Chapter 22 Deep Brain Stimulation
 Deep brain stimulation (DBS) is a neurosurgical intervention the efficacy, safety, and utility of which have been robustly demonstrated in the treatment of movement disorders.
Deep brain stimulation (DBS) is a neurosurgical intervention the efficacy, safety, and utility of which have been robustly demonstrated in the treatment of movement disorders. For the treatment of chronic pain refractory to medical therapies, many prospective case series have been reported, but few centers worldwide have published findings from patients treated during the last decade using current standards of neuroimaging and stimulator technology.
For the treatment of chronic pain refractory to medical therapies, many prospective case series have been reported, but few centers worldwide have published findings from patients treated during the last decade using current standards of neuroimaging and stimulator technology. With a clinical experience of DBS of the sensory thalamus and periventricular/periaqueductal gray matter now in over 70 patients treated throughout the last decade, we summarize the historical background, our scientific rationale, patient selection and assessment methods, surgical techniques, and clinical results.
With a clinical experience of DBS of the sensory thalamus and periventricular/periaqueductal gray matter now in over 70 patients treated throughout the last decade, we summarize the historical background, our scientific rationale, patient selection and assessment methods, surgical techniques, and clinical results. Several experienced centers continue DBS for chronic pain with considerable success in selected patients, in particular those with pain after amputation; plexopathies; stroke; and head and face pain, including anesthesia dolorosa.
Several experienced centers continue DBS for chronic pain with considerable success in selected patients, in particular those with pain after amputation; plexopathies; stroke; and head and face pain, including anesthesia dolorosa. Other carefully selected patient groups with visceral or genital pain or pain caused by multiple sclerosis, malignancy, and trauma may benefit from DBS; but efficacy in pain after spinal injury might be limited.
Other carefully selected patient groups with visceral or genital pain or pain caused by multiple sclerosis, malignancy, and trauma may benefit from DBS; but efficacy in pain after spinal injury might be limited. Complications are similar to those of other DBS indications, including small risks of stroke, seizures, hemorrhage, the need for implantable pulse generator revision surgery every 3 to 5 years for nonrechargeable devices, and treatment failure.
Complications are similar to those of other DBS indications, including small risks of stroke, seizures, hemorrhage, the need for implantable pulse generator revision surgery every 3 to 5 years for nonrechargeable devices, and treatment failure. Findings from studies using static and functional neuroimaging modalities and invasive neurophysiological insights from local field potential recording are discussed. Intensive and detailed prospective cohort studies translate into improved patient selection and consistent efficacy, encouraging larger clinical trials.
Findings from studies using static and functional neuroimaging modalities and invasive neurophysiological insights from local field potential recording are discussed. Intensive and detailed prospective cohort studies translate into improved patient selection and consistent efficacy, encouraging larger clinical trials.Clinical Pearls: Although not a new therapy, DBS has metamorphosed considerably over the last decade, concomitant with advances in both stimulator technology and neuroimaging techniques and by corollary improvements in efficacy and reductions in complications. Few centers have published detailed studies of patients treated during the last decade. Our results suggest that DBS gives analgesia most consistently to patients with pain after amputation, either phantom or stump; and cranial and facial pain, including anesthesia dolorosa and plexopathies. Our greater experience of pain after stroke reveals greatest efficacy for stroke patients complaining of burning hyperesthesia.1,2 Therefore our stroke case series illustrates how important patient selection is to outcome. To improve selection and thus efficacy, objective adjuncts to current pain assessments are desirable. Subjective patient preference for PVG/PAG stimulation over VPL/VPM in stroke together with correlations revealed between cardiovascular effects, analgesic efficacy of DBS and burning hyperesthesia point toward autonomical measures as potential objective markers.3
Sustained analgesia by DBS has been shown for myriad indications; our own experience includes multiple sclerosis and genital pain. Each case must be considered individually rather than relying on dogmatic distinctions between neuropathic and nociceptive pain. Consistent with the notion that chronic pain states confer specific central neuropathic changes are results showing poor DBS efficacy for spinal cord–related pain (e.g., from failed back surgery). Predominantly spinal injuries and hence spinal neuropathic changes are unlikely to respond favorably to central brain stimulation. Conversely, causes of chronic pain not traditionally treated by DBS (e.g., visceral pain in which PVG/PAG changes are described using functional neuroimaging) 4,5 have potential for amelioration by DBS worthy of further study.
Investigations both into the mechanisms of DBS and using deep brain recording to elucidate pain processing mechanisms have yielded considerable advances. Future insights will arise from complementary information gathered using new technologies. Diffusion tensor imaging (DTI) using MRI to trace neuronal connections has shown connectivity between PVG/PAG and thalamic structures and may elucidate differential somatotopic connections6,7; it also has clinical use to aid targeting in functional neurosurgery.8 MEG enables whole brain changes to be mapped, with spatial resolution comparable to functional MRI yet temporal resolution of the order of milliseconds, in contrast to functional neuroimaging technique.9 Our initial investigations have revealed activation of pain-processing neocortical areas during analgesic DBS after filtering out artifactual interference from stimulation.10,11 Therefore global MEG measurements combined with local deep brain recording holds promise for revealing much about pain processing and DBS-related mechanisms beyond wider neurosurgical applications,12 toward identifying predictors of efficacy and enhancing treatments. Nevertheless, complimentary functional neuroimaging modalities such as SPECT have roles in characterizing whole brain changes with DBS with excellent deep brain structure penetration compared to present MEG studies, albeit with more limited temporal resolution and characterization of metabolic correlates of neuronal function.13
Clinical Pitfalls: The large variability of results in case series of DBS for pain to date reflects not just limitations in pain assessment tools and study design and execution, but individual differences among patients as to what constitutes success. A positive outcome may be the removal of a particular component of pain (e.g., burning hyperesthesia) without quantitative reduction in pain scores. Such pain relief may serve to unmask other types or components of pain elsewhere such as muscular allodynia, as has been described after stroke.14 Conversely, complete pain eradication by DBS may even accompany unease, motor complications, or other sequelae precipitating intolerance of stimulation. Thus clinicians must characterize patients’ pain qualitatively and quantitatively, and investigators should endeavor to include quality of life measures in outcome assessment to overcome the limitations of using pain questionnaires alone.
Other contemporary case series suggest that between one third and one half of patients successful during trial stimulation do not experience long-term success beyond 1 year after surgery. To address the predicament and to improve case selection, further challenges are to identify predictors of long-term efficacy and investigate the putative phenomenon of tolerance. Progressive increases of stimulus amplitude or insertion of a second electrode have proven unhelpful.15 Our experience2 and that of others16 is that tolerance is often overcome by subtle alterations of either pulse width by 30 to 90 µs, frequency by 5 to 15 Hz, or both, and by either cycling stimulation or having stimulation breaks (i.e., periods off DBS lasting from days to months as required). Such experience is in contrast to DBS for movement disorders in which it has been established that rebound of tremor with time can be overcome by ramping up thalamic stimulation parameters.17 It is possible that the unmasking of other discomfort such as muscular allodynia by relief of burning hyperesthesia can be overcome by “deramping” DBS. A positive correlation found between frequency and amplitude in long-term follow-up of poststroke pain DBS and reduction in both amplitudes and frequencies of stimulation over time in patients achieving pain relief supports such a hypothesis.2
Introduction
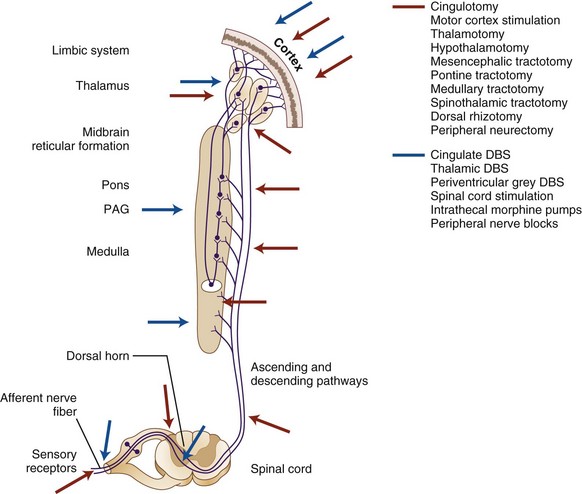
The pioneer neurosurgeon Lauri Laitinen once commented that “when one sets out to make a historical survey of surgical attempts to relieve the tremor and rigor in Parkinson disease, one cannot help feeling that it would have been a far easier task to list those nervous structures which have not been attacked.”18,19 Neurosurgical attempts to relieve intractable pain mirror his wry observation; all structures from peripheral nerve through dorsal root, spinal cord, midbrain, and thalamus to cingulate cortex have been first lesioned and later electrically stimulated or perfused with analgesics or anesthetics (Fig. 22-1). Yet chronic pain continues to present a considerable burden to society, transcending many debilitating medical diseases, including cancer, stroke, trauma, and failed surgery.20 Its prevalence may be over 20%.21
