28 Delirium
Pediatric delirium is an under-recognized and often reversible complication of critical illness that is considered a medical emergency demanding immediate management.1 New research on the clinical presentation of pediatric delirium and age-appropriate assessment tools for prompt identification have highlighted some of the differences between the adult and pediatric populations.2–6 In the context of palliative care, delirium is one of many crises, including pain, anxiety, and dyspnea, which require a thorough and collaboratively developed plan of care.7
In the adult palliative care setting, delirium has been carefully studied and identified as a sign of impending death.8,9 Among adult patients who are terminally ill, delirium is the most common neuropsychiatric disorder with a prevalence estimated as high as 85%.10 In a palliative care setting, researchers11 identified 42% of the patients with a diagnosis of delirium at the time of hospital admission, and an additional 45% who developed delirium during their hospital stay. These data suggest that delirium occurs in the majority of patients nearing the end of life and that improved methods of diagnosis and management are warranted for this highly distressing and often reversible condition. Therefore, in all critically ill patients of every age, mental status should be carefully evaluated in addition to conventional monitoring of pulse, temperature, respiratory rate, blood pressure, and pain.12
The study of delirium is at the confluence of a number of clinical disciplines including anesthesiology, critical care medicine, pediatrics, geriatrics, oncology, pain medicine, palliative medicine, neurology, psychiatry, psychology, nursing, pharmacy, and bioethics. Across these domains, there has been a recent groundswell of interest in delirium,13 and specifically pediatric delirium, after a long period of neglect between 1980 and 2003.2,3 Advances in medical care that prolong life place critically ill patients at greater risk for delirium. In addition, awareness of potential cognitive and psychological sequelae of delirium has led to efforts directed at early recognition and treatment. Goals of palliative and general clinical care target delirium as a symptom that necessitates immediate care.14
The aim of this chapter is to provide an overview of pediatric delirium and its clinical relevance in the palliative care setting. An additional goal includes underscoring the interdisciplinary nature of delirium. Any clinician or caregiver, including respiratory therapist, nurse, doctor, pharmacist, and parent, has the opportunity to screen patients for delirium. Delirium has many causes, occurs in both simple and complex clinical situations and should be suspected in all circumstances when mental status is altered. One long-term study has shown that nurse-directed delirium prevention and management programs, involving multidisciplinary education and collaboration, have led to a decrease in delirium and clinician workload.15 Every member of the treatment team serves as a resource in the prevention, recognition, and management of delirium.
Definition
According to the Diagnostic and Statistical Manual16 the core features of delirium include a disturbance of consciousness, change in cognition, and perceptual disturbance that develops over a short period, usually hours to days, and fluctuates over time (Box 28-1). Delirium has been associated with rates of morbidity and mortality that surpass those of all other psychiatric diagnoses.17 Across clinical disciplines and countries, there are many ambiguities in the terminology used to describe delirium.18 Such terms as acute brain failure, encephalopathy, acute confusional state, and intensive care unit (ICU) psychosis have been widely employed, and the critical care literature has now conformed to the recommendations of the American Psychiatric Association, and other experts, that the term delirium be used uniformly to describe this syndrome of brain dysfunction.19 The need for shared terminology reflects the challenges clinicians face in diagnosing delirium across clinical settings and age groups.
BOX 28-1 DSM-IV-TR Diagnostic Criteria for Delirium
Adapted from the American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition revised, (DSM-IV-TR).Washington, DC, American Psychiatric Press, 2000.
Clinical presentation
Although the clinical presentation of delirium in children and adolescents is considered to be similar to that of adults, there are observed differences in the type and frequency of symptoms across various age groups.4,5,6 Clinician-observed features of pediatric delirium varied across several studies. Pediatric patients are described as having more frequent fluctuation in symptoms, greater affective lability, impaired attention, and disturbances in their sleep-wake cycle.4 By contrast6 a study described pediatric patients as having lower rates of sleep disturbance, fewer cognitive deficits, and lower levels of symptom fluctuation.
With regard to psychotic symptoms, authors have described both lower and higher prevalence of perceptual disturbances and delusions among children compared to adults.4,6 Some4 have suggested that delusions and hallucinations may not be core symptoms of pediatric delirium. Alternatively, a study of pediatric patients emerging from anesthesia reported that younger children had higher rates of hallucinations and other perceptual disturbances, compared with older children.20 These differences may reflect variations in brain function across the age span, developmental immaturity in children, functional decline in old age, and differences in the expression of distress during hospitalization.21 The variance in observed clinical features is wide and may result from the fluctuating nature of the illness, the subjective nature of clinical assessment, lack of age-appropriate screening tools, and the various causes of delirium.
Subtypes of Delirium
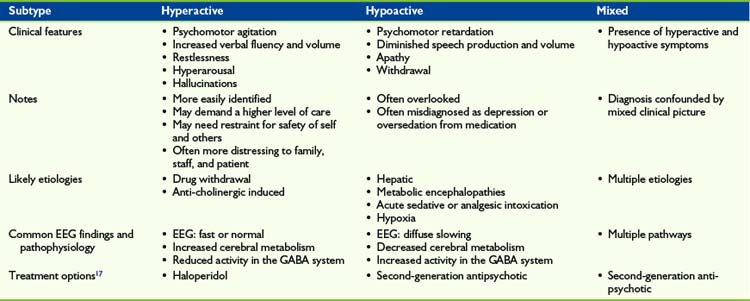
Three motoric subtypes of delirium–hypoactive, hyperactive, and mixed–have been well described in the adult literature.22 Delirium is also categorized on the basis of symptom severity, with terms such as pre-delirium, sub-syndromal, veiled, and full-blown used to designate differences in presentation.23 These additional terms reflect growing research into the phenomenology of delirium. Although few studies have focused on categorizing delirium temporally or by severity, the practical finding from this work is that delirium may be detectable earlier in its course with vigilant screening. In this chapter, a discussion of motoric subtypes is reviewed with vignettes (Table 28-1).
