Chapter 5
Development of the Nervous System
Development of the Neural Tube: General Concepts
Congenital Nervous System Defects of Primary Neurulation
Congenital Nervous System Defects of Secondary Neurulation
Diencephalon and Cerebral Hemispheres
Defects of Prosencephalization
Infectious Diseases Causing Congenital Nervous System Defects
Posterior (Dorsal) Root Ganglia
Relationship of Spinal Cord to Vertebral Column
Abnormalities of Cortical Development
Cellular Events in Brain Development
The central nervous system (CNS) develops from primitive ectoderm, one of the three germ layers of the embryo. From a few dozen cells that together weigh perhaps a microgram, the brain becomes an organ weighing about 800 g at birth, 1200 g at 6 years of age, and about 1400 g in the adult—about a billion-fold increase. Most but not all neurons undergo their last cell division before birth. The development of a fully functional nervous system requires division and migration of nerve cells, myelination, and the formation of synaptic connections.
OVERVIEW
In view of the complex embryology of the human CNS, it is remarkable that there are so few congenital CNS defects. Although 3% of births are associated with major malformations of the CNS, most fetuses and infants in this category do not survive. About 75% of spontaneously aborted fetuses and 40% of infants who die within the first year of life have major CNS malformations.
The basic form of the human CNS is complete by about the sixth week of gestation. The next phases, which include cellular proliferation and migration, are most prominent in the second trimester of gestation but continue until term. Myelination peaks during the third trimester but continues until adulthood. The development of synaptic connections between neurons and the response of the brain to its experiences result in its functional maturity. This developmental process continues throughout life.
From a few primordial cells, about 100 billion neurons develop, each with thousands of contacts with other neurons. Through this network of interconnecting neurons, the human brain is capable not only of directing the movement of the body and sensing the environment but also of thinking, reasoning, experiencing emotions, and dreaming.
DEVELOPMENT OF THE NEURAL TUBE: GENERAL CONCEPTS
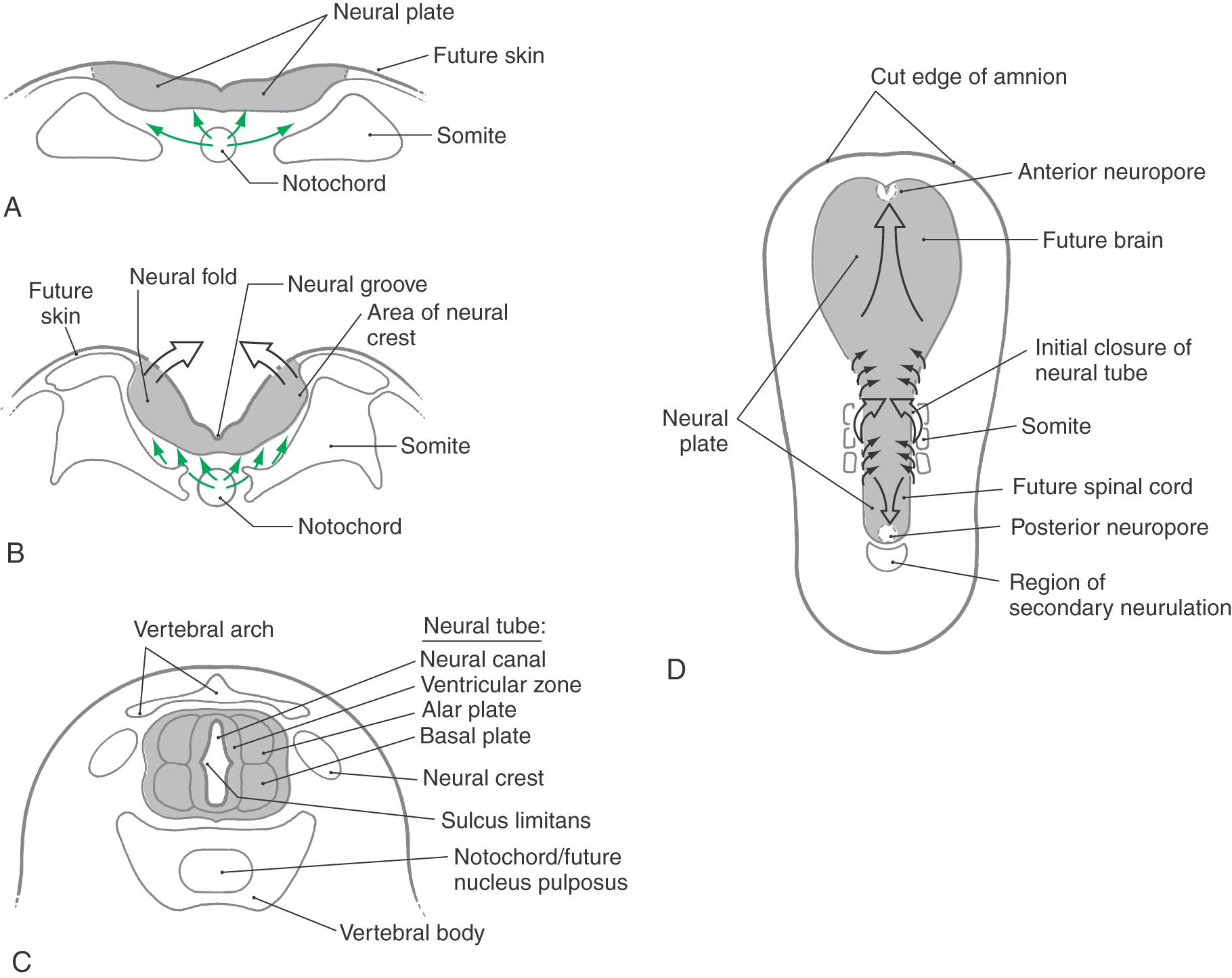
One of the first indicators of the developing nervous system to appear is the neural groove on the posterior aspect of the trilaminar embryo (Fig. 5-1A, B). The neural groove deepens and the neural folds at the lateral margins of the neural plate become obvious as they elevate and eventually join along the posterior midline to form the neural tube (Fig. 5-1C). This apposition and fusion of the neural folds and of the overlying ectoderm initially takes place at what will be the cervical levels of the spinal cord, then proceeds rostrally and caudally from this location (Fig. 5-1D). The anterior and posterior neuropores, as described later, are the last points at which the neural tube closes. After formation of the neural tube, three layers, the ventricular, marginal, and intermediate zones, appear in rapid succession. Although these zones are transient in their embryonic form, they give rise to important adult derivatives.
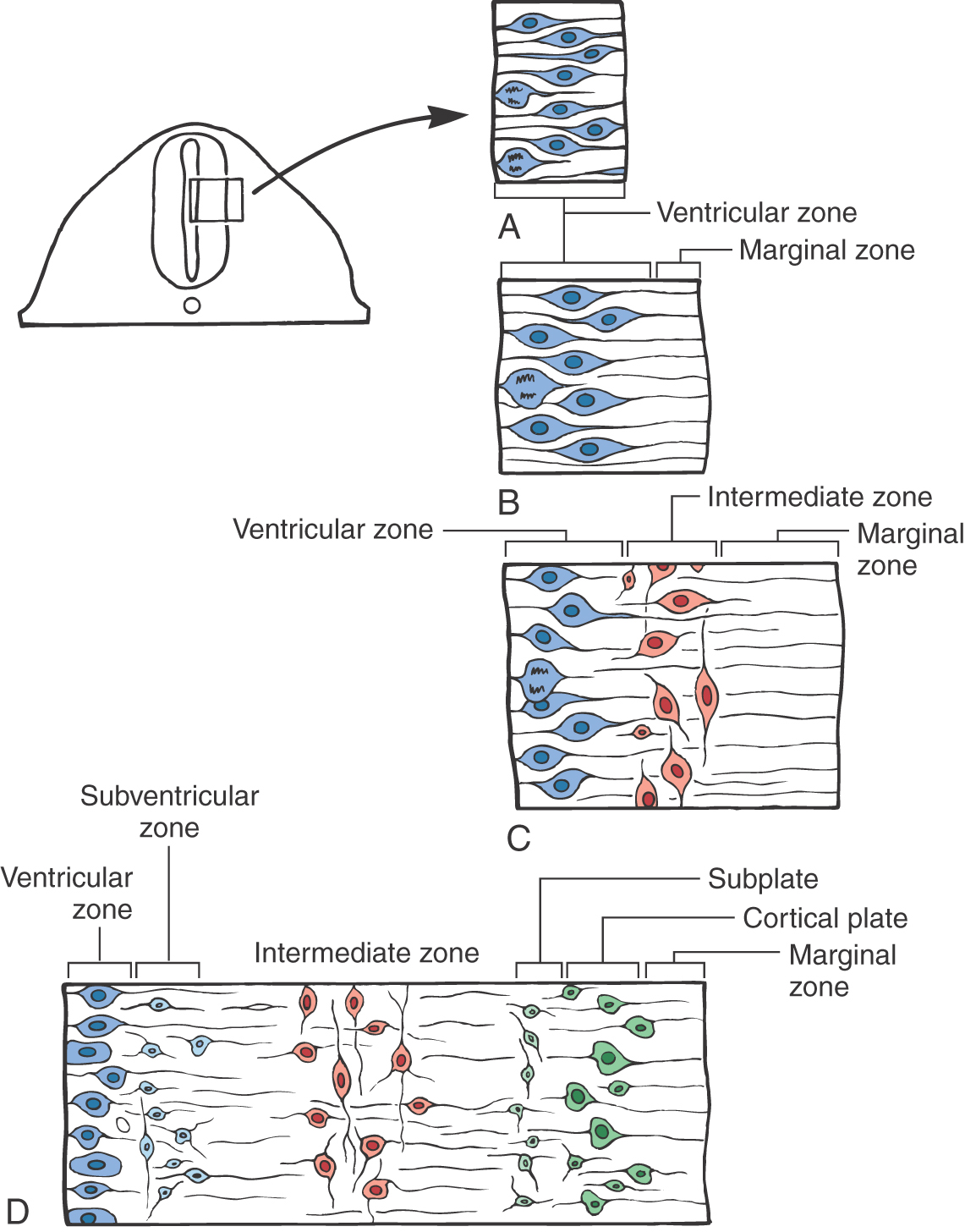
In the early stages, this closing neural plate and tube consist of a single layer, the ventricular zone, composed of a pseudostratified layer of fusiform cells undergoing DNA replication and cell division (mitosis). The nuclei in the cells of this zone migrate in a to-and-fro manner within the cell as mitosis takes place (Fig. 5-2A). The progenitor cells of this layer will give rise to the neurons and some glial cells of the mature nervous system and to the ependymal cells lining the ventricles.
Immediately after the ventricular zone is formed, the marginal zone appears (Fig. 5-2B). This zone is at the abluminal aspect of the neural tube and consists of the processes of cells located within the ventricular zone, but it does not contain their nuclei. The marginal zone contains almost no cell bodies. This zone will be invaded by axons of neurons that are located in the intermediate zone.
The third area to appear is the intermediate zone. The intermediate zone is formed between the ventricular and marginal zones as the progenitor cells from the ventricular zone give rise to immature postmitotic neurons (Fig. 5-2C). These immature neurons migrate into the area immediately external to the ventricular zone, where they set up residence. The processes of some intermediate zone neurons continue to grow into the marginal zone. Although the term is no longer appropriate, the intermediate zone generally corresponds to what was formerly called the mantle layer.
The subventricular zone forms at the interface of the ventricular and intermediate zones (Fig. 5-2D). Unlike the nuclei in the cells of the ventricular zone, nuclei of subventricular zone cells generally do not migrate. The progenitor cells of the subventricular zone give rise to the macroglial cells of the CNS and to specific populations of developing neurons in the brainstem and forebrain.
The concept of the alar plate and basal plate is best viewed by recognizing that the development of the posterior horn (alar plate derivative) and anterior horn (basal plate derivative) is a dynamic process. The immature intermediate zone neurons that give rise to mature posterior or anterior horn neurons are the product of cell division in one zone with migration into and further development in a subsequently formed zone. It is helpful to think of the alar and basal plates as consisting of the ventricular zone and adjacent intermediate zone, which are, of course, dynamically changing as development occurs. The posterior part of the ventricular zone and adjacent intermediate zone represents the alar plate, whereas the corresponding layers in the anterior part of the developing neural tube represent the basal plate. As development proceeds, the ventricular zone will essentially disappear, while the intermediate zone with its maturing neurons will progressively enlarge to form its adult derivatives. Consequently, the adult derivatives are the products of cell division in the ventricular zone, migration and formation of the intermediate zone, and maturation within this intermediate zone.
The three-zone configuration described previously—ventricular zone, intermediate zone, and marginal zone—is the basic organizational plan from which the brain and spinal cord will arise. To a large extent, the development of the brainstem and forebrain is just a more elaborate version of this basic plan. There are individual developmental events unique to these zones of the CNS. In the metencephalon, the basic plan of the neural tube is modified to accommodate development of the cerebellar cortex. In the forebrain, the basic plan of the neural tube is modified to accommodate the development of the cerebral cortex.
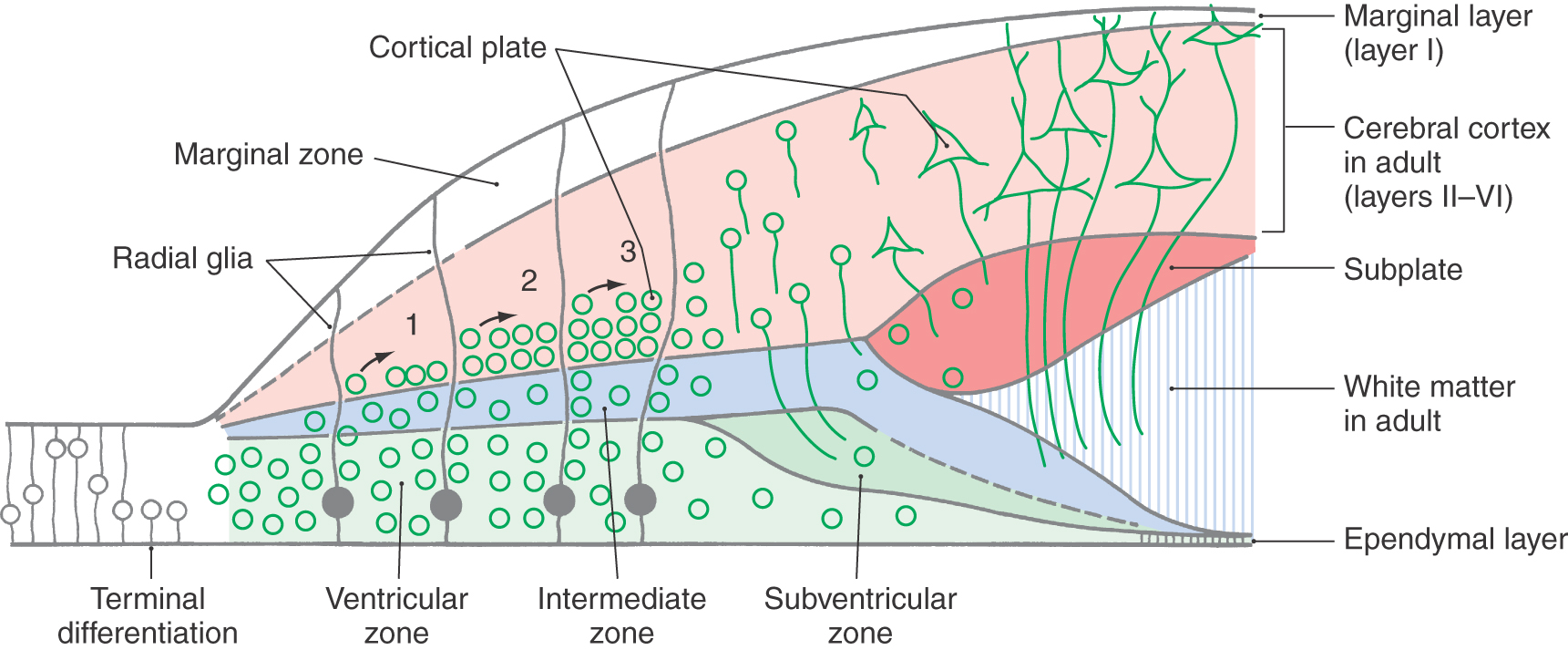
The modification to accommodate the cerebral cortex is the appearance of the cortical plate and the subplate (Fig. 5-2D). The cortical plate forms at the interface of the marginal zone and the intermediate zone and is composed of neurons that originate from the ventricular zone; these postmitotic immature neurons traverse the intermediate zone, using the radially oriented processes of radial glia as a scaffold, to take up their position as the cortical plate. (It is emphasized that cell migration on radial glia is characteristically seen in all portions of the developing nervous system.) The subplate is a narrow region located immediately internal to the cortical plate. The cerebral cortex develops from the cortical plate and the marginal zone. In those portions of the neural tube that form the cerebral cortex, the marginal zone gives rise to layer/lamina I of the cortex, the cortical plate to layers/laminae II to VI, and the subplate and intermediate zone to portions of the subcortical white matter. The histogenesis of the cerebellar cortex is a slight modification of this plan due to the presence of an external germinal layer. This layer originates from the rhombic lip (an alar plate derivative) and is located within the marginal layer. These relationships are described later in this chapter.
