CHAPTER 55 Cyberknife® Radiosurgical Ablation of Meningiomas
INTRODUCTION
Meningiomas are benign tumors of the central nervous system with an annual incidence of 2.3 to 4 in 100,000.1,2 The term was initially coined by Harvey Cushing in 1922 to describe a tumor originating from the meningeal covering of the brain and spinal cord.3 Intracranial meningiomas account for approximately 90% of these tumors and outnumber spinal meningiomas by 10-fold. Brain tumor series indicate meningiomas to be 20% of all intracranial tumors (second only behind primary glial neoplasms), and autopsy studies estimate an incidence of 30%.4 These neoplasms have also been described in the orbit, paranasal sinuses, the skin and subcutis, lung, mediastinum, and adrenal glands.5 Meningiomas originate from arachnoid cap cells, are often found in association with arachnoid villi at the dural venous sinus and veins, and commonly attach to the dural covering of the brain and spinal cord.4
The World Health Organization (WHO) classifies meningiomas into grade I (benign), grade II (atypical), and grade III (anaplastic), which account for 80%, 5% to 20%, and 1% to 2% of all meningiomas respectively.5,6 In large clinical series there is a strong association between outcome and grade. Patients with WHO grade I meningiomas have a greater than 80% chance of being progression free at 10 years, while only 40% to 60% of WHO grade II patients are progression free at 10 years. The median recurrence-free rate of patients with anaplastic meningioma is 2 years.7
Meningiomas often express hormonal receptors, which may explain the increased prevalence of meningiomas in females where the overall ratio is 2:1 in the brain and up to 10:1 in the spine.8 The best established of these receptors is the progesterone receptor, which is found in greater than two thirds of these tumors. In addition, greater than 30% of meningiomas also express estrogen receptors and approximately 40% express androgen receptors. Moreover, patients with meningiomas have been found to have a higher incidence of breast cancer and breast cancer patients have a higher incidence of meningiomas.9 The most frequent genetic alteration seen in meningiomas is the loss of the neurofibromatosis 2 gene (NF2) on chromosome 22q which encodes a tumor suppressor called merlin (also known as schwannomin). Merlin is related to molecules of the Protein 4.1 superfamily that are involved with cell growth and regulation.5,8
Meningiomas are slow growing and are often incidentally found on imaging. If symptomatic, they can present with headaches, seizures, mental status changes, or focal neurologic deficits depending on the location of the lesion. On magnetic resonance imaging (MRI), meningiomas classically present as an extra-axial mass that is hypointense on T1-weighted images, hyperintense on T2-weighted imaging, and uniformly contrast enhance with gadolinium. Other characteristic findings include an enhancing dural tail, cerebrospinal fluid/vascular cleft, and hyperostosis of nearby bone. However, 10% to 15% of meningiomas exhibit atypical MRI findings that can mimic brain metastases or primary glial tumors.10
TREATMENT: OVERVIEW
Gross total resection has traditionally been considered the gold standard treatment for meningiomas. Radiation historically was reserved for poor surgical candidates, subtotal resection, unresectable tumors, or high-grade and recurrent tumors. Chemotherapy has not been shown to be effective. A recent phase II study evaluating temozolomide for treatment of recurrent meningioma showed no benefit.11 Targeted therapies against the hormonal receptors have been disappointing,12 but other adjuvant therapies such as hydroxyurea and other targeted therapies are currently under evaluation.13
Simpson was the first to publish the prognostic importance of complete surgical resection and created a scale linking extent of resection and its relationship to risk of recurrence.14 Since then, many authors have confirmed this link. However, even after an apparent gross total resection, three large series with extended follow-up report 7% to 12%, 20% to 25%, and 24% to 32% rates of recurrence at 5, 10, and 15 years, respectively.15–17 Four series with extended follow-up of patients with subtotal resection alone reported 37% to 47%, 55% to 63%, and greater than 70% recurrence rate after an interval of 5, 10, and more than 15 years, respectively. In addition to high recurrence rates, complete excision is associated with a range of potential risks depending on the location of the tumor. Surgical resection of cavernous sinus, foramen magnum, and petroclival meningiomas is accompanied by great risk of cranial nerve dysfunction and cerebrospinal fluid leakage, while surgery for tumors that invade or occlude the posterior sagittal sinus and torcula not infrequently lead to venous infarction. Especially for meningiomas in such locations, the development of modern radiosurgical techniques has provided an adjunct, or in some cases, an alternative therapy to surgery.
RADIATION FOR MENINGIOMAS
Goldsmith and colleagues18 demonstrated that local control rates of subtotally resected meningiomas treated with conventional external beam radiation could rival that of gross-total resection. In this study, the 5-year progression free survival (PFS) in the post-1980 era when three-dimensional computed tomography (CT) planning and conformal radiation therapy became available was 98% compared with 77% in patients treated before 1980. With doses greater than 52 Gray (Gy), the 10-year PFS rates was 93% versus 65% in patients treated with less than 52 Gy. These results with traditional radiation therapy provide an important basis for evaluating ever newer radiation delivery techniques and in particular the application of stereotactic radiosurgery (SRS).