The concept of ameliorating persistent pain by deep brain stimulation (DBS) originates from clinical studies over half a century ago. On the basis of septal self-stimulation experiments in rodents22 and reports of analgesia in patients receiving septal DBS for psychiatric disorders,23,24 Heath and Mickle25 postulated that stimulating the same area that produced pleasure might relieve pain. They successfully reduced a patient’s cancer pain over several weeks with intermittent stimulation. The findings were replicated by a case series using improved equipment a decade later.26
Further impetus for DBS was provided in the mid-1960s by the theoretical paradigm shift initiated by Melzack and Wall’s gate control theory27 and advances in stimulator technology. The gate control theory was translated first into implantable peripheral nerve stimulators28 and then into spinal cord stimulation (SCS),29 developed by Medtronic (Minneapolis, Minn) into a commercially available permanently implantable device.30,31
Identification of the periventricular and periaqueductal gray (PVG/PAG) regions as a target for DBS has its origins in animal research. Mayer and associates32 and Reynolds and others33 were able to perform major surgery in awake rodents using analgesia induced by PAG stimulation alone. Pain relief by PVG/PAG DBS was first reported in patients by Richardson and Akil34–36 and then Hosobuchi, Adams, and Linchitz.37 Evidence supporting ventroposterolateral and ventroposteromedial (VPL/VPM) thalamic nuclei and adjacent structures as putative targets for DBS came from ablative surgery,38–41 leading Hosobuchi, Adams, and Rutkin42 to treat anesthesia dolorosa with VPM thalamic DBS. Several others pioneered thalamic DBS, including Mazars, Merienne, and Cioloca,43,44 Mazars,45 Mazars, Roge, and Mazars46 and Adams and Fields who, along with Hosobuchi,47–49 also targeted the internal capsule. Observations from inadvertent localization errors and investigations into current spread from the PVG/PAG led others to target more medial thalamic nuclei, including the centromedian-parafascicular complex (Cm-Pf).50–53
The rapid diffusion of electrical stimulation treatments for multifarious clinical indications by the mid-1970s led the U.S. Food and Drug Administration (FDA) to sponsor a symposium to evaluate their merits.54 Only pain treatments were documented to be both safe and effective,55,56 albeit in an era when the long-term complications of the then recently discovered levodopa on Parkinson disease were yet to be revealed. Crucial primate investigations elucidating basal ganglia functions and presaging a renaissance in DBS for movement disorders had also not yet been undertaken.19,57
Despite the consensus, the U.S. Medical Device Amendments of 1976 compelled the FDA to request DBS manufacturers to conduct further studies to show the benefits of DBS for pain, and an additional ruling in 1989 required clinical trials to demonstrate safety and efficacy. Two multicenter trials were conducted, first in 1976 using the Medtronic Model 3380 electrode (196 patients) and then in 1990 with the Model 3387 (50 patients) that superseded it.58 The two studies were an amalgam of prospective case series from participating neurosurgical centers, neither randomized nor case controlled, and both suffered from poor enrollment and high attrition. Other shortcomings included heterogeneous case mixes with underspecified patient selection criteria and subjective and unblinded assessment of patient outcomes. Confounds arose from inconsistencies in deep brain sites stimulated, numbers of electrodes used per patient, and stimulation parameters chosen. Improvements made to the later Model 3387 trial included limiting deep brain sites stimulated to two per patient and using visual analog scores (VASs) to rate pain intensity for outcome assessment; but its included cases per center were tiny, with a mean of five and median of three patients treated.
Neither trial satisfied study criteria for efficacy of at least half of patients reporting at least 50% pain relief 1 year after surgery. Therefore U.S. FDA approval for analgesic DBS was not sought by the device manufacturer. However, intriguingly, the large numbers of patients lost to follow-up resulted in a steady increase with time in the proportion of patients with at least 50% pain relief; 2 years after implantation they comprised 18 out of the 30 remaining patients (60%) followed up in the Model 3380 trial and 5 out of the 10 in the Model 3387 trial (50%). Nonetheless, the trials resulted in the U.S. FDA giving DBS for pain “off-label” status, thus precluding its approval by medical insurers.58–60 As a consequence, few clinical investigations into DBS for pain using current technology and techniques have been reported.
In the last decade to our knowledge only five centers, three European and two Canadian, have published case series of more than six patients.1,61–69 In contrast, both other centrally implantable neurostimulation treatments for pain, SCS and motor cortex stimulation (MCS), have continued to yield research publications, albeit mostly of uncontrolled case series,70–75 with small randomized controlled, clinical trials in SCS emerging.76–78 Over 1300 recipients of DBS for pain have been reported63,64,68,69,79,80 compared to nearly 400 patients with MCS73,81 and nearly 4000 with SCS.74,75
Our experience is that DBS is superior to MCS for selected refractory pain syndromes.82 Similarly we find DBS more appropriate than SCS for certain pain etiologies, although little published data exists comparing treatments by the same surgeon in the same group of patients. Two retrospective studies from the same group have compared all three modalities of central neurostimulation, but the results are obfuscated first by different treatments trialed both between and sequentially within patients and second by limited outcome information.83,84 A recent review has compared the three neurostimulatory therapies but did not include all contemporary DBS case series in its analysis.85 Over the last decade we have treated over 70 patients with analgesic DBS, regularly publishing results for many implanted and amenable to follow-up.1,62,65,67,68 Our experience is described, and results reviewed alongside other current studies to clarify the current status of DBS for pain.
Establishing Diagnosis and Indications/Contraindications
Historically, clinical approaches to DBS have sought to categorize patients first by cause of pain and second by dichotomizing the pain into such categories as nociceptive or deafferentation, epicritic or protopathic, peripheral or central. Such distinctions are largely unhelpful to patient selection since a gathering body of human functional neuroimaging and electrophysiological evidence confirms that chronic pain arises concomitant with centrally mediated changes related to neuronal plasticity, regardless of etiology.86–91 Thus it can be assumed that chronic pain refractory to medical treatment is largely central pain and thus neuropathic. The challenges to patient selection for DBS then become twofold: (1) the confirmation that the patient’s pain is neuropathic and neither factitious nor psychogenic, and (2) the selection of those with neuropathic pain who are likely to derive benefit from DBS.
Essential to the patient selection process is assessment by a multidisciplinary team consisting at a minimum of a pain specialist, neuropsychologist, and neurosurgeon. Comprehensive neuropsychological evaluation provides best practice in patient selection for DBS to exclude psychoses, addiction, and medically refractory psychiatric disorders and ensure minimal cognitive impairment.92–95 Quantitative assessment of the pain and health-related quality of life should be a requirement of the preoperative patient selection process. Our preference is to use both VAS (scale of 0 to 10) to rate pain intensity and the McGill Pain Questionnaire (MPQ) for pain evaluation,96,97 the latter giving additional qualitative information and including a quality of life assessment. We also assess quality of life using the Short Form 36 (SF-36) and VAS part of the Euroqol five-dimensional assessment tool (EQ-5D).98–100 The patient records his or her VAS twice daily in a pain diary over a period of 12 days. The 24 VAS scores are reviewed to ensure consistency and clarified with the patient if inconsistent. The EQ-5D, SF-36, and MPQ are administered by the pain specialist before surgery. The MPQ is repeated on a separate occasion independently by the neuropsychologist and scored using the ranked pain rating index. Our experience is that certain items of the MPQ can predict a positive response to DBS. In particular, over 80% of our patients who describe burning pain have found benefit from DBS, regardless of whether VPL/VPM, PVG/PAG or both are stimulated. We have published patient data linking resolution of burning pain with blood pressure reduction and long-term PVG/PAG DBS analgesia implicating cardiovascular changes in this type of pain3 and found heart rate variability changes consistent with sympathetic suppression and/or parasympathetic augmentation by PVG/PAG DBS101; but further multivariate analysis of other MPQ items, other brain targets, and other measurable cardiovascular parameters are required to elucidate further the phenomenon.
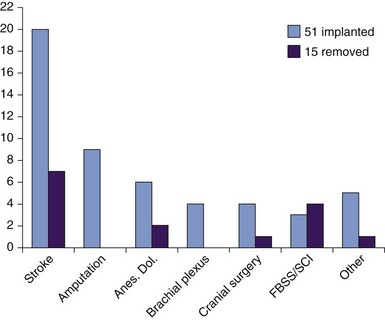
The greater body of clinical studies of SCS,71,72 coupled with ours and others’ lack of success with DBS for spinal injuries,63,65 favors SCS over DBS as a more appropriate first-line neurostimulatory intervention for spinal cord injury when central reorganization is likely to be mostly at a spinal level. However, our experience of DBS for pain after limb or plexor injury65,67 encourages us to consider DBS rather than SCS as first-line treatment for complex regional pain syndromes (CRPS). A 100% success rate in 13 implanted patients to date, together with a recent paradigm shift toward central brain reorganization with autonomic dysfunction as the mechanism underlying CRPS,102–106 supports the treatment both for brachial and lumbar plexus injuries and stump pain after amputation and for phantom limb pain.