BRAIN DEVELOPMENT
The first neural tissue appears at the end of the third week of embryonic development, when the embryonic disk is composed of ectoderm, mesoderm, and endoderm. A specialized part of the ectoderm, the neuroectoderm, gives rise to the brain, spinal cord, and peripheral nervous system (Fig. 5-1).
Induction
The notochord arises from axial mesoderm at about 16 days and is completely formed by the beginning of the fourth week. It defines the longitudinal axis of the embryo, determines the orientation of the vertebral column, and persists as the nucleus pulposus of the intervertebral disks. One important function of the notochord is induction: directing the overlying ectoderm to form the neural plate (Fig. 5-1A, B). Associated with this process is the production of cell adhesion molecules in the notochord. These molecules diffuse from the notochord into the neural plate and function to join the primitive neuroepithelial cells into a tight unit.
Within the neuroectoderm, some neuroepithelial cells elongate and become spindle shaped. This cellular elongation, also induced by the notochord, forms the neural plate and is completed by the end of the third week of gestation (Fig. 5-1A). The neural plate gives rise to most of the nervous system.
Primary Neurulation
The CNS develops from a hollow structure called the neural tube, which is produced by neurulation. There are two neurulation processes. Most of the neural tube forms from the neural plate by a process of infolding called primary neurulation. This part of the neural tube will give rise to the brain and to the spinal cord through lumbar levels. The most caudal portion of the neural tube, which will give rise to sacral and coccygeal levels of the cord, is formed by a process called secondary neurulation. Secondary neurulation is described in the next section. By about day 18 after fertilization, the neural plate begins to thicken at its lateral margins (Fig. 5-1B). This thickening elevates the edges of the neural plate to form neural folds. At about 20 days, the neural folds first contact each other to begin the formation of the neural tube. This fusion initially takes place on the dorsal midline at what will become cervical levels of the spinal cord and proceeds, zipper-like, in rostral and caudal directions (Fig. 5-1C, D). During the process, the lumen of the neural tube, called the neural canal, is open to the amniotic cavity both rostrally and caudally (Fig. 5-1D). The rostral opening, the anterior neuropore, closes at about 24 days, and the caudal opening, the posterior neuropore, closes about 2 days later.
Neurulation is brought about by morphologic changes in the neuroblasts, the immature and dividing future neurons in the ventricular zone. As mentioned previously, these cells are elongated and are oriented at right angles to the dorsal surface of the neural plate, which will be the inner wall of the neural canal. Microfilaments in each cell form a circular bundle parallel to the future luminal surface, whereas microtubules extend along the length of the cell. The contraction of the circular bundle of microfilaments causes the microtubules to splay out like the rays of a fan. This forms an elongated conical cell with its apex at the neural groove and its base at the edge of the neural fold. Neurulation does not occur in embryos exposed to colchicine, which depolymerizes microtubules, or to cytochalasin, which inhibits microfilament-based contraction.
Congenital malformations associated with defective neurulation are called dysraphic defects. The process of induction also means that the proper development of a structure is dependent on the proper development of its neighbors. There is an intimate relationship of neural tissue to the surrounding bone, meninges, muscles, and skin. Because of this relationship, a failure of neurulation often impairs the formation of these surrounding structures.
Several well-controlled clinical trials have proved that supplementation with the vitamin folic acid can reduce the incidence of neural tube defects. In the MRC Vitamin Study, carried out in Great Britain and published in 1991, women who had previously been delivered of a child with a dysraphic defect were assigned to either a folic acid supplementation group or a control group during a subsequent pregnancy. Folic acid supplementation reduced the incidence of neural tube defects by about 70% relative to that in untreated controls. The mechanism for this effect is not known at this time. It is thought that women who are delivered of infants with dysraphic defects have an inborn metabolic problem that is corrected by folate. One research group has suggested that the conversion of homocysteine to methionine, which requires folate as a cofactor, is the critical step. Dysraphic defects have also been observed in infants born to mothers who had circulating antibodies to the folate receptor. Because the neural plate and tube develop so early in pregnancy, it is important that physicians recommend folic acid supplementation (400 mcg/day) to all women who intend to have children, whether or not they are pregnant. In addition, drugs taken for epilepsy, such as valproic acid and carbamazepine, can cause dysraphic defects.
Congenital Nervous System Defects of Primary Neurulation
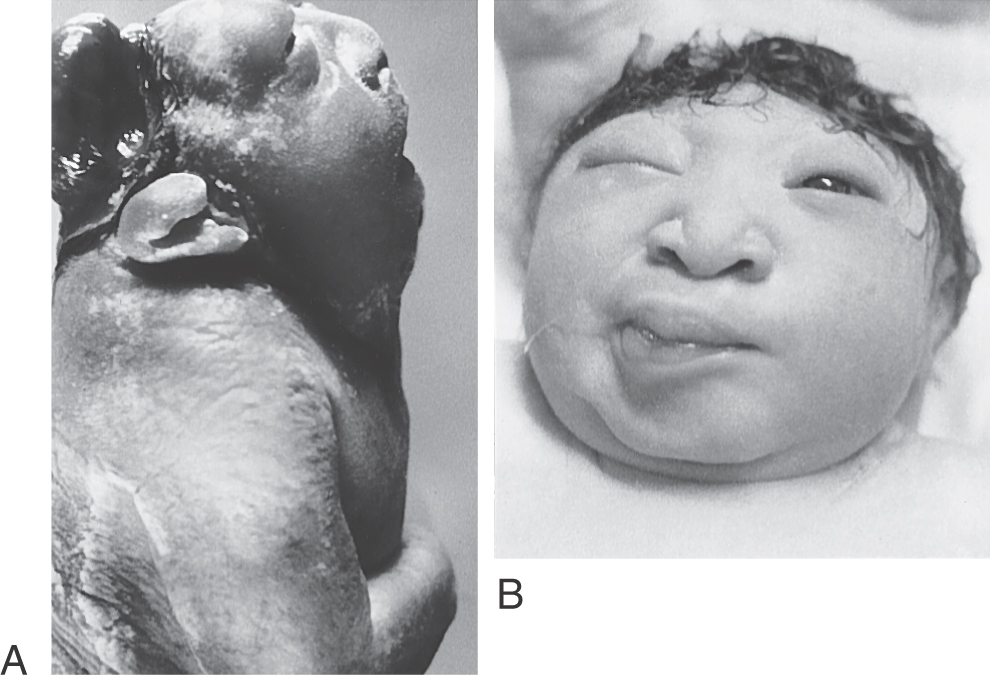
Most dysraphic disorders occur at the location of the anterior or posterior neuropore. Failure of the anterior neuropore to close results in anencephaly (Fig. 5-3). In this defect, the brain is not formed, the surrounding meninges and skull may be absent, and there are facial abnormalities. The defect extends from the level of the lamina terminalis, the site of anterior neuropore closure, to the region of the foramen magnum. Anencephaly occurs in about 5 of every 10,000 live births. Neonatal death is inevitable.
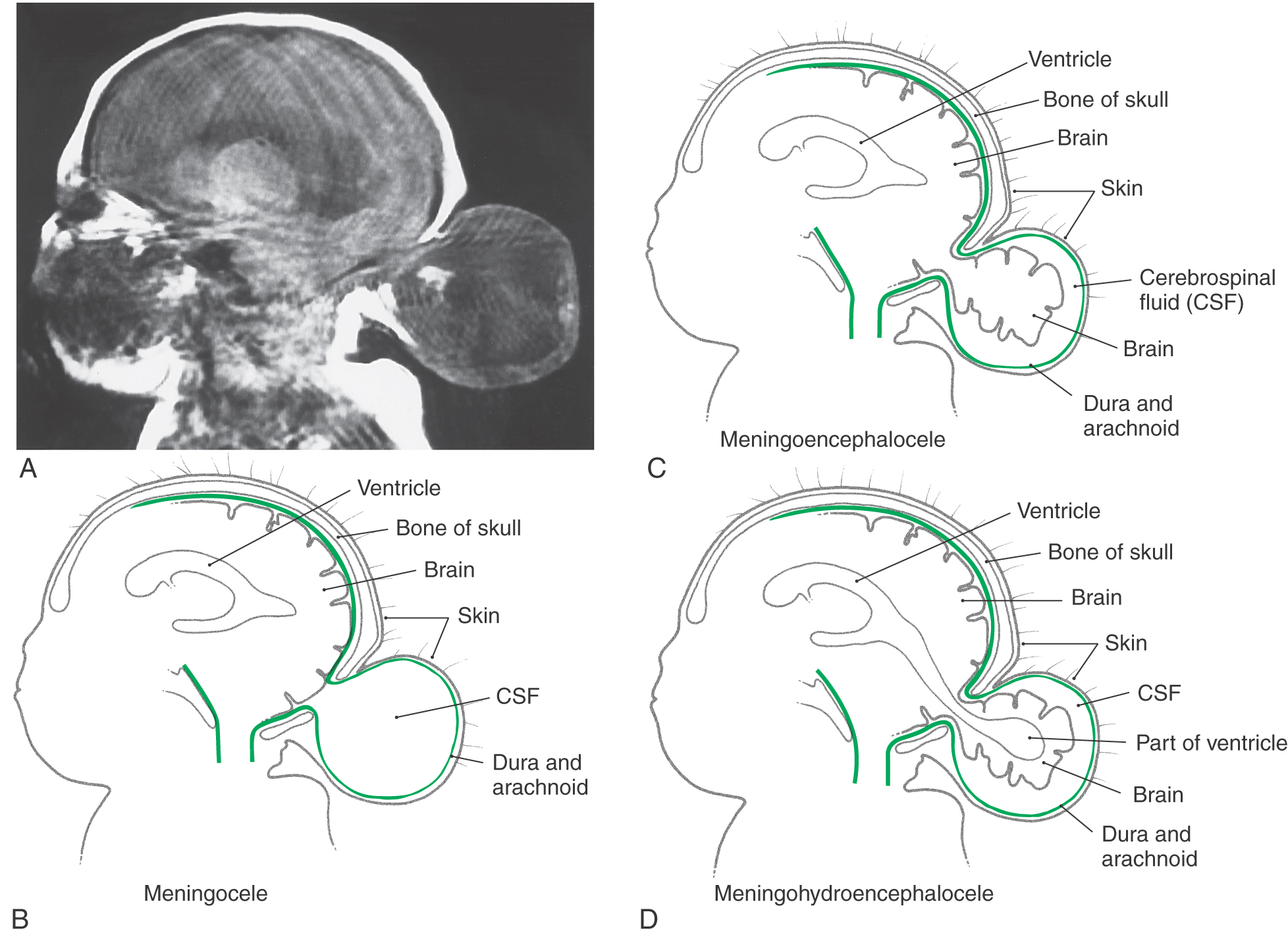
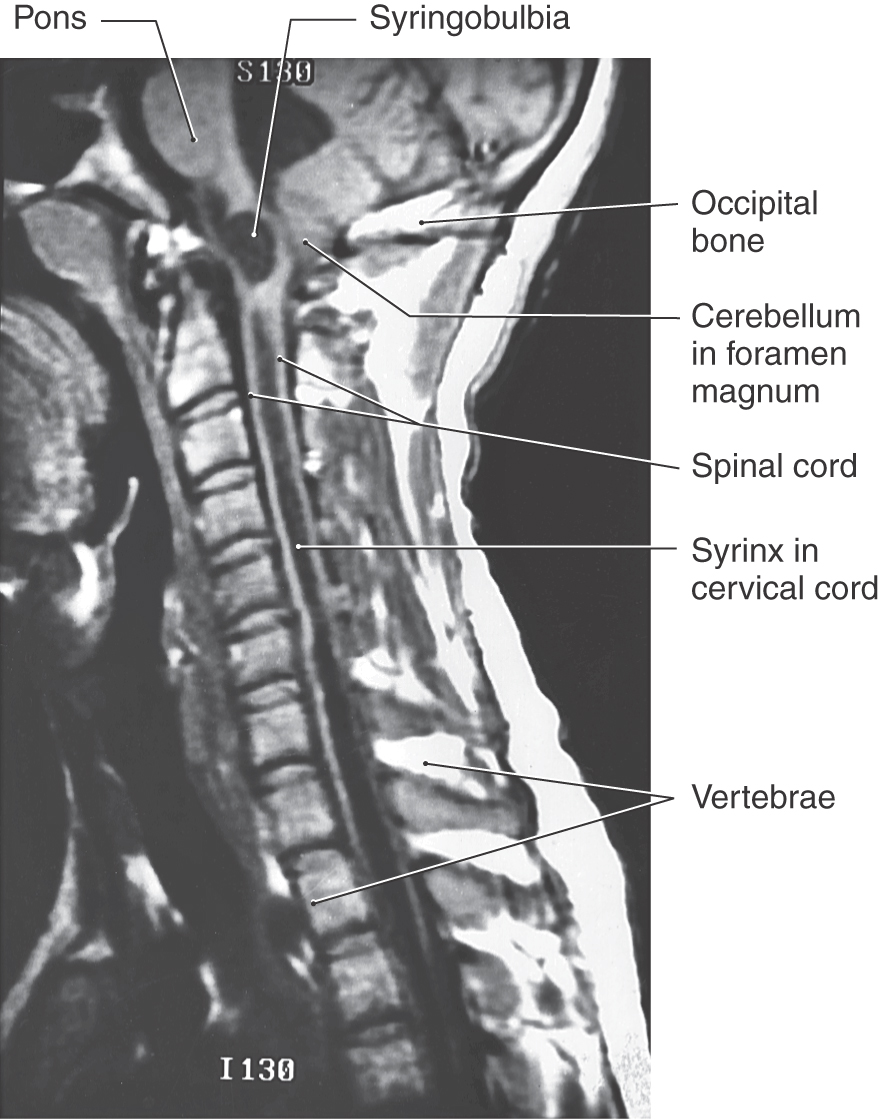
An encephalocele is a herniation of intracranial contents through a defect in the cranium (cranium bifidum) (Fig. 5-4A). The cystic structure may contain only meninges (meningocele), meninges plus brain (meningoencephalocele), or meninges plus brain and a part of the ventricular system (meningohydroencephalocele) (Fig. 5-4B-D). Encephaloceles are most common in the occipital region, but they may also occur in frontal and parietal locations. A more subtle defect in the same area is thought to be the cause of the Chiari I malformation, a congenital herniation of the cerebellar vermis through the foramen magnum, which may cause pressure on the medulla oblongata and cervical spinal cord (Fig. 5-5). This defect may go unnoticed until early adulthood and is often associated with a cavitation of the spinal cord (syringomyelia) or of the medulla (syringobulbia). The Chiari II malformation, also called the Arnold-Chiari malformation or deformity, is a similar defect associated with myelomeningocele (discussed later).
