Chapter 19 Diagnosis and Prognosis in Cardiac Disease Using Cardiac PET Perfusion Imaging
INTRODUCTION
There is a worldwide rising concern about the increasing morbidity and mortality rates related to coronary artery disease (CAD). This has motivated remarkable advances in the field of cardiovascular imaging that we have witnessed within the last 2 decades. The latest evidence underscores that functional imaging remains essential for an appropriate selection of therapy and for improving patient outcomes.1 The introduction of positron emission tomography (PET) represented a major breakthrough that ultimately has shed light on the pathophysiology and diagnosis of heart diseases. More recently, the concept of dual-modality imaging technology emerged,2 mainly spurred by the use of computed tomography (CT) to achieve accurate attenuation correction (AC). Currently, hybrid PET/CT systems allow simultaneous assessment of cardiac and coronary arterial structure together with myocardial perfusion and metabolism.
At first PET was regarded a powerful investigative tool that contributed to the understanding of several diseases.3,4 Nevertheless, the leading role of PET imaging in the field of oncology, along with the U.S. Food and Drug Administration (FDA) approval of radiotracers and changes in reimbursement, have all contributed to move the state-of-the-art technology from the research laboratory to the clinical arena. Currently, cardiac PET constitutes a well-developed means for detecting and tracking the progression of CAD, diagnosing microvascular dysfunction, and for the follow-up of different therapies, offering a comprehensive approach for the workup of CAD.
Cardiac PET has potential advantages in patients with multivessel CAD as well as in subjects with large body habitus, prone to attenuation artifacts. Taking into account risk stratification, a normal PET study indicates an excellent prognosis,5–8 and the risk of hard cardiac events increases with higher summed stress scores (SSS) and lower LVEF.3,9,10
Three-dimensional (3D)-mode PET imaging is becoming the standard with the latest brands of PET/CT systems. 3D-mode combines improved image quality and reduced patient radiation exposure, but this method increases scatter and presents certain technical challenges for cardiac PET.11–13
PRACTICAL ASPECTS OF IMAGING AND ANALYSIS
General Principles of PET Imaging
PET imaging is based on the use of radiotracers that decay by positron emission. A positron (a positively charged electron) is emitted from the nuclei of unstable isotopes during radioactive decay.14 Then, both the positron and an electron undergo a process known as positron annihilation and are converted into two coincident gamma-ray photons of 511 keV that travel in opposite directions. The distance traveled by the positron prior to annihilation constitutes the positron range. Detectors placed on either side of the active volume are connected in a coincidence circuit, so that if both detectors record an event within a very short interval, it is assumed that positron annihilation has occurred.12 This constitutes the basis of PET.
PET utilizes biological radiotracers labeled with positron-emitting isotopes such as carbon (11C), oxygen (15O), rubidium (82Rb), and fluorine (18F) that mimic natural substances. Thus, PET enables the measurement of several biological processes, including cardiac tissue blood flow, metabolism, and neurohormonal and receptor function. Many of the isotopes used for PET imaging have short physical half-lives (T1/2),14 making them readily applicable in studies requiring repetitive measurements in the same session.15
Attenuation effects are significantly higher with PET than with SPECT. Attenuation correction (AC) is relatively straightforward with PET because the length of the path of attenuation for the pair of 511-keV photons is constant and known for PET, whereas it is variable with SPECT.13 Traditionally, external transmission sources, such as germanium-68 (68Ge) or cesium-137 (137Cs), have been used as established means for AC of the PET emission data.13 Today this has been replaced exclusively by x-ray transmission scanning in the current generation of PET/CT systems, shortening the total transmission imaging time to under a minute. Since fast helical CT scans acquire images at a single point in the respiratory cycle, whereas PET data are averaged over many respiratory cycles, respiratory motion mismatch artifacts can be produced.11 These artifacts, when present, typically affect the anterior and anterolateral segments of the LV.16,17
In recent years, PET instrumentation has shown substantial evolution. New crystal materials, such as lutetium oxyorthosilicate (LSO) and gadolinium oxyorthosilicate (GSO) have become available.13 These crystals are attractive for PET, owing to faster light decay time and higher light yield than bismuth germanate (BGO) crystals.18 Furthermore, there is an increasing trend to apply 3D-imaging acquisition mode (septa out) instead of the traditional two-dimensional (2D) mode (septa in), with the potential to improve image quality and reduce injected doses but at the expense of increased background counts and higher reliance on scatter-correction accuracy.11,19 Notably, several new PET/CT scanners operate only in 3D mode.13 The current role of 3D mode versus 2D-mode will be fully described elsewhere in this textbook.
Cardiac PET has several technical advantages over traditional SPECT that should be appreciated:
Myocardial PET Perfusion Tracers
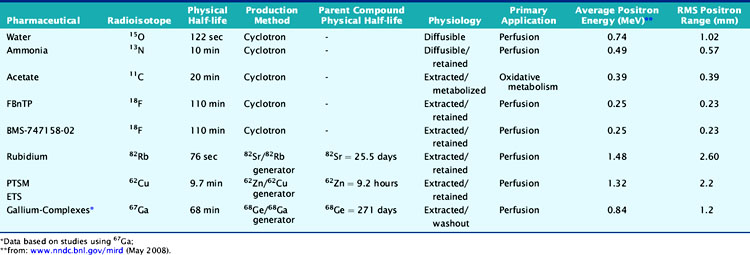
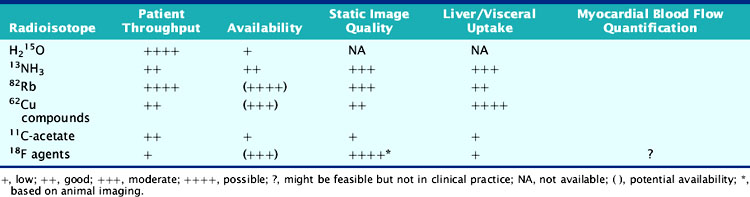
According to their physical properties, myocardial PET blood flow tracers fall into two basic categories: (1) inert, freely diffusible tracers like H215O and (2) physiologically retained tracers like 13NH3 and 82Rb.4,14 The main aspects of PET flow tracers have been summarized in Tables 19-1, 19-2, and 19-3.
Nitrogen-13 Ammonia
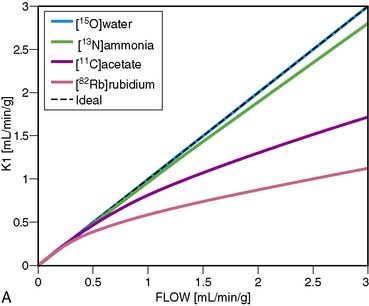
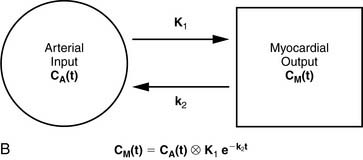
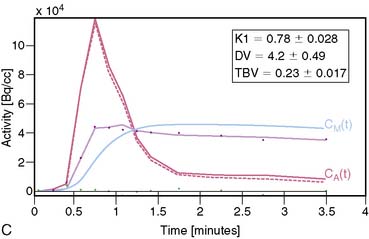
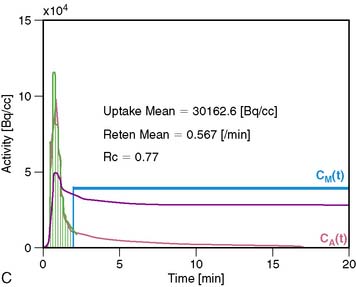
13NH3 (T1/2 = 9.96 minutes) requires an on-site cyclotron and radiochemistry synthesis capability.3,22 In the bloodstream, neutral 13NH3 is in equilibrium with the ionic form, ammonium (NH4+). 13NH3 diffuses freely across capillary and cell membranes and is retained in myocardial tissue,24 whereby it can be either incorporated into synthesis of 13N-glutamine, or it can diffuse back into the vascular space. The initial extraction is high, even at high flow rates. As such, the uptake rate constant K1 is a good estimate for quantitative blood flow (Fig. 19-1).
The net tissue retention is approximately 90% in the resting state. For 13NH3, the direct relationship between net tissue retention and blood flow is preserved for values of blood flow up to 2.5 mL/min/g, but at higher flow rates this linear relationship is lost (Fig. 19-2).25 Therefore, it is necessary to correct for flow-dependent changes in net tissue retention.
