Diabetes Mellitus and Disorders of Glucose Homeostasis
Diabetes Mellitus
Principles of Disease
Insulin.: Insulin receptors on the beta cells of the pancreas sense elevations in blood glucose concentration and trigger insulin release into the blood. For incompletely understood reasons, glucose taken by mouth evokes more insulin release than parenteral glucose does. Certain amino acids induce insulin release and even cause hypoglycemia in some patients. Sulfonylurea oral hypoglycemic agents work, in part, by stimulating the release of insulin from the pancreas.
Glucose Regulatory Mechanisms.: Maintenance of the normal plasma glucose concentration requires precise matching of glucose use and endogenous glucose production or dietary glucose delivery. The regulatory mechanisms that maintain systemic glucose balance involve hormonal, neurohumoral, and autoregulatory factors. Glucose regulatory hormones include insulin, glucagon, epinephrine, cortisol, and growth hormone. Insulin is the main glucose-lowering hormone. Insulin suppresses endogenous glucose production and stimulates glucose use. Insulin is secreted from the beta cells of the pancreatic islets into the hepatic portal circulation and has important actions on the liver and the peripheral tissues. Insulin stimulates glucose uptake, storage, and use by other insulin-sensitive tissues, such as fat and muscle.
Types of Diabetes
The American Diabetes Association (ADA) defines four major types of diabetes mellitus: type 1 diabetes mellitus, type 2 diabetes mellitus, gestational diabetes, and diabetes due to secondary disease processes or drugs. The 1997 National Diabetes Data Group report discontinued the use of the terms insulin-dependent diabetes mellitus and non–insulin-dependent diabetes mellitus because they are confusing and clinically inaccurate. In addition, use of Arabic numerals (1 and 2) instead of Roman numerals is the standard. The most recent update to the standards of care for diabetes was published in January 2011.1 The diagnostic criteria for diagnosis of diabetes were changed in 2010 from the previous standards of elevated fasting glucose concentration and abnormal result of the 2-hour oral glucose tolerance test (OGTT) to use of the hemoglobin A1c (HbA1c) value as the preferred confirmatory test.1 An HbA1c value above 6.5% is now considered diagnostic of diabetes. However, the fasting plasma glucose concentration and 2-hour OGTT are still considered valid, as is the presence of a random glucose measurement of more than 200 mg/dL in a nonfasting patient. In addition, the use of fasting plasma glucose concentration may help identify patients at risk for diabetes (if their glucose concentration is elevated but not crossing the threshold for diagnosis of diabetes).
Type 1 Diabetes Mellitus.: Type 1 diabetes is characterized by abrupt failure of production of insulin with a tendency to ketosis even in the basal state. Parenteral insulin is required to sustain life. From 85 to 90% of patients with type 1 diabetes demonstrate evidence of one or more autoantibodies implicated in the cell-mediated autoimmune destruction of the beta cells of the pancreas. Strong human leukocyte antigen (HLA) associations are also found in type 1 diabetes. The autoimmune destruction has multiple genetic predispositions and may be related to undefined environmental insults.
Type 2 Diabetes Mellitus.: Patients with type 2 diabetes may remain asymptomatic for long periods and show low, normal, or elevated levels of insulin because of insulin resistance. Ketosis is rare in type 2 disease. Patients have a high incidence of obesity. Hypertriglyceridemia is also frequently noted. No association exists with viral infections, islet cell autoantibodies, or HLA expression. Hyperinsulinemia may be related to peripheral tissue resistance to insulin because of defects in the insulin receptor. Defects in muscle glycogen synthesis have an important role in the insulin resistance that occurs in type 2.
Gestational Diabetes.: Gestational diabetes “mellitus” is characterized by an abnormal OGTT result that occurs during pregnancy and either reverts to normal during the postpartum period or remains abnormal. The clinical pathogenesis is thought to be similar to that of type 2. The clinical presentation is usually nonketotic hyperglycemia during pregnancy. Screening is performed around the 24th to 28th week with a 75-g oral glucose load in a woman with no prior history of diabetes.
