32 Dermoscopy
Advantages of and Evidence for Dermoscopy
In two separate meta-analyses, dermoscopy had significantly higher discriminating power than clinical examination for experienced users.1–3 It is intuitively obvious that this improved diagnostic accuracy translates into improved patient management. For example, in one study, the malignant-to-benign ratio improved in dermoscopy users from 1 : 18 to 1 : 4.4
Primary care physicians (PCPs) who were given a 1-day training course in skin cancer detection and dermoscopic evaluation were able to improve their sensitivity for melanoma diagnosis. The study randomly assigned PCPs to the dermoscopy evaluation arm or the naked-eye (no dermoscopy) evaluation arm. The study showed that 23 malignant skin tumors were missed by PCPs performing naked-eye observation, whereas only 6 were missed by PCPs using dermoscopy (P = 0.002). The authors concluded that the use of dermoscopy improves the ability of PCPs to triage lesions suggestive of skin cancer without increasing the number of unnecessary expert consultations.5
Equipment
Dermoscopes illuminate the skin via the use of light emitting diode (LED) lights with or without the use of polarizing filters. Although most units are either nonpolarized (no polarizing filters in place) or polarized (polarized filters used), a few newer units, known as hybrids, allow the operator to toggle between polarized and nonpolarized light within the same unit. Nonpolarized dermoscopes (NPDs) are manufactured by Heine and Welch Allyn (Figure 32-1A). 3Gen manufactures NPDs, polarized dermoscopes (PDs), and hybrid dermoscopes (Figure 32-1B). A number of new dermoscopes attach to smart phones for easy dermoscopic photography.
Nonpolarized dermoscopy works best for visualizing:
Principles of Dermoscopy
The colors seen on dermoscopy, which are based on the depth of the melanin and other skin structures, are illustrated in Figure 32-2.
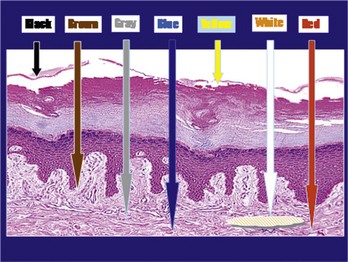
Figure 32-2 Colors seen on dermoscopy based on the depth of the melanin and other skin structures.
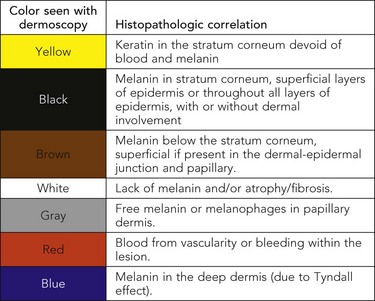
(Courtesy of Alfred W. Kopf, MD.)
Network Patterns
Pigment Network
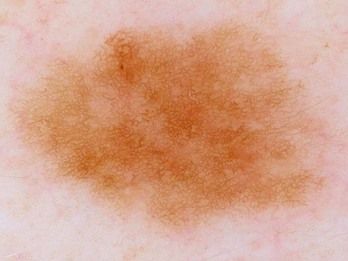
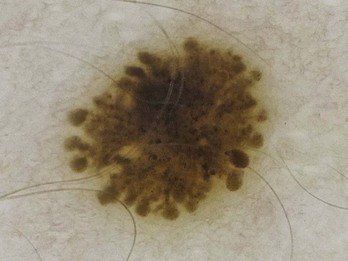
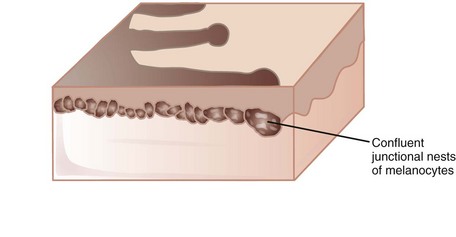
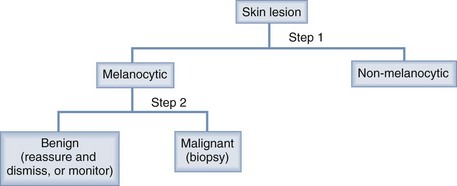
A reticulated pigment network (Figure 32-3) has the following characteristics:
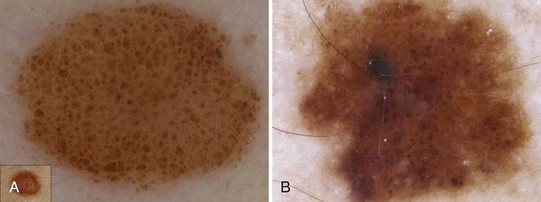
A typical pigment network in a benign nevus consists of (Figure 32-4):
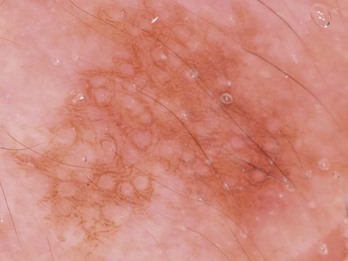
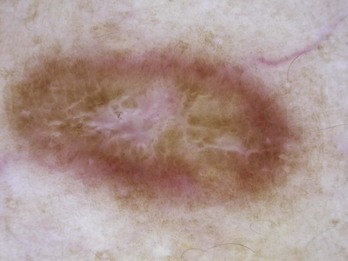
For an atypical pigment network (Figure 32-5),
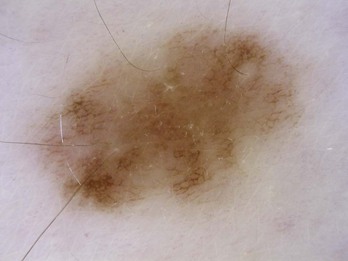
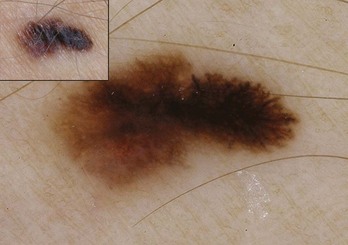
Figure 32-5 Atypical pigment network in a dysplastic nevus with variations in line thickness, darkness, and pattern.
(Copyright Richard P. Usatine, MD.)
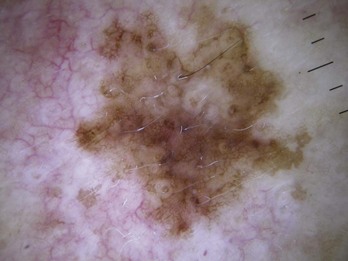
A pseudonetwork (Figure 32-6) is
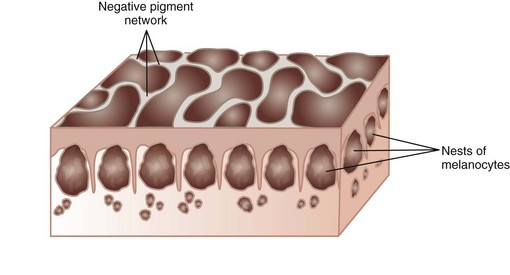
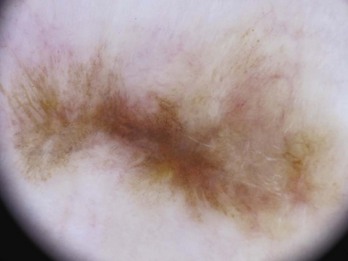
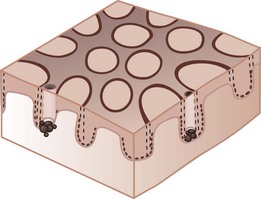
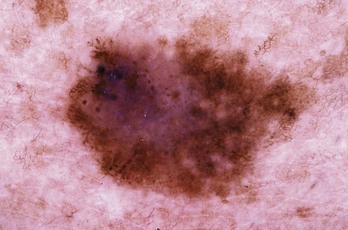
A negative network is a reverse network that is seen in melanoma (Figures 32-7 and 32-8). It consists of dark, elongated, and curved globular structures surrounded by relative hypopigmentation. This results in the impression that the lines of the network appear lighter in color with an almost serpiginous pattern with holes that are darker in color (and appear sausage shaped).
