13 Decision Making and Perioperative Transesophageal Echocardiography
All too often in medicine, critical decisions are made without the benefit of a thorough consideration of data, evidence, and framework. The paucity of clinical outcomes research in echocardiography, especially in the perioperative period, dampens the prospects for evidence-based decision making. In the absence of evidence-directed practice, decision making typically is based on anecdote, clinical impression, and tradition, with little effort devoted to the process of reaching an intelligent conclusion. The quantity of information is increasingly abundant in medicine, and the operating room is no exception. Its acquisition, interpretation, and application for decision making can be cumbersome, distracting, and misguided. In the era of increasing information, there is an imperative to develop a systematic process of handling data streams, organizing ideas and thoughts, defining and prioritizing problems, and effecting care through a well-thought-out decision. A formalized approach to the acquisition of data and decision making (Figure 13-1) enhances the quality of the intraoperative echocardiogram, its interpretation, and the confidence with which the findings are communicated to other members of the operative and nonoperative teams. Poor decisions are not made by the physician with bad intentions. Poor decisions are more commonly the result of individuals relying on limited medical knowledge (a database typically defined by the narrow bounds of their profession), narrow framing, and false or tenuous anchors. The echocardiographer who is overly confident in the abilities of his or her surgical counterpart may be falsely anchored to the prior performances of the surgeon, with little or no reliance on a formalized decision-making process. The lack of a structured paradigm for decision making is most worrisome when clinicians with lesser ability or experience are making the decisions. The least accomplished clinicians often have the most inflated estimate of their own abilities, thus lending themselves to the vulnerabilities of limited skills plus lack of a decision-making process.1
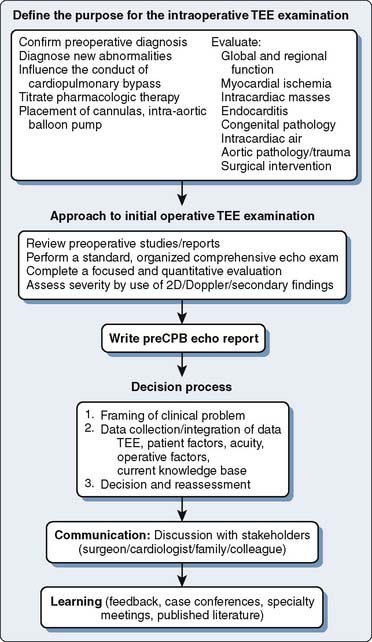
Figure 13-1 An algorithm for the decision-making process.
CPB, cardiopulmonary bypass; TEE, transesophageal echocardiography.
The intraoperative consultant in echocardiography is confronted with multiple channels of information (Boxes 13-1, 13-2, and 13-3). The broad database that is required to formulate an intelligent decision includes provider- and patient-specific data. Patient-specific data include history and demographics, preoperative diagnostic examinations, admitting diagnosis and comorbidities, the patient’s wishes, recommendations of referring physicians, and intraoperative data. Intraoperative data include hemodynamic data, visual inspection, surgical input, and the transesophageal echocardiographic (TEE) examination. A systematic TEE examination of the heart and great vessels permits the acquisition and interpretation of qualitative and quantitative echocardiographic data applied to intraoperative decision making. The provider-specific data are composed of an accumulated database of knowledge acquired from training, experience, and continuous medical education. Expertise is gained from experience and enhances the repertoire of experiences from which a practitioner can draw but does not alter the cognitive engine and does not immunize the practitioner from errors in decision making. Intuition and experience are not reliable predictors of success. Learning through methods of trial and error and self-education by exploratory problem solving have little role in the arena of cardiac surgery. Heuristic methods of decision making often create a systematic and predictable bias. It is acceptable to be wrong. It is unacceptable to be consistently wrong in the same direction. A structured process of assessing all the data and weighing various alternatives (cognitive engine) will enable the physician to formulate a concise, organized approach to problem solving, communicating the findings and management alternatives.
BOX 13-2. PATIENT DATA
BOX 13-3. OPERATIVE FACTORS
An intraoperative TEE examination can correct preoperative inaccuracies in diagnosis or detect occult disease. With increasing emphasis on decreasing preoperative testing, avoiding redundant testing, and decreasing costs, accurate diagnoses of disease may not occur until the time of surgery. The increased reliance on the intraoperative TEE is fiscally wise but places greater responsibility and impact on the intraoperative echocardiographer. The detection of occult disease not appreciated during the preoperative evaluation often impacts operative management. It is necessary to reframe a problem when the data acquired reveal new insights. For example, the detection of mobile atheroma in the ascending aorta may influence positioning of an aortic infusion cannula or cross-clamp, hence changing circulatory management and the operation.2–4 A change in clinical management in respect to otherwise asymptomatic and silent findings is often controversial. The change in the operation has typically not been discussed with the patient, as the findings were unanticipated. The intraoperative diagnosis of moderate aortic valve regurgitation (AR) that was not detected before surgery will create a clinical challenge for the surgical team regarding administration of cardioplegia and the decision whether to replace the aortic valve (AV). Hence, the decision to proceed with an unplanned aortic valve replacement (AVR) relies on the ability of the echocardiographer to establish the diagnosis and mechanism of valvular pathology, define the pertinent factors that sway the decision (preoperative symptoms of congestive heart failure [CHF], ventricular size and function, pulmonary hypertension), and communicate with the surgeon and the other pertinent stakeholders. It is important to realize that the decision to recommend AVR to a patient is not often guided by the degree of AR but rather by the degree of corresponding ventricular dilatation and dysfunction. The finding of moderate AR with normal ventricular systolic function and chamber size, normal left atrial pressure, and no preoperative history of CHF may sway the operative team to proceed with the originally planned surgery and to treat the occult finding medically with postoperative afterload reduction and follow-up serial echocardiograms. In contrast, in a patient with otherwise unexplained shortness of breath, pulmonary hypertension, and a dilated left ventricle (LV), the presence of moderate AR typically leads to AVR. The introduction of new findings to the operative team warrants a “time-out” approach to determine the impact of the findings on the intraoperative care.
Decision-Making Process
The process of decision making is, in essence, “deciding how to decide.”5 What is the primary issue that needs to be addressed? What are the pitfalls in the decision? What are the consequences of the decision? What tools and resources does the decision maker require? What information is needed to make an informed decision? Is there evidence to support one decision over another? How much time does the decision-maker need to make the decision? Rarely is there a valid reason for not taking enough time to make a well-thought-out decision, even in the high-productivity, high-throughput environment of the operating room. Does the decision-maker need help? The authors have applied the methods of Russo and Schoemaker5 to decision making in medicine:
The data should include echocardiographic and nonechocardiographic data (see Boxes 13-1 through 13-3). The importance and impact of TEE decisions have been generally recognized and accepted. The ability to make an appropriate decision is predicated on a comprehensive examination. Confirmatory information is useful, as is contradictory information. The process of decision making also includes defining what information “not to collect.” Collecting as much data as possible typically leads to confusion and loss of direction in the reasoning process. A common hazard for the echocardiographer is the performance of an abridged examination because of either increased clinical demands or reliance on a preoperative examination. The conclusions drawn from an intraoperative examination and associated decisions should not be hurried and should be based on all aspects of the examination. Although physical injury from the TEE examination is a serious matter, the greatest risk of TEE is that of errors of omission or misinterpretation, leading to mismanagement and poor outcome.6–11
Performance of the decision maker is judged based on final outcome. However, decision making should be based on the information the decision maker had at the time of the decision. A significant limitation of measuring performance during uncertainty is that it is often judged, not by the decision-making process, but by single case results. If the decision is followed by a good outcome, the decision maker often is applauded with little regard to the ability to reach an intelligent conclusion. A poor outcome does not necessarily imply a poor process or poor decision. High-risk surgery leads to poor outcomes in many cases despite robust decisions. In medicine, this often leads to individuals being reluctant to make any decision at all, knowing that a poor outcome is likely and that it will be linked to their decision making. The reality of performance assessment is that even if the decision to proceed with therapy is substantiated, a poor outcome often will reflect negatively on the abilities of the echocardiographer, anesthesiologist, surgeon, and the operative team. Conversely, a good outcome does not imply a good process or a good decision.5 The surgeon’s decision to perform a posterior sliding mitral valvuloplasty and quadrilateral resection of the posterior leaflet based on a dilated mitral annulus with normal leaflet motion as defined by TEE may result in a technically competent MV repair and good long-term results. The decision to perform a posterior sliding valvuloplasty may or may not have been a wise decision. Equally good results may have occurred with the insertion of an annular ring without leaflet resection. The measure of an outcome by recording a metric is a valid assessment of quality only if the metric is a function of the actions of the provider.12 The outcome cannot be random; otherwise, there is no basis for estimating quality.
INTRAOPERATIVE TRANSESOPHAGEAL ECHOCARDIOGRAPHY: INDICATIONS
The first decision by the echocardiographer is whether TEE is indicated. Application of intraoperative TEE in the care of the patient with mitral disease is widely accepted. Even in this area, however, there is a paucity of data supporting an improved outcome for intraoperative patients cared for with TEE compared with no TEE. The decision to perform TEE during cardiac surgery is substantiated by practice expectations and consensus opinion. In an attempt to develop an evidence-based approach to this expanding technology, the American Society of Anesthesiologists (ASA) and the Society of Cardiovascular Anesthesiologists (SCA) cosponsored a task force to develop guidelines for defining the indications for perioperative TEE. Despite the scarcity of outcome data to support the application of TEE in the perioperative period, TEE had rapidly been adopted by cardiac surgeons and cardiac anesthesiologists as a routine monitoring and diagnostic modality during cardiac surgery. In 1996, the task force published their guidelines, designed to establish the scientific merit of TEE and justification of its use in defined patient cohorts.13 The indications were grouped into three categories based on the strength of the supporting evidence/expert opinion that TEE improves outcome (Box 13-5). Category I indications suggested strong evidence/expert opinion that TEE was useful in improving clinical outcome. Category II indications suggested there was weak evidence/expert opinion that TEE improves outcome in these settings. Category III indications suggested there was little or no scientific merit or expert support for the application of TEE in these settings (see Chapters 12 and 41). These guidelines were further updated in 2010 to include virtually all adult cardiac surgery (Box 13-6).14
BOX 13-5. INDICATIONS FOR THE USE OF TRANSESOPHAGEAL ECHOCARDIOGRAPHY
Modified from the Practice guidelines for perioperative transesophageal echocardiography. A report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology 84:986, 1996.
BOX 13-6 2010 UPDATED RECOMMENDATIONS FOR TRANSESOPHAGEAL ECHOCARDIOGRAPHY
Modified from Practice guidelines for perioperative transesophageal echocardiography: An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology 112:1084, 2010.
INTRAOPERATIVE TRANSESOPHAGEAL ECHOCARDIOGRAPHY: PERFORMANCE OF THE INTRAOPERATIVE EXAMINATION
The ability to render a sound conclusion often is predicated on the performance of a complete and quantitative echocardiographic examination. Incomplete and qualitative assessments may be subject to missed or inaccurate diagnoses. The complete TEE examination has been described through a consensus opinion.15 The exact sequence of the comprehensive examination is less important than is adherence to it. A common practice is to perform a targeted examination, followed by the complete sequenced examination. This method allows for capture of the most important information should the patient become unstable and need rapid initiation of CPB before completion of the sequence of images that constitute the comprehensive TEE. The “soft” interpretation of “mild-to-moderate” is not always avoidable. The ability to “nail down” a diagnosis and establish a quantifiable measure of dysfunction allows for serial follow-up and comparison before and after treatment. The imperative for quantitative measures is directed at producing reproducible conclusions and trackable results.
Aliasing of color-flow Doppler in the LVOT is sensitive for detection of outflow obstruction but lacks specificity. Altered loading conditions, contractility, obstructive myopathy, and systolic anterior mitral motion may produce similar color Doppler findings. Spectral Doppler offers distinct advantages to color Doppler by measuring gradients and analysis of blood-flow velocity profiles, rendering the interpretation of abnormal flow patterns and pressure gradients more reliable and quantifiable. A similar approach is applied to assess abnormal blood-flow velocities across the MV (see Chapter 12).
Cardiac Function and Regional Wall Motion Abnormalities
Data Collection
Regional assessment provides an index of myocardial well-being that can be linked to coronary anatomy and blood flow. Although the measurement of coronary blood flow is not achieved by TEE, the perfusion beds and corresponding myocardium for the left anterior descending, left circumflex, and right coronary arteries are relatively distinct and can be scrutinized by TEE using multiplane imaging. The transgastric and long-axis imaging views of the LV are the most widely used for evaluating wall motion abnormalities. Digital archival systems have gained popularity for their ability to capture a single cardiac cycle that can then be examined more closely as a continuous cine loop. Cine loops also can permit side-by-side display of images obtained under varying conditions (e.g., prebypass and postbypass). Regional myocardial ischemia produces focal changes in the corresponding ventricular walls before changes occur on the ECG.16 Changes progress from normal wall motion to hypokinesis or akinesis. Dyskinesis, thinning, and calcification of the myocardium suggest a nonacute process, likely a prior infarction.
Ventricular failure can be caused by diastolic dysfunction: the compromised ability of the ventricle to accommodate diastolic filling. Diastolic dysfunction is assessed echocardiographically by examining volumetric filling of the ventricle at the mitral or tricuspid valves and annular excursion. Normal diastolic filling is biphasic with an early (passive) component that exceeds the late (active) inflow velocities. Abnormalities of ventricular filling (e.g., impaired ventricular relaxation, restriction, constriction) produce characteristic changes in the spectral recordings of Doppler inflow velocities. Abnormalities of diastolic function can lend insight into the mechanisms of circulatory instability (see Chapter 12).
Discussion
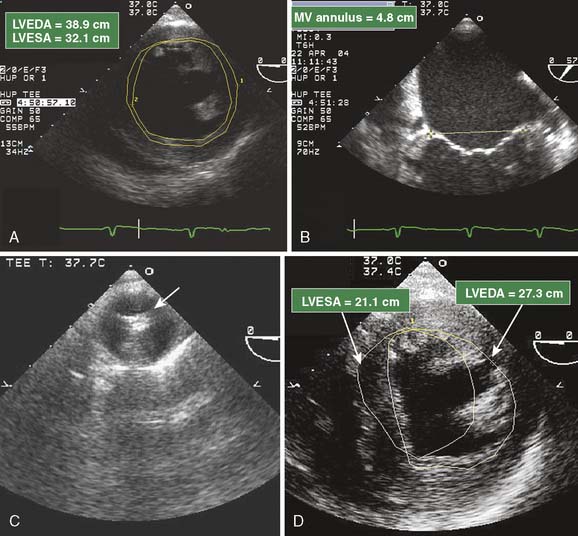
Preexisting ventricular dysfunction suggests increased risk for surgery and poorer long-term outcome. The presence of such ventricular dysfunction may deteriorate intraoperatively, requiring the need for marked pharmacologic or mechanical support. A patient with a preoperative EF of 10% scheduled for coronary artery bypass grafting (CABG) and MV repair is at increased risk for intraoperative ischemia, acute heart failure, and difficulty maintaining hemodynamic stability during the immediate postbypass period. Anticipating such problems, consider placement of an intra-aortic balloon pump or femoral arterial catheter during the prebypass period (Figure 13-2). The same patient is likely to benefit from the administration of inotropic agents (see Chapter 32).
Not all preexisting SWMAs benefit from coronary revascularization. Regions of akinesia and dyskinesia usually are the result of a myocardial infarction and may reflect nonviable myocardium, although “hibernating” myocardium is possible. Hypokinetic segments generally are viable and may represent active ischemia.17 Preoperative positron emission tomographic scanning can detect hibernating myocardium and may be cost-effective to guide CABG.18–20 The detection of hibernating myocardium in an area of chronic ischemia and regional hypokinesis will direct the surgeon to revascularize the corresponding stenosed coronary artery. In contrast, an occluded coronary artery with downstream infarction may not benefit from revascularization because contractile function may be irreversibly lost. However, in this latter scenario, revascularization postinfarction may provide some benefit in decreasing the risk for ventricular aneurysm formation.21
Diastolic dysfunction is associated with significant increases in mortality during long-term follow-up.22 Characterization of abnormalities of diastolic function lends insight into the mechanisms of circulatory instability and hypotension. Severe left ventricular hypertrophy with a noncompliant LV and hyperdynamic systolic function may produce severe heart failure if adequate loading is not achieved. Hemodynamic indices obtained from a pulmonary artery catheter (PAC) may be misleading. The findings of a small left ventricular chamber size, blunted transmitral filling velocities, and an increased FAC demonstrate the cause of the hypotension. The decision to administer volume may be appropriate despite the increased pulmonary artery pressures.
