Chapter 26 Diagnosis and Surveillance of Barrett’s Esophagus
Epidemiology
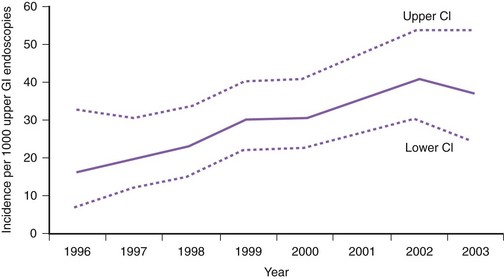
The incidence of Barrett’s esophagus has increased markedly since the 1970s. This increase was previously thought to be due to the increased use of diagnostic upper endoscopy combined with the change in the definition of Barrett’s esophagus to include shorter segments of columnar-lined epithelium.1 However, more recent data from the Netherlands suggest that the incidence of Barrett’s esophagus has increased from 14.3 per 100,000 person-years in 1997 to 23.1 per 100,000 person-years in 2002 in the general population independent of the number of upper endoscopies (Fig. 26.1).2
It is estimated that Barrett’s esophagus is found in approximately 5% to 15% of patients undergoing endoscopy for symptoms of GERD.3 A more recent study of a high-risk patient population (chronic GERD, white race, age >50 years) undergoing endoscopy for symptoms of GERD found Barrett’s esophagus in 13.2% of the subjects.3 Population-based studies suggest that the prevalence of Barrett’s esophagus is approximately 1.3% to 1.6%.4,5 Most of these patients in the general population have short-segment Barrett’s esophagus, and approximately 45% have no reflux symptoms.
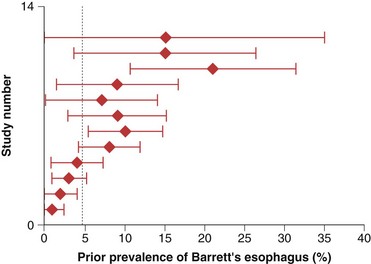
The prevalence of long-segment Barrett’s esophagus (≥3 cm of intestinal metaplasia) is approximately 5%, whereas the prevalence of short-segment Barrett’s esophagus (<3 cm of intestinal metaplasia) is approximately 6% to 12% in patients undergoing endoscopy in various settings.6–8 It is estimated that only 5% of patients undergoing resection for esophageal adenocarcinoma have a prior diagnosis of Barrett’s esophagus (Fig. 26.2).9
Barrett’s esophagus is predominantly a disease of middle-aged white men.10,11 However, approximately 25% of patients with Barrett’s esophagus are women or younger than 50 years of age.12,13 The prevalence of Barrett’s esophagus increases until a plateau is reached between the seventh and ninth decades.10,14 Various risk factors have been identified for the presence of Barrett’s esophagus, including frequent and long-standing reflux episodes, smoking, male gender, older age, and central obesity.15–19 Body mass index itself does not seem to be a risk factor for Barrett’s esophagus but rather the central obesity characteristic of male pattern obesity.17,18
Pathogenesis
Barrett’s esophagus is an acquired condition resulting from severe esophageal mucosal injury. It is unclear, however, why some patients with GERD develop Barrett’s esophagus whereas others do not. Animal studies suggest that the development of Barrett’s esophagus requires injury to the esophageal mucosa accompanied by an abnormal environment of epithelial repair.20 Epidemiologic data suggest that once injury occurs, Barrett’s esophagus develops to its full extent fairly rapidly with little subsequent change in length.10 The mechanism whereby injury triggers metaplasia and why this occurs in some but not all individuals is unknown. The cell of origin of columnar metaplasia is unclear. Candidates include dedifferentiation of squamous epithelium into columnar epithelium or stimulation of stem cells originating from the basal layer of the esophageal epithelium, esophageal submucosal glands, or bone marrow.21–23 The transcription factor CDX2, which can be induced by both acid and bile salts, seems to play a role in promoting the columnar epithelial differentiation pathway.24
Barrett’s esophagus is clearly associated with severe GERD. Compared with patients with erosive and nonerosive GERD without Barrett’s esophagus, patients with Barrett’s esophagus typically have greater esophageal acid exposure based on 24-hour pH monitoring.25,26 Part of the increase in acid exposure in patients with Barrett’s esophagus may be related to the almost uniform presence of a hiatal hernia, which is typically longer and associated with larger defects in the hiatus in patients with Barrett’s esophagus than in controls or patients with esophagitis alone.27,28 In addition, patients with Barrett’s esophagus have a lower basal lower esophageal sphincter pressure compared with GERD patients without Barrett’s esophagus.26 Reflux of duodenal contents is also increased in patients with Barrett’s esophagus compared with GERD patients without Barrett’s esophagus.29 Patients with short-segment Barrett’s esophagus tend to have pathophysiologic abnormalities intermediate to the abnormalities of patients with long-segment Barrett’s esophagus and normal controls.30,31 Esophageal pH monitoring studies suggest a correlation between the length of Barrett’s mucosa and the duration of esophageal acid exposure.31
Clinical Features
Patients with Barrett’s esophagus are difficult to distinguish clinically from patients with GERD uncomplicated by a columnar-lined esophagus.32 However, some observational studies suggest that features such as the development of reflux symptoms at an earlier age, increased duration of reflux symptoms, increased severity of nocturnal reflux symptoms, and increased complications of GERD (e.g., esophagitis, ulceration, stricture, bleeding) may distinguish patients with Barrett’s esophagus from GERD patients without Barrett’s esophagus.16 Similar clinical risk factors have been identified for esophageal adenocarcinoma.33 Identification of patients with Barrett’s esophagus may be hampered by the paradox that patients with Barrett’s esophagus have an impaired sensitivity to esophageal acid perfusion compared with patients with uncomplicated GERD.34 Many patients with Barrett’s esophagus are elderly, however, and this observation may be related to an age-related decrease in acid sensitivity.35 A subset of patients with Barrett’s esophagus may have an inherited predisposition; studies have reported families with multiple affected relatives over successive generations.36–38 These reports suggest an autosomal dominant pattern of inheritance in certain patients with Barrett’s esophagus.
Pathology
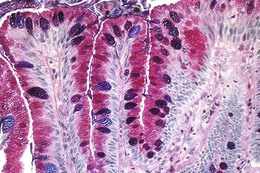
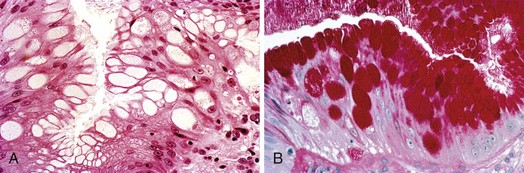
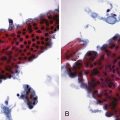
The columnar-lined esophagus is characterized by a mosaic of three different types of columnar epithelium above the lower esophageal sphincter zone: fundic-type epithelium, characterized by parietal and chief cells similar to the native gastric fundus; cardiac-type mucosa, characterized by mucous glands and no parietal cells; and specialized columnar epithelium, characterized by a villiform surface and alcian blue–staining intestinal-type goblet cells.39 At the present time, the diagnosis of Barrett’s esophagus is established if the squamocolumnar junction is displaced proximal to the EG junction and intestinal metaplasia, characterized by acid mucin–containing goblet cells is detected by biopsy (Fig. 26.3). The requirement of intestinal metaplasia for the diagnosis of Barrett’s esophagus has come under question more recently, however, as described later. In most cases, goblet cells are easily identified on routine hematoxylin and eosin preparations, and special stains such as alcian blue or periodic acid–Schiff (PAS) are unnecessary. However, alcian blue and PAS stain can help avoid overinterpretation of pseudogoblet cells characterized by distended surface foveolar-type cells that stain for PAS but do not contain alcian blue–positive acid mucins (Fig. 26.4).40
It is well recognized that pathologic interpretation of Barrett’s esophagus specimens is problematic in the community and in academic centers. In a community study, intestinal metaplasia without dysplasia was recognized correctly by only 35% of pathologists, and gastric metaplasia without intestinal metaplasia was identified as Barrett’s esophagus in 38% of the cases.41 Pathologic interpretation is also problematic for expert gastrointestinal (GI) pathologists; interobserver reproducibility is substantial at the ends of the spectrum of Barrett’s esophagus—negative for dysplasia and high-grade dysplasia and carcinoma but not especially good for low-grade dysplasia or indefinite for dysplasia.42 There are also problems with interobserver agreement among pathologists in distinguishing high-grade dysplasia from intramucosal cancer, even when evaluating esophagectomy specimens.43,44
Factors that contribute to some of the problems in pathologic interpretation include experience of the pathologist, quality of the slides, and size of the specimens.45 In an effort to improve pathologic interpretation, current practice guidelines now recommend endoscopic mucosal resection (EMR) of any nodularity in the Barrett’s segment before making final treatment decisions.46,47 More recent data support such an approach. Mino-Kenudson and colleagues45 found that interobserver agreement for Barrett’s esophagus–associated neoplasia on EMR specimens was higher than that for mucosal biopsy specimens, especially in distinguishing intramucosal cancer from submucosal cancer.
Differential Diagnosis
The diagnosis of Barrett’s esophagus has clear implications for patients. Patients are subject to surveillance endoscopy at regular intervals; worry about cancer risk, which they typically overestimate; face higher life insurance premiums; and are provided with conflicting information on how best to treat their condition.48,49 It is crucial to diagnose Barrett’s esophagus as accurately as possible given the downstream effects of such a diagnosis.
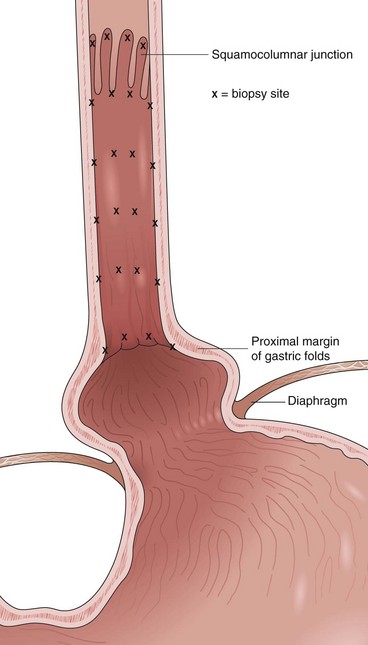
Barrett’s esophagus is defined as a metaplastic change in the lining of the tubular esophagus. Endoscopically, this metaplastic change is characterized by displacement of the squamocolumnar junction proximal to the EG junction defined by the proximal margin of gastric folds (Fig. 26.5). At the time of endoscopy, landmarks should be carefully identified, including the diaphragmatic pinch, the EG junction as best defined by the proximal margin of the gastric folds seen on partial insufflation of the esophagus, and level of the squamocolumnar junction. It is commonly accepted that the proximal margin of the gastric folds is the most useful landmark for the junction of the stomach and the esophagus.50 However, the precise junction of the esophagus and the stomach may be difficult to determine endoscopically because of the presence of a hiatal hernia, the presence of inflammation, and the dynamic nature of the EG junction, all of which may make targeting of biopsy specimens problematic.
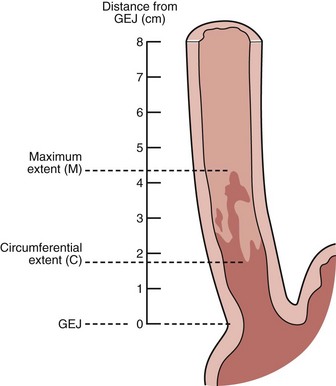
Endoscopists identify landmarks necessary for the diagnosis of the columnar-lined esophagus inconsistently (Fig. 26.6)51; this leads to inconsistencies in defining the length of the columnar-lined esophagus.52 The Prague classification scheme should help improve the description of the columnar-lined esophagus.53 This classification scheme describes the circumferential extent (C value) and maximum extent (M value) of columnar mucosa above the proximal margin of the gastric folds (Fig. 26.7). The Prague classification does not include columnar islands, however. Reliability coefficients for both criteria are excellent for segments greater than 1 cm in length. Recognition of less than 1 cm of columnar metaplasia even with this scoring system is still problematic, pointing out the difficulties in measuring such short segments.
If the squamocolumnar junction is above the level of the EG junction, as defined by the proximal margin of the gastric folds using partial insufflation, biopsy specimens should be obtained for confirmation of columnar metaplasia. There is ongoing debate regarding the presence of intestinal metaplasia for the diagnosis of Barrett’s esophagus.54 The professional societies of North America all require intestinal metaplasia for the diagnosis of Barrett’s esophagus, whereas the British Society of Gastroenterology and a global consensus group do not require the presence of intestinal metaplasia for the diagnosis.46,55–57 More recent evidence suggests that non–goblet cell columnar metaplasia shows DNA content abnormalities indicative of neoplastic risk similar to neoplasias encountered in intestinal metaplasia.58 The issue of intestinal metaplasia versus columnar metaplasia as a diagnostic criterion remains unsettled at the present time.
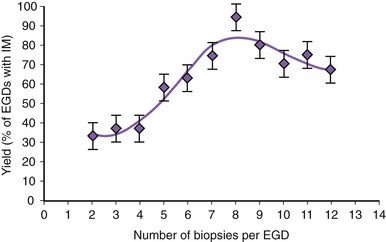
There is no agreement at the present time on the appropriate number of biopsy specimens to obtain to detect intestinal metaplasia. Detection of intestinal metaplasia seems to be related to many factors, including location of biopsies, length of columnar-lined segment, number of biopsy specimens obtained, male gender, and increasing age.59,60 Intestinal metaplasia is more commonly found in biopsy specimens obtained in the proximal portion of the columnar-lined esophagus where goblet cell density is also greater.61 More recent work suggests that the detection of intestinal metaplasia increases with increasing number of biopsy specimens per endoscopy: Four biopsy specimens had a yield of 34.7%, whereas eight biopsy specimens had a yield of 94% for intestinal metaplasia (Fig. 26.8).62 Taking more than eight biopsy specimens does not seem to enhance the yield of intestinal metaplasia.
It may be difficult to determine endoscopically where the esophagus ends and the stomach begins for the reasons outlined earlier. It is impossible to distinguish reliably columnar metaplasia of the distal esophagus from columnar metaplasia of the stomach. A biopsy specimen of the squamocolumnar junction should not be routinely obtained in clinical practice if it is at the level of the EG junction. Intestinal metaplasia may be seen in the cardia of normal individuals and in patients with chronic reflux disease. The prevalence of intestinal metaplasia at a normal-appearing EG junction varies from 5% to 36%.63 In contrast to Barrett’s esophagus, there is no clear gender predominance in patients with intestinal metaplasia of the EG junction and cardia because this condition is more common in older patients who are often infected with Helicobacter pylori and have evidence of gastritis or intestinal metaplasia or both elsewhere in the stomach.8,64 A subset of these patients may have GERD, however, and it is unclear if this condition is a sequela of aging, H. pylori infection, GERD, or some combination of these factors.
