Chapter 11 Dementia and Alzheimer’s disease
With contribution from Dr Andrew Pipingas and Ms Danielle Sestito
Introduction
The dementias are conditions that co-occur with, but are not necessarily caused by, increasing age. They cause marked deterioration of cognitive abilities such as memory, reasoning and judgment abilities and, often as a consequence, physical deterioration (such as severe weight loss in individuals who cannot care for themselves), and poor emotional control and mood problems. The dementias are distinct from normal cognitive impairment due to ageing, with many of the progressive dementias ultimately fatal as the brain deterioration eventually causes physical shutdown. Alzheimer’s dementia (AD) is the most common cause of senile dementia, accounting for about 50% of all cases.1 Other dementias include Lewy Body dementia, frontotemporal dementias, vascular dementia, and sub-cortical degenerative dementias. Dementia can also be caused by exposure to toxins (alcohol or toxic medications), brain trauma, and infections (such as Lyme disease and AIDS). Currently, although there are pharmaceutical therapies which help relieve some of the symptoms of AD, there is still no cure for the disease or definite prevention model. Due to the emotional and financial costs of the disorder to patients and society, as well as the increasing ageing population and limitations of care facilities available, it is important that more is understood about prevention and treatment for the dementias.
Table 11.1 summarises the cognitive, physical and emotional symptoms in dementias.
| Cognitive symptoms | Physical and emotional symptoms |
|---|---|
| Memory impairment — including prospective, remote, working, and recent memory | Personality change |
| Aggression | |
| Word-finding deficit | Depression and anxiety |
| Executive dysfunction | Agitation |
| Visuospatial problems (agnosias) | Paranoia and delusions |
| Apraxia | Wandering |
| Delirium | Sleep disturbances |
| Language impairment | Weight loss |
Incidence and prevalence
A recent (2007) study on an American cohort found that about 13% of those older than 70 years had dementia.3 The proportion of Americans over 70 years who had AD specifically was 9.7% (comprising almost 75% of all dementia cases within that cohort). They also found that the prevalence of dementia was related to age, with individuals aged between 71–79 years having a 5% prevalence, however, in individuals over 90 years the prevalence increased to 37.4%.
A recent Australian study, published in 2008, reported about 5.6% of males and 6% of females between 70–79 years have dementia. For those aged between 85–89 years, the prevalence in males is 12.8% and females, 20.2%.4 Similar to the American study, they found prevalence markedly increased again for those aged over 90 years, with dementia seen in 22.1% of males and 30.8% of females.4 Generally, the risk of developing dementia in middle age is very low (the risk is only about 1% for those who are 65) and then increases rapidly with increasing age.1 Consistent with many countries with ageing populations, in Australia the prevalence of dementia is expected to increase fourfold by the year 2050, impacting significantly on the community, both financially and socially.5
Risk factors
Lifestyle — general
Obesity and high body mass index
Two longitudinal population-based studies support the link between mid-life obesity and later life dementia. One study analysed health data from 10 276 men and women who were aged between 40–45 when they underwent detailed health evaluations during the period of 1964 to 1973.6 Upon re-evaluation in later life, it was found that the mid-life weight–dementia risk link appeared to be linear, with obese individuals (those with a Body Mass Index [BMI] calculation >30), having a 74% increased risk of dementia and overweight individuals (with a BMI range of 25–29) having a 35% greater dementia risk in comparison to individuals who were within the normal BMI range. Another study followed a sample of 7402 men who were aged between 47–55 when they attended medical exams during 1970–1973. They underwent a follow-up exam in 1998 and these results displayed a similar pattern to the previous research after controlling for smoking, socioeconomic status, blood pressure, diabetes and serum cholesterol.7 They found that those individuals with higher BMI (22.50–24.99) had a substantially increased dementia risk than those who had low to normal BMI (20.00–22.49) at mid-life. The results from these studies suggest that obesity in mid-life may pose a substantial risk for developing dementia in later life above and beyond other risk factors (such as smoking and cholesterol).
Interestingly, the results of an American cardiovascular health study yielded different results.8 While this study also measured the link between mid-life weight and dementia, it also compared obesity in late-life with dementia. While the 2798 participants initially evaluated were found to be free from dementia in mid-life, they observed, in line with other studies, that those who were overweight or obese initially had a higher risk of developing dementia in later life. However, the study prospectively followed participants for a follow-up evaluation and found that in later life, a BMI within the normal range was associated with a higher risk of dementia, while a higher BMI was unrelated to increased dementia risk. The authors concluded that this reverse effect in later life was a reflection of the physical changes that occur in demented individuals as they approach a disabled status. This finding is supported by other studies which show that higher baseline BMI and slower declining BMI during later life is associated with a reduced risk of dementia.9
Alcohol intake
A longitudinal study of an elderly community sample free of dementia at baseline looked at cognitive functions and self-reported drinking habits. Drinking habits were assessed every 2 years and were followed for an average of 7 years.10 Cognitive measures were compared across 3 groups categorised using self-reported drinking habits. The groups compared were: no drinking, minimal drinking and moderate drinking. Interestingly they observed that mild to moderate drinking was associated with lesser declines in learning and memory tests compared with those who abstained from alcohol completely. This relationship was found to be more pronounced when comparing those who had always consumed alcohol or those who no longer drank alcohol to those who had abstained from alcohol consumption over a lifetime.10
A recent systematic review and meta-analysis of 23 studies (mainly epidemiological in nature) also concluded that small amounts of alcohol in earlier adult life may be protective against developing dementia (risk ratio [RR] 0.63; 95% CI 0.53–0.75) and Alzheimer’s disease (RR 0.57; 0.44–0.74) later in life, and less for vascular dementia (RR 0.82; 0.50–1.35) or cognitive decline (RR 0.89; 0.67–1.17).11
Environment
Smoking
Researchers from The Netherlands studied the effect of smoking on cognitive decline over a 5-year period at middle age (43–70 years). They found that among smokers, as numbers of cigarettes smoked and number of years spent smoking increased, functioning in a number of cognitive indices declined.12 Another study found that having stiff lungs, which impairs an individual’s ability to blow out a large volume of air quickly, was associated with increased risk of dementia.13 Additionally, a recent stratified random sample study of non-smokers found a relationship between exposure to second-hand smoke and cognitive decline.14
Sunshine — vitamin D deficiency
Generally, low serum vitamin D levels have been shown to be linked to dementia and poorer performance on cognitive tasks. Vitamin D deficiency is common in the elderly, particularly as they spend more time indoors, especially with hospitalisation and institutionalisation.The majority of cross-sectional research has found a correlation between vitamin D serum levels and cognitive function with low serum 25-hydroxvitamin D concentration being associated with increased probability of cognitive impairment.15–18 Similarly, low vitamin D status has been linked to higher risk of dementia. Individuals with normal cognitive function had higher levels of serum 25(OH) D than those who were cognitively impaired. Those with lowest serum level concentrations were 4 times more likely to be demented. Interestingly, there has been mixed results with 1 cross-sectional study revealing that between different age ranges, the relationship between levels of vitamin D and cognitive performance is different.19 They found that for younger age groups there was no relationship between vitamin D status and cognition, however in elderly age groups they found an opposite effect; that those with the highest level of serum vitamin D had the largest impairment on the task (4809 participants were in this age group). It must be noted, however, that in this sample while the participants were representative of their age group, none had dementia. Recently, a study found that vitamin D status was a better predictor of dementia than the more commonly evaluated BMI or serum albumin.20 One very large scale study (the European Male Ageing Study, EMAS) which evaluated 3369 men aged 40–79 found that serum vitamin D was related to cognitive performance.21 They observed that those with higher serum 25-hydroxyvitamin D levels had better performance in a number of cognitive measures than individuals with lower serum levels, and this effect was observed particularity in older participants.
Generally, large-scale reviews of the relationships between vitamin D and dementia conclude Ethat findings remain unclear, biological plausibility of relationship is supported, and thus there is an argument for the need for long-term, well-designed trials22.
Heavy metal exposure
Metal exposure is of interest to researchers as a possible risk factor for dementia given that in AD accumulation of metals within the brain form characteristic amyloid plaques, a key pathological trait in the disorder. Additionally, studies have observed that certain metals, such as copper, aluminium and zinc have been implicated in the precipitation and cytotoxicity of amyloid protein.23 One recent study has found evidence that high exposure to aluminium in drinking water was significantly associated with increased dementia risk,24 and this finding has been replicated in a number of epidemiological studies.25
Mind—body medicine
Education
Research has found that attaining a higher education is an independent protective factor for dementia and cognitive decline, however this has been debated.26 On the other hand, studies investigating later-life cognitive stimulation practices for individuals who were already demented improved some cognitive domains as well as the individual’s quality of life when compared to individuals who received their regular treatment in the absence of cognitive stimulation.27 This result has been mirrored in prospective studies within non-clinical populations, where older individuals (75 years +) with increased cognitive activity during their leisure activities show reduced risk of developing mild cognitive impairment.28
Work complexity
A case-control study, controlling for age, gender, and level of education found that the more complex the work (either with people or with data) was associated with reduced risk of AD.29 The study included 10 079 members of the population-based Swedish Twin Registry who were participants in the HARMONY study. They analysed data with case-control and co-twin control designs. The co-twin control design provides control over genetic and familial factors.
Loneliness and socialisation
Social isolation in old age has been associated with the risk of developing dementia, but the risk associated with perceived isolation, or loneliness, is not well understood. A longitudinal clinicopathologic cohort study found that the lonely persons had almost twice the risk of developing dementia compared with those who were not classified as lonely.30 This effect remained apparent even after controlling for social isolation. This study observed that more lonely people showed lower indices of cognitive function at baseline and increased rapid cognitive decline at follow-up.
Pets
A recent review summarised 9 studies that investigated dog therapy for older people with dementia residing within residential aged care.31 The most common findings were substantial decreases in agitated episodes and increased socialisation during dog contact. Interestingly, the improvements seen in social behaviour were unrelated to dementia severity. Additionally, improvements on various measures of overall function were also observed in those receiving dog therapy.
Personality
Personality testing found Japanese centenarians have optimistic attitudes, adaptability, and an easy-going approach to life.32 Strong social integration and a deep spirituality were particularly evident among older women when in their prime of life. They scored low when it came to feelings of ‘time urgency’ and ‘tension’ and high in ‘self-confidence’ and ‘unyieldingness.’