If the intraoperative examination reveals new ventricular dysfunction, the intraoperative team must determine the cause and severity and then plan a treatment. Other causes of SWMAs such as conduction abnormalities (left bundle branch block or ventricular pacing) can be difficult to distinguish. Is the decrement in function potentially reversible with conservative therapy, or should additional intervention be considered? Treatment of myocardial ischemia may include optimizing hemodynamics; administering anticoagulants, nitrates, calcium channel blockers, or β-blockers; inserting an intra-aortic balloon pump; or instituting CPB and coronary revascularization. The presence of new-onset SWMAs after separation from CPB is worrisome for myocardial ischemia. Even the patient without coronary artery disease (CAD) remains at risk because of hypotension, a shower of air or debris into the coronary circulation, or coronary spasm. The patient with CAD undergoing CABG may have all the above risks, technical difficulties at the anastomotic site, injury to the native coronary artery (e.g., stitch caught the back wall or occlusion of the circumflex artery during MV surgery), or occlusion of the coronary graft by thrombosis or aortic dissection. The coronary arteries, grafts, and anastomoses should be carefully inspected for patency and flow. Graft patency in the operating room is difficult to determine. Techniques include manual stripping and refill, measuring coronary flow by handheld Doppler, or administration of echo contrast agents (see Chapter 12). Hybrid operating rooms have been increasing in number with the intent of providing advanced imaging of the coronary circulation at the time of surgery.23 A new SWMA in the distribution of a new coronary graft can prompt the decision-making strategies listed in Table 13-1 (see Chapters 18, 32, and 34).
TABLE 13-1 Management Strategies for New-Onset Myocardial Ischemia after Bypass
| Diagnosis | Plausible Treatment |
|---|---|
| Coronary graft occlusion | Revise coronary graft |
| Coronary air emboli | Increase coronary perfusion pressure, administer coronary dilators |
| Coronary calcium/atheroma emboli | Support circulation |
| Dissection of the aortic root | Repair dissection |
| Coronary spasm | Administer coronary dilators |
Transesophageal Echocardiography as a Rescue Device: Management of Marked Hemodynamic Instability
Discussion
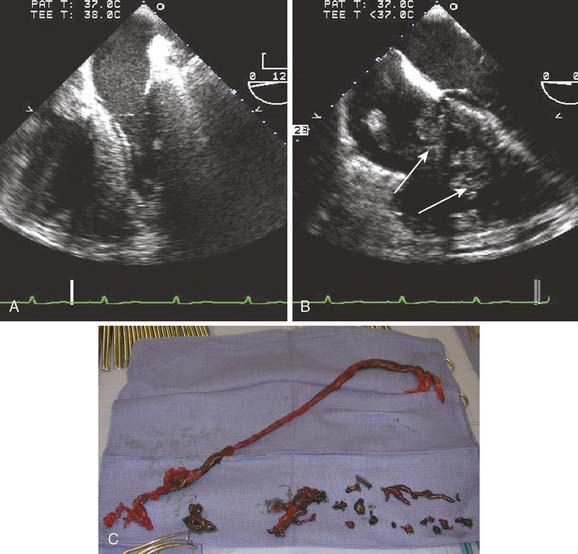
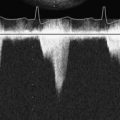
The common causes of intraoperative or perioperative hypotension include intravascular hypovolemia, myocardial ischemia, myocardial infarction, and systemic vasodilatation, either pathologic from infection or inflammation, or iatrogenic from drug administration (e.g., vancomycin). Mechanical causes of hypotension typically are related to compressive forces impairing the heart’s ability to fill or eject (e.g., pericardial fluid, tension pneumothorax). The MV is inspected for incompetence. Acute mitral regurgitation is rare in the absence of myocardial ischemia or infarction. A diagnosis of dynamic LVOT obstruction, an uncommon cause during the perioperative period, is difficult to establish in the absence of more invasive monitoring such as TEE (Figure 13-3). Systemic hypotension with a dilated RV and a small, underfilled LV implies either primary right ventricular failure (e.g., myocardial ischemia or infarction in the distribution of the right coronary artery) or secondary right ventricular failure from acute increases in pulmonary vascular resistance (e.g., pulmonary embolus [Figure 13-4], pneumothorax, or protamine reaction).
The distribution of the right coronary arterial system of most patients (right dominant system) includes the RV and the posterior descending coronary artery, which provides blood supply to the inferior and inferoseptal walls of the LV. Acute right ventricular dysfunction is not uncommon after the release of the aortic cross-clamp. Preservation of the RV is less reliable compared with the LV because of its exposure to ambient room temperature and variability in its coronary circulation. Open-chamber procedures increase the risk for right ventricular dysfunction because of retained intracardiac air. In the supine patient, the right coronary ostium is located in the least-dependent portion of the aortic root, predisposing it for the embolization of air bubbles. Air embolization to the right coronary artery produces acute ST-segment changes, marked global right ventricular dysfunction, and SWMAs of the inferior wall of the LV (Figure 13-5). Conservative treatment includes increasing the blood pressure to promote coronary perfusion while continuing CPB.
Pericardial Effusion and Tamponade
Discussion
Not all pericardial effusions require immediate intervention. Development of cardiac tamponade is related to the rate of accumulation of pericardial fluid and the capacity for the pericardium to stretch and accommodate fluid. Chronic pericardial effusions, which occur in cases of malignancy, uremia, connective tissue disease, Dressler syndrome, and postinfection pericarditis, uncommonly require emergent intervention. Acute pericardial effusions that occur postcardiotomy are usually more ominous and often result in hemodynamic compromise, requiring treatment (see Chapters 22 and 34).
Management of Ischemic Mitral Regurgitation
Framing
Ischemic heart disease is the most common cause of mitral insufficiency in the United States. Mechanisms of valve incompetence are varied and include annular dilatation, papillary muscle dysfunction from active ischemia or infarction, papillary muscle rupture, or ventricular remodeling from scar, often leading to a tethering effect of the subvalvular apparatus. Mitral regurgitation leads to pulmonary hypertension, pulmonary vascular congestion, and pulmonary edema with functional disability. Ventricular function deteriorates as the LV becomes volume overloaded with corresponding chamber dilatation. Left untreated, severe mitral regurgitation from ischemic heart disease has a poor prognosis, hence the imperative for diagnosis and treatment.24–26 Less certain is the impact of lesser degrees of mitral insufficiency on functional status and long-term morbidity and mortality. Patients presenting for CABG often have concomitant mitral regurgitation of a mild or moderate degree. The intraoperative team is confronted with the decision whether to surgically address the MV during the coronary operation.
Data Collection
Pertinent data, including preoperative functional status and evaluation, need to be considered to appropriately interpret and place the intraoperative data in context. The preoperative echocardiogram and ventriculogram need to be reviewed. The intraoperative hemodynamic data are coupled with TEE information to complete the dataset needed to move forward with the decision-making process. The severity of mitral regurgitation on TEE is measured by the vena contracta, maximum area of the regurgitant jet, regurgitant orifice area, and pulmonary vein blood-flow velocities. Valvular disease causes changes in other cardiac structures. Chronic mitral regurgitation may be associated with a dilated LA, pulmonary hypertension, and right ventricular dysfunction. Wall motion assessment and the ECG are used for detecting reversible myocardial dysfunction that may benefit from revascularization. The hemodynamic and TEE data are coupled with provocative testing of the MV in an attempt to emulate the working conditions of the MV in an awake, unanesthetized state. It is not uncommon that preoperative mild-to-moderate mitral regurgitation with a structurally normal valve totally resolves under the unloading conditions of general anesthesia.27–29
Discussion
Most cases of ischemic mitral regurgitation are categorized as “functional” rather than structural. In a study of 482 patients with ischemic mitral regurgitation, 76% had functional ischemic mitral regurgitation, compared with 24% having significant papillary muscle dysfunction.30 The mechanism of ischemic mitral regurgitation is attributed to annular dilatation, secondary to left ventricular enlargement and regional left ventricular remodeling with papillary muscle displacement, causing apical tethering and restricted systolic leaflet motion.31 The importance of local left ventricular remodeling with papillary muscle displacement as a mechanism for ischemic mitral regurgitation has been reproduced in an animal model.32
The mitral regurgitation is prioritized in accordance with the principal diagnosis (e.g., CAD), comorbidities, functional disability, and short- and long-term outcome. Ischemic mitral regurgitation is quantified and the mechanism of valve dysfunction is defined. Intraoperative mitral regurgitation is compared with preoperative findings. Discrepancies between the preoperative and intraoperative assessment of the valve may reflect the pressure and volume unloading effects of general anesthesia. In patients with functional ischemic 1 to 2+ mitral regurgitation, the MV often is not repaired or replaced. However, the need for surgical intervention in patients with 2+ mitral regurgitation under anesthesia remains a point of debate and has not been definitively answered by prospective studies. MV surgery typically is recommended to improve functional status and long-term outcome for patients with 3+ ischemic mitral regurgitation or greater.30 Ignoring significant ischemic mitral regurgitation at the time of CABG can limit the functional benefit derived from surgery.
The risks to the patient of not surgically altering the MV and anticipated residual regurgitation are weighted against the risk for atriotomy, mitral surgery, extending CPB and aortic cross-clamp times, and the likelihood that the CABG surgery will be successful at decreasing the severity of mitral regurgitation. Added risk includes commitment to a mechanical prosthesis should a reparative procedure prove unsuccessful. Mitral regurgitation caused by acute ischemia may resolve after restoration of coronary blood flow (Figure 13-6). The reversibility of the regurgitation is difficult to predict: Factors supporting reversibility (and, hence, no immediate need to surgically address the valve) include a structurally normal MV, normal left atrial and left ventricular dimensions, including the mitral annulus, and SWMAs associated with transient regurgitation and pulmonary edema. Revascularization of the culprit myocardium with improvement in regional function may be all that is necessary to restore normal mitral coaptation.33,34 Myocardial infarction with a fixed wall-motion defect or aneurysm, chronically dilated left-sided heart chambers, dilated annulus, or other structural abnormalities that are not reversible (ruptured papillary muscle or chordae, leaflet prolapse, leaflet perforation) suggest myocardial revascularization is unlikely to correct the valvular incompetence.
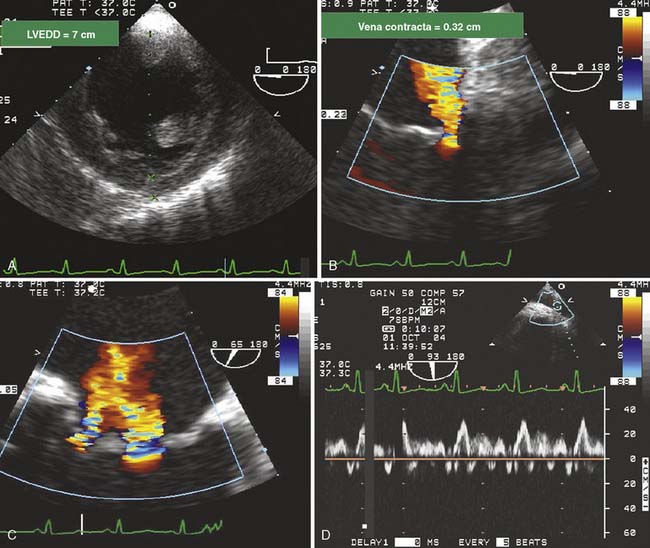
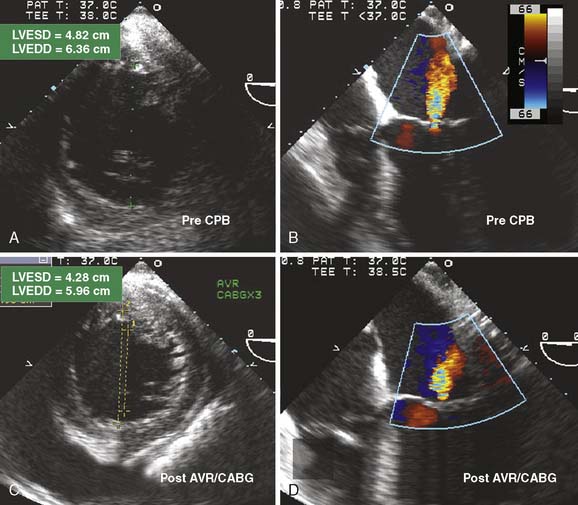
Figure 13-6 Evaluation of mitral regurgitation (MR) in a patient undergoing coronary artery bypass grafting.
The decision whether to proceed with mitral surgery in the setting of ischemic heart disease is institution and surgeon dependent. Centers may elect to surgically address any degree of mitral regurgitation detected during the preoperative or intraoperative work-up of a patient scheduled for CABG surgery. Less aggressive sites elect to proceed with coronary revascularization, followed by repeat scrutiny of the ventricular wall motion and MV. If revascularization has not corrected the mitral regurgitation, the surgeon proceeds with CPB and mitral surgery. With the advent of off-pump coronary artery bypass surgery, this process has gained another level of complexity because decisions to proceed with mitral repair will commit the patient to CPB. Off-pump mitral surgical procedures may be possible in the near future. A device that decreases the minor annular axis by adjusting the length of an artificial subvalvular chord that spans the ventricle between anterior and posterior epicardial pads has been used to treat functional mitral regurgitation.35,36 Likewise, percutaneous endovascular procedures to “clip” the free edges of the anterior and posterior mitral leaflets akin to the Alfieri surgical technique are aimed at noninvasively improving leaflet coaptation.37,38 Short- and long-term outcome data regarding such investigational techniques remain forthcoming (see Chapters 3, 18, 19, 26, and 27).
Myxomatous Degeneration of the Mitral Valve and Mitral Regurgitation
Data Collection
The intraoperative TEE examination targets the mitral regurgitation, LA, LV, and RV. Two-dimensional and color-flow Doppler remain the standard, although increasing application of three-dimensional echo has targeted mitral surgery as a niche. Three-dimensional echo has made significant advances in miniaturization, speed of data processing, and user interfaces. In the past, it was a technology seeking an application. Mitral surgery for myxomatous disease appears to be one of its prime applications. Myxomatous mitral regurgitation typically is amenable to valve repair. Typical findings of myxomatous valves include excessive leaflet motion, redundant leaflet tissue, and a dilated mitral annulus. Leaflets commonly prolapse into a dilated LA, the degree of which is based on the chronicity of the illness. Chordal rupture is common and leads to flail leaflets and severe mitral regurgitation. The imperative is to be exact in the descriptive anatomy of the MV. The accepted nomenclature of the anatomy of the MV is stated in a consensus guideline.15 The locus of flailed scallops or leaflets and regurgitant orifice, the width of the anterior and posterior leaflets, the bisecting widths of the mitral annulus (minor and major axes), the locus of a perforation, severity of annular calcification (less common in isolated ischemic or myxomatous disease), and the size of the LA all contribute significantly to the planned repair. The findings of long and redundant anterior and posterior leaflets increase the risk for postoperative SAM of the MV. Maslow et al39 examined the predictors of LVOT obstruction after MV repair. In this study of patients who were undergoing repair for myxomatous valve disease, 11 of 33 patients experienced development of SAM and outflow tract obstruction. The major predictive factors were smaller anteroposterior length ratio (annulus to coaptation; 0.99 vs. 1.95) and short distance from septum to MV coaptation point (2.53 vs. 3.00 cm). The surgeon may elect to perform a posterior sliding valvuloplasty to move the point of coaptation laterally, thereby decreasing the risk for outflow obstruction and mitral incompetence associated with SAM.
