CHAPTER 78 Development of the urogenital system
URINARY SYSTEM
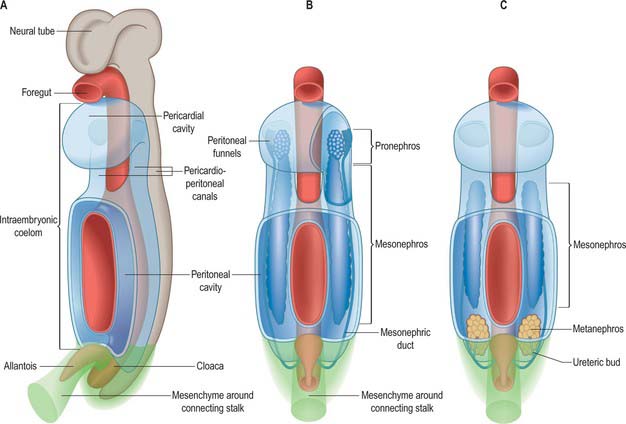
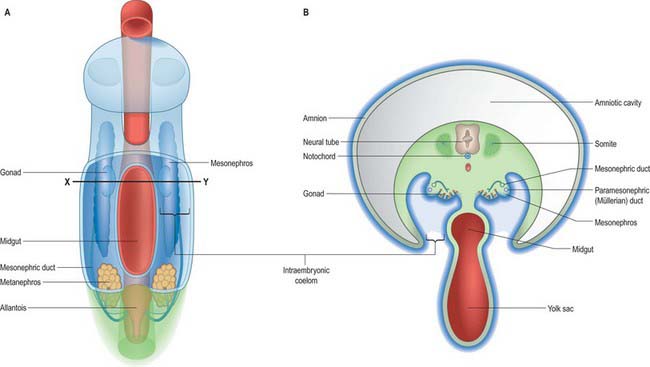
Intermediate mesenchyme is disposed longitudinally in the trunk, subjacent to the somites (in the folded embryo), at the junction between the splanchnopleuric mesenchyme (adjacent to the gut medially) and the somatopleuric mesenchyme (subjacent to the ectoderm laterally) (Fig. 78.1). In lower vertebrates, intermediate mesenchyme typically develops serial, segmental epithelial diverticuli termed nephrotomes. Each nephrotome encloses a cavity, the nephrocele, which communicates with the coelom through a peritoneal funnel, the nephrostome (Fig. 78.2). The dorsal wall of a nephrotome evaginates as a nephric tubule. The dorsal tips of the cranial nephric tubules bend caudally and fuse to form a longitudinal primary excretory duct, which grows caudally and curves ventrally to open into the cloaca. The more caudally placed, and therefore chronologically later, tubules open secondarily into this duct or into tubular outgrowths from it. Glomeruli, specific arrangements of capillaries and overlying coelomic epithelium, arise from the ventral wall of the nephrocele (internal glomeruli) or the roof of the coelom adjacent to the peritoneal funnels (coelomic or external glomeruli), or in both situations (Fig. 78.2).
It has been customary to regard the renal excretory system as three organs, the pronephros, mesonephros and metanephros, succeeding each other in time and space, such that the last to develop is retained as the permanent kidney (Figs 78.1, 78.2). However, it is difficult to provide reliable criteria by which to distinguish these stages or to define their precise limits in embryos.
Pronephros
The intermediate mesenchyme becomes visible in stage 10 embryos and can be distinguished as a nephrogenic cord when 10 somites are present. A pronephros is present in human embryos only as clusters of cells in the most cranial portions of the nephrogenic cord (Figs 78.1, 78.2). More caudally, similar groups of cells appear and become vesicular. The dorsal ends of the most caudal of the vesicles join the primary excretory duct. Their central ends are connected with the coelomic epithelium by cellular strands, which probably represent rudimentary peritoneal funnels. Glomeruli do not develop in association with these cranially situated nephric tubules, which ultimately disappear. It is doubtful whether external glomeruli develop in human embryos.
Primary excretory duct
In stage 11 embryos of approximately 14 somites, the primary excretory duct can be seen as a solid rod of cells in the dorsal part of the nephrogenic cord. Its cranial end is about the level of the ninth somite and its caudal tip merges with the undifferentiated mesenchyme of the cord. It differentiates before any nephric tubules, and when the latter appear it is at first unconnected with them. In older embryos the duct has lengthened and its caudal end becomes detached from the nephrogenic cord to lie immediately beneath the ectoderm. From this level it grows caudally, independent of the nephrogenic mesenchyme, and then curves ventrally to reach the wall of the cloaca. It becomes canalized progressively from its caudal end to form a true duct, which opens into the cloaca in embryos at stage 12 (Fig. 78.1C). Clearly, up to this stage the name ‘duct’ is scarcely appropriate.
Mesonephros
From stage 12 mesonephric tubules, which develop from the intermediate mesenchyme between somite levels 8–20, begin to connect to the primary excretory duct, which is now renamed the mesonephric duct. More caudally, a continuous ridge of nephrogenic mesenchyme extends to the level of somite 24. The mesonephric tubules (nephrons) are not metameric – there may be two or more mesonephric tubules opposite each somite.
By the end of the sixth week each mesonephros is an elongated, spindle-shaped organ that projects into the coelomic cavity, one on each side of the dorsal mesentery, from the level of the septum transversum to the third lumbar segment. This whole projection is called the mesonephric ridge, mesonephros, or Wolffian body (Figs 78.1B,C, 78.3). It develops subregions, and a gonad develops on its medial surface (p. 1311). There are striking similarities in structure between the mesonephros and the permanent kidney or metanephros, but the mesonephric nephrons lack a segment that corresponds to the descending limb of the loop of Henle. The mesonephros is believed to produce urine by stage 17. A detailed comparison of the development and function of the mesonephros and metanephros in staged human embryos is not available.

Fig. 78.3 Arrangement of mesonephric duct from mesonephros to urogenital sinus. The duct runs within the tubal fold with the paramesonephric duct. For later development, see Fig. 78.14.
(Redrawn with permission from Tuchmann-Duplessis H, Haegel P 1972 Illustrated Human Embryology, Vol 2 Organogenesis. London: Chapman and Hall. With kind permission of Springer Science+Business Media.)
In stage 18 embryos (13–17 mm) the mesonephric ridge extends cranially to about the level of rib 9. In both sexes the cranial end of the mesonephros atrophies, and in embryos 20 mm in length (stage 19) a mesonephros is found only in the first three lumbar segments, although it may still possess as many as 26 tubules. The most cranial one or two tubules persist as rostral aberrant ductules (see Fig. 78.13); the succeeding five or six tubules develop into either the efferent ductules of the testis and lobules of the head of the epididymis (male), or the tubules of the epoöphoron (female); the caudal tubules form the caudal aberrant ductules and the paradidymis (male), or the paroöphoron (female) (p. 1313).
