CHAPTER 65 Degenerative Spondylolisthesis
Degenerative spondylolisthesis (DS) was first described in 1930 by Junghanns, who coined the term pseudospondylolisthesis to describe the presence of forward slippage of a vertebral body in the presence of an intact neural arch.1 The clinical and pathologic features of this entity were further defined by MacNab, who described the condition as “spondylolisthesis with an intact neural arch.”2 The term degenerative spondylolisthesis was originally used by Newman and Stone3 and is the terminology most commonly used to describe the anterior slippage of one vertebral body on another in the presence of an intact neural arch. More recently, an etiology-based classification has been proposed that distinguishes between the pathologic processes that may cause spondylolisthesis.4 Under this classification, two broad types of spondylolisthesis have been defined: developmental and acquired. DS, as described initially by MacNab, and subsequently by Newman and Stone, is a subtype of the acquired form subsequently described by Marchetti and Bartolozzi.4,5 In that classification, DS may be either primary or secondary. Primary degenerative spondylolisthesis is typically seen in middle-aged women and usually presents with clinical spinal stenosis. Secondary degenerative spondylolisthesis occurs as a result of a predisposing factor such as adjacent segment degeneration and slip above a preexisting fusion.
Epidemiology and Biomechanics
DS is a condition of older people and rarely affects those younger than 40 years of age. It most commonly involves the L4-5 level, although other levels may be affected. It much less commonly involves the L5-S1 level, in contrast to isthmic spondylolisthesis, which most commonly occurs at L5-S1. Factors that have been reported to predispose to anterolisthesis at the lumbosacral junction include an L5 vertebral body that is less deeply seated within the pelvis, a more slender L5 transverse process, and increased sacral inclination, all of which are more common in women than men.6
DS is approximately four to five times more common in females than in males and is more common in black females than in white females.7 The female preponderance is thought to be due to greater ligamentous laxity and hormonal effects.8–10 A significantly increased expression of estrogen receptors has been found in facet joints of postmenopausal women having severe facet arthritis associated with DS compared with those with spinal stenosis only. It was not clear whether the higher expression of estrogen receptors aggravated the degenerative facet changes or was a causative factor for DS.9
In an epidemiologic survey of 4151 patients, the Copenhagen Osteoarthritis Study found the incidence of DS was 2.7% in males and 8.4% in females.11 The only factors associated with an increased risk of DS in women were elevated body mass index (BMI), increased age, and increased angle of lordosis. In men, only increased age was associated with a higher risk of degenerative spondylolisthesis. There was no association between DS and age at menopause, smoking, or occupational lifting exposure.
It is likely that the development of DS is multifactorial and dependent on anatomic factors such as intervertebral disc pathology, ligament laxity, posterior facet joint arthrosis, the amount of lumbar lordosis, hormonal factors, and prior pregnancy. Genetic factors are also a likely contributor to the development of spinal stenosis with DS in some patients. One study demonstrated that a type IX collagen gene polymorphism that introduced a tryptophan residue into the protein’s triple helix predisposed its carriers to development of spinal stenosis with DS.12
A cadaveric model investigating the relative contributions of disc integrity and anterior and posterior longitudinal ligament factors in the development of low-grade anterolisthesis found that integrity of the disc was more important than ligamentous factors, although disruption of both was necessary to produce significant destabilization.13 The effect of facet joint orientation has also been reported to be a potential factor in the development of DS. Specifically, more sagittally oriented L4-5 facet joints have been implicated as a cause of DS.14–16 In one study, individuals in whom both L4-5 facet joint angles were sagittally oriented more than 45 degrees were 25 times more likely to have DS than those with less than 45 degrees of facet angulation.14 Whether the sagittal orientation is developmental or acquired is unclear and is a matter of debate. One study reported that sagittal orientation is a result of facet joint remodeling associated with arthrosis rather than the cause of anterior subluxation.16
A retrospective, age- and sex-matched, case-control radiographic study of middle-aged women found that decreased anterior disc height and increased lumbar index (lumbar index = posterior vertebral body height/anterior vertebral body height) were two independent predictors of DS.17
Natural History
The natural history of DS, like many other spinal conditions, is not well characterized.18 A meta-analysis of the literature on DS between 1970 and 1993 found that only 25 of the 152 studies reviewed, representing 889 patients, satisfied their inclusion criteria.19 Only three of these studies, encompassing 278 patients, described the natural history of DS.20–22 Overall, 90 of these 278 patients (32%) achieved satisfactory results without treatment. The study by Matsunaga and colleagues21 represented the best of the three studies and was the only true natural history study. In that study 40 patients who received no treatment were followed from 5 to 14 years (mean, 8.25 years). Only 4 of 40 patients (10%) showed clinical deterioration over the course of the study, and all were in the group of 28 patients who exhibited no slip progression over the follow-up period. Progressive slip was noted in 12 patients (30%), although none of the 12 patients exhibited clinical deterioration. The majority of the patients in this study showed slight improvement in their clinical symptoms over time. In general, no correlation was noted between slip progression and clinical deterioration. No slip progression was noted in patients with intervertebral disc narrowing, spur formation, subcartilaginous sclerosis, or ligamentous ossification, suggesting that these anatomic factors were protective against further slip and represented a mechanism of spinal restabilization. The lack of correlation between slip progression and progression of symptoms has also been reported by other authors.18,23,24 The generally favorable prognosis of DS was confirmed by a North American Spine Society (NASS) work group consensus statement that summarized evidence-based clinical guidelines on the diagnosis and treatment of DS.18 Although it did not distinguish between natural history and conservative care, that committee reported that most patients without neurologic deficits did well without surgery.
Clinical Features
The clinical features of DS are the same as those of spinal stenosis. DS, like spinal stenosis, may be either asymptomatic or may produce low back and/or leg pain. Back pain with DS is typically mechanical and may be aggravated by back extension or by arising from a bent posture. It is to be distinguished from discogenic back pain, which is typically provoked by flexion or sitting. Only a small percentage of patients with DS may experience low back pain (LBP). A recent report that investigated a small cohort of patients from the Framingham Heart Study concluded that there did not appear to be an association between LBP and DS, as diagnosed by computed tomography (CT).25
Leg pain may be either radicular or referred in a characteristic pattern of neurogenic claudication. Neurogenic claudication, also known as pseudoclaudication, is a clinical condition consisting of leg pain associated with walking.26 Neurogenic claudication must be distinguished from vascular claudication, which has slightly different clinical features, a different etiology, and completely different treatment (Table 65–1). Neurogenic claudication is defined as lower extremity pain, paresthesias, or weakness associated with walking or standing.27,28 Pain is the predominant symptom, being present in up to 94% of patients with spinal stenosis, with numbness (63%) and weakness (43%) being less common.27,28 Bilateral involvement is common. Patients with neurogenic claudication may present with either unilateral radicular pain or with diffuse, nondermatomal symptoms beginning in the buttocks and extending a variable distance into the legs. Radicular pain is typically dermatomal in distribution and is often unilateral. It is the presenting type of symptom in 6% to 13% of symptomatic patients with stenosis.28 It is often seen with lateral recess stenosis, foraminal stenosis, or concomitant disc herniation. The clinical effects of spinal canal narrowing are magnified by the presence of a degenerative slip that further narrows the spinal canal.
