Chapter 16 Cutting balloon angioplasty
 Cutting balloon angioplasty (CBA) is similar in success to plain old balloon angioplasty (POBA) and can be used in the majority of cases.
Cutting balloon angioplasty (CBA) is similar in success to plain old balloon angioplasty (POBA) and can be used in the majority of cases.INTRODUCTION
POBA3 increases luminal size by controlled barotrauma causing plaque rupture and vessel wall expansion. It is established that while the final angiographic result of POBA is related to the extent and force of balloon dilatation it is the extent of vessel wall trauma that determines acute vessel closure and also initiates the mechanism that when exaggerated leads to restenosis. Part of the difficulty is that the balloon frequently exerts its maximal force on the more normal parts of the vessel wall causing stretching which is prone to early recoil and damaging normal endothelium rather than cracking more organised fibrous plaque.
The Barath cutting balloon1 was designed specifically to address the problems of barotrauma-related complications of POBA. The device is a non-compliant balloon which when expanded has three or four longitudinally mounted microtomes on the external surface. The balloon material is folded to shield the blades and protect the vessel wall as the catheter is passed to and from the lesion. When the balloon is expanded at the site of a coronary lesion the blades produce controlled incisions into plaque rather than a random dissection. This allows the balloon barotrauma pressure to be evenly distributed and force to be applied to plaque at least as effectively as to normal parts of the vessel wall. The reduction in hoop stress and lower pressures used to achieve an angiographically acceptable result (maximum of 6–8 atmospheres) are the main factors put forward as being likely to reduce both acute complications and restenosis.
Intravascular ultrasound (IVUS) studies have demonstrated that the different mechanism of lesion dilation with CBA achieves similar luminal dimensions to POBA but with larger plaque reduction and less vessel expansion in noncalcified lesions.2 In contrast in calcified lesions CBA achieves larger lumen gain than POBA with similar proportional effects on plaque compression and vessel expansion. The virtual absence of vessel recoil would appear to be a significant factor in reducing the restenosis rates.
PRACTICAL POINTS
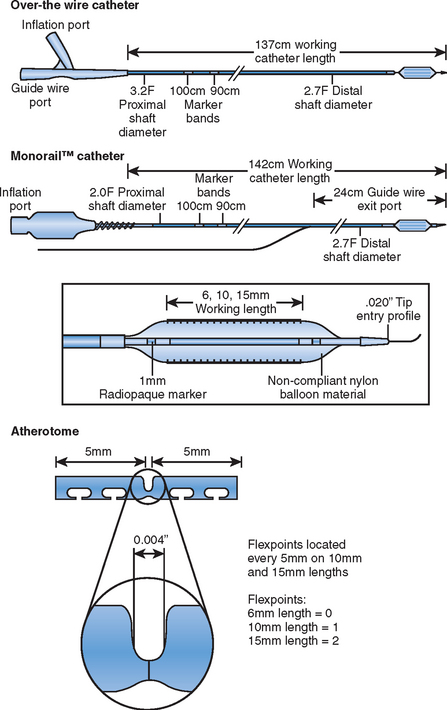
The Flextome Cutting Balloon (Boston Scientific, US) is the latest incarnation of the original Barath design (Fig. 16.1) and is sized from 2.0 to 4.0 mm in quarter sizes. The deflated profile is from 0.041 to 0.046”, the atherotome lengths are either 6, 10 or 15 mm and the distal shaft is 3.2 F (Fig. 16.2). The changes to balloon material and the incorporation of flex points every 5 mm for the 10 and 15 mm atherotomes has improved flexibility although its ability to cross areas of excessive tortuosity is inevitably inferior to the performance of modern compliant balloons. As with many interventional devices there are relative indications and contraindications, which are varied, in everyday use. Clear contraindications to its use are extensive vessel calcification and visible intraluminal thrombus. In practical terms excessive tortuosity and long lesions present difficulties though repeated inflations of the shorter balloon can achieve a good result. If there is difficulty crossing a lesion predilatation with a small balloon to