25 Dermal Fillers
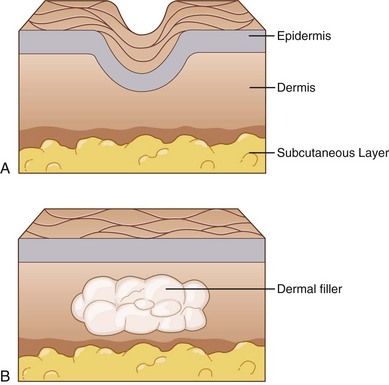
Soft-tissue augmentation, commonly referred to as dermal filler treatment, reduces facial lines and improves contour defects by temporarily restoring volume to the dermis and soft tissues through the use of injectable products.1 With the use of appropriate techniques and volumes, dermal filler treatments can enhance appearance in a subtle, natural way. Treatments require short recovery times and can be safely performed in the outpatient setting.2,3
Dermal filler treatments have become the second most commonly performed minimally invasive cosmetic procedure in the United States, according to statistics from the American Society for Aesthetic Plastic Surgery.4 The popularity of this procedure is largely due to increased patient demand for less invasive cosmetic treatment options and to recent product innovations that have prolonged treatment results.
Numerous dermal fillers (DFs) are available, each varying in composition, duration of action, palpability, ease of administration, complications, and other factors.5 In addition to the provider’s knowledge of DF products and injection skill, an appreciation for aesthetic facial proportions and symmetry is required to achieve desirable outcomes. Injection of dermal fillers has a steeper learning curve than botulinum toxin injections and requires practice to achieve desirable results.6 This chapter is intended to assist aesthetic providers in getting started with products and techniques that consistently achieve good results and have a low risk of complications. The focus is on the use of hyaluronic acid DF products for the FDA-approved treatment of nasolabial folds (NLFs).
Cosmetic Indications
Products Currently Available
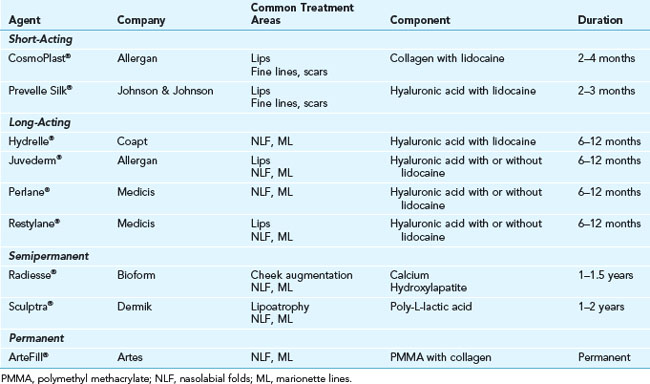
Dermal filler products can be categorized based on their duration of action as either short acting (less than 4 months), long acting (6 months to 1 year), semipermanent (1 to 2 years), and permanent (2 years or more) (see Table 25-1).7–9
Injectable hyaluronic acid (HA) products are one of the most versatile dermal fillers currently available. HA is a naturally occurring glycosaminoglycan in the dermal extracellular matrix that provides structural support and nutrients and, through its hydrophilic capacity, adds volume and fullness to the skin. Commercially available HAs vary in formulation, concentration, and degree of cross-linkage, which affects their duration of action as well as postprocedure risks of swelling and bruising.10,11
Contraindications
Anatomy
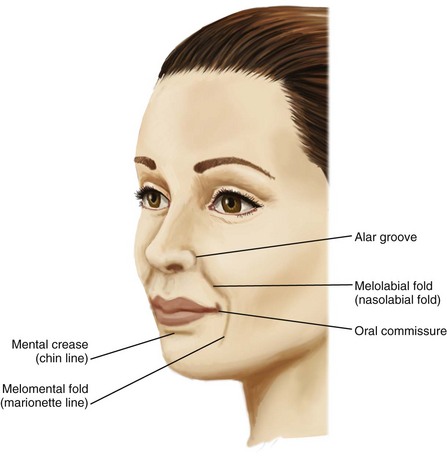
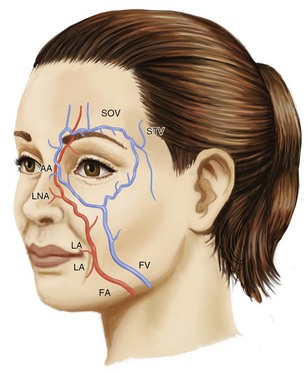
Facial wrinkles and folds of the lower two-thirds of the face commonly treated with dermal fillers are shown in Figure 25-1. Arterial and venous supply for these areas is shown in Figure 25-2. The lateral nasal artery is a noteworthy vessel for NLF treatments. It is found at the junction of the facial artery and angular artery, and is the main vascular supply for the nasal tip and ala. It is located 2 to 3 mm superior to the nasal alar groove.12
Procedure Preparation
Dermal Filler Treatment for Nasolabial Folds: Steps and Principles
The following recommendations are guidelines for treatment using hyaluronic acid dermal fillers.