Mixed delirium describes patients who fluctuate between hyperactive and hypoactive states.22,25 These critically ill patients present with an array of symptoms, in the context of possible pain, anxiety, and nausea, making it difficult to recognize delirium and identify the cause.
Prevalence and Epidemiology
Among adult studies, delirium has been reported in 15% to 18% of patients on acute medical and surgical wards, with higher rates in specific populations.17 One of the largest retrospective pediatric studies on delirium,3 reported a 9% prevalence of delirium in a sample of 1027 patients referred for psychiatric consultation (Table 28-2). Another study5 reported a lower prevalence of 4.6% in a pediatric sample of 877 critical care patients.
Among pediatric patients, age as a risk factor for delirium is not well known. The study of 1027 patients3 identified 84 patients with delirium ages 6 months to 18 years and there was no significant difference in mean and median age based on the cause of delirium. The study did not assess whether age itself was risk factor for delirium. According to the study5 that presented data stratified by age, the greatest incidence of delirium occurred among the oldest children (15 to 18 years), but these data were not analyzed for statistical significance. Conversely, another study26 found a negative correlation between age and delirium among post-anesthesia patients suffering with emergence delirium.
Although studies to assess the prevalence of delirium in children receiving end-of-life care have not yet been undertaken, data from adult studies suggest potentially much higher rates in this population. For example, immediately before death, delirium rates of 68% to 88% have been reported in adult oncology patients.11,27 More specifically, according to a review8 hypoactive delirium, which is easily overlooked, has been reported as high as 40% to 78% in adult palliative care patients.11,28,29
Attempts to quantify the prevalence of delirium by subtype among adults have resulted in a range of findings. For example, a study30 reported hypoactive delirium (43.5%) occurring considerably more often than “purely” hyperactive delirium (1.6%), and mixed delirium was the most frequently observed subtype (54.1%). The low number of cases with hyperactive delirium in this cohort is unusual when compared with other studies that report rates of hyperactive delirium ranging from 15% to 80%.22,31 On the other hand, the high number of cases in the cohort30 of 375 elderly diagnosed with hypoactive delirium suggests that careful screening may accurately identify more subtle presentations of delirium. The only comparison4 of the prevalence of hyperactive and hypoactive states in adults to children found across three adult studies there was an average of 59% hypoactive delirium, similar to a pediatric prevalence of 69%. Agitation was noted in the adult studies at a combined rate of 44%, which was significantly lower than the pediatric rate of hyperactive delirium of 68%.
In adult palliative care literature, the rates of hypoactive delirium are as high as 86%, mean prevalence 48% in one meta-analysis, while rates of hyperactive delirium vary between 13% to 46% of patients.29,32 Figures for the prevalence of delirium by subtype in the pediatric palliative care setting are not known.
Despite the varied estimates on prevalence, numerous studies suggest that the motoric subtypes of delirium differ beyond the degree of psychomotor activity. Studies show variation between hypoactive, hyperactive, and mixed delirium with regard to other non-motoric features of delirium,33 etiology and pathophysiology,34 ease of detection and assessment, response to treatment,24 and outcome.35 Notably, clinician observations of patient psychomotor disturbance were less reliable when compared with data from electronic motion detectors.36 Because recognition of delirium continues to be challenging and machine-assisted assessment is helpful, there is the potential that future technological innovations may enhance clinician assessment. For example, similar to the use of heart monitors in the ICU, motion detection may be used to help alert clinicians to the presence of delirium among high-risk groups (see Table 28-2).
Etiology
Causes and risk factors for delirium are often multifaceted and include vascular, infectious, neoplastic, degenerative, organ failure, toxic, deficiencies including vitamin, congenital, central nervous system pathologic, traumatic, endocrinological, metabolic, dehydration, heavy metal, and anoxic phenomena.17 Medication-related etiologies, as a result of toxic effects or withdrawal reactions, are also common. As with adults, certain classes of medications such as steroids, opiates, benzodiazepines, and anticholinergic agents are frequent precipitants.
In the palliative care setting, delirium often results from aggressive treatment of pain and suffering, which can lead to drug-induced change in mental status.9 A study of 40 critically ill children5 found that the most frequent causes of delirium in decreasing order included a change in analgosedative medication, neurological disorders, infections, and respiratory disorders. Often there were multiple causes for delirium. Infection was twice as often the precipitating factor compared with drug induced-delirium in one report.4 A thorough diagnostic workup to identify the cause is the standard of care37; however, in the palliative care setting where the goal of care is to decrease pain and suffering, this approach is often modified.