allow passage of the cutting balloon is accepted practice. Once positioned across the lesion inflation should be undertaken slowly to reach 6 atmospheres at 30 seconds and inflation maintained for up to 2 minutes provided patients can tolerate this. The slow unfolding of the microtomes prevents vessel damage and allows the movement of the artery to assist incision. It is important to allow full deflation to ensure involution of the microtomes prior to withdrawing the balloon. If full expansion is not achieved up to 8 atmospheres pressure may be applied. The balloon/artery ratio should be 1.2:1 and in practical terms this means using one-quarter size more than the normal vessel segment. The final appearance may be ‘stent-like’ but up to a 30% residual stenosis is counted as an acceptable result and it is well observed that further positive remodelling takes place after initial dilatation such that follow-up angiograms not infrequently look better than the final result. Small controlled linear dissections are not uncommon and provided there is no change in the appearance over 5–10 minutes, acute vessel closure is extremely unlikely.3 As stenting is undertaken in 90% lesions acute closure is not a clinical issue and small linear dissection may allow better stent expansion.
CLINICAL DATA
The hypothesis that controlled ‘surgical’ expansion using CBA would induce less vessel wall injury and reduce neointimal hyperplasia was explored by the CUBA investigators.4 This study examined the clinical effectiveness as a primary treatment in 304 patients randomised to CBA or POBA and demonstrated that restenosis rates were significantly lower at six months in the CBA arm (CBA 30% vs POBA 42%; p=0.03). These promising early findings, however, were not confirmed by the pivotal multi-center Global trial,5 which randomised 1,238 patents to either CBA or POBA. The study was limited to simple A/B1 lesions and used a single CBA inflation at a balloon artery ratio of 1.1:1. Restenosis rates were identical in the two groups (CBA 31.4% vs POBA 30.4%; p=0.75) with significantly more coronary perforations in the CBA group (CBA 5 vs POBA 0; p=0.03).
SMALL VESSEL PTCA
The CAPAS study randomised 232 patients (248 type B or C lesions) with a reference vessel diameters of <3 mm to CBA or POBA.6 The success rate was high with a stent implant rate of 6% in CBA and 11% in POBA. Angiographic follow-up was 95% complete and showed a significant reduction in restenosis after CBA (22% vs 41%; p <0.01). Although there was a reduction in TVR after CBA this did not reach statistical significance (22% vs 29%). A further randomised trial by Ergene and colleagues7 although small, confirmed similar findings with a restenosis rate significantly lower after CBA (27% vs 47%; p <0.05) and observed significantly fewer dissections after CBA. Iijima et al.8 corroborated these findings in a retrospective comparison of 316 patients, with lesions in <2.5 mm vessels, treated with POBA, CBA and BMS. The CBA group demonstrated greater acute gain than POBA and lower late loss than both other techniques with a significantly lower binary stenosis rate (CBA 31%, POBA 46.5%, Stent 43.9%; p=0.048).
OSTIAL AND RESISTANT LESIONS
The outcome of POBA of ostial lesions at whatever site is poor.9 Cutting balloon angioplasty has been reported in a wide variety of both aorto-ostial lesions of SVGs and the right coronary artery and of the ostia of major branches of the coronary tree though few series have been compiled.