Discussion
There is a growing imperative to repair instead of replace myxomatous MVs. The intraoperative echocardiographer soon becomes familiar with the abilities and limitations of his or her surgical counterparts. Outcomes may be quite dependent on the ability of the individual surgeon, more so than in valve replacement surgery. Hence it may be prudent to track short-term (intraoperative) results of these operations because outcomes may be less defined by national databases and more so by individual provider. In general, the likelihood of a successful repair is based on the severity and extent of involvement of the mitral leaflets. Isolated prolapse of the middle scallop of the posterior mitral leaflet associated with eccentric mitral regurgitation that overrides an otherwise normal anterior leaflet is associated with a high success rate. However, cases of extensive leaflet degeneration with bileaflet prolapse, multiple chordal ruptures from both leaflets, leaflet destruction from preceding endocarditis, two or more regurgitant orifices, and extensive calcification are associated with a significantly lower success rate for repair.40 Many patients leave the operating room with a prosthetic MVR with preservation of the subchordal apparatus with excellent long-term results. Retention of the subvalvular apparatus preserves longitudinal shortening of the LV and decreases the incidence of HF in the long term.41,42 In a comparative study, the EF of patients without chordal transfer decreased 24% after surgery compared with patients having chordal transfer who maintained preoperative function.42
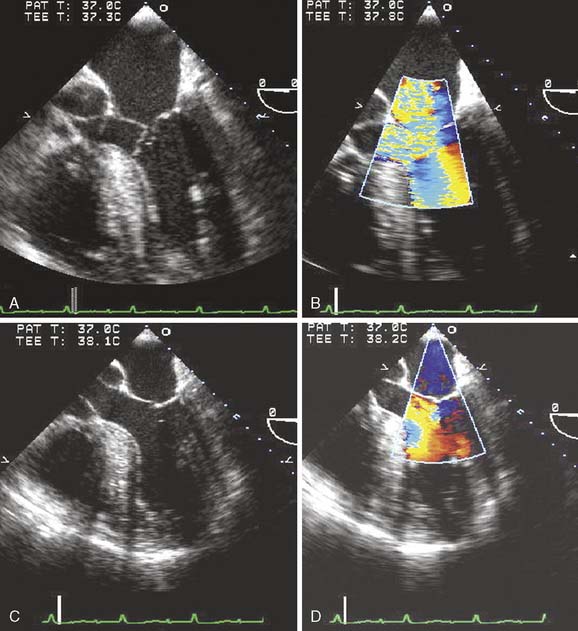
The finding of LVOT obstruction caused by SAM after mitral repair often is caused by the displacement of leaflet coaptation toward the septum, resulting in the anterior leaflet to paradoxically move into the LV, rather than toward the LA late in systole (Figure 13-7).43 Displacing the anterior leaflet into the LVOT during ventricular ejection (Venturi effect) produces outflow tract obstruction, early closure of the AV, and mitral regurgitation. The incidence of postoperative SAM and the need to revise the surgical repair have decreased as understanding of the predisposing factors and management strategies has improved. SAM may be intermittent and dependent on loading conditions. If possible, patients should be examined after adequate volume resuscitation and with minimal inotropic support. Most cases of SAM resolve with conservative measures including β-blockade, vasoconstriction, and fluid administration. In a single-center retrospective study of 2076 patients who underwent MV repair, the incidence rate of intraoperative SAM was 8.4% (174 cases).44 Revision of repair or valve replacement related to SAM during initial operation was undertaken in only two patients. However, in the case of persistent severe SAM with a high LV-to-aorta gradient, returning to CPB and revising the mitral repair (e.g., posterior sliding mitral valvuloplasty to “slide” the locus of coaptation laterally), performing an edge-to-edge repair or possibly replacing the MV should be considered. Patients who transiently demonstrate SAM or turbulence in the outflow tract in the operating room may be at increased risk during the immediate postoperative period (see Figure 13-3). An important role of the clinical echocardiographer is to recognize potentially important findings that may have an impact on subsequent patient care and long-term follow up (see Chapters 12, 19, and 22).
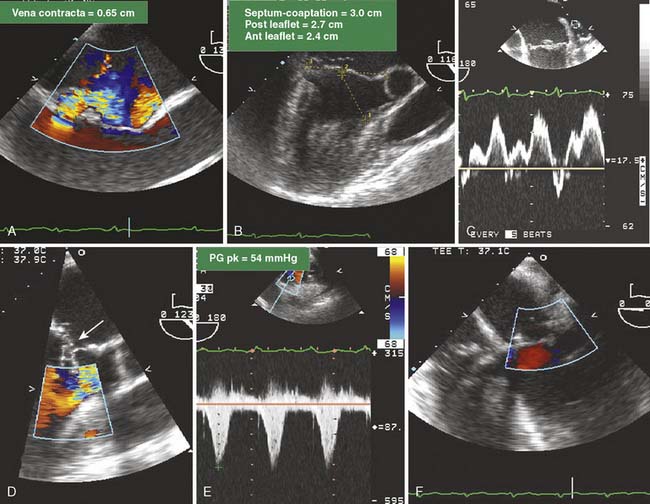
Figure 13-7 Complex mitral valve (MV) replacement/repair revision.
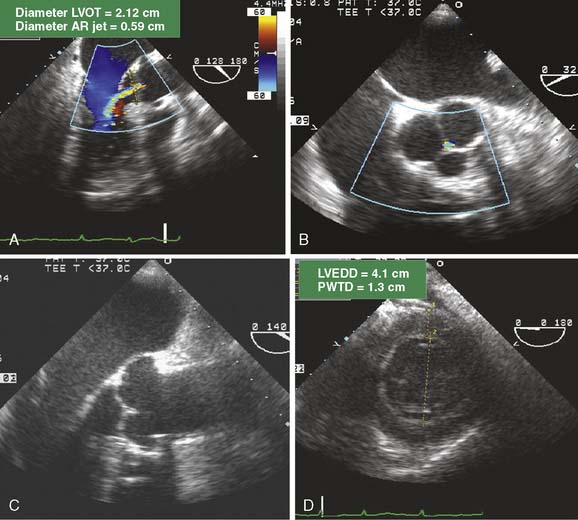
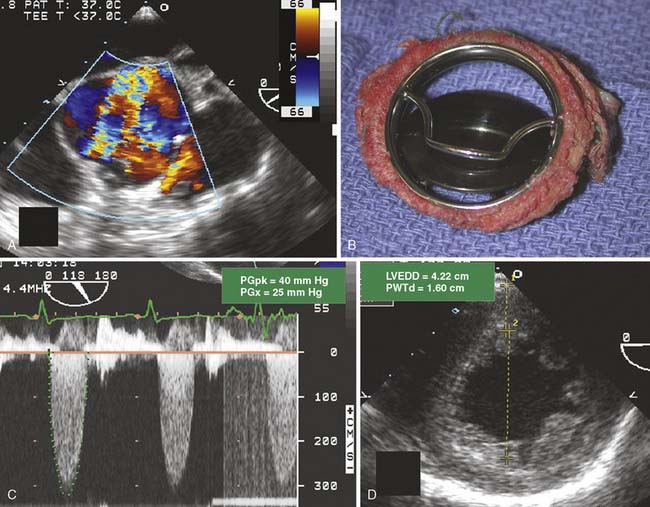
A 58-year-old woman with severe mitral regurgitation (MR) and a history of congestive heart failure, pulmonary edema, and hypertension was scheduled for surgical repair of mitral insufficiency. The prebypass transesophageal echocardiogram (TEE) characterized the MV as having severe MR with mildly thickened, myxomatous leaflets and several ruptured chordae. The markedly dilated annulus was consistent with the chronic disease process. Prolapse of the posterior leaflet resulted in the MR jet overriding the anterior leaflet (A). Although the distance from the septum to the coaptation point of the MV was greater than the value cited by Maslow as predictive of postbypass SAM, the ratio of the length of the anterior and posterior leaflets (0.89) suggested a risk for LV outflow obstruction (B).39 The midesophageal long-axis view demonstrates several ruptured chordae (B) that resulted in MR having a vena contracta of 0.65 cm. Blunting of the systolic component of pulmonary vein blood flow velocity corroborated the diagnosis of significant MR (C). The surgeon performed a quadrangular resection of the posterior leaflet and secured the annulus with a No. 30 Physio ring. The postbypass imaging of the midesophageal long-axis view demonstrated a coaptation defect, designated by the arrow, and nonlaminar flow in the LV outflow tract (D). Shift of the coaptation point medially created laxity of the redundant chordae that were drawn into the outflow tract by systolic LV ejection (D). The outflow tract obstruction was characterized by a pressure gradient of 54 mm Hg as determined by continuous-wave Doppler through the outflow tract using the transgastric long-axis view (E). Neither the MR nor the outflow tract obstruction was effectively addressed by volume loading and decreasing the inotropic support. Circulatory bypass was reinstituted, and the surgeon revised the previous repair by further resecting the posterior leaflet and enlarging the annular ring to a No. 34. The patient was successfully separated from bypass using minimal support and had resolution of LV outflow tract obstruction, return of normal hemodynamics, and reduction of MR to trace severity (F).
Occult Congenital Abnormalities: Persistent Left-Sided Superior Vena Cava
Data Collection
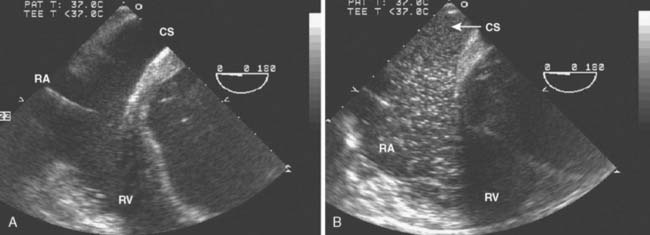
The echocardiographer should suspect a persistent left-sided SVC if the coronary sinus is significantly dilated or if significant difficulty was encountered while attempting to place a pulmonary artery catheter (PAC). Because the differential diagnosis of a dilated coronary sinus includes pathology associated with increased right-sided pressures, confirmation should be obtained by injecting agitated saline contrast into an intravenous catheter in the left arm. In the case of a persistent left-sided SVC, opacification of the coronary sinus occurs before that of the RA or RV (Figure 13-8). Once the diagnosis is confirmed, the echocardiographer should look for other associated congenital anomalies, including atrial septal defects (ASDs) or an unroofed coronary sinus with communication between the coronary sinus and the floor of the LA.
Occult Congenital Abnormalities: Atrial Septal Defects and Patent Foramen Ovale
Framing
Incidental detection of an ASD or a patent foramen ovale (PFO) is a common occurrence. The clinical implications of an intracardiac defect are shunting, stroke, headaches, pulmonary hypertension, right ventricular dysfunction, and paradoxic embolization. If transseptal flow is present, it is generally from left to right, because left atrial pressure is generally greater throughout the cardiac cycle. Bidirectional flow is possible with transient increases of right atrial pressure that can be observed during normal respiratory maneuvers (i.e., Valsalva, coughing, and physical straining).45 Routine physical activities associated with a Valsalva maneuver include heavy physical activity, lifting heavy objects, defecation, or vigorous coughing.46 Although uncommon, right-to-left shunting can produce episodes of relative hypoxia and paradoxic embolization. TEE is an extremely sensitive technique to detect an ASD or a PFO.47 ASD or PFO is generally well tolerated and often remains asymptomatic into adulthood; hence their detection often is incidental during a routine intraoperative TEE examination.
Data Collection
The application of two-dimensional echocardiography, color-flow Doppler imaging, and contrast administration in the midesophageal four-chamber and midesophageal bicaval views provide for high sensitivity of detection and diagnosis of ASDs and PFOs. The clinical implication of these findings is determined by the type of pathology. The PFO may be detected in about 25% of adults; it occurs when the secundum septum fails to close or is stretched open because of elevated pressures in the LA (Figure 13-9). Ostium secundums, which account for 70% of ASDs, are located in the area of the foramen ovale. The cause of this lesion is attributed to poor growth of the secundum septum or excessive absorption of the primum septum. MV prolapse is present in up to 70% of patients with this abnormality and may be related to a change in the left ventricular geometry resulting from right ventricular volume overload. The primum ASD develops when the septum fails to fuse with the endocardial cushion at the base of the interatrial septum. A primum ASD commonly is associated with cleft anterior leaflet of the MV and mitral regurgitation. The tricuspid valve also may be abnormal. The final type of ASD is a sinus venosus defect, which comprises only 10% of ASDs. It commonly is associated with abnormal insertion of the pulmonary veins into the RA or SVC.
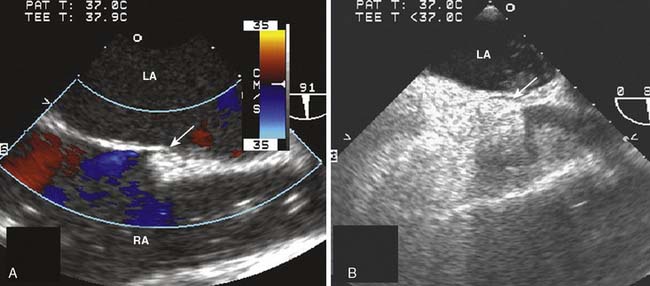
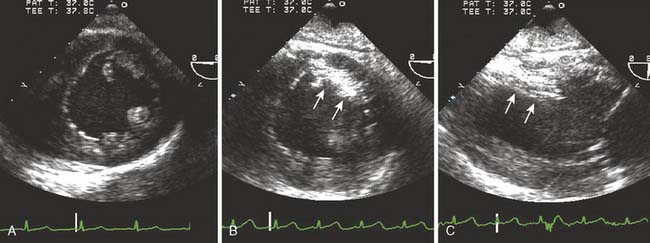
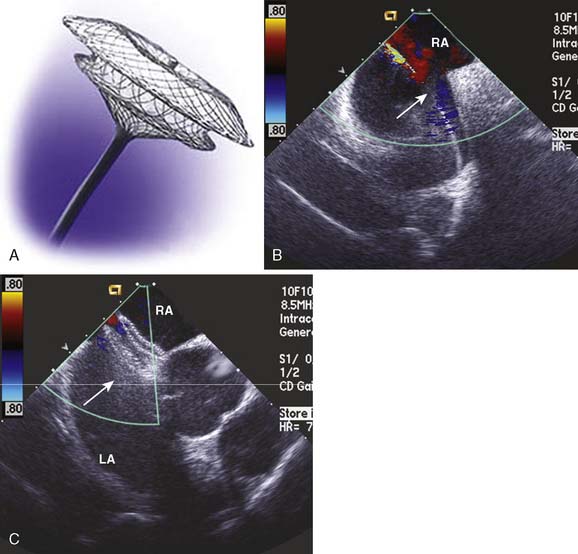
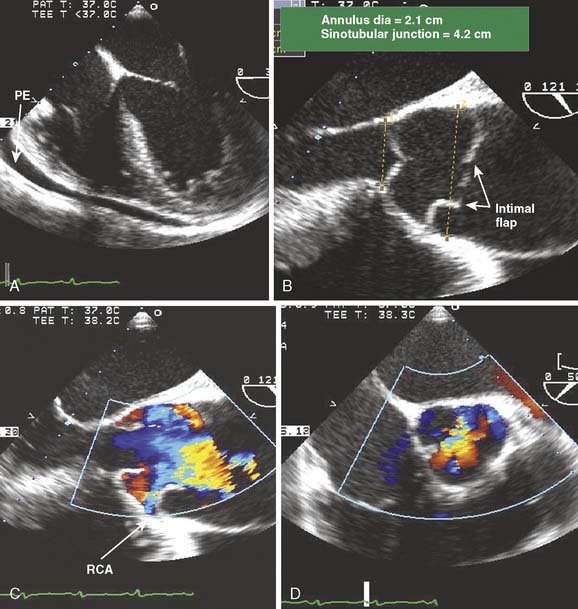
Figure 13-9 Detection of a patent foramen ovale in a patient undergoing cardiac surgery.
A 68-year-old patient was undergoing two-vessel off-pump coronary artery bypass grafting (OPCAB). In addition to coronary artery disease, the patient had a medical history of hypertension, non–insulin-dependent diabetes, and a stroke of unknown cause. The intraoperative transesophageal echocardiographic (TEE) examination that included an inspection of the interatrial septum in the midesophageal four-chamber and bicaval imaging planes showed a new finding of an aneurysmal interatrial septum with a patent foramen ovale (PFO, arrow). Color-flow Doppler showed minimal shunt flow from the left atrium (LA) to the right atrium (RA). The presence of the PFO, which was initially diagnosed by color-flow Doppler imaging (A), was confirmed by the transient flow of injected contrast (arrow) into the LA (B), after it was injected into the venous circulation. A provocative maneuver such as Valsalva transiently increases RA pressure more than that of the LA, increasing the sensitivity of PFO detection.47 Because of the patient’s history of a cryptogenic stroke, the surgical plan and circulatory management were altered. The patient was fully heparinized and circulation supported by an extracorporeal pump, while the CABG was performed and the PFO was closed.