TABLE 65–1 Characteristics of Vascular vs. Neurogenic Claudication
| Evaluation | Vascular | Neurogenic |
|---|---|---|
| Walking distance | Fixed | Variable |
| Palliative factors | Standing | Sitting/bending |
| Provocative factors | Walking | Walking/standing |
| Walking uphill | Painful | Painless |
| Bicycle test | Positive (painful) | Negative (painless) |
| Pulses | Absent | Present |
| Skin | Loss of hair/shiny | Normal |
| Weakness | Rarely | Occasionally |
| Back pain | Occasionally | Commonly |
| Back motion | Normal | Limited |
| Pain character | Cramping/distal-to-proximal | Numbness/aching/proximal-to-distal |
| Atrophy | Uncommon | Occasionally |
Typical neurogenic claudication is less dermatomal in character than is radicular pain. It is frequently bilateral and may have a radicular component to it. Symptoms are typically produced by standing or walking and are relieved by sitting or bending forward (see Table 65–1). Indeed, patients may preferentially assume a stooped-over posture when walking or standing to ameliorate symptoms (“grocery cart sign”). Other leg symptoms such as weakness or numbness may also occur in association with standing or walking. Night pain is an uncommon feature of spinal stenosis, although it has been described in patients with lateral stenosis (lateral recess stenosis or foraminal stenosis). Unusual symptoms of spinal stenosis such as priapism associated with intermittent claudication during walking have also been reported.
The relationship of symptoms to posture can be explained on the basis of variation in canal size with posture.29,30 Cadaveric studies have demonstrated that spinal canal cross-sectional area, midsagittal diameter, subarticular sagittal diameter, and foraminal size are significantly reduced in extension and are increased with flexion.29 Similarly, neural compression is greater in extension than in flexion. An association between posture and epidural pressure measurements has also been demonstrated. In vivo studies relating posture to epidural pressure measurements have shown that epidural pressures at the level of stenosis were higher in standing compared with lying and sitting and were increased with extension and decreased with flexion.29
Neurogenic claudication should be distinguished from vascular claudication because their causes and treatments are different (see Table 65–1). Although both conditions may present as leg pain associated with walking, only patients with neurogenic claudication typically have leg pain with standing. Leg pain associated with neurogenic claudication is highly position dependent, whereas vascular claudication is unaffected by back flexion or extension. Leg pain with cycling in a sitting position is common with vascular claudication but is unaffected by neurogenic claudication.31 Patients with vascular claudication will typically have more leg pain produced by walking uphill than downhill, whereas patients with a neurogenic claudication will typically have less pain walking uphill, owing to the slightly flexed posture of the lumbar spine that results in neuroforaminal widening and reduced neural compression. Patients with neurogenic claudication may actually have increased leg pain when walking down an incline owing to associated lumbar lordosis and consequent neuroforaminal narrowing.
Radiographic Diagnosis
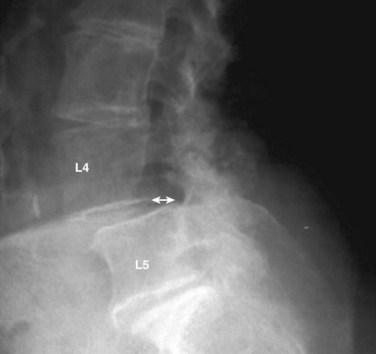
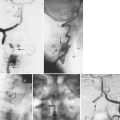
The diagnosis of DS is a radiographic diagnosis that is made on the lateral lumbar radiograph (Fig. 65–1). Although the slippage (anterolisthesis) may also be evident on a supine lateral radiograph, it is important that the lateral x-ray be performed in the standing position because there can be a dynamic component to the slip, causing it to reduce in the supine position and appear normal.32 It is not unusual for a patient to present with a normally aligned supine magnetic resonance imaging (MRI) study as the only radiographic study. Unless standing lumbar radiographs are obtained, however, the presence of a degenerative slip could be missed. In one recent study, 22% of L4-5 degenerative slips, as documented by standing lateral flexion-extension radiographs, were not detectable on supine MRI.33 Other dynamic radiographic views such as sitting or standing flexion-extension views and distraction-compression radiography may also be considered.
MRI may show increased signal within a facet joint at the level of the slip.33,34 Large facet effusions greater than 1.5 mm were found to be highly predictive of L4-5 DS, even in the absence of a measurable slip on the supine MRI.33 Therefore a patient who presents with only an MRI should be suspected of having a DS if a large facet effusion is detected, and standing lumbar radiographs should be obtained. Alternatively, the slip can be documented by upright or axial loaded MRI.34,35
Although the actual measurement of translation is generally straightforward, the distinction between what is normal dynamic translation and abnormal segmental motion (instability) is not.36,37 There is no consensus as to what constitutes clinically significant radiographic instability of the lumbar spine, nor even what is considered to be the normal range of translation between motion segments.36,37 As with routine radiographs, there exists a spectrum of normal translation that can exist in the absence of symptoms.36,37 One study showed that more than 90% of asymptomatic volunteers exhibited between 1 and 3 mm of translation on flexion-extension lateral lumbar radiographs and that a dynamic change of greater than 4 mm was therefore considered abnormal.36
Treatment
As mentioned previously, both the natural history of DS and, until recently, its optimal treatment are incompletely understood. The well-publicized Spine Patient Outcomes Research Trial (SPORT) was a prospective evaluation of the 2-year38 and 4-year18,39 outcomes of 607 patients with DS. Half of the patients were enrolled in a randomized cohort and half in an observational cohort. Pre-enrollment nonoperative care was not specified, and the type of surgery or nonoperative treatment during the study period was left to the discretion of the treating physicians. This study was hampered by a significant cross-over and nonadherence to treatment between the two groups, leading to both an as-treated and an intent-to-treat analysis of the data. When both the randomized and observational cohorts were combined, the as-treated analysis revealed that the surgically treated patients had significantly better outcome for both pain and function at 2-year and 4-year follow-ups. This study did not allow comparison of types of treatments, so it did not answer the question of which surgical treatments provided better outcomes.
That same SPORT trial examined radiographic predictors of outcome in both surgically and nonoperatively treated patients.40 Radiographic features examined included degree of slip (grade I vs. grade II), disc height (<5 mm vs. >5 mm), and mobility (stable vs. hypermobile). As noted previously, surgically treated patients had better outcomes than nonsurgically treated patients across all three radiographic parameters examined. For nonoperative patients, those with a grade I slip did better than those with a grade II slip, and those with a hypermobile slip did better than those with a stable slip.
In a long-term follow-up of patients with DS, progressive slip was noted in 34% of the 145 nonsurgically managed patients who were observed for a minimum of 10 years.41 This study was not a true natural history study because it included patients who had various nonsurgical interventions. Seventy-five percent of the patients were neurologically normal at the beginning of the study, and the majority (76%) remained so at final follow-up. Of the 34% who had neurologic symptoms, 83% experienced neurologic deterioration and had a poor outcome. There was no correlation between slip progression and clinical symptoms. This study suggested that conservative (nonsurgical) treatment in neurologically normal patients can result in satisfactory clinical outcome at an average of 10 years’ follow-up in the majority of patients.
Decompression Without Fusion
A recent review of Medicare patients undergoing surgery for stenosis, either with or without spondylolisthesis, from 2002 to 2007 found that only 21% of patients undergoing surgery for stenosis with spondylolisthesis in 2007 had simple decompression surgery, compared with 79% having some form of decompression with fusion.42 Although the trend in type of surgical procedure performed over this 6-year period was not examined for patients with stenosis associated with DS, the study noted that for patients with stenosis the trend was one of increasing complexity of surgical procedure. The rate of complex fusion, defined as fusion involving more than two levels or a 360-degree fusion, increased 15-fold from 2002 to 2007. It is likely that a similar increase in the rate of complex surgery occurred for patients with stenosis associated with DS.
