Chapter 16 Diabetes type 2
AETIOLOGY
Diabetes type 2, in contrast to type 1, is a lifestyle disease. It has become a public health burden worldwide, leading to premature morbidity and mortality. Abdominal obesity with deranged glucose tolerance (where glucose tissue uptake is impaired) leads to insulin resistance, hyperinsulinaemia, diabetes type 2 and cardiovascular abnormalities such as hypertension, hypertriglyceridaemia, low high-density lipoproteins (HDL), microvascular lesions and atherosclerosis.1,2 It is also a risk factor for polycystic ovarian syndrome, sleep apnoea and some hormone-sensitive cancers.3 It has been aptly called the ‘hyperactive fork and hypoactive foot’, a ‘deadly duet’.4
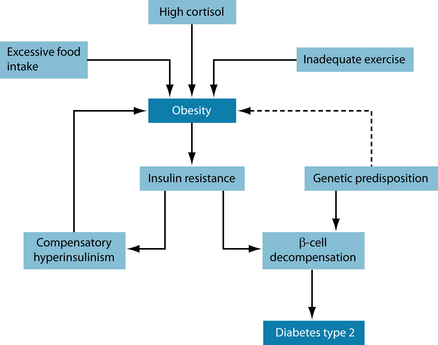
Two commonly encountered mechanisms seem to underlie the development of insulin resistance, leading to metabolic syndrome: high cortisol levels and adiposity. Increases in serum cortisol, such as is seen in stress, lead to gluconeogenesis (often derived from protein, leading to progressive muscle wasting) and therefore increased glucose levels in blood.5,6 The interconnection of cortisol and glucose could thus be the cause for the progressive nature of the disease.7 Up-regulation of cortisol due to stress (see also Chapter 15 on adrenal exhaustion) increases glucose and very low density lipoproteins VLDL secretion from the liver while inhibiting their reuptake, thus promoting hyperinsulinaemia, insulin resistance and storage of energy in the form of visceral fat.8,9 Disturbances in the hypothalamic–pituitary–adrenal (HPA) axis not only increase cortisol levels but also enhance immune activation.10 These disturbances exert their influence on other steroid hormones. Assaying these may give a better understanding of underlying wider-ranging pathology; it may also give clues as to their possible treatment.11
Increased inflammatory markers have been noted, linking the signs and symptoms of metabolic syndrome to an inflammatory process.12 One study10 hypothesises that the pathoaetiology of metabolic syndrome results from pro-inflammatory cytokines (IL-1, IL-6, TNF-α), due to inflammatory processes or emotional stress, enhancing sympathetic nervous system (SNS) activity, leading to obesity from enhanced feeding activity and increased leptin levels due to neuropeptide Y. A further indicator of inflammation in insulin resistance is an elevation in C-reactive protein (CRP).13 Genetic links have also been suggested, with glucocorticoid receptor polymorphism.14 Research echoes all of the above.15
Chronic elevations in blood glucose result in glycosylation of red blood cells and other biological substances, leading to oxidative tissue damage. It is suggested that this is the underlying mechanism that results in the common, late complications of diabetes, such as microvascular damage in blood vessels, eyes and kidneys.16,17 It has been suggested that oxidative stress could also be the cause, not just the result, of this syndrome.18
High dietary saturated fat intake, low omega-3 fatty acids and other polyunsaturated fatty acids, alcohol consumption, sedentary lifestyle and mental stress are all promoters of metabolic syndrome. Certain personality types have enhanced sympathetic nervous system activity, with increased secretion of catecholamines, cortisol and serotonin, which all appear to be involved in the pathogenesis of metabolic syndrome.19,20 It is estimated that approximately 40% of Europeans above the age of 60 years have metabolic syndrome, with rates in the US population climbing to approximately two-thirds.1,20 Figure 16.1 shows the interdependency of the variables described in the text above.21
Metabolism of glucose uptake into the cells and the role of insulin
In most tissues, except hepatic tissue, glucose relies on a transport system called GLUT4 to be able to enter the cell. This system requires insulin for optimal uptake of glucose by muscle and adipose tissue, where insulin enhances the translocation of GLUT4 from the intracellular pool to the cell membrane.22 The putative metabolic cause for diabetes type 2 lies in suboptimal functioning of these glucose transporters due to insulin resistance.21
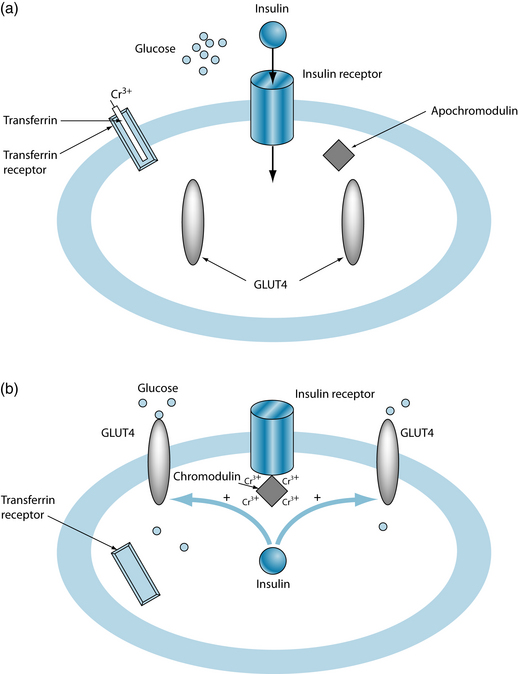
It has been found that insulin is more effective in the presence of chromium. The mechanism is not entirely elucidated, but it is suggested that chromium (Cr3+), bound to transferrin, enters the cells via transferrin receptors. Once inside the cell, chromium is released and four chromium ions bind with apochromodulin, forming holochromodulin (often simply called ‘chromodulin’). Chromodulin then binds to the insulin receptor, increasing receptor activity.21 Figures 16.2a and 16.2b illustrate the initial phase of insulin uptake (Figure 16.2a) and the resultant uptake of glucose into the cell (Figure 16.2b), and the role of chromodulin.
Testing for diabetes type 2 and metabolic syndrome
In-house testing comprises blood glucose, urinalysis (dipstick), blood pressure and anthropometric measurements. The latter includes body mass index (BMI∗), waist circumference† and body composition measured through skinfold thickness or bioimpedance.2
Metabolic syndrome is confirmed when there are abnormalities in three or more of the following:
Since multiple parameters are involved in diabetes type 2 and metabolic syndrome the combined results of in-house and pathology testing can give conclusive evidence. Table 16.1 shows information on each of the laboratory tests involved.
Table 16.1 Pathology tests recommended for suspected diabetes type 2 and metabolic syndrome23,24
| TEST | TISSUE | COMMENTS |
|---|---|---|
| Fasting glucose | Serum | Values > 6 mmol/L are indicative of diabetes type 2 or metabolic syndrome. |
| Fasting insulin | Serum | Normal values are 4–10 mU/L. |
| GTT (glucose tolerance test) | Serum | Commonly, the test is done over 2 hours, with blood samples taken pre-glucose loading and 2 hours after. Values > 7.8 mmol/L post-loading are indicative of DM2. However, this test will not show insulin and cortisol levels, nor the finer details of glucose control. Hence a 3-hour test with half-hourly measurements of glucose, insulin and cortisol is recommended. Insulin should not rise more than eightfold after glucose loading. |
| Glycosylated haemoglobin (HbA1c) | Serum |
Cortisol
Cortisol is subject to diurnal variations, being highest in the morning and lowest at night. The best time for measuring cortisol levels in serum are early morning and again around 4 p.m. Night-time or 24-hour urinary cortisol, or repeat salivary samples, may be even more advantageous.25
Lipid studiesSerumTriglycerides > 1.81 and 1.52 mmol/L and HDL < 0.75 and 0.91 in males and females, respectively, are indicative of diabetes type 2 or metabolic syndrome.Liver profileSerumElevations in alanine aminotransferase (ALT) have been found in insulin resistance and metabolic syndrome.26CRP, ESR (inflammatory markers)SerumThe ideal range of CRP and ESR is < 1. Elevation, even if still within the normal range, is indicative of inflammation, with the level of these markers proportional of the degree of inflammation.HomocysteineSerumHomocysteine is an inflammatory amino acid produced in the methylation cycle. It is commonly elevated in metabolic and heart diseases.27 Ideal range is < 10.Active B12SerumActive B12 or holotranscobalamin has been termed the best and earliest indicator of impending vitamin B12 deficiency.28 It needs to be > 35 pmol/L.FolateRBCFolate and vitamin B12 are both needed in the homocysteine-methionine (methylation) cycle. Values differ markedly between laboratories.Vitamin DSerumDespite the high amount of sunshine, vitamin D deficiency is high in Australia. It is needed for insulin secretion and found to be low in people not receiving much sunlight on their skin.29 Both the inactive (25-hydroxyvitamin D) and the active (1,25-dihydroxyvitamin D) forms can be measured. Ideal values for vitamins are in the upper half of the reference range.TSHSerumHypothyroidism is present when thyroid-stimulating hormone is elevated. It may explain some people’s inability to lose weight. However, subtle elevations (> 2.5 mIU/L) within the reference range (0.4–5.0 mIU/L) could indicate a ‘labouring’ thyroid or a pre-hypothyroid state.30,31
RISK FACTORS
Stress, a diet high in refined and simple carbohydrates and human-modulated fats, and low in fibre, antioxidants and polyunsaturated fatty acids, especially omega-3, as well as lack of exercise, are the most outstanding contributors to obesity and metabolic syndrome.
