circum-Mediterranean area, extending to the east through the Balkans into parts of China as well as into western Asia. Chagres virus is endemic in Panama. Sandflies are found in both rural and urban settings and are known for their short flight ranges and their small sizes; the latter enables them to penetrate standard mosquito screens and netting. Epidemics have been described in the wake of natural disasters and wars. After World War II, extensive spraying in parts of Europe to control malaria greatly reduced sandfly populations and sandfly fever Naples virus transmission; the incidence of sandfly fever continues to be low.
A common pattern of disease in endemic areas consists of high attack rates among travelers and military personnel and little or no disease in the local population, who are protected after childhood infection. Toscana virus infection is common during the summer among rural residents and vacationers, particularly in Italy, Spain, and Portugal; a number of cases have been identified in travelers returning to Germany and Scandinavia. The disease may manifest as an uncomplicated febrile illness but is often associated with aseptic meningitis, with virus isolated from the CSF.
Punta Toro virus is a phlebovirus that is not part of the sandfly fever serocomplex but that, like the members of this complex, is transmitted by sandflies. Punta Toro virus causes a sandfly fever–like disease in the Latin American tropical forest, where the vectors rest on tree buttresses. Epidemics have not been reported, but antibody prevalence among inhabitants of villages in endemic areas indicates a cumulative lifetime exposure rate of >50%.
Flaviviruses The most clinically important flaviviruses that cause the fever and myalgia syndrome are dengue viruses 1–4. In fact, dengue is probably the most important arthropod-borne viral disease worldwide, with 50–100 million infections occurring per year. Year-round transmission of dengue viruses 1–4 occurs between latitudes of 25°N and 25°S, but seasonal forays of the viruses into the United States and Europe have been documented. All four viruses have A. aegypti as their principal vector. Through increasing spread of mosquitoes throughout the tropics and subtropics and international travel of infected humans, large areas of the world have become vulnerable to the introduction of dengue viruses. Thus, dengue and severe dengue (see “Viral Hemorrhagic Fevers,” below) are becoming increasingly common. For instance, conditions favorable to dengue virus 1–4 transmission via A. aegypti exist in Hawaii and the southern United States. The range of a lesser dengue virus vector, A. albopictus, now extends from Asia to the United States, the Indian Ocean, parts of Europe, and Hawaii. A. aegypti typically breeds near human habitation, using relatively fresh water from sources such as water jars, vases, discarded containers, coconut husks, and old tires. The mosquito usually inhabits dwellings and bites during the day. Bursts of dengue cases are to be expected in the southern United States, particularly along the Mexican border, where containers of water may be infested with A. aegypti. Closed habitations with air-conditioning may inhibit transmission of many arboviruses, including dengue viruses 1–4.
Dengue begins after an incubation period averaging 4–7 days, when the typical patient experiences the sudden onset of fever, frontal headache, retroorbital pain, and back pain along with severe myalgias. These symptoms gave rise to the colloquial designation of dengue as “break-bone fever.” Often a transient macular rash appears on the first day, as do adenopathy, palatal vesicles, and scleral injection. The illness may last a week, with additional symptoms and clinical signs usually including anorexia, nausea or vomiting, and marked cutaneous hypersensitivity. Near the time of defervescence on days 3–5, a maculopapular rash begins on the trunk and spreads to the extremities and the face. Epistaxis and scattered petechiae are often noted in uncomplicated dengue, and preexisting gastrointestinal lesions may bleed during the acute illness.
Laboratory findings of dengue include leukopenia, thrombocytopenia, and, in many cases, elevations of serum aminotransferase concentrations. The diagnosis is made by IgM ELISA or paired serology during recovery or by antigen-detection ELISA or RT-PCR during the acute phase. Virus is readily isolated from blood in the acute phase if mosquito inoculation or mosquito cell culture is used.
Reoviruses Several orbiviruses (Lebombo, Kemerovo, Orungo, and Tribeč viruses) and coltiviruses (Colorado tick fever, Eyach, and Salmon River viruses) can cause fever and myalgia in humans. With the exception of Lebombo and Orungo viruses, all of these viruses are transmitted by ticks. The most important reoviral arthropod-borne disease is Colorado tick fever. Several hundred patients with this disease are reported annually in the United States. The infection is acquired between March and November through the bite of an infected ixodid tick, the Rocky Mountain wood tick (Dermacentor andersoni), in mountainous western regions at altitudes of 1200–3000 m. Small mammals serve as amplifying hosts. The most common presentation is fever and myalgia; meningoencephalitis is not uncommon, and hemorrhagic disease, pericarditis, myocarditis, orchitis, and pulmonary presentations have also been reported. Rash develops in a minority of patients. Leukopenia and thrombocytopenia are also noted. The disease usually lasts 7–10 days and is often biphasic. The most important differential diagnostic considerations since the beginning of the twentieth century have been Rocky Mountain spotted fever (although Colorado tick fever is much more common in Colorado) and tularemia. Colorado tick fever virus replicates for several weeks in erythropoietic cells and can be found in erythrocytes. This feature, detected in erythroid smears stained by immunofluorescence, can be diagnostically helpful and is important during screening of blood donors.
PULMONARY DISEASE
Hantavirus pulmonary syndrome (HPS) was first described in 1993, but retrospective identification of cases by immunohistochemistry (1978) and serology (1959) support the idea that HPS is a recently discovered rather than a truly new disease. The causative agents are hantaviruses of a distinct phylogenetic lineage that is associated with the cricetid rodent subfamily Sigmodontinae. Sin Nombre virus, which chronically infects North American deer mice (Peromyscus maniculatus), is the most important agent of HPS in the United States. Several other related viruses (Anajatuba, Andes, Araraquara, Araucária, Bayou, Bermejo, Black Creek Canal, Blue River, Castelo dos Sonhos, El Moro Canyon, Juquitiba, Laguna Negra, Lechiguanas, Maciel, Monongahela, Muleshoe, New York, Orán, Paranoá, Pergamino, Río Mamoré, and Tunari) cause the disease in North and South America, but Andes virus is unusual in that it has been implicated in human-to-human transmission. HPS particularly affects rural residents living in dwellings permeable to rodent entry or working in occupations that pose a risk of rodent exposure. Each type of rodent has its own particular habits; in the case of deer mice, these behaviors include living in and around human habitation.
HPS begins with a prodrome of ~3–4 days (range, 1–11 days) comprising fever, malaise, myalgia, and—in many cases—gastrointestinal disturbances such as abdominal pain, nausea, and vomiting. Dizziness is common and vertigo occasional. Severe prodromal symptoms/signs may bring some patients to medical attention, but most cases are recognized as the pulmonary phase begins. Typical signs are slightly lowered blood pressure, tachycardia, tachypnea, mild hypoxemia, thrombocytopenia, and early radiographic signs of pulmonary edema. Physical findings in the chest are often surprisingly scant. The conjunctival and cutaneous signs of vascular involvement seen in hantavirus VHFs (see below) are uncommon. During the next few hours, decompensation may progress rapidly to severe hypoxemia and respiratory failure.
The HPS differential diagnosis includes abdominal surgical conditions and pyelonephritis as well as rickettsial disease, sepsis, meningococcemia, plague, tularemia, influenza, and relapsing fever. A specific diagnosis is best made by IgM antibody testing of acute-phase serum, which has yielded positive results even in the prodrome. Tests using a Sin Nombre virus antigen detect antibodies to the related HPS-causing hantaviruses. Occasionally, heterotypic viruses will react only in the IgG ELISA, but such a finding is highly suspicious given the very low seroprevalence of these viruses in normal populations. RT-PCR is usually positive when used to test blood clots obtained in the first 7–9 days of illness and when used to test tissues; this assay is useful in identifying the infecting virus in areas outside the home range of deer mice and in atypical cases.
During the prodrome, the differential diagnosis of HPS is difficult, but by the time of presentation or within 24 h thereafter, a number of diagnostically helpful clinical features become apparent. Cough usually is not present at the outset. Interstitial edema is evident on a chest x-ray. Later, bilateral alveolar edema with a central distribution develops in the setting of a normal-sized heart; occasionally, the edema is initially unilateral. Pleural effusions are often seen. Thrombocytopenia, circulating atypical lymphocytes, and a left shift (often with leukocytosis) are almost always evident; thrombocytopenia is a particularly important early clue. Hemoconcentration, hypoalbuminemia, and proteinuria should also be sought for diagnosis. Although thrombocytopenia virtually always develops and prolongation of the partial thromboplastin time is the rule, clinical evidence for coagulopathy or laboratory indications of disseminated intravascular coagulation (DIC) are found in only a minority of severely ill patients. Patients with severe illness also have acidosis and elevated serum lactate concentrations. Mildly increased values in renal function tests are common, but patients with severe HPS often have markedly elevated serum creatinine concentrations. Some New World hantaviruses other than Sin Nombre virus (e.g., Andes virus) have been associated with more kidney involvement, but few such cases have been studied.