Other pain etiologies for which we and others have obtained positive outcomes using DBS are stroke1,2; facial and head pain, including postherpetic trigeminal neuralgia and anesthesia dolorosa62,107; multiple sclerosis63; genital pain; and malignancy.65,68 These are listed for reference by etiology with implantation success rates shown in Fig. 22-2; however, we reiterate our position that to select patients for DBS primarily by etiology rather than by findings on history and examination and use of assessment tools is to oversimplify their chronic pain and risk poor outcomes. Rather than using inflexible selection criteria, our preference in patient selection is expert opinion after multidisciplinary assessment demonstrating quantitatively severe pain refractory to medication for at least 1 year, with significantly impaired quality of life and qualitative pain suggestive of neuropathic changes without predominantly spinal involvement. We find little merit in opiate or naloxone administration to determine suitability for DBS, although an historical literature exists.80 Medical contraindications to DBS include uncorrectable coagulopathy obviating neurosurgery and ventriculomegaly sufficient to preclude direct electrode passage to the surgical target.
Considering cephalalgias, we have also successfully treated cluster headache with DBS of the posterior hypothalamic nuclei and investigated it using multiple translational methods as for chronic pain.108–110 As we review comprehensively elsewhere,111 this debilitating condition has responded impressively to DBS in our initial experience and others’ and is worthy of multicenter, randomized controlled clinical trials. Further consideration of cluster headache is beyond the scope of this review.
Anatomy
Moreover, our ultimate adjustment of intracerebral electrode position is directed by awake patient reports of somesthetic localization during intraoperative stimulation. Such subjective information may alter the final electrode site by up to several millimeters from preoperative target coordinates. A guiding principle is the established somatotopic organization of the somesthetic thalamic and PVG/PAG regions. Human microelectrode studies reveal a mediolateral somatotopy in the contralateral ventroposterior thalamus, the head of the homunculus being medial and the feet lateral.112 Subjective observation of a rostrocaudally inverted sensory homunculus in contralateral PVG/PAG113 has been confirmed objectively by our human macroelectrode recordings of somatosensory evoked potentials.114 The PVG/PAG target is found at a point 2 to 3 mm lateral to the third ventricle at the level of the posterior commissure, 10 mm posterior to the midcommissural point. Its pertinent anatomical boundaries in the midbrain include the medial lemniscus laterally, the superior colliculus inferoposteriorly, and the red nucleus inferoanteriorly. Sensory thalamic targets are found 10 to 13 mm posterior to the midcommissural point and from 5 mm below to 2 mm above it. The VPM is targeted for facial pain only and found midway between the lateral wall of the third ventricle and the internal capsule; the arm area of VPL is 2 to 3 mm medial to the internal capsule and the leg area of VPL 1 to 2 mm medial to the internal capsule. The sensory thalamus is bordered by Cm-Pf nuclei medially; the internal capsule laterally; the thalamic fasciculus, zona incerta, and subthalamic nucleus inferiorly; the thalamic nucleus ventralis intermedius anteriorly; and the pulvinar thalamic nucleus posteriorly.
Basic Science
A wealth of electrophysiological, anatomical, and radiological evidence in humans and animals, reviewed elsewhere, establishes both PVG/PAG and ventrobasal thalamus as structures important to pain perception and the pathophysiology of chronic pain syndromes.9,115–122 The subtleties of hierarchical position and behavioral function of individual brain structures, whether sensory-discriminative, attentional, motivational-affective, or hedonic, are much debated. However, the consensus is toward a pain neuromatrix also involving spinal cord; posterior hypothalamus; amygdala; and neocortical structure, including somatosensory, insular, anterior cingulate, and prefrontal cortex. Whether pain control is top-down or bottom-up in its hierarchy is unresolved and often depends on the experimental paradigm used. Our human electrophysiological studies of neuronal coherence have used somatosensory-evoked potentials and Granger causality predictive modeling to suggest that PVG/PAG exerts ascending modulation on VPM/VPL in a bottom-up model of pain processing,114 a finding that functional MRI would be unable to confirm because of insufficient temporal resolution.9,123–125
Central to the rationale for DBS is the concept of aberrant neuronal firing at the target sites concomitant with the chronic pain. Human and animal electrophysiological experiments show increased thalamic neuronal firing in pain.126 Comprehensive reviews of electrophysiological studies conclude that the mechanisms of analgesic stimulation are not clearly delineated.80,127–131 From insights revealed by basal ganglia microelectrode recordings and DBS for movement disorders reviewed elsewhere,132–134 we postulate that altered rhythmic activity in VPL/VPM and PVG/PAG neurons is likely to play an important role in the pathophysiology of central pain. At either target our clinical experience is that in general DBS at lower frequencies (≤50 Hz) is analgesic and at higher frequencies (>70 Hz) hyperalgesic,2,135,136 supporting a dynamic model whereby synchronous oscillations in discrete neuronal populations centrally modulate chronic pain perception. Therefore analgesic DBS may either disrupt pathological high-frequency synchronous oscillations or, more likely, augment pathologically diminished low-frequency synchronous oscillations in the thalamic and reticular components of a reticulothalamocorticofugal pain neuromatrix. We have shown a positive correlation between analgesic efficacy at either DBS site and the amplitude of slow frequency (<1 Hz) VPL/VPM local field potentials (LFPs),61,137 allowing for physiologically modulated artifact.138 We now also have early evidence that patients off DBS have characteristically enhanced low-frequency (8 to14 Hz) power spectra of both PVG/PAG and VPL/VPM LFPs when in pain.139 Further research is required to elucidate if such neuronal signatures could aid patient selection, in particular if combined with technical advances in noninvasive functional neuroimaging and electrophysiological techniques such as single photon emission computed tomography (SPECT) and magnetoencephalography (MEG) to characterize functional neuronal connectivity.10,11,13
The PVG/PAG is a structure optimally sited anatomically to integrate interoceptive function, both from adjacent mesencephalic cardiovascular centers and more distal pain processing areas. Its autonomic effects have been well studied in animals,121,140–143 and changes noted with DBS.131 We have demonstrated a positive correlation between degree of analgesia in patients receiving PVG/PAG DBS and magnitude of blood pressure reduction3 and shown that, whereas dorsal PAG stimulation can acutely elevate blood pressure, ventral stimulation reduces it.144,145 Such findings advance investigations for objective markers of chronic pain and also potential selection of patients who may respond best to PVG/PAG DBS. Indeed our investigations into heart rate variability changes and preliminary findings from ambulatory blood pressure monitoring that such blood pressure changes are sustained may provide objective somatic measures of efficacy that correlate to subjective rating scales.146,147 Detailed autonomic testing using such equipment as tilt-tables, Portapres, and real-time VAS recording is underway to elucidate such possibilities.
Current thinking is that ventral PVG/PAG DBS engages analgesia commensurate with passive coping behavior, whereas dorsal PVG/PAG DBS may involve “fight or flight” analgesia with associated sympathomimetic effect.3 However, evidence to substantiate the conjecture that PVG/PAG DBS acts via augmenting endogenous opioid release is contentious. The hypothesis arose from animal experiments revealing stimulation-produced analgesia reversed by naloxone148,149 and human studies that also showed elevated levels of cerebrospinal fluid enkephalins and endorphins with DBS.37,150,151 However, the cerebrospinal fluid measures were artifactual,152,153 and double-blinded investigation in humans has revealed no cross-tolerance between DBS and morphine and similar reversibility between naloxone and saline placebo,154 confirming others’ findings.52,155 Our preliminary human studies agree that naloxone and placebo effects on DBS are similar, questioning an opioid-dependent mechanism. Furthermore, naloxone and saline seem to have distinct and different effects on the low-frequency power spectra of PVG/PAG and VPL/VPM LFPs, suggesting that both opioids and contextual influences can modulate neuronal responses in the pain neuromatrix independent of DBS, in agreement with the dynamic rate model proposed previously. However, naloxone can potentiate acute withdrawal and psychosis in long-term users of opiate analgesia. Thus we advise against its administration during stereotactic neurosurgical procedures in awake patients.
An obstacle yet to be surmounted in the quest to understand the mechanisms of analgesic stimulation is the lack of adequate animal models of chronic pain.156,157 In addition to their limited homology in chronic pain paradigms, the smaller brains of rodent and murine models increase targeting inaccuracies, in particular for small brainstem structures such as PVG/PAG. Such experience emphasizes the important opportunities presented by patient-based translational research into DBS to study the mechanisms underlying its efficacious analgesia.
Guidelines
There are no recent North American guidelines for DBS for pain because of its off-label indication in the United States. We have contributed to the European Federation of Neurological Societies’ guidelines on neurostimulation therapy for neuropathic pain, which concludes that, because of the few recent case series published, DBS should be limited to specialist centers willing to study and report their outcomes.158
Imaging, Equipment, and Technique
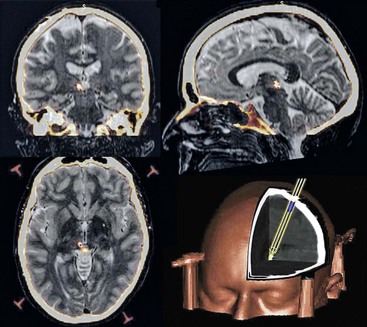
Informed written patient consent is obtained after detailed explanations of the risks and potential benefits of the procedure and counseling for its duration of approximately 2 hours under moderate sedation and local anesthesia with the head fixed and cranial stereotaxis applied (Fig. 22-3). Our operating theatre setup is shown in Fig. 22-4. A week or more before surgery, patients have a T1 weighted MRI scan. For surgery a Cosman-Roberts-Wells (CRW) base ring is applied to the patient’s head under local anesthesia. A stereotactic CT scan is then performed, and the MRI scan is volumetrically fused to it using computerized image fusion and stereotactic planning programs to eliminate spatial distortions that arise from magnetic field effects.159,160 The coordinates for the PVG/PAG and VPL or VPM and entry trajectory are then calculated (Fig. 22-5). A frontal trajectory avoiding the lateral ventricles is preferred. DBS targets are described in the previous anatomy section.