One reason for considering decompression without fusion in select patient populations is that it is less invasive than fusion and reduces the morbidity and mortality associated with spinal fusion in elderly patients.42–46 In the recent retrospective review of Medicare claims for patients undergoing surgery for spinal stenosis between 2002 and 2007, patients having a complex fusion had greater morbidity, more life-threatening complications, greater likelihood of rehospitalization within 30 days of surgery, and higher costs compared with patients having decompression alone or decompression with simple fusion (defined as one- or two-level fusion through a single surgical approach).42
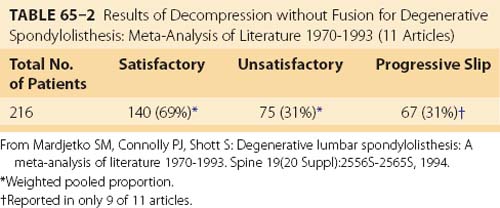
A meta-analysis of the literature on DS between 1970 and 1993 found only 11 papers, encompassing 216 patients, reporting outcome measures after decompression without fusion that met their inclusion criteria (Table 65–2).19 One of these studies was retrospective and nonrandomized,20 two were prospective and randomized,47,48 and the remaining eight were retrospective, nonrandomized, and uncontrolled. Overall, 69% of patients in this meta-analysis reported satisfactory outcome with decompression alone, with 31% having an unsatisfactory result.
TABLE 65–2 Results of Decompression without Fusion for Degenerative Spondylolisthesis: Meta-Analysis of Literature 1970-1993 (11 Articles)
One report that supported decompression without fusion for DS reviewed an elderly (average age, 67 years) population of 290 patients, 250 of whom had a one-level slip and 40 of whom had a two-level slip.49 The data from that study were self-reported by the surgeons and were retrospective. The decompressive procedures included laminectomy in 249 patients and fenestration procedures in 41 patients. Fenestration procedures typically involved bilateral laminotomy with partial medial facetectomy and foraminotomy. Only patients with a “stable” slip having less than 4-mm translation and less than 10 to 12 degrees of angulation on dynamic lateral radiographs were included. At an average follow-up of 10 years (range, 1 to 27 years), 69% of patients reported excellent outcome, 13% good outcome, 12% fair, and 6% poor. The authors concluded that 82% excellent/good outcome was acceptable in this elderly population, in whom fusion is associated with higher morbidity and mortality.
Similar results were reported in a recent retrospective review of 49 elderly patients (mean age, 68.7 years) with symptomatic degenerative lumbar spondylolisthesis, without evidence of hypermobility on flexion-extension radiographs, and who underwent decompression without fusion.50 At a mean follow-up of 3.73 years, 73.5% of the patients reported excellent or good results, although 10% underwent revision surgery with an instrumented fusion. The study concluded that limited decompression alone can be helpful in a select group of elderly patients without hypermobility.
Bilateral decompression through a unilateral approach has also been described in patients with spinal stenosis, both with and without DS. A retrospective study using that technique compared patients with and without spondylolisthesis and showed similar functional outcome between the two groups at 2 years.51 Although there was a statistically significant increase in the percentage slip postoperatively in the patients with spondylolisthesis, it did not appear to produce an adverse functional outcome. Nevertheless, the presence of an increased slip at 2 years is a cause for concern and caution.
One prospective study assigned a group of 67 patients with spinal stenosis to either laminectomy or multilevel laminotomy and included a small subgroup of patients with DS.52 Nine of the patients assigned to the laminotomy group crossed over to the laminectomy group, which allowed for some difficulty in interpreting the results. However, no patient who underwent multilevel laminotomies developed instability as a result of the surgery, compared with three patients who developed instability following laminectomy. The authors recommended multilevel laminotomies for patients with developmental stenosis, mild to moderate degenerative stenosis, or DS. Bilateral laminectomy was recommended for patients with severe degenerative stenosis or marked DS.
Another recent study prospectively evaluated 54 consecutive patients who underwent decompression without fusion for spinal stenosis.53 In the small subgroup of 15 patients who had concomitant DS, 87% (13 of 15 patients) showed no change in the amount of preoperative slip. Overall, 88% of the 54 patients reported good/excellent clinical outcome and the results were comparable between patients with and without DS. The study concluded that degenerative spinal stenosis including patients with DS can be decompressed effectively without the need for fusion.
Noninstrumented Posterolateral Fusion
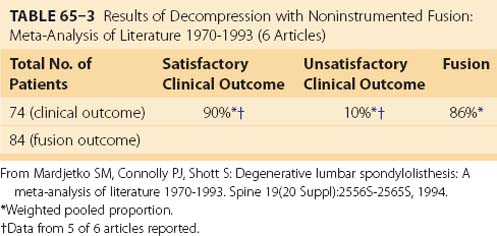
Although the beneficial role of fusion in the surgical treatment of spinal stenosis associated with DS is less controversial than the role of fusion in the treatment of other degenerative back conditions, incontrovertible evidence supporting fusion is sparse. An attempted meta-analysis of literature reported between 1970 and 1993 found only six studies meeting the inclusion criteria that reported results of decompression with noninstrumented fusion for DS.19 In that review, 90% of patients having decompression with noninstrumented fusion reported satisfactory clinical outcome and 86% achieved a solid arthrodesis, although the fusion rate varied widely, ranging from 3047 to 100% (Table 65–3).54 Patients undergoing decompression with noninstrumented fusion achieved a statistically significantly better clinical outcome than those treated with decompression alone (90% vs. 69%, respectively).
TABLE 65–3 Results of Decompression with Noninstrumented Fusion: Meta-Analysis of Literature 1970-1993 (6 Articles)
Many studies on the surgical treatment of DS report unfavorable outcome after decompression without fusion. One early, small study by two groups of surgeons from two different institutions included two populations of patients with spinal stenosis and DS: one group underwent decompression alone and the other had decompression and fusion.20 In the patients undergoing decompression alone, 5 of 11 (45%) were rated as good (satisfactory) and 6 of 11 (55%) as fair/poor (unsatisfactory). In contrast, 5 of 8 patients (63%) undergoing decompression with in situ posterolateral fusion achieved a satisfactory outcome. This study suggested that patients did better when their decompression was accompanied by noninstrumented fusion.
Several studies have supported the position that patients undergoing fusion with decompression for DS do clinically better than those undergoing decompression alone.20,48,55 It is difficult to gain a clear understanding of this issue from a review of existing literature, however, because well-done studies reporting surgical outcome after surgery for DS are uncommon.
Although most studies report no correlation between clinical outcome and the amount of slip progression, one study suggested that poor outcome was associated with slip progression.47 That study was a prospective randomized study that included a subgroup of 11 patients undergoing decompression and noninstrumented fusion for DS. Of the 10 patients available for follow-up, only 3 (30%) reported improved functional outcome and 7 had an increase in their preoperative spondylolisthesis, suggesting that slip progression was associated with poor clinical outcome.
A landmark prospective, randomized study comparing decompression alone with decompression and noninstrumented posterolateral spinal fusion in the treatment of L3-4 and L4-5 DS with spinal stenosis reported superior results when concomitant fusion was performed with the decompression.48 Satisfactory outcome was more than twice as common in the fused group compared with the unfused group (96% vs. 44%, respectively). Furthermore, the percentage of excellent results was significantly and dramatically greater in the fused group (44% excellent) than in the unfused group (8% excellent; P < 0.0001) (Table 65–4). This study concluded that the results of surgical decompression with in situ arthrodesis were superior to those of decompression alone in the treatment of spinal stenosis associated with L3-4 or L4-5 DS. Outcome was influenced by neither the age or sex of the patient nor the preoperative height of the disc space. The authors concluded that the decision for concomitant arthrodesis should be based purely on the presence or absence of a preoperative slip rather than on other preoperative factors such as the age or sex of the patient, disc height, or intraoperative factors such as the amount of bone resected during the decompression. This study showed that the results of decompression with an attempted arthrodesis produced superior results to decompression alone, even if the fusion was unsuccessful (pseudarthrosis). Although postoperatively there was a significant (P = 0.002) increase in the slip in patients not receiving an arthrodesis compared with those undergoing fusion, 36% of the arthrodesis group were also noted to have a pseudarthrosis, although all had an excellent or good result.
