Chapter 13 Diabetes
Introduction
Type 1 diabetes mellitus (T1DM) — prevalence and incidence
The prevalence of T1DM accounts for about 10% of all cases of diabetes, occurs most commonly in people of European descent and affects more than 2 million people in Europe and North America.1, 2 A recent update reported that in the US the prevalence of diagnosed and undiagnosed diabetes (all forms in all ages as at 2007) totalled 23.6 million people — 7.8% of the population had some form of the disease. That is, those diagnosed accounted for 17.9 million people and undiagnosed 5.7 million people.2
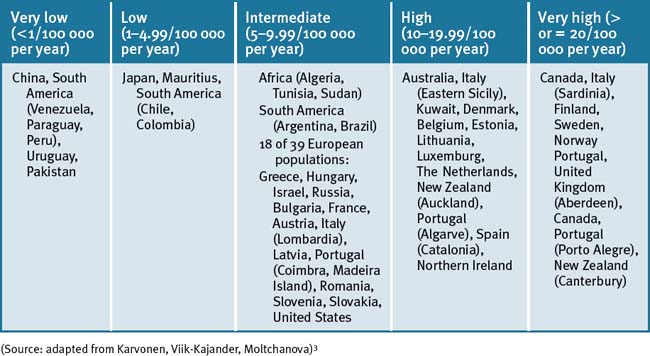
There is also a wide variation in incidence of T1DM from country to country that is evident globally.3 For example, the marked geographic variation in incidence is clearly evident from data that show that a child in Finland has approximately 400 times the likelihood than does a child in Venezuela to acquire the disease.2, 3, 4 Moreover, the incidence of T1DM in children is increasing in Western countries.2, 5, 6 The global incidence of T1DM in children less than or equal to 14 years of age as calculated per 100 000 population has marked variation within ethnic and racial distribution in the world population, as summarised in Table 13.1. The incidence increases with age, highest at 10–14 years of age.
There are also further sub-group variations of T1DM incidence within populations.2, 3, 4 For instance, in the US, non-Hispanic white youth have a higher risk of T1DM.2 A multiethnic, population-based study in the United States estimated the incidence of T1DM (per 100 000 person-years) was 24.3 in children (less than 19 years of age) with the highest rate observed in non-Hispanic white youth (18.6, 28.1, and 32.9 for age groups 0–4, 5–9, and 10–14 years, respectively).7
Type 2 diabetes mellitus (T2DM) — prevalence and incidence
As with T1DM the prevalence of T2DM also varies extensively for different populations from distinct geographical areas.8 At the end of the 20th century, it was estimated that 150 million people in the world had diabetes and the World Health Organization (WHO) predicts that, between 1997 and 2025, the number of patients with T2DM will double to reach about 300 million.9, 10 According to the WHO, T2DM affects up to 7% of Western populations and is increasing in newly industrialised and developing countries.11
The heightened susceptibility and high prevalence of T2DM of Micronesian and Polynesian Pacific Islanders,10, 12 Native Americans,13 Indigenous Australians and Torres Strait Islanders,10, 14 and Asian Indians14 has been well documented.
In Australia, the prevalence of T2DM has been reported to have doubled since 19816, 10 (Table 13.2).
| Men | Women | |
|---|---|---|
| Prevalence | 8% | 6.8% |
| Impaired glucose tolerance and/or impaired fasting glucose | 17.4% | 15.4% |
There are a number of risk factors that have been identified with the development of T1DM and T2DM (Table 13.3).
Table 13.3 Risk factors that have been reported to be associated with T1 and T2DM
| Risk factors for T1 and T2DM |
|---|
(Source: adapted from Adamo, Tesson)15
The number of adolescents with T2DM diagnosed yearly is also increasing globally and has been attributed to factors such as weight gain, poor nutrition and lack of exercise.16
Lifestyle — general, education and prevention of T2DM
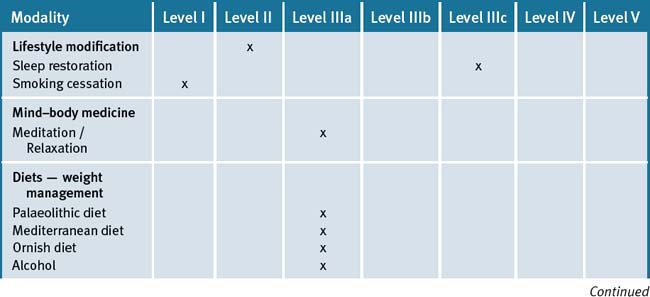
Intensive lifestyle interventions can reduce the incidence of diabetes in people with impaired glucose tolerance, so encouragement of positive lifestyle strategies is essential where social opportunities arise, such as with school education, religion or in any other social community situations. Lifestyle changes can lead to lifelong changes and reduce the incidence of diabetes or complications associated with diabetes, even after active counselling has ceased.17 A post-intervention follow-up study of participants found the risk of developing T2DM was reduced by 43% over a 7-year median period for those who received intensive lifestyle counselling compared with standard intervention.17
A systematic review and meta-analysis of the literature identified 17 randomised trials of 8084 participants and confirmed the value of intensive lifestyle intervention counselling to prevent and delay T2DM onset in people with impaired glucose tolerance.18 Healthy diet and exercise is much more effective at preventing T2DM than drug therapy. When comparing lifestyle intervention and metformin, a study of 3234 subjects over 3 years with impaired glucose tolerance were randomly assigned to a group of placebo, metformin, or a lifestyle-modification program involving diet and exercise over a 2.8 year follow-up. Lifestyle intervention reduced the incidence of developing T2DM by 58% compared with metformin by 31%.19
The aim of a healthy lifestyle to prevent T2DM:
T2DM is associated with a Westernised lifestyle. Lifestyle management is the most effective way of preventing T2DM, especially with dietary measures and exercise. A recent Cochrane review of the literature emphasises the importance that combined diet interventions and exercise significantly reduces the risk of developing T2DM by up to 37% compared with standard recommendations (RR 0.63) with favourable modest effects on reducing blood lipids and blood pressure compared with exercise or dietary changes alone.20
A study of 577 Chinese adults with impaired glucose tolerance without T2DM from 33 Chinese medical clinics, were randomised to either a control group, or 1 of 3 lifestyle intervention groups that included diet or exercise alone, or diet with exercise over a 6-year period and an assessment of the lifestyle intervention group again in the 20 year follow–up.21
The combined lifestyle intervention group significantly reduced the incidence of developing diabetes by up to 51% over the 6-year period and up to 43% in the 20-year follow-up compared with the control group, and significantly more than the diet and exercise groups alone. This study emphasises the importance of promoting intensive lifestyle changes to prevent diabetes onset, particularly in patients with reported impaired intolerance or at high risk of developing DM.21
Dietary factors and T1DM
Cow’s milk consumption in early infancy
Children fed cow’s milk during the first few days of life are twice as likely to develop T1DM than children who are breast fed.22 The association between T1DM may be explained by the generation of specific immune response to beta casein from cow’s milk exposure, triggering cellular and humoral anti- β casein immune response which may cross-react with beta-cell antigen.23 Overall high cow’s milk consumption (3 or more glasses of cow’s milk a day) was associated with a 5.4 fold increased risk of developing T1DM in young children, and this was higher in children who also had HLA genotype for diabetes.24
Dietary factors and T2DM
Obesity is the main cause of developing T2DM according to large scale studies. Whilst lack of physical activity also contributes, the risk of T2DM rises progressively with increasing Body Mass Index (BMI), increasing waist circumference, and obesity.25
Epidemiologic studies suggest that greater consumption of fruit and vegetables reduces the risk of diabetes mellitus. In a population-based prospective cohort (European Prospective Investigation of Cancer–Norfolk) study, 21 831 individuals aged 40–75 years at baseline were assessed for plasma vitamin C level and habitual intake of fruit and vegetables over a 12-year follow-up period. The researchers found a strong inverse association between plasma vitamin C level and T2DM risk. High plasma vitamin C level and, to a lesser degree, fruit and vegetable intake was associated with a substantially decreased risk of T2DM.26
Cultural factors and prevention
Minority ethnic groups found that upper-middle and high-income countries often suffer a higher prevalence of T2DM than the local population, due to cultural and communication barriers, being of lower socioeconomic backgrounds, and having less access to good quality health care.27
For instance, a UK study of a Bangladeshi community found the Islam norms and teachings offers many opportunities for supporting diabetes prevention. The authors noted that interventions designed for the white population needed to be adapted to be meaningful to the Bangladeshi community. So religion may play an important role in supporting health promotion in any sub-community. Working collaboratively between health educators, health professionals and religious leaders should be explored to promote positive lifestyle changes within communities.28
Breastfeeding and prevention
Women breastfeeding for over 6 months significantly reduces the woman’s risk of developing T2DM later in life. The longer the duration of breastfeeding, the greater the protection against T2DM.
A large scale prospective observational cohort study of 83 585 parous women identified a strong association of duration of breastfeeding and the incidence of T2DM. Whilst controlling for other relevant risk factors such as BMI, the study found that the longer the women breastfed the less likely they would develop T2DM. For each additional year of lactation, women with a birth in the prior 15 years had a decrease in the risk of diabetes of up to 15% amongst participants. Lactation improves glucose homeostasis.29
Gender factors and prevention
Studies indicate, in general, women with risk factors and a family history of diabetes were more likely to obtain diabetes education, gain benefits of self-management and reported higher levels of social support from their diabetes health care team than men did. This highlights the special needs for men with respect to diabetes education, understanding risk factors, prevention, self-care and self-management of T2DM.30
Genetics
Whilst studies suggest T1DM is associated with a wide spectrum of susceptibility and protective genotypes within the HLA class II system, it is likely that increasing environmental exposure is able to trigger these genes even in subjects who are less genetically susceptible.31
T2DM is the most common disease in the Western world today that has a genetic link.32 It is the phenotype for more than 150 genotypes.32, 33, 34 Each of these genotypes is characterised by impaired glucose tolerance and impairment of the control of intermediary metabolism.
Mind–body medicine
Meditation, biofeedback, and hypnosis have all been found to improve T2DM.35
Life stressors and/or depression
Stress may be a contributor to T1DM onset according to a prospective population-based study of almost 6000 children. The study demonstrated children had a threefold risk of developing autoimmunity T1DM if their parents divorced or experienced violence during the child’s infancy within 2.5 years of the event.36 The authors concluded that ‘maternal experiences of serious life events…seem to be involved in the induction or progression of diabetes-related autoimmunity in children’.36 Bullying, a form of stress for a child, is also known to adversely affect glycaemic control and self-management, and increase feelings of depression in T1DM children.37
Research demonstrates that regular use of stress management techniques can significantly reduce blood glucose levels in T2DM. After 1 year, compared with control, stress management reduced HbA1c by up to 1% which is clinically significant. Stress management would be a useful addition to a lifestyle intervention program for diabetics.38
Even daily hot baths for relaxation has been shown to lower fasting serum glucose in diabetics.39 The researchers postulated the benefits could result from increased blood flow to skeletal muscles.
Stress and depression both influence hormonal levels.40 Stress and depression increase cortisol, growth hormone and adrenaline. Cortisol and growth hormone increase insulin resistance, and adrenaline stimulate the breakdown of glycogen into glucose (glycogenolysis).41
Depression is associated with insulin resistance and with diabetes.35 Low mood, mild and moderate depression is 2 to 3 times more common in diabetics than in non-diabetics, and is frequently not diagnosed or treated.42 Insulin resistance is positively associated with the development of diabetes.43 A large prospective scale study of 4847 participants without depression symptoms at baseline, at 3 years, treated T2DM was significantly associated with depression with an odds ratio of 1.52, even after adjusting for BMI, socioeconomic status, lifestyle and diabetes severity, and after excluding T2DM patients on antidepressants possibly for diabetic neuropathy. It was felt that depression contributed to diabetes and diabetes also contributed to depression.44
In another large scale study of more than 4600 patients over 65 years or older, depression symptoms over time was associated with a higher incidence of T2DM after adjusting for other diabetic risk factors.45
In a study of patients with work-related depression, the authors found the presence of depression or depressive symptoms was associated with subsequent increased risk of developing T2DM. The relative risk was 1.25 (95% confidence interval [CI]:1.02–1.48) compared with workers not experiencing depression.46
There is a positive correlation between insulin resistance and severity of depressive symptoms with impaired glucose tolerance before the outbreak of T2DM.47
A recent study found that serotonin receptor agonists can improve glucose tolerance, and reduce plasma insulin levels.48
Hence, the proper management of depression and stress are key factors in reducing the risk and progression of diabetes (see also Chapter 12).
Sunshine and Vitamin D
Vitamin D deficiency plays an important role with both insulin synthesis and secretion by way of vitamin D receptors and vitamin D binding proteins in pancreatic tissues as demonstrated in both human and animal models. The mechanism of action of vitamin D is also thought to occur as a direct action on pancreatic beta-cell function and mediated through regulation of plasma calcium levels, which may explain why vitamin D deficiency may play a role in the causation in T1 and T2DM.49
Lack of sunshine exposure is contributing to vitamin D deficiency as most vitamin D forms in the skin as a result of ultraviolet radiation (UVB wavelength; 290–320 nm) exposure from sunlight.50
People living in the southern regions of the southern hemisphere or the northern regions of the northern hemisphere have a higher risk of vitamin D deficiency, particularly during the winter months. Certain ethnic groups are at increased risk of vitamin D deficiency, especially dark skinned people who cover their skin for religious or cultural reasons, the elderly, babies of vitamin D deficient mothers, and people who are housebound or are in institutional care.51
T1DM
There is a significant body of biologically plausible evidence that strongly suggests that vitamin D plays a role in the prevention of T1DM. Giving infants vitamin D supplementation could protect them from T1DM according to a systematic review of 5 observational studies that showed a reduced risk of T1DM by up to 30% compared with infants not supplemented.52
Sleep
A number of studies have demonstrated a strong link between lack of sleep or over-sleeping with T2DM. The odds of developing T2DM for subjects sleeping 5 hours or less or 9 hours or more was associated with a significant increase risk of developing T2DM or impaired glucose tolerance test. Factors such as more environmental lighting, long working hours, television and personal computers are contributing to shorter sleep durations in Western societies.54, 55
Obstructive sleep apnoea may also be a risk factor for T2DM.56
Insomnia, anxiety and depression may double the risk of diabetes in men by 2.2 times compared with those with low levels of psychological distress, independent of risk factors such as BMI, family history for diabetes, and smoking, according to a study of 2127 men and 31 000 women followed up over 8–10 years. Of interest, women with psychological distress and insomnia did not have a higher risk of diabetes.57
Environment
Smoking
Researchers have combined the best available information from 25 studies in a systematic review which followed the health of 1.2 million people for several years.58
There is an increased risk of T2DM of approximately 50% from smoking and the more a person smoked the higher the risk and vice-versa; smoking preceded the development of T2DM.58 Smoking has an adverse effect on pancreatic function. It may cause internal inflammation and increase abdominal fat, even in thin smokers, which could then lead to an increase in insulin resistance. Smoking becomes even more of a problem when combined with T2DM risk-enhancing behaviours such as obesity and inactivity.59
Further studies have confirmed this association. In a study of 906 participants free of DM at baseline and after multivariable adjustment, the researchers found of the current smokers, 25% developed T2DM at 5 years, compared with 14% of those who never smoked.60
Arsenic exposure
Environmental exposure to low levels of inorganic arsenic in drinking water has been associated with diabetes development. Millions of people world-wide are exposed to inorganic arsenic in water from natural mineral deposits. A cross-sectional USA study of 788 adults aged 20 years or older based on a 2003–2004 survey, urine arsenic levels, and after adjustment for biomarkers such as seafood intake, total urine arsenic was associated with increased prevalence of T2DM. Participants with T2DM had a 26% higher level of total arsenic in their urine than participants without T2DM. After similar adjustment, the odds of developing T2DM led to a 3.6 fold increase risk associated with exposure of arsenic. The authors speculated that arsenic could induce diabetes through oxidative stress, inflammation or apoptosis.61
Physical activity
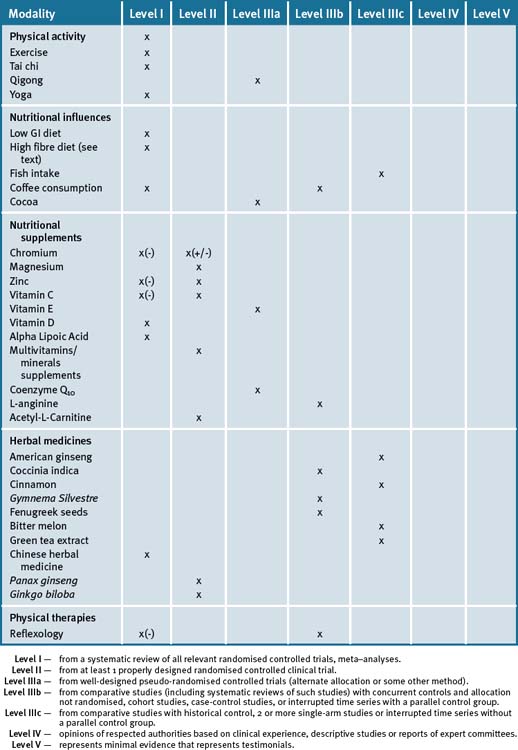
Physical activity is one of the key modifiable risk factors for hyperglycaemia. Evidence from population-based, cross-sectional studies report that both decreased physical activity and elevated sedentary behaviours, such as television viewing time, are independently associated with raised blood glucose levels in adults without a diagnosis of diabetes.62, 63 Regular exercise benefits T1DM and T2DM. Children with T1DM showed improved haemoglubin A1c (HbA1c) and glycaemic control with regular exercise of at least 3 times weekly compared with children who did not exercise.64, 65
Walking just 30 minutes daily can significantly reduce the incidence of T2DM.66 Walking 2 hours weekly can reduce mortality from all causes by 39% and by 34% from cardiovascular disease in patients with T2DM.67 A study of healthy young sedentary men demonstrated a high intensity aerobic exercise protocol comprising a total of 15 minutes of exercise (6 sessions; 4–6 30-second cycle sprints per session) substantially improved insulin action and glucose tolerance test, and supported the important role of exercise in preventing diabetes.68
The size of cities can influence the risk of diabetes. When a person migrates from a rural environment or third-world country to the city or a Western country the risk of T2DM is significantly elevated.69, 70 If that city happens to be of more than 1 million in population, as described in a recent study,69 then the risk of diabetes increases fourfold. It is thought that the design of cities can influence whether the people walk or not.