In another study, investigators linked high neuroticism, a personality trait, to dementia.33 They assessed the personality traits among 506 dementia-free individuals participating as part of the Kungsholmen Project (Sweden) and prospectively followed them for 6 years on average. The main trends observed were for participants scoring lowest in trait neuroticism and combined with high trait extraversion were associated with reduced risk of dementia. Additionally, it was also observed that in individuals who were socially isolated, even low neuroticism alone was associated with decreased dementia risk.
Physical activity
The results of a prospective cohort study published in Annals of Internal Medicine suggests that regular exercise may assist delaying the onset of dementia and AD.35 In this study, 1740 individuals aged 65 years and older were tested on cognitive domains, exercise and other health outcomes and re-tested after approximately 6 years. While 158 participants developed dementia during this period (107 developed AD), there was a reduced incidence of dementia and AD in participants who exercised more frequently (greater than 3 times a week).
Recently, researchers investigated whether late life physical activity in elderly men would have an effect on the risk of developing dementia. A key finding they uncovered was that that elderly men who had poor physical functionality and underwent high levels of physical activity, had half the risk of developing dementia than a matched group who underwent minimal physical exercise. This finding remained significant after controlling for other variables. They postulated that general physical activity may protect against or delay the onset of dementia.36 Outdoor exercise would gain the added benefits of sun exposure for vitamin D and fresh air.
Nutritional influences
Diet
Fruits and vegetables
A study tested whether consumption of fruit and vegetable juices, containing a high concentration of polyphenols, decreases the risk of incident-probable AD. This was a prospective-design study which followed 1836 participants who were dementia-free during baseline testing (between 1992–1994) through to 2001.37 They found that the hazard ratio for probable AD was much lower in those participants who reported consuming juices at least 3 times per week than those who reported consuming less than 3 serves of juice per week. Interestingly, this effect was more apparent in individuals who both carried an apolipoprotein E ε-4 allele and did not exercise often.
Another study which found a gene–diet relationship evaluated the results from 8085 non-demented participants aged 65 and over as part of the Three-City cohort study. While they also found that frequent consumption of fruits and vegetables was related with a lower dementia risk in all cases, those who consumed a diet high in omega-6 fats and did not carry the ApoE 4 gene had an increased risk of dementia and a diet high in fish and omega-3 rich oils was protective.38, 39
Mediterranean diet
There is converging evidence that composite dietary patterns such as the Mediterranean diet are related to lower risk for cardiovascular disease, several forms of cancer, and overall mortality. The Mediterranean diet is generally comprised of a high intake of fruit, vegetables, legumes and grains, preferring fish to red meat, mainly cooking with olive oil and the consumption of a moderate amount of red wine.40 A recent review concluded that essential components of the Mediterranean diet, including poly-unsaturated fatty acids (PUFA’s), cereals and wine, seem to be protective against cognitive decline.41 A study prospectively evaluated 2258 community-based non-demented individuals in New York every 1.5 years, and found that higher adherence to the Mediterranean diet is associated with a reduction in risk for AD.42 A multiethnic community study using Cox proportional hazards method found that higher adherence to the Mediterranean diet is associated with a trend for reduced risk of developing MCI and with reduced risk of MCI conversion to AD.43
Okinawa diet
Observing inhabitants of the Japanese island, Okinawa, they generally have a long life expectancy and demonstrate low prevalence of dementia. Outside of genetic factors, lifestyle appears to be a main contributor for longevity. Okinawan’s consume more nutrient-dense, calorie-starved foods such as soy, fruits, vegetables, wholegrains, sprouts, broth-based soups, seaweed, sweet potatoes, fish, beans, and yogurt, and infrequent consumption of breads, cheese, oil, nuts, meats and sweets. Other features of Okinawan’s include eating small frequent meals (till 80% full), drinking water, green and/or black tea, plenty of exercise through physical labour, martial arts, dance and maintaining a strong community bond that uplifts the spirit.33
Green tea
In analysis of cross-sectional data from a community-based Comprehensive Geriatric Assessment, Japanese researchers examined the association between green tea consumption and cognitive function in humans.44 The assessment comprised 1003 individuals aged 70 years or older, who answered self-report questions about the frequency of green tea consumption. The participants had their cognitive function evaluated, scored using the Mini-Mental State Examination (MMSE). The research observed that the higher consumption of green tea, the lower the prevalence of cognitive impairment. Interestingly, the findings for green tea were highly statistically significant whereas a similar analysis for black or oolong tea and coffee was not statistically significant. Currently, however, there is a lack of substantial clinical trials or long-term prospective design studies, which need to be carried out in order to make any definitive claims for green tea.
Caffeine
Caffeine is a drug which is very widely consumed, in the Western world. Previous experimental models have demonstrated some neuroprotective effects of caffeine at low doses when administered chronically.45 Subsequent research has investigated the association between caffeine consumption in midlife and AD in late life, with mixed results. One study reported mid-life caffeine consumption to be inversely related to AD while controlling for other variables.46, 47 In a more recent study, the lowest risk of Alzheimer’s disease was found in individuals who consumed on average 3–5 cups of coffee per day in midlife.46 A prospective study into the effects of caffeine consumption over a 2 and 4 year follow-up found that only in women was high daily consumption of caffeine associated with reduced cognitive decline in some measures.48 The research surrounding caffeine as a possible preventative for later life dementia is lacking and requires larger, long-term studies before any definitive claims can be made.
Nutritional deficiency
Nutrient deficiency is common in the elderly and may impact adversely on cognition and memory.49, 50, 51 For example, older individuals generally spend less time outdoors and are more likely to be deficient in vitamin D, but those individuals who maintain an outdoor life into older age retain serum vitamin D levels similar to individuals of younger ages.52 Additionally, research has shown that some essential nutrients are unable to be taken up through food source alone in older individuals, particularly for those in care.53 The elderly who have a limited range of foods (e.g. tea and toast), those with dentures limiting proper chewing and those on medication and suffering diseases are also more prone to nutrient deficiencies. Given the relationship between nutrient deficiency and cognitive decline it is therefore prudent to maintain adequate nutrient levels in middle to later life and blood screening is advisable to assess nutrient levels such as vitamin B6, B12, folate, vitamin D and magnesium.51
Magnesium deficiency
Previous studies have suggested that magnesium may be a useful dietary supplement in the treatment of Alzheimer’s disease. A recent study on a clinical sample compared severity of symptoms/clinical stage of the dementia with current magnesium levels.54 They found a relationship between serum magnesium levels and the degree of severity of dementia symptoms, with lower levels indicating significantly worse scores on measures of cognition.
Table 11.2 summarises the lifestyle risk factors that can contribute to cognitive decline, dementia and AD.
| Obesity |
| Work complexity |
| No or high alcohol intake |
| Low education |
| Loneliness and social isolation |
| High neuroticism and stress |
| High exposure to heavy metals in drinking water |
| Smoking |
| Lack of sunshine; vitamin D deficiency |
| Lack of exercise and/or physical activity |
| Dietary factors (e.g. high saturated fat intake) |
| Vitamin and mineral deficiencies (e.g. vitamin D, B6/12, folate and magnesium) |
Integrative management of dementia
Mind–body medicine
Meditation
Meditation practices have various health benefits including the possibility of preserving cognition and preventing dementia. While the exact mechanisms remain investigational, there is some evidence from a few cognitive, brain electrical (EEG), and structural neuro-imaging studies. In 1 cross-sectional study, meditation practitioners were found to have a lower age-related decline in the thickness of specific cortical regions.55 They found that, compared to non-mediators of the same age, the differences in cortical thickness were most pronounced in the older participants, suggesting that meditation may have contributed to offsetting age-related cortical thinning. Additionally, the thickness of 2 cortical regions correlated with meditation experience.
Cognitive behavioural therapy (CBT)
Anxiety is a common symptom in dementia, often contributing to limited independence and a greater need to be placed in nursing home care. One study assessed CBT as a means to treat dementia related anxiety, comparing the results from 2 patients who were treated with a dementia-specific modified version of CBT.56 The researchers made modifications in the content, structure, and learning strategies of CBT in the treatment process, with the patients receiving education and awareness training and were taught the skills of diaphragmatic breathing, coping self-statements, exposure, and behavioural activation. They found improvements in anxiety as measured by standardised rating scales. CBT has also been shown to be beneficial in treating anxiety in the caregivers and family members of dementia sufferers.57, 58
Additionally, positive belief-based CBT has been used for teaching mnemonic strategies to older adults who were still in the early stages of dementia as a means to improve cognitive function. A 7-week group intervention study used CBT to address unhelpful memory-related beliefs in 3 older men with mild/moderate dementia and associated low mood or anxiety.59 While results were promising, the researchers concluded that further research into the use of CBT is required.
Music and relaxation therapy
Relaxation can help lower the levels of agitation and stress in AD patients and their caregivers. Simple techniques such as massage, therapeutic and physical touching can help keep patients calm by allaying anxiety. A review of the literature of 8 research-based articles, found that demented older individuals who listened to their preferred music during the study intervention period had positive results, one of which was the reduction of some agitated behaviours.60 The review cautioned that while only the findings in 1 study did not reach statistical significance, the sample numbers were small and further investigation was necessary.
Aromatherapy
Aromatherapy is the use of pure essential oils from fragrant plants to help relieve health problems and improve the quality of life in general. Aggressiveness, restlessness and excitability are experienced by a large proportion of people with severe dementia, and can be distressing for patients and caregivers. Aromatherapy may play a role in helping to alleviate these symptoms.
One study of 72 people living in long-term care facilities in the UK were randomly assigned to aromatherapy (essential lemon balm oil) or placebo (sunflower oil), combined with a base lotion.61 Caregivers applied the oils onto the patients’ faces and arms twice a day. Over 4 weeks of treatment, the aromatherapy treatment group resulted in a 35% improvement in agitation compared to 11% with placebo treatment. Also, scores on quality of life indices also significantly improved. The lemon balm appeared to be well tolerated and safe with no serious adverse side-effects.
A Cochrane review identified 3 randomised control trials (RCTs) of aromatherapy for dementia, however, found several methodological difficulties with 2 of the studies. The review concluded ‘a statistically significant treatment effect in favour of the aroma therapy intervention on measures of agitation and neuropsychiatric symptoms’ in 1 of the trials.62 More research is required.
Brain training
A recent Cochrane review investigated whether cognitive training and cognitive rehabilitation improved symptomatology within individuals with dementia.63 The review concluded that no definite protective effects were observed and the authors argued that more studies of a larger power and of a better methodological design were required before concluding the efficacy of such training.