Aortic ostial lesions contain more fibrous and elastic components in comparison to lesions within the coronary tree and these lesions can be both resistant to plaque rupture and prone to substantial elastic recoil following POBA. The stronger radial force and more organised lesion disruption properties of CBA would, therefore, appear to offer a potential advantage in predilation prior to stent implantation in this area. Muramatsu and colleagues10 reported 37 patients undergoing CBA to ostial stenoses and compared them to a comparable group of patients having POBA with no patient being stented. The initial angiographic success rate was higher in the CBA group (95% vs 85%) with a much higher incidence of serious dissections following POBA 6.5% vs 0% for CBA. Angiographic restenosis occurred in 41% of CBA patients and 53% of POBA cases. Oversizing the cutting balloon led to a reduced restenosis rate of 31% but a higher dissection rate. A small series of patients reported by Kurbaan et al.11 showed the benefits of combined cutting balloon and stenting in ostial lesions. In these eight patients initial high pressure (18 atmospheres) ballooning led to a modest improvement in stenosis from 82% to 68% but adjunctive CBA left a residual stenosis of 44% which after stenting was reduced to 10%. During clinical follow up there was no evidence of restenosis. Combined CBA and stenting, therefore, appears the most appropriate treatment for aorto-ostial lesions although this strategy has not been tested in a randomised controlled trial.
Branch ostial lesions combine the problems of resistance and elastic recoil with added difficulty of potential plaque shift into the main vessel. Chung and colleagues investigated the role of CBA in comparison to BMS for ostial disease in 2.5–3 mm vessels in a nonrandomised retrospective study.12 Although the use of stents offered significantly enhanced acute gain, binary restenosis was lower in the CBA group at six months (CBA 41%, Stent 63%; p=0.05). Interestingly with CBA the MLD within the native vessel remained unaltered both immediately and at six-month follow up whilst significant lumen loss was seen at both time points in the stenting group, lending support to the theory of less plaque shift with CBA.
CBA has been demonstrated to be superior to POBA in the treatment of bifurcation lesions in retrospective analysis with increased procedural success (92% vs 76%; p=0.035), reduced bail out stenting (8% vs 24%; p=0.035) and reduced binary stenosis at three months (40% vs 67%; p=0.026). The incidence of restenosis with CBA is, therefore, comparable with bare metal stenting strategies but is likely to be inferior to the use of DES particularly with the utilization of final kissing balloon techniques.13
IN-STENT RESTENOSIS
Although stenting has significantly reduced the incidence of vessel restenosis and need for TVR when restenosis does occur within a stent the rate of re-restenosis remains high with repeat high-pressure ballooning having a recurrence rate of greater than 50%.15 IVUS studies have shown that in-stent restenosis is principally due to neo-intimal proliferation and hyperplasia as there is little scope for vessel recoil after stent deployment. It was, therefore, proposed that atherectomy by removing tissue would be the treatment of choice with or without adjunctive ballooning. However the results of atherectomy for restenosis have been disappointing with one year TLR rates of 26–31% with an average late loss of approximately 1 mm.15 The only randomised trial of rotablation (ARTIST) demonstrated a superior effect of POBA to rotational atherectomy and low-pressure adjunctive ballooning for in-stent restenosis.16
IVUS studies have demonstrated that the mechanism of lumen enlargement for balloon angioplasty in restenosis is equally dependent on stent expansion and extrusion of neo-intimal tissue through stent struts.17 The theoretical advantage for CBA over POBA in this setting is its greater ability to extrude tissue by separating the neo-intimal tissue into quadrants during balloon expansion as demonstrated in a recent IVUS study.18 It should be added that CBA also offers a practical advantage over POBA due to the avoidance of balloon displacement on inflation due to the ability of the blades to anchor the device on the slippery hyperplastic tissue surface. Initial clinical experience was encouraging with Adamian and colleagues19 retrospectively reporting 181 consecutive matched patients undergoing treatment for in-stent restenosis using POBA, high speed rotational atherectomy (HSRA) and CBA. In this study the final MLD was similar in all three groups with lower MACE at follow up found in the CBA group. With both angiographic re-restenosis and TVR significantly lower in the CBA group compared to HSRA and POBA. These finding were supported by other small observational studies demonstrating lower angiographic restenosis rates18 with CBA for in-stent restenosis in comparison to POBA and reduced TVR.20