This disease process brings with it a number of other risk factors, notably in cardiovascular health. Hypertension and dyslipidaemia are commonly encountered and in fact are part of metabolic syndrome. The derangement of steroid hormones, including sex hormones and dehydroepiandrosterone (see Figure 15.3 in Chapter 15 on adrenal exhaustion) can lead to lack of libido, erectile dysfunction and prostate problems in men, and menstrual irregularities, infertility and exacerbation of hormonal symptoms in females.
Due to the reduced life quality that accompanies any chronic health problem and possibly the decreased feelings of self-worth from being overweight, depression and anxiety should also be considered. Depression may also play a role in the aetiology of stress and metabolic syndrome (see also Chapter 12 on depression).
CONVENTIONAL TREATMENT
It is now commonly accepted that diet and lifestyle need to be considered in order to control this disease.30 A number of diets have been recommended, with no clear answers as yet (see the discussion below).
In addition, medical treatment aims at regulating blood sugar to gain control of diabetes type 2. The drugs mentioned here are suitable only if there is some function left in the pancreatic β-cells; otherwise insulin needs to be given.
In the first instance, biguanides (such as metformin) will be prescribed. The supposed mechanisms include reduced gluconeogenesis and increased insulin sensitivity. A possible side effect of this drug is pernicious anaemia. Therefore, vitamin B12 should be measured regularly and, if found to decline or be low, vitamin B12 treatment via monthly intramuscular injections should be initiated.33
Sulfonylureas are recommended to enhance the secretion of insulin in response to blood glucose. However, care needs to be taken in patients with liver or kidney disease. They also promote weight gain and are therefore not ideal in metabolic syndrome.
Another treatment option is α-glucosidase inhibitors. This class of drugs reduces the breakdown of carbohydrates in the intestines by inhibiting the enzymes involved in this process, thus reducing the rise in blood sugar levels following a meal. They are therefore useful in obesity. However, the remaining carbohydrates are likely to be fermented in the gastrointestinal tract, leading to unwelcome side effects such as bloating and flatulence.
The glitazones (thiazolidinediones) reduce insulin, but have no direct effect on glucose. Their beneficial action in diabetes type 2 may be the lowering of free fatty acids, thus increasing muscular glucose usage.
Various combinations of these drugs, notably metformin with sulfonylureas, are currently used, particularly when good glucose control (as assessed by HbA1c levels) is not achieved by one drug alone. Only as a last resort will insulin be used for diabetes type 2.
Cardiovascular risk factors are commonly controlled by blood pressure and lipid-lowering medication (see Section 3 on the cardiovascular system).
KEY TREATMENT PROTOCOLS
This needs to be a multi-pronged approach, including stress management, dietary and lifestyle changes, exercise, and nutritional and herbal supplements.
Modify lifestyle and diet
Fat loss, even if it is small, is paramount and has resulted in greater insulin sensitivity, lower blood pressure and improved lipid profile, thus reducing risk factors for both diabetes type 2 and heart disease.1 A hypocaloric diet rather than a total fast has been advocated, together with physical exercise and reduction in alcohol consumption. These lifestyle changes, although superior to drug treatment, are sometimes difficult to achieve and an extensive health-care program needs to be initiated to prevent relapse.20 A slow reduction in weight seems to be better to rapid weight-loss diets for lasting success as the weight is more likely to stay off. Protein needs to be emphasised as there is often a reduction in lean body mass due to the gluconeogenic effects of raised cortisol.
There has been much debate regarding the best diet for this condition: high complex-carbohydrate/low-fat (HC/LF) or high-protein/low-carbohydrate diet (HP/LC), or anything in between.44,45 It is feared that the HP/LC diet may be too high in saturated fats due to its high meat content and hence contribute to increased calorie consumption. Although weight loss after 1 year seems to be the same on either diet, the HC/LF dieters tends to have a better LDL profile. However, the HP/LC diet has led to greater fat loss and improved triglyceride and HDL values.44,46
Further, there is evidence that animal fat (in dairy and meat), particularly conjugated linoleic acid, can have a protective effect against obesity, diabetes, heart disease and cancer.47–50 With the low-fat diet recommendations, apparently people now consume less than half the amount of conjugated linoleic acid needed to reap the benefits.51 And the beneficial effects of fish consumption, especially those with high omega-3 content, are well known. Another advantage of a low-carbohydrate diet lies in its reduced insulin requirements.
Care needs to be taken, too, with low-fat products, as the fat is often replaced with carbohydrates such as olestra or a similar ‘fat substitute’. Believing that this is a low-fat (healthy) food, people may tend to eat more, thus making up the calories. Besides, these
‘Diabetic foot’ is the umbrella term for the numerous foot problems in diabetics that arise from arterial complications and peripheral neuropathy. This combination can lead in turn to poor circulation; poor wound healing and eventual ulcer development. Therapies such as corrective footwear, diabetic socks and regular podiatrist review are encouraged, as well as hyperbaric oxygen and various wound management techniques when or if ulceration does occur. However, one of the best patient recommendations may simply be that of increased vigilance—studies suggest that increasing awareness of the issue is effective in reducing serious lesions. Simple tips such as testing shoe tightness with fingers to reduce pressure—which many patients may not be aware of, due to neuropathy—may stave off serious issues in the future. Improving circulation may also reduce the possibility of ulcer formation by improving wound-healing ability. Considering that 10–15% of diabetic patients can expect complications, that over half of diabetic hospital admissions are related to foot complications and that diabetes is the most common reason for medical amputation, these recommendations need to be an integral part of diabetic patient management.
fat replacers also have calories. Carroll claims that this substitution has contributed markedly to the current obesity epidemic.52 In addition, olestra has been shown to reduce the absorption of fat-soluble vitamins, thus depriving the body further of essential nutrients.53
In order to achieve a feeling of satiety without overindulging in calorie-rich foods, fibre needs to be an essential dietary constituent of every person attempting to lose weight.45 Fibre will bind fats and lower the absorption of glucose through delaying gastric emptying. Studies with psyllium hulls (Plantago psyllium) have shown significant reductions in fasting plasma glucose, cholesterol, LDL and triglycerides, and elevations in HDL.54 However, care needs to be taken as too much of it will reduce nutrient absorption and can lead to flatulence.
Increase exercise
The positive influence of exercise on glucose control cannot be overemphasised,20 as a plethora of literature on this topic testifies. It facilitates the uptake of glucose into the cells and lowers insulin resistance, apart from the beneficial effect on body composition. Even small daily tasks, such as getting up to change the TV channel or taking the stairs rather than the lift, will be beneficial.55
In insulin resistance and diabetes type 2, high levels of fatty acids are stored in skeletal muscle, with exercise being able to reduce this through β-oxidation (fat burning), thus lowering triglycerides and improving glucose control.56 For people with impaired fasting glucose, before the development of diabetes type 2, even greater benefits can be achieved, as seen in improvements in glycolysis, aerobic metabolism, β-oxidation and mitochondrial biogenesis.57
In particular, strength training maximises fat loss and minimises the loss of lean body mass which usually accompanies weight loss diets.2 Sarcopenia is common in diabetics, particularly in the elderly.58 Since it is the muscles that use glucose to produce energy, lack of muscle mass further promotes the disease process.