Mesonephric duct
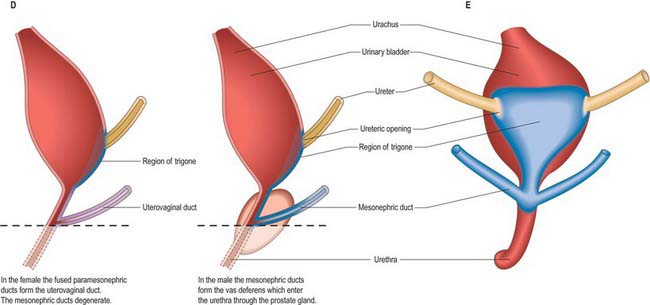
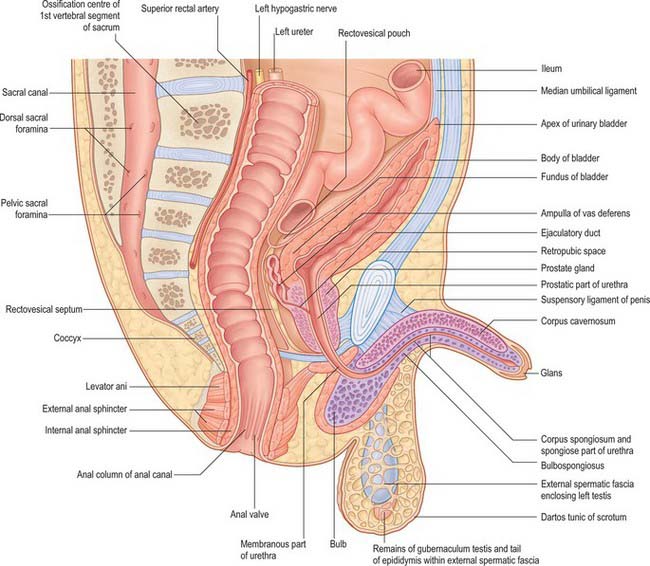
Once mesonephric nephrons connect to the primary excretory duct it is renamed the mesonephric duct. This runs caudally in the lateral part of the nephric ridge, and at the caudal end of the ridge it projects into the cavity of the coelom in the substance of a mesonephric fold (Fig. 78.3). As the mesonephric ducts from each side approach the urogenital sinus the two mesonephric folds fuse, between the bladder ventrally and the rectum dorsally, forming a transverse partition across the cavity of the pelvis, which is somewhat inappropriately called the genital cord (Fig. 78.3). In the male the peritoneal fossa between the bladder and the genital cord becomes obliterated, but it persists in the female as the uterovesical pouch. In the male the mesonephric duct itself becomes the canal of the epididymis, vas deferens and ejaculatory duct (p. 1317).
Urogenital sinus
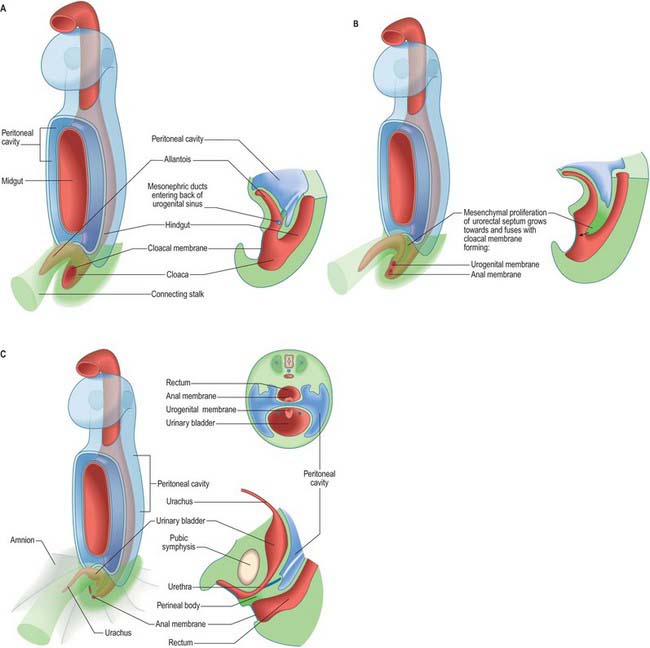
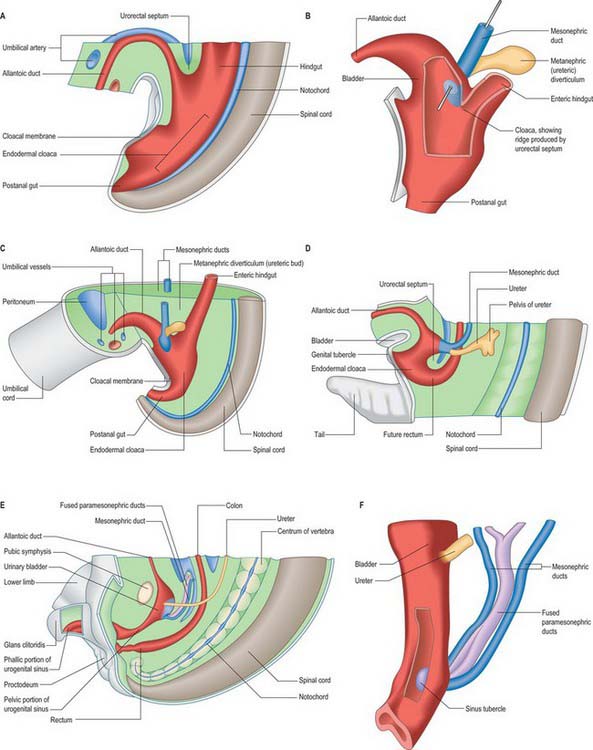
The primitive hindgut ends in a cloacal region. This is connected ventrally with a blind-ending diverticulum, the allantois, which is intimately related to the development of the caudal portion of the urinary system. The enteric and allantoic portions of the hindgut are separated by the proliferation of the urorectal septum, a partition of mesenchyme and endoderm in the angle of the junction of hindgut and allantois (Fig. 78.4; see Fig. 78.7). The endodermal epithelium beneath the mesenchyme of the urorectal septum approaches but does not fuse with the cloacal membrane: it effectively divides the membrane into anal (dorsal) and urogenital (ventral) membranes, and the cloacal region into dorsal and ventral portions. The dorsal portion of the cloacal region is the putative rectum. The ventral portion can be further divided into: a cranial vesicourethral canal, continuous above with the allantoic duct; a middle, narrow channel, the pelvic portion; and a caudal, deep, phallic section, which is closed externally by the urogenital membrane. The second and third parts together constitute the urogenital sinus.
Metanephros
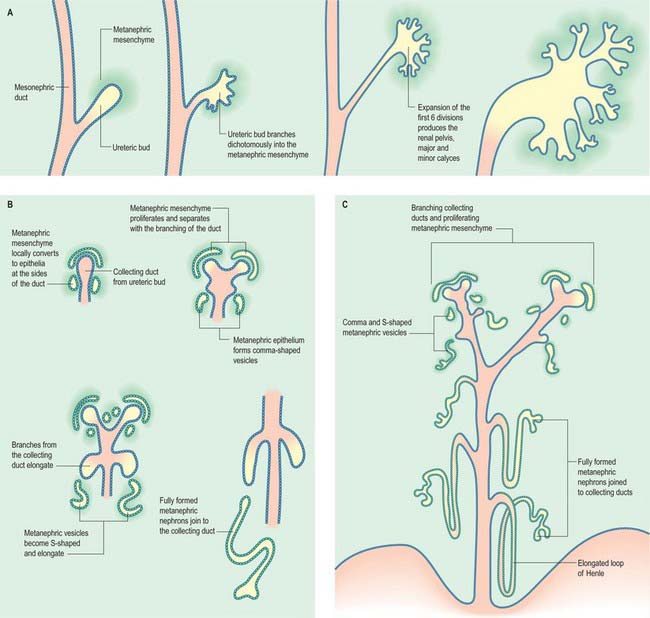
The metanephric kidney develops from three sources. An evagination of the mesonephric duct, the ureteric bud, and a local condensation of mesenchyme, the metanephric blastema, form the nephric structure (Fig. 78.5). Angiogenic mesenchyme migrates into the metanephric blastema slightly later to produce the glomeruli and vasa recta. It is possible that an intact nerve supply is also required for metanephric kidney induction.