Other Structures
Structureless Areas
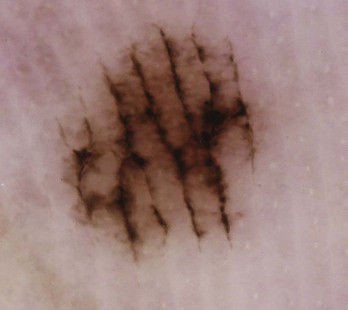
Dots
Typical dots in benign melanocytic lesions are seen in Figure 32-11. Brown dots on the network appear in normal nevi (Figure 32-12). Brown dots off the network may appear in dysplastic nevi or melanoma (Figure 32-13).
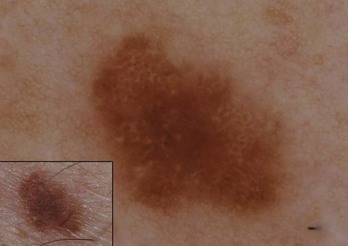
Globules
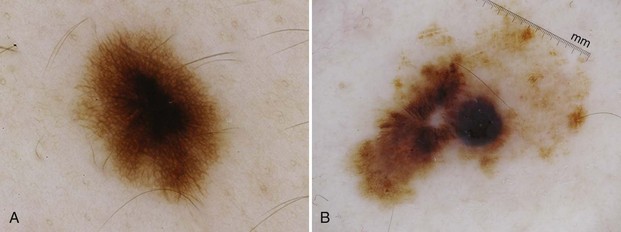
Figure 32-14A shows typical globules evenly distributed in a benign nevus; Figure 32-14B shows atypical globules aggregated in the lower left corner of a dysplastic nevus.
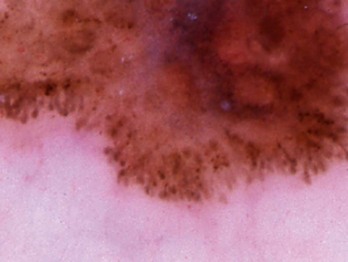
Pseudopods
Various types of pseudopods are shown in Figures 32-15 through 32-17.
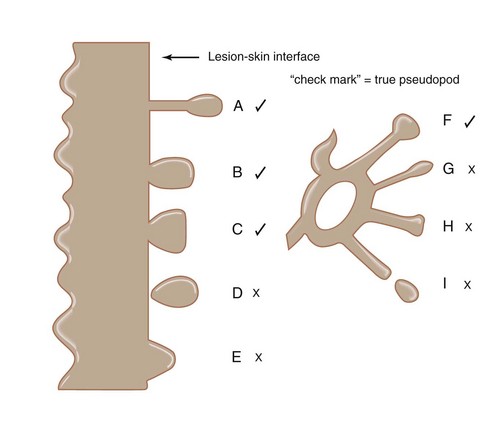
Figure 32-15 Pseudopods. A, B, C, and F are true pseudopods; the others are not.
(Adapted from Menzies SW, Crotty K, McCarthy W. The morphologic criteria of the pseudopod in surface microscopy. Arch Dermatol. 1995;131:436–440.)
Radial Streaming
The term radial streaming refers to radial, parallel, linear extensions that resemble pseudopods but do not have a knob at the ends (Figure 32-18). They are located at the periphery.
Streaks
Streaks is a term that encompasses both pseudopods and radial streaming. These are seen in melanoma and Spitz nevi (Figures 32-15 through 32-19).
Blotch (Black Lamella)
Blue White Veil, Raised
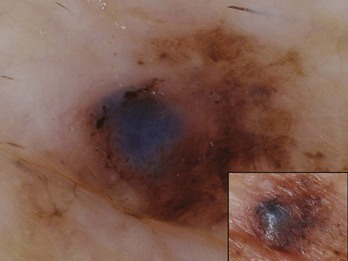
Figure 32-21 shows a blue-white veil over a raised area in a melanoma.
Blue White Veil, Flat
A flat blue-white veil is also called a white scar-like area with “peppering” or regression structures (Figure 32-22).
