CHAPTER 13 Degenerative Arthritis
Arthroscopic débridement for OA of the knee was initially reported by Burman and colleagues in 1934.1 In 1941, Magnuson introduced the term “joint debridement.”2 During and after World War II, arthroscopy waned and the open Magnuson procedure, consisting of total synovectomy, osteophyte resection, cruciate ligament excision (if torn), and patellectomy became the treatment of choice for arthritis of the knee until the resurgence of arthroscopy in the early 1970s.3
ANATOMY
In 1743, William Hunter stated, “From Hippocrates to the present age, it is universally allowed that ulcerated cartilage is a troublesome thing and that once destroyed it is not repaired.”4 In 1849, Leidy confirmed this principle, stating that “a rupture of cartilage fragments is never united and that articular cartilage lacks regenerative power and the joint becomes filled with tough fibrous tissue.”5 As noted by Mankin, superficial lacerations of cartilage neither heal nor progress to more serious disorders if they are small lesions, but deep lacerations may be clearly visible years after injury.6 When the subchondral bone is thus disrupted, interosseous blood vessels expose bone matrix growth factors, causing fibrin clot formation. Inflammation introduces new cells into the cartilage defect and these cells proliferate and begin matrix repair. The matrix of articular cartilage is a hyperhydrated tissue, with estimates of water content ranging as high as 80%; it contains type I collagen consisting of two alpha chains and one alpha-2 chain. It is this type I collagen that is formed when fibrous tissue regenerates when attempting to form normal hyaline articular cartilage.7 Furthermore, mature repair tissue has a relatively low proteoglycan concentration. These reparative cells do not produce tissue with the unique composition, structure, and biochemical properties of normal articular cartilage.8 After cartilage injury or during the progression of osteoarthritis, some chondrocytes proliferate but do not migrate through the matrix to enter the site of tissue injury. The repair tissue matrix, which is usually formed by undifferentiated cells containing primarily type I collagen, thus cannot restore normal articular cartilage properties. These reparative cells fail to organize the molecules that they produce to create a strong cohesive structure such as that of articular cartilage; rather, they produce other types of molecules that may interfere with the assembly of the cartilage matrix.
These alterations compromise the ability of cartilage to survive and function in the highly stressed mechanical environment found in load-bearing joints and may lead to further cartilage degeneration and osteoarthritis. Disruption of collagen cross-linking causes cartilage to lose its intrinsic tensile stiffness, strength, and shear stiffness, and this loss of proteoglycans and increased water content compromises its compressive and permeability properties.9,10 A number of treatments have been attempted to stimulate repair or reformation of the articular surface of the knee joint. Arthroscopically, these treatments include marrow stimulation procedures, débridement and shaving of fibrillated cartilage, and joint lavage.
TREATMENT
Arthroscopic Technique
Marrow Stimulation Procedures
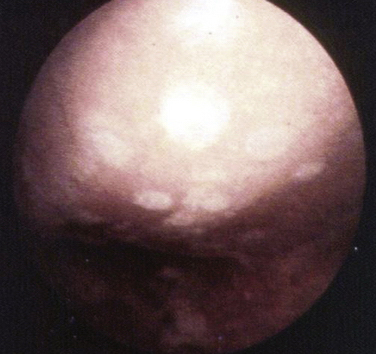
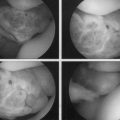
The concept of drilling through eburnated bone to stimulate reparative cartilage formation was originally described by Pridie in 1959 (Fig. 13-1).11 Laboratory animal studies attempted to confirm these findings. Akeson and associates12 drilled the subchondral bone of dog femoral heads and Mitchell and Shepard13 drilled holes into the subchondral bone of rabbit knee joints. Both groups noted deterioration of the repair tissue within 1 year.
