CHAPTER 1 Detection of Breast Cancer: Screening of Asymptomatic Patients
Breast cancer is the most common malignancy and the second leading cause of cancer deaths among American women. In 2005, it is estimated that more than 211,000 new cases will be diagnosed, and more than 40,000 women will die of the disease.1 Breast carcinoma mortality in the United States has declined substantially over the past 30 years, from 31.4 deaths per 100,000 women per year in 1975 to 25.9 deaths per 100,000 women per year in 2001.2 More recent analysis by the CDC of 1999–2003 data from the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) study and the CDC National Program of Cancer Registries (NPCR) indicated that age-adjusted incidence rates for invasive breast cancer decreased each year from 1999 to 2003, with the greatest decrease (6.1%) occurring from 2002 to 2003. For in situ cancers, rates increased each year from 1999 to 2002 and then decreased from 2002 to 2003, although the percentage decrease (2.7%) was smaller than that for invasive cancers (6.1%). In addition to advances in treatment options, the combination of increasing utilization of screening mammography and improved mammographic quality, allowing detection of cancers at an earlier stage, is likely to account for the reduction in breast cancer mortality.3–4
SCREENING MAMMOGRAPHY
To date, the benefits from screening mammography for women 40 to 70 years of age have been proven in eight randomized controlled trials (RCTs) conducted in Europe and the United States during the past 40 years.5–11 Reported reductions in breast cancer mortality range from 20% to 45%. Because of the relatively small numbers of women aged 40 to 49 years in the individual trials, the benefit from screening in this age range has been controversial. However, in 1997, a meta-analysis of women aged 40 to 49 years in all five Swedish trials found a 30% reduction in breast cancer deaths.12 In addition, long-term follow-up of three trials (Health Insurance Plan Project [HIP], Gothenburg, and Malmö) each found statistically significant reductions in breast cancer mortality.13–15 Therefore, for average risk women, most professional organizations in the United States recommend screening mammography beginning at the age of 40 years. For screening women younger than the age of 40 years, there are few RCT data. Therefore, decisions concerning screening practice in this age group must be based on less rigorous evidence and on a more individual basis. Given that younger women have a longer life expectancy and that cancers tend to grow more rapidly in younger women, earlier detection of these cancers may theoretically be advantageous. However, this must be balanced by the limitations of screening mammography in younger women, which include a lower frequency of breast cancer, reduced sensitivity of mammography, slightly increased radiation risk, and higher recall rates. Screening of younger women will be of most benefit in women at high risk, especially those known to carry the BRCA1 or BRCA2 gene mutation. Methods for estimating risk based on medical and family history include the Gail, Claus, and BRCAPRO mathematical models. Observational studies of high-risk women aged 30 to 39 years show a cancer detection rate similar to that for women aged 40 to 49 years.16–17 In general, screening of women aged less than 40 years is restricted to high-risk subgroups (women with a 20% lifetime breast cancer risk at or before the age of 30 years or breast cancer risk at a given age equivalent to that of the average woman at the age of 40 years). These include BRCA1 or BRCA2 gene mutation carriers, women with a personal history of breast cancer, women with a prior diagnosis of atypical ductal or lobular hyperplasia, women with previous radiation therapy to the chest before the age of 30 years, and women with a strong family history of breast cancer (usually involving one or more first-degree relatives with premenopausal breast cancer or breast cancer before the age of 50 years).
LIMITATIONS OF MAMMOGRAPHY
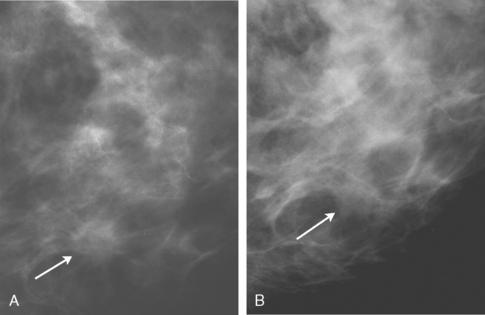
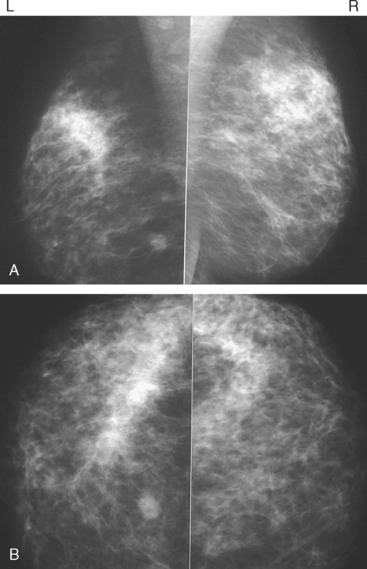
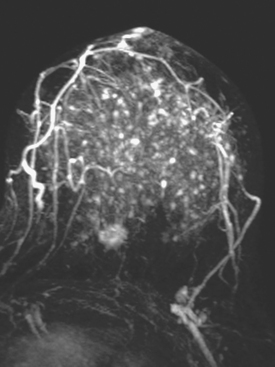
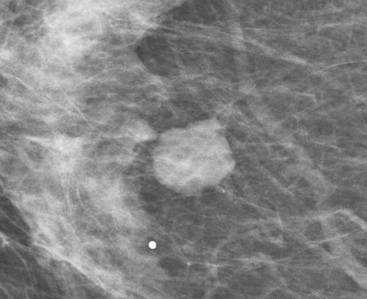
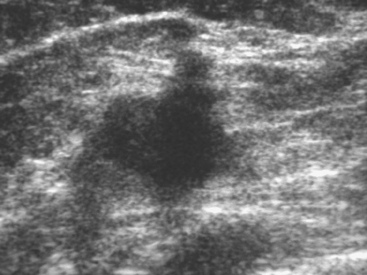
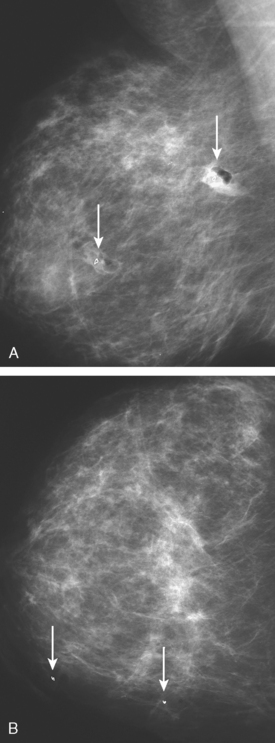
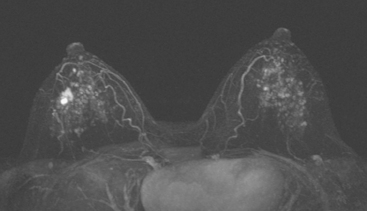
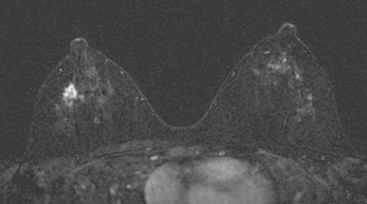
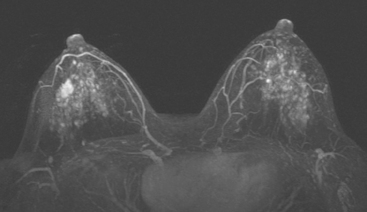
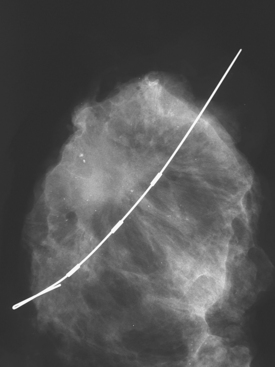
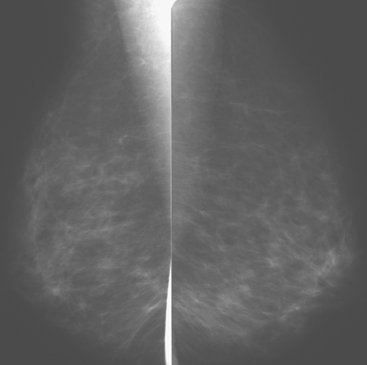
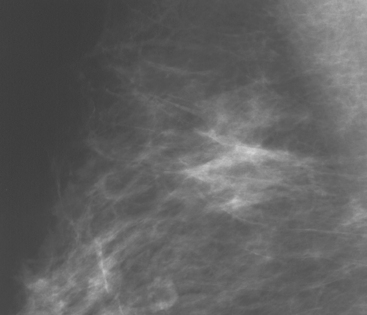