There have also been a number of studies evaluating the efficacy of brain training on cognitive processes and activities of daily living in older, cognitively healthy participants. Overall, the findings have been positive, with a number of studies investigating large cohorts in controlled clinical trials. In a study of 2832 persons living in 6 US cities, 10 sessions of computerised cognitive training were used that included specific cognitive domains such as episodic memory and reasoning.64 Each intervention improved the targeted cognitive ability compared with baseline. In addition, booster training improved specific cognitive functions that were sustained at a 2-year follow-up. Improvements due to training were of the same magnitude as the amount of age-associated decline expected over a 7–14 year period in individuals without dementia. In a follow-up study, brain training was shown to improve trained cognitive abilities and this was maintained after 5 years.65 The intervention included 10 sessions of training and then follow-up booster training at 11 and 35 months. Importantly, brain training also resulted in a reduced functional decline in activities of daily living over this time.
Two other recent studies have also shown benefits associated with cognitive training. In 1 of these studies a cohort of 487 participants used a cognitive training battery and showed improvements in memory and attention after 8 weeks (1 hour per day, 5 days per week).66 In the other study, improvements were found both in the cognitive domains being trained and also in non-related standardised neuropsychological tasks.67 The authors concluded that ‘intensive plasticity-engaging training can result in an enhancement of cognitive function in normal mature adults’.
There is also evidence of neurobiological changes that occur as a result of brain training suggesting possible neural underpinnings associated with brain training studies outlined above. In a neuro-imaging study, the density of Dopamine D1 receptors were assessed before and after 14 hours (5 weeks) of working memory training.68 There were significant changes in D1 binding potential in both prefrontal and parietal brain regions ‘demonstrating a reciprocal interplay between mental activity and brain biochemistry in vivo’.
Sleep
Insomnia
Insomnia is a common problem in AD. In a randomised-control trial of 36 community dwelling patients with AD and suffering sleep problems, 17 were randomised into active treatment with their caregivers receiving specific treatment about setting up a sleep hygiene program and training in behaviour management skills.69 They were also instructed to walk daily and to increase daytime light exposure with the use of a light box. Control subjects (n = 19) received general dementia education and caregiver support. The active group demonstrated a significant (p<.05) reduction in the number of night-time awakenings, total time awake at night, and depression. These benefits persisted at 6-month follow-up.
Sunshine
Sunshine is the main source of vitamin D produced by the body in response to direct skin exposure to UVB. This means that no or minimal exposure to sun can contribute to vitamin D deficiency as seen in community groups with dress codes (e.g. wearing veils), living in geographical prone areas (e.g. in high and low altitudes) especially over winter, working indoors (e.g. office work), institutionalisation, prolonged hospitalisation and bed-bound people, particularly in dark skin people who need longer sun exposure.70, 71
There is a growing body of evidence that vitamin D deficiency can contribute to the risk of dementia (see above under risk factors). Vitamin D appears to have a role in neuro-protection and reduce the risk of dementia.72 Interestingly, an Australian study has found that during heat waves, admissions to hospital for a variety of disorders, including dementias, were significantly increased, as well as increased mortality in individuals with dementia.73 Heat waves impact upon mental health.
Light therapy
Light therapy may be beneficial in improving some cognitive and non-cognitive symptoms of dementia. A 2008 study showed that light therapy reduced cognitive decline and ameliorated depressive symptoms in individuals with dementia.74 Moreover, in the same study mood was adversely affected with administration of melatonin, often administered to improve insomnia. The authors recommended that melotonin should only be taken in combination with light therapy. Their results suggested that the bright light therapy did demonstrate an improvement in some cognitive domains, especially in the participants who were in the initial stages of AD. Their results suggested that bright light therapy improved cognitive functions, especially in individuals who were in early stages of AD.75 However, a 2004 Cochrane review found that there was insufficient evidence to suggest that light therapy is effective in managing sleep, behaviour, cognitive, or mood disturbances associated with dementia.76 The authors found only 3 studies that fulfilled all inclusion criteria and recommended that more properly constructed studies are necessary to draw solid conclusions.
Physical and mental activity
Exercise
The relationship between physical exercise and cognition in older individuals has been investigated in a number of studies. One study showed that either memory-based cognitive exercises or cardiovascular exercises resulted in older individuals out-performing age-matched participants in tests of memory functioning.77 Additionally, the implementation of a 24-week physical activity program in adults who believed they had a memory impairment (but were dementia free) found adequate improvements in memory, even after an 18 month follow-up period.78 Another recent study found that dementia-free elderly individuals significantly improved in fluid intelligence measures after participation in 2 x 1 hour general exercise classes (conducted over a period of 6 months), compared to a no-exercise control group or a group who participated in a flexibility based relaxation class.79 Recent Cochrane reviews, however, indicated that the bank of research into the effects of exercise and dementia are still inconclusive due to insufficient research methods.80
Tai chi
A study compared healthy individuals aged 45–74 years from Fuyang city, 53 of whom practiced tai chi exercise for half a year or more, and 48 were ‘no exercise’ controls.81 The participants were compared across 3 age groups: 45–54 years, 55–64 years, and 65–74 years. Additionally, the tai chi group was subdivided into 3 groups according to experience in tai chi: ≤3 years, 4–6 years, and ≥7years. The cognitive function of both tai chi experienced and no exercise control groups declined with age, but this decline was slower in the tai chi group. In addition, people who had practiced tai chi for a longer duration had less cognitive decline than those with less experience. The researchers concluded that long-term tai chi exercise can possibly benefit cognitive function in middle-aged and old people.
A recent study examined performance on cognitive and physical tasks in 20 older adult subjects after participation in a 10-week tai chi program.82 They found that compared to baseline, 2 measures of executive cognition had improved. Collectively these studies suggest some beneficial effects of tai chi for cognition in older adults; however, more controlled randomised trials are required for conclusive evidence.
Nutritional influences
Diet
A high dietary intake of fruit, vegetables, legumes, wholegrains, fish and olive oil is protective of cognition and towards reducing the risk of dementia (refer to risk factor section above, under diet). Therefore, encouraging a Mediterranean diet may play a role in helping to reduce any further deterioration of cognition and memory in patients with established dementia and Alzheimer’s disease.40–43
Nutritional supplements
As outlined above (in the risk factor section, under nutrient deficiency), adequate nutrition is essential for maintaining health and cognition in old age. Research has shown that in older persons in particular it can be difficult to obtain optimum nutritional status through food sources alone, and thus additional supplementation may be required.53 Additionally, as outlined within the following section, research has uncovered evidence of certain vitamins, nutrients and herbs with potential to act as complementary therapeutic agents for dementia.
Vitamins B6, B12 and folate
The majority of conclusions drawn for the importance of substantial B vitamin status, including folic acid, are derived from studies finding that compromised brain functioning is linked to vitamin deficiencies.83 In the normal elderly population, vitamin deficiency is quite common, leading to the suggestion that B vitamin status is related to cognitive performance or possibly dementia, however, current findings do not paint a clear picture. One study postulates the link between B vitamin deficiency and impairment of methylation reactions leading to the damage of brain tissue.83 Another theory that relates to B vitamin deficiency postulates that elevated homocysteine levels are responsible for cognitive dysfunction given that elevated homocysteine has been linked to vascular disease, dementia and AD. One recent randomised placebo-controlled trial aimed to determine the effects of folic acid, vitamin B12, riboflavin, and vitamin B6 supplementation on homocysteine levels and cognitive function.84 The trial was comprised of 185 patients who suffered ischaemic vascular diseases and were aged 65 years or more. After 1 year, homocysteine levels were lower in the group receiving a combination of folic acid and B12 than patients not receiving the treatment. However, this folic acid/vitamin B12 combined treatment did not significantly alter cognitive performance; neither did a riboflavin/vitamin B6 combined treatment. However, a separate more recent cross-sectional study in the Japanese elderly population reported an independent positive association between reduced folate and vitamin B12 levels and cognitive decline.85
This prospective 5-year study measured markers of vitamin B12 status and brain volume loss in older individuals (aged 61–87 years). They found that individuals with lower B12 vitamin status displayed greater decreases in brain loss and the relationship remained significant after controlling for ‘age, sex, creatinine, education, initial brain volume, cognitive test scores, systolic blood pressure, ApoE 4 status, homocysteine and folate’.85 Another study revealed that elderly individuals with a pre-existing vitamin B12 deficiency and mild to moderate dementia did show an improvement in cognitive function with B12 supplementation, however those with advanced dementia displayed an improvement in neurological symptoms only.86
A cross-sectional and longitudinal analysis published in the American Journal of Medicine in 2005 in a cohort of 499, 70–79 year olds, demonstrated those with elevated homocysteine levels or low levels of folate or vitamin B6, demonstrated worse baseline cognitive function.87 After adjusting for variables, those with bottom quartile folate levels had a 1.6 fold increased risk of being in the worst quartile of 7-year cognitive decline. Reduced folate levels appear to be a greater risk factor for cognitive decline. Chan et al.88 examined the efficacy of a vitamin/nutraceutical formulation in a 12-month pilot trial in individuals with early-stage Alzheimer’s disease. The formulation contained a mixture of folate, vitamin B6, vitamin E, s-adenosyl methionine, N-acetyl cysteine and acetyl-L-carnitine. Those taking the formulation performed better on neuropsychiatric and daily living measures than the individuals who were taking pharmacological medications or placebo. While a larger clinical trial is required to confirm these preliminary findings, generally previous large cross section studies have found a trend between adequate folate intake (at or above the RDI) and reduced risk of AD.89, 90 A similar result was found by Wouters-Wesseling et al. (2005)90 who administered a vitamin-nutrient enriched drink to elderly individuals in their double-blind, randomised, placebo-controlled study. The 67 participants received either the drink or placebo for 6 months. They found significant differences after 6 months in a word learning test, and a category fluency test compared to the placebo group. Additionally, they observed that vitamin B12 levels significantly increased and homocysteine levels decreased in the supplement group compared to the placebo group.
Generally a large number of studies have observed that increasing folic acid and vitamin B6 and B12 concentrations decrease homocysteine levels.85, 92–100 Considering the findings of some improved cognition in elderly individuals with supplementation with B vitamins and folate there appears to be merit to the relationship between homocysteine and cognitive decline.