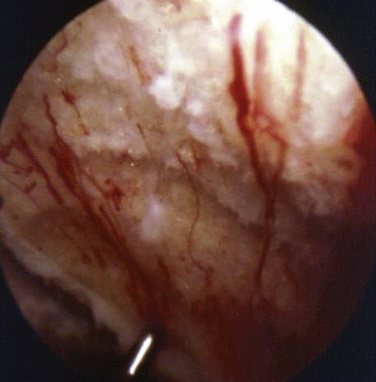
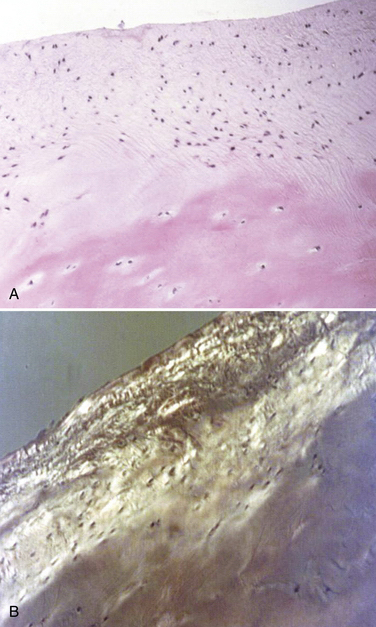
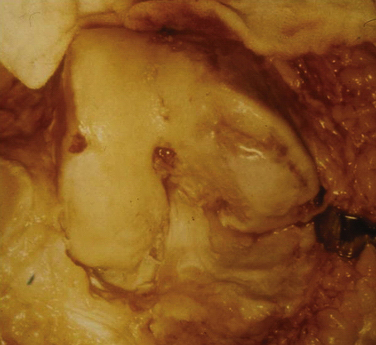
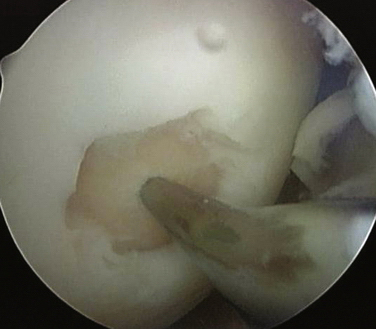
These experiments were the first to show that perforation of subchondral bone could stimulate repair of large areas of joint surface with fibrocartilaginous tissue, but the repair tissue lacked the proteoglycan concentration found in normal cartilage that was noted previously. Abrasion arthroplasty of grade IV eburnated chondral lesions using an arthroscopic burr to expose interosseus bleeding was introduced by Johnson14 in 1981 (Fig. 13-2). The resulting hemorrhagic exudate formed a fibrin clot, allowing for fibrous repair tissue formation over the eburnated bone (Fig. 13-3). Only one of eight biopsy specimens showed any type II collagen typical of hyaline cartilage at the time of arthroscopic review and biopsy, and the remainder had types I and III collagen. In a series of patients at our institution who had abrasion arthroplasty, at 5-year follow-up examinations, 15 had been converted to total knee replacement (TKR) and biopsies were obtained at the time of TKR. All patients had fibrocartilage and type I collagen on their biopsy specimens (Fig. 13-4). In this series of 126 patients who were treated for unicompartmental OA with abrasion arthroplasty plus débridement or arthroscopic débridement alone, only 51% had good to excellent results with abrasion arthroplasty at 5-year follow-up; 66% had good to excellent results with arthroscopic débridement alone.15 Coventry and Bowman16 have noted that formation of hyaline-like cartilage occurs in the unloaded medial compartment of several patients after valgus upper tibial osteotomy (Fig. 13-5). This finding was confirmed arthroscopically by Fujisawa and coworkers17 12 to 18 months after upper tibial osteotomies, which implies that regeneration of reparative cartilage can occur secondary to unloading of bone alone without additional surgery. Because adult articular cartilage does not heal and the regenerative tissue is not hyaline cartilage but fibrocartilage, it lacks normal amounts of proteoglycan. Thus, effective methods of repairing significant cartilage defects must provide cells that can migrate, proliferate, and differentiate in the chondral sites and produce and maintain a cartilage matrix. Furthermore, there must be a mechanical and biologic environment that promotes synthesis, assembly, and maintenance of articular cartilage matrix. This regenerative fibrocartilage has a difficult time transferring compressive loads and, therefore, cannot be expected to survive in an abnormal mechanical environment.7–10
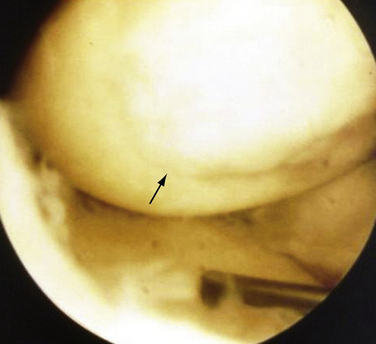
FIGURE 13-3 Arthroscopic view of a patient 4 years after abrasion arthroplasty showing resurfacing with fibrocartilage.