Management of HPS during the first few hours after presentation is critical. The goal is to prevent severe hypoxemia by oxygen therapy, with intubation and intensive respiratory management if needed. During this period, hypotension and shock with increasing hematocrit invite aggressive fluid administration, but this intervention should be undertaken with great caution. Because of low cardiac output with myocardial depression and increased pulmonary vascular permeability, shock should be managed expectantly with pressors and modest infusion of fluid guided by pulmonary capillary wedge pressure. Mild cases can be managed by frequent monitoring and oxygen administration without intubation. Many patients require intubation to manage hypoxemia and developing shock. Extracorporeal membrane oxygenation is instituted in severe cases, ideally before the onset of shock. The procedure is indicated in patients who have a cardiac index of 2.3 L/min/m2 or an arterial oxygen tension/fractional inspired oxygen (PaO2/FIO2) ratio of <50 and who are unresponsive to conventional support. Lethality rates remain at ~30–40% even with good management, but most patients surviving the first 48 h of hospitalization are extubated and discharged within a few days with no apparent long-term residua. The antiviral drug ribavirin inhibits hantaviruses in vitro but did not have a marked effect on patients treated in an open-label study.
VIRAL HEMORRHAGIC FEVER
VHF is a constellation of findings based on vascular instability and decreased vascular integrity. An assault, direct or indirect, on the microvasculature leads to increased permeability and (particularly when platelet function is decreased) to actual disruption and local hemorrhage (a positive tourniquet sign). Blood pressure is decreased, and in severe cases shock supervenes. Cutaneous flushing and conjunctival suffusion are examples of common, observable abnormalities in the control of local circulation. Hemorrhage occurs infrequently. In most patients, hemorrhage is an indication of widespread vascular damage rather than a life-threatening loss of blood volume. In some VHFs, specific organs may be particularly impaired. For instance, the kidneys are primary targets in hemorrhagic fever with renal syndrome (HFRS), and the liver is a primary target in yellow fever and filovirus diseases. However, in all of these diseases, generalized circulatory disturbance is critically important. The pathogenesis of VHF is poorly understood and varies among the viruses regularly implicated in the syndrome. In some viral infections, direct damage to the vascular system or even to parenchymal cells of target organs is an important factor; in other viral infections, soluble mediators are thought to play a major role in the development of hemorrhage or fluid redistribution.
The acute phase in most cases of VHF is associated with ongoing virus replication and viremia. VHFs begin with fever and myalgia, usually of abrupt onset. (Arenavirus infections are the exceptions as they often develop gradually.) Within a few days, the patient presents for medical attention because of increasing prostration that is often accompanied by abdominal or chest pain, anorexia, dizziness, severe headache, hyperesthesia, photophobia, and nausea or vomiting and other gastrointestinal disturbances. Initial examination often reveals only an acutely ill patient with conjunctival suffusion, tenderness to palpation of muscles or abdomen, and borderline hypotension or postural hypotension, perhaps with tachycardia. Petechiae (often best visualized in the axillae), flushing of the head and thorax, periorbital edema, and proteinuria are common. AST concentrations are usually elevated at presentation or within a day or two thereafter. Hemoconcentration from vascular leakage, which is usually evident, is most marked in HFRS and in severe dengue. The seriously ill patient progresses to more severe clinical signs and develops shock and other findings typical of the causative virus. Shock, multifocal bleeding, and CNS involvement (encephalopathy, coma, seizures) are all poor prognostic signs.
One of the major diagnostic clues to VHF is travel to an endemic area within the incubation period for a given syndrome. Except in infections with Seoul, dengue, and yellow fever viruses, which have urban hosts/vectors, travel to a rural setting is especially suggestive of a diagnosis of VHF. In addition, several diseases considered in the differential diagnosis—falciparum malaria, shigellosis, typhoid fever, leptospirosis, relapsing fever, and rickettsial diseases—are treatable and potentially lethal.
Early recognition of VHF is important because of the need for virus-specific therapy and supportive measures. Such measures include prompt, atraumatic hospitalization; judicious fluid therapy that takes into account the patient’s increased capillary permeability; administration of cardiotonic drugs; use of pressors to maintain blood pressure at levels that will support renal perfusion; treatment of the relatively common secondary bacterial (and the more rare fungal) infections; replacement of clotting factors and platelets as indicated; and the usual precautionary measures used in the treatment of patients with hemorrhagic diatheses. DIC should be treated only if clear laboratory evidence of its existence is found and if laboratory monitoring of therapy is feasible; there is no proven benefit of such therapy. The available evidence suggests that VHF patients have decreased cardiac output and will respond poorly to fluid loading as it is often practiced in the treatment of shock associated with bacterial sepsis. Specific therapy is available for several of the VHFs. Strict barrier nursing and other precautions against infection of medical staff and visitors are indicated when VHFs are encountered except when the illness is due to dengue viruses, hantaviruses, Rift Valley fever virus, or yellow fever virus.
Novel VHF-causing agents are still being discovered. Besides the viruses listed below, the latest addition may be the unclassified rhabdovirus Bas-Congo virus, which has been associated with three cases of VHF in the Democratic Republic of the Congo. However, Koch’s postulates have not yet been fulfilled to prove cause and effect.
Arenaviruses The most important arenaviruses causing VHF are Junín virus, Lassa virus, and Machupo virus. Chapare, Guanarito, Lujo, and Sabiá viruses have caused limited and/or infrequent outbreaks or individual cases.
JUNÍN/ARGENTINIAN AND MACHUPO/BOLIVIAN HEMORRHAGIC FEVERS These severe diseases (with fetal lethality rates reaching 15–30%) are caused by Junín virus and Machupo virus, respectively. Their clinical presentations are similar, but their epidemiology differs because of the distribution and behavior of the viruses’ rodent reservoirs. Junín/Argentinian hemorrhagic fever has thus far been recorded only in rural areas of Argentina, whereas Machupo/Bolivian hemorrhagic fever seems to be confined to rural Bolivia. Infection with the causative agents almost always results in disease, and all ages and both sexes are affected. Person-to-person or nosocomial transmission is rare but has occurred. The transmission of Junín/Argentinian hemorrhagic fever from convalescing men to their wives suggests the need for counseling of patients with arenavirus hemorrhagic fever concerning the avoidance of intimate contacts for several weeks after recovery. Compared with the pattern in Lassa fever (see below), thrombocytopenia—often marked—is the rule, hemorrhage is common, and CNS dysfunction (e.g., marked confusion, tremors of the upper extremities and tongue, and cerebellar signs) is much more common in disease caused by Junín virus and Machupo virus. Some cases follow a predominantly neurologic course, with a poor prognosis.
The clinical laboratory is helpful in diagnosis since thrombocytopenia, leukopenia, and proteinuria are typical findings. Junín/Argentinian hemorrhagic fever is readily treated with convalescent-phase plasma given within the first 8 days of illness. In the absence of passive antibody therapy, IV ribavirin in the dose recommended for Lassa fever is likely to be effective in all the South American VHFs caused by arenaviruses. A safe, effective, live attenuated vaccine exists for Junín/Argentinian hemorrhagic fever. After vaccination of more than 250,000 high-risk persons in the endemic area, the incidence of this VHF decreased markedly. In experimental animals, this vaccine is cross-protective against Machupo/Bolivian hemorrhagic fever.
LASSA FEVER Lassa virus is known to cause endemic and epidemic disease in Nigeria, Sierra Leone, Guinea, and Liberia, although it is probably more widely distributed in western Africa. In countries where Lassa virus is endemic, Lassa fever can be a prominent cause of febrile disease. For example, in one hospital in Sierra Leone, laboratory-confirmed Lassa fever is consistently responsible for one-fifth of admissions to the medical wards. In western Africa alone, probably tens of thousands of Lassa virus infections occur annually. Lassa virus can be transmitted by close person-to-person contact. The virus is often present in urine during convalescence and is suspected to be present in seminal fluid early in recovery. Nosocomial spread has occurred but is uncommon if proper sterile parenteral techniques are used. All ages and both sexes are affected; the incidence of disease is highest in the dry season, but transmission takes place year-round.
Among the VHF agents, only arenaviruses are typically associated with a gradual onset of illness, which begins after an incubation period of 5–16 days. Hemorrhage is seen in only ~15–30% of Lassa fever patients; a maculopapular rash is often noted in light-skinned patients. Effusions are common, and male-dominant pericarditis may develop late. Maternal lethality is higher than the usual 15–30% and is especially increased during the last trimester. The fetal death rate reaches 90%. Excavation of the uterus may increase survival rates of pregnant women, but data on Lassa fever and pregnancy are still sparse. These figures suggest that interruption of the pregnancy of Lassa virus–infected women should be considered. White blood cell counts are normal or slightly elevated, and platelet counts are normal or somewhat low. Deafness coincides with clinical improvement in ~20% of patients and is permanent and bilateral in some patients. Reinfection may occur but has not been associated with severe disease.
High-level viremia or a high serum AST concentration statistically predicts a fatal outcome. Thus, patients with an AST concentration of >150 IU/mL should be treated with IV ribavirin. This antiviral nucleoside analogue appears to be effective in reducing case–fatality rates from those documented among retrospective controls. However, possible side effects, such as reversible anemia (which usually does not require transfusion), dependent hemolytic anemia, and bone marrow suppression, need to be kept in mind. Ribavirin should be given by slow IV infusion in a dose of 32 mg/kg; this dose should be followed by 16 mg/kg every 6 h for 4 days and then by 8 mg/kg every 8 h for 6 days. Inactivated Lassa virus vaccines failed in preclinical studies.