An epithelial/mesenchymal interaction between the duct system and the surrounding mesenchyme occurs in both mesonephric and metanephric systems. In the mesonephric kidney, development proceeds in a craniocaudal progression, and cranial nephrons degenerate before caudal ones are produced. In the metanephric kidney a proportion of the mesenchyme remains as stem cells that continue to divide and which enter the nephrogenic pathway later when the individual collecting ducts lengthen. The temporal development of the metanephric kidney is patterned radially, such that the outer cortex is the last part to be formed. The following interactions occur in the development of the metanephric kidney (Fig. 78.5). The ureteric bud undergoes a series of bifurcations within the surrounding metanephric mesenchyme, and forms smaller ureteric ducts. At the same time the metanephric mesenchyme condenses around the dividing ducts to form S-shaped clusters, which transform into epithelia and fuse with the ureteric ducts at their distal ends. Blood vessels invade the proximal ends of the S-shaped clusters to form vascularized glomeruli.
The ureteric bud bifurcates when it comes into contact with the metanephric blastema in response to extracellular matrix molecules synthesized by the mesenchyme. Both chondroitin sulphate proteoglycan synthesis and chondroitin sulphate glycosaminoglycan processing are necessary for the dichotomous branching of the ureteric bud. In metanephric culture, incubation of fetal kidneys in β-D-xyloside, an inhibitor of chrondroitin sulphate synthesis, dramatically inhibits ureteric bud branching.
Subsequent divisions of the ureteric bud and associated mesenchyme define the gross structure of the kidney and the major and minor calyces, the distal branches of the ureteric ducts that will form the collecting ducts of the kidney. The proximal position of the ureteric bud elongates to form the developing ureter (Fig. 78.6). As the collecting ducts elongate the metanephric mesenchyme condenses around them. An adhesion molecule, syndecan, can be detected between the mesenchymal cells in the condensate. The cells switch off expression of N-CAM, fibronectin and collagen I, and start to synthesize L-CAM (also called E cadherin) and the basal lamina constituents laminin and collagen IV. The mesenchymal clusters are thus converted to small groups of epithelial cells, which undergo complex morphogenetic changes. Each epithelial group elongates, and forms first a comma-shaped, then an S-shaped, body, which continues to elongate and subsequently fuses with a branch of the ureteric duct at its distal end, while expanding as a dilated sac at its proximal end (Fig. 78.5). The latter involutes, and cells differentiate locally such that the outer cells become the parietal glomerular cells, while the inner ones become visceral epithelial podocytes. The podocytes develop in close proximity to invading capillaries derived from angiogenic mesenchyme outside the nephrogenic mesenchyme. This third source of mesenchyme produces the endothelial and mesangial cells within the glomerulus. The (metanephric-derived) podocytes and the angiogenic mesenchyme produce fibronectin and other components of the glomerular basement membrane. The isoforms of type-IV collagen within this layer follow a specific programme of maturation as the filtration of macromolecules from the plasma becomes restricted.
Platelet derived growth factor (PDGF) β-chain and the PDGF receptor β-subunit (PDGFR β) have been detected in developing human glomeruli between 54 and 109 days’ gestation. PDGF β-chain is localized in the differentiating epithelium of the glomerular vesicle during its comma and S-shaped stages, while PDGFR β is expressed in the undifferentiated metanephric blastema, vascular structures and interstitial cells. Both PDGF β-chain and PDGFR β are expressed by mesangial cells, which may promote further mesangial cell proliferation.
All stages of nephron differentiation are present concurrently in the developing metanephric kidney (Fig. 78.5). Antigens for the brush border of the renal tubule appear when the S-shaped body has formed. They appear first in the inner cortical area.
The metanephric kidney is lobulated throughout fetal life, but this condition usually disappears during the first year after birth (see Fig. 78.8). Varying degrees of lobulation occasionally persist throughout life.
Endocrine development of the kidney
The kidney functions not only as an excretory organ, but also as an endocrine organ, secreting hormones that are concerned with renal haemodynamics. Before birth homeostasis is controlled by the placenta. The fetal kidney produces amniotic fluid. The kidneys of premature babies of less than 36 weeks are immature. They contain incompletely differentiated cortical nephrons, which compromise their ability to maintain homeostasis. Problems of immaturity are further compounded by the effects of hypoxia and asphyxia, which modify renal hormones.
Urinary bladder
The urinary bladder develops from the cranial vesicourethral canal, which is continuous above with the allantoic duct (Figs 78.4, 78.6, 78.7). The mesonephric ducts open into the urogenital sinus early in development. The ureters develop as branches of the mesonephric ducts, which attain their own access to the developing bladder, and their orifices open separately into the bladder on the lateral side of the opening of the mesonephric ducts. Later the two orifices become separated still further and, although the ureter retains its point of entry into the bladder, the mesonephric duct opens into that part of the urogenital sinus that subsequently becomes the prostatic urethra (Fig. 78.6). The triangular region of absorption of the mesonephric ducts contributes to the trigone of the bladder and dorsal wall of the proximal half of the prostatic urethra, i.e. as far as the opening of the prostatic utricle and ejaculatory ducts, or its female homologue, the whole female urethral dorsal wall. The remainder of the vesicourethral canal forms the body of the bladder and urethra, and its apex is prolonged to the umbilicus as a narrow canal, the urachus.
Neonatal urinary system
At birth the two kidneys weigh approximately 23 g. They function early in development and produce the amniotic fluid that surrounds the fetus. The lobulated appearance of fetal kidneys is still present at birth (Fig. 78.8; see Fig. 14.4). Addition of new cortical nephrons continues in the first few months of postnatal life after which general growth of the glomeruli and tubules results in the disappearance of lobulation. The renal blood flow is lower in the neonate; adult values are attained by the end of the first year. The glomerular filtration rate at birth is approximately 30% of the adult value, which is attained by 3–5 months of age.
The neonatal urinary bladder is egg-shaped and the larger end is directed downwards and backwards (Figs 78.9, 78.10; see Figs 14.4, 14.5). Although described as an abdominal organ, nearly one half of the neonatal bladder lies below a line drawn from the promontory of the sacrum to the upper edge of the pubic symphysis, i.e. within the cavity of the true pelvis. From the bladder neck, the bladder extends anteriorly and slightly upwards in close contact with the pubis until it reaches the anterior abdominal wall. The apex of the contracted bladder lies at a point midway between the pubis and the umbilicus. When the bladder is filled with urine the apex may extend up to the level of the umbilicus. It is therefore possible to obtain urine by inserting a needle, connected to a syringe, into the bladder through the abdominal wall about 2 cm above the symphysis pubis and aspirating the contents into the sterile syringe. The success rate of the procedure is variable and depends upon the bladder being full: a much higher success rate has been reported by using an ultrasound scanner to locate the bladder and confirm that it contains urine prior to the insertion of the needle.
There is no true fundus in the fetal bladder as there is in the adult. Although the anterior surface is not covered with peritoneum, peritoneum extends posteriorly as low as the level of the urethral orifice. Because the apex of the bladder is relatively high, pressure on the lower abdominal wall will express urine from an infant bladder. Moreover, because the bladder remains connected to the umbilicus by the obliterated remains of the urachus (see Fig. 14.5), stimulation of the umbilicus can initiate micturition in babies. The elongated shape of the bladder in neonates means that the ureters are correspondingly reduced in length and they lack a pelvic portion. The bladder does not gain its adult, pelvic, position until about the sixth year. A distinct interureteric fold is present in the contracted neonatal bladder.