The position statements from both the American Diabetes Association and the American College of Sports Medicine, has recommended the use of resistance training as part of a physical activity program to reduce the risk of developing diabetes.71, 72
A Cochrane meta-analysis of 14 randomised controlled trials (RCTs) comparing exercise against no exercise in 377 participants with T2DM found that compared with control and independent of weight loss, exercise intervention significantly improved glycaemic control as indicated by a significant decrease in glycated haemoglobin levels of 0.6% (−0.6% HbA1c, 95%CI −0.9 to –0.3; P < 0.05), reduced visceral adipose tissue, plasma triglycerides but not cholesterol levels.73
Of interest, studies indicate aerobic and resistance training alone improves glycaemic control in T2DM, but improvements were greatest with a combined exercise training of both aerobic with resistance training.74
Tai chi
Tai chi is a Chinese system of slow meditative physical exercise designed for relaxation, balance and health. Tai chi has demonstrated improved balance, gait speed, muscle strength, cardiorespiratory fitness, and quality of life in older adults.75–80
However, recent studies including a systematic review have demonstrated that there is some controversy as to its efficacy for patients with T2DM.81
Recently reported RCTs have demonstrated a change in fasting blood glucose levels.79–82 One RCT demonstrated no significant intergroup differences in fasting blood glucose levels compared with a placebo intervention.83
One Chinese clinical trial84 reported superior effects of tai chi on fasting blood glucose levels compared with walking or running, while 2 other Chinese clinical trials85, 86 reported that there was no difference compared with a self-management program, or no treatment.
Three clinical trials reported assessing the effectiveness of tai chi on HbA1c.83, 84, 85 Of these only 1 RCT85 suggested no effectiveness of tai chi on HbA1c compared with a sham exercise regimen whereas 2 Chinese clinical trials83, 84 failed to report intergroup differences compared with no treatment, self-management, walking or running exercise activities. One recent Chinese clinical trial with women diagnosed with T2DM reported that the tai chi group had significantly improved glycaemic control and serum triglyceride levels.86
Qigong
Qigong is another Chinese health practice that is associated with a system of healing and energy medicine. Preliminary studies show positive effects for qigong and reflexology in T2DM.87, 88
A recent review has concluded that although qigong has beneficial effects on some of the metabolic risk factors for T2DM, there exist methodologic limitations that hence make it difficult to draw firm conclusions about the purported benefits.89 Further clinical trials are warranted.
Further, a recent clinical study adds significant strength to the role of tai chi and/or qigong in the management of the metabolic syndrome and glycaemic control in pre-T2DM.90
Yoga
There are few studies that have investigated the efficacy of yoga in T2DM.
Three small studies from India have demonstrated and suggested that yoga asanas and pranayama can better manage glycaemic control and stabilise autonomic functions in T2DM cases.91, 92, 93 Moreover, in 1 of these studies it was reported that yoga improved sub-clinical neuropathy.93
A recent community-based study of yoga classes for T2DM aimed to demonstrate benefits of yoga for diabetic patients but unfortunately experienced ‘recruitment challenges; practical and motivational barriers to class attendance; physical and motivational barriers to engaging in the exercises; inadequate intensity and/or duration of yoga intervention; and insufficient personalisation of exercises to individual needs’ and these factors need to be considered in any future research.94 It also highlights how research in yoga can be difficult.
A systematic review of the literature identified 25 eligible studies, including 15 uncontrolled trials, 6 non-RCTs and 4 RCTs. Overall, the studies demonstrate yoga showing benefit in several risk indices, including ‘glucose tolerance and insulin sensitivity, lipid profiles, anthropometric characteristics, blood pressure, oxidative stress, coagulation profiles, sympathetic activation and pulmonary function, as well as improvement in specific clinical outcomes’.95 Again, the authors acknowledge the limitations of these studies when drawing firm conclusions and encourage additional high-quality trials to confirm the benefits of yoga in diabetics.95
Diet and nutrition
For both Type 1 and Type 2DM, a low-GI diet can ‘improve glycaemic control in diabetes without compromising hypoglycaemic events’ according to a recent Cochrane review of the literature of 11 relevant RCTs involving 402 participants, although no study reported on mortality, morbidity or costs.96 Low glycaemic diet led to a significant decrease in the glycated haemoglobin A1c (HbA1c) and less episodes of hypoglycaemia compared to high GI diet.96
T1DM
A study of 1776 young children (less than 11.5 years of age) who were at increased risk of genetic type 1 diabetes demonstrated that high dietary GI (hazard ratio [HR]: 2.20, 95% CI: 1.17–4.15) and high glycaemic load (HR: 1.59, 95% CI: 0.96–2.64) were associated with rapid progression of islet autoimmunity (IA) to T1DM. Furthermore it was reported that this was possible due to an increased demand on pancreas beta-cell release of insulin and that the development of IA was not associated with high GI foods.97
High fish consumption in the diet may also play an important role in the prevention of T1DM onset. A 12-year study reported a diet rich in omega-3 oils, primarily from fish and other seafood, can reduce the chances of high-risk children developing pancreatic islet beta cell autoimmunity and T1DM by up to 55%. The researchers speculated that omega-3 fatty acids may accentuate T-helper 1 activity associated with autoimmunity and its anti-inflammatory effects may contribute to its benefits.98
A recent study of 532 people over a 5-year period demonstrated that diets high in fat, saturated fat and lower in carbohydrates were associated with worse glycaemic control in T1DM, independent of exercise and BMI.99
T2DM
A low GI diet is as effective as medication in improving glycaemic control in diabetics. Patients with T2DM should be under the care of a dietician or diabetic educator to continually monitor their diet. Dietary compliance is fundamental in the management of T2DM.100
A recent study has reported that in those individuals that are at risk for developing T2DM a change in lifestyle that includes dietary manipulation with a significant decrease in fat intake and a concomitant increase in vegetable and fruit consumption resulted in marked inhibition of the transition from the pre-diabetic state to manifest T2DM.40
It has been documented that a diet rich in wholegrains and seafood will be high in magnesium.41, 101
There is a strong inverse relationship to T2DM with magnesium.102 Also, such diets may be protective against the development of T2DM.103 Wholegrain intake is inversely associated with homocysteine and markers of glycaemic control, and studies suggest a lower risk of diabetes and heart disease in persons who consume diets high in wholegrains.104
Foods rich in soluble fibre such as oats, legumes, guar gum and psyllium are more slowly absorbed and produce lower blood glucose levels.105 Increasing dietary fibre up to 50g daily (such as raisins and oranges) can improve glycaemic control in patients with T2DM.106 There is increasing evidence that inflammation plays an important role in the pathogenesis of diabetes, as obesity is associated with a pro-inflammatory state.107
A Cochrane review that investigated the potential effects of wholegrain foods for the prevention of T2DM has concluded that studies have consistently demonstrated that a high intake of wholegrain foods or cereal fibre is associated with a lower risk of the development of T2DM.108 However, the evidence for a protective effect coming from prospective cohort studies only has to be considered as weak as with this design no cause and effect relationship could be recognised. Hence, it was further concluded that well-designed RCTs are warranted that are able to draw definite conclusions about the preventive effects of wholegrain consumption on the development of T2DM.108
Fish
Fish is protective towards the development of T2DM apart from having a number of health benefits by preventing cardiovascular disease. In a study of insulin-resistant participants, a high fish diet improved insulin sensitivity compared with participants not eating fish.109 Greater fish intake was associated with a lower risk of macroalbuminuria in a self-defined diabetic population.109
An anti-inflammatory diet can include fish, nuts, flaxseeds, fruits and vegetables, ginger and turmeric.110, 111 Partially hydrogenated oils, as in vegetable oils and margarines, are pro-inflammatory and can increase the risk of T2DM.112
Other protective foods
Garlic, green tea, chilli and onions have all been shown to have beneficial effects in DM. Onion and garlic can decrease blood glucose, cholesterol and blood pressure.113, 114
Two RCTs have found that supplementation with soy protein in T2DM improves fasting insulin, insulin resistance, total and low-density lipoprotein (LDL) cholesterol,115, 116, 117 which also showed soy improved glucose control. They also found that regular consumption of chilli could attenuate postprandial hyperinsulinemia.118
Diets with low GI and glycaemic load will modulate swings in blood glucose levels and insulin secretion because of slower absorption and have been shown to improve haemoglobin A1c (HbA1c).115
Other food considerations
Rice consumption
There are also cultural dietary differences that may contribute to high prevalence of diabetes in specific population groups. For instance, in a cohort of 64 227 Chinese women with no history of diabetes or other chronic disease over a 4.6 year period the authors noted dietary carbohydrate intake and consumption of rice (high GI and load) was positively associated with increased risk of developing T2DM.119
Sugary drinks
T2DM is a common problem amongst African American women. A prospective follow-up study of 59 000 African American women reported on food and beverage consumption between 1995 and 2001 and found, after adjusting for relevant confounders such as BMI, the incidence of T2DM was associated with higher intake of both sugar-sweetened soft drinks and fruit drinks. Two or more soft drinks per day increased the risk by 1.24 (95% confidence interval, 1.06–1.45) and for fruit drinks, the comparable incidence rate ratio was 1.31 (95% CI, 1.13–1.52).120
Artificial flavours
A recent animal study with rats found foods using artificial sweeteners resulted in ‘increased caloric intake, increased body weight, and increased adiposity, as well as diminished caloric compensation and blunted thermic responses to sweet-tasting diets’. These results suggest that consumption of foods containing artificial sweeteners may lead to increased body weight and obesity, and so should be avoided.121
Meat intake
High intake of red meat significantly increases the risk of fatal coronary heart disease (CHD) in women with T2DM. Women who consumed the highest levels of haeme iron and red meat also had a 50% increased risk of developing CHD compared with women who consumed the lowest intake.122
Low fat dairy intake
A large scale prospective women’s health study of 37 183 middle-aged non-diabetic women found low fat dairy and yoghurt consumption significantly reduced the risk of developing T2DM.123
A diet rich in low fat dairy food in T2DM helps reduce weight in the overweight and the researchers found no association between dairy calcium intake and other diabetes or CVD disease indexes.124
Vegetarian, plant-based diet
A review of the literature demonstrates a strong link between vegetarian and plant-based diets in reducing the risk of obesity, T2DM, heart disease and some cancers.125 A diet high in vegetables and vegetarianism can significantly reduce the risk of diabetes, improve glycaemic and lipid control and reduce cardiovascular complications.126, 127
Body weight
Obesity is the main predisposing risk factor for the development of diabetes.128
The prevalence of obesity varies among different racial groups and it is associated with approximately 30% of Chinese and Japanese patients with T2DM. Obesity is found in 60–70% of people in Western countries and, although estimates vary, it is thought that the prevalence of T2DM could be as high as 30% of aboriginal people.129 Pima Indians, Pacific Islanders from Nauru or Samoa will all almost certainly develop T2DM with obesity.130
In a study of 1079 participants, intensive lifestyle intervention for overweight patients with impaired glucose tolerance reduced the 3-year incidence of overt T2DM from 35% to 15% by losing 5 kg compared with the control intervention. Every 1 kg of weight loss resulted in a relative risk reduction of developing TSDM by 16%.131
A number of dietary regimes have been recently reported as alternatives for macronutrient intake and the prevention of chronic diseases.132 A comparative study that included the macronutrient contents of 7-day menu plans from the Omni-Heart Study, Dietary Approaches to Stop Hypertension (DASH), Zone, Atkins, Mediterranean, South Beach, and Ornish diets were evaluated for consistency with the US Food and Nutrition Board’s Acceptable Macronutrient Distribution Ranges and with the dietary recommendations of several health organisations for the prevention of cardiovascular disease, cancer and metabolic syndrome. The intent of the study/analysis was to evaluate the healthfulness of the 3 Omni-Heart Trial diets high-carbohydrate (Omni-Carb), high-protein (Omni-Protein), and high unsaturated fat (Omni-Unsat) diets — and were compared with that of the DASH diet and 5 other popular diets, namely the Atkins, Ornish, South Beach, Mediterranean, and Zone diets. The resultant analysis reported that all 3 Omni study diets were consistent with US national guidelines to reduce chronic disease risk. Further, that, popular diets vary in their nutritional adequacy and consistency with guidelines for risk reduction.132
The Mediterranean diet
The Mediterranean diet with its emphasis on vegetables, grains, fish and olive oil has been shown to reduce the risk of atherosclerosis in diabetic patients.133, 134 There is a significant amount of evidence that strongly suggests that the Mediterranean diet could serve as an anti-inflammatory dietary pattern.135 This could help prevent diseases that are related to chronic inflammations that include visceral obesity, T2DM and the metabolic syndrome.135 The rationale for this evidence is further supported by a recent study that demonstrates that adherence to the Mediterranean diet is inversely associated with risk of developing diabetes and the clustering of hypertension, diabetes, obesity, and hypercholesterolemia among high-risk patients.136, 137
Monounsaturated fatty acids (MUFA) — olive oil and nuts
The MUFA (especially olive oil) in the Mediterranean diet appear to play an important preventative role for T2DM, even in healthy subjects. Insulin sensitivity reduced in healthy subjects on diets high in MUFA compared with diets high in saturated fatty acid. Subjects with the Ala 54 allele of the fatty acid-binding protein 2 gene demonstrated improved insulin sensitivity when the saturated fats were replaced by the MUFA.137
A large prospective cohort study involving over 83 000 university graduates without diabetes at baseline demonstrated high adherence to the Mediterranean diet with an 83% reduced risk of developing T2DM.138
Consumption of nuts and peanuts is related inversely with diabetes risk.139 Nuts may also confer cardiovascular benefit and improve lipid profile for patients with T2DM due to their high content of MUFA and nutritional value.140
After 6 months of adding 30g of walnut to a low-fat diet of T2DM patients, compared with those on no walnuts, the LDL cholesterol dropped by 10% and HDL cholesterol increased.141
| Oils and margarines | Vegetables | Nuts |
|---|---|---|
| Polyunsaturated fats: | ||
| Oils and margarines | Fish and other seafood | Nuts and seeds |
| Canola, olive, macadamia, sunflower oil, peanut | Avocados, olives | Almonds, peanuts, cashews, hazelnuts, macadamias, pecans |
| Sunflower, safflower, corn, soybean, sesame, cottonseed, grapeseed, flaxseed oil or linseed | ||
(Source: adapted from Phillips & Carapetis.)142
The Palaeolithic diet
A randomised control study of 29 patients with ischaemic heart disease plus either glucose intolerance or T2DM were randomised to receive (1) a Palaeolithic (‘Old Stone Age’) diet based on lean meat, fish, fruit, vegetables, root vegetables, eggs and nuts or (2) a Mediterranean-like diet based on wholegrains, low-fat dairy products, vegetables, fruits, fish, oils and margarines. The study demonstrated that the Palaeolithic diet — the basis of diet by humans before the introduction of grains, dairy, salt and refined fats and sugars — improved glucose tolerance with a 26% decrease of GI compared with 7% decrease in patients on the Mediterranean diet and independent of waist circumference.143
The Ornish diet
The Ornish diet was originally designed as a therapy to reverse cardiovascular disease and is also applicable to patients with diabetes. The Ornish diet consists of a low fat, high fibre, basically vegetarian diet with 75% of the kilojoules (calories) obtained from carbohydrates.144–146
Critical components of the Ornish dietary plan also include physical activity, yoga and relaxation therapy. In a group of T2DM patients requiring insulin who had adhered to the plan, 60% no longer required insulin at the end of the study.144
Caffeine
A systematic review of the literature identified coffee consumption as being protective towards the development of T2DM, reducing the risk by up to 35%.147
A large-scale longitudinal study demonstrated a significant reduction in incidence of glucose intolerance and T2DM of up to 64% in white middle-class people who drank regular coffee consumption compared with people who never drank coffee.148 This benefit persisted in those even after ceasing coffee consumption.