Bunyaviruses The most important VHF-causing bunyaviruses are Crimean-Congo hemorrhagic fever virus, hantaviruses, Rift Valley fever virus, and “severe fever with thrombocytopenia syndrome virus.” Other bunyaviruses—e.g., the Garissa variant of Ngari virus and Ilesha virus—have caused sporadic VHF outbreaks in Africa.
CRIMEAN-CONGO HEMORRHAGIC FEVER (CCHF) This severe VHF has a wide geographic distribution, potentially emerging wherever virus-bearing ticks occur. Because of the propensity of CCHF virus–transmitting ticks to feed on domestic livestock and certain wild mammals, veterinary serosurveys are the most effective mechanism for the monitoring of virus circulation in a particular region. Human infections are acquired via tick bites or during the crushing of infected ticks. Domestic animals do not become ill but do develop viremia. Thus, there is risk of acquiring CCHF during sheep shearing, slaughter, and contact with infected hides or carcasses from recently slaughtered infected animals. Nosocomial epidemics are common and are usually related to extensive blood exposure or needlesticks.
Although generally similar to other VHFs, CCHF causes extensive liver damage, resulting in jaundice in some patients. Clinical laboratory values indicate DIC and show elevations in concentrations of AST, creatine phosphokinase, and bilirubin. Patients who do not survive generally have more distinct changes than survivors in the concentrations of these markers, even in the early days of illness, and also develop leukocytosis rather than leukopenia. In addition, thrombocytopenia is more marked and develops earlier in patients who do not survive than in survivors. The benefit of IV ribavirin for treatment remains hotly debated, but clinical experience and retrospective comparison of patients with ominous clinical laboratory values suggest that ribavirin may be efficacious. No human or veterinary vaccines are recommended.
HEMORRHAGIC FEVER WITH RENAL SYNDROME HFRS is the most important VHF today, with more than 100,000 cases of severe disease in Asia annually and milder infections numbering in the thousands in Europe. The disease is widely distributed in Eurasia. The major causative viruses are Puumala virus (Europe), Dobrava-Belgrade virus (the Balkans), and Hantaan virus (eastern Asia). Amur/Soochong, Gou, Kurkino, Muju, Saaremaa, Sochi, and Tula viruses also cause HFRS but much less frequently and in more geographically confined areas determined by the distribution of reservoir hosts. Seoul virus is exceptional in that it is associated with brown rats (Rattus norvegicus); therefore, the virus has a worldwide distribution because of the migration of these rodents on ships. Despite the wide distribution of Seoul virus, only mild or moderate HFRS occurs in Asia, and human disease has been difficult to identify in many areas of the world. Most cases of HFRS occur in rural residents or vacationers; the exception is Seoul virus infection, which may be acquired in an urban or rural setting or from contaminated laboratory-rat colonies. Classic Hantaan virus infection in Korea and in rural China is most common in the spring and fall and is related to rodent density and agricultural practices. Human infection is acquired primarily through aerosols of rodent urine, although virus is also present in rodent saliva and feces. Patients with HFRS are not infectious.
Severe cases of HFRS evolve in four identifiable stages. The febrile stage lasts 3 or 4 days and is identified by the abrupt onset of fever, headache, severe myalgia, thirst, anorexia, and often nausea and vomiting. Photophobia, retroorbital pain, and pain on ocular movement are common, and the vision may become blurred with ciliary body inflammation. Flushing over the face, the V area of the neck, and the back is characteristic, as are pharyngeal injection, periorbital edema, and conjunctival suffusion. Petechiae often develop in areas of pressure, the conjunctivae, and the axillae. Back pain and tenderness to percussion at the costovertebral angle reflect massive retroperitoneal edema. Laboratory evidence of mild to moderate DIC is present. Other laboratory findings of HFRS include proteinuria and active urinary sediment. The hypotensive stage lasts from a few hours to 48 h and begins with falling blood pressure and sometimes shock. The relative bradycardia typical of the febrile phase is replaced by tachycardia. Kinin activation is marked. The rising hematocrit reflects increasing vascular leakage. Leukocytosis with a left shift develops, and thrombocytopenia continues. Atypical lymphocytes—which in fact are activated CD8+ and, to a lesser extent, CD4+ T cells—circulate. Proteinuria is marked, and the urine’s specific gravity falls to 1.010. Renal circulation is congested and compromised from local and systemic circulatory changes resulting in necrosis of tubules, particularly at the corticomedullary junction, and oliguria. During the oliguric stage, hemorrhagic tendencies continue, probably in large part because of uremic bleeding defects. Oliguria persists for 3–10 days before the return of renal function marks the onset of the polyuric stage (diuresis and hyposthenuria), which carries the danger of dehydration and electrolyte abnormalities.
Mild cases of HFRS may be much less stereotypical. The presentation may include only fever, gastrointestinal abnormalities, and transient oliguria followed by hyposthenuria. Infections with Puumala virus, the most common cause of HFRS in Europe (nephropathia epidemica), result in a much-attenuated picture but the same general presentation. Bleeding manifestations are found in only 10% of patients, hypotension rather than shock is usually documented, and oliguria is present in only about half of patients. The dominant features may be fever, abdominal pain, proteinuria, mild oliguria, and sometimes blurred vision or glaucoma followed by polyuria and hyposthenuria in recovery. The lethality rate is <1%.
HFRS should be suspected in patients with rural exposure in an endemic area. Prompt recognition of the disease permits rapid hospitalization and expectant management of shock and renal failure. Useful clinical laboratory parameters include leukocytosis, which may be leukemoid and is associated with a left shift; thrombocytopenia; and proteinuria. HFRS is readily diagnosed by an IgM-capture ELISA that is positive at admission or within 24–48 h thereafter. The isolation of hantaviruses is difficult, but RT-PCR of a blood clot collected early in the clinical course or of tissues obtained postmortem should give positive results. Such testing is usually undertaken if definitive identification of the infecting virus is required.
Mainstays of therapy are management of shock, reliance on vasopressors, modest crystalloid infusion, IV human serum albumin administration, and treatment of renal failure with prompt dialysis to prevent overhydration that may result in pulmonary edema and to control hypertension that increases the possibility of intracranial hemorrhage. Use of IV ribavirin has reduced lethality and morbidity in severe cases, provided treatment is begun within the first 4 days of illness. Lethality may be as high as 15% but with proper therapy should be <5%. Sequelae have not been definitively established.
RIFT VALLEY FEVER The natural range of Rift Valley fever virus was previously confined to sub-Saharan Africa, with circulation of the virus markedly enhanced by substantial rainfall. The El Niño Southern Oscillation phenomenon of 1997 facilitated subsequent spread of Rift Valley fever to the Arabian Peninsula, with epidemic disease in 2000. The virus has also been found in Madagascar and has been introduced into Egypt, where it caused major epidemics in 1977–1979, 1993, and thereafter. Rift Valley fever virus is maintained in nature by transovarial transmission in floodwater Aedes mosquitoes and presumably also has a vertebrate amplifier. Increased transmission during particularly heavy rains leads to epizootics characterized by high-level viremia in cattle, goats, or sheep. Numerous types of mosquitoes then feed on these animals and become infected, thereby increasing the possibility of human infections. Remote sensing via satellite can detect the ecologic changes associated with high rainfall that predict the likelihood of Rift Valley fever virus transmission. High-resolution satellites can also detect the special depressions in floodwaters from which the mosquitoes emerge. In addition, the virus can be transmitted by contact with blood or aerosols from domestic animals. Transmission risk is therefore high during birthing, and both abortuses and placentas need to be handled with caution. Slaughtered animals are not infectious because anaerobic glycolysis in postmortem tissues results in an acidic environment that rapidly inactivates bunyaviruses. Neither person-to-person nor nosocomial transmission of Rift Valley fever has been documented.
Rift Valley fever virus is unusual in that it causes several clinical syndromes. Most infections are manifested as the fever–myalgia syndrome. A small proportion of infections result in VHF with especially prominent liver involvement. Renal failure and DIC are also common features. Perhaps 10% of otherwise mild infections lead to retinal vasculitis, and some patients have permanently impaired vision. Funduscopic examination reveals edema, hemorrhages, and infarction of the retina as well as optic nerve degeneration. In a small proportion of patients (<1 in 200), retinal vasculitis is followed by viral encephalitis.
No proven therapy exists for Rift Valley fever. Both retinal disease and encephalitis occur after the acute febrile syndrome has resolved and serum neutralizing antibody has developed—events suggesting that only supportive care need be given. Epidemic disease is best prevented by vaccination of livestock. The ability of this virus to propagate after introduction into Egypt suggests that other potentially receptive areas, including the United States, should develop response plans. Rift Valley fever, like Venezuelan equine encephalitis, is likely to be controlled only with adequate stocks of an effective live attenuated vaccine, but such global stocks are unavailable. A formalin-inactivated vaccine confers immunity in humans, but quantities are limited and three injections are required; this vaccine is recommended for potentially exposed laboratory workers and for veterinarians working in sub-Saharan Africa. A new live attenuated vaccine, MP-12, is being tested in humans and may soon become available for general use. The vaccine is safe and licensed for use in sheep and cattle.