Myocardial retention of 13NH3 may be heterogeneous,26 and the lateral LV wall uptake can be 10% lower than that of other segments. Also, image quality can be hampered by the occasional intense liver activity, which could interfere with the evaluation of the inferior wall. Finally, in patients with severe left ventricular ejection fraction (LVEF) impairment, chronic obstructive pulmonary disease (COPD), or smoking, the sequestration of 13NH3 in the lungs can be abnormally increased. Then it would be necessary to delay the time between injection and image acquisition to enhance image quality.13
Rubidium-82
82Rb is produced from a strontium-82 (82Sr)/82Rb generator, which can be eluted every 10 minutes.27 The T1/2 of 82Sr is 25.5 days, which results in a generator life of 6 to 8 weeks. The short T1/2 of 82Rb (76 seconds) allows repeated and sequential perfusion studies but requires rapid image acquisition shortly after tracer administration.3,12 82Rb is a monovalent cationic analog of potassium and has similar biological activity to thallium-201 (201Tl). Myocardial uptake of 82Rb requires active transport via the sodium/potassium adenosine triphosphate transporter. In animal models, the net retention is approximately 50% at rest and decreases to 30% at peak flow. The retention fraction can be altered by acidosis and acute hypoxia.4
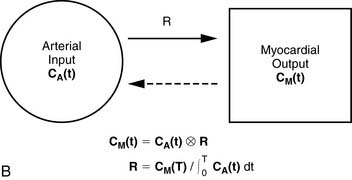
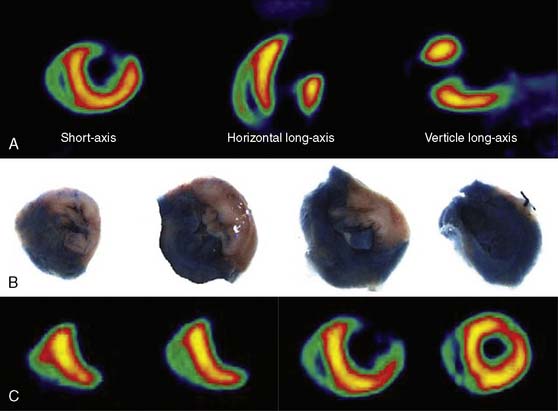
A small and mobile generator infusion system is used for eluting 82Rb every 10 to 15 minutes with low radiation exposure.27 Quantitative assessment with 82Rb is quite feasible and clinically practical with this generator as compared to cyclotron-produced compounds (Figs. 19-3, 19-4, and 19-5).28–31
Oxygen-15 Water
H215O (T1/2 = 2.04 minutes) is a cyclotron product and is considered the gold standard for absolute flow quantification.14 Since H215O is a freely diffusible agent, the extraction fraction is not affected by flow rates and is independent of the metabolic state of the myocardium.22 Cardiac imaging with H215O can be demanding because of its high concentration in the blood pool, entailing subtraction of the blood pool counts from the original image to visualize the myocardium.32 As such, it does not usually produce clinically interpretable perfusion images. Despite the success of H215O for research purposes, its clinical applications remain limited.18,22
Carbon-11-Acetate
Currently, PET using 11C-acetate (T1/2 = 20.4 minutes), a cyclotron product, is considered to be the most accurate and commonly used noninvasive method for measuring myocardial oxygen consumption (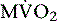 ). As well, 11C-acetate has been proposed as a potential myocardial blood flow tracer33 because of its relatively high initial extraction fraction. Van den Hoff et al.34 reported that good MBF estimates can be obtained by fitting a simple compartmental model to regional acetate kinetics, providing similar quantitative accuracy relative to 13NH3-based blood flow methods. In addition, a potential advantage of 11C-acetate is its ability to simultaneously assess MBF and oxidative metabolism under resting conditions in a single tracer administration.35
). As well, 11C-acetate has been proposed as a potential myocardial blood flow tracer33 because of its relatively high initial extraction fraction. Van den Hoff et al.34 reported that good MBF estimates can be obtained by fitting a simple compartmental model to regional acetate kinetics, providing similar quantitative accuracy relative to 13NH3-based blood flow methods. In addition, a potential advantage of 11C-acetate is its ability to simultaneously assess MBF and oxidative metabolism under resting conditions in a single tracer administration.35
Cu-62 PTSM, Cu-62 ETS
62Cu-pyruvaldehyde bis (N4-methylthiosemicarbazone) (PTSM) (T1/2 = 9.7 minutes36) is another generator-produced PET perfusion tracer and is produced from 62Zn/62Cu generator.37,38 62Cu-PTSM is a promising tracer for assessing myocardial and cerebral perfusion.36,39 Approximately 5% to 10% of the injected dose of 62Cu-PTSM remains in the circulation due to binding to red blood cells. Therefore, the quantitative measurement of regional MBF using the microsphere model requires correction of the arterial blood time-activity curve for blood-pool binding.40
Liver uptake may be problematic, but this appears less with newer related compounds such as 62Cu-ETS. Also, 62Cu has high positron energy similar to 82Rb, which may reduce image resolution.41,42
Recent Advances in PET Imaging: Novel Myocardial Blood Flow Tracers
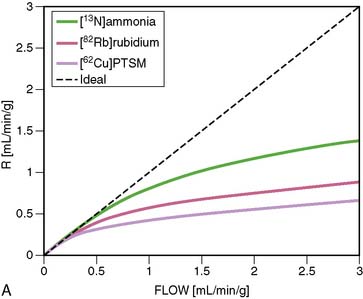
18F-labeled compounds have relatively long physical T1/2 (110 minutes). Among these, 18F-p-fluorobenzyl triphenyl phosphonium cation (18F-FBnTP) is a member of a new class of positron-emitting lipophilic cations that may act as myocardial perfusion PET tracers.43,44 18F-BMS-747158-02 constitutes another emerging agent that is an analog of the insecticide pyridaben, an inhibitor of mitochondrial complex I (MC-1).45 Mitochondria constitute 20% to 30% of the myocardial intracellular volume. Consequently, molecules that target mitochondrial proteins may be enriched and retained selectively in the myocardium. With the longer 18F T1/2, these compounds have the potential for wide distribution. However, they may require reinjection or 2-day stress/rest imaging protocol similar to technetium-99 (99mTc) SPECT agents. On the other hand, this feature may enable routine exercise stress, which has not been widely developed with PET imaging to date. Recent data suggest kinetics may be suitable for quantification (Fig. 19-6).46
A 68Ge/68Ga generator can provide a convenient source of PET tracers because of the long physical half-life of 68Ge (T1/2 = 271 days) and a suitable daughter half-life (68Ga; T1/2 = 67.7 minutes). Recently, a ligand has been successfully labeled and tested with 67Ga for SPECT.47 The biodistribution of this novel complex has been assessed in normal and infarcted rat models, whereby it showed a high heart uptake, albeit low cardiac retention and high liver uptake. Further studies of other gallium complexes may improve the feasibility of measuring regional myocardial perfusion using similar ligands labeled with the PET isotope 68Ge.
MYOCARDIAL IMAGING PROTOCOLS AND ACQUISITION
Patient Preparation
Patients should be instructed to fast for at least 6 hours, to abstain from caffeine-containing products at least 12 hours, and to avoid theophylline-containing medications for 48 hours prior to the test.13 Diabetic patients should be guided on how to administer insulin before the study. Patients undergoing dobutamine stress tests should discontinue beta-blockers 48 hours prior to the test (only for diagnostic purposes and if it is clinically safe to do so).
Stress Testing Protocols
PET perfusion imaging is usually performed with pharmacologic stress. Adenosine, dipyridamole, and adenosine triphosphate block transport of adenosine into the cells and/or increase extracellular levels of adenosine, which causes coronary vasodilatation by interacting with the adenosine A2 receptors in the cell membrane.13 Adenosine and dipyridamole increase MBF without increasing oxygen demand. Side effects are somewhat greater with adenosine than with dipyridamole, but the latter more often require reversal with aminophylline (which is routine in some laboratories). More selective agonism of the adenosine A2A receptor subtype should theoretically result in a similar degree of coronary vasodilation with fewer and less severe side effects. Binodenoson,48 regadenoson,49 and apadenoson50 are highly selective agonists for the adenosine A2A receptor and are under investigation for clinical use for vasodilator stress imaging.
Dobutamine stress is a feasible alternative in those situations where adenosine, dipyridamole, or ATP are contraindicated.13,51 Dobutamine increases MBF to meet increasing myocardial oxygen demand. In segments supplied by diseased vessels, the increase in flow is attenuated during high-dose dobutamine administration (20 to 40 μg/kg/min).
Exercise stress may be a valid alternative and provide clinical information unobtainable with pharmacologic stress that is helpful in decision making, particularly for patients unable to tolerate pharmacologic stress. 13NH3 can be used in conjunction with treadmill exercise (TEX) or upright bicycle test. The tracer is administrated at peak exercise, and exercise should be continued for an additional 30 to 60 seconds after tracer injection. The patient is then repositioned in the PET camera to start the acquisition within 4 to 6 minutes.52 Accurate repositioning is of key importance to minimize artifact due to incorrect AC. Exercise stress is also available for the 82Rb imaging13,53,54 but can be logistically demanding; patients must be moved to and positioned in the scanner within 3 minutes of completing exercise.
Myocardial Perfusion Imaging Protocols
The most widely applied tracers in clinical practice are13NH3 and 82Rb.
13N Ammonia
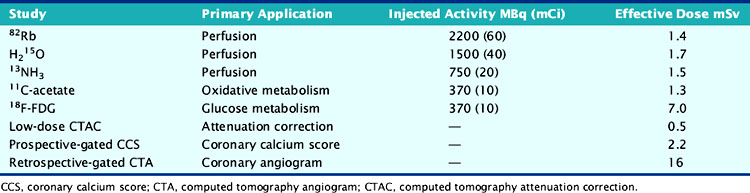
For relative perfusion imaging, 13NH3 is injected as a bolus of 370 to 740 MBq (10 to 20 mCi), and static images are acquired 2 to 3 minutes after tracer administration for an imaging time of 5 to 15 minutes.3,13 For patients undergoing PET/CT, two separate CT-based transmission scans should be performed for correction of rest and stress (after each emission scan is preferred to prevent misregistration artifacts).3
For flow quantification, a dynamic acquisition is required. This can be accomplished by performing separate dynamic and gated acquisitions with the same injection or through a single list-mode acquisition. From the dynamic images, time-activity curves are generated for the myocardium and the blood pool.13 Global and regional MBF can be measured with use of one- or two-tissue-compartment tracer kinetic modeling fit to myocardial activity data and corrected for spillover from the arterial input function.22
82Rubidium
A larger amount of tracer can be administered with similar absorbed dose to the patient because of the short physical half-life of 82Rb. In 2D acquisition mode, approximately 1500 to 2200 MBq (40 to 60 mCi) of 82Rb can be injected as an infusion over 30 seconds, with serial dynamic imaging acquisition starting at onset of the infusion. Between 70 and 150 seconds after completion of tracer infusion, a 3- to 7.5-minute image acquisition is initiated.8,13 Alternatively, newer-generation PET cameras can acquire data in 3D mode,28 which enables lower doses of 82Rb (750 to 1100 MBq [20 to 30 mCi]).
For quantitative assessment of global and regional MBF, one- and two-tissue-compartment models have been used. These account for activity in the vascular space and within the tissue compartment.55 Following bolus injection of the tracer, predominantly unidirectional transport is assumed from the vascular space into the tissue space.56 A simplified approach using tracer retention that involves a summed late image corrected for the input function, which can subsequently be corrected for the net retention,57,58 and similar methods have been developed for 13NH3.59 This approach enables a robust means to quantify perfusion that may be easier to apply in the clinical setting (see Figs. 19-1 and 19-2).28,30
Image Evaluation for Technical Sources of Errors: Quality Control
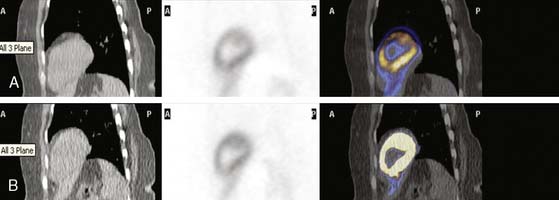
This step is of extreme importance for an accurate interpretation of the images. Patient body movements can lead to blurring of contours. Acquisition of a brief scan or scout image may facilitate accurate patient positioning. Body motion or respiratory movement can lead to transmission-emission misalignment and potential AC-induced artifacts (Fig. 19-8). However, most PET/CT systems should include software tools to correct transmission-emission misalignments. Finally, other potential sources of reconstruction artifacts encompass streak artifacts seen in large patients with arms-down imaging, metal implants, and IV contrast, as well as residual radioactivity in the IV line within the field of view (FOV).3,13
Image Analysis and Interpretation of Perfusion Images
Myocardial perfusion defects are usually identified by visual analysis of the reconstructed slices and compared between stress and rest images. Perfusion defect description, including extent, severity, reversibility, location, and specific coronary territories, should be reported routinely.60 Defects are typically defined as fixed, suggesting scar formation; reversible, indicating ischemia; or partly reversible, indicating a mixture of scar and ischemia. ACC/AHA/ASNC guidelines recommend semiquantitative analysis using a 17-segment model.13,61 Using this approach, summed perfusion defect score can be calculated, and this score is useful for cardiac risk stratification.61,62
Gated images are also acquired and interpreted. Contour definitions used for EF and volume estimates should be confirmed prior to interpreting gated EF data. The change in EF may have diagnostic value in terms of the extent of disease (discussed later).63 Wall-motion abnormalities at rest represent myocardial injury, either scar or otherwise, as may occur in a dilated cardiomyopathy. Wall-motion abnormalities that appear or are worse on stress imaging indicate ischemia-induced wall-motion abnormalities. This is in contrast to post-stress SPECT imaging acquired at least 30 minutes after the tracer injection, whereby wall-motion abnormalities are not reflecting concurrent ischemia but rather postischemic dysfunction or stunning. PET wall motion is acquired shortly after tracer injection, and thus changes reflect ischemic wall-motion abnormalities.