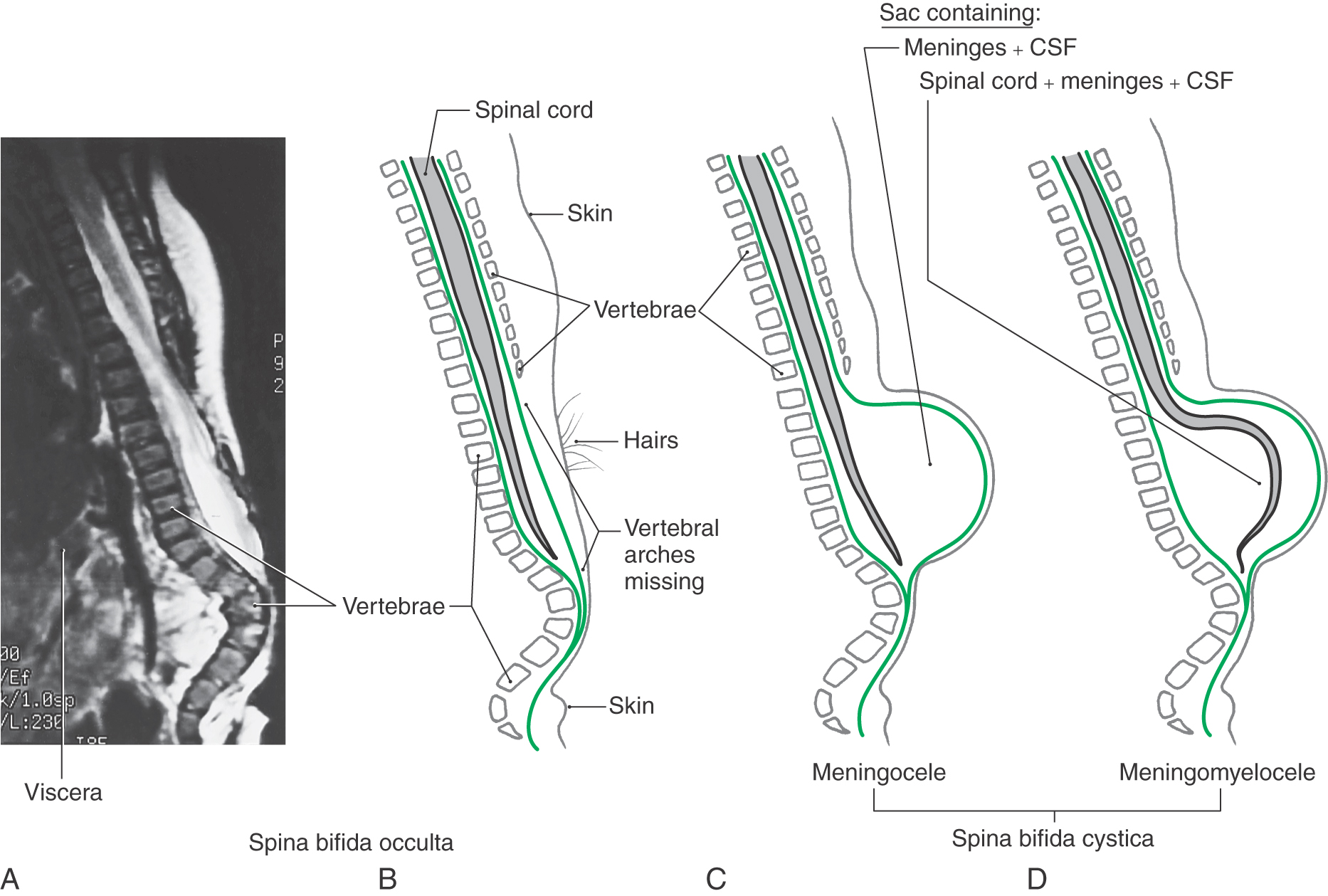
Defects in the closure of the posterior neuropore cause a range of malformations known collectively as myeloschisis. The defect always involves a failure of the vertebral arches at the affected levels to form completely and fuse to cover the spinal cord (spina bifida). If that is the only defect and the skin is closed over it, the unseen condition is called spina bifida occulta (Fig. 5-6A, B). The site of the defect is usually marked by a patch of dark, coarse hairs. If the skin is not closed over the vertebral defect, leaving a patent aperture, the malformation is called spina bifida aperta.
As with occipital encephaloceles, a cystic mass (spina bifida cystica) may also accompany spina bifida (Fig. 5-6C, D). This saccular structure may contain only meninges and cerebrospinal fluid (CSF) (meningocele) or meninges and CSF plus spinal neural tissue (meningomyelocele). In the latter case, the neural tissue may be the lower part of the spinal cord or, more commonly, a portion of the cauda equina. Infants with meningomyelocele may be unable to move their lower limbs or may not perceive pain sensations from skin innervated by nerves passing through the lesioned area. These infants may also have other CNS malformations, such as hydrocephalus and the Arnold-Chiari malformation. The incidence of meningomyelocele is approximately 5 per 10,000 births.
Secondary Neurulation
The sacral and coccygeal segments of the spinal cord and their corresponding dorsal and ventral roots are formed by secondary neurulation (Fig. 5-1D). This process begins on day 20 and is complete by about day 42. A cell mass, the caudal eminence, appears just caudal to the neural tube and then enlarges and cavitates. The caudal eminence joins the neural tube, and its cavity becomes continuous with the neural canal.
Congenital Nervous System Defects of Secondary Neurulation
Myelodysplasia refers to malformations of the parts of the neural tube formed by secondary neurulation. The malformation is covered with skin in most cases, but the site may be marked by unusual pigmentation, hair growth, telangiectases (large superficial capillaries), or a prominent dimple. A common abnormality is tethered cord syndrome, in which the conus medullaris and filum terminale are abnormally fixed to the defective vertebral column. The sustained traction damages the cord and causes variable weakness, sensory loss and asymmetric growth of the legs and feet, and problems with bowel and bladder control. Infants born to mothers with diabetes mellitus can have the caudal regression syndrome, which affects the development of the embryonic structures in the caudal region, including the spinal cord.
Primary Brain Vesicles
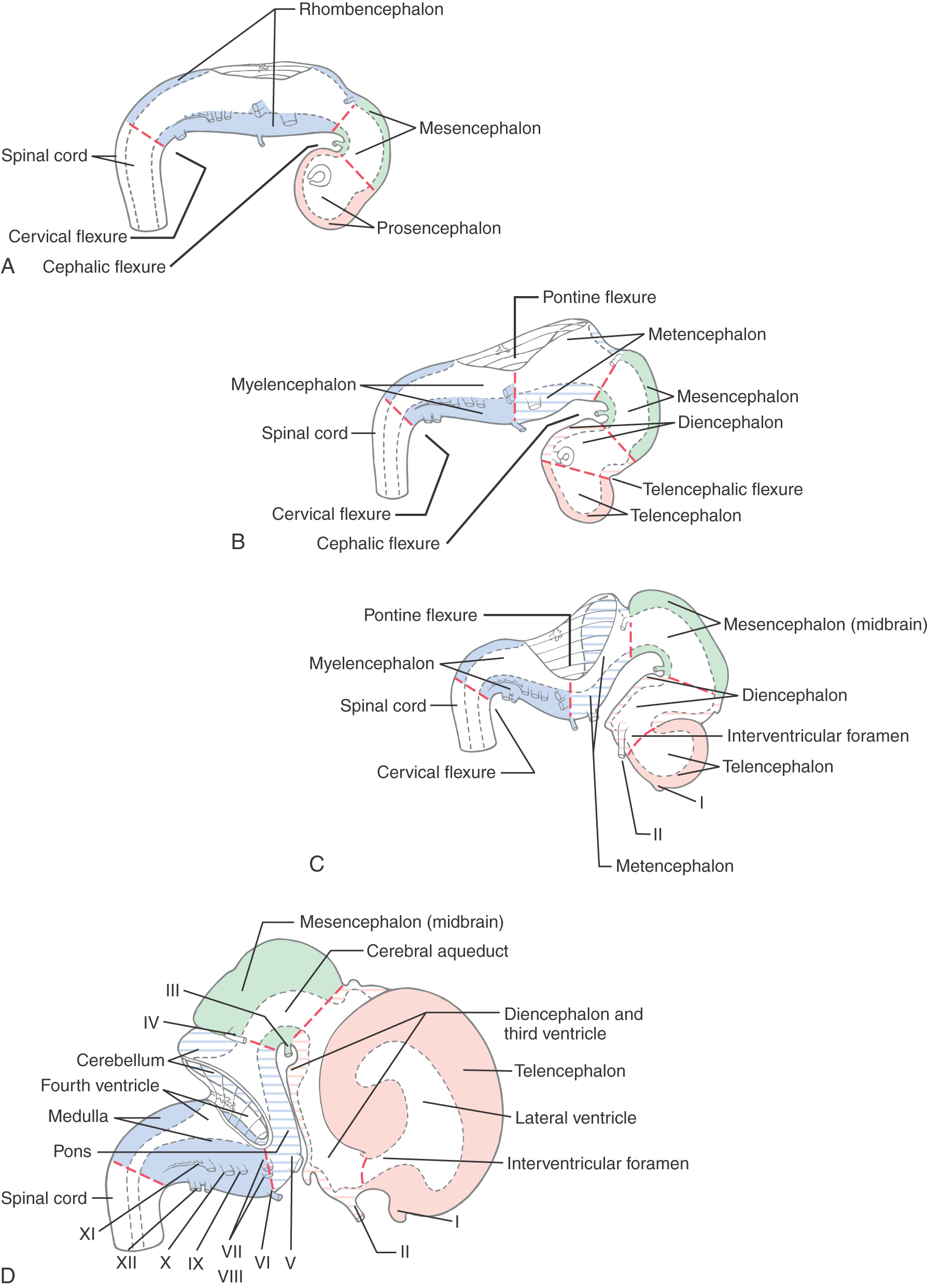
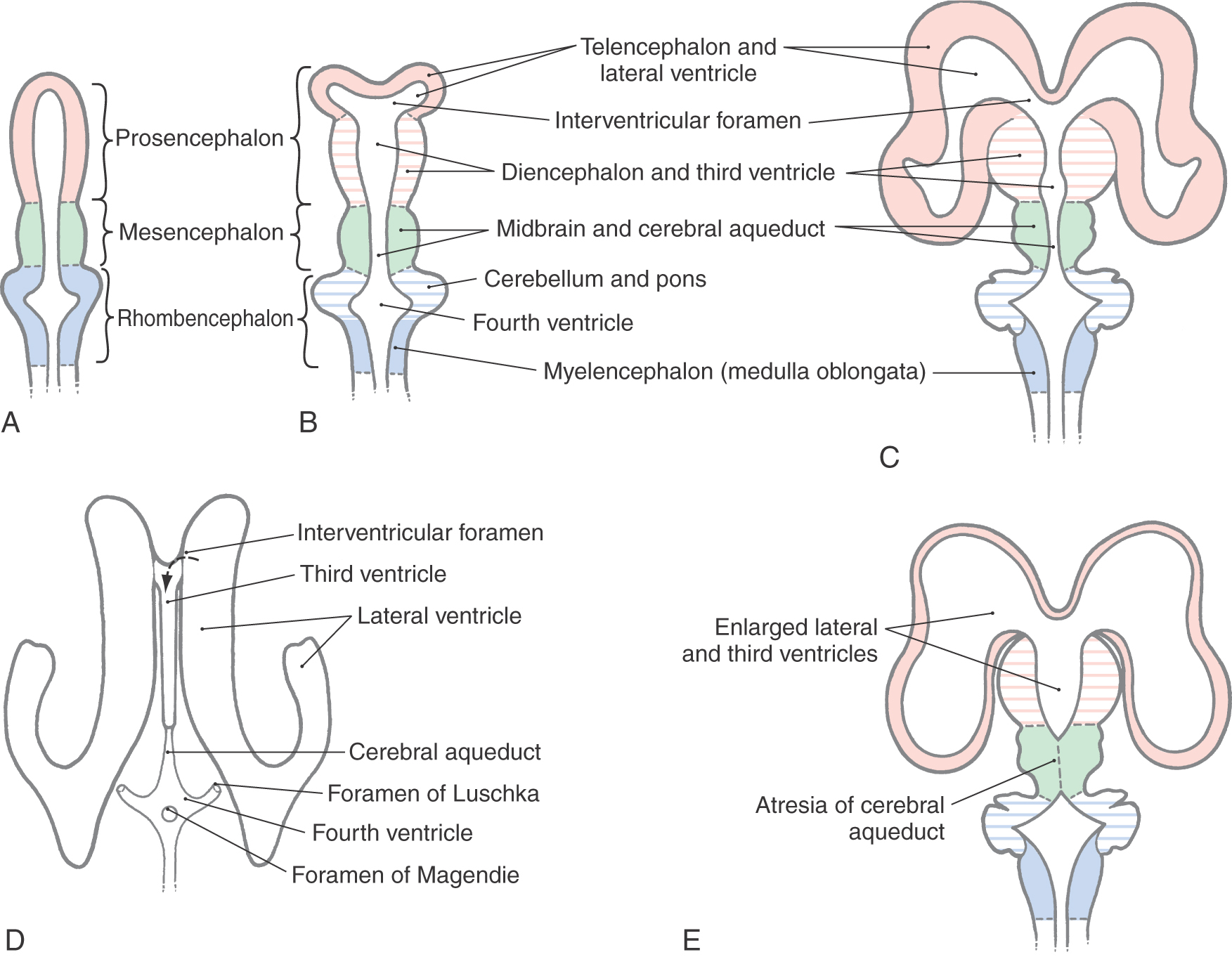
During the fourth week after fertilization, in which the anterior neuropore closes, there is rapid growth of neural tissue in the cranial region. The three primary brain vesicles formed are prosencephalon (forebrain), mesencephalon (midbrain), and rhombencephalon (hindbrain) (Fig. 5-7A, B). At the rhombencephalon–spinal cord junction, there is a slight bend in the developing neural tube; this is the cervical flexure. A second bend in the neural tube at the level of the mesencephalon is the mesencephalic (or cephalic) flexure.
Secondary Brain Vesicles
During the fifth week, the three primary brain vesicles are divided into five secondary brain vesicles (Fig. 5-7C, D). This requires two additional flexures. The pontine flexure divides the hindbrain into the myelencephalon caudally and the metencephalon rostrally. The mesencephalon does not partition further. The telencephalic flexure (shortened here from the longer term diencephalic-telencephalic sulcus) divides the forebrain into the diencephalon caudally and the telencephalon rostrally (Fig. 5-7C, D). The telencephalon (meaning “end-brain”) forms as an outpocketing of the forebrain and expands enormously, with its complex lobes, gyri, and sulci, to become the largest part of the brain.
Diencephalon and Cerebral Hemispheres
The main structures of the forebrain develop during the second month of gestation. Because the mesoderm in this region is simultaneously forming facial structures, abnormalities of forebrain development are often associated with facial defects (Fig. 5-3). The process of forebrain development is referred to as central induction.
At about the end of the fifth week, the telencephalon gives rise to two lateral expansions called the telencephalic (cerebral) vesicles (Fig. 5-7C, D). These are the primordia of the cerebral hemispheres. Their adult derivatives include the cerebral cortex and the subcortical white matter (including the internal capsule), the olfactory bulb and tract, portions of the basal ganglia, the amygdala, and the hippocampus. The diencephalon develops into the thalamic nuclei and associated structures and also gives rise to the optic cup, which eventually forms the optic nerve and retina. Septooptic dysplasia (the de Morsier syndrome) is a congenital dysgenesis of the diencephalon with hypoplasia of the optic nerve and hypothalamus-pituitary and midline structural defects, especially absence of the septum pellucidum and agenesis of the corpus callosum. Infants have congenital blindness and may have hypothyroidism, diabetes insipidus, and hypoadrenocorticism. By 10 weeks of development, the major structures of the CNS are clearly recognizable by their morphologic features, and immature versions of all structures in the brain are present by the end of the first trimester.