Discussion
In general, patients benefit from ASD closure. Although often asymptomatic, ASDs may present with atrial arrhythmias, heart murmur, abnormal ECG, dyspnea, cerebrovascular injury or stroke, or migraine headaches. Medical management and surgical closure of secundum ASDs were compared in randomized trials; surgical closure was associated with significantly decreased morbidity and mortality.48 However, there are little data concerning treatment strategies and outcomes for the occult ASD or PFO detected intraoperatively. In the absence of recognized consensus guidelines, the decision to proceed with definitive closure should be based on the following factors: history of neurologic event without a definite cause, recurrent stroke while receiving anticoagulation, significant shunting through the defect, previous episode of hypoxia that may be related to intracardiac shunting, right ventricular dysfunction, or previous paradoxic embolism. Detection of a primum or sinus venosus defect requires a more involved surgical procedure, and associated anomalies must be addressed. Alternatives to operative closure are increasing as transvenous percutaneous closure devices become increasingly applicable. Transvenous catheter–based closure of an ASD by interventional cardiologists can be performed only on a secundum ASD or PFO (Figure 13-10). Approximately 30% of secundum ASDs are amenable to percutaneous closure.49 The technique optimally requires a defect with limited size and a rim of tissue surrounding the defect of at least 5 mm to prevent obstruction of the coronary sinus or impingement of the AV (see Chapters 3 and 20).
A PFO is the most common congenital finding and usually is asymptomatic. However, a number of studies have found an increased prevalence of PFO and atrial septal aneurysms in patients having cryptogenic strokes (i.e., no identified cardioembolic or large vessel source).50–56 Atrial septal aneurysm is a congenital outpouching of the interatrial septum at the fossa ovalis and is strongly associated with PFOs.50,51,53 The occurrence of atrial septal aneurysms detected by echocardiography is about 10% in the general population and up to 28% in patients with a history of stroke or transient ischemic episode.52–54,57 A meta-analysis of these case-control studies found that PFO, atrial septal aneurysms, or both were significantly associated with ischemic stroke in patients younger than 55 years.58 However, the relation between septal pathology and neurologic events in patients older than 55 is less clear. The data on treating patients with atrial septal abnormalities for either primary or secondary prevention of stroke are limited. The Patent Foramen Ovale in Cryptogenic Stroke Study and a meta-analysis by Oregera found that warfarin treatment was superior to other antiplatelet therapy and comparable with surgical PFO closure for the prevention of recurrent cerebral events.59 Chronic anticoagulation is associated with complications. The decision regarding closure of an incidental PFO considers the presence of right ventricular dysfunction that may increase the risk for right-to-left transatrial blood flow and cryptogenic stroke. Clinical practices typically are based on individual surgical preferences. A survey of cardiothoracic surgeons in the United States noted a high degree of variability in management of intraoperatively discovered PFO.60 During planned on-pump CABG surgery, 27.9% of responders stated they always closed intraoperatively discovered PFOs, whereas 10.3% did not. Only 11% of surgeons converted a planned off-pump procedure to an on-pump procedure to close the defect, but the rate of closure increased to 96% if the patient had a history of possible paradoxic embolism. A single-institution retrospective study of 2277 patients from 1995 to 2006 confirmed that incidental detection of PFO is common, and that repair was not associated with a survival benefit. The authors found that propensity modeling of the data detected an increased incidence of postoperative stroke in patients having PFO repair. Several case reports describe postoperative complications related to hypoxia and neurologic events in patients with persistent PFOs.60–63
Management of Previously Undiagnosed Aortic Valve Disease
Data Collection and Characterization of the Aortic Valve
Multiplane TEE permits an accurate assessment of AV area, valvular pathology, severity of regurgitation and stenosis, and detection of secondary cardiac changes. In the case of AS, the severity of valvular dysfunction is determined by measuring the transvalvular pressure gradient, calculating the AV area using the continuity equation, and by planimetry of the AV systolic orifice. Planimetry of the AV orifice with TEE is more closely correlated with the catheterization-determined valve area (using the Gorlin formula) than the value derived from TTE (r = 0.91 vs. 0.84).64 The severity of AR by TEE generally is graded with color-flow Doppler imaging with measurement of the width of the regurgitant jet relative to the width of the LVOT. TEE is sensitive to even the most trivial amount of AR. Jet areas measured by TEE tend to be larger, and their severity is graded as greater compared with AR assessed by TTE.65 Determining the clinical significance of AR typically requires assessment of more than just regurgitant grade, although severe 4+ AR is never left unaddressed. A more challenging decision is whether to surgically address lesser degrees of AR in patients requiring cardiac surgery for another reason (see Chapters 3, 19, and 21).
The cause and extent of AV disease can be delineated best by TEE, as shown in Figure 13-11. The relatively high resolution of the AV and associated structures in the near field of the midesophageal short- and longitudinal-axis views permits an accurate assessment of the severity and mechanism of valvular disease. The aortic leaflets should be inspected in the midesophageal long-axis view for the presence of vegetations, perforation, restriction, thickening/calcification, malcoaptation, and leaflet prolapse. The presence of subvalvular disease, such as a discrete fibrous subaortic membrane, also can be reliably excluded. The ascending aorta from the valve to the right pulmonary artery also should be viewed in long axis. This view is usually optimal for examining associated pathology of the aortic root and ascending aorta (e.g., aortoannular ectasia, bicuspid valve, type A aortic dissection).
Bicuspid Aortic Valve
Bicuspid AVs are common in the general population, with an estimated incidence rate between 0.9% and 2.25%. The natural history of bicuspid valves is variable. Patients can go late into adulthood without AV or aortic disease. Bicuspid AVs can function without major hemodynamic abnormality well into the seventh decade of life.66 In contrast, a significant percentage of patients experience progressive dilatation of the aortic root, producing AR, or premature calcification of the valve, producing AS.67–71 For patients in their fourth decade of life, the predominant lesion at surgery is AR. With increasing age, the predominant lesion associated with bicuspid AVs becomes AS.72 The architectural makeup of the wall of the aorta is abnormal in patients with a bicuspid AV, predisposing for aneurysm formation.73–76 The decision-making process in regard to surgical correction of a bicuspid AV must account for the size of the aortic root and the likelihood the patient will return for aortic root surgery in the not-too-distant future. The rate of ascending aortic dilatation in patients with a bicuspid AV may be as high as 0.9 mm/year.77 Even though patients with a bicuspid AV are at increased risk for cardiac surgery at a later time in life, surgical intervention is not recommended for a bicuspid AV in the absence of aortic root aneurysm, AS, or AR. A normally functioning bicuspid AV may have a longer duration than a bioprosthetic artificial valve. Some centers in North America have adopted a more aggressive stance toward repairing a mildly dilated ascending aorta (≥4-cm diameter) because of the increased risk for acute aortic dissection and ruptures.78
The majority of young patients with a bicuspid AV have a regurgitant lesion. If found as an incidental finding, valvular dysfunction is usually minimal and patients do not have a significantly increased risk for heart failure and death. Although AVR is associated with a low risk for mortality, the lifelong cumulative risk for valve replacement is not insignificant. Mechanical heart valves are associated with low but significant rates of valve thrombosis, thromboembolism, and hemorrhagic complications from lifelong anticoagulation. Biologic prosthetic valves are limited by degeneration of the bioprosthetic material. The use of a homograft or pulmonary autograft provides alternatives for patients who do not want to be or are at high risk for anticoagulation. Bicuspid AVs that can be rendered competent and nonstenotic may be “repaired” in the setting of surgery for aortic dissection or aneurysm. Reconstruction of an isolated, severely regurgitant bicuspid AV has been attempted and described79–82 but is not in the mainstream of cardiac surgery in the United States because of the risk for recurrent AR. Surgical advances of AV repair have raised the hope of increased valve longevity that rivals or exceeds that of a biologic prosthesis (see Chapter 19).
Natural Course of Aortic Stenosis
The natural course of AS in the adult begins with a prolonged asymptomatic period associated with minimal mortality. Progression of the disease is manifested by a reduction in the valve area and an increase in the transvalvular systolic pressure gradient. The progression is quite variable, exhibiting a decrease in effective valve area ranging from approximately 0.1 to 0.3 cm2/year. AV calcification, as depicted by echocardiography, has been suggested to be an independent predictor of outcome. Patients with no or mild valvular calcification, compared with those with moderate or severe calcification, had significantly increased rates of event-free survival at 1 and 4 years (92% vs. 60% and 75% vs. 20%, respectively).83 Decisions regarding valve replacement for mild or moderate AV disease in the setting of cardiac surgery for another cause are complicated by the variability in the natural progression of the disease. The pathogenesis of AS is an active process having many similarities to the progression of atherosclerosis.84 AV calcification is not a random degenerative process but an actively regulated disease associated with hypercholesterolemia, inflammation, and osteoblast activity. More aggressive medical control of these processes might be expected to have a positive impact on outcome by retarding the degenerative process. Statin therapy has been proposed to slow the progression of disease.85 The definitive evidence that such therapies attenuate the progress of AV stenosis remains unclear but should be forthcoming. If the progression of AV stenosis can be attenuated, the need for AVRs would decrease significantly (see Chapter 19).
Assessment of Mild and Moderate Aortic Stenosis
A review of 1,344,100 patients in the national database of the Society of Thoracic Surgeons having CABG, CABG/AVR, or AVR alone culminated in a decision paradigm recommendation.86 The study assumed rates of AV disease progression (pressure gradient of 5 mm Hg/yr), valve-related morbidity, and age-adjusted mortality rates that were obtained from published reports. The authors proposed three factors in the consideration of CABG or AVR/CABG: age (life expectancy), peak pressure gradient, and rate of progression of the AS (if known). Because the latter is difficult to discern, the analysis assumed an average rate of disease progression and recommended patients should undergo AVR/CABG when the pressure gradient exceeds 30 mm Hg. The threshold (AS pressure gradient) to perform both procedures is increased for patients older than 70 years because the reduced life expectancy diminishes the likelihood that they will become symptomatic from the AV disease. Whether to perform a concomitant AVR at the time of revascularization was also addressed by Rahimtoola,87 who advocated a less aggressive approach. One problem with both studies is that they analyzed the transvalvular pressure gradient, which may be a misleading measure of the degree of stenosis of the AV because its value is dependent on CO. A low CO and flow rate will produce a low transvalvular pressure gradient, even in the setting of a severely stenotic AV. However, in the setting of preserved ventricular systolic function and mild or moderate AS, a pressure gradient is a useful metric. The variable rate of disease progression and the controversy regarding the indications for “prophylactic” AVR preclude a simple algorithm for dealing with this patient cohort. Increased age, lack of symptoms, minimal left ventricular hypertrophy, a valve area suggesting milder disease, and a pressure gradient less than 30 mm Hg would sway the decision not to replace the AV. In an asymptomatic young patient, a severely calcified valve, bicuspid valve, and left ventricular hypertrophy in the setting of moderate stenosis, as well as a pressure gradient greater than 30 mm Hg would suggest that an AVR might be beneficial in the long term. The considerations regarding the progression of AS and need for a subsequent, highly invasive redo surgery on older and presumably more ill patients must be reconsidered in the advent of the emerging technologies of the transcatheter AVR. Initial results seem promising; there is no evidence of early stenosis or prosthetic valve dysfunction. It often is useful to include the patient’s primary cardiologist and family in the decision-making process.
Assessment of Low-Pressure Gradient Aortic Stenosis
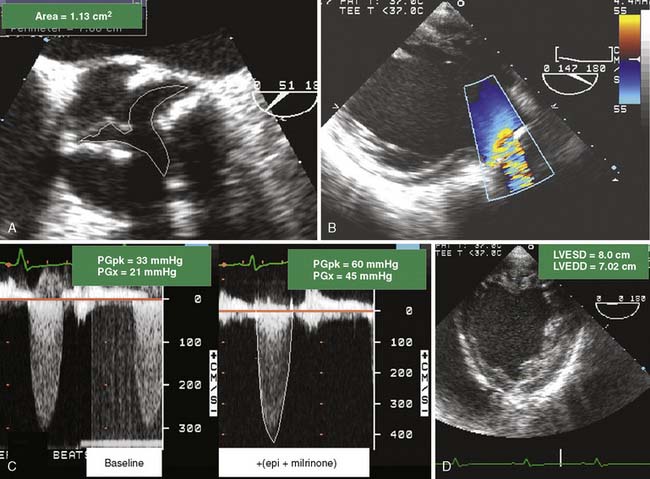
Patients with left ventricular dysfunction and decreased CO in the setting of AS often present with only modest transvalvular pressure gradients(< 30 mm Hg). Distinguishing patients with a low CO and severe AS from patients with mild-to-moderate AS can be challenging (Figure 13-12). The standard for assessing severity of AS is AV area, typically calculated using either a continuity method or by planimetry. Patients with low-gradient AS with severe left ventricular dysfunction who received an AVR had improved survival and functional status compared with patients who did not have a valve replacement.88–90
A low-pressure gradient related to left ventricular dysfunction may not open the AV to its maximum capacity. Dobutamine challenge in a patient with low-pressure gradient AS can be useful in establishing true AV area. The ability to distinguish between true AV stenosis and a state of “pseudostenosis” relies on characteristic changes in hemodynamic and structural measurements in response to the augmented CO. The test is not usually performed in the operative setting but rather as a preoperative evaluation. The increase in calculated AV area is related to the increase in the CO and is attributed to partial reversal of primary cardiac dysfunction.91–94 If dobutamine improves CO and increases AV area, it is likely the baseline calculations overestimated the severity of the AS. The dobutamine challenge is conducted as follows: patients with low-gradient AS receive intravenous dobutamine at 5 μg/kg/min with stepwise increases in dose.92 Patients may exhibit a significant increase in AV area (0.8 to 1.1 cm2) and a decline in valve resistance after dobutamine challenge. Patients with fixed, high-grade AS would demonstrate no change in valve area and an increase in valve resistance. The 2003 ACC/AHA/ASE Task Force gave a Class IIb recommendation (usefulness/efficacy is less well-established by evidence/opinion) for the use of dobutamine echocardiography in the evaluation of patients with low-gradient AS and ventricular dysfunction.95 In addition to its role in distinguishing between true stenosis and pseudostenosis, low-dose dobutamine echocardiography is helpful in risk-stratifying of patients with severe true AS. Patients with augmented contractile function after dobutamine administration have an improved outcome after surgery.96,97
Aortic Regurgitation: Natural Course and Management
Chronic AR generally evolves in a slow and insidious manner with a very low morbidity during a long asymptomatic phase. Some patients with mild AR may remain asymptomatic for decades. Others exhibit progressive worsening of the regurgitant lesion and develop left ventricular systolic dysfunction, leading eventually to heart failure. Evaluation of left ventricular size and function is important because of the poor correlation between symptoms and severity of cardiac pathology. The nature of the transition between the compensated and uncompensated periods is poorly understood. Clinicians should be reluctant to consider valve replacement in an asymptomatic patient with preserved left ventricular function (Figure 13-13). Early surgery exposes the patient to perioperative mortality and morbidity, as well as to the long-term complications of a prosthetic valve. Guidelines for AV surgery in patients with AR were published in 1998 by the ACC/AHA Task Force and in 2002 by the Working Group on Valvular Heart Disease of the European Society of Cardiology.98,99 AVR is clearly indicated for symptomatic patients with chronic AR and left ventricular dysfunction. However, the decision to perform valve replacement is less apparent for asymptomatic patients. The published recommendations suggest serial examinations to detect the progression of left ventricular dysfunction. In a strategy similar to that applied for patients with low-gradient AS, the induction of stress exercise testing may be helpful for assessing functional capacity and timing of surgery. However, intraoperative detection of occult AR does not provide for the luxury of serial examinations.