ABSOLUTE MYOCARDIAL BLOOD FLOW QUANTIFICATION
Advantages of Absolute Quantitative Analysis
Atherosclerotic vascular disease has become a leading cause of death worldwide, and there is a need to identify the presence of CAD before the onset of symptoms. PET technology has the potential to image and measure pathophysiologic and molecular processes in vivo and is now recognized as the best noninvasive means by which to quantify MBF in absolute terms (mL/min/g) and CFR. Table 19-4 describes normal values of MBF and CFR in healthy subjects.
Table 19-4 Normal Values in Baseline Myocardial Blood Flow and Coronary Flow Reserve in Normal Subjects
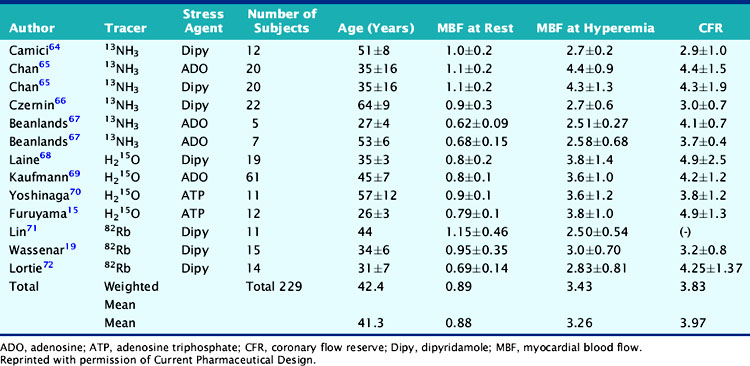
Parkash et al. demonstrated the clinical importance of CFR over conventional visual analysis in patients with three-vessel CAD using 82Rb PET. In this study, the perfusion defect sizes were larger using quantification in comparison with the conventional relative uptake evaluation.29 Yoshinaga and colleagues compared the clinical value of CFR by PET to the relative assessment of MPI by SPECT in a population of 27 patients with overt CAD. Approximately two-thirds of regions with angiographic lesions more than 50% showed normal SPECT perfusion scans but significant impaired CFR by PET.70 Further investigations in larger sample size populations are needed to fully understand the clinical utility and added value of this approach. Only a few studies have assessed the prognostic value of abnormal MBF and CFR.73,74
Models for Flow Quantification
A mathematical model is constructed with parameters that represent the flux of the radiotracer between the compartments. Various tracer kinetic models have been applied and validated for MBF quantification with H215O, 13NH3, 82Rb, and 11C-acetate. Simple one-tissue-compartment models can be applied for flow quantification with each,25,32,34,56,75,76 providing a reasonable approach for estimating absolute tissue perfusion (see Fig. 19-1).11
A further simplified retention model assumes that the tracer is retained completely without subsequent washout. A flow-dependent correction for net tracer retention is required to obtain quantitative perfusion estimates from the retention rates. The net retention model has been shown to produce precise estimates with 13NH377 and 82Rb62,78 in normal subjects and in patients with CAD, but the results depend in part on the time at which the model is evaluated and on the accuracy of retention measurement.11 3D PET studies in normal subjects have yielded retention fractions similar to those reported in 2D mode11,59,62 with 13NH3 and 82Rb.11,57
Three-Dimensional Imaging Mode for Absolute Blood Flow Quantification
It is important to realize that MBF quantification studies have generally applied 2D-mode PET technology. However, with the widespread application of PET/CT for oncology imaging, there has been a shift from 2D to 3D imaging also being applied to cardiac imaging. Currently, the available evidence supports the use of 3D cardiac PET for flow quantification, which offers the potential to measure MBF simultaneously with routine ECG-gated perfusion imaging. The ideal requirements for clinical 3D-mode imaging include a wide dynamic-range scanner along with robust tracer kinetic models, regional partial volume correction and lastly, simultaneous dynamic, static, and ECG-gated imaging through list-mode acquisition and processing.11
Radiotracers and Myocardial Blood Flow Quantification
Oxygen-15-Water
The biological behavior of H215O can be modeled with a simple one-compartment model as originally described by Kety et al.32,79
Iida et al.80 proposed a mathematical model to correct the input function for the tissue-to-blood spillover and partial volume effect. This model permits estimation of the perfusable tissue fraction, defined as the water-perfusable tissue divided by total extravascular anatomic tissue. Katoh et al. developed an automatic algorithm to calculate regional MBF using a semiautomatic region of interest algorithm and uniform input function.81
Nitrogen-13-Ammonia
The most common approach assumes a two-tissue-compartment model with the compartments being the extravascular and metabolic spaces.25 The initial unidirectional extraction is assumed to be 100%.82 K1 representing the transport of the tracer from the vascular space into the extravascular space, is an accurate estimate of MBF. These PET measurements have been well validated with microsphere measurements in experimental preparations confirming that regional MBF can be quantitatively measured over a wide blood-flow range using 13NH3 and the compartmental approach.83–85 Simplified approaches have been used to quantify regional MBF with 13NH3 as described earlier.86 The simplest method is the net retention approximation of the “microsphere model” that requires one static scan and arterial input function and correction for the net retention fraction,59 but the quantitative value is quite variable, depending on the time of measurement after tracer administration. To reduce this effect, Patlak graphic analysis of the early uptake phase has been applied for the quantitative estimation of MBF with parametric imaging.87
Rubidium-82
A few groups have examined the possibility of quantifying MBF with 82Rb. The use of a retention model along with an extraction correction to obtain estimates of MBF has been proposed.58,78 Other investigators55,88 have applied a two-compartment model to describe the kinetics of 82Rb in myocardium. Although the two-compartment model was found to provide acceptable estimates of MBF under noise-free conditions, large estimation errors prevented adequate differentiation of flow at more realistic noise levels. Coxson et al.88 suggested that clinically relevant differences in flow could be detected with a simple one-compartment model. Moreover, fits to experimental data obtained with the two-compartment and the one-compartment models were of comparable quality. A recent study by Lortie et al.76 has shown that it is possible to obtain accurate estimates of MBF in normal myocardium by using a one-tissue-compartment model of 82Rb kinetics and a nonlinear extraction function. This has been subsequently confirmed in CAD patients.56
Physiologic Parameters for Absolute Quantification
The coronary circulation is a dynamic vascular bed that can accommodate marked increases in blood flow through changes in arteriolar resistance in response to increasing metabolic tissue demands and during periods of activation of the sympathetic nervous system. Vasodilators induce a mixed response with both smooth muscle vasodilation and endothelial-dependent vasodilation at the resistance vessels. The magnitude of the flow response, that is, the CFR, is defined as the ratio of near-maximal MBF during pharmacologically induced hyperemia to MBF at rest (effectively, the relative flow reserve).4,89 The MBF difference, also termed the absolute flow reserve, represents the difference in stress/rest flow and may also be a useful parameter.29,90
MBF and CFR are considered to reflect the functional status of both the macrocirculation and the microcirculation. Normal values of CFR with PET range between 2 and 5.4,14 H215O and 13NH3 have been validated in animal studies by microspheres and shown comparable results.85,91 The CFR measured by PET is well correlated with intracoronary Doppler guide wire.92
H215O and 13NH3 have good short-term and long-term reproducibility at both rest and hyperemic MBF in normal controls.93,94 Using an appropriate noise-reduction approach, MBF measured by 82Rb has shown good correlation with H215O.94 Recent data also suggest a good correlation between 82Rb and H215O MBF measured using the one-compartment approach.95–97
FUSION OF STRUCTURE AND FUNCTION: INSIGHTS OF INTEGRATED PET/CT SYSTEMS
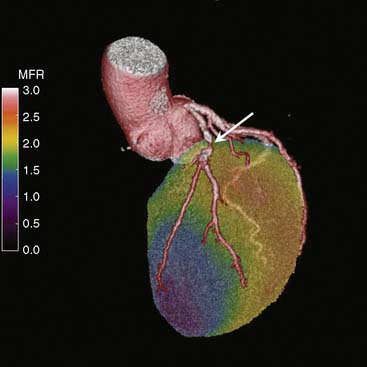
Combining images from different modalities could offer significant diagnostic advantages. This has given rise to sophisticated software techniques that allow a true integration of cardiac structure and function. State-of-the-art PET/CT enables simultaneous assessment of cardiac and coronary arterial structure together with myocardial perfusion and metabolism (Fig. 19-9).21 The synergistic effect of dual-modality imaging potentially offers a more in-depth evaluation for patients with suspected CAD, and may ultimately facilitate appropriate selection of therapy to optimize patient outcomes. Of note, ischemia measured on PET imaging is observed less frequently than stenoses are observed on CTA. CTA tends to define stenoses as more severe relative to quantitative coronary angiography or perfusion imaging. As such, PET can be used to assess the functional significance of CTA findings and to determine the presence of ischemia in patients with high coronary calcium scores. Figures 19-8, 19-9, and 19-10 show examples of hybrid PET/CT imaging. For more details on hybrid PET/CT imaging in this textbook, see Chapter 23.
CLINICAL APPLICATIONS FOR MYOCARDIAL PERFUSION IMAGING WITH PET
Cardiac PET MPI provides accurate regional myocardial perfusion information in patients with suspected or established CAD.98 PET MPI may be useful for obese patients, women, patients with nondiagnostic findings using other diagnostic tests, and patients with poor LV function.13,61 Although SPECT MPI remains the traditional approach, there is increasing interest in the use of PET for this purpose.
