CHAPTER 51 Development of the limbs
Development of the limb depends on a series of complex cell–cell interactions that have been identified in experiments on amphibian, avian and reptilian species; the range demonstrates the remarkable conservation of developmental processes. Fate maps have revealed which structures in the limb arise from particular cells or groups of cells in the embryo and considerable progress has been made in identifying the genetic basis of limb development. The finding that the same molecules are involved in developing embryos of both model organisms and humans has promoted an increasing convergence between developmental biology and clinical genetics.
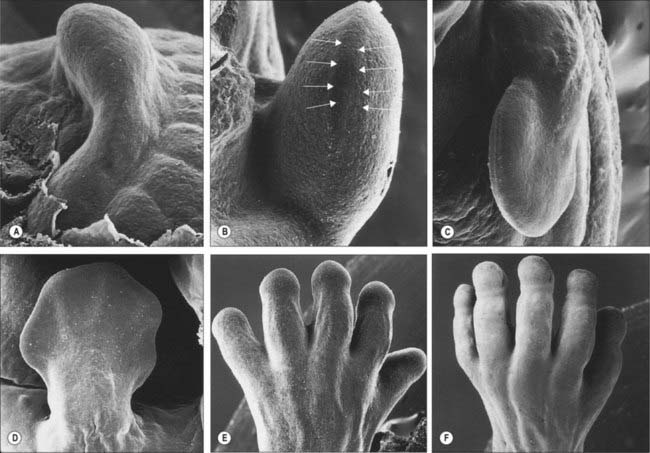
The limbs develop from somatopleuric mesenchyme in the lateral body wall. Regions of the somatopleuric mesenchyme at specific positions along the main body axis proliferate extensively to give rise to limb buds. These buds are the first visible signs of limb development and are rimmed by a longitudinal ridge of high columnar epithelial cells, the apical ectodermal ridge (Fig. 51.1).
AXES OF LIMBS
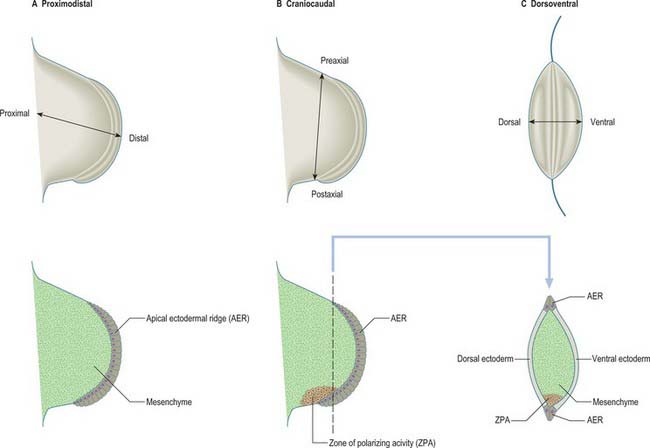
For descriptive, experimental and conceptual purposes, it has been necessary to define and name various ‘axes’, borders, surfaces and lines in relation to the developing limb bud (Fig. 51.2). An imaginary line from the centre of the elliptical base of the bud, through the centre of its mesenchymal core, to the centre of the apical ectodermal ridge, defines the proximodistal axis of the limb bud (previously known in descriptive embryology simply as the axis). Named in relation to the latter, the cranially placed limb border is the preaxial border, and the caudally placed limb border is the postaxial border. (In tetrapods and birds, the last two are termed anterior and posterior borders, respectively.) Any line that passes through the limb bud from preaxial to postaxial border, orthogonal to the proximodistal axis, constitutes a craniocaudal axis. The dorsal and ventral ectodermal surfaces thus clothe their respective aspects from preaxial to postaxial borders, and any line that passes from dorsal to ventral aspect, orthogonal to both proximodistal and craniocaudal axes, constitutes a dorsoventral axis. It should be noted here that the terms dorsal and ventral axial lines are to be used exclusively in relation to developing and definitive patterns of cutaneous innervation of the limbs and their associated levels of the trunk.