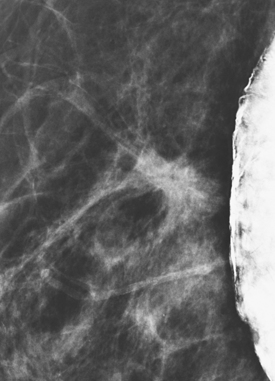
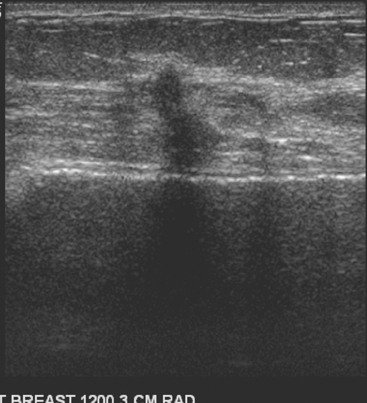
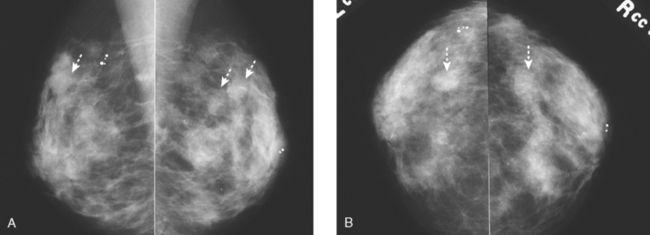
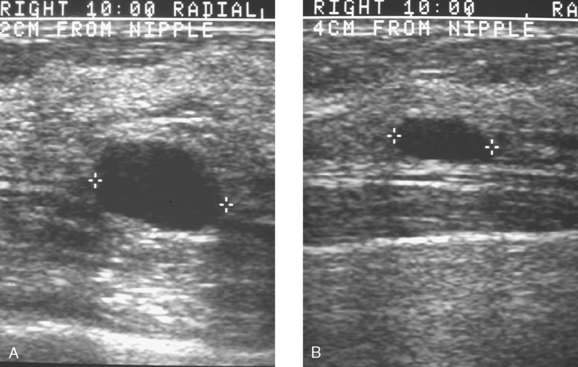
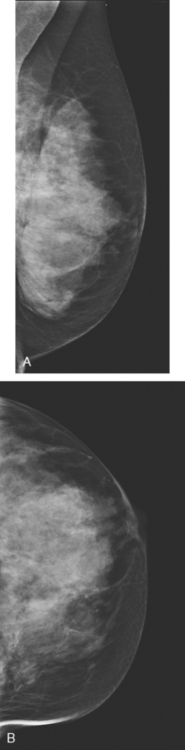
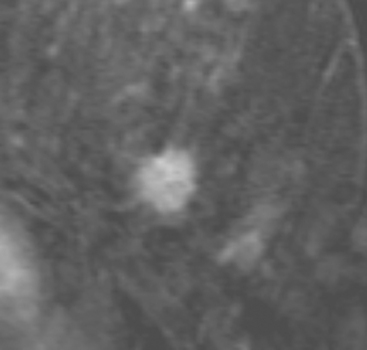
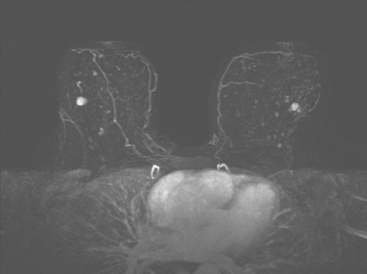
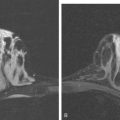
Although there have been significant improvements in mammographic technique over the past 50 years, fundamental limitations remain. These include the low inherent contrast differences between tissue structures in the breast and the fact that mammographic detection of breast cancer (sensitivity) relies on the ability to visualize cancer through the background of overlying normal tissue (Figure 1). Mammographic specificity relies on the ability to distinguish benign from malignant breast lesions based on their margins and morphologic features.18–19 However, malignant and benign lesions may have similar appearances, thereby reducing specificity. Data from the Breast Cancer Surveillance Consortium demonstrated that on average, screening mammography programs had a callback rate of 6.4% to 13.3% and a positive predictive value of 3.4% to 6.2%.20 The mean cancer detection rate was 4.7 per 1000, and the mean size of invasive cancers was 13 mm. The main mammographic signs of breast cancer include clustered microcalcifications, masses, architectural distortions, and asymmetrical densities. Comparison to prior mammograms is essential because some cancers may only be detected by perceiving them as a subtle change from prior studies. In addition, the availability of prior mammograms for comparison can reduce the number of unnecessary callbacks for stable benign findings.21
DIGITAL MAMMOGRAPHY AND COMPUTER-AIDED DETECTION
Screen-film mammography (SFM) has been the standard method used for breast cancer screening since the end of xeromammography some 20 years ago. Advances in screen-film technology and film-processing techniques have contributed to major improvements in the quality of mammographic images. In addition to high contrast, the strength of SFM lies in its extremely high spatial resolution, often greater than 10 line pairs per millimeter (lp/mm). This allows the detection of exceedingly small clusters of microcalcifications, one of the earliest signs of breast cancer. The limitations of SFM include the detection of subtle soft tissue lesions, especially in the presence of dense glandular tissues, and the fact that the film serves simultaneously as the image receptor, display medium, and long-term storage medium. Recent technologic advances have led to the development of full-field digital mammography (FFDM). One of the initial concerns of FFDM is its inherent lower spatial resolution of 5 to 10 lp/mm. However, several studies have demonstrated that despite the limited spatial resolution, the visibility of calcifications on FFDM is not significantly different from that on SFM.22–24 The higher contrast resolution of FFDM may account for its comparable detection rates. In a recent multicenter trial of 49,528 women, Pisano and colleagues24 reported that the overall diagnostic accuracy of FFDM was comparable to SFM as a means of screening for breast cancer. The study also found that digital mammography was more accurate in women younger than 50 years, women with radiographically dense breasts, and premenopausal or perimenopausal women.
CAD programs were developed to assist a radiologist in the interpretation of screening mammograms, so that cancer detection rates could be improved. These programs rely on neural networks to analyze the images and highlight potentially suspicious findings that may have been overlooked by the radiologist. Several studies have shown improved cancer detection by radiologists using CAD versus radiologists alone, without significantly increasing callback rates.24–27 However, other studies have shown less promising results.28–29 A recent study by Fenton and colleagues29 reported that CAD decreased the accuracy of screening mammogram interpretation. This was not due to a decrease in cancer detection, but rather to an increase in false-positive results. To be effective, CAD programs should not increase callback rates and should not significantly prolong interpretation times for screening mammograms. It is important to remember that CAD programs are tools that must be used in an appropriate manner. They should not be used to override a suspicious finding detected by a radiologist. In general, these systems tend to be more helpful for low-volume or inexperienced readers.