Pathogenesis
Delirium is a neuropsychiatric disorder involving global en-cephalopathy dysfunction caused by multiple impaired neural pathways and physiologic compromises. The neurotransmitters that have been implicated in the pathophysiology of delirium include acetylcholine, dopamine, glutamine, gamma aminobutyric acid (GABA), and serotonin.34 Dopamine is thought to modulate mood and cognitive function, and in excess can lead to psychotic disorders. Similarly, acute alterations in dopamine levels may contribute to the characteristic symptoms of delirium. Antipsychotic medications that inhibit the dopamine pathway are effective in treating delirium.38 Alteration of GABA, an inhibitory neurotransmitter, may also cause changes in cerebral functioning, possibly by affecting sleep patterns. In the critical and palliative care setting medications such as benzodiazepines and propofol, which directly affect GABA receptors, are frequently used and have been found to contribute to the development of delirium in the ICU setting.39 Likewise, acetylcholine deficiency is associated with delirium and the use of anticholinergic drugs has been found to precipitate and worsen symptoms of delirium.40 These neurotransmitter perturbations are thought to cause neuronal membrane hyperpolarization, thus spreading neuronal depression, which is seen on EEG as a diffuse slowing.41
Other insults to cerebral functioning such as inflammation, hypoxia, metabolic encephalopathies, and drugs or toxins, are implicated in the development of delirium. Inflammation, caused by infection, surgery or other tissue injury, heightens blood-brain barrier permeability leading to translocation of inflammatory mediators, such as cytokines and chemokines, into the CNS, causing encephalopathy dysfunction.42 Cytokines such as interleukin (IL)-1, IL-2, interferon (IFN) and tumor necrosis factor (TNF) can affect neuronal pathways by inhibiting acetylcholine, leading to agitation, perceptual disturbances, seizures, and delirium.43 There are reports of chemokine elevations in patients with delirium, and one study found IL-6 increase in children with influenza that developed delirium.44 Inability to meet oxygen needs, either because of increased demand or decreased delivery, contributes to development of delirium because of disruption of ionic gradients, neurotransmitter homeostasis, and neurotoxic byproduct elimination. The compromise of oxygen metabolism can be due to hypoglycemia, hyper- or hypothermia, and vitamin or amino acid deficiencies. Impaired oxygen metabolism leads to reduced neurotransmitter synthesis, causing a deregulation of sleep-wake cycle, behavior and mood, and psychomotor activity, all of which are clinical manifestations of delirium.38,45
Assessment
The diagnosis of delirium is primarily made on clinical examination, which should always include an interview with parents and primary caregivers. There should also be a review of the medical chart, including laboratory and imaging test results. Nursing staff and parents’ observations are particularly important to evaluate fluctuations in levels of consciousness and sleep disturbance. The Nursing Staff Delirium Screening Scale (NDSS) provides a quick and consistent measure for the clinicians who have the most frequent clinical contact with patients.46 Although not generally standard of care, EEG findings may offer diagnostic support of delirium and perhaps subtype.41 Additionally, family members serve as a valuable clinical resource regarding the patient’s state of arousal, activity, and orientation, especially in the pediatric population. In palliative care, the education of patients and family members in recognizing signs of delirium can aid in assessment and early intervention, minimize distress to the patient and family, and support clinical decision making in end-of-life care.
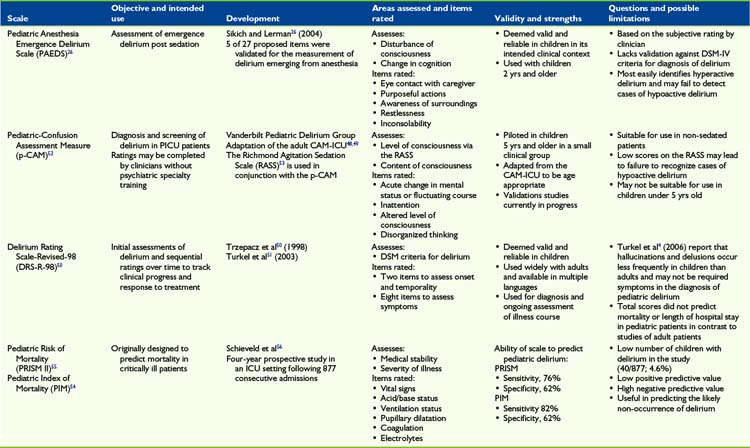
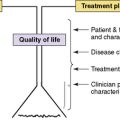
To supplement and help guide the direct clinical evaluation, there are a number of structured assessments and rating scales to diagnose and track symptoms related to delirium (Table 28-3). These tools include assessment for cognitive impairment, such as Mini Mental Status Exam47; screening for delirium using DSM-IV-TR diagnostic criterias including the Confusion Assessment Measure48,49; and delirium-specific numeric scales to rate severity, that is, Delirium Rating Scale-Revised-98 (DRS-R-98).50 The DRS-R-98 is widely used to assess delirium in adults using a 16-item scale with items that include disturbances of cognition, perception, thought, language, sleep, affect, and psychomotor function. Items are rated based on direct observation, data from the medical record, and accounts from caregivers. The DRS-R-98 has been proved reliable for both the diagnosis and the serial assessment of delirium, and has been used and validated for pediatric patients.51 The Pediatric Anesthesia Emergence Delirium Scale (PAEDS) is a measure designed to rate post-surgical emergence delirium and has been validated for use in children.26 Unlike other scales, the PAEDS is a clinician report of observed behaviors and responses to stimuli, which differentiate it from the DRS-R-98. The pediatric Confusion Assessment Measure (p-CAM) is a two-part assessment tool that screens for overall cognitive impairment using the Richmond Agitation Sedation Scale (RASS) and then distinguishes delirium from other causes of cognitive impairment.52,53 Unlike the DRS-R-98, the p-CAM is designed to diagnose delirium rather than to rate symptom severity. The Pediatric Index of Mortality (PIM)54 and the Pediatric Risk of Mortality (PRISM II)55 are scales used in the pediatric intensive care setting to predict outcome. Using PIM and PRISM, one study56 found the positive predictive value was low mostly due to few identified cases of delirium, 40 out of 877 patients, the negative predictive value was very high at 99%, suggesting that these scales can be used to rule out non-occurrence of delirium.56 A 2009 study suggested an algorithm for diagnosing and managing pediatric delirium using the RASS and PAEDS.23 As the authors point out, further research is required to validate any proposed methods. Likewise, the overview (see Fig. 28-1, p. 262) included in this chapter highlights important aspects of identifying and treating pediatric delirium. Further research will be needed to establish best evidenced-based practices (see Table 28-3).
