Chapter 85 Diabetic Foot
Risk Factors for Foot Problems in Diabetic Patients
Peripheral Neuropathy
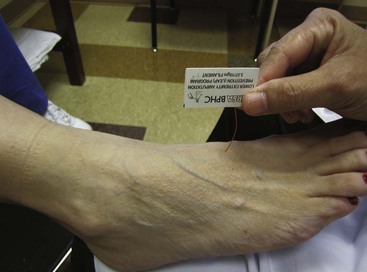
Peripheral neuropathy can be diagnosed by physical examination and can be confirmed with electromyography/nerve conduction studies. Sixty to 70 percent of patients with diabetes have some neural manifestation, and almost 30% of patients with diabetes who are 40 years or older have loss of sensation in the feet. Loss of protective sensation can be determined by use of a 5.07-mm Semmes-Weinstein filament and is thought to be the threshold at which complications such as neuropathic ulcers and Charcot arthropathy occur (Fig. 85-1).
Peripheral Vascular Disease
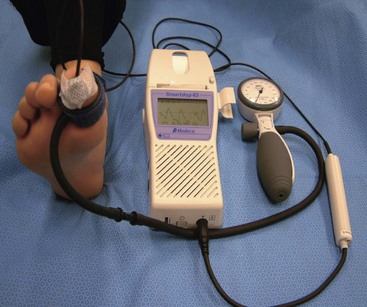
The ankle-brachial index can be unreliable for the diagnosis of peripheral arterial disease in diabetic patients, because calcification of arteries can lead to elevated results, masking the severity of the disease. Toe pressure or TcpO2 is thought to be more reliable in diabetic patients (Fig. 85-2). Angiography is the gold standard for diagnosing arterial lesions that may require intervention; however, it may be complicated by the fact that many diabetics have renal disease, which can be worsened by the intravenous contrast medium used in such studies.
Diabetic Ulcers and Infections
Pathophysiology
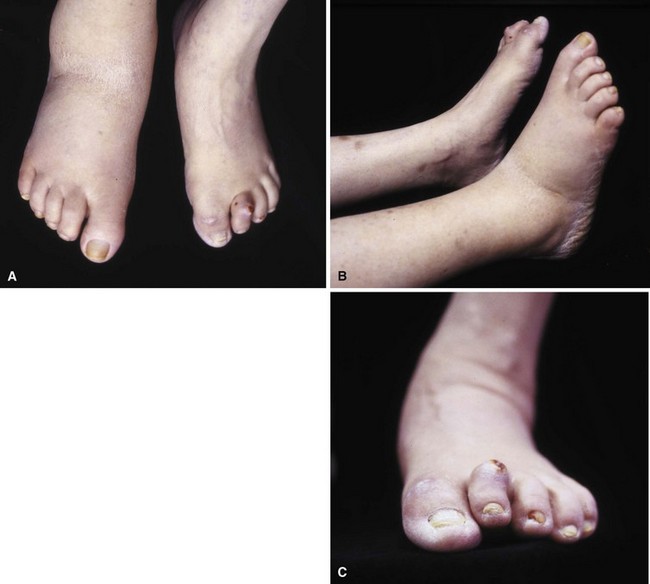
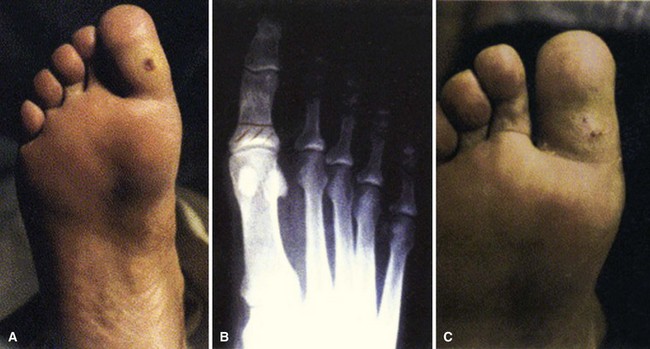
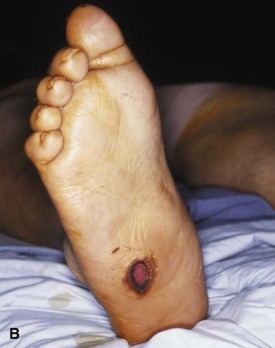
Patients who develop ulcers usually have a combination of peripheral neuropathy, deformity or joint contracture, increased plantar pressure, and peripheral arterial disease. Because of the loss of sensation, patients do not realize that undue pressure is placing their skin at risk. Motor neuropathy can lead to deformity such as claw toes; the bony prominences from the deformity make the skin more vulnerable to breakdown (Fig. 85-3). Achilles contracture can lead to increased forefoot pressures, which increases the chances of forefoot ulceration. There are known structural changes in the Achilles tendon in diabetic patients, including disorganization of the tendon fibers and calcification within the tendon. These changes are more prevalent in older patients and can explain the increased stiffness that is known to occur in the Achilles tendons in diabetics. Peripheral arterial disease also places the skin at risk and leads to impairment of healing once ulceration does occur. The presence of peripheral arterial disease increases the risk of ulceration ninefold.
Classification
The Wagner classification (Box 85-1) is the most commonly used for grading foot ulcers. The University of Texas San Antonio classification system adds the criteria of infection and ischemia and correlates more closely with prognosis because increased severity (higher grade) is associated with longer healing times and amputation.
Treatment
Nonoperative Treatment
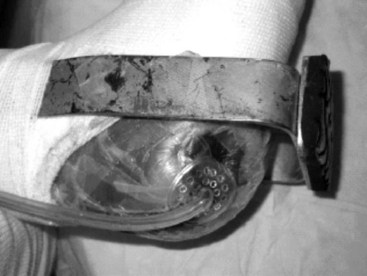
Total contact casting is the standard of care, because it reduces plantar loads better than a well-molded shoe cast and, by extrapolation, better than shoes with custom insoles (Fig. 85-7). Complications may arise from the use of total contact casts, but most are minor and reversible new areas of ulcerations. The risk for complications of total contact casting is lower after deformity-correcting surgery, as well as when the patient is non–weight bearing. Trepman et al. described a method for total contact casting (Fig. 85-8). In a patient who cannot be kept non–weight bearing, the addition of a metal stirrup that extends beyond the foot-plate of the cast takes pressure off the plantar surface of the foot and transmits it to the shank of the cast (Fig. 85-9). Healing rates after total contact casting can be high; however, the recurrence rate also can be high unless severe deformities are surgically corrected.
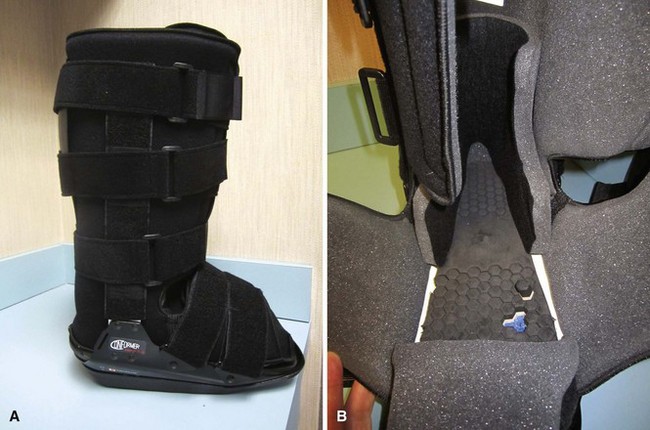
Removable diabetic boots (Fig. 85-10) have been shown to be as efficacious as total contact casting in some studies; however, in one study, whereas the boot demonstrated better forefoot unloading than a total contact cast, healing rates were better in the cast, presumably secondary to lack of patient compliance with the boot. Wrapping the diabetic boot to make it less removable does lead to healing rates that are higher than boots that are not wrapped, again suggesting that patient compliance is an issue with the removable boot.
Operative Treatment
According to guidelines based on a systematic review by the International Working Group on the Diabetic Foot, the indications for urgent surgical intervention include necrotizing infections and gangrene or deep abscesses (Fig. 85-11). Less urgent surgery may be required if there is a substantially compromised soft tissue envelope, loss of mechanical function of the foot, or bone involvement that is limb threatening or if the patient prefers to avoid prolonged antibiotic therapy. Surgical débridement of osteomyelitis is not always required.