Similarly, another large-scale population-based study of approximately 2400 subjects found that coffee consumption was found to be significantly and inversely associated with fasting glucose, 2-hour plasma glucose, and fasting insulin in both men and women compared with non-drinkers of coffee.149
A prospective, community-based cohort of 12 204 non-diabetic, middle-aged men and women assessed risk of caffeine consumption as part of the Atherosclerosis Risk in Communities (ARIC) Study. After adjusting for potential confounders, increased coffee consumption was significantly associated with a decreased risk of diagnosed T2DM in community-based US adults.150
Cocoa
Based on epidemiological data, diets rich in flavanols are associated with reduced cardiovascular risk and may play a role in reversing vascular dysfunction in diabetics. Cocoa is rich in flavanols. In a study of 41 medicated diabetic patients over a 30-day period, dietary intervention with flavanol-rich cocoa (321mg flavanols per dose) significantly increased circulating flavanols, vascular function and flow-mediated dilation of the brachial artery compared with a nutrient-matched control (25mg flavanols per dose). The benefits of flavanol-rich cocoa (321mg flavanols per dose) were dose dependent up to 3 times daily.151
Alcohol
A large-scale US cross-sectional study of 32 000 people found an inverse relationship between alcohol intake and glycaemic control. On average, T2DM patients who drank alcohol in moderate amounts had HbA1c levels 1.2 less than non-drinkers.152
A multicenter trial randomly assigned 109 patients (41–74 years old) with T2DM who abstained from alcohol to receive 150 ml wine (13g alcohol) at dinner or non-alcoholic diet beer (control) each day during a 3-month period. The study found initiation of moderate daily alcohol consumption reduced fasting plasma glucose but not postprandial glucose and improved sleep in T2DM patients. The authors concluded alcohol may have a favourable glycaemic effect on HbA1c. There were no significant changes on liver function tests.153
Nutritional supplements
Vitamins
Antioxidant micronutrients may play a role in the prevention of diabetic complications.154
Vitamin C
A large population study has reported an inverse relationship between serum vitamin C levels and HbA1c.155 Vitamin C also improves endothelium-dependent vasodilatation in T2DM.156 A recently reported trial on the combined use of chromium and vitamins C and E as well as chromium on its own was effective for reducing metabolic dysfunction and improvement of glucose metabolism in T2DM patients.157
A study of 84 patients with T2DM were randomly assigned to either 500mgs or 1000mgs of vitamin C for 6 weeks. Whilst the dose of 500mgs daily vitamin C had no impact on blood parameters, the group taking 1000mgs of vitamin C daily demonstrated significant decrease in fasting blood sugars, triglyceride and LDL cholesterol levels, HbA1c and serum insulin. These results suggest 1000mgs vitamin C may be beneficial for patients with T2DM and reducing the risk of complication.158
A recent Cochrane review has concluded that there were no relevant clinical trials identified for the effective treatment diabetic eye disease with vitamin C and superoxide dismutase.159
Vitamin E
Vitamin E has been reported to be useful in preventing the long-term complications of diabetes through its synergistic antioxidant effect with vitamin C, improvement of insulin sensitivity and reduction of LDL cholesterol oxidation.160 Vitamin E also has been reported in an early study to reduce platelet aggregation and hence the risk of thrombosis.161
Vitamin D and calcium
Our best source of vitamin D is from direct sun exposure.
Vitamin D deficiency is common in T1 and T2DM has been shown to alter insulin synthesis and secretion in animal models of the disease, a result that has also been demonstrated in humans.53, 162
There is a significant body of biologically plausible evidence that strongly suggests that vitamin D plays a role in the prevention of T1DM and T2DM development and management.53, 163
Furthermore, a recent meta-analysis of the literature has noted an increased association between vitamin D and calcium deficiency and postulate combined supplementation with both nutrients may be beneficial in optimising glucose metabolism in both T1DM and T2DM.49, 164
Vitamin B1 thiamine
A recent randomised placebo-controlled pilot study has demonstrated that high dose thiamine supplementation may be beneficial for patients diagnosed with T2DM.165 This small pilot study compared a thiamine supplement administered at a dose of 300mg per day for 3 months to placebo. There was a 2-month washout follow-up. The primary endpoint was a change in urinary albumin excretion. Other markers of renal and vascular dysfunction and plasma concentrations of thiamine were also determined. The study concluded that high-dose thiamine therapy produced a regression of urinary albumin excretion in T2DM patients with microalbuminuria. Thiamine supplements at high dose could provide improved therapy for early-stage diabetic nephropathy. There was, however, no effects observed in glycaemic control, dyslipidaemia or blood pressure. There were no reported adverse effects due to thiamine therapy.165
Vitamin B3 nicotinic acid
A 16 week trial of 148 patients trialled on low dose extended nicotinic acid of 1000 milligrams and 1500md/day and when compared with placebo led to increases in HDL cholesterol by 19 and 24% and reduction in triglycerides by 13 and 28% respectively, with only a slight reduction of LDL cholesterol with the higher dose.166 Nicotinic acid may play a role in diabetics for lipid management. Only 1 subject withdrew from the trial due to flushing from nicotinic acid.
Vitamin K
Vitamin K may be potentially beneficial for insulin resistance. A trial assessing the role of vitamin K supplementation (500 μg/d of phylloquinone) on bone loss, also found that Vitamin K supplementation for 36 months at doses attainable in the diet may reduce progression of insulin resistance in older men, but not women.167
Vitamin B12
Whilst vegan diets may help in the prevention and management of T2DM, there is a risk of patients developing vitamin B12 deficiency especially those taking metformin which interferes with vitamin B12 absorption.168
The risk for vitamin B12 deficiency was accentuated with prolong metformin use or when used in higher doses. Patients on metformin should have their vitamin B12 levels checked annually. The situation is magnified in diabetics on vegan diets, coupled with metformin use needing to have their vitamin B12 levels carefully monitored.169
Multivitamin/mineral supplements
Elevated C–reactive protein levels have been associated with the risk of cardiovascular disease and T2DM. An analysis of the data from a randomised, double-blind, placebo-controlled study was carried out at the end of the trial and it was reported that multivitamin use was associated with lower C–reactive protein levels.172 Recently, it was demonstrated in an RCT that the effects of vitamins C and E, and also when in combination with magnesium and zinc, were a significant improvement of glomerular, but not tubular, renal function in T2DM patients.173 Furthermore, in randomised placebo-control trials after 3 months of using the combined multivitamin and mineral supplements, the treatment group had significant increased HDL cholesterol and apolipoproteins (apo) A1 enzyme levels, and reduced blood pressure in T2DM patients but no change in serum levels of total cholesterol, LDL cholesterol, triglyceride, and apo B levels. This suggests that the multivitamin and mineral supplements may have a favourable cardiovascular benefit by raising HDL levels and reducing blood pressure in T2DM. Supplementation with vitamin C and E and Zn when used alone did not show any benefit.174, 175
Other studies have not been able to demonstrate any benefit of antioxidants on fasting blood glucose levels in T2DM.176
Middle-aged and elderly patients with T2DM experienced a significant reduction of infections such as flu-like illnesses respiratory, gastrointestinal and urinary tract infections, and days off work (17% of diabetics) in those who took a daily multivitamin supplement compared with those who took placebo (90% of diabetics).177 Researchers postulated patients with diabetes were more likely to have suboptimal intake of micronutrients which would explain the positive effect.
Minerals
Chromium
Chromium has been the most widely studied supplement for diabetes as it is essential for the metabolism of glucose and blood lipids by the body. Low levels of chromium are associated with increased risk of T2DM.178
Numerous in vitro and in vivo studies suggest that chromium supplements, particularly niacin-bound chromium or chromium-nicotinate may be effective in attenuating insulin resistance and lowering plasma cholesterol levels.179 Chromium functions as a co-factor for insulin, regulating the activity of insulin. It has been reported that T2DM patients, who are chromium-deficient, are likely to benefit from chromium supplementation.180–189
A recent review has concluded that there was no significant effect of chromium on lipid or glucose metabolism observed in people without diabetes.190 However, chromium supplementation significantly improved glycaemia among patients with diabetes.190 A further review reported that there was greater bioavailability of chromium picolinate compared with other forms of chromium, such as niacin-bound or chromium trichloride. This may explain the comparative superior efficacy in glycemic and lipidemic control.191 The pooled data from the studies that were investigated that used chromium picolinate supplementation for T2DM patients demonstrated substantial reductions in hyperglycaemia and hyperinsulinemia, which then equates to a significant reduced risk for disease complications.191 Collectively, the data supported the safety and therapeutic value of chromium picolinate for the management of cholesterolemia and hyperglycaemia in those patients diagnosed with T2DM.191
Recent studies with selected populations presenting with insulin resistance have also reported significant benefits with chromium picolinate supplementation either alone or in combination with biotin.192–195 In 1 study it was reported that 1000 μg/day of chromium picolinate therapy improved insulin resistance in some HIV-positive patients, however, highlighting that extra caution was warranted in this population.192 Chromium in combination with biotin may have a beneficial effect on reducing atherosclerosis in patients with T2DM.196
Moreover, clinical trials suggest positive effects of chromium in T2DM patients with hypercholesterolaemia, with hypercholesterolemic patients (prescribed statins), moderately obese to obese T2DM patients (prescribed anti glycemic medications) benefited significantly from the combined use of chromium picolinate and biotin190, 194, 195, 196 as ascertained by improved blood lipid and glucose profiles.
There have also been negative findings of chromium on T2DM. A double-blind trial of chromium picolinate 800mcg/day showed no difference in impaired glucose tolerance progression compared with placebo197 and a systematic review of the literature found chromium had no effect on glucose or insulin concentrations in non-diabetics and was inconclusive for diabetics.198
Magnesium
Magnesium is the second most abundant intracellular divalent cation and has been established as a cofactor for over 300 metabolic reactions in the body.199 Magnesium is also essential in glucose metabolism.199 Magnesium deficiency is common in patients with diabetes200–204 and a growing body of research suggests a significant inverse association between magnesium intake and diabetes risk.205–214
A meta-analysis of prospective cohort studies including more than 286 000 subjects demonstrated that magnesium dietary intake, either dietary or supplemental magnesium (100mg daily), were equally inversely associated with significantly reducing incidence of T2DM and suggest increased consumption of magnesium-rich foods such as wholegrains, beans, nuts, and green leafy vegetables may reduce the risk of T2DM.202 In another meta-analysis study of the literature, dietary fibre especially high in cereal fibre and magnesium intake is strongly associated with reduced risk of T2DM. The meta-analysis incorporating data from 9 cohort studies on fibre intake and 8 from magnesium intake, showed 33% reduction in diabetes risk with high cereal intake and 23% reduction of diabetes risk with magnesium intake.215 Another meta-analysis has confirmed this finding.216
Magnesium intake is clearly inversely correlated with the risk of developing T2DM.216–222
Two large prospective epidemiological studies,202, 203 demonstrated that magnesium had a significant inverse association between magnesium intake and T2DM. Moreover, 2 RCTs223, 224 with magnesium supplementation reported that significant reduced homeostasis model analysis for insulin resistance (HOMA-IR) index compared to controls on 1 study (2.5 gm/day for 12 weeks versus placebo)223 and significantly improved insulin sensitivity and metabolic control (lower HOMA-IR index), lower fasting blood glucose levels, and lower HbA1c compared to controls in the other (50 gm of magnesium chloride/1,000mL of solution for 16 weeks versus placebo).224
Zinc
The mineral zinc plays a key role in the synthesis and action of insulin, both physiologically and in T2DM. Zinc seems to stimulate insulin action and insulin receptor tyrosine kinase activity.225
Diabetics excrete more zinc in their urine than normal amounts 224 and often serum zinc levels are lower in diabetic patients than in healthy controls.226 A number of studies indicate oral supplementation of zinc can improve T2DM and metabolic syndromes and reduce cardiovascular and neurological complications.226–231 A double-blind placebo-controlled study of T2DM patients who received either zinc sulfate (30mg elemental zinc daily) or placebo for 3 months found the treatment group demonstrated elevated zinc levels, improved glycaemic control as evidenced by a decrease in HbA1c levels.226 Patients diagnosed with T2DM have demonstrated less metabolic dysfunctions when supplemented with 30mg of zinc daily.231, 232
A Cochrane review has concluded that there were no significant differences favouring people receiving zinc supplementation compared to placebo. Hence there is no evidence to suggest that the use of zinc supplementation is effective in the prevention of T2DM.233
Vanadium
A number of in vitro studies demonstrate vanadium salts to mimic the effects of insulin on main target tissues of the hormone, and in animal studies vanadium can induce a sustained fall in blood glucose levels in insulin-deficient diabetic rats, and improve glucose homeostasis in obese, insulin-resistant diabetic rodents suggesting therapeutic potential of vanadium salts in the treatment of T2DM.234, 235
References and further reading may be available for these articles by Brichard and Henquin,234 and by Preet et al.235
Other supplements
Alpha lipoic acid
Alpha lipoic acid is a potent antioxidant which enhances glucose uptake, prevents glycosylation and is useful in diabetic neuropathy.236 A recent meta-analysis has reported that treatment with alpha-lipoic acid (600mg/day IV) over 3 weeks was safe and significantly improved both positive neuropathic symptoms and neuropathic deficits to a clinically meaningful degree in T2DM patients with symptomatic polyneuropathy.237
Arginine — amino acid
L-Arginine is an essential amino acid that is used by endothelial cells to produce nitric oxide (NO). Disturbance of the arginine to NO pathway can lead to endothelial dysfunction, resulting in vascular wall damage and directly leading to cardiovascular risk factors such as atherosclerosis and hypertension. Factors that may disturb this pathway include inflammation caused by insulin resistance, hyperglycaemia, elevated visceral fat, smoking and high cholesterol. Studies also indicate that arginine supplementation may benefit patients with cardiovascular risk factors by improving small vessel endothelial function.238–240
Research suggests oral supplementation with up to 3g of arginine can help to restore endothelial function and improve insulin sensitivity. For instance, a double-blind trial on T2DM patients demonstrated that a dose of 3g of arginine 3 times daily over 1 month improved cardiovascular risk and insulin sensitivity significantly as evidenced by an increase in baseline forearm blood flow by 36%, increased glucose disposal by 34%, reduced systolic blood pressure by 14% and reduced endogenous glucose production by 29%.241
Coenzyme Q10 (CoQ10)
CoQ10 may improve glycaemic control and insulin requirement in patients with T1DM and also blood pressure control in patients with T2DM.242, 243
A recent double-blind crossover study of 23 statin-treated T2DM patients with LDL cholesterol <2.5mmol/L and endothelial dysfunction (brachial artery flow-mediated dilatation [FMD] <5.5%) were randomised to oral CoQ10 200mg/day or placebo for 12 weeks. Compared with placebo, CoQ10 supplementation increased brachial artery FMD, improving endothelial dysfunction in statin-treated T2DM patients, possibly by altering local vascular oxidative stress.244
Fish oils and/or omega-3 fatty acid
A trial of 162 healthy individuals randomly assigned to a 3-month diet rich in monounsaturated fats or saturated fats and a second group randomisation to fish oil (n-3 fatty acids 3.6 g/day or placebo) found fish oil supplements or high dietary intake of n-6 and n-3 fatty acids of did not affect insulin sensitivity, insulin secretion, beta-cell function or glucose tolerance.245
Type 1 DM
Supplementing the diets of children who are at increased risk of developing T1DM with omega-3 fatty acid may reduce the risk by up to 37% by helping to prevent the development of autoimmune antibodies towards islet cell within the pancreas.246
Type 2 DM
A review of the literature inclusive of 18 randomised trials explored the use of fish oil supplementation in patients with T2DM as they are at increased risk of cardiovascular disease.247 Whilst blood sugar levels were not affected, the meta-analysis demonstrated a statistically significant effect of fish oil in lowering triglycerides by 0.56mmol/l (95% CI, −0.71 to −0.40mmol/l) and raising LDL cholesterol by 0.21mmol/l (95% CI, 0.02 to 0.41mmol/l). No effect was seen on HbA1c, total or HDL cholesterol. Despite these findings fish oil supplementation may still play a role in patients with T2DM as it has a clear preventative role in patients with cardiovascular disease risk factors according to the National Heart Foundation of Australia.248 A recent Cochrane review249 has concluded that although some types of fat in the blood are reduced through omega-3 fatty acid supplementation, others including LDL cholesterol were increased. A concern as it may predispose to CV disease. Moreover, the control of blood sugar levels was not affected by the treatment.
