Chapter 25 Diagnosis and Staging of Esophageal Carcinoma
Introduction
The incidence of esophageal carcinoma is increasing in many countries, and the disease remains highly lethal because most patients present with advanced disease. Squamous cell carcinoma (SCC) and adenocarcinoma are the most common subtypes, whereas verrucous carcinoma (a variant of SCC) and small cell carcinoma occur rarely. Nonepithelial tumors of the esophagus are discussed in Chapter 29. Diagnosis of early SCC and its precursors is discussed in Chapter 30. Diagnosis and surveillance of Barrett’s esophagus is described in Chapter 26. The use of emerging endoscopic technologies in the diagnosis and assessment of early esophageal neoplasia is discussed in Chapter 27.
Epidemiology
Carcinoma of the esophagus ranked as the eighth most common carcinoma worldwide in 1990 and accounts for 7% of gastrointestinal (GI) malignancies.1 Most patients in the Western world present after the age of 65 years. Approximately 13,900 people in the United States are affected annually with an overall age-adjusted incidence of 4.5 per 100,000, ranging from 2.1 per 100,000 in women to 7.7 per 100,000 in men.2,3 Overall 5-year survival has improved in recent years to 14%, which is still low. There are significant epidemiologic differences between the two main carcinoma subtypes, and these are considered separately.
Squamous Cell Carcinoma
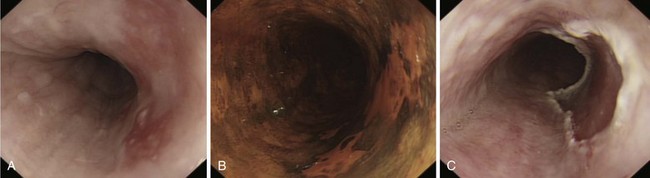
SCC can arise anywhere in the esophagus with an approximately equal distribution throughout (Fig. 25.1). Globally, SCC is more common than adenocarcinoma with an overall incidence of 2.5 to 5.0 per 100,000 for men and 1.5 to 2.5 per 100,000 for women.4 There are, however, striking geographic variations in incidence. High-risk areas include the Transkei region of South Africa and the so-called “Asian esophageal cancer belt” comprising eastern Turkey, India, northern Iran, and northern China, where the incidence is greater than 100 per 100,000 population.5 In the United States, African American men have the highest incidence of any ethnic group (16.8 per 100,000), and men are generally affected two to three times more often than women,6 although the incidence is similar in the two groups in high-risk areas of the world. The disease is most prevalent among lower socioeconomic groups.
Variations in incidence have been attributed to numerous etiologic factors, including environmental exposure, dietary habits, infection, radiation exposure, and associated high-risk conditions. The most important risk factors for SCC (in the Western world) are tobacco and alcohol use,7,8 the risk being greatest in individuals who both smoke and drink. Many carcinogens, including nitrosamines and polycyclic hydrocarbons, exist in both tobacco smoke and alcohol, and the risk may be compounded in alcohol abusers by concomitant nutritional and immunologic deficiencies.5
Numerous high-risk conditions have been described. Achalasia may predispose to SCC in association with chronic stasis of food and debris in the dilated esophagus, with an incidence of SCC of up to 9% after 15 to 20 years.5 Lye strictures of the esophagus are associated with a 1000-fold increased risk of SCC compared with controls, with patients often presenting 40 to 50 years after lye ingestion. Up to 16% of patients with Plummer-Vinson syndrome (iron deficiency anemia, dysphagia, and postcricoid webs in elderly women) may develop pharyngeal or esophageal carcinoma.9 Long-standing celiac disease is rarely associated with esophageal and pharyngeal SCC in addition to enteropathy-associated T-cell lymphoma and adenocarcinoma.10 Tylosis is a rare autosomal dominant disease consisting of palmar and plantar hyperkeratosis along with thickening and fissuring of the skin. Up to 95% of patients develop esophageal SCC by age 65 years.11 Patients with SCC of the head and neck have been reported to develop synchronous esophageal carcinoma in up to 8% of cases12 with tobacco and alcohol as common risk factors for both.
Adenocarcinoma
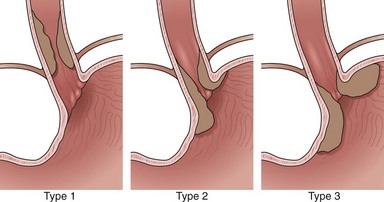
Adenocarcinomas arise in the distal esophagus and at the esophagogastric (EG) junction (Fig. 25.2). Tumors at the EG junction and gastric cardia were previously considered as primary gastric carcinomas invading the distal esophagus. These tumors have been reclassified (Fig. 25.3) as type 1 when they arise from the distal esophagus, type 2 when the origin is the gastric cardia, and type 3 when they are subcardial.13 Although this classification should allow more accurate characterization of tumors at the EG junction into primary esophageal or primary gastric types, changes in terminology and classification over time must be borne in mind when interpreting data from relevant studies.
Current evidence suggests that type 1 (and possibly type 2) carcinomas of the EG junction have a similar clinical course to distal esophageal tumors and should be staged as for esophageal carcinoma.14 Epidemiologic differences between esophageal adenocarcinoma and type 3 adenocarcinoma of the EG junction suggest that these diseases are separate entities,15 and type 3 tumors are usually staged according to criteria for gastric cancer.
In contrast to SCC, the incidence of esophageal adenocarcinoma has been increasing in many parts of the developed world since the 1970s, a consistent finding across the United States, Europe, and Australasia.16–18 The most rapid increases have been reported in the United States at 10% per year,19 especially in white men, and adenocarcinoma is now more common than SCC.16 Current incidence in the developed world is approximately 4.0 per 100,000 population, varying from less than 1.0 in Eastern Europe to 5.0 to 8.7 per 100,000 in Great Britain.3,4 Similar trends have not been observed to date in Asian populations.20
The predominant risk factors for adenocarcinoma are gastroesophageal reflux (GER) and Barrett’s esophagus. In contrast to SCC, lower socioeconomic status and tobacco and alcohol use are not strongly associated with increased incidence,21 although smoking has been found to be a risk factor in some studies.22,23 Chronic GER predisposes to Barrett’s esophagus24 but was also associated independently with the development of adenocarcinoma in a large study of Swedish patients.25 In this population, the development of adenocarcinoma correlated with duration, frequency, and severity of GER. Obesity has also been implicated as an independent risk factor in the development of adenocarcinoma, perhaps because of an influence on GER. A meta-analysis of eight studies reported an odds ratio for esophageal adenocarcinoma of 1.52 (95% confidence interval 1.147 to 2.009) in patients with a body mass index of 25 to 30 kg/m2, increasing to 2.78 (95% confidence interval 1.850 to 4.164) in patients whose BMI was greater than 30 kg/m2.26 There are wide variations in the reported relative risk of cancer development in Barrett’s esophagus, but it is estimated to be approximately 0.5% per year (i.e., 1 in 200 patient-years).24,27 There is a weak correlation between length of the Barrett’s segment and malignant potential.28
Clinical Features
The esophagus lacks a serosal covering, and early tumor growth causes asymptomatic dilation of the smooth muscle. In most patients, dysphagia results only when the lumen has narrowed to 50% to 75% of its normal circumference. By this time, local or nodal spread has often occurred, and the tumor is incurable. Cancers can manifest early with dysphagia, but this is uncommon. Dysphagia may be present for several months before medical advice is sought, and subjective localization of the site of obstruction is a poor indicator of the actual site of disease in the esophagus.29 Symptoms are commonly gradual in onset, with progressive difficulty swallowing solids and then liquids. In rare cases, EG junction tumors infiltrating the submucosa affect motility and result in “pseudoachalasia” with no obvious endoscopic mucosal abnormality and clinical features similar to achalasia.
Anorexia and weight loss, which often precede the onset of dysphagia, are common, and odynophagia may occur with ulcerated tumors. Persistent retrosternal pain, unrelated to swallowing, and back pain are sinister symptoms suggesting mediastinal invasion.29 Cough worsened by swallowing or recurrent pneumonia suggests esophagobronchial fistulization. This complication occurs in 5% to 10% of patients and is associated with poor outcome resulting in a median survival time of 1.5 to 4 months.30 Hematemesis is uncommon.31 Exsanguination resulting from aortoesophageal fistula is rare. Tumor involvement of the left recurrent laryngeal nerve results in hoarseness.