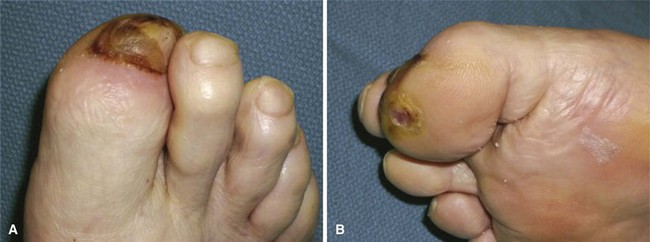
FIGURE 85-11 Cellulitis, abscess, and osteomyelitis arising from ulcer over fifth toe caused by tight shoe.
(From Brodsky JW: The diabetic foot. In Coughlin MJ, Mann RA, Saltzman CL, editors: Surgery of the foot and ankle, ed 8, Philadelphia, 2007, Elsevier.)
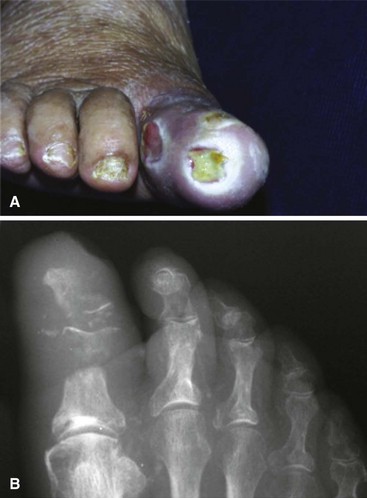
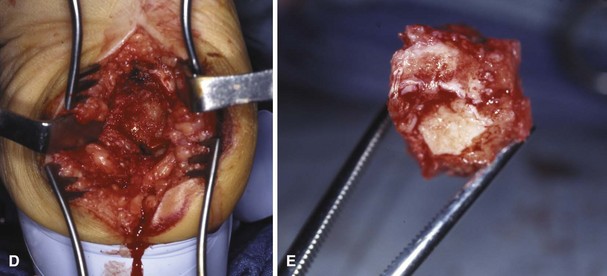
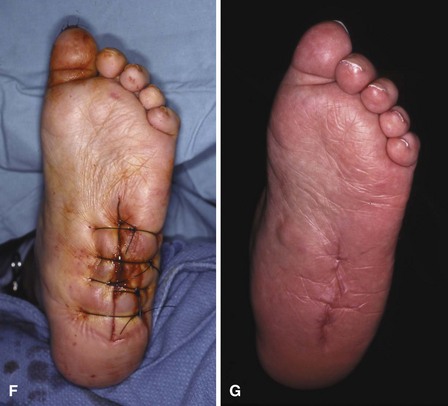
For severe infections with abscess formation, incision and drainage with thorough débridement may be required. In cases of chronic osteomyelitis that has failed to respond to intravenous antibiotics, surgical débridement may be necessary. Infected bone should be completely excised; however, every effort should be made to preserve as much bone as possible. For osteomyelitis in the toes, amputation may be required. Preservation of part of the proximal phalanx may help keep the adjacent toes from drifting (Fig. 85-12).
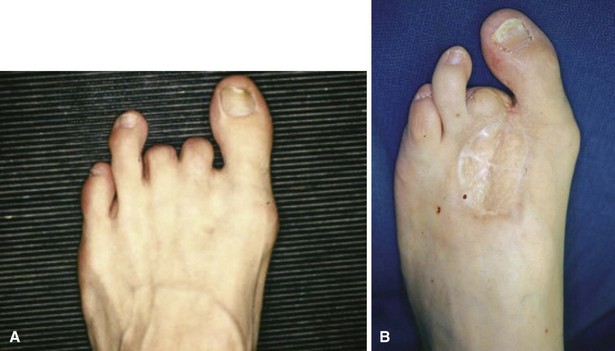
FIGURE 85-12 A and B, Drift of toes to fill defect created by amputation of toe.
(From Brodsky JW: The diabetic foot. In Coughlin MJ, Mann RA, Saltzman CL, editors: Surgery of the foot and ankle, ed 8, Philadelphia, 2007, Elsevier.)
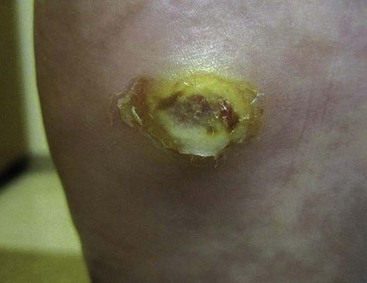
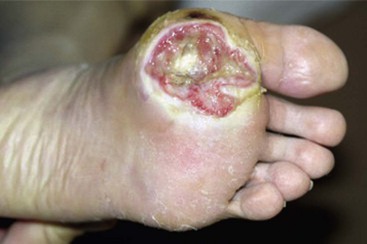
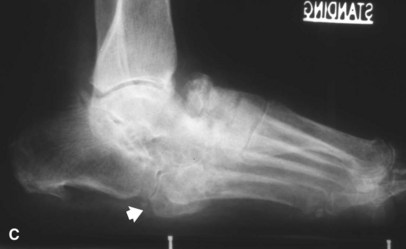
Osteomyelitis of the metatarsal heads is relatively common (Fig. 85-13) because many ulcers occur in this area.Metatarsal head resection may be indicated. If multiple metatarsal heads are involved, resection of all lesser metatarsal heads or transmetatarsal amputation may be required. Ray resection may be needed if the osteomyelitis involves more than the metatarsal head. Midfoot osteomyelitis can be treated with exostectomy if stability can be preserved. Hindfoot infections occasionally can be treated with amputation at that level, but a below-knee amputation often is more functional. Partial calcanectomy may be attempted if osteomyelitis affects the calcaneus secondary to a heel ulcer and can avoid a below-knee amputation.
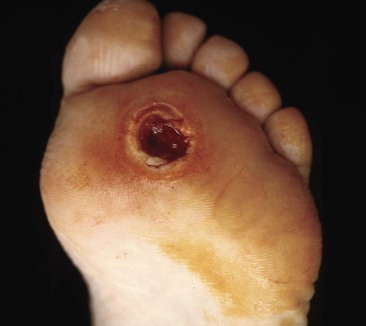
FIGURE 85-13 Grade 3 ulcer under second metatarsal head extends into metatarsal head with presumptive contiguous osteomyelitis.
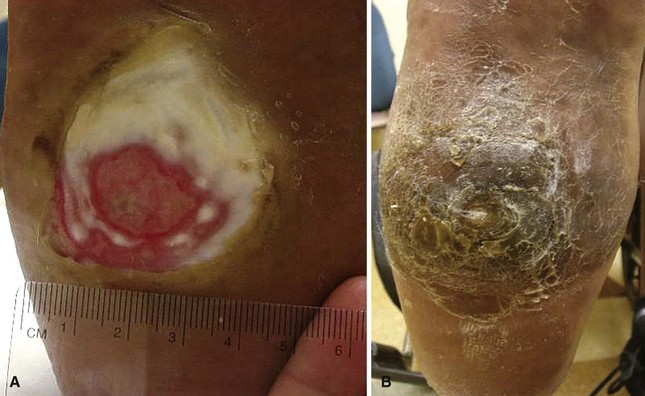
Modified resection arthroplasty after débridement of infected tissue can avoid toe amputation in patients with chronically infected ulcers and claw toe deformities (Fig. 85-14), and toe flexor tenotomies can lead to healing of ulcers at the tip of the toe if the claw toe deformity is flexible and wounds are Wagner grade 1, 2, or 3. For chronic infected ulcers under the first metatarsal head, ray resection may be avoided with resection of the first metatarsophalangeal joint and pin stabilization.