Treatment
An interdisciplinary approach for the clinical management of delirium combines the identification of risk factors, patient and family education, environmental modification, treatment of underlying etiologies, and use of appropriate medications. In the setting of comfort care, the management of delirium is further complicated by the goal of providing relief from suffering while avoiding medication-induced delirium when treating pain and other discomforts. For example, patients with terminal illness often receive, or are withdrawn from, high doses of opiates and benzodiazepines, which may cause delirium. Another challenge in the pediatric population is differentiating between agitation, delirium, fear, anxiety, and pain. Few age-appropriate validated assessments tools are available that easily make the distinction in young patients.57,58 Parents often are the best indicators of the nature and intensity of their child’s distress. Parent opinion regarding symptoms of discomfort should be sought by clinical staff. The interdisciplinary treatment team, the patient, and the family should create repeated opportunities to discuss goals of care regarding treatment and comfort. When illness cure is no longer the goal, symptomatic control remains the sole aim; refractory symptoms of discomfort may necessitate deeper or palliative sedation.8
The first step in the management of delirium should be to treat the underlying cause. In the palliative population this approach requires prudence and collaboration. More simple, less invasive interventions may include restoring electrolyte imbalances, ensuring adequate hydration and oxygenation, judicious tapering of medications, controlling pain, and treating infections.1 Clinicians should conduct a diagnostic workup taking into account the treatment goals of the specific patient and weighing such factors as distress related to invasive procedures as well as the potential efficacy of the intervention.37 In an effort to minimize suffering, it should be noted that in one prospective adult study59 the cause of delirium was identified in less than 50% of terminally ill patients. Advanced care planning involving the family, and the patient when age appropriate, is essential in managing palliative care crises such as delirium. Anticipating delirium, which can occur in 85% of adult palliative care patients,10 should be an essential step in end-of-life treatment planning. Children as young as 10 years old have demonstrated the ability to express preferences regarding end-of-life treatment, an understanding of possible consequences to their decisions, and the capacity to weigh complex issues.60 Education about delirium before onset is an opportunity for pediatric patients and their families to participate in care, especially those with chronic illness, and thereby minimize distress and promote a sense of agency.
Prevention
Awareness of the major risk factors for delirium is critical for prevention and early recognition. Although current research focuses on risk factors in adults, there is overlap with the pediatric population. Risk factors can be divided into three categories: predisposing, precipitating, and environmental factors (Table 28-4).
| Predisposing factors | Precipitating factors | Environmental factors |
|---|---|---|
Medications, which are often major contributors to the development of delirium, should be chosen with care and with appropriate evaluation of possible unwanted drug-drug interactions. In the palliative care setting, careful attention to the dosing and scheduling of sedatives, analgesics, anticholinergics, including some low-potency antipsychotics, corticosteroids, and anticonvulsants, is essential in reducing the incidence of delirium. In particular, tapering medications should be carefully managed because withdrawal from drugs such as opioids and benzodiazepines can trigger or worsen symptoms of delirium. Pain should also be frequently assessed and appropriately managed. In the palliative care setting, avoiding medications that may exacerbate delirium is often the first and only step taken to treat delirium, particularly when it is not possible to treat the primary immediate cause, such as cancer.61
Nonpharmacologic treatment
Nonpharmacologic treatment of delirium should focus on correction of underlying causes, reorientation, and patient safety. Interventions such as restoring electrolyte imbalances, ensuring adequate hydration and oxygenation, improving bowel and bladder function, slow taper of medications, controlling pain, and treating infections have been shown to improve the rate of delirium recovery.1,62
Environmental modifications are also beneficial. An effort should be made to orient the patient using calendars, clocks, and familiar objects such as toys and pictures.5 There should be a clear distinction between night and day to help restore disturbances in the sleep-wake cycle. Some options include the minimization of noise and light at night, organizing vital sign checks, procedures, and medications at a time that does not interrupt sleep, and massage and music for relaxation. During the day, social interactions that are soothing and familiar should be encouraged. Every effort should be made to minimize the use of catheters, intravenous lines, and other items that limit physical mobility. Family, visitors, and clinical staff should attempt to speak in short simple sentences, avoiding confrontation and provide reassurance whenever possible. Patients should ideally be placed in a single-patient room with either one-on-one nursing or a family member present. This direct observation is important to ensure patients do not harm themselves or others. If the patient is highly agitated, physical restraints may be necessary to prevent dislodgement of lines or catheters. However, physical restraints have been reported as an independent risk factor for delirium and should be used only with careful monitoring and a clear, time-limited plan.62 The appropriate use of the nonpharmacologic measures should include a thoughtful and supportive discussion with family members (Table 28-5).
TABLE 28-5 Nonpharmacologic Management Guideline for Pediatrics
| Intervention type | Potential activities |
|---|---|
| Sensory and environmental modification |
Pharmacologic interventions
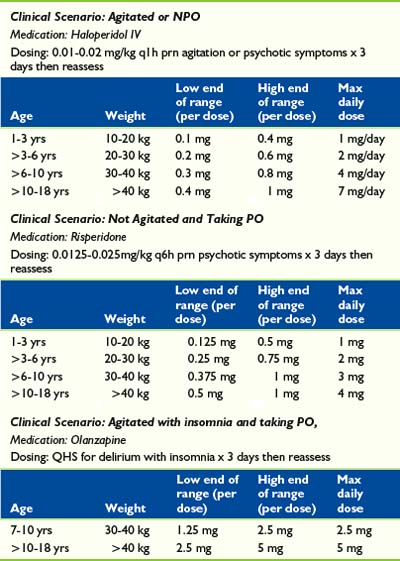
While a diagnostic workup of potential causes is being conducted, pharmacologic interventions may still be required to manage symptoms in patients with delirium (Table 28-6). Antipsychotic and sedative agents can be used to diminish levels of agitation, correct disturbances in the sleep-cycle, and minimize perceptual disturbances. Haloperidol, second-generation antipsychotics, and benzodiazepines have all been used in the treatment of delirium with varying degrees of success. The side effect profile of each agent should be considered and individualized to each patient. Moreover, treatments should always be accompanied by adequate pain management and monitoring of potential medication withdrawal reactions.