Short-segment Barrett’s esophagus is clearly associated with some risk of developing dysplasia and esophageal cancer, which is not substantially lower than the risk in patients with long-segment Barrett’s esophagus. Dysplasia and carcinoma have been reported in patients with intestinal metaplasia of the EG junction or cardia, but the magnitude of that risk seems to be less than the risk of short-segment Barrett’s esophagus.65 A reliable biomarker to distinguish between intestinal metaplasia of the cardia versus intestinal metaplasia of the esophagus would be beneficial. Techniques such as the Das-1 antibody and cytokeratin immunohistochemical staining patterns do not reliably distinguish between these two entities.66 However, some features such as mucosal and submucosal esophageal glands, squamous epithelium overlying columnar crypts with intestinal metaplasia, and hybrid glands characterized by intestinal metaplasia confined to the superficial aspect of cardia-type mucus glands are more often associated with the columnar-lined esophagus than intestinal metaplasia of the cardia.67 Precise targeting of biopsy specimens above the proximal margin of the gastric folds and communication of this information to pathologists are crucial from a quality perspective.
Barrett’s Esophagus and Esophageal Adenocarcinoma
Barrett’s esophagus is a clearly recognized risk factor for the development of esophageal adenocarcinoma compared with the general population.68 Studies show that the incidence of this cancer has increased by approximately sixfold between 1975 and 2001, a rate greater than that of any other cancer in the United States during that time.69 This increase has been accompanied by an increase in mortality rates from 2 to 15 deaths per 1 million during that same time period.69 Similar findings are occurring elsewhere in the Western world. However, the overall burden of esophageal adenocarcinoma remains relatively low. It was estimated that there would be 16,470 new cases of esophageal cancer (not all of which would be adenocarcinoma) in the United States in 2009.70
Despite the alarming increase in the incidence of esophageal adenocarcinoma, the precise incidence of adenocarcinoma in patients with Barrett’s esophagus is uncertain, with rates varying from approximately 1 in 52 to 1 in 694 years of follow-up.71 It is estimated that the risk of developing cancer in a patient with Barrett’s esophagus is approximately 0.5% to 0.7% annually with no clear evidence of geographic variation.71,72 Evolving epidemiologic data suggest that despite the alarming increase in the incidence of esophageal adenocarcinoma, most patients with Barrett’s esophagus never develop esophageal cancer and die of causes other than cancer.73–75 The survival of patients with Barrett’s esophagus is similar to survival of the general population.74
The reason for the increase in the incidence of esophageal adenocarcinoma is unknown. Barrett’s esophagus is clearly a risk factor for adenocarcinoma of the esophagus. Various epidemiologic factors have been identified that either increase or decrease the risk for the development of esophageal adenocarcinoma. The well-accepted risk factors for the development of esophageal adenocarcinoma include increasing age; male gender; white ethnicity; obesity, especially male pattern central obesity; and smoking.76–79 Protective factors include aspirin and nonsteroidal antiinflammatory drug (NSAID) ingestion and a diet high in fruits and vegetables.80–82 Factors of uncertain significance include family history, infection with H. pylori, alcohol consumption, antireflux therapy (surgical or pharmacologic), and dietary supplements.79
Cancer Biology
Compelling evidence exists for a dysplasia-carcinoma sequence in Barrett’s esophagus whereby nondysplastic columnar epithelium progresses to low-grade dysplasia, high-grade dysplasia, and finally to carcinoma. Foci of carcinoma typically appear adjacent to dysplasia.83 The time course for this progression is highly variable, and most patients never progress to dysplasia.
It is hypothesized that cancer develops in a subset of patients who have acquired genomic instability in Barrett’s epithelium.84 This genomic instability predisposes to the development of abnormal clones of cells that accumulate progressively more genetic errors, which include numerical and structural chromosomal rearrangements, gene mutations, loss of normal cell cycle control, and increased cell proliferation rates.85,86 Among the most frequently described molecular changes that precede the development of adenocarcinoma in Barrett’s esophagus are alterations in p53 (mutation, deletion, or loss of heterozygosity [LOH]) and p16 (mutation, deletion, promoter hypermethylation, or LOH) and aneuploidy.87–90 However, there is no clearly predictable sequence of genetic abnormalities that leads to the development of cancer. Upregulation of cyclooxygenase-2 (COX-2) expression also occurs in the metaplasia-dysplasia-carcinoma sequence.91,92 Increased COX-2 expression is associated with increased cellular proliferation and decreased apoptosis in vitro, and administration of selective COX-2 inhibitors can decrease cell growth and increase apoptosis in esophageal adenocarcinoma cell lines.93 This finding may have implications for chemoprevention strategies under investigation.
Screening and Surveillance Strategies for Barrett’s Esophagus
Esophageal adenocarcinoma is a lethal disease with a 5-year survival of approximately 12% to 14%.94,95 Survival depends on the stage, and early spread before the onset of symptoms is characteristic of this tumor. Early invasive cancer may be classified as intramucosal when neoplastic cells penetrate through the basement membrane to the lamina propria or muscularis mucosa and submucosal when neoplastic cells infiltrate into the submucosa. The prognosis for these two lesions is very different because the risk of lymph node metastasis is approximately 0% to 7% for intramucosal cancer but increases to 5% to 50% for submucosal cancer.96–99 Lymph node metastases are a clear prognostic factor for decreased survival.100 Approximately 95% of esophageal adenocarcinomas are diagnosed in patients without a prior diagnosis of Barrett’s esophagus.9 The best hope for improved survival of patients with esophageal adenocarcinoma is detection of cancer at an early and potentially curable stage.
Screening
One potential strategy to decrease the mortality rate of esophageal adenocarcinoma further is to identify more patients at risk, such as patients with Barrett’s esophagus. Population-based studies suggest that in patients with newly diagnosed esophageal adenocarcinoma, a prior endoscopy and diagnosis of Barrett’s esophagus was associated with both early stage cancer and improved survival.101 Only a few patients with esophageal adenocarcinoma have undergone prior endoscopy, however.102 Current professional society practice guidelines equivocate on screening patients with chronic GERD symptoms for Barrett’s esophagus.46,47,56 The 2009 American Cancer Society cancer screening guidelines does not include any recommendation for screening of either esophageal cancer or Barrett’s esophagus.103
Endoscopy with biopsy is still the only validated technique to diagnose Barrett’s esophagus. It has clear limitations as a screening tool, however, including cost, risk, and complexity. If screening with endoscopy and biopsy were applied to the estimated 20% of the population with regular GERD symptoms, the cost implications would be staggering.104 Unsedated upper endoscopy using small-caliber instruments still has the potential to change the economics of endoscopic screening because this technique may decrease sedation-related complications and costs. Unsedated small-caliber endoscopy detects Barrett’s esophagus and dysplasia with sensitivity comparable to conventional endoscopy.105 Although both procedures are well tolerated by patients, a major hurdle for unsedated endoscopy is patient resistance to undergoing a test without sedation. It is uncertain if endoscopy without sedation would meet with patient acceptance given the cultural preference for sedation in the United States. Otherwise, there are still no validated alternative techniques to screen for Barrett’s esophagus that overcome the cost and risks associated with conventional upper endoscopy.
There has been considerable interest in esophageal capsule endoscopy as a screening alternative to conventional upper endoscopy. Studies to date show a sensitivity of 60% to 79% and a specificity of 75% to 100% compared with conventional upper endoscopy.106–108 Modeling studies suggest that capsule endoscopy is not a cost-effective alternative to conventional endoscopy either.109 Adoption of esophageal capsule endoscopy as a screening alternative to upper endoscopy is unlikely in the near future.
After a normal initial upper endoscopy, some clinicians wonder if a repeat screening upper endoscopy should be undertaken in symptomatic GERD patients at a later date. Several studies have addressed this point with consistent results. In patients with nonerosive reflux disease at the index endoscopy, Barrett’s esophagus is rarely found if the repeat endoscopy is performed within 5 years.110,111 Barrett’s esophagus may be present in 9% to 12% of patients with erosive esophagitis at the time of index endoscopy, and higher grades of esophagitis are associated with a higher case finding rate of Barrett’s esophagus on repeat endoscopy.112,113 Screening for Barrett’s esophagus in GERD patients should take place only after initial therapy with a proton pump inhibitor (PPI). A negative endoscopy at baseline makes it highly unlikely to find Barrett’s esophagus if endoscopy is repeated.
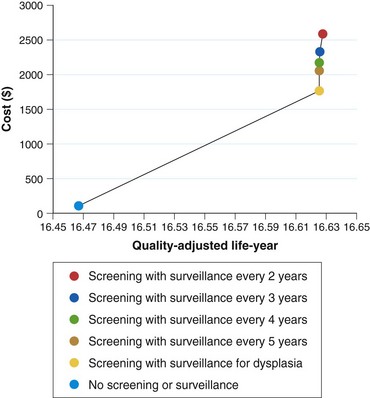
There are still no data from randomized controlled trials or observational studies to evaluate the strategy of screening. A decision analysis model by Inadomi and colleagues114 examined screening of 50-year-old white men with chronic GERD symptoms for Barrett’s esophagus and found that one-time screening is probably cost-effective if subsequent surveillance is limited to patients with dysplasia on initial examination (Fig. 26.9). This strategy would result in a cost of $10,440 per quality-adjusted life year saved compared with a strategy of no screening or surveillance. Other modeling studies support screening in patients with chronic GERD symptoms as well but only if the following conditions are met: patients at high risk for Barrett’s esophagus, high-grade dysplasia, or adenocarcinoma; high sensitivity and specificity of endoscopy with biopsy; and little or no reduction in quality of life with esophagectomy.115,116 Any variation of these ideal conditions quickly made this strategy cost-ineffective.
There is clearly a need to develop either a better profile of patients at high risk for Barrett’s esophagus and high-grade dysplasia or a far less expensive tool to provide mass population screening. A simple questionnaire and nomogram has been described in an effort to predict Barrett’s esophagus in patients with GERD symptoms.117 The sensitivity of this questionnaire for predicting Barrett’s esophagus was 77% with a specificity of only 63%. Although clearly cost saving, this questionnaire would miss patients with Barrett’s esophagus with GERD symptoms and not account for individuals without any symptoms of GERD.
Surveillance
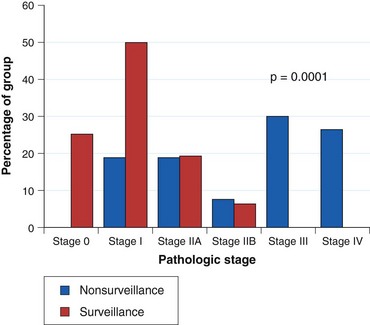
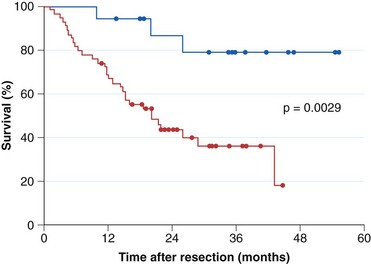
Current practice guidelines recommend endoscopic surveillance of patients with Barrett’s esophagus in an attempt to detect cancer at an early and potentially curable stage.46,47,55,56 Numerous observational studies suggest that patients with Barrett’s esophagus in whom adenocarcinoma was detected in a surveillance program have their cancers detected at an earlier stage (Fig. 26.10), with markedly improved 5-year survival compared with similar patients not undergoing routine endoscopic surveillance (Fig. 26.11).102,118–121 Nodal involvement is far less likely in patients undergoing surveillance compared with patients not undergoing surveillance.120 Because survival in esophageal cancer is stage-dependent, these studies suggest that survival may be enhanced by endoscopic surveillance. Several decision-analysis models support the concept of endoscopic surveillance.114,122,123 The model of Provenzale and coworkers122 suggests that surveillance every 5 years is the most effective strategy to increase length and quality of life, whereas the model of Inadomi and colleagues114 suggests that surveillance should be limited only to individuals with dysplasia at the time of initial endoscopy.
Other authors argue that because most patients with Barrett’s esophagus do not die of esophageal cancer, the benefit for surveillance remains uncertain, and endoscopic surveillance is not warranted until substantiated by prospective studies.124 Design flaws such as selection bias, healthy volunteer bias, lead time bias, and length time bias are inherent in the observational studies that support endoscopic surveillance. The resources encumbered by vigorous endoscopic surveillance are considerable. Despite the concern regarding the esophageal cancer “epidemic,” the overall burden of disease is limited in the Western world compared with other malignancies such as colon cancer. A randomized controlled trial of surveillance versus no surveillance in Barrett’s esophagus has not been performed and is not likely to be performed in the future.
Candidates for Endoscopic Surveillance
Patients with documented Barrett’s esophagus are candidates for surveillance. Before entering into a surveillance program, patients should be advised about risks and benefits, including the limitations of surveillance endoscopy and the importance of adhering to appropriate surveillance intervals.47,56,125 Other considerations include age, likelihood of survival over the next 5 years, and ability to tolerate either endoscopic or surgical interventions for early esophageal adenocarcinoma.
Surveillance Techniques
The aim of surveillance is to detect dysplasia. The description of dysplasia should use a standard five-tier system: (1) negative for dysplasia, (2) indefinite for dysplasia, (3) low-grade dysplasia, (4) high-grade dysplasia, and (5) carcinoma.42 Active inflammation makes it more difficult to distinguish dysplasia from reparative changes. It is essential that surveillance endoscopy is performed only after any active inflammation related to GERD is controlled with antisecretory therapy. The presence of ongoing erosive esophagitis is a contraindication to performing surveillance biopsies.