Esophageal carcinoma progresses through direct extension, lymphatic spread, or hematogenous metastasis or a combination of these. Lack of a serosal covering facilitates direct extension and invasion of structures in the neck or chest. Early lymphatic spread via rich interconnecting lymphatic networks in the esophageal submucosa is common. Tumors at any site may involve nodes in the neck or the mediastinum, although proximal lesions metastasize more commonly to cervical nodes, and distal lesions metastasize to abdominal nodes.32 Depth of invasion is a major determinant of lymph node metastasis. Disease confined to the mucosa is associated with nodal involvement in less than 5% of cases,33 whereas penetration into the submucosa is associated with a 15% to 50% risk of metastases.34,35 Hematogenous spread occurs to the lungs, liver, adrenals, kidneys, pancreas, peritoneum, bones, and brain.
Diagnosis
Esophagogastroduodenoscopy
Indications and Contraindications
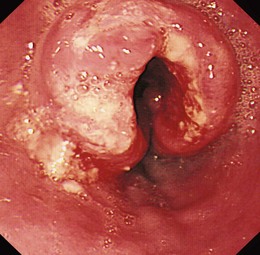
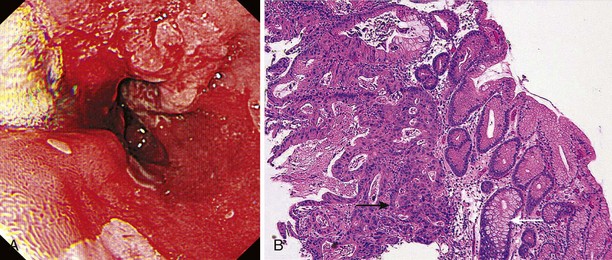
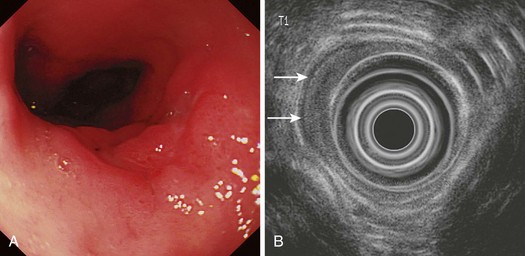
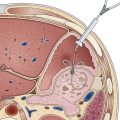
Flexible videoendoscopy with biopsy is the “gold standard” investigation for the diagnosis of esophageal carcinoma (see Figs. 25.1 and 25.2). Endoscopy is more sensitive and specific than double-contrast barium for the diagnosis of upper GI cancer,36 and when biopsy and cytology are combined, the accuracy of endoscopy for diagnosis approaches 100%.37 Rigid esophagoscopy is rarely necessary and is no longer recommended on the grounds of safety and cost-effectiveness.38 In rare patients with pseudoachalasia and repeated negative mucosal biopsy results, endoscopic ultrasound (EUS) with or without fine needle aspiration (FNA) biopsy from within the esophageal wall may provide supportive evidence for malignancy and a tissue diagnosis.39
Equipment
Good-quality videoendoscopes are essential. For assessment of dysplasia or early neoplasia, high-resolution instruments with magnification and instruments with enhanced imaging techniques (e.g., autofluorescence, narrow band imaging, or confocal endomicroscopy) can provide additional useful information.40 Biopsy forceps and cytology brushes are essential, and either balloon or Savary bougie dilators (see Chapter 17) should be available if dilation is necessary to facilitate endoscope passage and completion of the procedure.
Anatomy
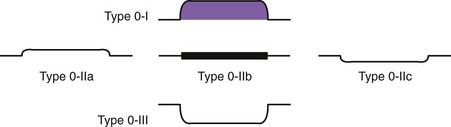
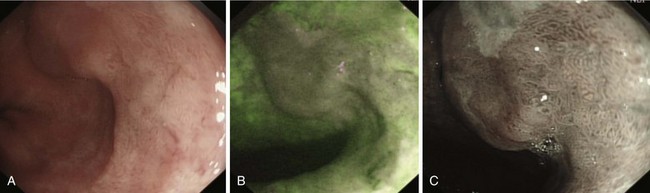
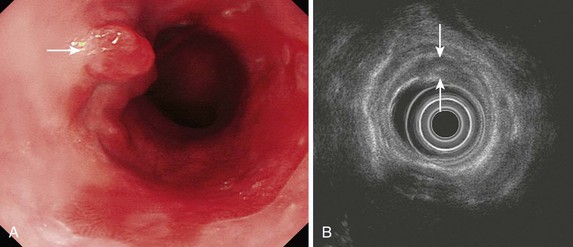
Early lesions may appear as minor irregularities of the mucosa; areas of erythema; or depressed, raised, or ulcerated areas (Figs. 25.5 and 25.6; see also Figs. 25.1 and 25.4). A high index of suspicion is required, and biopsy specimens should be obtained of any tissue with these abnormalities. A more recent study has suggested that characterization of early lesions in Barrett’s segments according to the Paris classification may offer useful information to aid decisions regarding the likelihood of submucosal invasion and the suitability for endoscopic mucosal resection (EMR) (see Figs. 25.4 and 25.6C). Elevated lesions (type 0-I) are associated with a higher prevalence of submucosal involvement, and ulcerated or depressed lesions (type 0-III) are technically unsuitable for EMR.41 This study also showed that most early cancers in Barrett’s esophagus were flat lesions that were difficult to visualize. Dye staining of the mucosa improves the detection rate and is discussed subsequently. More advanced lesions are usually obvious polypoid or ulcerating masses. Stenosis of the esophageal lumen may be present because of tumor bulk or involvement of the muscular layers. A tightly closed distal esophageal sphincter with normal mucosal appearance and resistance to the passage of the endoscope suggests pseudoachalasia.
Procedure
Chromoendoscopy
Lugol’s iodine reacts with glycogen in squamous epithelial cells to produce a uniform dark brown coloration. Inflamed, dysplastic, and malignant cells are glycogen-depleted and consequently appear minimally stained or unstained.42,43 Using a standard washing catheter inserted through the biopsy channel of the endoscope, 1% to 3% Lugol’s iodine is sprayed onto the mucosa. Most highly dysplastic or malignant lesions remain unstained (see Fig. 25.6), and clinical trials have shown that biopsy of these areas enhances detection of high-grade dysplasia and early carcinoma.44,45 The technique is simple and does not require specialized equipment or additional staff; it is described in more detail in Chapter 30.
Methylene blue is a vital stain taken up by the cytoplasm of absorptive cells. These include goblet cells present in Barrett’s epithelium, in addition to normal cells of the colon and small intestine.46 Methylene blue staining was first shown to stain selectively areas of specialized intestinal metaplasia in Barrett’s esophagus in 1996.47 Neoplastic change is associated with a reduction in goblet cell numbers, an increasing nuclear-to-cytoplasm ratio, and reduced absorption of methylene blue. Increasing grades of dysplasia may appear as heterogeneous or unstained areas, allowing targeted rather than random biopsy specimens to be taken.48,49 However, the emergence of electronic chromoendoscopy in the setting of Barrett’s esophagus has eclipsed methylene blue dye staining.
Biopsy and Cytology
Biopsy samples are obtained using forceps inserted via the biopsy channel of the endoscope. The cup volume of standard forceps is 12.4 mm3. The cup volume of the larger jumbo forceps is 30.4 mm3 allowing larger biopsy samples to be taken, but an endoscope with a 3.7-mm channel is required. A nonendoscopic balloon device for obtaining esophageal cytology has been developed in China mainly for use in screening high-risk populations.50
Jumbo forceps may provide better tissue samples for detection of high-grade dysplasia or early carcinoma in Barrett’s esophagus.51 Biopsy specimens are taken from the edge of ulcerated lesions to avoid necrotic tissue, and at least six samples are required to confirm the diagnosis in 100% of patients.52 Larger biopsy specimens can also be obtained by a “turn and suction” technique, in which the forceps are inserted, opened, and withdrawn until flush with the endoscope tip. The tip is turned onto the esophageal wall or lesion while suctioning air from the lumen; this draws tissue into the forceps, which are closed and withdrawn in the usual way.53
Brush cytology alone may detect malignancy and in some studies has been shown to improve the diagnostic yield when combined with standard biopsy.38,54 For best results, brushing should be performed before biopsy to minimize contamination with blood. Samples are obtained by passing the brush catheter into the lumen and drawing the brush across the lesion several times until minor mucosal bleeding is noted. Smears are made on slides and fixed in alcohol before staining using Papanicolaou’s technique.