Charcot Arthropathy
Pathophysiology
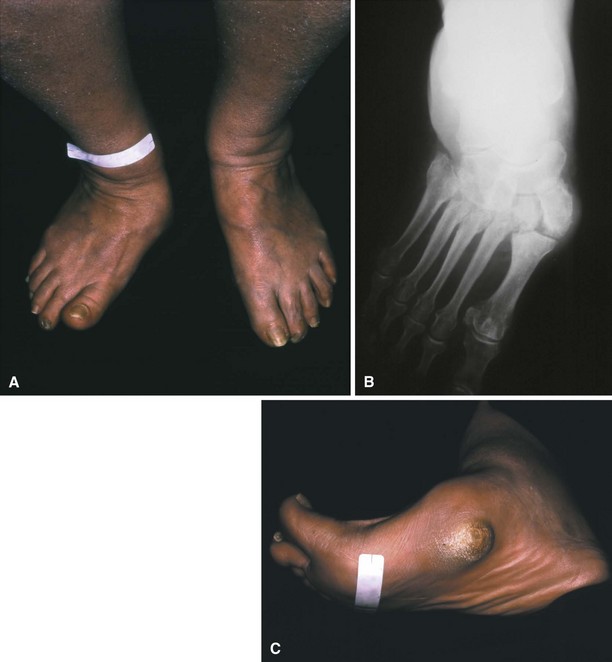
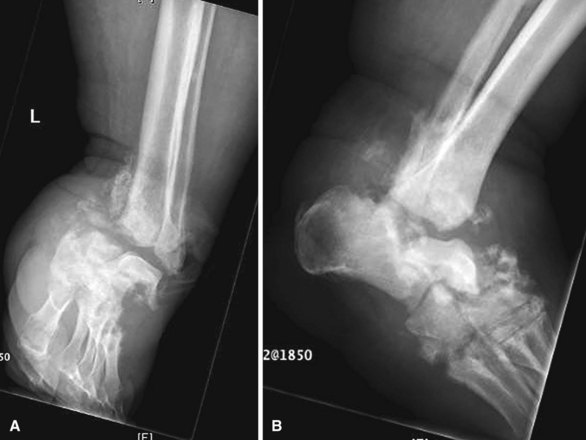
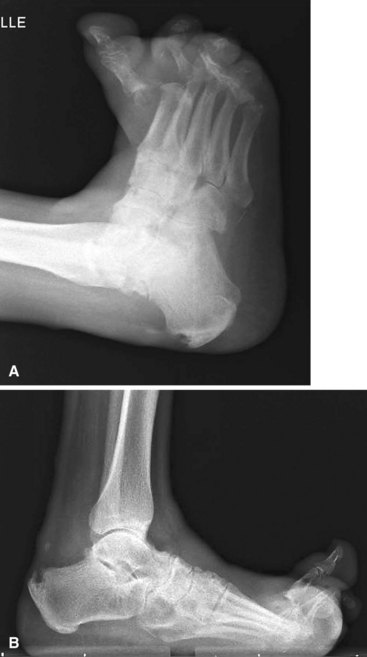
The most plausible explanation for the development of neuropathic arthropathy in a diabetic foot is loss of autonomic control of the vasculature. Resting blood flow in patients with diabetic neuropathy may be five times normal values. Arteriovenous shunting also has been documented in these feet. This high flow rate results in osteopenia, and, combined with somatosensory loss of pain and proprioception, multiple small mechanical insults unrecognized by the patient and occurring in osteopenic bone set the stage for bony dissolution and loss of structural integrity, followed by a collapse deformity (Fig. 85-15). Even with minor trauma to the foot (sprains, contusions, minor fractures), neuropathic skeletal changes may develop in a patient with diabetes mellitus.
Evaluation
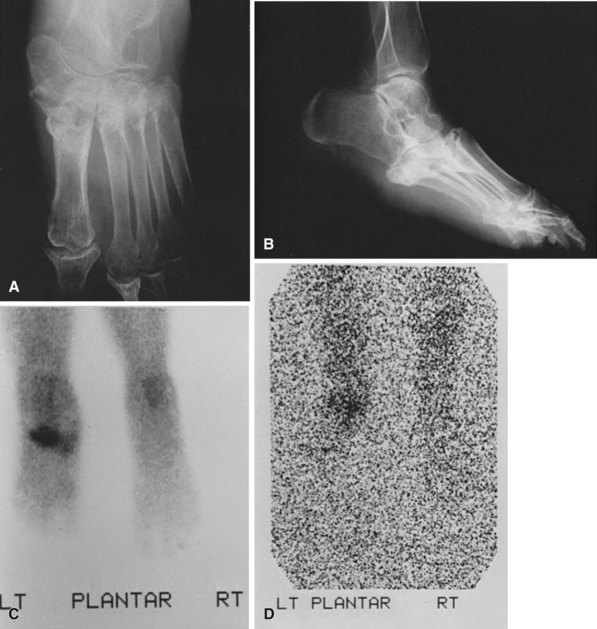
The diagnostic dilemma occurs when Charcot arthropathy is accompanied by ulceration and possible infection. Imaging studies such as plain radiographs, CT scans, and bone scans can be positive in both Charcot arthropathy and osteomyelitis (Fig. 85-16). White blood cell scans can be helpful, especially when combined with sulfur colloid bone marrow imaging. If infection is present, the tagged white cells will accumulate at the site but the sulfur colloid scan will be negative because bone marrow activity will be depressed by the infection. It does, however, take about 1 week for the sulfur colloid scan to be negative after the onset of infection. Positron emission tomography can be very sensitive and specific, but this test is not widely available. It also can be difficult to differentiate between Charcot arthropathy and infection on MRI; MRI characteristics of sinus tract, replacement of soft tissue fat, fluid collection, and extensive bone marrow abnormality may indicate infection in a patient with Charcot arthropathy. Thin rim enhancement of effusion, subchondral cysts, and intraarticular bodies suggest a lack of infection.
Classification
Stages of Charcot arthropathy as originally described by Eichenholtz with modifications are:
Stage 0: No radiographic changes, marked warmth and swelling after injury.
Stage 1: Fragmentation. Erythema, warmth and swelling of the extremity, with subluxation/dislocation of joints and bony debris and fragmentation of subchondral bone.
Stage 2: Coalescence. Decreased erythema, warmth and swelling of the extremity, with absorption of fine debris, new bone formation, and coalescence of larger fragments.
Stage 3: Consolidation. Resolution of swelling; however, residual deformity is present, with remodeling of bone seen on radiographs.
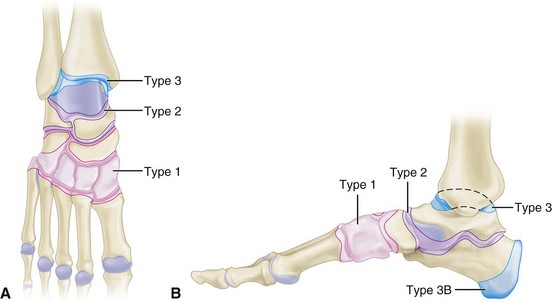
The anatomical classification as described by Brodsky with modifications includes five types (Fig. 85-17):
Type 1: Involvement of the tarsometatarsal joints. This is the most common type and often leads to the rocker-bottom deformity and plantar ulcerations secondary to bony prominences in this area.
Type 2: Involvement of the Chopart and subtalar joints. The second most common affected area, type 2 involvement also can lead to a rocker-bottom deformity with plantar flexion of the talar head. Marked varus/valgus of the hindfoot also can develop.
Type 3A: Involvement of the ankle. Marked varus or valgus usually develops, with ulceration over the prominent malleoli. This rarely can be controlled without surgery because of the degree of deformity that develops.
Type 3B: Involvement of the calcaneus. This can be an avulsion of the posterior tuberosity of the calcaneus and can lead to ulceration of the overlying skin. Another type involves joint depression, which can result in varus or valgus deformity and flattening of the talar declination angle. A third type includes fracture of the anterior process of the calcaneus, which can lead to instability of the lateral column that causes instability of the talonavicular joint.
Type 4: Involvement of multiple regions.
Type 5: Involvement of the forefoot. This is a rare form, and dislocation of the metatarsophalangeal joint can lead to ulcerations over metatarsal heads.
Treatment
Operative Treatment
Exostectomy should be done only if it does not lead to further instability (Fig. 85-18). Incisions should be made away from the ulcer and should be full thickness down to bone, avoiding undermining of the superficial soft tissue. Appropriate removal of bone should be done without affecting stability, reattaching important tendons as needed. Achilles tendon lengthening often is necessary.
Amputation may be necessary as a last resort in the presence of severe infection, severe instability, or loss of bone stock that precludes adequate fixation (Fig. 85-19). As for all other surgical procedures, adequate medical and vascular workup should be done preoperatively to maximize healing.