Selection of Patients
ACC/AHA/ASNC clinical guidelines from 2003 and the joint position statement by CCS/CAR/CANM/CNCS/CanSCMR from 2007 agree that diagnosis and risk stratification of CAD patients with nondiagnostic or equivocal previous tests are important indications for PET MPI (class 1 indication and evidence level B).9,61
Left bundle branch block (LBBB) or ventricular pacing rhythm may also benefit from PET MPI (ACC/AHA/ASNC class IIa and CCS: Class I [level of evidence B]).9,61 Obese patients or other patients prone to equivocal results could take advantage of PET imaging evaluation as well (CCS et al. Class I [level of evidence B]).9
PET MPI can also be used as the initial first test for detection of the extent and location of ischemia. (ACC/AHA/ASNC 2003 class IIa and more recent evaluation by CCS 2007: Class I [level of evidence B]).9,61
Diagnosis of Coronary Artery Disease
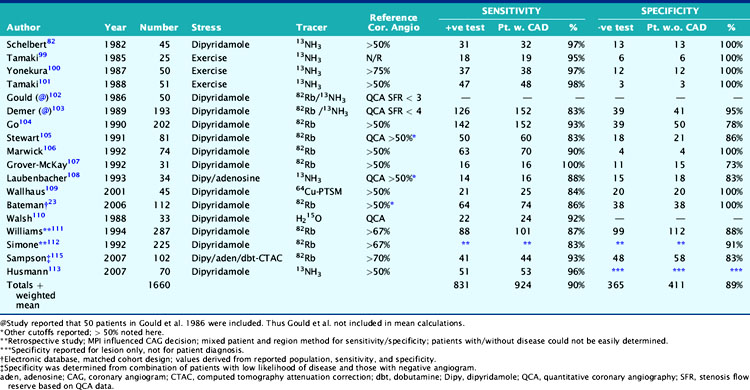
The mean sensitivity and specificity of PET MPI for diagnosis of CAD have been found to be 89% and 90%, with ranges from 83% to 100% and 73% to 100%, respectively (Table 19-5).9,23,82,99–113,115
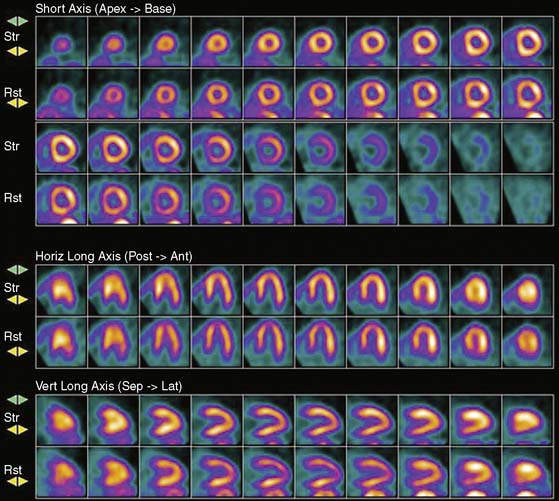
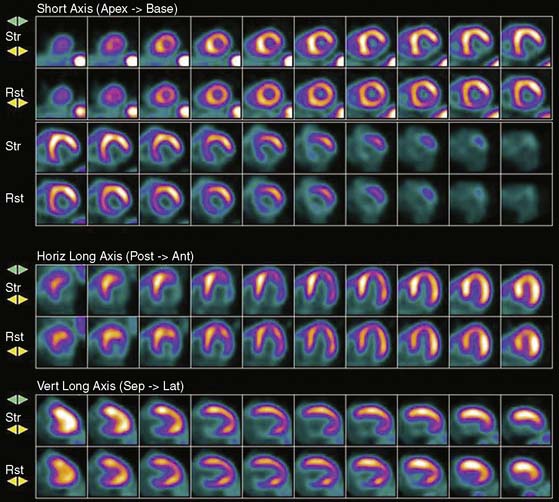
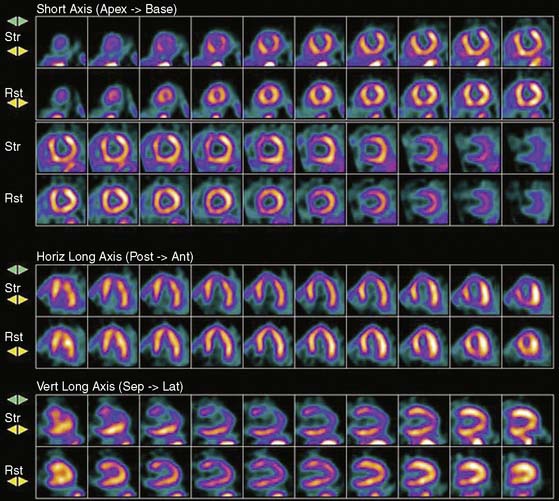
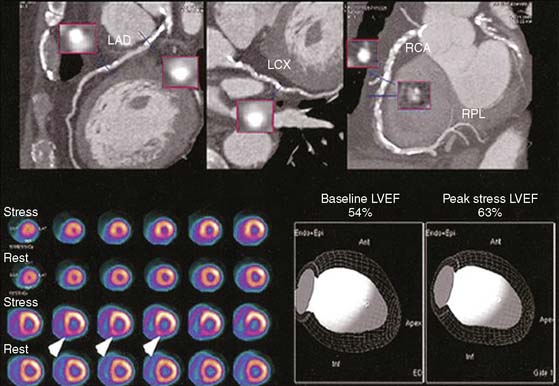
Previous studies comparing CAD diagnostic accuracy demonstrated superiority of 82Rb or 13NH3 PET MPI over 201Tl SPECT imaging.82,101,104,105 More recently, Bateman et al.23 showed greater diagnostic accuracy using 82Rb PET versus gated 99mTc-sestamibi SPECT (89% versus 79%, P = 0.03). This study also demonstrated a significant improvement in diagnostic certainty in favor of PET. Husmann et al.113 also showed superiority of PET compared to SPECT regarding sensitivity. Namdar et al.114 evaluated the diagnostic accuracy of 13NH3 using hybrid PET/CT systems for detecting disease in 100 coronary lesions in 25 patients with CAD. The sensitivity and specificity of PET/CT were 90% and 98%, while PPV and NPV were 82% and 99%, respectively. Sampson et al.115 reported that 82Rb PET/CT MPI correctly identified CAD in patients with sensitivity of 93% and specificity of 83%. However, the agreement between stress PET MPI findings and coronary stenosis in assessing the anatomic CAD showed relatively low agreement in patients with multivessel disease (58%). Thus PET MPI has excellent ability to define patients with underlying CAD, but conventional relative perfusion approaches (as also used with SPECT) may have limitations in defining extent of disease. MBF quantification provides additional information that may improve diagnostic accuracy in this setting.29,116 The Study of Perfusion versus Anatomy’s Role in CAD (SPARC) is expected to clarify the relative value of the new approaches in relation to SPECT and CT angiography imaging (see examples in Figs. 19-3 through 19-5 and 19–9).117
PET is able to estimate LV function at baseline and during peak stress.21,63 An increase in LVEF of more than 5% yielded an NPV of 97% for ruling out three-vessel CAD.63 In addition, a recent study suggested that the magnitude of LVEF increase is determined in part by stress perfusion/reversible perfusion defects (r = −0.25; P = 0.009).10
Prognosis of Coronary Artery Disease
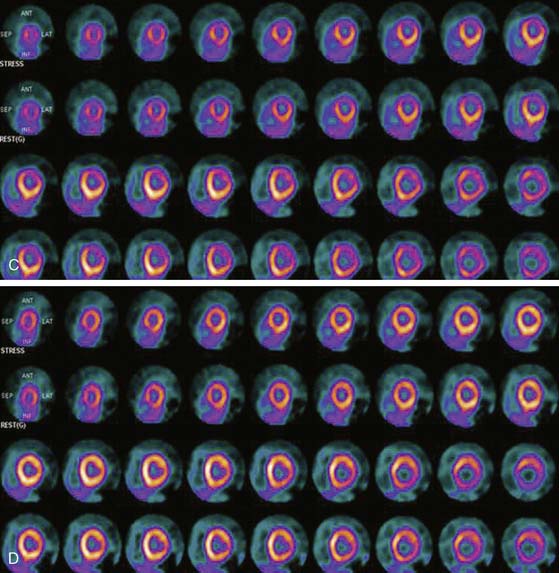
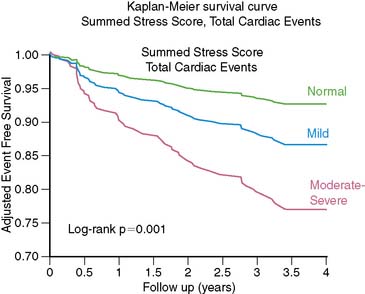
SPECT MPI has well-established utility for determining prognosis of patients with known or suspected CAD. Patients with normal stress perfusion imaging have a low hard cardiac event rate and hence an excellent prognosis.61 There have been some limited data assessing the prognostic value of PET MPI. An early report by Marwick et al. reported that the results of PET perfusion imaging yielded incremental prognostic information in comparison with clinical and angiographic findings alone.6 Chow et al. described a very low hard cardiac event rate (0.09%/year) in a group of patients with normal 82Rb PET scans.5 Yoshinaga et al. reported that a normal 82Rb PET MPI scan also indicates an excellent prognosis, and cardiac events are associated with stress perfusion defect severity (Fig. 19-11).8 This study also reported that 82Rb PET MPI has prognostic value in patients whose diagnosis remains uncertain after SPECT MPI and in obese patients (Table 19-6). Further studies of the prognostic value of PET MPI are underway.
POTENTIAL CLINICAL APPLICATIONS OF PET ABSOLUTE QUANTIFICATION
Clinical applications of MBF and CFR measurements can be categorized into four main areas: (1) early detection of atherosclerotic disease, (2) established CAD, (3) nonatherosclerotic microvascular disease, and (4) assessment of therapeutic approaches.4,89
Preclinical Diagnosis of Cardiovascular Disease
Significance of Endothelial and Microvessel Dysfunction
Microvascular disease underlies the involvement of the small coronary arteries in early phases of several cardiovascular conditions that usually precede the onset of symptoms. Microvascular disease or dysfunction is considered to be an independent prognostic value.120 Coronary endothelial vasodilator dysfunction has been observed in patients with traditional cardiovascular risk factors.
Endothelial cells protect the coronary artery as mechanical barriers and produce vasoactive substances, cytokines, and other active biological compounds to maintain vascular homeostasis.121 The biochemical hallmark of coronary endothelial dysfunction is a reduction in the synthesis or bioactivity of NO, with reduced endothelium-dependent vasodilatation.
Methods to Assess Microvascular Structure and Function
Several techniques are available to estimate endothelial function, such as coronary angiography, ultrasonographic evaluation of brachial arteries, or PET.122 Clinical studies of endothelial function involve local infusion of acetylcholine, with measurement of the change in vessel diameter by quantitative coronary angiography or Doppler flow wires. These catheter-based approaches are considered the gold standard, but being invasive limits their routine use and their use in repeated testing for serial follow-up.
The cold pressor test induces a mixed vascular response with adrenergically mediated vasoconstrictor and vasorelaxant effects. In healthy individuals, the adrenergically mediated coronary vasoconstriction is offset by flow-mediated endothelium-dependent relaxation, whereas in patients with endothelial dysfunction, cold-induced flow responses can be diminished, absent, or even paradoxical. Several observations support the validity of noninvasively measured changes in MBF during CPT as indicators of endothelium-dependent coronary vasomotion.89,123,124 The protocol for CPT involves immersing the patient’s foot or forearm in ice water for at least 3 to 4 minutes.15 MBF increases 30% to 60% of baseline in normal volunteers.15 Reduced MBF response or reductions in flow indicate endothelial dysfunction.15
Mental stress also induces catecholamine release from the adrenal medulla and cardiac nerve terminals. Schoder et al. reported that during mental stress, MBF increases by about 30% in normal, healthy individuals, using measured 13NH3 and correlated with changes in cardiac work. The increase in MBF under mental stress is reduced in patients with CAD but to a lesser degree (14%).125
The methods discussed consider the global impairments in flow or flow reserve measured using PET in response to a stressor. Johnson and Gould have also observed that heterogeneity of resting flow with improvement during dipyridamole, and base-to-apex perfusion gradient suggests the presence of microvascular dysfunction and early atherosclerosis.126,127 These may also become useful parameters for microvascular disease characterization in addition to flow quantification.