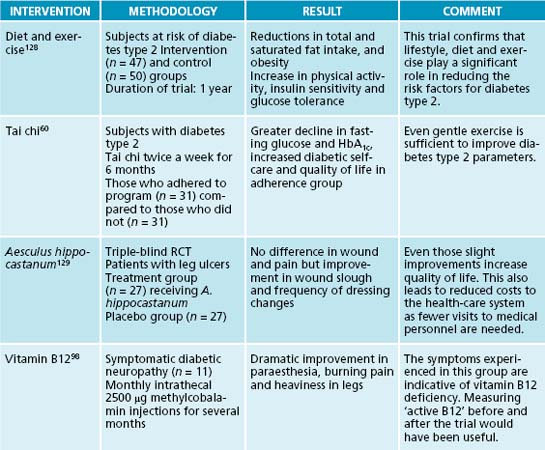
Tai chi has shown to significantly improve glycaemic control and lower triglyceride levels in Chinese diabetic women.59 In a 6-month trial in Korea those people adhering to the activity schedule experienced much better glucose control and quality of life.60
As exercise usually has a glucose-lowering effect, blood glucose needs to be monitored carefully regarding possible hypoglycaemia, and medication may need to be adjusted (see also Chapter 19 on Endometriosis).58
Increase insulin release and receptor sensitivity, and reduce hyperinsulinaemia
The precursor to diabetes type 2 is insulin resistance and compensatory hyperinsulinaemia. Although insulin is overproduced and secreted by the pancreas it is not facilitating glucose uptake into the cell. Fat loss results in increased insulin sensitivity and should be pursued.61 Certain nutrients and herbs can aid insulin sensitivity, thus reducing hyperinsulinaemia.
Nutritional interventions
A whole range of vitamins, minerals, amino acids and bioactive substances have been explored in the treatment of diabetes type 2 and insulin resistance. Nutrients which have shown a positive effect include vitamins A, B1, B3 and biotin, the minerals calcium, potassium, zinc and manganese and fructo-oligosaccharides. Some amino acids, such as taurine (needed for liver detoxification pathways and control of heart rhythm and blood pressure) and L-arginine (for nitric oxide production to maintain endothelial function) may also help.3,4,21,38,45 However, there are nutrients that are very specific for diabetes type 2, as demonstrated below.
Most animals produce their own vitamin C from glucose. The two molecules are therefore structurally similar, and competition for cellular uptake between vitamin C and glucose has been suggested as a possible mechanism by which this vitamin exerts its positive influence on glucose metabolism. Further, vitamin C is needed to stimulate the release of insulin following glucose ingestion. As little as 500 mg/day has been shown to be effective. Vitamin C also acts as an antioxidant, reduces blood pressure and protects blood vessels.4
Chromium is needed to facilitate the entry of glucose into the cells by stimulating insulin uptake and enhancing its activity (Figure 16.2).21 Supplementation has had a beneficial effect on metabolic syndrome by lowering fasting blood glucose (FBG), postprandial glucose (PPG) and corresponding insulin levels, and by improving insulin sensitivity, HbA1c values and lipid profiles. The recommended dosage is 200–1000 μg/day. Increased benefits have been noted with higher doses.45,62 Another possible effect of chromium is its action on down-regulating pancreatic β-cell activity, thus increasing glucagon levels.63
Magnesium is essential for all reactions requiring energy as it is needed to stabilise ATP. Low levels have been found in cardiovascular disease and diabetes, and in people with high alcohol intake. Normalisation through supplementation up to 400 mg/day has enhanced insulin sensitivity, reduced fasting glucose and improved HbA1c.21,45,64 Trials with small amounts of magnesium have yielded mixed results. However, reductions in FBG and HbA1c, and increases in postprandial insulin, in glucose uptake and in glucose oxidation have been noted.62
Enhanced insulin sensitivity with reductions in FBG, HbA1c and hepatic glucose production, and better insulin-mediated glucose uptake, have been found with vanadium.62 The usual dose is 100 μg/day. Vanadium mimics the action of insulin, as seen by its similar effects on the translocation of GLUT4 to the cell membrane.21
Coenzyme Q10 has been shown to reduce blood glucose, hyperinsulinaemia, high blood pressure, triglycerides and lipid peroxidation.4 Dosage range is 30–120 mg/day. It is particularly important for people on statin drugs as some of these have a depleting effect on this nutrient.65
N-acetyl carnitine, made from lysine by methylation, is instrumental in the transport of fatty acids across the mitochondrial membrane for β-oxidation. It is therefore essential for energy production from fat burning.38 Administration of this nutrient has enhanced glucose uptake, storage and utilisation, and insulin sensitivity.62 A relatively high amount, ≥ 3 g/day, is needed to achieve these effects.
Vitamin E tends to decrease HbA1c, FBG and PPG.62
The bioactive nutrient α-lipoic acid is both water and fat soluble and has been shown to enhance cellular glucose uptake and utilisation. The daily amount is up to 1200 mg/day. In conjunction with exercise, this nutrient has shown to enhance glucose transport and insulin signalling in skeletal muscle cells.66
Herbal medicines
In diabetes type 2, insulin resistance and metabolic syndrome, a range of herbal supplements have been proven to be of help in blood glucose and insulin regulation.67 In particular, bitters have been indicated.68 Galega officinalis allegedly increases the action of insulin. Its active ingredient, galegine, was used as a model for metformin.62,69 Coccinia indica seems to mimic the action of insulin, Bauhinia forficata has been dubbed ‘vegetable insulin’, garlic and onion can decrease fasting serum glucose levels as well as exerting a beneficial effect on cardiovascular risk factors, Opuntia streptacantha has reportedly insulin-sensitising properties and Aloe vera juice has hypoglycaemic effects.62,69 Berberis-containing herbs have shown regulatory effects not only on glucose control but also on cardiovascular pathology.68 Coleus forskohlii has putative beneficial effects on insulin resistance and diabetes type 2. Inula racemosa has shown increased insulin sensitivity in an animal model,3 and Ocimum sanctum (holy basil) is effective in lowering FBG, PPG and glucosuria.62,69 The use of Cinnamomum cassia (cinnamon) shows in in vitro studies that it can potentiate the action of insulin.70
Gymnema sylvestre has glucose-lowering ability without causing hypoglycaemia. Decreases in FBG, HbA1c and urinary glucose excretion have been found, leading to a reduced need for conventional medication. G. sylvestre is thought to delay glucose uptake in the small intestines, and through its restorative action on β-cells the release of insulin from the pancreas has been increased.62,71 Lipid studies have also shown favourable effects.70 If given at least 30 minutes before eating, it reduces appetite and the cravings for sweets by anaesthetising the taste buds (1–2 mL/day). Therefore it may aid in weight loss.69,70 Thus, G. sylvestre has the ability to affect a number of factors linked to the aetiology and pathophysiology of diabetes type 2 simultaneously, something that no other single hypoglycaemic agent is able to exert.72
The various forms of ginseng (Panax ginseng and P. quinquefolium, and Eleutherococcus senticosus) have beneficial effects in diabetes type 2.62 Decreases in PPG, FBG and HbA1c have been noted with P. quinquefolium.62,73
Supplementation with Trigonella foenum-graecum carbohydrate has led to lowered FBG, PPG, postprandial insulin, urine glucose and absorption, with modulation of peripheral glucose utilisation, thus increasing glycaemic control.62,69,70
Momordica charantia has hypoglycaemic (for both PPG and FBG) and antidiabetic effects. It has the ability to stimulate the pancreas to release insulin; to increase glucose uptake, glycogen synthesis, and glucose oxidation; and to decrease hepatic gluconeogenesis.62,69,71
Tinospora cordifolia can avert blood sugar elevation by reducing glycogenolysis (the breakdown of glycogen to form free glucose) from liver and muscle, thus preventing excess lactic acid being converted to glucose in the liver, and increasing glucose utilisation in muscle tissue.71
The Indian herb Salacia oblonga has been used in trials to treat diabetes and it was found to be as effective as prescription drugs. A maximum of 1000 mg has been used.74
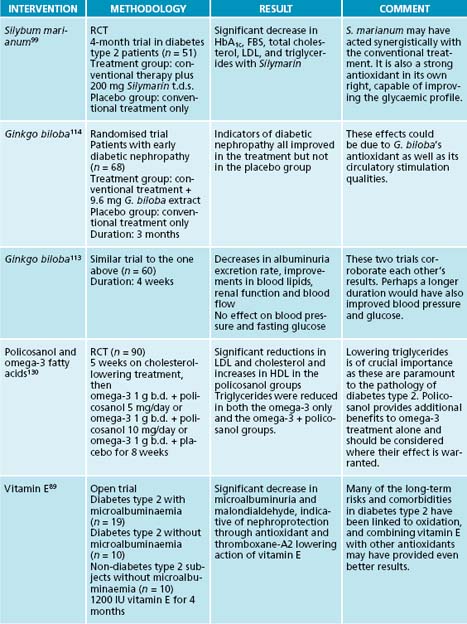
Apart from working as an antioxidant by restoring glutathione levels, Silybum marianum can lower blood and urinary glucose, and HbA1c. It also has beneficial effects on insulin, lowering its requirement.62 In other studies, the positive effect was mainly on diabetic complications and lipid parameters.68
Reduce immune system activation and inflammation
Inflammation plays an important part in the pathophysiology of chronic and lifestyle-induced disease such as diabetes type 2, and polyunsaturated fatty acids (PUFAs) play an important role in regulating pro-inflammatory cytokines.75,76 Not only the omega-3 but also the omega-6 series have positive effects, leading to anti-inflammatory prostaglandin E3 and E1, respectively. Low levels of γ-linolenic acid have been found in diabetes type 2 due to the reduced activity of the enzymes delta 5 and delta 6 desaturase,75,77 as insulin is needed for their activation.78 Research has therefore focused on the beneficial effects of fish oils to bypass these enzymes. In addition to the triglyceride-lowering and anti-inflammatory effects that have been noted with the administration of fish oils,19,45 reductions in serum lipids, lipoproteins, platelet aggregation and blood pressure, and increases in cell membrane fluidity, have been found.76,79
Due to the inflammatory component of metabolic syndrome, antioxidants are paramount. Vitamin E has been investigated with mixed results as to its effects on inflammation, diabetes and heart disease.45 However, antioxidants need to be administered in conjunction with each other, due to their mutual recycling. It is important, too, to use mixed tocopherols, not the synthetic d-α-tocopherol. Further, N-acetyl cysteine has shown to have beneficial effects in insulin resistance due to its antioxidant properties17 and being a cofactor for glutathione production.