The intraoperative decision for asymptomatic patients should be based on the severity of AR and the presence of left ventricular dysfunction. Patients with a left ventricular end-systolic dimension less than 50 mm would be expected to remain asymptomatic for the next few years; the risk for development of symptoms or left ventricular dysfunction would range from 1% to 2% per year to as high as 6% per year.100,101 However, the yearly rate of becoming symptomatic or developing significant left ventricular dysfunction increases to greater than 20% when the left ventricular end-systolic dimension exceeds 50 mm.100,102
Choice of Surgical Intervention
Discussion
The development of reparative procedures for the AV has lagged behind those implemented for the MV or for the development of prosthetic devices. Some of the major factors are the apparently irreversible changes produced by calcification of the cusps, the retraction of cusp tissue in AR, and the lack of durable biologic material suitable for supplementing the valvular apparatus. It is unlikely that surgical repair of stenotic and highly calcified valves will achieve much success within the near future. However, aortic valvular repair has been successfully applied for the resuspension of the AV to correct acute AR resulting from an aortic dissection. Yacoub, Cohn, and others reported improved short- and long-term results for minimizing residual AR and freedom from reoperation.103–105
A diseased valve characterized by thickened, calcified leaflets and having restricted mobility is not a candidate for valve repair and would require valve replacement. Alternatively, remodeling of the aortic root and AV to improve the geometry of the tricuspid AV has been used successfully in cases of acute type A aortic dissection. In addition, a select cohort of patients having AR as the primary valvular diagnosis may be candidates for surgical repair. In the case of bicuspid AVs or isolated AR, successful repair of the AV was more likely when the anatomy demonstrated left coronary/right coronary cusp fusion and when the primary mechanism of AR was leaflet prolapse or commissural separation, compared with poor leaflet coaptation of a “central defect.”103 Although a complete review of the technical aspects and controversy of AV-sparing surgeries are beyond the scope of this chapter, it should be noted that aortic dilatation has a negative prognostic implication for successful long-term outcome. A more detailed discussion of surgical repair of the AV can be found in Chapter 19 and in literature reviews.104,105
Valve repair and replacement surgery for patients having symptomatic valve disease have been heralded as significant successes. Advances in surgical technique and perioperative care have enabled the morbidity and mortality of valve replacement to decrease despite the increasing proportion of high-risk patients. The choice of prosthetic heart valve replacement generally is based on patient age, size, and wishes106 (Figure 13-14). A joint decision with the patient, the cardiologist, and the cardiac surgeon is indicated before surgery.
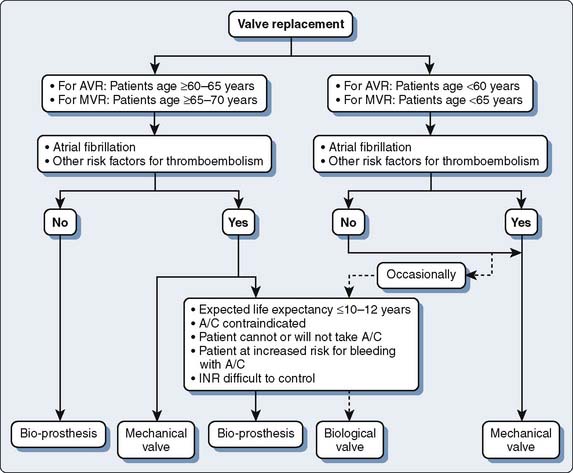
Figure 13-14 Algorithm for choice of prosthetic heart valve.
(Redrawn from Rahimtoola SH: Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol 41:893, 2003, Figure 8.)
Compared with nature’s own heart valves, prosthetic valves are relatively stenotic. Prosthetic valves are sized based on their external diameter, even though their transvalvular gradient reflects the internal diameter and orifice size. Each valve has an “effective orifice area” (EOA) that is based on in vitro studies performed by the manufacturer. The clinical importance of the disparity between prosthesis annular size and EOA becomes evident when relatively small prosthetic valves are inserted into comparatively large patients. Prosthetic valve/patient mismatch results when transvalvular flow through a prosthetic valve is limited and produces clinically significant stenosis (Figure 13-15). The increase in transvalvular blood-flow velocity necessary to accommodate and sustain an acceptable SV in a large patient with a prosthetic AV can produce surprisingly high transvalvular pressure gradients.
An objective of valve replacement surgery is to provide the patient with the largest EOA. Prosthetic valve mismatch is more likely to occur in older patients, those with increased body surface area (BSA), smaller prosthetic valve size, and preoperative diagnosis of valvular stenosis.107 The incidence of AV mismatch increases with prosthetic annular size less than 21 mm. In larger patients, mismatch can occur in patients receiving prosthetic AVs greater than 21 mm because dominant factors in determining the pressure gradient are the SV and CO, which are related to body size and level of activity. The association of mismatch with stenotic native AVs is a result of calcified native valves, a calcified annulus, and a smaller annular size. The treatment of AS through AVR in the setting of preserved ventricular systolic function may result in dramatic increases in SV and CO. The resultant increase in transvalvular flow produces a large transprosthetic pressure gradient. In contrast, valvular insufficiency is usually associated with annular dilatation and decreased systolic function. Hence patients with annuloectasia often receive larger prosthetic valves, and functional AS after AVR rarely is a problem.
Increased postoperative transvalvular gradients have been implicated in increasing long-term morbidity and mortality.108–110 The short-term clinical significance of prosthetic valve size mismatch is controversial and may be associated with immediate postoperative risk for cardiovascular complications and mortality. Controversy stems, in part, from studies with limited sample populations and the failure to normalize specific valvular EOA to a metric of patient size. Nonetheless, the concept of prosthetic valve mismatch adversely affecting outcome is generally accepted. The long-term risk for prosthetic valve mismatch is inability to restore or improve functional capacity. A consequence of an increased residual transvalvular pressure gradient is to prevent or attenuate regression of left ventricular hypertrophy.111 Left ventricular hypertrophy is associated with diastolic dysfunction, decreased exercise capacity, and is a predictor of increased mortality. Significant differences in regression of left ventricular hypertrophy occur in patients who received prosthetic AVs larger than 21 mm (−21%) compared with patients with prosthetic valves that were less than 21 mm (−8%).111,112 Hence it would be expected that a corresponding difference in functional capacity would parallel these differences in hypertrophy regression. A decreased indexed EOA (EOA/BSA = cm2/m2) was an independent predictor of long-term morbidity after aortic and MV replacement.113
In general, an indexed EOA for a prosthetic AV should be greater than 0.85 cm2/m2.113 For a similar external rim size, the EOA can vary considerably between mechanical and bioprosthetic AVs and by manufacturer. Bioprosthetic valves tend to have a significantly larger EOA compared with mechanical valves. Biologic aortic roots have even larger EOAs. If the anatomy of the aortic root precludes implantation of a suitably sized prosthesis, the surgeon may perform a supra-annular implantation, allowing the placement of a larger valve. Alternatively, creation of a larger EOA may require enlargement of the aortic root, a biologic aortic root, a pulmonic valve autograft (Ross procedure), or implantation of a stentless valve (see Chapters 19 and 21).
Aortic Valve: Concomitant Mitral Regurgitation
Framing
Valvular diseases do not often occur in isolation. Other anatomic structures may be influenced, either by the same pathophysiology or as a secondary consequence of the primary valvular lesion. Mitral insufficiency is a common finding, occurring in about two thirds of the patients having significant AS.114 Patients presenting for AVR commonly have mitral regurgitation and pose the question of whether to repair or replace the MV in addition to the AV (Figure 13-16). Such an undertaking is not without risk. Patients undergoing double-valve replacement have increased mortality compared with isolated AVR.
Discussion
Mitral regurgitation is a maladaptive consequence of increasing AS. More significant mitral regurgitation is associated with greater trans-AV pressure gradients, as well as more progressive dilatation and worsening systolic function.115,116 If MV anatomy is markedly abnormal and the severity of regurgitation is severe, the decision is relatively obvious: MV repair or replacement at the time of AVR. If the MV is anatomically normal (no leaflet prolapse, no perforation, no rheumatic changes) and the regurgitation is trace or mild, the decision is also relatively obvious: Correct the AS and do not surgically address the MV. In the setting of rheumatic AS, it is common to have other valves involved in the disease process. Hence mitral calcification, thickening, and leaflet fusion are common in patients with rheumatic AS. The MV is rarely replaced based on anatomic abnormality alone in the absence of significant stenosis or regurgitation.
Mitral regurgitation with an anatomically normal MV is referred to as “functional mitral regurgitation.” Increased left ventricular afterload, driving pressure, and left ventricular remodeling have been implicated in the development of functional mitral regurgitation in patients with severe AS. The natural history and clinical impact of mild-to-moderate mitral regurgitation in patients undergoing AVR for AS suggest that concurrent MV surgery may not be warranted in all cases. In the cohort of patients with functional mitral regurgitation and severe AS, the decrease in mitral regurgitation after AVR alone was variable; between 39% and 90% of patients having moderate mitral regurgitation experienced a regression in its severity after AVR.117–122 In general, patients with functional mitral regurgitation of moderate grade had significant improvement (decrease) in the mitral regurgitation after AVR, suggesting that leaving the MV alone in this setting is not unreasonable. Conversely, a significant number of patients, between 8% and 64%, had either no change or an increase in mitral regurgitation severity. In a study of 196 patients who had concomitant mitral regurgitation, Moazami et al121 noted a decrease in 3-year survival for those patients whose moderate-to-severe mitral regurgitation persisted after AVR and recommended surgical intervention for this cohort of patients.
In general, functional mitral regurgitation of moderate severity usually will regress at least one grade after AVR for severe AS (see Figure 13-16). Mild-to-moderate mitral regurgitation that improves after AVR for AS seems to be a lasting phenomenon.117 Unfortunately, there has been little control for the severity of concurrent AR, which is a common finding associated with AS. Although the physiologic load of patients with combined AS and AR is different, the particular pathology of the AV did not affect regression of mitral regurgitation after AVR.121 Harris et al120 ascribed the decrease in the severity of mitral regurgitation after AVR to several anatomic changes: decreases in mitral annular area, left atrial size, and left ventricular length. Together with the decrease in driving pressure that occurs with resolution of AS, all three of these anatomic changes alter the architecture of the MV and ventricle and contribute to decreasing functional mitral regurgitation. The greater the decrease in systolic area and increase in FAC of the LV after AVR, the greater is the reduction in postoperative mitral regurgitation.120
It is difficult to define the negative predictors for the regression of mitral regurgitation after AVR. The presence of valvular abnormalities, such as rheumatic leaflet thickening, calcification or prolapse (i.e., myxomatous degeneration), and chordal pathology that could contribute to a “nonfunctional” cause of mitral regurgitation would most likely persist. Mitral annular calcification is a potential complicating factor and its impact is unclear. Annular calcification would be expected to impede the reduction in MV area that normally occurs during systole. This reduction appears to be important in the mechanism of mitral regurgitation reduction after AVR for AS. Likewise, mitral annular calcification renders mitral surgery more difficult and the implantation of a mitral prosthesis more problematic, likely increasing the risk for paravalvular leak and AV groove disruption. Many patients with severe AS have a calcified AV with concomitant MV annular calcification. Mitral annular calcifications may be predictive of fixed mitral regurgitation after AVR.123 An enlarged left atrium (>5 m) has been reported to be a significant predicator of persistent mitral regurgitation after AVR.124 Other factors to consider include presence of CHF, CAD, pulmonary hypertension, and atrial fibrillation. Ischemic mitral regurgitation may improve after revascularization as a more favorable balance of myocardial oxygen supply/demand is produced after AVR.118 “Functional” mitral regurgitation of moderate-to-severe grade that is not operated on during AVR may remain unchanged after AVR and require reinstitution of CPB for MV surgery. In the absence of more predictable criteria to define this group, patients with moderate-to-severe mitral regurgitation caused by intrinsic valvular pathology should be considered for surgical intervention. Reports suggest that AVR combined with MV repair can be accomplished with excellent long-term results and minimal effect on mortality.118
Ascending Aorta, a Source of Embolization
Framing
The most disabling complication after cardiac surgery is stroke. Major focal and nonfocal neurologic deficits, cognitive decline, and coma after surgery are common. The pathogenesis of cerebral damage is multifactorial, with embolism considered a major contributor. Other factors include hypotension, low flow, reperfusion injury, and inflammation. Embolic events are strongly associated with the severity of atherosclerotic disease, characterized by plaque thickness of greater than 4 mm, ulcerated plaques, and mobile protruding plaques in the aorta.125,126 The severity of atherosclerosis of the descending aorta, as determined by TEE, is a significant risk factor and an independent predictor of adverse cardiac and neurologic outcome in patients undergoing CABG.127 Surgical manipulation of the thoracic aorta may liberate debris from diseased aortic tissue. The process of microembolization has been detected by transcranial Doppler during aortic cannulation, application and removal of the aortic cross-clamp, commencement of CPB, and initiation of ventricular ejection. The clinical consequence of distal embolization is dependent on the number, composition (e.g., air bubbles, fat particles, platelet aggregates, and calcium deposits), size, and location of the emboli (see Chapters 12, 16, 18, 19, 21, 28, and 36).
Data Collection
TEE imaging of the anterior wall of the ascending aorta is limited by far-field imaging resolution and its juxtaposition to air in the open chest. Imaging of the mid and distal ascending aorta by TEE is limited by the interposition of the airway with the esophagus and aorta. Epiaortic ultrasound can provide high-definition imaging of these otherwise hidden portions of the aorta. Epiaortic scanning offers higher sensitivity for detection of atheroma of the ascending aorta, as compared with TEE, especially in the mid and distal segments.3,128 The descending aorta is immediately adjacent to the esophagus and easily imaged using conventional TEE. A common practice is the interrogation of the descending thoracic aorta for high-grade atheroma. In the absence of atheromatous disease in the descending aorta, the ascending aorta and locus of aortic cannulation are significantly less likely to have high-grade disease. If the descending aorta contains high-grade or mobile atheroma, it may be prudent to examine the ascending aorta with epiaortic scanning for potential sites for aortic cannulation and clamping (Figure 13-17).
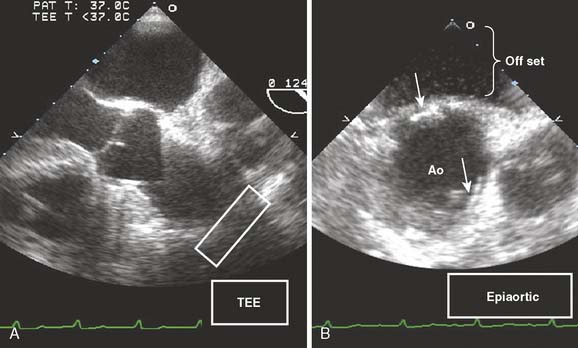
Figure 13-17 Detection of atheromatous plaque by epiaortic ultrasound.
A 67-year-old patient was scheduled to undergo coronary artery bypass grafting × 3. The transesophageal echocardiographic (TEE) examination was performed to evaluate the mitral valve for mitral regurgitation that might necessitate surgical intervention. Routine examination of the descending thoracic aorta detected severe atherosclerotic disease characterized by several ulcerated areas and plaques with a thickness of 4 mm. Because the severity of atherosclerotic disease in the descending aorta predicts significant disease in the ascending aorta,127 examination of the ascending aorta was attempted. TEE examination of the ascending aorta at the site of aortic cross-clamp and cannulation (white rectangle) was limited by poor resolution in the far field and interposition of the trachea (A). A handheld probe that was placed in a sterile sleeve was used to examine the aorta (B). The bottom of the sleeve was filled with saline as an offset to better image the anterior surface of the aorta (Ao). Several calcified plaques (arrows) were detected. Based on findings of the epiaortic scanning, the surgeon modified the site of cannulation and used a single cross-clamp technique to avoid a second clamping of the aorta during the proximal anastomosis.
Discussion
Historically, surgeons palpate the ascending aorta to determine the extent of intraluminal atherosclerotic disease in an effort to choose an appropriate location for cannulation, cross-clamping, and proximal graft anastomosis. However, palpation is a notoriously poor predictor of atheromatous disease.129–131 The occurrence of atheroma in the ascending aorta of cardiac surgery patients may be as high as 60% to 90%.130,132 Advanced age, hypertension, and diabetes are risk factors for atheromatous disease of the aorta and stroke after cardiac surgery.3,133 The information provided by ultrasound imaging of the aorta defines the location and severity of atheromatous disease, which can guide the surgeon to more strategically choose the cannulation, cross-clamp, and anastomotic sites.