Use of Esophageal Dilation
In 20% to 40% of cases, a malignant stricture prevents the passage of a standard adult endoscope.55 This situation may prevent both a complete examination and the performance of an adequate biopsy from within the main body of the tumor, but a cytology brush passed into the stricture may be a useful adjunct to obtain the biopsy sample from the proximal end of the lesion.56 Dilation facilitates biopsy, relieves symptoms, and enables subsequent staging with EUS. Biopsy immediately after dilation is safe57; for dilation of malignant strictures, the complications of hemorrhage and perforation occur in 2.5% to 10% of patients.58,59 Dilation provides only short-term palliation of malignant dysphagia, however, with the effects lasting days or at the most a couple of weeks. The issue of dilation before EUS staging is discussed later (see also Chapter 17).
Staging of Esophageal Carcinoma
Prognosis depends on tumor stage at diagnosis. Patients with early disease (T1N0M0; stage I) have 5-year survival rates greater than 90% with surgery alone, but prognosis worsens with advancing stage at diagnosis; patients with stage IV disease have 5-year survival rates of less than 5%. In patients without nodal involvement at surgery (N0), 5-year survival is approximately 40% to 60%, but 5-year survival is only 5% to 17% in patients with nodal involvement (N1). Patients undergoing surgery alone for T3N1 disease have 5-year survival rates of 8% to 10%,60,61 underlining the reality that although these tumors may be technically resectable, they are rarely curable. Accurate staging is essential in determining prognosis and in identifying patients for whom surgery alone is likely to be curative and patients with advanced disease for whom surgery has little to offer and for whom medical therapy or palliation should be the goal of therapy.
The benefits of neoadjuvant therapy have not yet been conclusively shown.62–64 A full discussion of the relative benefits of neoadjuvant therapy is beyond the scope of this chapter; however, given its increasing use and the considerable morbidity associated with it, it is imperative that patients undergo accurate staging to guide stage-specific therapy with the hope of improving survival. These strategies vary according to local practice and must be understood by clinicians staging such patients. In addition, to obtain clinically useful and important information from future trials of neoadjuvant therapy, it is essential that study groups are well matched, and highly accurate staging is crucial in this regard.
Staging System
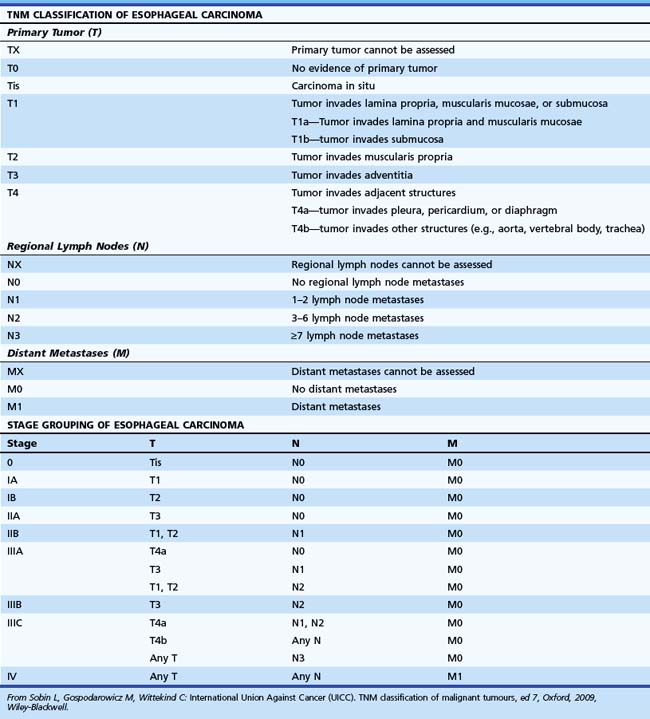
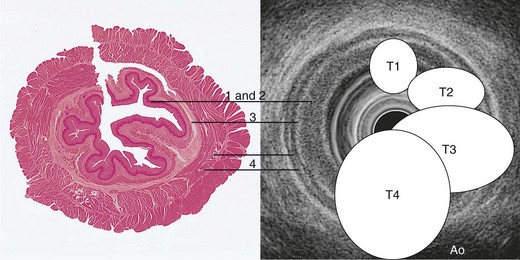
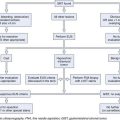
Tumors are staged using the tumor, nodes, and metastases (TNM) staging system developed by the International Union against Cancer (UICC) and American Joint Committee on Cancer (AJCC).65 This system, revised in the seventh edition (TNM-7) in 2009, describes the anatomic extent of cancer at the time of diagnosis and before therapy and allows a classification of the stages of cancer for estimation of prognosis and comparison of the results of different treatments. The definition of TNM for all esophageal cancer subtypes and stage groupings are detailed in Table 25.1, based on the depth of invasion of the tumor into the esophageal wall or beyond, the presence or absence of regional lymph node involvement, the number of involved lymph nodes, and identification of distant metastasis.
For the UICC TNM-7 classification of malignant tumors,65 a tumor with an epicenter within 5 cm of the EG junction and extending into the esophagus is staged as esophageal; a tumor with an epicenter in the stomach that is greater than 5 cm from the EG junction or is less than 5 cm from the junction but does not involve the esophagus is staged according to the gastric system.
Controversy exists over how best to classify patients with involvement of distant lymph nodes. In TNM-7, regardless of the site of the primary tumor, regional lymph nodes are nodes in the esophageal drainage area, and this includes nodes in the celiac axis and paraesophageal neck nodes but not supraclavicular nodes. TNM-7 also takes account of newer data, including a more recent study from the United Kingdom proposing a new N stage classification based on number of involved nodes and location (one or both sides of the diaphragm) as a more powerful predictor of prognosis.66 In TNM-7, the presence of one or two involved regional nodes is classified as N1 disease, the presence of three to six involved nodes is classified as N2 disease, and the presence of seven or more nodes is classified as N3 disease. Further studies corroborating this approach are necessary; however, the presence of a high nodal burden or involved nodes in two compartments is associated with a poor prognosis.
Assessment of Distant Metastases (M)
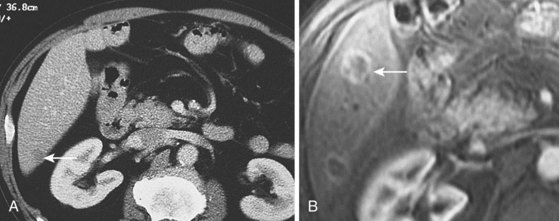
Distant metastases are present in approximately 20% of patients at diagnosis61,67 and most commonly involve nonregional abdominal or supraclavicular lymph nodes. CT scanning is widely available and noninvasive and is the mainstay of initial staging in esophageal cancer. Its role in T and N staging is discussed later; for distant metastasis, CT has overall sensitivity of 52% (33% to 71%), overall specificity of 91% (86% to 96%), and accuracy of 63% to 90%.68,69 Sensitivity for detecting solid organ metastasis greater than 1 cm in size is approximately 80% but significantly less for smaller lesions (Fig. 25.7).
Diminutive liver lesions are assessed better by laparoscopy, which can also assess peritoneal involvement more accurately than CT (sensitivity 95% vs. 21% for CT).70 Because of its noninvasive nature, relatively low cost, and availability, however, CT remains the primary modality for assessment of metastatic disease.