Vitamin D plays a role in immunomodulation and insulin secretion. There are correlations between insulin resistance, diabetes type 2 and heart disease, and supplementation of ≥ 1000 IU/day (regardless of body status) has shown to improve these parameters.6,38,29,80
Chronic immune activation, as is the case in obesity, increases tryptophan breakdown, potentially leading to serotonin deficit and symptoms of mood disorder, depression and impaired satiety. The latter increases caloric intake, especially from carbohydrates, thus perpetuating a vicious circle.81
Reduce stress
Stress increases cortisol through HPA axis activation (see Chapter 15 on adrenal exhaustion). Elevated cortisol promotes visceral adiposity, insulin resistance and loss of glycaemic control,82–85 thus contributing to metabolic syndrome.15,86 The vicious cycle of visceral fat storage, gluconeogenesis from muscle protein and increased demand for insulin with resultant increase in cortisol promotes carbohydrate cravings, which in turn leads to further abdominal fat gain.
To break this cycle, stress management is paramount. The following herbs do not only have adaptogenic properties with balancing effects on stress hormones; they also have shown direct benefits in treating insulin resistance and diabetes type 2.
Eleutherococcus senticosus is known for its adaptogenic properties during stress. It is used during periods of fatigue and debility. It has shown positive effects on blood pressure, total and LDL cholesterol, triglycerides and glucose. There may be reduction of stress-related symptoms and faster recovery in chronic illness due to increased aerobic metabolism.70,87 Due to its potential hypoglycaemic effects, diabetics should monitor their blood sugar levels when taking this herb.73
Panax ginseng is another adaptogen used in stress, in chronic diseases and to replenish depleted energy stores.70 The rate of carbohydrate absorption is reduced, with increased glucose uptake, transport and storage in form of glycogen. Insulin secretions can be modulated.62 Although small drops in blood glucose have been reported, one study68 claims that further results in diabetes can be achieved only when the herb has been injected.
Prevent/address diabetic complications
Despite medication, chronic elevations and fluctuations in blood glucose in both diabetes type 1 and diabetes type 2 cannot be avoided entirely. Oxidative stress and inflammation have been found not only in diabetic patients but also in obese people and those with insulin resistance. This, plus the increase in free fatty acids and possibly other metabolites, is responsible for the oxidative stress that leads to diabetic complications such as damage to the nerves, the vascular endothelium and the kidneys. Various antioxidant treatments have been used to alleviate oxidative damage and thus delay or prevent these complications.19,28,29
Nutritional interventions
Essential fatty acids, including evening primrose oil, can alleviate diabetic complications due to their anti-inflammatory properties (as outlined above).68,79 N-acetyl cysteine has been recommended as being useful to alleviate oxidative stress.16,17 It is the limiting amino acid for glutathione production, and whey is an excellent source of this nutrient.88 Supplementation with either whey or N-acetyl cysteine may therefore increase the availability of the endogenous antioxidant glutathione.
Vitamin E improves the action of other antioxidants, especially vitamin C and glutathione. It reduces LDL and the risk of cardiovascular complications of insulin resistance. It may help to preserve pancreatic β-cells. Since vitamin E works closely with selenium in endogenous antioxidant systems, the combined use has resulted in greater benefits.4 Its use in nephropathy has resulted in decreased microalbuminaemia due to reductions in thromboxane A2.89
The renin-angiotensin system is crucial in the development of diabetic nephropathy. It is negatively regulated by vitamin D, which suppresses renin expression. Research on mice has shown a protective effect of vitamin D on the renal system by modulating the renin-angiotensin system.90 The active form of vitamin B6, pyridoxyl 5’-phosphate, has shown in rats to prevent advanced glycation end-products being formed, thus favourably influencing diabetic nephropathy. This may yet prove to be a valuable treatment in this condition for diabetic patients.91
The mechanism by which α-lipoic acid exerts its benefits in diabetes are thought to be due to its ability to facilitate the translocation of GLUT4 to the cell membrane, in readiness for glucose uptake into the cell.39 It is especially useful to prevent or reduce diabetic complications resulting from oxidative damage,92 such as cataract formation, polyneuropathy and damage to blood vessels. As an antioxidant it also helps to recycle other antioxidants, such as vitamins C and E, and glutathione.4,39,62
Diabetic neuropathy is characterised by demyelinisation with resultant reductions in nerve conduction velocity.93 A review94 found that α-lipoic acid in conjunction with vitamin E reduced diabetic neuropathy and corrected nerve conduction velocity. Although some conflicting data exist regarding what exactly α-lipoic acid improves in diabetic neuropathy, there is no doubt that it does have a beneficial effect in this condition.95 Its application is safe and effective.96
One of the most troublesome symptoms of diabetic neuropathy is pain. Acetyl L-carnitine at a daily dose of 1000 mg has been effective in regenerating nerve fibres and significantly reducing pain.97 Vitamin B12, in its coenzyme form of methylcobalamin, has been injected intrathecally (injection into the subarachnoid space) with dramatic improvements in leg pain, heaviness and paraesthesia (tingling, numbness, and pins and needles).98 This suggests that vitamin B12 deficiency plays a role in diabetic neuropathy.
Herbal medicines
Silybum marianum has been trialled in diabetes type 2 due to its antioxidant profile and beneficial effects on diabetes. An improvement in glycaemic control has been found; this could reduce the risk of diabetic complications.99,100
Gymnema sylvestre has wide-ranging actions on blood glucose metabolism and is therefore able to alleviate a range of diabetic complications, including cardiovascular risk factors such as hypertension, hyperlipidaemia and atherosclerosis, and the diabetic complications trio nephropathy, neuropathy and retinopathy, as well as susceptibility to infection and erectile dysfunction.72
Leg ulcers are a common complication of diabetes and are often difficult to heal. Aesculus hippocastanum, due to its venous toning, antioxidant and anti-inflammatory effects, has been used to treat this condition.101 Significant improvements have been noted in wound slough and in reduced necessity to change wound dressings.102,103
Diabetic retinopathy has been treated with traditional Chinese medicine and antioxidants. Chinese yin-nourishing, kidney-tonifying and blood-activating herbs have been used to enhance blood flow to the eyes, resulting in improved visual acuity.104 However, there have been no conclusive trials as yet on the use of antioxidants in this condition,105–107 although it would be reasonable to concur that antioxidants are called for since this condition is the result of oxidative damage.
Similarly, nephropathy is thought to be a result of oxidative stress. Several combinations of traditional Chinese herbal medicine remedies to nourish Qi with Western medicine have been used with good results.108–110 A traditional Chinese medicine formulation to remove blood stasis, stomach heat and heart fire, while supporting the stomach and kidneys, has proven superior to drug treatment.111 One study used a combination of Fructus arctii and Astragalus membranaceus, which reduced proteinuria and albuminuria, and improved blood glucose and lipids.112
Ginkgo biloba is known as an antioxidant, circulatory stimulant and neuroprotectant,70 and its use in diabetic nephropathy seems therefore logical. In the early stages of the disease G. biloba has been found to be effective in increasing renal function with resultant decreased albuminuria and other improvements.113,114 Benefits have also been shown with G. biloba with respect to intercellular and vascular adhesion molecules.115
Address comorbidities
Insulin resistance, diabetes type 2, heart disease and metabolic syndrome are closely related. Due to insulin resistance, the risk of heart disease is increased in diabetes type 2 as part of the metabolic syndrome.27 Hypertension and dyslipidaemia (high triglycerides, high LDL and low HDL) are part of the symptom picture in metabolic syndrome and need to be addressed conjointly with insulin resistance and diabetes type 2.1,2 Please refer to Section 3 on the cardiovascular system for more detailed interventions.