Herbal medicines
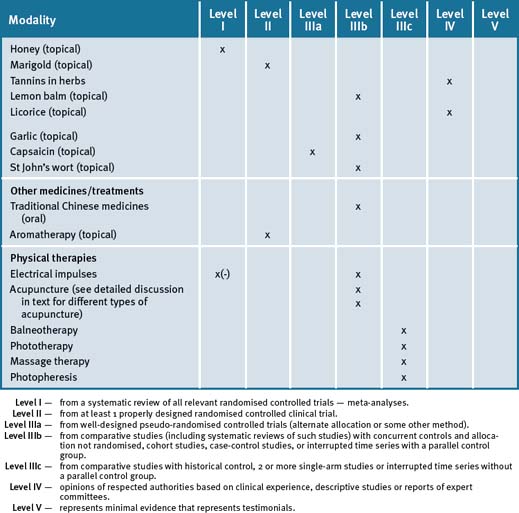
A systematic review of the published literature on the efficacy and safety of herbal therapies and vitamin/mineral supplements for glucose control in patients with diabetes identified a total of 108 trials examining 36 herbs (single or in combination) and 9 vitamin/mineral supplements, involving 4565 patients with diabetes or impaired glucose tolerance.250 There were 58 controlled clinical trials involving individuals with mostly T2DM or impaired glucose tolerance. The direction of the evidence for the 58 trials was for improved glucose control positive in 76% (44 of 58) with very few adverse effects reported. The authors conclude that there is still insufficient evidence to draw definitive conclusions about the efficacy of individual herbs and supplements for diabetes but several supplements warrant further study. The review identified the best evidence for efficacy from adequately designed RCTs is available for Coccinia indica (Ivy gourd) and Panax quinquefolius (American ginseng). Other herbs with positive preliminary results include Gymnema sylvestre, Aloe vera, Momordica charantia (bitter melon), and nopal (prickly pear cactus).250
American ginseng (Panax quinquefolius)
Doses of 1–3g of American ginseng reduced postprandial glycaemia, fasting glucose and HbA1c in healthy and T2DM subjects in 2 trials.251, 252
Korean or Asian Red Ginseng (Panax ginseng)
Numerous studies have demonstrated the benefits of Panax ginseng as a potential to improve diabetic control. A randomised control trial (RCT) of T2DM who received 2g of ginseng 6 times daily (6g/day) 40 minutes before meals, improved glycaemic control, glucose and insulin levels compared with placebo.253
Korean and American ginseng are known to possess sulfonylurea-like activity.
Cinnamon
Cinnamon has been reported to enhance insulin sensitivity.254 Cinnamon has also been reported to improve blood glucose, triglyceride, HDL cholesterol and LDL cholesterol levels.255 Chromium and polyphenols from cinnamon may explain its potential benefit for diabetes and improving insulin resistance.256
A recent meta-analysis reported on 5 RCTs that had reported data on HbA1c, fasting blood glucose or lipid parameters.257 The prospective RCTs258–262 covered 282 participants and it was concluded that the use of cinnamon did not significantly alter on HbA1c, fasting blood glucose or lipid parameters.
Despite these findings a recent small study demonstrated that cinnamon could play an important role as a dietary supplement for poorly controlled diabetics. Ingestion of 3g of cinnamon on 300g of rice pudding daily reduced serum insulin levels after mealtime and increased the concentration of glucagon-like peptide 1 (GLP–1) in 15 healthy Swedish men (n = 9) and women (n = 6) when compared with 1g or no cinnamon after a period of fasting.263
Gymnema silvestre
Preliminary studies support the use of Gymnema silvestre.264, 265 Gymnema silvestre has been reported to suppress sweet taste in liquid form, which can result in reduced calorie consumption.264–273
Fenugreek (Trigonella foenum)
This herb is a soluble fibre and hence slows gastric emptying, delaying glucose absorption. Fenugreek also raises the rate of insulin release plus increasing insulin sensitivity.274 There is an extensive body of in vitro and in vivo animal evidence for the biological plausible effect of fenugreek in controlling blood glucose levels.273–306
Fenugreek seeds has been documented to lower fasting blood glucose in both T1 and T2DM compared to controls.306, 307 Reduction in triglyceride levels has also been noted.307
Bitter melon (Momordica charantia)
Bitter melon has been documented to contain several insulin-like polypeptides with other substances that lower glucose levels and it also has been reported to increase insulin sensitivity.308 Moreover, it has been in involved regulating glucose uptake in the jejunum and stimulates glucose uptake into skeletal muscle.
A number of clinical and preclinical trials have documented the hypoglycaemic effects of bitter lemon in T2DM.309 A recent small controlled RCT trial with outpatients who were either newly diagnosed or poorly controlled T2DM with HbA1c levels between 7% and 9% were randomised to either bitter melon capsules or placebo in addition to standard therapy. The study targeted a 1% decline in HbA1c at the outset with an estimated power of 88%. With the observed decline of 0.24%, the study achieved a power of only 11%. Hence no definite conclusions as to the effectiveness of bitter melon were possible.310 Larger controlled studies though are warranted.
Green tea extract
There have been conflicting studies using green tea extract for T2DM. One study demonstrated favourable effects with a green tea extract demonstrating a significant reduction of haemoglobin A1c levels and borderline reduction in diastolic blood pressure in patients with T2DM.311
Another study found green tea extract in obese middle-aged male subjects had no effect on insulin sensitivity, insulin secretion or glucose tolerance, but did reduce diastolic blood pressure and had a positive effect on mood.312
Ginkgo (Ginkgo biloba)
Whilst it is not clear if ginkgo may help T2DM, and there have been mixed results on insulin sensitivity in animal studies, the herb may have a cardiovascular benefit for patients.313–331
Ginkgo is recognised to assist in improving peripheral circulation and intermittent claudication and some studies suggest it may play a role in alleviating early diabetic neuropathic pain.330, 331
Other potentially hypoglycaemic herbal medicines
The following herbal medicines may also be useful in the treatment of T2DM. These include bilberry, milk thistle, pycnogenol, Inolter, Ginkgo biloba, aloe vera, ginseng, mulberry, chamomile, cranberry, Chinese herbal medicines, Tibetan herbal medicine332 and red clover isoflavone although more studies are required.319, 331–336
Chinese herbal medicine
Chinese herbal medicines have been investigated in 2 recent systematic reviews for the treatment of T2DM.337, 338 A Cochrane review337 included 66 randomised trials that involved 8302 participants that met the inclusion criteria. Sixty-nine different Chinese herbal medicines were tested in the included trials, which compared herbal medicines with placebo, hypoglycaemic drugs, or herbal medicines plus hypoglycaemic drugs.
In the trials that compared herbs with placebo, Holy basil leaves, Xianzhen Pian, Qidan Tongmai, traditional Chinese formulae, Huoxue Jiangtang Pingzhi, and Inolter demonstrated a significant hypoglycaemic response. In the trials that compared herbs with hypoglycaemic drugs that included glibenclamide, tolbutamide, or gliclazide, 7 herbal medicines demonstrated a significant better metabolic control, including Bushen Jiangtang Tang, Composite Trichosanthis, Jiangtang Kang, Ketang Ling, Shenqi Jiangtang Yin, Xiaoke Tang, and Yishen Huoxue Tiaogan. In 29 trials that assessed herbal medicines combined with hypoglycaemic drugs, 15 different herbal preparations demonstrated additional enhanced effects than hypoglycaemic drugs monotherapy. Two herbal therapies combined with diet and behaviour change showed better hypoglycaemic effects than diet and behaviour change alone. No serious adverse effects from the herbal medicines were reported in these trials.337 Generally methodological quality was reported to be of a low quality.
In a second review338 it was reported that the use of Chinese herbal medicines for the treatment of T2DM was promising but there was a strong requisite for further studies.
Ayurvedic herbs
Ayurveda is a traditional healing system which originated in India. Some Ayurvedic herbs have glucose-lowering effects and deserve further study.339 A systematic review of the literature identified 54 articles reporting the results of 62 studies. The most-studied herbs were Gymnema sylvestre, Coccinia indica, fenugreek, and Eugenia jambolana. A number of herbal formulas were tested, but Ayush-82 and D-400 were most often studied. There were large variations in the quality of the studies. Of the 10 RCTs, case-control trials or natural experiments included in the review, 12 were case series or cohort studies. The study concluded there was evidence to suggest that the following herbs C. indica, holy basil, fenugreek, and G sylvestre, and the herbal formulas Ayush-82 and D-400 had a glucose-lowering effect and that further studies were warranted.340
Physical therapies
Reflexology
Reflexology (or zone therapy as it is also known) is the practice of massaging, squeezing, or pushing on parts of the feet, or the hands and ears, with the goal of encouraging a beneficial health effect on other parts of the body, thereby improving overall general health.343 An early study344 suggested that foot reflexo-therapy was an effective treatment for T2DM. However, a recent review of the scientific literature has concluded that there was no clinical evidence that reflexology had any beneficial effect on T2DM.345
T2DM peripheral neuropathy
Diabetic neuropathy is a common late complication of diabetes and is usually associated with neuropathic pain. Its prevalence is estimated at 15% of people with diabetes. There are several different presentations of diabetic neuropathy, the commonest being chronic diabetic peripheral neuropathy characterised by pain in the feet and ankles. The National Prescribing Service recommends non-drug strategies as part of any management plan to include stress reduction, sleep hygiene, physiotherapy, psychological support and transcutaneous electrical nerve stimulation (TENS).346
Vitamin D
Vitamin D supplementation may also be an effective treatment of neuropathic pain in T2DM patients. A study of 51 patients with vitamin D deficiency (mean serum 25 hydroxy-vitamin D concentration of 18 ng/mL) and T2DM, and suffering typical neuropathic pain after 3 months, vitamin D supplementation with cholecalciferol (vitamin D3) tablets resulted in a significant reduction in pain scores, with pain severity reducing by up to 50%. The researchers suggest that vitamin D insufficiency may potentiate diabetic nerve damage and impair nociceptor function.349
Alpha lipoic acid
In a study of 443 diabetic patients with painful neuropathy, after receiving daily supplementation of 600mg daily alpha–lipoic acid over an average 5-year period, patients were switched onto 600–2400mg daily gabapentin or no treatment. In the no-treatment group 73% developed neuropathic symptoms 2 weeks after cessation of alpha-lipoic acid and 55% of the gabapentin treatment group, did not respond to doses up to 2400mgs daily, requiring other pain relief medication. Moreover, 45% of the gabapenitn group developed intolerable side-effects, requiring outpatient visits up to 7.9 times in 3 months compared with only 3.8 times per 3 months whilst taking alpha-lipoic acid. The authors concluded that alpha–lipoic acid was an effective, safer and more cost-effective method of treating diabetic neuropathy.350
Studies also suggest alpha-lipoic acid supplementation in T2DM may assist in accelerating chronic wound healing in patients undergoing hyperbaric oxygen therapy.351
Acetyl–L–Carnitine (ALC)
Research supports the role of carnitine for the treatment of diabetic peripheral neuropathic pain.352 Carnitine is produced from the amino acids lysine and methionine and is essential for the breakdown of fat into energy. Carnitine may improve the utilisation of fats for energy and may be beneficial in heart disease, enhancing physical performance and diabetes. ALC is often deficient in diabetes.
Two randomised placebo-controlled clinical diabetic neuropathy trials over a 52-week period tested 2 doses of ALC: 500 or 1000mg/day given 3 times daily demonstrated that supplementation with ALC, especially at 1000mgs, is efficacious in alleviating symptoms, particularly pain, and improving nerve fibre regeneration and vibration perception in patients with established diabetic neuropathy. Nerve conduction velocities and amplitudes did not improve.353, 354
In another review of the literature that identified 2 large clinical trials involving 1679 subjects, those who received at least 2g daily of ALC showed significant reduction in pain scores in patients with diabetic neuropathic pain. One study showed improvements in electrophysiologic factors, such as nerve conduction velocities, and nerve regeneration was documented in 1 trial. ALC was well tolerated and the authors suggested it should be recommended to patients early in the disease process to provide maximal benefit.355
Conclusion
Often it is difficult for patients to take on the responsibility for their illnesses. Taking responsibility is essential for the best outcome in patients diagnosed with T1DM and T2DM, particularly as lifestyle plays an important role in the prevention and management of diabetes. T2DM is the leading cause of death from cardiovascular disease associated complications such as macrovascular and microvascular disease. An integrative approach to management is essential (see Table 13.5). (For management of gestational diabetes, see Chapter 32.)
Table 13.5 Levels of evidence for lifestyle and complementary medicines/therapies in the management of type 2 diabetes mellitus
The most important risk factors in this patient population are stressors, depression, poor diet, obesity and lack of exercise. An integrative approach which highlights patient participation as a key factor in their care is most applicable and would be most beneficial. A diet which is high in unrefined carbohydrates and restricts fats, except for fish fats plus monounsaturated fats, would be ideal. Foods with a GI less than 55 are desirable, combined with exercise, preferably outdoors to maximise sun exposure for vitamin D and stress management.
Clinical tips handout for patients with cardiovascular disease
1 Lifestyle advice
Sunshine
2 Physical activity/exercise
3 Mind–body medicine
5 Dietary changes
7 Supplements
Fish oils
Vitamin B’s especially B6 and B12
Vitamin D3 (cholecalciferol 1000 international units)
Doctors should check blood levels and suggest supplementation if levels are low.
Vitamin C and vitamin E (best given together)
Magnesium and calcium (best provided together)
Zinc
Herbs
Bitter melon
Fenugreek
Panax ginseng (Korean)
American ginseng
1 Eurodiab Ace Study Group. Variation and trends in incidence of childhood diabetes in Europe. Lancet. 2000;355:873-876.
2 http://diabetes.niddk.nih.gov/dm/pubs/statistics/#allages Accessed December 2008.
3 Karvonen M., Viik-Kajander M., Moltchanova E., et al. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23:1516-1526.
4 Gillespie K.M. Type 1 diabetes: pathogenesis and prevention. CMAJ. 2006;175(2):165-170.
5 Taplin C.E., Craig M.E., Lloyd M., et al. The rising incidence of childhood type 1 diabetes in New South Wales, 1990-2002. MJA. 2005;183:243-246.
6 Australian Institute of Health and Welfare (AIHW). Online. Available: www.aihw.gov.au/publications/cvd/daf08/daf08pdf (accessed December 2008).
7 The SEARCH for Diabetes in Youth Study Writing Group. Incidence of Diabetes in Youth in the United States. JAMA. 2007;297:2716-2724.
8 Amos A., McCarty D., Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14:S1-S85.
9 King H., Aubert R., Herman W. Global burden of diabetes, 1995–2025: prevalence, numerical estimates and projections. Diab Care. 1998;21:1414-1431.