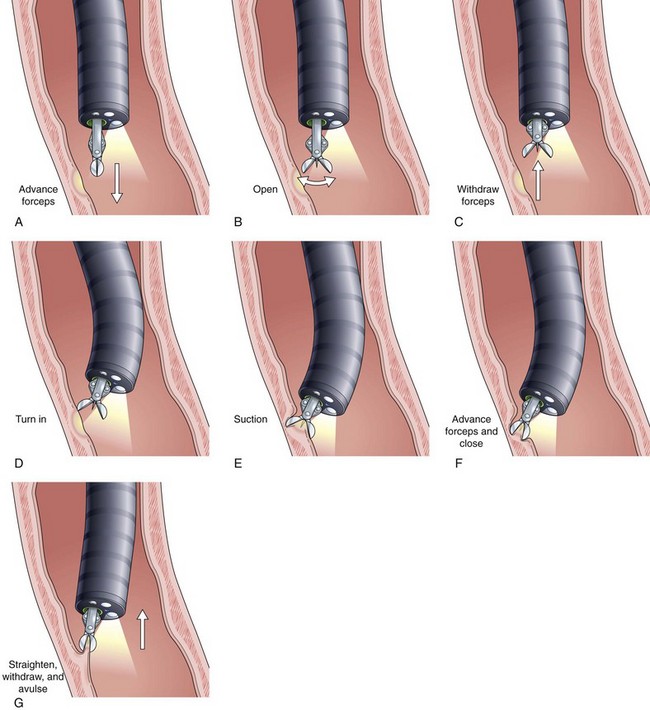
Current guidelines suggest obtaining systematic four-quadrant biopsy specimens at 2-cm intervals along the entire length of the Barrett’s segment after inflammation related to GERD is controlled with antisecretory therapy (Fig. 26.12).46,47,55,56 A systematic biopsy protocol clearly detects more dysplasia and early cancer compared with ad hoc random biopsies.126,127 Extensive biopsies of subtle mucosal abnormalities, no matter how trivial, such as ulceration, erosion, plaque, nodule, stricture, or other luminal irregularity in the Barrett’s segment, should also be performed because there is an association of such lesions with underlying cancer.128 Current guidelines also recommend, however, that patients with mucosal abnormalities, especially in the setting of high-grade dysplasia, should undergo EMR.46,47 Studies suggest that EMR changes the diagnosis in approximately 50% of patients, given the larger tissue sample available for review by the pathologist.129 The “turn and suction” technique (Fig. 26.13) allows acquisition of biopsy specimens that are significantly larger than the specimens obtained by the traditional techniques of advancing an open biopsy forceps into the lumen and then closing it to obtain the biopsy sample.130 The safety of systematic endoscopic biopsy protocols has been shown.131
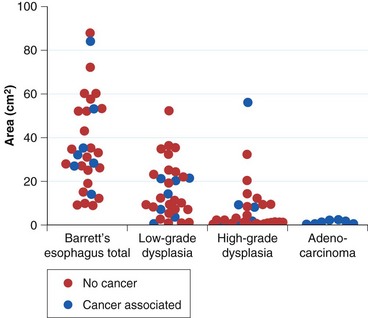
The rationale for such a comprehensive biopsy program comes from observations that high-grade dysplasia and early carcinoma in Barrett’s esophagus often occur in the absence of endoscopic abnormalities and from the focal nature of dysplasia. Systematic esophagectomy mapping studies show just how focal dysplasia and superficial cancer may be. In 30 esophagectomy specimens from patients undergoing surgery for either high-grade dysplasia or early invasive adenocarcinoma with no endoscopic evidence of cancer, the median surface area of total Barrett’s esophagus was found to be 32 cm2; low-grade dysplasia, 13 cm2; high-grade dysplasia, 1.3 cm2; and adenocarcinoma, 1.1 cm2 (Fig. 26.14).132 The three smallest cancers had surface areas of 0.02 cm2, 0.3 cm2, and 0.4 cm2.
Because of the focal nature of dysplasia and cancer, some experts recommend that endoscopic surveillance should use a large particle (jumbo) forceps to obtain biopsy specimens.133 However, other authors have found that jumbo forceps biopsies still miss cancer in patients with high-grade dysplasia if the biopsies are performed at both 1-cm and 2-cm intervals.134 This technique requires passage of a therapeutic endoscope, and the generalizability of this technique to clinical practice is problematic. Large-capacity forceps are available for passage through standard diameter endoscopes, which should render this issue less important than in the past. The extensive use of EMR has changed biopsy sampling considerably. Current guidelines suggest that available evidence does not support the routine use of the jumbo biopsy forceps.47
Surveillance Intervals
Surveillance intervals, determined by the presence and grade of dysplasia, are based on the limited understanding of the biology of esophageal adenocarcinoma. The most recently published recommendations from the American College of Gastroenterology are shown in Table 26.1. These intervals are arbitrary, however; they have never been subject to a clinical trial and likely never will be. Guidelines from various professional societies are not in agreement on surveillance intervals or techniques. The American College of Gastroenterology46 and the American Society for Gastrointestinal Endoscopy55 both recommend surveillance every 3 years as adequate in patients without dysplasia after two negative examinations. The American Gastroenterological Association47 recommends extending the surveillance interval up to 5 years, whereas the British Society of Gastroenterology56 recommends continued surveillance at 2-year intervals in this setting.
Table 26.1 2008 American College of Gastroenterology Practice Guidelines for Endoscopic Surveillance of Barrett’s Esophagus
| Dysplasia Grade | Interval |
|---|---|
| None | Every 3 yr after two endoscopies are negative within 1 yr |
| Low-grade | Expert GI pathologist confirmation |
| Repeat endoscopy within 6 mo to ensure no higher grade of dysplasia | |
| Every year until no dysplasia on two consecutive endoscopies | |
| High-grade | Expert GI pathologist confirmation |
| If Barrett’s segment flat—redo endoscopy and biopsies within 3 mo | |
| If mucosal abnormality—endoscopic mucosal resection | |
| Counsel patient with options | |
| Intensive surveillance | |
| Endoscopic therapy | |
| Esophagectomy |
GI, gastrointestinal.
Adapted from Wang KK, Sampliner RE: Updated guidelines 2008 for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 103:788–797, 2008.
If low-grade dysplasia is found, the diagnosis should first be confirmed by an expert GI pathologist owing to the marked interobserver variability in interpretation of these biopsy specimens. Data suggest that if there is a consensus diagnosis by two or three expert GI pathologists, the risk of progression is greater than if there is no such agreement.135 These patients should receive aggressive antisecretory therapy for reflux disease with a PPI to decrease the chances of regeneration that make pathologic interpretation of this category so difficult. A repeat endoscopy should be performed within 6 months of the initial diagnosis. If low-grade dysplasia is confirmed, annual surveillance is recommended when low-grade dysplasia is present until two examinations in a row are negative.46 There is no agreement on the biopsy protocol to use, although a protocol of four-quadrant biopsy specimens at 1-cm intervals as would be used for high-grade dysplasia makes sense. EMR should be performed if any mucosal abnormality is present in these patients.
If high-grade dysplasia is found, the diagnosis should first be confirmed by an experienced GI pathologist as well. If the segment is flat and without any mucosal abnormalities, the endoscopic biopsy protocol should be repeated within 3 months to exclude an unsuspected carcinoma using careful inspection with high-quality white light endoscopy.46 It is still unclear how much enhanced imaging techniques add to careful inspection with high-resolution or high-definition white light endoscopy (see later). The presence of any mucosal abnormality warrants EMR in an effort to maximize staging accuracy.
If high-grade dysplasia is confirmed, there is no consensus on the appropriate management of these patients. Options include continued surveillance, endoscopic therapy, or esophagectomy. Although continued surveillance has been compared with endoscopic approaches in randomized controlled trials, esophagectomy has not been compared with endoscopic ablative therapy in any randomized controlled trials.136 Observational studies suggest, however, that survival and cancer-free survival are comparable in patients treated either surgically or endoscopically for high-grade dysplasia.137 If continued surveillance is chosen, one proposed option is surveillance at 3-month intervals for 1 year.138 If there is no high-grade dysplasia on two consecutive endoscopies for the first year, endoscopy frequency is lengthened to every 6 months for the second year and then to annually thereafter as long as high-grade dysplasia is not encountered again. If high-grade dysplasia persists, continued short-interval endoscopy is warranted.
The extent of high-grade dysplasia is thought by some authors to be a risk factor for the subsequent development of adenocarcinoma.139 However, there are currently no uniform criteria for defining the extent of high-grade dysplasia, and there are conflicting data on the clinical significance of extent of high-grade dysplasia in biopsy specimens and risk for unsuspected carcinoma.139,140 Mucosal abnormalities in patients with multifocal high-grade dysplasia may also be a risk factor for adenocarcinoma.141,142 High-grade dysplasia remains a worrisome lesion, although progression to carcinoma may take many years and is not inevitable. The ultimate approach to a patient with high-grade dysplasia should consider factors such as available surgical and endoscopic expertise; age of the patient; length of Barrett’s epithelium that would require biopsy to eliminate sampling error; compliance with endoscopic surveillance; future need for multiple surveillance endoscopies; and suspicious lesions such as plaques, nodules, and strictures.
Limitations of Surveillance
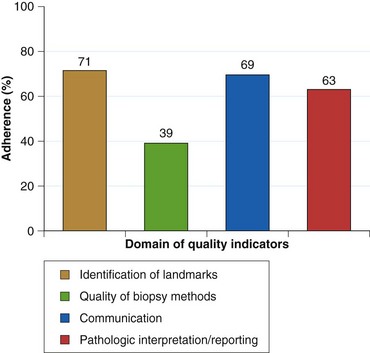
As currently practiced, endoscopic surveillance of Barrett’s esophagus has numerous shortcomings. Dysplasia and early adenocarcinoma are endoscopically indistinguishable from intestinal metaplasia without dysplasia. The distribution of dysplasia and cancer is highly variable, and even the most thorough biopsy surveillance program has the potential for sampling error. There are considerable interobserver variability and quality control problems in the interpretation of dysplasia in both community and academic settings. Current surveillance programs are expensive and time-consuming. Survey data indicate that although surveillance is widely practiced, there is considerable variability in the technique and interval of surveillance because practice guidelines are not widely followed in the community (Fig. 26.15).143–146 However, education programs can enhance compliance with guidelines.145
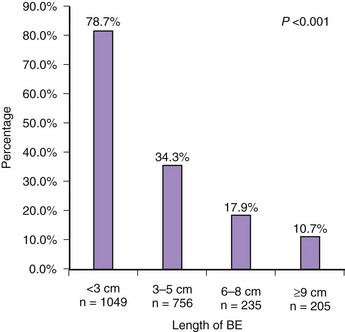
Fig. 26.15 Adherence to Seattle biopsy protocol in the community setting by length of Barrett’s esophagus.
(From Abrams JA, Kapel RC, Lindberg GM, et al: Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol 7:736–742, 2009.)
Potential Strategies to Enhance Surveillance
Chromoendoscopy
Methylene blue is a vital stain that selectively diffuses into the cytoplasm of absorptive epithelium of the small intestine and colon. The presence of staining in the esophagus indicates the presence of intestinal metaplasia.147 Some studies have suggested that methylene blue chromoendoscopy increases the efficiency of detecting dysplasia; fewer biopsies are required, and more patients are identified with dysplasia compared with four-quadrant biopsy specimens obtained at 2-cm intervals.148 However, other studies were unable to detect any differences in detection of dysplasia between methylene blue–directed biopsies compared with a standard biopsy protocol.149 Chromoendoscopy is appealing because it is simple, inexpensive, and safe. However, there is no agreement on application technique in terms of the concentration, volume, and “dwell time” of various reagents, and interpretation of staining is subjective. Methylene blue chromoendoscopy also adds additional procedure time. A meta-analysis found that methylene blue chromoendoscopy resulted in no incremental yield compared with random biopsies for the detection of intestinal metaplasia, high-grade dysplasia, or early cancer.150
Acetic acid has also been used as a chromoendoscopy technique. Application of 3% acetic acid in 100 patients with Barrett’s esophagus showed a normal uniform pattern in 85 patients, none of whom had dysplasia, and an abnormal irregular pattern in 15, of whom 87% had dysplasia.151 Other authors showed that acetic acid–enhanced endoscopy improves image quality but does not increase the detection rate of dysplasia or adenocarcinoma.152
Optical Contrast Endoscopy
Various optical imaging enhancements have been developed to enable detailed inspection of the mucosal and vascular surface patterns. Narrow band imaging involves the placement of optical filters that narrow the band width of white light to blue light. This technique allows for detailed imaging of the mucosal and vascular surface patterns in Barrett’s esophagus without the need for chromoendoscopy. Two postprocessing software driven systems are available to accomplish similar visualization (iScan System and FICE). Almost all of the published literature to date has examined narrow band imaging.153
Studies of narrow band imaging with optical zoom found that irregular mucosal and vascular patterns were highly predictive of early neoplasia.154,155 A tandem endoscopy study that compared standard resolution white light endoscopy with narrow band imaging combined with high-definition white light endoscopy found the latter to be superior to conventional white light endoscopy for the detection of early neoplasia and required fewer biopsy specimens to accomplish this.156 Other studies suggest that although narrow band imaging improves overall image quality, it does not lead to enhanced detection of early neoplasia compared with high-resolution white light endoscopy.152,157
A more recent systematic review found that narrow band imaging has a sensitivity of 77% to 100% and a specificity of 79% to 94% for the detection of intestinal metaplasia.158 The sensitivity for the detection of high-grade dysplasia or cancer is 77% to 100% with a specificity of 58% to 100%. There are many unresolved issues regarding narrow band imaging, however, including multiple classification schemes for the mucosal and vascular patterns, image interpretation based on still images instead of real-time endoscopy, the use of optical versus electronic zoom, and the use of study populations enriched with early neoplasia in tertiary care centers. Despite these limitations, narrow band imaging is a promising technique for distinguishing intestinal metaplasia from gastric columnar metaplasia and early neoplasia from nonneoplastic tissue. It also may be useful for detecting residual columnar epithelium after ablative therapies. All classification schemes concentrate on regular versus irregular mucosal and vascular patterns.
Spectroscopy
Initial work with laser-induced fluorescence spectroscopy in a group of 36 patients had a sensitivity of 100% for high-grade dysplasia and a specificity of 70% for no dysplasia, but all six patients with low-grade dysplasia were classified as benign by laser-induced fluorescence spectroscopy.159 A spectroscopic probe that combined the techniques of fluorescence, reflectance, and light scattering spectroscopy in 16 patients with Barrett’s esophagus had a sensitivity and specificity of 100% for separating high-grade dysplasia from low-grade dysplasia and no dysplasia and a sensitivity of 93% with a specificity of 100% for separating any dysplasia from no dysplasia.160 Elastic scattering spectroscopy has also shown promise.161 However, spectroscopic techniques, as currently configured, require a “point and shoot” method of touching the mucosa with the probe followed by biopsy. To be clinically helpful, these techniques need to image a larger field by “spraying light” followed by targeted biopsies of abnormal optical regions.
Optical Coherence Tomography
Optical coherence tomography uses infrared light to produce high-resolution images of mucosal tissue in vivo. Current technology is limited to a “touch and image” technique and is unable to sample large areas rapidly. Data to date suggest that the accuracy of current systems is insufficient for clinical application.162
Autofluorescence Endoscopy
Autofluorescence endoscopy is an imaging technique with the potential to examine a large surface area of GI mucosa rapidly to detect small areas of dysplasia or cancer. Autofluorescence endoscopy is based on the principle that normal, metaplastic, and dysplastic tissues have different autofluorescence colors visible to the naked eye. It involves illumination of tissue of interest with short-wavelength light, which leads to excitation of endogenous substances known as fluorophores and emission of fluorescence light of longer wavelengths.163
Early work with autofluorescence endoscopy involved fiberoptic technology. Despite some promising preliminary studies, a randomized crossover trial of fiberoptic autofluorescence endoscopy versus white light video endoscopy in Barrett’s esophagus found no difference in the detection rates for high-grade dysplasia or adenocarcinoma between the two techniques.164 Fiberoptic autofluorescence endoscopy was limited by poor image quality and poor maneuverability of the bulky imaging platform, making this technique of limited clinical value.