MAGNETIC RESONANCE IMAGING
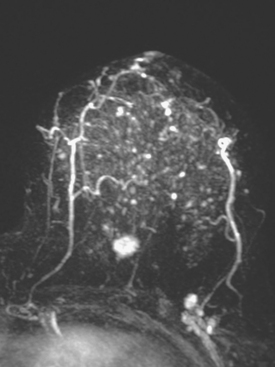
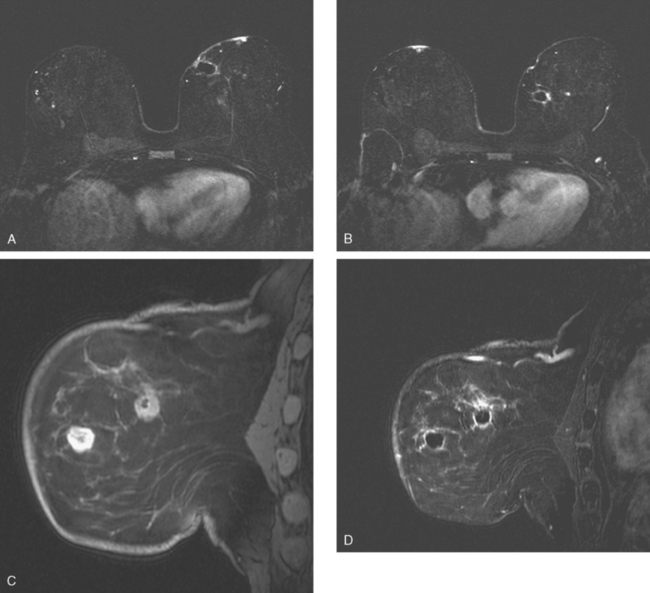
Dynamic, contrast-enhanced MRI of the breast has been shown to be extremely sensitive in the detection of invasive breast cancer and is not limited by the density of the breast tissue. However, because the reported sensitivity of MRI for ductal carcinoma in situ (DCIS) ranges between 45% and 100%, MRI is currently not recommended as a replacement for mammography.30 A more recent single institution study suggests that MRI may have a higher sensitivity than mammography for DCIS than previously thought, particularly for high-grade DCIS.31 The use of MRI in the general population has been limited by its moderate specificity. Therefore, its use has been focused on studying patients in whom the yield from MRI is likely to be higher. Multiple studies have shown that MRI is a useful tool as an adjuvant to screening mammography in women at high risk for breast cancer.32–34 The American Cancer Society (ACS) recommends annual screening MRI for women with a 20% to 25% lifetime risk for breast cancer.35 This includes women with the BRCA1 or BRCA2 breast cancer genes, as well as women with multiple family members with breast or ovarian cancer, and women who have undergone mediastinal irradiation for Hodgkin’s disease. Women whose benefit from screening MRI was considered questionable by the ACS because of insufficient data included women with a personal history of breast cancer, prior biopsy yielding atypia, or extremely dense breasts on mammography. The decision to perform screening MRI in these women should be made on a case-by-case basis. Several models may be used to calculate lifetime risk for breast cancer, including the Gail, Claus, and Tyrer-Cusick models.
ULTRASOUND
Early studies of breast ultrasound for cancer screening were disappointing because of its poor detection of small cancers and excessively high false-positive rates.36–40 With the advent in the early 1990s of technical improvements in ultrasound, including improved spatial and contrast resolution utilizing higher-megahertz (MHz) transducers, the potential of whole-breast ultrasound as a screening tool has been revisited. Several recent singleinstitution studies have demonstrated a prevalence detection rate of 3 to 4 mammographically occult cancers per 1000 women screened.41–50 However, the biopsy positive predictive values were less than 20%, lower than accepted for mammography.51 These initial studies have focused mainly on women with mammographically higher-density breasts. Screening ultrasound appears to be more sensitive in detecting early invasive cancer, whereas mammography is more sensitive in the detection of DCIS. Of the invasive cancers, ultrasound found a higher percentage of invasive lobular carcinomas than that usually found on mammography. Therefore, whole-breast ultrasound should supplement mammographic screening, rather than replace it.
The initial studies were performed mostly by radiologists with a high ultrasound skill level and in some studies were not blinded to the mammographic findings. Therefore, the initial results of screening ultrasound may not extrapolate to its use in general clinical practice.52 Other concerns include the lack of standardized exam techniques, interpretation criteria, false-positive results, and unnecessary biopsies. In addition, most of the initial studies on whole-breast ultrasound have evaluated prevalence (initial) detection rates, rather than incidence (subsequent) detection rates. The benefit from subsequent yearly screening ultrasound is uncertain, but likely to be less. The American College of Radiology Imaging Network (ACRIN) is currently conducting a randomized multicenter trial evaluating whole-breast bilateral screening ultrasound in high-risk, asymptomatic women with dense breasts. The study will evaluate both prevalence (year 1) and incidence (years 2 and 3) screening ultrasound detection rates as compared with mammography. Interpretation of the examinations will be performed by radiologists trained in mammographic and ultrasound interpretation, using standardized interpretive criteria.
1 Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30.
2 Ries LAG, Eisner MP, Kosary CL, et al. SEER cancer statistics review, 1975–2001. Bethesda, MD: National Cancer Institute, 2004.
3 Elkin EB, Hudis C, Begg CB, Schrag D. The effect of changes in tumor size on breast carcinoma survival in the U.S.: 1975–1999. Cancer. 2005;104(6):1149-1157.
4 Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
5 Smith RA, Duffy SW, Gabe R, et al. The random-ized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793-806. v.
6 Shapiro S, Venet W, Strax P, Venet L. Periodic screening for breast cancer: the Health Insurance Plan Project and its Sequelae, 1963–1986. Baltimore: Johns Hopkins University Press, 1988.
7 Tabar L, Vitak B, Chen HH, et al. The Swedish Two-County Trial twenty years later. Radiol Clin North Am. 2000;38:625-652.
8 Alexander FE, Anderson TJ, Brown HK, et al. 14 years of follow-up from Edinburgh randomized trial of breast cancer screening. Lancet. 1999;353:1903-1908.
9 Andersson I, Aspegren K, Janzon L, et al. Mammographic screening and mortality from breast cancer: the Malmo mammographic screening trial. BMJ. 1988;297:943-948.
10 Frisell J, Lidbrink E, Hellstrom L, Rutqvist LE. Follow-up after 11 years: update of mortality results in the Stockholm mammographic screening trial. Breast Cancer Res Treat. 1997;45:263-270.
11 Bjurstam N, Bjorneld L, Duffy SW. The Gothenburg Breast Screening Trial: first results on mortality, incidence, and mode of detection for women ages 39–49 years at randomization. Cancer. 1997;80:2091-2099.
12 Hendrick RE, Smith RA, Rutledge JH3rd, Smart CR. Benefit of screening mammography in women aged 40–49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87-92.
13 Chu KC, Smart CR, Tarone RE. Analysis of breast cancer mortality and stage distribution by age for the Health Insurance Plan clinical trial. J Natl Cancer Inst. 1988;80:1125-1132.
14 Bjurstam N, Bjorneld L, Warwick J, et al. The Gothenburg Breast Screening Trial. Cancer. 2003;97:2387-2396.
15 Andersson I, Janzon L. Reduced breast cancer mortality in women under 50: updated results from the Malmo Mammographic Screening Program. J Natl Cancer Inst Monogr. 1997;22:63-68.
16 Liberman L, Dershaw DD, Deutch BM, et al. Screening mammography: value in women 35–39 years old. AJR Am J Roentgenol. 1993;161:53-56.
17 Curpen BN, Sickles EA, Sollitto RA, et al. The comparative value of mammographic screening for women 40–49 years old versus women 50–64 years old. AJR Am J Roentgenol. 1995;164:1099-1103.
18 Sickles EA. Breast masses: mammographic evaluation. Radiology. 1989;173(2):297-303. Review
19 Sickles EA. Mammographic features of malignancy found during screening. Recent Results Cancer Res. 1990;119:88-93. Review
20 Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology. 2006;241:55-66.
21 Frankel SD, Sickles EA, Curpen BN, et al. Initial versus subsequent screening mammography: comparison of findings and their prognostic significance. AJR Am J Roentgenol. 1995;164:1107-1109.
22 Fischer U, Baum F, Obenauer S, et al. Comparative study in patients with microcalcifications: full-field digital mammography vs screen-film mammography. Eur Radiol. 2002;12:2679-2683.
23 Lewin JM, Hendrick RE, D’Orsi CJ, et al. Comparison of full-field digital mammography to screen-film mammography for cancer detection: results of 4945 paired examinations. Radiology. 2001;218:873-880.
24 Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773-1783.
25 Freer TW, Ulissey MJ. Screening mammography with computer-aided detection: prospective study of 12,860 patients in a community breast center. Radiology. 2001;220(3):781-786.
26 Birdwell RL, Bandodkar P, Ikeda DM. Computer-aided detection with screening mammography in a university hospital setting. Radiology. 2005;236(2):451-457.
27 Morton MJ, Whaley DH, Brandt KR, Amrami KK. Screening mammograms: interpretation with computer-aided detection—prospective evaluation. Radiology. 2006;239(2):375-383.
28 Gur D, Sumkin JH, Rockette HE, et al. Changes in breast cancer detection and mammography recall rates after the introduction of a computer-aided detection system. J Natl Cancer Inst. 2004;96(3):185-190.