These promising initial results, however, have not been replicated in subsequent randomised trials. The RESCUT21 (REStenosis CUTting balloon evaluation study) was a multicenter, prospective trial in which 428 patients with a wide range of ISR subtypes were randomised to either POBA or CBA. At 30 days and seven months no differences in clinical outcomes were discernable with a 7 month TLR rate in the CBA arm of 13.5% in comparison to 13.1% in the POBA arm (p=0.99). Angiographic characteristics were also comparable with no differences found in late loss (CBA 0.56 mm, POBA 0.62 mm; p=0.42) or binary restenosis (CBA 29.8%, POBA 31.4%; p=0.82). One issue in the trial was the low rate of ISR with POBA. Also the trial design did not allow additional high pressure POBA in the CBA group which would be usual practice. The equivalence of the two techniques in terms of MACE and angiographic outcome in restenotic lesions would appear to be confirmed by the second randomised trial in this area. REDUCE II (REstenosis reDUction by Cutting balloon Evaluation) randomised 416 to CBA and POBA and although the study is yet to be published early reports suggest that binary stenosis and TLR rates are not significantly different between the two arms of the study at six months follow up. Despite this disappointing data interest has remained in the combination of CBA with intravasular brachytherapy in the treatment of ISR supported by pooled nonrandomised data that has consistently reported good angiographic results in terms of late loss and binary restenosis. Unfortunately this combination, however, failed to demonstrate benefit in angiographic characteristics at seven months over brachytherapy and POBA in a recent randomised clinical trial (BETACUT).22
The use of DES for ISR has shown promise in small observational trials23,24 and supported in a recently published retrospective matched comparison25 of DES treated lesions with a control group consisting of individuals from RESCUT. The use of DES in this report was associated with a benefit in angiographic characteristics at six months with significant differences seen in acute lumen gain (CBA 1.36 mm, DES 1.69 mm; p<0.001), late loss (CBA 0.56 mm, DES 0.35 mm; p=0.028) and binary restenosis (CBA 29.8%, POBA 12.8%; p=0.016).
VESSEL PREPARATION PRIOR TO STENTING
Stent placement has made enormous impact on the need for reintervention but stent implantation alone does not guarantee long-term procedural success. Unless the stent is fully deployed with complete lesion coverage there remains a risk of both restenosis and sub acute thrombosis. This is particularly a problem in the presence of calcified or fibrotic lesions where inadequate vessel wall preparation can lead to under expansion despite both high pressure deployment and aggressive post dilation with a non-compliant balloon. Even in the era of DES the majority of target vessel failure has been attributed to stent under expansion.26 Vessel wall preparation using CBA is a straight forward method of increasing vessel compliance during stent deployment. CBA expands the lesion using low pressure dilatation and longitudinal incisions weakening areas that will then allow correct strut apposition and expansion.
The use of CBA for vessel preparation prior to stent implantation has been tested in REDUCE III, a large multicentre trial that randomised 520 patients to predilation with either CBA or POBA and these groups were further randomised to IVUS guided coronary stenting. At six-month follow up the IVUS guided CBA arm demonstrated significant lower restenosis than the other techniques (CBA IVUS 10.7%, CBA 18%, POBA IVUS 20%, POBA 18%; p=0.016). Failure to use CBA was an independent predictor of restenosis. In an era of concern for subacute and late thrombosis in DES this improvement in BMS results has increasing relevance. The question as to whether CBA has a role in reducing MACE post DES implantation has jet to be tested.
1 Barath P, Fishbein MC, Vari S, Forrester JS. Cutting balloon: a novel approach to percutaneous angioplasty. Am J Cardiol. 1991;68:1249-1252.
2 Okura H, Hayase M, Hosokawa H, et al. Acute lumen gain after cutting balloon angioplasty in calcified and non calcified lesions: intravascular ultrasound analysis of the REDUCE study. JACC February. 1999;33((2) Suppl. A):101A. 886–3.