Although fiberoptic autofluorescence endoscopy seems to provide no advantage over conventional white light imaging, video-based technology, especially when combined with narrow band imaging, offers potential for enhancing endoscopic surveillance of Barrett’s esophagus. Kara and associates165 found that video-based autofluorescence endoscopy increased the detection of high-grade dysplasia and early carcinoma lesions that were not seen with high-resolution white light video endoscopy. When used as a standalone technique, however, autofluorescence endoscopy was still associated with a high number of false-positive areas of abnormal fluorescence. In this study, 28 of 81 regions (35%) of abnormal fluorescence were associated with no dysplasia.
The combination of high-resolution white light, narrow band imaging, and autofluorescence in one endoscope is known as trimodal imaging. In one study, the combination of the three techniques decreased the false-positive rate from 40% to 10%, whereas in a second study, the false-positive rate was decreased from 81% to 26%, and the detection rate of intramucosal carcinoma or high-grade dysplasia lesions was increased compared with high-resolution white light endoscopy alone.166,167 Taken together, these studies show the promise of autofluorescence endoscopy when combined with narrow band imaging and high-resolution white light endoscopy for surveillance of Barrett’s esophagus. For this technology to have future clinical application, image quality still needs to be improved, and the false-positive rate needs to be decreased further.
Confocal Laser Endomicroscopy
Confocal laser endomicroscopy is a new endoscopic imaging technique that allows for subsurface imaging and in vivo histologic assessment of the mucosal layer during standard white light endoscopy.168 It is a potentially ideal small field imaging technique that optimally should be used with a “red flag” method to target image acquisition. The goal of endomicroscopy is to distinguish neoplastic from nonneoplastic tissue and provide the potential for decreased number of biopsies. Two different platforms are available: an endoscope-based device that is integrated into the distal tip of the endoscope and a probe-based device that can be inserted through a standard endoscope. Both devices require administration of fluorescein, an intravenous fluorescence agent.
Several studies have examined confocal laser endomicroscopy in Barrett’s esophagus. Using a scope-based device, a confocal Barrett’s esophagus classification scheme involving cellular and vascular architecture was developed to predict histopathology in the columnar-lined segment. The sensitivity and specificity for the prediction of nondysplastic Barrett’s epithelium were 98% and 94% and for Barrett’s esophagus–associated neoplasia were 93% and 98%. The κ value for interobserver agreement was .843.169 A similar pilot study of probe-based confocal laser microscopy yielded a sensitivity of 75% to 89% and specificity of 75% to 91%.170 More recent work also suggests that confocal endomicroscopy with the scope-based technique improves the diagnostic yield of endoscopically inapparent neoplasia compared with standard white light endoscopy and surveillance biopsies.171
Risk Stratification
Numerous clinical and biologic markers may define patients at increased risk for the development of adenocarcinoma. Clinical risk factors for the development of high-grade dysplasia or adenocarcinoma include gender, ethnicity, age, dysplasia, hiatal hernia size, length of Barrett’s segment, body mass index, and smoking.79 Although esophageal cancer develops in both short and long segments of Barrett’s esophagus, segment length does not seem to be a strong risk factor for the development of cancer.71,172
Dysplasia is still the best available marker of cancer risk. Dysplasia is recognized adjacent to and distant from Barrett’s esophagus–associated adenocarcinoma in resection specimens from patients with Barrett’s esophagus. Patients with Barrett’s esophagus progress through a phenotypic sequence of no dysplasia, low-grade dysplasia, high-grade dysplasia, and adenocarcinoma, although the time course is highly variable, and this stepwise sequence is not preordained.120,173 Some patients may progress directly to cancer without prior detection of dysplasia of any grade.174 Dysplasia is the only factor at the present time that is useful in clinical practice for identifying patients at increased risk for the development of esophageal adenocarcinoma.
The natural history of low-grade dysplasia is poorly understood. First, the diagnosis is often transient175,176; this may be due in part to the high degree of interobserver variability in establishing this diagnosis and the variable biopsy protocols by which these patients are followed, resulting in issues related to tissue sampling. Although most patients with low-grade dysplasia do not progress to adenocarcinoma or high-grade dysplasia, a subset of these patients do progress to a higher grade lesion. More recent studies suggest an intermediate risk of progression to cancer with a weighted average incidence rate of 1.69% per year.177 Factors associated with progression to cancer include consensus agreement among two or more pathologists and extent of low-grade dysplasia.135,178
High-grade dysplasia in Barrett’s esophagus is a well-recognized risk factor for the development of adenocarcinoma.138,139,179 Unsuspected carcinoma is detected at esophagectomy in approximately 40% of patients with high-grade dysplasia (range 0% to 73%).180 More recent studies using EMR before esophagectomy suggest that finding unsuspected cancer is reduced considerably to approximately 13%.137 Several studies have improved understanding of the natural history of high-grade dysplasia. Buttar and colleagues139 followed 100 patients with high-grade dysplasia with continued endoscopic surveillance and found cancer at 1 year and 3 years in 38% and 56% of individuals with diffuse high-grade dysplasia and 7% and 14% of individuals with focal high-grade dysplasia. Reid and associates179 followed 76 patients for 5 years and encountered cancer in 59%. Schnell and colleagues138 found cancer in 5% of 79 patients during the first year of surveillance and in 16% of the remaining patients followed for a mean of 7 years (20% of the total group developed cancer). Other studies have reported regression of high-grade dysplasia over time as well.138,181 A more recent meta-analysis found that the incidence of adenocarcinoma in patients with high-grade dysplasia was approximately 6.58% annually.182 Mucosal abnormalities in patients with multifocal high-grade dysplasia may also be a risk factor for adenocarcinoma.139,183 Although progression to carcinoma may take many years and is not inevitable, high-grade dysplasia remains a worrisome lesion.
Biomarkers of Increased Risk
Numerous molecular markers may define patients at increased risk for the development of esophageal adenocarcinoma. Among the most frequently described molecular changes that precede the development of adenocarcinoma in Barrett’s esophagus are alterations in p53 (mutation, deletion, or LOH)87,88,184,185 and p16 (mutation, deletion, promoter hypermethylation, or LOH)89,90,186 and aneuploidy by flow cytometry.187,188 Neoplastic progression in Barrett’s esophagus is accompanied by flow cytometric abnormalities such as aneuploidy or increased G2/tetraploid DNA contents, and these abnormalities may precede the development of high-grade dysplasia or adenocarcinoma. The potential importance of flow cytometry as a prognostic biomarker was illustrated in work by Reid and colleagues,189 who found that for patients with no flow cytometric abnormalities at baseline and with histology that showed no dysplasia, indefinite dysplasia, or low-grade dysplasia, the 5-year incidence of cancer was 0%. In contrast, aneuploidy, increased 4N fractions, or high-grade dysplasia was detected in each of the 35 patients who went on to develop cancer within 5 years.
Mutations of p53 and 17p LOH have been reported in 92% and 100% of esophageal adenocarcinomas.190 Both abnormalities have been detected in Barrett’s epithelium before the development of carcinoma. Reid and colleagues88 found that the prevalence of 17p (p53) LOH at baseline increased from 6% in patients negative for dysplasia to 20% in patients with low-grade dysplasia and to 57% in patients with high-grade dysplasia. More importantly, the 3-year incidence of cancer was 38% for individuals with 17p (p53) LOH compared with 3.3% for individuals with two 17p alleles. However, techniques to detect p53 mutations and 17p LOH are labor-intensive and have not achieved widespread acceptance in clinical practice to date. Similarly, p16 LOH and inactivation of the p16 gene by promoter region hypermethylation have been reported frequently in esophageal adenocarcinoma.90 9p LOH is commonly encountered in premalignant Barrett’s epithelium and can be detected over large regions of the Barrett’s mucosa.90 It is hypothesized that clonal expansion occurs in conjunction with p16 abnormalities creating a field in which other genetic lesions leading to esophageal adenocarcinoma can arise.
Epigenetic changes in the form of hypomethylation and hypermethylation and alteration to histone complexes have also been implicated in the progression of Barrett’s esophagus to adenocarcinoma. Hypermethylation of p16, RUNX3, and HPP1 all are independently associated with an increased risk of progression of Barrett’s esophagus to high-grade dysplasia or esophageal adenocarcinoma.191
Given the complexity and diversity of alterations observed to date in the metaplasia-dysplasia-carcinoma sequence, a panel of biomarkers may be required for risk stratification. Two studies examined the use of biomarkers with promising results. The combination of 17p LOH, 9p LOH, and DNA content abnormality has been shown to predict 10-year adenocarcinoma risk better than any single biomarker alone. Patients with a combination of these abnormalities had a markedly increased risk of developing cancer compared with patients with no baseline abnormalities (relative risk 38.7, 95% confidence interval 10.8 to 138.5). In patients with no abnormalities of any of these biomarkers at baseline, 12% developed adenocarcinoma at 10 years. In contrast, patients with the combination of 17p LOH, 9p LOH, and DNA content abnormality had a cumulative incidence of adenocarcinoma of 79% over the same period.192 A risk stratification model using a methylation index constructed from the methylation values for p16, HPP1, and RUNX3 also showed potential for prediction of progression to high-grade dysplasia or adenocarcinoma.193 Although these studies show the potential for biomarkers to predict risk of esophageal adenocarcinoma, none of these biomarkers have been validated in large-scale clinical trials to date and are not yet useful for clinical decision making.
Treatment
Medical Therapy
Because Barrett’s esophagus has the most severe pathophysiologic abnormalities of GERD, PPIs are the cornerstone of medical therapy for Barrett’s esophagus. Studies show that PPIs consistently result in symptom relief and heal esophagitis in patients with Barrett’s esophagus.194–196 However, even at high doses, PPIs result in either no regression of the Barrett’s segment or modest regression that is of uncertain clinical importance.197–199 PPIs typically increase squamous islands in the Barrett’s segment, but biopsy specimens taken from such islands often show underlying intestinal metaplasia.200
Alleviation of reflux symptoms in Barrett’s esophagus is not equivalent to normalization of esophageal acid exposure, despite the use of high-dose PPI therapy. Persistent abnormal acid exposure is encountered in approximately 25% of patients with Barrett’s esophagus despite use of twice-daily PPIs.201,202 The importance of complete control of esophageal acid exposure in patients with Barrett’s esophagus is unknown. Some studies provide support, however, for the concept of aggressive acid suppression in these patients. Normalization of intraesophageal acid exposure in patients with Barrett’s esophagus decreases cellular proliferation rates and increases cellular differentiation rates over 6 months, whereas inability to normalize intraesophageal acid exposure results in no difference in proliferation or differentiation rates.203 However, this change is not accompanied by any effect on apoptosis or COX-2 levels.204
A more recent Veterans Administration cohort study suggested that PPI therapy, especially long duration use, was associated with a decreased risk for the development of dysplasia.205 However, most of the cases of dysplasia were low-grade—a lesion with an intermediate and highly variable risk for development of cancer. Similar observational data on reduction of dysplasia risk with administration of PPIs have been obtained in Australia.206 However, there are no randomized controlled trials that have examined the issue of dysplasia or cancer prevention and administration of PPIs as standalone therapy.
Antireflux Surgery
Antireflux surgery effectively alleviates GERD symptoms in patients with Barrett’s esophagus.207–209 A randomized controlled trial that was completed more recently found that the outcome of laparoscopic antireflux surgery was similar in GERD patients with and without Barrett’s esophagus and comparable to use of esomeprazole, although antireflux surgery afforded better intraesophageal acid control.210 The indications for surgery in patients with Barrett’s esophagus are the same as the indications for GERD patients without Barrett’s esophagus.
Some authors have hypothesized that antireflux surgery provides protection from progression of Barrett’s esophagus to adenocarcinoma.211 However, two lines of evidence suggest that antireflux surgery does not protect patients from developing esophageal adenocarcinoma. A large population-based cohort study of GERD patients from Sweden who underwent antireflux surgery found that surgery did not protect against the development of esophageal adenocarcinoma.212 The standardized incidence ratio of esophageal adenocarcinoma in the surgically treated group was 14.1 (95% confidence interval 8.0 to 22.8) compared with 6.3 (95% confidence interval 4.5 to 8.7) in the medically treated group.
A Veterans Administration cohort study also found no attenuation of the risk for developing esophageal adenocarcinoma in GERD patients treated surgically compared with patients treated medically (0.072%/yr vs. 0.04%/yr).213 Similar findings are seen in patients with Barrett’s esophagus. A meta-analysis of surgical versus medical therapy of Barrett’s esophagus found no difference in the risk of esophageal adenocarcinoma between the two groups.214 A subsequent systematic review by Chang and coworkers215 found no difference in the incidence of esophageal adenocarcinoma in medically treated versus surgically treated patients and found that any evidence suggesting otherwise was driven by uncontrolled case series. The best available evidence suggests that antireflux surgery does not decrease cancer risk in patients with GERD or Barrett’s esophagus.
Chemoprevention
Chemoprevention is pharmacologic intervention for either the prevention of cancer or the treatment of identifiable precancerous lesions.216 Various chemoprevention agents have been proposed for patients with Barrett’s esophagus, including PPIs, celecoxib, aspirin, lyophilized black raspberries, antioxidants, green tea, retinoids, ursodeoxycholic acid, statins, and curcumin.217–219
Most attention at the present time is directed toward the use of aspirin and NSAIDs. Observational studies suggest that NSAIDs, including aspirin, may play a protective role against esophageal adenocarcinoma by inhibiting COX-1 and COX-2 enzymes, which regulate prostaglandin E2 production.80,81,220,221 Animal studies also suggest that administration of selective and nonselective cyclooxygenase inhibitors decreases the development of esophageal adenocarcinoma in experimentally induced Barrett’s esophagus.222 One possible mechanism that is involved in reflux-associated carcinogenesis in Barrett’s esophagus is COX-2 activation and high levels of prostaglandin E2 production induced by acid and bile salts. A systematic review suggested that the protective effect of aspirin and NSAIDs was greater with more regular use, an observation supported in a cohort study as well.80,81
A single clinical trial examined the effect of celecoxib at a dose of 200 mg twice daily given for 48 weeks in patients with low-grade and high-grade dysplasia on change in proportion of biopsy samples with dysplasia between patients treated with celecoxib compared with a placebo.223 No differences were found between the two groups. A small crossover study showed that high-dose PPI therapy in conjunction with aspirin at a dose of 325 mg daily can decrease mucosal prostaglandin E2 content in mucosal biopsy specimens from patients with Barrett’s esophagus.224 These findings led to a large randomized clinical trial in the United Kingdom (ASPECT) and a smaller clinical trial in the United States in an effort to examine the potential for chemoprevention with aspirin in conjunction with a PPI as a clinical strategy in patients with Barrett’s esophagus. However, it is premature to administer aspirin or NSAIDs routinely to these patients until more data become available.