The three developmental axes (proximodistal, craniocaudal, and dorsoventral) can be identified in the developing limb bud by stage 13. Experiments in chick embryos have shown that development of structures in relation to each of these three principal axes seem to be specified by different mechanisms (Fig. 51.2). Outgrowth along the proximodistal axis is controlled by the apical ectodermal ridge and subjacent somatopleuric mesenchyme; outgrowth of the limb bud is accompanied by the sequential formation of limb structures along the proximodistal axis. The craniocaudal axis is controlled by a small population of mesenchymal cells on the postaxial border of the limb bud; this region of mesenchyme is termed the zone of polarizing activity or polarizing region. The dorsoventral axis of the limb appears to be controlled by the surface ectodermal covering of the limb bud.
APICAL ECTODERMAL RIDGE
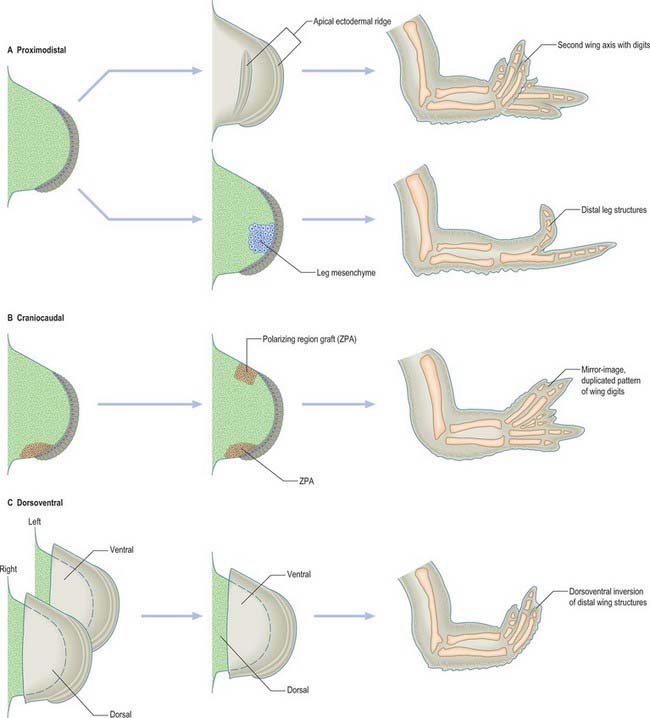
The fundamental epithelial–mesenchymal interactions seen in limb development are discussed in detail in Hinchcliffe & Johnson (1980) and the more recently discovered genetic basis of these interactions in Ferretti & Tickle (2006). The knowledge of limb embryology gained from studying chicks may be summarized as follows (Fig. 51.3). The apical ectodermal ridge provides the orientating influence for limb bud outgrowth. Its removal results in cessation of limb development, whereas grafting a second apical ectodermal ridge results in two axes of development, and duplication of distal structures (Fig. 51.3). There is evidence that the limb mesenchyme beneath the apical ectodermal ridge provides an apical ectodermal ridge maintenance factor that is essential to the function of the ridge. Thus, when leg bud cells are grafted to the tip of a wing bud beneath the apical ectodermal ridge, the ridge is maintained and bud outgrowth continues (it should be noted that the leg cells, even though placed in a wing bud, still form distal leg structures) (Fig. 51.3). In addition, the leg mesenchyme will pass information to the local ectoderm eliciting the appropriate epidermal development, which in this case is the formation of scales rather than feathers.
ZONE OF POLARIZING ACTIVITY
The zone of polarizing activity is a small region of somatopleuric mesenchyme on the postaxial border of the limb. When a second zone of polarizing activity is grafted beneath or adjacent to the apical ectodermal ridge at the preaxial border of a chick wing bud, duplication of distal limb structures occurs, generating a mirror-image pattern of six digits (Fig. 51.3). The digit closest to the zone of polarizing activity is always the most postaxial digit (in the chick wing this is digit 4), while more preaxial digits develop further away from the zone of polarizing activity. Thus signalling from the zone of polarizing activity appears to control both digit number and digit pattern. It has been suggested that digit number is controlled by regulation of the production of the apical ectodermal ridge maintenance factor, while digit pattern is regulated by production of a diffusible morphogen that acts in a concentration dependent fashion.
