Cultured Limbal Epithelial Stem Cells for Reconstruction of the Corneal Epithelium
Introduction
The possibility to serially propagate limbal epithelial stem cells (LESCs) in the cell culture laboratory has made these cells amenable to further study in vitro, but has also been used as a therapeutic approach. Cultivated sheets of LESCs can be transplanted with the aim of reconstructing a stem cell-deficient corneal surface. A recent study followed 112 patients with ocular surface disease due to corneal burns which received transplantation of autologous LESCs expanded on a fibrin gel. Clinical success (defined as a transparent, avascular and stable corneal surface) was seen in more than 75% of the study eyes.1 Cultured limbal epithelium has also been used to treat other causes of limbal stem cell deficiency with varying success rates.2 Although there is currently a lack of evidence to show that transplantation of cultivated LESCs is superior to conventional techniques involving whole limbal tissue transplants, there are theoretical advantages. In this chapter, the authors outline the concept and practice of cultured LESCs for reconstruction of the corneal surface and provide an overview of its current clinical significance and potential.
History and Rationale
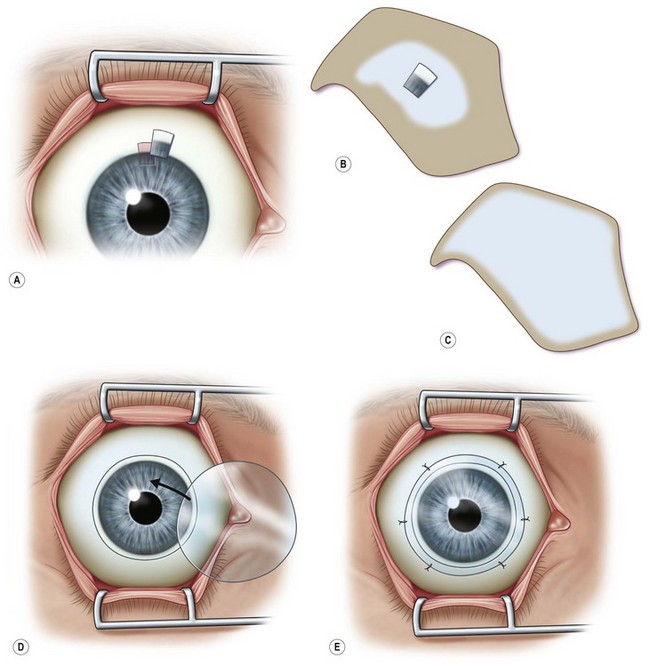
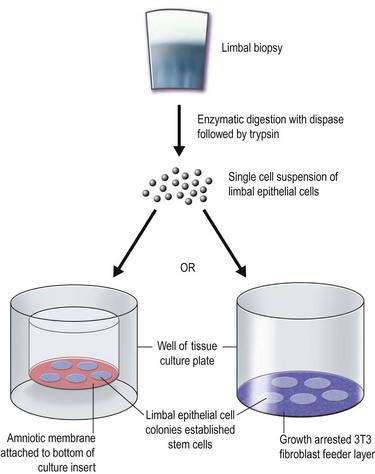
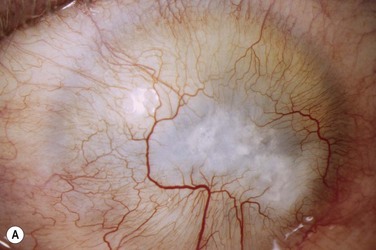
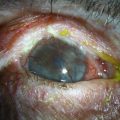
Transplantation of healthy limbal tissue for treatment of total limbal stem cell deficiency can be obtained from the contralateral eye,3,4 a relative of the patient, or from the eye of a cadaveric donor. There is a very low risk for the healthy eye of a living donor unless repeated limbal biopsies are required. Therefore, the concept of ex vivo expansion of LESCs is regarded as a potential beneficial to increase cell yields prior to transplantation, allowing the size of the limbal biopsy to be reduced (Figs 44.1, 44.2). In exceptional cases, using this technique, autologous LESCs can also be obtained from a small, healthy region of a partially stem cell-deficient eye.5 In addition, cultured sheets of allogeneic epithelium have been purported to be less prone to immune rejection, since antigen-presenting cells are deemed to be absent following cell culture.