A recent Cochrane review of 8 RCTs found nothing significant or consistent enough to suggest that folic acid, (either with or without vitamin B12), had a beneficial effect on cognitive function in older people, unless possibly when baseline homocysteine levels were high.101
A very recent Australian double-blind, placebo-controlled, randomised trial administered 2mg of folate, plus 25mg of B6 and 400 μg of B12, or placebo to 299 men aged 75 years or older, for a period of 2 years.102 They found that those in the treatment group had significantly lower levels of Aβ protein 1–40 than the placebo group. While the participants in this sample were dementia free, the study demonstrated the possibility of a simple AD prevention strategy given that Aβ protein was lowered significantly with B vitamin supplementation.102
Antioxidants
A review of this subject by the Lancet in 1997 suggests protective effects of fruit and vegetables against stroke and vascular dementia may be related to their antioxidant content.103 Recently, research has provided significant findings to link mitochondrial oxidative damage or the ‘free radical theory’ of ageing and neurodegenerative diseases such as AD. Researchers in the AD field are beginning to recognise the possible involvement of a mutant APP and its derivatives in causing mitochondrial oxidative damage in AD, and are thus looking at the role of dietary antioxidants in older populations. However, there are conflicting reports about the potential role of vitamin antioxidants.
Vitamin antioxidants
Antioxidants such as vitamins C and E may have an important role through scavenging of increased free-radical formation and repair impaired antioxidant defences. Longitudinal data examined from the Canadian Study of Health and Ageing, a population-based, prospective 5-year investigation of the epidemiology of dementia among Canadians aged 65+ years, suggest a possible protective effect for antioxidant vitamins in relation to cognitive decline.104 The study included 894 subjects with no dementia. Over a 5-year period, subjects who were consuming combined vitamin E and C supplements and/or multivitamin consumption at baseline were significantly less likely to experience significant cognitive decline during the 5-year follow-up period. Subjects reporting any antioxidant vitamin use at baseline also showed a significantly lower risk for vascular cognitive impairment but no evidence for reduced risk of dementia or AD.
A larger scale study of 4740 elderly people, published in Archives of Neurology, yielded similar results.105 This study demonstrated the use of combined vitamin C and E supplements, but not if used alone, was associated with reduced AD prevalence. Additionally, the study revealed that individuals who were taking antioxidant vitamins E and C in combination with non-steroidal anti-inflammatory drugs at baseline declined in Modified-MMSE scores 0.96 points less every 3 years than non-users, and this effect was entirely attributed to individuals who carried the APOE e4 allele (whose scores declined by 2.25 points fewer on the M-MMSE than non-users).
However, in a double-blind study published in the NEJM of 769 subjects, 212 who developed AD, compared with placebo, there were no significant differences in the risk of progression to AD in the vitamin E group or the anti-cholinesterase inhibitor donepezil group during 3 years of treatment.106 On the other hand, Sano et al. (1997)107 conducted a double-blind, placebo-controlled, randomised trial including patients with moderately severe AD who received either Selegiline (a selective monoamine oxidase inhibitor), or vitamin E or a combination of both for 2 years. The authors concluded from the study that either treatment appeared to slow the progression of the disease in terms of ‘functional deterioration’ leading to nursing home placement relative to placebo; however the authors noted that possible problematic methodology may have contributed to the positive findings.107, 108
Another study of 2889 elderly residents published in Archives of Neurology, found higher vitamin E intake from foods or supplements, is associated with less cognitive decline with age and slowing the progression of AD.109 After adjusting for other factors that can influence cognitive status, the researchers found a 36% reduction in the rate of cognitive decline among persons consuming the highest amount of total vitamin E (combined food and supplement sources) compared to those getting the least. This finding has been replicated in other previous age-matched cross sectional research.104
Subsequent meta analysis of placebo-controlled, randomised trials have yet to find significant cognitive differences with vitamin E supplementation alone despite cross-sectional observations.110 Vitamin E combined with vitamin C may be the critical factor in demonstrating benefit.104,105 Additionally, subjects who reported taking antioxidant multivitamins at baseline also displayed a lower risk of vascular cognitive impairment, however no reduced risk for incident dementia or AD was observed.111
Other supplements
Melatonin
A small double-blind study that examined the effects of 3mg/day of melatonin in AD patients found that melatonin administration improved sleep time and reduced night activity.112 Cognitive and non-cognitive functions were also improved.
However, a recent Cochrane review that included 3 studies exploring the effects of melatonin in patients with dementia or cognitive impairment, found currently the available evidence is insufficient to support the effectiveness of melatonin in managing the cognitive and -cognitive symptoms of dementia.113
Herbal supplements
Ginkgo (Ginkgo biloba)
Extracts of the leaves of Ginkgo biloba tree have been used in traditional Chinese medicine for thousands of years. Studies suggest several mechanisms for its benefits, including vasodilation, reducing blood viscosity, and modifying neurotransmitter systems. In a recent Cochrane review (2009)35 controlled clinical trials were included that investigated Ginkgo biloba in the treatment of cognitive decline and dementia.114 The authors concluded that Ginkgo biloba appears to be safe compared with placebo. Early trials prone to highly variable quality of preparations and use of unsatisfactory methods showed mixed results. The authors commented on 4 recent trials; 1 of these showing positive effects on cognition, activities of daily living, mood, depression and carer burden. The other 3 were negative. Overall the authors conclude: ‘The evidence that Ginkgo biloba has predictable and clinically significant benefit for people with dementia or cognitive impairment is inconsistent and unreliable’.114 Examples of findings from a number of these trials will now be discussed.
One 42-month pilot trial found no significant effect of Ginkgo biloba to either alter the risk of progression from normal functioning to clinically demented or protect against memory decline, however the authors noted that when participant compliance was taken into account, those with high compliance did show a significant effect although the compliant sample size was too small to be conclusive.115 A randomised, double-blind placebo-controlled study which was conducted for 26 weeks compared the effects of both 120mg/day and 240mg/day of Ginkgo biloba supplementation with placebo. This study also failed to yield any significant results overall, however did report that in a subgroup of patients with neuropsychiatric symptoms there was a significant beneficial effect of the treatment on cognitive and global functioning observed in comparison to no treatment.116 The researchers suggested that the null findings overall could have been due to the lack of decline in the placebo group, lessening the sensitivity of the results. Another study matched non-demented 60–70 year olds participants on education level and compared 8 months of Ginkgo biloba supplementation on both neuropsychological tests as well as tests of blood viscosity and regional cerebral perfusion117. The authors reported that those taking Ginkgo biloba displayed a reduction in blood viscosity, improved cerebral blood flow in specific areas and improved global cognitive functioning. Very recently, a study compared the effects of 160mg daily dose of a Ginkgo biloba special extract, EGb 761; a pharmaceutical used often for the treatment of dementia, Donepezil (5mg/day) and placebo over a period of 24 weeks.118 The patients (aged between 50–80 years) in the study were all diagnosed as having mild to moderate Alzheimer’s dementia. They found that the clinical efficacy of the Ginkgo biloba was comparable to the Donepezil, and concluded that it would be an efficient alternative treatment for those in the mild/moderate stages.
Brahmi (Bacopa monniera)
Brahmi is an Indian herb and traditional Ayurvedic medicine with reported memory-enhancing, anti-inflammatory, antioxidative, analgesic, sedative, antipyretic and antiepileptic properties. The memory-enhancing properties of the herb however have drawn a greater deal of research attention, with a number of relatively recent randomised placebo-controlled trials finding positive memory effects of the herb in adult and elderly populations. Brahmi contains Bacoside A and Bacoside B which are steroidal saponins believed to be essential for the clinical efficacy of the product and may have several modes of action in the brain all of which may be useful in ameliorating cognitive decline in the elderly which include: (1) direct cholinergic properties; (2) antioxidant (flavonoid) properties; (3) metal chelation; (4) anti-inflammatory; (5) increases blood circulation; (6) adaptogenic activity; and (7) removal of β-amyloid deposits.
One study investigated the effects of 90 days supplementation with 300mg Bacopa monniera (BM) compared to placebo.119 They found that individuals taking the supplement had significantly improved performance on a spatial working memory task. In addition, participants also responded with less false positives in a rapid visual processing task. Another study which utilised the same BM supplement, employing the same dosage but with neuropsychological testing after supplementation of only 5 and 12 weeks also found that, compared to placebo, that those taking the BM supplement performed significantly better on a visual processing inspection time task and demonstrated improved learning rate and memory consolidation.120 However, previous somewhat conflicting evidence has also been observed in a separate placebo-controlled, randomised independent groups study. Roodenrys et al. (2002)121 investigated learning and memory following 3 months of supplementation utilising the same dosage and same BM branded supplement as Stough et al. (2001)120 and Stough et al. (2008),119 however only found significant differences between experimental and placebo group on 1 measure of delayed recall. They found that less was forgotten in the BM group compared to placebo after about 90 day’s supplementation in a paired-word recall task however they found no effects of reduced forgetting in a story reconstruction memory task, or in any other learning indices.
Other studies on elderly individuals and individuals with age-associated memory impairments have found significant improvements following BM supplementation. Calabrese et al. (2008)122 tested 48 older men without clinical signs of dementia, with 24 individuals in each experimental and control group. They were supplemented with 300mg/day BM or placebo for 12 weeks. The findings revealed improved performance in BM group relative to placebo after 12 weeks on word recall scores and performance on the Stroop task (filtering of irrelevant information). Another study investigated the effects of only 125mg/day supplementation for 12 weeks on a group of individuals with age-associated memory impairments compared to a matched placebo group. They found that the BM supplement improved performance on mental control, logical memory and paired associated learning tasks.
Overall the results look promising for the use of Brahmi supplementation for improved memory however human clinical trials are required in order to investigate whether the herb is efficacious in dementia patients. While some preliminary animal models of dementia have shown promising results with BM supplementation,123 whether the same effects can be seen in human patients is not yet known.
Lemon balm (Melissa officinalis)
Melissa officinalis or lemon balm is a traditional herbal medicine which can be used as a mild sedative, muscle relaxant and as an antibacterial agent. Due to studies in vitro which display the cholinergic binding properties of the herb, it has been postulated that it may be effective in the treatment of AD. One study has investigated the effects of lemon balm extract compared to placebo on 42 patients (aged between 65–80) with mild to moderate AD.124 The randomised, double-blind trial went over a 4-month period, and at 4 months, those taking the lemon balm extract performed significantly better on cognitive tasks than placebo.