ECTODERMAL INTERACTION
It is possible to remove the mesenchymal core of an early chick limb bud leaving the surface ectodermal epithelium. When the ectodermal epithelium from a left limb bud is recombined with the mesenchymal core from a right limb bud, keeping the cranio-caudal axis in register, the distal part of the limb that subsequently develops conforms to the polarity of the ectoderm rather than that of the mesenchyme (Fig. 51.3). This can be seen in the pattern of muscles, orientation of the joints and eventual characteristic differentiation of the epidermis. Thus signals from the ectoderm control the pattern along the dorso-ventral axis.
MOLECULAR BASIS OF CELL–CELL INTERACTIONS
The apical ectodermal ridge secretes fibroblast growth factors that act on the underlying mesenchyme and maintain the zone of polarizing activity. The latter expresses Sonic hedgehog (Shh), a gene encoding another class of secreted protein that mediates cell–cell signalling. Sonic Hedgehog protein acts on adjacent mesenchyme cells and maintains the expression of the gene encoding Gremlin (an extracellular bone morphogenetic protein antagonist), which functions as an apical ectodermal ridge maintenance factor. Sonic hedgehog also plays a pivotal role in digit patterning: it diffuses across the limb bud and responding cells transduce the signal through the Gli family of transcription factors. (The Gli proteins are bifunctional effectors of Shh signalling and can lead to either activation or repression of expression of gene targets.) Dorsal limb ectoderm expresses Wnt7a, which acts as a dorsalizing signal.
SKELETAL ELEMENTS OF THE LIMB
It has been suggested that formation of the cartilaginous elements of a limb is related to the shape of the limb and the conditions necessary for chondrogenesis. An antichondrogenic zone beneath the ectoderm of a developing limb prevents chondrogenesis within the dermis and myogenic zones. Foci of chondrogenesis occur in the centre of the limb bud where cell density is greatest. One centre of chondrogenesis forms in the proximal region of an early limb bud. As the limb bud lengthens it also widens, forming next two centres of chondrogenesis and five centres that appear further distally. The Sox9 transcription factor is essential for chondrocyte differentiation: expression of the Sox9 gene is coincident with expression of the gene that encodes collagen type II, the collagen characteristic of cartilage extracellular matrix. Sox9 gene transcripts are expressed in the humeral and forearm skeletal elements of human embryos at approximately 44 days, and in carpals, metacarpals and phalanges, in addition to more proximal elements, at approximately 52 days (Fig. 51.4).
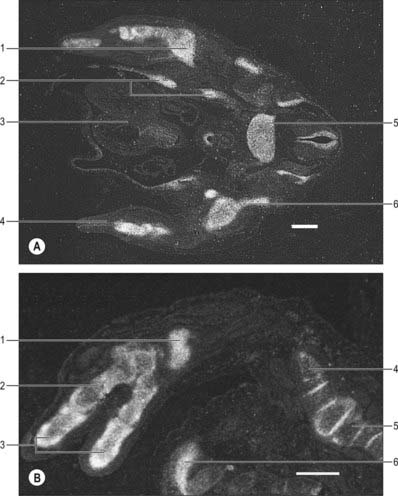
(The human tissue was provided by the joint MRC-Wellcome Human Developmental Biology Resource (http://www.hdbr.org) at the Institute of Human Genetics, Newcastle upon Tyne, UK. By courtesy of Dr Susan Lindsay, Institute of Human Genetics, University of Newcastle upon Tyne, UK.)
For further details describing the development of cartilage see page 81, and the development of bone see page 92. For further details about the development of specific limb bones see the appropriate regional chapter.