29 Fenton JJ, Taplin SH, Carney PA, et al. Influence of computer-aided detection on performance of screening mammography. N Engl J Med. 2007;356(14):1399-1409.
30 Bazzocchi M, Zuiani C, Panizza P, et al. Contrast-enhanced breast MRI in patients with suspicious microcalcifications on mammography: results of a multicenter trial. AJR Am J Roentgenol. 2006;186:1723-1732.
31 Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370(9586):485-492.
32 Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427-437.
33 Lehman CD, Blume JD, Weatherall P, et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103:1898-1905.
34 Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469-8476.
35 Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75-89.
36 Cole-Beuglet C, Goldberg BB, Kurtz AB, et al. Clinical experience with a prototype realtime dedicated breast scanner. AJR Am J Roentgenol. 1982;139:905-911.
37 Sickles EA, Filly RA, Callen PW. Breast cancer detection with sonography and mammography: comparison using state-of-the-art equipment. AJR Am J Roentgenol. 1983;140:843-845.
38 Egan RL, Egan KL. Automated water-path full-breast sonography: correlation with histology of 176 solid lesions. AJR Am J Roentgenol. 1984;143:499-507.
39 Egan RL, McSweeney MB, Murphy FB. Breast sonography and the detection of breast cancer. Recent Results Cancer Res. 1984;90:90-100.
40 Kopans DB, Meyer JE, Lindfors KK. Whole-breast US imaging: four-year follow-up. Radiology. 1985;157:505-507.
41 Gordon PB, Goldenberg SL. Malignant breast masses detected only by ultrasound. Cancer. 1995;76:626-630.
42 Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: detection with screening US—diagnostic yield and tumor characteristics. Radiology. 1998;207:191-199.
43 Buchberger W, DeKoekkoek-Doll P, Springer P, et al. Incidental findings on sonography of the breast: clinical significance and diagnostic workup. AJR Am J Roentgenol. 1999;173:921-927.
44 Buchberger W, Niehoff A, Obrist A, et al. Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR. 2000;21:325-336.
45 Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology. 2001;221:641-649.
46 Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175.
47 Leconte I, Feger C, Galant C, et al. Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol. 2003;180:1675-1679.
48 Crystal P, Strano S, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol. 2003;181:177-182.
49 Cortesi L, Turchetti D, Marchi I, et al. Breast cancer screening in women at increased risk according to different family histories: an update of the Modena Study Group experience. BMC Cancer. 2006;6:210.
50 Corsetti V, Ferrari A, Ghirardi M, et al. Role of ultrasonography in detecting mammographically occult breast carcinoma in women with dense breasts. Radiol Med (Torino). 2006;111(3):440-448.
51 Quality Determinants of Mammography Guideline Panel. Quality determinants of mammography. AHCPR Publication no. 95–0632. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, 1994.
52 Berg WA, Blume JD, Cormack JB, Mendelson EB. Operator dependence of physician-performed whole-breast US: lesion detection and characterization. Radiology. 2006;241(2):355-365.
CASE 1 Breast cancer presenting as a small new mass on mammography
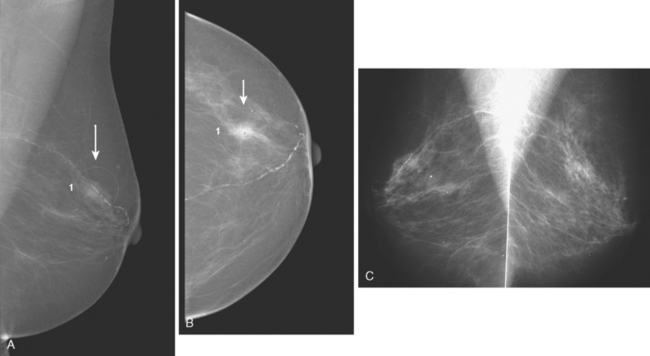
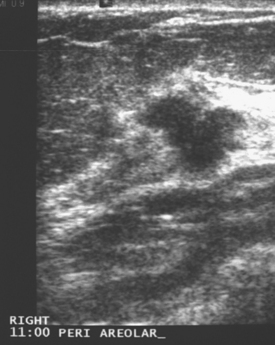
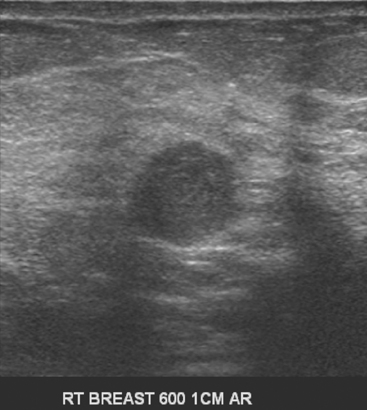
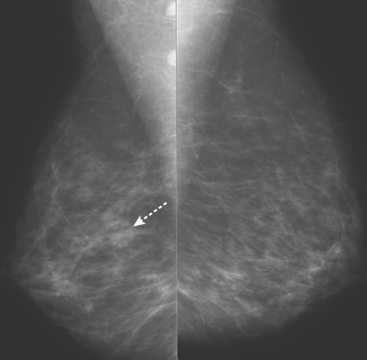
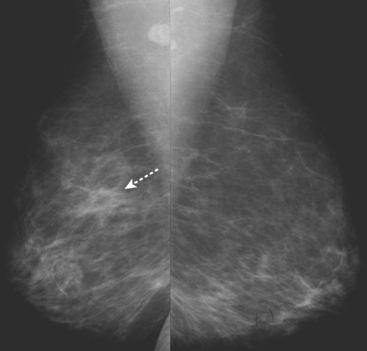
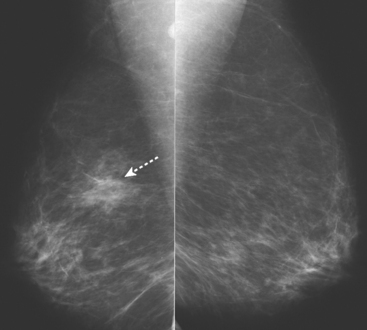
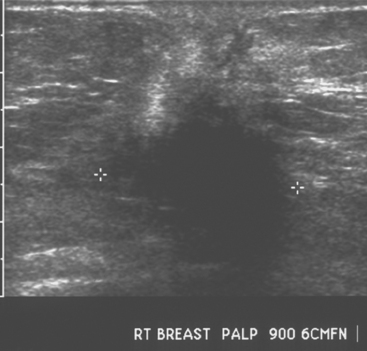
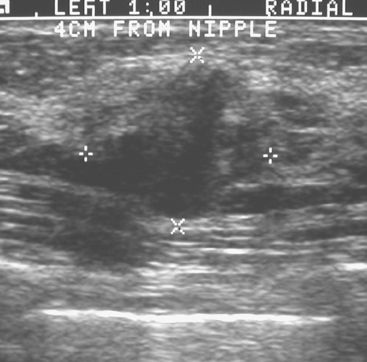
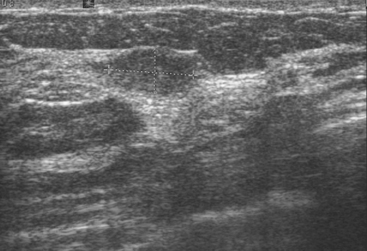
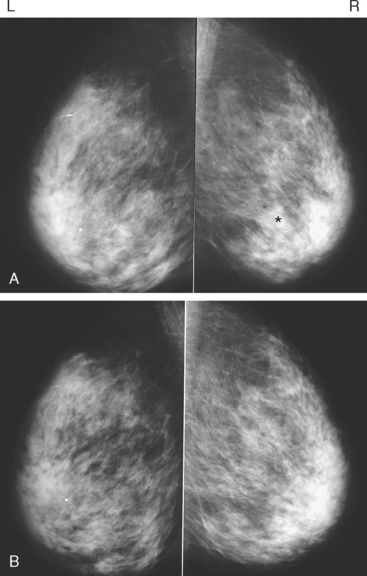
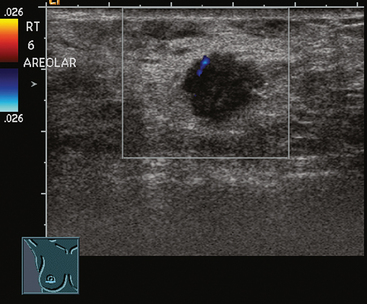
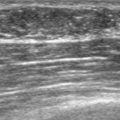
An asymptomatic 57-year-old woman underwent screening mammography, which showed a new finding of a 6-mm round mass in the anterior upper outer quadrant (UOQ) of the left breast (Figures 1 and 2). The patient had undergone a prior benign left breast needle biopsy with placement of a marker clip in the posterior upper outer left breast. Ultrasound confirmed the presence of a somewhat ill-defined 6-mm solid mass (Figure 3). Ultrasound-guided core needle biopsy yielded a diagnosis of low-grade ductal carcinoma in situ.