10 Dunstan D.W., Zimmet P., Welborne T.A., et al. The rising prevalence of diabetes and impaired glucose tolerance. Diab Care. 2002;25:829-834.
11 World Health Organization. http://www.who.int/mediacentre/factsheets/fs312/en/(accessed May 2009).
12 Serjeantson S.W., Owerbach D., Zimmet P., et al. Genetics of diabetes in Nauru: effects of foreign admixture, HLA antigens and the insulin-gene-linked polymorphism. Diabetologia. 1983;25:13-17.
13 Knowler W.C., Williams R.C., Pettitt D.J., et al. Gm3;5,13,14 and type 2 diabetes mellitus: an association in American Indians with genetic admixture. Am J Hum Genet. 1988;43:520-526.
14 Simmons D., Williams D.R., Powell M.J. The Coventry Diabetes Study: prevalence of diabetes and impaired glucose tolerance in Europids and Asians. Q J Med. 1991;81:1021-1030.
15 Adamo K.B., Tesson F. Gene-environment interaction and the metabolic syndrome. Novartis Found Symp. 2008;293:103-119.
16 Pinhas-Hamiel O., Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146(5):693-700.
17 Lindström J., Ilanne-Parikka P., Peltonen M., et al. Finnish Diabetes Prevention Study Group. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673-1679.
18 Gillies C.I., Abrams K.R., Lambert P.C., et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meat-analysis. BMJ. 2007;334:299.
19 Knowler W.C., Barrett-Connor E., Fowler S.E., et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. NEJM. 2002;346:393-403.
20 Orozco L.J., Buchleitner A.M., Gimenez-Perez G., et al. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. (Issue 3):2008. Art. No.: CD003054. doi: 10.1002/14651858.CD003054.pub3
21 Li G., Zhang P., Wang J., et al. The long-term effect of lifestyle interventions to preventdiabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lifestyle intervention, diabetes, and cardiovascular disease. Lancet. 2008;371:1731-1733.
22 Gimeno S.G., de Souza J.M. IDDM and milk consumption. A case-control study in São Paulo, Brazil. Diabetes Care. 1997;20(8):1256-1260.
23 Cavallomg, Fava D., Monetini L., et al. Cell-mediated immune response to β casein in recent-onset insulin-dependent diabetes: implications for disease pathogenesis. Lancet. 1996;348:926-928.
24 Virtanen S.M., Läärä E., Hyppönen E., et al. Cow’s milk consumption, HLA-DQB1 genotype, and type 1 diabetes: a nested case-control study of siblings of children with diabetes. Childhood diabetes in Finland study group. Diabetes. 2000;49(6):912-917.
25 Rana J.S., Li T.Y., Manson J.E., et al. Adiposity compared with physical inactivity and risk of type 2 diabetes in women. Diabetes Care. 2007;30(1):53-58.
26 Harding A.H., Wareham N.J., Bingham S.A., et al. Plasma Vitamin C Level, Fruit and Vegetable Consumption, and the Risk of New-Onset Type 2 Diabetes Mellitus. The European Prospective Investigation of Cancer–Norfolk Prospective Study. Arch Intern Med. 2008;168(14):1493-1499.
27 Cochrane PEARLS. http://www.cochraneprimarycare.org/en/newPage1.html Practical Evidence About Real Life Situations. No. 95, September 2008.
28 Grace C., Begum R., Subhani S., et al. Prevention of type 2 diabetes in British Bangladeshis: qualitative study of community, religious, and professional perspectives BMJ. 2008 November 4;337:a1931.
29 Stuebe Alison M., Rich-Edwards Janet W., et al. Duration of Lactation and Incidence of Type 2 Diabetes. JAMA. 2005;294:2601-2610.
30 Gucciardi E., Chi-Tyan Wang S., De Melo M., et al. Characteristics of men and women with diabetes. Observations during patients’ initial visit to a diabetes education centre. Can Fam Phys. 2008;54:219-227.
31 Vehik K., Hamman R.F., Lezotte D., et al. Trends in High-Risk HLA Susceptibility Genes Among Colorado Youth With Type 1 Diabetes. Diabetes Care. 2008;31:1392-1396. doi: 10.2337/dc07-2210
32 Barroso I. Genetics of Type 2 diabetes. Diabet Med. 2005;22(5):517-535.
33 Lammert E. The vascular trigger of type II diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116(Suppl 1):S21-S25.
34 Moore A.F., Florez J.C. Genetic susceptibility to type 2 diabetes and implications for antidiabetic therapy. Annu Rev Med. 2008;59:95-111.
35 McGrady A.V., et al. Spencer J.W., Jacobs J.J., editors. Complementary and Alternative Medicine: an evidence-based approach. St. Louis: Mosby, 2003.
36 Sepa A., Frodi A., Ludvigsson J. Mothers’ experiences of serious life events increase the risk of diabetes-related autoimmunity in their children. Diabetes Care. 2005;28(10):2394-2399.
37 Storch E.A., Heidgerken A.D., Geffken G.R., et al. Bullying, regimen self-management, and metabolic control in youth with type I diabetes. J Pediatr. 2006;148(6):784-787.
38 Surwit R.S., van Tilburg M.A.L., Zucker N., et al. Stress Management Improves Long-Term Glycemic Control in Type 2 Diabetes. Diab Care. 2002;25:30-34.
39 Hooper P.L. Hot-tub therapy for type 2 diabetes mellitus. NEJM. 1999;341(12):924-925.
40 Haslam D. Understanding obesity in the older person: prevalence and risk factors. Br J Community Nurs. 2008;13(3):115-122.
41 Keller U. From obesity to diabetes. Int J Vitam Nutr Res. 2006;76(4):172-177.
42 Anderson R.J., Freedland K.E., et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069-1078.
43 Lawlor D.A., Ebrahim S., Smith G.D. Association of insulin resistance with depression: cross-sectional findings from the British Women’s Heart and Health Study. BMJ. 2003;327:383-384.
44 Golden S.H., Lazo M., Carnethon M., et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299:2751-2759.
45 Carnethon M.R., Biggs M.L., Barzilay J.I., et al. Longitudinal association between depressive symptoms and incident type 2 diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2007;167(8):802-807.
46 MartinCosgrove P., Lincoln A., et al. Does depression increase the risk of developing type 2 diabetes? Occupational Medicine. 2008;58(1):7-14.
47 Timonen M., Laakso M., Jokelainen J., et al. Insulin resistance and depression: cross-sectional study. BMJ. 2005;330:17-18.
48 Zhou L., Sutton G.M., Rochford J.J., et al. Serotonin 2C receptor agonists improve Type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398-405.
49 Pittas A.G., Lau J., Hu F., et al. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017-2029.
50 Vitamin D and adult bone health in Australia and New Zealand: a position statement. Working Group of the Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia and Osteoporosis Australia MJA 2005;182:281–285.
51 National Prescribing Service, Australia. Published 1 December 2008. http://www.nps.org.au/health_professionals/publications/nps_radar/issues/current/december_2008/alendronate_with_cholecalciferol?SQ_BACKEND_PAGE=main&backend_section=am&am_section=edit_asset&assetid=65713&sq_asset_path=1,43,72,360,686, 24894, 25055, 65656, 65712&asset_ei_screen=contents&sq_link_path=,0,43,93,526, 1081, 33528, 33753, 128772, 128852#revhistory] (accessed December 2008)
52 Zipitis C.S., Akobeng A.K. Vitamin D Supplementation in Early Childhood and Risk of Type 1 Diabetes: a Systematic Review and Meta-analysis. Archives of Disease in Childhood. 2008. doi:10.1136/adc.2007.128579
53 Palomer X., Gonzalez-Clemente J.M. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10(3):185-197.
54 Chaput J.P., Després J.P., Bouchard C., et al. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetol. 2007;50:2298-2304.
55 Gottlieb D.J., Naresh Punjabi N.M., Newman A.B., et al. Association of Sleep Time With Diabetes Mellitus and Impaired Glucose Tolerance. Arch Intern Med. 2005;165:863-867.
56 West S.D., Nicoll D.J., Stradling J.R. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax. 2006;61(11):945-950.
57 Eriksson A.K., Ekbom A., Granath F., et al. Psychological distress and risk of pre-diabetes and Type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabetic Medicine. 2008;25:834-842.
58 Willi C., et al. Active smoking and the risk of Type 2 diabetes. A systematic review and meta-analysis. JAMA. 2007;289:2654-2664.
59 Kruger J., Ham S.A., Prohaska T.R. Behavioural risk factors associated with overweight and obesity among older adults: the 2005 National Health Interview Survey. Prev Chronic Dis. 2009;6(1):A14. Epub 2008 Dec 15
60 Foy C.G., Bell R.A., Farmer D.F., et al. Smoking and incidence of diabetes among U.S. adults: findings from the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2005;28(10):2501-2507.
61 Navas-Acien A., Silbergeld E.K., Pastor-Barriuso R., et al. Arsenic Exposure and Prevalence of Type 2 Diabetes in US Adults. JAMA. 2008;300(7):814-822.
62 Healy G.N., Dunstan D.W., Shaw J.E., et al. Beneficial associations of physical activity with 2-h but not fasting blood glucose in Australian adults: Aus Diab Study. Diabetes Care. 2006;29:2598-2604.
63 Dunstan D.W., Salmon J., Healy G.N., et al. Association of television viewing with fasting and 2-hr post-challenge plasma glucose levels in adults without diagnosed diabetes. Diabetes Care. 2007;30:516-522.
64 Herbst A., Bachran R., Kapellen T., et al. Physical activity, also, has a major influence on obesity control and is a significant predictor of mortality in T2DM. Arch Pediatr Adolesc Med. 2006;160:573-577.
65 Jeon C.Y., Lokken R.P., Hu F.B., et al. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30(3):744-752.
66 Kriska A.M., Saremi A., Hanson R.L., et al. Physical activity, obesity, and the incidence of type 2 diabetes in a high-risk population. Am J Epidemiol. 2003;158(7):669-675.
67 Gregg E.W., Gerzoff R.B., Caspersen C.J., et al. Relationship of Walking to Mortality Among US Adults With Diabetes. Arch Intern Med. 2003;163:1440-1447.
68 Babraj J.A., Vollaard N.B.J., Keast C., et al. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocrine Disorders. 2009;9:3. doi:10.1186/1472-6823-9-3
69 Silink M. http://www.abc.net.au/am/content/2006/s17869.
70 Hosper K., Deutekom M., Stronks K. The effectiveness of ‘Exercise on Prescription’ in stimulating physical activity among women from ethnic minority groups in the Netherlands: protocol for a randomised controlled trial. BMC Public Health. 2008;8(1):406.
71 American Diabetes Association. Diabetes mellitus and exercise (position statement). Diabetes Care. 2000;23(Suppl. 1):S50-S54.
72 Albright A., et al. American College of Sports Medicine position stand: exercise and Type 2 diabetes. Med Sci Sports Exerc. 2000;32:1345-1360.
73 Thomas D.E., Elliott E.J., Naughton G.A. Exercise for type 2 diabetes mellitus. The Cochrane Database of Systematic Reviews. (3):2006.
74 Sigal R.J., Kenny G.P., Boulé N.G., et al. Effects of Aerobic Training, Resistance Training, or Both on Glycemic Control in Type 2 Diabetes. A Randomised Trial. Ann Intern Med. 2007;147:357-369.
75 Christou E.A., Yang Y., Rosengren K.S. Taiji training improves knee extensor strength and force control in older adults. J Gerontol A Biol Sci Med Sci. 2003;58:763-766.
76 Lan C., Lai J.S., Chen S.Y., et al. Tai Chi Chuan to improve muscular strength and endurance in elderly individuals: a pilot study. Arch Phys Med Rehabil. 2000;81:604-607.
77 Tsang W.W., Hui-Chan C.W. Effect of 4-and 8-wk intensive Tai Chi training on balance control in the elderly. Med Sci Sports Exerc. 2004;36:648-657.
78 Hong Y., Li J.X., Robinson P.D. Balance control, flexibility, and cardiorespiratory fitness among older Tai Chi practitioners. Br J Sports Med. 2000;34:29-34.
79 Richerson S., Rosendale K. Does Tai Chi improve plantar sensory ability? A pilot study. Diabetes Technol Ther. 2007;9(3):276-286.
80 Taylor-Piliae R.E., Haskell W.L., et al. Improvement in balance, strength, and flexibility after 12 weeks of Tai chi exercise in ethnic Chinese adults with cardiovascular disease risk factors. Altern Ther Health Med. 2006;12(2):50-58.
81 Lee M.S., Pittler M.H., Kim M.S., et al. Tai chi for Type 2 diabetes: a systematic review. Diabet Med. 2008;25(2):240-241.
82 Orr R., Tsang T., Lam P., et al. Mobility impairment in type 2 diabetes: association with muscle power and effect of Tai Chi intervention. Diabetes Care. 2006;29(9):2120-2122.
83 Song R., Lee E.O., Bae S.C., et al. Effects of tai chi self-help program on glucose control, cardiovascular risks and quality of life in type II diabetic patients (in Korean). J Muscle Joint Health. 2007;14:13-25.
84 Wang J., Cao Y. Effects of tai chi exercise on plasma neuropeptide Y of type 2 diabetes mellitus with geriatric obesity (in Chinese). J Sports Sci. 2003;24:67-68.
85 Tsang T., Orr R., Lam P., et al. Effects of Tai Chi on glucose homeostasis and insulin sensitivity in older adults with type 2 diabetes: a randomised double-blind sham-exercise-controlled trial. Age Ageing. 2008;37(1):64-71. Epub 2007 Oct 25
86 Zhang Y., Fu F.H. Effects of 14-week Tai Ji Quan exercise on metabolic control in women with type 2 diabetes. Am J Chin Med. 2008;36(4):647-654.
87 Jeong I.S., Lee H.J., Kim M.H. The effect of the Taeguk Gi-gong exercise on insulin resistance and blood glucose in patients with type II diabetes mellitus (in Korean). J Korean Acad Fundam Nurs. 2007;14:44-52.
88 Tsujiuchi T., Kumano H., Yoshiuchi K., et al. The effect of Qi-gong relaxation exercise on the control of Type 2 diabetes mellitus: a randomised controlled trial. Diab Care. 2002;25:241-242.
89 Xin L., Miller Y.D., Brown W.J. A qualitative review of the role of qigong in the management of diabetes. J Altern Complement Med. 2007;13(4):427-433.
90 Liu X., Brown W., Miller Y. Effects of Chinese medical exercise on indicators of metabolic syndrome in Australian adults with elevated blood glucose level: A randomised controlled trial. J of Sci and Med in Sport. 2007;10(6):54.
91 Singh S., Malhotra V., Singh K.P., et al. Role of yoga in modifying certain cardiovascular functions in type 2 diabetic patients. J Assoc Phys India. 2004;52:203-206.
92 Malhotra V., Singh S., Singh K.P., et al. Study of yoga asanas in assessment of pulmonary function in NIDDM patients. Indian J Phys Pharm. 2002;46(3):313-320.
93 Malhotra V., Singh S., Tandon O.P., et al. Effect of Yoga asanas on nerve conduction in type 2 diabetes. Indian J Physiol Pharmacol. 2002;46:298-306.
94 Skoro-Kondza L., Tai S.S., Gadelrab R., et al. Community based yoga classes for type 2 diabetes: an exploratory randomised controlled trial. BMC Health Services Research. 2009;9:33. doi:10.1186/1472-6963-9-33
95 Innes K.E., Vincent H.K. Review: The Influence of Yoga-Based Programs on Risk Profiles in Adults with Type 2 Diabetes Mellitus: A Systematic Review. Advance Access Publication 11 December 2006. eCAM. 2007;4(4):469-486.
96 Thomas D., Elliott E.J. Low glycaemic index, low glycaemic load diets for diabetes mellitus. Cochrane Database of Systematic Reviews. (issue 1):2009. Art. No: CD006296. Do1: 10.1002/14651858 CD 006296. pub2
97 Lamb M.M., Yin X., Barriga K., et al. Dietary Glycemic Index, Development of Islet Autoimmunity, and Subsequent Progression to Type 1 Diabetes in Young Children. J Clin Endocrin Metabol Accepted. July 24, 2008.
98 Norris J.M., Yin X., Lamb M.M., et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298(12):1420-1428.
99 Delahanty L.M., Nathan D.M., Lachin J.M., et al. Association of diet with glycated hemoglobin during intensive treatment of type 1 diabetes in the Diabetes Control and Complications Trial. America Journal of Clinical Nutrition. 2009;89:518-524.