SEVERE FEVER WITH THROMBOCYTOPENIA SYNDROME This is a recently described tick-borne disease caused by a previously unknown and still-unclassified phlebovirus. Numerous human infections have been reported during the past few years from China, and several cases have also been detected in Japan and South Korea. The clinical presentation ranges from mild nonspecific fever to severe VHF with a high (>12%) lethality rate.
Flaviviruses The most important flaviviruses that cause VHF are the mosquito-borne dengue viruses 1–4 and yellow fever viruses. These viruses are widely distributed and cause tens to hundreds of thousands of infections each year. Kyasanur Forest disease virus and Omsk hemorrhagic fever virus are geographically very restricted but important tick-borne flaviviruses that cause VHF, sometimes with subsequent viral encephalitis. Tick-borne encephalitis virus has caused VHF in a few patients. There is currently no therapy for these VHFs, but an inactivated vaccine has been used in India to prevent Kyasanur Forest disease.
SEVERE DENGUE Several weeks after convalescence from infection with dengue virus 1, 2, 3, or 4, the transient protection conferred by that infection against reinfection with a heterotypic dengue virus usually wanes. Heterotypic reinfection may result in classic dengue or, less commonly, in severe dengue. In the past 20 years, A. aegypti has progressively reinvaded Latin America and other areas, and frequent travel by infected individuals has introduced multiple variants of dengue viruses 1–4 from many geographic areas. Thus the pattern of hyperendemic transmission of multiple dengue virus serotypes established in the Americas and the Caribbean has led to the emergence of severe dengue as a major problem. Among the millions of dengue virus 1–4 infections, ~500,000 cases of severe dengue occur annually, with a lethality rate of ~2.5%. The induction of vascular permeability and shock depends on multiple factors, such as the presence or absence of enhancing and nonneutralizing antibodies, age (susceptibility to severe dengue drops considerably after 12 years of age), sex (females are more often affected than males), race (whites are more often affected than blacks), nutritional status (malnutrition is protective), or sequence of infections (e.g., dengue virus 1 infection followed by dengue virus 2 infection seems to be more dangerous than dengue virus 4 infection followed by dengue virus 2 infection). In addition, considerable heterogeneity exists among each dengue virus population. For instance, Southeast Asian dengue virus 2 variants have more potential to cause severe dengue than do other variants.
Severe dengue is identified by the detection of bleeding tendencies (tourniquet test, petechiae) or overt bleeding in the absence of underlying causes, such as preexisting gastrointestinal lesions. Shock may result from increased vascular permeability. In milder cases of severe dengue, restlessness, lethargy, thrombocytopenia (<100,000/μL), and hemoconcentration are detected 2–5 days after the onset of typical dengue, usually at the time of defervescence. The maculopapular rash that often develops in dengue may also appear in severe dengue. In more severe cases, frank shock is apparent, with low pulse pressure, cyanosis, hepatomegaly, pleural effusions, and ascites; in some patients, severe ecchymoses and gastrointestinal bleeding develop. The period of shock lasts only 1 or 2 days.
A virologic diagnosis of severe dengue can be made by the usual means. However, multiple flavivirus infections result in broad immune responses to several members of the genus, and this situation may result in a lack of virus specificity of the IgM and IgG immune responses. A secondary antibody response can be sought with tests against several flavivirus antigens to demonstrate the characteristic wide spectrum of reactivity.
Most patients with shock respond promptly to close monitoring, oxygen administration, and infusion of crystalloid or—in severe cases—colloid. The case–fatality rates reported vary greatly with case ascertainment and quality of treatment; however, most patients with severe dengue respond well to supportive therapy, and the overall lethality rate at an experienced center in the tropics is probably as low as 1%.
The key to control of both dengue and severe dengue is the control of A. aegypti, which also reduces the risk of urban yellow fever and chikungunya virus circulation. Control efforts have been handicapped by the presence of nondegradable tires and long-lived plastic containers in trash repositories (perfect mosquito breeding grounds when filled with water during rainfall) and by insecticide resistance. Urban poverty and an inability of the public health community to mobilize the populace to respond to the need to eliminate mosquito breeding sites are also factors in lack of mosquito control. A tetravalent live attenuated dengue vaccine based on the attenuated yellow fever virus 17D platform is currently being evaluated in phase 3 clinical trials in Latin America, Asia, and Australia. At least two other live attenuated candidate vaccines based on modified recombinant dengue viruses have been evaluated in phase 1 clinical studies, but the results have not been promising.
YELLOW FEVER Yellow fever virus had caused major epidemics in Africa and Europe before its transmission by A. aegypti mosquitoes was discovered in 1900. Urban yellow fever became established in the New World as a result of colonization with A. aegypti, originally an African mosquito. Subsequently, different types of mosquitoes and nonhuman primates were found to maintain yellow fever virus in Africa and also in Central and South American jungles. Transmission to humans is incidental, occurring via bites from mosquitoes that have fed on viremic monkeys. After the identification of A. aegypti mosquitoes as vectors of yellow fever, containment strategies were aimed at increased mosquito control. Today, urban yellow fever transmission occurs only in some African cities, but the threat exists in the great cities of South America, where reinfestation by A. aegypti has taken place and dengue virus 1–4 transmission by the same mosquito is common. Despite the existence of a highly effective and safe vaccine, several hundred jungle yellow fever cases occur annually in South America, and thousands of jungle and urban cases occur each year in Africa (29,000–60,000 estimated for 2013).
Yellow fever is a typical VHF accompanied by prominent hepatic necrosis. A period of viremia, typically lasting 3 or 4 days, is followed by a period of “intoxication.” During the latter phase in severe cases, characteristic jaundice, hemorrhages, black vomit, anuria, and terminal delirium occur, perhaps related in part to extensive hepatic involvement. Blood leukocyte counts may be normal or reduced and are often high in terminal stages. Albuminuria is usually noted and may be marked. As renal function fails in terminal or severe cases, the concentration of blood urea nitrogen rises proportionately. Abnormalities detected in liver function tests range from modest elevations of AST concentrations in mild cases to severe derangement.
Urban yellow fever can be prevented by the control of A. aegypti. The continuing sylvatic cycles require vaccination of all visitors to areas of potential transmission with live attenuated variant 17D vaccine virus, which cannot be transmitted by mosquitoes. With few exceptions, reactions to the vaccine are minimal; immunity is provided within 10 days and lasts for at least 25–35 years. An egg allergy mandates caution in vaccine administration. Although there are no documented harmful effects of the vaccine on fetuses, pregnant women should be immunized only if they are definitely at risk of exposure to yellow fever virus. Because vaccination has been associated with several cases of encephalitis in children <6 months of age, it is contraindicated in this age group, nor is it recommended for infants 6–8 months of age unless the risk of exposure is very high. Rare, serious, multisystemic adverse reactions (occasionally fatal) have been reported, particularly affecting the elderly, and risk-to-benefit should be weighed prior to vaccine administration to individuals ≥60 years of age. Nevertheless, the number of deaths of unvaccinated travelers with yellow fever exceeds the number of deaths from vaccination, and a liberal vaccination policy for travelers to involved areas should be pursued. Timely information on changes in yellow fever distribution and yellow fever vaccine requirements can be obtained from the U.S. Centers for Disease Control and Prevention (http://www.cdc.gov/vaccines/vpd-vac/yf/default.htm).
234 |
Ebolavirus and Marburgvirus Infections |
Several viruses of the family Filoviridae cause severe and frequently fatal viral hemorrhagic fevers in humans. Introduction of filoviruses into human populations is an extremely rare event that most likely occurs by direct or indirect contact with healthy mammalian filovirus hosts or by contact with infected, sick, or deceased nonhuman primates. Filoviruses are highly infectious but not very contagious. Natural human-to-human transmission takes place through direct person-to-person (usually skin-to-skin) contact or exposure to infected bodily fluids and tissues; there is no evidence of such transmission by aerosol or respiratory droplets. Infections progress rapidly from influenza-like to hemorrhagic manifestations and typically culminate in multiple-organ dysfunction syndrome and shock. Treatment of filovirus infections is of necessity entirely supportive because no specific efficacious antiviral agents or vaccines are yet available.
Filoviruses are categorized as World Health Organization (WHO) Risk Group 4 Pathogens. Consequently, all work with material suspected of containing filoviruses should be conducted only in maximal containment (biosafety level 4) laboratories. Experienced personnel handling these viruses must wear appropriate personal protective gear (see “Prevention,” below) and follow rigorous standard operating procedures. The proper authorities and WHO reference laboratories should be contacted immediately when filovirus infections are suspected.