Defects of Prosencephalization
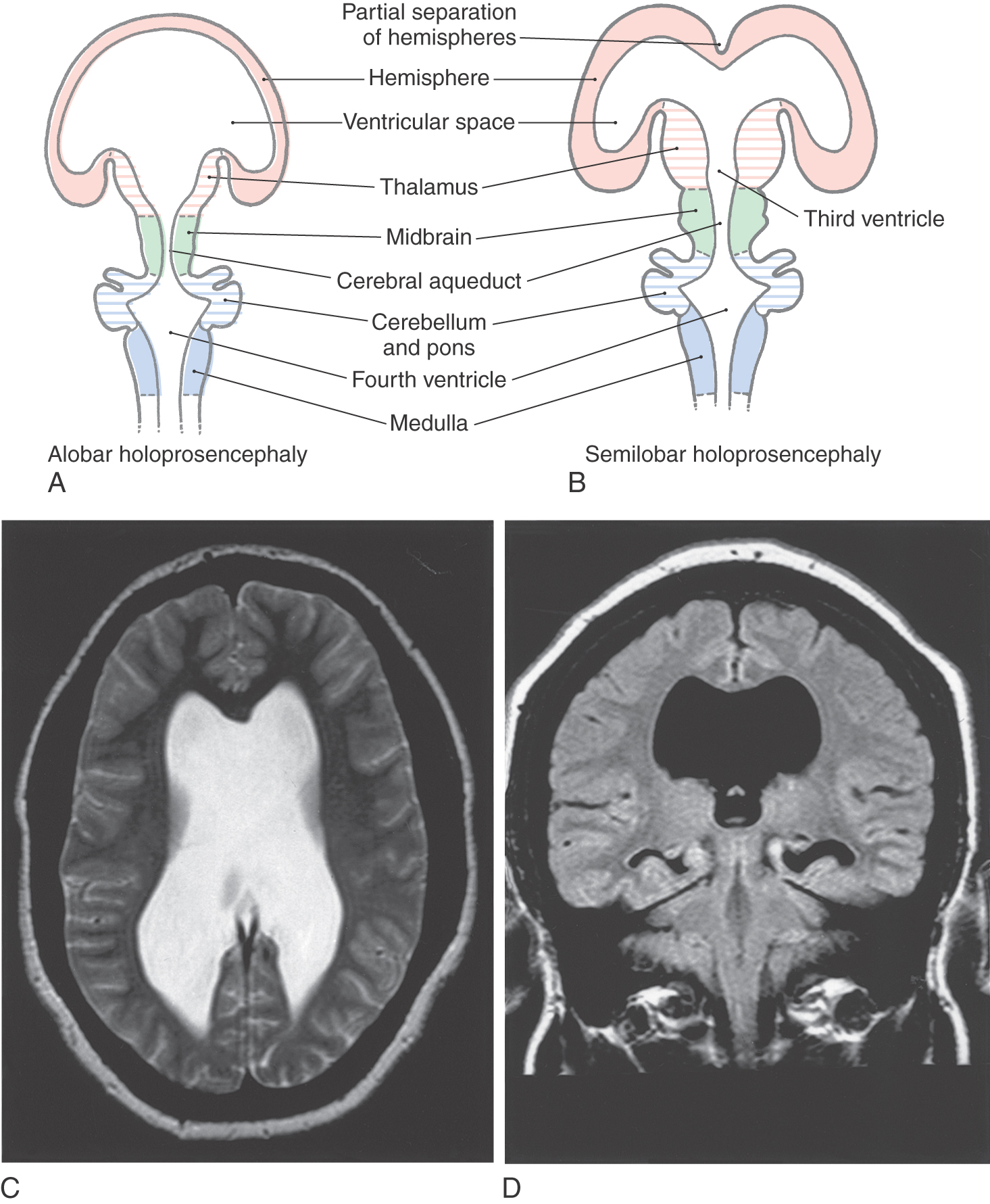
The sequence of events by which the primitive prosencephalon differentiates into the diencephalic and telencephalic vesicles is called prosencephalization. Failure of the prosencephalon to undergo cleavage results in a malformation called holoprosencephaly (Fig. 5-8A). In its most severe form (alobar holoprosencephaly), no discernible lobes develop. There is a large single forebrain ventricle, the thalamus is poorly developed, and many structures (corpus callosum, longitudinal cerebral fissure and falx cerebri, olfactory structures) are lacking. In semilobar holoprosencephaly (Fig. 5-8B-D), there is some separation of the forebrain into two discernible lobes (more prominent in occipital areas) and partial development of the falx cerebri. The hemispheres have some visible lobes and gyri, and there are rudimentary but enlarged lateral and third ventricles. These large ventricles are continuous one with the other, and midline structures, such as the septum pellucidum, that normally separate the ventricles are missing (Fig. 5-8C, D). Most infants with holoprosencephaly also have facial malformations. These may be as subtle as mild hypotelorism (unusually close-set eyes) or as obvious as the presence of only a single, midline eye (cyclops) accompanied by a rudimentary nasal structure (proboscis). In general, the more severe the brain malformation, the more severe the facial defect.
Holoprosencephaly is associated with a number of environmental exposures, including alcohol, retinoic acid, and maternal diabetes. It is also associated with a number of syndrome disorders and trisomy 13 and trisomy 18. A number of gene defects have been implicated as a cause of holoprosencephaly. SHH (7q36) is one such gene whose disruption has caused similar congenital defects in animal models.
Infectious Diseases Causing Congenital Nervous System Defects
Fetal exposure to several common infectious diseases can cause congenital nervous system defects. The acronym TORCH is often used for the more common etiologic agents: toxoplasmosis, other agents (syphilis), rubella, cytomegalovirus, and herpes simplex virus. Human immunodeficiency virus (HIV) infection has also been associated with congenital CNS defects.
Nervous system defects include cataracts, retinitis and blindness, deafness, cerebral calcifications, cerebral atrophy, and microcephaly. Infants actively infected at birth can also have rash, fever, anemia, bleeding, and other organ system disease.
Ventricular System
The ventricular system is an elaboration of the lumen of cephalic portions of the neural tube, and its development parallels that of the brain (Figs. 5-7 and 5-9A-D). This process, also discussed in Chapter 6, is summarized here. The cavities of the telencephalic vesicles become the lateral ventricles; the diencephalic cavity becomes the third ventricle; and the rhombencephalic cavity becomes the fourth ventricle. The cavity of the mesencephalon becomes the narrow cerebral aqueduct (of Sylvius) connecting the third and fourth ventricles, and the openings between the lateral ventricles and the third ventricle become the intraventricular foramina (of Monro).
The ventricular system is lined with ependymal cells. Each ventricle originally has a thin roof composed of an internal layer of ependyma and an outer layer of delicate connective tissue (pia mater). In each ventricle, blood vessels invaginate this membrane to form the choroid plexus.
Openings that arise in the caudal roof of the fourth ventricle during development form a communication between the ventricular system and the subarachnoid space. These are the midline medial aperture (foramen of Magendie) and the paired lateral foramina of Luschka. Although these foramina develop slowly, they are patent by the end of the first trimester. CSF is produced mainly by the choroid plexuses of the lateral and third ventricles. It escapes the ventricular system through foramina of the fourth ventricle and passes into the subarachnoid space. From there, it is absorbed into the venous system through the arachnoid villi located primarily in the superior sagittal sinus.
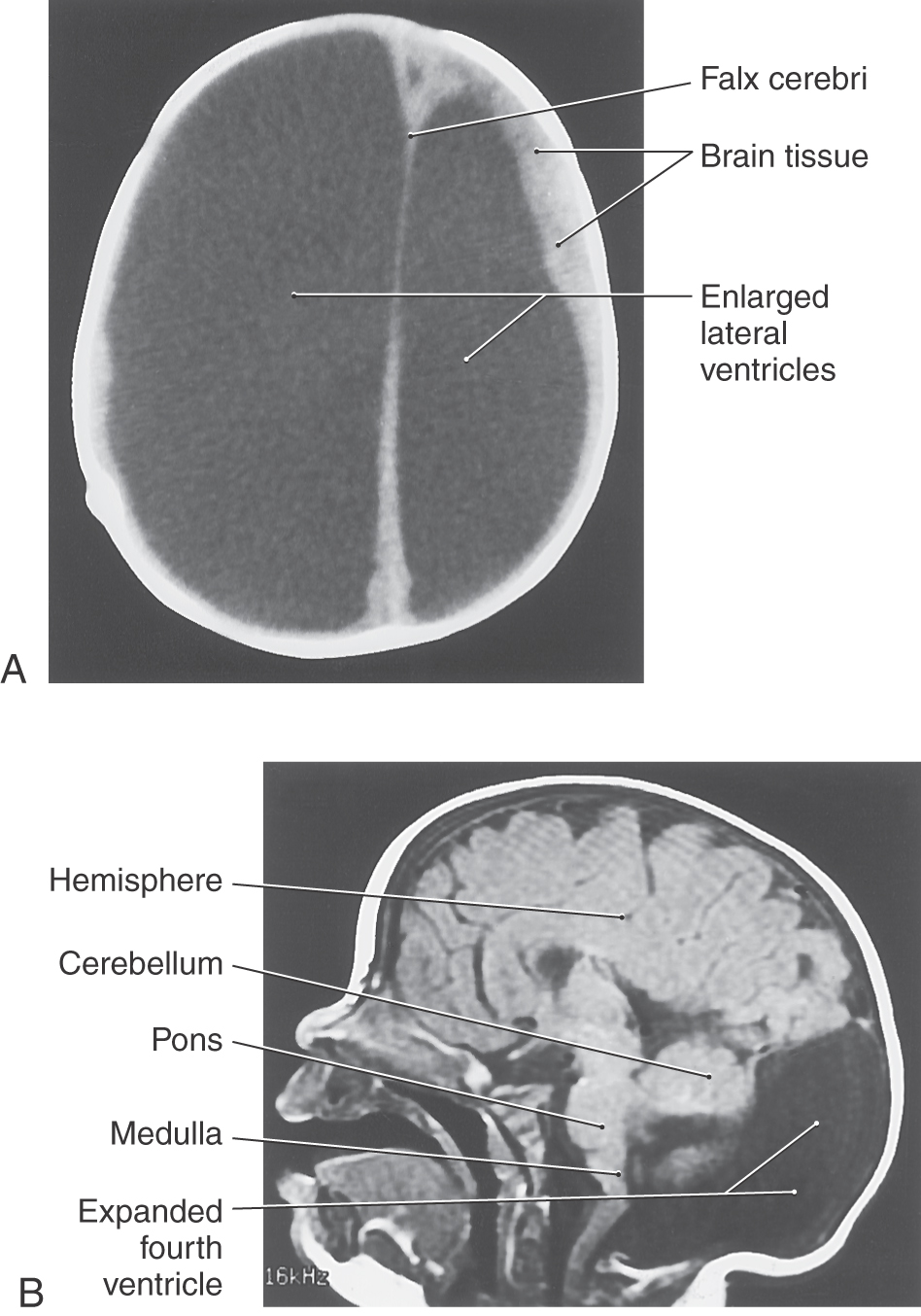
If the flow of CSF through the ventricles is obstructed during prenatal development, the ventricular system can become markedly dilated, a condition called congenital hydrocephalus (Fig. 5-10A). The cerebral aqueduct, only 0.5 mm in diameter, is a likely site for such a blockage. Congenital atresia (failure to form) of the aqueduct can occur as an isolated event, can be inherited, or can be associated with CNS deformities (Fig. 5-9E). Stenosis, or total obstruction, from cellular debris associated with an infection or from an intraventricular hemorrhage may also occlude this narrow passage.
PERIPHERAL NERVOUS SYSTEM
Neural Crest
The peripheral nervous system develops mostly from cells of the neural crest (Table 5-1; see also Fig. 5-1). These cells, which arise from the lateral edge of the neural plate, detach and move to locations lateral to the neural tube. The neural crest gives rise to most of the peripheral nervous system as well as to a number of other structures (Table 5-1).
Table 5-1 Principal Structures Derived from Neural Crest Cells
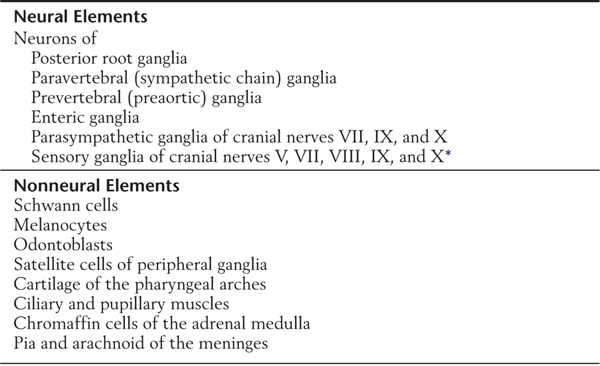
*Some of the sensory cells in these ganglia arise from placodes.
Placodes
Specialized epidermal cells called placodes are found in the developing head region. These will join neural crest cells, and together placodes and neural crest form the ganglia of cranial nerves V, VII, VIII, IX, and X.
Cranial Nerve Ganglia
Cranial nerves V (trigeminal nerve), VII (facial nerve), IX (glossopharyngeal nerve), and X (vagus nerve) have sensory ganglia that originate from neural crest and placode cells and contain pseudounipolar cell bodies. These are the trigeminal or semilunar (V) ganglion, the geniculate ganglion (VII), the superior and inferior (IX) ganglia of the glossopharyngeal nerve, and the jugular and nodose (X) ganglia of the vagus nerve. It is also common to refer to the jugular and nodose ganglia as, respectively, the superior and inferior ganglia of the vagus nerve. The distal processes of these cranial nerves travel as the sensory components of the corresponding cranial nerve, whereas the proximal processes innervate the appropriate cranial nerve nuclei in the brainstem. A notable exception to this pattern is the mesencephalic nucleus of the trigeminal nerve. In this cranial nerve nucleus, pseudounipolar cells have failed to migrate with the neural crest and remain inside the CNS. They form, in essence, a “ganglion” ectopically trapped inside the mesencephalon.
The cell bodies of the ganglia of cranial nerve VIII (the vestibulocochlear nerve) arise primarily from the otic placode, with a small contribution from neural crest. These ganglion cells retain a bipolar shape in the adult.
