25
Dermatitis Herpetiformis and Linear IgA Bullous Dermatosis
This is the third chapter, along with Chapters 23 and 24, that deals with autoimmune bullous diseases (see Table 23.1).
Dermatitis Herpetiformis (DH)
• In predisposed individuals (e.g. those with HLA-DQ2), IgA antibodies form against gliadin cross-linked to tissue tranglutaminase; the presumed autoantigen in the skin is epidermal transglutaminase (Fig. 25.1).
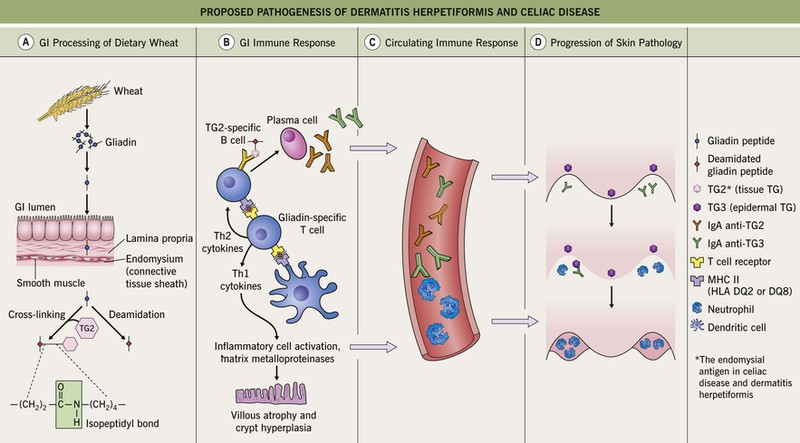
Fig. 25.1 Proposed pathogenesis of dermatitis herpetiformis and celiac disease. A Dietary wheat, barley, or rye is processed by digestive enzymes into antigenic gliadin peptides, which are transported intact across the mucosal epithelium. Within the lamina propria, tissue transglutaminase (TG2) (1) deamidates glutamine residues within gliadin peptides to glutamic acid and (2) becomes covalently cross-linked to gliadin peptides via isopeptidyl bonds (formed between gliadin glutamine and TG2 lysine residues). B CD4+ T cells in the lamina propria recognize deamidated gliadin peptides presented by HLA-DQ2 or -DQ8 molecules on antigen-presenting cells, resulting in the production of Th1 cytokines and matrix metalloproteinases that cause mucosal epithelial cell damage and tissue remodeling. In addition, TG2-specific B cells take up TG2–gliadin complexes and present gliadin peptides to gliadin-specific helper T cells, which stimulate the B cells to produce IgA anti-TG2. C Over time, IgA directed against TG3 (IgA anti-TG3) forms as a result of epitope spreading and both IgA anti-TG2 and IgA anti-TG3 circulate in the bloodstream. D When IgA anti-TG3 antibodies reach the dermis, they complex with TG3 antigens which have been produced by keratinocytes (epidermal TG) and then have diffused into the dermis. That is, IgA/TG3 immune complexes are formed locally within the papillary dermis. This leads to neutrophil chemotaxis (with formation of neutrophilic abscesses), proteolytic cleavage of the lamina lucida, and subepidermal blister formation.
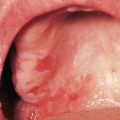
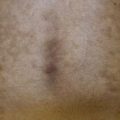
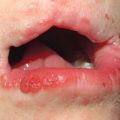
• Primary lesions consist of pruritic vesicles on an erythematous base and edematous erythematous papules that are often grouped (i.e. herpetiform); however, due to scratching, only excoriated papules and hemorrhagic crusts may be present (Fig. 25.2).
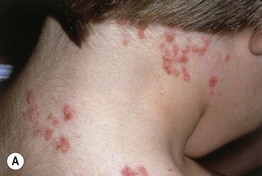
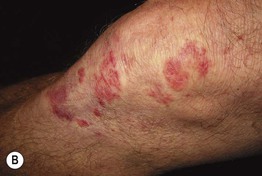
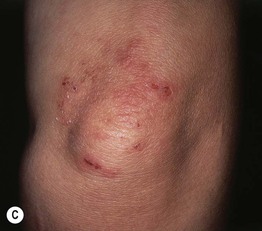
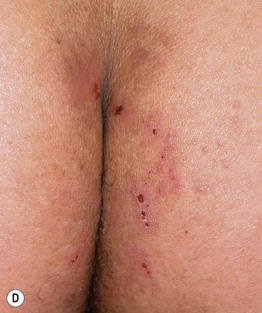
Fig. 25.2 Dermatitis herpetiformis. A Grouped vesicles and pink papulovesicles on the upper back, neck, and scalp of a child. B Pink plaques on the knee composed of grouped papules and papulovesicles. C Pink papulovesicles admixed with erosions and hemorrhagic crusts on the elbow. D Pruritic pink papules of the buttocks, some of which have central hemorrhagic crusts. C, Courtesy, Thomas Horn, MD; D, Courtesy, Louis A. Fragola, Jr., MD.