Whether conventional linear accelerator, Gamma Knife® (GKS), or CyberKnife®-based, SRS has been increasingly utilized instead of external-beam radiotherapy for managing meningiomas. The tumors being treated with SRS are generally recurrent, partially resected, surgically inaccessible, or in patients deemed poor surgical candidates or who refuse surgery. Several authors have hypothesized that a therapeutic gain may be achieved by treating slowly proliferating tumors, such as meningiomas, with larger-sized fractions, a treatment that is possible only with radiosurgical techniques.2,19,20 Since the early 1990s many reports have described the efficacy of adjunctive or primary GKS radiosurgery. These studies demonstrate 5-year local control rates between 86% and 99%, tumor regression rates between 28% and 70%, and symptom improvement in 8% to 65%; meanwhile, complications occur in 2.5% to 13% of all cases.21 Further analysis of a subgroup of these patients by Pollock and colleagues22 looked at 7-year progression-free survival after a median follow-up of 64 months. There was no significant difference between patients treated with GKS alone (95%) or patients who underwent Simpson grade 1 resection (96%); Simpson grade 1 resection represents gross total resection of all tumor and involved dura and bone. Meanwhile, the Pollock study also showed that patients treated with GKS alone had increased tumor control at 7 years when compared to patients with Simpson grade 2 (82%) and grade 3 to 4 (34%) resections. Finally, when comparing the outcomes between standard radiation therapy and radiosurgery, Metellus and colleagues demonstrated excellent local tumor control with both, but clearly superior tumor shrinkage with radiosurgery.23 After more than two decades of experience, SRS has become a widely accepted tool for managing a broad spectrum of meningiomas in different locations.
CYBERKNIFE RADIOSURGERY
Stereotactic radiosurgery is a radiation technique delivered in one to five sessions and defined by a high degree of spatial accuracy and rapid radiation dose fall off at the periphery of the target lesions. Since Lars Leksell first conceived of radiosurgery in the 1950s,24 this procedure has advanced with technologic improvements in instrumentation, computing, and imaging. For three decades, stereotactic targeting that utilized rigid skeletal fixation was at the center of all radiosurgical systems, and especially the gold standard GKS. However, in the 1990s, a novel frameless radiosurgery system that uses the patient’s bony anatomy for image guidance was developed and termed the CyberKnife radiosurgical system. After more than a decade of improvements, this technology is now widely commercially available.
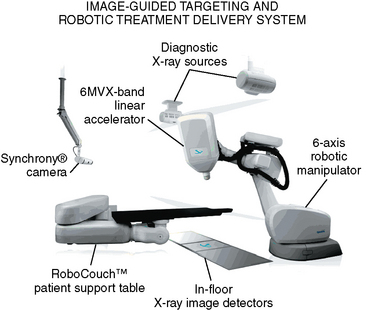
The CyberKnife is a frameless, image-guided, robotic radiosurgical instrument. Therapeutic radiation is emitted from a compact 6 MV linear accelerator (LINAC) mounted on a robotic manipulator that is able to bring in beams from greater than 1200 directions (Fig. 55-1). This allows for non-isocentric radiation planning that can optimize both dose conformality and dose homogeneity. The need for rigid fixation is circumvented by image guidance. For intracranial targets, patients are relatively immobilized with an Aquaplast mask. Flat panel X-ray detectors are mounted on either side of the treatment table and obtain orthogonal X-ray images in real time during the treatment. These images are automatically referenced to digitally reconstructed radiographs (DRRs) created during treatment planning; computer algorithms establish the patient’s position by analyzing adjacent bony anatomy and/or implanted fiducials. The image guidance software compares spatial differences in three translational and three rotational axes and minute adjustments are then made with the treatment couch and robotic manipulator. This process is continuously updated throughout the radiosurgical treatment to maintain accuracy.25
Unlike radiosurgical instruments that require rigid skeletal fixation, the CyberKnife can be used to ablate extracranial targets. For spinal targets, the X-Sight image guided targeting system utilizes nonrigid deformation modeling and hierarchical mesh tracking to maintain the spatial accuracy of target location within 0.6 mm. The X-Sight system has been shown to be accurate with system error ranging from 0.52 mm to 0.61 mm depending on testing methods and conditions.26 This image-guided frameless system also has sufficient flexibility to perform radiosurgery in either a single day or over multiple days. Such fractionation or multi-session delivery of the radiation dose can be especially important when treating lesions near radiation sensitive structures such as the spinal cord. As detailed in the text that follows, the CyberKnife affords users unique advantages over other radiosurgical methods when treating certain types of intracranial and spinal meningiomas.