Anomalies of the urinary system
It was thought that renal cysts arose from clumps of vesicular cells, which persisted when the tips of branches from the ureteric diverticulum failed to fuse with metanephrogenic cap tissue. It is now believed that they are wide dilatations of a part of otherwise continuous nephrons. In most cases, autosomal dominant polycystic kidney disease results from mutations of PKD1 or PKD2 genes which are expressed in human embryos from 5–6 weeks of development within the mesonephros and later the metanephros (Chauvet et al 2002). In this condition the cystic dilatation may affect any part of the nephron, from Bowman’s capsule to collecting tubules. Less common is infantile cystic renal disease, inherited as a recessive trait, where the proximal and distal tubules are dilated to some degree but the collecting ducts are grossly affected.
Abnormalities of the ventral body wall caudal to the umbilicus, especially with inappropriate siting of the genital tubercle can result in exstrophy of the bladder (Fig. 78.11). In this condition the urorectal septum (internal) is associated with the genital tubercle (external), which means that the urogenital and anal membranes are widely separated. When the urogenital membrane involutes, the posterior surface of the bladder is exposed to the anterior abdominal wall. The lower part of the abdominal wall is therefore occupied by an irregularly oval area, covered with mucous membrane, on which the two ureters open. The periphery of this extroverted area, which is covered by urothelium, becomes continuous with the skin.
The volume of amniotic fluid is used as an indicator of renal function, but, because other sources produce amniotic fluid in early gestation, amniotic volume does not reflect fetal urinary output until the second trimester. Too little amniotic fluid is termed oligohydramnios, too much, polyhydramnios. Although variation in the amount of amniotic fluid may suggest abnormalities of either the gut or kidneys, it is not always possible to correlate even severe oligohydramnios with renal dysfunction. There is an important relationship between the volume of amniotic fluid, lung development and maturity, and oligohydramnios has been shown to be associated with pulmonary hypoplasia (see Ch. 59).
REPRODUCTIVE SYSTEM
Early gonadal development (ambisexual or indifferent stage)
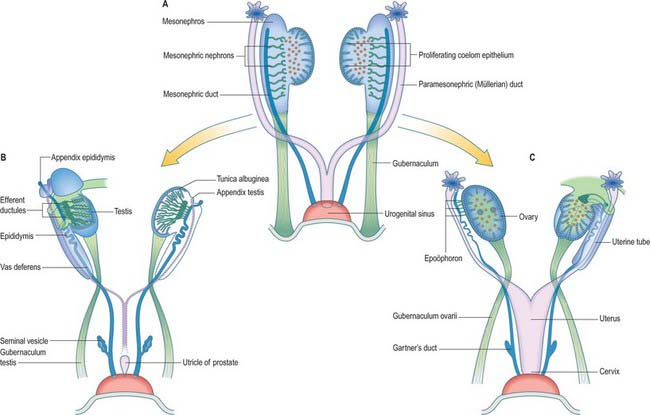
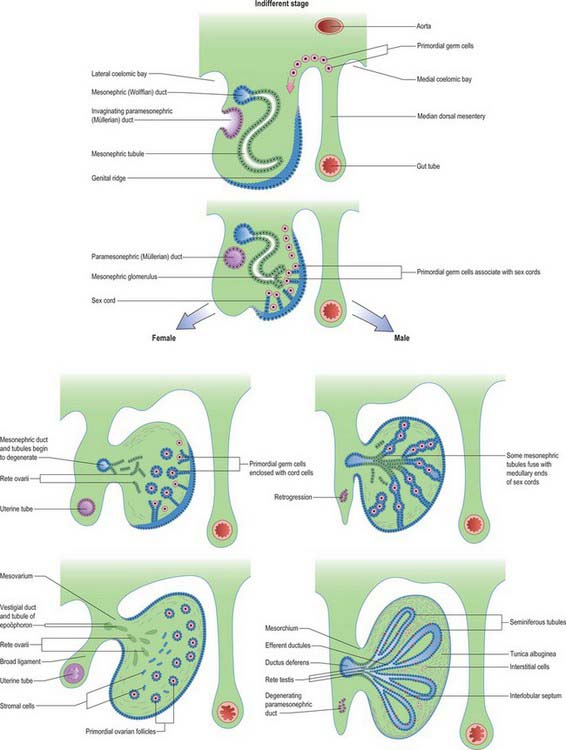
The formation of the gonads is first indicated by the appearance of an area of thickened coelomic epithelium on the medial side of the mesonephric ridge in the fifth week, stage 16 (Figs 78.12, 78.13; see Fig. 78.16). Elsewhere on the surface of the ridge the coelomic epithelium is one or two cells thick, but over this gonadal area it becomes multilayered. Thickening rapidly extends in a longitudinal direction until it covers nearly the whole of the medial surface of the ridge. The thickened epithelium continues to proliferate, displacing the renal corpuscles of the mesonephros in a dorsolateral direction and forms a projection into the coelomic cavity, the gonadal ridge. Surface depressions form along the limits of the ridge, which is thus connected to the mesonephros by a broad mesentery, the mesogenitale. In this way the mesonephric ridge becomes subdivided into a lateral part, the tubal fold, containing the mesonephric and paramesonephric ducts, and a medial part, the gonadal fold. The tubal fold also contains the nephric tubules and glomeruli at its base (Fig. 78.3).
Up to the seventh week the ambisexual gonad possesses no sexually differentiating feature. From stage 16 the proliferating coelomic epithelium forms a number of cellular epithelial cords (sometimes called primary sex cords), separated by mesenchyme. The cords remain at the periphery of the primordium and form a cortex. Proliferation and labyrinthine cellular condensation of the mesonephric mesenchyme, including angiogenic mesenchyme, produces a central medulla (see Fig. 78.16).
Reproductive ducts
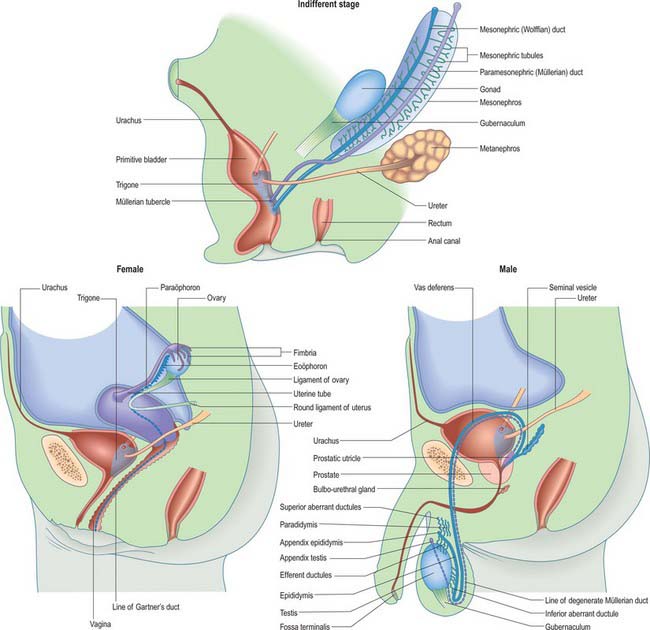
The paramesonephric, Müllerian, ducts develop in embryos of both sexes, but become dominant in the development of the female reproductive system. They are not detectable until the embryo reaches a length of 10–12 mm (early sixth week). Each begins as a linear invagination of the coelomic epithelium, the paramesonephric groove, on the lateral aspect of the mesonephric ridge near its cranial end. The blind caudal end continues to grow caudally into the substance of the ridge as a solid rod of cells, which becomes canalized as it lengthens. Throughout the extent of the mesonephros it is lateral to the mesonephric duct, which acts as a guide for it. The paramesonephric duct reaches the caudal end of the mesonephros in the eighth week. It turns medially and crosses ventral to the mesonephric duct to enter the genital cord, where it bends caudally in close apposition with its fellow from the opposite side (Fig. 78.13). The two ducts reach the dorsal wall of the urogenital sinus during the third month, and their blind ends produce an elevation called the Müllerian sinus tubercle (Fig. 78.7F; see Fig. 78.19).