DEVELOPMENT OF JOINTS
In fibrous joints, the interzone is converted into collagen, which is the definitive medium connecting the bones involved. In synchondroses it becomes (growth) cartilage of the modified hyaline type, and in symphyses it is predominantly fibrocartilage. The interzonal mesenchyme of developing synovial joints becomes trilaminar when a more tenuous intermediate zone appears splitting the mesenchyme into two dense strata. As the skeletal elements chondrify, and in part ossify, the dense strata of the interzonal mesenchyme also become cartilaginous; subsequent cavitation of the intermediate zone establishes the cavity of the joint. The loose mesenchyme around the cavity forms the synovial membrane and probably also gives rise to all other intra-articular structures, such as tendons, ligaments, discs and menisci. In joints containing discs or menisci, and in compound articulations, more than one cavity may appear initially, sometimes merging later into a complex single cavity. As development proceeds, thickenings in the fibrous capsule can be recognized as the specializations peculiar to a particular joint. (In some joints, such accessions to the fibrous capsule are derived from neighbouring tendons, muscles or cartilaginous elements.) Although the initial stages in the process of cavitation of joints is independent of movements, a true joint cavity can form only in the presence of movements (Pitsillides 2006). The literature suggests that all musculoskeletal elements are in their appropriate positions by 10 weeks. For detailed accounts of the process, consult O’Rahilly & Gardner (1975) and Uhthoff (1990).
Musculature of the limb
It is now well established that all limb myogenic precursor cells originate from the somites. These cells are committed at an early stage and can be identified in the lateral halves of the somites. After the mesenchymal sclerotome cells have migrated from the epithelial somite, the remaining dorsolateral portion is termed the epithelial plate or dermomyotome of the somite (Fig. 44.3). Cells from the dorsomedial edge of the dermomyotomes form the axial musculature, whereas at limb levels cells de-epithelialize and migrate from the ventrolateral edges of the dermomyotome into the limb bud. These precursor limb myoblasts migrate through a non-random, structured network of extracellular fibrils. At their leading ends the migrating cells exhibit filopodia which are in contact with the extracellular fibrils or with other cells: it is believed that the orientation of the extracellular fibrils may direct the migration of the cells. The precursor muscle cells do not differentiate into muscle before their migration into the limb, probably in response to inhibitory signals produced by the somatopleuric mesenchyme.
EMBRYONIC MOVEMENTS
Embryonic movements are vital for development of the musculoskeletal system (reviewed by Pitsillides, 2006). They have effects on the developing muscle, and are necessary to align trabeculae within bones, and to ensure the correct attachments of the tendons and the appropriate coiling of the constituent collagen fibres within the tendons. Simple movements of an extremity have been observed sporadically as early as the seventh week of gestation. Combined movements of limb, trunk and head commence between 12 and 16 weeks of gestation in human embryos. Movements of the embryo and fetus encourage normal skin growth and flexibility, in addition to the progressive maturation of the musculoskeletal system.
VASCULATURE OF THE LIMB
ARTERIES
The early limb bud receives blood via intersegmental arteries that contribute to a primitive capillary plexus. At the tip of the limb bud, there is a terminal plexus which is constantly renewed in a distal direction as the limb grows (see Figs 52.1, 85.1). Later, one main vessel, the axial artery, supplies the limb and the terminal plexus.
Ferretti P, Tickle C. The limbs. In: Ferretti P, Copp A, Tickle C, Moore G. Embryos. Genes and Birth Defects. 2nd edn. Chichester: Wiley; 2006:123-166.
Presents the most recently identified genes involved in the control of limb development..
Hinchcliffe JR, Johnson DR. The Development of the Vertebrate Limb. In: An Approach through Experiment, Genetics and Evolution. Oxford: Clarendon Press; 1980.
Presents the classic experiments on limb development..
Lane EB, Tickle C. How to make a hand: a 3 day symposium held at the University of Dundee. J Anat. 202(1), 2003.
Includes reviews on limb development and human limb malformations..
O’Rahilly R, Gardner E. The timing and sequence of events in the development of the limbs in the human embryo. Anat Embryol. 1975;148:1-23.
Pitsillides AA. Early effects of embryonic movement: ‘a shot out of the dark’. J Anat. 2006;208:417-431.
Uhthoff HK. The Embryology of the Human Locomotor System. Berlin: Springer-Verlag, 1990.