Functional Significance of Cardiovascular Risk Factors on MBF and CFR
Dayanikli et al.128 first described a linear relationship between coronary flow measurement and serum cholesterol levels in asymptomatic patients at risk for developing CAD. Yokoyama et al.129 confirmed these results by describing reduction of CFR in asymptomatic patients with familial hypercholesterolemia.
Czernin et al.130 evaluated the acute effect of smoking on myocardial vasculature reactivity to vasodilator stimulation. Campisi et al. demonstrated an altered response of MBF during a CPT despite normal CFR in long-term smokers, suggesting endothelial dysfunction.124
PET studies have shown that individuals with diabetes mellitus have consistently higher MBF at rest131 than controls. Diabetic patients do have altered myocardial vasodilation responses to either pharmacologic agents131 or CPT.132 It has been suggested that chronic hyperglycemia may play a leading role in the pathogenesis of vascular dysfunction in diabetic patients.
Arterial hypertension with or without LVH can reduce CFR by altering the coronary vasculature and resistance.12 This reduction of CFR may be dependent on impaired maximal vasodilator capacity. It has been proposed that hemodynamic pressure overload and local vasoactive substances may play a key role in the genesis of regional perfusion abnormalities.89
Myocardial Blood Flow Parameters in Established Cardiovascular Disease
The assessment of MBF parameters represents a more physiologic evaluation of cellular perfusion than coronary angiography.133 Gould et al.134 described the value of CFR as an index of the functional severity of CAD. MBF at rest remains normal until there is an 80% to 85% diameter coronary stenosis, while hyperemic coronary flow measured after maximal vasodilatation begins to decrease progressively if the stenosis is more than about 40%.
Demer et al.135 first reported a significant relationship between the severity of relative perfusion abnormalities on PET perfusion images and CFR measurements from quantitative coronary angiography. Uren et al.98 showed a relationship between coronary artery stenosis on angiography and CFR data obtained by H215O PET perfusion studies. Similar results were obtained with 13NH3 PET perfusion data.67 Notably, since multiple factors other than lesion diameter influence the measured CFR, interpretation of CFR may require consideration of dynamic characteristics of both epicardial coronary artery and resistance vessels22 and/or the concomitant definition of coronary anatomy.
MBF and CFR can also help detect extensive epicardial disease, since overt perfusion defects diagnostic of multivessel CAD may be apparent in only a third of these patients due to the “balanced ischemia” phenomenon.136 Finally, quantitative PET can assist distinguishing the presence of collaterals29 and early hibernating myocardium.137
Myocardial Blood Flow Parameters in Nonatherosclerotic Microvascular Disease
In patients with hypertrophic cardiomyopathy (HCM), Camici et al. reported that CFR is markedly impaired not only in the hypertrophied septum but also in the less hypertrophied LV free wall.138 In the absence of coronary stenoses, this is indicative of a diffuse microvascular dysfunction, which is associated with poor clinical outcomes.139 This study was the first to evaluate the prognostic value of PET blood flow measurements.
Rajappan et al. observed that the subendocardial CFR was lower than subepicardial CFR in patients with aortic valve stenosis (AS), despite normal coronary arteries.20 Burwash et al. reported that very low CFR in patients with AS had an accuracy of 85% for distinguishing true low-flow AS from pseudoaortic stenosis.88,140 This study suggested CFR would be a useful parameter for the differential diagnosis of truly severe, compared to pseudosevere, AS in patients with poor LV function.
Myocardial Blood Flow Parameters as Surrogate Markers
MBF and CFR for the Follow-up of Current Medical Managements
Guethlin et al.141 evaluated the CFR before, at 3 months, and at 6 months after initiation of therapy with fluvastatin. The investigator verified that CFR increased only after 6 months of therapy. Combined therapy with conventional lipid-lowering therapy and the insulin sensitizer pioglitazone improved resting MBF in patients with familial combined hyperlipidemia.142 On the other hand, Ling et al.143 observed that simvastatin improved endothelial function measured using brachial blood flow but did not significantly improve MBF measured using dipyridamole PET imaging. This was possibility related to dose and/or the patient population or the imaging parameter used. This is exemplified by Sdringola et al., who recently demonstrated that the severity of quantified myocardial perfusion defects on PET imaging did not improve after 6 months of atorvastatin versus placebo. However, visual change scores did significantly improve compared to placebo.144
Short-term intravenous angiotensin-converting enzyme inhibitor (ACEI) treatment improves stress flow and flow reserve in ischemic regions.145 Akinboboye et al.146 compared the long-term effects of the ACEI, lisinopril and the angiotensin receptor blocker (ARB) losartan. The ACEI improved hyperemic MBF, but the ARB did not. In contrast, another ARB, olmesartan, reduced coronary resistance during the cold pressor test, indicating olmesartan may benefit coronary endothelial function.90 The discrepancies between the two studies may be due to the method of coronary microcirculation assessment. The latter study by Naya et al. evaluated coronary microcirculation using the cold pressor test, and the former study used pharmacologic stress. Pharmacologic stress evaluates mixed vascular function, including endothelial function and vascular smooth cell function.4,147 Thus, detailed coronary microvascular evaluation in the ideal setting may require both the cold pressor test and vasodilator stress. This has been and will continue to be a limitation to its routine and clinical use.
Diabetes mellitus patients have an increased coronary event risk, and clinical trials have shown the possibility of risk reduction by appropriate therapeutic interventions. Pitkänen et al. reported reduced CFR even in young men with insulin-dependent diabetes mellitus (IDDM) with or without minimum microvascular complications.148 Di Carli et al. compared the coronary vascular function between type 1 diabetes and type 2 diabetes. Both groups showed similar vascular dysfunction.149 Yokoyama et al. found that CFR reduction was inversely correlated with the over-5-year average hemoglobin A1c (r = −0.55; P < 0.01) but not age.150 These studies may indicate the importance of the glycemic control for coronary vascular dysfunction. In patients with diabetes mellitus, perindoprilat improved endothelial function measured using CPT/PET imaging.151 The insulin sensitizer pioglitazone improved glycemic control in patients with insulin-dependent type 2 diabetes. However, pioglitazone did not lead to improvement in either adenosine-induced hyperemia or CFR.152
Yoshinaga et al. evaluated the effect of exercise training effect on regional MBF in patients with stable CAD. The exercise training increased hyperemic MBF in diseased segments compared to the sedentary lifestyle group.31 Furthermore, Sdringola et al. has also applied relative PET perfusion imaging to demonstrate that combined intensive lifestyle and target-driven lipid-lowering drug therapy reduce perfusion abnormalities as well as cardiac events when compared to usual care or cholesterol-lowering drugs.153
MBF and CFR for the Assessment of Interventional Therapies
Van Tosh et al.154 demonstrated that abnormal CFR after angioplasty on PET imaging identifies increased risk for future coronary restenosis. Scott et al. used PET perfusion imaging to demonstrate the benefit of stent therapy in acute myocardial infarction.155 Such surrogate endpoint data have helped lead to larger clinical trials that support the routine use of PCI in STEMI.155
Rechavia et al.156 demonstrated higher resting MBF with reduction of CFR in patients with cardiac transplantation. Chan et al.157 described a decrease in hyperemic flow with an increase in resting flow in excess of cardiac work in patients with transplant rejection. During a follow-up study after successful treatment, patients with transplant rejection had significant improvement of vascular function, suggesting the role and possible importance of serial noninvasive flow measurements by PET.158
Studies that investigated the effect of cardiac resynchronization therapy (CRT) on MBF159,160 demonstrated a more homogeneous resting blood flow distribution and a significant increase in LVEF after 3 months of CRT, compared with baseline.
Myocardial Blood Flow Parameters and Novel Therapeutic Approaches
PET is well suited for tracking of transplanted cells by use of labeling with radionuclides or reporter-genes. Preliminary investigations have been performed by either FDG PET or SPECT MPI.161,162 Reporter-gene approaches163 are expected to shed more light on mechanisms of therapy on the tissue level, but significant advances in this technology are still required.
PET perfusion imaging and flow quantification is likely to play a key role in the evaluation of these cell- and gene-based therapies. Recently, in a randomized trial, Ruel et al. applied 13NH3 PET quantification and was able to demonstrate that the combination of vascular endothelial growth factor angiogenesis and endothelium modulator l-arginine improved perfusion in patients with surgical multivessel CAD and severe diffuse disease in the LAD.164
Hybrid Imaging: Fusion Images of CTA and MBF
Hybrid PET/CT systems would afford mechanistic insights into atherothrombotic processes and may also enable identification of vulnerable plaques by enabling image fusion of structure and function (see Fig. 19-10). Sites with these capabilities are now exploring the role of such a comprehensive evaluation that includes traditional relative uptake perfusion images at stress and rest, gated studies for regional and global function, flow and reserve quantification, and combining these with calcium scoring and coronary CT angiography. Further discussion on hybrid methodologies is found in Chapter 23.
1. Shaw L.J., Berman D.S., Hartigan P.H., et al. Differential improvement in stress myocardial perfusion ischemia following percutaneous coronary intervention as compared with optimal medical therapy alone: nuclear substudy results from the clinical outcomes using revascularization and aggressive drug evaluation (COURAGE) trial. Circulation. 2007;116:2628.
2. Townsend D.W., Cherry S.R. Combining anatomy and function: the path to true image fusion. Eur Radiol. 2001;11:1968-1974.
3. Di Carli M.F., Dorbala S., Moore S., et al. Clinical myocardial perfusion PET/CT. J Nucl Med. 2007;48:783-793.
4. Yoshinaga K., Chow B., deKemp R.A., Beanlands R.S., et al. Application of cardiac molecular imaging using positron emission tomography in evaluation of drug and therapeutics for cardiovascular disorders. Curr Pharm Des. 2005;11:903-932.
5. Chow B.J., Williams K., Yoshinaga K., et al. Prognostic significance of dipyridamole-induced ST depression in patients with normal 82Rb PET myocardial perfusion imaging. J Nucl Med. 2005;46:1095-1101.
6. Marwick T., Go R.T., Patel S., et al. Incremental value of rubidium-82 positron emission tomography for prognostic assessment of known or suspected coronary artery disease. Am J Cardiol. 1997;80:865-870.
7. Yoshinaga K., Chow B., deKemp R.A., et al. Prognostic value of rubidium-82 perfusion positron emission tomography: Preliminary results from 153 consecutive patients. J Am Coll Cardiol. 2004;43:338A.
8. Yoshinaga K., Chow B.J., deKemp R.A., Beanlands R.S., et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol. 2006;48:1029-1039.