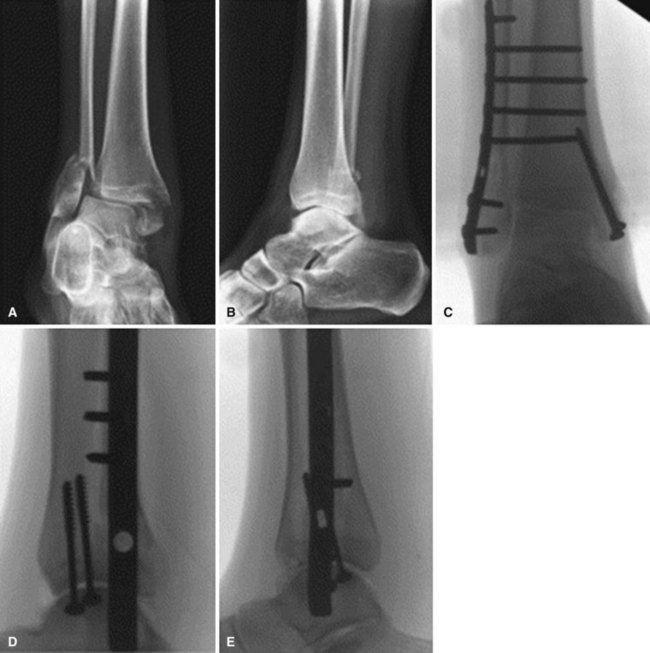
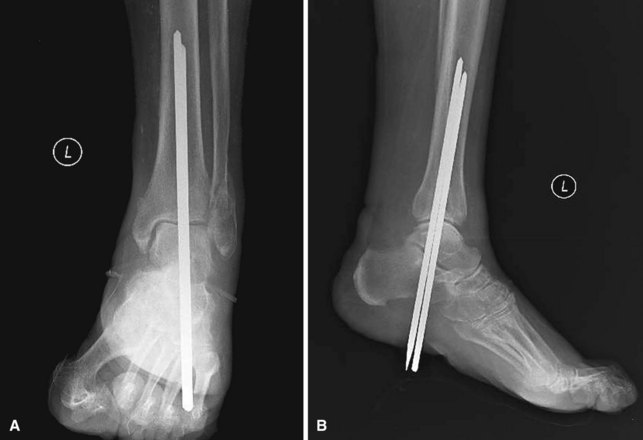
For Charcot arthropathy of the ankle, tibiotalocalcaneal arthrodesis with an intramedullary nail often is required to control the severe instability that occurs (see Chapter 11). Occasionally, talectomy may be required to correct deformity before arthrodesis. If talectomy is required, however, the postoperative complication rate is higher (Fig. 85-20).
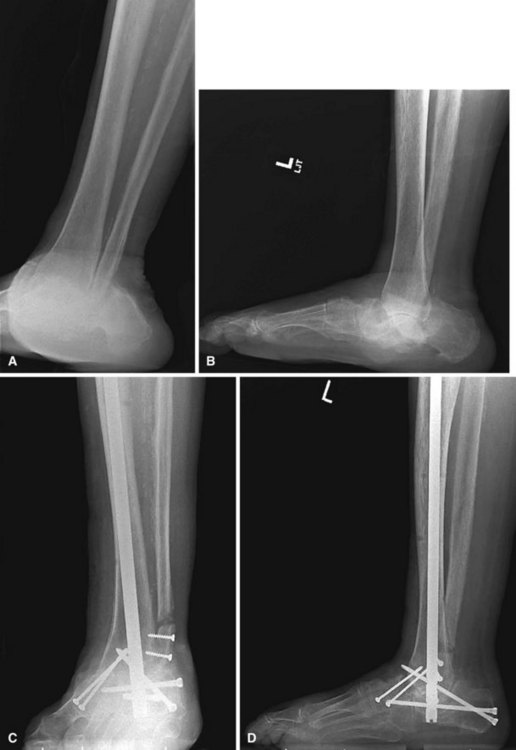
FIGURE 85-20 A and B, Charcot arthropathy. C and D, After tibiotalocalcaneal arthrodesis with intramedullary nail.
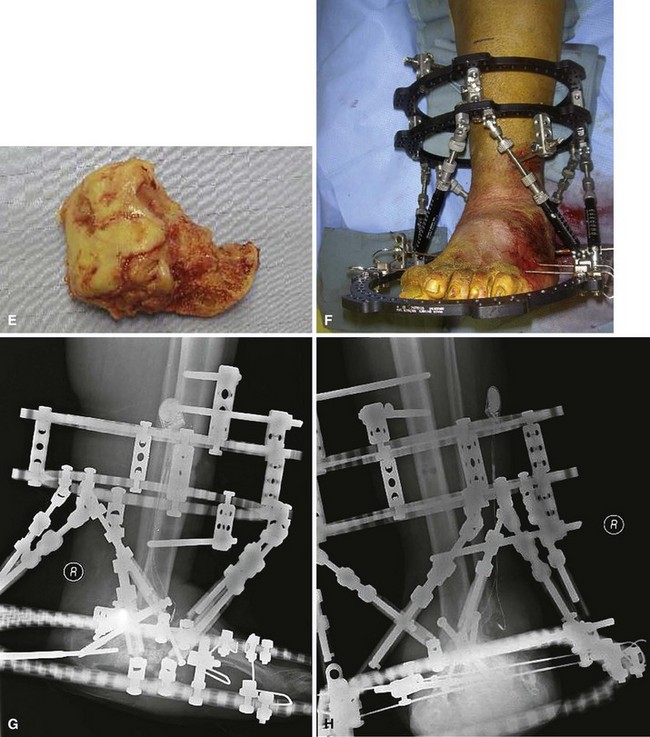
Hybrid external fixation may be attempted for patients with Charcot arthropathy of the ankle and osteomyelitis to prevent amputations. Infected tissue should be débrided, followed by arthrodesis preparation and application of the fixator (Fig. 85-21). Intravenous antibiotics should be administered, and open wounds can be treated with negative pressure wound therapy to try to avoid the need for free flap coverage.
Surgical treatment of hindfoot and midfoot arthropathy includes arthrodesis: triple arthrodesis for type 2 and midfoot fusion for type 1. Resection of wedges of bone may be necessary to obtain deformity correction, and bone grafting may be required to fill in gaps after deformity correction. Large screws (6.5 to 7.3 mm) should be used in the hindfoot, and a combination of plates and screws may be necessary in the midfoot. For the midfoot, placing a plate on the plantar surface of the bones takes advantage of the tension band principle and may provide stronger fixation (Fig. 85-22). Internal fixation for midfoot Charcot arthropathy can include a medial column screw, which is an 8.0-mm cannulated screw placed from the posterior talus into the first metatarsal (Fig. 85-23). Adjunctive fixation can be added for further stabilization. Achilles tendon lengthening is almost always needed, because most of these patients have an equinus contracture.
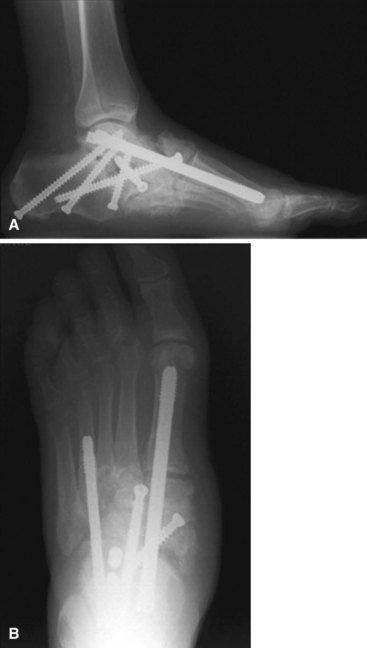
FIGURE 85-23 A and B, Midfoot arthrodesis with use of medial column screw.
(From Lusser R, Hintermann B: Midfoot arthrodesis in Charcot foot deformity with “Charcot screws.” In Pfeffer GB, Easley ME, Frey C, et al, editors: Operative techniques in foot and ankle surgery, Philadelphia, 2010, Elsevier.)
Patients at risk for complications from midfoot Charcot arthropathy include obese patients, those with severe bony deformity or a chronic ulcer with osteomyelitis, and those who are immunocompromised. Determining appropriate treatment for Charcot arthropathy of the midfoot first involves determining if the foot is plantigrade. If the foot is plantigrade, the patient can be treated with a weight-bearing total contact cast for 8 to 16 weeks, followed by commercially available extra-depth shoes and custom orthoses. If the foot is not plantigrade, surgical treatment is recommended. For a low-risk patient, this includes Achilles tendon lengthening, débridement of infected or nonviable tissue, deformity correction, and internal fixation. For poor surgical candidates, a thin-wire fixator can be used instead of internal fixation. Frames can be left on for 8 weeks, followed by total contact casting and therapeutic footwear. Platelet-rich concentrate and bone marrow aspirate can be used to augment the fusion. For forefoot Charcot arthropathy, if total contact casting has not been successful, treatment may include cheilectomy, resection arthroplasty, or arthrodesis (Fig. 85-24).