Nutritional interventions
Essential fatty acids (EFAs) prevent organ damage in diabetes type 242 through their effect on dyslipidaemia and the ability to lower triglycerides, blood pressure and atherogenesis.76 Brain function has been improved on omega-3 fatty acids,19 evidenced by the fact that people with diabetes type 2 have reported less depression when supplementing with omega-3 fatty acids. This may be linked to the beneficial effects omega-3 fatty acids have on cardiovascular disease.116
Vitamin B12 and folate are both needed for the remethylation of homocysteine to methionine (the universal methyl group donor). Vitamin B6 facilitates the breakdown of homocysteine via the transsulfuration pathway.21 Together, they are needed to reduce homocysteine levels, which can pose an independent risk factor for heart disease and diabetes type 2.27 Recent research has cast doubt on the link of homocysteine to heart disease. However, the fact remains that it is also linked to reduced bioavailability of nitric oxide, thus indirectly effecting cardiovascular disease.117
A derivative of vitamin B5, pantethine, has shown promising results in the prevention of diabetes-induced angiopathy, due to its triglyceride-, lipid- and apolipoprotein-lowering effects.118 It is particularly useful in dyslipidaemia when this is not amenable to dietary or other means.119
The reader should be aware that the evidence stated under nutritional interventions has been mostly derived from trials where single substances have been used. As is the case with antioxidants, combinations of the above may yield even better results. Nutrients and herbs that match the individual patient’s profile best need to be chosen.
Herbal medicines
One of the early manifestations of heart disease is hypertension. This can be improved by Crataegus monogyna. This herb has also shown benefits in other areas of heart disease, such as congestive heart failure70 and decreased cardiac output, circulatory disturbances and arrythmias.120 Coleus forskohlii has blood pressure lowering as well as platelet inhibiting effects.70
Trigonella foenum-graecum, apart from its beneficial effects on blood glucose, has been shown to lower atherosclerotic parameters.70 Similarly, Cynara scolymus can reduce hyperlipidaemic parameters such as cholesterol and triglycerides.70
INTEGRATIVE MEDICAL CONSIDERATIONS
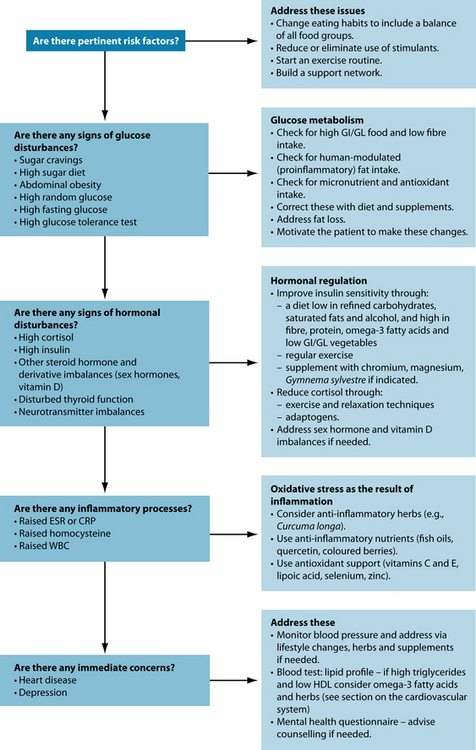
Relaxation exercises such as meditation have shown beneficial effects not only on mood and depression, but also on insulin receptors and the HPA axis.19 Since metabolic syndrome is a multifaceted disease a whole range of parameters need to be considered and attended to. Therefore, a close working relationship with the patient’s GP would be advisable.
Homoeopathy has been used successfully in cases of diabetes type 2121 as well as its complications.122 Although some remedies tend to have a greater positive effect on this disease than others,123 it is still important to repertorise and thus individualise,124 as more obscure remedies may be the ones needed for a particular individual.125,126
PATHOGENESIS AND CONVENTIONAL TREATMENT FOR DIABETES TYPE 134,32,21
Diabetes type 1 is primarily an autoimmune disease where the β-cells of the pancreas, which produce insulin, are destroyed by leukocyte infiltration.35 The starting age is usually during childhood or adolescence. The disease is characterised mainly by ketoacidosis with the typical acetone breath, polyuria with resultant thirst, postural hypotension and anorexia due to uncontrolled hyperglycaemia, which interferes with electrolyte and osmotic balance. This is a catabolic disease with elevated glucagon and muscle wasting due to gluconeogenesis, and can lead to coma and death if untreated. Lifelong exogenous insulin by injection is the usual treatment. In recent years the transplant of a whole pancreas or pancreatic islet cells has been trialled, with several patients not requiring insulin for some time after the procedure, but cytotoxic drugs are then needed for life.30 This treatment is still in its infancy and not yet common practice.
Diagnosis is made from either urine or fasting blood glucose levels. Reference ranges are the same as for diabetes type 2. A glucose tolerance test may be performed to corroborate the results of the urine or blood test. To ascertain long-term control, HbA1c will be measured every 3 to 6 months.
Acute diabetic complications include hypoglycaemia due to too much insulin administration which can lead to coma. Long term complications involve damage to the micro- and macrovascular systems, nervous tissue, skin and eyes and are the result of fluctuations in blood glucose levels that are part of the disease picture. Common conditions include cataracts, retinopathy leading to blindness, nephropathy leading to renal failure, neuropathy, myocardial infarction and stroke, and gangrene of the feet leading to amputations.
TREATMENT PROTOCOLS AND POSSIBLE INTERVENTIONS FOR DIABETES TYPE 1
A nutritious, balanced diet is recommended, with regular meals, and sugar in particular needs to be restricted. The use of artificial sweeteners, so often recommended as sugar substitutes and considered safe for diabetics, may have harmful effects long-term and should be avoided or at least limited.
Vitamin D deficiency has been linked to the pathogenesis of autoimmune diseases, including diabetes type 1.6,36 Early intervention with vitamin D may be able to arrest the pro-inflammatory cytokine production and thus avoid or alleviate the disease if supplementation is commenced early. Oxidative stress is part of DT1 and its complications, and antioxidants should be considered.37,38 Lipoic acid is particularly beneficial for the prevention and treatment of diabetic complications.39 It has also been used, in conjunction with vitamin E, in diabetic neuropathy.40 For glaucoma and raised intraocular pressure, high dose vitamin C, lipoic acid, vitamin B12, magnesium, melatonin, Ginkgo biloba and Coleus forskohlii have been suggested.41 Essential fatty acids have also been shown to play a role in diabetes type 1.38,42
The Indian system of Ayurveda has a wide range of herbal medicines for many diseases. In diabetes type 2, Pterocarpus marsupium (kino tree) has decreased elevated blood glucose and glycosylated haemoglobin levels.127
Other useful modalities and therefore referrals include acupuncture, fitness training (gym), grief counselling and creativity (art and music).
Case Study
A 67-year-old male presents with inability to stop weight gain. His last blood test showed a fasting blood sugar level of 11.3 mmol/L and a cortisol level of 720 nmol/L. He has put on 20 kg over the past 5 years, since his wife died of cancer. Most of this weight gain happened since his retirement 2 years ago. He finds it hard to cope on his own; he has isolated himself from his friends and family and spends most of his days at home watching television (for distraction). With little cooking skills his meals consist mainly of sandwiches (white bread) with cheese or jam, and biscuits in between. Occasionally he fries an egg or some sausages, or buys some take-aways. Fluid intake is mainly derived from four cups of coffee a day (with milk and 2 teaspoons of sugar), two cans of soft drink and three or four cans of beer at night.
Example treatment
The tentative diagnosis for this patient is hypercortisolism due to prolonged stress, combined with an imbalanced high-glycaemic-index diet, excess alcohol consumption and a sedentary lifestyle, resulting in insulin resistance, diabetes type 2 and metabolic syndrome.
The first consultation, after thorough case taking, physical examination and in-house testing (see Table 16.2), was spent educating the patient about how stress, his diet and his lifestyle have contributed to his current health problems. He knew he was not feeling 100%, but had no idea how much at risk of major health problems he was until his medical practitioner had told him he had diabetes and wanted him to take medication. This was a ‘big wake-up call’ for him—he had rarely taken medication in his whole life.
Table 16.2 Physical examination at first consultation
| MEASUREMENT | ACTUAL | IDEAL |
|---|---|---|
| BP | 145/90 | 120/80 |
| Height | 1.71 m | Not applicable |
| Weight | 99.5 kg | 73 kg |
| BMI | 34 | 25 (maximum) |
| Lean body mass | 49.5 kg | 60 kg |
| Fat mass | 50 kg | 13 kg |
| Urinalysis | Glucose 1+ | None |
It was deemed important to explain the connections between stress, lifestyle and diet in detail and in terms he could relate to. Since he used to be a builder the analogy of mortar and bricks to build a house, and what happens when materials are missing or are of poor quality, was used. This understanding on the part of the patient was considered of utmost importance to achieve compliance.