TABLE 65–4 Prospective, Randomized Comparison of Decompression vs. Decompression and Noninstrumented Spinal Fusion for Degenerative Spondylolisthesis
| Outcome | Arthrodesis (n = 25) | No Arthrodesis (n = 25) |
|---|---|---|
| Excellent | 11 (44%) | 2 (8%) |
| Good | 13 (52%) | 9 (36%) |
| Fair | 1 (4%) | 12 (48%) |
| Poor | 0 (0%) | 2 (8%) |
| Mean increase in slip (preoperative to postoperative) | 0.5 mm | 2.6 mm (P = .002) |
From Herkowitz HN, Kurz LT: Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am 73:802-808, 1991.
A long-term review of 96 patients undergoing decompressive surgery for spinal stenosis followed for at least 5 years included a subset of patients with associated DS.56 Although this subgroup was not fully analyzed separately and the study itself was retrospective, nonrandomized, and uncontrolled, some important trends were noted. Twenty-six patients (27%) of the entire group were considered failures: 16 because of recurrent neural symptoms and 10 because of low back pain. The incidence of DS was significantly greater in that surgical failure group (12 of 26 patients [46%]) than in the surgical successes (16 of 64 [25%]). The authors concluded that because of the higher incidence of recurrent symptoms in patients with preexisting DS, all patients with an associated slip should be fused.
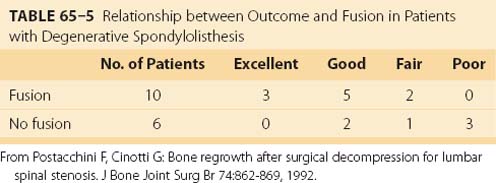
One well-recognized cause of long-term failure of decompression for spinal stenosis is subsequent bone regrowth causing recurrent neural compression. One study reported the relationship between bone regrowth, occurring an average of 8.6 years after surgical decompression for spinal stenosis, and long-term outcome.54 Of the 40 patients in the study, 16 had preoperative DS, 10 of whom had concomitant arthrodesis. Although all 16 patients with preexisting DS showed some bone regrowth, the degree of regrowth was less severe in the 10 patients undergoing arthrodesis than in the 6 patients who were not fused (Table 65–5). Furthermore, the proportion of satisfactory results was significantly higher in patients who had spinal fusion. Although this study was retrospective and not randomized, it suggested that arthrodesis stabilized the spine, resulting in less bone regrowth causing recurrent stenosis, and produced superior long-term results.
One problem associated with noninstrumented in situ fusion is the difficulty, if not inability, to restore normal lumbar lordosis. This is particularly true with a multilevel noninstrumented fusion, which can produce a flatback deformity, although it can occur even with a single-level noninstrumented fusion. It has been demonstrated that an L4-5 in situ fusion that produces kyphosis or hypolordosis results in increased motion at the adjacent L3-4 level.57 Such hypermobility may be one factor in adjacent-level degeneration after fusion.
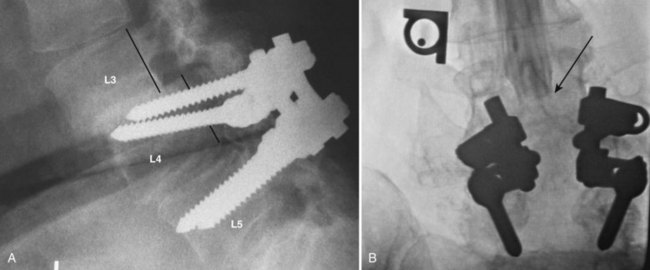
An important issue with the use of spinal instrumentation in the elderly patient is its potential biomechanical effect on adjacent, unfused levels. There is concern and evidence that the rigidity produced by a solid fusion, particularly with instrumentation, may cause significant stresses at adjacent levels above or below the fusion with the potential for adjacent-level failure (Fig. 65–2). Such failure may be manifested by symptomatic or asymptomatic degeneration or by adjacent level vertebral compression fracture or stress fracture because of the osteoporotic nature of the bone. The use of instrumentation may also produce direct injury to the superior facet by either capsular disruption or articular facet damage. These are arguments against pedicle screw fixation in the elderly patient with osteoporosis. Therefore the use of less rigid instrumentation, or no instrumentation, may be preferable to rigid instrumentation because of theoretically reduced stresses on adjacent levels by the presence of a less rigid fusion or even a stable pseudarthrosis or by less risk to the superior facet joint by a pedicle screw.
Posterior Instrumented Fusion
The long-term clinical outcome of surgical decompression with instrumented spinal fusion for DS, particularly when compared with the outcome of decompression with noninstrumented fusion, is not completely known. A comprehensive literature search of the English literature on lumbar or lumbosacral fusion from 1979 to 200058 identified only two prospective and randomized studies that were limited to DS.48,59 Although that study identified a nonsignificant trend toward greater use of instrumentation technology for lumbar fusion, generally, the clinical benefit of that pattern was unclear.
A Cochrane review of surgery of lumbar surgery found that there was limited evidence that fusion produced a better outcome, or resulted in less slip progression, than decompression alone. Although there was strong evidence that the use of adjunct instrumentation produced a higher fusion rate than noninstrumented fusion with decompression, superior outcome was not demonstrated.60
A recent randomized, controlled trial comparing surgery with nonsurgical treatment for spinal stenosis found that patients undergoing fusion with decompression had less pain and better functional outcome at 2-year follow-up than patients undergoing decompression alone.61 This was also true for a smaller subset of patients with DS who underwent instrumented fusion. Although the number of patients undergoing instrumented fusion was too small to permit extensive analysis, the authors felt that instrumented fusion should be considered for stenosis associated with DS.
A prospective, nonrandomized study of patients with grade I DS compared decompression alone with decompression with instrumented fusion with 1-year follow-up.62 The study demonstrated statistically significant functional improvement in the fusion group compared with the decompression group by Oswestry Disability Index (ODI) and Short Form-36 (SF-36). The type of surgery was at the discretion of the treating surgeon and was done at two institutions by two surgeons. The nonrandom nature of the study and its restriction to patients with grade I slips only limited the generalization of the conclusions.
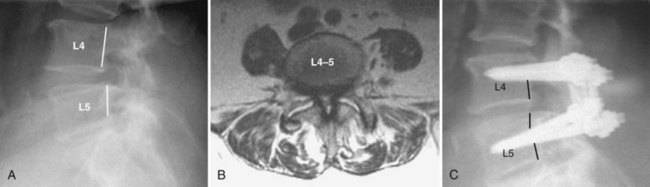
Most studies looking at fusion with instrumentation in the treatment of DS involve concomitant decompression (Fig. 65–3). One recent study, however, investigated the role of instrumented fusion with slip reduction and minimal decompression for DS.63 The decompression involved only bilateral foraminotomies to safely visualize and mobilize the exiting nerve root. At a mean month follow-up of 33 months, 82% of patients with leg pain and 75% of those with back pain showed relief. The degree of anterolisthesis was reduced by 90% at follow-up. The authors concluded that the clinical, functional, and radiographic outcome produced results comparable with the published outcomes of in situ fusion after formal laminectomy and that formal laminectomy may not always be necessary in the treatment of degenerative lumbar spinal stenosis with spondylolisthesis.