Endoscopic Therapy
Each of the endoscopic techniques is able to eliminate much or all of the Barrett’s epithelium while the esophagus remains in situ, and the risks of surgery for high-grade dysplasia or cancer are avoided. More recent data with these techniques are encouraging, showing low morbidity and mortality and excellent 5-year survival.225 Each of these techniques has disadvantages as well, however, including the need for continued meticulous surveillance, the potential for “at-risk” mucosa remaining after therapy, and diagnostic uncertainty. The decision to perform endoscopic therapy in patients with Barrett’s esophagus is complex. Many factors enter into the decision-making process, including grade of dysplasia, characteristics of the lesion in question, patient characteristics, and institutional factors including expertise in pathology, surgery, and interventional endoscopy.
Thermal Ablation Techniques
Thermal ablation of Barrett’s esophagus can be accomplished by various techniques, including laser, multipolar electrocoagulation, heater probe, argon plasma coagulation, radiofrequency ablation, and cryotherapy. Randomized controlled trials have been conducted to compare various thermal ablation techniques with each other. These clinical trials have highlighted the difficulty in obtaining complete endoscopic and histologic ablation with argon plasma coagulation and multipolar electrocoagulation.226–228 Studies of these techniques routinely found incomplete macroscopic regression of the Barrett’s segment and buried intestinal metaplasia beneath the neosquamous epithelium, which led to reports of subsquamous cancers developing in patients with previously nondysplastic Barrett’s epithelium.229–231 Consequently, techniques such as multipolar electrocoagulation, heater probe, and argon plasma coagulation all seem to have fallen by the wayside.
Radiofrequency Ablation
Radiofrequency ablation involves the application of high-power radiofrequency energy using bipolar electrodes, causing rapid heating of the tissue with ablation to a depth of approximately 0.5 mm. Studies on radiofrequency ablation have involved a stepwise progression from animal studies; to human studies before esophagectomy; to human dosimetry studies, single-center studies, multicenter nonrandomized studies, and multicenter randomized controlled trials.232–234 This process also led to modifications in the radiofrequency ablation technique to include both a 360-degree balloon-based device and a focal ablation device.
Radiofrequency ablation has been studied for nondysplastic and dysplastic Barrett’s esophagus. Sharma and colleagues234 described their 12-month results with circumferential radiofrequency ablation of nondysplastic Barrett’s epithelium in 70 patients; complete elimination of intestinal metaplasia was achieved without any buried glands in 69% of subjects. Buried intestinal metaplasia was not encountered in any of the biopsy specimens. Subsequently, longer term follow-up for up to 30 months after additional focal radiofrequency ablation showed complete elimination of metaplasia in 97% of patients at 30 months.235 No buried intestinal metaplasia was encountered in any of the biopsy specimens at 12 months and 30 months. Adverse events were believed to be minor and were encountered in 15.1% after the circumferential ablation phase of the study and 2.6% after the focal ablation phase of the study. Any postablation symptoms resolved within 4 days of the procedure.
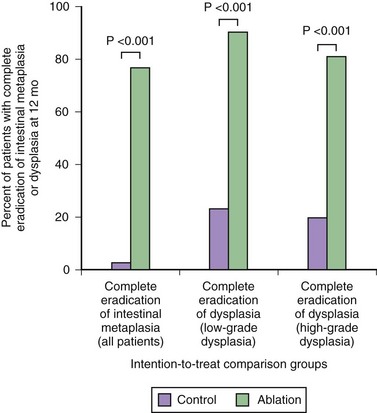
A randomized sham control study evaluated radiofrequency ablation for low-grade dysplasia and high-grade dysplasia.236 This study showed complete resolution of high-grade dysplasia in 81% of the treatment group compared with 19% of the sham group and complete resolution of low-grade dysplasia in 91% of the treatment group compared with 23% of the sham group using a combination of the circumferential and focal probes at 1-year follow-up (Fig. 26.16). Among patients with high-grade dysplasia, progression to cancer occurred in 2.4% of the treatment group compared with 19% of the sham group. Complete elimination of intestinal metaplasia occurred in 77% of the treatment group compared with 2% of the sham group. Adverse events were encountered in 3 of 298 treatments including bleeding and chest pain; 6% developed strictures that were easily dilated.
Taken together with other data on radiofrequency ablation, it is known that a combination of circumferential and focal probes provides optimal results, that this technique can be safely combined with EMR, that preexisting genetic abnormalities resolve, and that buried intestinal metaplasia seems to be rare.237 This method does not completely eliminate cancer risk or progression of low-grade dysplasia to high-grade dysplasia. As with other ablative techniques except for EMR, radiofrequency ablation does not allow tissue confirmation of efficacy, leaving some uncertainty for each patient. Only a few patients have been studied to date, and long-term results beyond 2.5 years are still unknown.
Cryotherapy
Cryotherapy freezes the gut mucosa to induce cell death. There are two techniques: carbon dioxide and liquid nitrogen. However, very limited data are available regarding efficacy in Barrett’s esophagus, and there are no randomized controlled trials to date. Johnston and colleagues238 treated 11 patients with liquid nitrogen, with complete endoscopic and histologic reversal in 7 of 11 patients at 6 months. A case series reported 26 patients with high-grade dysplasia treated with liquid nitrogen, of which 32% were free of dysplasia at 12 months of follow-up.239 The concern with this technique, besides lack of published data to date, is the uneven application inherent in spraying of the cryogen rather than direct balloon-based application to isolated segments of the esophagus.
Photodynamic Therapy
A randomized controlled study evaluated photodynamic therapy with porfimer sodium compared with a strategy of continued surveillance for patients with high-grade dysplasia.240 At 2 years, complete ablation of high-grade dysplasia occurred in 77% of patients in the photodynamic therapy group compared with 39% of patients in the surveillance group with progression to cancer in 13% in the photodynamic therapy group and 28% in the surveillance group. Complete elimination of all intestinal metaplasia and dysplasia occurred in 52% of the photodynamic therapy group and 7% of the surveillance group. Complications were common—strictures in 36% and photosensitivity in 69%. At 5 years, the probability of complete ablation of high-grade dysplasia after photodynamic therapy was 48%, and progression to cancer occurred in 15%.241 At the Mayo Clinic, patients with high-grade dysplasia treated with photodynamic therapy with or without concomitant EMR had long-term survival comparable to patients treated with esophagectomy and low rates of cancer-associated death.242
Photodynamic therapy has the advantages of leaving the esophagus in situ, evidence from randomized controlled trials that it is superior to continued surveillance and evidence from cohort studies that survival is comparable to esophagectomy. Disadvantages include the considerable capital expense of the equipment required, high rate of strictures, prolonged photosensitivity, lack of tissue confirmation, and problems in attaining complete ablation of intestinal metaplasia. Persistent genetic abnormalities have been noted in residual dysplastic and nondysplastic epithelium after photodynamic therapy with reports of subsequent redevelopment of high-grade dysplasia.243–246
Endoscopic Mucosal Resection
EMR is a therapeutic option for patients with either high-grade dysplasia or intramucosal carcinoma in the setting of appropriate risk stratification. As described earlier, EMR permits accurate histologic staging of neoplasia arising in Barrett’s epithelium compared with esophageal resection specimens. Negative margins on EMR specimens correlate well with absence of residual disease at the time of surgery, but submucosal involvement is associated with both residual disease at the time of surgery and lymph node metastases.247 As emphasized by the Wiesbaden group, EMR for superficial cancer with curative intent should be attempted only for lesions with the following criteria: lesion diameter less than 20 mm and macroscopically type I (polypoid), IIa (flat and slightly elevated), IIb (flat and level), or IIc (flat, depressed, and <10 mm); well or moderately differentiated histologic grade; lesions limited to the mucosa proven by histology of the resected specimens; and no invasion of lymph vessels or veins. The issue of submucosal cancer limited to the superficial layer is an evolving area of debate.
The pioneering work of the Wiesbaden group with EMR in 100 patients with high-grade intraepithelial neoplasia or intramucosal carcinoma resulted in complete local remission in 99 after a mean of 1.47 EMRs with no strictures and only minor bleeding in 11 patients.248 However, there were 11 metachronous lesions in 11 patients for a recurrence rate of 11%, characterized by local recurrence in 6 and disease at a different location in 5. There were two deaths in the series: One patient with CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, scleroderma, and telangiectases) died of pneumonia, and one patient died of carcinoma of the oral cavity. The 5-year life-table survival of these patients was 98%.
It is important to emphasize some key methodologic aspects of the work of the Wiesbaden group. Before entry into the study, patients with confirmed adenocarcinoma were meticulously staged with the following techniques: high-resolution white light endoscopy, methylene blue chromoendoscopy, biopsy specimens of all macroscopically visible lesions and unstained areas on chromoendoscopy, four-quadrant biopsy specimens every 1 to 2 cm of the Barrett’s segment, and endoscopic ultrasound. All patients with proven adenocarcinoma underwent chest radiography, computed tomography scan of the abdomen and chest, and ultrasound of the abdomen. There was no standard approach to residual Barrett’s epithelium, although 49 patients underwent thermal ablation with either argon plasma coagulation for short-segment Barrett’s esophagus or aminolevulinic acid photodynamic therapy for long-segment Barrett’s esophagus. Follow-up examinations were rigorous and involved four-quadrant biopsy specimens and biopsy specimens of any visual lesions at 1 month, 2 months, 3 months, 6 months, 9 months, and 12 months followed by every 6 months for 5 years along with endoscopic ultrasound and computed tomography scans at every other visit. Residual or metachronous disease, defined as high-grade epithelial neoplasia or early cancer after complete local remission, was treated by EMR. Although the work by Ell and associates248 makes a very strong case for the safety of EMR in superficial adenocarcinoma of the esophagus meeting low-risk criteria, their work also points out the problem of at-risk mucosa that remains after therapy because recurrent or metachronous lesions were found in 11% of patients.
Studies to date suggest that circumferential EMR results in complete remission of high-grade dysplasia and intramucosal adenocarcinoma in 75% to 100% of patients.249–253 A single-center series of circumferential EMR by Chennat and colleagues254 showed complete elimination of all Barrett’s epithelium, dysplasia, and early cancer in 31 of 32 patients at a mean follow-up of 23 months with a stricture rate of 37% and no bleeding or perforation. Complication rates vary in other series, but early bleeding, occasional perforation, and late strictures remain issues.
Combination Therapy
More recent studies indicate that complete ablation of Barrett’s esophagus with EMR in combination with radiofrequency ablation is feasible. The Amsterdam group described the technique of circumferential and focal radiofrequency ablation in a few patients with Barrett’s esophagus with residual dysplasia after EMR of visible lesions.255,256 Gondrie and colleagues255 found complete absence of Barrett’s epithelium, dysplasia, cancer, and buried intestinal metaplasia in all 12 patients treated by EMR of all visible lesions followed by radiofrequency ablation of the residual Barrett’s esophagus segment at a median follow-up of 14 months. Other investigators have described excellent long-term results with combinations of EMR and photodynamic therapy.242,257
Comparisons with Surgical Therapy
Although there are no randomized controlled trials that have compared endoscopic with surgical approaches for the management of high-grade dysplasia and superficial carcinoma, numerous observational studies suggest that long-term survival of the two techniques is comparable.242,257–259 Studies extending over 5 years are available on EMR, photodynamic therapy, and a combination of the two showing long-term survival comparable to esophageal surgery for high-grade dysplasia or intramucosal adenocarcinoma and low rates of cancer-associated death. A population-based study of patients with early esophageal cancer found comparable long-term survival for patients managed with endoscopic therapy compared with patients treated with surgical resection.258 Although the 5-year survival is comparable between the two treatment modalities, cancer develops during follow-up in approximately 6% to 12% of patients treated endoscopically.
Unresolved Issues in Endoscopic Therapy
There are many unresolved issues in ablation therapy. Assuming equal endoscopic skills, it is important to know which endoscopic therapy should be applied to a given patient. Should EMR be limited to focal lesions only? What is the length threshold for circumferential EMR? Who should receive thermal techniques, and what parameters should be used to determine which patient should get which combination techniques? What factors predict if a patient will respond to a given therapy? Possible variables include segment length, hiatal hernia size, adequacy of acid suppression, and biomarkers. One study to date has evaluated biomarkers to predict response to photodynamic therapy. Prasad and coworkers260 found that p16 loss, detected by fluorescence in situ hybridization of cytology specimens obtained before photodynamic therapy for high-grade dysplasia or intramucosal carcinoma, predicted a lesser response to photodynamic therapy. A multivariate analysis by Pech and associates,261 based on the long-term results of the Wiesbaden group’s approach to patients with high-grade intraepithelial neoplasia and intramucosal adenocarcinoma with EMR with or without photodynamic therapy, identified the following as risk factors for disease recurrence after ablation therapy: long-segment Barrett’s esophagus, multifocal neoplasia, piecemeal resection, and no ablative therapy of the residual Barrett’s segment after a complete response by EMR.
Although early data with radiofrequency ablation are promising, it is difficult to conceive of any technique reliably eliminating all subsquamous intestinal metaplasia. Biomarker abnormalities persist in this subsquamous epithelium, and it is still unknown what degree of subsquamous columnar epithelium, if any, can be tolerated after ablation. Studies in a few patients with buried intestinal metaplasia after photodynamic therapy found that buried Barrett’s epithelium had reduced crypt proliferation and near-normal DNA content compared with Barrett’s epithelium before treatment, raising the question of the neoplastic potential of the buried Barrett’s epithelium.262 Better techniques of detecting buried columnar epithelium are needed.
Several reports suggest that the cardia behaves in unexpected and potentially undesirable ways after ablation therapy. Nodules with high-grade dysplasia or cancer may develop months to years after therapy.263,264 The reason for this development is unknown. Although squamous epithelium may develop below the EG junction after ablation, it is unclear what the natural history of that metaplastic mucosa is.265 Not only can problems develop at the cardia, but also techniques such as radiofrequency ablation are difficult to apply to the cardia, even with the focal probe, owing to positioning and the anatomic alterations in the setting of a large hiatal hernia.
Finally, despite the ready availability of various ablation techniques, it is difficult to justify a decision to perform ablation for all patients with Barrett’s esophagus without dysplasia at this time for the following reasons: (1) Cancer risk for an individual patient is low, (2) the need for surveillance is unchanged, (3) all of the techniques involve considerable financial cost, and (4) adverse events still occur. In regard to any treatment of patients with nondysplastic Barrett’s epithelium, even if one assumes a risk reduction of 50% for the development of cancer, from an estimated 0.50% per year to 0.25% per year, the number needed to treat to prevent 1 cancer in nondysplastic Barrett’s epithelium would be approximately 400 patients.266
1 Conio M, Cameron AJ, Romero Y, et al. Secular trends in the epidemiology and outcome of Barrett’s oesophagus in Olmsted County, Minnesota. Gut. 2001;48:304-309.