100 Schulze M.B., Schulz M., Heidemann C., et al. Fiber and Magnesium Intake and Incidence of Type 2 Diabetes A Prospective Study and Meta-analysis. Arch Intern Med. 2007;167:956-965.
101 Brand-Miller J., Hayne S., Petocz P., et al. Low–Glycemic Index Diets in the Management of Diabetes: A meta-analysis of randomised controlled trials Diabetes Care. 2003;26:2261-2267.
102 Meyer K.A., et al. Carbohydrates, dietary fiber, and incident Type 2 diabetes in older women. Am J Clin Nutr. 2000;71:921-930.
103 Feldeisen S.E., Tucker K.L. Nutritional strategies in the prevention and treatment of metabolic syndrome. Appl Physiol Nutr Metab. 2007;32(1):46-60.
104 Jensen M.K., Koh-Banerjee P., Franz M., et al. Wholegrains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation. AJCN. 2006;83:275-283.
105 McKeown N.M. Wholegrain intake and insulin sensitivity: evidence from observational studies. Nutr Rev. 2004;62(7 Pt 1):286-291.
106 Chandalia M., Garg A., Lutjohann D. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. NEJM. 2000;342(19):1392-1398.
107 Wellenet al K.L. Inflammation, stress and diabetes. J Clin Invest. 2005;115:1111-1119.
108 Priebemg, van Binsbergen J.J., de Vos R., et al. Whole grain foods for the prevention of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. (Issue 1):2008. Art. No.: CD006061. doi: 10.1002/14651858.CD006061.pub2
109 Lee C.T., Adler A.I., Forouhi N.G., et al. Cross-sectional association between fish consumption and albuminuria: the European Prospective Investigation of Cancer-Norfolk Study. Am J Kidney Dis. 2008;52(5):876-886.
110 Santangelo C., Varì R., Scazzocchio B., et al. Polyphenols, intracellular signalling and inflammation. Ann Ist Super Sanita. 2007;43(4):394-405.
111 Ordovas J. Diet/genetic interactions and their effects on inflammatory markers. Nutr Rev. 2007;65(12 Pt 2):S203-S207.
112 Salmeron J., et al. Dietary fat intake and risk of Type 2 diabetes in women. Am Clin Nutr. 2001;73:1019-1026.
113 Sharma K.K., et al. Anti-hyperglycemic effect of onion: effect on fasting blood sugar and induced hyperglycemia in man. Ind J Med Res. 1977;65:422-429.
114 Sheela C.G., et al. Anti-diabetic effects of S-allylcysteine sulphoxide isolated from garlic (Allium sativum). Ind J Exp Biol. 1992;192:523-526.
115 Jaimenez-Cruz A., Bacardi-Gascon M., Turnbull W.H., et al. A flexible low glycemic index Mexican-style diet in overweight and obese subjects with Type 2 diabetes improves metabolic parameters during a 6-week treatment period. Diabetes Care. 2003;26:1967-1970.
116 Teixeira S.R., Tappenden K.A., Carson L., et al. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J Nutr. 2004;134(8):1874-1880.
117 Jayagopal V, Albertazzi P, Kilpatrick ES, et-al. Beneficial Effects of Soy Phytoestrogen Intake in Postmenopausal Women With Type 2 Diabetes. Diabetes Care 25:1709–14.
118 Ahuja K.D., Robertson I.K., Geraghty D.P., et al. Effects of chili consumption on postprandial glucose, insulin, and energy metabolism. AJCN. 2006;84:63-69.
119 Villegas R., Liu S., Gao Y.T., et al. Prospective Study of Dietary Carbohydrates, Glycemic Index, Glycemic Load, and Incidence of Type 2 Diabetes Mellitus in Middle-aged Chinese Women. Arch Intern Med. 2007;167:2310-2316.
120 Palmer J.R., Boggs D.A., Krishnan S., et al. Sugar-Sweetened Beverages and Incidence of Type 2 Diabetes Mellitus in African American Women. Arch Intern Med. 2008;168:1487-1492.
121 Swithers S.E., Davidson T.L. A Role for Sweet Taste: Calorie Predictive Relations in Energy Regulation by Rats. Behavioural Neuroscience. 2008;122:161-173.
122 Qi Lu, van Dam Rob M., Rexrode Kathryn, et al. Hu Heme Iron From Diet as a Risk Factor for Coronary Heart Disease in Women With Type 2 Diabetes Diabetes Care. 2007;30:101-106.
123 Liu S., Choi H.K., Ford E., et al. A prospective study of dairy intake and risk of type 2 diabetes in women. Diabetes Care. 2006;29:1579-1584.
124 Shahar D.R., Abel R., Elhayany A., et al. Does Dairy Calcium Intake Enhance Weight Loss Among Overweight Diabetic Patients? Diabetes Care. 2007;30:485-489.
125 Teixeira Rde C., Molina Mdel C., et al. Cardiovascular risk in vegetarians and omnivores: a comparative study. Arq Bras Cardiol. 2007;89(4):237-244.
126 Barnard N.D., Cohen J., Jenkins D.J.A., et al. A Low-Fat Vegan Diet Improves Glycemic Control and Cardiovascular Risk Factors in a Randomised Clinical Trial in Individuals With Type 2 Diabetes. Diabetes Care. 2006;29:1777-1783.
127 Turner-McGrievy G.M., Barnard N.D., Cohen J., et al. Changes in nutrient intake and dietary quality among participants with type 2 diabetes following a low-fat vegan diet or a conventional diabetes diet for 22 weeks. J Am Diet Assoc. 2008;108(10):1636-1645.
128 Wing S.S. The UPS in diabetes and obesity. BMC Biochem. 2008;9(Suppl 1):S6.
129 Rowley K., et al. Diabetes in Australian aborigines and Torres Strait islander peoples. PNG Med J. 2001;44:164-170.
130 Wild S., et al. Global prevalence of diabetes: estimates for the year 2000, and projections for 2030. Diabetes Care. 2004;27:1047-1053.
131 Knowler W.C., Barrett-Connor E., Fowler S.E., et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Eng J Med. 2002;346:393-403.
132 Dansinger M.L., Tatsioni A., Wong J.B., et al. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147(1):41-50.
133 Jossa F.A. Mediterranean diet in the prevention of arteriosclerosis. Recent Prog Med. 1996;87:175-181.
134 Martinez-Gonzalez M.A., Estruch R., Bulló M., et al. Adherence to a Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ. 2008;336:1348-1351.
135 Giugliano D., Esposito K. Mediterranean diet and metabolic diseases. Curr Opin Lipidol. 2008;19(1):63-68.
136 Martínez-González M.A., de la Fuente-Arrillaga C., Nunez-Cordoba J.M., et al. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ. 2008;336(7657):1348-1351.
137 Sánchez-Taínta A., Estruch R., Bulló M., et al. Adherence to a Mediterranean-type diet and reduced prevalence of clusteredcardiovascular risk factors in a cohort of 3,204 high-risk patients. Eur J Cardiovasc Prev Rehabil. 2008;15(5):589-593.
138 Marin C., Perez-Jimenez F., et al. The Ala54Thr polymorphism of the fatty acid-binding protein 2 gene is associated with a change in insulin sensitivity after a change in the type of dietary fat. AJCN. 2005;82(1):196-200.
139 Jiang R., Manson J.E., Stampfer M.J., et al. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA. 2002;288:2554-2560.
140 Jenkins D.J.A., Hu F.B., Tapsell L.C., et al. Possible Benefit of Nuts in Type 2 Diabetes. J Nutr. 2008;138:1752S-1756S.
141 Tapsell L.C., Gillen L.J., Patch C.S., et al. Including Walnuts in a Low-Fat/Modified-Fat Diet Improves HDL Cholesterol-to-Total Cholesterol Ratios in Patients With Type 2 Diabetes. Diabetes Care. 2004;27:2777-2783.
142 Phillips M., Carapetis M. Six steps to a healthy lifestyle- the keystone in managing type 2 diabetes. Medicine Today. 2008;8:51-58.
143 Lindeberg S., Jönsson T., Granfeldt Y., et al. A Palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
144 Ornish D.M. Can lifestyle changes reverse coronary heart disease? Lancet. 1990;336:129-133.
145 Pischke C.R., Scherwitz L., Weidner G., et al. Long-term effects of lifestyle changes on well-being and cardiac variables among coronary heart disease patients. Health Psychol. 2008;27(5):584-592.
146 Pischke C.R., Weidner G., Elliott-Eller M., et al. Lifestyle changes and clinical profile in coronary heart disease patients with an ejection fraction of <or=40% or >40% in the Multicenter Lifestyle Demonstration Project. Eur J Heart Fail. 2007;9(9):928-934.
147 van Dam R.M., Hu F.B. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294(1):97-104.
148 Smith B., Wingard D.L., Smith T.C., et al. Does coffee consumption reduce the risk of type 2 diabetes in individuals with impaired glucose? Diabetes Care. 2006;29:2385-2390.
149 Bidel S., Hu G., et al. Effects of coffee consumption on glucose tolerance, serum glucose and insulin levels-a cross-sectional analysis. Horm Metabol Res. 2006;38:38-43.
150 Paynter N.P., Yeh H.C., Voutilainen S., et al. Coffee and sweetened beverage consumption and the risk of type 2 diabetes mellitus: the atherosclerosis risk in communities study. Am J Epidemiol. 2006;164:1075-1084.
151 Balzer J., Rassaf T., Heiss C., et al. Sustained Benefits in Vascular Function Through Flavanol-Containing Cocoa in Medicated Diabetic Patients. A Double-Masked, Randomised, Controlled Trial. J Am Coll Cardiol. 2008;51:2141-2149.
152 Mackenzie T., Brooks B., O’Connor G. Beverage intake, diabetes, and glucose control of adults in America. Ann Epidemiol. 2006;16:688-691.
153 Shai I., Wainstein J., Harman-Boehm I., et al. Glycemic Effects of Moderate Alcohol Intake Among Patients With Type 2 Diabetes. A multicenter, randomised, clinical intervention trial. Diabetes Care. 2007;30:3011-3016.
154 Bonnefont-Rousselot D. The role of antioxidant micronutrients in the prevention of diabetic complications. Treat Endocrinol. 2004;3(1):41-52.
155 Sargeant L.A., Wareham N.J., Bingham S., et al. Vitamin C and hyperglycemia in the European Prospective Investigation into Cancer–Norfolk (EPIC- Norfolk) study: a population-based study. Diabetes Care. 2000;23:726-732.
156 Ting H.H., Timimi F.K., Boles K.S., et al. Vitamin C improves endothelium-dependent vasodilatation in patients with non-insulin dependent diabetes mellitus. J Clin Invt. 1996;97:22-28.
157 Lai M.H. Antioxidant effects and insulin resistance improvement of chromium combined with vitamin C and e supplementation for type 2 diabetes mellitus. J Clin Biochem Nutr. 2008;43(3):191-198.
158 Afkhami-Ardekani M., Shojaoddiny-Ardekani A. Effect of vitamin C on blood glucose, serum lipids and serum insulin in type 2 diabetes payients. Indian J Med Res. 2007;126(5):471-474.
159 Lopes de Jesus C.C., Atallah A.N., Valente O., et al. Vitamin C and superoxide dismutase (SOD) for diabetic retinopathy. Cochrane Database of Systematic Reviews. (Issue 1):2008. Art. No.: CD006695. doi: 10.1002/14651858.CD006695.pub2
160 Paolisso G., D’Amore A., Galzerano D., et al. Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly Type 2 diabetic patients. Diabetes Care. 1993;16:1433-1437.
161 Huijgens P.C., van den Berg C.A., Imandt L.M., et al. Vitamin E and platelet aggregation. ACTA Haematol. 1981;65:217-218.
162 Luong K., Nguyen L.T.H., Nguyen D.N.P. The role of vitamin D in protecting type 1 diabetes mellitus. Diabetes Metab Res Rev. 2005;21:338-346.
163 Mathieu C., Gysemans C., Guilietti A., et al. Vitamin D and diabetes. Diabetologia. 2005;48:1247-1257.
164 Zipitis C.S., Akobeng A.K. Vitamin D Supplementation in Early Childhood and Risk of Type 1 Diabetes: a Systematic Review and Meta-analysis. Archives of Disease in Childhood. 2008;93:512-517.
165 Rabbani N., Alam S.S., Riaz S., et al. High-dose thiamine therapy for patients with type 2 diabetes and microalbuminuria: a randomised, double-blind placebo-controlled pilot study. Diabetologia. 2008 Dec 5. Epub ahead of print
166 Janssen I., Powell L.H., Crawford S., et al. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2002;162:1568-1575.
167 Yoshida M., Jacques P.F., Meigs J.B., et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care. 2008;31:2092-2096.
168 Sahin M., Tutuncu N.B., Ertugrul D., et al. Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitus. J Diabetes Complications. 2007;21(2):118-123.
169 Ting R.Z., Szeto C.C., Chan M.H., et al. Risk factors of vitamin B12 deficiency inpatients receiving metformin. Archives of Internal Medicine. 2006;166:1075-1079.
170 Bursell S.E., Clermont A.C., Aiello L.P., et al. High-dose vitamin E supplementation normalises retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999;22:1245-1251.
171 Skyrme-Jones R.A., O’Brien R.C., et al. Vitamin E supplementation improves endothelial function in type I diabetes mellitus: a randomised, placebo-controlled study. J Am Coll Cardiol. 2000;36(1):94-102.
172 Church T.S., Earnest C.P., Wood K.A., et al. Reduction of C-reactive protein levels through use of a multivitamin. Am J Med. 2003;115(9):702-707.
173 Farvid M.S., Jalali M., Siassi F., et al. Comparison of the effects of vitamins and/or mineral supplementation on glomerular and tubular dysfunction in type 2 diabetes. Diabetes Care. 2005;28(10):2458-2464.
174 Farvid M.S., Siassi F., Jalali M., et al. The impact of vitamin and/or mineral supplementation on lipid profiles in type 2 diabetes. Diabetes Res Clin Pract. 2004;65(1):21-28.
175 Farvid M.S., Jalali M., Siassi F., et al. The impact of vitamins and/or mineral supplementation on blood pressure in type 2 diabetes. J Am Coll Nut. 2004;23(3):272-279.
176 Czernichow S., Couthouis A., Bertrais S., et al. Antioxidant supplementation does not affect fasting plasma glucose in the SU.VI.MAX study in France: association with dietary intake and plasma concentrations. AJCN. 2006;84(2):395-399.
177 Barringer T.A., Kirk J.K., Santaniello A.C., et al. Effect of a multivitamin and mineral supplement on infection and quality of life. A randomised, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(5):365-371.
178 Anderson R.A. Chromium in the prevention and control of diabetes. Diabetes Metab. 2000;26(1):22-27.
179 Lau F.C., Bagchi M., Sen C.K., et al. Nutrigenomic basis of beneficial effects of chromium(III) on obesity and diabetes. Mol Cell Biochem. 2008;317(1-2):1-10.
180 Finney L.S., et al. Dietary chromium and diabetes: is there a relationship? Clin Diabetes. 1997;15:6-10.
181 Roussel A.M., Andriollo-Sanchez M., Ferry M., et al. Food chromium content, dietary chromium intake and related biological variables in French free-living elderly. Br J Nutr. 2007;98(2):326-331.
182 Vladeva S.V., Terzieva D.D., Arabadjiiska D.T. The effect of chromium on the insulin resistance in patients with type II DM. Folia Med. 2005;47(3-4):59-62.
183 Aguilar M.V., Saavedra P., Arrieta F.J., et al. Plasma mineral content in Type II diabetic patients and their association with the metabolic syndrome. Ann Nutr Metab. 2007;51:402-406.
184 Vladeva S.V., Terzieva D.D., Arabadjiiska D.T. The effect of chromium on the insulin resistance in patients with type II DM. Folia Med. 2005;47(3-4):59-62.
185 Mark D.A. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type II DM. Diab Care. 2006;29:2764.
186 Sreekanth R., Pattabhi V., Rajan S.S. Molecular basis of chromium insulin interactions. Biochem Biophys Res Commun. 2008;369(2):725-729.
187 Singer G.M., Geohas J. The effect of chromium picolinate and biotin supplementation on glycaemic control in poorly controlled patients with type II DM: A placebo-controlled, double-blinded, randomised trial. Diabetes Technol Ther. 2006;8:636-643.