Possible modifications to the conduct of cardiac surgery and CPB include (1) altering the site of cannulation, (2) aborting CPB alto-gether and performing the CABG surgery as an off-bypass procedure, (3) performing the surgery on a fibrillating heart without aortic cross-clamping, (4) performing aortic atherectomy or replacement of the ascending aorta, or (5) attempting a “no-touch” technique using deep hypothermic circulatory arrest. Epiaortic scanning combined with modification of surgery techniques in patients undergoing CABG decreased the number of emboli as detected by transcranial Doppler and significantly reduced neurologic behavioral changes at 1 week and 1 month after surgery.134 Modifications in the methods used in CABG revascularization, including the avoidance of proximal aortic graft anastomoses after detection of atheroma by echocardiography, resulted in a lower incidence of late neurologic complications.2
The application of epiaortic scanning for all patients undergoing cardiac surgery is controversial. Epiaortic scanning takes time and expertise in its interpretation and poses the potential risk for wound contamination. There are insufficient data to support its use in all cardiac patients, although high-risk patients (advanced age, diabetes, hypertension) are the most likely to benefit.135,136 As the profile of patients undergoing cardiac surgery ages, they have a greater incidence of atherosclerosis of the aorta and the presence of concomitant risk factors for postoperative complications. Echocardiographers working in the operating room need the skill set to interrogate the aorta by handheld ultrasound and the ability to guide the surgeon in performing the examination. It could be potentially life-saving in high-risk patients. A diagnostic scan of the ascending aorta and arch to evaluate the location of possible cannulation and clamp sites can be performed in several minutes. Standardized approaches to a comprehensive organized intraoperative epiaortic and epicardial examination are useful guides.137
Intracardiac Clot, Calcium, and Air
Framing
The incidence of postoperative neurologic dysfunction is greater in patients undergoing open cardiac procedures. The sources of microemboli include atheroma, calcified debris, tissue, and entrapped air. The particulate matter can embolize to the coronary circulation (see Figure 13-5), causing acute right ventricular or left ventricular dysfunction; to the cerebral circulation, producing postoperative neurologic deficits; and/or to distal vital organs.138–140
Aortic Pathology and Transesophageal Echocardiography
Framing
The utility of TEE has expanded beyond simply examining cardiac performance and pathology. TEE is well suited as a quick, highly sensitive, and specific tool to detect the presence of aortic pathology and some of its life-threatening sequelae. The detection and differentiation of true aneurysms and pseudoaneurysms, aortic dissections and transections, intraluminal atherosclerotic disease, and abnormalities of the aortic root are often the challenge of the intraoperative team (see Chapter 21).
Data Collection
Data acquired in the assessment of the patient with aortic disease include a targeted history for evidence of malperfusion or vital organ injury (e.g., stroke) and examination of blood pressure and pulse in each of the extremities, carotid arteries, and abdominal aorta. A history of chest or back pain is common with aortic dissection or aneurysm, with tearing of the aorta and its adventitia. Increasing shortness of breath may occur with AR, heart failure, or a left pleural effusion. Patients may present with hoarseness related to recurrent laryngeal nerve damage causing vocal cord dysfunction. The preoperative blood work may detect renal insufficiency with an increased blood creatinine concentration. The chest radiograph often shows a widened mediastinum or calcification of the thoracic aorta. The TEE data are aimed at dimensional and anatomic assessment of the aortic root, ascending arch, and descending aorta. The echocardiographer measures and reports the size of the aortic annulus, sinus of Valsalva, sinotubular junction, and ascending aorta (see Figure 13-11). Left ventricular hypertrophy may suggest long-lasting hypertension. A dilated LV may accompany chronic AR. Dilatation of the aortic root is common in the setting of AS and AR. Patients with a congenital predisposition for aortic aneurysm, such as Marfan syndrome or bicuspid AV, often develop a dilated and aneurysmal ascending aorta. Epiaortic scanning increases the sensitivity of detecting abnormalities of the ascending aorta because it is in the far field from a TEE window and may be hidden by the interposition of the trachea and left mainstem bronchus, which obstruct the distal half of the ascending aorta.128
Discussion
Aneurysm rupture occurred at a stunningly high frequency of 32% to 68% in patients with thoracic aortic aneurysms managed by conservative medical treatment and accounted for 32% to 47% of deaths. The one-, three-, and five-year survival of unoperated thoracic aneurysms was 65%, 36%, and 20%, respectively.141–143 The risk for aortic rupture is a function of the size and rate of growth of the aneurysm. The risk for rupture for aneurysms less than 40, 40 to 59, and greater than 60 mm was 0%, 16%, and 31%, respectively.144 The diameter of aneurysms tends to grow at a rate of approximately 5 mm/year. However, the rate of aneurysm expansion is variable. In general, the change in diameter of thoracic aortic aneurysms is a function of the initial diameter and increases more quickly in larger aneurysms.
Disease of the AV often is accompanied by abnormalities of the thoracic aorta. Aortic root dilatation may require ascending aortic replacement. The decision is based on size of the aortic root diameter and presence of abnormalities of the aortic wall, such as thrombus, dissection, or focal aneurysm. There are no strict guidelines that dictate practice. The surgeon might choose to replace the entire root or just the AV and ascending aorta, sparing the sinuses of Valsalva. The Wheat procedure is a replacement of the AV and ascending aorta, while preserving the native sinuses of Valsalva and coronary ostia. Alternatively, in the setting of a dilated or aneurysmal sinus, the surgeon may elect to replace the entire aortic root. Choice of implanting a biologic root or composite AVR is a surgical decision based on many of the factors presented in Table 13-1. Implantation of a root or composite graft necessitates reattachment of the coronary arteries, increasing the risk for postoperative bleeding and coronary ischemia.145 Despite a history of normal coronary arteries, the close scrutiny of regional wall motion after bypass is aimed at detecting newly created abnormalities of coronary blood flow. Air into the coronary circulation or compromise of coronary blood flow through the newly constructed coronary buttons may produce severe myocardial dysfunction and SWMAs within the specific coronary vascular distribution. Kinking of the coronaries or stenosis at the ostia often produce transmural ischemia that is not subtle. Enlargement of the sinus segment may facilitate coronary anastomoses. A prosthetic graft with an enlarged area to mimic the sinus of Valsalva has been developed. Enlargement of the sinus segment may facilitate coronary anastomoses and, thus, may decrease the risk for such complications, but this remains to be proved.
No consensus guidelines exist on the specific dimensions of the aortic root beyond which a root replacement is deemed necessary. However, distortion of the root producing severe AR or disruption in its integrity requires surgical attention. The echocardiographer reports the size of the aortic annulus, sinus of Valsalva, sinotubular junction, and ascending aorta (see Figure 13-11). These measurements are obtained using the AV long-axis view. The short- and longitudinal-axis views from TEE can define the severity and mechanism of AR, as well as the success of AV repair. Patients scheduled for aorta surgery with an intrinsically normal AV with AR caused by a correctable aortic lesion (incomplete leaflet closure, leaflet prolapse, or dissection flap prolapse) can undergo AV repair.104,146 In contrast, valvular pathology that includes connective tissue degeneration (Marfan or bicuspid valve), aortitis, or severe calcification would most likely require valve replacement. Surgical management is dependent on the skill set of the surgeon and individual patient factors.
Acute Aortic Syndromes
Framing
The unstable patient with suspected acute aortic disease or injury is often the most challenging of TEE cases. Few more crucially important decisions are posed to the intraoperative echocardiographer than to quickly and accurately diagnose the nature and extent of acute aortic injury. Hypotension and respiratory distress may prevent a complete and comprehensive evaluation before surgery (Figure 13-18). History often is unobtainable. The echocardiographer becomes a detective. Clues are quickly gathered from the available clinical presentation, history, and associated physical findings. The TEE is often the only modality used to establish the diagnosis and define the surgical plan.
CASE STUDY: “WHAT INCISION DO I NEED TO MAKE? HURRY PLEASE, THE PATIENT IS DYING!”
The sensitivity and specificity of TEE to detect and diagnose injury or disease of the thoracic aorta are significantly better than the sensitivity and specificity of TTE, and are comparable with CT scan and MRI.147,148 TEE provides information regarding cardiac performance and the presence of other critically important sequelae that may be important in determining the approach and timing for surgical intervention. Hence TEE is indicated even if MRI or CT scanning has confirmed the diagnosis.
Data Collection
Because the diagnosis and cause for instability are not established, the entire mediastinum, including the left pleural space, is interrogated before definitive therapy is initiated. Rarely is there not enough time to do a complete TEE examination. The operative team can often proceed with confidence in the management of these critically ill patients with only TEE to guide the treatment. The primary event in aortic dissection is a tear and separation of the aortic intima. It is uncertain whether the inciting event is a primary rupture of the intima with secondary dissection of the media or hemorrhage within the media and subsequent rupture of the overlying intima. Systolic ejection forces blood into the aortic media through a tear that leads to the separation of the intima from the surrounding media, creating a false lumen. Blood flow may exist in both the false and true lumens through communicating fenestrations. Aortic dissections are classified by one of two anatomic schemes (the DeBakey and Stanford classifications). Transection is diagnosed through the detection of para-aortic hematoma near the isthmus and a “step-up” in the internal media wall (see Chapter 21).
Discussion
Acute dissections (Stanford type A or DeBakey type I or type II) involving the ascending aorta or arch are considered acute surgical emergencies. In contrast, dissections confined to the descending aorta (distal to the left subclavian artery; Stanford type B or DeBakey type III) are treated medically unless the patient demonstrates proximal extension, hemorrhage, or malperfusion. From the International Registry of Acute Aortic Dissection, 73% of the 384 patients with type B dissections were managed medically; in-hospital mortality rate was 10%.149 The long-term survival rate after applying medical therapy was approximately 60% to 80% at 4 to 5 years149–152 and approximately 40% to 45% at 10 years.151,152 Survival was best in patients with noncommunicating and retrograde dissections.153 From the International Registry of Acute Aortic Dissection, in-hospital mortality rate for surgical patients was significantly greater (32%).149 The increased rate of mortality for surgically treated patients likely was influenced by selecting a cohort of patients with more advanced disease and complicated course (malperfusion, leakage, extension). The overall reported short- and long-term outcomes were similar for medically treated patients with type B dissections.152 Of 142 patients with type B aortic dissections, there was a trend toward lower mortality with medical therapy compared with surgical treatment at 1 year (15% vs. 33%). Both groups had similar survival rates at 5 and 10 years (60% and 35%).152
Endovascular stent grafting for descending thoracic aortic dissections is gaining momentum as a less invasive alternative to surgery in stable patients with type B dissections.154,155 The stent graft is positioned to cover the intimal flap and seal the entry site of the dissection, resulting in thrombosis of the false lumen. In one nonrandomized evaluation of 24 consecutive patients with a subacute or chronic thoracic type B dissection, there was no morbidity (paraplegia, stroke, embolization, malperfusion syndrome, or infection) or mortality with stent grafting.154 In contrast, surgical intervention was associated with a 33% mortality rate and a 42% incidence rate of adverse events within a 12-month period. Other studies are promising but less positive.156 In a series of 19 patients with acute dissections (15 type B and 4 type A dissections), the major morbidity rate was 21% (small-bowel and renal infarction and lower extremity ischemia) and 30-day mortality rate was 16%. However, there were no additional deaths or instances of aneurysm/aortic rupture during the subsequent 13-month follow-up period.
Ascending aortic dissections (involving the aortic root, ascending aorta, or arch) are acute surgical emergencies because of the high risk for a life-threatening complication such as AR, cardiac tamponade, myocardial infarction, rupture, and stroke. The mortality rate is as high as 1% to 2% per hour early after symptom onset.157 Neither acute myocardial ischemia nor cerebral infarction should contraindicate urgent intervention. Although patients with stroke in progress may be at increased risk for hemorrhagic cerebral infarction because of intraoperative anticoagulation, leading to hemorrhagic stroke, the authors have seen several patients who experienced dramatic neurologic recovery. Operative mortality for ascending aortic dissections at experienced centers varied from 7% to 36%, well below the greater than 50% mortality with medical therapy.150,157–164
Traumatic aortic rupture is a life-threatening vascular injury that often results in lethal hemorrhage. In a multicenter trial of 274 patients, the overall mortality rate reached 31%, with 63% of deaths attributable to aortic rupture.165 Aortic transection and rupture usually occurred at the aortic isthmus (between the left subclavian and the first intercostal arteries) and resulted from shear forces generated by unrestrained frontal collisions.166 Although aortography had been considered the gold standard for the diagnosis of transection, TEE and contrast-enhanced spiral CT and MRI are currently favored, especially for patients with renal insufficiency.167–171 Intravascular ultrasonography has been proposed as a potential diagnostic tool for the identification of limited aortic injuries.172 Traumatic aortic rupture needs to be distinguished from an aortic dissection. Imaging of a dissected aorta typically reveals true and false lumens at multiple levels. The focal aortic injury of aortic transection is quite localized and may be overlooked when performing a cursory examination. A second potential diagnostic problem is that protuberant atherosclerotic changes of the aorta may be difficult to differentiate from partial aortic tears. The thick and irregular intraluminal flap, which corresponds to disruption of both intimal and medial aortic layers, can be imaged in both the short- and long-axis planes in the vicinity of the isthmus. In the longitudinal view, the medial flap is nearly perpendicular to the aortic wall because traumatic lesions are usually confined within a few centimeters distal to the left subclavian. The formation of a localized contained rupture of the false aneurysm is common.168,173 Color-flow Doppler imaging and spectral Doppler can be used to detect turbulence associated with nonlaminar flow at the aortic defect and the presence of a pressure gradient. Traditional treatment includes immediate surgical invention using a right lateral decubitus approach and resection of the aorta with insertion of a tube graft. Deployment of endovascular stent grafts has been successful. Two series that included a total of 16 patients having aortic transection reported successful repair with no mortality or serious morbidity.174,175 However, the application of this device under such conditions poses a high risk for left subclavian malperfusion and paraplegia.176 The decision regarding appropriate management and time course of therapy will depend on the technical availability and expertise within the institution and the forthcoming results of clinical trials that use newer, less invasive technologies.
Intramural Hematoma
Framing
The presence of an IMH is a subtle finding that may be missed when evaluating the thoracic aorta for a dissection or rupture. IMH is distinct from a classic aortic dissection but has been considered by some as being a variant.177
Data Collection
Aortic IMH is often considered a variant of an aortic dissection.177 Although many of the patients may present with chest or back pain, many of the associated sequelae, such as pericardial or pleural effusions, stroke, and myocardial infarction, are absent.179–180 It is characterized by the absence of a detectable intimal tear. The hematoma probably is produced by a hemorrhage of the vaso vasorum into the aortic wall. In a meta-analysis, acute aortic IMH was most often associated with long-standing hypertension; traumatic cause was also a significant factor for 6% of cases.178 In the meta-analysis of 143 cases, 81% were diagnosed by CT, and the remaining patients by MRI, TEE, or both.178 The sensitivity and specificity of TEE to detect an IMH were reported to be 100% and 91%, respectively.180 The diagnosis is characterized by the absence of a dissecting flap or intimal disruption with specific regional thickening of the aortic wall of greater than 7 mm in a crescent or circular shape. On MRI or CT, this crescent-shaped or circular area in the aortic wall demonstrates high attenuation that does not enhance with contrast.181 Although IMHs can be detected in any portion of the aorta, the location appears to be a factor in its cause and prognosis. The ascending aorta, which was involved in 33% to 57% of cases, appeared to represent the early stage of a classic dissection,178,179,181–183 whereas traumatic IMHs typically involved the descending thoracic aorta.182
Discussion
Acute management is similar to that of a classic aortic dissection. In the meta-analysis of 143 patients with aortic IMH, lesions of the ascending aorta were associated with a lower early mortality with surgical intervention than medical treatment (14% vs. 36%); lesions of the descending aorta had a similar mortality with medical or surgical therapy (14% vs. 20%).178 The force of left ventricular systolic blood pressure, the driving force for disease progression, is reduced by the administration of β-blockers. Patients with an IMH in the descending thoracic aorta should be medically treated using β-blockers. Management of patients having IMH of the ascending aorta is controversial. A comprehensive evidence-based approach is not practical at present. Research publications have been limited to case reports, single-institutional series, multicenter registries, and meta-analyses. The experience seems to vary between the Asian centers (Japan and Korean), which have more success with medical management, and the Western centers (European and American), which have advocated a more aggressive surgical management. In a recent single-institutional study from Korea of 357 patients having acute aortic syndrome, 101 patients had IMH, 16% of whom were unstable receiving surgery and the rest were medically treated.183 Of the patients who initially received medical management, the aortic pathology progressed in 37% of patients, lending to delayed surgery or death. Prognostic factors of impending deterioration include IMH greater than 15 mm or increased aortic diameter. The difference in response to management strategies also may reflect population genetics, comorbid diseases (atherosclerosis, hypertension, environmental factors), or experience in accurately diagnosing this variant. The periaortic thickness of IMH, which can be small and subtle, may be underdiagnosed in the West.184 The risk for sudden deterioration and death related to pursuing medical management must be balanced by the potential morbidity/mortality of surgery. In a center having low surgical mortality, a surgical approach might be favored. A high-risk patient at a surgical center of lesser experience may consider aggressive medical management.
1 Kruger J., Dunning D. Unskilled and unaware of it: How difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J Person Soc Psychol. 1999;77:1121.
