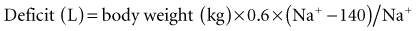167 Diabetes Insipidus
Diabetes insipidus is a disorder of water metabolism associated with polyuria, urine hypotonicity, and hypernatremia.1–3 The quantitative criteria include urine output greater than 200 mL/h or 3 mL/kg/h, urine osmolality less than 150 mOsm/kg, and plasma sodium greater than 145 mEq/L. If urine osmolality measurement is not available, hypotonicity can be assessed from a urine specific gravity less than 1.005.
 Central Diabetes Insipidus
Central Diabetes Insipidus
Neurogenic or central diabetes insipidus is characterized by a lack of antidiuretic hormone (ADH) that may result from any injury to the anterior hypothalamus, pituitary stalk, or posterior pituitary gland. In acute critically ill patients, the most common causes of diabetes insipidus are surgery for pituitary tumors, cerebral trauma, intracranial hypertension, and brain death (Box 167-1). Diabetes insipidus also may occur as a complication of bacterial meningitis or encephalitis, vascular aneurysm or thrombosis, drug administration, or alcohol intoxication. Injuries to the hypothalamus most often yield permanent diabetes insipidus because ADH is synthesized in the hypothalamus itself. Injuries to the pituitary stalk and neurohypophysis more commonly cause transient diabetes insipidus, because hypothalamic ADH secretion can be effective even in the absence of anatomic pathways to the normal site of release. Chronic diabetes insipidus in critically ill patients generally results from tumors of the pituitary region and from the sequelae of cerebral trauma.
 Clinical Picture
Clinical Picture
Clinical signs of hypernatremia usually appear only when the plasma sodium concentration increases to greater than 155 to 160 mEq/L or plasma osmolality increases to greater than 330 mOsm/kg.4 Signs may appear sooner if hypernatremia is associated with other metabolic disorders, particularly with disorders that also increase plasma osmolality. Symptoms mainly include confusion and lethargy. Severe hypernatremia results in coma and sometimes seizures. Acute and severe dehydration and hypernatremia may lead to cerebral shrinkage, sometimes associated with subdural or intraparenchymal hemorrhages.
The formula assumes that only free water has been lost and that sodium stores are normal. Most often, some sodium has been lost together with additional water, and the total water deficit is even higher than that estimated from the formula. A moderate level of hypernatremia (e.g., 155 mEq) already is associated with a free water deficit of more than 4 L and a total water deficit that may be much higher if sodium has been lost.
 Treatment
Treatment
Management of diabetes insipidus includes two components: (1) reduction of excessive urine output and (2) correction of water deficit (Box 167-2). The polyuria of central diabetes insipidus is treated effectively by vasopressin (ADH) or by its synthetic analog, desmopressin acetate (DDAVP [1-deamino-8-D-arginine vasopressin]).5–7 As indicated by its multiple names, vasopressin not only has antidiuretic but also vasoconstrictive and oxytocic effects, whereas desmopressin essentially retains the antidiuretic action. The effects of aqueous vasopressin (4-10 units subcutaneously or intramuscularly) on diuresis begin rapidly but last for only a few hours. Vasopressin must be repeated every 4 to 6 hours, and it has been recommended only for diagnostic purposes or in acute conditions (e.g., trauma) in which the diabetes insipidus might be transient. The effects of vasopressin tannate in oil emulsion (2-5 units intramuscularly) last 48 to 96 hours, but the preparation requires close attention to warming and mixing the suspension before injection. Vasopressin tannate was once standard therapy in patients with central diabetes insipidus, but now it has been abandoned in favor of desmopressin. Where still available, vasopressin tannate may be used in patients who are refractory to desmopressin or who experience significant side effects of the drug. Desmopressin has prolonged effects (8-20 hours) and is appropriate for intravenous (IV), subcutaneous, and intranasal routes. Lypressin is another ADH analog that is appropriate for intranasal use, but its effectiveness is limited by its duration of action of only 4 to 6 hours. Desmopressin is known to increase factor VIII and von Willebrand factor levels and is sometimes used for this purpose in patients with coagulation disorders and in surgical procedures associated with significant bleeding; however its efficacy in the absence of von Willebrand syndrome is doubtful. In the ICU and for acute central diabetes insipidus, desmopressin is initially given as 10 to 20 µg intranasally and repeated every 30 to 60 minutes until urine output is reduced to less than 100 mL/h. The initial dose required to maintain a normal urine volume ranges from 10 to 60 µg in most patients. The total appropriate dose is repeated when urine output again increases to greater than 200 mL/h (i.e., after 8-24 hours). The dosage must be reduced if urine output is excessively decreased. Systematic administration is not recommended because most cases of diabetes insipidus seen in ICUs are associated with acute events and may be incomplete or intermittent or both. The subcutaneous route is seldom used, because absorption may be erratic in vasoconstricted patients and an IV line is virtually always available in ICU patients. Desmopressin is injected IV when the intranasal route is not available (i.e., in cases of rhinorrhea and facial trauma). The required initial dose ranges from 2 to 20 µg and is given as repeated 2- to 4-µg boluses.