Structures in Nonmelanocytic Lesions
Basal Cell Carcinoma (BCC)
Leaf-Like Areas (Figures 32-25 and 32-26)
Blue-Gray Ovoid Nests (Figure 32-27)
Spoke-Wheel-Like Structures (Figure 32-27)
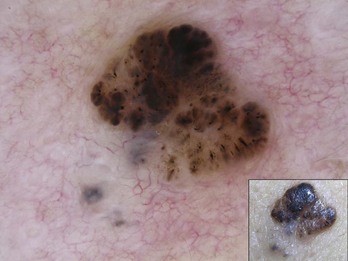
Figure 32-26 Leaf-like structures and blue-gray ovoid nest in bottom left-hand corner of this BCC.
(Copyright Richard P. Usatine, MD.)
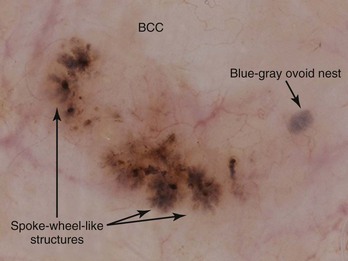
Figure 32-27 Spoke-wheel-like structures and isolated blue-gray ovoid nest on the left.
(Copyright Ashfaq A. Marghoob, MD.)
Arborizing blood vessels are seen in BCC (Figure 32-28).
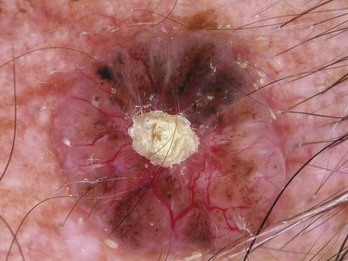
Figure 32-28 Arborizing blood vessels like branching trees in a BCC.
(Copyright Richard P. Usatine, MD.)
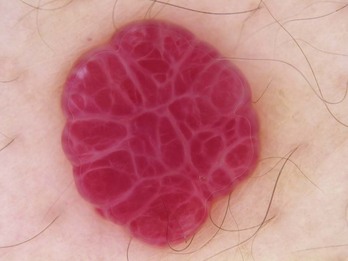
Angioma/Angiokeratoma
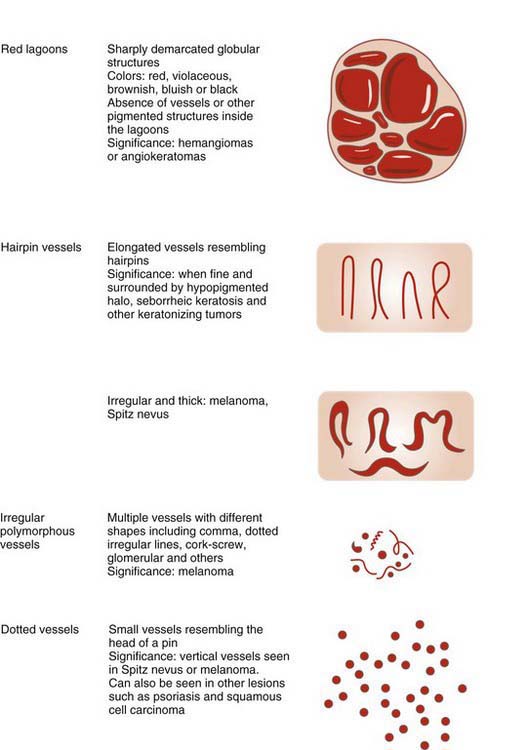
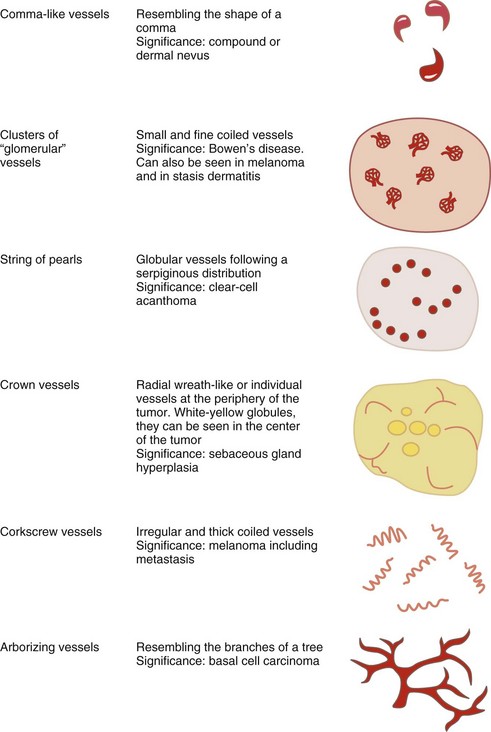
Angiomas have red lacunae (lagoons) (Figure 32-29). Angiokeratomas have red, maroon, blue, and black lacunae (lagoons) (Figure 32-30). Their vascular architecture is described in Figure 32-31.