Uterus and uterine tubes
In the female the mesonephric duct is vestigial. Cranially it becomes the longitudinal duct of the epoöphoron, while caudally it is referred to as Gartner’s duct (Table 78.1). The cranial part of the paramesonephric ducts forms the uterine tubes, and the original coelomic invagination remains as the pelvic opening of the tube. The fimbriae become defined as the cranial end of the mesonephros degenerates. The caudal vertical parts of the two ducts fuse with each other to form the uterovaginal primordium (Figs 78.7, 78.13, 78.14; see Fig. 78.19). This gives rise to the lower part of the uterus and, as it enlarges, it takes in the horizontal parts to form the fundus and most of the body of the adult uterus. A constriction between the body of the uterus and the cervix can be found at 9 weeks. The stroma of the endometrium and the uterine musculature develop from the surrounding mesenchyme of the genital cord.
Table 78.1 Homologies of the parts of the urogenital system in male and female.
| Gonad | Testis | Ovary |
|---|---|---|
| Gubernacular cord | Gubernaculum testis | Ovarian and round ligaments |
| Mesonephros (Wolffian body) | Appendix of epididymis (?) | Appendices vesiculosae (?) |
| Efferent ductules | Epoöphoron | |
| Lobules of epididymis | ||
| Paradidymis | Paroöphoron | |
| Aberrant ductules | ||
| Mesonephric duct (Wolffian duct) | Duct of epididymis | Duct of epoöphoron (Gartner’s duct) |
| Vas deferens | ||
| Ejaculatory duct | ||
| Part of bladder and prostatic urethra | Part of bladder and urethra | |
| Paramesonephric (Müllerian) duct | Appendix of testis | Uterine tube |
| Uterus | ||
| Prostatic utricle | Vagina (?) | |
| Allantoic duct | Urachus | Urachus |
| Cloaca: | ||
| Dorsal part | Rectum and upper part of anal canal | Rectum and upper part of anal canal |
| Ventral part | Most of bladder | Most of bladder and urethra |
| Part of prostatic urethra | ||
| Urogenital sinus | Prostatic urethra distal to utricle | |
| Bulbo-urethral glands | Greater vestibular glands | |
| Rest of urethra to glans | Vestibule | |
| Genital folds | Ventral penis | Labia minora |
| Genital tubercle | Penis | Clitoris |
| Urethra in glans |

Fig. 78.14 Relative movements of the gonads and associated tubes. A, Gonads and mesonephros move caudally, and the metanephros ascends. B, Posterior view of the mesonephric ducts (ureters) and the fused paramesonephric ducts (uterovaginal canal) in the female. For earlier development, see Fig. 78.3.
(Redrawn from Tuchmann-Duplessis H, Haegel P 1972 Illustrated Human Embryology, Vol 2 Organogenesis. London: Chapman and Hall. With kind permission of Springer Science+Business Media.)
Failure of fusion of the two paramesonephric ducts can lead to a range of anomalies summarized in Fig. 78.15. These fusions can also contribute to anomalies of vaginal development.

Fig. 78.15 Uterovaginal malformations.
(Redrawn from Tuchmann-Duplessis H, Haegel P 1972 Illustrated Human Embryology, Vol 2 Organogenesis. London: Chapman and Hall. With kind permission of Springer Science+Business Media.)
At birth the uterus is 2.5–5 cm long (average 3.5 cm), 2 cm wide between the uterine tubes, and a little over 1 cm thick (Fig. 78.9; see Figs 14.4, 14.5). The body of the uterus is smaller than the uterine cervix, which forms two-thirds or more of the length. The isthmus between the body and the cervix is absent. The fetal female reproductive tract is affected by maternal hormones and undergoes some enlargement in the fetus. The endocervical glands are active before birth and the cervical canal is usually filled with mucus. The uterus is relatively large at birth, and subsequently involutes to about one-third of its length and more than half of its weight; it does not regain its neonatal size and weight until puberty. The uterine tubes are relatively short and wide.
The position of the uterus in the pelvic cavity depends to a great extent on the state of the bladder anteriorly and the rectum posteriorly. If the bladder contains only a small amount of urine the uterus may be anteverted but it is often in a direct line with the vagina (Fig. 78.9; see Fig. 78.19).
Vagina
At approximately 60 mm crown rump (CR) length an epithelial proliferation, the sinuvaginal bulb, arises from the dorsal wall of the urogenital sinus in the region of the sinus tubercle (Figs 78.7F, 78.14B),. Its origin marks the site of the future hymen. The proliferation gradually extends cranially as a solid anteroposteriorly flattened plate inside the tubular condensation of the uterovaginal primordium, which will eventually become the fibromuscular vaginal wall. The caudal tip of the paramesonephric duct epithelium recedes until, at about 140 mm (CR), its junction with the epithelial proliferation lies in the cervical canal.
Starting from its caudal end, and gradually extending cranially through its whole extent, the solid plate formed by the sinus proliferation enlarges into a cylindrical structure. Thereafter the central cells desquamate to establish the vaginal lumen. The extent to which mesonephric and paramesonephric ducts contribute directly to the formation of the vagina is controversial. As the upper end of the vaginal plate enlarges it grows up to embrace the cervix, and then is excavated to produce the vaginal fornices. Anomalies of paramesonephric duct fusion can produce related vaginal anomalies (Fig. 78.15). The urogenital sinus undergoes relative shortening craniocaudally to form the vestibule, which opens on the surface through the cleft between the genital folds. The lower end of the vaginal plate grows caudally so that in 109 mm embryos the vaginal rudiment approaches the vestibule. In fetuses of 162 mm the vaginal lumen is complete except at the cephalic end where the fornices are still solid; they are hollow by 170 mm. At approximately half way through gestation (180 mm) the genital canal is continuous with the exterior. During the later months of fetal life the vaginal epithelium is greatly hypertrophied, apparently under the influence of maternal hormones, but after birth it assumes the inactive form of childhood.
In the neonate, the vagina is 2.5–3.5 cm long and 1.5 cm wide at the fornices, and the uterine cervix extends into the vagina for about 1 cm. The posterior vaginal wall is longer than the anterior wall, giving the vagina a distinct curve (Fig. 78.9; see Fig. 78.19 and Fig. 14.5). The cavity is filled with longitudinal columns covered with a thick layer of cornified, stratified squamous epithelium. These cells slough off after birth when the effect exerted by maternal hormones is removed.
The orifice of the vagina is surrounded by a thick elliptical ring of connective tissue, the hymen (Fig. 78.9). During childhood the hymen becomes a membranous fold along the posterior margin of the vaginal lumen. Should the fold form a complete diaphragm across the vaginal lumen it is termed an imperforate hymenal membrane.
Reproductive ducts in the male
In the male, most of the paramesonephric ducts atrophy (Fig. 78.13) under the influence of anti-Müllerian hormone (AMH), which is released into the mesonephric duct by the Sertoli cells of the testis. Vestigial structures are therefore most likely to persist cranially and/or caudally, at the limits of the local exocrine effects of AMH. A vestige of the cranial end of the duct persists as the appendix testis (Fig. 78.13; see Fig. 78.19, Table 78.1). The fused caudal ends of the two ducts are connected to the wall of the urogenital sinus by a solid utricular cord of cells, which soon merges with a proliferation of sinus epithelium, the sinu-utricular cord. The latter is similar to, but less extensive than, the sinus proliferation in the female. The proliferating epithelium is claimed to be an intermingling of the endoderm of the urogenital sinus with the lining epithelia of the mesonephric and paramesonephric ducts, which have extended on to the surface of the sinus tubercle. As the sinu-utricular cord grows, so the utricular cord recedes from the tubercle. In the second half of fetal life the composite cord acquires a lumen and becomes dilated to form the prostatic utricle, the lining of which consists of hyperplastic stratified squamous epithelium (Fig. 78.13; see Fig. 78.19). The sinus tubercle becomes the colliculus seminalis.