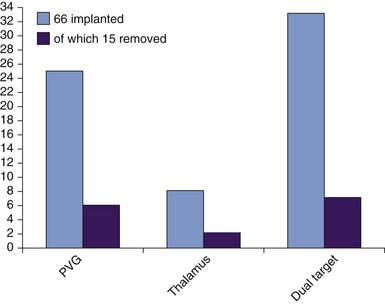
After a 3-cm parasagittal scalp incision and separate 2.7-mm twist drill craniotomy per electrode, both VPL/VPM and PVG/PAG have been implanted to date with Medtronic Model 3387 quadripolar electrodes. PVG/PAG is implanted first; excellent intraoperative analgesia obviates implantation of a second electrode in VPL/VPM in up to half of patients, in particular those with marked thalamic damage (e.g., following stroke). Thus most of our cohorts have received PVG/PAG or dual-target DBS (Fig. 22-6). The minority who have received VPL/VPM DBS alone have either not experienced efficacy or had side effects with a trial of PVG/PAG DBS. Final electrode position is determined by intraoperative clinical assessment reliant on subjective reporting by the awake patient; microelectrode recording is not routinely used. Bipolar 5- to 30-Hz stimulation is performed initially, pulse width 100 to 450 µs, amplitude 0.1 to 3 V. VPL/VPM stimulation aims to supplant painful sensation by pleasant paresthesia, and PVG/PAG stimulation seeks to induce a sensation of warmth or analgesia in the painful area. Adjustment is primarily somatotopic so as to evoke appropriate topographic responses, but the assessor should be alert to pyramidal signs suggesting capsular involvement with VPL/VPM DBS and with PVG/PAG DBS for oscilliopsia and reports of visual disturbances caused by superior collicular involvement or facial paresthesia arising from medial lemniscus stimulation. Each electrode is fixed to the skull by a miniplate, and its leads externalized parietally via temporary extensions. Immediately after surgery a further stereotactic CT is performed and co-registered as before to confirm electrode position. MRI may be performed after surgery during the week before implantable pulse generator (IPG) insertion for further anatomical target corroboration.
After a week of postoperative clinical assessment, a decision is made whether to permanently implant the electrodes in a second operation under general anesthesia. They are connected to an IPG implanted subcutaneously, usually infraclavicularly or alternatively intraabdominally in subcutaneous fascia. Medtronic Synergy or Kinetra has been used to date. Our surgical technique is detailed further elsewhere.136,161,162
Patient Management/Evaluation
Another method favored for evaluating analgesia in single cases and small groups of patients is the N-of-1 trial.162–164 A randomized, placebo-controlled intrapatient trial is conducted whereby the patient receives pairs of treatment periods during which each intervention, be it DBS on or off or different stimulation targets or parameters, occurs once. The order of treatments is randomized, and the effects of treatment or placebo can be compared between treatment periods. We have demonstrated the validity of N-of-1 trials using the VAS, and their concordance with overall MPQ has been demonstrated for VPL/VPM, PVG/PAG, and dual-target DBS.165 Blinding and randomization methodologies have also been adopted by others to investigate the efficacy of thalamic DBS.66 However, the process is labor intensive for the clinician and thus not routinely practicable with limited clinical resources.
Outcomes Evidence
Several reviews of DBS for chronic pain have been published, many expert, some commentaries, several systematic.* Our systematic searches have identified a number of primary studies.†
†See reference numbers 15, 34–37, 43, 45, 50, 51, 61–66, 68, 69, 82, 95, 131, 150, 151, 154, 169, and 187–209.
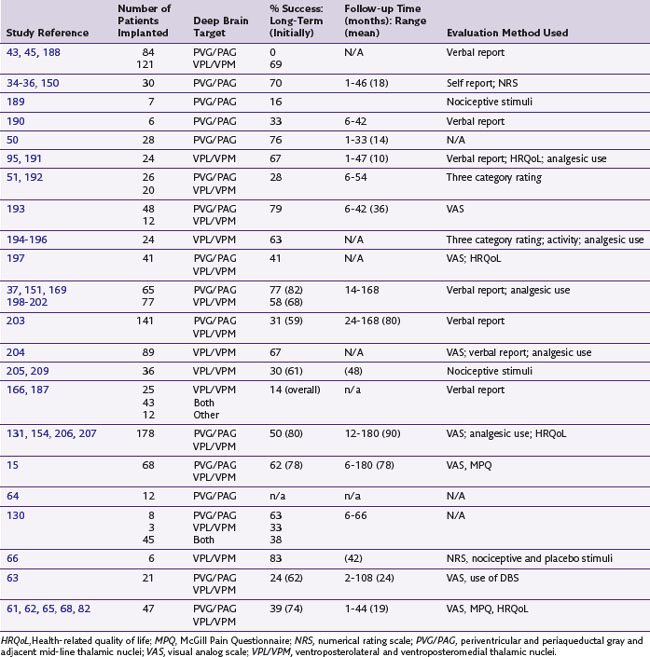
Published case series of at least six patients using current DBS targets are listed in Table 22-1 and their efficacy summarized. When the same authors reviewed their clinical data more than once, only their latest or largest patient series was considered. Pain relief scores showing 50% or more improvement or verbal ratings of good or excellent after surgery were considered successful outcomes, and patients not permanently implanted included as failed outcomes. However, not all authors reported such failures, leading to overestimation of efficacy in some reports. The literature is also obfuscated by varying and often simplistic or subjective outcome measures with a paucity of double-blind, placebo-controlled studies. To our knowledge only four groups have published studies of at least six patients using current standards of target localization and currently available models of deep brain stimulators in all patients with adequate follow-up and description of outcome.63,66,68,69 All other primary studies are based on cases first implanted more than a decade ago, some using electrodes now no longer commercially available, and some targeting the internal capsule.
Table 22-1 Summary of Prospective Case Series of Thalamic and Periventricular Deep Brain Stimulation for Pain
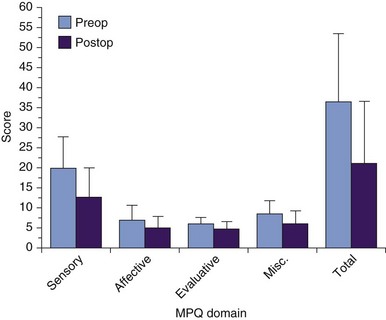
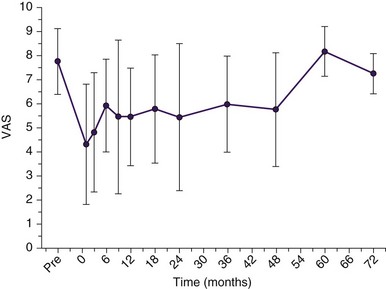
The etiologies and brain targets of our 66 patient-prospective case series are summarized in Figs. 22-2 and 22-6. A fifth of all patients had failed other invasive neuromodulatory treatment, including MCS, SCS, and analgesic pump implantation. Fifty-one patients (77%) gained pain relief during the week after procedure and proceeded to full DBS implantation. Mean MPQ-ranked pain indices for the cohort before and after surgery showed significant improvement (p < 0.05) in sensory domains and overall (Fig. 22-7). Average improvements in VAS for the cohort of 80% were seen at 3 months after surgery and maintained at 35% to 43% for 4 years thereafter (Fig. 22-8). VAS scores frequently did not mirror patients’ considerable subjective improvements in function and quality of life, suggesting its limitations as an assessment tool. Other explanations include emergence of a late-tolerance phenomenon or unmasking of other types or regions of pain with restoration of patient activity.