Microfracture
Blevens and colleagues18 have recommended the microfracture (MF) technique, in which they use an arthroscopic awl to create multiple perforations into the subchondral bone. They reported 266 patients between 1985 and 1990, with 3.7-year follow-up, using a grading system similar to that of the Outerbridge classification. After chondral surface débridement, the bone is perforated to a depth of 3 to 4 mm using an awl and the holes are placed approximately 4 to 5 mm apart in a full-thickness lesion with exposed subchondral bone (Fig. 13-6). Blood should be seen emanating from the microfracture holes after perforation is complete. A postoperative rehabilitation program was used to provide motion without applying high load stress to the treated chondral defect. Repeat arthroscopies were performed in 80 patients. In most chondral defects, subchondral bone was covered with cartilage of varying quality, and the term hyaline-like was introduced to describe the fibrocartilage surface. There was no evidence that hyaline cartilage was present at second-look arthroscopy, and the authors confirmed that the only type of tissue that was seen to regenerate over these surfaces was fibrocartilaginous repair tissue. They stated further that the “biochemical composition and durability of the presumed fibrocartilage repair tissue is unanswered.” Clearly, there is no evidence that hyaline cartilage is regenerated by marrow stimulation.
Arthroscopic Débridement
Arthroscopic débridement as a treatment option for OA was reported by Sprague in 1981.19 At 1-year follow-up, 74% of patients stated that their “knee was improved and more functional” than before surgery. The extent of arthritis, however, was not correlated clinically or roentgenographically with good success rates. In the early to mid-1980s, some studies reported that the results of arthroscopic débridement were not correlated with age or extent of arthritis either roentgenographically or arthroscopically, with up to 11-year follow-up.20,21 Gross and associates22 and Ogilvie-Harris and Fitsialos23 concluded in the early 1990s that OA severity was the best predictor of success after arthroscopic débridement, and that normally aligned knees with mild arthritis had the best results at 8-year follow-up. It is certainly not clear, however, that shaving a damaged articular cartilage relieves pain. Bentley24 has reported that chondroplasty during arthrotomy produces unpredictable results, and only 25% of patients treated with patellar chondroplasty had satisfactory results beyond 1 year. In 1990, Timoney and coworkers25 retrospectively reviewed 109 patients who had arthroscopic débridement for degenerative arthritis of the knee at 4.2-year follow-up. Using the Harris hip score (HHS), only 45% reported good results and 21% of the patients experienced worsened symptoms and subsequently had TKR. Moseley and colleagues,26 in 1996, randomized 10 patients with osteoarthritis of the knee into a placebo group, an arthroscopic lavage group, and an arthroscopic débridement group. All 10 patients at 6 months reported improvement in their pain scores and satisfaction with their surgery, with the exception of one placebo patient, after their 6-month follow-up. This study was repeated in 200227 with a larger patient group at a VA hospital; 70% of these patients had moderate to severe OA. No significant differences were found among the three groups who had arthroscopy with débridement, arthroscopy with lavage, or placebo knee surgery. Of interest was that those patients who had positive magnetic resonance imaging (MRI) scans with meniscal tears were excluded from his study. It was concluded that there is no clear role for arthroscopy in knees with OA. Steadman and associates28 recently reported a success rate of 71% using the WOMAC and Lysholm scoring system with 2-year follow-up results. Wai and coworkers29 and Hawker and colleagues30 have reported that up to 9.2% of patients had a TKR after 1 year and 18.4% had a TKR after 3 years subsequent to arthroscopic débridement. A number of studies have claimed that arthroscopic débridement and shaving help relieve the symptoms of osteoarthritis of the knee.14,15,19-23,31 However, it is not clear why these patients improved and equally unclear as to why they stay improved for as long as 5 years postoperatively.