Acute Fractures
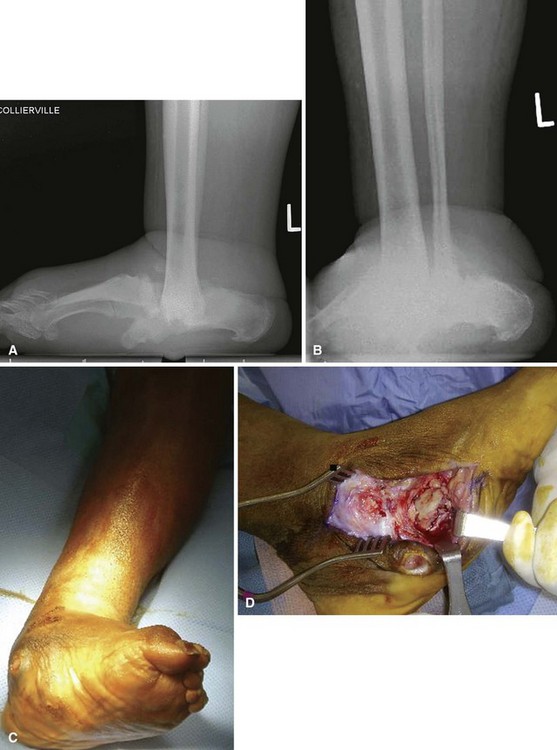
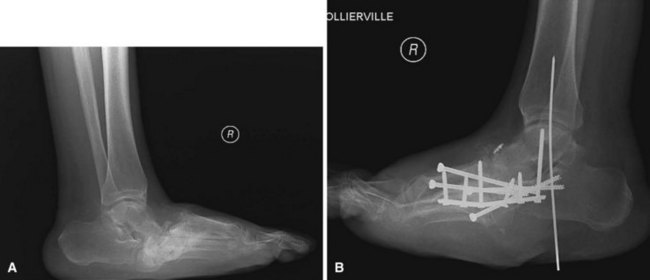
Because operative treatment has higher complication rates in diabetic patients, especially in those with comorbidities such as peripheral arterial disease and peripheral neuropathy, it is tempting to treat ankle fractures in diabetic patients without surgery. Ankle fractures in diabetic patients, however, should be treated with operative fixation similar to that done in nondiabetics, because nonoperative treatment can have significant morbidity. Stronger fixation should be used in diabetic patients, including locking plates, supplemental fixation with Kirschner wires, and augmentation of fixation with multiple syndesmotic screws or pins across the tibiotalar joint placed from the heel (Fig. 85-25). External fixation also can be used as adjunctive fixation. Percutaneous fixation may be considered if appropriate for the fracture pattern and there are concerns about wound healing (Fig. 85-26). Longer periods of immobilization are necessary, as well as an increased period of protected weight bearing. Careful blood glucose control can decrease the incidence of complications.
Abidia A, Laden G, Kuhan G, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomized-controlled trial. Eur J Vasc Endovasc Surg. 2003;25:513.
Ahmadi ME, Morrison WB, Carrino JA, et al. Neuropathic arthropathy of the foot with and without superimposed osteomyelitis: MR imaging characteristics. Radiology. 2006;238:622.
Anderson LB, DiPreta J. Charcot of the calcaneus. Foot Ankle Clin North Am. 2006;11:824.
Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 2001;24:1799.
Armstrong DG, Lavery LA, Wu S, Boulton AJM. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds. Diabetes Care. 2005;28:551.
Armstrong DG, Nguyen HC, Lavery LA, et al. Off-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care. 2001;24:1019.
Assal M, Ray A, Stern R. Realignment and extended fusion with use of a medial column screw for midfoot deformities secondary to diabetic neuropathy: surgical technique. J Bone Joint Surg. 2010;92A(Suppl 1 Pt 2):20.
Assal M, Stern R. Realignment and extended fusion with use of a medial column screw for midfoot deformities secondary to diabetic neuropathy. J Bone Joint Surg. 2009;91A:812.
Aulivola B, Craig RM. Decision making in the dysvascular lower extremity. Foot Ankle Clin North Am. 2010;15:391.
Baglioni P, Malik M, Okosieme OE. Acute Charcot foot. BMJ. 2012;344:e1397.
Batista F, Nery C, Pinzur M, et al. Achilles tendinopathy in diabetes mellitus. Foot Ankle Int. 2008;29:498.
Beam HA, Parsons JR, Lin SS. The effects of blood glucose control upon fracture healing in the BBWistar rat with diabetes mellitus. J Orthop Res. 2001;20:1210.
Besse JL, Leemrijse T, Deleu PA. Diabetic foot: the orthopedic surgery angle. Orthop Traumatol Surg Res. 2011;97:314.
Bibbo C, Lin SS, Beam HA, et al. Complications of ankle fractures in diabetic patients. Orthop Clin North Am. 2001;32:113.
Bibbo C, Patel DV. Diabetic neuropathy. Foot Ankle Clin North Am. 2006;11:753.
Blume PA, Walters J, Payne W, et al. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcer: a multicenter, randomized, controlled trial. Diabetes Care. 2008;31:631.
Bollinger M, Thordarson DB. Partial calcanectomy: an alternative to below-knee amputation. Foot Ankle Int. 2002;23:927.
Campanaro NR, Gurr JM, Murray RJ. Percutaneous bone biopsy to distinguish osteomyelitis from Charcot osteoarthropathy: two case reports. Foot Ankle Int. 2009;30:1219.
Capobianco CM, Stapleton JJ, Zgonis T. Soft tissue reconstruction pyramid in the diabetic foot. Foot Ankle Spec. 2010;3:241.
Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. Plast Reconstr Surg. 2011;127(Suppl 1):248S.
Chen CE, Ko JY, Fong CY, Juhn RJ. Treatment of diabetic foot infection with hyperbaric oxygen therapy. Foot Ankle Surg. 2010;10:91.
Costigan W, Thordarson DB, Debnath UK. Operative management of ankle fractures in patients with diabetes mellitus. Foot Ankle Int. 2007;28:32.
Crerand S, Dolan M, Laing P, et al. Diagnosis of osteomyelitis in neuropathic foot ulcers. J Bone Joint Surg. 1996;78B:51.
Cuttica DJ, Philbin TM. Surgery for diabetic foot infections. Foot Ankle Clin North Am. 2010;15:465.
Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30:1065.
Dissanayake SU, Bowling FL, Jude EB. The diabetic Charcot foot. Curr Diabetes Rev. 2012. Mar 8. [Epub ahead of print]
Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 20, 2010. CD003556
Fablia E, Caravaggi C, Clerici G, et al. Effectiveness of removable walker cast versus nonremovable fibreglass off-bearing cast in the healing of diabetic plantar foot ulcer. Diabetes Care. 2010;33:1419.
Flynn JM, Rodriguez-del Rio F, Piza PA. Closed ankle fractures in the diabetic patients. Foot Ankle Int. 2000;21:311.
Frigg A, Pagenstert G, Schafer D, et al. Recurrence and prevention of diabetic foot ulcers after total contact casting. Foot Ankle Int. 2007;28:64.
Game FL, Hinchliffe RJ, Apelqvist J, et al. A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2012;28(Suppl 1):119.
Ganesh SP, Pietrobon R, Cecilio WA, et al. The impact of diabetes on patient outcomes after ankle fracture. J Bone Joint Surg. 2005;87A:1712.
Goodridge D, Trepman E, Sloan J, et al. Quality of life of adults with unhealed and healed diabetic foot ulcers. Foot Ankle Int. 2006;27:274.
Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26:1790.
Gutekunst DJ, Hastings MK, Bohnert KL, et al. Removable cast walker boots yield greater forefoot off-loading than total contact casts. Clin Biomech (Bristol, Avon). 2011;26:649.
Guyton GP. An analysis of iatrogenic complications from the total contact cast. Foot Ankle Int. 2005;26:903.
Hastings MK, Sinacore DR, Mercer-Bolton N, et al. Precision of foot alignment measures in Charcot arthropathy. Foot Ankle Int. 2011;32:867.