Sage (Salvia officinalis)
Sage is a herbal extract which has displayed pharmacological actions in humans which are relevant to AD; for example, antioxidant effects, anti-inflammatory activity, estrogenic activity, and cholinesterase inhibition.125
Sage appears to be well tolerated by Alzheimer’s patients. An open-label pilot study administered oral Salvia lavandulaefolia (part of the Salvia officinalis family) to AD patients for 6 weeks and found that generally the herb was well tolerated and improvements in memory were seen.125 A double-blind, randomised placebo-controlled trial of Salvia officinalis has also been conducted in patients with mild to moderate AD.126 The study lasted 4 months and patients were administered 60 drops/day of the treatment. The researchers observed significantly improved memory measures in the treatment group compared with placebo group and no significant differences were observed in side-effects, with the only exception being that the placebo group did appear to experience agitated episodes more often than the experimental group.
Ginseng (Panax ginseng)
Ginseng is a herbal remedy which has been used in eastern Asian cultures for several thousand years. It has been used to treat many different disorders, and recently has been implicated with enhancing mental performance and influencing the ageing process.127 While there are some clinical trials on cognitive benefits of ginseng within younger populations, to date not much is known about the benefits of chronic ginseng administration and dementia. The limited clinical research indicates that while it is relatively safe to use in small doses, the current evidence for efficacy of the herb for long-term clinical benefit is not well supported.128 A very recent study investigated the effects of Panax ginseng administered 4.5g/day for 12 weeks to Alzheimer’s patients. At follow-up, those within the treatment group displayed significant improvements in the ADAS-cog and MMSE scores. However, after discontinuing treatment scores returned to control levels. Overall, the limited recent clinical data appears promising for the use of ginseng in AD as long as the use is continued. There is a need however for larger trials of a longer duration.
Korean Red Ginseng
Clinical data relating ginseng to dementia specifically has found Korean Red Ginseng (KRG) to be beneficial for cognition in patients with AD.129 They administered either 4.5g/day or 9g/day of KRG or placebo for 12 weeks, and found improvement within the high-dose group compared to control in all cognitive measures. Another study administered a standardised ginseng vitamin complex daily for 9 months and found improvements in memory for the treatment group.130 All participants within this study had age-associated memory impairment, but an MMSE score no less than 24.
Turmeric (Curcuma longa)
Turmeric has been used in India for at least 2500 years, and is known for possessing antiseptic, anti-inflammatory, detoxifying, and carminative properties. Various studies and research results have displayed that the prevalence of AD in India among individuals aged between 70–79 years is 4.4 times less than that US adults of the same age range.131 Studies have shown that curcumin (polyphenols contained in turmeric) inhibits the formation of beta-amyloid oligomers and fibrils by binding to plaques in vitro and in vivo.132 Tze-Ping Ng et.al (2006)133 conducted an epidemiologic study investigating dietary curry consumption and cognition. They found that in individuals who reported consuming both low and moderate amounts of curry in their diet, better cognitive performance was observed. Additionally, the amount of curry consumed, whether ‘low’ or ‘moderate’ was not significantly related to better cognitive performance between groups. This is consistent with biological data which demonstrates that low but not high doses of curcumin reduced beta-amyloid and plaque burden.134
Other epidemiologic data shows that among cultures which regularly consume curry (Chinese, Malay, Indian), the difference in MMSE scores are more pronounced in the Indian cultures, who consume curry more frequently than other cultures.133 Overall however, there is a great need for clinical trials to investigate the causal effects of curcumin in cognition and dementia.
To date, only 1 randomised, placebo-controlled, double-blind clinical trial pilot has been conducted to investigate the effects of curcumin on AD.135 The study duration was 6 months and included individuals who were 50 years or older and had a diagnosis of either AD or probable AD. Only 27 participants finished the trial, which had 3 arms — 8 subjects on placebo, 8 subjects on 1 gm/day curcumin and 11 subjects taking 8gm of curcumin/day. Overall, the researchers were not able to find a significant decline in the placebo group over the 6 month period, and suggested that this was probably the reason for the nullified results as the curcumin is thought to work by slowing progression of the cognitive decline rather than improving cognitive function. The researchers argue that their findings warrant a larger, longer term study into the effects of curcumin.
Resveratrol (resveratrol-grape skin extract; trans-3,4,5-trihydroxystilbene)
Epidemiological studies have suggested that moderate consumption of red wine, which is enriched in resveratrol, is related to a lower incidence of dementia and Alzheimer’s disease.136 A recent study found that resveratrol significantly lowered levels of amyloid-beta peptides, a finding the authors suggest may have therapeutic potential in AD.137 Additionally, rodent study findings have suggested theoretical implications for wine in treatment of dementia in humans.138, 139
Other supplements
Omega-3 fatty acids/fish oil
There is a plethora of evidence to support the use of dietary omega-3 polyunsaturated fatty acid (PUFA) for reducing the risk of dementia and cardiovascular disease in the elderly. Postulated mechanisms include its anti-atherogenic, anti-inflammatory, antioxidant, anti-amyloid and neuroprotective properties. A recent Cochrane review (2006) found no randomised trials in the literature, but acknowledged ‘a growing body of evidence from biological, observational and epidemiological studies that suggests a protective effect of omega-3 PUFAs against dementia’.140 This review concluded that until data from randomised trials becomes available that there is no compelling evidence to support the use of omega-3 PUFA for the prevention of cognitive impairment or dementia.
Several systematic review papers suggest an increase of saturated fatty acids could have negative effects on cognitive function, with a clear reduction of risk of cognitive decline found in populations with a high intake of PUFAs, particularly weekly fish consumption.141, 142
More recently, several epidemiological studies have demonstrated that regular fish consumption is associated with a decreased risk of dementia and Alzheimer’s disease, as well as better cognitive performance overall in the older population.143, 144 Additionally, total omega-3 PUFAs within the plasma or erythrocyte membranes is related to slower cognitive decline and a reduced dementia risk,145, 146, 147 and dietary supplementation with PUFAs has been associated with improved cognitive function in individuals with mild cognitive impairment or organic brain impairment.148
Recent double-blind, randomised, placebo-controlled trials have yielded some interesting but mixed results. One study supplemented 174 patients diagnosed with AD with 1.7g/day of docosahexaenoic acid and 0.6g/day of eicosapentaenoic acid (ω-3 fatty acid treatment group) or a placebo for 6 months duration.149 They found that only within a subgroup of patients who had only a mild cognitive dysfunction (an MMSE score >27), compared to the untreated group, a reduction in the MMSE decline rate was observed. In another study 64 mild or moderate AD patients were supplemented with 1.8g/day total omega-3 PUFAs or placebo for a period of 24 weeks.150 Overall, the treatment group observed better improvement as rated by the Clinician’s Interview-Based Impression of Change Scale than the placebo group at 24 weeks, however only the subgroup of individuals with mild cognitive impairment showed improvements on cognitive measures. A third study with a similar study design but employing elderly but cognitively healthy individuals receiving either 1800mg, or 400mg of omega-3 EPA-DHA or placebo for 26 week did not improve on the measures of cognition.151 Overall, the clinical results show the ω-3 supplementation appears beneficial for patients with quite mild cognitive impairment.
A very recent (2009) systematic review published in Nature Reviews found that overall the current findings provide significant evidence that long-chain omega-3 fatty acids slow cognitive decline in elderly individuals without dementia, however does not prevent dementia.152 They point out however that this may be due to limitations of the current dementia-omega-3 studies.
Coenzyme Q10
Bragin et al. (2005) investigated an integrative treatment approach on cognitive performance.153 The study sample comprised of 35 individuals (mean age = 71) who were diagnosed with both mild dementia and depression. Patients were given treatment of antidepressants, cholinesterase inhibitors, as well as vitamins and supplements (including CoQ10). The integration of the treatments in conjunction with healthy eating and regular exercise was found to improve cognition, especially executive activity and memory. However, the specific role that CoQ10 played in this improvement is unknown.
Physical therapies
Musculoskeletal
One study found that 6 months of a physiotherapeutic intervention improved participants’ balance, but overall there were no improvements observed in global cognition with the intervention.154 However, when the physiotherapeutic intervention was carried out on a multidisciplinary basis, attenuation in the decline of global cognition in specific cognitive domains was observed.
Massage
Agitation in individuals with dementia living in the nursing home environment affects care and quality of life. Relaxation techniques such as music and massage are showing promise to decrease agitation and improve quality of life in individuals with dementia. Massage has been found to significantly decrease agitation in AD patients when combined with the individual’s favourite music.155
Acupuncture
Acupuncture is often used as a treatment for dementia and is claimed to be effective in improving intelligence. A recent review found that the few studies which have been carried out to date do not demonstrate significant effectiveness of acupuncture for AD.156 A recent prospective study investigated the effect of electro-acupuncture (EA) in the treatment of vascular dementia compared with a medication group.157, 158 They found improvements from baseline across measures in the ‘acupuncture only’ group however far greater improvements were seen in the medication group. Acupuncture could be explored as an adjunctive therapy, however more research is required.
Conclusion
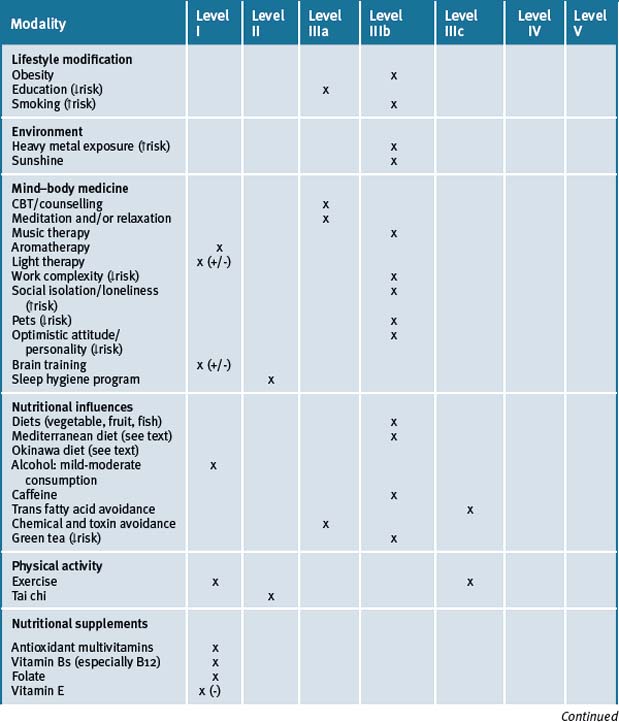
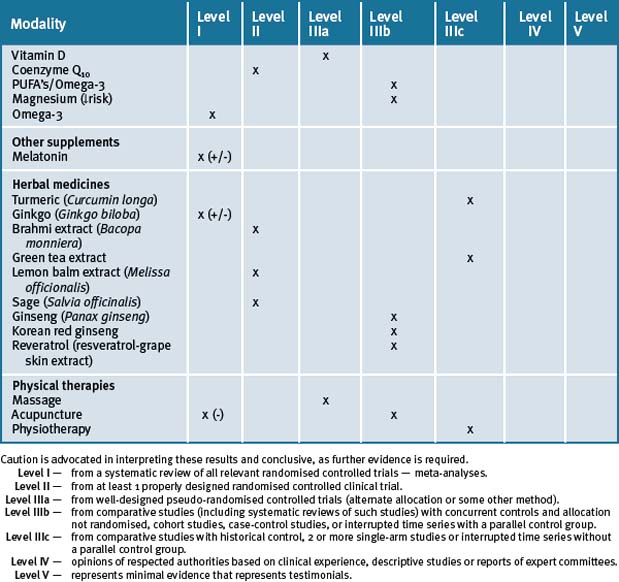
Overall it is evident that there are many options when considering complementary strategies for treatment of the dementias. Currently, while there is good scientific rationale for a complementary medicinal approach using vitamins and herbal extracts, more randomised, double-blind clinical trials are required to confirm safety and efficacy (see Table 11.3). Additionally, it appears that a number of lifestyle and behavioural changes can be considered in middle age for prevention and management of dementia in older age.