Although there is little argument that segmental instrumentation produces a more solid arthrodesis than noninstrumented fusion, there are conflicting data relating a solid arthrodesis to better clinical outcome. The multicenter historical cohort study of spinal fusion using pedicle screw fixation involved a retrospective review of 2684 patients with DS.64 Solid radiographic fusion was noted in 89% of patients undergoing pedicle screw fixation compared with 70% of those without instrumentation. Clinical outcome was also better in the group of patients undergoing instrumented fusion. This report, however, was a retrospective, historical review rather than a prospective randomized study, and the validity of its conclusions is therefore limited.
A prospective, randomized study followed 124 patients for 1 year after either instrumented or noninstrumented fusion for various diagnoses including DS.55 Two types of spinal instrumentation were employed: a rigid system and a semirigid system. Outcome was based primarily on radiographic fusion rate. The overall fusion rate was 65% for the noninstrumented group, 77% for the semirigid fixation group, and 95% for the rigid fixation group. For the subgroup of patients with DS, fusion was achieved in 65% of the noninstrumented patients, 50% of the semirigid fixation group, and 86% of the rigid fixation group. A trend for better clinical outcome with increasing rigidity of fixation was also observed: 71% of the noninstrumented group, 89% of the semirigid group, and 95% of the rigid group reported excellent or good results.
A retrospective review of 30 patients undergoing decompression and instrumented fusion for DS reported both radiographic outcome by fusion rate and functional outcome by patient questionnaire and the SF-36 survey.65 Both fusion rate and patient satisfaction were 93%. However, 13 patients (43%) had complications including dural tears (3 patients), excessive blood loss (2 patients), pseudarthrosis (2 patients), pulmonary embolus (1 patient), deep infection (1 patient), urinary tract infections (3 patients), and unstable angina (1 patient). Patients with complications were found to have poorer outcomes. The study concluded that patients treated with decompression and fusion for DS had improved patient-reported functional outcomes but a significant risk of complications.
Some studies have concluded that the addition of spinal instrumentation to a fusion did not necessarily improve outcome. A randomized prospective study of patients undergoing posterolateral lumbar fusion, with and without pedicle screw instrumentation, for a variety of conditions concluded that the addition of instrumentation did not produce a significant incremental clinical benefit to that obtained from noninstrumented fusion, although there was a slight but nonsignificant trend toward higher fusion rate in the instrumented fusion group.66 Overall, there was no statistical difference in patient-reported outcome between the two groups. Although there was a slight nonsignificant trend toward an increased radiographic fusion rate in the group with instrumentation, this did not correlate with increased patient-reported improvement. For the entire group, the results did not show a clinical benefit from the addition of instrumentation in elective lumbar fusions. For a small subgroup of 10 patients who had DS, 5 underwent instrumented fusion and 5 underwent noninstrumented in situ fusion. Four of the five patients with DS undergoing instrumented fusion achieved excellent/good outcome, compared with two of five of those undergoing noninstrumented fusion. For this small subgroup of patients with DS, the clinical outcome appeared to be better than that of the overall population studied, although this subgroup was too small to achieve statistical significance.
A prospective, randomized study of 68 patients with spinal stenosis and DS compared decompression and arthrodesis without instrumentation to decompression with segmental transpedicular instrumentation.59 At an average of 2 years’ follow-up, successful fusion was significantly more common in the instrumented group than in the noninstrumented group (83% vs. 45%, respectively), although there was no significant improvement in clinical outcome between the two groups (76% vs. 85% excellent/good outcome, respectively). The authors concluded that the presence of successful fusion did not predict or influence short-term clinical outcome at 2 years.
In a long-term follow-up of 58 patients previously reported and prospectively randomized to decompression with noninstrumented fusion,48,59 47 were available for review at an average of 7 years, 8 months postoperatively (range, 5 to 14 years).67 Excellent and good clinical outcome was reported in 86% of patients achieving a solid arthrodesis but in only 56% of those patients having a pseudarthrosis. Patients with a solid fusion had significantly less back pain and better function than those with a pseudarthrosis. This study demonstrated a clear benefit of a solid arthrodesis on clinical outcome for patients undergoing decompression for spinal stenosis with DS. The short-term improvement in outcome noted at 2 years’ follow-up in Herkowitz and Kurz’s48 and Fischgrund and colleagues’59 initial studies deteriorated at final follow-up in patients who did not achieve a solid arthrodesis. The finding of better clinical outcome associated with solid fusion was also confirmed in another retrospective comparison of patients having a solid uninstrumented arthrodesis compared with patients with a pseudarthrosis.68
A 7-year follow-up of 47 patients treated with decompression and noninstrumented fusion found that long-term outcome was better in patients having a solid arthrodesis compared with those with a pseudarthrosis.67 The authors inferred that the use of adjunct instrumentation might produce better long-term clinical outcome than fusion without instrumentation. However, they did not compare the long-term clinical outcome of the uninstrumented but solidly fused patients from their initial study with instrumented patients from their subsequent study, so a definitive conclusion on the value of instrumentation on clinical outcome could not be made.59,69 Therefore the issue of whether the addition of spinal instrumentation confers long-term clinical outcome that is superior to, or worse than, or the same as that of a solid noninstrumented arthrodesis has not been answered by this or any other study.70–72
Posterior Fusion with Anterior Column Support
Some authors have recommended the use of concomitant posterior fusion with anterior column support in the surgical management of some types of spondylolisthesis. Most commonly, the anterior column support is provided by either a posterior lumbar interbody fusion (PLIF) or a transforaminal lumbar interbody fusion (TLIF), although anterior lumbar interbody fusion (ALIF) has also been used. Anterior column support has been more commonly recommended for isthmic spondylolisthesis, although its use for DS has also been advocated.73–76 As with posterior instrumented fusion, generally, convincing data comparing posterior fusion with anterior column augmentation with other types of fusion do not exist. Furthermore, there is no evidence to suggest that the biomechanics and potential mechanism of failure for isthmic spondylolisthesis are the same as those for DS.
Purported advantages of interbody fusion with PLIF or TLIF compared with posterior instrumented fusion without an interbody fusion include greater likelihood of fusion, better indirect foraminal decompression, better reduction of the spondylolisthesis, and better lordosis.73,74,76 In a one-level spondylolisthesis, however, it is not clear whether a slight improvement in slip reduction or lordosis produces a better clinical outcome. Nor is it known whether the potential for a slight incremental improvement in sagittal alignment or an increase in lordosis is worth the risk of potential nerve root injury as a result of interbody fusion.
Options for interbody fusion devices include metallic cages, carbon fiber cages, polyetheretherketone (PEEK) cages, or bone. A study comparing combined anterior and posterior lumbar reconstruction using anterior cages to posterior pedicle screw fixation alone investigated the biomechanical effects of interbody cages on construct stiffness, pedicle-screw strain, and adjacent-level changes.77 This study found that for spinal instability with preserved anterior load sharing, pedicle screw fixation alone was biomechanically adequate and recommended that interbody cages not be used because they further increased segmental motion at the adjacent level. Where anterior column support was deficient, however, posterior stabilization with pedicle screws alone provided inadequate stability and resulted in a high level of implant strain. Under such circumstances, the addition of an interbody cage significantly increased the construct stiffness and decreased hardware strain, although it resulted in increased motion at the adjacent segment. Similar adverse effects on the adjacent level were demonstrated in another biomechanical study that showed that rigid fusion using posterior pedicle screw fixation and an interbody cage produced higher loads at the superior adjacent level than posterior instrumented fusion without an interbody cage because of the increased stiffness of the fixed segments using the cage.78
From a societal perspective, fusion, particularly instrumented fusion, adds significantly to the incremental costs of treating spinal stenosis with degenerative spondylolisthesis. Kuntz and colleagues79 looked at the 10-year costs, quality-adjusted life-years (QALY), and incremental cost-effectiveness ratios (reported as dollars per quality-adjusted year of life gained) for patients undergoing decompressive surgery, with or without spinal fusion, for spinal stenosis with DS. Laminectomy with noninstrumented fusion was found to cost $56,500 per quality-adjusted year of life versus laminectomy without fusion. The cost-effectiveness ratio of instrumented fusion, compared with noninstrumented fusion, was $3,112,800 per quality-adjusted year of life. A cost-effectiveness ratio of $82,400 per quality-adjusted year of life was calculated if the proportion of patients experiencing symptom relief after instrumented fusion was 90% as compared with 80% for patients with noninstrumented fusion. The study concluded that the cost-effectiveness of laminectomy with noninstrumented fusion compared favorably with other surgical interventions such as lumbar discectomy for treatment of herniated lumbar disc or coronary artery bypass grafting for triple-vessel coronary artery disease. The cost-effectiveness, however, depended greatly on the true effectiveness of the surgery to alleviate symptoms and also on how patients valued the quality-of-life effect of relieving severe stenosis symptoms. Instrumented fusion was expensive compared with the incremental gain in health outcome. The study further concluded that better data on the effectiveness of this and other alternative procedures were necessary to justify their incremental cost.