Alignment And Arthroscopic Débridement
Correlation of preoperative angular deformity with the results of arthroscopically débrided knees was originally reported by Salisbury and associates31 in 1985. In patients with residual varus deformity, 32% noted improvement in pain at a minimum 1-year follow-up. Normal knee alignment was considered to be 1 to 7 degrees of femoral tibial valgus alignment preoperatively. Harwin32 and Baumgartner33 concluded that abnormal varus or valgus angulation was a statistically significant factor in predicting a failed result after arthroscopic irrigation and débridement. Similar findings were reported by Ogilvie-Harris and Fitsialos.23 Those patients who have varus or significant valgus knee deformities with medial or lateral compartment disease, respectively, will have worse results than those with postoperative neutral or mild valgus alignment.
Arthroscopy And Tidal Lavage
In 1978, Bird and Ring34 reported on a series of 14 patients who had arthroscopic lavage of the knee and 93% of patients improved by 1 week, but by 4 weeks only 50% had noted mild to moderate improvement. Jackson and colleagues35 have reported on more than 207 patients with femoral tibial arthritic disease in the medial or lateral compartment who had lavage versus arthroscopic débridement with 2-year follow-ups in 1988. They found that débridement results in 68% improvement and lavage alone results in 45% symptomatic improvement. In 1991, Livesley and associates36 compared osteoarthritic knees treated by arthroscopic lavage and physiotherapy with a control group treated by physiotherapy alone. The joint lavage group improved to a greater degree than the control group and the improvement lasted longer. in 1992, Ike and coworkers37 compared a group of patients treated with standard medical treatment (nonsteroidal anti-inflammatory drugs, steroid injections, physical therapy, and analgesics) with those receiving tidal lavage in the office using local anesthesia. At 12 weeks, 62% of the tidal irrigation group patients and 36% of the medically managed patients were improved functionally and symptomatically. In 1993, Chang and colleagues38 reported on two groups of patients, one that received arthroscopic surgery and débridement and the other needle joint lavage only. At 1 year, they concluded that the removal of soft tissue abnormalities via arthroscopic surgery does not generally improve pain and knee dysfunction associated with non–end-stage osteoarthritis any more than simple joint lavage, unless a meniscal tear was present.
Various explanations for symptomatic relief secondary to arthroscopic lavage have been postulated, such as removal of cartilage debris, crystals, and inflammatory factors. Temporary improvement in signs of inflammation may support the hypothesis that lavage removes inflammatory agents, although it is not clear what role these inflammatory agents contribute to pain.39,40
PEARLS& PITFALLS
PEARLS
CONCLUSIONS
Arthroscopic débridement of the degenerative knee is a worthwhile procedure for younger patients and for older patients who desire symptomatic improvement and do not wish to risk the morbidity of a total knee replacement. Success rates for arthroscopic débridement vary between 50% and 67% depending on many factors, including patient age, degree of arthritis, activity level, and extent of follow-up. Arthroscopic lavage success rates vary between 45% and 51% and do not appear to have the same longevity of success as arthroscopic débridement. It is apparent from at least two studies comparing arthroscopic débridement with marrow stimulation in conjunction with débridement that marrow stimulation does not offer any greater benefit in the treatment of degenerative arthritis of the knee than débridement alone.15,41
There does not appear to be any advantage in performing arthroscopy in conjunction with upper tibial osteotomy compared with upper tibial osteotomy alone. The results are similar. Furthermore, the results of upper tibial osteotomy in conjunction with abrasion arthroplasty were identical to those in a similar series of patients who had upper tibial osteotomy alone.42 Therefore, arthroscopic débridement or marrow stimulation in conjunction with upper tibial osteotomy (UTO) seems to be of limited value for improvement of symptoms compared with UTO alone. Furthermore, the prognostic value of the arthroscope in determining whether to proceed with UTO is of minimal value, as noted by Fujisawa and coworkers17 and Keene and Dyreby.43 They concluded there was no correlation in terms of prognosis and arthroscopic evaluation prior to UTO compared with the clinical results subsequent to UTO.