SRS TOXICITY
Radiosurgical ablation of meningiomas is well tolerated, with symptomatic complication rates ranging from 2.5% to 14%.27–29 As radiosurgical techniques have improved and doses to normal anatomy have been reduced, the risk of postradiosurgical complications has decreased. Patients treated since 1991 experience less toxicity (5.3% vs. 22.9%), largely because radiosurgery prescription doses were gradually decreased from a median marginal dose of 17 Gy (range 10–20 Gy) between 1987 and 1991 to 14 Gy (range 8.9 Gy–20 Gy) between 1991 and 2000.30
Peritumoral edema after SRS has been reported in 16% of cases and is related to tumor location.31 In a study of 179 patients with meningiomas, 9.3% of the 140 patients with MRI follow-up had symptomatic imaging changes that were manifested by headaches in most, but also seizures (4/33 patients) and other neurologic deficits (3/33 patients). In their analysis, only tumor location was found to be a significant predictor for peritumoral edema. Those tumors arising from the convexity (18%), parasagittal region (40%), and falx (6.7%) developed more edema than skull-base lesions (1.3%).28 Ganz and colleagues also found that a sagittal sinus or midline location or a marginal dose greater than 18 Gy increased the risk of edema as well.32 Other studies have not found this relationship to dose. Kondziolka and colleagues reported a 16% actuarial 5-year rate of symptomatic edema in patients with parasagittal meningiomas treated with SRS; this complication rate was unrelated to dose but was influenced by the presence of pretreatment neurologic deficit.31 After conservative management, all of Kondziolka and colleagues’ patients had symptom resolution after a median of 15 months.
Cranial nerve damage, particularly optic neuropathy, is a well documented complication of all skull-base irradiation including SRS. Tischler and colleagues studied the risk of injury to cranial nerves II to VI in 62 patients (42/62 meningiomas) treated with radiosurgical doses between 10 and 40 Gy near the cavernous sinus. With a median follow-up of 19 months, 12 patients developed cranial nerve injury 3 to 41 months after treatment. Injury to cranial nerves III to VI was not related to dose. But in the four patients with optic nerve injury, there was increased risk in patients receiving greater than 8 Gy to any portion of the optic apparatus as compared to those receiving less than 8 Gy (24% vs. 0%, P = 0.009).33 Another study reported that patients treated with larger doses (10–15 Gy) had 26.7% incidence of optic neuropathy.34 Intriguingly, Adler and colleagues reported on 49 patients (27/49 meningiomas) with perioptic lesions treated with multisession fractionated radiosurgery to an average marginal dose of 20.3 Gy in two to five sessions. After a mean follow-up of 49 months,35 the visual acuity of only three patients (6%) deteriorated, but was associated with tumor progression in two of the three cases. This suggests that dose escalation near the optic region can be safely achieved by using a multisession technique. Cranial nerves III to VI appear to have a higher maximal tolerated dose with no complications reported even after doses as high as 30 Gy.34 However, the trigeminal nerve in Meckel’s cave may be more sensitive with a maximum tolerated dose of 19 Gy.36
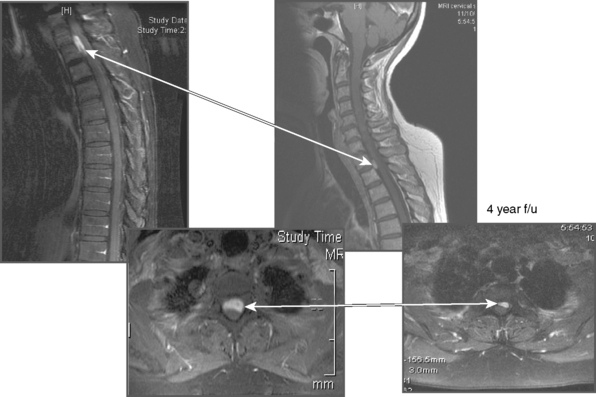
Maximal tolerable spinal radiosurgical doses are still being established. Dodd and colleagues reported on 51 patients (16/51 meningiomas) with benign, extramedullary tumors treated with CyberKnife radiosurgery after a mean follow-up of 36 months. One patient with a C7/T1 meningioma (Fig. 55-2) developed radiation myelopathy 8 months after treatment with a marginal dose of 24 Gy in three fractions and a maximum dose of 34 Gy.37 The largest published spinal radiosurgery study of any kind, which included 500 vertebral metastases in 393 patients, reported no clinical or radiographical evidence of radiation myelopathy after median follow-up of 21 months.38
Vascular complications occur in approximately 1% to 2% of patients treated with SRS.39–41 In a series of 80 patients with 30.5 months of median follow-up, there was one case of carotid occlusion 14 months after treatment with GKS. The estimated dose to the carotid was 36 Gy.42 Stafford and colleagues reported on 190 patients with meningioma (77% skull-base tumors) treated with GKS. Two patients (1%) developed symptomatic carotid stenosis 35 and 60 months after doses greater than 25 Gy to the carotid.39 Meanwhile, intratumoral bleeding has been reported in 2.3% of meningiomas treated (4/173 tumors) one to eight years after radiosurgery.41
In otherwise healthy meningioma patients with a long life-expectancy, the potential risk of secondary malignancies from radiation exposure is an important consideration when comparing surgical and radiotherapeutic options. While there have been case reports of secondary malignancies after SRS,43 a large retrospective study demonstrated no increased risk.44 However, the theoretical potential for causing a new cancer should be incorporated into the decision process.