9. Beanlands R., Chow B.J., Dick A., et al. CCS / CAR / CANM / CNCS / CanSCMR Joint Position Statement on Advanced Non-invasive Cardiac Imaging using Positron Emission Tomography, Magnetic Resonance Imaging and Multi-Detector Computed Tomographic Angiography in the Diagnosis and Evaluation of Ischemic Heart Disease Abbreviated Report. Can J Cardiol. 2007;23:107-119.
10. Brown T.L., Bengel F.M., Merrill J., Volokh L. Determinants of the response of left ventricular ejection fraction to vasodilator stress in electrocardiographically gated 82rubidium myocardial perfusion PET. Eur J Nucl Med Mol Imaging. 2008;35:336-342.
11. deKemp R.A., Yoshinaga K., Beanlands R.S. Will 3-dimensional PET-CT enable the routine quantification of myocardial blood flow? J Nucl Cardiol. 2007;14:380-397.
12. Machac J. Cardiac positron emission tomography imaging. Semin Nucl Med. 2005;35:17-36.
13. Machac J., Beanlands R.S., Di Carli M.F., et al. Positron emission tomography perfusion and glucose metabolism imaging. J Nucl Cardiol. 2006;13:e121-e151.
14. Camici P.G. Positron emission tomography and myocardial imaging. Heart. 2000;83:475-480.
15. Furuyama H., Katoh C., Odagawa Y., et al. Assessment of coronary function in children with a history of Kawasaki disease using (15) O-water positron emission tomography. Circulation. 2002;105:2878-2884.
16. Loghin C., Gould K.L., Sdringola S. Common artifacts in PET myocardial perfusion images due to attenuation-emission misregistration: clinical significance, causes, and solutions. J Nucl Med. 2004;45:1029-1039.
17. Martinez-Moller A., Schwaiger M., Souvatzoglou M., et al. Artifacts from misaligned CT in cardiac perfusion PET/CT studies: frequency, effects, and potential solutions. J Nucl Med. 2007;48:188-193.
18. Schwaiger M., Ziegler S., et al. PET/CT: Challenge for nuclear cardiology. J Nucl Med. 2005;46:1664-1678.
19. Wassenaar R.W., Beanlands R.S., deKemp R.A., Ruddy T.D. Three dimensional cardiac positron emission tomography. Res Adv Nuc Med. 2002;1:51-60.
20. Rajappan K., Dutka D.P., Rimoldi O.E., et al. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation. 2002;105:470-476.
21. Di Carli M.F., Hachamovitch R. New Technology for Noninvasive evaluation of coronary artery disease. Circulation. 2007;115:1464-1480.
22. Yoshinaga K: Principles and Practice in Positron Emission Tomography. In Wahl R., editor: Chapter 8: Cardiac Applications, ed 2, 2007.
23. Bateman T.M., Friedman J.D., Heller G.V., McGhie A.I., et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13:24-33.
24. Krivokapich J., Huang S.C., MacDonald N.S., Phelps M.E., et al. Dependence of 13NH3 myocardial extraction and clearance on flow and metabolism. Am J Physiol. 1982;242:H536-H542.
25. Hutchins G.D., Krivokapich J., Rosenspire K.C., Schelbert H., Schwaiger M., et al. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15:1032-1042.
26. Beanlands R.S., Hutchins G.D., Muzik O., et al. Heterogenicity of regional nitrogen 13-labeled ammonia tracer distribution in the normal human heart: comparison with rubidium 82 and copper 62-labeled PTSM. J Nucl Cardiol. 1994;1:225-235.
27. Alvarez-Diez T.M., Beanlands R.S., deKemp R., Vincent J. Manufacture of strontium-82/rubidium-82 generators and quality control of rubidium-82 chloride for myocardial perfusion imaging in patients using positron emission tomography. Appl Radiat Isot. 1999;50:1015-1023.
28. deKemp R.A., Hewitt T., Ruddy T.D., Beanlands R.S., et al. Detection of serial changes in absolute myocardial perfusion with 82Rb PET. J Nucl Med. 2000;41:1426-1435.
29. Parkash R., deKemp R.A., Ruddy T.D., Beanlands R.S., et al. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol. 2004;11:440-449.
30. Scott N., deKemp R., LeMay M., et al. Evaluation of myocardial perfusion using rubidium-8/82 positron emission tomography after myocardial infarction in patients receiving primary stent implantation or thrombolytic therapy. Am J Cardiol. 2001;88:886-889.
31. Yoshinaga K., deKemp R.A., Beanlands R.S., et al. Effect of exercise training on myocardial blood flow in patients with stable coronary artery disease. Am Heart J. 2006;151(1324):e11-e18.
32. Bergmann S.R., Fox K.A.A., Rond A.L., et al. Quantification of regional myocardial blood flow in vivo with H215O. Circulation. 1984;70:724-733.
33. Gropler R.J., Geltman E.M., Siegel B.A. Myocardial uptake of carbon-11-acetate as an indirect estimate of regional myocardial blood flow. J Nucl Med. 1991;32:245-251.
34. van den Hoff J., Börner A.R., Burchert W., et al. [1–11C]acetate as a quantitative perfusion tracer in myocardial PET. J Nucl Med. 2001;42:1174-1182.
35. Sun K.T., Buxton D.B., Yeatman L.A., et al. Simultaneous measurement of myocardial oxygen consumption and blood flow using 11-carbon acetate. J Nucl Med. 1998;39:272-280.
36. Beanlands R.S., Mintun M., Muzik O., et al. The kinetics of copper-62-PTSM in the normal human heart. J Nucl Med. 1992;33:684-690.
37. Green M.A., Mathias C.J., Welch M.J., et al. Copper-62-labeled pyruvaldehyde bis(N4-methylthiosemicarbazonato)copper(II): synthesis and evaluation as a positron emission tomography tracer for cerebral and myocardial perfusion. J Nucl Med. 1990;31:1989-1996.
38. Shelton M.E., Bergmann S.R., Green M.A., Mathias C.J., Welch M.J. Assessment of regional myocardial and renal blood flow with copper-PTSM and positron emission tomography. Circulation. 1990;82:990-997.
39. Wallhaus T.R., Green M.A., Nickles R.J., Stone C.K., et al. Human biodistribution and dosimetry of the PET perfusion agent copper-62-PTSM. J Nucl Med. 1998;39:1958-1964.
40. Mathias C.J., Bergmann S.R., Green M.A. Development and validation of a solvent extraction technique for determination of Cu-PTSM in blood. Nucl Med Biol. 1993;20:343-349.
41. Haynes N.G., Lacy J.L., Nayak N., et al. Performance of a 62Zn/62Cu generator in clinical trials of PET perfusion agent 62Cu-PTSM. J Nucl Med. 2000;41:309-314.
42. Ackerman L.J., Green M.A., Mathias C.J., West D.X. Synthesis and evaluation of copper radiopharmaceuticals with mixed bis (thiosemicarbazone) ligands. Nucl Med Biol. 1999;26:551-554.
43. Madar I., Du Y., Hilton J., Volokh L., et al. Characterization of uptake of the new PET imaging compound18F-fluorobenzyl triphenyl phosphonium in dog myocardium. J Nucl Med. 2006;47:1359-1366.
44. Madar I., DiPaula A., Ravert H., et al. Assessment of severity of coronary artery stenosis in a canine model using the PET agent 18F-fluorobenzyl triphenyl phosphonium: Comparison with 99mTc-tetrofosmin. J Nucl Med. 2007;48:1021-1030.
45. Yalamanchili P., Hayes M., Wexler e., et al. Mechanism of uptake and retention of F-18 BMS-747158–02 in cardiomyocytes: a novel PET myocardial imaging agent. J Nucl Cardiol. 2007;14:782-788.
46. Yu M., Guaraldi M.T., Mistry M., Robinson S.P., et al. BMS-747158–02: A novel PET myocardial perfusion imaging agent. J Nucl Cardiol. 2007;14:789-798.
47. Plössla K., Chandraa R., Qua W., et al. A novel gallium bisaminothiolate complex as a myocardial perfusion imaging agent. Nucl Med Biol. 2008;35:83-90.
48. Udelson J.E., Heller G.V., Wackers F.J., et al. Randomized, controlled dose-ranging study of the selective adenosine A2A receptor agonist binodenoson for pharmacological stress as an adjunct to myocardial perfusion imaging. Circulation. 2004;109:457-464.
49. Iskandrian A.E., Bateman T.M., Hendel R.C., et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: Results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14:645-658.
50. Cerqueira M.D. Advances in pharmacologic agents in imaging: new A2A receptor agonists. Curr Cardiol Rep. 2006;8:119-122.
51. American Society of Nuclear Cardiology. Imaging guidelines for nuclear cardiology procedures, part 2. J Nucl Cardiol. 1999;6:G47-G84.
52. Chow B.J., Lee A., Beanlands R.S., et al. Treadmill exercise produces larger perfusion defects than dipyridamole stress N-13 ammonia positron emission tomography. J Am Coll Cardiol. 2006;47:411-416.
53. Chow B.J., Ananthasubramaniam K., deKemp R.A., Beanlands R.S., et al. Feasibility of exercise rubidium-82 positron emission tomography myocardial perfusion imaging. J Am Coll Cardiol. 2003;41:428A.
54. Chow B.J., deKemp R.A., Ruddy T.D., Beanlands R.S., et al. Comparison of treadmill exercise versus dipyridamole stress with myocardial perfusion imaging using rubidium-82 positron emission tomography. J Am Coll Cardiol. 2005;45:1227-1234.
55. Herrero P., Bergmann S.R., Markham J., Shelton M.E. Implementation and evaluation of a two-compartment model for quantification of myocardial perfusion with rubidium-82 and positron emission tomography. Circ Res. 1992;70:496-507.
56. Lortie M., Beanlands R., DaSilva J., deKemp R. Quantification of myocardial blood flow in subjects with coronary artery disease with 82Rb dynamic PET imaging. J Nucl Med. 48, 2007.
57. Renaud J., Beanlands R.S., deKemp R.A., Lortie M., et al. Quantifying the normal range of myocardial blood flow with 13N-ammonia and 82Rb dynamic PET imaging. J Nucl Med. 2006;47:67P.
58. Yoshida K., Gould K.L., Mullani N. Coronary flow and flow reserve by PET simplified for clinical applications using rubidium-82 or nitrogen-13-ammonia. J Nucl Med. 1996;37:1701-1712.
59. Bellina C., Camici P., Parodi O., Salvadori P.A., et al. Simultaneous in vitro and in vivo validation of nitrogen-13-ammonia for the assessment of regional myocardial blood flow. J Nucl Med. 1990;31:1335-1343.
60. Hendel R.C., Berman D.S., Wackers F.J., et al. American Society of Nuclear Cardiology consensus statement: reporting of radionuclide myocardial perfusion imaging studies. J Nucl Cardiol. 2003;10:705-708.
61. Klocke F.J., Baird M.G., Lorell B.H., et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging: executive summary-a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol. 2003;42:1318-1333.