3 Martai V, Martin V, Carcia J, Guiteras P, Auge JM. Significance of angiographic coronary dissection after cutting balloon angioplasty. Am J Cardiol. 1998;81:1349-1352.
4 Mainar V, Auge JM, Dominguez J, et al. Randomised comparison of cutting balloon vs conventional balloon angioplasty: final report and the in-hospital results of the CUBA study. Circulation. 1997;96((8) Suppl.):1526-1527.
5 Mauri L, Bonan R, Weiner BH, Legrand V, Bassand JP, Popma JJ, Niemyski P, Prpic R, Ho KK, Chauhan MS, Cutlip DE, Bertrand OF, Kuntz RE. Cutting balloon angioplasty for the prevention of restenosis: results of the Cutting Balloon Global Randomized Trial. Am J Cardiol. 2002;90:107-983.
6 Izumi M, Tsuchikane E, Fumamoto F, et al. One year clinical and 3-month angiographic follow-up of cutting balloon angioplasty versus plain old balloon angioplasty randomised study in small coronary artery (CAPAS). JACC. 1999;33((2) Suppl. A):47. 810–15.
7 Ergene O, Seyithanogula BY, Tastan A, et al. Comparison of angiographic and clinical outcome after cutting balloon and conventional balloon angioplasty in vessels smaller than 3 mm in diameter: a randomized trial. J Invasive Cardiol. 1998;10:70-75.
8 Iijima R, Ikari Y, Wada M, Shiba M, Nakamura M, Hara K. Cutting balloon angioplasty is superior to balloon angioplasty or stent implantation for small coronary artery disease. Coron Artery Dis. 2004;7:435-440.
9 Topol EJ, Ellis SG, Fishman J, Leimgruber P, Myler RK, Stertzer SH, O’Neill WW, Douglas JS, Roubin GS, King SB3rd. Multicenter study of percutaneous transluminal angioplasty for right coronary artery ostial stenosis. J Am Coll Cardiol. 1987 Jun;9(6):1214-1218.
10 Muramatasu T, Tsukahara R, Ho M, et al. Efficacy of cutting balloon angioplasty for lesions at the ostium of the coronary arteries. J Invas Cardiol. 1999;11:201-206.
11 Kurbaan AS, Kelly PA, Sigwart U. Cutting balloon angioplasty and stenting for aorto-ostial lesions. Heart. 1997 Apr;77(4):350-352.
12 Chung CM, Nakamura S, Tanaka K, Tanigawa J, Kitano K, Akiyama T, Matoba Y, Katoh O. Comparison of cutting balloon vs stenting alone in small branch ostial lesions of native coronary arteries. Circ J. 2003 Jan;67(1):21-25.
13 Ge L, Airoldi F, Iakovou I, Cosgrave J, Michev I, Sangiorgi GM, Montorfano M, Chieffo A, Carlino M, Corvaja N, Colombo A. Clinical and angiographic outcome after implantation of drug-eluting stents in bifurcation lesions with the crush stent technique: importance of final kissing balloon post-dilation. J Am Coll Cardiol. 2005 Aug 16;46(4):613-620.
14 Baim DS, Levine MJ, Leon MB, Levine S, Ellis SG, Schatz RA. Management of restenosis within the Palmaz-Schatz coronary stent (the U.S. multicenter experience. The U.S. Palmaz-Schatz Stent Investigators. Am J Cardiol. 1993 Feb 1;71(4):364-366.
15 Almeda FQ, Klein LW. Cutting balloon angioplasty: to cut is to cure? J Invasive Cardiol. 2002 Dec;14(12):725-727.
16 vom Dahl J, Dietz U, Haager PK, Silber S, Niccoli L, Buettner HJ, Schiele F, Thomas M, Commeau P, Ramsdale DR, Garcia E, Hamm CW, Hoffmann R, Reineke T, Klues HG. Rotational atherectomy does not reduce recurrent in-stent restenosis: results of the angioplasty versus rotational atherectomy for treatment of diffuse in-stent restenosis trial (ARTIST). Circulation. 2002 Feb 5;105(5):583-588.