CYBERKNIFE RADIOSURGERY FOR INTRACRANIAL MENINGIOMAS
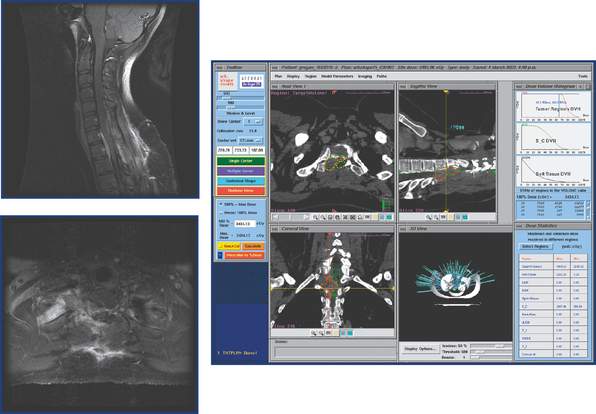
Several published series report the efficacy and safety of stereotactic radiosurgery for treatment of a broad spectrum of intracranial lesions. However, there are no published reports solely focusing on CyberKnife radiosurgical ablation of intracranial meningiomas. Therefore, we retrospectively reviewed the charts from patients treated with CyberKnife radiosurgery at Stanford University from 1999 to 2005. In that time 306 intracranial meningiomas were treated. The most common location was falcine/parasagittal (58 tumors), followed by cavernous sinus (48 tumors), and then petrous/petroclival (37 tumors). Fifty-one percent of the lesions involved the skull base. Two hundred and sixteen patients were women and 90 were men. Most of the treated lesions were either benign meningiomas or tumors treated primarily with radiosurgery and in patients in whom no definitive histologic diagnosis had been performed. Nevertheless, 8% of the meningiomas were malignant (22 atypical and 3 anaplastic). The median tumor volume ablated with SRS was 6.37 cm3. The overall tumor control rate was 94% after a median follow-up of 32.1 months (range 3–100). Of the 19 tumors that grew despite radiosurgery, 8 were atypical meningiomas and 3 were anaplastic meningiomas. Therefore, the local control rate was 97%, 64%, and 0 for benign, atypical, and anaplastic meningiomas respectively. Although the follow-up is limited, these preliminary results are comparable to other series of meningiomas treated with SRS.39,45,46
CYBERKNIFE RADIOSURGERY FOR SPINAL MENINGIOMAS
Spinal meningiomas represent 25% to 46% of tumors of the spine.47 Most arise from arachnoid cap cells embedded in dura near the nerve root sleeve, and as a result are predominantly lateral in location.48 Surgical resection of spinal meningiomas is generally safe and successful, but patients with neurofibromatosis, recurrent tumors, multiple lesions, or medical problems that place them at higher surgical risk may benefit from alternative treatments. Moreover, when spinal meningiomas lie anterior to the spinal cord, particularly in the thoracic area, meningiomas provide a significant challenge to open surgical resection. In the past, spinal meningiomas could not be treated with traditional radiosurgical devices that relied on rigid skeletal fixation. The advent of the CyberKnife made it possible for the first time to ablate these tumors with radiosurgery.
Dodd and colleagues published Stanford University’s experience of CyberKnife radiosurgery for ablation of benign intradural extramedullary spinal tumors37 (Fig. 55-3). Of the 16 patients who had spinal meningiomas, 53% presented with pain, 53% presented with radicular sensory loss, and 35% had radicular weakness. The mean radiosurgical dose was 20.31 Gy (range 16–30 Gy), which was administered to a mean tumor volume of 2.441 cm3 (range 0.136–7.569 cm3), using an average of two sessions (range 1–5). The mean follow-up interval in this cohort was 27.2 months (median 25 months). After radiosurgery, the majority of patients reported improvement in pain and strength but sensory loss was no better. However, in 30% of cases, a minor degree of worsening in pain, numbness, or subjective weakness was described after radiosurgical ablation. All but one of the patients had radiologic follow-up. None of the tumors enlarged after CyberKnife SRS, 67% remained stable, and 33% decreased in size.