188 Martin J., Wang Z.Q., Zhang X.H., et al. Chromium picolinate supplementation attenuates Body weight gain and increases insulin sensitivity in subjects with type II DM. Diabetes Care. 2006;29:1826-1832.
189 Yang X., Li S.Y., Dong F., et al. Insulin-sensitising and cholesterol-lowering effects of chromium (D-phenylalanine). J Inorg Biochem. 2006;100(7):1187-1193.
190 Balk E.M., Tatsioni A., Lichtenstein A.H., et al. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomised controlled trials. Diabetes Care. 2007;30(8):2154-2163.
191 Broadhurst C.L., Domenico P. Clinical studies on chromium picolinate supplementation in diabetes mellitus—a review. Diabetes Technol Ther. 2006;8(6):677-687.
192 Feiner J.J., McNurlan M.A., Ferris R.E., et al. Chromium picolinate for insulin resistance in subjects with HIV disease: a pilot study. Diabetes Obes Metab. 2008;10(2):151-158.
193 Komorowski J.R., Greenberg D., Juturu V. Chromium picolinate does not produce chromosomal damage. Toxicol in Vitro. 2008;22(3):819-826.
194 Albarracin C., Fuqua B., Geohas J., et al. Combination of chromium and biotin improves coronary risk factors in hypercholesterolemic type 2 diabetes mellitus: a placebo-controlled, double-blind randomised clinical trial. J Cardiometab Syndr. 2007;2(2):91-97.
195 Albarracin C.A., Fuqua B.C., Evans J.L., et al. Chromium picolinate and biotin combination improves glucose metabolism in treated, uncontrolled overweight to obese patients with type 2 diabetes. Diab Metab Res Rev. 2008;24(1):41-51.
196 Geohas J., Daly A., Juturu V., et al. Chromium picolinate and biotin combination reduces atherogenic index of plasma in patients with type 2 diabetes mellitus: a placebo-controlled, double-blinded, randomised clinical trial. Am J Med Sci. 2007;333(3):145-153.
197 Gunton J.E., Cheung W.N., Hitchman R., et al. Chromium Supplementation Does Not Improve Glucose Tolerance, Insulin Sensitivity, or Lipid Profile: A randomised, placebo-controlled, double-blind trial of supplementation in subjects with impaired glucose tolerance. Diabetes Care. 2005;28:712-713.
198 Althius M.D., Jordan N.E., Ludington E.A., et al. Glucose and insulin responses to dietary chromium supplements: a meta-analysis. AJCN. 2002;76:148-155.
199 Takaya J., Higashino H., Kobayashi Y. Intracellular magnesium and insulin resistance. Magnes Res. 2004;17(2):126-136.
200 Volpe S.L. Magnesium, the metabolic syndrome, insulin resistance, and type 2 diabetes mellitus. Crit Rev Food Sci Nutr. 2008;48(3):293-300.
201 Wells I.C. Evidence that the etiology of the syndrome containing type 2 diabetes mellitus results from abnormal magnesium metabolism. Can J Physiol Pharmacol. 2008;86(1-2):16-24.
202 Larsson S.C., Wolk A. Magnesium intake and risk of type 2 diabetes: a meta-analysis. J Intern Med. 2007;262(2):208-214.
203 Lopez-Ridaura, Willett W.C., Rimm E.B., et al. Magnesium intake and risk of Type 2 diabetes in men and women. Diabetes Care. 2004;27:134-140.
204 Song Y., Ridker P.M., Manson J.E., et al. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28(6):1438-1444.
205 Huertamg, Rascón-Pacheco R.A., Rodríguez-Morán M., et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care. 2005;28(5):1175-1181.
206 Chambers E.C., Heshka S., Gallagher D., et al. Serum magnesium and Type II DM in African-Americans and Hispanics: A New York cohort. J Am Coll Nutr. 2006;25(6):509-513.
207 Van Dam R.M., Hu F.B., Rosenberg L., et al. Dietary calcium and magnesium major food sources, and risk of Type II DM in US black women. Diab Care. 2006;29(10):2238-2243.
208 Sharma A., Dabla S., Agrawal R.P., et al. Serum magnesium: An early predictor of course and complications of DM. J Indian Med Assoc. 2007;105(1):16-20.
209 Mayer-Davis E.J., Nichols M., Liese A.D., et al. Dietary intake among youth with DM: the SEARCH for DM in youth study. J Am Diet Assoc. 2006;106(5):689-697.
210 He K., Liu K., Daviglus M.L., et al. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation. 2006;113(13):1675-1682.
211 Pham P.C., Pham P.M., Pham S.V. Hypomagnesemia in patients with type 2 diabetes mellitus. Clin J Am Soc Nephrol. 2007;2(2):366-373.
212 Brabagallo M., Dominguez L.J. Magnesium metabolism in Type II DM, metabolic syndrome and insulin resistance. Arch Biochem Biophys. 2007;458(1):40-47.
213 Volpe S.L. Magnesium, the metabolic syndrome, insulin resistance and type II DM. Crit Rev Food Sci Nutr. 2008;48(93):293-300.
214 Longstreet D.A., Heath D.L., Panaretto K.S., et al. Correlations suggest low magnesium may lead to higher rates of type II DM in indigenous Australians. Rural Remote Health. 2007;7:843.
215 Schulze M.B., Schulz M., Heidemann C., et al. Fiber and Magnesium Intake and Incidence of Type 2 Diabetes A Prospective Study and Meta-analysis. Arch Intern Med. 2007;167:956-965.
216 Larsson S.C., Wolk A. Magnesium intake and risk of Type II DM: A meta-analysis. J Intern Med. 2007;262(2):208-214.
217 Sales C.H., Pedrosa Lde F. Magnesium and DM: Their relation. Clin Nutr. 2006;25(4):554-562.
218 Corica F., Corsonello A., Ientile R., et al. Serum ionised magnesium levels in relation to metabolic syndrome in Type II DM patients. J Am Coll Nutr. 2006;25(3):210-215.
219 Rumawas M.E., McKeown N.M., Rogers G., et al. Magnesium intake is related to improved insulin homeostasis in the Framingham Offspring cohort. J Am Coll Nutr. 2006;25(6):486-492.
220 Bo S., Durazzo M., Guidi S., et al. Dietary magnesium and fibre intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am J Clin Nutr. 2006;84(5):1062-1069.
221 Ma B., Lawson A.B., Liese A.D., et al. Dairy, magnesium and calcium intake in relation to insulin sensitivity: Approaches to modeling a dose-dependent association. Am J Epidemiol. 2006;164(5):449-458.
222 Song Y., Buring J., Manson A.E., et al. Dietary magnesium intake in relation to plasma insulin levels and risk of Type 2 Diabetes in women. Diabetes Care. 2004;27:59-65.
223 Guerrero-Romero F., Tamez-Perez H.E., Gonzalez-Gonzalez G., et al. Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance. A double-blind placebo-controlled randomised trial. Diabetes Metab. 2004;30(3):253-258.
224 Rodrıguez-Moran M., Guerrero-Romero F. Oral Magnesium supplementation improves insulin sensitivity and metabolic control in Type 2 diabetic subjects. A randomised, double-blind controlled trial. Diabetes Care. 2003;26:1147-1152.
225 Beletate V., El Dib R.P., Atallah A.N. Zinc supplementation for the prevention of type 2 diabetes mellitus. Cochrane Database Syst Rev. (1):2007. CD005525
226 Al-Maroof R.A., Al-Shaarbatti S.S. Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med J. 2006;27(3):344-350.
227 Adachi Y., Yoshida j, kodera Y., et al. Oral administration of a zinc complex improves type II DM and metabolic syndromes. Biochem Biophys Res Commun. 2006;8351(1):165-170.
228 Yoshikawa Y., Adachi Y., Sakurai H. A new type of orally active anti-diabetic Zn(II)-dithiocarbamate complex. Life Sci. 2007;80(8):759-766.
229 Soinio M., Marniemi J., Laakso M., et al. Serum zinc level and coronary heart disease events in patients with type II DM. Diabetes Care. 2007;30(3):523-528.
230 Hayee M.A., Mohammed Q.D., Haque A. Diabetic neuropathy and zinc therapy. Bangladesh Med Res Counc Bull. 2005;31(2):62-67.
231 Blostein-Fujii A., DiSilvestro R.A., Frid D., et al. Short-term zinc supplementation in women with non-insulin-dependent diabetes mellitus: effects on plasma 5′-nucleotidase activities, insulin-like growth factor I concentrations, and lipoprotein oxidation rates in vitro. Am J Clin Nutr. 1997;66:639-642.
232 Anderson R.A., Roussel A.M., Zouari N., et al. Potential antioxidant effects of zinc and chromium supplementation in people with Type 2 diabetes mellitus. J Am Col Nutr. 2001;20:212-218.
233 Beletate V., El Dib R.P., Atallah A.N. Zinc supplementation for the prevention of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. (Issue 1):2007. Art. No.: CD005525. doi: 10.1002/14651858.CD005525.pub2
234 Brichard S.M., Henquin J.C. The role of vanadium in the management of diabetes. Trends in Pharmacological Sciences. 1995;16:265-270.
235 Preet A., Gupta B.L., Yadava P.K., et al. efficacy of lower doses of vanadium in restoring altered glucose metabolism and anti-oxidant status in diabetic rat lenses. J Biosci. 2005;30(2):221-230.
236 Bartlett H.E., Eperjesi F. Nutritional supplementation for type 2 diabetes: a systematic review. Ophthalmic Physiol Opt. 2008;28(6):503-523.
237 Ziegler D., Hanefeld M., Ruhnau K.J., et al. Treatment of symptomatic diabetic neuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diab Med. 21, 2004. 114–12
238 Boger R.H., Ron E.S. L–Arginine improves vascular function by overcoming deleterious effects of ADMA, a novel CV risk factor. Altern Med Rev. 2005;10(1):14-23.
239 Piatti P.M., et al. Long term oral L–arginine administration improves peripheral and hepatic insulin sensitivity in Type 2 diabetic patients. Diabetes Care. 2001;24(5):875-880.
240 Lerman A., et al. Long term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97(21):2123-2128.
241 Piatti P.M., Monti L.D., Valsecchi G., et al. Long term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in Type 2 diabetic patients. Diabetes Care. 2001;24(5):875-880.
242 Hodgson J.M., Watts G.F., Playford Da, et al. Coenzyme Q10 improves blood pressure and glycemic control: a controlled trial of subjects with type 2 diabetes. Eur J Clin Nutr. 2002;56(11):1137-1142.
243 Henriksen J.E., et al. Impact of ubiquinone (coenzyme Q10) treatment on glycemic control, insulin requirement and well-being in patients with Type 1 diabetes mellitus. Diabet Med. 1999;16(4):312-318.
244 Hamilton S.J., Chew G.T., Watts G.F. Co Enzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009 February 19. doi: 10.2337/dc08-1736
245 Giacco R., Cuomo V., Vessby B., et al. Fish oil, insulin sensitivity, insulin secretion and glucose tolerance in healthy people: Is there any effect of fish oil supplementation in relation to the type of background diet and habitual dietary intake of n-6 and n-3 fatty acids? Nutr Metabol Cardiovasc Dis. 2007;17(572):e580.
246 Norris J.M., Yin X., Lamb M.M., et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298:1420-1428.
247 Farmer A., Montori V., Dinneen S., et al. Fish oil in people with type 2 diabetes mellitus. The Cochrane Database of Systematic Reviews. (3):2006.
248 Heart Foundation Australia. Online. Available: www.heartfoundation.org.au/ http://www.heartfoundation.org.au/SiteCollectionDocuments/HW_FS_FishOils_PS_FINAL_web.pdf (accessed December 2008)
249 Hartweg J., Perera R., Montori V., et al. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. (Issue 1):2008. Art. No.: CD003205. doi: 10.1002/14651858.CD003205.pub2
250 Yeh G.Y., Eisenberg D.M., Kaptchuk T.J., et al. Systematic Review of Herbs and Dietary Supplements for Glycemic Control in Diabetes. Diab Care. 2003;26:1277-1294.
251 Vuksan V., Sievenpiper J.L., Wong J., et al. American ginseng (Panax quinquefolius L) attenuates postprandial glycemia in a time-dependent but not dose-dependent manner in healthy individuals. AJCN. 2001;73(4):753-758.
252 Vuksan V., Sievenpiper J.L., Koo V.Y., et al. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects wit type 2 diabetes mellitus. Arch Int Med. 2000;160:1009-1013.
253 Vuksan V., Sung M.K., Sievenpiper J.L., et al. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomised, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18(1):46-56.
254 Khan A., Bryden N., Polansky M., et al. Cinnamon improves glucose and lipids of people with Type 2 diabetes. Diabetes Care. 2003;26:3215-3218.
255 Yeh G.Y., Eisenberg D.M., Kaptchuk T.J., et al. Systematic review of herbs and dietary supplements for glycaemic control in diabetes. Diabetes Care. 2003;26:1277-1294.
256 Anderson R.A. Chromium and polyphenols from cinnamon improve insulin sensitivity. Proc Nutr Soc. 2008;67(1):48-53.
257 Baker W.L., Gutierrez-Williams G., White C.M., et al. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care. 2008;31(1):41-43.
258 Altschuler J.A., Casella S.J., MacKenzie T.A. The effect of cinnamon on A1C among adolescents with type 1 diabetes. Diabetes Care. 2007;30(4):813-816.
259 Blevins S.M., Leyva M.J., Brown J., et al. Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diab Care. 2007;30(9):2236-2237.
260 Khan A., Safdar M., Ali Khan M.M., et al. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215-3218.
261 Mang B., Wolters M., Schmitt B., et al. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36(5):340-344.
262 Vanschoonbeek K., Thomassen B.J., Senden J.M., et al. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr. 2006;136(4):977-980.
263 Hlebowicz J., Hlebowicz A., Lindstedt S., et al. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. AJCN. 2009;89:815-821.
264 Porchezhian E., Dobriyal R.M. An overview on the advances of Gymnema sylvestre: chemistry, pharmacology and patents. Pharmazie. 2003;58(1):5-12.
265 Persaud S.J., Al-Majed H., Raman A., et al. Gymnema silvestre stimulates insulin release in vitro by increasing membrane permeability. J Endocrinol. 1999;163:207-213.
266 Brala P.M., Hagen R.L. Effects of sweetness perception and calorie value of a preload on short-term intake. Physiol Behav. 1983;30:1-9.
267 Kanetkar P., Singhal R., Kamat M. Gymnema sylvestre: A Memoir. J Clin Biochem Nutr. 2007;41(2):77-81.
268 Chattopadhyay R.R. A comparative evaluation of some blood sugar lowering agents of plant origin. J Ethnoopharmacol. 1999;30;67(3):367-372.
269 Leach M.J. Gymnema sylvestre for DM: a systematic review. J Alt Comp Med. 2007;13(9):977-983.
270 Kanetkar P.V., Singhal R.S., Laddha K.S., et al. Extraction and quantification of gymnemic acids through gymnemagenin from callus cultures of Gymnema sylvestre. Phytochem Anal. 2006;17(6):409-413.
271 Kimura I. Medical benefits of using natural compounds and their derivatives having multiple pharmacological actions. Yakugaku Zasshi. 2006;126(3):133-143.
272 Baskaran K., Kisar Ahamath B., Radha Shanmugasundarum K., et al. Anti-diabetic effects of a leaf extract from gymnema sylvestre in NIDDM patients. J Ethnopharmacol. 1990;30(3):295-300.
273 Al Habori M., Raman A., Lawrence M.J., et al. In vitro effect of fenugreek extracts on intestinal sodium-dependent glucose uptake and hepatic glycogen phosphorylase A. Int J Exp Diabetes Res. 2001;2:91-99.
274 Raju J., Patiolla J.M., Swamy M.V., et al. Diosgenin, a steroid saponin of Trigonella foenum graecum inhibits azoxymethane-induce aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol biomarkers Prev. 2004 Aug;13(8):1392-1398.
275 Broca C., Breil V., Cruciani-Guglielmacci C., et al. Insulinotropic agent ID-1101 (4-hydroxyleucine) activates insulin signaling in rat. Am J Physiol Endocrinol Metab. 2004 Sept;287(3):E463-E471.
276 Flammang A.M., Cifone M.A., Erexson G.L., et al. Genotoxicity testing of a fenugreek extract. Food Chem Toxicol. 2004 Nov;42(11):1769-1775.