In December 2008 mean efficacious DBS parameters for the entire 51-patient cohort at last clinical follow-up were frequency 18.5 Hz (range 5 to 35 Hz, SD 15 Hz), pulse width 295 µs (range 90-450 µs, SD 93 µs), and amplitude 2.5 V (range 0.5 to 5.2 V, SD 1.2 V). Sixty-seven percent of patients received bipolar stimulation. Eleven patients (22% of those implanted for at least 1 year) required IPG changes, with a mean time between changes of 25 months in this minority, the mean follow-up of the remaining 78% without IPG changes being 42 months. Mean follow-up of all patients was 55 months (range 1 month to 10 years). Five patients from the cohort died of other causes more than 1 year after their surgery. Four patients developed wound infections, one resolving with antibiotics, one requiring scalp debridement, one IPG site debridement, and one complete system removal. Four patients required DBS lead replacement, two following fall-related fractures and two because of lead tethering; one patient had lead adjustment to improve efficacy. Other complications included visual disturbances in three patients with PVG/PAG DBS and one with VPL DBS and dysarthria in one patient with PVG/PAG DBS. Detailed outcomes by etiology from our patient cohort, including quality of life outcomes, are described elsewhere with comprehensive analyses also in progress.1,62,65,67,68,135,165
Risk and Complication Avoidance
Specific DBS complications accounted for are stroke (<1%), seizures (<1%), hemorrhage (0.3%), death (0.1%), and the need for IPG revision surgery every 3 to 5 years because of limited battery life and infection (3%); a small proportion of cases required complete removal of the DBS system. Although our overall complication rates are lower than these percentages (e.g., 0.05% hemorrhage; 0.02% death) and IPG changes have been required in a fifth of patients receiving DBS for pain in the last decade, it appears appropriate to quote figures from prevailing published literature.161,210,211 Patients are also counseled for the possibility that they may derive no benefit from DBS or not tolerate it well, again necessitating its removal; no specific percentage is quoted since each case is best considered individually, but analysis of our case series suggests that explantation rates caused by poor efficacy have remained constant at 23% during a decade of experience of 66 patients with refining case selection, surgery, and postoperative parameter adjustment (see Fig. 22-4).
Conclusion
Advances in stimulator technology such as the development of rechargeable and demand-driven stimulators may not only obviate the need for IPG replacement and thus improve cost-effectiveness but also enable demand-driven patient controlled analgesia and potentially overcome tolerance or the unmasking of other pain with successful DBS.212 The characterization by LFP recording in awake patients of neural signatures for pain to which such stimulators could respond forms a firm foundation for research into smart DBS for pain.139
LFP recording and diffusion tensor imaging are beginning to objectively characterize objective somatotopy in the PVG/PAG as has previously been described in the thalamus.112–114 Establishment of deep brain target somatotopy gives objective support to intraoperative electrode position adjustments and can enable the development of intraoperative somatosensory-evoked potential monitoring together with microelectrode or macroelectrode recording to guide electrode positioning, in particular in the anesthetized patient.
There remain groups of patients presently refractory to thalamic or PVG/PAG DBS or whose pain (e.g., whole body pain lacking distinct somatotopy or pain after spinal cord injury) makes them poor candidates for the procedure. We have successfully implanted DBS into the anterior cingulate cortex in such patients with the rationale of reducing the emotional saliency of pain perception while not seeking to alter its nociceptive component. Such work draws on a wealth of literature and our own positive clinical experience of anterior cingulotomy for cancer pain.213,214 We expect that anterior cingulate DBS will not only become established as a viable novel target in DBS for chronic pain but that its use and related translational investigations will yield many neuropsychological insights into emotion, attention, and executive function.
Further challenges in our DBS for pain service with its international referral patterns are poor recruitment to trials and loss of patients to follow-up. History has demonstrated the potential for unconstrained application of functional neurosurgery in psychiatric disorders; thus patients must be enrolled in formal studies and not lost to clinical follow-up.215
An admirable feature of contemporary clinical trials is the a priori standardization of clinical outcomes by collaborative multicenter groups and inclusion of quality of life measures. As DBS for chronic pain illustrates, meta-analyses and systematic reviews become hamstrung in the conclusions that can be drawn from them if the outcome measures of the primary studies analyzed are not standardized.80,158,162,172 Novel evidence-based methods are being considered and adapted to factor in risks of surgery such as application of a signal-to-noise ratio of treatment effect of DBS compared to expected prognosis of the chronic pain without treatment.162,216 Treatment effects can be inferred from well-designed case series and nonrandomized cohort studies and justified when a rapid response is seen against a stable or progressively declining disease natural history using such paradigms.
Key Issues
 DBS for the treatment of chronic pain refractory to drug therapy has been undertaken for half a century, but few centers have reported contemporary findings using current technology. Its use presently remains restricted to experienced, specialist centers willing to publish results, exemplifying the many challenges to demonstrating evidence of efficacy in functional neurosurgery.
DBS for the treatment of chronic pain refractory to drug therapy has been undertaken for half a century, but few centers have reported contemporary findings using current technology. Its use presently remains restricted to experienced, specialist centers willing to publish results, exemplifying the many challenges to demonstrating evidence of efficacy in functional neurosurgery. DBS for pain is typically used when all other treatment has failed or is inadequate. This context should be taken into account in evaluating outcomes. Alongside a move to multicenter clinical trials, intrapatient N-of-1 trials and signal-to-noise evidence-based methodology can be applied to DBS for pain to yield robust measures of efficacy from intensively studied case series.
DBS for pain is typically used when all other treatment has failed or is inadequate. This context should be taken into account in evaluating outcomes. Alongside a move to multicenter clinical trials, intrapatient N-of-1 trials and signal-to-noise evidence-based methodology can be applied to DBS for pain to yield robust measures of efficacy from intensively studied case series. Our experience over a decade of DBS for pain in 66 patients emphasizes symptomatology rather than etiology in patient selection but reveals positive outcomes after stroke and excellent outcomes for pain after amputation, plexopathies, and head and face pain, including anesthesia dolorosa and postoperative pain.
Our experience over a decade of DBS for pain in 66 patients emphasizes symptomatology rather than etiology in patient selection but reveals positive outcomes after stroke and excellent outcomes for pain after amputation, plexopathies, and head and face pain, including anesthesia dolorosa and postoperative pain. PVG/PAG and VPL/VPM remain our targets of choice in DBS for intractable pain, with PVG/PAG assessed first and anterior cingulate cortex DBS considered in refractory or selected cases.
PVG/PAG and VPL/VPM remain our targets of choice in DBS for intractable pain, with PVG/PAG assessed first and anterior cingulate cortex DBS considered in refractory or selected cases. As with other neuromodulatory therapies, DBS efficacy declines with time when measured by VAS alone, suggesting either a tolerance or unmasking phenomenon likely to be common in neuropathic pain and that may be overcome by stimulation parameter alteration or deramping.
As with other neuromodulatory therapies, DBS efficacy declines with time when measured by VAS alone, suggesting either a tolerance or unmasking phenomenon likely to be common in neuropathic pain and that may be overcome by stimulation parameter alteration or deramping. Translational insights from invasive electrophysiology, MEG, SPECT, DTI, and autonomic testing aim to advance our understanding of DBS for intractable pain and improve patient selection, surgical targeting, and efficacy alongside advances in DBS technology such as the prospect of demand-driven “smart” stimulation.
Translational insights from invasive electrophysiology, MEG, SPECT, DTI, and autonomic testing aim to advance our understanding of DBS for intractable pain and improve patient selection, surgical targeting, and efficacy alongside advances in DBS technology such as the prospect of demand-driven “smart” stimulation.1 Owen SL, et al. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain. 2006;120(1-2):202-206.
2 Pereira EA, et al. Deep brain stimulation for central post-stroke pain-relating outcomes and stimulation parameters in 21 patients. Acta Neurochir. 2008;150(9):968.
3 Green AL, et al. Stimulating the human midbrain to reveal the link between pain and blood pressure. Pain. 2006;124(3):349-359.
4 Dunckley P, et al. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25(32):7333-7341.
5 Chang L. Brain responses to visceral and somatic stimuli in irritable bowel syndrome: a central nervous system disorder? Gastroenterol Clin North Am. 2005;34(2):271-279.
6 Owen SL, et al. Preoperative DTI and probabilistic tractography in four patients with deep brain stimulation for chronic pain. J Clin Neurosci. 2008;15(7):801-805.
7 Sillery E, et al. Connectivity of the human periventricular-periaqueductal gray region. J Neurosurg. 2005;103(6):1030-1034.
8 Johansen-Berg H, Behrens TE. Just pretty pictures? What diffusion tractography can add in clinical neuroscience. Curr Opin Neurol. 2006;19(4):379-385.
9 Kupers R, Kehlet H. Brain imaging of clinical pain states: a critical review and strategies for future studies. Lancet Neurol. 2006;5(12):1033-1044.
10 Kringelbach ML, et al. Deep brain stimulation for chronic pain investigated with magnetoencephalography. Neuroreport. 2007;18(3):223-228.
11 Ray NJ, et al. Abnormal thalamocortical dynamics may be altered by deep brain stimulation: using magnetoencephalography to study phantom limb pain. J Clin Neurosci. 2009;16(1):32-36.
12 Makela JP, et al. Magnetoencephalography in neurosurgery. Neurosurgery. 2006;59(3):493-511. discussion 493-511
13 Pereira EA, et al. Regional cerebral perfusion differences between periventricular grey, thalamic and dual target deep brain stimulation for chronic neuropathic pain. Stereotact Funct Neurosurg. 2007;85(4):175-183.
14 Kamano S. Author’s experience of lateral medullary infarction—thermal perception and muscle allodynia. Pain. 2003;104(1-2):49-53.
15 Kumar K, Toth C, Nath R. Deep brain stimulation for intractable pain: a 15-year experience. Neurosurgery. 1997;40(4):736-746. discussion 746-747
16 Romanelli P, Heit G. Patient-controlled deep brain stimulation can overcome analgesic tolerance. Stereotact Funct Neurosurg. 2004;82(2-3):77-79.