The Spine Patient Outcomes Research Trial (SPORT) looked at the cost-effectiveness of spine surgery at 2-year follow-up.80 This study evaluated the 61% of patients with DS who had surgery. Ninety-three percent of these surgical patients had fusion, most (78%) with instrumentation. The study found that surgery significantly improved quality of life compared with nonoperative treatment, with an average cost of $115,000 per QALY gained. The authors concluded that surgery was not highly cost-effective compared with other elective orthopedic surgeries over the 2-year follow-up period but did compare favorably with many other health interventions. Whether or not surgery for DS is cost-effective in the long run depends on long-term benefits and the ongoing costs associated with fusion surgery (e.g., the potential for future revision surgery).
The cost-effectiveness of fusion, generally, was also examined in a Swedish study that compared a group of patients who were randomized to one of four treatment groups for chronic low back pain: noninstrumented posterolateral fusion, instrumented posterolateral fusion, instrumented posterolateral fusion with interbody fusion, and a nonsurgical control group.81 This study did not specifically examine patients with spinal stenosis and DS but concluded that the cost of treatment of chronic low back pain at 2-year follow-up was significantly higher if fusion was performed. Although the treatment effect of all surgical groups was found to be better than the control (nonoperative) group, the added (incremental) cost per quality-adjusted year of life for fusion compared with nonoperative care ranged from $52,000 to $157,000 in the United States, depending on the magnitude of the assumed average annual quality-adjusted year of life.
A recent study that reviewed major medical complications and charges associated with surgery for spinal stenosis in Medicare patients from 2002 to 2007 found that adjusted mean hospital charges for complex fusion procedures, defined as fusion involving more than two levels or a 360-degree (interbody) fusion, cost $80,888 compared with $23,724 for decompression alone.42
Besides the added cost of instrumented fusion, other adverse effects of fusion have also been noted. It has been demonstrated that lumbar fusion is associated with greater morbidity than decompression alone.43,44,46 In addition, a greater potential for complications exists with instrumentation than without it. A retrospective 6.5-year follow-up (range, 5 to 10.75 years) of 36 patients undergoing instrumented fusion for DS reported a patient satisfaction rate of 83%.82 Although there were no neurologic deficits, pseudarthroses, recurrent stenosis at the fused segment, or progression of deformity at the fused level, five patients had symptomatic adjacent-level degeneration (transition syndrome) and an additional seven patients had asymptomatic radiographic transition syndromes. The authors concluded that although the rate of major complications (2%), implant failures (2%), and symptomatic pseudarthroses (0%) was low, radiographic degeneration at levels adjacent to the fused levels (transition syndrome) was common.
Alternative Surgical Strategies
Indirect spinal canal and foraminal decompression via interspinous process distraction has been proposed as an alternative to decompression for spinal stenosis with DS.83 A randomized controlled study of 42 patients with spinal stenosis associated with DS was compared with 33 control patients treated nonoperatively.83 That study found that the use of an interspinous process distraction device produced a better functional outcome than nonoperative management in patients with neurogenic claudication associated with degenerative spondylolisthesis. The question of whether or not that device was as good as, worse, or better than traditional decompression or decompression and fusion was not addressed by that study.
A contrary view of interspinous process distraction was reported in a study of 12 consecutive patients with DS and DS treated by interspinous process distraction and followed for a mean of 30 months.84 That study reported a high failure rate: although two thirds of patients had complete relief of their preoperative symptoms, one third had no relief and 58% underwent surgical decompression and fusion within 24 months of their index procedure.
Significant reduction in total sagittal range of motion has been reported with one interspinous device that used both a mechanical blocking component and a tension band.85 The tension band resulted in a significant additional restriction in total motion including flexion compared with the device without the tension band (43% reduction in motion compared with 16% reduction).
The use of motion-sparing technology has been advocated by some authors as an effective alternative to fusion that can reduce the potential for adjacent-level degeneration.51,86–88 One prospective, minimum 4-year follow-up study of 26 consecutive patients with DS reported significantly improved pain and walking distance without progression of the spondylolisthesis.51,87 Three patients demonstrated radiographic screw loosening and one patient had screw breakage. Nearly half of the patients showed some degeneration at adjacent levels. Comparable clinical outcome using the same device was reported in a prospective, randomized, multicenter U.S. Food and Drug Administration investigational device exemption (IDE) trial with a 1-year follow-up.88 Early results from that study showed significant improvement in both back and leg pain, as well as function, but the study cautioned that further follow-up was necessary.
Summary of Treatment Options
Currently there does not appear to be a clear consensus as to the optimal way to treat patients with symptomatic DS. Some studies suggest that patients undergoing surgery do better when the decompression is accompanied by fusion. It is less clear, however, whether the fusion should be augmented with instrumentation. Although a fusion is more robust and solid with instrumentation than without it, the incremental benefits of instrumentation on clinical outcome are less clear. It seems reasonable that when clear evidence of instability on flexion-extension radiographs exists, the immediate stability provided by instrumentation warrants the additional time, expense, and potential morbidity associated with its use. This is especially appropriate for young, active patients with good bone stock. On the other hand, the indication for the use of hardware in a patient with a collapsed disc space, no motion at the spondylolisthetic level, or the presence of osteoporotic bone is less clear. A 2005 focus issue in Spine on lumbosacral fusion contained a combined position statement by Spine and the Scoliosis Research Society. It noted a positive long-term correlation between fusion and improved clinical outcome. It also noted a clear association between the use of instrumentation and higher fusion rates. Accordingly, the use of spinal instrumentation was recommended for spinal stenosis associated with DS. The position statement concluded by stating that the effectiveness of other posterior or anterior techniques (e.g., interbody fusion techniques) has yet to be established.89
Summary
Pearls and Pitfalls
1 Macnab I. Spondylolisthesis with an intact neural arch: The so-called pseudo-spondylolisthesis. J Bone Joint Surg Br. 1950;32:325-333.
2 Newman P, Stone K. The etiology of spondylolisthesis. J Bone Joint Surg Br. 1963;45:39-59.
3 Herkowitz H, Kurz L. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802-808.
4 Fischgrund J, Mackay M, Herkowitz H, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807-2812.
5 Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726-733.
6 Mardjetko S, Connolly P, Shott S. Degenerative lumbar spondylolisthesis: A meta-analysis of literature, 1970-1993. Spine. 1994;19(20 Suppl):2256S-2265S.