General Treatment Technique
TIP: If injecting at the incorrect level, withdraw the needle to the skin insertion and retry.
Methods for Dermal Filler Injection
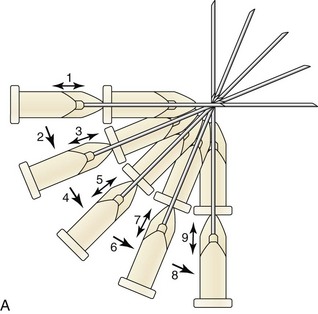
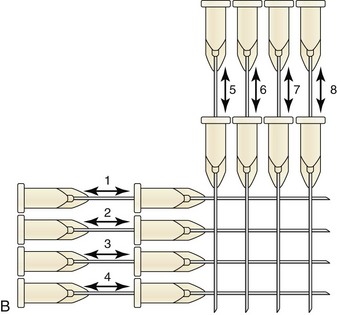
The two main methods for injecting dermal filler in the dermis are (Figure 25-4):
Planning and Designing
Figure 25-5 shows a grading scale for the severity of NLFs. Patients with mild, moderate, and severe NLFs are candidates for dermal filler treatments. Deep folds with excess laxity may have less satisfactory outcomes with dermal filler treatments and may require surgical interventions for significant improvement.
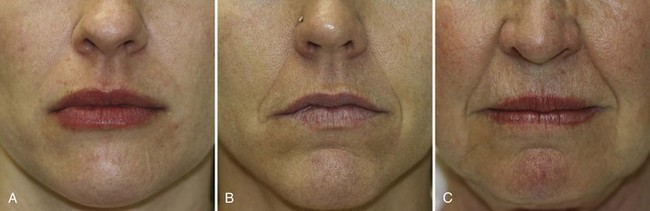
FIGURE 25-5 Nasolabial fold grading scale of (A) mild, (B) moderate, and (C) severe.
(Copyright Rebecca Small, MD.)
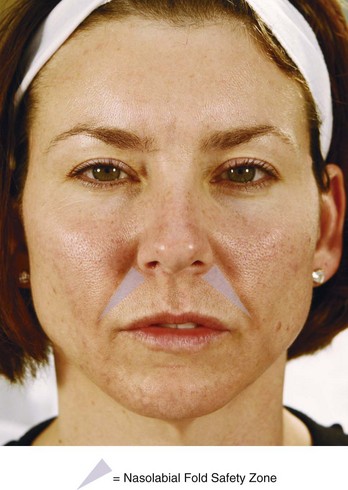
Dermal filler is placed within the Nasolabial Fold Safety Zone, which is bounded laterally by the nasolabial fold superiorly by the edge of the nasal ala and extends to the inferior-most portion of the nasolabial fold (Figure 25-6).
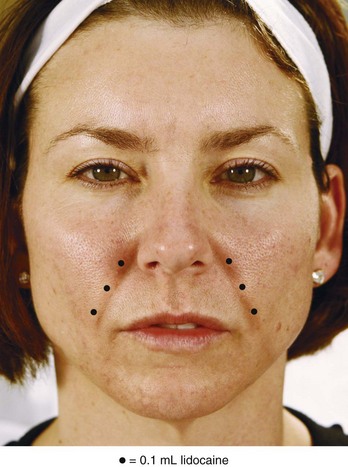
An overview of injection points for treatment of NLFs using an HA dermal filler is shown in Figure 25-7. All injections are placed medial to the NLFs. Injections proceed superiorly from number 1 to 3, with the filler product fanned medially at the nasal ala.