17 Hariz MI, et al. Tolerance and tremor rebound following long-term chronic thalamic stimulation for Parkinsonian and essential tremor. Stereotact Funct Neurosurg. 1999;72(2-4):208-218.
18 Laitinen LV. Surgical treatment, past and present, in Parkinson’s disease. Acta Neurol Scand Suppl. 1972;51:43-58.
19 Pereira EA, Aziz TZ. Surgical insights into Parkinson’s disease. J R Soc Med. 2006;99(5):238-244.
20 Ashburn MA, Staats PS. Management of chronic pain. Lancet. 1999;353(9167):1865-1869.
21 Gureje O, et al. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA. 1998;280(2):147-151.
22 Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat bra. J Comp Physiol Psychol. 1954;47(6):419-427.
23 Pool JL, et al. Steroid Hormonal response to stimulation of electrodes implanted in the subfrontal parts of the brain. Springfield, Ill: Charles C Thomas; 1956.
24 Heath R. Studies in schizophrenia. Cambridge, Mass: Harvard University Press; 1954.
25 Heath RG, Mickle WA. Evaluation of seven years’ experience with depth electrode studies in human patients. New York: Paul B Hoeber; 1960.
26 Gol A. Relief of pain by electrical stimulation of the septal area. J Neurol Sci. 1967;5(1):115-120.
27 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(699):971-979.
28 Sweet WH, Wepsic JG. Treatment of chronic pain by stimulation of fibers of primary afferent neuron. Trans Am Neurol Assoc. 1968;93:103-107.
29 Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46(4):489-491.
30 Mullett K. Electrical brain stimulation for the control of chronic pain. Med Instrum. 1978;12(2):88-91.
31 Mullett K. State of the art in neurostimulation. Pacing Clin Electrophysiol. 1987;10(1 Pt 2):162-175.
32 Mayer DJ, et al. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174(16):1351-1354.
33 Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164(878):444-445.
34 Richardson DE, Akil H. Long-term results of periventricular gray self-stimulation. Neurosurgery. 1977;1(2):199-202.
35 Richardson DE, Akil H. Pain reduction by electrical brain stimulation in man. Part 1. Acute administration in periaqueductal and periventricular sites. J Neurosurg. 1977;47(2):178-183.
36 Richardson DE, Akil H. Pain reduction by electrical brain stimulation in man. Part 2. Chronic self-administration in the periventricular gray matter. J Neurosurg. 1977;47(2):184-194.
37 Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197(4299):183-186.
38 White JC, Sweet WH. Pain and the neurosurgeon. Springfield, Ill: Charles C Thomas; 1969.
39 Ervin FR, Brown CE, Mark VH. Striatal influence on facial pain. Confin Neurol. 1966;27(1):75-90.
40 Mark VH, Ervin FR. Role of thalamotomy in treatment of chronic severe pain. Postgrad Med. 1965;37:563-571.
41 Mark VH, Ervin FR, Hackett TP. Clinical aspects of stereotactic thalamotomy in the human. Part I. The treatment of chronic severe pain. Arch Neurol. 1960;3:351-367.
42 Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic stimulation for the control of facial anesthesia dolorosa. Arch Neurol. 1973;29(3):158-161.
43 Mazars G, Merienne L, Cioloca C. Treatment of certain types of pain with implantable thalamic stimulators. Neurochirurgie. 1974;20(2):117-124.
44 Mazars G, Merienne L, Ciolocca C. Intermittent analgesic thalamic stimulation: preliminary note. Rev Neurol (Paris). 1973;128(4):273-279.
45 Mazars GJ. Intermittent stimulation of nucleus ventralis posterolateralis for intractable pain. Surg Neurol. 1975;4(1):93-95.
46 Mazars G, Roge R, Mazars Y. Results of the stimulation of the spinothalamic fasciculus and their bearing on the physiopathology of pain. Rev Prat. 1960;103:136-138.
47 Adams JE, Hosobuchi Y, Fields HL. Stimulation of internal capsule for relief of chronic pain. J Neurosurg. 1974;41(6):740-744.
48 Fields HL, Adams JE. Pain after cortical injury relieved by electrical stimulation of the internal capsule. Brain. 1974;97(1):169-178.
49 Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic and internal capsule stimulation for the control of central pain. Surg Neurol. 1975;4(1):91-92.
50 Ray CD, Burton CV. Deep brain stimulation for severe, chronic pain. Acta Neurochir (Wien). 1980;30(suppl):289-293.
51 Thoden U, et al. Medial thalamic permanent electrodes for pain control in man: an electrophysiological and clinical study. Electroencephalogr Clin Neurophysiol. 1979;47(5):582-591.
52 Boivie J, Meyerson BA. A correlative anatomical and clinical study of pain suppression by deep brain stimulation. Pain. 1982;13(2):113-126.
53 Andy OJ. Parafascicular-center median nuclei stimulation for intractable pain and dyskinesia (painful-dyskinesia). Appl Neurophysiol. 1980;43(3-5):133-144.
54 Gildenberg PL. Symposium on the safety and clinical efficacy of implanted neuroaugmentive devices. Appl Neurophysiol. 1977;40:69-240.
55 Gildenberg PL. Neurosurgical statement on neuroaugmentive devices. Appl Neurophysiol. 1977;40:69-71.
56 Gildenberg PL. History of electrical neuromodulation for chronic pain. Pain Med. 2006;7(suppl 1):S7-S13.
57 Pereira EA, Aziz TZ. Parkinson’s disease and primate research: past, present, and future. Postgrad Med J. 2006;82(967):293-299.
58 Coffey RJ. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Med. 2001;2(3):183-192.
59 Long DM. The current status of electrical stimulation of the nervous system for the relief of chronic pain. Surg Neurol. 1998;49(2):142-144.
60 Long DM. Conquering pain. Neurosurgery. 2000;46(2):257-259.
61 Nandi D, et al. Thalamic field potentials in chronic central pain treated by periventricular gray stimulation—a series of eight cases. Pain. 2003;101(1-2):97-107.
62 Green AL, et al. Deep brain stimulation for neuropathic cephalalgia. Cephalalgia. 2006;26(5):561-567.
63 Hamani C, et al. Deep brain stimulation for chronic neuropathic pain: long-term outcome and the incidence of insertional effect. Pain. 2006;125(1-2):188-196.
64 Krauss JK, et al. Deep brain stimulation of the centre median-parafascicular complex in patients with movement disorders. J Neurol Neurosurg Psychiatry. 2002;72(4):546-548.
65 Owen SLF, et al. Deep brain stimulation for neuropathic pain. Neuromodulation. 2006;9(2):100-106.
66 Marchand S, et al. Analgesic and placebo effects of thalamic stimulation. Pain. 2003;105(3):481-488.
67 Bittar RG, et al. Deep brain stimulation for phantom limb pain. J Clin Neurosci. 2005;12(4):399-404.
68 Owen SL, et al. Deep brain stimulation for neuropathic pain. Acta Neurochir Suppl. 2007;97(Pt 2):111-116.
69 Rasche D, et al. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006;21(6):E8.
70 Rasche D, et al. Motor cortex stimulation for long-term relief of chronic neuropathic pain: a 10 year experience. Pain. 2006;121(1-2):43-52.
71 Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006;31(4 Suppl):S13-S19.
72 Turner JA, et al. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108(1-2):137-147.
73 Brown JA, Barbaro NM. Motor cortex stimulation for central and neuropathic pain: current status. Pain. 2003;104(3):431-435.
74 Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100(3 suppl Spine):254-267.
75 Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factor. Spine. 2005;30(1):152-160.
76 Kumar K, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63(4):762-770. discussion 770
77 Kemler MA, et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;08(2):292-298.
78 Manca A, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain. 2008;12(8):1047-1058.
79 Gybels J. Brain stimulation in the management of persistent pain, ed 4. Philadelphia, London: Saunders; 2000.
80 Levy RM. Deep brain stimulation for the treatment of intractable pain. Neurosurg Clin North Am. 2003;14(3):389-399. vi
81 Smith H, et al. Motor cortex stimulation for neuropathic pain. Neurosurgical focus (electronic resource). 2001;11(3):E2.
82 Nandi D, et al. Peri-ventricular grey stimulation versus motor cortex stimulation for post stroke neuropathic pain. J Clin Neurosci. 2002;9(5):557-561.
83 Katayama YY, et al. Motor cortex stimulation for post-stroke pain: comparison of spinal cord and thalamic stimulation. Stereotact Funct Neurosurg. 2001;77(1-4):183-186.
84 Katayama Y, et al. Motor cortex stimulation for phantom limb pain: comprehensive therapy with spinal cord and thalamic stimulation. Stereotact Funct Neurosurg. 2001;77(1-4):159-162.
85 Coffey RJ, Lozano AM. Neurostimulation for chronic noncancer pain: an evaluation of the clinical evidence and recommendations for future trial designs. J Neurosurg. 2006;105:175-189.