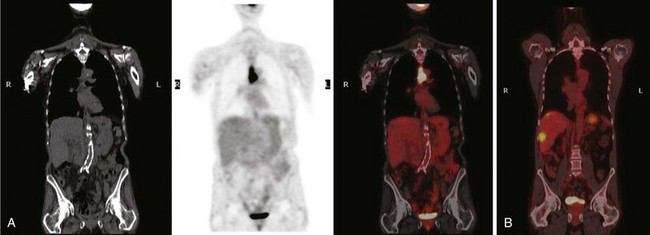
18F-Fluorodeoxyglucose-enhanced PET in combination with CT (CT/PET) may be superior to CT alone, but its routine use in the staging of esophageal cancer is unclear. Data suggest that PET may be superior to CT for detection of distant (e.g., celiac axis) lymph nodes (Fig. 25.8), but it is inaccurate for staging regional nodal disease. Limited spatial resolution impairs the ability to differentiate nodal involvement from the high signal in the adjacent primary tumor (see Fig. 25.8), and the presence of reactive inflammatory nodes can lead to false-positive results. Sensitivity for detection of regional nodes is also significantly less than that of EUS (30% to 40% vs. approximately 80%). PET may upstage a few patients owing to its greater sensitivity for distant metastases compared with CT alone,68,71 but data on its utility in esophageal carcinoma are conflicting, and its use is decided on a case-by-case basis in many centers.
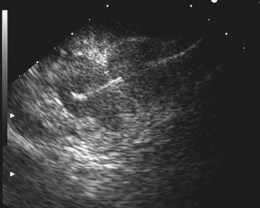
Because EUS is able to visualize only the GI wall layers and structures immediately adjacent to the gut wall, it is not primarily used to perform M staging, but it can provide useful M stage information in some patients without evidence of distant metastasis on CT. Small (<1 cm) metastases in the left lobe of the liver can be detected and are often amenable to EUS-guided FNA for tissue confirmation.72 EUS is also highly sensitive for assessing the celiac axis for nodal involvement (Fig. 25.9). The excellent sensitivity and the potential for near 100% specificity with EUS-guided FNA have been shown in several studies.69,73–79 In one retrospective study, high-quality thin-slice helical CT detected only 53% of celiac lymph nodes that were proven to be involved by EUS-guided FNA.80 Of 48 patients, 12 who were thought to have resectable lesions by helical CT had either metastatic lymph node involvement or T4 disease by EUS-guided FNA. EUS can play a complementary role to CT in improving the accuracy of staging. EUS assesses morphologic features in addition to size and offers the potential for cytologic proof of malignancy through EUS-FNA cytology. EUS may allow detection of low-volume ascites not visible on other imaging modalities. A study from the United Kingdom found that EUS detected low-volume ascites in 6.5% of 802 patients with EG cancer.81 Of these, only half had operable disease at laparoscopy, and only half of the patients undergoing laparotomy achieved R0 resection—that is, 76% of patients with low-volume ascites at EUS had incurable disease.
Regional Lymph Node Staging (N)
Early spread to locoregional nodes is common because of the rich supply of interconnecting submucosal lymphatic channels; this applies equally to SCC and adenocarcinoma. The prevalence of nodal involvement increases with increasing T stage such that less than 5% of patients with T1m tumors have nodal involvement, increasing to approximately 25% in T1sm tumors, 60% in T2 tumors, and more than 80% in T3 or T4 tumors. Although the presence of peritumoral nodes does not prevent successful tumor resection, it has a major negative impact on prognosis, and cure rates after surgery are only 5% to 10%.82,83 In a study of 94 patients, the prognosis of patients with N1 disease was equally poor regardless of the T stage of the tumor, highlighting the overriding importance of accurate detection of nodal involvement so that such patients can be considered for neoadjuvant therapy.84 In addition to the presence of nodes, the number detected is prognostically important; patients with three or more involved regional nodes fare particularly poorly.82–85
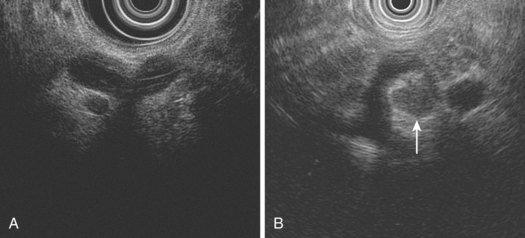
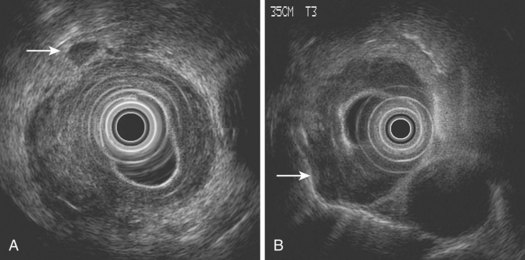
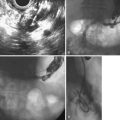
EUS not only assesses lymph node size but also assesses morphologic features, such as shape, margin, and internal echo features. Normal or reactive nodes in the mediastinum are usually flat or triangular in shape with indistinct borders and an echogenic center, whereas malignant involvement is suggested by a size of greater than 10 mm, round shape, distinct outer border, and hypoechoic echo features (Fig. 25.10).86,87 Although the presence of all of these features is 80% accurate for malignant involvement, this occurs in only 25% to 40% of malignant nodes. Overall, the sensitivity of EUS for detecting nodal involvement is 85%, increasing to 97% with EUS-guided FNA,88 and accuracy is approximately 65% to 70%,69,79,88 the latter declining with increasing distance from the primary tumor site. The number of nodes detected at EUS correlates well with the number detected histologically and with prognosis in SCC; it is valuable to document these carefully at EUS.82 In a multivariate analysis, the detection of malignant-looking nodes by EUS was a statistically significant predictor of poor prognosis with a median survival of 13.5 months compared with more than 25 months in patients without nodes.83
As is the case with CT, size remains a problem despite the ability of EUS to visualize lymph nodes 2 to 3 mm in size. The addition of EUS-guided FNA can improve the accuracy of lymph node staging, and the overall accuracy of EUS-guided FNA for detecting malignant involvement in periintestinal lymph nodes is high (85% to 93%).88–90 In one retrospective study, the accuracy improved from 70% to 93% with the addition of FNA; this was the result of an improvement in both sensitivity and, to a lesser extent, specificity.91 Although safe, the addition of FNA prolongs procedure time, and performance of FNA may be impossible without traversing the primary tumor, risking contamination of the sample and obtaining false-positive results. However, FNA is useful if the information gained would upstage the patient and affect subsequent management. As discussed previously, FNA is particularly relevant in the assessment of distal nodal involvement, especially at the celiac axis, where conclusive cytologic proof of involvement usually results in a change in management to a nonsurgical approach in most cases.
Tumor Stage (T)
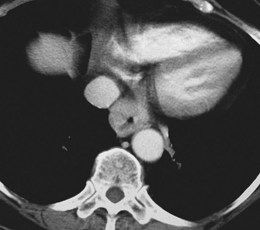
CT is incapable of showing individual wall layers, invasion of which forms the basis of assessment of T stage. Esophageal carcinomas are usually seen on CT as areas of either focal wall thickening or circumferential irregular thickening of the esophageal wall (Fig. 25.11). Proximal and distal extent of the tumor can also be difficult to measure, and it is often difficult to assess tumors of the EG junction accurately, unless the stomach is adequately distended. The presence of a hiatal hernia can also lead to overstaging. Although invasion of the periesophageal fat may suggest T3 disease, and T4 involvement may be suggested by such features as anterior bowing of the posterior wall of the trachea or loss of the triangular fat pad between the esophagus, aorta, and spine, these features lack sufficient accuracy to be reliable. Sometimes clear T4 disease can be seen on CT, but the main value of CT lies in assessment of visceral metastatic disease and possibly involvement of nonregional lymph nodes. It is also useful in radiotherapy planning and measurement of initial tumor bulk for subsequent assessment of response to chemoradiotherapy or chemotherapy protocols.