2 van Soest EM, Dieleman JP, Siersema PD, et al. Increasing incidence of Barrett’s oesophagus in the general population. Gut. 2005;54:1062-1066.
3 Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc. 2005;61:226-231.
4 Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: An endoscopic study. Gastroenterology. 2005;129:1825-1831.
5 Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: The Loiano-Monghidoro study. Gut. 2008;57:1354-1359.
6 Spechler SJ, Zeroogian JM, Antonioli DA, et al. Prevalence of metaplasia at the gastro-oesophageal junction. Lancet. 1994;344:1533-1536.
7 Nandurkar S, Talley NJ. Barrett’s esophagus: The long and the short of it. Am J Gastroenterol. 1999;94:30-40.
8 Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: Prevalence and clinical data. Gastroenterology. 1999;116:277-285.
9 Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: A systematic review. Gastroenterology. 2002;122:26-33.
10 Cameron AJ, Lomboy CT. Barrett’s esophagus: Age, prevalence, and extent of columnar epithelium. Gastroenterology. 1992;103:1241-1245.
11 Abrams JA, Fields S, Lightdale CJ, et al. Racial and ethnic disparities in the prevalence of Barrett’s esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol. 2008;6:30-34.
12 Falk GW, Thota PN, Richter JE, et al. Barrett’s esophagus in women: Demographic features and progression to high-grade dysplasia and cancer. Clin Gastroenterol Hepatol. 2005;3:1089-1094.
13 Guardino JM, Khandwala F, Lopez R, et al. Barrett’s esophagus at a tertiary care center: Association of age on incidence and prevalence of dysplasia and adenocarcinoma. Am J Gastroenterol. 2006;101:2187-2193.
14 van Blankenstein M, Looman CW, Johnston BJ, et al. Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol. 2005;100:568-576.
15 Smith KJ, O’Brien SM, Smithers BM, et al. Interactions among smoking, obesity, and symptoms of acid reflux in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:2481-2486.
16 Eisen GM, Sandler RS, Murray S, et al. The relationship between gastroesophageal reflux disease and its complications with Barrett’s esophagus. Am J Gastroenterol. 1997;92:27-31.
17 Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology. 2007;133:34-41.
18 Edelstein ZR, Farrow DC, Bronner MP, et al. Central adiposity and risk of Barrett’s esophagus. Gastroenterology. 2007;133:403-411.
19 Smith KJ, O’Brien SM, Green AC, et al. Current and past smoking significantly increase risk for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2009;7:840-848.
20 Falk GW. Barrett’s esophagus. Gastroenterology. 2002;122:1569-1591.
21 Milano F, van Baal JW, Buttar NS, et al. Bone morphogenetic protein 4 expressed in esophagitis induces a columnar phenotype in esophageal squamous cells. Gastroenterology. 2007;132:2412-2421.
22 Colleypriest BJ, Palmer RM, Ward SG, et al. Cdx genes, inflammation and the pathogenesis of Barrett’s metaplasia. Trends Mol Med. 2009;15:313-322.
23 Sarosi G, Brown G, Jaiswal K, et al. Bone marrow progenitor cells contribute to esophageal regeneration and metaplasia in a rat model of Barrett’s esophagus. Dis Esophagus. 2008;21:43-50.
24 Peters JH, Avisar N. The molecular pathogenesis of Barrett’s esophagus: Common signaling pathways in embryogenesis metaplasia and neoplasia. J Gastrointest Surg. 2010;14(Suppl 1):S81-S87.
25 Neumann CS, Cooper BT. 24 hour ambulatory oesophageal pH monitoring in uncomplicated Barrett’s oesophagus. Gut. 1994;35:1352-1355.
26 Singh P, Taylor RH, Colin-Jones DG. Esophageal motor dysfunction and acid exposure in reflux esophagitis are more severe if Barrett’s metaplasia is present. Am J Gastroenterol. 1994;89:349-356.
27 Cameron AJ. Barrett’s esophagus: Prevalence and size of hiatal hernia. Am J Gastroenterol. 1999;94:2054-2059.
28 Wakelin DE, Al-Mutawa T, Wendel C, et al. A predictive model for length of Barrett’s esophagus with hiatal hernia length and duration of esophageal acid exposure. Am J Gastroenterol. 2003;58:350-355.
29 Champion G, Richter JE, Vaezi MF, et al. Duodenogastroesophageal reflux: Relationship to pH and importance in Barrett’s esophagus. Gastroenterology. 1994;107:747-754.
30 Loughney T, Maydonovitch CL, Wong RK. Esophageal manometry and ambulatory 24-hour pH monitoring in patients with short and long segment Barrett’s esophagus. Am J Gastroenterol. 1998;93:916-919.
31 Fass R, Hell RW, Garewal HS, et al. Correlation of oesophageal acid exposure with Barrett’s oesophagus length. Gut. 2001;48:310-313.
32 Eloubeidi MA, Provenzale D. Clinical and demographic predictors of Barrett’s esophagus among patients with gastroesophageal reflux disease: A multivariable analysis in veterans. J Clin Gastroenterol. 2001;33:306-309.
33 Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825-831.
34 Johnson DA, Winters C, Spurling TJ, et al. Esophageal acid sensitivity in Barrett’s esophagus. J Clin Gastroenterol. 1987;91:23-27.
35 Grade A, Pulliam G, Johnson C, et al. Reduced chemoreceptor sensitivity in patients with Barrett’s esophagus may be related to age and not to the presence of Barrett’s epithelium. Am J Gastroenterol. 1997;92:2040-2043.
36 Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut. 2002;51:323-328.
37 Chak A, Ochs-Balcom H, Falk G, et al. Familiality in Barrett’s esophagus, adenocarcinoma of the esophagus, and adenocarcinoma of the gastroesophageal junction. Cancer Epidemiol Biomarkers Prev. 2006;15:1668-1673.
38 Drovdlic CM, Goddard KA, Chak A, et al. Demographic and phenotypic features of 70 families segregating Barrett’s oesophagus and oesophageal adenocarcinoma. J Med Genet. 2003;40:651-656.
39 Paull A, Trier JS, Dalton D, et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med. 1976;295:476-480.
40 Weinstein WM, Ippoliti AF. The diagnosis of Barrett’s esophagus: Goblets, goblets, goblets. Gastrointest Endosc. 1996;44:91-94.
41 Alikhan M, Rex D, Khan A, et al. Variable pathologic interpretation of columnar lined esophagus by general pathologists in community practice. Gastrointest Endosc. 1999;50:23-26.
42 Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia Barrett esophagus: A reaffirmation. Hum Pathol. 2001;32:368-378.
43 Ormsby AH, Petras RE, Henricks WH, et al. Observer variation in the diagnosis of superficial oesophageal adenocarcinoma. Gut. 2002;51:671-676.
44 Downs-Kelly E, Mendelin JE, Bennett AE, et al. Poor interobserver agreement in the distinction of high-grade dysplasia and adenocarcinoma in pretreatment Barrett’s esophagus biopsies. Am J Gastroenterol. 2008;103:2333-2340.
45 Mino-Kenudson M, Hull MJ, Brown I, et al. EMR for Barrett’s esophagus-related superficial neoplasms offers better diagnostic reproducibility than mucosal biopsy. Gastrointest Endosc. 2007;66:660-666.
46 Wang KK, Sampliner RE. Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788-797.
47 Wang KK, Wongkeesong M, Buttar NS. American Gastroenterological Association technical review on the role of the gastroenterologist in the management of esophageal carcinoma. Gastroenterology. 2005;128:1471-1505.
48 Shaheen NJ, Green B, Medapalli RK, et al. The perception of cancer risk in patients with prevalent Barrett’s esophagus enrolled in an endoscopic surveillance program. Gastroenterology. 2005;129:429-436.
49 Shaheen NJ, Dulai GS, Ascher B, et al. Effect of a new diagnosis of Barrett’s esophagus on insurance status. Am J Gastroenterol. 2005;100:577-580.
50 McClave SA, Boyce HW, Gottfied MR. Early diagnosis of the columnar-lined esophagus: A new endoscopic criterion. Gastrointest Endosc. 1987;33:413-416.
51 Ofman JJ, Shaheen NJ, Desai AA, et al. The quality of care in Barrett’s esophagus: Endoscopist and pathologist practices. Am J Gastroenterol. 2001;96:876-881.
52 Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett’s esophagus. Gastroenterology. 1994;107:945-949.
53 Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: The Prague C & M criteria. Gastroenterology. 2006;131:1392-1399.
54 Riddell RH, Odze RD. Definition of Barrett’s esophagus: Time for a rethink—is intestinal metaplasia dead? Am J Gastroenterol. 2009;104:2588-2594.
55 Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: The role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570-580.
56 British Society of Gastroenterology. Guidelines for the diagnosis and management of Barrett’s columnar-lined oesophagus. Available at www.BSG.org.uk
57 Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920.
58 Liu W, Hahn H, Odze RD, et al. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell-containing epithelium. Am J Gastroenterol. 2009;104:816-824.
59 Wang A, Mattek NC, Corless CL, et al. The value of traditional upper endoscopy as a diagnostic test for Barrett’s esophagus. Gastrointest Endosc. 2008;68:859-866.
60 Csendes A, Smok G, Burdiles P, et al. Prevalence of intestinal metaplasia according to the length of the specialized columnar epithelium lining the distal esophagus in patients with gastroesophageal reflux. Dis Esophagus. 2003;16:24-28.
61 Chandrasoma PT, Der R, Dalton P, et al. Distribution and significance of epithelial types in columnar-lined esophagus. Am J Surg Pathol. 2001;25:1188-1193.
62 Harrison R, Perry I, Haddadin W, et al. Detection of intestinal metaplasia in Barrett’s esophagus: An observational comparator study suggests the need for a minimum of eight biopsies. Am J Gastroenterol. 2007;102:1154-1161.
63 Sharma P, McElhinney C, Topalovski M, et al. Detection of cardia intestinal metaplasia: Do the biopsy number and location matter? Am J Gastroenterol. 2004;99:2424-2428.
64 Goldblum JR, Vicari JJ, Falk GW, et al. Inflammation and intestinal metaplasia of the gastric cardia: The role of gastroesophageal reflux and H. pylori infection. Gastroenterology. 1998;114:633-639.
65 Sharma P, Weston AP, Morales T, et al. Relative risk of dysplasia for patients with intestinal metaplasia in the distal esophagus and in the gastric cardia. Gut. 2000;46:9-13.
66 Morales CP, Spechler SJ. Intestinal metaplasia at the gastroesophageal junction: Barrett’s, bacteria, and biomarkers. Am J Gastroenterol. 2003;98:759-762.
67 Srivastava A, Odze RD, Lauwers GY, et al. Morphologic features are useful in distinguishing Barrett esophagus from carditis with intestinal metaplasia. Am J Surg Pathol. 2007;31:1733-1741.
68 Solaymani-Dodaran M, Logan RF, West J, et al. Risk of oesophageal cancer in Barrett’s oesophagus and gastro-oesophageal reflux. Gut. 2004;53:1070-1074.
69 Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142-146.
70 Jemal A, Siegel R, Ward E, et al. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225-249.
71 Thomas T, Abrams KR, De Caestecker JS, et al. Meta analysis: Cancer risk in Barrett’s oesophagus. Aliment Pharmacol Ther. 2007;26:1465-1477.
72 Shaheen NJ, Crosby MA, Bozymski EM, et al. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus? Gastroenterology. 2000;119:333-338.
73 Anderson LA, Murray LJ, Murphy SJ, et al. Mortality in Barrett’s oesophagus: Results from a population based study. Gut. 2003;52:1081-1084.
74 Moayyedi P, Burch N, Akhtar-Danesh N, et al. Mortality rates in patients with Barrett’s oesophagus. Aliment Pharmacol Ther. 2008;27:316-320.
75 van der Burgh A, Dees J, Hop WC, et al. Oesophageal cancer is an uncommon cause of death in patients with Barrett’s oesophagus. Gut. 1996;39:5-8.
76 van Blankenstein M, Looman CW, Hop WC, et al. The incidence of adenocarcinoma and squamous cell carcinoma of the esophagus: Barrett’s esophagus makes a difference. Am J Gastroenterol. 2005;100:766-774.
77 Kubo A, Corley DA. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am J Gastroenterol. 2004;99:582-588.
78 Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:35235-35238.
79 Falk GW. Risk factors for esophageal cancer development. Surg Oncol Clin N Am. 2009;18:469-485.
80 Corley DA, Kerlikowske K, Verma R, et al. Protective association of aspirin/NSAIDs and esophageal cancer: A systematic review and meta-analysis. Gastroenterology. 2003;124:47-56.
81 Vaughan TL, Dong LM, Blount PL, et al. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett’s oesophagus: A prospective study. Lancet Oncol. 2005;6:945-952.
82 Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404-1413.
83 McArdle JE, Lewin KJ, Randall G, et al. Distribution of dysplasias and early invasive carcinoma in Barrett’s esophagus. Hum Pathol. 1992;23:479-482.
84 Reid BJ, Barrett MT, Galipeau PC, et al. Barrett’s esophagus: Ordering the events that lead to cancer. Eur J Cancer Prev. 1996;5(Suppl 2):57-65.
85 Maley CC. Multistage carcinogenesis in Barrett’s esophagus. Cancer Lett. 2007;245:22-32.
86 Paulson TG, Maley CC, Li X, et al. Chromosomal instability and copy number alterations in Barrett’s esophagus and esophageal adenocarcinoma. Clin Cancer Res. 2009;15:3305-3314.
87 Galipeau PC, Prevo LJ, Sanchez CA, et al. Clonal expansion and loss of heterozygosity at chromosomes 9p and 17p in premalignant esophageal (Barrett’s) tissue. J Natl Cancer Inst. 1999;91:2087-2095.
88 Reid BJ, Prevo LJ, Galipeau PC, et al. Predictors of progression in Barrett’s esophagus. II: Baseline 17p (p53) loss of heterozygosity identifies a patient subset at increased risk for neoplastic progression. Am J Gastroenterol. 2001;96:2839-2848.
89 Bian YS, Osterheld MC, Fontolliet C, et al. P16 inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in Barrett’s esophagus. Gastroenterology. 2002;122:1113-1121.
90 Wong DJ, Paulson TG, Prevo LJ, et al. p16INK4a lesions are common, early abnormalities that undergo clonal expansion in Barrett’s metaplastic epithelium. Cancer Res. 2001;61:8284-8289.
91 Shirivani VN, Ouatu-Lascar R, Kaur BS, et al. Cyclooxygenase 2 expression in Barrett’s esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487-496.