Posterior (Dorsal) Root Ganglia
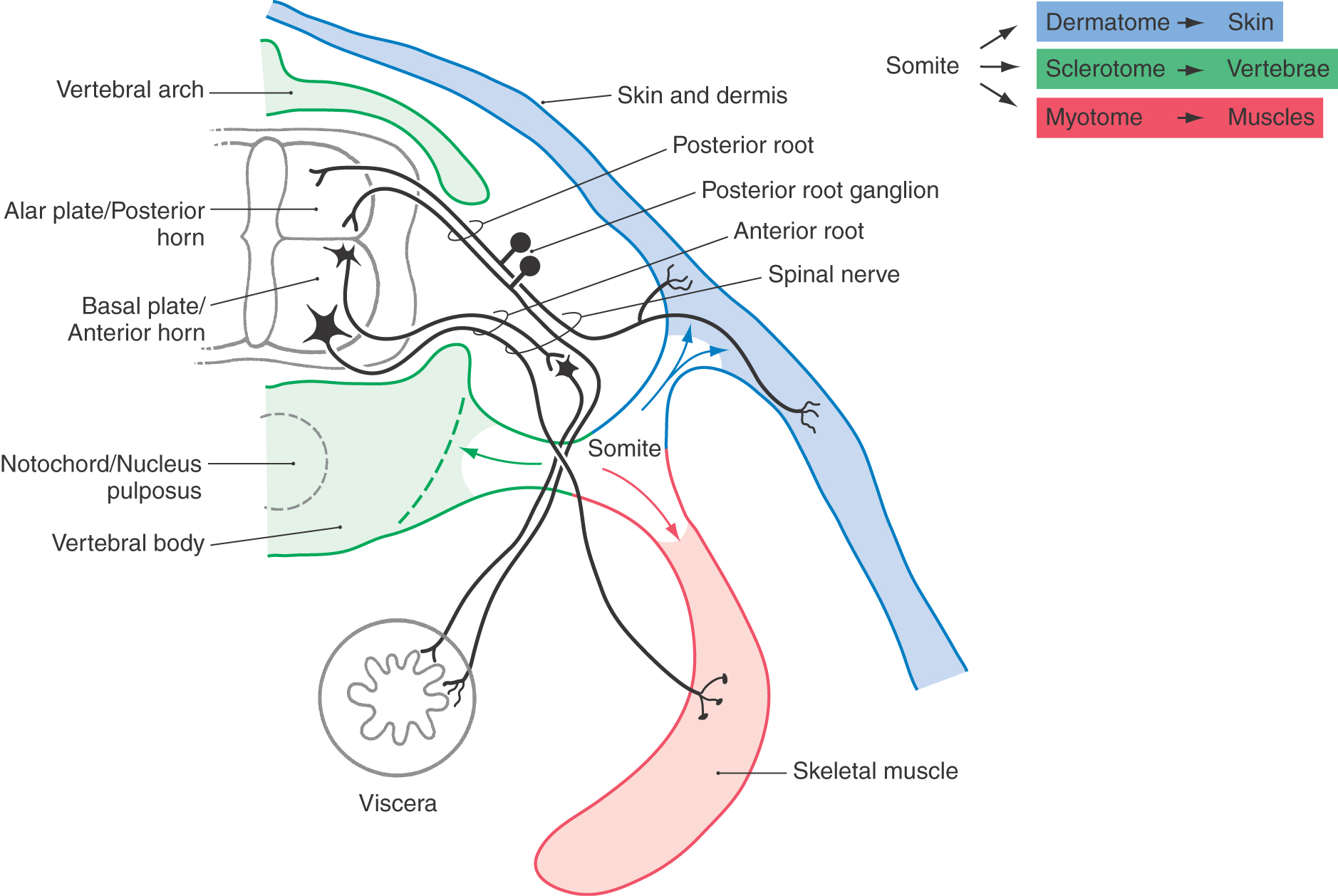
Pseudounipolar cells of posterior, or dorsal, root ganglia are derived from the neural crest. Each spinal nerve and its corresponding ganglion are associated with a segment (or somite) of the developing embryo (Fig. 5-11). As the somites grow out to form portions of the body’s connective tissue and musculature, the peripheral processes of the developing pseudounipolar cells of the corresponding dorsal root ganglia grow distally, using the extracellular matrix of the underlying tissue as a guide (Fig. 5-11).
The matrix molecules fibronectin and laminin contain the amino acid sequence arginine-glycine-aspartate (called the RGD sequence after the one-letter abbreviations for these amino acids). This sequence is recognized by proteins known as integrins on the surface of neural crest cells. The selective adhesion of the peripheral process of a neural crest cell to the RGD sequence of the extracellular matrix is probably involved in guiding the distal processes to their correct targets.
The segmental nature of the embryo is reflected in the segmental sensory innervation of the body surface (Fig. 5-11; see also Fig. 18-4). These segments, known as dermatomes, are important in the diagnosis of many neurologic disorders.
Visceral Motor System
The postganglionic sympathetic and parasympathetic neurons of the visceral motor system are also derived from the neural crests. Some of these cells remain near their site of origin to form the sympathetic chain ganglia adjacent to the vertebral column. Other cells migrate with branches of the aorta to form the sympathetic prevertebral ganglia.
Most of the autonomic (visceromotor) neurons of the digestive tract (Auerbach and Meissner plexuses) are formed by neural crest cells that migrate from the area of the rhombencephalon. Consequently, these cells receive vagal innervation in the adult. Visceromotor (autonomic) neurons of the descending colon and pelvic structures are derived from neural crest cells that arise from sacral cord levels during secondary neurulation.
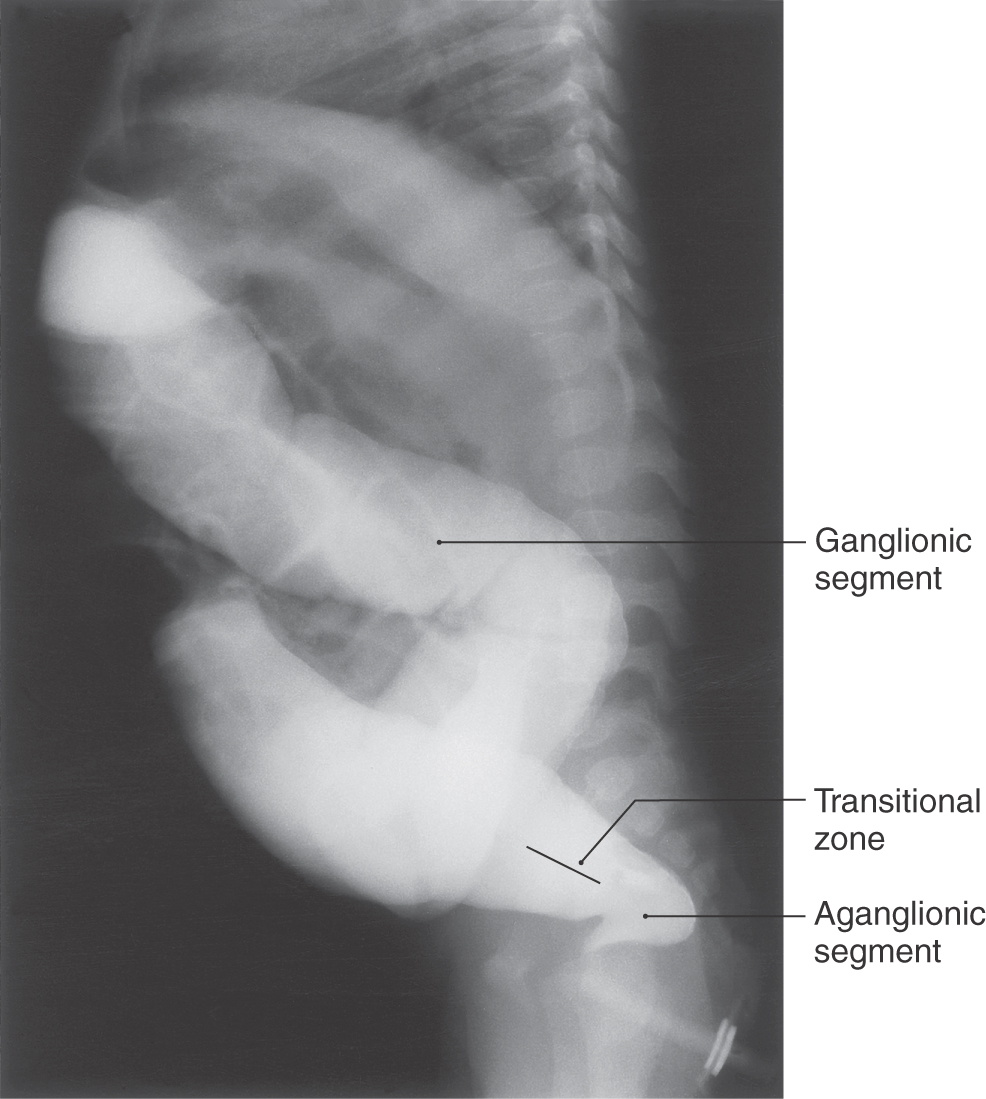
The human syndrome congenital megacolon (Hirschsprung disease) (Fig. 5-12) closely resembles an animal model in which excess extracellular matrix molecules are present in the colon. This results in aberrant migration of neural crest–derived cells. In this animal model, and in Hirschsprung disease in humans, neurons forming the enteric ganglia fail to migrate into the lower bowel. In the absence of these cells, no sensory signal indicating the presence of feces in the colon is sent to the CNS. Therefore, no motor signal is sent to control defecation.
Another clinical entity, familial dysautonomia, also reflects aberration in the development of neural crest derivatives. Patients with this disorder have both sensory symptoms (impaired pain and temperature perception) and autonomic symptoms (cardiovascular instability, gastrointestinal dysfunction).
Schwann Cells
The Schwann cells, which ensheath and myelinate axons in the peripheral nervous system, are also derived from neural crest cells (see Chapter 2). Schwann cells migrate in a segmental fashion, accompanying the growing processes of the peripheral nerve fibers they will eventually ensheath.
CENTRAL NERVOUS SYSTEM
Basic Features
In general, CNS neuroblasts arise at the ventricular surface of the developing brain, that is, the luminal surface of the neural tube. Just after the neural tube forms, there is no apparent cell differentiation in a cross section at any level. At this time, the neural tube is a pseudostratified columnar epithelium. As development proceeds and the wall of the neural tube thickens, however, dividing cells cluster at the ventricular surface, leaving a zone without cell bodies at the abluminal surface. This region, with few cell nuclei, is called the marginal zone.
As cells undergo their last division, they begin to migrate away from the luminal (ventricular) surface on transient glial cell guides called radial glia. As they migrate, they form a moving front of cell bodies between the marginal and ventricular zones called the intermediate zone. These features are common to all parts of the developing neuraxis; other elaborations are possible and are outlined in the following sections.
After cells migrate and take up their final positions in the developing brain, they begin to extend processes and form connections with other neurons or muscle cells. Dendritic processes begin to receive information from other developing cells. Meanwhile, an axonal process, tipped by a spade-like extension called the growth cone, begins to drive its way through intervening regions to reach distant targets.
Although the idea is controversial, both neurons and glia seem to originate from a single precursor cell population. Two main lineages arise: a neuroblastic lineage that generates neurons and a glioblastic lineage that includes precursors of radial glial cells, astroglial cells, and oligodendrocytes. The glioblastic lineage is believed to split into three main branches: (1) the type 1 astrocyte progenitor, (2) the oligodendrocyte/type 2 astrocyte precursor (called the O2A progenitor), and (3) the radial glia progenitor. Whereas all other cell types persist into adulthood, radial glial cells in most regions of the brain appear to be converted to astrocytes, ependymal cells, or tanycytes. There are two notable exceptions. In the cerebellum, radial glial cells retain most of their features as Bergmann glial cells, and in the retina, they are seen as Müller cells. Differentiation of glial cells is influenced by a variety of growth factors, such as platelet-derived growth factor, ciliary neurotrophic factor, and fibroblast growth factor, which are secreted by neighboring glia and neurons.
Spinal Cord
The adult spinal cord gray matter is butterfly shaped and consists of anterior (ventral) and posterior (dorsal) horns. At some levels of the spinal cord, an intermediate zone and a lateral horn of the gray matter lie halfway between posterior and anterior horns.
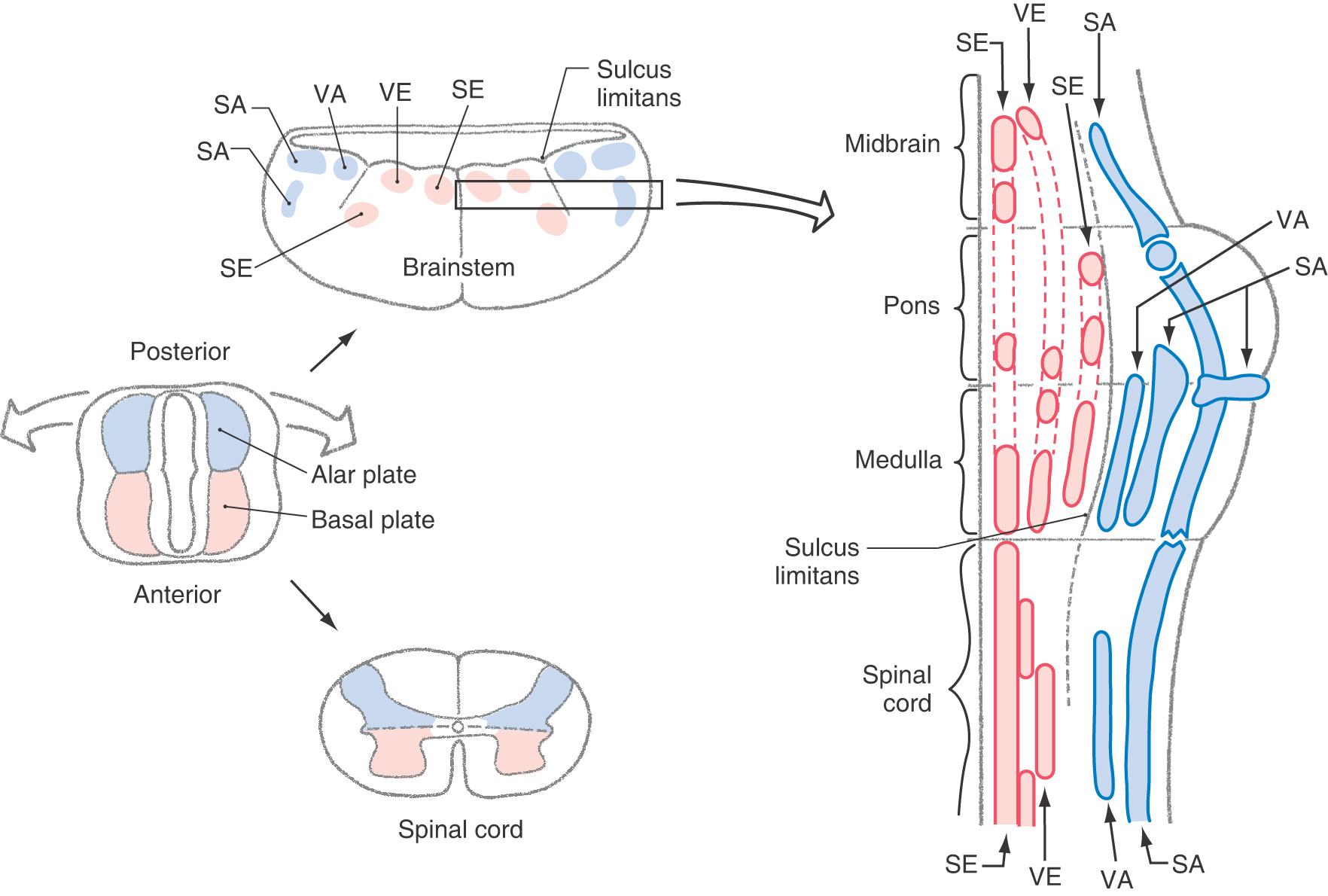
The spinal cord develops from caudal portions of the neural tube (Fig. 5-1). The neural canal in this region will become the central canal of the spinal cord (Fig. 5-7). Neuroblasts that give rise to spinal cord neurons are produced between the fourth and twentieth weeks of development by a burst of proliferation in the ventricular layer lining the neural canal. These cells migrate peripherally to form four longitudinal plates, which will become the gray matter of the spinal cord: a pair of anteriorly located cell masses, which constitute the basal plate, and a pair of posteriorly located masses, which constitute the alar plate. The basal and alar plates on each side are separated by a longitudinal groove called the sulcus limitans in the lateral wall of the central canal. The basal plate develops into the anterior (ventral) horn of the spinal cord, and the alar plate will become the posterior (dorsal) horn of the spinal cord (Fig. 5-11). Development in the basal plate somewhat precedes that in the alar plate; postmitotic neurons are clearly evident in the basal plate during week 20 of development. That portion of the adult spinal cord, commonly called the intermediate zone (and the lateral horn), originates from the interface of the alar and basal plates.