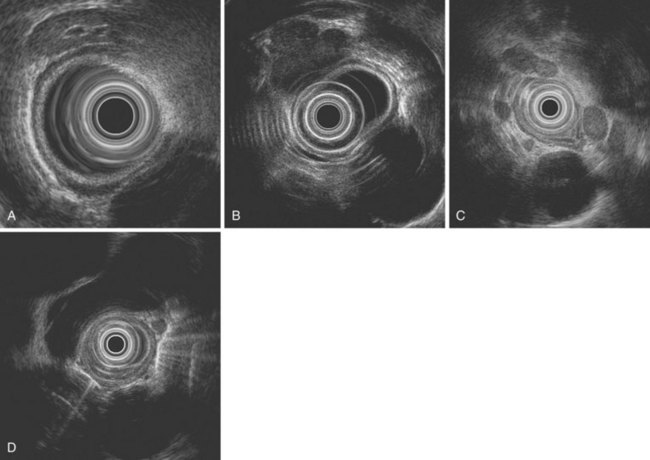
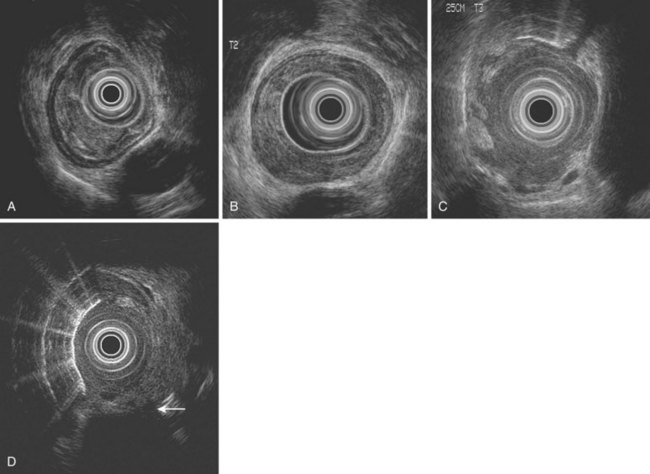
The ability of EUS to image the intestinal wall as a series of concentric layers makes it ideally suited to T staging, especially in the esophagus (Figs. 25.12 and 25.13). Multiple studies over the years have repeatedly shown the accuracy of EUS for assessment of T stage in esophageal carcinoma, and quoted overall accuracy rates are approximately 80% to 85%.69,73–81 In a meta-analysis, the sensitivity and specificity of EUS for T1 disease were 81.6% and 99.4%; the corresponding results for T4 stage were 92.4% and 97.4%.88 However, accuracy varies within each T stage and is generally best for T3 and T4 tumors (Fig. 25.14). Accuracy has generally been reported to be poorest for T2 tumors, for which it ranges from 65% to 73%69,80 possibly because of difficulty detecting microscopic invasion beyond the muscularis propria.
EUS is the only technique currently available for assessing patients with superficial (T1) tumors because these are rarely evident on CT (Figs. 25.15 and 25.16). Staging T1 tumors is especially important with the increasing use of local endoscopic therapies such as EMR, photodynamic therapy, or radiofrequency ablation as less invasive alternatives to surgical resection. Tumors confined to the mucosa (T1a) or perhaps the superficial one-third of the submucosa (T1b, T1sm1) may be suitable for EMR.91,92 The accuracy of EUS for T1 disease has been reported to be approximately 85%. High-frequency ultrasound catheter probes may be useful in this setting with greater accuracy reported in some studies,93,94 but imaging with these miniprobes can be technically challenging. Data from Japanese groups suggest that ultra-high-frequency catheter probes (30 MHz) can accurately distinguish superficial submucosal involvement (T1sm1) from deeper involvement (T1sm2 or T1sm3), but experience in Western countries is less encouraging, and results are not as good as the results obtained by high-resolution endoscopy alone or diagnostic EMR (see Fig. 25.5).95,96 Greater availability and more simplified techniques for EMR have largely restricted the role of EUS in early tumors to looking for locoregional lymph nodes that have not been detected on CT.
At the opposite extreme, 20% to 30% of patients with esophageal cancer have esophageal stenosis of such severity that it is impossible to traverse the stricture with a standard echoendoscope.55,97–99 Failure to do so and to complete the procedure is associated with significant understaging; however, early studies of EUS reported unacceptably high rates of esophageal perforation, either resulting from the procedure itself or with prior dilation to 16 to 17 mm.55 Since these reports, echoendoscope technology has advanced significantly, and modern instruments are slimmer, have less bulky ultrasonic transducers at the tip and better video optics, and are rarely associated with esophageal perforation. In a large study of 132 patients, 32% required dilation up to 14 to 16 mm to complete the procedure in almost all patients, and only one perforation occurred.100 In this study, dilation allowed detection of advanced disease (either T4 or M1a) in 19% of patients undergoing dilation.
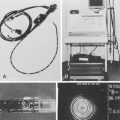
If the information gained from completing the EUS procedure is likely to affect patient management, careful dilation should be undertaken, in stages if necessary, to allow completion of the procedure. An alternative is the use of the 7.8-mm esophagoprobe (Olympus MH-908) (Fig. 25.17). This conical-tipped instrument lacks endoscopic optics and is passed through strictures over a monorail guidewire placed endoscopically. With this instrument, it is possible to pass all but the tightest stenoses with no or minimal dilation. Several studies have shown the equivalent accuracy of this instrument compared with standard echoendoscopes with a reported T staging accuracy of 89%.97,99 Catheter ultrasound probes have also been used with some success but are not routinely recommended in this situation because of the lack of penetration and suboptimal nodal imaging. If nodes in the celiac axis are detected by these methods, FNA may be necessary, and this is one argument in favor of the use of dilation and a standard echoendoscope in preference to other methods.

Fig. 25.17 Nonoptical 8-mm esophagoprobe (Olympus MH-908) for use in patients with tight, stenosing carcinomas.
Accurate staging of tumors of the EG junction is a challenge for all imaging techniques, especially for type III lesions. Although EUS has been shown to be superior to CT for both T and N staging of type II tumors, neither EUS nor CT was very accurate for type III cancers with both understaging and overstaging occurring.101 This poor accuracy may relate to the change of direction and oblique course of the intraabdominal esophagus, and this needs to be kept in mind when staging tumors in this location.
Endoscopic Ultrasound Staging of Esophageal Carcinoma
Procedure
Other Staging Techniques
Although CT and EUS are the mainstays of esophageal cancer staging, other modalities have an important role to play in certain patients. Patients with distal esophageal tumors or tumors of the EG junction may have significant intraabdominal disease (tumor extending down the lesser curve, lymphadenopathy, or peritoneal deposits), and careful laparoscopy with or without laparoscopic ultrasound has been shown to be useful in such cases. Several studies have shown the ability of laparoscopy to upstage a significant percentage of patients and reduce open-closed laparotomy rates.68,70 Few studies have compared the information provided by a combined approach of CT, EUS, and laparoscopy, but this approach may improve further the detection of patients with unresectable tumors.
Although not widely available, video-assisted thoracoscopy is a sensitive and accurate means of detecting mediastinal lymph node involvement and assessing T4 involvement, especially of the airways. Few studies have directly compared this form of minimally invasive staging with CT and, in particular, EUS. Video-assisted thoracoscopy is significantly more invasive than EUS, has a small but significant complication rate, and requires general anesthesia. Finally, patients with SCC may have clinically undetectable involvement of supraclavicular lymph nodes, and several studies have shown the ability of ultrasound of the neck with FNA biopsy to detect these nodes with high sensitivity and specificity.102,103 The exact place of cervical ultrasound in the staging algorithm of thoracic esophageal carcinoma remains to be defined, but it is a simple, safe, and relatively inexpensive procedure and one that merits consideration in patients with disease at this location.
Outcomes and Evidence
Do Endoscopic Ultrasound Findings Correlate with Prognosis?
In recent years, numerous studies have assessed the prognostic value of EUS findings in patients with esophageal cancer. The presence of celiac axis lymphadenopathy (especially when proven by EUS-guided FNA) is associated with a poor prognosis regardless of whether or not patients undergo surgical resection.104–106 In a multivariate analysis of 203 patients, the presence of malignant-looking regional lymph nodes was also a statistically significant predictor of survival.83 In another study, median survival was only 8 months in patients with EUS features of malignant lymph node involvement compared with more than 28 months in patients with no EUS evidence or nodal involvement.107 In a large Japanese study of 339 patients, the number of lymph nodes identified by EUS and transcutaneous ultrasound correlated well with prognosis. In this study, 5-year survival rates for patients with zero, one to three, four to seven, and eight or more detected nodes were 53.3%, 33.8%, 17%, and 0%.82
Two retrospective studies also identified T4 stage by EUS as a marker of poor survival regardless of subsequent therapy and showed that survival in these patients was not improved by surgery.108,109 The findings of celiac lymph node, regional nodal, or T4 involvement at EUS not only influences management but also provides useful prognostic information. The more recent suggestion that staging should be modified to take account of node number and location in combination needs to be thoroughly tested in prospective studies.66
Can the Accuracy of Nodal Staging Be Improved?
