Chapter 32 Deep fungal infections
| SUBCUTANEOUS FUNGAL INFECTIONS | SYSTEMIC OR RESPIRATORY FUNGAL INFECTIONS | OPPORTUNISTIC FUNGAL INFECTIONS |
|---|---|---|
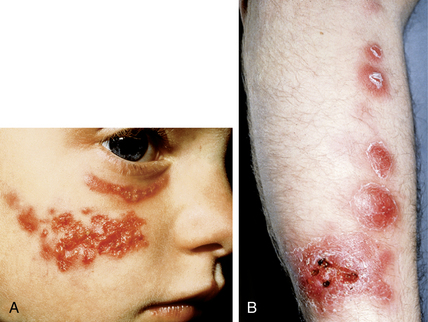
Subcutaneous fungal infections
Wheat LJ, Kauffman CA: Histoplasmosis, Infect Dis Clin North Am 17:1–19, 2003.
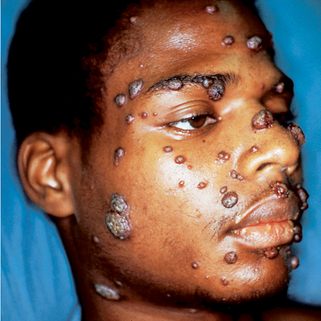
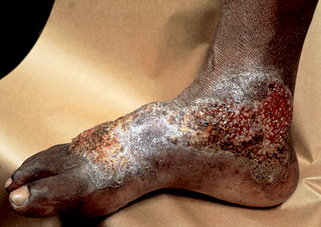
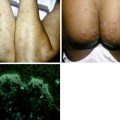
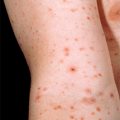
Figure 32-9. Disseminated coccidioidomycosis. Discrete verrucous papules, plaques, and nodules.
(Courtesy of James E. Fitzpatrick, MD.)
DiCaudo DJ: Coccidioidomycosis: a review and update, J Am Acad Dermatol 55(6):929–942, 2009.
Kullavanijaya P: Penicilliosis in AIDS, J Dermatol 28:667–670, 2001.
| RARE | RHINOSCLEROMA |
|---|---|
Opportunistic fungal infections
Table 32-3. Fungal Pathogens in HIV Infection
| ORGANISM | CLINICAL FEATURES |
|---|---|
| Candida albicans | Thrush, vaginal, and esophageal candidiasis |
| Cryptococcus neoformans | Pulmonary and disseminated disease, meningitis, skin, eye, prostate |
| Histoplasma capsulatum | Disseminated disease with fever, weight loss, and predilection for reticuloendothelial system, adrenal glands, and CNS |
| Coccidioides immitis | Disseminated and pulmonary disease. Predilection for skin, lymph nodes, bones/joints, and CNS |
| Blastomyces dermatitidis | Disseminated and pulmonary disease. Predilection for lung, skin, bone, CNS, and prostate |
| Aspergillus fumigatus | Disseminated and pulmonary disease |
| Penicillium marneffei | Disseminated disease with fever, anemia, weight loss Mucocutaneous lesions are common |
| Sporotrichosis schenckii | Disseminated disease. Sites of predilection: joints/bones, eyes, and meninges |
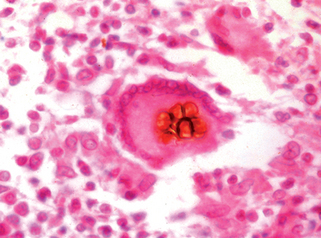
Perfect JR, Casadevall A: Cryptococcosis, Infect Dis Clin North Am 16:837–874, 2002.
Key Points: Deep Fungal Infections
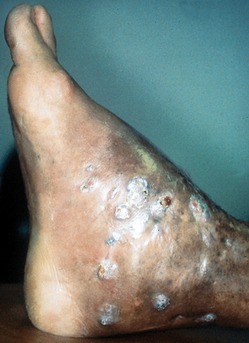
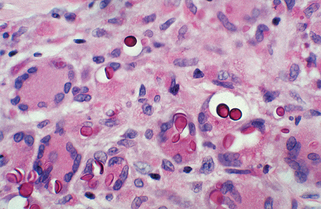
Figure 32-10. Disseminated cryptococcosis. Multiple papules and nodules that resemble molluscum contagiosum.
(Courtesy of James E. Fitzpatrick, MD.)
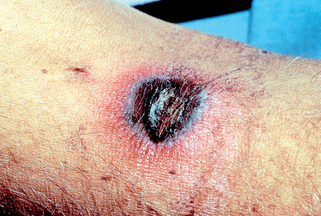
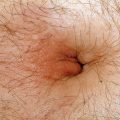
Figure 32-12. Aspergillosis cellulitis at the site of adhesive tape demonstrating erythematous plaques with pustules.
Dignani MC, Anaissie E: Human fusariosis, Clin Microbiol Infect 10:67–75, 2004.
Gonzalez CE, Rinaldi MG, Sugar AM: Zygomycosis, Infect Dis Clin North Am 16:895–914, 2002.
Eucker J, Sezer O, Graf B, Possinger K: Mucormycoses, Mycoses 44:253–260, 2001.
Centers for Disease Control and Prevention: National Select Agent Registry, Available at: http://www.selectagents.gov/. Accessed July 25, 2010.