Human corneal epithelium was first grown successfully in culture in 1977. This was made possible by the use of the 3T3 co-culture method, which had been described 2 years earlier by Rheinwald and Green.3,4 They reported that in the presence of mouse embryonic 3T3 fibroblasts, which had been growth-arrested by irradiation, single epithelial cells showed clonal expansion and could be serially propagated in vitro. However, it was not until 20 years later that transplantation of autologous, cultured limbal epithelial cells was shown to be beneficial in clinical cases of limbal stem cell deficiency. In 1997, Pellegrini and coworkers successfully transplanted cultured cell sheets obtained from autologous limbal tissue in two patients with alkali burns. Since then, numerous studies have focused on transplantation of cultured limbal epithelium.6,7 These interventions were performed in a number of different conditions, using different culture systems and yielding varying, yet promising results (see below).
Currently it remains unclear what the precise mechanisms are by which grafting cultivated LESCs contributes to ocular surface healing. One would expect transplanted LESCs to improve the corneal surface by replenishing the depleted stem cell pool. However, Daya et al.8 determined the genotype of corneal epithelial cells following their transplantation to the corneal surface in humans, and were unable to detect donor DNA from as early as 1 month postoperatively. This may mean that rather than integrating into the recipient corneal surface (re-integration theory), transplanted corneal epithelium may allow or actively stimulate the recovery of an endogenous stem cell population (biological bandage theory).
Isolation Methods
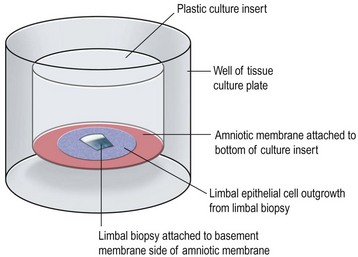
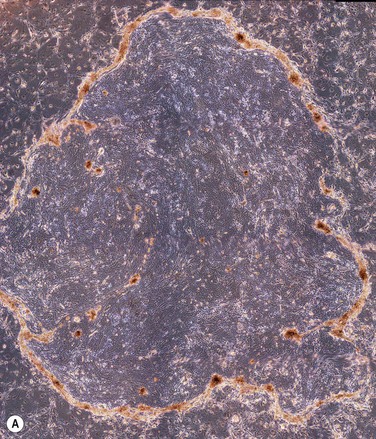
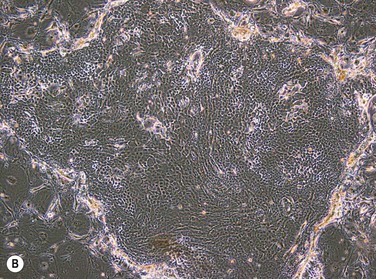
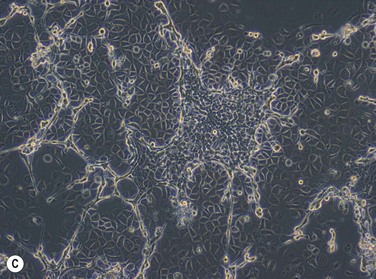
The two main techniques available for isolation and ex vivo expansion of corneal epithelium are the explant culture system and the somewhat more common suspension culture system. The former consists of placing a piece of limbal tissue (e.g. 2×2 mm) in culture as a whole (Fig. 44.3). The latter uses enzymatic digestion to remove the epithelial cells from the limbal tissue (Fig. 44.4). In this system, dispase is most frequently used to digest the epithelial basement membrane, while trypsin is employed subsequently to disrupt intercellular adhesions, producing a suspension of epithelial cells. Epithelial outgrowth from the explant or clonal growth around single isolated LESCs can be observed when appropriate culture conditions are being used (see below). Favorable clinical results have been obtained in a number of studies using either isolation strategy, and although limbal epithelial cell suspensions may contain a higher proportion of stem cells, clinical superiority of either of the two methods has not been formally established.2
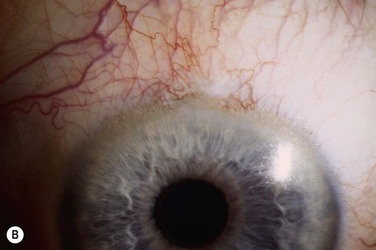
Baylis et al. have proposed that impression cytology should be performed on the donor eye to rule out subclinical LESC deficiency, which could be made manifest by performing a limbal biopsy or which may predict culture failure.6 Also, several studies have suggested an uneven distribution of LESCs along the limbus.9 Knowledge regarding the localization of LESCs is important for targeting limbal biopsies to stem cell-rich regions. Most frequently, LESCs have been proposed to be more abundant in the superior and inferior limbal regions. However, the lack of definitive markers for unequivocal identification of LESCs continues to hamper the development of efficient methods not only for isolation, but also for propagation and delivery of LESCs. When cells are harvested from a living donor, the limbal palisades should be identified, and a specimen of 1–6 mm2 is obtained under local anesthesia (see Fig. 44.2). Although this technique maximizes the yield of clonogenic LESCs and therefore, the chances of successfully initiating an LESC culture, it remains unknown whether it also has a positive effect on the clinical performance of the graft.