Given the important prognostic implications of nodal involvement and the potential impact this may have in selecting patients for neoadjuvant therapy, it is essential to improve on the modest accuracy of EUS imaging alone. EUS-guided FNA of identified nodes (Fig. 25.18) is logical to improve specificity and accuracy; however, despite numerous reports of its performance, there have been relatively few randomized, prospective studies comparing EUS imaging alone with EUS-guided FNA of nodes in esophageal cancer.
One retrospective study assessed the impact of EUS-guided FNA in 64 patients.91 The addition of FNA increased the accuracy of nodal staging to 93% largely by increasing the sensitivity and, to a lesser degree, specificity. The benefits were not as great for celiac nodes probably because enlarged lymph nodes at this location in esophageal cancer are rarely benign and reactive, a finding confirmed in other studies. The data available from these studies show that EUS-guided FNA reduces the rate of both false-positive and false-negative results, and the routine use of FNA sampling of detected nodes in all cases has been advocated by some authors. A meta-analysis of the performance of EUS in T and N staging of esophageal cancer supports this suggestion.88 Studies from the Mayo Clinic have sounded a note of caution, however, showing that it is possible to obtain false-positive lymph node cytology at EUS-guided FNA as a result of contamination by suction of tumor cells shed into the GI tract lumen into the endoscope accessory channel and by traversing abnormal tissue to reach nodes.110
Does Endoscopic Ultrasound Have an Impact on Clinical Outcome?
Although EUS is the most accurate locoregional staging modality, a major impact of EUS staging on patient management and outcomes has not been shown, and properly designed outcome studies in this area are relatively few. A prospective study from the United Kingdom examined the effect of EUS on the management of 100 consecutive cases of esophageal carcinomas and carcinomas of the EG junction.111 Three specialist surgeons were asked in a blinded fashion to select a management plan after reviewing full staging information before EUS and again after EUS. The additional EUS information led to a significant change in treatment plan in 16%, 18%, and 32% of cases among the three surgeons. Giovannini and coworkers112 reported that EUS showed distant lymphadenopathy in 40 of 198 patients (20%) with esophageal cancer. FNA was performed with a sensitivity of 97% and a specificity of 100%, and the findings led to a change in treatment in 77.5% of this subgroup of patients (i.e., 16% of all patients). In a prospective study of 108 patients, EUS detected nodal or metastatic involvement in 16.5% of patients, in whom FNA was positive in 86%. When interpreted on an “intention to biopsy” basis, EUS-guided FNA was relevant to only 8.3% of the entire study population, and the overall impact was 13% in terms of changing the therapeutic strategy.113 Further large studies of this type are needed, as are carefully designed economic studies to bolster the decision-modeling analyses that have already suggested the cost-effectiveness of an EUS-based strategy for staging esophageal cancer.
Is Endoscopic Ultrasound Useful for Restaging after Neoadjuvant Therapy?
Initial studies evaluating the accuracy of EUS restaging after neoadjuvant chemoradiotherapy were disappointing and highlighted the inability to differentiate residual tumor from inflammatory or fibrotic changes.114–116 However, these studies reported T and N stage accuracy rates and were not surprisingly disappointing. Alternatively, documenting a reduction in maximal tumor cross-sectional area by EUS may be a promising means of predicting response to therapy. Several small studies have reported that a 50% or greater reduction in cross-sectional area is relatively accurate at predicting response.117,118 In one study, EUS correctly predicted a tumor response to chemoradiation in 20 of 23 patients (87%) who had pathologic tumor regression, and overall the positive predictive value of EUS for pathologic regression was 80%.119 Meta-analyses of available evidence found that EUS and PET were both reasonably accurate for restaging and superior to CT with maximum joint values for sensitivity and specificity of 85% to 86% compared with 54% for CT.120 Whether or not the use of three-dimensional EUS imaging to estimate tumor volumes would be valuable in assessing responses to neoadjuvant therapy is unknown at the present time. As for nodal involvement, case reports have suggested the potential of EUS-guided FNA to document nodal downstaging after therapy, but the utility of this approach remains largely unexplored.121
Future Trends
Detection of esophageal cancer at an earlier stage is the most likely means by which significant improvements in survival would be achieved, and new imaging techniques in endoscopy are constantly being developed to achieve this.40 Many of these techniques are now available, and the next few years are likely to see further developments and clarification of which (if any) will translate into routine practice.
1 Pisani P, Parkin DM, Bray F, et al. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18-29.
2 . SEER Cancer Statistics Review, 1975–2000. Ries LAG, Eisner MP, Kosary C. et al. Bethesda, MD: National Cancer Institute. 2003. Available at http://seer.cancer.gov/csr/1975_2000
3 Bollshweiler E, Wolfgarten E, Gutschow C, et al. Demographic variations in the rising incidences of esophageal adenocarcinoma in white males. Cancer. 2001;92:549-555.
4 Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72-81.
5 Ribeiro U, Posner MC, Safatle-Ribeiro AV, et al. Risk factors for squamous cell carcinoma of the esophagus. Br J Surg. 1996;83:1174-1185.
6 Kirkby TJ, Rice TW. The epidemiology of esophageal cancer: The changing face of a disease. Chest Surg Clin North Am. 1994;4:217-225.
7 Wynder L, Mabuchi K. Cancer of the esophagus: Etiological and environmental factors. JAMA. 1973;226:1546-1548.
8 Pottern LM, Morris LE, Blot WJ, et al. Esophageal cancer among black men in Washington, DC: Alcohol, tobacco, and other risk factors. J Natl Cancer Inst. 1981;67:777-783.
9 Wynder EL, Hultberg S, Jacobsson F, et al. Environmental factors in cancer of the upper alimentary tract: A Swedish study with special reference to Plummer-Vinson (Paterson-Kelly) syndrome. Cancer. 1957;10:470-487.
10 Ferguson A, Kingstone K. Coeliac disease and malignancies. Acta Paediatr Suppl. 1996;412:78-81.
11 Harper PS, Harper RM, Howel-Evans AW. Carcinoma of the oesophagus with tylosis. QJM. 1970;39:317-333.
12 Hashimoto C, Iriya K, Baba E, et al. Lugol’s dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005;100:275-282.
13 Siewert JR, Stein HJ. Carcinoma of the cardia: Carcinoma of the gastroesophageal junction—classification, pathology and extent of resection. Dis Esoph. 1996;9:173-182.
14 van Sandick JW, van Lanschot JJ, Tytgat GN, et al. Barrett oesophagus and adenocarcinoma: An overview of epidemiologic, conceptual and clinical issues. Scand J Gastroenterol. 2001;36(Suppl 234):51-60.
15 El-Serag HB, Mason AC, Petersen N, et al. Epidemiological differences between adenocarcinoma of the oesophagus and adenocarcinoma of the gastric cardia in the USA. Gut. 2002;50:368-372.
16 Devesa SS, Blot WJ, Fraumeni JFJr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053.
17 Botterweck AA, Schouten LJ, Volovics A, et al. Trends in incidence of adenocarcinoma of the esophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29:645-654.
18 Lord RV, Law MG, Ward RL, et al. Rising incidence of oesophageal adenocarcinoma in men in Australia. J Gastroenterol Hepatol. 1998;13:356-362.
19 Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289.
20 Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729-735.
21 Levi F, Ollyo JB, La Vecchia C, et al. The consumption of tobacco, alcohol and the risk of adenocarcinoma in Barrett’s oesophagus. Int J Cancer. 1990;45:852-854.
22 Wu A, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus. Cancer Causes Control. 2001;12:721-732.
23 Gammon MD, Schoenberg JB, Ahsan H, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277-1284.
24 Spechler SJ. Esophageal columnar metaplasia (Barrett’s esophagus). Gastrointest Endosc Clin N Am. 1997;7:1-18.
25 Lagergren J, Bergstrom R, Lingren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal carcinoma. N Engl J Med. 1999;340:825-831.