ETIOLOGY
The family Filoviridae includes three genera: Cuevavirus, Ebolavirus, and Marburgvirus (Table 234-1 and Fig. 234-1). The available data suggest that the only known cuevavirus, Lloviu virus (LLOV), and one ebolavirus, Reston virus (RESTV), are not pathogenic for humans. The remaining four ebolaviruses—Bundibugyo virus (BDBV), Ebola virus (EBOV), Sudan virus (SUDV), and Taï Forest virus (TAFV)—cause Ebola virus disease (EVD; International Classification of Disease, Tenth Revision [ICD-10], code A98.4). The two marburgviruses, Marburg virus (MARV) and Ravn virus (RAVV), are the etiologic agents of Marburg virus disease (MVD; ICD-10 code A98.3).
|
FILOVIRUS TAXONOMY |
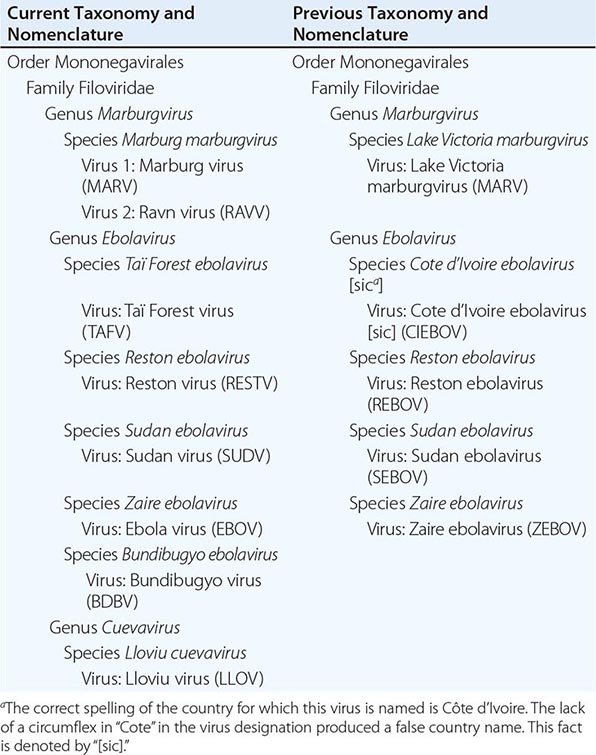
FIGURE 234-1 Filovirus phylogeny/evolution. Bayesian coalescent analysis of representative variants of all known filovirus clades (represented by underlined GenBank accession numbers). The maximal clade credibility tree is shown with the most recent common ancestor (MRCA) at each node. Posterior probability values are shown beneath MRCA estimates in years. Scale is in substitutions/site based on an analysis performed by Dr. Serena Carroll, Centers for Disease Control and Prevention. BDBV, Bundibugyo virus; EBOV, Ebola virus; LLOV, Lloviu virus; MARV, Marburg virus; RAVV, Ravn virus; RESTV, Reston virus; SUDV, Sudan virus; TAFV, Taï Forest virus.
![]() Filoviruses have linear, nonsegmented, single-stranded, negative-sense RNA genomes that are ~19 kb in length. These genomes contain six or seven genes that encode the following seven structural proteins: nucleoprotein, polymerase cofactor (VP35), matrix protein (VP40), glycoprotein (GP1,2), transcriptional cofactor (VP30), secondary matrix protein (VP24), and RNA-dependent RNA polymerase (L protein). Cuevaviruses and ebolaviruses, but not marburgviruses, also encode three nonstructural proteins of unknown function (sGP, ssGP, and Δ-peptide). Filovirions are unique among human virus particles in that they are predominantly pleomorphic filaments but also assume torus- or 6-like shapes (width, ~80 nm; average length, ≥790 nm). These enveloped virions contain helical ribonucleocapsids and are covered with GP1,2 spikes (Fig. 234-2).
Filoviruses have linear, nonsegmented, single-stranded, negative-sense RNA genomes that are ~19 kb in length. These genomes contain six or seven genes that encode the following seven structural proteins: nucleoprotein, polymerase cofactor (VP35), matrix protein (VP40), glycoprotein (GP1,2), transcriptional cofactor (VP30), secondary matrix protein (VP24), and RNA-dependent RNA polymerase (L protein). Cuevaviruses and ebolaviruses, but not marburgviruses, also encode three nonstructural proteins of unknown function (sGP, ssGP, and Δ-peptide). Filovirions are unique among human virus particles in that they are predominantly pleomorphic filaments but also assume torus- or 6-like shapes (width, ~80 nm; average length, ≥790 nm). These enveloped virions contain helical ribonucleocapsids and are covered with GP1,2 spikes (Fig. 234-2).
FIGURE 234-2 Ebola virus particle: the first transmission electron micrograph of an Ebola virion in a culture of Vero cells inoculated with a blood sample from a patient from the 1976 Zaire outbreak of Ebola virus disease. Shown is the typical and unique filamentous and pleomorphic structure of filovirions. (PHIL ID#1833, taken by Dr. Fredrick A. Murphy, Centers for Disease Control and Prevention.)
EPIDEMIOLOGY
![]() To date (i.e., as of December 3, 2014), a total of 20,012 human filovirus infections and 8058 fatalities have been recorded (Fig. 234-3). These numbers emphasize both the high degree of lethality (number of deaths per number of sick people; 40.3%) and the overall low mortality (impact on healthy population) of filovirus infections. At least for the moment, natural filovirus infections do not pose a global threat. Filoviruses pathogenic for humans appear to be exclusively endemic to Equatorial Africa, although this distribution may change if natural or artificial environmental alterations lead to filovirus host migration and increased contacts between nonhuman hosts and humans (Fig. 234-4). The majority of recorded EVD and MVD outbreaks can be traced back to single index cases who transmitted the infection to others. These chains of contacts suggest that only around 50 natural host-to-human spillover events have occurred since the discovery of filoviruses in 1967. Outbreak frequency, case numbers, and overall lethality probably depend on the particular etiologic agent, the geographic location and socioeconomic conditions of the affected country, and local customs. In particular, the availability of personal protective gear and reusable medical equipment, such as syringes and needles, has affected overall case numbers in the past, and outbreaks have been contained when local burial practices, such as ritual washing, have been either prevented or altered by the use of gloves. The incidence of EVD and MVD may have increased over the past two decades (Figs. 234-3 and 234-4), but researchers debate whether the observed change is due to increased filovirus activity, more frequent contact between filovirus hosts and humans, or continuous improvement in surveillance capabilities.
To date (i.e., as of December 3, 2014), a total of 20,012 human filovirus infections and 8058 fatalities have been recorded (Fig. 234-3). These numbers emphasize both the high degree of lethality (number of deaths per number of sick people; 40.3%) and the overall low mortality (impact on healthy population) of filovirus infections. At least for the moment, natural filovirus infections do not pose a global threat. Filoviruses pathogenic for humans appear to be exclusively endemic to Equatorial Africa, although this distribution may change if natural or artificial environmental alterations lead to filovirus host migration and increased contacts between nonhuman hosts and humans (Fig. 234-4). The majority of recorded EVD and MVD outbreaks can be traced back to single index cases who transmitted the infection to others. These chains of contacts suggest that only around 50 natural host-to-human spillover events have occurred since the discovery of filoviruses in 1967. Outbreak frequency, case numbers, and overall lethality probably depend on the particular etiologic agent, the geographic location and socioeconomic conditions of the affected country, and local customs. In particular, the availability of personal protective gear and reusable medical equipment, such as syringes and needles, has affected overall case numbers in the past, and outbreaks have been contained when local burial practices, such as ritual washing, have been either prevented or altered by the use of gloves. The incidence of EVD and MVD may have increased over the past two decades (Figs. 234-3 and 234-4), but researchers debate whether the observed change is due to increased filovirus activity, more frequent contact between filovirus hosts and humans, or continuous improvement in surveillance capabilities.
FIGURE 234-3 Characteristics of outbreaks of human filovirus disease. Six of eight known filoviruses have caused disease in humans in the past. Outbreaks are listed by virus in chronological order. Laboratory infections are shaded gray and italicized. Arrows indicate international case exportation. Total number of cases and total number of lethal cases are summarized in the middle column (2014 EBOV infections as of December 3). The lethality/case–fatality rate (black dots) for each outbreak is plotted on a 0–100% scale along with 99% confidence intervals (black horizontal lines). The overall case–fatality rate for disease caused by a particular virus is delineated by vertical bold-colored lines, with vertical bold-colored dashed lines indicating the corresponding 99% confidence intervals; the overall case–fatality rate for all ebolavirus infections, all marburgvirus infections, and all filovirus infections are shown by vertical gray bars. BDBV, Bundibugyo virus; COD, Democratic Republic of the Congo (formerly Zaire); COG, Republic of the Congo; EBOV, Ebola virus; MARV, Marburg virus; RAVV, Ravn virus; SUDV, Sudan virus; TAFV, Taï Forest virus; UK, United Kingdom; USSR, Union of Soviet Socialist Republics (today Russia).
FIGURE 234-4 Geographic distribution of human filovirus disease outbreaks and years of occurrence. Arrows indicate international case exportation. BDBV, Bundibugyo virus; COD, Democratic Republic of the Congo (formerly Zaire); COG, Republic of the Congo; EBOV, Ebola virus; MARV, Marburg virus; RAVV, Ravn virus; SUDV, Sudan virus; TAFV, Taï Forest virus.