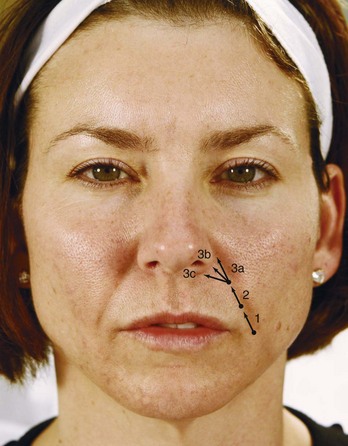
FIGURE 25-7 Overview of nasolabial fold injection points and technique.
(Copyright Rebecca Small, MD.)
The estimated HA dermal filler volume necessary for treatment is based on patients’ observed facial anatomy and volume loss in the treatment area (see Figure 25-5):
Anesthesia
The four main methods for providing anesthesia to the nasolabial folds are as follows:
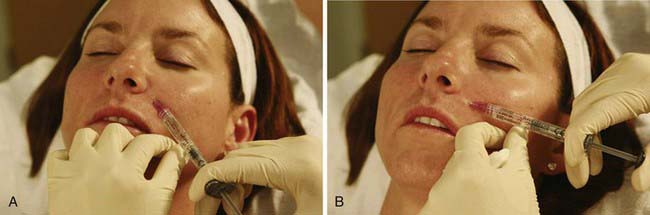
Performing the Dermal Filler Injection
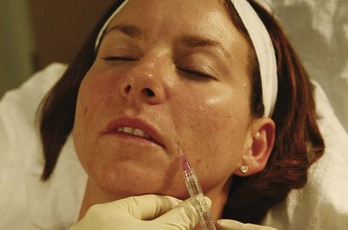
FIGURE 25-9 First injection point for nasolabial fold dermal filler treatment.
(Copyright Rebecca Small, MD.)
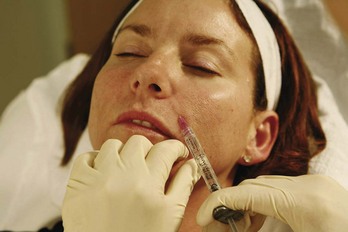
FIGURE 25-10 Second injection point for nasolabial fold dermal filler treatment.
(Copyright Rebecca Small, MD.)
Results
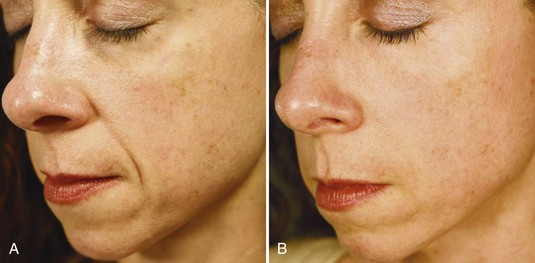
Immediate correction of lines and contour defects are seen with dermal filler injections. Figure 25-12 shows a 38-year-old patient with moderate nasolabial folds (A) before and (B) 1 week after treatment with an HA dermal filler, Juvederm Ultra Plus®, using 0.8 mL (one syringe) in each nasolabial fold for a total volume of 1.6 mL.
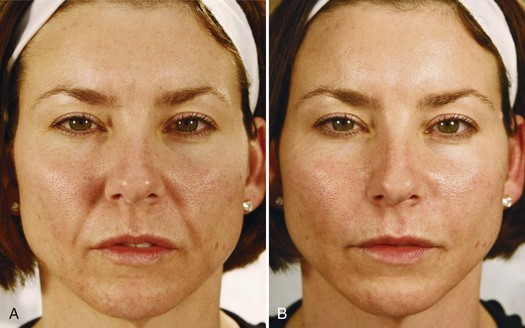
FIGURE 25-12 (A) Before and (B) after hyaluronic acid dermal filler treatment of the nasolabial folds.
(Copyright Rebecca Small, MD.)
Figure 25-13 shows a 46-year-old patient with severe nasolabial folds (A) before and (B) 4 weeks after layering treatment with 1.5 mL of a calcium hydroxylapatite filler, Radiesse®, and 0.8 mL of a hyaluronic acid filler, Juvederm Ultra Plus®, in the nasolabial folds.
Complications
Some degree of transient postinjection bruising, swelling, erythema and tenderness is common and expected with any dermal filler treatment. These typically resolve within a few days to a few weeks.13,14 Specific side effects are associated with each filler product15 and the following discussion focuses on complications reported with hyaluronic acid fillers and their management.