Jani MM, Ricci WM, Borrelli J, Jr., et al. A protocol for treatment of unstable ankle fractures using transarticular fixation in patients with diabetes mellitus and loss of protective sensibility. Foot Ankle Int. 2003;24:838.
Johnson JE, Anderson SA. One-stage resection and pin stabilization of first metatarsophalangeal joint for chronic plantar ulcer with osteomyelitis. Foot Ankle Int. 2010;31:973.
Jones KB, Maiers-Yeldon KA, Marsh JL, et al. Ankle fractures in patients with diabetes mellitus. J Bone Joint Surg. 2005;87B:489.
Jude EB, Selby PL, Burgess J, et al. Bisphosphonates in the treatment of Charcot neuroarthropathy: a double-blind randomized controlled trial. Diabetologia. 2001;44:2032.
Karatepe O, Eken I, Acet E, et al. Vacuum assisted closure improves the quality of life in patients with diabetic foot. Acta Chir Belg. 2011;111:298.
Keegan TH, Schwartz AV, Bauer DC, et al. Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care. 2004;27:1547.
Kim BS, Choi WJ, Baek MK, et al. Limb salvage in severe diabetic foot infection. Foot Ankle Int. 2011;32:31.
Kim JY, Kim TW, Park YE, Lee YJ. Modified resection arthroplasty for infected non-healing ulcers with toe deformity in diabetic patients. Foot Ankle Int. 2008;29:493.
Kranke P, Bennett MH, Martyn-St James M, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 4, 2012. CD004123
Laborde JM. Neuropathic plantar forefoot ulcers treated with tendon lengthenings. Foot Ankle Int. 2008;29:378.
Laborde JM. Neuropathic toe ulcers treated with toe flexor tenotomies. Foot Ankle Int. 2007;28:1160.
Lavery LA, Peters EJ, Williams JR, et al. Reevaluating the way we classify the diabetic foot: restructuring the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care. 2008;31:154.
Leibner ED, Brodsky JW, Pollo FE, et al. Unloading mechanism in the total contact cast. Foot Ankle Int. 2006;27:281.
Lipsky BAS, Peters EJG, Berendt AR, et al. Specific guidelines for the treatment of diabetic foot infections 2011. Diabetes Metab Res Rev. 2012;28(Suppl 1):234.
Marks RM. Complications of foot and ankle surgery in patients with diabetes. Clin Orthop Relat Res. 2001;391:153.
Marx RC, Mizel MS. What’s new in foot and ankle surgery. J Bone Joint Surg. 2010;92A:512.
Mehta SK, Breitbart EA, Berberian WS, et al. Bone and wound healing in the diabetic patients. Foot Ankle Clin. 2010;15:411.
Mendonca DA, Cosker T, Makwana NK. Vacuum-assisted closure to aid wound healing in foot and ankle surgery. Foot Ankle Int. 2005;26:761.
Mohler ER, 3rd. Therapy insight: peripheral arterial disease and diabetes—from pathogenesis to treatment guidelines. Nat Clin Pract Cardiovasc Med. 2007;4:151.
Mueller MJ, Sinacore DR, Hastings MK, et al. Effect of Achilles tendon lengthening on neuropathic plantar ulcers: a randomized clinical trial. J Bone Joint Surg. 2003;85A:1436.
Oyibo SO, Jude EB, Tarawneh I, et al. A comparison of two diabetic foot ulcer classification systems: the Wager and the University of Texas wound classification systems. Diabetes Care. 2001;24:84.
Paola LC, Ceccacci T, Ninkovic S, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30:1065.
Perry MD, Taranow WS, Manoli A, 2nd., et al. Salvage of failed neuropathic ankle fractures: use of large-fragment fibular plating and multiple syndesmotic screws. J Surg Orthop Adv. 2005;14:85.
Pino AE, Taghva S, Chapman C, Bowker jH. Lower-limb amputations in patients with diabetes mellitus. Orthopedics. 2011;34:e885.
Pinzur MS. Neutral ring fixation for high-risk non-plantigrade Charcot midfoot deformity. Foot Ankle Int. 2007;28:961.
Pinzur MS. Use of platelet-rich concentrate and bone marrow aspirate in high-risk patients with Charcot arthropathy of the foot. Foot Ankle Int. 2009;30:124.
Pinzur MS. Diabetic peripheral neuropathy. Foot Ankle Clin. 2011;16:345.
Pinzur MS, Lio T, Posner M. Treatment of Eichenholtz stage I Charcot foot arthropathy with a weight-bearing total contact cast. Foot Ankle Int. 2006;27:324.
Pitocco D, Ruotolo V, Caputo S, et al. Six-month treatment with alendronate in acute Charcot neuroarthropathy: a randomized controlled trial. Diabetes Care. 2005;28:1214.
Rao SR, Saltzman CL, Wilken J, Yak J. Increased passive ankle stiffness and reduced dorsiflexion range of motion in individuals with diabetes mellitus. Foot Ankle Int. 2006;27:617.
Robinson AH, Pasapula C, Brodsky JW. Surgical aspects of the diabetic foot. J Bone Joint Surg. 2009;91B:1.
Romanos MT, Raspovic A, Perrin BM. The reliability of toe systolic pressure and the toe brachial index in patients with diabetes. Foot Ankle Res. 2010;3:31.
Sella EJ. Current concepts review: diagnostic imaging of the diabetic foot. Foot Ankle Int. 2009;30:568.
Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;29:510.
Simon SR, Tejwani SG, Wilson DL, et al. Arthrodesis as an early alternative to nonoperative management of Charcot arthropathy of the diabetic foot. J Bone Joint Surg. 2001;82A:939.
Stone C, Smith N. Resection arthroplasty, external fixation, and negative pressure dressing for first metatarsophalangeal joint ulcers. Foot Ankle Int. 2011;32:272.
Stone NC, Daniels TR. Midfoot and hindfoot arthrodeses in diabetic Charcot arthropathy. Can J Surg. 2000;43:449.
Strauss MB. Hyperbaric oxygen as in intervention for managing wound hypoxia: its role and usefulness in diabetic foot wounds. Foot Ankle Int. 2005;26:15.
Tamir E, Daniels TR, Finestone A, Nof M. Off-loading of hindfoot and midfoot neuropathic ulcers using a fiberglass cast with a metal stirrup. Foot Ankle Int. 2007;28:1048.
Taylor SM, Johnson BL, Samies NL, et al. Contemporary management of diabetic neuropathic foot ulceration: a study of 917 consecutively treated limbs. J Am Coll Surg. 2011;212:532.
Topping RE, Bolander ME, Balian G. Type X collagen in fracture callus and the effects of experimental diabetes. Clin Orthop Relat Res. 1994;308:220.
Trepman E, Pinzur MS, Shields NN. Application of the total contact cast. Foot Ankle Int. 2005;26:108.
van der Ven A, Chapman CB, Bowker JH. Charcot neuroarthropathy of the foot and ankle. J Am Acad Orthop Surg. 2009;17:562.
Wang CJ, Kuo YR, Wu RW, et al. Extracorporeal shockwave treatment for chronic diabetic foot ulcers. J Surg Res. 2009;152:96.
Wukich DK, Joseph A, Ryan M, et al. Outcomes of ankle fractures in patients with uncomplicated versus complicated diabetes. Foot Ankle Int. 2011;32:120.
Ahmed M, Le Quesne PM. Quantitative sweat test in diabetics with neuropathic foot lesions. J Neurol Neurosurg Psychiatry. 1986;49:1059.
Apelqvist J, Ragnarson-Tennvall G, Larsson J, et al. Long-term costs for foot ulcers in diabetic patients in a multidisciplinary setting. Foot Ankle Int. 1995;16:388.
Armstrong DG. Loss of protective sensation: a practical evidence-based definition. J Foot Ankle Surg. 1999;38:79.
Armstrong DG, Lavery LA. Elevated peak plantar pressures in patients who have Charcot arthropathy. J Bone Joint Surg. 1998;80A:365.
Armstrong DG, Stacpoole-Shea S, Nguyen H, et al. Lengthening of the Achilles tendon in diabetic patients who are at high risk for ulceration of the foot. J Bone Joint Surg. 1999;81A:535.
Armstrong DG, Todd WF, Lavery LA, et al. The natural history of acute Charcot’s arthropathy in a diabetic foot specialty clinic. Diabet Med. 1997;14:357.