Diabetes due to Other Causes.: Myriad other causes of diabetes have been identified; these include chronic pancreatitis, cystic fibrosis, genetic defects in the beta cell or in insulin receptors, and chemical induced (such as Vacor and chemotherapeutic, antipsychotic, or antiretroviral medications). The management of diabetes due to these conditions is cause specific and depends on whether the underlying pathophysiologic process more closely resembles type 1 or type 2 diabetes.
Impaired Glucose Tolerance.: Impaired glucose tolerance (IGT) and its analogue, impaired fasting glucose (IFG), are considered to identify individuals at high risk for development of diabetes. This group is composed of persons whose plasma glucose levels are between normal and diabetic and who are at increased risk for the development of diabetes and cardiovascular disease. The pathogenesis is thought to be related to insulin resistance. Presentations of IGT/IFG include nonketotic hyperglycemia, insulin resistance, hyperinsulinism, and often obesity.
IGT/IFG differs from the other classes in that it is not associated with the same degree of complications of diabetes mellitus. Many of these patients even spontaneously have normal glucose tolerance. One should not be complacent about the patient with IGT because the decompensation of this group into the category of diabetes mellitus is 1 to 5% per year.1
Epidemiology
The prevalence of diabetes is difficult to determine because many standards have been used. Regardless, the most recent data estimate that 8.3% of Americans of all ages and 11.3% of all adults older than 20 years have diabetes.1 Approximately 215,000 Americans younger than 20 years have diabetes. Of these, 5 to 10% have type 1, and 90 to 95% have type 2; other types account for 1 to 5% of cases.
The peak age at onset of type 1 diabetes is 10 to 14 years. Approximately 1 of every 600 schoolchildren has this disease. In the United States the prevalence of type 1 is approximately 0.26% by the age of 20 years, and the lifetime prevalence approaches 0.4%. The annual incidence among persons from birth to 16 years of age in the United States is 12 to 14 per 1 million population. The incidence is age dependent, increasing from near-absence during infancy to a peak occurrence at puberty and another small peak at midlife.1
Diagnostic Strategies
As a rule, any random plasma glucose level above 200 mg/dL, HbA1c value above 6.5%, fasting plasma glucose concentration above 126 mg/dL, or 2-hour postload OGTT is sufficient to establish the diagnosis of diabetes. In the absence of hyperglycemia with metabolic decompensation, these criteria should be confirmed by repeated testing on a different day. Confirmation can be made by the same test or two different tests (fasting plasma glucose and HbA1c, for example). A value of 150 mg/dL is likely to distinguish diabetic from nondiabetic patients more accurately. Formal OGTTs are unnecessary except during pregnancy or in patients who are thought to have diabetes but who do not meet the criteria for a particular classification. The World Health Organization and ADA provide protocols for performance of the OGTT.1
Hypoglycemia
Hypoglycemia is a common problem in patients with type 1 diabetes, especially if tight glycemic control is practiced; it may be the most dangerous acute complication of diabetes. The estimated incidence of hypoglycemia in diabetic patients is 9 to 120 episodes per 100 patient-years. As significant efforts continue to keep both fasting and postprandial glucose concentrations within the normal range, the incidence of hypoglycemia may increase. The most common cause of coma associated with diabetes is an excess of administered insulin with respect to glucose intake. Hypoglycemia may be associated with significant morbidity and mortality.2 Severe hypoglycemia is usually associated with a blood glucose level below 40 to 50 mg/dL and impaired cognitive function.
Principles of Disease
Hypoglycemia without warning symptoms, or hypoglycemia unawareness, is a dangerous complication of type 1 diabetes probably caused by previous exposure to low blood glucose concentrations.2 Even a single hypoglycemic episode can reduce neurohumoral counter-regulatory responses to subsequent episodes. Other factors associated with recurrent hypoglycemic attacks include overaggressive or intensified insulin therapy, longer history of diabetes, autonomic neuropathy, and decreased epinephrine secretion or sensitivity.