Treatment options
To reduce stress and elevated cortisol levels, he was advised to:
To reduce elevated glucose levels and obesity, and increase insulin sensitivity, he should:
These options were discussed to find out what he could do differently, with emphasis on eliciting realistic ideas from him.
With respect to dietary changes, he agreed to add some apples and carrots to his shopping list. As far as other vegetables were concerned, he did not know how to prepare them. Some healthy pre-packaged as well as take-away meal options were discussed. He was prepared to try some wholemeal bread and buy some cans of fish (in spring water) for his lunch. He had a blender at home and was instructed in how to use this for making daily protein smoothies (containing protein powder and mixed berries for antioxidant support, made up with rice milk).
He was advised to return to his medical practitioner for some further testing (see above), which he agreed to do.
He was prescribed a range of supplements (see the prescription box), consisting of chromium and magnesium to increase insulin sensitivity and an antioxidant with α-lipoic acid to minimise oxidative damage. To reduce the high homocysteine and ensure optimal methylation, folate and vitamin B12 were given as both vitamin levels were low, together with vitamin B6, which breaks down homocysteine in the transsulfuration pathway. His vitamin D status was most likely low because he spent so much time inside. To address this deficiency 5000 IU/day was added. Lastly, fish oils were recommended for cardiovascular and brain health and to reduce inflammation, particularly since in diabetes plant oils are not readily converted to EPA and DHA due to delta 6 desaturase deficiency.
The herbal prescription in form of a tincture included Curcuma longa for liver phase I and II detoxification support,70 and its antioxidant, anti-inflammatory and hypolipidaemic properties. Gymnema sylvestre was chosen for its ability to reduce sugar cravings and control blood sugar levels. Eleutherococcus senticosus also has the ability to regulate blood sugar as well as blood pressure, apart from its adaptogenic effect on stress. Similarly, Trigonella foecum-gracum has positive effects on blood sugar control and cardiovascular parameters.
Expected outcomes and follow-up protocols
As the patient is still a ‘young retiree’ there is hope that some, if not all, parameters can be improved and perhaps even normalised if he maintains his adherence to the protocols and prescriptions given to him, and if he manages to lose some weight. Counselling and joining a social club for outings could be considered.
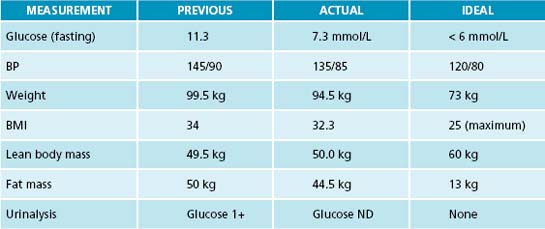
In the second consultation the patient brought with him the results of the tests requested previously (Table 16.3). He had started to exercise by playing bowls three times a week. His diet had improved somewhat as he was now eating proper meals (meat, potatoes and vegetables). His beer consumption had gone down to one or two cans per night, and he had replaced soft drinks with water. He also was enjoying the protein smoothies. It was decided that further dietary changes were premature and would probably result in failure.
Table 16.3 Pathology test results
| TEST | PATIENT RESULTS | REFERENCE RANGE∗ |
|---|---|---|
| ALT | 56 U/L | 5–40 U/L |
| Homocysteine | 14 μmol/L | 0–15 μmol/L, but ideally < 10 μmol/L |
| Active B12 | 36 pmol/L | > 35 pmol/L |
| R/C folate | 263 nmol/L | > 250 nmol/L |
| ESR | 25 mm/h | 1–30 mm/h, but ideally < 1 mm/h |
| CRP | 8 mg/L | 0–10 mg/L, but ideally <1 mg/L |
| Triglycerides | 3.4 mmol/L | 0.6–2 mmol/L |
| HbA1c | 7.8% | < 6% |
| HDL | 0.9 mmol/L | 1.1–1.9 mmol/L, ideally at the upper end |
| Hydroxycalciferol (vitamin D) | 45 nmol/L | 50–150 nmol/L, but ideally > 100 nmol/L |
∗ The difference between the reference values given in Table 16.1 and those given here are due to the latter being from the laboratory and the former from the textbook.
With these encouraging improvements, and due praise from the practitioner, the next step could be tackled: supplementation. None of these nutrients had been contained in his diet (at time of first consultation), so their levels were expected to be low, further evidenced by his health problems and blood-test results. The supplements prescribed were based on signs and symptoms, diet and lifestyle analysis, and on the test results in Table 16.3 (which showed suboptimal values in most parameters).
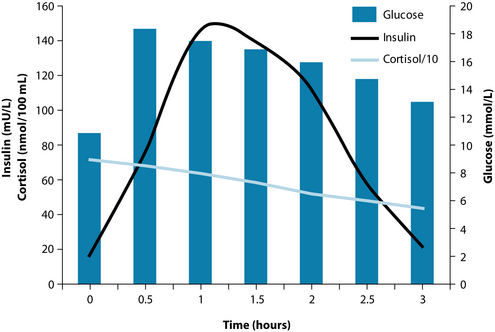
The glucose tolerance test results are displayed in Figure 16.4. The test revealed hyperglycaemia, hyperinsulinaemia, hypercortisolism and insulin resistance (the right-shift of the insulin peak to the glucose peak denotes delayed insulin response—that is, insulin resistance). The results were explained to the patient; this made him realise how imbalanced his whole body had become. It meant either medication or compliance with the recommendations of the naturopath. He chose the latter.
There are more possibilities regarding nutrients and herbs, and diet could be further refined. Vitamin B12 may need to be given intramuscularly if the oral supplementation fails to raise this. Decreasing vitamin B12 values may also indicate reduced gastric secretions, as is often the case with high stress as well as with age. In that case digestive enzymes (hydrochloric acid, pepsin and pancreatic enzymes) should be added to the regimen.
When the patient returned 4 weeks later he had lost 2 kg. He had been compliant with the instructions given to him and had taken his supplements and herbs assiduously. His diet had further improved as he was now cooking meat, fish and vegetables at home. Instead of drinking beer at night he had one or two glasses of dry red wine.
It was deemed appropriate to talk about low glycaemic index/glycaemic load foods and the balance of macronutrients at this point. He was advised to reduce the carbohydrates (bread and potatoes in particular) and replace them with low glycaemic load vegetables (a handout was given), and to include protein with every meal. It would also be advantageous to replace some of his coffee with green tea, and to use stevia instead of sugar for sweetening.
Again, the patient had been very compliant when he returned 4 weeks later. His weight had gone down a further 3 kg. He was still playing bowls three times a week, and he was going for a walk twice a week with his neighbour. He felt more motivated and had completed many of the small tasks at home. The patient commented that he felt he had a new lease on life.
These positive changes were also reflected in his physical values (Table 16.4). He was delighted and felt encouraged by these results. The prescriptions were repeated and a date for a follow-up consultation made in 3 months.
Bone K. Phytotherapy for diabetes and the key role of Gymnema. Modern Phytotherapist. 2002;7(1):7-11.
Gropper S., et al. Advanced nutrition and human metabolism, 5th edn. Australia: Wadsworth: Cengage Learning; 2009. 276–277
Kelly G.S. Insulin resistance: lifestyle and nutritional interventions. Altern Med Rev. 2000;5(2):109-132.
Mota M., et al. The metabolic syndrome—a multifaced disease. Rom J Intern Med. 2004;42(2):247-255.
Roberts K., et al. Syndrome X: medical nutrition therapy. Nutr Rev. 2000;58(5):154-160.
Shils M.E., et al. Modern nutrition in health and disease. Philadelphia: Lippincott Williams & Wilkins; 2006. 1004–1066, Chapters 62–65
Yeh G.Y., et al. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26(4):1277-1294.
1. Mota M., et al. The metabolic syndrome—a multifaced disease. Rom J Intern Med. 2004;42(2):247-255.
2. Shils M.E., et al. Modern nutrition in health and disease. Philadelphia: Lippincott Williams & Wilkins; 2006.
3. Kelly G.S. Insulin resistance: lifestyle and nutritional interventions. Altern Med Rev. 2000;5(2):109-132.
4. Roberts K., et al. Syndrome X: medical nutrition therapy. Nutr Rev. 2000;58(5):154-160.
5. Khani S., Tayek J.A. Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin Sci. 2001;101(6):739-747.
6. Holick M.F. Diabetes and the vitamin D connection. Curr Diab Rep. 2008;8(5):393-398.
7. Vinson G.P. Angiotensin II, corticosteroids, type II diabetes and the metabolic syndrome. Med Hypotheses. 2007;68(6):1200-1207.
8. Brindley D.N. Role of glucocorticoids and fatty acids in the impairment of lipid metabolism observed in the metabolic syndrome. Int J Obes Relat Metab Dis. 1995;19(Suppl 1):S69-S75.