Haloperidol, one of the first-generation antipsychotics, is the most frequently used pharmacologic treatment of delirium.63 Although not approved by the Federal Drug Administration (FDA) for treatment of delirium, reasons for its use include considerations of efficacy, cost, and intravenous route of administration. Intravenous use results in more reliable absorption, decreased incidence of extrapyramidal reactions, and minimal effects on blood pressure, respiration, and heart rate.64,65 The intravenous dose is twice as potent as oral administration. This high-potency agent is preferred to low-potency antipsychotics because of the decreased likelihood of hypotensive and anticholinergic effects, and has been found to be safe and effective in managing cases of pediatric delirium.66 In the palliative care population, subcutaneous and intramuscular injection are also convenient options. The primary mechanism of haloperidol’s action is inhibition of dopaminergic pathways that may be overstimulated in states of hyperactive delirium, thus helping restore normal thought patterns and sensorium. The cautious use of haloperidol is recommended in patients with hypoactive delirium, who may already have dopaminergic suppression and consequently its use may exacerbate symptoms of delirium.39 A double-blinded, randomized control trial compared haloperidol, chlorpromazine, and lorazepam for the treatment of delirium reports both haloperidol and chlorpromazine as effective in improving symptoms of delirium and cognitive function, while lorazepam was not only ineffective but also worsened symptoms in some patients.
All antipsychotics have side effects and patients must be closely monitored. Serious adverse reactions requiring im- mediate medical attention include malignant hyperthermia. Also, extrapyramidal movement disorders including laryngeal spasm, hypotension, and glucose and lipid dysregulation; anticholinergic effects, such as constipation, urinary retention, and dry mouth; and cardiac effects such as QTc prolongation and torsades de pointes, need to be monitored. Electrocardiogram monitoring is strongly recommended for those on intravenous haloperidol. Significant complications such as dystonia, hypotension, and hyperpyrexia in more than 20% of pediatric burn patients treated with haloperidol were reported.68
Second-generation antipsychotics, such as risperidone, olanzapine, and quetiapine, is another option for the treatment of delirium. Most second-generation antipsychotics act on both dopaminergic and serotonergic receptors. Their side effect profile is commonly reported as more favorable than that of the first-generation antipsychotics. Although there have been few efficacy studies directly comparing the second-generation antipsychotic agents and haloperidol, there may be some benefits in terms of the second-generation antipsychotic side effect profile.62,69 A review on the topic concluded that second-generation antipsychotics are effective in treating delirium and have fewer side effects compared with high-dose haloperidol. In addition, in the case of hypoactive delirium, second-generation antipsychotics may be indicated because their mechanisms of action extend beyond dopamine blockade and affect other pathways likely implicated in the pathophysiology of delirium.24 Disadvantages of second-generation antipsychotic agents include the lack of an intravenous formulation. Although most of these agents must be given orally, some, such as risperidone and olanzapine, have intramuscular and sublingual formulations (see Table 28-6).
Like haloperidol, second-generation antipsychotics also carry an FDA warning of increased risk of death, mostly cardiovascular related, in elderly patients. Controlled studies for delirium in the pediatric population, comparing both classes of antipsychotics for efficacy and safety, have yet to be undertaken. Of note, all of the antipsychotics are employed off-label when used to treat delirium. The second-generation antipsychotics have been tested in children with approval for other indications. For example, risperidone has been approved for use in children ages 5 to 16 for management of aggressive behavior related to autism. Olanzapine is indicated for the treatment of schizophrenia and manic or mixed episodes of bipolar disorder in 13- to 17-year-olds. These medications can be safely used at low doses in young children with appropriate monitoring when treating delirium.70
Benzodiazepines, such as lorazepam and diazepam, are GABA agonists that treat distress related to anxiety and sleep-wake cycle disturbance in delirious patients. They have amnesic properties and consequently can worsen cognitive impairment. There are also reports of paradoxical effects, such as aggression, violence, and irritability in pediatric patients.71 In general, there appears to be some consensus that benzodiazepines should not be used as first-line agents in the treatment of pediatric delirium. In addition, a longer duration of benzodiazepines and opiate use in critically ill children was associated with increased reports of delusional memory, which is associated with greater risk for post-traumatic stress disorder.72 In some cases when a benzodiazepine is required, lorazepam combined with haloperidol for severe agitation in older adolescent and adult patients has been beneficial.62
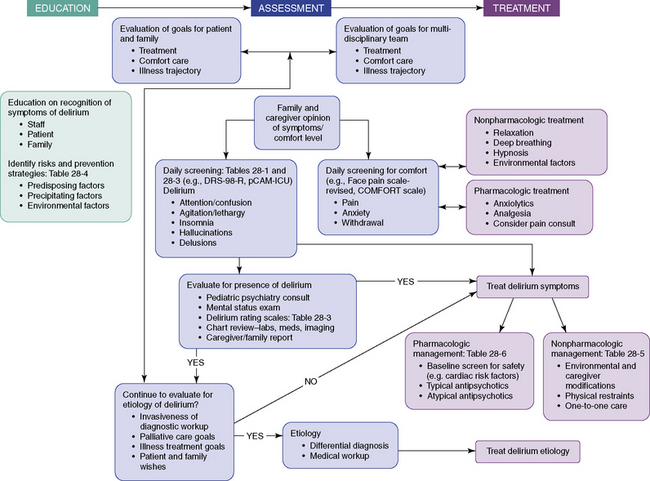
Figure 28-1 describes an algorithm that depicts the key factors and decision points salient to education, assessment, and treatment of delirium in the pediatric palliative care setting. Although this algorithm is not an evidenced-based protocol, it does suggest a paradigm for understanding delirium and its management.