Visible filler lumpiness occurring at the time of, or shortly after treatment, can usually be treated with aggressive compression by the provider. This may require local anesthetic infiltration prior to compression for patient comfort, and a bruise may form as a result of compression. In addition, hyaluronidase has been used by some providers for treatment of HA bumps, but it is not FDA approved for this use (see below).16 Some providers report lancing large filler collections with a scalpel and expressing the product.17
Prolonged or excessive swelling can be treated with regular use of ice, as described above, and oral antihistamines (e.g., cetirizine 10 mg, 1 tablet daily until swelling resolves). Severe swelling and allergic hypersensitivity reactions, such as angioedema, have been reported, and oral or intramuscular steroids are indicated in these rare cases.18
Prolonged erythema is often due to tissue hypervascularity above the dermal filler. Discrete telangiectasias may be present with the erythema. Hypervascularity may be due to vascular congestion from filling the tissue, or an inherent tissue response to the dermal filler product.19 Lasers or intense pulsed light devices specific for reduction of vascularities are useful treatment modalities to reduce erythema associated with hypervascularity. Prolonged erythema may also be due to irritation and/or inflammation in the treatment area, particularly when treatments are performed in highly mobile areas, such as the oral commissures.
Prolonged erythema may stimulate postinflammatory hyperpigmentation, which can be treated with topical lightening products such as hydroquinone (see Chapter 24, Skin Care Products).
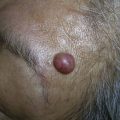
Erythematous tender bumps and nodules (also called inflammatory nodules) are treated as bacterial infections. These can occur immediately after treatment or be delayed, up to a year or more.19 Management consists of empiric antibiotics with a macrolide (e.g., clarithromycin 500 mg BID) or tetracycline (e.g., minocycline 100 mg BID).20,21 A 6-week course of antibiotics is common; however, the duration of treatment depends on the intensity of infection. If fluctuant, it is advisable to incise, drain, and culture the nodule. HA fillers may be degraded by injecting hyaluronidase into the nodular area (10 to 30 units, based on the volume to be removed). New evidence is emerging to suggest that late-onset inflammatory nodules (and perhaps granulomas) may be due to biofilms, which are aggregates of microorganisms within adhesive protective coverings that commonly adhere to foreign bodies, and can be elusive to standard culture methods and highly resistant to antibiotics. Once the dermal filler foreign body is gone, the biofilm is presumed to resolve.20
Granulomas are a delayed complication, appearing 6 months to 2 years after dermal filler treatment, which are much more common with permanent and certain semipermanent fillers such as poly-L-lactic acid. A minority of granulomas spontaneously resolve, however, intralesional steroid injection or excision is often required.15
A Tyndall effect with bluish skin discoloration may occur with superficial placement of HA dermal fillers in thin-skinned areas.17 Product can either be reduced using compression, hyaluronidase, or expressed as described above.22
Ischemia with possible impending tissue necrosis may present with pain or painlessly, and may be visible as a violaceous reticular pattern or white blanching. With NLF treatments, intravascular injection of the angular artery may occur (see Figure 25-2), with ischemic signs visible on the nose and/or nasolabial fold. Ischemia is managed urgently because it can rapidly progress to tissue necrosis. If ischemia occurs, attempt to revascularize by massaging the ischemic area, and if intravascular injection of the artery is suspected, apply heat packs, inject 10 to 30 units of hyaluronidase in the area, and administer aspirin 2 tablets of 325 mg orally. In addition, reports of applying of a vasodilator such as nitropaste to the affected area have also been used successfully.12,23 It is advisable to contact the local emergency room and/or plastic surgeon if ischemia does not rapidly resolve.
Financial Considerations
Cosmetic dermal filler treatments are generally not covered by insurance. In rare instances, coverage for filler treatments to scars may be possible. Dermal filler fees are based on the type of filler used, size and number of syringes, injector skill, and vary according to local prices in different geographic regions. Prices range from $500 to $650 per syringe of hyaluronic acid filler (0.8 mL). Box 25-1 lists CPT codes for DF procedures.