Baumhauer JF, Wervey R, McWilliams J, et al. A comparison study of plantar foot pressure in a standardized shoe, total contact cast, and prefabricated pneumatic walking brace. Foot Ankle Int. 1997;18:26.
Bono JV, Roger DJ, Jacobs RL. Surgical arthrodesis of the neuropathic foot. Clin Orthop Relat Res. 1993;296:14.
Boulton AJM, Ward JD. Diabetic neuropathies and pain. Clin Endocrinol Metab. 1986;15:917.
Brindley GW, Cofield RH. Local treatment for neuropathic foot ulceration and osteoarthropathy. Foot Ankle. 1985;5:245.
Brodsky JW. Outpatient diagnosis and care of the diabetic foot. Instr Course Lect. 1993;42:121.
Brodsky JW, Rouse AM. Exostectomy for symptomatic bony prominences in diabetic Charcot feet. Clin Orthop Relat Res. 1993;296:21.
Brodsky JW, Schneidler C. Diabetic foot infections. Orthop Clin North Am. 1991;22:473.
Brown NL. Neuropathic osteoarthropathy. Orthopedics. 1987;10:1607.
Chang BB, Shah DM, Darling RC, et al. Treatment of the diabetic foot from a vascular surgeon’s viewpoint. Clin Orthop Relat Res. 1993;296:27.
Chew JT, Tan SB, Sivathasan C, et al. Vascular assessment in the neuropathic diabetic foot. Clin Orthop Relat Res. 1995;320:95.
Clohisy DR, Thompson RC. Fractures associated with neuropathic arthropathy in adults who have juvenile-onset diabetes. J Bone Joint Surg. 1988;70A:1192.
Cofield RH, Morrison MJ, Beabout JW. Diabetic neuropathy in the foot: patient characteristics and patterns of radiographic change. Foot Ankle. 1983;4:15.
Cohn BT, Brahms MA. Diabetic arthropathy of the first metatarsal cuneiform joint: introduction of a new surgical fusion technique. Orthop Rev. 1987;16:465.
Connolly JF, Csencsitz T. Limb-threatening neuropathic complications from ankle fractures in patients with diabetes. Clin Orthop Relat Res. 1998;348:212.
Cundy TF, Edmonds ME, Watkins PJ. Osteopenia and metatarsal fractures in diabetic neuropathy. Diabet Med. 1985;2:461.
Delbridge L, Appleberg M, Reeve TS. Factors associated with development of foot lesions in the diabetic. Surgery. 1983;93:78.
Delmaire M, Maugendre D, Moreno M, et al. Impaired leukocyte function in diabetic patients. Diabet Med. 1997;14:29.
Duckworth T, Boulton AJM, Betts RP, et al. Plantar pressure measurements and the prevention of ulceration in the diabetic foot. J Bone Joint Surg. 1985;67B:79.
Early JS, Hansen ST. Surgical reconstruction of the diabetic foot: a salvage approach for midfoot collapse. Foot Ankle Int. 1996;17:325.
Edmonds ME. The diabetic foot: pathophysiology and treatment. Clin Endocrinol Metab. 1986;15:889.
Edmonds ME, Clarke MB, Newton S, et al. Increased uptake of bone radiopharmaceutical in diabetic neuropathy. QJM. 1985;57:843.
Eichenholtz SN. Charcot joints. Springfield, IL: Charles C Thomas; 1966.
Ewing DJ, Clarke BF. Autonomic neuropathy: its diagnosis and prognosis. Clin Endocrinol Metab. 1986;15:855.
Gillon KRW, King RHM, Thomas PK. The pathology of diabetic neuropathy and the effects of aldose reductase inhibitors. Clin Endocrinol Metab. 1986;15:837.
Harrelson JM. Management of the diabetic foot. Orthop Clin North Am. 1989;20:605.
Harris JR, Brand PW. Patterns of disintegration of the tarsus in the anaesthetic foot. J Bone Joint Surg. 1966;48B:4.
Harvey J, Cohen MM. Technetium-99-labeled leukocytes in diagnosing diabetic osteomyelitis in the foot. J Foot Ankle Surg. 1997;36:209.
Hauser CJ, Klein SR, Mehringer CM, et al. Assessment of perfusion in the diabetic foot by regional transcutaneous oximetry. Diabetes. 1984;33:527.
Herbsma H, Powers JC, Hirschman A, et al. Retardation of fracture healing in experimental diabetes. J Surg Res. 1968;8:424.
Holewski JJ, Moss KM, Stess RM, et al. Prevalence of foot pathology and lower-extremity complications in a diabetic outpatient clinic. J Rehabil Res Dev. 1989;26:35.
Holstein P, Lassen NA. Healing of ulcers on the feet correlated with distal blood pressure measurements in occlusive arterial disease. Acta Orthop Scand. 1980;51:995.
Horibe S, Tada K, Nagano J. Neuroarthropathy of the foot in leprosy. J Bone Joint Surg. 1988;70B:481.
Hosch J, Quiroga C, Bosma J, et al. Outcomes of transmetatarsal amputations in patients with diabetes mellitus. J Foot Ankle Surg. 1997;36:430.
Jacobs RL, Karmody A. Charcot foot. In: Jahss, MH, ed. Disorders of the foot. Philadelphia: Saunders, 1988.
Johnson JE. Operative treatment of neuropathic arthropathy of the foot and ankle. J Bone Joint Surg. 1998;80A:1700.
Johnson JE, Kennedy EJ, Shereff MJ, et al. Prospective study of bone, indium-111-labeled white blood cell, and gallium-67 scanning for the evaluation of osteomyelitis in the diabetic foot. Foot Ankle Int. 1996;17:10.
Kim EE, Pjura GA, Lowry PA, et al. Osteomyelitis complicating fracture: pitfalls of 111In leukocyte scintigraphy. AJR Am J Roentgenol. 1987;148:927.
Koval KJ, Petraco DM, Kummer FJ, et al. A new technique for complex fibula fracture fixation in the elderly: a clinical and biomechanical evaluation. J Orthop Trauma. 1997;11:28.
Kristiansen B. Ankle and foot fractures in diabetics provoking neuropathic joint changes. Acta Orthop Scand. 1980;51:975.
Kuck EM, Bouter KP, Hoekstra JB, et al. Tissue concentrations after a single-dose, orally administered ofloxacin in patient with diabetic foot infections. Foot Ankle Int. 1998;19:38.
Kumagi SG, Mahoney CR, Fitzgibbons TC. Treatment of diabetic (neuropathic) foot ulcers with two-stage debridement and closure. Foot Ankle Int. 1998;19:160.
Larsson J, Agardh CD, Apelqvist J, et al. Clinical characteristics in relation to final amputation level in diabetic patients with foot ulcers: a prospective study of healing below or above the ankle in 187 patients. Foot Ankle. 1995;16:69.
Larsson J, Agardh CD, Apelqvist J, et al. Long-term prognosis after healed amputation in patients with diabetes. Clin Orthop Relat Res. 1998;350:149.
Lee BY, McCann WJ, Jr., DelGuercio LRM, et al. Optimal management of perforating ulcers of the foot. Contemp Orthop. 1988;17:41.
Lesko P, Maurer RC. Talonavicular dislocations and midfoot arthropathy in neuropathic diabetic feet: natural course and principles of treatment. Clin Orthop Relat Res. 1989;240:226.
Leventen EO. Charcot foot: a technique for treatment of chronic plantar ulcer by saucerization and primary closure. Foot Ankle. 1986;6:295.
Levin ME, O’Neal LW. The diabetic foot, ed 4. St. Louis: Mosby; 1988.
Levine SE, Neagle CE, Esterhai JL, et al. Magnetic resonance imaging for the diagnosis of osteomyelitis in the diabetic patient with a foot ulcer. Foot Ankle. 1994;15:151.
Lin SS, Lee TH, Wapner KL. Plantar forefoot ulceration with equinus deformity of the ankle in diabetic patients: the effect of tendo-Achilles lengthening and total contact casting. Orthopedics. 1996;19:465.
Loder RT. The influence of diabetes mellitus on the healing of closed fractures. Clin Orthop Relat Res. 1988;232:210.
LoGerfo FW, Coffman JD. Vascular and microvascular disease of the foot in diabetes: implications for foot care. N Engl J Med. 1984;311:1615.