Management
Treatment of hypoglycemia secondary to oral hypoglycemic agents depends on the agent. Metformin and the thiazolidinedione agents rarely cause significant or prolonged hypoglycemia, whereas sulfonylureas, which are insulin secretagogues, do cause hypoglycemia. Sulfonylurea oral hypoglycemic agents pose special problems because the hypoglycemia they induce tends to be prolonged and severe. Patients with an overdose of sulfonylurea hypoglycemic agents should have a minimum observation period of 24 hours if hypoglycemia is recurrent in the ED after management of the initial episode. Patients at risk for hypoglycemia from oral sulfonylureas include patients with impaired renal function, pediatric patients, and patients who are naïve to hypoglycemic agents. Although symptoms may occur after an overdose, several case reports in patients with renal failure and pediatric patients describe refractory hypoglycemia after ingestion of a single pill. One case series of pediatric patients presenting with sulfonylurea ingestion who were euglycemic initially demonstrated an average time to onset of 8 hours to the initial hypoglycemic episode.3 However, onset of symptoms was delayed up to 18 hours in some patients. As a result, we recommend 23 hours of observation for patients with known or suspected ingestion of hypoglycemic agents.
A patient with hypoglycemia from sulfonylureas, in addition to standard glucose replacement, frequently requires treatment with an agent to inhibit further insulin release, such as octreotide, a somatostatin analogue. Several case series have described the use of octreotide in both adult and pediatric patients suffering from sulfonylurea-induced hypoglycemia, frequently reporting successful results with a significant decrease in the number of episodes of recurrent hypoglycemia. A randomized clinical trial concluded that patients receiving octreotide had a decreased glucose supplementation requirement.4 No single set protocol for use has been described; however, typical adult doses have ranged from 50 to 100 µg intravenously or subcutaneously every 12 hours, and pediatric dosages have ranged from 25 to 50 µg intravenously or subcutaneously. Whereas experience thus far with octreotide has been positive, it does not obviate the need for prolonged observation and serial glucose measurements.
Diabetic Ketoacidosis
Pathophysiology
DKA is a syndrome in which insulin deficiency and glucagon excess combine to produce a hyperglycemic, dehydrated, acidotic patient with profound electrolyte imbalance. All derangements producing DKA are interrelated and are based on insulin deficiency (Fig. 126-1). DKA may be caused by cessation of insulin intake or by physical or emotional stress despite continued insulin therapy.
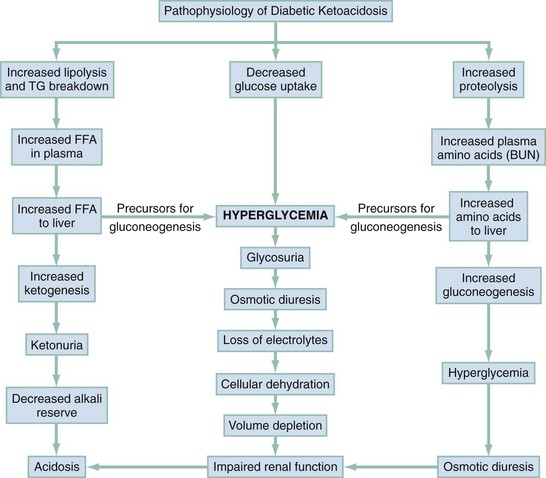
Figure 126-1 Syndrome of diabetic ketoacidosis. BUN, blood urea nitrogen; FFA, free fatty acids; TG, total glucose concentration.