26 Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883-890.
27 Cameron AJ. Epidemiology of columnar lined esophagus and adenocarcinoma. Gastroenterol Clin North Am. 1997;26:487-494.
28 Rudolph RE, Vaughan TL, Storer BE, et al. Effect of segment length on risk for neoplastic progression in patients with Barrett’s esophagus. Ann Intern Med. 2000;132:612-620.
29 Postlethwait RW. Carcinoma of the esophagus. Curr Probl Cancer. 1978;2:1-44.
30 Altorki NK, Migliore M, Skinner DB. Esophageal carcinoma and airway invasion: Evolution and choices of therapy. Chest. 1994;104:742-745.
31 Barrie JR, Goodner JT. Hematemesis from cancer of the esophagus. J Thorac Cardiovasc Surg. 1968;56:289-292.
32 Akiyama H, Masahiko T, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220:364-373.
33 Stein HJ, Feith M, Mueller J, et al. Limited resection for early adenocarcinoma in Barrett’s esophagus. Ann Surg. 2000;232:733-742.
34 Nigro JJ, Hagen JA, DeMeester TR, et al. Occult esophageal adenocarcinoma: Extent of disease and implications for effective therapy. Ann Surg. 1999;230:433-440.
35 Nigro JJ, Hagen JA, DeMeester TR, et al. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: Implications for therapy. J Thorac Cardiovasc Surg. 1999;117:16-25.
36 Dooley CP, Larson AW, Stace NH, et al. Double contrast barium meal and upper gastrointestinal endoscopy: A comparative study. Ann Intern Med. 1984;101:538-545.
37 O’Donoghue J, Waldron R, Gough D, et al. An analysis of the diagnostic accuracy of endoscopic biopsy and cytology in the detection of oesophageal malignancy. Eur J Surg Oncol. 1992;18:332-334.
38 Glaws WR, Etzkorn KP, Wenig BL, et al. Comparison of rigid and flexible esophagoscopy in the diagnosis of esophageal disease: Diagnostic accuracy, complications and cost. Ann Otol Rhinol Laryngol. 1996;105:262-266.
39 Faigel DO, Deveney C, Phillips D, et al. Biopsy-negative malignant esophageal stricture: Diagnosis by endoscopic ultrasound. Am J Gastroenterol. 1998;93:2257-2260.
40 Curvers WL, Kiesslich R, Bergman JJ. Novel imaging modalities in the detection of oesophageal neoplasia. Best Pract Res Clin Gastroenterol. 2008;22:687-720.
41 Pech O, Gossner L, Manner H, et al. Prospective evaluation of the macroscopic types and location of early Barrett’s neoplasia in 380 lesions. Endoscopy. 2007;39:588-593.
42 Schiller W. Early diagnosis of carcinoma of the cervix. Surg Gynecol Obstet. 1933;56:210-222.
43 Sugimachi K, Kitamura K, Baba K, et al. Endoscopic diagnosis of early carcinoma of the esophagus using Lugol’s solution. Gastrointest Endosc. 1992;38:657-661.
44 Yokoyama A, Ohmori T, Makuuchi H, et al. Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer. 1995;76:919-921.
45 Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220-231.
46 Carr-Locke DL, Al-Chaws FH, Branch MS, et al. Technology assessment status evaluation: Endoscopic tissue staining and tattooing. Gastrointest Endosc. 1996;43:652-656.
47 Canto MI, Setrakian S, Petras RE, et al. Methylene blue selectively stains intestinal metaplasia in Barrett’s esophagus. Gastrointest Endosc. 1996;44:1-7.
48 Canto MI, Setrakian S, Willis J, et al. Methylene blue directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2000;51:560-568.
49 Canto MI, Setrakian S, Willis J, et al. Methylene blue staining of dysplastic and non-dysplastic Barrett’s esophagus: An in vivo and ex vivo study. Endoscopy. 2001;33:391-400.
50 Yang H, Berner A, Mei Q, et al. Cytologic screening for esophageal cancer in a high-risk population in Anyang county, China. Acta Cytol. 2002;46:445-452.
51 Levine DS, Haggitt RC, Blount PL, et al. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus. Gastroenterology. 1993;105:40-50.
52 Lal N, Bhasin DK, Malik AK, et al. Optimal number of biopsy specimens in the diagnosis of carcinoma of the oesophagus. Gut. 1992;33:724-726.
53 Levine DS, Reid BJ. Endoscopic biopsy technique for acquiring larger mucosal samples. Gastrointest Endosc. 1991;37:332-337.
54 Zargar SA, Khuroo MS, Jan GM, et al. Prospective comparison of the value of brushings before and after biopsy in the endoscopic diagnosis of gastroesophageal malignancy. Acta Cytol. 1991;35:549-552.
55 Van Dam J, Rice TW, Catalano MF, et al. High grade malignant stricture is predictive of tumor stage: Risks of endosonographic evaluation. Cancer. 1993;71:2910-2917.
56 Kobayashi S, Kasugai T. Brushing cytology for the diagnosis of gastric cancer involving the cardia or the lower esophagus. Acta Cytol. 1978;22:155-157.
57 Barkin JS, Taub S, Rogers AI. The safety of combined endoscopy, biopsy and dilation in esophageal strictures. Am J Gastroenterol. 1981;76:23-26.
58 Moses FM, Peura DA, Wong RK, et al. Palliative dilation of esophageal carcinoma. Gastrointest Endosc. 1985;31:61-63.
59 Lundell L, Leth R, Lind T, et al. Palliative endoscopic dilatation in carcinoma of the esophagus and esophagogastric junction. Acta Chir Scand. 1989;155:179-184.
60 Faivre J, Forman D, Esteve J, et al. Survival of patients with oesophageal and gastric cancers in Europe. Eur J Cancer. 1998;34:2167-2175.
61 Newnham A, Quinn MJ, Babb P, et al. Trends in oesophageal and gastric cancer incidence, mortality and survival in England and Wales 1971–1998/1999. Aliment Pharmacol Ther. 2003;17:655-664.
62 Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet. 2002;359:1727-1733.
63 Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localised esophageal cancer. N Engl J Med. 1998;339:1979-1984.
64 Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodality therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462-467.
65 Sobin L, Gospodarowicz M, Wittekind C. International Union Against Cancer (UICC). TNM Classification of malignant tumours, ed 7. Oxford: Wiley-Blackwell; 2009.
66 Peters CJ, Hardwick RH, Vowler SL, et al. Generation and validation of a revised classification for oesophageal and junctional adenocarcinoma. Br J Surg. 2009;96:724-733.
67 Luketich JD, Friedman DM, Weigel TL, et al. Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg. 1999;68:1133-1136.
68 van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: A meta-analysis. Br J Cancer. 2008;98:547-557.
69 Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49:534-539.
70 Bemelman WA, van Delden OM, van Lanschot JJ, et al. Laparoscopy and laparoscopic ultrasonography in staging of carcinoma of the esophagus and gastric cardia. J Am Coll Surg. 1995;181:421-425.
71 Wong WL, Chambers RJ. Role of PET/PET CT in the staging and restaging of thoracic oesophageal cancer and gastrooesophageal cancer: A literature review. Abdom Imaging. 2008;33:183-190.
72 Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357-361.
73 Tio TL, Cohen P, Coene PP, et al. Endosonography and computed topography of esophageal carcinoma. Gastroenterology. 1989;96:1478-1486.
74 Sugimachi K, Ohno S, Fujishima H, et al. Endoscopic ultrasonographic detection of carcinomatous invasion and of lymph nodes in the thoracic esophagus. Surgery. 1990;107:366-371.
75 Vilgrain V, Mompoint D, Palazzo L, et al. Staging of esophageal carcinoma: Comparison of results with endoscopic sonography and CT. AJR Am J Roentgenol. 1990;155:277-281.
76 Ziegler K, Sanft C, Zeitz M, et al. Evaluation of endosonography in TN staging of oesophageal cancer. Gut. 1991;32:16-20.
77 Grimm H. Endoscopic ultrasonography with the ultrasonic esophagoprobe. Endoscopy. 1994;26:818-821.
78 Souquet JC, Napoleon B, Pujol B, et al. Endoscopic ultrasonography in the preoperative staging of esophageal cancer. Endoscopy. 1994;26:764-766.