1. Small R. Aesthetic procedures in office practice. Am Fam Physician. 2009;80(11):1231-1237.
2. Wise JB, Greco T. Injectable treatments for the aging face. Facial Plast Surg.. 2006;22(2):140-146.
3. Small R. Aesthetic procedures introduction. In: Mayeaux E, editor. The Essential Guide to Primary Care Procedures. Philadelphia: Lippincott Williams & Wilkins; 2009:195-199.
4. Cosmetic Surgery National Data Bank 2008 Statistics. American Society for Aesthetic Plastic Surgery; 2008.
5. Eppley BL, Dadvand B. Injectable soft-tissue fillers: clinical overview. Plast Reconstr Surg.. 2006;118(4):98e-106e.
6. Small R. Dermal fillers for facial rejuvenation. In: Mayeaux E, editor. The Essential Guide to Primary Care Procedures. Philadelphia: Lippincott Williams & Wilkins; 2009:214-233.
7. Goldman MP. Optimizing the use of fillers for facial rejuvenation: the right tool for the right job. Cosm Dermatol. 2007;20(7 S3):14-26.
8. Johl SS, Burgett RA. Dermal filler agents: a practical review. Curr Opin Ophthalmol. 2006;17(5):471-479.
9. Sadick NS. Soft tissue augmentation: selection, mode of operation, and proper use of injectable agents. Cosm Dermatol. 2007;20(5 S2):8-13.
10. Monheit GD, Coleman KM. Hyaluronic acid fillers. Dermatol Ther. 2006;19(3):141-150.
11. Green MS. Not all hyaluronic acid dermal fillers are equal. Cosm Dermatol. 2007;20(11):724-729.
12. Grunebaum LD, Allemann IB, Dayan S, et al. The risk of alar necrosis associated with dermal filler injection. Derm Surg. 2009;35:1635-1640.
13. Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Derm Surg. 2005;31(11 Pt 2):1616-1625.
14. Gladstone HB, Cohen JL. Adverse effects when injecting facial fillers. Semin Cutan Med Surg. 2007;26(1):34-39.
15. Zielke H, Wolber L, Wiest L, et al. Risk profiles of different injectable fillers: results from the Injectable Filler Safety Study (IFS Study). Derm Surg. 2008;34(3):326-335.
16. Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Derm Surg. 2005;31(8 Pt 1):893-897.
17. Cohen JL. Understanding, avoiding, and managing dermal filler complications. Derm Surg. 2008;34(Suppl 1):S92-S99.
18. Geisler D, Shumer S, Elson M. Delayed hypersensitivity reaction to Restylane. Cosm Dermatol. 2007;20(12):784-786.
19. Narins RS, Jewell M, Rubin M, et al. Clinical conference: Management of rare events following dermal fillers—focal necrosis and angry red bumps. Derm Surg. 2006;32(3):426-434.
20. Narins RS, Coleman WPIII, Glogau RG. Recommendations and treatment options for nodules and other filler complications. Derm Surg. 2009;35(Suppl 2):1667-1671.
21. Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Derm Surg. 2009;35(Suppl 2):1672-1680.
22. Cox SE. Clinical experience with filler complications. Derm Surg. 2009;35:1661-1666.
23. Hirsch RJ, Cohen JL, Carruthers JD. Successful management of an unusual presentation of impending necrosis following a hyaluronic acid injection embolus and a proposed algorithm for management with hyaluronidase. Derm Surg. 2007;33(3):357-360.
Elson M. Dermal fillers. In Pfenninger JL, Fowler GC, editors: Pfenninger and Fowler’s Procedures for Primary Care, 3rd ed, Philadelphia: Mosby/Elsevier, 2011.
Small R, Hoang D. Nasolabial folds. In: Small R, Hoang D, editors. A Practical Guide to Dermal Filler Procedures. Philadelphia: Lippincott Williams & Wilkins, 2011.
Small R, Hoang D. Dermal Filler Introduction and foundation concepts. In: Small R, Hoang D, editors. A Practical Guide to Dermal Filler Procedures. Philadelphia: Lippincott Williams & Wilkins, 2011.
Small R, Hoang D. Anesthesia. In: Small R, Hoang D, editors. A Practical Guide to Dermal Filler Procedures. Philadelphia: Lippincott Williams & Wilkins, 2011.
Small R, Hoang D. Complications. In: Small R, Hoang D, editors. A Practical Guide to Dermal Filler Procedures. Philadelphia: Lippincott Williams & Wilkins, 2011.