In a similar vein, where cadaveric corneal tissue needs to be relied upon, the duration of storage of ocular tissue in the eye bank has been suggested to negatively influence the rate of successfully initiating LESC cell culture; however, this has not been shown to affect the suitability of successfully established cell sheets for transplantation.2 Likewise, characteristics of tissue donors may influence attachment, survival and proliferation of LESCs. It has been reported that donor age has an influence on putative limbal stem cell markers, as well as morphological niche parameters, and that colony forming efficiency of LESCs declines with age.10,11 Additionally, it has been shown that corneal wound healing is impaired in diabetes mellitus, and limbal basal cells from diabetic human donors have been suggested to express fewer putative stem cell markers than those from normal subjects.12 This exemplifies that LESCs may be affected by systemic disease present in the donor. Again, whether this is likely to affect culture and transplantation of human limbal epithelial cells remains to be determined.
The Limbal Stem Cell Niche in Culture
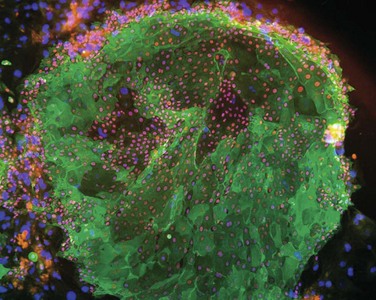
Transplantation of limbal epithelium is deemed a stem cell therapy. This notion is supported, for instance, by the observation that a minimum of 3000 transplanted putative limbal stem cells was associated with a higher success rate in patients with ocular surface disease due to chemical and thermal burns.1 However, expanded limbal epithelial cell sheets contain a considerable fraction of differentiated daughter cells (Fig. 44.5). Also, LESCs have only a finite life span when propagated in culture, although one of the most important features describing stem cell populations is that of indefinite self-renewal. The current conception is that the maintenance of limbal stem cells is governed by a number of intrinsic, but also extrinsic factors, the latter of which are provided in vivo by the local microenvironment present at the corneoscleral limbus. Rather than being a mere transition zone between cornea and sclera, the limbus provides some unique anatomical specializations that are believed to be important for maintaining the stem cell population (see Chapter 5). In addition to a protective limbal anatomy, signals received from extracellular matrix and surrounding cells are believed to contribute to regulating LESC function and fate. For instance, extracellular matrix components were found to be expressed differentially in different areas of the ocular and corneal surface and may play a role in regulating LESC proliferation and differentiation.13 Likewise, soluble factors secreted by limbal fibroblasts have been proposed to have regulatory effects on limbal basal epithelium. The notion of adjacent structures and cells influencing stem cell behavior corresponds well to what has been termed the ‘stem cell niche.’ A system which removes LESCs from their niche to expand the cells for transplantation will need to replicate the stem cell niche in order to retain ‘stemness’ of the cells.