1 Junghanns H. Spondylolisthesen ohne Spalt in Zwischengelenkstueck. Arch Orthop Unfallchir. 1930;29:118-127.
2 Macnab I. Spondylolisthesis with an intact neural arch: The so-called pseudo-spondylolisthesis. J Bone Joint Surg Br. 1950;32:325-333.
3 Newman P, Stone K. The etiology of spondylolisthesis. J Bone Joint Surg Br. 1963;45:39-59.
4 Marchetti PG, Bartolozzi P. Spondylolisthesis: Classification of spondylolisthesis as a guideline for treatment. In: Bridwell R, Dewald R, editors. The Textbook of Spinal Surgery. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1997:1211-1254.
5 Hammerberg KW. New concepts on the pathogenesis and classification of spondylolisthesis. Spine. 2005;30(Suppl):S4-11.
6 Hosoe H, Ohmori K. Degenerative lumbosacral spondylolisthesis: Possible factors which predispose the fifth lumbar vertebra to slip. J Bone Joint Surg Br. 2008;90:356-359.
7 Rosenberg N. Degenerative spondylolisthesis: Surgical treatment. Clin Orthop Rel Res. 1976;117:112-120.
8 Bird HA, Eastmond CJ, Hudson A, Wright V. Is generalized joint laxity a factor in spondylolisthesis? Scand J Rheumatol. 1980;9:203-205.
9 Ha Kee-Yong, Chang Cheong-Ho, Kim Ki-Won, et al. Expression of estrogen receptor of the facet joints in degenerative spondylolisthesis. Spine. 2005;30:562-566.
10 Sanderson PL, Fraser RD. The influence of pregnancy on the development of degenerative spondylolisthesis. J Bone Joint Surg Br. 1996;78:951-954.
11 Jacobsen S, Sonne-Holm S, Rovsing H, et al. Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine.. 2007;32:120-125.
12 Matsui Y, Mirza SK, Wu JJ, et al. The association of lumbar spondylolisthesis with collagen IX tryptophan alleles. J Bone Joint Surg Br. 2004;86:1021-1026.
13 Crawford NR, Cagli S, Sonntag VK, et al. Biomechanics of grade 1 degenerative spondylolisthesis: I. In vitro model. J Neurosurg Spine. 2001;94:51-60.
14 Boden SD, Riew KD, Yamaguchi K, et al. Orientation of the lumbar facet joints: Association with degenerative disc disease. J Bone Joint Surg Am. 1996;78:403-411.
15 Grobler LJ, Robertson PA, Novotny JE, Pope MH. Etiology of spondylolisthesis: Assessment of the role played by lumbar facet joint morphology. Spine. 1993;18:80-91.
16 Love TW, Fagan AB, Fraser RD. Degenerative spondylolisthesis: Developmental or acquired? J Bone Joint Surg Br. 1999;81:670-674.
17 hen I-Ru, Wei Ta-Sen. Disc height and lumbar index as independent predictors of degenerative spondylolisthesis in middle-aged women with low back pain. Spine. 2009;34:1402-1409.
18 Watters WC, Bono CM, Gilbert TJ, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. The Spine Journal. 2009;9:609-614.
19 Mardjetko S, Connolly P, Shott S. Degenerative lumbar spondylolisthesis: A meta-analysis of literature, 1970-1993. Spine. 1994;19(20 Suppl):2256S-2265S.
20 Feffer H, Weisel S, Cuckler JM, Rothman RH. Degenerative spondylolisthesis: To fuse or not to fuse. Spine. 1985;10:286-289.
21 Matsunaga S, Sakou T, Morizono Y, et al. Natural history of degenerative spondylolisthesis: Pathogenesis and natural course of the slippage. Spine. 1990;15:1204-1210.
22 Saal JA, Saal JA, Parthasarathy R. The natural history of lumbar spinal stenosis. The Results of Non-operative Treatment. Presented at 10th annual meeting of the North American Spine Society (NASS) Washington, DC. 1995.
23 Cinotti G, Postacchini F, Fassari F, et al. Predisposing factors in degenerative spondylolisthesis: A radiographic and CT study. Int Orthop. 1997;21:337-342.
24 Grob D, Humke T, Dvorak J. Degenerative lumbar spinal stenosis decompression with and without arthrodesis. J Bone Joint Surg Am. 1995;77:1036-1041.
25 Kalichman L, Kim DH, Li L, et al. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34:199-205.
26 Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36:230-237.
27 Katz JN, Dalgas M, Stucki G, et al. Degenerative lumbar spinal stenosis: Diagnostic value of the history and physical examination. Arthritis Rheum. 1995;38:1236-1241.
28 Katz J, Lipson S, Larson M, et al. The outcome of decompressive laminectomy for degenerative lumbar stenosis. J Bone Joint Surg Am. 1991;73:809-816.
29 Inufusa A, An HS, Lim TH. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine. 1996;21:2412-2420.
30 Yoshida M, Shima K, Taniguchi Y. Hypertrophied ligamentum flavum in lumbar spinal canal stenosis: Pathogenesis and morphologic and immunohistochemical observation. Spine. 1992;17:1353-1360.
31 Dyck P, Doyle JBJr. “Bicycle test” of van Gelderen in diagnosis of intermittent cauda equina compression syndrome: Case report. J Neurosurg. 1977;46:667-670.
32 Bendo JA, Ong B. Importance of correlating static and dynamic imaging studies in diagnosing degenerative lumbar spondylolisthesis. Am J Orthop. 2001;30:247-250.
33 Chaput C, Padon D, Rush J, et al. The significance of increased fluid signal on magnetic resonance imaging in lumbar facets in relationship to degenerative spondylolisthesis. Spine.. 2007;32:1883-1887.
34 Ben-Galim P, Reitman CA. The distended facet sign: an indicator of position-dependent spinal stenosis and degenerative spondylolisthesis. Spine Journal. 2007;7:245-248.
35 Jayakumar P, Nnadi C, Saifuddin A, et al. Dynamic degenerative lumbar spondylolisthesis: diagnosis with axial loaded magnetic resonance imaging. Spine. 2006;31:E298-301.
36 Boden SD, Wiesel SW. Lumbosacral segmental motion in normal individuals: Have we been measuring instability properly? Spine. 1990;5:571-576.
37 Hayes MA, Howard TC, Gruel CR, et al. Roentgenographic evaluation of lumbar spine flexion-extension in asymptomatic individuals. Spine. 1989;14:327-331.
38 Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257-2270.
39 Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. J Bone Joint Surg. 2009;91-A:1295-1304.
40 Pearson AM, Lurie JD, Blood EA, et al. Spine patient outcomes research trial: radiographic predictors of clinical outcomes after operative or nonoperative treatment of degenerative spondylolisthesis. Spine. 2008;33:2759-2766.
41 Matsunaga S, Ijiri K, Hayashi K. Nonsurgically managed patients with degenerative spondylolisthesis: A 10- to 18-year follow-up study. J Neurosurg. 2000;93(2 Suppl):194-198.
42 Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259-1265.
43 Deyo R, Cherkin D, Loeser J, et al. Morbidity and mortality in association with operations on the lumbar spine. J Bone Joint Surg Am. 1992;74:536-543.
44 Deyo R, Ciol M, Cherkin D, et al. Lumbar spinal fusion: A cohort study of complications, reoperations, and resource use in the Medicare population. Spine. 1993;18:1463-1470.
45 Oldridge N, Yuan Z, Stoll J, Rimm A. Lumbar spine surgery and mortality among Medicare beneficiaries, 1986. Am J Public Health. 1994;84:1292-1298.
46 Turner JA, Ersek M, Herron L, et al. Patient outcomes after lumbar spinal fusions. JAMA. 1992;268:907-911.
47 Bridwell K, Sedgewick TA, O’Brien MF, et al. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993;6:461-472.
48 Herkowitz H, Kurz L. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802-808.
49 Epstein N, Epstein J. Decompression in the surgical management of degenerative spondylolisthesis: Advantages of a conservative approach in 290 patients. J Spinal Disord. 1998;11:116-122.