Despite no change on MRI, one patient in the Dodd series had a resection of her spinal meningioma because of persistent myelopathy. The only radiosurgery-related complication occurred in a 29-year-old woman with a recurrent C7/T1 7.56 cm3 spinal meningioma, who developed posterior column dysfunction eight months after radiosurgery (see Fig. 55-2). In this case of presumed radiation myelopathy, the peripheral dose was 24 Gy, administered in three sessions, and the maximum dosage was 34.35 Gy. The dose volume histogram revealed that approximately 1.7 cm3 of the spinal cord received greater than 8 Gy per fraction.
The above experience, which came from our institution, demonstrates both the relative safety and early evidence of efficacy, for spinal radiosurgery and provides a rough framework for treating benign tumor patients going forward. Although spinal meningiomas recur at lower rates when compared to their intracranial counterparts,49 the duration of this study is limited and further follow-up is required before long-term local control is established. Nevertheless, the present study does suggest that CyberKnife stereotactic radiosurgery could someday serve as a tool in the neurosurgical armamentarium for managing selected spinal meningiomas.
CYBERKNIFE RADIOSURGERY FOR PERIOPTIC MENINGIOMAS
Surgically unresectable meningiomas that are within 2 mm of or even displacing the anterior visual pathways are most commonly managed with conventional radiotherapy. Treatment of such perioptic lesions with doses of radiation between 45 and 55 Gy using 1.8- to 2-Gy fractions results in good local control rates and 10-year local control ranges from 68% to 89%.18,50,51 Although this treatment has been shown to be safe and effective, there remain inherent limitations. Because of set-up inaccuracies, the treatment field includes a margin that results in the irradiation of normal structures such as the optic nerve, medial temporal lobe, hypothalamus, and pituitary gland. Conventional radiotherapy to this region is occasionally complicated by injury to the anterior visual pathways and more commonly complicated by pituitary failure.52,53 Much less frequently, brain necrosis and secondary malignancy formation can complicate the treatment of lesions involving the parasellar region with conventionally fractionated radiotherapy. Long-term follow-up of conventional radiation for benign lesions in younger patients is needed as the risk of secondary malignancy with a wide field in this region has not been well defined. An additional shortcoming to conventional radiotherapy is the inability for a second treatment after recurrence of the tumor or with patients who have had radiation to adjacent cranial base lesions. Finally, a 6-week course of therapy is often inconvenient for many patients and an equally effective, yet shorter, treatment is attractive to most patients.
Radiosurgery has the capacity to minimize the irradiation of nearby critical structures, thereby restricting collateral damage. Moreover, SRS allows for radiation to be used in patients who suffer meningioma recurrence, while at the same time providing the convenience of a shorter course of treatment. However, previous studies have demonstrated an increased risk of visual injury when a segment of the optic nerve or chiasm is irradiated with more than 8 to 10 Gy in a single fraction.33,34,54 Consequently, when the distance between tumor and anterior visual pathways is less than 2 to 3 mm, radiosurgery is likely to result in the optic apparatus receiving more than 10 Gy and is therefore usually contraindicated. The emergence of image-guided technology now makes it practical to incorporate the concept of multiple sessions into radiosurgery. Such a procedure blends the anatomic precision and conformality of radiosurgery with the biological advantages of fractionation. By taking advantage of the volume effect, one can, theoretically, use larger doses per session for treating perioptic neoplasms than was previously possible with conventional radiation therapy. The above advantages provided the foundation for using multisession radiosurgery to manage perioptic lesions.55,56
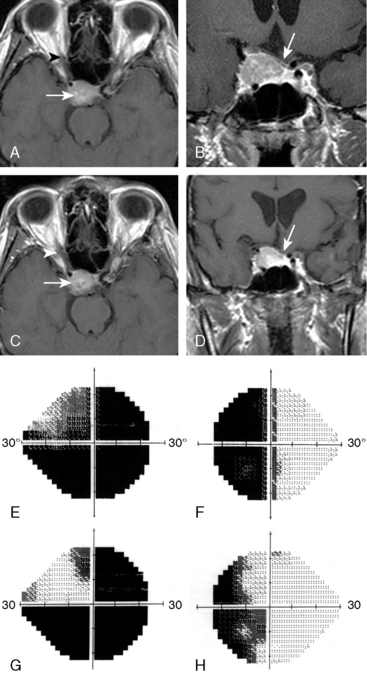
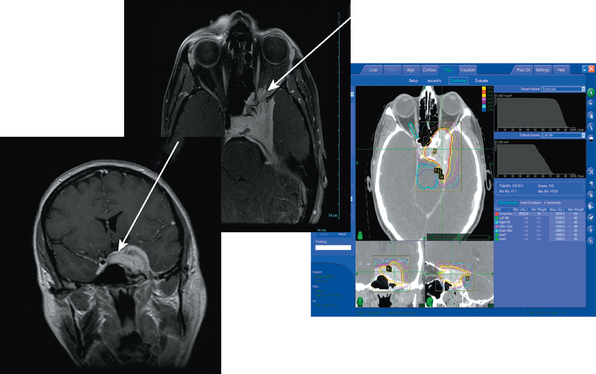
Adler and colleagues35 described Stanford’s experience of treating 49 consecutive lesions within 2 mm of a “short segment” of the optic apparatus with multisession CyberKnife radiosurgery (Fig. 55-4). Thirty-nine of these patients had previous subtotal surgical resection, and six had previously been treated with conventional fractionated radiotherapy. CyberKnife radiosurgery was delivered in two to five sessions to an average tumor volume of 7.7 cm3 and a cumulative average marginal dose of 20.3 Gy. Of the 49 patients, 27 were meningiomas. After a mean visual field follow-up of 49 months (range, 6–96 months), vision was unchanged postradiosurgery in 38 patients, improved in 8 (16%), and worse in 3 (6%). Each instance of visual deterioration was accompanied by tumor progression that ultimately resulted in patient death. After a mean MRI follow-up period of 46 months, tumor volume was stable or smaller in all other cases. Two patients died of unrelated extracranial causes. This Stanford experience suggests that multisession radiosurgery for perioptic meningiomas results in high rates of tumor control and preservation of visual function.