The effects of insulin deficiency may be mimicked in peripheral tissues by a lack of either insulin receptors or insulin sensitivity at receptor or postreceptor sites. When the hyperglycemia becomes sufficiently marked, the renal threshold is surpassed and glucose is excreted in the urine. The hyperosmolarity produced by hyperglycemia and dehydration is the most important determinant of the patient’s mental status.5
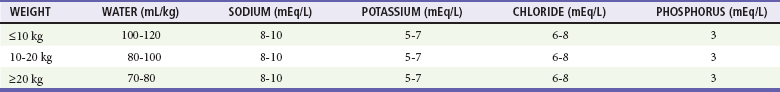
Glucose in the renal tubules draws water, sodium, potassium, magnesium, calcium, phosphorus, and other ions from the circulation into the urine. This osmotic diuresis, combined with poor intake and vomiting, produces the profound dehydration and electrolyte imbalance associated with DKA (Table 126-1). Exocrine pancreatic dysfunction closely parallels endocrine beta cell dysfunction, producing malabsorption that further limits the body’s intake of fluid and exacerbates electrolyte loss.
Diagnostic Strategies
Laboratory Tests
Initial tests allow preliminary confirmation of the diagnosis and immediate initiation of therapy (Table 126-2).4 Subsequent tests are made to determine more specifically the degree of dehydration, acidosis, and electrolyte imbalance and to reveal the precipitant of DKA.
Table 126-2
Typical Laboratory Values in Diabetic Ketoacidosis (DKA) and Hyperglycemic Hyperosmolar State (HHS)
| DKA | HHS | |
| Glucose (mg/dL) | >350 | >700 |
| Sodium (mEq) | low 130s | 140s |
| Potassium (mEq) | ≈4.5-6.0 | ≈5 |
| Bicarbonate (mEq) | <10 | >15 |
| Blood urea nitrogen (mg/dL) | 25-50 | >50 |
| Serum ketones | Present | Absent |
All laboratory determinations must be interpreted with caution. Serum creatinine determinations made by autoanalyzer may be falsely elevated. Leukocytosis more closely reflects the degree of ketosis than the presence of infection. Only the elevation of band neutrophils has been demonstrated to indicate the presence of infection, with a sensitivity of 100% and a specificity of 80% from a single small retrospective study.6 Historically, the diagnosis of pancreatitis in a patient with DKA could be confounded by elevation of amylase levels in DKA. Given the strength of the current literature demonstrating greater specificity of lipase for diagnosis of pancreatitis, lipase should be the blood test of choice if pancreatitis is a concern.
Management
The diagnosis of DKA is generally simple. When hyperglycemia, ketosis, and acidosis have been established, begin fluid, electrolyte, and insulin therapy (Box 126-1).
Insulin
DKA cannot be reversed without insulin, and insulin therapy should be initiated as soon as the diagnosis is certain. There are no randomized trials comparing insulin with placebo or other therapies in DKA. However, the mortality from DKA was 90% in historical controls before development of exogenous insulin and 50% after insulin was introduced; with appropriate supportive therapy, it has reached the current levels of 5 to 7%.5 In the past, very high dosages of insulin were administered to diabetic patients in DKA because they were thought to be extremely insulin resistant. However, several clinical trials done in the 1970s showed that low-dose insulin therapy is as effective as high-dose therapy.
Whereas the dosing of insulin infusions has been established, the utility of an intravenous bolus before the infusion remains controversial and is no longer routinely recommended. More recently, in selected patients with mild DKA, the subcutaneous or intramuscular administration of insulin has been proved safe and as effective as the intravenous administration of insulin. In selected cases with good outpatient follow-up, treatment of DKA with intermittent bolus dosing of regular insulin by the subcutaneous or intramuscular routes without admission has also been shown to be safe. However, this strategy has not been used in sicker patients. Poor perfusion may hamper the absorption of intramuscular or subcutaneous insulin, resulting in erratic absorption.1,5 The current therapy of choice as recommended by the ADA is regular insulin infused at 0.1 unit/kg/hr up to 5 to 10 units/kg/hr, mixed with the intravenous fluids.