Sequelae of Delirium
The psychological and cognitive impact of delirium on patients, families, and caregivers of critically ill adults at the end of life has been well explored and reviewed.8 Delirium is related to longer hospital stay, higher rates of morbidity and mortality, and negative outcome post recovery.73,74 Post-traumatic stress disorder (PTSD) in adults following the experience of delirium has also been reported.75,76,77
Post-traumatic stress disorder
The recognition of post-traumatic stress disorder in medically ill patients, including those receiving treatment in ICUs, is now well recognized. These symptoms include flashbacks, nightmares, avoidance of reminders, anger, and hypervigilance.16 More recently, there have also been a number of studies examining the relationship between PTSD and the experience of delirium. This is particularly relevant because there is evidence that a great number of patients with mild to moderate delirium (43% of those with hypoactive delirium and 66 % of patients with hyperactive features), recall their experience.75 This vignette describes the traumatic experience of delirium and its impact.
Adult and pediatric studies have explored the association between delirium and PTSD in intensive care patients and have found that those with delusional memories have increased risk of developing PTSD symptoms compared with those with factual memories.72,78 Because alterations in memory and perception are common presentations of delirium, children with delirium may be at greater risk of developing PTSD. Such sequelae should not be taken lightly because it can cause significant social impairment and can negatively impact academic performance.79
If pediatric patients experience delirium, when they recover it is appropriate to ask what they remember. Some children do not recall the experience or report positive associations.72 The specifics of delusions should not be over-interpreted (e.g., intravenous lines being perceived as snakes) rather, themes of fear, anxiety, loss, and mortality can be considered and explored. Further psychological support is recommended both for patient and the family if delirium has been distressing whether for themselves or for anyone else, including caregivers and staff.
Impact on caregivers
Delirium affects not only patients but also family members and medical staff. Mean distress levels regarding delirium on a 4.0 scale were highest among spouses (3.75), nurses (3.09), and then patients (3.02).77 For families, hyperactive delirium and severe physical debilitation were the strongest predictors of distress in contrast to nurses who rated the severity of the delirium and perceptual disturbances more highly.77 Interviews conducted of bereaved family members who witnessed delirium revealed they were often troubled by feelings of guilt and helplessness, struggled with making proxy decisions, and worried about the burden of care.80 Families often equate signs of delirium with pain or discomfort, going crazy, and death anxiety.80 In addition, family members of patients with delirium reported both concerns about inadequate medical care as well as a desire for more information about the condition.81
These accounts by families likely reflect the challenge that medical staff face when caring for patients with delirium. Nurses commonly reported stress from increased workload, safety concerns for themselves and their patients, and difficulty finding a balance between connecting with the patient and being on guard.82 Long-term consequences for caregivers are yet to be fully investigated. Similarly, parents who have children hospitalized in intensive care are at increased risk of developing PTSD.83 All caregivers, including parents, siblings, nurses, doctors, other clinicians, social workers, and clergy are at risk of psychological sequelae from traumatic experiences with critically ill patients.
Summary
Pediatric patients experiencing delirium constitute a high-risk population because of developmental vulnerabilities associated with their neurophysiology, challenges in assessment, and advancements in life-prolonging medical therapies. Delirium is associated with a number of indices of medical morbidity that include increased length of hospital stay and elevated rates of both morbidity and mortality.84,85 In addition, research has shown associations with poor functional outcome, cognitive decline, and patient and family emotional distress in patients with delirium.77,86,87 Research in adult intensive care patients has shown a threefold increase in mortality in patients diagnosed with delirium independent of other risk factors.88 Similarly, studies in pediatric patients have reported mortality rates as high as 20%.3 Because pediatric delirium is an often under-diagnosed and reversible complication of serious illness with significant negative impact, further research has the potential to improve patient care and outcome across all disciplines.
1 Smith H.A., Fuchs D.C., Pandharipande P.P., et al. Delirium: an emerging frontier in the management of critically ill children. Crit Care Clin. 2009;25(3):593-614.
2 Prugh D.C., Wagonfield S., Metcalf D., et al. A clinical study of delirium in children and adolescents. Psychosom Med. 1980;42:177-195.
3 Turkel S.B., Tavaré C.J. Delirium in children and adolescents. J Neuropsychiatry Clin Neurosci. 2003;15:431-435.
4 Turkel S.B., Trzepacz P.T., Tavare C.J. Comparison of delirium in adults and children. Psychosomatics. 2006;47:320-324.
5 Schieveld J.N., Leroy P.L., van O.S.J., et al. Pediatric delirium in critical illness: phenomenology, clinical correlates and treatment response in 40 cases in the pediatric intensive care unit. Intensive Care Med. 2007;33:1033-1040.
6 Leentjens A.F., Schieveld J.N., Leonard M., et al. A comparison of the phenomenology of pediatric, adult, and geriatric delirium. Psychosomatics. 2008;64:219-223.
7 Nauck F., Alt-Epping B. Crises in palliative care: a comprehensive approach. Lancet Oncol. 2008;9:1086-1091.
8 Leonard M., Agar M., Mason C., et al. Delirium issues in palliative care settings. J Psychosom Res. 2008;65:289-298.
9 Breitbart W., Lawlor P. Delirium in the terminally ill. In: Chochinov M., editor. Oxford handbook of psychiatry in palliative care medicine. ed 2. Oxford: Oxford University Press; 2009:81-100.
10 Massie M.J., Holland J., Glass E. Delirium in terminally ill cancer patients. Am J Psychiatry. 1983;140:1048-1050.
11 Lawlor P.G., Gagnon B., Mancini I.L., et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160:786-794.
12 Flaherty J.H., Rudolph J., Shay K., et al. Delirium is a serious and under-recognized problem: why assessment of mental status should be the sixth vital sign. J Am Med Dir Assoc. 2007;8(5):273-275.
13 Leentjens A.F.G., MacLullich A.F., Meagher D.J. Delirium, Cinderella no more … ? J Psychosom Res. 2008;65:205. Epub 2008 Jul 24
14 Institute of Medicine of the National Academies. When children die: improving palliative and end of life care for children and their families. New York: The National Academies Press, 2003.