92 Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol. 2001;96:990-996.
93 Souza RF, Shewmake K, Beer DG, et al. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res. 2000;60:5767-5772.
94 Sihvo EI, Luostarinen ME, Salo JA. Fate of patients with adenocarcinoma of the esophagus and the esophagogastric junction: A population-based analysis. Am J Gastroenterol. 2004;99:419-424.
95 Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252.
96 Westerterp M, Koppert LB, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497-504.
97 Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: Pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566-573.
98 Nigro JJ, Hagen JA, DeMeester TR, et al. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: Implications for therapy. J Thorac Cardiovasc Surg. 1999;117:16-23.
99 Bollschweiler E, Baldus SE, Schröder W, et al. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy. 2006;38:149-156.
100 Rice TW, Blackstone EH, Goldblum JR, et al. Superficial adenocarcinoma of the esophagus. J Thorac Cardiovasc Surg. 2001;122:1077-1090.
101 Cooper GS, Kou TD, Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: A population-based study with temporal trends. Am J Gastroenterol. 2009;104:1356-1362.
102 Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett’s adenocarcinomas: A population-based study. Gastroenterology. 2002;122:633-640.
103 Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: A review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009;59:27-41.
104 Shaheen NJ, Provenzale D, Sandler RS. Upper endoscopy as a screening and surveillance tool in esophageal adenocarcinoma: A review of the evidence. Am J Gastroenterol. 2002;97:1319-1327.
105 Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: A randomized and blinded comparison. Am J Gastroenterol. 2006;101:2693-2703.
106 Sharma P, Wani S, Rastogi A, et al. The diagnostic accuracy of esophageal capsule endoscopy in patients with gastroesophageal reflux disease and Barrett’s esophagus: A blinded, prospective study. Am J Gastroenterol. 2008;3:525-532.
107 Galmiche JP, Sacher-Huvelin S, Coron E, et al. Screening for esophagitis and Barrett’s esophagus with wireless esophageal capsule endoscopy: A multicenter prospective trial in patients with reflux symptoms. Am J Gastroenterol. 2008;103:538-545.
108 Lin OS, Schembre DB, Mergener K, et al. Blinded comparison of esophageal capsule endoscopy versus conventional endoscopy for a diagnosis of Barrett’s esophagus in patients with chronic gastroesophageal reflux. Gastrointest Endosc. 2007;65:577-583.
109 Gerson L, Lin OS. Cost-benefit analysis of capsule endoscopy compared with standard upper endoscopy for the detection of Barrett’s esophagus. Clin Gastroenterol Hepatol. 2007;5:319-325.
110 Stoltey J, Reeba H, Ullah N, et al. Does Barrett’s oesophagus develop over time in patients with chronic gastro-oesophageal reflux disease? Aliment Pharmacol Ther. 2007;25:83-91.
111 Rodriguez S, Mattek N, Lieberman D, et al. Barrett’s esophagus on repeat endoscopy: Should we look more than once? Am J Gastroenterol. 2008;103:1892-1897.
112 Hanna S, Rastogi A, Weston AP, et al. Detection of Barrett’s esophagus after endoscopic healing of erosive esophagitis. Am J Gastroenterol. 2006;101:1416-1420.
113 Modiano N, Gerson LB. Risk factors for the detection of Barrett’s esophagus in patients with erosive esophagitis. Gastrointest Endosc. 2009;69:1014-1020.
114 Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high risk groups: A cost-utility analysis. Ann Intern Med. 2003;138:176-186.
115 Soni A, Sampliner RE, Sonnenberg A. Screening for high-grade dysplasia in gastroesophageal reflux disease: Is it cost effective? Am J Gastroenterol. 2000;95:2086-2093.
116 Gerson LB, Groeneveld PW, Triadafilopoulos G. Cost-effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2004;2:868-879.
117 Gerson L, Edson R, Lavori PW, et al. Use of a simple symptom questionnaire to predict Barrett’s esophagus in patients with symptoms of gastroesophageal reflux. Am J Gastroenterol. 2001;96:2005-2012.
118 Inacarbone R, Bonavina L, Saino G, et al. Outcome of esophageal adenocarcinoma detected during endoscopic biopsy surveillance for Barrett’s esophagus. Surg Endosc. 2002;16:263-266.
119 Ferguson MK, Durkin A. Long-term survival after esophagectomy for Barrett’s adenocarcinoma in endoscopically surveyed and nonsurveyed patients. J Gastrointest Surg. 2002;6:29-36.
120 Van Sandick JW, Lanschot JJ, Kuiken BW, et al. Impact of endoscopic biopsy surveillance of Barrett’s esophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut. 1998;43:216-222.
121 Peters JH, Clark GW, Ireland AP, et al. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. Thorac Cardiovasc Surg. 1994;108:813-822.
122 Provenzale D, Schmitt C, Wong JB. Barrett’s esophagus: A new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043-2053.
123 Sonnenberg A, Soni A, Sampliner RE. Medical decision analysis of endoscopic surveillance of Barrett’s oesophagus to prevent oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2002;16:41-50.
124 Conio M, Blanchi S, Lapertosa G, et al. Long-term endoscopic surveillance of patients with Barrett’s esophagus: Incidence of dysplasia and adenocarcinoma: A prospective study. Am J Gastroenterol. 2003;98:1931-1939.
125 Wang KK, Wongkeesong M, Buttar NS. American Gastroenterological Association medical position statement: Role of the gastroenterologist in the management of esophageal carcinoma. Gastroenterology. 2005;128:1468-1470.
126 Fitzgerald RC, Saeed I, Khoo D, et al. Rigorous surveillance protocol increases detection of curable cancers associated with Barrett’s esophagus. Dig Dis Sci. 2001;46:1892-1898.
127 Abela JE, Going JJ, Mackenzie JF, et al. Systematic four-quadrant biopsy detects Barrett’s dysplasia in more patients than nonsystematic biopsy. Am J Gastroenterol. 2008;103:850-855.
128 Reid BJ, Blount PL, Feng Z, et al. Optimizing endoscopic biopsy detection of early cancers in Barrett’s high-grade dysplasia. Am J Gastroenterol. 2000;95:3089-3096.
129 Peters FP, Brakenhoff KP, Curvers WL, et al. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett’s esophagus. Gastrointest Endosc. 2008;67:604-609.
130 Levine DS, Reid BJ. Endoscopic biopsy technique for acquiring larger mucosal samples. Gastrointest Endosc. 1991;37:332-337.
131 Levine DS, Blount PL, Rudolph RE, et al. Safety of a systematic endoscopic biopsy protocol in patients with Barrett’s esophagus. Am J Gastroenterol. 2000;95:1152-1157.
132 Cameron AJ, Carpenter HA. Barrett’s esophagus, high-grade dysplasia and early adenocarcinoma. Am J Gastroenterol. 1997;92:586-591.
133 Levine DS, Haggitt RC, Blount PL, et al. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus. Gastroenterology. 1993;105:40-50.
134 Kariv R, Plesec TP, Goldblum JR, et al. The Seattle protocol does not more reliably predict the detection of cancer at the time of esophagectomy than a less intensive surveillance protocol. Clin Gastroenterol Hepatol. 2009;7:653-658.
135 Skacel M, Petras RE, Gramlich TL, et al. The diagnosis of low-grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol. 2000;95:3383-3387.
136 Green S, Tawil A, Barr H, et al: Surgery versus radical endotherapies for early cancer and high grade dysplasia in Barrett’s oesophagus. Cochrane Database Syst Rev (2):CD007334, 2009.
137 Prasad GA, Wang KK, Buttar NS, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2007;132:1226-1233.
138 Schnell TG, Sontag SJ, Chejfec G, et al. Long-term nonsurgical management of Barrett’s esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607-1619.
139 Buttar NS, Wang KK, Sebo TJ, et al. Extent of high-grade dysplasia in Barrett’s esophagus correlates with risk of adenocarcinoma. Gastroenterology. 2001;120:1630-1639.
140 Dar M, Goldblum JR, Rice TW, et al. Can extent of high-grade dysplasia predict the presence of adenocarcinoma at esophagectomy? Gut. 2003;52:486-489.
141 Tharavej C, Hagen JA, Peters JH, et al. Predictive factors of coexisting cancer in Barrett’s high-grade dysplasia. Surg Endosc. 2006;20:439-443.
142 Nigro JJ, Hagen JA, DeMeester TR, et al. Occult esophageal adenocarcinoma: Extent of disease and implications for effective therapy. Ann Surg. 1999;230:433-438.
143 Falk GW, Ours TM, Richter JE. Practice patterns for surveillance of Barrett’s esophagus in the United States. Gastrointest Endosc. 2000;52:197-203.
144 Gross CP, Canto MI, Hixson J, et al. Management of Barrett’s esophagus: A national study of practice patterns and their cost implications. Am J Gastroenterol. 1999;94:3440-3447.
145 Das D, Ishaq S, Harrison R, et al. Management of Barrett’s esophagus in the UK: Overtreated and underbiopsied but improved by the introduction of a national randomized trial. Am J Gastroenterol. 2008;103:1079-1089.
146 Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol. 2009;7:736-742.
147 Canto MI, Setrakian S, Petras RE, et al. Methylene blue selectively stains intestinal metaplasia in Barrett’s esophagus. Gastrointest Endosc. 1996;44:1-7.
148 Canto MI, Setrakian S, Willis J, et al. Methylene blue-directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2000;51:560-568.
149 Lim CH, Rotimi O, Dexter SP, et al. Randomized crossover study that used methylene blue or random 4-quadrant biopsy for the diagnosis of dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2006;64:195-199.
150 Ngamruengphong S, Sharma VK, Das A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett’s esophagus: A meta-analysis. Gastrointest Endosc. 2009;69:1021-1028.
151 Vázquez-Iglesias JL, Alonso-Aguirre P, Diz-Lois MT, et al. Acetic acid allows effective selection of areas for obtaining biopsy samples in Barrett’s esophagus. Eur J Gastroenterol Hepatol. 2007;19:187-193.
152 Curvers W, Baak L, Kiesslich R, et al. Chromoendoscopy and narrow-band imaging compared with high-resolution magnification endoscopy in Barrett’s esophagus. Gastroenterology. 2008;134:670-679.
153 Song LM, Adler DG, Conway JD, et al. Narrow band imaging and multiband imaging. Gastrointest Endosc. 2008;67:581-589.
154 Kara MA, Ennahachi M, Fockens P, et al. Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett’s esophagus by using narrow band imaging. Gastrointest Endosc. 2006;64:155-166.
155 Sharma P, Bansal A, Mathur S, et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest Endosc. 2006;64:167-175.
156 Wolfsen HC, Crook JE, Krishna M, et al. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s esophagus. Gastroenterology. 2008;135:24-31.
157 Curvers WL, Bohmer CJ, Mallant-Hent RC, et al. Mucosal morphology in Barrett’s esophagus: Interobserver agreement and role of narrow band imaging. Endoscopy. 2008;40:799-805.
158 Curvers WL, van den Broek FJ, Reitsma JB, et al. Systematic review of narrow-band imaging for the detection and differentiation of abnormalities in the esophagus and stomach (with video). Gastrointest Endosc. 2009;69:307-317.
159 Panjehpour M, Overholt BF, Vo-Dinh T, et al. Endoscopic fluorescence detection of high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 1996;111:93-101.
160 Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light-scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2001;120:1620-1629.
161 Lovat LB, Johnson K, Mackenzie GD, et al. Elastic scattering spectroscopy accurately detects high grade dysplasia and cancer in Barrett’s oesophagus. Gut. 2006;55:1078-1083.
162 Wallace MB. Detecting dysplasia with optical coherence tomography. Clin Gastroenterol Hepatol. 2006;4:36-37.
163 Kara MA, DaCosta RS, Streutker CJ, et al. Characterization of tissue autofluorescence in Barrett’s esophagus by confocal fluorescence microscopy. Dis Esophagus. 2007;20:141-150.
164 Kara MA, Smits ME, Rosmolen WD, et al. A randomized crossover study comparing light-induced fluorescence endoscopy with standard videoendoscopy for the detection of early neoplasia in Barrett’s esophagus. Gastrointest Endosc. 2005;61:671-678.
165 Kara MA, Peters FP, Ten Kate FJ, et al. Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett’s esophagus. Gastrointest Endosc. 2005;61:679-685.
166 Kara MA, Peters FP, Fockens P, et al. Endoscopic video-autofluorescence imaging followed by narrow band imaging for detecting early neoplasia in Barrett’s esophagus. Gastrointest Endosc. 2006;64:176-185.
167 Curvers WL, Singh R, Song LM, et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus: A multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2008;57:167-172.
168 Kantsevoy SV, Adler DG, Conway JD, et al. Confocal laser endomicroscopy. Gastrointest Endosc. 2009;70:197-200.
169 Kiesslich R, Gossner L, Goetz M, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979-987.
170 Pohl H, Rösch T, Vieth M, et al. Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett’s oesophagus. Gut. 2008;57:1648-1653.
171 Dunbar KB, Okolo P3rd, Montgomery E, et al. Confocal laser endomicroscopy in Barrett’s esophagus and endoscopically inapparent Barrett’s neoplasia: A prospective, randomized, double-blind, controlled, crossover trial. Gastrointest Endosc. 2009;70:645-654.
172 Rudolph RE, Vaughan TL, Storer BE, et al. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann Intern Med. 2000;132:612-620.
173 Hameeteman W, Tytgat GN, Houthoff HJ, et al. Barrett’s esophagus: Development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249-1256.
174 Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:566-572.
175 Offman JJ, Lewin K, Ramers C, et al. The economic impact of the diagnosis of dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2000;95:2946-2952.
176 Conio M, Blanchi S, Lapertosa G, et al. Long-term endoscopic surveillance of patients with Barrett’s esophagus: Incidence of dysplasia and adenocarcinoma: A prospective study. Am J Gastroenterol. 2003;98:1931-1939.
177 Wani S, Puli SR, Shaheen NJ, et al. Esophageal adenocarcinoma in Barrett’s esophagus after endoscopic ablative therapy: A meta-analysis and systematic review. Am J Gastroenterol. 2009;104:502-513.
178 Srivastava A, Hornick JL, Li X, et al. Extent of low-grade dysplasia is a risk factor for the development of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol. 2007;102:483-493.
179 Reid BJ, Levine DS, Longton G, et al. Predictors of progression to cancer in Barrett’s esophagus: Baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669-1676.
180 Pellegrini CA, Pohl D. High-grade dysplasia in Barrett’s esophagus: Surveillance or operation? J Gastrointest Surg. 2000;4:131-134.
181 Weston AP, Sharma P, Topalovski M, et al. Long-term follow-up of Barrett’s high-grade dysplasia. Am J Gastroenterol. 2000;95:1888-1893.
182 Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: A meta-analysis. Gastrointest Endosc. 2008;67:394-398.