79 Rösch T. Endosonographic staging of esophageal cancer: A review of literature results. Gastrointest Endosc Clin N Am. 1995;5:537-547.
80 Romagnuolo J, Scott J, Hawes RH, et al. Helical CT versus EUS with fine needle aspiration for celiac nodal assessment in patients with esophageal cancer. Gastrointest Endosc. 2002;55:648-654.
81 Sultan J, Robinson S, Hayes N, et al. Endoscopic ultrasonography-detected low-volume ascites as a predictor of inoperability for oesophagogastric cancer. Br J Surg. 2008;95:1127-1130.
82 Pfau PR, Ginsberg GG, Lew RJ, et al. EUS predictors of long-term survival in esophageal carcinoma. Gastrointest Endosc. 2001;53:463-469.
83 Natsugoe S, Yoshinaka H, Shimada M, et al. Number of lymph node metastases determined by presurgical ultrasound and endoscopic ultrasound is related to prognosis in patients with esophageal carcinoma. Ann Surg. 2001;234:613-618.
84 Killinger WA, Rice TW, Adelstein DJ, et al. Stage II esophageal carcinoma: The significance of T and N. J Thorac Cardiovasc Surg. 1996;111:935-940.
85 Wang LS, Chow K-C, Chi KH, et al. Prognosis of esophageal squamous cell carcinoma: Analysis of clinicopathological and biological factors. Am J Gastroenterol. 1999;94:1933-1940.
86 Catalano MF, Sivak MVJr, Rice T, et al. Endosonographic features predictive of lymph node metastases. Gastrointest Endosc. 1994;40:442-446.
87 Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479.
88 Puli SR, Reddy JBK, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: A meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479-1490.
89 Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095.
90 Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experience. Gut. 1999;44:720-726.
91 Vazquez-Sequerios E, Norton ID, Clain JE, et al. Impact of EUS-guided fine needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc. 2001;53:751-757.
92 Holscher AH, Bollschweiler E, Schneider PM, et al. Early adenocarcinoma in Barrett’s oesophagus. Br J Surg. 1997;84:1470-1473.
93 Hasegawa N, Niwa Y, Arisawa T, et al. Preoperative staging of superficial esophageal carcinoma: Comparison of an ultrasound probe and standard endoscopic ultrasonography. Gastrointest Endosc. 1996;44:388-393.
94 Menzel J, Domscke W. Gastrointestinal miniprobe sonography: The current status. Am J Gastroenterol. 2000;95:605-616.
95 Waxman I, Raju GS, Critchlow J, et al. High-frequency probe ultrasonography has limited accuracy for detecting invasive adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia or intramucosal carcinoma: A case series. Am J Gastroenterol. 2006;101:1773-1779.
96 May A, Gunter E, Roth F, et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: A comparative, prospective, and blinded trial. Gut. 2004;53:634-640.
97 Mallery S, Van Dam J. Increased rate of complete EUS staging of patients with esophageal cancer using the nonoptical, wire-guided echoendoscope. Gastrointest Endosc. 1999;50:53-57.
98 Pfau PR, Ginsberg GG, Lew RJ, et al. Esophageal dilation for endosonographic evaluation of malignant esophageal strictures is safe and effective. Am J Gastroenterol. 2000;95:2813-2815.
99 Binmoeller KF, Seifert H, Seitz U, et al. Ultrasonic esophagoprobe for TNM staging of highly stenosing esophageal carcinoma. Gastrointest Endosc. 1995;41:547-552.
100 Wallace MB, Hawes RH, Sahai AV, et al. Dilation of malignant esophageal stenosis to allow EUS-guided fine-needle aspiration: Safety and effect on patient management. Gastrointest Endosc. 2000;51:309-313.
101 Blackshaw G, Lewis WG, Hopper AN, et al. Prospective comparison of endosonography, computed tomography, and histopathological stage of junctional esophagogastric cancer. Clin Radiol. 2008;63:1092-1098.
102 van Overhagen H, Lameris JS, Zonderland HM, et al. Ultrasound and ultrasound-guided fine needle aspiration biopsy of supraclavicular lymph nodes in patients with esophageal carcinoma. Cancer. 1991;67:585-587.
103 Doldi SB, Lattuada E, Zappa MA, et al. Ultrasonographic evaluation of the cervical lymph nodes in preoperative staging of esophageal neoplasms. Abdom Imaging. 1998;23:275-277.
104 Catalano MF, Alcocer E, Chak A, et al. Evaluation of metastatic celiac axis lymph nodes in patients with esophageal carcinoma: Accuracy of EUS. Gastrointest Endosc. 1999;50:352-356.
105 Reed CE, Mishra G, Sahai AV, et al. Esophageal cancer staging: Improved accuracy by endoscopic ultrasound of celiac lymph nodes. Ann Thorac Surg. 1999;67:319-321.
106 Eloubeidi MA, Wallace MB, Reed CE, et al. The utility of EUS and EUS-guided fine needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer: A single center experience. Gastrointest Endosc. 2001;54:714-719.
107 Hiele M, De Leyn P, Schurmans P, et al. Relation between endoscopic ultrasound findings and outcome of patients with tumors of the esophagus or esophagogastric junction. Gastrointest Endosc. 1997;45:381-386.
108 Chak A, Canto M, Gerdes H, et al. Prognosis of esophageal cancers preoperatively staged to be locally invasive (T4) by endoscopic ultrasound (EUS): A multicenter retrospective cohort study. Gastrointest Endosc. 1995;42:501-506.
109 Fockens P, Kisman K, Merkus MP, et al. The prognosis of esophageal carcinoma staged irresectable (T4) by endosonography. J Am Coll Surg. 1998;186:17-23.
110 Levy MJ, Campion MB, Caudill JL, et al. Prospective cytologic assessment of gastrointestinal luminal fluid acquired during EUS: Potential source of false positive FNA and needle tract seeding. Gastrointest Endosc. 2008;67:AB205.
111 Preston SR, Clark GW, Martin IG, et al. Effect of endoscopic ultrasonography on the management of 100 consecutive patients of oesophageal and junctional carcinoma. Br J Surg. 2003;90:1220-1224.
112 Giovannini M, Monges G, Seitz JF, et al. Distant lymph node metastases in esophageal cancer: Impact of endoscopic ultrasound-guided biopsy. Endoscopy. 1999;31:536-540.
113 Mortensen MB, Pless T, Durup J, et al. Clinical impact of endoscopic ultrasound-guided fine needle aspiration biopsy in patients with upper GI tract malignancies: A prospective study. Endoscopy. 2001;33:478-483.
114 Zuccaro GJr, Rice TW, Goldblum J, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol. 1999;94:906-912.
115 Pfau PR, Kochman ML. Pretreatment staging by endoscopic ultrasonography does not predict complete response to neoadjuvant chemoradiation in patients with esophageal carcinoma. Gastrointest Endosc. 2000;52:583-586.
116 Hirata N, Kawamoto K, Ueyama T, et al. Using endosonography to assess the effects of neoadjuvant therapy in patients with advanced esophageal cancer. AJR Am J Roentgenol. 1997;169:485-491.
117 Isenberg G, Chak A, Canto MI, et al. Endoscopic ultrasound in restaging of esophageal cancer after neoadjuvant chemoradiation. Gastrointest Endosc. 1998;48:158-163.
118 Willis J, Cooper GS, Isenberg G, et al. Correlation of EUS measurement with pathologic assessment of neoadjuvant therapy response in esophageal carcinoma. Gastrointest Endosc. 2002;55:655-661.
119 Chak A, Canto MI, Cooper GS, et al. Endosonographic assessment of multimodality therapy predicts survival of esophageal carcinoma patients. Cancer. 2000;88:1788-1795.
120 Westerterp M, Van Westreenen HL, Reitsma JB, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy—systematic review. Radiology. 2005;236:841-851.
121 Penman ID, Williams DB, Sahai AV, et al. Ability of EUS with fine-needle aspiration to document nodal staging and response to neoadjuvant chemoradiotherapy in locally advanced esophageal cancer: A case report. Gastrointest Endosc. 1999;49:783-786.