The main reproductive ducts in the male are derived from the mesonephric ducts; the latter persist under the action of androgens that are probably secreted down the ducts themselves, and are subsumed into the male reproductive system as the metanephric kidney develops. The mesonephric duct gives rise to the canal of the epididymis, vas deferens and ejaculatory duct. The seminal vesicle and the ampulla of the vas deferens appear as a common swelling at the end of the mesonephric duct during the end of the third and into the fourth month. Their appearance coincides with degeneration of the paramesonephric ducts, although no causal relation between the two events has been established. Separation into two rudiments occurs at about 125 mm crown–heel length. The seminal vesicle elongates, its duct is delineated and hollow diverticula bud from its wall. About the sixth month (300 mm crown–heel length) the growth rate of both vesicle and ampulla is greatly increased. Figure 78.10 shows the position of the ampulla of the vas deferens in the neonate, possibly in response to increased secretion of prolactin by the fetal or maternal hypophysis, or to the effects of placental hormones. The tubules of the prostate show a similar increase of growth rate at this time.
Primordial germ cells
The primordial germ cells are formed very early from the epiblast (see Fig. 10.3). They are larger (12 to 20 μm in diameter) than most somatic cells, and are characterized by vesicular nuclei with well-defined nuclear membranes and by a tendency to retain yolk inclusions long after these have disappeared from somatic cells. It is not yet clear whether the primordial germ cells are derived from particular blastomeres during cleavage, if they constitute a clonal line from a single blastomere, or if they are the product of a progressive concentration of the nucleus of the fertilized ovum by unequal partition at successive mitoses. Primordial germ cells spend the early stages of development within the extraembryonic tissues near the end of the primitive streak and in the connecting stalk (see Fig. 10.4). In this situation they are away from the inductive influences to which the majority of the somatic cells are subjected during early development.
Primordial germ cells can be identified in human embryos in stage 11 when the number of cells is probably not more than 20–30. When the tail fold has formed they appear within the endoderm and the splanchnopleuric mesenchyme and epithelium of the hindgut as well as in the adjoining region of the wall of the yolk sac. They migrate dorsocranially in the mesentery, by amoeboid movements and by growth displacement, and pass around the dorsal angles of the coelom (medial coelomic bays) to reach the genital ridges from stage 15 (Fig. 78.16). It is believed that the genital ridges exert long-range effects on the migrating primordial germ cells, in terms of controlling their direction of migration and supporting the primordial germ-cell population.
Development of the gonads
The factors that lead to formation of either testis or ovary are described below and in Fig. 78.16. The morphological events which occur in each type of gonadal development are presented first.
Testis
Most studies support the hypothesis that the seminiferous tubules are formed from cords of epithelial cells derived from the proliferating coelomic epithelium (Figs 78.13, 78.16). The cords lengthen, partly by addition from the coelomic epithelium, and encroach on the medulla, where they unite with a network of cells derived from the mesonephric mesenchyme destined to become the rete testis. Primordial germ cells are incorporated into the cords, which later become enlarged and canalized to form the seminiferous tubules. The cells derived from the surface of the early gonad form the supporting Sertoli cells. The latter proliferate throughout development and perhaps into childhood; when they stop dividing they mature and cannot be reactivated. Each Sertoli cell can only support a fixed number of germ cells during their development into spermatozoa, i.e. the number of Sertoli cells produced at this time determines the maximal limit of sperm output. Because the germ cells make up the bulk of the adult testis, the number of Sertoli cells is a major determinant of the size to which the testes will grow (factors which impair the process of spermatogenesis, resulting in the loss of germ cells, will also affect testicular size). Variation in Sertoli cell number is probably the most important factor in accounting for the enormous variation in sperm counts between individual men, whether fertile or infertile. Indeed, the available data for adult men indicate that Sertoli cell numbers vary across a fifty-fold range. Although some of this variation may result from attrition of Sertoli cell numbers because of ageing, the major differences in Sertoli cell numbers will have been determined by events in fetal and/or childhood life.
Ovary
The ovary closely resembles the testis early in development, except that its characteristically female features are slower to differentiate (Figs 78.13, 78.16). Few, if any, of the epithelial cords invade the medulla. The majority remain in the cortex, where they may be joined by a second proliferation from the coelomic epithelium overlying the gonad. In histological sections of ovaries from the third and subsequent months, the epithelial cords appear as clusters of cells, which may contain primitive germ cells, separated by fine septa of undifferentiated mesenchyme. An ovarian rete condenses in the medullary mesenchyme and some of its cords may join mesonephric glomeruli. The medulla subsequently regresses, and connective tissue and blood vessels from this region invade the cortex to form the ovarian stroma. During this invasion the clusters of epithelial cortical cells break into individual groups of supporting cells (now identified as granulosa cells), which surround the primordial germ cells (now identified as primary oocytes) that have entered the prophase of the first meiotic division. Primary oocytes are derived from a mitotic division of the primordial germ cells (naked oogonia). Their epithelial capsules consist of flattened pregranulosa cells derived from proliferations of coelomic epithelium. The ovary now has its full complement of primary oocytes. The majority undergo atresia, a hormonally controlled apoptotic process, but the remainder resume development after puberty, when they complete the first meiotic division shortly before ovulation (p. 1296). The granulosa cells at this time enlarge and multiply to form the stratum granulosum; as they do so, they become surrounded by thecal cells, which differentiate from the stroma.
Sex determination in the embryo
The possession of a Y chromosome is usually associated with a male developmental pathway. Deletion mapping of the Y chromosome in a class of XX males arising from abnormal X:Y interchange at meiosis showed that the male-determining region of the chromosome was a conserved sequence located near its tip, termed the testis-determining factor (TDF). This sequence contains a gene that is regarded by some as the ‘master switch’ that programmes the direction of sexual development, the so-called SRY gene (sex-determining region on Y chromosome). It is believed to be genetically and functionally equivalent to the TDF. The SRY gene acts initially within the epithelial cords of cells derived from the coelomic epithelium of the ambisexual gonad. These cells can potentially differentiate into either Sertoli or granulosa cells (the supporting cells for the germ cells in the testis and ovary respectively). Studies indicate that SRY initiates testis formation from the indifferent gonad by directing the development of supporting-cell precursors as Sertoli rather than granulosa cells (Albrecht & Eicher 2001). Gene expression is seen first in cells designated as pre-Sertoli located centrally in the developing gonad and then later in the cranial and caudal poles. Once the developmental pathway of Sertoli cells is directed this influences the differentiation of the other cell types in the testicular pathway, so that Leydig cells appear later, and the connective tissue becomes organized into a male pattern. The germ cells are also affected by this environment. When they arrive they become enclosed within the Sertoli cells and enter mitotic arrest (which is characteristic of spermatogenesis), instead of entering meiosis and meiotic arrest (which characterizes oogenesis). Thus the development of male characteristics follows the expression of SRY, and female characteristics develop in its absence.
Although fetuses of either sex are exposed to maternal hormones, the accelerated development of the testis at early embryological stages ensures arrest of meiosis of the germ cells, and the production of hormones that masculinize the male embryo before the development of the reproductive tract and ovaries of the female. The range of intersex conditions, of phenotypic sex that is not correlated to genotype, and the effect of multiple X chromosomes in males, suggests that the male developmental pathway involves many testis determining genes, whereas only a single X chromosome determines the female default pathway. Once testicular differentiation and male hormone secretion have begun, other Y-chromosomal genes are required to maintain spermatogenesis and complete spermiogenesis. The consequences of impairment of oogenesis by other chromosomal abnormalities are much less severe than the impairment of spermatogenesis.