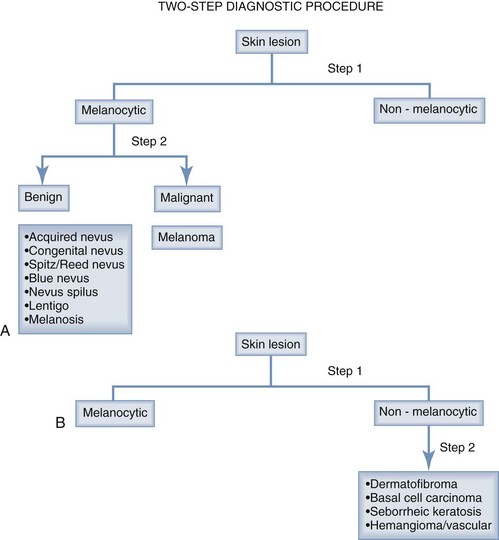
Two-Step Dermoscopy Algorithm
The two-step dermoscopy algorithm forms the foundation for the dermoscopic evaluation and differentiation of skin lesions (Figure 32-32). If a lesion manifests any of the structures listed below, it is considered to be a melanocytic lesion:
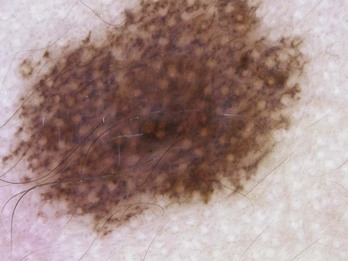
Figure 32-35 Pseudonetwork pattern is seen on the face in this congenital nevus.
(Copyright Richard P. Usatine, MD.)
Note, however, an exception: a pattern consisting of a delicate network surrounding a scar-like area is associated with dermatofibroma. This particular pattern trumps the presence of a network structure; thus, despite the presence of a network, this type of lesion is not considered to be melanocytic (Figures 32-37 and 32-38).
If the lesion does not manifest any of the structures mentioned above, look for features of:
BCC has at least one of these:
Seborrheic keratoses exhibits these characteristics (Figure 32-39):
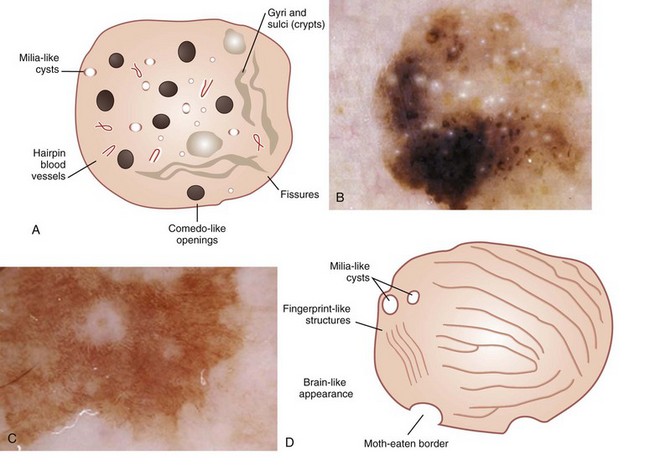
Figure 32-39 A seborrheic keratosis can have all or any of these structures.
(A, D: Adapted from Malvehy J, Braun RP, Puig S, et al. Handbook of Dermoscopy. London: Informa Healthcare; 2006:83, Figure A-21.)
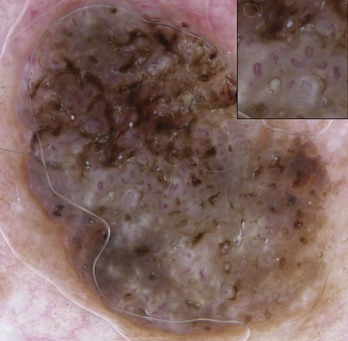
Figure 32-40 SK with hairpin vessels surrounded by a whitish halo.