Clinical tips handout for patients — dementia and Alzheimer’s disease
1 Lifestyle advice
Sunshine
3 Mind–body medicine
Brain training
4 Environment
5 Dietary changes
7 Supplements
Multivitamin especially B-group containing vitamins B6, B12, and folate
Folate
Vitamin B12
Multivitamins/antioxidants
Vitamin D (cholecalciferol)
90% of vitamin D sources come from sunshine, however, supplementation may still be required.
Doctors should check blood levels and suggest supplementation if levels are low.
Minerals
Magnesium and calcium (best provided together)
CoEnzyme Q10
Fish oils
Herbal medicines
Ginkgo (Ginkgo biloba)
Turmeric (Curcuma longa)
Lemon balm (Melissa officinalis)
Other supplements
Melatonin
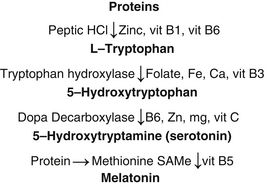
Melatonin is naturally produced by the body from tryptophan and requires various minerals and vitamins for its production. Deficiency of any of these may lead to reduced production of melatonin.
For a diagrammatic representation of how melatonin is produced in the human brain see Figure 11.1
1 Dementia Kempler D.. Brace-Thompson J., editor. Neurocogntive Disorders in Aging. Sage Publications, California, USA, 2005;179-201.
2 Kwok J., Schofield. The Molecular basis of Alzheimer’s Disease. In: Sachdev, editor. The Ageing Brain; The Neurobiology and neuropsychology of Ageing. Lisse, The Netherlands: Swets & Zeitlinger Publishers; 2003:173-186.
3 Plassman B.L., Langa K.M., Fisher G.G., et al. Prevalence of Dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29(1-2):125-132.
4 Nepal B., Brown L., Ranmuthugala G. Years of life lived with and without dementia in Australia, 2004-2006: A population health measure. Australian and New Zealand Journal of Public Health. 2008;32(6):565-568.
5 Access Economics. Dementia prevalence and incidence among Australians who do not speak English at home. Alzheimer’s Australia. 2006:1-27.
6 Whitmer R.A., Gunderson E.P., Barrett-Connor E., Quesenberry C.P.Jr., Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360.
7 Rosengren A., Skoog I., Gustafson D., Wilhelmsen L. Body Mass Index, Other Cardiovascular Risk Factors, and Hospitalization for Dementia. Arch Intern Med. 2005;165(3):321-326.
8 Fitzpatrick A.L., Kuller L.H., Lopez O.L., et al. Midlife and Late-Life Obesity and the Risk of Dementia: Cardiovascular Health Study. Arch Neurol. 2009;66(3):336-342.
9 Hughes T.F., Borenstein A.R., Schofield E., Wu Y., Larson E.B. Association between late-life body mass index and dementia: The Kame Project. Neurology. 2009;72(20):1741-1746.
10 Ganguli M., Bilt J.V., Saxton J.A., Shen C., Dodge H.H. Alcohol consumption and cognitive function in late life: A longitudinal community study. Neurology. 2005;65(8):1210-1217.
11 Peters R., Peters J., Warner J., Beckett N., Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 2008;37(5):505-512.
12 Wang H.X., Karp A., Herlitz A., et al. Personality and lifestyle in relation to dementia incidence. Neurology. 2009;72(3):253-259.
13 Nooyens A.C.J., van Gelder B.M., Verschuren W.M.M. Smoking and Cognitive Decline Among Middle-Aged Men and Women: The Doetinchem Cohort Study. Am J Public Health. 2008;98(12):2244-2250.
14 Simons L.A., Simons J., McCallum J., Friedlander Y. Lifestyle factors and risk of dementia: Dubbo study of the elderly. Medical Journal of Australia. 2006;184(2):68-70.
15 Llewellyn D.J., Lang I.A., Langa K.M., Naughton F., Matthews F.E. Exposure to secondhand smoke and cognitive impairment in non-smokers: national cross sectional study with cotinine measurement. BMJ. 2009;338(feb12_2):b462.
16 Przybelski R.J., Binkley N.C. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Archives of Biochemistry and Biophysics. 2007;460(2):202-205.
17 Wilkins C.H., Sheline Y.I., Roe C.M., Birge S.J., Morris J.C. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. American Journal of Geriatric Psychiatry. 2006;14(12):1032-1040.
18 Oudshoorn C., Mattace-Raso F.U.S., Van Der Velde N., Colin E.M., Van Der Cammen T.J.M. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2008;25(6):539-543.
19 Jorde R., Waterloo K., Saleh F., Haug E., Svartberg J. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels: The Tromsø study. Journal of Neurology. 2006;253(4):464-470.
20 McGrath J., Scragg R., Chant D., Eyles D., Burne T., Obradovic D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology. 2007;29(1-2):49-54.
21 Ravaglia G., De Ronchi D., Forti P., et al. Nutritional status and dementia in oldest-old women. Archives of Gerontology and Geriatrics. 1998;26(Suppl1):427-430.
22 Lee D.M., Tajar A., Ulubaev A., et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
23 Barnham K.J., Bush A.I. Metals in Alzheimer’s and Parkinson’s Diseases. Current Opinion in Chemical Biology. 2008;12(2):222-228.
24 Domingo J.L. Aluminum and other metals in Alzheimer’s disease: A review of potential therapy with chelating agents. Journal of Alzheimer’s Disease. 2006;10(2):331-341.
25 Rondeau V., Jacqmin-Gadda H., Commenges D., Helmer C., Dartigues J- F. Aluminum and Silica in Drinking Water and the Risk of Alzheimer’s Disease or Cognitive Decline: Findings From 15-Year Follow-up of the PAQUID Cohort. Am J Epidemiol. 2009;169(4):489-496.
26 Shcherbatykh I., Carpenter D.O. The Role of Metals in the Etiology of Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2007;11(2):191-205.
27 Ngandu T., von Strauss E., Helkala E.L., et al. Education and dementia: What lies behind the association? Neurology. 2007;69(14):1442-1450.
28 Knapp M., Thorgrimsen L., Patel A., et al. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. The British Journal of Psychiatry. 2006;188(6):574-580.
29 Verghese J., LeValley A., Derby C., et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66(6):821-827.
30 Andel R., Crowe M., Pedersen N.L., et al. Complexity of Work and Risk of Alzheimer’s Disease: A Population-Based Study of Swedish Twins. J Gerontol B Psychol Sci Soc Sci. 2005;60(5):P251-P258.
31 Wilson R.S., Krueger K.R., Arnold S.E., et al. Loneliness and risk of Alzheimer disease. Archives of General Psychiatry. 2007;64(2):234-240.
32 Perkins Jacqueline, HBCT J.R. Dog-assisted therapy for older people with dementia: A review. Australasian Journal on Ageing. 2008;27(4):177-182.
33 Willcox B., Willcox D., Todoriki H., et al. Caloric Restriction, the Traditional Okinawan Diet, and Healthy Aging. Annals of the New York Academy of Sciences. 2007;1114(Healthy Aging and Longevity Third International Conference):434-455.
34 Buell J.S., Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: Preventing “D”ecline? Molecular Aspects of Medicine. 2008;29(6):415-422.
35 Larson E.B., Wang L., Bowen J.D., et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Annals of Internal Medicine. 2006;144(2):73-81.
36 Taaffe D.R., Irie F., Masaki K.H., et al. Physical Activity, Physical Function, and Incident Dementia in Elderly Men: The Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2008;63(5):529-535.
37 Qi D., Amy R.B., Yougui W., James C.J., Eric B.L. Fruit and Vegetable Juices and Alzheimer’s Disease: The Kame Project. The American Journal of Medicine. 2006;119(9):751-759.
38 Barberger-Gateau P., Raffaitin C., Letenneur L., et al. Dietary patterns and risk of dementia: The Three-City cohort study. Neurology. 2007;69(20):1921-1930.
39 Yochum L., Kushi L.H., Meyer K., Folsom A.R. Dietary Flavonoid Intake and Risk of Cardiovascular Disease in Postmenopausal Women. Am J Epidemiol. 1999;149(10):943-949.
40 Eckert G.P. The Mediterranean diet to prevent Alzheimer’s disease. Mediterrane ernährung zur prophylaxe der Alzheimer demenz. 2008;55(8):480-485.
41 Panza F., Solfrizzi V., Colacicco A.M., et al. Mediterranean diet and cognitive decline. Public Health Nutrition. 2004;7(07):959-963.
42 Nikolaos Scarmeas YSM-XTRMJAL. Mediterranean diet and risk for Alzheimer’s disease. Annals of Neurology. 2006;59(6):912-921.
43 Scarmeas N., Stern Y., Mayeux R., Manly J.J., Schupf N., Luchsinger J.A. Mediterranean Diet and Mild Cognitive Impairment. Arch Neurol. 2009;66(2):216-225.
44 Kuriyama S., Hozawa A., Ohmori K., et al. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr. 2006;83(2):355-361.
45 de Mendonca A., Sebastiao A.M., Ribeiro J.A. Adenosine: does it have a neuroprotective role after all? Brain Res Brain Res Rev. 2000;33(2-3):258-274.
46 Maia L., De Mendonça A. Does caffeine intake protect from Alzheimer’s disease? European Journal of Neurology. 2002;9(4):377-382.