Descent of the gonads
Descent of the testis
Transabdominal phase
Each testis initially lies on the dorsal abdominal wall. As it enlarges, its cranial end degenerates and the remaining organ therefore occupies a more caudal position. It is attached to the mesonephric fold by the mesorchium (the mesogenitale of the undifferentiated gonad), a peritoneal fold that contains the testicular vessels and nerves and a quantity of undifferentiated mesenchyme. It also acquires a secondary attachment to the ventral abdominal wall, which has a considerable influence on its subsequent movements. At the point where the mesonephric fold bends medially to form the genital cord (Fig. 78.3), it becomes connected to the lower part of the ventral abdominal wall by an inguinal fold of peritoneum. The mesenchymal cells occupying the core of the inguinal fold condense as another cord, the gubernaculum (Figs 78.3, 78.13, 78.17). This extends from the inguinal abdominal wall, at the site of the future inguinal canal, through the inguinal fold and the mesorchium to the caudal pole of the testis. The future inguinal canal is formed around it by the differentiating muscles of the abdominal wall. At the end of the second month the caudal part of the ventral abdominal wall is horizontal but, after the return of the intestine to the peritoneal cavity, it grows in length and becomes progressively more vertical. As the umbilical artery runs ventrally from the dorsal to the ventral wall, it pulls up a falciform peritoneal fold, which forms the medial boundary of a peritoneal fossa, the saccus vaginalis or lateral inguinal fossa, into which the testis projects. This lower end of the fossa protrudes down the inguinal canal, within the ventrosuperior aspect of the gubernaculum, as the processus vaginalis (Fig. 78.17; see Fig. 78.19).
Inguinoscrotal phase
The testis remains in apposition with the deep inguinal ring, held by the gubernaculum during the fourth to sixth months. During the inguinoscrotal phase the testis descends, surrounded by the elongating processus vaginalis, within the migrating gubernaculum. This occurs relatively rapidly about the seventh month, the left testis usually descending ahead of the right. In full-term male neonates over 95% have descended testes, although in premature babies descent may not be complete. After descent the distal processus vaginalis persists as a ‘tiny satellite peritoneal cavity’ around the testis as the tunica vaginalis, which becomes firmly attached to the surrounding scrotum within a few weeks. The proximal part of the processus vaginalis then obliterates, and there is evidence that this also is controlled by androgens indirectly via calcitonin gene-related peptide (CGRP) released from the genitofemoral nerve. Both exogenous CGRP and hepatocyte growth factor can cause obliteration in vitro of the processus vaginalis in hernial sacs excised from babies with indirect inguinal hernia.
Descent of the ovary
The relative movements of the ovary are less extensive than those of the testis and are not hormonally regulated. Like the testis, the ovary ultimately reaches a lower level than it occupies in the early months of fetal life, but it does not leave the pelvis to enter the inguinal canal, except in certain anomalies. The ovary is connected to the medial aspect of the mesonephric fold by the mesovarium (homologous with the mesorchium), and to the ventral abdominal wall by the inguinal fold (Figs 78.13, 78.18). A mesenchymatous gubernaculum develops in this fold but, as it traverses the mesonephric fold, it acquires an additional attachment to the lateral margin of the uterus near the entrance of the uterine tube. Its lower part, caudal to this uterine attachment, becomes the round ligament of the uterus and the part cranial to this becomes the ovarian ligament. Collectively these structures are homologous with the gubernaculum testis in the male (Figs 78.17–78.19). This new uterine attachment may be correlated with the restricted ovarian descent. At first the ovary is attached to the medial side of the mesonephric fold but, in accordance with the manner in which the two mesonephric folds form the genital cord, it is finally connected to the posterior layer of the broad ligament of the uterus (Figs 78.3, 78.13, 78.14B). The gubernacula thus develop in the female, unlike the male, as bilateral fibrous bands or ligaments in the absence of Insl3. They do not extend into the labia majora, but end in the connective tissue just external to the external ring of the inguinal canal, because, in the absence of androgens, there is no migratory phase analogous to inguinoscrotal migration in the male. The saccus vaginalis is present in the female. Its prolongation into the inguinal canal (sometimes termed the canal of Nuck) is normally completely obliterated, but may remain patent and form the sac of a potential indirect inguinal hernia.
In the neonate the ovaries lie in the lower part of the iliac fossae. The long axis of the ovary is almost vertical. It becomes temporarily horizontal during descent, but regains the vertical when it reaches the ovarian fossa. The ovaries complete their descent into the ovarian fossae in early childhood. Thus, at birth the ovary and the lateral end of the corresponding uterine tube lie above the pelvic brim. They do not sink into the lesser pelvis until the latter enlarges sufficiently to contain both of them and the other pelvic viscera, including the bladder. The combined weight of the ovaries at birth is 0.3 g, which is relatively large, and much larger than the combined weight of the testes (Fig. 78.9; see Fig. 14.4). The ovaries double in weight during the first 6 postnatal weeks. They bear surface furrows, which disappear during the second and third postnatal months. All of the primary oocytes for the reproductive life of a female are present in her ovaries by the end of the first trimester of pregnancy. Of the 7,000,000 primary oocytes estimated to be present at the fifth month of gestation, 1,000,000 remain at birth, 40,000 by puberty, and only 400 are ovulated during reproductive life.
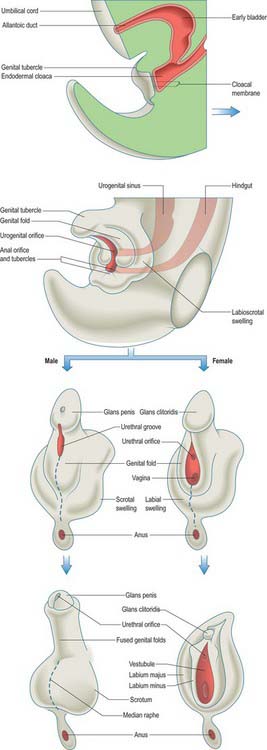
Cloaca and external genitalia
The cloaca is that region at the end of the primitive hindgut which is continuous with the allantois (Figs 78.4, 78.7). The allantois, a ventral diverticulum, passes into the connecting stalk of the early embryo prior to tail folding and is then drawn into the body cavity after stage 10. It retains an extension into the connecting stalk, and later into the umbilicus, throughout embryonic life. The cloaca is a slightly dilated cavity lined with endoderm. It is initially connected cranially to the enteric hindgut, and ventrocaudally is in contact with overlying ectoderm at the cloacal membrane. Proliferation of mesenchyme at the angle of the junction of the hindgut and allantois produces a urorectal septum, which grows caudally promoting the movement, but not the fusion, of the endodermal epithelium toward the cloacal membrane (Figs 78.4, 78.7, 78.20). The cloaca becomes separated into a presumptive rectum and anal canal dorsally, and a presumptive urinary bladder and urogenital sinus ventrally, and the cloacal membrane is divided into anal and urogenital parts respectively. The nodal centre of division is the site of the future perineal body. The urogenital sinus receives the mesonephric and paramesonephric ducts.
Anomalies of cloacal development may result in a range of defects. In extroversion of the cloaca (ectopia cloacae), the urorectal septum does not develop and there is failure of mesenchymal migration around the ventral body wall to support the umbilical cord: this results in a large abdominal defect with a central colonic portion and bilateral bladder components. With only partial development of the urorectal septum, the urogenital sinus may remain with a high confluence of bladder, vagina and rectum. The cloacal membrane may be abnormally elongated and prematurely ruptured throughout its whole extent, prior to the formation of the urorectal septum, or, in some cases, there may be only a small sinus opening externally at the skin. The anal musculature is often present but not associated with the anal canal.