(Copyright Richard P. Usatine, MD.)
Vascular lesions have red or blue-black lacunae (see Figures 32-29 and 32-30).
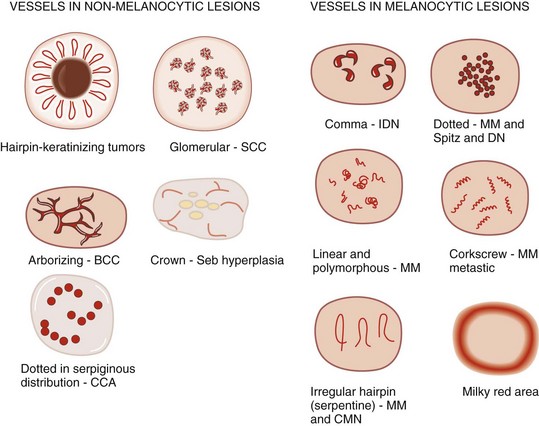
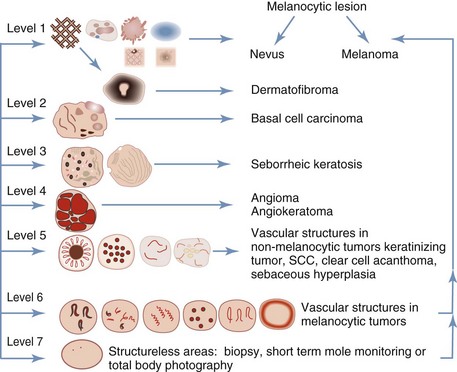
If the lesion has no melanocytic features and it has no features seen in one of the four common nonmelanocytic lesions, then search for any vascular structures/patterns as seen in Figures 32-31 and 32-41. If the lesion has no features of a melanocytic lesion, DF, BCC, SK, or hemangioma and it also lacks diagnostic vascular structures, then the lesion is considered structureless. All structureless lesions are by default considered to be melanocytic and one needs to consider the diagnosis of melanoma. These lesions should be biopsied or monitored very closely. Figure 32-42 puts this all together in one algorithm.
Pattern Analysis
Practitioners should look for the “ugly duckling” or the “Beauty and the Beast” sign. The ugly duckling is the lesion that stands out as different compared to surrounding nevi. Another way of looking at this is that the ugly lesion is the “Beast” and the other benign lesions are the “Beauty.”7
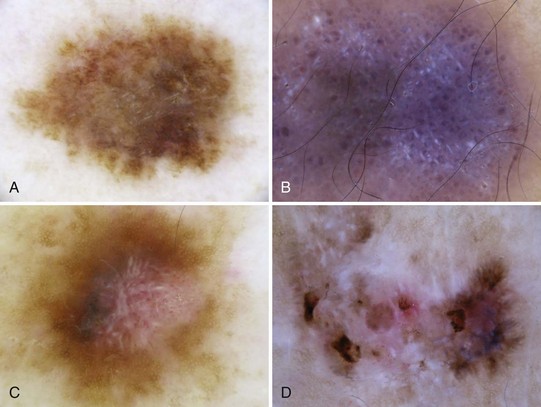
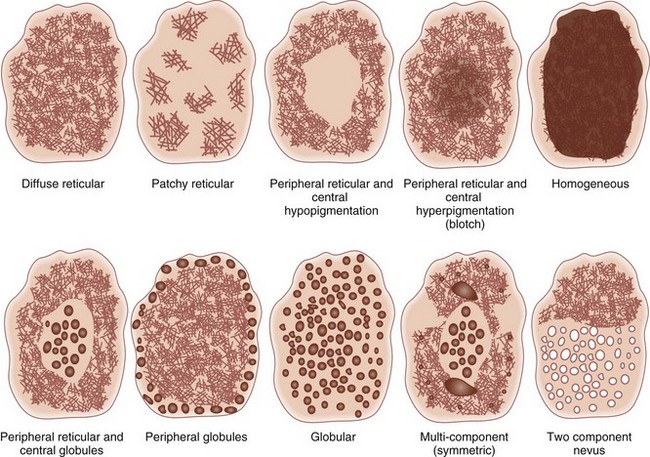
Melanoma deviates from global benign patterns and has at least one of these local features (Figure 32-44):
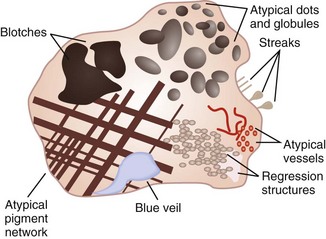
Figure 32-44 Melanoma-specific structures.