86 Anderson WS, et al. Chapter 21 Plasticity of pain-related neuronal activity in the human thalamus. Prog Brain Res. 2006;157:353-364.
87 Melzack R, et al. Central neuroplasticity and pathological pain. Ann N Y Acad Sci. 2001;2001(933):157-174.
88 Coderre TJ, et al. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52(3):259-285.
89 Schweinhardt P, Lee M, Tracey I. Imaging pain in patients: is it meaningful? Curr Opin Neurol. 2006;19(4):392-400.
90 Stern J, Jeanmonod D, Sarnthein J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage. 2006;31(2):721-731.
91 Apkarian AV, et al. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463-484.
92 Saint-Cyr JA, Trepanier LL. Neuropsychologic assessment of patients for movement disorder surgery. Mov Disord. 2000;15(5):771-783.
93 Voon V, et al. Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord. 2006;21(suppl 14):S305-S327.
94 Lang AE, et al. Deep brain stimulation: preoperative issues. Mov Disord. 2006;21(suppl 14):S171-S196.
95 Shulman R, Turnbull IM, Diewold P. Psychiatric aspects of thalamic stimulation for neuropathic pain. Pain. 1982;13(2):127-135.
96 Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87-101.
97 Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277-299.
98 Ware JE, et al. SF-36 Health survey manual and interpretation guide. Boston, Mass: New England Medical Centre, The Health Institute; 1993.
99 EuroQol—a new facility for the measurement of health-related quality of life, The EuroQol Group. Health Policy. 1990;16(3):199-208.
100 Medical Outcomes Trust. How to Score the SF-36 Health Survey. Boston, Mass: Medical Outcomes Trust; 1991.
101 Pereira EA, et al. Sustained blood pressure changes with periventricular grey but not posterior hypothalamic deep brain stimulation. Acta Neurochir. 2008;150(9):933.
102 Ramachandran VS, Rogers-Ramachandran D. Phantom limbs and neural plasticity. Arch Neurol. 2000;57(3):317-320.
103 Janig W, Baron R. Complex regional pain syndrome is a disease of the central nervous system. Clin Auton Res. 2002;12(3):150-164.
104 Janig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol. 2003;2(11):687-697.
105 Janig W, Baron R. Is CRPS I a neuropathic pain syndrome? Pain. 2006;120(3):227-229.
106 Ramachandran VS. Plasticity and functional recovery in neurology. Clin Med. 2005;5(4):368-373.
107 Green AL, et al. Post-herpetic trigeminal neuralgia treated with deep brain stimulation. J Clin Neurosci. 2003;10(4):512-514.
108 Brittain J-S, et al. Local field potentials reveal a distinctive neural signature of cluster headache in the hypothalamus. Cephalalgia. 2009;29(11):1165-1173.
109 Owen SL, et al. Connectivity of an effective hypothalamic surgical target for cluster headache. J Clin Neurosci. 2007;14(10):955-960.
110 Ray NJ, et al. Using magnetoencephalography to investigate brain activity during high frequency deep brain stimulation in a cluster headache patient. Biomed Imag Intervent J. 2007;3(1):25.
111 Grover PJ, et al. Deep brain stimulation for cluster headache. J Clin Neurosci. 2009;16(7):861-866.
112 Lenz FA, et al. Single-unit analysis of the human ventral thalamic nuclear group: somatosensory responses. J Neurophysiol. 1988;59(2):299-316.
113 Bittar RG, et al. Somatotopic organization of the human periventricular gray matter. J Clin Neurosci. 2005;12(3):240-241.
114 Pereira EAC, et al. Stimulating the brain to relive pain: from homunculi to consciousness and deep brain recording to magnetoencephalography. Neurosurgery. 2007;61(1):221.
115 Romanelli P, Esposito V. The functional anatomy of neuropathic pain. Neurosurg Clin North Am. 2004;15(3):257-268.
116 Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain: a review and meta-analysis (2000). Neurophysiol Clin. 2000;30(5):263-288.
117 Tracey I. Nociceptive processing in the human brain. Curr Opin Neurobiol. 2005;15(4):478-487.
118 Sewards TV, Sewards MA. The medial pain system: neural representations of the motivational aspect of pain. Brain Res Bull. 2002;59(3):163-180.
119 Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87(2):251-258.
120 Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14(1):2-31.
121 Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46(6):575-605.
122 Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1-30.
123 Garcia-Larrea L, et al. Functional imaging and neurophysiological assessment of spinal and brain therapeutic modulation in humans. Arch Med Res. 2000;31(3):248-257.
124 Zambreanu L, et al. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114(3):397-407.
125 Pereira EA, et al. Regional cerebral perfusion differences between periventricular grey, thalamic and dual target deep brain stimulation for chronic neuropathic pain. Stereotact Funct Neurosurg. 2007;85(4):175-183.
126 Yamashiro K, et al. Neurons with spontaneous high-frequency discharges in the central nervous system and chronic pain. Acta Neurochir Suppl. 2003;87:153-155.
127 Duncan GH, Bushnell MC, Marchand S. Deep brain stimulation: a review of basic research and clinical studies. Pain. 1991;45(1):49-59.
128 Gybels JM, Sweet WH. Neurosurgical treatment of persistent pain. physiological and pathological mechanisms of human pain. Basel: Karger; 1989.
129 Weigel R, Krauss JK. Center median-parafascicular complex and pain control. Review from a neurosurgical perspective. Stereotact Funct Neurosurg. 2004;82(2-3):115-126.
130 Tronnier VM. Deep brain stimulation. Amsterdam London: Elsevier; 2003.
131 Young RF, Rinaldi PC. Brain stimulation. New York: Springer-Verlag; 1997.
132 Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Movement Disorders. 2003;18(4):357-363.
133 Engel AK, et al. Invasive recordings from the human brain: clinical insights and beyond. Nat Rev Neurosci. 2005;6(1):35-47.
134 Hutchison WD, et al. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. J Neurosci. 2004;24(42):9240-9243.
135 Nandi D, Aziz TZ. Deep brain stimulation in the management of neuropathic pain and multiple sclerosis tremor. J Clin Neurophysiol. 2004;21(1):31-39.
136 Bittar RG, et al. Deep brain stimulation for movement disorders and pain. J Clin Neurosci. 2005;12(4):457-463.
137 Nandi D, et al. Thalamic field potentials during deep brain stimulation of periventricular gray in chronic pain. Pain. 2002;97(1-2):47-51.
138 Xie K, et al. The physiologically modulated electrode potentials at the depth electrode-brain interface in humans. Neurosci Lett. 2006;402(3):238-243.
139 Green AL, et al. Neural signatures in patients with neuropathic pain. Neurology. 2009;72(6):569-571.
140 Rossi F, Maione S, Berrino L. Periaqueductal gray area and cardiovascular function. Pharmacol Res. 1994;29(1):27-37.
141 Bandler R, et al. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53(1):95-104.
142 Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58(1-2):27-47.
143 Bandler R, Carrive P, Zhang SP. Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization. Prog Brain Res. 1991;87:269-305.
144 Green AL, et al. Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport. 2005;16(16):1741-1745.
145 Green AL, et al. Controlling the heart via the brain: a potential new therapy for orthostatic hypotension. Neurosurgery. 2006;58(6):1176-1183. discussion 1176-1183
146 Pereira EA, et al. Sustained reduction of hypertension by deep brain stimulation. J Clin Neurosci. 2010;17(1):124-127.
147 Pereira EA, et al. Ventral periaqueductal grey stimulation alters heart rate variability in humans with chronic pain. Exp Neurol. 2010;223(2):574-581.
148 Akil H, Liebeskind JC. Monoaminergic mechanisms of stimulation-produced analgesia. Brain Res. 1975;94(2):279-296.
149 Akil H, Mayer DJ, Liebeskind JC. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976;191(4230):961-962.
150 Akil H, et al. Enkephalin-like material elevated in ventricular cerebrospinal fluid of pain patients after analgetic focal stimulation. Science. 1978;201(4354):463-465.
151 Hosobuchi Y, et al. Stimulation of human periaqueductal gray for pain relief increases immunoreactive beta-endorphin in ventricular fluid. Science. 1979;203(4377):279-281.
152 Dionne RA, et al. Contrast medium causes the apparent increase in beta-endorphin levels in human cerebrospinal fluid following brain stimulation. Pain. 1984;20(4):313-321.
153 Fessler RG, et al. Elevated beta-endorphin in cerebrospinal fluid after electrical brain stimulation: artifact of contrast infusion? Science. 1984;224(4652):1017-1019.
154 Young RF, Chambi V. Pain relief by electrical stimulation of the periaqueductal and periventricular gray matter: evidence for a non-opioid mechanism. J Neurosurg. 1987;66(3):364-371.
155 Meyerson BA. Biochemistry of pain relief with intracerebral stimulation: few facts and many hypotheses. Acta Neurochir (Wien). 1980;30(suppl):229-237.
156 Blackburn-Munro G. Pain-like behaviours in animals—how human are they? Trends Pharmacol Sci. 2004;25(6):299-305.
157 Oliveras JL, Besson JM. Stimulation-produced analgesia in animals: behavioural investigations. Prog Brain Res. 1988;77:141-157.