183 Tharavej C, Hagen JA, Peters JH, et al. Predictive factors of coexisting cancer in Barrett’s high-grade dysplasia. Surg Endosc. 2006;20:439-443.
184 Reid BJ. P53 and neoplastic progression in Barrett’s esophagus. Am J Gastroenterol. 2001;96:1321-1323.
185 Prevo LJ, Sanchez CA, Galipeau PC, et al. P53-mutant clones and field effects in Barrett’s esophagus. Cancer Res. 1999;59:4784-4787.
186 Wong DJ, Barrett MT, Stoger R, et al. p16INK4a promoter is hypermethylated at a high frequency in esophageal adenocarcinomas. Cancer Res. 1997;57:2619-2622.
187 Reid BJ, Haggitt RC, Rubin CE, et al. Barrett’s esophagus: Correlation between flow cytometry and histology in detection of patients at risk for adenocarcinoma. Gastroenterology. 1987;93:1-11.
188 Reid BJ, Blount PL, Rubin CE, et al. Flow-cytometric and histological progression to malignancy in Barrett’s esophagus: Prospective endoscopic surveillance of a cohort. Gastroenterology. 1992;102:1212-1219.
189 Reid BJ, Levine DS, Longton G, et al. Predictors of progression to cancer in Barrett’s esophagus: Baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669-1676.
190 Reid BJ. P53 and neoplastic progression in Barrett’s esophagus. Am J Gastroenterol. 2001;96:1321-1323.
191 Schulmann K, Sterian A, Berki A, et al. Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett’s-associated neoplastic progression and predicts progression risk. Oncogene. 2005;24:4138-4148.
192 Galipeau PC, Li X, Blount PL, et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med. 2007;4:e67.
193 Sato F, Jin Z, Schulmann K, et al. Three-tiered risk stratification model to predict progression in Barrett’s esophagus using epigenetic and clinical features. PLoS ONE. 2008;3:e1890.
194 Triadafilopoulos G. Proton pump inhibitors for Barrett’s oesophagus. Gut. 2000;46:144-146.
195 Sampliner RE. Effect of up to 3 years of high-dose lansoprazole on Barrett’s esophagus. Am J Gastroenterol. 1994;89:1844-1848.
196 Neumann CS, Iqbal TH, Cooper BT. Long term continuous omeprazole treatment of patients with Barrett’s oesophagus. Aliment Pharmacol Ther. 1995;9:451-454.
197 Cooper BT, Chapman W, Neumann CS, Gearty JC. Continuous treatment of Barrett’s oesophagus patients with proton pump inhibitors up to 13 years: Observations on regression and cancer incidence. Aliment Pharmacol Ther. 2006;23:727-733.
198 Wilkinson SP, Biddlestone L, Gore S, et al. Regression of columnar-lined (Barrett’s) oesophagus with omeprazole 40 mg daily: Results of 5 years of continuous therapy. Aliment Pharmacol Ther. 1999;13:1205-1209.
199 Sharma P, Sampliner RE, Camargo E. Normalization of esophageal pH with high-dose proton pump inhibitor therapy does not result in regression of Barrett’s esophagus. Am J Gastroenterol. 1997;92:582-585.
200 Sharma P, Morales TG, Bhattacharyya A, et al. Squamous islands in Barrett’s esophagus: What lies underneath? Am J Gastroenterol. 1998;93:332-335.
201 Spechler SJ, Sharma P, Traxler B, et al. Gastric and esophageal pH in patients with Barrett’s esophagus treated with three esomeprazole dosages: A randomized, double-blind, crossover trial. Am J Gastroenterol. 2006;101:1964-1971.
202 Wani S, Sampliner RE, Weston AP, et al. Lack of predictors of normalization of oesophageal acid exposure in Barrett’s oesophagus. Aliment Pharmacol Ther. 2005;22:627-633.
203 Ouatu-Lascar R, Triadafilopoulos G. Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal acid reflux in patients with Barrett’s esophagus. Am J Gastroenterol. 1998;93:711-716.
204 Lao-Sirieix P, Roy A, Worrall C, et al. Effect of acid suppression on molecular predictors for esophageal cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:288-293.
205 El-Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2004;99:1877-1883.
206 Hillman LC, Chiragakis L, Shadbolt B, et al. Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett’s oesophagus. Med J Aust. 2004;180:387-391.
207 Abbas AE, Deschamps C, Cassivi SD, et al. Barrett’s esophagus: The role of laparoscopic fundoplication. Ann Thorac Surg. 2004;77:393-396.
208 O’Riordan JM, Byrne PJ, Ravi N, et al. Long-term clinical and pathologic response of Barrett’s esophagus after antireflux surgery. Am J Surg. 2004;188:27-33.
209 Hofstetter WL, Peters JH, DeMeester TR, et al. Long-term outcome of antireflux surgery in patients with Barrett’s esophagus. Ann Surg. 2001;234:532-538.
210 Attwood SE, Lundell L, Hatlebakk JG, et al. Medical or surgical management of GERD patients with Barrett’s esophagus: The LOTUS trial 3-year experience. J Gastrointest Surg. 2008;12:1646-1654.
211 DeMeester S, DeMeester T. Columnar mucosa and intestinal metaplasia of the esophagus: Fifty years of controversy. Ann Surg. 2000;231:303-321.
212 Ye W, Chow WH, Lagergren J, et al. Risk of adenocarcinomas of the esophagus and gastric cardia in patients with gastroesophageal reflux diseases and after antireflux surgery. Gastroenterology. 2001;121:1286-1293.
213 Tran T, Spechler SJ, Richardson P, et al. Fundoplication and the risk of esophageal cancer in gastroesophageal reflux disease: A Veterans Affairs cohort study. Am J Gastroenterol. 2005;100:1002-1008.
214 Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. Am J Gastroenterol. 2003;98:2390-2394.
215 Chang EY, Morris CD, Seltman AK, et al. The effect of antireflux surgery on esophageal carcinogenesis in patients with Barrett esophagus: A systematic review. Ann Surg. 2007;246:11-21.
216 Kelloff GJ, Lippman SM, Dannenberg AJ, et al. Progress in chemoprevention drug development: The promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer—a plan to move forward. Clin Cancer Res. 2006;12:3661-3697.
217 Ogunwobi OO, Beales IL. Statins inhibit proliferation and induce apoptosis in Barrett’s esophageal adenocarcinoma cells. Am J Gastroenterol. 2008;103:825-837.
218 Bozikas A, Marsman WA, Rosmolen WD, et al. The effect of oral administration of ursodeoxycholic acid and high-dose proton pump inhibitors on the histology of Barrett’s esophagus. Dis Esophagus. 2008;21:346-354.
219 Kubo A, Corley DA. Meta-analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. Am J Gastroenterol. 2007;102:2323-2330.
220 Jayaprakash V, Menezes RJ, Javle MM, et al. Regular aspirin use and esophageal cancer risk. Int J Cancer. 2006;119:202-207.
221 Anderson LA, Johnston BT, Watson RG, et al. Nonsteroidal anti-inflammatory drugs and the esophageal inflammation-metaplasia-adenocarcinoma sequence. Cancer Res. 2006;66:4975-4982.
222 Buttar NS, Wang KK, Leontovich O, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett’s esophagus. Gastroenterology. 2002;122:1101-1112.
223 Heath EI, Canto MI, Piantadosi S, et al. Chemoprevention for Barrett’s Esophagus Trial Research Group. Secondary chemoprevention of Barrett’s esophagus with celecoxib: Results of a randomized trial. J Natl Cancer Inst. 2007;99:545-557.
224 Triadafilopoulos G, Kaur B, Sood S, et al. The effects of esomeprazole combined with aspirin or rofecoxib on prostaglandin E2 production in patients with Barrett’s oesophagus. Aliment Pharmacol Ther. 2006;23:997-1005.
225 Falk GW. The future of endoscopic treatment of early Barrett neoplasia: The endoscopist’s view. Endoscopy. 2008;40:1041-1047.
226 Kelty CJ, Ackroyd R, Brown NJ, et al. Endoscopic ablation of Barrett’s oesophagus: A randomized-controlled trial of photodynamic therapy vs. argon plasma coagulation. Aliment Pharmacol Ther. 2004;20:1289-1296.
227 Dulai GS, Jensen DM, Cortina G, et al. Randomized trial of argon plasma coagulation vs. multipolar electrocoagulation for ablation of Barrett’s esophagus. Gastrointest Endosc. 2005;61:232-240.
228 Sharma P, Wani S, Weston AP, et al. A randomised controlled trial of ablation of Barrett’s oesophagus with multipolar electrocoagulation versus argon plasma coagulation in combination with acid suppression: Long term results. Gut. 2006;55:1233-1239.
229 Van Laethem JL, Peny MO, Salmon I, et al. Intramucosal adenocarcinoma arising under squamous re-epithelialisation of Barrett’s oesophagus. Gut. 2000;46:574-577.
230 Shand A, Dallal H, Palmer K, et al. Adenocarcinoma arising in columnar lined oesophagus following treatment with argon plasma coagulation. Gut. 2001;48:580-581.
231 Mino-Kenudson M, Ban S, Ohana M, et al. Buried dysplasia and early adenocarcinoma arising in Barrett esophagus after porfimer-photodynamic therapy. Am J Surg Pathol. 2007;31:403-409.
232 Ganz RA, Utley DS, Stern RA, et al. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: A phased evaluation in the porcine and in the human esophagus. Gastrointest Endosc. 2004;60:1002-1010.
233 Dunkin BJ, Martinez J, Bejarano PA, et al. Thin-layer ablation of human esophageal epithelium using a bipolar radiofrequency balloon device. Surg Endosc. 2006;20:125-130.
234 Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185-195.
235 Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic ablation of Barrett’s esophagus: A multicenter study with 2.5-year follow-up. Gastrointest Endosc. 2008;68:867-876.
236 Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288.
237 Pouw RE, Gondrie JJ, Rygiel AM, et al. Properties of the neosquamous epithelium after radiofrequency ablation of Barrett’s esophagus containing neoplasia. Am J Gastroenterol. 2009;104:1366-1373.
238 Johnston MH, Eastone JA, Horwhat JD, et al. Cryoablation of Barrett’s esophagus: A pilot study. Gastrointest Endosc. 2005;62:842-848.
239 Dumot JA, Vargo JJ, Falk GW, et al. An open-label, prospective trial of cryospray ablation for Barrett’s esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc. 2009;70:635-644.
240 Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: International, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488-498.
241 Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc. 2007;66:460-468.
242 Prasad GA, Wang KK, Buttar NS, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2007;132:1226-1233.
243 Krishnadath KK, Wang KK, Taniguchi K, et al. Persistent genetic abnormalities in Barrett’s esophagus after photodynamic therapy. Gastroenterology. 2000;119:624-630.
244 Hage M, Siersema PD, Vissers KJ, et al. Molecular evaluation of ablative therapy of Barrett’s oesophagus. J Pathol. 2005;205:57-64.
245 Hage M, Siersema PD, Vissers KJ, et al. Genomic analysis of Barrett’s esophagus after ablative therapy: Persistence of genetic alterations at tumor suppressor loci. Int J Cancer. 2006;118:155-160.
246 Dvorak K, Ramsey L, Payne CM, et al. Abnormal expression of biomarkers in incompletely ablated Barrett’s esophagus. Ann Surg. 2006;244:1031-1036.
247 Prasad GA, Buttar NS, Wongkeesong LM, et al. Significance of neoplastic involvement of margins obtained by endoscopic mucosal resection in Barrett’s esophagus. Am J Gastroenterol. 2007;102:2380-2386.
248 Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc. 2007;65:3-10.
249 Seewald S, Akaraviputh T, Seitz U, et al. Circumferential EMR and complete removal of Barrett’s epithelium: A new approach to management of Barrett’s esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc. 2003;57:854-859.
250 Giovannini M, Bories E, Pesenti C, et al. Circumferential endoscopic mucosal resection in Barrett’s esophagus with high-grade intraepithelial neoplasia or mucosal cancer: Preliminary results in 21 patients. Endoscopy. 2004;36:782-787.
251 Peters FP, Kara MA, Rosmolen WD, et al. Stepwise radical endoscopic resection is effective for complete removal of Barrett’s esophagus with early neoplasia: A prospective study. Am J Gastroenterol. 2006;101:1449-1457.
252 Larghi A, Lightdale CJ, Ross AS, et al. Long-term follow-up of complete Barrett’s eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy. 2007;39:1086-1091.
253 Lopes CV, Hela M, Pesenti C, et al. Circumferential endoscopic resection of Barrett’s esophagus with high-grade dysplasia or early adenocarcinoma. Surg Endosc. 2007;21:820-824.
254 Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: An effective treatment modality for high-grade dysplasia and intramucosal carcinoma—an American single-center experience. Am J Gastroenterol. 2009;104:2684-2692.
255 Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett’s neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy. 2008;40:370-379.
256 Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Stepwise circumferential and focal ablation of Barrett’s esophagus with high-grade dysplasia: Results of the first prospective series of 11 patients. Endoscopy. 2008;40:359-369.
257 Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology. 2009;137:815-823.
258 Das A, Singh V, Fleischer DE, et al. A comparison of endoscopic treatment and surgery in early esophageal cancer: An analysis of surveillance epidemiology and end results data. Am J Gastroenterol. 2008;103:1340-1345.
259 Schembre DB, Huang JL, Lin OS, et al. Treatment of Barrett’s esophagus with early neoplasia: A comparison of endoscopic therapy and esophagectomy. Gastrointest Endosc. 2008;67:595-601.
260 Prasad GA, Wang KK, Halling KC, et al. Utility of biomarkers in prediction of response to ablative therapy in Barrett’s esophagus. Gastroenterology. 2008;135:370-379.
261 Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200-1206.
262 Hornick JL, Mino-Kenudson M, Lauwers GY, et al. Buried Barrett’s epithelium following photodynamic therapy shows reduced crypt proliferation and absence of DNA content abnormalities. Am J Gastroenterol. 2008;103:38-47.
263 Weston AP, Sharma P, Banerjee S, et al. Visible endoscopic and histologic changes in the cardia, before and after complete Barrett’s esophagus ablation. Gastrointest Endosc. 2005;61:515-521.
264 Sampliner RE, Camargo E, Prasad AR. Association of ablation of Barrett’s esophagus with high grade dysplasia and adenocarcinoma of the gastric cardia. Dis Esophagus. 2006;19:277-279.
265 Fass R, Garewal HS, Hayden CW, et al. Preferential repair by squamous epithelium of thermal induced injury to the proximal stomach in patients undergoing ablation of Barrett’s esophagus. Gastrointest Endosc. 2001;53:711-716.
266 Sharma P, Falk GW, Sampliner R, et al. Management of nondysplastic Barrett’s esophagus: Where are we now? Am J Gastroenterol. 2009;104:805-808.