In 2002 and 2008, the American Academy of Orthopaedic Surgeons discussed the role of arthroscopy for OA of the knee.44,45 It was concluded that there are clinical variables associated with improvement after arthroscopic débridement. These include preoperative mechanical symptoms, including locking, catching, or giving way, indicative of loose bodies, chondral flaps, and unstable meniscal tissue. Furthermore, on clinical examination, the patient should have medial joint line tenderness and a positive Steinmann test, indicating a symptomatic torn meniscus, as well as the presence of an unstable meniscal tear at the time of arthroscopy.46 The arthroscope is therefore useful for the treatment of degenerative arthritis of the arthritic knee when these conditions are met.
1. Burman M, Finkelstein H, Mayer L. Arthroscopy of the knee joint. J Bone Joint Surg. 1934;16:255.
2. Magnuson P. Joint debridement: Surgical treatment of degenerative arthritis. Surg Gynecol Obstet. 1941;73:1.
3. Isserlin LB. Joint debridement for osteoarthritis of the knee. J Bone Joint Surg Br. 1950;32:302-306.
4. Hunter W. On the structure and diseases of articulating cartilage. Philos Trans R Soc Lond B Biol Sci. 1743;9:267.
5. Leidy J. On the intimate structure and history of articular cartilage. Am J Med Sci. 1849;17:277.
6. Mankin HJ. Response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460-466.
7. Buckwalter JA, Cruess R. Healing of musculoskeletal tissues. In: Rockwood CA, Green DP, Bucholz RW, editors. Fractures in Adults. 3rd ed. Philadelphia: JB Lippincott; 1991:181.
8. Mow VC, Rosenwasser MP. Articular cartilage: biomechanics. In: Woo SL, Buckwalter JA, eds. Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge, Ill: American Academy of Orthopaedic Surgeons; 1988: 427.
9. Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age: degeneration of water content. J Bone Joint Surg Am. 1982;64:88-94.
10. Woo SL-Y, Mow VC, Lai W. Biomechanical properties for articular cartilage. In: Skalik R, Chein S, editors. Handbook of Bioengineering. New York: McGraw-Hill; 1987:41.
11. Pridie AH. The method of resurfacing osteoarthritic knee joints. J Bone Joint Surg Br. 1959;41:618.
12. Akeson WH, Miyashita C, Taylor TK, et al. Experimental cup arthroplasty of the canine hip. Extracellular matrix composition in cup arthroplasty. J Bone Joint Surg Am. 1969;51:149-164.
13. Mitchell N, Shepard N. Resurfacing of adult rabbit articular cartilage by multiple perforations of the subchondral bone. J Bone Joint Surg Am. 1976;58:230-233.
14. Johnson LL. Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. J Arthrosc. 1986;2:54-69.
15. Bert JM, Maschka K. The arthroscopic treatment of unicompartmental gonarthrosis: A five-year follow-up study of abrasion arthroplasty plus arthroscopic debridement and arthroscopic debridement alone. Arthroscopy. 1989;5:25-32.
16. Coventry MB, Bowman PW. Long-term results of upper tibial osteotomy for degenerative arthritis of the knee. Acta Orthop Belg. 1982;48:139-156.
17. Fujisawa Y, Masuhara K, Shiomi S. The effect of high tibial osteotomy in osteoarthritis of the knee: an arthroscopic study in 54 knee joints. Orthop Clin North Am. 1979;10:585-608.
18. Blevens FT, Steadman R, Rodrigo J, Silliman J. Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. J Orthop. 1998;21:761-767.
19. Sprague NFIII. Arthroscopic debridement for degenerative knee joint disease. Clin Orthop Relat Res. 1981;(160):118-123.
20. Jackson RW, Silver R, Marans R. The arthroscopic treatment of degenerative joint disease. J Arthrosc. 1986;2:11.
21. Shahriaree H, O’Connor RF, Nottage W. Seven years follow-up arthroscopic debridement of degenerative knee. Filed View. 1982;1:1.
22. Gross DE, Brenner SL, Esformes I, Gross ML. The arthroscopic treatment of degenerative joint disease in the knee. J Orthop. 1991;14:1317-1321.