158 Cruccu G, et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14(9):952-970.
159 Papanastassiou V, et al. Use of the Radionics Image FusionTM and StereoplanTM programs for target localization in functional neurosurgery. J Clin Neurosc. 1998;5(1):28-32.
160 Orth RC, et al. Development of a unique phantom to assess the geometric accuracy of magnetic resonance imaging for stereotactic localization. Neurosurgery. 1999;45(6):1423-1431.
161 Joint C, et al. Hardware-related problems of deep brain stimulation. Movement Disorders. 17(suppl 3), 2002.
162 Pereira EA, et al. Deep brain stimulation: indications and evidence. Expert Rev Med Devices. 2007;4(5):591-603.
163 McLeod RS, et al. Single-patient randomised clinical trial. Use in determining optimum treatment for patient with inflammation of Kock continent ileostomy reservoir. Lancet. 1986;1(8483):726-728.
164 McQuay H. N-of-1 Trials. New York: Raven Press; 1990.
165 Green AL, et al. N-of-1 Trials for assessing the efficacy of deep brain stimulation in neuropathic pain. Neuromodulation. 2004;7(2):76-81.
166 Kaplitt MG, et al. Deep brain stimulation for chronic pain, ed 5. Philadelphia: Saunders; 2004.
167 Bendok BR, Levy RM, Onibukon A. Deep Brain Stimulation for the treatment of intractable pain. In: Batjer HH, Loftus CM, editors. Textbook of neurological surgery: principles and practice. Philadelphia, London: Lippincott Williams & Wilkins; 2003:2673-2681.
168 Meyerson BA. Problems and controversies in PVG and sensory thalamic stimulation as treatment for pain. Prog Brain Res. 1988;77:175-188.
169 Adams JE, Hosobuchi Y. Technique and technical problems. Neurosurgery. 1977;1(2):196-199.
170 Adams JE, Hosobuchi Y, Linchitz R. The present status of implantable intracranial stimulators for pain. Clin Neurosurg. 1977;24:347-361.
171 Burchiel KJ. Deep brain stimulation for chronic pain: the results of two multi-center trials and a structured review. Pain Med. 2001;2(3):177.
172 Bittar RG, et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12(5):515-519.
173 Garonzik I, et al. Deep brain stimulation for the control of pain. Epilepsy Behav. 2001;2(suppl 3):3.
174 Gybels J. Thalamic stimulation in neuropathic pain: 27 years late. Acta Neurol Belg. 2001;101(1):65-71.
175 Gybels J, et al. Neuromodulation of pain: a consensus statement prepared in Brussels 16-18 January 1998 by the following task force of the European Federation of IASP Chapters (EFIC). Eur J Pain. 1998;2(3):203-209.
176 Raslan AM. Deep brain stimulation for chronic pain: can it help? Pain. 2006;120(1-2):1-2.
177 Tasker RR, Filho OV. Deep brain stimulation for neuropathic pain. Stereotactic Functional Neurosurg. 1995;65(1-4):122-124.
178 Wallace BA, Ashkan K, Benabid A. Deep brain stimulation for the treatment of chronic, intractable pain. Neurosurg Clin North Am. 2004;15(3):343-357. vii
179 Simpson BA. Spinal cord and brain stimulation. In: Wall PD, Melzack R, editors. Textbook of pain,. ed 4. Edinburgh: Churchill Livingstone; 1999:1353-1382.
180 Simpson BA. Spinal cord and brain stimulation, ed 5. Edinburgh: Churchill Livingstone; 2003.
181 Siegfried J. Therapeutical neurostimulation—indications reconsidered. Acta Neurochir. 1991;52(suppl):112-117. Wien
182 North RB, Levy RM. Consensus conference on the neurosurgical management of pain. Neurosurgery. 1994;34(4):756-760. discussion 760-761
183 Osenbach R. Neurostimulation for the treatment of intractable facial pain. Pain Med. 2006;7(suppl 1):S126-S136.
184 Hosobuchi Y. The current status of analgesic brain stimulation. Acta Neurochir. 1980;30(suppl):219-227. Wien
185 Hosobuchi Y. Current issues regarding subcortical electrical stimulation for pain control in humans. Prog Brain Res. 1988;77:189-192.
186 Stojanovic MP. Stimulation methods for neuropathic pain control. Current Pain Headache Rep. 2001;5(2):130-137.
187 Tasker RR, Vilela Filho O. Deep brain stimulation for neuropathic pain. Stereotact Funct Neurosurg. 1994;65(1-4):122-124.
188 Mazars G, Merienne L, Cioloca C. Comparative study of electrical stimulation of posterior thalamic nuclei, periaqueductal gray, and other midline mesencephalic structures in man. New York: Raven Press; 1979.
189 Gybels J. Electrical stimulation of the brain for pain control in human. Verh Dtsch Ges Inn Med. 1980;86:1553-1559.
190 Schvarcz JR. Chronic self-stimulation of the medial posterior inferior thalamus for the alleviation of deafferentation pain. Acta Neurochir. 1980;30(suppl):295-301. Wien
191 Turnbull IM, Shulman R, Woodhurst WB. Thalamic stimulation for neuropathic pain. J Neurosurg. 1980;52(4):486-493.
192 Dieckmann G, Witzmann A. Initial and long-term results of deep brain stimulation for chronic intractable pain. Appl Neurophysiol. 1982;45(1-2):167-172.
193 Plotkin R. Results in 60 cases of deep brain stimulation for chronic intractable pain. Appl Neurophysiol. 1982;45(1-2):173-178.
194 Tsubokawa T, et al. Thalamic relay nucleus stimulation for relief of intractable pain: clinical results and beta-endorphin immunoreactivity in the cerebrospinal fluid. Pain. 1984;18(2):115-126.
195 Tsubokawa T, et al. Clinical results and physiological basis of thalamic relay nucleus stimulation for relief of intractable pain with morphine tolerance. Appl Neurophysiol. 1982;45(1-2):143-155.
196 Tsubokawa T, et al. Deafferentation pain and stimulation of the thalamic sensory relay nucleus: clinical and experimental study. Appl Neurophysiol. 1985;48(1-6):166-171.
197 Meyerson BA. Electrostimulation procedures: Effects, presumed rationale, and possible mechanisms. Adv Pain Res Ther. 1983;5:495-534.
198 Hosobuchi Y. Chronic brain stimulation for the treatment of intractable pain. Res Clin Stud Headache. 1978;5:122-126.
199 Hosobuchi Y. Dorsal periaqueductal gray-matter stimulation in humans. Pacing Clin Electrophysiol. 1987;10(1 Pt 2):213-216.
200 Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans: report of 122 cases (1970-1984). J Neurosurg. 1986;64(4):543-553.
201 Hosobuchi Y. Combined electrical stimulation of the periaqueductal gray matter and sensory thalamus. Appl Neurophysiol. 1983;46(1-4):112-115.
202 Baskin DS, et al. Autopsy analysis of the safety, efficacy and cartography of electrical stimulation of the central gray in humans. Brain Res. 1986;371(2):231-236.
203 Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery. 1987;21(6):885-893.
204 Siegfried J. Sensory thalamic neurostimulation for chronic pain. Pacing Clin Electrophysiol. 1987;10(1 Pt 2):209-212.
205 Gybels J, Kupers R. Deep brain stimulation in the treatment of chronic pain in man: where and why? Neurophysiol Clin. 1990;20(5):389-398.
206 Young RF, et al. Electrical stimulation of the brain in treatment of chronic pain: experience over 5 years. J Neurosurg. 1985;62(3):389-396.
207 Young RF, Brechner T. Electrical stimulation of the brain for relief of intractable pain due to cancer. Cancer. 1986;57(6):1266-1272.
208 Katayama Y, et al. Deep brain and motor cortex stimulation for post-stroke movement disorders and post-stroke pain. Acta Neurochir. 2003;87(suppl):121-123.
209 Gybels J, Kupers R, Nuttin B. Therapeutic stereotactic procedures on the thalamus for pain. Acta Neurochir (Wien). 1992;124(1):19-22.
210 Lyons KE, et al. Surgical and hardware complications of subthalamic stimulation: a series of 160 procedures. Neurology. 2004;63(4):612-616.
211 Yianni J, et al. The costs and benefits of deep brain stimulation surgery for patients with dystonia: an initial exploration. Neuromodulation. 2005;8(3):155-161.
212 Toward a demand driven deep-brain stimulator for the treatment of movement disorders: third IEE International Seminar on Medical Applications of Signal Processing, London, UK, 2005.
213 Hassenbusch SJ. Cingulotomy for cancer pain. In: Gildenberg P, Tasker R, editors. Textbook of stereotactic and functional neurosurgery. New York: McGraw Hill; 1997:1447-1451.
214 Cosgrove GR, Rauch SL. Stereotactic cingulotomy. Neurosurg Clin North Am. 2003;14(2):225-235.
215 Pereira EAC. The lobotomist. J R Soc Med. 2005;98(8):181-182.
216 Glasziou P, et al. When are randomised trials unnecessary? Picking signal from noise. Br Med J. 2007;334(7589):349-351.