62. Yoshinaga K., Chow B.J., deKemp R.A., Beanlands R.S., et al. Prognostic value of rubidium-82 perfusion positron emission tomography in patients referred after SPECT imaging. J Nucl Cardiol. 2005;12:S43.
63. Dorbala S., Di Carli M.F., Kwong R., et al. Value of left ventricular ejection fraction reserve in assessment of severe left main/three-vessel coronary artery disease: a rubidium-82 PET-CT Study. J Nucl Med. 2007;48:349-358.
64. Camici P., Chiriatti G., Lorenzoni R., et al. Coronary vasodilation is impaired in both hypertrophied and nonhypertrophied myocardium of patients with hypertrophic cardiomyopathy: a study with nitrogen-13 ammonia and positron emission tomography. J Am Coll Cardiol. 1991;17:879-886.
65. Chan S.Y., Brunken R.C., Czernin J., et al. Comparison of maximal myocardial blood flow during adenosine infusion with that of intravenous dipyridamole in normal men. J Am Coll Cardiol. 1992;20:979-985.
66. Czernin J., Chan S., Muller P., et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation. 1993;88:62-69.
67. Beanlands R.S., Hutchins G.D., Schwaiger M., et al. Noninvasive quantification of regional myocardial flow reserve in patients with coronary atherosclerosis using nitrogen-13 ammonia positron emission tomography: determination of extent of altered vascular reactivity. J Am Coll Cardiol. 1995;26:1465-1475.
68. Laine H., Niinikoski H., Raitakari O.T., et al. Early impairment of coronary flow reserve in young men with borderline hypertension. J Am Coll Cardiol. 1998;32:147-153.
69. Kaufmann P.A., Gnecchi-Ruscone T., Schafers K.P., et al. Low density lipoprotein cholesterol and coronary microvascular dysfunction in hypercholesterolemia. J Am Coll Cardiol. 2000;36:103-109.
70. Yoshinaga K., Katoh C., Noriyasu K., et al. Reduction of coronary flow reserve in areas with and without ischemia on stress perfusion imaging in patients with coronary artery disease: a study using oxygen 15-labeled water PET. J Nucl Cardiol. 2003;10:275-283.
71. Lin J.W., Chou R.L., Laine A.F., Sciacca R.R., et al. Quantification of myocardial perfusion in human subjects using 82Rb and wavelet-based noise reduction. J Nucl Med. 2001;42:201-208.
72. Lortie M., Kelly C., Mostert K., et al. Quantification of myocardial blood flow with rubidium-82 dynamic PET imaging. J Nucl Med. 2005;46:60P.
73. Cecchi F., Camici P.G., Gistri R., Olivotto I., et al. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027-1035.
74. Neglia D., Michelassi C., Pratali L., Trivieri M.G., et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation. 2002;105:186-193.
75. DeGrado T.R., Hanson M.W., Turkington T.G., Vallee J.P., et al. Estimation of myocardial blood flow for longitudinal studies with 13N-labeled ammonia and positron emission tomography. J Nucl Cardiol. 1996;3:494-507.
76. Lortie M., Beanlands R.S., Yoshinaga K., deKemp R.A., et al. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging. 2007;34:1765-1774.
77. Choi Y., Huang S.C., Hawkins R.A., Kim J.Y., Kim B.T., Hoh C.K., et al. Quantification of myocardial blood flow using 13N-ammonia and PET: comparison of tracer models. J Nucl Med. 1999;40:1045-1055.
78. Herrero P., Bergmann S.R., Markham J., Shelton M.E., et al. Noninvasive quantification of regional myocardial perfusion with rubidium-82 and positron emission tomography: exploration of a mathematical model. Circulation. 1990;82:1377-1386.
79. Kety S.S. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol Rev. 1951;3:1-41.
80. Iida H., Kanno I., Takahashi A., et al. Measurement of absolute myocardial blood flow with H215O and dynamic positron-emission tomography. Strategy for quantification in relation to the partial-volume effect. Circulation. 1988;78:104-115.
81. Katoh C., Morita K., Shiga T., Tamaki N., et al. Improvement of algorithm for quantification of regional myocardial blood flow using 15O-water with PET. J Nucl Med. 2004;45:1908-1916.
82. Schelbert H.R., Phelps M.E., Wisenberg G., et al. Noninvasive assessment of coronary stenoses by myocardial imaging during pharmacologic coronary vasodilation. VI. Detection of coronary artery disease in human beings with intravenous N-13 ammonia and positron computed tomography. Am J Cardiol. 1982;49:1197-1207.
83. Bol A., Melin J.A., Vanoverschelde J.L., et al. Direct comparison of [13N]ammonia and [15O]water estimates of perfusion with quantification of regional myocardial blood flow by microspheres. Circulation. 1993;87:512-525.
84. Kuhle W.G., Huang S.C., Porenta G., et al. Quantification of regional myocardial blood flow using 13N-ammonia and reoriented dynamic positron emission tomographic imaging. Circulation. 1992;86:1004-1017.
85. Muzik O., Beanlands R.S., Hutchins G.D., Mangner T.J., et al. Validation of nitrogen-13-ammonia tracer kinetic model for quantification of myocardial blood flow using PET. J Nucl Med. 1993;34:83-91.
86. Choi Y., Hawkins R.A., Huang S.C., et al. A simplified method for quantification of myocardial blood flow using nitrogen-13-ammonia and dynamic PET. J Nucl Med. 1993;34:488-497.
87. Tadamura E., Tamaki N., Yonekura Y., et al. Assessment of coronary vasodilator reserve by N-13 ammonia PET using the microsphere method and Patlak plot analysis. Ann Nucl Med. 1995;9:109-118.
88. Burwash I., deKemp R., Pibarot P., et al. Myocardial blood flow in patients with low flow gradient aortic stenosis: differences between true and pseudo severe aortic stenosis. Results from the multicenter TOPAS study [abstract]. Circulation. 2005;112:718.
89. Kaufmann PA, Camici P.G. Myocardial blood flow measurement by PET: Technical aspects and clinical applications. J Nucl Med. 2005;46:75-88.
90. Naya M., Katoh C., Morita K., Tsukamoto T., et al. Olmesartan, but not amlodipine, improves endothelium-dependent coronary dilation in hypertensive patients. J Am Coll Cardiol. 2007;50:1144-1149.
91. Schafers K.P., Camici P.G., Spinks T.J., et al. Absolute quantification of myocardial blood flow with H215O and 3-dimensional PET: an experimental validation. J Nucl Med. 2002;43:1031-1040.
92. De Bruyne B., Baudhuin T., Melin J.A., et al. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation. 1994;89:1013-1022.
93. Nagamachi S., Czernin J., Kim A.S., et al. Reproducibility of measurements of regional resting and hyperemic myocardial blood flow assessed with PET. J Nucl Med. 1996;37:1626-1631.
94. Kaufmann P.A., Camici P.G., Gnecchi-Ruscone T., Rimoldi O., et al. Assessment of the reproducibility of baseline and hyperemic myocardial blood flow measurements with 15O-labeled water and PET. J Nucl Med. 1999;40:1848-1856.
95. Lortie M., Mostert K., Renaud J., et al. Myocardial blood flow quantification with Rb-82 and N-13-ammonia PET in healthy volunteers. Can J Cardiol. 2004.
96. Yoshinaga K., Beanlands R.S., deKemp R.A., et al. Measurement of coronary endothelial function with rubidium-82 PET. Comparison with oxygen 15-labeled water PET. J Nucl Med. 2008;49(Suppl 1):75P.
97. Prior J.O., Allenbach G., Valenta L., et al. Myocardial blood flow quantification using Rb-82: Validation to O-15-Water in healthy volunteer and CAD patients. J Nucl Med. 2008. In press
98. Uren N.G., De Bruyne B., Camici P.G., Melin J.A. Relation between myocardial blood flow and the severity of coronary artery stenosis. N Engl J Med. 1994;330:1782-1788.
99. Tamaki N., Senda M., Yonekura Y., et al. Myocardial positron computed tomography with 13N-ammonia at rest and during exercise. Eur J Nucl Med. 1985;11:246-251.
100. Yonekura Y., Senda M., Tamaki N., et al. Detection of coronary artery disease with 13N ammonia and high resolution positron-emission computed tomography. Am Heart J. 1987;113:645-654.
101. Tamaki N., Koide H., Saji H., Yamashita K., et al. Value and limitation of stress thallium-201 single photon emission computed tomography: comparison with nitrogen-13 ammonia positron tomography. J Nucl Med. 1988;29:1181-1188.
102. Gould K.L., Goldstein R.A., Mullani N.A., et al. Noninvasive assessment of coronary stenoses by myocardial perfusion imaging during pharmacologic coronary vasodilation. VIII. Clinical feasibility of positron cardiac imaging without a cyclotron using generator-produced rubidium-82. J Am Coll Cardiol. 1986;7:775-789.
103. Demer L.L., Gould K.L., Goldstein R.A., et al. Assessment of coronary artery disease severity by positron emission tomography. Comparison with quantitative arteriography in 193 patients. Circulation. 1989;79:825-835.
104. Go R.T., Marwick T.H., MacIntyre W.J., et al. A prospective comparison of rubidium-82 PET and thallium-201 SPECT myocardial perfusion imaging utilizing a single dipyridamole stress in the diagnosis of coronary artery disease. J Nucl Med. 1990;31:1899-1905.
105. Stewart R.E., Molina E., Popma J., Squicciarini S., et al. Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am J Cardiol. 1991;67:1303-1310.
106. Marwick T.H., Nemec J.J., Salcedo E.E., Stewart W.J. Diagnosis of coronary artery disease using exercise echocardiography and positron emission tomography: comparison and analysis of discrepant results. J Am Soc Echocardiogr. 1992;5:231-238.
107. Grover-McKay M., Ratib O., Schwaiger M., et al. Detection of coronary artery disease with positron emission tomography and rubidium 82. Am Heart J. 1992;123:646-652.
108. Laubenbacher C., Rothley J., Sitomer J., et al. An automated analysis program for the evaluation of cardiac PET studies: initial results in the detection and localization of coronary artery disease using nitrogen-13-ammonia. J Nucl Med. 1993;34:968-978.
109. Wallhaus T.R., Lacy J., Stewart R., Stone C.K., et al. Copper-62-pyruvaldehyde bis(N-methyl-thiosemicarbazone) PET imaging in the detection of coronary artery disease in humans. J Nucl Cardiol. 2001;8:67-74.
110. Walsh M.N., Bergmann S.R., Kenzora J.L., Steele R.L., et al. Delineation of impaired regional myocardial perfusion by positron emission tomography with 15OH2O. Circulation. 1988;78:612-620.
111. Williams B.R., Jansen D.E., Mullani N.A., et al. A retrospective study of the diagnostic accuracy of a community hospital-based PET center for the detection of coronary artery disease using rubidium-82. J Nucl Med. 1994;35:1586-1592.
112. Simone G.L., Mullani N.A., Page D.A., et al. Utilization statistics and diagnostic accuracy of a nonhospital-based positron emission tomography center for the detection of coronary artery disease using rubidium-82. Am J Physiol Imaging. 1992;7:203-209.