9. Ginsberg H.N., et al. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. 2005;36(3):232-240.
10. Hristova M., Aloe L. Metabolic syndrome—neurotrophic hypothesis. Med Hypotheses. 2006;66(3):545-549.
11. Walker B.R. Steroid metabolism in metabolic syndrome X. Best Pract Res Clin Endocrinol Metab. 2001;15(1):111-122.
12. Pickup J.C., et al. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40(11):1286-1292.
13. Clifton P.M. Diet and C-reactive protein. Curr Atheroscler Rep. 2003;5(6):431-436.
14. Rosmond R. The glucocorticoid receptor gene and its association to metabolic syndrome. Obes Res. 2002;10(10):1078-1086.
15. Björntorp P., Rosmond R. The metabolic syndrome—a neuroendocrine disorder? Br J Nutr. 2000;83(Suppl 1):S49-S57.
16. Evans J.L., et al. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23(5):599-622.
17. Evans J.L., et al. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7(7–8):1040-1052.
18. Sebeková K., et al. Association of metabolic syndrome risk factors with selected markers of oxidative status and microinflammation in healthy omnivores and vegetarians. Mol Nutr Food Res. 2006;50(9):858-868.
19. Singh R.B., et al. Can brain dysfunction be a predisposing factor for metabolic syndrome? Biomed Pharmacother. 2004;58(Suppl 1):S56-S68.
20. Wirth A. [Non-pharmacological therapy of metabolic syndrome]. Herz. 1995;20(1):56-69.
21. Gropper S., et al. Advanced nutrition and human metabolism, 5th edn. Australia: Wadsworth: Cengage Learning; 2009.
22. Devlin T.M. Textbook of biochemistry with clinical correlations, 5th edn. New York: Wiley-Liss; 2002.
23. Pagana K.D., Pagana T.J. Mosby’s manual of diagnostic and laboratory tests, 3rd edn. USA: CV Mosby; 2006.
24. Upfal J., O’Callaghan J. Your medical tests. what do they really mean?. Melbourne: Black Ink; 2001.
25. Pasquali R., et al. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann N Y Acad Sci. 2006;1083:111-128.
26. Schindhelm R.K., et al. Liver alanine aminotransferase, insulin resistance and endothelial dysfunction in normotriglyceridaemic subjects with type 2 diabetes mellitus. Eur J Clin Invest. 2005;35(6):369-374.
27. Hayden M.R., Tyagi S.C. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: the pleiotropic effects of folate supplementation. Nutr J. 2004;3:4.
28. Herbert V., et al. Low holotranscobalamin II is the earliest serum marker for subnormal vitamin B12 (cobalamin) absorption in patients with AIDS. Am J Hematol. 1990;34:132-139.
29. Boucher B.J..Inadequate vitamin D status: does it contribute to the disorders comprising syndrome ‘X’?. [erratum appears in Br J Nutr 1998 Dec;80(6):585]. Br J Nutr 1998;79(4):315-327.
30. Kumar P., Clark M. Clinical medicine, 5th edn. London: W.B. Saunders; 2002.
31. Dickey R.A., et al. Optimal thyrotropin level: normal ranges and reference intervals are not equivalent. Thyroid. 2005;15(9):1035-1039.
32. Tierney L.M., et al. Medical diagnosis and treatment, 44th edn. New York: McGraw-Hill; 2005.
33. MIMS annual. Australian edition. CMPMedica, June 2006.
34. Watkins P. The diabetic foot. BMJ. 2003;326(7396):977-979.
35. Giarratana N., et al. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J Immunol. 2004;173(4):2280-2287.
36. Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92(1):4-8.
37. Chertow B. Advances in diabetes for the millennium: vitamins and oxidant stress in diabetes and its complications. MedGenMed. 2004;6(3 Suppl):4.
38. Triggiani V., et al. Role of antioxidants, essential fatty acids, carnitine, vitamins, phytochemicals and trace elements in the treatment of diabetes mellitus and its chronic complications. Endocr Metab Immune Disord Drug Targets. 2006;6(1):77-93.
39. Packer L., et al. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition. 2001;17(10):888-895.
40. van Dam P.S. Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectives. Journal of the Peripheral Nervous System [Article]. 2002;7(4):246-248.
41. Head K.A. Natural therapies for ocular disorders, part two: cataracts and glaucoma. Altern Med Rev. 2001;6(2):141-166.
42. Das U.N. Essential fatty acids in health and disease. J Asso Physicians India. 1999;47(9):906-911.
43. Giurini J., Lyons T. Diabetic foot complications: diagnosis and management. Int J Low Extrem Wounds. 2005;4:171-182.
44. Brehm B.J., D’Alessio D.A. Weight loss and metabolic benefits with diets of varying fat and carbohydrate content: separating the wheat from the chaff. Nat Clin Pract Endocrinol Metab [serial on the Internet]. 4(3), 2008. Online. Available: http://www.medscape.com/viewprogram/8640_pnt
45. Neff L.M. Evidence-based dietary recommendations for patients with type 2 diabetes mellitus. Nutr Clin Care. 2003;6(2):51-61.
46. Pelkman C.L., et al. Effects of moderate-fat (from monounsaturated fat) and low-fat weight-loss diets on the serum lipid profile in overweight and obese men and women. Am J Clin Nutr. 2004;79(2):204-212.
47. Belury M.A. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Ann Rev of Nutr. 2002;22:505-531.
48. Belury M.A., et al. The conjugated linoleic acid (CLA) isomer, t10c12-CLA, is inversely associated with changes in body weight and serum leptin in subjects with type 2 diabetes mellitus. J Nutr. 2003;133(1):257S-260S.
49. Gaullier J-M., et al. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr. 2005;135(4):778-784.
50. Gaullier J-M., et al. Conjugated linoleic acid supplementation for 1 y reduces body fat mass in healthy overweight humans. Am J Clin Nutr. 2004;79(6):1118-1125.
51. Dhiman T.R., et al. Factors affecting conjugated linoleic acid content in milk and meat. Crit Rev Food Sci Nutr. 2005;45(6):463-482.
52. Carroll J. Attack on the food pyramid. Wall Street Journal. 2002. Jun 13;Sect. B.1
53. Abboud L., Cairns A. Even a diet of fat-free foods can pose a weighty problem. Wall Street Journal. 2002. Jun 11;Sect. D.4
54. Rodríguez-Morán M., et al. Lipid- and glucose-lowering efficacy of Plantago psyllium in type II diabetes. J Diabetes Complications. 1998;12(5):273-278.
55. Do Just. It: Diabetes and exercise. Clin Diabetes. 2008;26(3):140-141.
56. Turcotte L.P., Fisher J.S. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther. 2008;88(11):1279-1296.
57. Earnest C.P. Exercise interval training: an improved stimulus for improving the physiology of pre-diabetes. Med Hypotheses. 2008;71(5):752-761.
58. Gulve A.E. Exercise and glycemic control in diabetes: benefits, challenges, and adjustments to pharmacotherapy. Phys Ther. 2008;88(11):1297-1321.
59. Ying Z., Fu F.H. Effects of 14-week Tai Ji Quan exercise on metabolic control in women with type 2 diabetes. Am J Chin Med. 2008;36(4):647-654.
60. Song R., et al. Adhering to a t’ai chi program to improve glucose control and quality of life for individuals with type 2 diabetes. J Altern Complement Med. 2009;15(6):627-632.
61. Crowley L.V. An introduction to human disease: pathology and pathophysiology correlations, 6th edn. Sudbury: Jones and Bartlett Publishers; 2004.
62. Yeh G.Y., et al. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26(4):1277-1294.
63. McCarty M.F. Chromium and other insulin sensitizers may enhance glucagon secretion: implications for hypoglycemia and weight control. Med Hypotheses. 1996;46(2):77-80.
64. Higdon J. An evidence-based approach to vitamins and minerals. New York: Thieme; 2003.
65. Hargreaves I.P., et al. The effect of HMG-CoA reductase inhibitors on coenzyme Q10: possible biochemical/clinical implications. Drug Saf. 2005;28(8):659-676.
66. Henriksen E.J., Saengsirisuwan V. Exercise training and antioxidants: relief from oxidative stress and insulin resistance. Exerc Sport Sci Rev. 2003;31(2):79-84.
67. Yeh G.Y., et al. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26(4):1277.
68. Mills S., Bone K. Principles and practice of phytotherapy. Sydney: Churchill Livingstone; 2000.
69. Bone K. Phytotherapy for diabetes and the key role of Gymnema. Modern Phytotherapist. 2002;7(1):7-11.
70. Bone K. A clinical guide to blending liquid herbs. USA: Elsevier; 2003.
71. Gormley J.J. Herbal approaches to diabetes and hyperglycemia. Better Nutrition. 1997;59(10):24.
72. Leach M.J. Gymnema sylvestre for diabetes mellitus: a systematic review. J Altern Complement Med. 2007;13(9):977-983.