Amniotic Membrane as a Culture Substrate
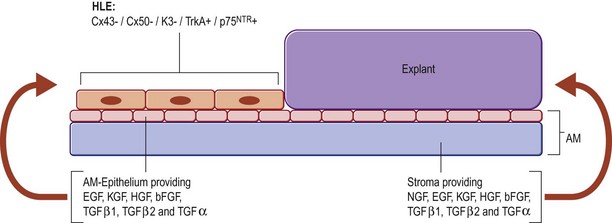
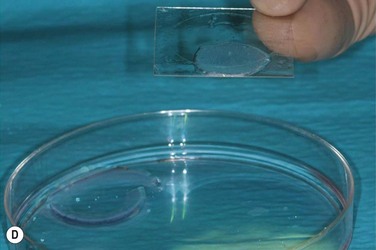
In the first two cases of cultured LESC transplantation reported by Pellegrini and coworkers, transfer of the cultured epithelial sheet to the recipient ocular surface was performed using petroleum gauze in one patient, while a soft contact lens served as a carrier in the other. Subsequent works have frequently used fibrin gels. These gels have been shown to support LESC growth and attachment in culture, while they are degraded within 24 hours following transplantation (Fig. 44.6). However, these matrices do not actively contribute to the preservation of stem cell properties during expansion and engraftment. To provide a surrogate niche for LESCs throughout ex vivo culture and after transfer to the ocular surface, amniotic membrane (AM) has become a popular substrate. AM is the innermost membrane of the fetal sac, which can be obtained from healthy volunteers during routine caesarean sections. Properties which make AM well suited for ocular surface reconstruction are its avascularity, relative transparency, low immunogenicity, expression of anti-inflammatory and antiangiogenic proteins, as well as growth factors (see Chapter 37). AM can therefore, act both as a culture substrate in vitro and as a carrier for LESC transfer and attachment to the corneal surface. AM has been used successfully as a substrate for LESC expansion and transplantation for more than a decade. Growth factors that convey favorable properties for LESC maintenance and expansion are produced by the AM stroma and particularly the amniotic epithelium. Among these growth factors are keratocyte, hepatocyte, epidermal, nerve and basic fibroblast growth factor, as well as members of the transforming growth factor family (Fig. 44.7).
Frequently, prior to applying limbal epithelial cells, the amniotic epithelium is removed by physical scraping or enzymatically to expose the underlying thick basement membrane. Basement membrane components that are present in AM and which may support LESC growth and adhesion include collagen IV and laminin isoforms, as well as fibronectin.14 These properties aid in the formation of confluent, well-attached, stratified sheets of epithelium of a corneal phenotype.15 However, it has been suggested that AM with an intact epithelial layer may be more suitable for maintaining LESCs in their undifferentiated state, as it appears to result in propagation of smaller cells (putative progenitor cells)16 and preservation of a limbal phenotype.14
Hence, the optimal method of preparing AM for LESC culture remains unclear. This extends also to methods used for AM preservation. It has been argued that fresh AM (stored at 4°C) may be able to contribute more of its bioactive factors; however, also cryopreserved and lyophilized membrane have been used successfully.17 While glycerol is used widely as a cryoprotective agent, Shortt et al. have suggested that it results in poor retention of putative stem cell markers and epithelial cell morphology. Instead, it has been proposed that the use of balanced saline solution may be superior with respect to the mentioned parameters.18 Protocols for the preparation of AM also differ in terms of the method used for reducing infectious risk. Here, antibiotic treatment, peracetic acid, and gamma irradiation have all been used in the context of LESC expansion.19
Although certainly useful for ocular surface reconstruction, AM does carry infectious risks which, despite costly screening of donors plus disinfection/sterilization, cannot be fully eliminated. Additional shortcomings, such as biological variability, inconsistent supply and only relative transparency have encouraged efforts to develop alternative, biomimetic substrates for expansion of LESCs. These substrates may be engineered to incorporate additional, more specific limbal niche components (see Chapter 43).
Culture Media
The growth potential of human epidermal cells has been characterized by the type of colony that forms in culture: holoclones contain cells showing high clonogenic capacity, meroclones include a larger fraction of cells that have a more limited capacity for proliferation, and paraclones consist of cells that are still closer to terminal differentiation. This typical growth pattern is also seen in epithelial cells obtained from the corneoscleral limbus (Fig. 44.8). In addition to the culture substrates discussed above, the media used for ex vivo expansion have been shown to play an important role in preserving stem cell properties of cultured LESCs.10 Optimal culture conditions for LESCs would be expected to yield a high proportion of stem cell-containing holoclones. A typical composition of limbal epithelial stem cell culture medium is listed in Table 44.1. Different variations of this formulation have been used in a number of clinical trials to evaluate LESC grafts. However, it has been proposed that the high calcium content of DMEM/F12 promotes rapid growth but leads to early differentiation.10 Since LESCs have been described as slow-cycling cells, it seems logical that culture conditions which achieve high rates of proliferation may not be expanding stem cells, but rather supporting the growth of transient amplifying progenitors. Instead, a growth medium containing low calcium concentration of 0.03 mM was shown to support an undifferentiated phenotype. This culture medium, however, contains bovine pituitary gland extract and may therefore, not be suitable for clinical use due to the potential risk of transmitting infectious agents of animal origin. Other investigators have proposed that the medium supplement B-27 is suitable for cultivating LESCs in a serum-free culture system without unknown xenobiotic factors to reduce this risk.20.