As the basal plate develops, axons of nascent motor neurons form the developing anterior (ventral) roots that will innervate peripheral structures. Anterior horn motor neurons innervate skeletal muscle and are classified as somatic efferent (SE). The lateral horn motor neurons project to autonomic (visceromotor) ganglia and are classified as visceral efferent (VE). The categories SE and VE are referred to as functional components. The cord regions devoted to these functional components can be thought of as constituting distinct longitudinal cell columns in the gray matter (Fig. 5-13). The SE column runs the full length of the spinal cord. The VE column extends from T1 through L2, where it is called the intermediolateral cell column, and from S2 through S4, where it is called the sacral visceromotor nucleus.
Neurons of the alar plate receive the central processes of developing posterior root ganglion (sensory) cells. Sensory neurons whose peripheral processes innervate the skin and receptors in joint capsules, tendons, and muscles are classified as somatic afferent (SA). Those that innervate receptors in visceral structures, such as the stomach, are classified as visceral afferent (VA). Like the SE and VE regions, the SA and VA functional components constitute separate columns (Fig. 5-13).
As each somite develops, it subdivides into a sclerotome, which forms vertebrae; a dermatome, which forms skin and dermis; and a myotome, which forms muscles (Fig. 5-11). The derivatives of the dermatomes and myotomes are innervated, respectively, by the axons of the posterior (sensory) and anterior (motor) roots of the corresponding spinal cord levels. These roots join at about the level of the future intervertebral foramina to form the spinal nerves (Fig. 5-11). The spinal nerves thus show the same segmental pattern as that for the dermatomes and myotomes they innervate. By contrast, the vertebrae develop between the spinal nerves and are thus intersegmental in position, even though they originate from the segmental sclerotomes. This situation comes about because the sclerotomes each split into cranial and caudal halves, and the vertebral rudiments are formed by the union of the caudal half of one sclerotome with the cranial half of the next posterior sclerotome.
Relationship of Spinal Cord to Vertebral Column
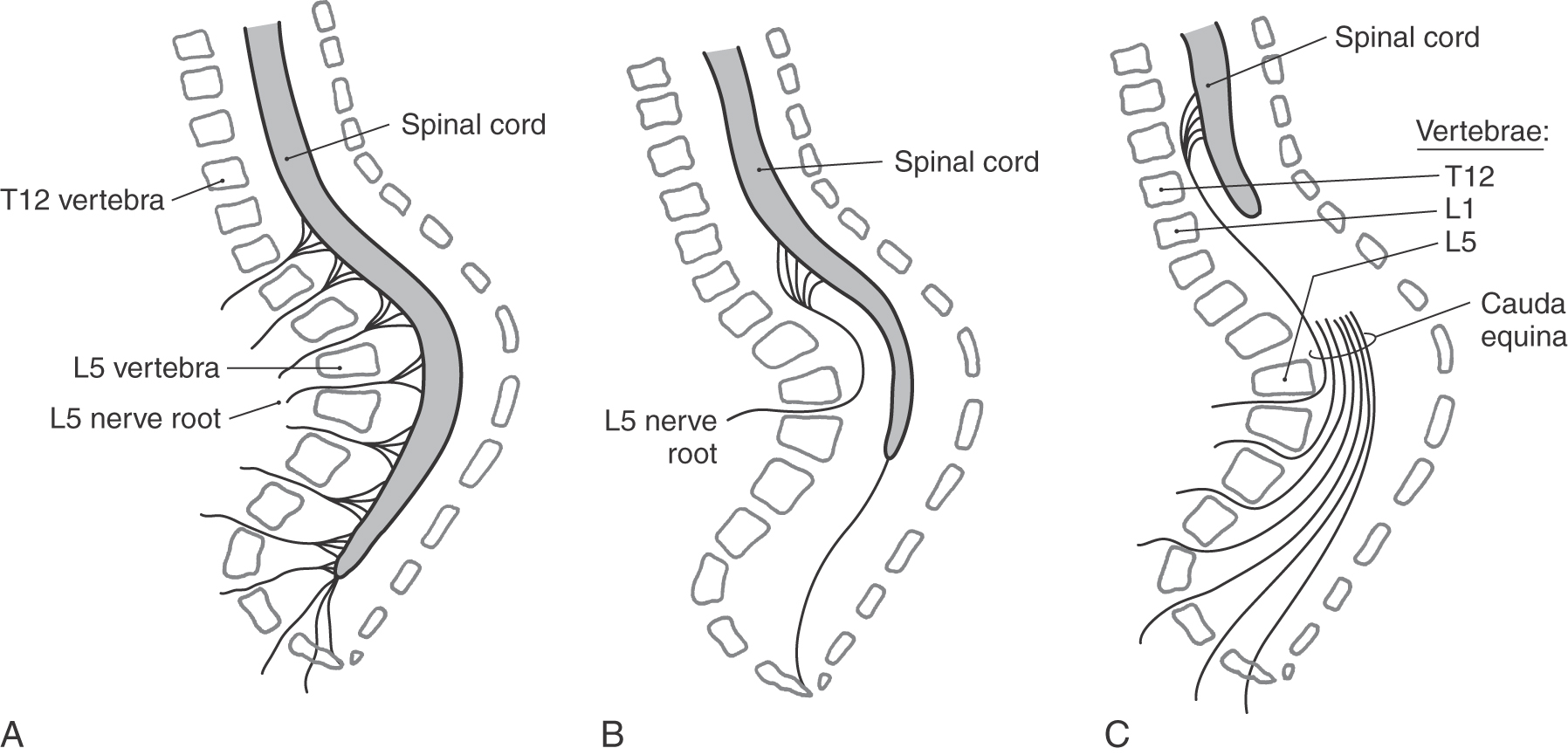
Although the spinal cord retains its general shape from the third trimester into adulthood, its physical relationship to the vertebral column alters dramatically (Fig. 5-14). By the end of the first trimester, the spinal cord, its meningeal coverings, and the surrounding vertebral arches are fully formed. The spinal nerves exit at about right angles to the spinal cord and pass through the intervertebral foramina. As development proceeds, the vertebral column and the spinal cord both grow caudally. However, the vertebral column grows slightly faster than does the spinal cord. The net result is that the cord seems to be drawn rostrally by its attachment to the brain. The intervertebral foramina, containing the spinal nerves, move caudally; and the posterior and anterior roots from lumbar, sacral, and coccygeal levels are significantly lengthened to form a bundle called the cauda equina (Fig. 5-14).
Brainstem
The brainstem consists of the myelencephalon (medulla oblongata), the pons (a part of the metencephalon), and the mesencephalon (midbrain). Although developmentally the cerebellum is a part of the metencephalon, it is considered a “suprasegmental” structure and not a part of the brainstem.
As one travels from the rostral part of the spinal cord to the caudal part of the brainstem (medulla oblongata), two features are notable. First, the appearance of the cerebellum and the flaring open of the central canal into the fourth ventricle force the dorsal portion of the neural tube (alar plate) to rotate dorsolaterally (Fig. 5-13). This rotation results in a lateral to medial orientation of sensory (alar plate) versus motor (basal plate) areas of the developing brainstem, in contrast to their dorsoventral relationship in the spinal cord. Second, the sulcus limitans, which disappears in the spinal cord during development, is retained as an important landmark in the floor of the fourth ventricle (Fig. 5-13).
The basal plate in the brainstem gives rise to motor cranial nerve nuclei, and the alar plate gives rise to sensory cranial nerve nuclei. As in the spinal cord, these portions of the basal and alar plates differentiate into rostrocaudally oriented cell columns that are associated with functional components.
Derivatives of the basal plate ultimately give rise to cell columns that form the motor nuclei of the brainstem (see Chapter 10 for details). Muscles that arise from paraxial mesoderm, either directly or through the pharyngeal arches, are innervated by somatic efferent (SE) motor neurons located in the brainstem. Within the medulla, these SE nuclei are the hypoglossal nucleus (XII, tongue musculature) and the nucleus ambiguus (IX, X, stylopharyngeus, laryngeal and pharyngeal musculature) (Fig. 5-13). The SE motor nuclei of the pons include the abducens nucleus (VI, lateral rectus muscle); the facial nucleus, commonly called the motor nucleus of the facial nerve (VII, muscles of facial expression); and the trigeminal motor nucleus (V, muscles of mastication).
In addition to SE motor cells that innervate skeletal muscle (striated muscle), the basal plate also gives rise to visceral efferent (VE) motor neurons. These neurons provide preganglionic parasympathetic innervation to peripheral ganglia that serve visceral structures, structures composed of smooth muscle, cardiac muscle, or glandular epithelium or combinations thereof. The VE motor neurons of the medulla are found in the dorsal motor vagal nucleus (X) and project to the intermural ganglia of thoracic and abdominal viscera (to the splenic flexure) and the inferior salivatory nucleus (IX), which projects to the otic ganglion. In the caudal pons, the VE motor neurons are found in the superior salivatory nucleus (VII), exit via the facial nerve, and project to the pterygopalatine and submandibular ganglia. The VE cells in the midbrain are located in the Edinger–Westphal preganglionic cell group (EWpg); these cells specifically project to the ciliary ganglion.
Neurons relaying sensory information conveyed on cranial nerves of the brainstem originate from the alar plate. Sensory afferent (SA) input from the vestibular labyrinth (SA proprioception, vestibular ganglion) and from the cochlea (SA exteroception, spiral ganglion) is conveyed into the vestibular and cochlear nuclei of the caudal pons and medulla. SA information originating from receptors in the skin of the face and portions of the scalp, lining of the oral cavity, nasal membranes, teeth, anterior two thirds of tongue, cornea and conjunctiva, membranes of the maxillary and frontal sinuses, masticatory muscles, periodontal ligament, and dura of anterior and middle fossae centrally terminate in the various portions of the trigeminal nuclei. The spinal trigeminal nucleus receives exteroceptive input, predominantly pain and thermal sense, conveyed on the trigeminal (V) and, to a much lesser degree, on the facial, glossopharyngeal, and vagus nerves. The cell bodies of these primary sensory fibers are in the sensory ganglia on these respective nerves. The fibers conveying SA discriminative touch terminate in the principal sensory nucleus, and those conveying SA proprioception enter the brain and form the mesencephalic tract and have their cell bodies in the mesencephalic nucleus.
Along with the posterolateral-anteromedial division of the brainstem into alar and basal plates, there is a rostral-caudal segmentation of the developing rhombencephalon into rhombomeres. These are clusters of immature neurons separated from each other by thin, transversely oriented bands of neuroepithelial cells. Cells in one rhombomere give rise to a specific motor nucleus (or nuclei) but will not migrate into adjacent rhombomeres. In general, the cell clusters forming the rhombomeres represent the rostral continuation of the alar and basal plates of the developing spinal cord. Indeed, motor nuclei originate from specific rhombomeres, and input from the corresponding sensory ganglia enters the corresponding rhombomere.
Rhombomeres are also sites of homeobox gene expression. These genes (abbreviated Hox) are “master switches” that control the formation of large blocks of tissue. For example, the gene Hox-2.1 is expressed only in the rhombomeres that give rise to cranial nerves X and XII; Hox-2.9 is expressed only in the region of the developing facial nerve. One example of a congenital defect affecting developmental sequences within the rhombomeres is the Möbius syndrome. This syndrome is characterized by a variety of developmental defects, including (1) aplasia (poor development of, or absence of) of the abducens and facial motor nuclei, with the correlated motor deficits of facial and eye movement; (2) weakness of the muscles innervated by the oculomotor and trochlear nerves; (3) atrophy or weakness of the tongue and weakness of the masticatory muscles; and (4) a variety of skeletal defects affecting the face and body. A rare disorder, pontocerebellar hypoplasia, is a congenital but progressive atrophy of the pons and cerebellum that causes hypotonia, poor feeding, growth retardation, and profound mental retardation.
Cerebellum
The cerebellum arises from the rhombic lip, an alar plate structure that forms part of the wall of the fourth ventricle. The rostral part of the rhombic lip forms the cerebellum, whereas the caudal part gives rise to the inferior olivary, cochlear, and pontine nuclei.
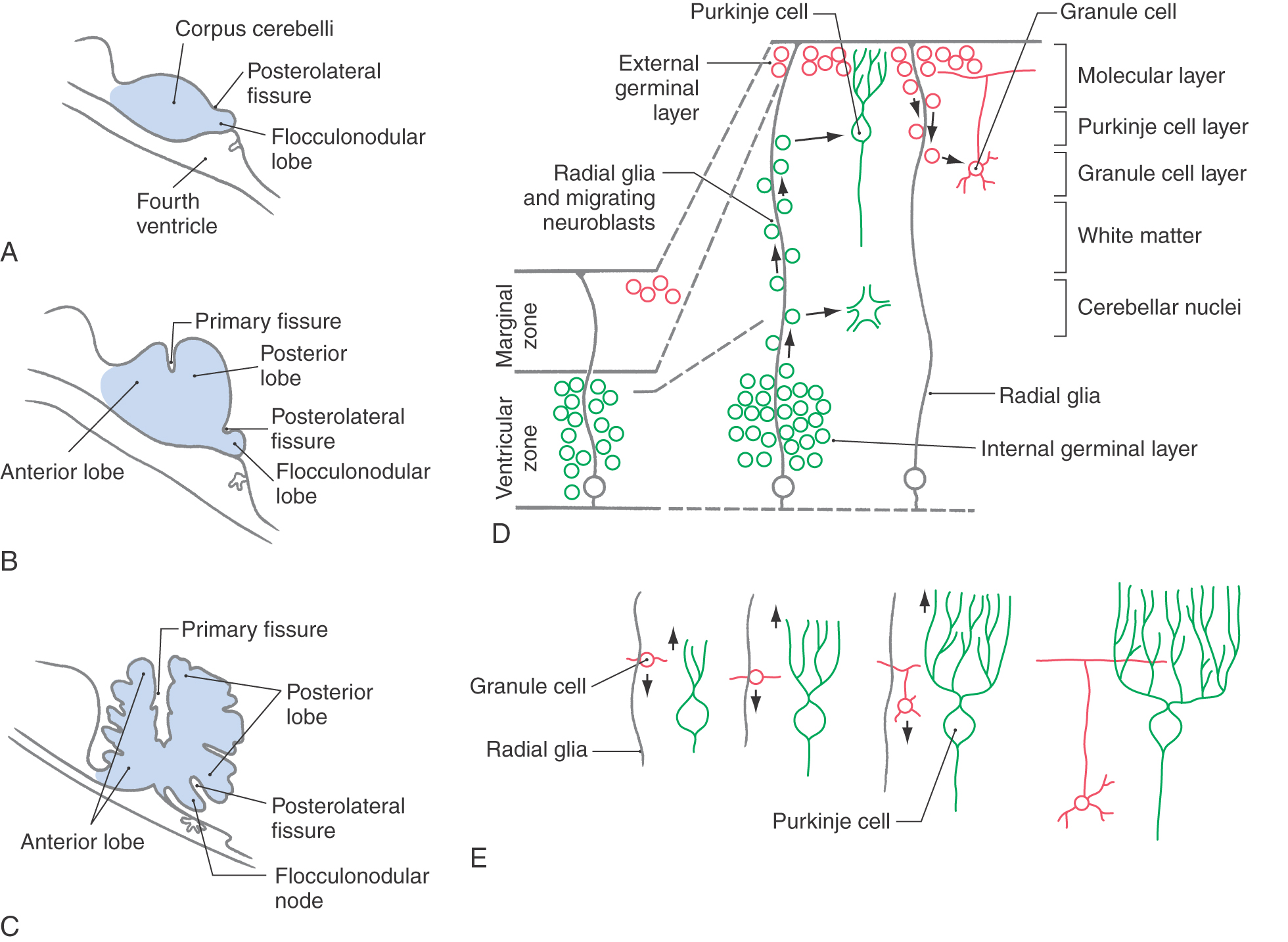
The rhombic lips join dorsal to the developing fourth ventricle to form the cerebellar plate. During the histogenesis of the cerebellar cortex, fissures appear that divide the cerebellum into its main lobes (Fig. 5-15A-C). The first, the posterolateral fissure, divides the cerebellar plate into the flocculonodular lobe and the corpus cerebelli. Despite its name, the primary fissure is the second to appear, and it divides the corpus cerebelli into anterior and posterior lobes. The advent of additional fissures divides the anterior and posterior lobes into the lobules characteristic of the adult brain.