Dehydration
Fluid resuscitation alone may help lower hyperglycemia. Because a low level of circulating insulin may be present even in DKA, increased perfusion may transport insulin to previously unreached receptor sites. In addition, a large volume of glucose may be cleared by the kidneys in response to improved renal perfusion. The mean plasma glucose concentration has been noted to drop 18% after administration of saline solution without insulin.1 Whereas fluid administration decreases serum glucose concentration and improves acidosis, the underlying insulin deficiency in DKA still requires administration of insulin for correction of ketoacidosis. Thus, fluid resuscitation is an important factor in management of DKA but is not sufficient therapy by itself.
Acidosis
1. Bicarbonate worsens the inhibition of oxygen release from red blood cells caused by the 2,3-diphosphoglycerate deficiency seen in phosphorus-depleted patients with DKA.
2. Overly rapid correction of acidosis is contraindicated because the blood-brain barrier is much more permeable to carbon dioxide than to bicarbonate. Thus, the correction of intravascular acidosis terminates Kussmaul’s respiration, further augmenting the blood carbon dioxide available to cross the blood-brain barrier. Slowly, sufficient bicarbonate crosses the blood-brain barrier to provide adequate buffering. In the short term, however, as the blood acidosis is corrected, the acidity of the fluid surrounding the brain increases, causing paradoxical cerebrospinal fluid acidosis. The clinical significance of an acidic cerebrospinal fluid pH is unclear.
3. The administration of bicarbonate increases the potassium requirement, both immediately by driving potassium into the cell and more gradually by affecting the kidney, making iatrogenic hypokalemia more likely. When bicarbonate is used, serum potassium levels need to be followed even more closely.
4. The overaggressive use of bicarbonate may produce alkalosis, which induces dysrhythmias largely through its effect on the distribution of electrolytes. Alkalemia occurring late in the course of therapy is more common in patients who have received bicarbonate because ketones are metabolized to carbon dioxide, water, and bicarbonate.
5. Evidence suggests that lowered pH produces a feedback mechanism that directly inhibits ketogenesis. Bicarbonate can increase ketonuria and delay the fall in serum ketones compared with saline infusion alone.
6. Patients treated with bicarbonate fare no better and possibly fare worse than patients treated without bicarbonate. Studies indicate that bicarbonate worsens the prognosis even in patients with severe acidosis and pH values in the range 6.9 to 7.1. It is possible to manage severe DKA with fluids and insulin alone. When this is done, pH normalization is similar to that in a bicarbonate control group.
Complications
Consider cerebral edema when the patient in DKA remains comatose or lapses into coma after the reversal of acidosis. It generally occurs 6 to 10 hours after the initiation of therapy. There are no warning signs, and the associated mortality rate is currently 90%.5 Cerebral edema is rare in adults or children older than 5 years and appears to be most strongly associated with severity of illness (acidemia and azotemia). Subclinical cerebral edema in children is probably common. Furthermore, subclinical cerebral edema may either precede or follow the onset of therapy, raising the question of whether this entity is caused by therapy or is simply a manifestation of the basic pathophysiologic mechanism of DKA. The treatment of cerebral edema is largely supportive and outcomes are poor. No large clinical trials have identified effective treatments for this entity, although some authors recommend mannitol. Steroids have not been shown to be effective.
Hyperglycemic Hyperosmolar State
HHS represents a syndrome of acute diabetic decompensation characterized by marked hyperglycemia, hyperosmolarity and dehydration, and decreased mental functioning that may progress to frank coma. The terminology has changed recently from the former hyperglycemic hyperosmolar nonketotic coma, as some patients have mild degrees of ketosis and coma is not universally present.1,5 Ketoacidosis is generally minimal or absent, although metabolic acidosis from another source, such as lactic acidosis from sepsis or uremia from acute renal failure, may be present. Focal neurologic signs may be present, or there may be a global encephalopathy. DKA and HHS may occur together.