EVD and MVD outbreaks are associated with distinct meteorologic and geographic conditions and are probably associated with distinct hosts or reservoirs. The four ebolaviruses that cause disease in humans are endemic in humid rainforests. EVD outbreaks have often been linked to hunting or contact with bush meat (i.e., meat from apes, other nonhuman primates, duikers, or bush pigs) in forests. Ecologic studies indicate that EBOV may be the etiologic agent of extensive and frequently fatal epizootics among wild chimpanzee and gorilla populations. However, replicating isolates of ebolaviruses from wild nonhuman primates have thus far been obtained only in the case of TAFV, which was isolated from a succumbed western chimpanzee in Côte d’Ivoire in 1994. The marburgviruses MARV and RAVV, on the other hand, seem to infect hosts inhabiting arid woodlands. MVD outbreaks have almost always been epidemiologically linked to visits to or work in natural or artificial caves or mines. A pteropid (fruit) bat, the cave-dwelling Egyptian rousette (Rousettus aegyptiacus), serves as a natural and subclinically infected reservoir for both MARV and RAVV. Although bats are suspected to be the hosts for ebolaviruses as well, definitive proof is still lacking. In fact, thus far, only EBOV and RESTV have been loosely connected to frugivorous and insectivorous bats by means of antibody or genome fragment detection, whereas the hosts of BDBV, SUDV, and TAFV remain unclear.
PATHOGENESIS
Human infections typically occur through direct exposure of skin lesions or mucosal surfaces to contaminated bodily fluids or material or by parenteral inoculation (e.g., via accidental needlesticks or reuse of needles in poorly equipped hospitals). Numerous studies, both in vitro and in vivo (in several animal models of human disease), have shed light on key pathogenetic events that evolve subsequent to filovirion exposure. The GP1,2 spikes on the surface of filovirions determine their cell and tissue tropism by engaging yet-unidentified cell-surface molecules and the intracellular receptor Niemann-Pick C1.
One of the pathogenetic hallmarks of filovirus infection is a pronounced suppression of the immune system. The first targets of filovirions are local macrophages, monocytes, and dendritic cells. Several structural proteins of filovirions, in particular VP35, VP40, and VP24, then suppress cellular innate immune responses by, for instance, inhibiting the interferon pathway and thereby enabling a productive filovirus infection. The result is the secretion of copious numbers of progeny virions, as evidenced by high titers in the bloodstream (>106 plaque-forming units [pfu]/mL of serum in humans) and the lymphatics, and dissemination to most tissues. Filovirions then infect additional phagocytic cells, such as other macrophages (alveolar, peritoneal, pleural), Kupffer cells in the liver, and microglia, as well as other targets, such as adrenal cortical cells, fibroblasts, hepatocytes, endothelial cells, and a variety of epithelial cells. Infection leads to the secretion of soluble signaling molecules (varying with the cell type) that most likely are crucial factors in immune response modulation and development of multiorgan dysfunction syndrome. For instance, infected macrophages react by secreting proinflammatory cytokines, a response that leads to further recruitment of macrophages to the site of infection. In contrast, infected dendritic cells are not activated to secrete cytokines, and expression of major histocompatibility class II antigens is partially suppressed. Immunosuppression occurs in part by massive lymphoid depletion in lymph nodes, spleen, and thymus in the absence of reactive inflammatory cellular responses. Results from animal studies suggest that depletion is a direct consequence of considerable bystander apoptosis of lymphocytes; this explanation would also account for the severe lymphopenia that develops in patients. The consequence of these events is not only florid filovirus dissemination but also a proclivity of the patient for secondary bacterial and fungal infections.
Other pathogenetic hallmarks of filovirus infections are a severe disturbance of the clotting system and the impairment of vascular integrity. Disseminated intravascular coagulation is the cause of the severe imbalance in the clotting system of filovirus-infected patients. Thrombocytopenia, increased concentrations of tissue factor, consumption of clotting factors, increased concentrations of fibrin degradation products (D-dimers), and declining concentrations of protein C are typical features of infection. Consequently, the occlusion of small vessels by widely distributed microthrombi leads to extensive necroses/hypoxic infarcts in target tissues (particularly the gonads, kidneys, liver, and spleen) in the absence of marked inflammatory responses. In addition, petechiae, ecchymoses, extensive visceral effusions, and other hemorrhagic signs are observed in internal organs, mucous membranes, and skin. Actual severe blood loss, however, is a rare event. Aberrance in cytokines or other factors such as nitric oxide and direct infection and activation of endothelial cells most likely are responsible for upregulated permeability of the endothelia of blood vessels. This upregulation leads to fluid redistribution (third spacing); interstitial and myocardial edema and hypovolemic shock are common developments. Clinical improvement is rare and is usually characterized by falling viral titers during the development of a virus-specific immune response.
CLINICAL MANIFESTATIONS
MVD and EVD cannot be differentiated by mere observation of clinical manifestations. The incidence of clinical signs does not differ significantly among infections caused by disparate filoviruses (Table 234-2). The incubation period ranges from 3 to 25 days, after which infected people develop a biphasic syndrome with a 1- to 2-day relative remission separating the two phases. The first phase (disease onset until around day 5–7) resembles influenza and is characterized by sudden onset of fever and chills, severe headaches, cough, myalgia, pharyngitis, arthralgia of the larger joints, development of a maculopapular rash, and other signs/symptoms (Table 234-2). The second phase (approximately 5–7 days after disease onset and thereafter) involves the gastrointestinal tract (abdominal pain with vomiting and/or diarrhea), respiratory tract (chest pain, cough), vascular system (postural hypotension, edema), and central nervous system (confusion, coma, headache). Hemorrhagic manifestations such as subconjunctival injection, nosebleeds, hematemesis, hematuria, and melena are typical (Table 234-2).
|
DISTRIBUTION OF CLINICAL SIGNS/SYMPTOMS OF FILOVIRUS-INFECTED PATIENTS IN THREE REPRESENTATIVE OUTBREAKS |
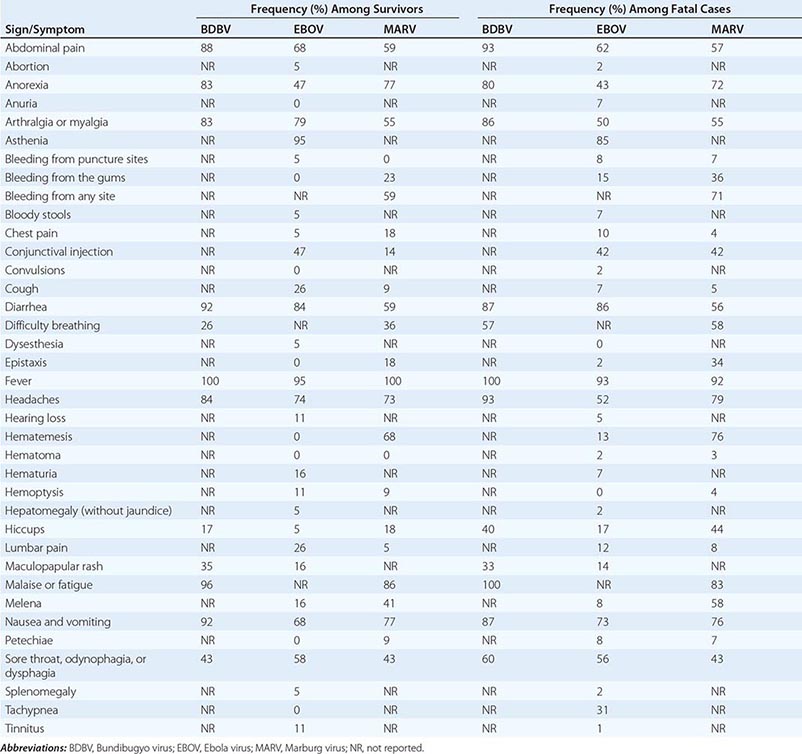
Typical laboratory findings are leukopenia (with cell counts as low as 1000/μL) with a left shift prior to leukocytosis, thrombocytopenia (with counts as low as 50,000/μL), increased concentrations of liver and pancreatic enzymes (aspartate aminotransferase > alanine aminotransferase, γ-glutamyltransferase, serum amylase), hypokalemia, hypoproteinemia, increased creatinine and urea concentrations with proteinuria, and prolonged prothrombin and partial thromboplastin times.
Patients usually succumb to disease 4–14 days after infection. Patients who survive experience prolonged and sometimes incapacitating sequelae such as arthralgia, asthenia, iridocyclitis, hearing loss, myalgia, orchitis, parotitis, psychosis, recurrent hepatitis, transverse myelitis, or uveitis. Temporary hair loss and desquamation of skin areas previously affected by a typical maculopapular rash are visible consequences of the disease. Rarely, filoviruses can persist in the liver, eyes, or testicles of survivors and may cause recurrent disease months after convalescence.