2 Royse A.G., Royse C.F., Ajani A.E., et al. Reduced neuropsychological dysfunction using epiaortic echocardiography and the exclusive Y graft. Ann Thorac Surg. 2000;69:1431.
3 Sylivris S., Calafiore P., Matalanis G., et al. The intraoperative assessment of ascending aortic atheroma: Epiaortic imaging is superior to both transesophageal echocardiography and direct palpation. J Cardiothorac Vasc Anesth. 1997;11:704.
4 Ura M., Sakata R., Nakayama Y., et al. Extracorporeal circulation before and after ultrasonographic evaluation of the ascending aorta. Ann Thorac Surg. 1999;67:478.
5 Russo J.E., Schoemaker P.J.H. The power of frames, winning decisions. New York: Doubleday, 2002.
6 Kallmeyer I.J., Collard C.D., Fox J.A., et al. The safety of intraoperative transesophageal echocardiography: A case series of 7200 cardiac surgical patients. Anesth Analg. 2001;92:1126.
7 MacGregor D.A., Zvara D.A., Treadway R.M.Jr, et al. Late presentation of esophageal injury after transesophageal echocardiography. Anesth Analg. 2004;99:41.
8 Olenchock S.A.Jr, Lukaszczyk J.J., Reed J.III, Theman T.E. Splenic injury after intraoperative transesophageal echocardiography. Ann Thorac Surg. 2001;72:2141.
9 Pong M.W., Lin S.M., Kao S.C., et al. Unusual cause of esophageal perforation during intraoperative transesophageal echocardiography monitoring for cardiac surgery—a case report. Acta Anaesthesiol. 2003;41:155.
10 Savino J.S., Hanson C.W.III, Bigelow D.C., et al. Oropharyngeal injury after transesophageal echocardiography. J Cardiothorac Vasc Anesth. 1994;8:76.
11 Venticinque S.G., Kashyap V.S., O’Connell R.J. Chemical burn injury secondary to intraoperative transesophageal echocardiography. Anesth Analg. 2003;97:1260.
12 Silber J.H. Using outcomes analysis to assess quality of care: Applications for cardiovascular surgery, outcome measurements. In: Tuman K.J., editor. Cardiovascular medicine. Philadelphia: Lippincott Williams & Wilkins; 1999:1.
13 Practice guidelines for perioperative transesophageal echocardiography. A report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 1996;84:986.
14 Practice guidelines for perioperative transesophageal echocardiography: An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 2010;112:1084.
15 Shanewise J.S., Cheung A.T., Aronson S., et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: Recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. Anesth Analg. 1999;89:870.
16 Battler A., Froelicher V.F., Gallagher K.P., et al. Dissociation between regional myocardial dysfunction and ECG changes during ischemia in the conscious dog. Circulation. 1980;62:735.
17 Foster E., O’Kelly B., LaPidus A., et al. Segmental analysis of resting echocardiographic function and stress scintigraphic perfusion: Implications for myocardial viability. Am Heart J. 1995;129:7.
18 Jacklin P.B., Barrington S.F., Roxburgh J.C., et al. Cost-effectiveness of preoperative positron emission tomography in ischemic heart disease. Ann Thorac Surg. 2002;73:1403.
19 Landoni C., Lucignani G., Paolini G., et al. Assessment of CABG-related risk in patients with CAD and LVD. Contribution of PET with [18F]FDG to the assessment of myocardial viability. J Cardiovasc Surg (Torino). 1999;40:363.
20 Kozman H., Cook J.R., Wiseman A.H., et al. Presence of angiographic coronary collaterals predicts myocardial recovery after coronary bypass surgery in patients with severe left ventricular dysfunction. Circulation. 1998;98:II-57.
21 Premaratne S., Razzuk A., Koduru S., et al. Incidence of postinfarction aneurysm within one month of infarct: Experience with 16 patients in Hawaii. J Cardiovasc Surg. 1999;40:473.
22 Redfield M.M., Jacobsen S.J., Burnett J.C.J., et al. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194.
23 Nollert G., Wich S. Planning a cardiovascular hybrid operating room: The technical point of view. Heart Surg Forum. 2009;12:E125.
24 Grigioni F., Enriquez-Sarano M., Zehr K.J., et al. Ischemic mitral regurgitation: Long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759.
25 Feinberg M.S., Schwammenthal E., Shlizerman L., et al. Prognostic significance of mild mitral regurgitation by color Doppler echocardiography in acute myocardial infarction. Am J Cardiol. 2000;86:903.
26 Grossi E.A., Goldberg J.D., LaPietra A., et al. Ischemic mitral valve reconstruction and replacement: Comparison of long-term survival and complications. J Thorac Cardiovasc Surg. 2001;122:1107.
27 Bach D.S., Deeb G.M., Bolling S.F. Accuracy of intraoperative transesophageal echocardiography for estimating the severity of functional mitral regurgitation. Am J Cardiol. 1995;76:508.
28 Choi H., Lee K., Lee H., et al. Quantification of mitral regurgitation using proximal isovelocity surface area method in dogs. J Vet Sci. 2004;5:163.
29 Grewal K.S., Malkowski M.J., Piracha A.R., et al. Effect of general anesthesia on the severity of mitral regurgitation by transesophageal echocardiography. Am J Cardiol. 2000;85:199.
30 Gillinov A.M., Wierup P.N., Blackstone E.H., et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2001;122:1125.
31 Yiu S.F., Enriquez-Sarano M., Tribouilloy C., et al. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation. 2000;102:1400.
32 Gorman J.H.III, Gorman R.C., Jackson B.M., et al. Annuloplasty ring selection for chronic ischemic mitral regurgitation: Lessons from the ovine model. Ann Thorac Surg. 2003;76:1556.
33 Guy T.S., Moainie S.L., Gorman J.H.III, et al. Prevention of ischemic mitral regurgitation does not influence the outcome of remodeling after posterolateral myocardial infarction. J Am Coll Cardiol. 2004;43:377.
34 Miller D.C. Ischemic mitral regurgitation redux—to repair or to replace? J Thorac Cardiovasc Surg. 2001;122:1059.
35 Inoue M., McCarthy P.M., Popovic Z.B., et al. Mitral valve repair without cardiopulmonary bypass or atriotomy using the Coapsys device: Device design and implantation procedure in canine functional mitral regurgitation model. Heart Surg Forum. 2004;7:E117.
36 Fukamachi K., Popovic Z.B., Inoue M., et al. Changes in mitral annular and left ventricular dimensions and left ventricular pressure-volume relations after off-pump treatment of mitral regurgitation with the Coapsys device. Eur J Cardiothorac Surg. 2004;25:352.
37 Fann J.I., St Goar F.G., Komtebedde J., et al. Beating heart catheter-based edge-to-edge mitral valve procedure in a porcine model: Efficacy and healing response. Circulation. 2004;110:988.
38 Condado J.A., Velez-Gimon M. Catheter-based approach to mitral regurgitation. J Interv Cardiol. 2003;16:523.
39 Maslow A.D., Regan M.M., Haering J.M., et al. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease. J Am Coll Cardiol. 1999;34:2096.
40 Hellemans I.M., Pieper E.G., Ravelli A.C., et al. Prediction of surgical strategy in mitral valve regurgitation based on echocardiography. Interuniversity Cardiology Institute of the Netherlands. Am J Cardiol. 1997;79:334.
41 Lee E.M., Shapiro L.M., Wells F.C. Superiority of mitral valve repair in surgery for degenerative mitral regurgitation. Eur Heart J. 1997;18:655.
42 Rozich J.D., Carabello B.A., Usher B.W., et al. Mitral valve replacement with and without chordal preservation in patients with chronic mitral regurgitation. Mechanisms for differences in postoperative ejection performance. Circulation. 1992;86:1718.
43 Charls L.M. SAM—systolic anterior motion of the anterior mitral valve leaflet postsurgical mitral valve repair. Heart Lung. 2003;32:402.
44 Brown M.L., Abel M.D., Click R.L., et al. Systolic anterior motion after mitral valve repair: Is surgical intervention necessary? J Thorac Cardiovasc Surg. 2007;133:136.
45 Movsowitz C., Podolsky L.A., Meyerowitz C.B., et al. Patent foramen ovale: A nonfunctional embryological remnant or a potential cause of significant pathology? J Am Soc Echocardiogr. 1992;5:259.
46 Langholz D., Louie E.K., Konstadt S.N., et al. Transesophageal echocardiographic demonstration of distinct mechanisms for right to left shunting across a patent foramen ovale in the absence of pulmonary hypertension. J Am Coll Cardiol. 1991;18:1112.
47 Augoustides J.G., Weiss S.J., Weiner J., et al. Diagnosis of patent foramen ovale with multiplane transesophageal echocardiography in adult cardiac surgical patients. J Cardiothorac Vasc Anesth. 2004;18:725.
48 Attie F., Rosas M., Granados N., et al. Surgical treatment for secundum atrial septal defects in patients >40 years old. A randomized clinical trial. J Am Coll Cardiol. 2001;38:2035.
49 Ferreira S.M., Ho S.Y., Anderson R.H. Morphological study of defects of the atrial septum within the oval fossa: Implications for transcatheter closure of left-to-right shunt. Br Heart J. 1992;67:316.
50 Mugge A., Daniel W.G., Angermann C., et al. Atrial septal aneurysm in adult patients. A multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91:2785.
51 Belkin R.N., Kisslo J. Atrial septal aneurysm: Recognition and clinical relevance. Am Heart J. 1990;120:948.
52 Cabanes L., Mas J.L., Cohen A., et al. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke. 1993;24:1865.
53 Mattioli A.V., Aquilina M., Oldani A., et al. Atrial septal aneurysm as a cardioembolic source in adult patients with stroke and normal carotid arteries. A multicentre study. Eur Heart J. 2001;22:261.
54 Agmon Y., Khandheria B.K., Meissner I., et al. Frequency of atrial septal aneurysms in patients with cerebral ischemic events. Circulation. 1999;99:1942.
55 Pearson A.C., Nagelhout D., Castello R., et al. Atrial septal aneurysm and stroke: A transesophageal echocardiographic study. J Am Coll Cardiol. 1991;18:1223.
56 Lechat P., Mas J.L., Lascault G., et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148.
57 Burger A.J., Sherman H.B., Charlamb M.J. Low incidence of embolic strokes with atrial septal aneurysms: A prospective, long-term study. Am Heart J. 2000;139:149.
58 Overell J.R., Bone I., Lees K.R. Interatrial septal abnormalities and stroke: A meta-analysis of case-control studies. Neurology. 2000;55:1172.
59. Orgera M.A., O’Malley P.G., Taylor A.J. Secondary prevention of cerebral ischemia in patent foramen ovale: Systematic review and meta-analysis,. South Med J. 2001;94:699.
60 Sukernik M.R., Goswami S., Frumento R.J., et al. National survey regarding the management of an intraoperatively diagnosed patent foramen ovale during coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2005;19:150.
61 Kollar A., Reames M.K., Coyle J.P. Patent foramen ovale and pulmonary embolism. An underestimated co-morbidity following cardiac surgery. J Cardiovasc Surg (Torino). 1998;39:355.
62 Yasu T., Fujii M., Saito M. Long-term follow-up of an interatrial right-to-left shunt that appeared after cardiac surgery—evaluation by transesophageal Doppler echocardiography. Jpn Circ J. 1996;60:70.
63 Akhter M., Lajos T.Z. Pitfalls of undetected patent foramen ovale in off-pump cases. Ann Thorac Surg. 1999;67:546.
64 Stoddard M.F., Arce J., Liddell N.E., et al. Two-dimensional transesophageal echocardiographic determination of aortic valve area in adults with aortic stenosis. Am Heart J. 1991;122:1415.
65 Smith M.D., Harrison M.R., Pinton R., et al. Regurgitant jet size by transesophageal compared with transthoracic Doppler color flow imaging. Circulation. 1991;83:79.
66 Fenoglio J.J.Jr, McAllister H.A.Jr, DeCastro C.M., et al. Congenital bicuspid aortic valve after age 20. Am J Cardiol. 1977;39:164.
67 Osler W. The bicuspid condition of the aortic valves. Trans Assoc Am Physicians. 1886;1:185.
68 Grant R.T., Wood J.E.Jr, Jones T.D. Heart valve irregularities in relation to subacute bacterial endocarditis. Heart. 1928;14:247.
69 Keith J.D. Bicuspid aortic valve. In: Keith J.D., Rowe R.D., Vlad P., editors. Heart disease in infancy and childhood. London: MacMillan; 1978:728.
70 Roberts W.C. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. 1970;26:72.
71 Larson E.W., Edwards W.D. Risk factors for aortic dissection: A necropsy study of 161 cases. Am J Cardiol. 1984;53:849.
72 Novaro G.M., Tiong I.Y., Pearce G.L., et al. Features and predictors of ascending aortic dilatation in association with a congenital bicuspid aortic valve. Am J Cardiol. 2003;92:99.
73 Borger M.A., Preston M., Ivanov J., et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004;128:677.
74 Olearchyk A.S. Congenital bicuspid aortic valve and an aneurysm of the ascending aorta. J Card Surg. 2004;19:462.
75 Olearchyk A.S. Congenital bicuspid aortic valve disease with an aneurysm of the ascending aorta in adults: Vertical reduction aortoplasty with distal external synthetic wrapping. J Card Surg. 2004;19:144.
76 Fedak P.W., de Sa M.P., Verma S., et al. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: Implications for aortic dilatation. J Thorac Cardiovasc Surg. 2003;126:797.
77 Ferencik M., Pape L.A. Changes in size of ascending aorta and aortic valve function with time in patients with congenitally bicuspid aortic valves. Am J Cardiol. 2003;92:43.
78 Borger M.A., Preston M., Ivanov J., et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004;128:677.
79 Cosgrove D.M., Rosenkranz E.R., Hendren W.G., et al. Valvuloplasty for aortic insufficiency. J Thorac Cardiovasc Surg. 1991;102:571.
80 Haydar H.S., He G.W., Hovaguimian H., et al. Valve repair for aortic insufficiency: Surgical classification and techniques. Eur J Cardiothorac Surg. 1997;11:258.
81 Moidl R., Moritz A., Simon P., et al. Echocardiographic results after repair of incompetent bicuspid aortic valves. Ann Thorac Surg. 1995;60:669.
82 Casselman F.P., Gillinov A.M., Akhrass R., et al. Intermediate-term durability of bicuspid aortic valve repair for prolapsing leaflet. Eur J Cardiothorac Surg. 1999;15:302.
83 Rosenhek R., Binder T., Porenta G., et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611.
84 Rajamannan N.M., Subramaniam M., Springett M., et al. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660.
85 Bellamy M.F., Pellikka P.A., Klarich K.W., et al. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002;40:1723.
86 Smith W.T., Ferguson T.B.Jr, Ryan T., et al. Should coronary artery bypass graft surgery patients with mild or moderate aortic stenosis undergo concomitant aortic valve replacement? A decision analysis approach to the surgical dilemma. J Am Coll Cardiol. 2004;44:1241.
87 Rahimtoola S.H. “Prophylactic” valve replacement for mild aortic valve disease at time of surgery for other cardiovascular disease? J Am Coll Cardiol. 1999;33:2009.
88 Pereira J.J., Lauer M.S., Bashir M., et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol. 2002;39:1356.
89 Brogan W.C.III, Grayburn P.A., Lange R.A., Hillis L.D. Prognosis after valve replacement in patients with severe aortic stenosis and a low transvalvular pressure gradient. J Am Coll Cardiol. 1993;21:1657.
90 Connolly H.M., Oh J.K., Schaff H.V., et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: Result of aortic valve replacement in 52 patients. Circulation. 2000;101:1940.
91 Ford L.E., Feldman T., Chiu Y.C., Carroll J.D. Hemodynamic resistance as a measure of functional impairment in aortic valvular stenosis. Circ Res. 1990;66:1.
92 deFilippi C.R., Willett D.L., Brickner M.E., et al. Usefulness of dobutamine echocardiography in distinguishing severe from nonsevere valvular aortic stenosis in patients with depressed left ventricular function and low transvalvular gradients. Am J Cardiol. 1995;75:191.
93 Bermejo J., Garcia-Fernandez M.A., Torrecilla E.G., et al. Effects of dobutamine on Doppler echocardiographic indexes of aortic stenosis. J Am Coll Cardiol. 1996;28:1206.
94 Lin S.S., Roger V.L., Pascoe R., et al. Dobutamine stress Doppler hemodynamics in patients with aortic stenosis: Feasibility, safety, and surgical correlations. Am Heart J. 1998;136:1010.