73. Blumenthal M. The ABC clinical guide to herbs. Austin: American Botanical Council; 2003.
74. Anonymous. Diabetes herb as effective as drugs. Better Nutrition. 2005;67(5):12.
75. Kapoor R., Huang Y.S. Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol. 2006;7(6):531-534.
76. Braun L., Cohen M. Herbs and natural supplements, 2nd edn. Sydney: Churchill Livingstone; 2007.
77. König D., et al. Mehrfach ungesättigte Fettsäuren, koronare Herzerkrankung und Diabetes mellitus Typ II—Hinweise fur den Stellenwert von Gamma-Linolensäure. Forsch Komplementärmed. 1997;4(2):94.
78. Brenner R.R. Hormonal modulation of delta6 and delta5 desaturases: case of diabetes. Prostaglandins Leukot Essent Fatty Acids. 2003;68(2):151-162.
79. Malasanos T.H., Stacpoole P.W. Biological effects of omega-3 fatty acids in diabetes mellitus. Diabetes Care. 1991;14(12):1160-1179.
80. Coyne D.W. Vitamin D and the diabetic patient. Medscape Nephrology [serial on the Internet].. April 2008. Online. Available: http://www.medscape.com/viewarticle/573383_print. Accessed 28
81. Brandacher G., et al. Chronic immune activation underlies morbid obesity: is IDO a key player? Curr Drug Metab. 2007;8(3):289-295.
82. Tsigos C., Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865-871.
83. Vanitallie T.B. Stress: a risk factor for serious illness. Metabolism. 2002;51(6 Suppl 1):40-45.
84. McEwen B.S. Mood disorders and allostatic load. Biol Psychiatry. 2003;54(3):200-207.
85. Mechanick J.I. Metabolic mechanisms of stress hyperglycemia. JPEN J Parenter Enteral Nutr. 2006;30(2):157-163.
86. Nieuwenhuizen A.G., Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav. 2008;94(2):169-177.
87. Szolomicki S., et al. The influence of active components of Eleutherococcus senticosus on cellular defence and physical fitness in man. Phytother Res. 2000;14(1):30-35.
88. Bounous G. Whey protein concentrate (WPC) and glutathione modulation in cancer treatment. Anticancer Res. 2000;20(6C):4785-4792.
89. Hirnerová E., et al. [Effect of vitamin E therapy on progression of diabetic nephropathy]. Vnitr Lék. 2003;49(7):529-534.
90. Li Y.C. Vitamin D and diabetic nephropathy. Curr Diab Rep. 2008;8(6):464-469.
91. Nakamura S., et al. Pyridoxal phosphate prevents progression of diabetic nephropathy. Nephrol Dial Transplant. 2007;22(8):2165-2174.
92. Ruhe R.C., McDonald R.B. Use of antioxidant nutrients in the prevention and treatment of type 2 diabetes. J Am Coll Nutr. 2001;20(5 Suppl):363S-369S. discussion 81S–83S
93. Tong H.I. Influence of neurotropic vitamins on the nerve conduction velocity in diabetic neuropathy. Ann Acad Med Singapore. 1980;9(1):65-70.
94. van Dam P.S. Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2002;18(3):176-184.
95. Foster T.S. Efficacy and safety of alpha-lipoic acid supplementation in the treatment of symptomatic diabetic neuropathy. Diabetes Educ. 2007;33(1):111-117.
96. Becić F., et al. [Pharmacological significance of alpha lipoic acid in up to date treatment of diabetic neuropathy]. Med Arh. 2008;62(1):45-48.
97. Sima A.A., et al. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28(1):89-94.
98. Ide H., et al. Clinical usefulness of intrathecal injection of methylcobalamin in patients with diabetic neuropathy. Clin Ther. 1987;9(2):183-192.
99. Huseini H.F., et al. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res. 2006;20(12):1036-1039.
100. Huseini H.F., et al. The clinical trial of Silybum marianum seed extract (Silymarin) on type II diabetic patients with hyperlipidemia. Iranian Journal of Diabetes & Lipid Disorders. 3, 2004. 78
101. Leach M. Aesculus hippocastanum. Australian Journal of Medical Herbalism. 2001;13(4):136-140.
102. Leach M.J. Evidence-based practice: a framework for clinical practice and research design. Int J Nurs Pract. 2006;12(5):248-251.
103. Leach M.J. Integrative health care: a need for change? J Complement Integr Med. 2006;3(1):1-11.
104. Deng Y.P., Xie X.J. [Preliminary study on the treatment of diabetic retinopathy utilizing with nourishing yin, tonifying kidney and blood-activating herbs]. Chinese Journal of Integrated Traditional and Western Medicine. 1992;12(5):270.
105. Lopes de Jesus C., et al. Vitamin C and superoxide dismutase (SOD) for diabetic retinopathy. Cochrane Database Syst Rev. (1):2008;. CD006695
106. Millen A.E., et al. Relations of serum ascorbic acid and alpha-tocopherol to diabetic retinopathy in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158(3):225-233.
107. Millen A.E., et al. Relation between intake of vitamins C and E and risk of diabetic retinopathy in the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2004;79(5):865-873.
108. Bian F., et al. [Clinical study on treatment of incipient diabetic nephropathy by integrated traditional Chinese and Western medicine]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2000;20(5):335-337.
109. Zou L.H., et al. [Clinical observation on Qidi Yiqi Yangyin Huoxue Recipe in treating diabetic nephropathy at stage III and IV]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26(11):1023-1026.
110. Zhao L., et al. [Integrated treatment of traditional Chinese medicine and western medicine for early- and intermediate-stage diabetic nephropathy]. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27(7):1052-1055.
111. Wu S., et al. Treatment of incipient diabetic nephropathy by clearing away the stomach-heat, purging the heart fire, strengthening the spleen and tonifying the kidney. J Tradit Chin Med. 2000;20(3):172-175.
112. Wang H.Y., et al. [Clinical observation on treatment of diabetic nephropathy with compound fructus arctii mixture]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(7):589-592.
113. Lu J., He H. Clinical observation of Ginkgo biloba extract injection in treating early diabetic nephropathy. Chin J Integr Med. 2005;11(3):226-228.
114. Zhu H.W., et al. [Effect of extract of Ginkgo biloba leaf on early diabetic nephropathy]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25(10):889-891.
115. Li X.S., et al. [Effect of extract of Ginkgo biloba on soluble intercellular adhesion molecule-1 and soluble vascular cell adhesion molecule-1 in patients with early diabetic nephropathy]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(5):412-414.
116. Pouwer F., et al. Fat food for a bad mood. Could we treat and prevent depression in type 2 diabetes by means of omega-3 polyunsaturated fatty acids? A review of the evidence. Diabet Med. 2005;22(11):1465-1475.
117. Romerio S.C., et al. Acute hyperhomocysteinemia decreases NO bioavailability in healthy adults. Atherosclerosis. 2004;176(2):337-344.
118. Eto M., et al. Lowering effect of pantethine on plasma beta-thromboglobulin and lipids in diabetes mellitus. Artery. 1987;15(1):1-12.
119. Arsenio L., et al. Effectiveness of long-term treatment with pantethine in patients with dyslipidemia. Clin Ther. 1986;8(5):537-545.
120. Blumenthal M. The complete German commission E monographs – therapeutic guide to herbal medicines [CD-ROM]. Austin: American Botanical Council; 1999.
121. Paul S. Dare to be non-diabetic. Natl J Homoeopath. 2008;10(10):13.
122. Paul S. Homoeopathic approach to diabetes mellitus and its complications. Natl J Homoeopath. 2008;10(10):56.
123. Anonymous. Important remedies in diabetes. Homoeopath Update. 2000;8(1):40.
124. Bhapkar C. Treat the man, not the disease. Natl J Homoeopath. 2008;10(10):48.
125. Hariharan R. The diabetic breakthrough. Natl J Homoeopath. 2006;8(2):109.
126. Soldner G., et al. Magnesium chloratum. Merkurstab. 2006;59(2):112.
127. Lodha R., Bagga A. Traditional Indian systems of medicine. Ann Acad Med Singapore. 2000;29(1):37-41.
128. Corpeleijn E., et al. Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: the SLIM study. Diabetologia. 2006;49(10):2392-2401.
129. Leach M.J., et al. Clinical efficacy of horsechestnut seed extract in the treatment of venous ulceration. J Wound Care. 2006;15(4):159-167.
130. Castano G., et al. Effects of addition of policosanol to omega-3 fatty acid therapy on the lipid profile of patients with type II hypercholesterolaemia. Drugs R D. 2005;6(4):207-219.