CASE 2 Breast cancer presenting as a new mass on mammography
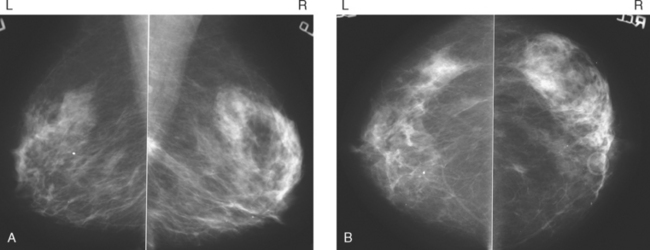
A new left lower inner quadrant mass with ill-defined margins was identified on a screening mammogram performed on an asymptomatic 67-year-old woman (Figure 1). Sonography confirmed a suspicious, 1 cm, irregularly marginated, hypoechoic, solid mass, which was taller than wide (Figure 2). A mammographically questioned second abnormality, architectural distortion in the left upper breast, had no sonographic confirmation. MRI was recommended to further assess the question of possible multicentric disease. The suspected cancer mass was highly suspicious by MRI criteria, showing irregular margination (Figures 3 and 4) and a washout pattern of enhancement (Figure 5). No correlate was found on MRI for the questioned left upper architectural distortion. There was no evidence of multicentricity by MRI.
CASE 3 Small growing breast cancer presenting as a contour change on mammography
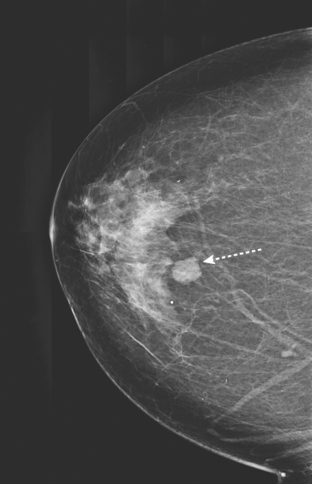
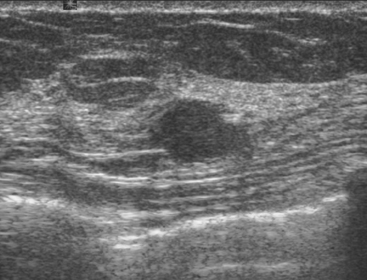
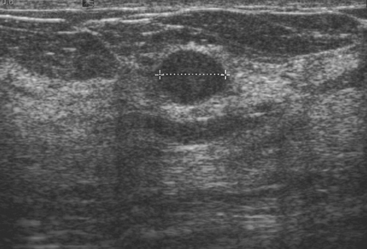
Routine digital screening mammography in an asymptomatic 62-year-old woman suggested an interval change compared with prior years’ studies, with a 1-cm upper outer quadrant mass suggested (Figure 1). This persisted on spot compression, and ultrasound identified a corresponding suspicious 9-mm hypoechoic, solid mass, with irregular margins (Figure 2).
CASE 4 Slow-growing microlobulated colloid carcinoma
An asymptomatic 65-year-old female underwent screening mammography, which showed an enlarging circumscribed mass in the central right breast (Figures 1 and 2). Ultrasound demonstrated a corresponding hypoechoic solid mass (Figure 3). Subsequent ultrasound-guided core needle biopsy was performed and yielded a diagnosis of invasive colloid carcinoma.
TEACHING POINTS
This case illustrates the importance of prior mammograms in the evaluation of subtle change. A new or enlarging mass should prompt further workup. In this case, because this is an enlarging mammographic mass, the workup can proceed directly to ultrasound. Evaluation of the margins of the mass on magnification mammographic views would not change clinical management. Any new or enlarging solid mass should prompt a biopsy, regardless of its appearance mammographically. The role of ultrasound in this instance is to determine whether the finding represents a cyst or solid mass. In this case, the mass was solid and demonstrated a relatively benign appearance. Some cancers, including colloid carcinoma, papillary carcinoma, and medullary carcinoma, may have relatively smooth margins.
CASE 5 Breast cancer presenting as a new posterior mass on mammography: Importance of inclusion of posterior breast tissue on mammography
A 70-year-old woman with a prior history of stage II ovarian carcinoma was noted on a routine screening mammogram to have a partially visualized, asymmetric density in the posterior right breast, seen only on the MLO view (Figure 1). She was recalled for additional evaluation. On spot compression, a mass was confirmed, with ill-defined margins (Figure 2). The mass was sufficiently far posterior that it was difficult to visualize entirely by mammography. Ultrasound showed highly suspicious characteristics, including being taller than wide, with irregular, angular margins (Figure 3). Ultrasound-guided core needle biopsy of the mass proved that the lesion was infiltrating mammary carcinoma. MRI confirmed that the lesion was solitary, without evidence of additional disease sites (Figure 4). Surgical therapy consisted of partial mastectomy and sentinel lymph node sampling. Final pathology showed a 1.8-cm infiltrating ductal carcinoma, with clear margins and one negative sentinel lymph node.
CASE 6 DCIS presenting as a microcalcification cluster
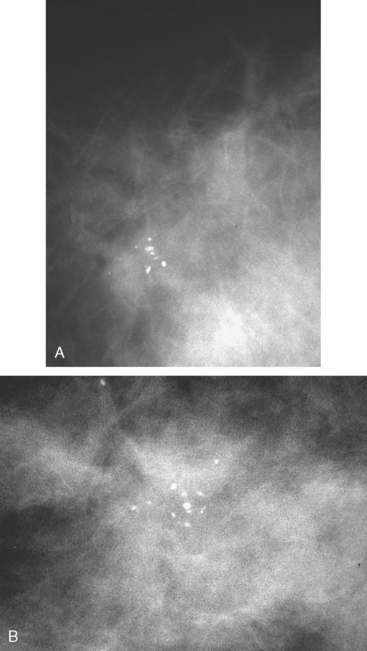
A new microcalcification cluster was identified on screening mammography in this 82-year-old woman. Magnification views showed pleomorphic forms (Figure 1), and stereotactic biopsy was recommended. Specimen radiography confirmed that the calcifications were sampled, and histology identified infiltrating ductal carcinoma and comedo ductal carcinoma in situ (DCIS). The patient opted for surgical therapy with mastectomy and was treated with tamoxifen for 5 years thereafter.
CASE 7 DCIS presenting as multiple microcalcification clusters along a ductal ray
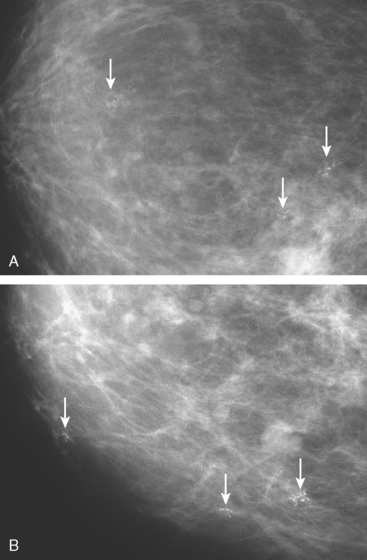
New microcalcification clusters were noted in the medial left breast on routine mammographic screening of a 61-year-old asymptomatic woman. The most recent comparison mammogram was 3 years earlier. A suspicious, segmental distribution of the microcalcifications was noted, and magnification was performed to better visualize them. Magnification views of the medial breast confirmed three separate, suspicious clusters of pleomorphic microcalcifications, aligned along an axis toward the nipple (Figure 1). Stereotactic biopsy was performed on two of the three clusters, including the most anterior and posterior (Figure 2). Intermediate- to high-grade ductal carcinoma in situ (DCIS), with necrosis, was obtained from both sites. Pathology commented that the DCIS obtained from the posterior site was variably associated with microcalcifications. Based on the distance between the sampled sites (4 cm), it seemed likely that there was extensive intraductal disease, suggesting the patient was not a suitable candidate for breast-conserving surgery. Breast MRI was performed to assess for additional, mammographically occult disease (Figure 3). No MRI findings particularly suggestive of unsuspected or occult invasive disease were seen.