95 Cheitlin M.D., Armstrong W.F., Aurigemma G.P., et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography—summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Coll Cardiol. 2003;42:954.
96 Nishimura R.A., Grantham J.A., Connolly H.M., et al. Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: The clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation. 2002;106:809.
97 Monin J.L., Quere J.P., Monchi M., et al. Low-gradient aortic stenosis: Operative risk stratification and predictors for long-term outcome: A multicenter study using dobutamine stress hemodynamics. Circulation. 2003;108:319.
98 ACC/AHA guidelines for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association. Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). J Am Coll Cardiol. 1998;32:1486.
99 Lung B., Gohlke-Barwolf C., Tornos P., et al. Recommendations on the management of the asymptomatic patient with valvular heart disease. Eur Heart J. 2002;23:1252.
100 Bonow R.O., Rosing D.R., McIntosh C.L., et al. The natural history of asymptomatic patients with aortic regurgitation and normal left ventricular function. Circulation. 1983;68:509.
101 Bonow R.O., Lakatos E., Maron B.J., Epstein S.E. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation. 1991;84:1625.
102 Henry W.L., Bonow R.O., Rosing D.R., Epstein S.E. Observations on the optimum time for operative intervention for aortic regurgitation. II. Serial echocardiographic evaluation of asymptomatic patients. Circulation. 1980;61:484.
103 Nash P.J., Vitvitsky E., Cosgrove D.M., et al. Echocardiographic predictors of aortic valve repair in patients with bicuspid valves and aortic regurgitation. J Am Coll Cardiol. 2003;41:516.
104 Yacoub M.H., Cohn L.H. Novel approaches to cardiac valve repair: From structure to function: Part II. Circulation. 2004;109:1064.
105 Carr J.A., Savage E.B. Aortic valve repair for aortic insufficiency in adults: A contemporary review and comparison with replacement techniques. Eur J Cardiothorac Surg. 2004;25:6.
106 Rahimtoola S.H. Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol. 2003;41:893.
107 Girard S.E., Miller F.A.Jr, Orszulak T.A., et al. Reoperation for prosthetic aortic valve obstruction in the era of echocardiography: Trends in diagnostic testing and comparison with surgical findings. J Am Coll Cardiol. 2001;37:579.
108 Rao V., Jamieson W.R., Ivanov J., et al. Prosthesis-patient mismatch affects survival after aortic valve replacement. Circulation. 2000;102:III-5.
109 He G.W., Grunkemeier G.L., Gately H.L., et al. Up to thirty-year survival after aortic valve replacement in the small aortic root. Ann Thorac Surg. 1995;59:1056.
110 Yazdanbakhsh A.P., van den Brink R.B., Dekker E., de Mol B.A. Small valve area index: Its influence on early mortality after mitral valve replacement. Eur J Cardiothorac Surg. 2000;17:222.
111 Barner H.B., Labovitz A.J., Fiore A.C. Prosthetic valves for the small aortic root. J Card Surg. 1994;9:154.
112 Gonzalez-Juanatey J.R., Garcia-Acuna J.M., Vega F.M., et al. Influence of the size of aortic valve prostheses on hemodynamics and change in left ventricular mass: Implications for the surgical management of aortic stenosis. J Thorac Cardiovasc Surg. 1996;112:273.
113 Pibarot P., Dumesnil J.G. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol. 2000;36:1131.
114 Come P.C., Riley M.F., Ferguson J.F., et al. Prediction of severity of aortic stenosis: Accuracy of multiple noninvasive parameters. Am J Med. 1988;85:29.
115 Palta S., Gill K.S., Pai R.G. Role of inadequate adaptive left ventricular hypertrophy in the genesis of mitral regurgitation in patients with severe aortic stenosis: Implications for its prevention. J Heart Valve Dis. 2003;12:601.
116 Brener S.J., Duffy C.I., Thomas J.D., Stewart W.J. Progression of aortic stenosis in 394 patients: Relation to changes in myocardial and mitral valve dysfunction. J Am Coll Cardiol. 1995;25:305.
117 Absil B., Dagenais F., Mathieu P., et al. Does moderate mitral regurgitation impact early or mid-term clinical outcome in patients undergoing isolated aortic valve replacement for aortic stenosis? Eur J Cardiothorac Surg. 2003;24:217.
118 Christenson J.T., Jordan B., Bloch A., Schmuziger M. Should a regurgitant mitral valve be replaced simultaneously with a stenotic aortic valve? Tex Heart Inst J. 2000;27:350.
119 Goland S., Loutaty G., Arditi A., et al. Improvement in mitral regurgitation after aortic valve replacement. Isr Med Assoc J. 2003;5:12.
120 Harris K.M., Malenka D.J., Haney M.F., et al. Improvement in mitral regurgitation after aortic valve replacement. Am J Cardiol. 1997;80:741.
121 Moazami N., Diodato M.D., Moon M.R., et al. Does functional mitral regurgitation improve with isolated aortic valve replacement? J Card Surg. 2004;19:444.
122 Tunick P.A., Gindea A., Kronzon I. Effect of aortic valve replacement for aortic stenosis on severity of mitral regurgitation. Am J Cardiol. 1990;65:1219.
123 Tassan-Mangina S., Metz D., Nazeyllas P., et al. Factors determining early improvement in mitral regurgitation after aortic valve replacement for aortic valve stenosis: A transthoracic and transesophageal prospective study. Clin Cardiol. 2003;26:127.
124 Ruel M., Kapila V., Price J., et al. Natural history and predictors of outcome in patients with concomitant functional mitral regurgitation at the time of aortic valve replacement. Circulation. 2006;114(Suppl 1):I541.
125 Amarenco P., Cohen A., Tzourio C., et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474.
126 Davila-Roman V.G., Barzilai B., Wareing T.H., et al. Atherosclerosis of the ascending aorta. Prevalence and role as an independent predictor of cerebrovascular events in cardiac patients. Stroke. 1994;25:2010.
127 Hartman G.S., Yao F.S., Bruefach M.III, et al. Severity of aortic atheromatous disease diagnosed by transesophageal echocardiography predicts stroke and other outcomes associated with coronary artery surgery: A prospective study. Anesth Analg. 1996;83:701.
128 Konstadt S.N., Reich D.L., Quintana C., Levy M. The ascending aorta: How much does transesophageal echocardiography see? Anesth Analg. 1994;78:240.
129 Katz E.S., Tunick P.A., Rusinek H., et al. Protruding aortic atheromas predict stroke in elderly patients undergoing cardiopulmonary bypass: Experience with intraoperative transesophageal echocardiography. J Am Coll Cardiol. 1992;20:70.
130 Marshall W.G.Jr, Barzilai B., Kouchoukos N.T., Saffitz J. Intraoperative ultrasonic imaging of the ascending aorta. Ann Thorac Surg. 1989;48:339.
131 Hosoda Y., Watanabe M., Hirooka Y., et al. Significance of atherosclerotic changes of the ascending aorta during coronary bypass surgery with intraoperative detection by echography. J Cardiovasc Surg (Torino). 1991;32:301.
132 Ohteki H., Itoh T., Natsuaki M., et al. Intraoperative ultrasonic imaging of the ascending aorta in ischemic heart disease. Ann Thorac Surg. 1990;50:539.
133 Davila-Roman V.G., Barzilai B., Wareing T.H., et al. Intraoperative ultrasonographic evaluation of the ascending aorta in 100 consecutive patients undergoing cardiac surgery. Circulation. 1991;84:III-47.
134 Hammon J.W.Jr, Stump D.A., Kon N.D., et al. Risk factors and solutions for the development of neurobehavioral changes after coronary artery bypass grafting. Ann Thorac Surg. 1997;63:1613.
135 Wilson M.J., Boyd S.Y., Lisagor P.G., et al. Ascending aortic atheroma assessed intraoperatively by epiaortic and transesophageal echocardiography. Ann Thorac Surg. 2000;70:25.
136 Konstadt S.N., Reich D.L., Kahn R., Viggiani R.F. Transesophageal echocardiography can be used to screen for ascending aortic atherosclerosis. Anesth Analg. 1995;81:225.
137 Eltzschig H.K., Kallmeyer I.J., Mihaljevic T., et al. A practical approach to a comprehensive epicardial and epiaortic echocardiographic examination. J Cardiothorac Vasc Anesth. 2003;17:422.
138 Chandraratna A., Ashmeg A., Pasha H.C. Detection of intracoronary air embolism by echocardiography. J Am Soc Echocardiogr. 2002;15:1015.
139 Baker R.C., Graham A.N., Phillips A.S., Campalani G. An unusual iatrogenic cause of right coronary air embolism. Ann Thorac Surg. 1999;68:575.
140 Ohnishi Y., Uchida O., Hayashi Y., et al. [Relationship between retained microbubbles and neuropsychologic alterations after cardiac operation]. Masui. 1995;44:1327.
141 Bickerstaff L.K., Pairolero P.C., Hollier L.H., et al. Thoracic aortic aneurysms: A population-based study. Surgery. 1982;92:1103.
142 Pressler V., McNamara J.J. Thoracic aortic aneurysm: Natural history and treatment. J Thorac Cardiovasc Surg. 1980;79:489.
143 Crawford E.S., DeNatale R.W. Thoracoabdominal aortic aneurysm: Observations regarding the natural course of the disease. J Vasc Surg. 1986;3:578.
144 Clouse W.D., Hallett J.W.Jr, Schaff H.V., et al. Improved prognosis of thoracic aortic aneurysms: A population-based study. JAMA. 1998;280:1926.
145 Sioris T., David T.E., Ivanov J., et al. Clinical outcomes after separate and composite replacement of the aortic valve and ascending aorta. J Thorac Cardiovasc Surg. 2004;128:260.
146 Feindel C.M., David T.E. Aortic valve sparing operations: Basic concepts. Int J Cardiol. 2004;97:61.
147 Nienaber C.A., von Kodolitsch Y., Nicolas V., et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328:1.
148 Roudaut R.P., Billes M.A., Gosse P., et al. Accuracy of M-mode and two-dimensional echocardiography in the diagnosis of aortic dissection: An experience with 128 cases. Clin Cardiol. 1988;11:553.
149 Suzuki T., Mehta R.H., Ince H., et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: Lessons from the International Registry of Aortic Dissection (IRAD). Circulation. 2003;108(Suppl II):II-312.
150 Doroghazi R.M., Slater E.E., DeSanctis R.W., et al. Long-term survival of patients with treated aortic dissection. J Am Coll Cardiol. 1984;3:1026.
151 Bernard Y., Zimmermann H., Chocron S., et al. False lumen patency as a predictor of late outcome in aortic dissection. Am J Cardiol. 2001;87:1378.
152 Umana J.P., Lai D.T., Mitchell R.S., et al. Is medical therapy still the optimal treatment strategy for patients with acute type B aortic dissections? J Thorac Cardiovasc Surg. 2002;124:896.
153 Erbel R., Oelert H., Meyer J., et al. Effect of medical and surgical therapy on aortic dissection evaluated by transesophageal echocardiography. Implications for prognosis and therapy. The European Cooperative Study Group on Echocardiography. Circulation. 1993;87:1604.
154 Nienaber C.A., Fattori R., Lund G., et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539.
155 Nienaber C.A., Eagle K.A. Aortic dissection: New frontiers in diagnosis and management: Part II: Therapeutic management and follow-up. Circulation. 2003;108:772.
156 Dake M.D., Kato N., Mitchell R.S., et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340:1546.
157 Nienaber C.A., Eagle K.A. Aortic dissection: New frontiers in diagnosis and management: Part I: From etiology to diagnostic strategies. Circulation. 2003;108:628.
158 Mehta R.H., Suzuki T., Hagan P.G., et al. Predicting death in patients with acute type A aortic dissection. Circulation. 2002;105:200.
159 Miller D.C., Mitchell R.S., Oyer P.E., et al. Independent determinants of operative mortality for patients with aortic dissections. Circulation. 1984;70:I-153.
160 Haverich A., Miller D.C., Scott W.C., et al. Acute and chronic aortic dissections—determinants of long-term outcome for operative survivors. Circulation. 1985;72:II-22.
161 Pansini S., Gagliardotto P.V., Pompei E., et al. Early and late risk factors in surgical treatment of acute type A aortic dissection. Ann Thorac Surg. 1998;66:779.
162 Sabik J.F., Lytle B.W., Blackstone E.H., et al. Long-term effectiveness of operations for ascending aortic dissections. J Thorac Cardiovasc Surg. 2000;119:946.
163 Kawahito K., Adachi H., Yamaguchi A., Ino T. Preoperative risk factors for hospital mortality in acute type A aortic dissection. Ann Thorac Surg. 2001;71:1239.
164 Lai D.T., Robbins R.C., Mitchell R.S., et al. Does profound hypothermic circulatory arrest improve survival in patients with acute type A aortic dissection? Circulation. 2002;106:I-218.
165 Fabian T.C., Richardson J.D., Croce M.A., et al. Prospective study of blunt aortic injury: Multicenter Trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42:374.
166 Fisher R.G., Hadlock F. Laceration of the thoracic aorta and brachiocephalic arteries by blunt trauma. Report of 54 cases and review of the literature. Radiol Clin North Am. 1981;19:91.
167 Ben Menachem Y. Assessment of blunt aortic-brachiocephalic trauma: Should angiography be supplanted by transesophageal echocardiography? J Trauma. 1997;42:969.
168 Vignon P., Lang R.M. Use of transesophageal echocardiography for the assessment of traumatic aortic injuries. Echocardiography. 1999;16:207.
169 Goarin J.P., Cluzel P., Gosgnach M., et al. Evaluation of transesophageal echocardiography for diagnosis of traumatic aortic injury. Anesthesiology. 2000;93:1373.
170 Vignon P., Boncoeur M.P., Francois B., et al. Comparison of multiplane transesophageal echocardiography and contrast-enhanced helical CT in the diagnosis of blunt traumatic cardiovascular injuries. Anesthesiology. 2001;94:615.
171 Patel N.H., Stephens K.E.Jr, Mirvis S.E., et al. Imaging of acute thoracic aortic injury due to blunt trauma: A review. Radiology. 1998;209:335.
172 Malhotra A.K., Fabian T.C., Croce M.A., et al. Minimal aortic injury: A lesion associated with advancing diagnostic techniques. J Trauma. 2001;51:1042.
173 Goarin J.P., Catoire P., Jacquens Y., et al. Use of transesophageal echocardiography for diagnosis of traumatic aortic injury. Chest. 1997;112:71.
174 Ott M.C., Stewart T.C., Lawlor D.K., et al. Management of blunt thoracic aortic injuries: Endovascular stents versus open repair. J Trauma. 2004;56:565.
175 Scheinert D., Krankenberg H., Schmidt A., et al. Endoluminal stent-graft placement for acute rupture of the descending thoracic aorta. Eur Heart J. 2004;25:694.
176 Lorenzen H.P., Geist V., Hartmann F., et al. Endovascular stent-graft implantation in acute traumatic aortic dissection with contained rupture and hemorrhagic shock. Z Kardiol. 2004;93:317.
177 Svensson L.G., Labib S.B., Eisenhauer A.C., Butterly J.R. Intimal tear without hematoma: An important variant of aortic dissection that can elude current imaging techniques. Circulation. 1999;99:1331.
178 Maraj R., Rerkpattanapipat P., Jacobs L.E., et al. Meta-analysis of 143 reported cases of aortic intramural hematoma. Am J Cardiol. 2000;86:664.
179 Moizumi Y., Komatsu T., Motoyoshi N., Tabayashi K. Clinical features and long-term outcome of type A and type B intramural hematoma of the aorta. J Thorac Cardiovasc Surg. 2004;127:421.
180 Kang D.H., Song J.K., Song M.G., et al. Clinical and echocardiographic outcomes of aortic intramural hemorrhage compared with acute aortic dissection. Am J Cardiol. 1998;81:202.
181 Song J.K., Kim H.S., Song J.M., et al. Outcomes of medically treated patients with aortic intramural hematoma. Am J Med. 2002;113:181.
182 Vilacosta I., San Roman J.A., Ferreiros J., et al. Natural history and serial morphology of aortic intramural hematoma: A novel variant of aortic dissection. Am Heart J. 1997;134:495.
183 Song J.K., Yim J.H., Ahn J.M., et al. Outcomes of patients with acute type A aortic intramural hematoma. Circulation. 2009;120:2046.
184 Evangelista A., Eagle K.A. Is the optimal management of acute type A aortic intramural hematoma evolving? Circulation. 2009;120:2029.