CYBERKNIFE RADIOSURGERY FOR PARASAGITTAL MENINGIOMAS
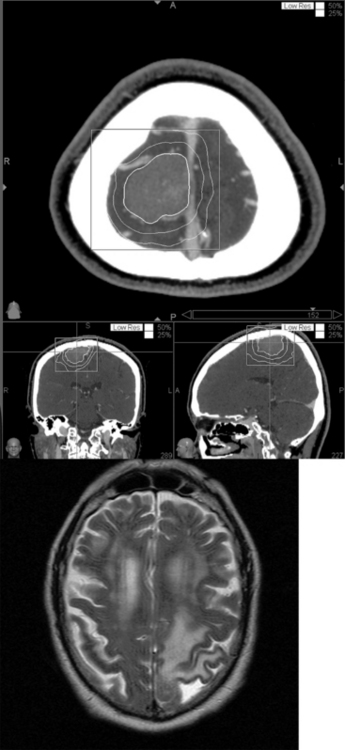
Meningiomas are prone to developing peritumoral edema after stereotactic radiosurgery.31,32,57–60 Based on a retrospective chart review of patients with supratentorial meningiomas, the overall risk of this phenomenon after CyberKnife stereotactic radiosurgery is approximately 15%. In our CyberKnife series, patients with parasagittal meningiomas or meningiomas in close proximity to a venous sinus were four times more likely to develop symptomatic postradiosurgical peritumoral edema than patients with meningiomas in other supratentorial locations. Despite a multivariate analysis, location was the only significant risk factor for developing symptomatic peritumoral edema. Edema appeared at a median of 7 months (range 4–20 months) after radiosurgery (Fig. 55-5). The symptoms and deficits included severe new onset headache, seizures, hemiparesis, and aphasia. The majority of patients were managed with glucocorticoids while a few patients with mild deficits were managed simply with serial imaging and clinical follow-up. All patients had resolution of their presenting symptoms and deficits without permanent neurologic sequlae.
One possible mechanism for the development of postradiosurgical edema is radiation injury to the small collateral bridging veins draining into the adjacent venous sinuses.60 The thrombosis of these damaged bridging veins would then cause a backward transmission of increased hydrostatic pressure into the peritumoral cortex thus, resulting in edema. It is a reasonable speculation that more detailed imaging of pre- and postradiosurgical venous anatomy using MR venography could be used to investigate such venous outflow problems as a factor in postradiosurgical edema. Given the fourfold increased risk of peritumoral edema, patients with parasagittal meningiomas require close postradiosurgical follow-up. In addition, this finding emphasizes the need to develop strategies that reduce postradiosurgical peritumoral edema. Multisession CyberKnife radiosurgery has been successfully utilized to treat perioptic lesions.35 A similar multisession strategy that minimizes the damage to the adjacent venous vasculature may be able to decrease the risk of postradiosurgical edema in patients with a parasagittal meningioma. The frameless design of the CyberKnife is well suited for such a multisession strategy, and studies to evaluate this approach are planned.