Management
The fluid, electrolyte, and insulin regimens for the initial resuscitation in HHS are subject to the same controversies as the therapies for DKA (see Box 126-1). There are varying recommendations about which intravenous fluids to administer, generally based on calculations of water deficits. There are no well-done randomized trials comparing isotonic versus hypotonic fluid resuscitation, and use of an isotonic crystalloid is a reasonable choice in the volume-depleted patient. Cerebral edema has been noted in isolated case reports in adults, especially with glucose levels above 700 mg/dL. An association between intravenous fluid resuscitation and cerebral edema has not been shown in the literature; previous reports of this association may have been due to the confounder that it is seen in sicker patients who often receive more aggressive fluid resuscitation.
Other Considerations
A vigorous search for the underlying precipitant of HHS should be pursued. Response to therapy should be followed in the manner described for patients in DKA. Phenytoin (Dilantin) is contraindicated for the seizures of HHS because it is often ineffective and may impair endogenous insulin release.1 Low-dose subcutaneous heparin may be indicated to lessen the risk of thrombosis, which is increased by the volume depletion, hyperviscosity, hypotension, and inactivity associated with HHS.
Complications
Reasons for high morbidity and mortality rates are not always clear, but many patients with HHS are elders who have underlying cardiac and renal disease. Pediatric HHS differs from adult HHS in that children have a much higher incidence of fatal cerebral edema. Other causes of morbidity and mortality are similar to those described for DKA. The mortality rate of treated HHS patients has been 40 to 70% in the past but now ranges from 8 to 25%.1,5
Late Complications of Diabetes
Late complications of diabetes cause significant morbidity and mortality. They develop approximately 15 to 20 years after the onset of overt hyperglycemia. The Diabetes Control and Complications Trial showed that tight glycemic control significantly reduces the risk of microvascular disease, such as microalbuminuria (the earliest sign of nephropathy), neuropathy, and retinopathy, but at the expense of greatly increasing the risk of recurrent hypoglycemia.1
Vascular Complications
Diabetes is associated with an increased risk for atherosclerosis and thromboembolic complications, which are a major cause of morbidity and premature death. The cause of accelerated atherosclerosis is unknown, although it is probably related to oxidated low-density lipoprotein and increased platelet activity. Atherosclerotic lesions are widespread, causing symptoms in many organ systems. Coronary artery disease and stroke are common. Diabetic patients have an increased incidence of “silent” myocardial infarction, complicated myocardial infarctions, and congestive heart failure. Peripheral vascular disease is noted clinically by claudication, nonhealing ulcers, gangrene, and impotence. In addition, standard treadmill stress tests have a decreased sensitivity in the detection of coronary artery disease in diabetics. For this reason, exercise or pharmacologic stress echocardiography or a nuclear medicine imaging study should be considered when a provocative test is needed to evaluate the diabetic patient for acute coronary syndrome.1
Neuropathy
Several distinct types of neuropathy have been recognized in diabetes. Peripheral symmetrical neuropathy is a slowly progressive, primary sensory disorder manifested bilaterally with anesthesia, hyperesthesia, or pain. The pain is often severe and worse at night. It affects upper and lower extremities, although lower extremities and the most distal sections of the involved nerves are most often affected. There may be a motor deficiency as well. The pain may be very difficult to control; opioid analgesics have been used, but nonopioid medications such as gabapentin, pregabalin, and amitriptyline are preferred. Pregabalin is the newest of these agents and seems to hold the most promise when it is used at higher doses (up to 600 mg/day). Duloxetine at doses of 60 mg/day is also effective. Both pregabalin and duloxetine achieve significant pain control in at least 50% of patients. Gabapentin at 300 mg three times daily has some efficacy, achieving significant pain relief in about a third of patients; amitriptyline 25 mg daily demonstrates similar results. Aldose reductase inhibitors have not proved to be better than placebo. A reasonable approach for the emergency physician is initiation of duloxetine or pregabalin as these have demonstrated the best efficacy in pain control, with the understanding that it may take several days for peak effect to be reached.7
The Diabetic Foot.: Approximately 20% of hospitalizations in diabetic patients are related to foot problems. Sensory neuropathy, ischemia, and infection are the principal contributors to diabetic foot disease. Loss of sensation leads to pressure necrosis from poorly fitting footwear and small wounds going unnoticed. The most common cause of injury is pressure on plantar bone prominences. Assess all neuropathic foot ulcers for infection, débride devitalized tissue, and obtain radiographs to evaluate for the presence of foreign bodies, soft tissue gas, or bone abnormalities.8 Weight bearing must be eliminated by total-contact casting.