DIAGNOSIS
Filovirus infections cannot be diagnosed on the basis of clinical presentation alone. Numerous diseases typical for Equatorial Africa need to be considered in the differential diagnosis of a febrile patient. Almost all of these diseases occur at a much higher incidence than filovirus infections and are therefore the more likely candidates during differential diagnostic deliberations. The most important of the infectious diseases that closely mimic EVD and MVD are falciparum malaria and typhoid fever; also important are enterohemorrhagic Escherichia coli enteritis, gram-negative septicemia (including shigellosis), meningococcal septicemia, rickettsial infections, fulminant viral hepatitis, leptospirosis, measles, and all other viral hemorrhagic fevers (in particular, yellow fever). Other ailments, such as venomous snakebites, warfarin intoxication, and the many transient or inherited platelet and vascular disorders, also must be considered. Visits to caves or mines and direct contact with bats, nonhuman primates (especially apes), or bush meat should raise suspicion of filovirus infection, as should admission to or treatment in rural hospitals or direct contact with severely ill local residents.
If EVD or MVD is suspected on the basis of epidemiologic history, exposure history, and/or clinical manifestations, infectious disease specialists and the proper public health authorities, including the WHO, should be notified immediately. Laboratory diagnosis of EVD and MVD is relatively straightforward but requires maximal containment (biosafety level 4), which usually is not available in filovirus-endemic countries, or the involvement of on-site personnel trained in the use of diagnostic assays adapted for field use. Consequently, diagnostic samples should be collected with great caution and with use of proper personal protective equipment and strict barrier nursing techniques. With adherence to established biosafety precautionary measures, samples should be sent in suitable transport media to national or international WHO reference laboratories. Acute-phase blood/serum is the preferred diagnostic specimen because it usually contains high titers of filovirions and filovirion-specific antibodies.
The current methods of choice for the diagnosis of filovirus infection are reverse-transcription polymerase chain reaction (detection limit, 1000–2000 virus genome copies per milliliter of serum) and antigen capture enzyme-linked immunosorbent assay (ELISA) for the detection of filovirus genomes and filovirion components, respectively. Direct IgM and IgG or IgM capture ELISA is used for the detection of filovirion-targeting antibodies from patients in later stages of disease—i.e., those who have been able to mount a detectable immune response, including survivors. All these assays can be conducted on samples treated with guanidinium isothiocyanate (for polymerase chain reaction) or cobalt-60 irradiation (for ELISA) or subjected to other effective measures that render filoviruses noninfectious. Virus isolation in cell culture and plaque assays for quantification or diagnostic confirmation is relatively easy but must be performed in maximal-containment laboratories. If available, electron microscopic examination of properly inactivated samples or cultures can confirm the diagnosis because filovirions have unique filamentous shapes (Fig. 234-2). Formalin-fixed skin biopsies can be useful for safe postmortem diagnoses.
COMPLICATIONS
Given the severe immunosuppression induced by filovirus infection, secondary infections should be kept in mind and appropriately treated as early as possible. Pregnancy and labor cause severe and frequently fatal complications in filovirus infections due to clotting factor consumption, fetal loss, and/or severe blood loss during birth.
PROGNOSIS
The prognosis of filovirus infections is generally poor, although outcome probably depends somewhat on which particular virus causes the infection (Fig. 234-3). Convalescence may take months, with skin peeling, alopecia, prostration, weight loss, orchitis, amnesia, confusion, and anxiety as typical sequelae. Rarely, filoviruses persist in apparently healthy survivors and are either reactivated by unknown means at a later point or transmitted sexually. Condom use or abstinence from sexual activity for at least 3 months after disappearance of clinical signs is therefore recommended for survivors.
CONTROL AND PREVENTION
Currently, filovirus vaccines are not available. Prevention of filovirus infection in nature is difficult because the ecology of the viruses is not completely understood. As stated above, frugivorous cave-dwelling pteropid bats (Egyptian rousettes) have been identified as healthy carriers of MARV and RAVV. Avoidance of direct or indirect contact with these bats is therefore useful advice to people entering or living in areas where the animals can be found. Prevention seems to be more difficult in the case of ebolaviruses, for which definite reservoirs have not yet been pinpointed. EVD outbreaks have been associated not with bats but rather with hunting or consumption of nonhuman primates. The mechanism of introduction of ebolaviruses into nonhuman primate populations is unclear. Therefore, the best advice to locals and travelers is to avoid contact with bush meat, nonhuman primates, and bats.
Relatively simple barrier nursing techniques, vigilant use of proper personal protective equipment, and quarantine measures usually suffice to terminate or at least contain filovirus disease outbreaks. Isolation of filovirus-infected people and avoidance of direct person-to-person contact without proper personal protective equipment usually suffice to prevent further spread as the pathogens are not transmitted through droplets or aerosols under natural conditions. Typical protective gear sufficient to prevent filovirus infections consists of disposable gloves, gowns, and shoe covers and a face shield and/or goggles. If available, N-95/N-100 respirators may be used to further limit infection risk. Positive air pressure respirators should be considered for high-risk medical procedures such as intubation or suctioning. Medical equipment used in the care of a filovirus-infected patient, such as gloves or syringes, should never be reused unless safety-tested sterilization or disinfection methods are properly applied. Because filovirions are enveloped, disinfection with detergents, such as 1% sodium deoxycholate, diethyl ether, or phenolic compounds, is relatively straightforward. Bleach solutions of 1:100 and 1:10 are recommended for surface disinfection and application to excreta/corpses, respectively. Whenever possible, potentially contaminated materials should be autoclaved, irradiated, or destroyed.
SECTION 16 |
FUNGAL INFECTIONS |
235 |
Diagnosis and Treatment of Fungal Infections |
TERMINOLOGY AND MICROBIOLOGY
Traditionally, fungal infections have been classified into specific categories based on both anatomic location and epidemiology. The most common general anatomic categories are mucocutaneous and deep organ infection; the most common general epidemiologic categories are endemic and opportunistic infection. Although mucocutaneous infections can cause serious morbidity, they are rarely fatal. Deep organ infections also cause severe illness in many cases and, in contrast to mucocutaneous infections, are often fatal. The endemic mycoses (e.g., coccidioidomycosis) are caused by fungal organisms that are not part of the normal human microbiota but rather are acquired from environmental sources. In contrast, opportunistic mycoses are caused by organisms (e.g., Candida and Aspergillus) that commonly are components of the normal human microbiota and whose ubiquity in nature renders them easily acquired by the immunocompromised host (Table 235-1). Opportunistic fungi cause serious infections when the immunologic response of the host becomes ineffective, allowing the organisms to transition from harmless commensals to invasive pathogens. Frequently, the diminished effectiveness of the immune system is a result of advanced modern therapies that coincidentally either cause an imbalance in the host’s microbiota or directly interfere with immunologic responses. Endemic mycoses cause more severe illness in immunocompromised patients than in immunocompetent individuals.
|
ENDEMIC AND OPPORTUNISTIC MYCOSES |
Patients acquire deep organ infection with endemic fungi almost exclusively by inhalation. Cutaneous infections result either from hematogenous dissemination or, more often, from direct contact with soil—the natural reservoir for the vast majority of endemic mycoses. The dermatophytic fungi may be acquired by human-to-human transmission, but the majority of infections result from environmental contact. In contrast, the opportunistic fungus Candida invades the host from normal sites of colonization, usually the mucous membranes of the gastrointestinal tract. In general, innate immunity is the primary defense mechanism against fungi. Although antibodies are formed during many fungal infections (and even during commensalism), they generally do not constitute the primary mode of host defense. Nevertheless, in selected infections, as discussed below, measurement of antibody titers may be a useful diagnostic test.
Three other terms frequently used in clinical discussions of fungal infections are yeast, mold, and dimorphic fungus. Yeasts are seen as rounded single cells or as budding organisms. Candida and Cryptococcus are traditionally classified as yeasts. Molds grow as filamentous forms called hyphae both at room temperature and in invaded tissue. Aspergillus, Rhizopus (the genus that causes mucormycosis, also known as zygomycosis), and fungi commonly infecting the skin to cause ringworm and related cutaneous conditions are classified as molds. Variations occur within this classification of yeasts and molds. For instance, when Candida infects tissue, both yeasts and filamentous forms may be present (except with C. glabrata, which forms only yeasts in tissue); in contrast, Cryptococcus exists only in yeast form. Dimorphic is the term used to describe fungi that grow as yeasts or large spherical structures in tissue but as filamentous forms at room temperature in the environment. Classified in this group are the organisms causing blastomycosis, paracoccidioidomycosis, coccidioidomycosis, histoplasmosis, and sporotrichosis.
The incidence of nearly all fungal infections has risen substantially. Opportunistic infections have increased in frequency as a consequence of intentional immunosuppression in organ and stem cell transplantation and other disorders, the administration of cytotoxic chemotherapy for cancers, the liberal use of antibacterial agents, and, more recently, the increasing use of monoclonal antibodies.
![]() Within a global context, the incidence of endemic mycoses has increased in geographic locations where there has been substantial population growth. When advances in medical care (e.g., more aggressive treatment of cancer or organ transplantation) are introduced into a given area, the opportunistic mycoses increase in incidence.
Within a global context, the incidence of endemic mycoses has increased in geographic locations where there has been substantial population growth. When advances in medical care (e.g., more aggressive treatment of cancer or organ transplantation) are introduced into a given area, the opportunistic mycoses increase in incidence.