The patient underwent mastectomy and sentinel lymph node sampling. The mastectomy specimen pathology showed residual foci of intermediate-grade cribriform DCIS with central necrosis, including four foci (ranging in size from < 1 mm to 4 mm) adjacent to the anterior biopsy site and one 2-mm residual focus adjacent to the posterior biopsy site. Four sentinel lymph nodes were negative for malignancy.
CASE 8 Breast cancer presenting as architectural distortion in extremely dense breasts
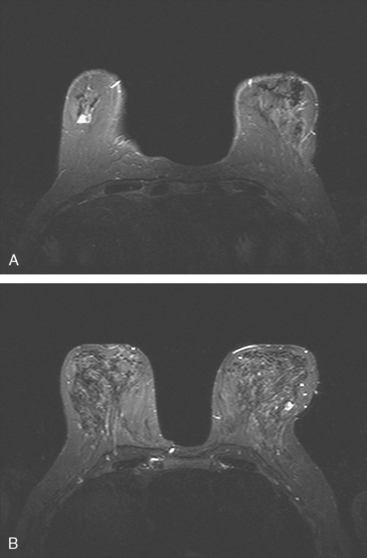
A 46-year-old woman with very dense breasts was called back for bilateral magnification views of microcalcifications noted on screening mammography. These proved to be widely scattered, with morphology consistent with milk of calcium. However, right upper outer quadrant (UOQ) increased breast density was noted with architectural distortion (Figure 1), and ultrasound was performed for further evaluation. Sonography showed a 1.2-cm hypoechoic, shadowing, irregularly marginated mass in the UOQ, corresponding to the mammogram (Figure 2). Ultrasound-guided core needle biopsy confirmed infiltrating ductal carcinoma (IDC). Because of the extreme density of the patient’s breast parenchyma, breast MRI was performed as an aid to presurgical staging. The known IDC was visualized as a spiculated, enhancing 1.5-cm mass in a background of diffuse fibrocystic enhancement, with innumerable scattered small foci of less intense enhancement (Figures 3, 4, 5).
After ultrasound-guided needle localization (Figure 6, specimen radiograph), a partial mastectomy was performed. The pathology showed a 1.5-cm IDC with mucinous and clear cell features, without angiolymphatic invasion, estrogen receptor and progesterone receptor positive, with margins clear for invasion and notable only for a focal close (1 mm) inferior medial margin for DCIS. Five sentinel lymph nodes were negative.
The final stage was stage I, T1N0M0 disease. The patient was treated with four cycles of doxorubicin (Adriamycin) and cyclophosphamide (Cytoxan) chemotherapy and radiation therapy, with a boost to the lumpectomy bed. Tamoxifen was started after completion of chemotherapy.
CASE 9 ILC presenting as a growing amorphous density
An asymptomatic 61-year-old woman underwent screening mammography over a 14-year period. Over the course of the patient’s screening examinations, an amorphous density and focal asymmetry developed within the upper outer right breast (Figures 1, 2, 3, and 4). Although this was recalled for spot compression views, the finding was interpreted as asymmetric glandular tissue because it partially dispersed on compression and contained fat density areas (Figure 5). The area subsequently became palpable, prompting an ultrasound examination. The ultrasound demonstrated an irregular solid mass with posterior acoustic shadowing (Figure 6). Needle biopsy of the mass revealed an invasive lobular carcinoma (ILC).
TEACHING POINTS
This case illustrates several teaching points. It is important to compare screening mammograms with several prior years’ mammograms. As in this case, a slowly growing cancer may not appear to change much from year to year. Comparing the current mammogram with films from several years before may reveal a developing mass. Although the mammographic finding could have been asymmetric glandular tissue, an unexplained enlarging density should prompt a biopsy. In this case, we see that invasive lobular carcinoma can mimic normal tissue, and ultrasound can help in differentiation. Invasive lobular carcinoma represents about 10% of invasive breast cancers.
CASE 10 Small cancer in implant patient, well seen only on implant-displaced views
An asymptomatic 51-year-old woman with bilateral silicone implants underwent annual screening mammography. The right standard views showed no definite abnormality (Figure 1). The implantdisplaced MLO view demonstrated a focal asymmetry in the upper right breast (Figure 2). Spot compression MLO view confirmed a spiculated mass in the upper right breast (Figure 3). Ultrasound showed a corresponding irregular 11-mm solid mass in the 12-o’clock position (Figure 4). Subsequent biopsy confirmed an invasive ductal carcinoma.
CASE 11 Importance of a complete workup of new mammographic masses
A screening mammogram on an asymptomatic 49-year-old woman showed bilateral, similar-appearing upper outer quadrant breast masses (Figure 1), which were new from a prior mammogram taken 2 years earlier. Ultrasound was performed to further evaluate these masses (Figures 2 and 3). On the right, where mammography suggested two masses, ultrasound confirmed two simple cysts. On the left, where mammography showed a single new mass (similar in appearance to the right-sided findings), sonography identified a vascular, solid mass with indeterminate features. Biopsy was recommended. Excision of the lesion demonstrated a 1.4-cm infiltrating ductal carcinoma. In addition to lumpectomy and a negative sentinel lymph node sampling, the patient was treated with radiation therapy.
TEACHING POINTS
The case illustrates the importance of complete workups. Mammographers often refer to the “rule of multiplicity,” which suggests that if there are multiple breast masses, they are all likely benign. It is difficult to correlate sonographic to mammographic masses, one for one, when the lesions are numerous. However, at least when beginning screening, it is useful to clearly establish by ultrasound that a patient with multiple masses has multiple cysts, multiple solid masses, or some combination. With this information, subsequent mammographic changes, such as waxing and waning of multiple cysts, can be more accurately assessed. However, development of a new mass or masses in a patient without such prior findings warrants a sonographic evaluation. In this case, virtually identical new bilateral mammographic findings resulted in a diagnosis of benign cysts on one side and cancer on the other.
CASE 12 MRI high-risk screening for occult breast cancer
An intensely enhancing small (5-mm) left breast nodule was noted to be new on high-risk screening MRI (Figure 1). After a 3-month delay during which a prior comparison MRI was obtained from another facility (confirming the lesion to be new), a sonographic correlate was identified and biopsied, confirming infiltrating ductal carcinoma (IDC) (Figure 2). By ultrasound, the mass measured 8 × 6 mm. The patient elected to undergo bilateral mastectomies for treatment and received postoperative chemotherapy with docetaxel (Taxotere) and cyclophosphamide (Cytoxan), as well as chest wall and supraclavicular radiation therapy. The left mastectomy specimen showed a 1.6-cm, T1N1 tumor with angiolymphatic invasion and negative margins, with one of 19 lymph nodes positive. No additional tumor was found on the right.
TEACHING POINTS
The high lifetime risk for developing breast cancer faced by women with the BRCA1 and BRCA2 gene mutations is well recognized. Lifetime breast cancer risks of up to 85% have been predicted for BRCA mutation patients. As illustrated by this case, there is a substantial risk (30%) in these patients of developing a contralateral breast cancer within 5 years after diagnosis of breast cancer.
Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
Morris EA, Liberman L, Ballon DJ, et al. MRI of occult breast carcinoma in a high-risk population. AJR Am J Roentgenol. 2003;181:619-626.
Stoutjesdijk MJ, Boetes C, Jager GJ, et al. Magnetic resonance imaging and mammography in women with a hereditary risk of breast cancer. J Natl Cancer Inst. 2001;93:1095-1102.
Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292:1317-1325.
Warner E, Plewes DB, Shumak RS, et al. Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer. J Clin Oncol. 2001;19(15):3524-3531.