CYBERKNIFE RADIOSURGERY FOR LARGE SKULL-BASE MENINGIOMAS
Large skull-base meningiomas can represent one of the greatest challenges in neurosurgery; gross total resection is often technically difficult and fraught with risks, while primary radiosurgical ablation can be complicated by the proximity of critical anatomy. With larger skull base meningioma (>3.5 cm in diameter) the potential for complication from SRS is great enough to preclude its use under most circumstances. In such cases the risks of radiosurgery includes major cranial nerve deficits, radiation necrosis or peritumoral edema, as well as less common carotid artery stenosis, and hypothalamic dysfunction. The very real potential for such complications has resulted in a trend toward lower and potentially less effective radiation doses. One commonly employed strategy for managing such lesions is to combine conservative microsurgical resection with radiosurgical ablation of the smaller tumor remnant. However, many elderly or medically infirm patients are poor candidates for any open surgery, and even this more conservative approach is not an option. Therefore, as with perioptic lesions, multisession radiosurgery can be used to ablate larger skull base meningiomas with a biologically greater radiation dose, while simultaneously minimizing the risk to adjacent structures. At Stanford, most large skull-base lesions are now routinely treated with a multisession radiosurgical approach rather than conventional radiation therapy (Fig. 55-6). We are currently evaluating the local control and complication rate that stems from this strategy.
[1] Rohringer M., Sutherland G.R., Louw D.F., Sima A.A. Incidence and clinicopathological features of meningioma. J Neurosurg. 1989;71:665-672.
[2] Chin L.S., Szerlip N.J., Regine W.F. Stereotactic radiosurgery for meningiomas. Neurosurg Focus. 2003;14:e6.
[3] Cushing H. The meningiomas (dural endotheliomas). Their source and favoured seats of origin. Brain. 1922;45:282-316.
[4] Rockhill J., Mrugala M., Chamberlain M.C. Intracranial meningiomas: an overview of diagnosis and treatment. Neurosurg Focus. 2007;23:E1.
[5] Simon M., Bostrom J.P., Hartmann C. Molecular genetics of meningiomas: from basic research to potential clinical applications. Neurosurgery. 2007;60:787-798.
[6] Louis D.N., Ohgaki H., Wisteler O.D., Cavenee W.K. WHO classification of tumours of the central nervous system. World Health Organization, 2007.
[7] Rogers L., Mehta M. Role of radiation therapy in treating intracranial meningiomas. Neurosurg Focus. 2007;23:E4.
[8] Lusis E., Gutmann D.H. Meningioma: an update. Curr Opin Neurol. 2004;17:687-692.
[9] Wahab M., Al-Azzawi F. Meningioma and hormonal influences. Climacteric. 2003;6:285-292.
[10] Buetow M.P., Buetow P.C., Smirniotopoulos J.G. Typical, atypical, and misleading features in meningioma. Radiographics. 1991;11:1087-1106.
[11] Chamberlain M.C., Tsao-Wei D.D., Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004;62:1210-1212.
[12] Ragel B., Jensen R.L. New approaches for the treatment of refractory meningiomas. Cancer Control. 2003;10:148-158.
[13] Norden A.D., Drappatz J., Wen P.Y. Targeted drug therapy for meningiomas. Neurosurg Focus. 2007;23:E12.
[14] Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
[15] Mirimanoff R.O., Dosoretz D.E., Linggood R.M., et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18-24.
[16] Stafford S.L., Perry A., Suman V.J., et al. Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc. 1998;73:936-942.
[17] Condra K.S., Buatti J.M., Mendenhall W.M., et al. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427-436.
[18] Goldsmith B.J., Wara W.M., Wilson C.B., Larson D.A. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195-201.
[19] Withers H.R., Thames H.D.Jr, Peters L.J. Biological bases for high RBE values for late effects of neutron irradiation. Int J Radiat Oncol Biol Phys. 1982;8:2071-2076.
[20] Thames H.D.Jr, Withers H.R., Peters L.J., Fletcher G.H. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219-226.
[21] Elia A.E.H., Shih H.A. Stereotactic radiation treatment for benign meningiomas. Neurosurg Focus. 2007;23:E5.
[22] Pollock B.E., Stafford S.L., Utter A., et al. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003;55:1000-1005.
[23] Metellus P., Regis J., Muracciole X., et al. Evaluation of fractionated radiotherapy and gamma knife radiosurgery in cavernous sinus meningiomas: treatment strategy. Neurosurgery. 2005;57:873-886.
[24] Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
[25] Hara W., Soltys S.G., Gibbs I.C. CyberKnife robotic radiosurgery system for tumor treatment. Expert Rev Anticancer Ther. 2007;7:1507-1515.
[26] Ho A.K., Fu D., Cotrutz C., et al. A study of the accuracy of cyberknife spinal radiosurgery using skeletal structure tracking. Neurosurgery. 2007;60:ONS147-ONS156.
[27] Kreil W., Luggin J., Fuchs I., et al. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry. 2005;76:1425-1430.
[28] Chang J.H., Chang J.W., Choi J.Y., et al. Complications after gamma knife radiosurgery for benign meningiomas. J Neurol Neurosurg Psychiatry. 2003;74:226-230.
[29] Kobayashi T., Kida Y., Mori Y. Long-term results of stereotactic gamma radiosurgery of meningiomas. Surg Neurol. 2001;55:325-331.