The process of cerebellar cortical development involves the migration of immature neurons to form the cells characteristic of the adult brain (Fig. 5-15D). Initially the cerebellar primordium is composed of the ventricular zone, the intermediate zone, and the marginal zone. By the end of the first trimester, a second layer of immature neurons has appeared in the outer part of the marginal layer. This is called the external germinal (or granular) layer, and the intermediate zone is now called the internal germinal (or granular) layer.
Radial glial cells extend from the ventricular zone to the surface of the marginal layer and are necessary for the proper migration of developing neurons (Fig. 5-15D). Immature neurons of the internal germinal layer migrate outward along the radial glia to form the cerebellar nuclei and the Purkinje cells, Golgi cells, and unipolar brush cells of the cerebellar cortex. Immature neurons of the external germinal layer migrate inward along the radial glia to form the granule cells. Other cells of the external germinal layer congregate just external to the Purkinje cell layer, where they will differentiate into the stellate cells and basket cells of the molecular layer.
Developing cerebellar neurons participate in other important cell interactions in addition to those with the radial glial cells. For example, as the granule cells migrate inward, they sprout axons that form synaptic contacts with the Purkinje cell dendrites that are growing into the molecular layer (Fig. 5-15E). Continued growth and development of Purkinje cell dendrites depend on these contacts with granule cell axons. Purkinje cell dendrites are stunted in the mutant mouse weaver, in which the granule cells die during development. These and other experimental mutant animals with cerebellar defects show the characteristic manifestations of cerebellar disease in humans: ataxia, hypotonia, and tremor.
The Dandy-Walker malformation consists of hypoplasia of the cerebellar vermis, cystic dilation of the fourth ventricle, and often hydrocephalus (Fig 5-10B); clinical manifestations include macrocephaly, ataxia, and developmental delay. The malformation has been associated with a number of syndromic and genetic disorders. Joubert syndrome is a similar disorder without the cystic dilation of the fourth ventricle.
Thalamus
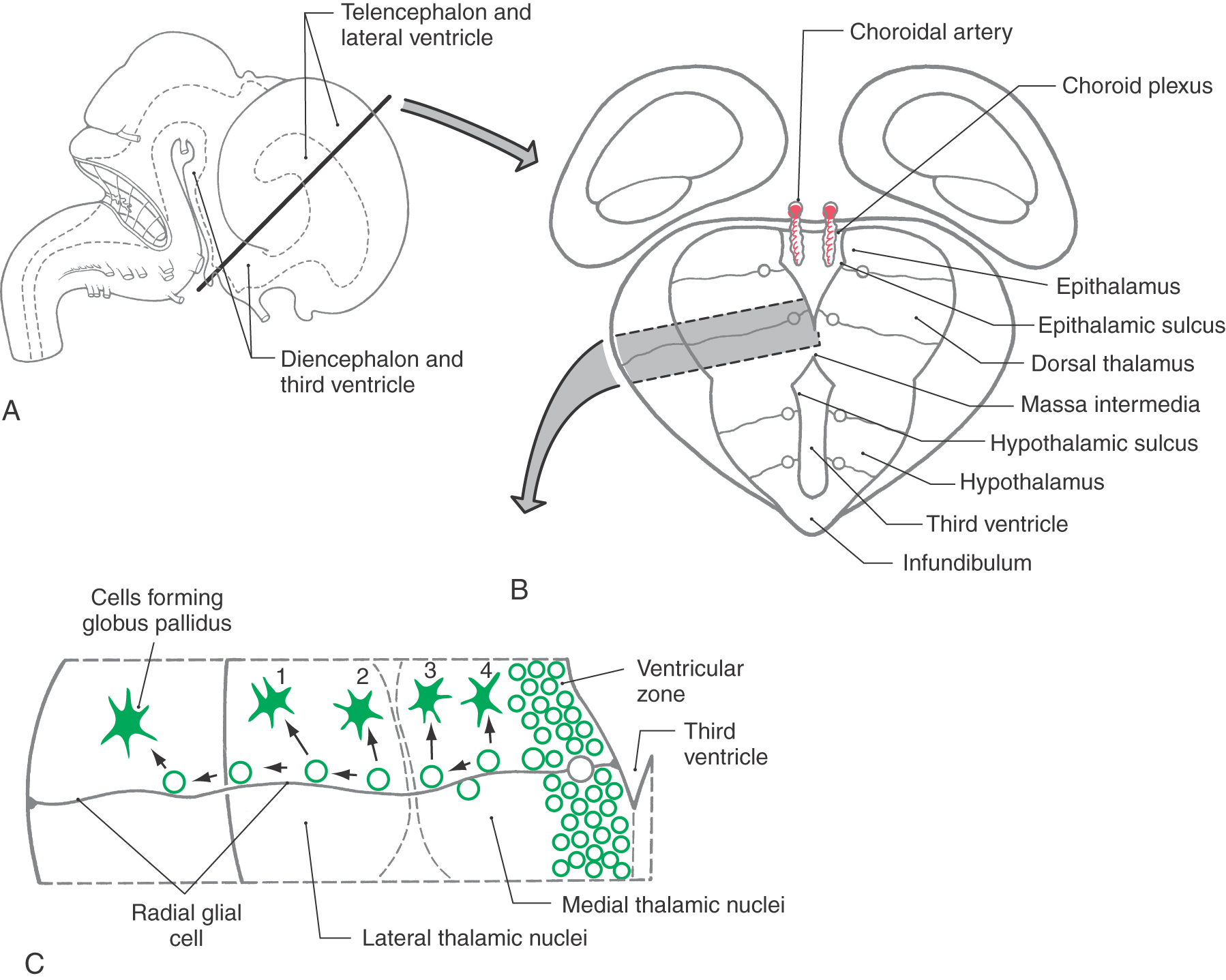
The gray matter of the diencephalon develops from a continuation of the brainstem alar plates; there is no homologue of the basal plate in the diencephalon. This alar plate is recognizable at 4 weeks of gestation; by 6 weeks of gestation, it has differentiated into three main areas of the diencephalon: the epithalamus, thalamus (dorsal thalamus), and hypothalamus (Fig. 5-16A, B). These structures are visible as swellings in the wall of the third ventricle, where they are separated from each other by the epithalamic and hypothalamic sulci. As development progresses, the epithalamic area remains small, whereas the hypothalamus and especially the thalamus enlarge. In about 80% of individuals, the two thalami fuse across the third ventricle to form the interthalamic adhesion (massa intermedia).
The basic concepts of the development of the thalamus are the same as for other CNS regions. Radial glia extend from the third ventricle to the pial surface, and developing neurons migrate along this guide (Fig. 5-16B, C). The development of the thalamus occurs in an “outside-first” sequence. That is, the first neurons to undergo their final cell division migrate to the outermost portion of the thalamus, where they mature. This means that the most lateral of the thalamic nuclei, such as the geniculate nuclei and the lateral and ventral nuclei, are generated first. The most medial thalamic nuclei, such as the dorsomedial nucleus, are the last to develop.
An important process in the development of the thalamic relay nuclei is the establishment of orderly maps of the sensory world. For example, a retinotopic (vision) map is formed in the lateral geniculate nucleus. As retinal ganglion cells send axons to the lateral geniculate nucleus, the arrangement of axonal contacts on cells in this visual relay center must accurately reflect the positions of ganglion cells in the retina. In this way, the map of visual space on the retina is maintained in the lateral geniculate nucleus and ultimately in the visual cortex. Similar maps are formed in the medial geniculate nucleus (tonotopic mapping) and ventral posterolateral nucleus (somatotopic mapping).
Cerebral Cortex
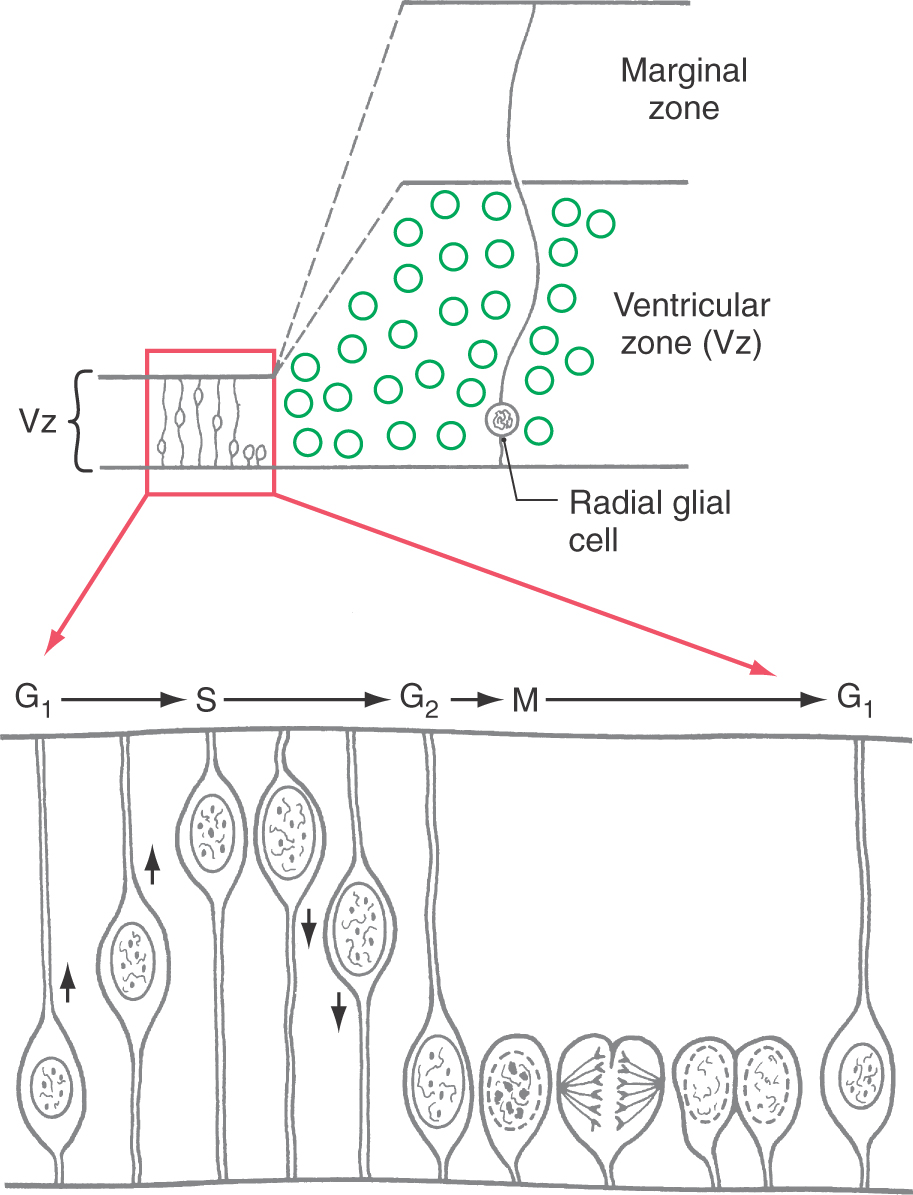
The cerebral cortex is generated by the basic mechanisms described previously for other regions. Except during mitosis and cytokinesis, cortical neuroblasts retain connections to both the ventricular and the pial surfaces of the developing brain and thus have a fusiform shape (Fig. 5-17). The nucleus engages in a peculiar cycle of migration within the cell, however. During the G1 phase of the cell cycle (before DNA replication), the nucleus travels from near the ventricular pole of the cell to near the pial pole. During the G2 phase (after DNA replication), it reverses direction and migrates back to a ventricular position. At the start of mitosis, the cell loses contact with the pial surface; after cytokinesis, the daughter cells grow processes that reconnect with the pial surface (Fig. 5-17). The cycle is then ready to repeat.
Unlike in other regions of the brain, in the cerebral cortex the first cells to migrate will disembark from the radial glial cells and take up positions close to the ventricular surface. Successive “waves” of immature neurons, migrating along radial glia, force their way through the differentiated cell layers to take up positions progressively closer to the pial surface. This sequence is called an “inside-out” pattern of development. These ranks of cells form the cortical plate as the axons of previously settled cells grow toward their targets.
The cerebral cortex proper is formed from expansion of the superficial part of the intermediate zone, the subplate and cortical plate (Fig. 5-18). Developing axons, originating in regions such as the thalamus that innervate the cortex, send out and form transient synaptic contacts in the subplate. The subplate is a transient structure that does not persist into adulthood. Neuronal cell bodies vacate the area between the subplate and the ventricular surface; most of the remaining cell bodies are glial cells. This region forms the white matter of the adult nervous system. The ventricular zone is reduced to a single layer of ependymal cells that line the lateral ventricles in the adult.
During peak periods of cellular migration, the hemispheric fissures appear and mold the telencephalic surface into the gyri and sulci characteristic of the adult brain. By the end of the first trimester, the longitudinal cerebral, sylvian, and transverse cerebral fissures are recognizable. The secondary sulci are completed by 32 weeks of development, with tertiary sulci completed during the last month of gestation.
Abnormalities of Cortical Development
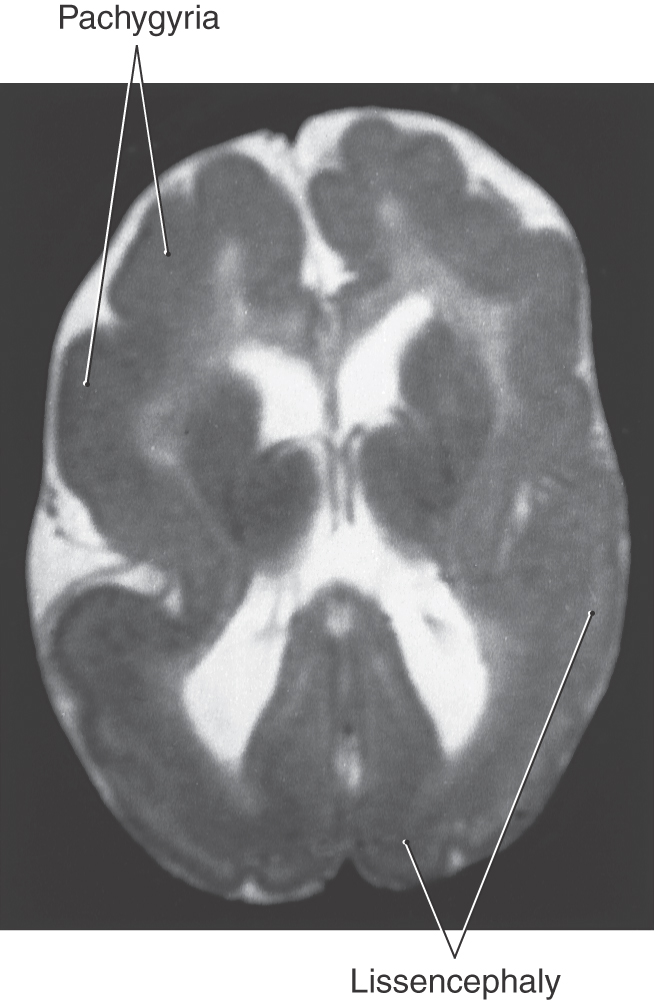
Abnormal patterns of gyri and sulci may be caused by disorders of cell migration in the developing cerebral cortex (Fig. 5-19). If gyri fail to form, the cerebral cortex will have a smooth surface, a condition called lissencephaly. Unusually large gyri constitute pachygyria, and unusually small gyri constitute microgyria. Any of these conditions may affect the whole cerebrum or may be localized, and they may coexist in the same patient. For example, a young patient may have lissencephaly and pachygyria in different regions of the same cerebral hemisphere (Fig. 5-19). All lissencephalic disorders have genetic defects, and 80% can be detected by current technology. The most common defects are related to the LIS family of genes that can be identified by fluorescence in situ hybridization. Most infants with lissencephaly and similar migration defects have epilepsy, which is often retractable.