15 Manuela Pretto M., Spirig R., Milisen K., et al. Effects of an interdisciplinary nurse-led Delirium Prevention and Management Program (DPMP) on nursing workload: a pilot study. Int J Nurs Stud. 2009;46(6):804-812. Epub 2009 Feb 27
16 American Psychiatric Association. ed 4 revised. Diagnostic and statistical manual of mental disorders. 2000. American Psychiatric Press: Washington, DC. (DSM-IV-TR)
17 Wise M.G., Trzepacz P.T. Delirium (Confusional States). In: Rundell J.R., Wise M.G., editors. Textbook of consultation-liaison psychiatry. Washington, DC: American Psychiatric Press; 1996:258-275.
18 Morandi P., Pandharipande M., Trabucchi, et al. Understanding international differences in terminology for delirium and other types of acute brain dysfunction in critically ill patients. Intensive Care Med. 2008;34:1907-1915.
19 Girard T.D., Pandharipande P.P., Ely E.W. Delirium in the intensive care unit. Crit Care. 2008;12(Suppl 3):S3.
20 Przybylo H.J., Martini D.R., Mazurek A.J., et al. Assessing behaviour in children emerging from anaesthesia: can we apply psychiatric diagnostic techniques? Paediatr Anaesth. 2003;13:609-616.
21 Gupta N., de Jongheb J., Schieveld J., et al. Delirium phenomenology: what can we learn from the symptoms of delirium? J Psychosom Res. 2008;65:215-222.
22 Liptzin B., Levkoff S.E. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843-845.
23 Schieveld J.N.M., van der Valk J.A., Smeets I., et al. Diagnostic considerations regarding pediatric delirium: a review and a proposal for an algorithm for pediatric intensive care units. Intensive Care Med. 2009;35:1843-1849.
24 Karnik N.S., Joshi S.V., Paterno C., Shaw R. Subtypes of pediatric delirium: a treatment algorithm. Psychosomatics. 2007;48(3):253-257.
25 Gupta A.K., Saravay S.M., Trzepacz P.T., et al. Delirium motoric subtypes. Psychosomatics. 2005;46:158.
26 Sikich N., Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100(5):1138-1145.
27 Morita T., Tei Y., Tsunoda J., et al. Underlying pathologies and their associations with clinical features in terminal delirium of cancer patients. J Pain Symptom Manage. 2001;22:997-1006.
28 Lam P.T., Tse C.Y., Lee C.H. Delirium in a palliative care unit. Prog Palliat Care. 2003;11:126-133.
29 Spiller J.A., Keen J.C. Hypoactive delirium: assessing the extent of the problem for inpatient specialist palliative care. Palliat Med. 2006;20:17-23.
30 Peterson J.F., Pun B.T., Dittus R.S., et al. Delirium and its motoric subtypes: A study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479-484.
31 Kobayashi K., Takeuchi O., Suzuki M., et al. A retrospective study on delirium type. Jpn J Psychiatry Neurol. 1992;46:911-917.
32 Ross C.A., Peyser C.E., Shapiro I., et al. Delirium: phenomenologic and etiologic subtypes. Int Psychogeriatr. 1991;3(2):135-147.
33 Meagher D.J., O’Hanlon D., O’Mahony E., et al. Relationship between symptoms and motoric subtype of delirium. J Neuropsychiatry Clin Neurosci. 2000;12:51-56.
34 Maldonado J. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24:789-856.
35 O’Malley G., Leonard M., Meagher D., et al. The delirium experience: a review. Psychosom Res. 2008;65(3):223-228.
36 Godfrey A., Conway R., Leonard M., et al. A classification system for delirium subtyping with the use of a commercial mobility monitor. Gait Posture. 2009;30(2):245-252.
37 Trzepacz P.T., Breitbart W., Franklin J., et al. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156:11.
38 Van der Mast R.C. Pathophysiology of delirium. Geriatr Psychiatr Neurol. 1998;11:138-145.
39 Maldonado J.R. Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin. 2008;24(4):657-722. vii
40 Rummans T.A., Evans J.M., Krahn L.E., et al. Delirium in elderly patients: evaluation and management. Mayo Clin Proc. 1995;70(10):989-998.
41 Jacobson S., Jerrier H. EEG in delirium. Semin Clin Neuropsychiatry. 2000;5:86-92.
42 Rudolph J.L. Chemokines are associated with delirium after cardiac surgery. J Gerontol Med Sci. 2008;63A:184-189.
43 Dunlop R.J., Campbell C.W. Cytokines and advanced cancer. J Pain Symptom Management. 2000;20:214-232.
44 Fukumoto Y., Okumura A., Hayakawa F., et al. Serum levels of cytokines and EEG findings in children with influenza associated with mild neurological complications. Brain Dev. 2007;29(7):425-430. Epub 2007 Feb 6
45 Eikelenboom P., Hoogendijk W.J.G., Jonker C., et al. Immunologic mechanisms and the spectrum of psychiatric syndromes in Alzheimer’s disease. J Psychiatric Res. 2002;36:269-280.
46 Gaudreau J.D., Gagnon P., Harel F., et al. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage. 2005;29:368-375.
47 Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
48 Inouye S.K., Vandyck C., Alessi C., et al. Clarifying confusion: the confusion assessment method: a new method for the detection of delirium. Ann Intern Med. 1990;113(12):941-948.
49 Ely E.W., Margolin R., Francis J., et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379.
50 Trzepacz P.T., Mittal D., Torres R., et al. Validation of the Delirium Rating Scale-Revised-98 (DRS-R-98). J Neuropsychiatry Clin Neurosci. 2001;12:156.
51 Turkel S.B., Braslow K., Tavare C.J., et al. The Delirium Rating Scale in children and adolescents. Psychosomatics. 2003;44:126-129.