47 Eskelinen M.H., Ngandu T., Tuomilehto J., Soininen H., Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. Journal of Alzheimer’s Disease. 2009;16(1):85-91.
48 Ritchie K., Carrière I., De Mendonça A., et al. The neuroprotective effects of caffeine: A prospective population study (the Three City Study). Neurology. 2007;69(6):536-545.
49 Bartali B., Frongillo E.A., Guralnik J.M., et al. Serum Micronutrient Concentrations and Decline in Physical Function Among Older Persons. JAMA. 2008;299(3):308-315.
50 Loikas S., Koskinen P., Irjala K., et al. Vitamin B12 deficiency in the aged: a population-based study. Age Ageing. 2007;36(2):177-183.
51 Deanne G., Lucinda J.B., Elisabeth A.I., Stacey H., Fran S., Judith D.B. Malnutrition prevalence and nutrition issues in residential aged care facilities. Australasian Journal on Ageing. 2008;27(4):189-194.
52 Scragg R., Camargo C.A.Jr. Frequency of Leisure-Time Physical Activity and Serum 25-Hydroxyvitamin D Levels in the US Population: Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;168(6):577-586.
53 Grieger J.A., Nowson C.A. Nutrient intake and plate waste from an Australian residential care facility. Eur J Clin Nutr. 2006;61(5):655-663.
54 Çilliler A.E., Öztürk S., Özbakir S. Serum Magnesium Level and Clinical Deterioration in Alzheimer’s Disease. Gerontology. 2007;53(6):419-422.
55 Lazar S.W., Kerr C.E., Wasserman R.H., et al. Meditation experience is associated with increased cortical thickness. NeuroReport. 2005;16(17):1893-1897.
56 Kraus C.A., Seignourel P., Balasubramanyam V., et al. Cognitive-behavioral treatment for anxiety in patients with dementia: Two case studies. Journal of Psychiatric Practice. 2008;14(3):186-192.
57 Akkerman R.L., Ostwald S.K. Reducing anxiety in Alzheimer’s disease family caregivers: The effectiveness of a nine-week cognitive-behavioral intervention. American Journal of Alzheimer’s Disease and other Dementias. 2004;19(2):117-123.
58 Secker D.L., Brown R.G. Cognitive behavioural therapy (CBT) for carers of patients with Parkinson’s disease: A preliminary randomised controlled trial. Journal of Neurology,. Neurosurgery and Psychiatry. 2005;76(4):491-497.
59 Kipling T., Bailey M., Charlesworth G. The feasibility of a cognitive behavioural therapy group for men with mild/moderate cognitive impairment. Behavioural and Cognitive Psychotherapy. 1999;27(2):189-193.
60 Sung H.C., Chang A.M. Use of preferred music to decrease agitated behaviours in older people with dementia: A review of the literature. Journal of Clinical Nursing. 2005;14(9):1133-1140.
61 Ballard C., O’Brien J., Reichelt K., Perry E. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind, placebo-controlled trial with Melissa. Journal of Clinical Psychiatry. 2002;63(7):553-558.
62 Thorgrimsen L., Spector A., Wiles A., Orrell M. Aroma therapy for dementia. Cochrane database of systematic reviews (Online). (3):2003.
63 Clare L., Woods R.T., Moniz Cook E.D., Orrell M., Spector A., et al. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. (4):2003.
64 Ball K., Berch D.B., Helmers K.F., et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271-2281.
65 Willis S.L., Tennstedt S.L., Marsiske M., et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805-2814.
66 Smith G.E., Housen P., Yaffe K., et al. A cognitive training program based on principles of brain plasticity: results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. J Am Geriatr Soc. 2009;57(4):594-603.
67 Mahncke H.W., Connor B.B., Appelman J., et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci U S A. 2006;103(33):12523-12528.
68 McNab F., Varrone A., Farde L., et al. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323(5915):800-802.
69 McCurry S.M., Gibbons L.E., Logsdon R.G., Vitiello M.V., Teri L., et al. Nighttime Insomnia Treatment and Education for Alzheimer’s Disease: A randomized, controlled trial. Journal of the American Geriatrics Society. 2005;53(5):793-802.
70 Caroline A.B., Hodan Y.A., Dianne E.C., Angela V., John D.W., et al. Vitamin D deficiency: a study of community beliefs among dark skinned and veiled people. International Journal of Rheumatic Diseases. 2008;11(1):15-23.
71 Diamond T., Levy S., Smith A., Day P., et al. High bone turnover in Muslim women with vitamin D deficiency. The Medical Journal of Australia. 2002;177:139-141.
72 Grant W.B. Does Vitamin D Reduce the Risk of Dementia? Journal of Alzheimer’s Disease. 2009;17(1):151-159.
73 Hansen A., Bi P., Nitschke M., Ryan P., Pisaniello D., Tucker G. The effect of heat waves on mental health in a temperate Australian City. Environmental Health Perspectives. 2008;116(10):1369-1375.
74 Riemersma-van Der Lek R.F., Swaab D.F., Twisk J., Hol E.M., Hoogendijk W.J.G., Van Someren E.J.W. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA – Journal of the American Medical Association. 2008;299(22):2642-2655.
75 Ito T., Yamadera H., Ito R., Endo S. Effects of bright light on cognitive disturbances in Alzheimer-type dementia. Nippon Ika Daigaku zasshi. 1999;66(4):229-238.
76 Forbes D., Morgan D.G., Bangma J., Peacock S., Pelletier N., Adamson J. Light therapy for managing sleep, behaviour, and mood disturbances in dementia. Cochrane database of systematic reviews (Online). (2):2004.
77 Chan A.S., Ho Y.C., Cheung M.C., Albert M.S., Chiu H.F.K., Lam L.C.W. Association between mind-body and cardiovascular exercises and memory in older adults. Journal of the American Geriatrics Society. 2005;53(10):1754-1760.
78 Lautenschlager N.T., Cox K.L., Flicker L., et al. Effect of Physical Activity on Cognitive Function in Older Adults at Risk for Alzheimer Disease: A Randomized Trial. JAMA. 2008;300(9):1027-1037.
79 Brown A.K., Liu-Ambrose T., Tate R., Lord S. The Effect of Group-Based Exercise on Cognitive Performance and Mood in Seniors Residing in Intermediate Care and Self-Care Retirement Facilities: A Randomized Controlled Trial. Br J Sports Med. 2008. bjsm.2008.049882
80 Forbes D., Forbes S., Morgan D.G., Markle-Reid M., Wood J., Culum I. Physical activity programs for persons with dementia. Cochrane Database of Systematic Reviews. (3):2008.
81 Zhang N.N., La X.B., Ni W., Mao W.Q. Effect of long-term Tai Chi exercise on cognitive function of middle-aged and old people. Chinese Journal of Clinical Rehabilitation. 2006;10(26):7-9.
82 Matthews M., Williams H. Can Tai chi enhance cognitive vitality? A preliminary study of cognitive executive control in older adults after A Tai chi intervention. Journal of the South Carolina Medical Association. 2008;104(8):255-257.
83 Selhub J., Bagley L.C., Miller J., Rosenberg I.H. B vitamins, homocysteine, and neurocognitive function in the elderly1. Am J Clin Nutr. 2000;71(2):614s-6620.
84 Stott D.J., MacIntosh G., Lowe G.D.O., et al. Randomized controlled trial of homocysteine-lowering vitamin treatment in elderly patients with vascular disease. Am J Clin Nutr. 2005;82(6):1320-1326.
85 Vogiatzoglou A., Refsum H., Johnston C., et al. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71(11):826-832.
86 Leischker A.H., Kolb G.F. Vitamin-B12 deficiency in the elderly. Vitamin-B12-mangel im alter. 2002;4(3):120-126.
87 Kado D.M., Karlamangla A.S., Huang M.-H., et al. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. The American Journal of Medicine. 2005;118(2):161-167.
88 Chan A., Paskavitz J., Remington R., Rasmussen S., Shea T.B. Efficacy of a vitamin/nutriceutical formulation for early-stage Alzheimer’s disease: A 1-year, open-label pilot study with an 16-month caregiver extension. American Journal of Alzheimer’s Disease and other Dementias. 2009;23(6):571-585.
89 Luchsinger J.A., Tang M.X., Miller J., Green R., Mayeux R. Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Archives of Neurology. 2007;64(1):86-92.
90 Corrada M.M., Kawas C.H., Hallfrisch J., Muller D., Brookmeyer R., et al. Reduced risk of Alzheimer’s disease with high folate intake: The Baltimore Longitudinal Study of Aging. Alzheimer’s and Dementia. 2005;1(1):11-18.
91 Wouters-Wesseling W., Wagenaar L.W., Rozendaal M., et al. Effect of an enriched drink on cognitive function in frail elderly persons. Journals of Gerontology – Series A Biological Sciences and Medical Sciences. 2005;60(2):265-270.
92 Aisen P.S., Schneider L.S., Sano M., et al. High-Dose B Vitamin Supplementation and Cognitive Decline in Alzheimer Disease: A Randomized Controlled Trial. JAMA. 2008;300(15):1774-1783.
93 Schulz R.J. Homocysteine as a biomarker for cognitive dysfunction in the elderly. Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10(6):718-723.
94 Frick B., Gruber B., Schroecksnadel K., Leblhuber F., Fuchs D. Homocysteine but not neopterin declines in demented patients on B vitamins. Journal of Neural Transmission. 2006;113(11):1815-1819.
95 Troen A., Rosenberg I. Homocysteine and cognitive function. Seminars in Vascular Medicine. 2005;5(2):209-214.
96 McMahon J.A., Green T.J., Skeaff C.M., Knight R.G., Mann J.I., Williams S.M. A controlled trial of homocysteine lowering and cognitive performance. New England Journal of Medicine. 2006;354(26):2764-2772.
97 Clarke R., Smith A.D., Jobst K.A., Refsum H., Sutton L., Ueland P.M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Archives of Neurology. 1998;55(11):1449-1455.
98 Zhang C.E., Tian Q., Wei W., et al. Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiology of Aging. 2008;29(11):1654-1665.
99 Scott T.M., Tucker K.L., Bhadelia A., et al. Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. American Journal of Geriatric Psychiatry. 2004;12(6):631-638.
100 Brain shrinkage from homocysteine?. Health news, 9(3). Waltham, Mass, 2003. 8
101 Malouf R., Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane database of systematic reviews (Online). (4):2008.
102 Flicker L., Martins R.N., Thomas J., et al. B-vitamins reduce plasma levels of beta amyloid. Neurobiology of Aging. 2008;29(2):303-305.