Deeper, limb-threatening infections—as evidenced by full-thickness ulceration, cellulitis more than 2 cm in diameter with or without lymphangitis, bone or joint involvement, or systemic toxicity—are usually polymicrobial in origin and caused by aerobic gram-positive cocci, gram-negative bacilli, and anaerobes. These patients require hospitalization and, after culture, broad-spectrum intravenous empirical antimicrobial therapy (Table 126-3), strict non–weight-bearing status, tight glycemic control, early surgical intervention for débridement, and meticulous wound care. Consider occult osteomyelitis in all cases of neuropathic ulceration.8 Hyperbaric oxygen has been shown to have some efficacy in treatment of complicated infection, especially with anaerobic organisms. Up to one third of patients eventually undergo amputation.
Table 126-3
Common Serious Infections in Diabetics and Their Antimicrobial Therapy
| INFECTIOUS CONDITION | ANTIMICROBIALS |
| Diabetic foot infection | Mild: consider trimethoprim-sulfamethoxazole 800/160 twice daily or clindamycin 300 mg q6h Moderate to severe: clindamycin 600 mg IV q6h ± piperacillin-tazobactam (Zosyn) 3.375 g IV q6h and vancomycin 15 mg/kg IV q12h |
| Malignant otitis externa | Oral: ciprofloxacin 500 mg PO twice daily for 10-14 days IV: ceftazidime 2 g IV q8h ± gentamicin 2 mg/kg IV q8h |
| Mucormycosis | Amphotericin B 1-1.5 mg/kg/day Posaconazole 400 mg twice daily |
| Mucocutaneous candidiasis | Ketoconazole 200 mg PO daily; may need several weeks of therapy |
| Nonclostridial gas gangrene (including Fournier’s) | Clindamycin 600 mg q6h + third-generation cephalosporin + vancomycin 15 mg/kg q12h |
Infections
Diabetic patients are more susceptible to complications of infections because of their inability to limit microbial invasion with effective polymorphonuclear leukocytes and lymphocytes. They have an increased incidence of extremity infections and pyelonephritis compared with the general population. In addition, they are particularly susceptible to certain other infections, such as tuberculosis, mucocutaneous candidiasis, intertrigo, mucormycosis, soft tissue infections, nonclostridial gas gangrene, osteomyelitis, and malignant Pseudomonas otitis externa. Treatment of diabetic patients with infection includes rapid culture and antibiotics (see Table 126-3), glycemic control, and generally hospitalization.