50 Kristof RA, Aliashkevich AF, Schuster M, et al. Degenerative lumbar spondylolisthesis-induced radicular compression: Nonfusion-related decompression in selected patients without hypermobility on flexion-extension radiographs. J Neurosurg. 2002;97(3 Suppl):S281-S286.
51 Sasai K, Umeda M, Maruyama T, et al. Microsurgical bilateral decompression via a unilateral approach for lumbar spinal canal stenosis including degenerative spondylolisthesis. J Neurosurg Spine. 2008;9:554-559.
52 Postacchini F, Cinotti G, Perugia D, et al. The surgical treatment of central lumbar stenosis: Multiple laminotomy compared with total laminectomy. J Bone Joint Surg Br. 1993;75:386-392.
53 Kleeman TJ, Hiscoe AC, Berg EE. Patient outcomes after minimally destabilizing lumbar stenosis decompression: The “port-hole” technique. Spine. 2000;25:865-870.
54 Postacchini F, Cinotti G. Bone regrowth after surgical decompression for lumbar spinal stenosis. J Bone Joint Surg Br. 1992;74:862-869.
55 Zdeblick T. A prospective, randomized study of lumbar fusion: Preliminary results. Spine. 1993;18:983-991.
56 Caputy A, Luessenhop A. Long-term evaluation of decompressive surgery for degenerative lumbar stenosis. J Neurosurg. 1992;77:669-676.
57 Akamaru T, Kawahara N, Tim Yoon S, et al. Adjacent segment motion after a simulated lumbar fusion in different sagittal alignments: A biomechanical analysis. Spine. 2003;28:1560-1566.
58 Bono CM, Lee CK. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: Influence of techniques on fusion rate and clinical outcome. Spine. 2004;29:455-463.
59 Fischgrund J, Mackay M, Herkowitz H, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807-2812.
60 Gibson JN, Grant IC, Waddell G. The Cochrane review of surgery for lumbar disc prolapse and degenerative lumbar spondylosis. Spine. 1999;24:1820-1832.
61 Malmivaara A, Slätis P, Heliövaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32:1-8.
62 Ghogawala Z, Benzel EC, Amin-Hanjani S, et al. Prospective outcomes evaluation after decompression with or without instrumented fusion for lumbar stenosis and degenerative Grade I spondylolisthesis. J Neurosurgery Spine. 2004;1:267-272.
63 Bednar DA. Surgical management of lumbar degenerative spinal stenosis with spondylolisthesis via posterior reduction with minimal laminectomy. J Spinal Disord Tech. 2002;15:105-109.
64 Yuan HA, Garfin SR, Dickman CA, et al. A historical cohort study of pedicle screw fixation in thoracic lumbar, and sacral spinal fusions. Spine. 1994;19(20 Suppl):2279-2296.
65 Nork SE, Serena SH, Workman KL, et al. Patient outcomes after decompression and instrumented posterior spinal fusion for degenerative spondylolisthesis. Spine. 1999;24:561-569.
66 France JC, Yaszemski MJ, Lauerman WC, et al. A randomized prospective study of posterolateral lumbar fusion outcomes with and without pedicle screw instrumentation. Spine. 1999;24:553-560.
67 Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726-733.
68 Tsutsumimoto T, Shimogata M, Yoshimura Y, et al. Union versus nonunion after posterolateral lumbar fusion: a comparison of long-term surgical outcomes in patients with degenerative lumbar spondylolisthesis. Eur Spine Journal. 2008;17:1107-1112.
69 Katz JN. Point of view. Spine. 2004;29:733-734.
70 Fischgrund JS. The argument for instrumented posterolateral fusion for patients with spinal stenosis and degenerative spondylolisthesis [Editorial]. Spine. 2004;29:173-174.
71 McLain RF. Instrumented fusion for degenerative spondylolisthesis: Is it necessary [Editorial]? Spine. 2004;29:170.
72 Phillips FM. The argument for noninstrumented posterolateral fusion for patients with spinal stenosis and degenerative spondylolisthesis [Editorial]. Spine. 2004;29:170-172.
73 Jagannathan J, Sansur C, Oskouian RJr., et al. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurg. 2009;64:955-963. discussion 963-4
74 Park P, Foley KT. Minimally invasive transforaminal lumbar interbody fusion with reduction of spondylolisthesis: technique and outcomes after a minimum of 2 years’ follow-up. Neurosurg Focus. 2008;25:E16.
75 Xu H, Tang H, Li Z. Surgical treatment of adult degenerative spondylolisthesis by instrumented transforaminal lumbar interbody fusion in the Han nationality. J Neurosurg Spine. 2009;10:496-499.
76 McAfee PC, DeVine JG, Chaput CD, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine. 2005;30(Suppl):S60-S65.
77 Oda I, Abumi K, Yu BS, et al. Types of spinal instability that require interbody support in posterior lumbar reconstruction: An in vitro biomechanical investigation. Spine. 2003;28:1573-1580.
78 Sudo H, Oda I, Abumi K, et al. In vitro biomechanical effects of reconstruction on adjacent motion segment: Comparison of aligned/kyphotic posterolateral fusion with aligned posterior lumbar interbody fusion/posterolateral fusion. J Neurosurg. 2003;99(2 Suppl):221-228.
79 Kuntz KM, Snider RK, Weinstein JN, et al. Cost-effectiveness of fusion with and without instrumentation for patients with degenerative spondylolisthesis and spinal stenosis. Spine. 2000;25:1132-1139.
80 Tosteson AN, Lurie JD, Tosteson TD, et al. Surgical treatment of spinal stenosis with and without degenerative spondylolisthesis: cost-effectiveness after 2 years. Ann Intern Med.. 2008;149:845-853.
81 Fritzell P, Hägg O, Jonsson D, Nordwall A. Cost-effectiveness of lumbar fusion and nonsurgical treatment for chronic low back pain in the Swedish Lumbar Spine Study: A multicenter, randomized controlled trial from the Swedish lumbar spine study group. Spine. 2004;29:421-434.
82 Booth KC, Bridwell KH, Eisenberg BA, et al. Minimum 5-year results of degenerative spondylolisthesis treated with decompression and instrumented posterior fusion. Spine. 1999;24:1721-1727.
83 Anderson P, Tribus C, Kitchel S. Treatment of neurogenic claudication by interspinous decompression: application of the X STOP device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine.. 2006;4:463-471.
84 Verhoof O, Bron J, Wapstra F, et al. High failure rate of the interspinous distraction device (X-Stop) for the treatment of lumbar spinal stenosis caused by degenerative spondylolisthesis. Eur Spine Journal. 2008;17:188-192.
85 Gunzburg R, Szpalski M, Callary SA, et al. Effect of a novel interspinous implant on lumbar spinal range of motion. Eur Spine J. 2009;18:696-703.
86 Kanayama M, Hashimoto T, Shigenobu K, et al. Non-fusion surgery for degenerative spondylolisthesis using artificial ligament stabilization: surgical indication and clinical results. Spine. 2005;30:588-592.
87 Schaeren S, Broger I, Jeanneret B. Minimum four-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization. Spine. 2008;33:E636-E642.
88 Welch W, Cheng B, Awad T, et al. Clinical outcomes of the Dynesys dynamic neutralization system: 1-year preliminary results. Neurosurg Focus. 2007;22:E8.
89 Mardjetko S, Albert T, Andersson G, et al. Spine/SRS Spondylolisthesis Summary Statement. Spine. 2005;30(6 Suppl):S3.