103 Rosemary L., Martin O.. The Lancet. 1997, 1189-1190.
104 Maxwell C.J., Hicks M.S., Hogan D.B., Basran J., Ebly E.M. Supplemental use of antioxidant vitamins and subsequent risk of cognitive decline and dementia. Dementia and Geriatric Cognitive Disorders. 2005;20(1):45-51.
105 Zandi P.P., Anthony J.C., Khachaturian A.S., et al. Reduced Risk of Alzheimer Disease in Users of Antioxidant Vitamin Supplements: The Cache County Study. Arch Neurol. 2004;61(1):82-88.
106 Petersen R.C., Thomas R.G., Grundman M., et al. Vitamin E and Donepezil for the Treatment of Mild Cognitive Impairment. N Engl J Med. 2005;352(23):2379-2388.
107 Sano M., Ernesto C., Thomas R.G., et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. New England Journal of Medicine. 1997;336(17):1216-1222.
108 Grundman M.. Vitamin E and Alzheimer disease: The basis for additional clinical trials. American Journal of Clinical Nutrition. 2000;2:71..
109 Morris M.C., Evans D.A., Bienias J.L., Tangney C.C., Wilson R.S. Vitamin E and Cognitive Decline in Older Persons. Arch Neurol. 2002;59(7):1125-1132.
110 Mgekn Isaac, Quinn R., Tabet N. Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database of Systematic Reviews. (3):2008.
111 Gray S.L., Anderson M.L., Crane P.K., et al. Antioxidant vitamin supplement use and risk of dementia or Alzheimer’s disease in older adults. Journal of the American Geriatrics Society. 2008;56(2):291-295.
112 Asayama K., Yamadera H., Ito T., Suzuki H., Kudo Y., Endo S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. Journal of Nippon Medical School. 2003;70(4):334-341.
113 Jansen S.L., Forbes D.A., Duncan V., Morgan D.G. Melatonin for cognitive impairment. Cochrane database of systematic reviews (Online). (1):2006.
114 Birks J., Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. (1):2009. CD003120
115 Dodge H.H., Zitzelberger T., Oken B.S., Howieson D., Kaye J. A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology. 2008;70(19_Part_2):1809-1817.
116 Schneider L.S., DeKosky S.T., Farlow M.R., Tariot P.N., Hoerr R., Kieser M. A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer’s type. Current Alzheimer Research. 2005;2(5):541-551.
117 Santos R.F., GaldurÃz J.C.F.3, Barbieri A., Castiglioni M.L.V., Ytaya L.Y., Bueno O.F.A. Cognitive Performance, SPECT, and Blood Viscosity in Elderly Non-demented People Using Ginkgo Biloba. Pharmacopsychiatry. 2003;36(4):127-133.
118 Mazzaa M., Capuanob A., Briaa P., Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer’s dementia in a randomized placebo-controlled double-blind study. European Journal of Neurology. 2006;13:981-985.
119 Stough C., Downey L.A., Lloyd J., et al. Examining the nootropic effects of a special extract of Bacopa monniera on human cognitive functioning: 90 Day double-blind placebo-controlled randomized trial. Phytotherapy Research. 2008;22(12):1629-1634.
120 Stough C., Lloyd J., Clarke J., et al. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology. 2001;156(4):481-484.
121 Roodenrys S., Booth D., Bulzomi S., Phipps A., Micallef C., Smoker J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology. 2002;27(2):279-281.
122 Calabrese C., Gregory W.L., Leo M., Kraemer D., Bone K., Oken B. Effects of a Standardized Bacopa monnieri Extract on Cognitive Performance, Anxiety, and Depression in the Elderly: A Randomized, Double-Blind, Placebo-Controlled Trial. The Journal of Alternative and Complementary Medicine. 2008;14(6):707-713.
123 Bhattacharya S.K., Kumar A., Ghosal S. Effect of Bacopa Monniera on animal models of Alzheimer’s disease and perturbed central cholinergic markers of cognition in rats. Research Communications in Pharmacology and Toxicology. 1999;4(3-4):II1-II12.
124 Akhondzadeh S., Noroozian M., Mohammadi M., Ohadinia S., Jamshidi A.H., Khani M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomised, placebo controlled trial. Journal of Neurology Neurosurgery and Psychiatry. 2003;74(7):863-866.
125 Perry N.S.L., Bollen C., Perry E.K., Ballard C. Salvia for dementia therapy: Review of pharmacological activity and pilot tolerability clinical trial. Pharmacology Biochemistry and Behavior. 2003;75(3):651-659.
126 Akhondzadeh S., Noroozian M., Mohammadi M., Ohadinia S., Jamshidi A.H., Khani M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomized and placebo-controlled trial. Journal of Clinical Pharmacy and Therapeutics. 2003;28(1):53-59.
127 Glenn M.B., Lexell J. Ginseng. The Journal of Head Trauma Rehabilitation. 2003;18(2):196-200.
128 Vogler B., Pittler M.H., Ernst E. The efficacy of ginseng. A systematic review of randomised trials. European Journal of Clinical Pharmacology. 1999;55:567-575.
129 Heo J., Lee S., Chu K., et al. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer’s disease. European Journal of Neurology. 2008;15(8):865-868.
130 Neri M., Andermarcher E., Pradelli J.M., Salvioli G. Influence of a double blind pharmacological trial on two domains of well-being in subject’s with age associated memory impairment. Archives of Gerontology and Geriatrics. 1995;21(3):241-252.
131 Mishra S., Palaniveru K. The effect of curcumin (tumeric) on Alzheimer’s disease:an overview. Annals of Indian Academy of Neurology. 2008;11:13-19.
132 Yang F., Lim G.P., Begum A.N., et al. Curcumin inhibits formation of amyloid Î2 oligomers and fibrils, binds plaques, and reduces amyloid in vivo. Journal of Biological Chemistry. 2005;280(7):5892-5901.
133 Ng T.P., Chiam P.C., Lee T., Chua H.C., Lim L., Kua E.H. Curry consumption and cognitive function in the elderly. American Journal of Epidemiology. 2006;164(9):898-906.
134 Lim G.P., Chu T., Yang F., Beech W., Frautschy S.A., Cole G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. Journal of Neuroscience. 2001;21(21):8370-8377.
135 Baum L., Lam C.W.K., Cheung S.K.-K., et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients With Alzheimer Disease. Journal of Clinical Psychopharmacology. 2008;28(1):110-113. 10.1097/jcp.0b013e318160862c
136 Vingtdeux V., Dreses-Werringloer U., Zhao H., Davies P., Marambaud P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl 2):S6.
137 Marambaud P., Zhao H. Davies. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280(45):37377-37382.
138 Ho L., Chen L.H., Wang J., et al. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. Journal of Alzheimer’s Disease. 2009;16(1):59-72.
139 Wang J., Ho L., Zhao Z., et al. Moderate consumption of Cabernet Sauvignon attenuates AÎ2 neuropathology in a mouse model of Alzheimer’s disease. FASEB Journal. 2006;20(13):2313-2320.
140 Lim W.S., Gammack J.K., Van Niekerk J., Dangour A.D. Omega 3 fatty acid for the prevention of dementia. Cochrane database of systematic reviews (Online). (1):2006.
141 Issa A.M., Mojica W.A., Morton S.C., et al. The Efficacy of Omega–3 Fatty Acids on Cognitive Function in Aging and Dementia: A Systematic Review. Dementia and Geriatric Cognitive Disorders. 2006;21(2):88-96.
142 Solfrizzi V., D’Introno A., Colacicco A.M., et al. Dietary fatty acids intake: possible role in cognitive decline and dementia. Experimental Gerontology. 2005;40(4):257-270.
143 Nurk E., Drevon C.A., Refsum H., et al. Cognitive performance among the elderly and dietary fish intake: the Hordaland Health Study. Am J Clin Nutr. 2007;86(5):1470-1478.
144 van Gelder B.M., Tijhuis M., Kalmijn S., Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007;85(4):1142-1147.
145 Conquer J., Tierney M., Zecevic J., Bettger W., Fisher R. Fatty acid analysis of blood plasma of patients with alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids. 2000;35(12):1305-1312.
146 Schaefer E.J., Bongard V., Beiser A.S., et al. Plasma Phosphatidylcholine Docosahexaenoic Acid Content and Risk of Dementia and Alzheimer Disease: The Framingham Heart Study. Arch Neurol. 2006;63(11):1545-1550.
147 Samieri C., Feart C., Letenneur L., et al. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr. 2008;88(3):714-721.
148 Kotani S., Sakaguchi E., Warashina S., et al. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neuroscience Research. 2006;56(2):159-164.
149 Freund-Levi Y., Eriksdotter-Jonhagen M., Cederholm T., et al. {omega}-3 Fatty Acid Treatment in 174 Patients With Mild to Moderate Alzheimer Disease: Omega AD Study: A Randomized Double-blind Trial. Arch Neurol. 2006;63(10):1402-1408.
150 Chiu C.C., Su K.P., Cheng T.C., et al. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(6):1538-1544.
151 van de Rest O., Geleijnse J.M., Kok F.J., et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71(6):430-438.
152 Majid F., Payam M., Kristine Y. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nature Reviews Neurology. 2009;5(3):140-152.
153 Bragin V., Chemodanova M., Dzhafarova N., Bragin I., Czerniawski J., Aliev G. Integrated treatment approach improves cognitive function in demented and clinically depressed patients. American Journal of Alzheimer’s disease and Other Dementias. 2005;20(1):21-26.
154 Christofoletti G., Oliani M.M., Gobbi S., Stella F., Bucken Gobbi L.T., Canineu P.R. A controlled clinical trial on the effects of motor intervention on balance and cognition in institutionalized elderly patients with dementia. Clinical Rehabilitation. 2008;22(7):618-626.
155 Hicks-Moore S.L., Robinson B.A. Favorite music and hand massage: Two interventions to decrease agitation in residents with dementia. Dementia. 2008;7(1):95-108.
156 Lee M.S., Shin B.C., Ernst E. Acupuncture for Alzheimer’s disease: A systematic review. International Journal of Clinical Practice. 2009;63(6):874-879.
157 Liu Z.B., Niu W.M., Yang X.H., Niu X.M. Clinical investigation on electroacupuncture treatment of vascular dementia with “Xiusanzhen”. Zhen ci yan jiu = Acupuncture research/[Zhongguo yi xue ke xue yuan Yi xue qing bao yan jiu suo bian ji]. 2008;33(2):131-134.