17 Mehran R, Mintz GS, Popma JJ, Pichard AD, Satler LF, Kent KM, Griffin J, Leon MB. Mechanisms and results of balloon angioplasty for the treatment of in-stent restenosis. Am J Cardiol. 1996 Sep 15;78(6):618-622.
18 Muramatsu T, Tsukahara R, Ho M, Ito Y, Hirano K, Ishimori H, Matushita M, Nakano M. Efficacy of cutting balloon angioplasty for in-stent restenosis: an intravascular ultrasound evaluation. J Invasive Cardiol. 2001 Jun;13(6):439-444.
19 Adamian MG, Marisco F, Brigouri C, et al. Cutting balloon treatment for in stent restenosis, a matched comparison with conventional angioplasty and rotational atherectomy. Circulation. 1999;100:1-305.
20 Nakamura M, Anzai H, Asahara T, et al. Cutting balloon angioplasty for stent restenosis. Jpn J Intero Cardiol. 1999;14:15-20.
21 Albiero R, Silber S, Di Mario C, Cernigliaro C, Battaglia S, Reimers B, Frasheri A, Klauss V, Auge JM, Rubartelli P, Morice MC, Cremonesi A, Schofer J, Bortone A, Colombo A, RESCUT Investigators. Cutting balloon versus conventional balloon angioplasty for the treatment of in-stent restenosis: results of the restenosis cutting balloon evaluation trial (RESCUT). J Am Coll Cardiol. 2004 Mar 17;43(6):943-949.
22 Schluter M, Tubler T, Lansky AJ, Kahler S, Berger J, Mathey DG, Schofer J. Angiographic and clinical outcomes at 8 months of cutting balloon angioplasty and beta-brachytherapy for native vessel in-stent restenosis (BETACUT): Results from a stopped randomized controlled trial. Catheter Cardiovasc Interv. 2005 Oct 7;66(3):320-326.
23 Sousa JE, Costa MA, Abizaid A, Sousa AG, Feres F, Mattos LA, Centemero M, Maldonado G, Abizaid AS, Pinto I, et al. Sirolimus-eluting stent for the treatment of in-stent restenosis: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2003;107:24-27.
24 Degertekin M, Lemos PA, Lee CH, Tanabe K, Sousa JE, Abizaid A, Regar E, Sianos G, van der Giessen WJ, de Feyter PJ, et al. Intravascular ultrasound evaluation after sirolimus eluting stent implantation for de novo and in-stent restenosis lesions. Eur Heart J. 2004;25:32-38.
25 Airoldi F, Rogacka R, Briguori C, Chieffo A, Carlino M, Montorfano M, Mikhail G, Iakovou I, Michev I, Vitrella G, Albiero R, Colombo A. Comparison of clinical and angiographic outcome of sirolimus-eluting stent implantation versus cutting balloon angioplasty for coronary in-stent restenosis. Am J Cardiol. 2004;94(10):1297-1300.
26 Takebayashi H, Kobayashi Y, Mintz GS, Carlier SG, Fujii K, Yasuda T, Moussa I, Mehran R, Dangas GD, Collins MB, et al. Intravascular ultrasound assessment of lesions with target vessel failure after siroli-mus eluting stent implantation. Am J Cardiol. 2005;95:498-502.
27 Ozaki Y, Yamaguchi T, Suzuki T, Nakamura M, Kitayama M, Nishikawa H, Inoue T, Hara K, Usuba F, Sakurada M, Awano K, Matsuo H, Ishiwata S, Yasukawa T, Ismail TF, Hishida H, Kato O. Impact of cutting balloon angioplasty (CBA) prior to bare metal stenting on restenosis. Circ J. 2007 Jan;71(1):1-8.