277 Vijayakumar M.V., Singh S., Chhipa R.R., et al. The hypoglycaemic activity of fenugreek seed extract is mediated through the stimulation of an insulin signaling pathway. Br J Pharmacol. 2005;146(1):41-48.
278 Puri D., Prabhu K.M., Murthy P.S. Mechanism of action of a hypoglycaemic principle isolated from fenugreek seeds. Indian J Physiol Pharmacol. 2002;46(4):457-462.
279 Vijayakumar M.V., Bhat M.K. Hypoglycaemic effect of a novel dialysed fenugreek seeds extract is sustainable and is mediated, in part, by the activation of hepatic enzymes. Phytother Res. 2008;22(4):500-505.
280 Hannan J.M., Ali L., Rokeya B., et al. Soluble dietary fibre fraction of trigonella foenum graecum seed improves glucose homeostasis in animal models of Type I and II DM by delaying carbohydrate carbohydrate digestion and absorption, and enhancing insulin action. Br J Nutr. 2007;97(3):514-521.
281 Kumar G.S., Shetty A.K., Salimath P.V. Modulatory effect of fenugreek seed mucilage and spent turmeric on intestinal and renal disaccharidases in STZ induced diabetic rats. Plant Foods Hum Nutr. 2005;60(2):87-91.
282 Mohammed S., Taha A., Akhtar K., et al. In vivo effect of trigonella on the expression of pyruvate kinase, phosphoenolpyruvate carboxykinase, and distribution of glucose transporter (GLUT4) in alloxan-diabetic rats. Can J Physiol Pharmacol. 2006;84(6):647-654.
283 Raju J., Gupta D., Rao A.R., et al. Trigonella seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes. Mol Cell Biochem. 2001;224(1-2):45-51.
284 Vats V., Yadav S.P., Grover J.K. Effect of Trigonella on glycogen contant of tissues of tissues and the key enzymes of carbohydrate metabolism. J Ethnopharmacol. 2003;85(2-3):237-242.
285 Pathak P., Srivastava S., Grover S. Development of food products based on millets, legumes and fenugreek seeds and their suitability in the diabetic diet. Int J Food Sci Nutr. 2000 Sept;51(5):409-414.
286 Mohamed S., Taha A., Bamezai R.N., et al. Lower doses of vanadate in combination with trigonella restore altered carbohydrate metabolism and anti-oxidant status in alloxan-diabetic rats. Clin Chem Acta. 2004;342(1-2):105-114.
287 Siddiqui M.R., Taha A., Moorthy K., et al. Amelioration of altered antioxidant status and membrane linked functions by vanadium and Trigonella in alloxan diabetic rat brains. J Biosci. 2005 Sept;30(4):483-490.
288 Mohamed S., Taha A., Bamezai R.N., et al. Modulation of glucose transporter (GLUT4) by vanadate and Trigonella in alloxan diabetic rats. Life Sci. 2006;78(8):820-824.
289 Siddiqui M.R., Taha A., Moorthy K., et al. Low doses of vanadate and Trigonella synergistically regulate Na/K and ATPase activity and GLUT4 translocation in alloxan diabetic rats. Mol cell Biochem. 2006;285(1-2):17-27.
290 Xue W.L., li X.S., Zhang J., et al. effect of Trigonella extract on blood glucose, blood lipids and haemorrheological properties in STZ-induced diabetic rats. Asia Pac J Clin Nutr. 2007;16(Suppl 1):422-426.
291 Kaviarasan S., Vijayalakshmi K., anuradha C.V. Polyphenol rich extract of fenugreek seeds protect erythrocytes from oxidative damage. Plant Foods Hum Nutr. 2004;Fall;59(4):143-147.
292 Petit P.R., Sauvaire Y.D., Hillaire-buys D.M., et al. Steroid saponins from fenugreek seeds: extraction, purification and pharmacological investigation on feeding behaviour and plasma cholesterol. Steroids. 1995;60(10):674-680.
293 Valette G., Sauvaire Y., Baccou J.C., et al. Hypocholesterolaemic effect of fenugreek seeds in dogs. Atherosclerosis. 1984;50(1):105-111.
294 Sauvaire Y., Ribes G., Baccou J.C., et al. Implication of steroid saponins and sapogenins in the hypocholesterolaemic effect of fenugreek. Lipids. 1991;26(3):191-197.
295 Ravikumar P., Anuradha C.V. Effect of fenugreek seeds on blood lipid peroxidation and antioxidants in diabetic rats. Phytother Res. 1999;13(3):197-201.
296 Annida B., Stanely Mainzen Prince P. Supplementation of fenugreek leaves lower lipid profile in STZ-induced diabatic rats. J Med Food. 2004;Summer;7(2):152-156.
297 Narender T., Puri A., Shweta A., et al. 4-Hydroxyleucine an unusual amino acid as antidyslipidaemic and antihyperglycaemic agent. Bioorg Med Chem Lett. 2006;16(2):293-296.
298 Hannan J.M., Rokeya B., Faruque O., et al. Effect of soluble dietary fibre fraction of Trigonella on glycaemic, insulinaemic, lipidaemic and platelet aggregation status of type II diabetic model rats. J Ethnopharmacol. 2003;88(1):73-77.
299 Gupta A., Gupta R., Lai B. Effect of Trigonella seeds on glycaemic control and insulin resistance in Type II DM: A double-blind placebo-controlled study. J Assoc Physicians India. 2001;49:1057-1061.
300 Bordia A., Verma S.K., Srivastava K.C. Effect of ginger and fenugreek on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot Fatty Acids. 1997;56(5):379-384.
301 Genet S., Kale R.K., Baquer N.Z. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: effect of vanadate and fenugreek. Mol Cell biochem. 2002;236(1-2):7-12.
302 Anuradha C.V., Ravikumar P. restoration on tissue antioxidants by fenugreek seeds in alloxan diabetic rats. Indian J Physiol Pharmacol. 2001;45(4):408-420.
303 Preet A., Siddiqui M.R., taha A., et al. Long-term effect of trigonella and its combination with sodium orthovanadate in preventing histopathological and biochemical abnormalities in diabetic rat ocular tissues. Mol Cell Biochem. 2006;289(1-2):137-147.
304 Vats V., yadav S.P., Biswas N.R., et al. Anti-cataract activity of Pterocarpus marsupium bark and Trigonella seed extract in alloxan diabetic rats. J Ethnopharmacol. 2004;93(2-3):289-294.
305 Preet A., Gupta B.L., Siddiqui M.R., et al. Restoration of ultrastructural and biochemical changes in alloxan-induced diabetic rat sciatic nerve on treatment with Na3VO4 and Trigonella- a promising anti-diabetic agent. Mol Cell Biochem. 2005;27891-2:21-31.
306 Sharma R.D., Raghuram T.C., Rao N.S. Effect of fenugreek seeds on blood glucose and serum lipids in Type 1 diabetes. Eur J Clin Nutr. 1990;44:301-308.
307 Gupta A., Gupta R., Lal B. Effect of Trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in Type 2 diabetes mellitus: a double-blind placebo-controlled study. J Assoc Phys India. 2001;49:1057-1061.
308 Grover J.K., Yadav S.P. Pharmacological actions and potential uses of Momordica charantia: a review. J Ethnopharmacol. 2004;93:123-132.
309 Basch E. Bitter lemon (Momordica charantia): a review of the efficacy and safety. Am J Health Syst Pharm. 2003;60:356-359.
310 Dans A.M., Villarruz M.V., Jimeno C.A., et al. The effect of Momordica charantia capsule preparation on glycemic control in type 2 diabetes mellitus needs further studies. J Clin Epidemiol. 2007;60(6):554-559.
311 Fukino Y., Ikeda A., Maruyama K., et al. Randomised controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. European Journal of Clinical Nutrition. 2008;62:953-960.
312 Brown A.L., Lane J., Coverly J., et al. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomised controlled trial. Br J Nutr. 2008;19:1-9.
313 Kudulo G.B. The effect of 3 month ingestion of Ginkgo biloba extract (EGb761) on pancreatic beta-cell function in response to glucose loading in individuals with NIDDM. J Clin Phrmacol. 2001;41(6):600-611.
314 Kudulo G.B., Delaney D., Blodgett J. Short-term ingestion of Ginkgo biloba extract (EGb761) reduces malondialdehyde levels in washed platelets of Type II DM subjects. Diabetes res Clin Pract. 2005;68(1):29-38.
315 Kudolo G.B., Wang W., Javors M., et al. The effect of the ingestion of EGb761 on the pharmacokinetics of metformin in non-diabetic and Type II DM subjects – a double-blind, placebo-controlled, crossover study. Clin Nutr. 2006;25(4):606-616.
316 Kudulo G.B., Dorsey S., Blodgett J. Effect of the ingestion of Ginkgo biloba extract on platelet aggregation and urinary prostanoid excretion in healthy and Type II DM subjects. Thromb Res. 2002;108(2-3):151-160.
317 Kudulo G.B., Wang W., Elrod R., et al. Short-term ingestion of Ginkgo biloba extract does not alter whole body insulin sensitivity in non-diabetic, pre-diabetic or type II diabetic subjects –a randomised double-blind placebo-controlled crossover study. Clin Nutr. 2006;25(1):123-134.
318 Tanaka S., Han L.K., Zheng Y.N., et al. Effects of the flavonoid fraction from Ginkgo biloba extraction on the postprandiol blood glucose elevation in rats. Yakugaku Zasshi. 2004;124(9):605-611.
319 Fodor J.I., Keve T. New phytotherapical opportunity in the prevention and treatment of Type II DM. Acta Pharm Hung. 2006;76(4):200-207.
320 Welt K., Fitzl G., Schepper A. Experimental hypoxia of STZ-diabetic rat myocardium and protective effects of Ginkgo biloba extract. Ultrastructural investigation of microvascular endothelium. Exp Toxic Pathol. 2001;52(6):503-512.
321 Fitzl G., Welt K., Martin R., et al. The influence of hypoxia on the myocardium of experimentally diabetic rats with and without protection by ginkgo biloba extract. Ultrastructural and biochemical investigations on cardiomyocytes. Exp toxicol pathol. 2000;52(5):419-430.
322 Welt K., Weiss J., Martin R., et al. Ultrastructural, immunohistochemical and biochemical investigations of the rat liver exposed to experimental diabetes and acute hypoxia with and without application of Ginkgo extract. Exp Toxicol Pathol. 2004;55(5):331-345.
323 Witte S., Anadere I., Walitza E. Improvement of haemorrheology with ginkgo biloba extract. Decreasing a cardiovascular risk factor. Fortschr Med. 1992;110(13):247-250.
324 Huang S.Y., Jeng C., Kao S.C., et al. Improved haemorrheological properties by Ginkgo biloba extract (EGb761) in Type II DM complicated by retinopathy. Clin Nutr. 2004;23(4):615-621.
325 Bernardczyk-Meller J., Siwiec-Proscinska J., Stankiewicz W., et al. Influence of EGb761 on the function of the retina in children and adolescent with long-lasting DM-preliminary report. Klin Oczna. 2004;106(4-5):569-571.
326 Welt K., Weiss J., Martin R., et al. Ginkgo biloba extracts protect rat kidney from diabetic and hypoxic damage. Phytomedicine. 2007;14(2-3):196-203.
327 Punkt K., Psinia I., Welt K., et al. Effects on skeletal muscle fibres of diabetes and ginkgo biloba extract treatment. Acta Histochem. 1999;101(1):53-69.
328 Wang G.X., Cao F.L., Chen J. Progress in researches on the pharmaceutical mechanism and clinical application of Ginkgo biloba extract on various kinds of diseases. Chin J Integr Med. 2006;12(3):324-329.
329 Lu J., He H. Clinical observation of Ginkgo biloba extract injection in treating early diabetic nephropathy. Chin J Integr Med. 2005;11(3):226-228.
330 Zhu H.W., Shi Z.F., Chen Y.Y. Effect of ginkgo biloba on early diabetic nephropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25(10):889-891.
331 Ernst E., et al. The Desktop Guide to Complementary and Alternative Medicine: an evidence-based approach. Philadelphia: Mosby; 2006.
332 Namdul T., Sood A., Ramakrishnan L., et al. Efficacy of Tibetan medicine as an adjunct in the treatment of type 2 diabetes. Diabetes Care. 2001;24:176-177.
333 Campbell M.J., Woodside J.V., Honour J.W., et al. Effect of red clover-derived isoflavone supplementation on insulin-like growth factor, lipid and antioxidant status in healthy female volunteers: a pilot study. EJCN. 2004;58:173-179.
334 Kato A., Minoshima Y., Yamamoto J., et al. Protective effects of dietary chamomile tea on diabetic complications. J Agric Food Chem. 2008;56(17):8206-8211.
335 McKay D.L., Blumberg J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother Res. 2006;20(7):519-530.
336 Apostolidis E., Kwon Y.I., Kalidas Shetty K. Potential of cranberry-based herbal synergies for diabetes and hypertension management. Asia Pac J Clin Nutr. 2006;15:433-441.
337 Liu J.P., Zhang M., Wang W.Y., et al. Chinese herbal medicines for type 2 diabetes mellitus. Cochrane Database Syst Rev. (3):2004. CD003642
338 Wang E., Wylie-Rosett J. Review of selected Chinese herbal medicines in the treatment of type 2 diabetes. Diabetes Educ. 2008;34(4):645-654.
339 Grover J.K., Yadav S., Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81(1):81-100.
340 Moghaddam M.S., Kumar P.A., Reddy G.B., et al. Effect of Diabecon on sugar-induced lens opacity in organ culture: mechanism of action. J Ethnopharmacol. 2005;97(2):397-403.
341 Brown L., Rosner B., Willett W.W., et al. Cholesterol-lowering effects of dietary fibre: a meta-analysis. Am J Clin Nutr. 1999;69:30-42.
342 Anderson J.W., Allgood L.D., Turner J., et al. Effects of psyllium on glucose and serum lipid responses in men with Type 2 diabetes and hypercholesterolaemia. AJCN. 1999;70:466-473.
343 Hodgson D.M., Nakamura T., Walker A.K. Prophylactic role for complementary and alternative medicine in perinatal programming of adult health. Forsch Komplementmed. 2007;14(2):92-101.
344 Wang X.M. Treating Type 2 diabetes mellitus with foot reflexotherapy. Zhongguo Zhong Zxi Yi Jie He Za Zhi. 1993;13:536-538.
345 Wang M.Y., Tsai P.S., Lee P.H., et al. The efficacy of reflexology: systematic review. J Adv Nurs. 2008;62(5):512-520.
346 National Prescribing Service NPS Newsletter 60, October 2008 ISSN 1441–7421.
347 Sowerby Centre for Health Informatics at Newcastle (SCHIN). Diabetes Type 1 and 2- foot disease. Clinical Knowledge Summaries. Newcastle upon Tyne, UK: National Library for Health; 2007.
348 Neal J.M. Diabetic neuropathic cachexia: a rare manifestation of diabetic neuropathy. South Med J. 2009;102(3):327-329.
349 Lee P., Chen R. Vitamin D as an analgesic for patients with type 2 diabetes and neuropathic pain. Arch Int Med. 2008;168:771-772.
350 Reussmann H.J. On behalf of the German Society of outpatient diabetes centres AND. Switching from pathogenetic treatment with alpha-lipoic acid to gabapentin and other analgesics in painful diabetic neuropathy: a real world study in outpatients. J Diabetes Complications. 2008 April 8.
351 Alleva R., Nasole, et al. Alpha-lipoic acid supplementation inhibits oxidative damage, accelerating chronic wound healing in patients undergoing hyperbaric oxygen therapy. Biochem Res Commun. 2005;333(2):404-410.
352 Cha Y.S. Effects of L-carnitine on obesity, diabetes, and as an ergogenic aid Asia Pac J Clin Nutr. 2008;17(Suppl 1):306-308.
353 Sima A.A., Calvani M., Mehra M., et al. Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomised placebo-controlled trials. Diabetes Care. 2005;28(1):89-94.
354 De Grandis D., Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R D. 2002;3(4):223-231.
355 Evans J.D., Jacobs T.F., Evans E.W. Role of acetyl-L-carnitine in the treatment of diabetic peripheral neuropathy. Ann Pharmacother. 2008;42(11):1686-1691.
356 Weintraub M.I., Wolfe G.I., Barohn R.A., et al. Static magnetic field therapy for symptomatic diabetic neuropathy: a randomised, double-blind, placebo-controlled trial. Arch Phys Med Rehabil. 2003;84:736-746.