23. Ogilvie-Harris DJ, Fitsialos DP. Arthroscopic management of the degenerative knee. J Arthrosc. 1991;7:151-157.
24. Bentley G. The surgical treatment of chondromalacia of the patellae. J Bone Joint Surg Am. 1978;60:74-81. J Bone Joint Surg Br
25. Timoney JM, Kneisl JS, Barrack RL, Alexander AH. Arthroscopy update #6. Arthroscopy in the osteoarthritic knee. Orthop Rev. 1990;19:376-379. Long-term follow-up
26. Moseley JBJr, Wray NP, Kuykendall D, et al. Arthroscopic treatment of osteoarthritis of the knee: a prospective, randomized, placebo-controlled trial. Am J Sports Med. 1996;24:28-34. Results of a pilot study
27. Moseley B, O’Malley K, Petersen N, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88.
28. Steadman R, Ramappa A, Maxwell B, Briggs K. An arthroscopic treatment regimen for osteoarthritis of the knee. Arthroscopy. 2007;23:948-955.
29. Wai E. Kreder J, Williams J. Arthroscpic debridement of the knee for osteoarthritis in patients fifty years of age or older: utilization and outcomes in the Province of Ontario. J Bone Joint Surg Am. 2002;84:17-22.
30. Hawker G, Guan J, Judge A, Dieppe P. Knee arthroscopyin England and Ontario: patterns of use, changes over time and relationship to total knee replacement. J Bone Joint Surg Am. 2008;90:2337-2345.
31. Salisbury RB, Nottage WM, Gardner D. The effect of alignment on results in arthroscopic debridement of the degenerative knee. Clin Orthop Relat Res. 1985;198:268-272.
32. Harwin S. Arthroscopic debridement for osteoarthritis of the knee: predictors of patient satisfaction. Arthroscopy. 1999;15:142-146.
33. Baumgartner M, Cannon W, Vittori J, et al. Arthroscopic debridement of the arthritic knee. Clin Orthop Relat Res. 1990;253:197-202.
34. Bird HA, Ring EF. Therapeutic value of arthroscopy. Ann Rheum Dis. 1978;37:78.
35. Jackson RW, Marans HJ, Silver RS. The arthroscopic treatment of degenerative arthritis of the knee. J Bone Joint Surg Br. 1988;70:332.
36. Livesley P, Doherty M, Needoff M, et al. Arthroscopic lavage of osteoarthritic knees. J Bone Joint Surg Br. 1991;73:922-926.
37. Ike RW, Arnold WJ, Rothschild EW, Shaw HL. Tidal irrigation versus conservative medical management in patients with osteoarthritis of the knee: a prospective randomized study. J Rheumatol. 1992;19:772-779. Tidal Irrigation Cooperating Group
38. Chang RW, Falconer J, Stulberg SD, et al. A randomized controlled trial of arthroscopic surgery versus closed-needle joint lavage for patients with osteoarthritis of the knee. Arthritis Rheum. 1993;36:289-296.
39. Dieppe PT, Muskinson BC, Willoughby DA. The inflammatory component of osteoarthritis. In: Nuki ED, editor. An Etiopathogenesis of Osteoarthritis. London: Pitman Medical; 1980:117.
40. Goldenberg DL, Egan MS, Cohen AS. Inflammatory synovitis in degenerative joint disease. J Rheumatol. 1982;9:204-209.
41. Rand J. Role of arthroscopy in osteoarthritis of the knee. Arthroscopy. 1991;7:358-363.
42. Fanelli GC, Rogers VP. High tibial valgus osteotomy combined with arthroscopic abrasion arthroplasty. Contemp Orthop. 1989;19:547.
43. Keene J, Dyreby JRJr. High tibial osteotomy in the treatment of osteoarthritis of the knee. J Bone Joint Surg Am. 1983;65:36-42. The role of preoperative arthroscopy
44. American Academy of Orthopaedic Surgeons (AAOS). Arthroscopic Surgery and Osteoarthritis of the Knee: A Report for the Centers for Medicare and Medicaid Services. Coverage Analysis Group December 2002 Available at http://www.cms.hhs.gov/determinationprocess/downloads/id7a.pdf Accessed January 14, 2010
45. American Academy of Orthopaedic Surgeons (AAOS). Treatment of Osteoarthritis of the Knee (Non-Arthroplasty). Rosemont, Ill: American Academy of Orthopaedic Surgeons; December 2008.
46. Dervin G, Stiell I, Rody K, Grabowski J. The effect of arthroscopic debridement for osteoarthritis of the knee on health-related quality of life. J Bone Joint Surg Am. 2003;85:10-19.