113. Husmann L., Kaufmann P.A., Valenta L., Wiegand M., et al. Diagnostic accuracy of myocardial perfusion imaging with single photon emission computed tomography and positron emission tomography: a comparison with coronary angiography. Int J Cardiovasc Imaging. 2007. In press
114. Namdar M., Hany T.F., Koepfli P., et al. Integrated PET/CT for the assessment of coronary artery disease: a feasibility study. J Nucl Med. 2005;46:930-935.
115. Sampson U.K., Di Carli M.F., Dorbala S., Kwong R., et al. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007;49:1052-1058.
116. Yoshinaga K., Katoh C., Noriyasu K., et al. Reduction of coronary flow reserve in areas with and without ischemia on stress perfusion imaging in patients with coronary artery disease: a study using oxygen 15-labeled water PET. J Nucl Cardiol. 2003;10:275-283.
117. Study of Myocardial Perfusion and Coronary Anatomy Imaging Roles in CAD (SPARC). http://www.sparctrial.org. Web page Available at:
118. Marwick T.H., Go R.T., Lauer M.S., MacIntyre W.J., et al. Use of positron emission tomography for prediction of perioperative and late cardiac events before vascular surgery. Am Heart J. 1995;130:1196-1202.
119. MacIntyre W.J., Go R.T., King J.L., et al. Clinical outcome of cardiac patients with negative thallium-201 SPECT and positive rubidium-82 PET myocardial perfusion imaging. J Nucl Med. 1993;34:400-404.
120. Schächinger V., Britten M., Zeiher A.M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899-1906.
121. Deanfield E., Halcox J.P., Rabelink T.J. Contemporary reviews in cardiovascular medicine. Endothelial function and dysfunction testing and clinical relevance. Circulation. 2007;115:1285-1295.
122. Struijker-Boudier H.A., Bruneval P., Camiri P.G., Rosei A., et al. Evaluation of the microcirculation in hypertension and cardiovascular disease. Eur Heart J. 2007;28:2834-2840.
123. Iwado Y., Yoshinaga K., Furuyama H., et al. Decreased endothelium-dependent coronary vasomotion in healthy young smokers. Eur J Nucl Med Mol Imaging. 2002;29:984-990.
124. Campisi R., Czernin J., Schoder H., et al. Effects of long-term smoking on myocardial blood flow, coronary vasomotion, and vasodilator capacity. Circulation. 1998;98:119-125.
125. Schoder H., Campisi R., Silverman D.H., et al. Effect of mental stress on myocardial blood flow and vasomotion in patients with coronary artery disease. J Nucl Med. 2000;41:11-16.
126. Gould K.L., Nakagawa K., Parker N., et al. Frequency and clinical implications of fluid dynamically significant diffuse coronary artery disease manifest as graded, longitudinal, base-to-apex myocardial perfusion abnormalities by noninvasive positron emission tomography. Circulation. 2000;101:1931-1939.
127. Johnson N.P., Gould K.L. Clinical evaluation of a new concept: Resting myocardial perfusion heterogeneity quantified by Markovian analysis of PET identifies coronary microvascular dysfunction and early atherosclerosis in 1,034 subjects. J Nucl Med. 2005;46:1427-1437.
128. Dayanikli F., Grambow D., Muzik O., Schwaiger M., et al. Early detection of abnormal coronary flow reserve in asymptomatic men at high risk for coronary artery disease using positron emission tomography. Circulation. 1994;90:808-817.
129. Yokoyama I., Murakami T., Ohtake T., et al. Reduced coronary flow reserve in familial hypercholesterolemia. J Nucl Med. 1996;37:1937-1942.
130. Czernin J., Brunken R., Schelbert H., Sun K., et al. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897.
131. Di Carli M.F., Grunberger G., Janisse J., et al. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41:1387-1393.
132. Schindler T.H., Facta A.D., Prior J.O., Schelbert H.R., et al. Improvement in coronary vascular dysfunction produced with euglycaemic control in patients with type 2 diabetes. Heart. 2007;93:345-349.
133. Muzik O., Beanlands R.S.B., Duvernoy C., Sawada S., et al. Assessment of diagnostic performance of quantitative flow measurements in normal subjects and patients with angiographically documented coronary artery disease by means of nitrogen-13 ammonia and positron emission tomography. J Am Coll Cardiol. 1998;31:534-540.
134. Gould K.L., Hamilton G.W., Lipscomb K. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33:87-94.
135. Demer L.L., Goldstein R.A., Gould K.L., et al. Assessment of coronary artery disease severity by positron emission tomography. Comparison with quantitative arteriography in 193 patients. Circulation. 1989;79:825-835.
136. Seth R., deKemp R.A., Hart B., Ruddy T.D., et al. Quantification of absolute perfusion reserve using 82Rb positron emission tomography defines greater extent of disease in three vessel coronary atherosclerosis. J Am Coll Cardiol. 2001;37:387A.
137. Beanlands R.S., deKemp R., Iwanochko R.M., Ruddy T., et al. Positron emission tomography and recovery following revascularization (PARR-1): the importance of scar and the development of a prediction rule for the degree of recovery of left ventricular function. J Am Coll Cardiol. 2002;40:1735-1743.
138. Camici P.G., Lorenzoni R., Marraccini P., et al. Coronary hemodynamics and myocardial metabolism in patients with syndrome X: response to pacing stress. J Am Coll Cardiol. 1991;17:1461-1470.
139. Knaapen P., Camici P.G., Germans T., et al. Determinants of coronary microvascular dysfunction in symptomatic hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;294:H986-H993.
140. Burwash I.G., Beanlands R.S., deKemp R.A., Lortie M., et al. Myocardial blood flow in patients with low Flow, low gradient aortic stenosis: Differences between true and pseudo-severe aortic stenosis. Results from the multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) Study. Heart. 2008. In press
141. Guethlin M., Coppenrath K., Kasel A.M., Schwaiger M., Ziegler S., et al. Delayed response of myocardial flow reserve to lipid-lowering therapy with fluvastatin. Circulation. 1999;99:475-481.
142. Naoumova R.P., Camici P.G., Kindler H., et al. Pioglitazone improves myocardial blood flow and glucose utilization in nondiabetic patients with combined hyperlipidemia. J Am Coll Cardiol. 2007;50:2051-2058.
143. Ling M.C., deKemp R.A., Ruddy T.D., et al. Early effects of statin therapy on endothelial function and microvascular reactivity in patients with coronary artery disease. Am Heart J. 2005;149(1137):e9-e16.
144. Sdringola S., Gould K.L., Zamarka L.G., et al. A 6 month randomized, double blind, placebo controlled, multi-center trial of high dose atorvastatin on myocardial perfusion abnormalities by positron emission tomography in coronary artery disease. Am Heart J. 2008;155:245-253.
145. Schneider C.A., Moka D., Voth E., et al. Improvement of myocardial blood flow to ischemic regions by angiotensin-converting enzyme inhibition with quinaprilat IV: a study using [15O] water dobutamine stress positron emission tomography. J Am Coll Cardiol. 1999;34:1005-1011.
146. Akinboboye O.O., Bergmann S.R., Chou R.L. Augmentation of myocardial blood flow in hypertensive heart disease by angiotensin antagonists: a comparison of lisinopril and losartan. J Am Coll Cardiol. 2002;40:703-709.
147. Camici P.G., Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830-840.
148. Pitkänen O.P., Nuutila P., Raitakari O.T., et al. Coronary flow reserve is reduced in young men with IDDM. Diabetes. 1998;47:248-254.
149. Di Carli M.F., Ager J., Grunberger G., et al. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41:1387-1393.
150. Yokoyama I., Momomura S., Ohtake T., et al. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1997;30:1472-1477.
151. Kjaer A., Meyer C., Nielsen F.S., Parving H.H., et al. Dipyridamole, cold pressor test, and demonstration of endothelial dysfunction: a PET study of myocardial perfusion in diabetes. J Nucl Med. 2003;44:19-23.
152. McMahon G.T., Di Carli M.F., Plutzky J., et al. Effect of a peroxisome proliferator-activated receptor agonist on myocardial blood flow in type 2 diabetes. Diabetes Care. 2005;28:1145-1150.
153. Sdringola S., Nakagawa K., Nakagawa Y., Gould K.L., et al. Combined intense lifestyle and pharmacologic lipid treatment further reduce coronary events and myocardial perfusion abnormalities compared with usual-care cholesterol-lowering drugs in coronary artery disease. J Am Coll Cardiol. 2003;41:263-272.
154. van Tosh A., Garza D., Roberti R., et al. Serial myocardial perfusion imaging with dipyridamole and rubidium-82 to assess restenosis after angioplasty. J Nucl Med. 1995;36:1553-1560.
155. Scott N.S., Beanlands R.S., de Kemp R., Ruddy T.D., et al. Evaluation of myocardial perfusion using rubidium-82 positron emission tomography after myocardial infarction in patients receiving primary stent implantation or thrombolytic therapy. Am J Cardiol. 2001;88:886-889.
156. Rechavia E., Araujo L.I., De Silva R., et al. Dipyridamole vasodilator response after human orthotopic heart transplantation: quantification by oxygen-15-labeled water and positron emission tomography. J Am Coll Cardiol. 1992;19:100-106.
157. Chan S.Y., Kobashigawa J., Stevenson L.W., Schelbert H.R., et al. Myocardial blood flow at rest and during pharmacological vasodilation in cardiac transplants during and after successful treatment of rejection. Circulation. 1994;90:204-212.
158. Zanco P., Gambino A., Livi U., et al. Changes in myocardial blood flow and coronary reserve evaluated by PET early and late heart transplant. J Nucl Med. 2000;41:41P.
159. Knaapen P., van Campen L.M., de Cock C.C., et al. Effects of cardiac resynchronization therapy on myocardial perfusion reserve. Circulation. 2004;110:646-651.
160. Lindner O., Kammeier A., Vogt J., et al. Effect of cardiac resynchronization therapy on global and regional oxygen consumption and myocardial blood flow in patients with non-ischaemic and ischaemic cardiomyopathy. Eur Heart J. 2005;26:70-76.
161. Chang G.Y., Xie X., Wu J.C. Overview of stem cells and imaging modalities for cardiovascular diseases. J Nucl Cardiol. 2006;13:554-569.
162. Zhou R., Acton P.D., Ferrari V.A. Imaging stem cells implanted in infarcted myocardium. J Am Coll Cardiol. 2006;48:2094-2106.
163. Bengel F.M., Anton M., Richter T., et al. Noninvasive imaging of transgene expression by use of positron emission tomography in a pig model of myocardial gene transfer. Circulation. 2003;108:2127-2133.
164. Ruel M., Beanlands R.S., deKemp R.A., Lortie M., et al. Concomitant treatment with oral L-arginine improves the efficacy of surgical angiogenesis in patients with severe diffuse coronary artery disease: the Endothelial Modulation in Angiogenic Therapy randomized controlled trial. J Thorac Cardiovasc Surg. 2008;135:762-770.