Diabetes in Pregnancy
Before the discovery of insulin in 1922, diabetes in pregnancy was associated with a fetal death rate of 60 to 72% and maternal morbidity of approximately 30%.9 In 1977, a linear relationship between glycemic control and perinatal mortality was discovered. Strict metabolic control is now a goal in all diabetic pregnancies.5
Hypoglycemia is common in pregnancy, in part because of intensive insulin treatment to maintain euglycemia. The effects of hypoglycemia on the fetus are unclear. Ketoacidosis is associated with a 50 to 90% fetal mortality rate.5
New-Onset Hyperglycemia
Patients often present to the ED with typical diabetic symptoms such as polyuria, polydipsia, and polyphagia. Many have serum glucose concentrations above 200 mg/dL but are not ketotic. Patients with newly diagnosed hyperglycemia with normal electrolyte values may be treated with intravenous hydration alone or with insulin, often reducing the glucose concentration to 150 mg/dL. In reliable patients whose initial glucose concentration is greater than 400 mg/dL, initiation of oral hypoglycemic therapy may be appropriate, with lifestyle modification. Obtain an HbA1c value before initiation of therapy to confirm a diagnosis of diabetes and to establish a baseline. Initial therapy with sulfonylureas is appropriate; glyburide (2.5-5 mg once daily) or glipizide (5 mg once daily) is recommended.1,10 Metformin may be initiated as well at a dose of 500 mg daily; however, it lowers blood glucose on average only about 100 mg/dL, and newly diagnosed diabetics frequently require additional agents to control their glucose concentration. Patients with kidney disease may have complications from use of either sulfonylureas or metformin and will likely need insulin therapy. Follow-up should be stressed and warning signs of hypoglycemia discussed.
Control of Diabetes
The common oral antidiabetic agents are presented in Table 126-4. There are several preparations of insulin available as well. The major recent advances in insulin preparations (in addition to regular insulin and NPH insulin) are ultra-long-acting and ultra-short-acting insulins. These agents are coming into favor because of better basal insulin levels (long acting) and more rapid onset and shorter duration of action (Humalog) to provide more rapid and predictable sliding scale coverage. Other newer agents include the incretin mimetics, such as exenatide, which is used for type 2 diabetes; it is injectable and used in conjunction with oral agents but not with insulin. Amylin analogues, such as pramlintide, are used with insulin but significantly increase the risk of hypoglycemia.
Table 126-4
Common Oral Diabetic Medications
| Biguanides (metformin) | Decrease hepatic glycogenolysis | 500-1000 mg twice daily |
| Sulfonylureas (glipizide, glimepiride) | Stimulate pancreatic insulin release | 2.5-5 mg daily |
| Thiazolidinediones (pioglitazone, rosiglitazone) | Insulin sensitizers, decrease hepatic gluconeogenesis | Increased risk of adverse cardiac events |
| Meglitinides (repaglinide, nateglinide) | Stimulate postprandial insulin release | Take with meals only |
| Dipeptidyl peptidase 4 inhibitors (sitagliptin) | Decrease insulin degradation and gluconeogenesis | Once a day; can be found in multiple combination medications |
| α-Glucosidase inhibitors (acarbose, miglitol) | Delay breakdown of carbohydrates in the intestines | Major side effect is diarrhea |
References
1. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34:S11–S62.
2. Halimi, S. Acute consequences of hypoglycaemia in diabetic patients. Diabetes Metab. 2010;36:S54–S58.
3. Lung, DD, Olson, KR. Hypoglycemia in pediatric sulfonylurea ingestion: An 8 year poison center retrospective study. Pediatrics. 2011;127:e1558–e1564.
4. Fasano, CJ, O’Malley, G, Dominici, P, Aguilera, E, Latta, DR. Comparison of octreotide and standard therapy versus standard therapy alone for the treatment of sulfonylurea-induced hypoglycemia. Ann Emerg Med. 2008;51:400–406.
5. Kitabchi, AE, et al. Thirty years of personal experience in hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endocrinol Metab. 2008;93:1541–1552.
6. Slovis, CM, et al. Diabetic ketoacidosis and infection: Leukocyte count and differential as early predictors of serious infection. Am J Emerg Med. 1987;5:1–5.
7. Lipsky, BA, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39:885–910.
8. Bril, V, et al. Evidence based guideline: Treatment of painful diabetic neuropathy. Neurology. 2011;76:1758–1765.
9. Castroino, K, Jovanovic, L. Pregnancy and diabetes management: Advances and controversies. Clin Chem. 2011;57:221–230.
10. Waugh, N, et al. Newer agents for blood glucose control in type 2 diabetes: Systematic review and economic evaluation. Health Technol Assess. 2010;14:1–248.