CASE 13 MRI high-risk screening for occult breast cancer
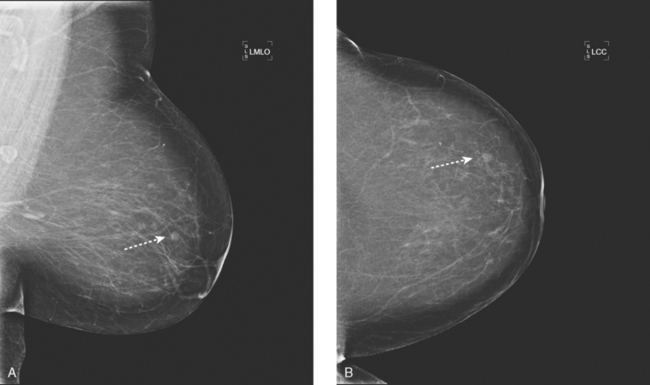
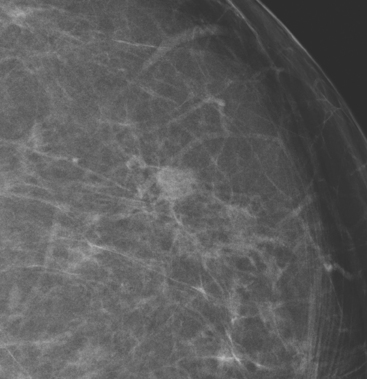
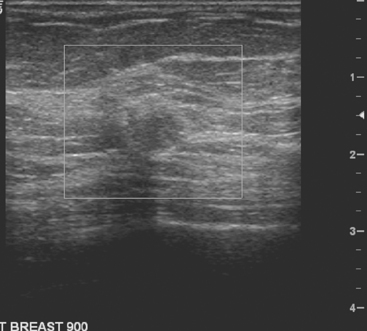
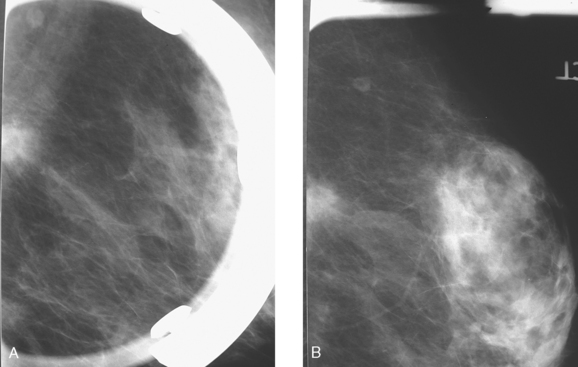
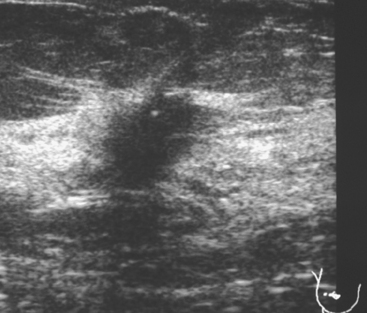
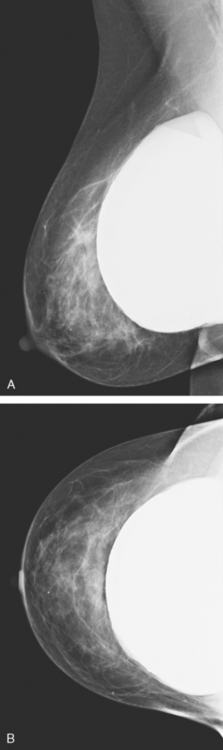
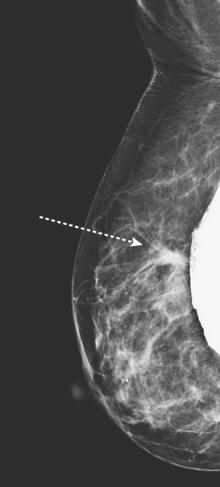
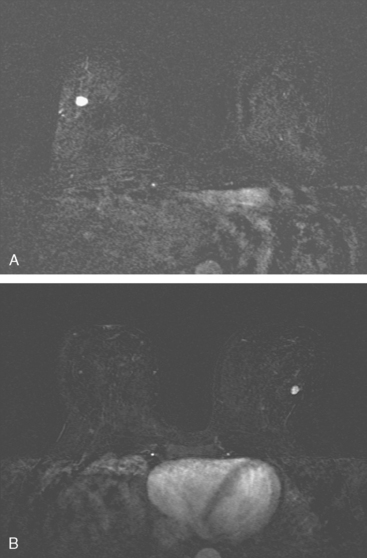
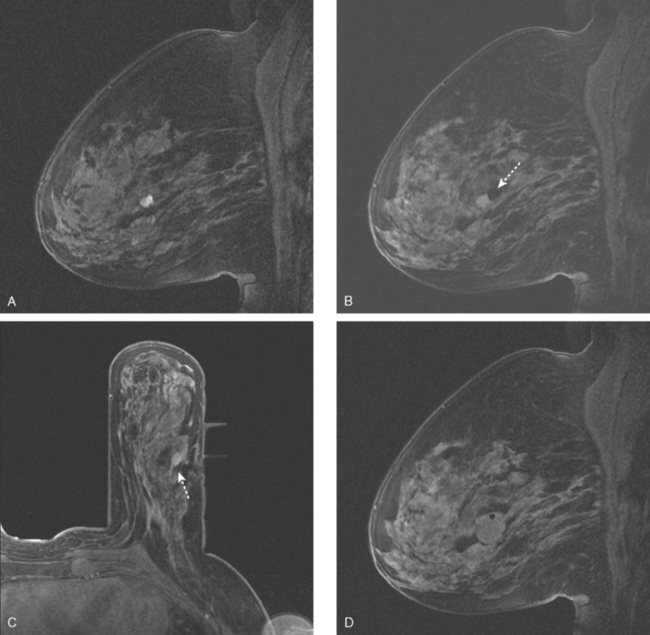
An asymptomatic 57-year-old high-risk woman underwent screening mammography and screening MRI. The patient was considered high risk because of breast cancer history in multiple family members. The patient had heterogeneously dense breasts, and the mammogram was unrevealing (Figure 1). Subsequent MRI showed a suspicious enhancing mass in the upper outer left breast (Figures 2 and 3). Directed ultrasound confirmed the presence of an irregular, 12-mm, hypoechoic solid mass (Figure 4). Ultrasound-guided core needle biopsy yielded a diagnosis of intermediate-grade invasive ductal carcinoma (IDC).
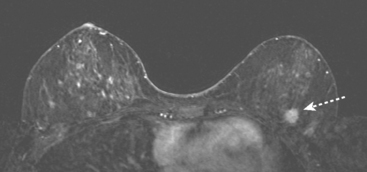
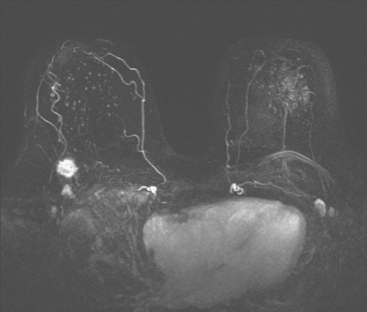
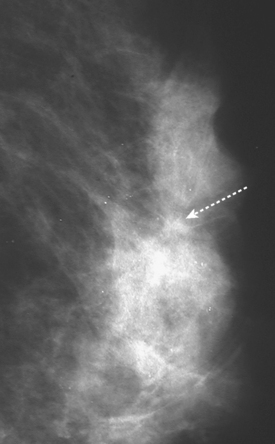
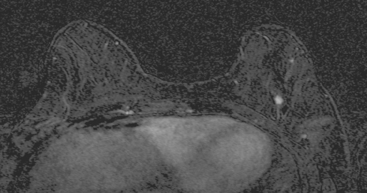
FIGURE 2 High-risk screening breast MRI shows an enhancing mass in the upper outer left breast (arrow).
TEACHING POINTS
There is no established role for breast MRI in screening of the general population. However, breast MRI has gained credence in recent years for screening of higher-risk patients. Recently published American Cancer Society guidelines recommend annual screening MRI as an adjuvant to mammography in high-risk patients. High-risk women were defined as women with an approximately 20% to 25% or greater lifetime risk for breast cancer. These patients include those with BRCA genetic mutations, strong family history (especially premenopausal breast cancer or breast cancer in two or more primary relatives), or prior treatment for Hodgkin’s disease. Increased breast density has recently been recognized as an independent risk factor for development of breast cancer and limits the efficacy of mammographic screening, as in this case. It should be emphasized that MRI is recommended as an adjuvant rather than a replacement for mammography. Given the variable sensitivity of MRI for DCIS, mammography may find areas of DCIS not detected by MRI. Conversely, because DCIS may not be calcified in up to two thirds of cases, MRI can identify DCIS that is mammographically occult.