[30] Flickinger J.C., Kondziolka D., Maitz A.H., Lunsford L.D. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003;56:801-806.
[31] Kondziolka D., Flickinger J.C., Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43:405-413.
[32] Ganz J.C., Schrottner O., Pendl G. Radiation-induced edema after Gamma Knife treatment for meningiomas. Stereotact Funct Neurosurg. 1996;66(Suppl. 1):129-133.
[33] Tishler R.B., Loeffler J.S., Lunsford L.D., et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215-221.
[34] Leber K.A., Bergloff J., Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88:43-50.
[35] Adler J.R.Jr, Gibbs I.C., Puataweepong P., Chang S.D. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery. 2006;59:244-254.
[36] Morita A., Coffey R.J., Foote R.L., et al. Risk of injury to cranial nerves after gamma knife radiosurgery for skull base meningiomas: experience in 88 patients. J Neurosurg. 1999;90:42-49.
[37] Dodd R.L., Ryu M.R., Kamnerdsupaphon P., et al. CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58:674-685.
[38] Gerszten P.C., Burton S.A., Ozhasoglu C. CyberKnife radiosurgery for spinal neoplasms. Prog Neurol Surg. 2007;20:340-358.
[39] Stafford S.L., Pollock B.E., Foote R.L., et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029-1037.
[40] Pollock B.E., Stafford S.L. Results of stereotactic radiosurgery for patients with imaging defined cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2005;62:1427-1431.
[41] Kwon Y., Ahn J.S., Jeon S.R., et al. Intratumoral bleeding in meningioma after gamma knife radiosurgery. J Neurosurg. 2002;97:657-662.
[42] Roche P.H., Régis J., Dufour H., et al. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg. 2000;93(Suppl. 3):68-73.
[43] Balasubramaniam A., Shannon P., Hodaie M., et al. Glioblastoma multiforme after stereotactic radiotherapy for acoustic neuroma: Case report and review of the literature. Neuro Oncol. 2007;9:447-453.
[44] Rowe J., Grainger A., Walton L., et al. Risk of malignancy after gamma knife stereotactic radiosurgery. Neurosurgery. 2007;60:60-65.
[45] Hakim R., Alexander E., Loeffler J.S., et al. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery. 1998;42:446-453.
[46] Kondziolka D., Levy E.I., Niranjan A., et al. Long-term outcomes after meningioma radiosurgery: physician and patient perspectives. J Neurosurg. 1999;91:44-50.
[47] Helseth A., Mork S.J. Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg. 1989;71:842-845.
[48] Perry A., Gutmann D.H. Molecular pathogenesis of meningiomas. J Neurooncol. 2004;70:183-202.
[49] King A.T., Sharr M.M., Gullan R.W., Bartless J.R. Spinal meningiomas: a 20–year review. Br J Neurosurg. 1998;12:521-526.
[50] Taylor B.W.Jr, Marcus R.B.Jr, Friedman W.A., et al. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299-304.
[51] Barbaro N.M., Gutin P.H., Wilson C.B., et al. Radiation therapy in the treatment of partially resected meningiomas. Neurosurgery. 1987;20:525-528.
[52] Cantore W.A. Neural orbital tumors. Curr Opin Ophthalmol. 2000;11:367-371.
[53] Estrada J., Boronat M., Mielgo M., et al. The long-term outcome of pituitary irradiation after unsuccessful transsphenoidal surgery in Cushing’s disease. N Engl J Med. 1997;336:172-177.
[54] Girkin C.A., Comey C.H., Lunsford L.D., et al. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology. 1997;104:1634-1643.
[55] Mehta V.K., Lee Q.T., Chang S.D., et al. Image guided stereotactic radiosurgery for lesions in proximity to the anterior visual pathways: a preliminary report. Technol Cancer Res Treat. 2002;1:173-180.
[56] Pham C.J., Chang S.D., Gibbs I.C., et al. Preliminary visual field preservation after staged CyberKnife radiosurgery for perioptic lesions. Neurosurgery. 2004;54:799-810.
[57] Inamura T., Nishio S., Takeshita I., et al. Peritumoral brain edema in meningiomas–influence of vascular supply on its development. Neurosurgery. 1992;31:179-185.
[58] Kalapurakal J.A., Silverman C.L., Akhtar N., et al. Intracranial meningiomas: factors that influence the development of cerebral edema after stereotactic radiosurgery and radiation therapy. Radiology. 1997;204:461-465.
[59] Kan P., Liu J.K., Wendland M.M., et al. Peritumoral edema after stereotactic radiosurgery for intracranial meningiomas and molecular factors that predict its development. J Neurooncol. 2007;83:33-38.
[60] Singh V.P., Kansai S., Vaishya S., et al. Early complications following gamma knife radiosurgery for intracranial meningiomas. J Neurosurg. 2000;93(Suppl. 3):57-61.