Macey LR, Kana SM, Jingushi S, et al. Defects of early fracture-healing in experimental diabetes. J Bone Joint Surg. 1989;71A:722.
Marks RM, Parks BG, Schon LC. Midfoot fusion technique for neuroarthropathic feet: biomechanical analysis and rationale. Foot Ankle. 1998;19:507.
Masson EA, Hay EM, Stockley I, et al. Abnormal foot pressures alone may cause ulceration. Diabet Med. 1989;6:426.
McCormack RG, Leith JM. Ankle fractures in diabetics: complications of surgical management. J Bone Joint Surg. 1998;80B:689.
McDermott JE. The diabetic foot: diagnosis and prevention. Instr Course Lect. 1993;42:117.
McDermott JE. The diabetic foot: evolving technologies. Instr Course Lect. 1993;42:169.
Mehta SK, Breitbart EA, Berberian WS, et al. Bone and wound healing in the diabetic patients. Foot Ankle Clin. 2010;15:411.
Mizel MS. Diabetic foot infections. Orthop Rev. 1989;18:572.
Myerson MS, Henderson MR, Saxby T, et al. Management of the midfoot diabetic neuroarthropathy. Foot Ankle. 1994;15:233.
O’Connor BL, Palmoski MJ, Brandt KD. Neurogenic acceleration of degenerative joint lesions. J Bone Joint Surg. 1985;67A:562.
Papa J, Myerson M, Girard P. Salvage, with arthrodesis, in intractable diabetic neuropathic arthropathy of the foot and ankle. J Bone Joint Surg. 1993;75A:1056.
Parkhouse N, Le Quesne PM. Impaired neurogenic vascular response in patients with diabetes and neuropathic foot lesions. N Engl J Med. 1988;318:1306.
Perry JE, Ulbrecht JS, Derr JA, et al. The use of running shoes to reduce plantar pressures in patients who have diabetes. J Bone Joint Surg. 1995;77A:1819.
Pinzur MS. Benchmark analysis of diabetic patients with neuropathic (Charcot) foot deformity. Foot Ankle Int. 1999;20:564.
Pinzur MS, Kelikian A. Charcot ankle fusion with a retrograde locked intramedullary nail. Foot Ankle Int. 1997;18:699.
Pinzur MS, Shields NN, Goelitz B, et al. American Orthopaedic Foot and Ankle Society shoe survey of diabetic patients. Foot Ankle Int. 1999;20:703.
Pinzur MS, Slovenkai MP, Trepman E. Guidelines for diabetic foot care. The Diabetes Committee of the American Orthopaedic Foot and Ankle Society. Foot Ankle Int. 1999;20:695.
Pinzur MS, Smith D, Osterman H. Syme ankle disarticulation in peripheral vascular disease and diabetic foot infection: the one-stage versus two-stage procedure. Foot Ankle. 1995;16:124.
Pinzur MS, Stuck R, Sage R, et al. Benchmark analysis on diabetics at high risk for lower-extremity amputation. Foot Ankle Int. 1996;17:695.
Pittet D, Wyssa B, Herter-Clavel C, et al. Outcome of diabetic foot infections treated conservatively: a retrospective cohort study with long-term follow-up. Arch Intern Med. 1999;159:851.
Prieskorn D. The insensate foot. In: Mizel, MS, Miller, RA, Scioli, MW. Orthopaedic knowledge update, foot and ankle 2. Chicago: American Academy of Orthopaedic Surgeons, 1998.
Ray D, Nimityongskul P, Anderson LD. Percutaneous intramedullary fixation of lateral malleolus fractures: technique and report of early results. J Trauma. 1994;36:669.
Resnick D. Neuroarthropathy. In: Resnick, D, Niwayama, G. Diagnosis of bone and joint disorders. Philadelphia: Saunders, 1988.
Ross R. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis. 1981;1:293.
Sammarco GJ, Conti SF. Surgical treatment of neuroarthropathic foot deformity. Foot Ankle Int. 1998;19:102.
Santori FS, Ghera S, Sadeh H, et al. Diabetic neuroarthropathy of the foot. Ital J Orthop Traumatol. 1984;10:411.
Sapico FL, Canawati HN, White JL, et al. Quantitative aerobic and anaerobic bacteriology of infected diabetic feet. J Clin Microbiol. 1980;12:413.
Schauwecker DS, Park HM, Burt RW, et al. Combined bone scintigraphy and indium 111 leukocyte scans in neuropathic foot disease. J Nucl Med. 1988;29:1651.
Schon LE, Easley ME, Weinfeld SB. Charcot neuroarthropathy of the foot and ankle. Clin Orthop Relat Res. 1998;349:116.
Schon LE, Marks RM. The management of neuroarthropathic fracture-dislocations in the diabetic patient. Orthop Clin North Am. 1995;26:375.
Seabold JE, Flickinger FW, Kao SCS, et al. Indium-111-leukocyte/technetium-99m-MDP bone and magnetic resonance imaging: difficulty of diagnosing osteomyelitis in patients with neuropathic osteoarthropathy. J Nucl Med. 1990;31:549.
Sinacore DR. Acute Charcot arthropathy in patients with diabetes mellitus: healing times by foot location. J Diabetes Complications. 1998;12:287.
Smith DG, Barnes BC, Sands AK, et al. Prevalence of radiographic foot abnormalities in patients with diabetes. Foot Ankle Int. 1997;18:342.
Smith DG, Boyko EJ, Ahroni JH, et al. Paradoxical transcutaneous oxygen response to cutaneous warming on the plantar foot surface: a caution for interpretation of plantar foot TC Po2 measurements. Foot Ankle Int. 1995;16:787.
Smith DG, Stuck RM, Ketner L, et al. Partial calcanectomy for the treatment of large ulcerations of the heel and calcaneal osteomyelitis: an amputation of the back of the foot. J Bone Joint Surg. 1992;74A:571.
Spanheimer RG. Direct inhibition of collagen production in vitro by diabetic rat serum. Metabolism. 1988;37:479.
Spanheimer RG. Correlation between decreased collagen production in diabetic animals and in cells exposed to diabetic serum: response to insulin. Matrix. 1992;12:101.
Spanheimer RG, Umpierez GE, Stump FV. Decreased collagen production in diabetic rats. Diabetes. 1988;37:371.
Stanwood W, Pinzur MS. Risk of contamination of the wound in a hydrotherapeutic tank. Foot Ankle Int. 1998;19:173.
Stemmer EA. Vascular complications of diabetes mellitus. In: Moore, WS, ed. Vascular surgery. New York: Grune & Stratton, 1983.
Stokes IAF, Faris IB, Hutton W. The neuropathic ulcer and loads on the foot in diabetic patients. Acta Orthop Scand. 1975;46:839.
Tenebaum MM, Rayfield E, Junior J, et al. Altered pressure-flow relationship in the diabetic foot. J Surg Res. 1981;31:307.
Thompson RC, Jr., Clohisy DR. Deformity following fracture in diabetic neuropathic osteoarthropathy: operative management of adults who have type-I diabetes. J Bone Joint Surg. 1993;75A:1765.
Tisdel CL, Marcus RE, Heiple KG. Triple arthrodesis for diabetic peritalar neuroarthropathy. Foot Ankle Int. 1995;16:332.
Van Nostrand D, Abreu SH, Callaghan JJ, et al. In-111-labeled white blood cell uptake in noninfected closed fracture in humans: prospective study. Radiology. 1988;167:495.
Veves A, Murray JH, Young MJ, Boulton AJM. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetes. 1992;35:660.
Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1982;2:64.
Wagner FW, Jr. The diabetic foot. Orthopedics. 1987;10:163.
Weinstein D, Wang A, Chambers R, et al. Evaluation of magnetic resonance imaging in the diagnosis of osteomyelitis in diabetic foot infections. Foot Ankle. 1993;14:18.
Wray JB, Stunkle E. The effect of experimental diabetes upon the breaking strength of the healing fracture in the rat. J Surg Res. 1965;5:479.
Wright DG. Diabetic foot disease. Foot Ankle. 1995;16:105.
Yue JJ, Marcus RE. The role of internal fixation in the treatment of Jones fractures in diabetics. Foot Ankle Int. 1996;17:559.
Yuh WTC, Corson JD, Baraniewski HM, et al. Osteomyelitis of the foot in diabetic patients: evaluation with plain film, 99mTc-MDP bone scintigraphy, and MR imaging. AJR Am J Roentgenol. 1989;152:795.