52 Smith H, Ely W: A multi-site validation study for the Pediatric Confusion Assessment Measure: Vanderbilt delirium group, Nashville, Tenn, in process via personal communication with Dr. Heidi Smith.
53 Sessler C.N., Gosnell M.S., Grap M.J. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344.
54 Shann F., Pearson G., Slater A., et al. Pediatric index of mortality (PIM): a mortality prediction model for children in intensive care. Intensive Care Med. 1997;23:201-207.
55 Pollack M.M., Ruttimann U.E., Getson P.R. The Pediatric Risk of Mortality (PRISM) score. Crit Care Med. 1988;16:1110-1116.
56 Schieveld J.N.M., Lousberg R., Berghmans E., et al. Pediatric illness severity measures predict delirium in a pediatric intensive care unit. Crit Care Med. 2008;36(6):1933-1936.
57 Hicks C.L., von Baeyer C.L., Spafford P., et al. The Faces Pain Scale—Revised: Toward a common metric in pediatric pain measurement. Pain. 2001;93:173-183.
58 Ambuel, et al. Assessing distress in pediatric intensive care environments: The COMFORT Scale. J Pediatr Psychol. 1992;17:95-109.
59 Breura E., Miller L., McCallion J. Cognitive failure in patients with cancer: a prospective study. J Pain Symptom Manage. 1992;7(4):192-195.
60 Hinds P.S., Drew D., Oakes L.L., et al. End-of-life care preferences of pediatric patients with cancer. J Clin Oncol. 2005;23(36):9146-9154.
61 Gagon R. Treatment of delirium in supportive and palliative care. Curr Opin Support Palliative Care. 2008;2:60-66.
62 Breitbart W., Alici Y. Agitation and delirium at the end of life. JAMA. 2008;300(24):2898-2910.
63 Ely E.W., Stephens R.K., Jackson J.C., et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med. 2004;32(1):106-112.
64 Beliles K.E. Alternative routes of administration of psychotropic medications. In: Stoudemire A., editor. Psychiatric care of the medical patient. ed 2. Oxford: Oxford University Press; 2000:395-405.
65 Menza M.A., Murray G.B., Holmes V.F., et al. Decreased extrapyramidal symptoms with intravenous haloperidol. J Clin Psychiatry. 1987;48:278-280.
66 Brown R.L., Henke A., Greenhalgh, et al. The use of haloperidol in the agitated, critically ill pediatric patient with burns. J Burn Care Rehabil. 1996;17:34-38.
67 Breitbart W., Marotta R., Platt M., et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231-237.
68 Ratcliff S.L., Meyer W.J., Cuervo L.J., et al. The use of haloperidol and associated complications in the agitated, acutely ill pediatric burn patient. J Burn Care Rehabil. 2004;25(6):472-478.
69 Liu C.Y., Juang Y.Y., Liang H.Y., et al. Efficacy of risperidone in treating the hyperactive symptoms of delirium. Int Clin Psychopharmacol. 2004;19(3):165-168.
70 Turkel S.B. Delirium. In: Shaw R.J., DeMaso D.R., editors. Textbook of pediatric psychosomatic medicine. Washington, DC: American Psychiatric Publishing, Inc; 2010:63-75.
71 Saïas T., Gallarda T. Paradoxical aggressive reactions to benzodiazepine use: a review. Encephale. 2008;34(4):330-336. Epub 2007 Dec 26
72 Colville G., Kerry S., Pierce C. Children’s factual and delusional memories of intensive care. Am J Respir Crit Care Med. 2008;177(9):976-982. Epub 2008 Jan 31
73 Levkoff S.E., Evans D.A., Liptzin B., et al. Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med. 1992;152(2):334-340.
74 Newman M.F., et al. Report of the sub-study assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke. 2001;32(12):2874-2881.
75 DiMartini A., Dew M.A., Kormos R., et al. Posttraumatic stress disorder caused by hallucinations and delusions experienced in delirium. Psychosomatics. 2007;48(5):436-439.
76 Dew M.A., Kormos R.L., DiMartini A.F., et al. Prevalence and risk of depression and anxiety-related disorders during the first three years after heart transplantation. Psychosomatics. 2001;42(4):300-313.
77 Breitbart W., Gibson C., Tremblay A. The delirium experience: delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics. 2002;43:183-194.
78 Jones C., Griffiths R.D., Humphris G., et al. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001;29(3):573-580.
79 Yasik A.E., Saigh P.A., Oberfield R.A., et al. Posttraumatic stress disorder: memory and learning performance in children and adolescents. Psychiatry. 2007;61(3):382-388. Epub 2006 Aug 22
80 Morita T., Akechi T., Ikenaga M., et al. Terminal delirium: recommendations from bereaved families’ experiences. J Pain Symptom Manage. 2007;34(6):579-589. Epub 2007 Jul 26
81 Namba M., Morita T., Imura C., et al. Terminal delirium: families’ experience. Palliat Med. 2007;21(7):587-594.
82 Lou M.F., Dai Y.T. Nurses’ experience of caring for delirious patients. J Nurs Res. 2002;10(4):279-290.
83 Balluffi A., Kassam-Adams N., Kazak A., et al. Traumatic stress in parents of children admitted to the pediatric intensive care unit. Pediatr Crit Care Med. 2004;5(6):547-553.
84 Inouye S.K., Rushing J.T., Foreman M.D., et al. Does delirium contribute to poor hospital outcome? J Gen Intern Med. 1998;13:234-242.
85 McCusker J., Cole M., Abrahamowicz M., et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162:457-463.
86 Trzepacz P.T., Meagher D.J. Delirium. In: Levenson J.L., editor. Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005:91-130.
87 Morita T., Akechi T., Ikenaga M., et al. Terminal delirium: recommendations from bereaved families’ experiences. J Pain Symptom Manage. 2007;34:579-589.
88 Ely E.W., Shintani A., Truman B., et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762.