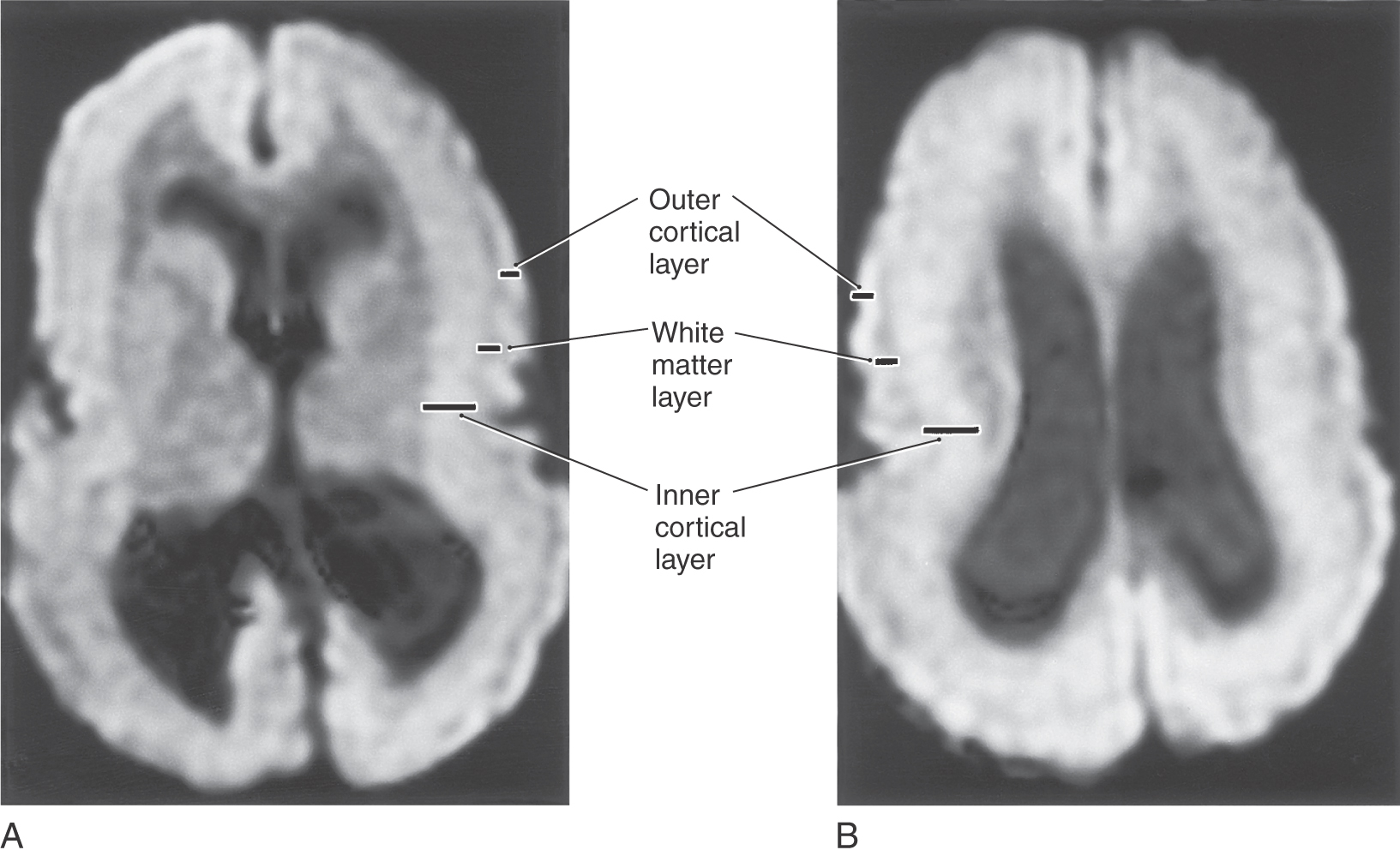
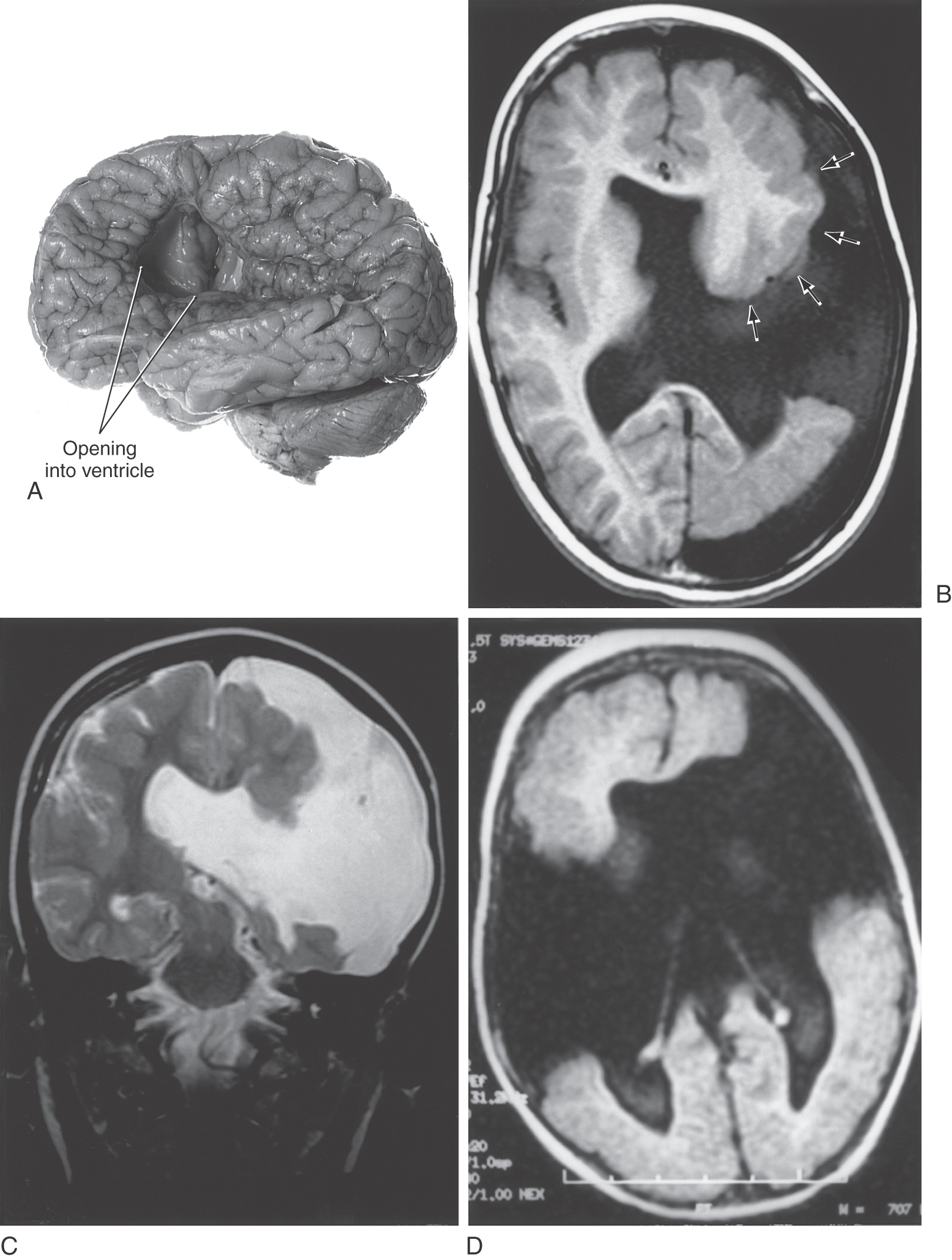
The migration of immature neurons from the ventricular surface can be disrupted, causing mature neurons to take up residence in the intermediate zones. The term heterotopia is used to describe this defect. Heterotopias are usually in the periventricular region on neuroimaging. The degree of disruption varies from mild, microscopic clusters of neurons in the white matter and deeper cortical layers to large, macroscopic clusters of neurons that can be seen grossly and on neuroimaging (Fig. 5-20). In the most severe case, an entire cellular migration wave is disrupted. This causes layers of gray matter alternating with layers of white matter (band heterotopia; Fig. 5-20A, B). In patients with band heterotopia, the cellular layers of the cortex are excessively thick and disordered, the ventricles may be quite large, and the gyral and sulcal patterns of the cortex are grossly abnormal (Fig. 5-20). Heterotopias can be found in isolation or in association with other congenital defects of the nervous system. For example, a brain may have heterotopia with lissencephaly or pachygyria. Heterotopias can be associated with epilepsy and developmental disorders. Abnormal patterns of sulcal and gyral development are seen in schizencephaly, a condition in which there are unilateral or bilateral clefts in the cerebral hemispheres (Fig. 5-21A) of almost any size. Small defects may consist of a thin slit in the hemisphere with communication between the ventricle and the surface of brain (closed lip schizencephaly). In severe cases the defect is large, resulting in a substantial loss of brain tissue and producing a large open channel between the ventricular cavity and the subarachnoid space (open lip schizencephaly) (Fig. 5-21B, C). One of the important characteristics of this type of developmental deficit is the continuity of cortex from the surface of the brain into the channel of the defect (Fig. 5-21B, arrows). In especially severe cases, the schizencephaly may be bilateral with a significant loss of brain tissue (Fig. 5-21D). These patients have a variety of mental deficits. Schizencephaly may result from a profound failure of cell migration. An alternative explanation, which may apply particularly to severe cases, is that the affected region did not receive an adequate blood supply during development. The result would be a central area of necrosis, which would become a thin spot or an open channel, surrounded by a zone of abnormal neuroblast migration. Cystic lesions caused by tissue destruction are called porencephalic cysts.
CELLULAR EVENTS IN BRAIN DEVELOPMENT
The organization of the brain ultimately determines its function. Three important parameters in brain organization are (1) the density of neurons, (2) the pattern of axon and dendrite branching, and (3) the pattern of synaptic contacts. These characteristics begin to develop toward the end of the peak period of neuronal migration at the sixth month of gestation. Although neuronal density and the basic patterns of axonal and dendritic growth are determined within the first 2 to 3 years after birth, remodeling of synaptic connections continues throughout life.
Overproduction of Neurons and Apoptosis
Embryogenesis produces 1½ to 2 times more neurons than are present in the mature brain. By 24 weeks of gestation, almost all of these neurons have been produced. Subsequent to this, there is selective death of neurons.
Genetically programmed cell death (apoptosis) of neurons is a feature of cellular development in many areas of the brain. In contrast to necrosis (cell death resulting from injury), apoptosis requires protein synthesis and therefore is an active cellular process.
Some growth factors interrupt the normal process of apoptosis. Nerve growth factor, brain-derived neurotrophic factor, and fibroblast growth factor are known to limit cell death. This finding has led to the idea that the administration of growth factors may block the neuronal cell death that occurs in certain degenerative diseases.
Axonal Outgrowth
After neuroblasts complete their final cell division and migrate to their final location, they begin to extend a single axon with one or more distal elaborations known as growth cones. This spade-shaped extension of the growing axon is capable of driving through fields of developing nervous or mesenchymal tissue to reach distant targets. The guidance of growth cones is influenced by both tropic factors (which guide a cell toward a particular target) and trophic factors (which maintain the metabolism of a cell or its processes). As an axon grows, it may send out branches, each with its own growth cone. Some branches may terminate in sites that will not ultimately be innervated by the cell. For example, cells of the motor cortex that send axons into the spinal cord to innervate motor neurons also send transient branches to structures of the brainstem; these ectopic connections are not normally maintained.
Synaptogenesis
Once an axonal growth cone arrives at its site of termination, it undergoes biochemical and morphologic changes to become a presynaptic terminal. Similarly, the area of the target neuron contacted by the presynaptic process begins expressing the characteristic postsynaptic machinery, such as neurotransmitter receptors and second messenger molecules.
One view of synapse development is that there is competition for the synaptic space available on target neurons. A synapse that forms will persist only if it exchanges the right cues with the target cell. Many synapses that form are subsequently lost and are replaced by other synapses that may be more successful. Thus, only a subset of the large number of synapses that form are ultimately retained. This is the concept of synaptic stabilization, and it requires (1) a signal generated by the presynaptic cell, possibly the neurotransmitter to be used at the adult synapse; (2) a means for the postsynaptic cell to respond to the presynaptic signal; and (3) a “retrograde signal” from the postsynaptic cell to the presynaptic cell to indicate which contacts are to remain in maturity.
Plasticity and Competition
An important process related to the development of neuronal organization is plasticity. The developing brain is not as vulnerable to injury as is the mature brain. Infants who suffer significant cortical injury in the prenatal or early postnatal period may show surprising functional recovery, ending up with few or no obvious deficits. The mechanism of plasticity relates to alterations in selective neuronal death and axonal simplification and to the retention of transient axonal branches and synapses that would otherwise be lost, as discussed previously.
One example of plasticity and the competition for synaptic space is the development of visual cortical connections. Fibers conveying visual input from each eye arrive in overlapping territories in the visual cortex during the fetal period. The synaptic space within this region normally is equivalently distributed to terminals carrying input from each eye. However, if the input from one eye is lost or if it is not functionally equivalent to the input from the other eye, the terminals from the “good” eye will experience a competitive advantage and occupy a larger share of the available synaptic space. The time during which these types of plastic changes can occur is called the critical period. Other areas of cortex have their own critical periods; the duration and time of occurrence of the critical period vary from region to region.
The concept of a critical period has clinical implications. If the input from one eye is dysfunctional during the critical period for visual system development (e.g., if one eye is severely myopic), the axon terminals carrying information from that eye are at a disadvantage as they compete for synaptic space. If the causative disorder goes untreated, the “good” eye has exclusive access to the visual cortex, and input from the “bad” eye is ignored. This condition is called amblyopia. If the myopia is corrected later in life, no signals can pass from the retina to the visual cortex because the appropriate synaptic connections were not formed during the critical period. As a result, the eye remains functionally blind. This blindness can be avoided by implementation of clinical interventions that equalize competition for synaptic territory during the critical period.
Synaptic development occurs in parallel with cellular proliferation and migration. Dendritic spines are the site of many synaptic contacts, especially in cortical neurons. The rate of spine formation varies in different parts of the brain but is usually maximal during the sixth month after birth. Many children with mental retardation, including those with Down syndrome, have fewer and less complex axonal and dendritic ramifications and fewer dendritic spines than in normal children. In some patients, there is a disturbance of the cytoskeletal structure that supports the architecture of axonal processes. Axonal and synaptic development are especially vulnerable to perinatal hypoxia, malnutrition, and environmental toxins.
Myelination
Oligodendrocytes myelinate neuronal axons in the CNS. Myelination begins at about the sixth month of development and peaks between birth and the first year of life, but it continues into adulthood. A delay in myelination can result in a delay in functional development. The best example is a congenital cortical blindness that resolves during the first year of life.
There is a definite hierarchy in the regional maturation of myelin formation. The motor and sensory tracts throughout the nervous system mature early, whereas the association tracts mature relatively late.
Several neurodegenerative diseases (leukodystrophies) affect the formation of myelin. Many other inborn errors of amino and organic acid metabolism impair myelination, notably phenylketonuria. Finally, inadequate nutrition also can impair myelination.
Sources and Additional Reading
Barkovich AJ. Pediatric Neuroimaging. ed 2 New York: Raven Press; 1995.
Evans OB. Manual of Child Neurology. New York: Churchill Livingstone; 1987.
Jacobson M. Developmental Neurobiology. ed 3 New York: Plenum Press; 1991.
Purves D, Lichtman JW. Principles of Neural Development. Sunderland, Mass: Sinauer Associates; 1985.
Rakic P. Principles of neural cell migration. Experientia. 1990;46:882–891.