• Histologically, collections of neutrophils are seen within the dermal papillae of involved skin; by direct immunofluorescence (DIF), granular deposits of IgA are detected within dermal papillae of adjacent, normal-appearing skin in at least 90% of patients (see Figs. 23.1 and 23.3).
Linear IgA Bullous Dermatosis (LABD)
• Autoimmune bullous disease that occurs in both children and adults and can be drug-induced (Table 25.1); in children, it is sometimes referred to as ‘chronic bullous disease of childhood’.
Table 25.1
Drug-induced linear IgA bullous dermatosis.
Based on case reports, additional drugs have been implicated and they are listed in Table 31.5 of Dermatology, Third Edition.

* Unusual variants include toxic epidermal necrolysis-like and morbilliform.
ACE, angiotensin-converting enzyme; NSAIDs, nonsteroidal anti-inflammatory drugs.
• Vesicles and bullae arise on the trunk and extremities and in children often favor the lower trunk and groin; lesions can assume a figurate arrangement and have been likened to a crown of jewels (Figs. 25.3–25.5).
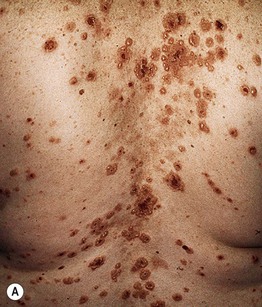
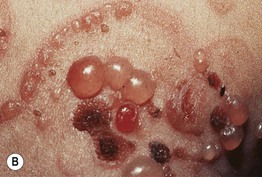
Fig. 25.3 Linear IgA bullous dermatosis. A Grouped and annular bullae on an erythematous base. B The figurate array of the bullae is characteristic of this disorder. A, B, Courtesy, John J. Zone, MD.
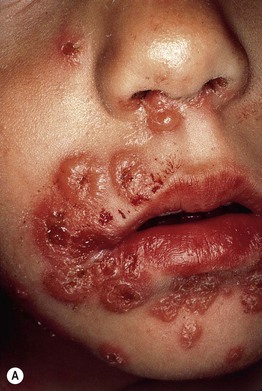
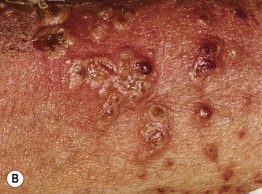
Fig. 25.5 Linear IgA bullous dermatosis. A Annular and herpetiform vesiculobullae on the face of a child. B Vancomycin-induced variant with a similar arrangement of vesiculopustules. Note the central hemorrhagic crusts in both patients.
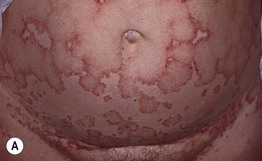
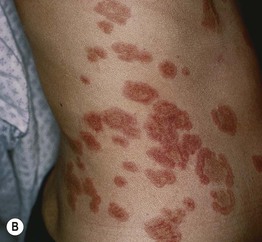
Fig. 25.4 Linear IgA bullous dermatosis. A, B In two women with the disorder, annular and polycyclic plaques of the trunk, with varying degrees of edema. A, B, Courtesy, John J. Zone, MD.
• Histologically, a subepidermal bulla is accompanied by an infiltrate of neutrophils; by DIF, linear deposits of IgA are detected at the basement membrane zone of perilesional skin (see Figs. 23.1 and 23.3).
• By indirect immunofluorescence (IIF), circulating IgA autoantibodies are detected in ~70% of patients with LABD (see Fig. 23.2); the autoantigen in LABD is a cleavage product of one of the two autoantigens of bullous pemphigoid (BP180; see Chapter 24).
• Important to review medications and withdraw possible culprits before instituting systemic therapy.
For further information see Ch. 31. From Dermatology, Third Edition.