Urethra
In the male, the prostatic urethra proximal to the orifice of the prostatic utricle is derived from the vesicourethral part of the cloaca and the incorporated caudal ends of the mesonephric ducts. The remainder of the prostatic part, the membranous part, and probably the part within the bulb, are all derived from the urogenital sinus. The anterior urethra, as far as the glans, is formed by canalisation of the urethral plate (see below). Secondary ingrowth of mesenchyme fuses the genital folds over the urethra (Fig. 78.20). The short section within the glans may be formed from ectoderm, which invaginates into the glans.
In the female, the urethra is derived entirely from the vesicourethral region of the cloaca, including the dorsal region derived from the mesonephric ducts. It is homologous with the part of the prostatic urethra proximal to the orifices of the prostatic utricle and the ejaculatory ducts. The region of the early urethra remains open to form the vestibule into which the definitive urethra and vagina open (Fig. 78.20). It is believed that these regions are invaded by ectoderm because they are innervated by somatic nerves.
Prostate gland
The prostate gland arises during the third month from interactions between the urogenital sinus mesenchyme and the endoderm of the proximal part of the urethra. Early outgrowths, some 14 to 20 in number, arise from the endoderm around the whole circumference of the tube, but mainly on its lateral aspects and excluding the dorsal wall above the utricular plate. They give rise to the outer glandular zone of the prostate. Later outgrowths from the dorsal wall above the mesonephric ducts arise from the epithelium of mixed urogenital, mesonephric and possibly paramesonephric, origin that covers the cranial end of the sinus tubercle. They produce the internal zone of glandular tissue. The outgrowths, which are at first solid, branch, become tubular and invade the surrounding mesenchyme. The latter differentiates into smooth muscle, associated blood and lymphatic vessels and connective tissue and is invaded by autonomic nerves.
External genitalia
The external genitalia, like the gonads, pass through an indifferent state before distinguishing sexual characters appear (Fig. 78.20). From stage 13, primordia of the external genitalia, composed of underlying proliferating mesenchyme covered with ectoderm, arise around the cloacal membrane, between the primitive umbilical cord and the caudal limit of the embryo. During stage 15 the cloacal membrane is divided by the urorectal septum into a cranial urogenital membrane and a caudal anal membrane (Figs 78.4, 78.14). Local ectodermal/mesenchymal interactions give rise to the anal sphincter, which will develop even without the presence of the anal canal. A surface elevation, the genital tubercle, appears at the cranial end of the urogenital membrane and two lateral ridges, the genital or urethral folds, form each side of the membrane (Fig. 78.20). The genital tubercle forms a distinct primordium, which will become the glans of either the penis or the clitoris. Elongation of the genital folds and urogenital membrane produces a primitive phallus. As this structure grows it is described as having a cranial surface analogous to the dorsum of the penis, and a caudal surface analogous to the perineal surface of both sexes. The urogenital sinus, contiguous with the internal aspect of the urogenital membrane, becomes attenuated within the elongating phallus forming the primitive urethra. The urogenital membrane breaks down at about stage 19, allowing communication of ectoderm and endoderm at the edges of the disrupted membrane and continuity of the urogenital sinus with the amniotic cavity. Urine can escape from the urinary tract from this time. The endodermal layer of the attenuated distal portion of the urogenital sinus, which is now displayed on the caudal aspect of the phallus, is termed the urethral plate. As mesenchyme proliferates within the genital folds, the urethral plate sinks into the body of the phallus forming a primary urethral groove. The genital folds meet proximally in a transverse ridge immediately ventral to the anal membrane.
While these changes are in progress two labioscrotal (genital) swellings appear, one on each side of the base of the phallus, and extend caudally, separated from the genital folds by distinct grooves (Figs 78.20, 78.21).
Homologies of the parts of the urogenital system are shown in Table 78.1.
Male genitalia
The growth of male external characteristics is stimulated by androgens regardless of the genetic sex. The male phallus enlarges to form the penis. The genital swellings meet each other ventral to the anus and unite to form the scrotum (Fig. 78.20). The genital folds fuse with each other from behind forwards, enclosing the phallic part of the urogenital sinus behind to form the bulb of the urethra and closing the definitive urethral groove in front to form the greater part of the spongiose urethra. Fusion of the folds results in the formation of a median raphe and occurs in such a way that the lining of the postglandular urethra is mainly, perhaps wholly, endodermal in origin, formed by canalisation of the urethral plate. Thus, as the phallus lengthens, the urogenital orifice is carried onwards until it reaches the base of the glans at the apex of the penis. From the tip of the phallus an ingrowth of surface ectoderm occurs within the glans to meet and fuse with the penile urethra. Subsequent canalisation of the ectoderm permits a continuation of the urethra within the glans.
Female genitalia
The female phallus, which exceeds the male in length in the early stages, becomes the clitoris. The genital swellings remain separate as the labia majora and the genital folds also remain separate, forming the labia minora (Fig. 78.20). The perineal orifice of the urogenital sinus is retained as the cleft between the labia minora, above which the urethra and vagina open. The prepuce of the clitoris develops in the same way as its male homologue. By the fourth month the female external genitalia can no longer be masculinized by androgens.
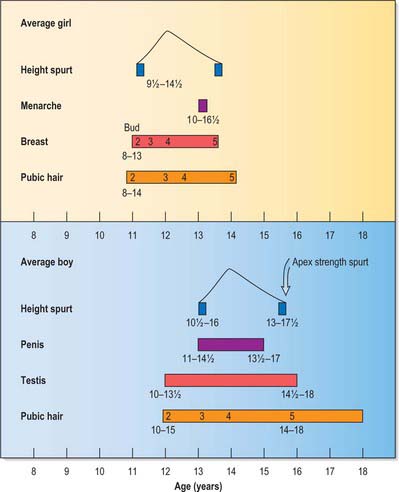
Maturation of the reproductive organs at puberty
Until the adolescent growth spurt the reproductive organs grow very slowly. Generally the changes occur over a time period termed puberty. The sequence of these events is much less variable than the age at which they take place. The sequence of puberty in girls and boys is shown in Fig. 78.22.
Albrecht KH, Eicher EM. Evidence that SRY is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92-107.
Barteczko KJ, Jacob MI. The testicular descent in human. Origin development and fate of the gubernaculum Hunteri, processus vaginalis peritonei and gonadal ligaments. Adv Anat Embryol Cell Biol. 2000;156:III-IIX. 1–98. Springer, Heidelberg
Chauvet V, Qian F, Boute N, et al. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am J Pathol. 2002;160:973-983.
Favorito LA, Klojda CA, Costa WS, Sampaio FJ. Is there a relationship with anomalous insertions of the distal gubernaculum testis and testicular ectopia? Analysis in human fetuses and patients with cryptorchidism. J Urol. 2003;170:554-557.
Heyns CF. The gubernaculum during testicular descent in the human fetus. J Anat. 1987;153:92-112.
Hutson JM, van der Putte SCJ, Penington E, Kluth D, Fiegel H. The embryology of anorectal malformations. In: Holschneider AM, Hutson JM. Anorectal Malformations in Children. Embryology, Diagnosis, Surgical Treatment, Follow-up. Heidelberg: Springer, 2006.
Hutson JM, Hasthorpe S. Testicular descent and cryptorchidism: the state of the art in 2004. J Pediatr Surg. 2005;40:297-302.
O’Connell HE, Hutson JM, Anderson CR, Plenter RJ. Anatomical relationship between urethra and clitoris. J Urol. 1998;159:1892-1897.
Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and the relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769-784.