 Nephrogenic Diabetes Insipidus
Nephrogenic Diabetes Insipidus
Nephrogenic diabetes insipidus is characterized by the inability of the renal parenchyma to concentrate urine in response to ADH.7–9 The disorder is seldom diagnosed in the ICU and is usually more severe when it is congenital. Hereditary forms generally result from mutations to the AVP-2 receptors or AQP-2 water channels. Acquired forms are due to vasopressin resistance of the distal tubule and collecting duct, or to markedly reduced renal concentrating capacity. Most of them are attributed to electrolyte disturbances and lithium therapy, but many other drugs have been implicated. Nephrogenic diabetes insipidus may be treated with a low-sodium, low-protein regimen that reduces the solute load, thiazide diuretics that induce a mild volume depletion and help reduce urine volume to acceptable values, and nonsteroidal antiinflammatory drugs such as indomethacin that inhibit prostaglandin synthesis.
Key Points
Verbalis JG. Diabetes insipidus. Rev Endocr Metab Disord. 2003;4:177-185.
A general review on diabetes insipidus.
Maghnie M. Diabetes insipidus. Horm Res. 2003;59:42-54.
A general review that focuses on etiology and clinical and radiologic features.
Bagshaw SM, Towsend DR. Disorders of sodium and water balance in hospitalized patients. Can J Anesth. 2009;56:151-167.
Sands JM, Bichet DG. Nephrogenic diabetes insipidus. Ann Intern Med. 2006;144:186-194.
A updated review of nephrogenic diabetes insipidus.
Garofeanu CG, Weir M, Rosas-Arellano MP, Henson G, Garg AX, Clark WF. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45:626-637.
A review of the many causes of acquired nephrogenic diabetes insipidus.
1 Bagshaw SM, Towsend DR. Disorders of sodium and water balance in hospitalized patients. Can J Anesth. 2009;56:151-167.
2 Maghnie M. Diabetes insipidus. Horm Res. 2003;59(Suppl 1):42-54.
3 Verbalis JG. Diabetes insipidus. Rev Endocr Metab Disord. 2003;4:177-185.
4 Adrogue HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493-1499.
5 Fukuda I, Hizuka N, Takano K. Oral DDAVP is a good alternative therapy for patients with central diabetes insipidus: experience of five-year treatment. Endocr J. 2003;50:437-443.
6 Vande Walle J, Stockner M, Raes A, Norgaard JP. Desmopressin 30 years in clinical use: a safety review. Curr Drug Saf. 2007;2:232-238.
7 Garofeanu CG, Weir M, Rosas-Arellano MP, Henson G, Garg AX, Clark WF. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45:626-637.
8 Linshaw MA. Congenital nephrogenic diabetes insipidus. Pediatr Rev. 2007;28:372-380.
9 Sands JM, Bichet DG. Nephrogenic diabetes insipidus. Ann Intern Med. 2006;144:186-194.