(Adapted from Malvehy J, Braun RP, Puig S, et al. Handbook of Dermoscopy. London: Informa Healthcare; 2006:75, Figure A-4.)
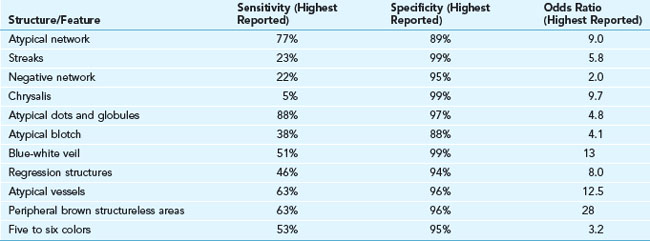
The sensitivity and specificity of melanoma structures and features are shown in Table 32-1. A three-point checklist can be applied to pigmented lesions only:
Final score and recommended treatment:
Special Conditions
Face
Three important dermoscopic features are:
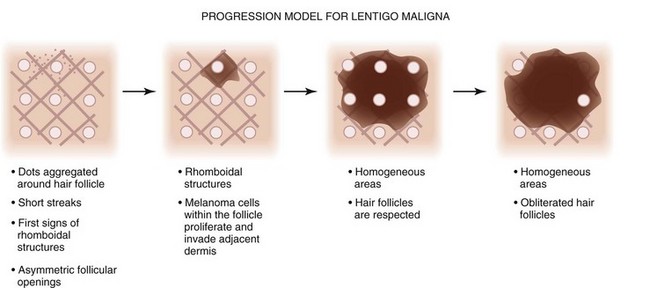
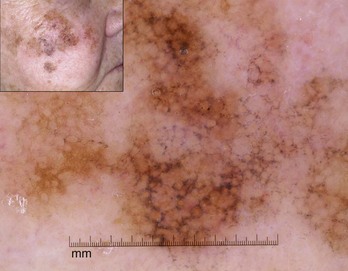
Schiffner et al. described the progression model for lentigo maligna that is detailed in Figure 32-45.12 Figure 32-46 shows actual lentigo maligna with rhomboidal structures.
Acral Areas—Palms and Soles
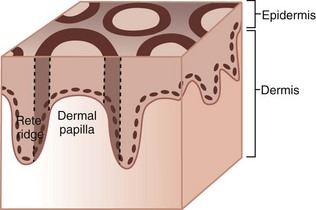
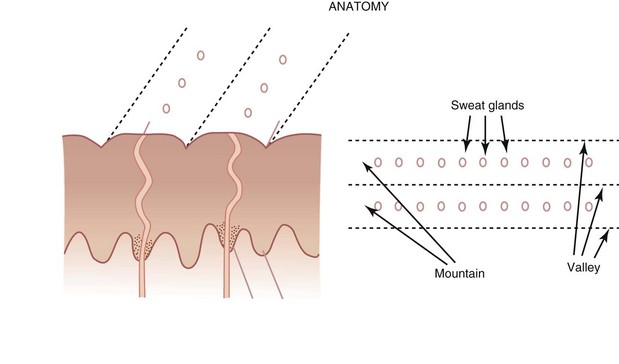
To understand how to differentiate melanoma from other pigmented lesions on the palms or soles, the practitioner must understand the anatomy of the acral skin. The dermatoglyphics on acral skin consists of mountains and valleys (ridges and furrows) with eccrine sweat glands that are found on the mountains (Figure 32-47). The mountains are wider than the valleys.
Benign nevi have three common patterns:
Melanomas also have three common patterns:
Figure 32-48 shows the pigment of a benign nevus in the furrows or valleys. There is also a lattice-like pattern and the pigment is seen running in the furrows with a cross network between the furrows.