CASE 14 Breast cancer presenting as a growing small mass on screening MRI
A 57-year-old woman who was due for routine screening mammography requested breast MRI as an alternative because of concerns about radiation. She had previously had a benign right stereotactic breast biopsy for new microcalcifications, 1.5 years before.
The breast MRI showed small, scattered bilateral enhancing nodules, thought to be benign (Figure 1). The largest was 8 × 6 mm in the right lower outer quadrant (LOQ), with a similar lesion in the left LOQ (Figure 2). A workup with bilateral mammography and ultrasound showed no suspicious findings or definite corresponding lesions. A repeat breast MRI in 6 months was recommended.
The follow-up breast MRI showed interval growth of both of the lower outer quadrant small masses (Figures 3, 4, and 5). They were both subcentimeter in size, and similar in appearance, being bright on short tau inversion recovery (STIR) imaging, with faint nonenhancing internal septa, suggesting fibroadenomas. A sonographic correlate was found on ultrasound for the larger (9 × 9 mm) nodule in the right LOQ (Figure 6). Ultrasound-guided core needle biopsy revealed invasive ductal carcinoma (IDC). Sonographic evaluation of the left breast showed a possible correlate at 3 o’clock, a hypoechoic, round, 7-mm complex cyst versus solid mass (Figure 7). However, it aspirated, proving it was a complex cyst and not a correlate for the enhancing (and thereby solid) nodule on MRI. No solid sonographic correlate was found for the left LOQ lesion, which by MRI was nearly identical in appearance to the contralateral proven cancer.
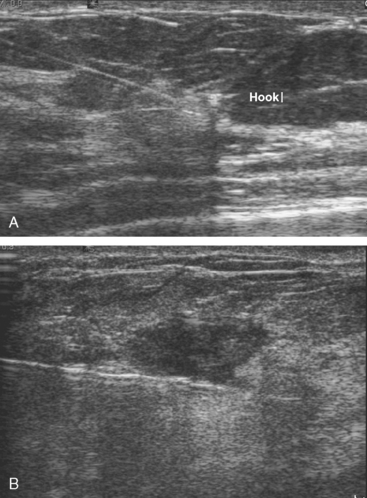
MRI-guided biopsy (Figure 8) of the left LOQ enhancing mass initially returned a diagnosis of cribriform atypical ductal hyperplasia and atypical lobular hyperplasia. A clip was placed to mark the site. The pathologic diagnosis was revised subsequently (after review by Dr. David Page of Vanderbilt) to atypical lobular hyperplasia.
Surgical therapy was accomplished by bilateral ultrasound-guided needle localizations of the residual mass on the right and the clip and postbiopsy hematoma site on the left (Figure 9). A sentinel lymph node procedure was performed on the right.
TEACHING POINTS
There are a variety of teaching and discussion points raised by this case. This patient requested MRI as an alternative to mammography because of concerns about radiation. She had had a prior benign biopsy of calcifications but had no prior pathologic diagnosis of atypical ductal hyperplasia (ADH) or other borderline histology to suggest she was a higher-risk patient. There are no data supporting the use of breast MRI for breast cancer screening in the general (non-high-risk) population. Thus, in a perfect world (in which patients heed the advice of medical professionals), this patient would not have undergone breast MRI as an alternative to mammography, which remains the only imaging modality that excels at detecting microcalcifications as a harbinger of potential malignancy. In cases of extreme patient anxiety (cancer phobia), a better but not completely supportable case could be made for supplementing mammographic surveillance with periodic breast MRI. That said, there likely are patients in many practices who may insist, for radiation-related or other reasons, on having an imaging alternative to mammography. If, after appropriate counseling, these patients cannot be dissuaded, we generally take the view that some imaging screening is better than none.
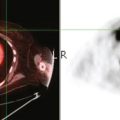
CASE 15 CT identification of unknown breast cancer in an asymptomatic patient
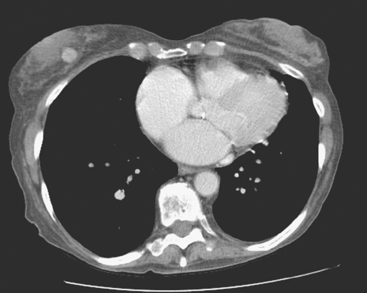
An 83-year-old woman had an abdomen and pelvis CT for left lower quadrant (LLQ) pain. An enhancing nodule was noted in the right breast on the most superior image (Figure 1). The patient had not had a mammogram for 4 years. A diagnostic workup was performed, including mammography and ultrasound.
On mammography, extreme breast density was noted, attributable to the patient’s use of conjugated estrogens (Premarin) for more than 40 years (Figure 2). The new mammogram showed a change in contour at the posterior margin of dense retroareolar tissue, suggesting a poorly visualized new mass. Ultrasound readily demonstrated a corresponding suspicious mass (Figure 3). Multiple features of malignancy were noted of the solid, vascular mass, including marked hypoechogenicity, taller-than-wide dimensions, and microlobulation of the margins. Ultrasound-guided biopsy confirmed infiltrating ductal carcinoma (IDC).
TEACHING POINTS
CT is an uncommon modality to be the first indication of breast cancer, but it does happen on occasion. Cross-sectional imagers interpreting chest and abdominal CT scans should include in their search pattern a review of the breast parenchyma for density, symmetry, and masses. Although breast cancers can often be seen on enhanced CT scans, the contrast generally achievable between an enhancing cancer and the surrounding breast tissue is not as advantageous as the contrast routinely obtained with fat-saturated or enhanced-subtracted breast MRI. For this reason, CT is not generally relied on for initial identification of breast lesions or local staging of known cancers, although it is an imaging mainstay in identifying axillary, interpectoral (Rotter’s), and supraclavicular lymph nodes in initial staging of locally advanced disease, often in conjunction with positron emission tomography.
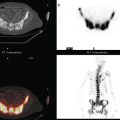
CASE 16 PET identification of occult breast cancer in an asymptomatic patient*
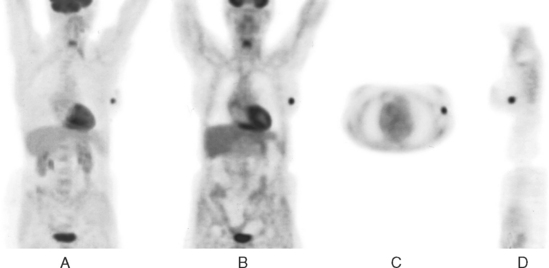
A 73-year-old woman was referred for positron emission tomography (PET) imaging for suspicion of recurrent lung cancer. Eighteen months after undergoing right lower lobe resection for lung cancer, she developed shortness of breath and a small right pleural effusion. PET scan demonstrated an unexpected hypermetabolic left breast focus (Figure 1). Subsequent evaluations confirmed breast cancer, which was excised by lumpectomy.
TEACHING POINTS
A series reported by Agress and Cooper addresses the frequency and significance of such unexpected PET findings. These investigators identified 58 FDG-avid unexpected sites of uptake in 53 patients on review of 1850 whole-body PET scans. Of these, 45 were followed up with correlative imaging, and histopathologic confirmation was obtained in 42. Of these, 30 (71%) were malignant or premalignant; colonic adenomas (18) and colonic adenocarcinomas (3) were the most frequently encountered unexpected diagnoses. Other previously unsuspected carcinomas diagnosed as a result of unexpected PET findings in this series included two breast carcinomas, two laryngeal squamous cell carcinomas, one gallbladder carcinoma, one endometrial adenocarcinoma, one ovarian adenocarcinoma, one fallopian tube adenocarcinoma, and one papillary thyroid carcinoma. Nine benign diagnoses were also confirmed to explain unexpected PET uptake encountered in this series, including three with clinical significance (one case each of cholecystitis, knee pigmented villonodular synovitis, and Hashimoto’s thyroiditis).
* Case from: Kipper MS, Tartar M. Case 1: Focal breast activity due to an unsuspected breast cancer. In Clinical Atlas of PET. Philadelphia, WB Saunders, 2004.