DIAGNOSIS
The definitive diagnosis of any fungal infection requires histopathologic identification of the fungus invading tissue and accompanying evidence of an inflammatory response. The identification of an inflammatory response has been especially important with regard to Aspergillus infection. Aspergillus is ubiquitous and can float in the air onto biopsy material. Therefore, in rare but important instances, this fungus is an ex vivo contaminant during processing of a specimen for microscopy, with a consequent incorrect diagnosis. The stains most commonly used to identify fungi are periodic acid–Schiff and Gomori methenamine silver. Candida, unlike other fungi, is visible on gram-stained tissue smears. Hematoxylin and eosin stain is not sufficient to identify Candida in tissue specimens. When positive, an india ink preparation of cerebrospinal fluid (CSF) is diagnostic for cryptococcosis. Most laboratories now use calcofluor white staining coupled with fluorescent microscopy to identify fungi in fluid specimens.
Extensive investigations of the diagnosis of deep organ fungal infections have yielded a variety of tests with different degrees of specificity and sensitivity. The most reliable tests are the detection of antibody to Coccidioides immitis in serum and CSF; of Histoplasma capsulatum antigen in urine, serum, and CSF; and of cryptococcal polysaccharide antigen in serum and CSF. These tests have a general sensitivity and specificity of 90%; however, because of variability among laboratories, testing on multiple occasions is advisable. The test for galactomannan has been used extensively in Europe and is now approved in the United States for diagnosis of aspergillosis. Sources of concern regarding galactomannan are the incidence of false-negative results and the need for multiple serial tests to reduce this incidence. The β-glucan test for Candida is also under evaluation but, like the galactomannan test, still requires additional validation; this test has a negative predictive value of ~90%. Both of these tests are being used with increasing frequency, especially for guiding the timing of initiation and duration of therapy. The galactomannan test is being evaluated in both serum and bronchoalveolar lavage fluid. Numerous polymerase chain reaction assays to detect antigens are in the developmental stages, as are nucleic acid hybridization techniques; currently, these tests are not widely available.
Of the fungal organisms, Candida is by far the most frequently recovered from blood. Although Candida species can be detected with any of the automated blood culture systems widely used at present, the lysis-centrifugation technique increases the sensitivity of blood cultures for Candida and for less common organisms (e.g., H. capsulatum). Lysis-centrifugation should be used when disseminated fungal infection is suspected.
Except in the cases of coccidioidomycosis, cryptococcosis, and histoplasmosis, there are no fully validated and widely used tests for serodiagnosis of disseminated fungal infection. Skin tests for the endemic mycoses are no longer available.
|
TREATMENT |
FUNGAL INFECTIONS |
This discussion is intended as a brief overview of general strategies for the use of antifungal agents in the treatment of fungal infections. Regimens, schedules, and strategies are detailed in the chapters on specific mycoses that follow in this section. The doses cited here are standard doses for adults with invasive infection.
Since fungal organisms are eukaryotic cells that contain most of the same organelles (with many of the same physiologic functions) as human cells, the identification of drugs that selectively kill or inhibit fungi but are not toxic to human cells has been highly problematic. Far fewer antifungal than antibacterial agents have been introduced into clinical medicine.
AMPHOTERICIN B
The introduction of amphotericin B (AmB) in the late 1950s revolutionized the treatment of fungal infections in deep organs. Before AmB became available, cryptococcal meningitis and other disseminated fungal infections were nearly always fatal. For nearly a decade after AmB was introduced, it was the only effective agent for the treatment of life-threatening fungal infections. AmB remains the broadest-spectrum antifungal agent but carries several disadvantages, including significant nephrotoxicity, lack of an oral preparation, and unpleasant side effects (fever, chills, and nausea) during treatment. To circumvent nephrotoxicity and infusion side effects, lipid formulations of AmB were developed and have virtually replaced the original colloidal deoxycholate formulation in clinical use (although the older formulation is still available). The lipid formulations include liposomal AmB (L-AmB; 3–5 mg/kg per day) and AmB lipid complex (ABLC; 5 mg/kg per day). A third preparation, AmB colloidal dispersion (ABCD; 3–4 mg/kg per day), is rarely used because of the high incidence of side effects associated with infusion.
The lipid formulations of AmB have the disadvantage of being considerably more expensive than the deoxycholate formulation. Experience is still accumulating on the comparative efficacy, toxicity, and advantages of the different formulations for specific clinical fungal infections, including central nervous system (CNS) infection. Whether there is a clinically significant difference in these drugs with respect to CNS penetration or nephrotoxicity remains controversial. Despite these issues and despite the expense, the lipid formulations are now much more commonly used than AmB deoxycholate in developed countries. In developing countries, AmB deoxycholate is still preferred because of the expense of the lipid formulations.
AZOLES
This class of antifungal drugs offers important advantages over AmB: the azoles cause little or no nephrotoxicity and are available in oral formulations. Early azoles included ketoconazole and miconazole, which have been replaced by newer agents for the treatment of deep organ fungal infections. The azoles’ mechanism of action is inhibition of ergosterol synthesis in the fungal cell wall. Unlike AmB, these drugs are considered fungistatic, not fungicidal.
Fluconazole Since its introduction, fluconazole has played an extremely important role in the treatment of a wide variety of serious fungal infections. Its major advantages are the availability of both oral and IV formulations, a long half-life, satisfactory penetration of most body fluids (including ocular fluid and CSF), and minimal toxicity (especially relative to that of AmB). Its disadvantages include (usually reversible) hepatotoxicity and—at high doses—alopecia, muscle weakness, and dry mouth with a metallic taste. Fluconazole is not effective for the treatment of aspergillosis, mucormycosis, or Scedosporium apiospermum infections. It is less effective than the newer azoles against Candida glabrata and Candida krusei.
Fluconazole has become the agent of choice for the treatment of coccidioidal meningitis, although relapses have followed therapy with this drug. In addition, fluconazole is useful as both consolidation and maintenance therapy for cryptococcal meningitis. This agent has been shown to be as efficacious as AmB in the treatment of candidemia. The effectiveness of fluconazole in candidemia and the drug’s relatively minimal toxicity, in conjunction with the inadequacy of diagnostic tests for widespread hematogenously disseminated candidiasis, have led to a change in the paradigm for candidemia management. The standard of care is now to treat all candidemic patients with an antifungal agent and to change all their intravascular lines, if feasible, rather than merely removing a singular suspect intravascular line and then observing the patient. The usual fluconazole regimen for treatment of candidemia is 400 mg/d given until 2 weeks after the last positive blood culture.
Fluconazole is considered effective as fungal prophylaxis in bone marrow transplant recipients and high-risk liver transplant patients. Its general use for prophylaxis in patients with leukemia, in AIDS patients with low CD4+ T cell counts, and in patients on surgical intensive care units remains controversial. Because of concerns about the possibility of infection due to resistant Candida species and of infection with Aspergillus species, many clinicians are initiating therapy with an echinocandin, which is then replaced by flucon-azole once a susceptible Candida species is recovered and concern about Aspergillus is diminished.
Voriconazole Voriconazole, which is available in both oral and IV formulations, has a broader spectrum than fluconazole against Candida species (including C. glabrata and C. krusei) and is active against Aspergillus, Scedosporium, and Fusarium. It is generally considered the first-line drug of choice for treatment of aspergillosis. A few case reports have shown voriconazole to be effective in individual patients with coccidioidomycosis, blastomycosis, and histoplasmosis, but because of limited data this agent is not recommended for primary treatment of the endemic mycoses. Among the disadvantages of voriconazole (compared with fluconazole) are its more numerous interactions with many of the drugs used in patients predisposed to fungal infections. Hepatotoxicity, skin rashes (including photosensitivity), and visual disturbances are relatively common. Skin cancer surveillance is now recommended for patients taking voriconazole. In addition, voriconazole is considerably more expensive than fluconazole. Moreover, it is advisable to monitor voriconazole levels in certain patients since (1) this drug is completely metabolized in the liver by CYP2C9, CYP3A4, and CYP2C19; and (2) human genetic variability in CYP2C19 activity exists. Dosages should be reduced accordingly in patients with liver failure. Dose adjustments for renal insufficiency are not necessary; however, because the IV formulation is prepared in cyclodextrin, it should not be given to patients with severe renal insufficiency.
Itraconazole Itraconazole is available in IV and oral (capsule and suspension) formulations. Varying blood levels among patients taking oral itraconazole reflect a disadvantage compared with the other azoles. Itraconazole is the drug of choice for mild to moderate histoplasmosis and blastomycosis and has often been used for chronic mucocutaneous candidiasis. It has been approved by the U.S. Food and Drug Administration (FDA) for use in febrile neutropenic patients. Itraconazole has also proved useful for the treatment of chronic coccidioidomycosis, sporotrichosis, and S. apiospermum infection. The mucocutaneous and cutaneous fungal infections that have been treated successfully with itraconazole include oropharyngeal candidiasis (especially in AIDS patients), tinea versicolor, tinea capitis, and onychomycosis. Disadvantages of itraconazole include its poor penetration into CSF, the use of cyclodextrin in both the oral suspension and the IV formulation, the variable absorption of the drug in capsule form, and the need for monitoring of blood levels in patients taking capsules for disseminated mycoses. Reported cases of severe congestive heart failure in patients taking itraconazole have been a source of concern. Like the other azoles, itraconazole can cause hepatic toxicity.