Figure 32-48 Pigment of a benign nevus on the sole of the foot in the furrows (valleys).
(Copyright Richard P. Usatine, MD.)
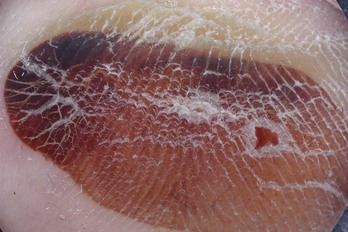
Ninety-eight percent of melanomas on the palms and soles have a parallel ridge pattern in which the pigment is on the mountains and there is hypopigmentation of the valleys. Zero percent of acral nevi have this pattern. Figure 32-49 shows an acrolentiginous melanoma on the foot with this parallel ridge pattern. Sometimes the eccrine sweat gland openings are visible in the middle of the ridges of hyperpigmentation.
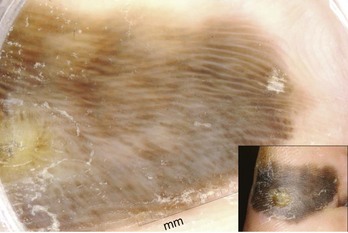
Figure 32-49 Acrolentiginous melanoma on the foot with a parallel ridge pattern.
(Copyright Ashfaq A. Marghoob, MD.)
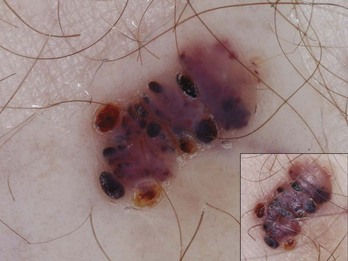
It is important not to confuse hemorrhagic lesions on the palms or soles with melanoma to avoid unnecessary biopsies. Frequently the history alone will help to distinguish pigmentation caused by trauma or ill-fitting shoes from a benign or malignant pigmented lesion. These subcorneal hemorrhages can appear yellow-red to red-black in color and may have globules at the periphery or look like pebbles on the ridges. The heel and bottom of the big toe are common locations for subcorneal hemorrhages seen with sports or ill-fitting shoes. Figure 32-50 is an example of a benign subcorneal hemorrhage.
Scabies
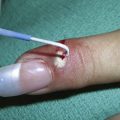
The dermatoscope can also be used for the diagnosis of scabies. Look for burrows in the skin and put the dermoscope over the burrow. Figure 32-51 shows scabies mites at the ends of the burrows. The mites are visible and resemble an arrowhead pointing away from the burrow (Figure 32-51). That is because the mite is in the process of burrowing through the skin using its mouth and legs to extend the tunnel. The round shape of the mite is also visible. This finding should be sufficient to confirm a suspected case of scabies. Once the mite has been identified with a dermoscope, a directed scraping may be used to see the mite, its eggs, or its feces under the microscope.
Resources
1. Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343-1350.
2. Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
3. Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
4. Carli P, De Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997–2001. Br J Dermatol. 2004;150:687-692.
5. Argenziano G, Puig S, Zalaudek I, et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J Clin Oncol. 2006;24:1877-1882.
6. Venuto-Andrade C, Marghoob AA. Ten reasons why dermoscopy is beneficial for the evaluation of skin lesions. Exp Rev Dermatol. 2006;1:369-374.
7. Marghoob AA, Korzenko AJ, Changchien L, et al. The beauty and the beast sign in dermoscopy. Dermatol Surg. 2007;33:1388-1391.
8. Menzies SW, Kreusch J, Byth K, et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch Dermatol. 2008;144:1120-1127.
9. Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679-693.
10. Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Res. 1996;6:55-62.
11. Annessi G, Bono R, Sampogna F, et al. Sensitivity, specificity, and diagnostic accuracy of three dermoscopic algorithmic methods in the diagnosis of doubtful melanocytic lesions: the importance of light brown structureless areas in differentiating atypical melanocytic nevi from thin melanomas. J Am Acad Dermatol. 2007;56:759-767.
12. Schiffner R, Schiffner-Rohe J, Vogt T, et al. Improvement of early recognition of lentigo maligna using dermatoscopy. J Am Acad Dermatol. 2000;42:25-32.