Table 44.1
Example of Limbal Epithelial Stem Cell Growth and Expansion Medium2,4
| Medium Constituent | Final Concentration | Description |
| Dulbecco’s modification of Eagle’s medium (DMEM) | Basal medium | |
| Ham’s nutrient mixture F-12 | 25% | DMEM and F-12 have also been used at a ratio of 1 : 1 |
| Fetal bovine serum | 10% | The use of human autologous serum has also been reported30 |
| Insulin | 5 µg/mL | Reduces serum requirements |
| Hydrocortisone | 0.4 µg/mL | Improves epithelial cell growth and morphology |
| Cholera toxin | 0.1 nM | Stimulates DNA synthesis |
| Adenine | 0.18 mM | Enhances colony formation |
| Transferrin | 5 µg/mL | Stimulates stem cell replication |
| Tri-iodothryronine | 2 nM | Reduces serum requirements |
| Epidermal growth factor | 10 ng/mL | Promotes migration and cell spreading; inhibits differentiation |
| Antibiotics/antimycotics | e.g. penicillin (100 IU/mL), streptomycin (100 µg/mL), amphotericin B (25 µg/mL) | Optional |
(From Shortt AJ, Tuft SJ, Daniels JT. Ex vivo cultured limbal epithelial transplantation. A clinical perspective. Ocul Surf 2010;8:80–90. and Osei-Bempong C, Henein C, Ahmad S. Culture conditions for primary human limbal epithelial cells. Regen Med 2009;4:461–70.)
Airlifting has been used by some investigators to produce coherent sheets of multilayered epithelium for transfer to the ocular surface. This technique consists of raising the monolayer of epithelial cells that forms during submersion in culture medium to the air – liquid interface to induce stratification. However, this procedure may result in the loss of stem cells within the cultured sheet due to increased differentiation cues. Other investigators have, therefore, proposed the transfer of a single-cell suspension to the recipient ocular surface.21 The idea of stem cell-containing eye drops illustrates that the development of cell-based therapies for the treatment of ocular surface disease falls within the scope of activities of regulatory authorities that supervise the development and distribution of medicinal products. Regulatory issues surrounding the transition of cultured LESCs from bench to bedside are discussed in the following section.
Regulatory Requirements
To ensure product safety and consistency, routine use of cultured cells for therapeutic purposes in humans underlies strict regulation from the relevant authorities in many countries. In the European Union, the legal framework for procurement and processing of tissues and cells for the production of biological therapeutics is provided by the EU Tissue and Cells Directive, with a national regulatory authority in each member state being responsible for implementing the exigencies of this directive. In the United States, the Food and Drug Administration is responsible for enforcing Good Manufacturing Practice (GMP) in the production of cell-based therapies. To comply with regulatory requirements, informed consent must be obtained from the donor (or next of kin), and serological screening must be performed for transmissible diseases.22 The need for serological testing is extended also to patients planned for autologous treatment to avoid pathogen transmission to laboratory staff or other specimens during processing. To minimize any risk of transmitting zoonotic diseases, of tumorigenesis or of precipitating immunologic rejection, it has repeatedly been postulated that products intended for cell therapy at the ocular surface should be free from xenobiotics.23 However, the actual risk is difficult to quantitate, and other authors argue that fetal calf serum and murine 3T3 feeder cells may be suitable to provide highly reproducible culture conditions for delivery of cultured LESC of consistent quality.24
Clinical and Surgical Management and Outcomes
It has been suggested that the local tissue environment may be an important factor determining graft success.24 This notion is supported by the somewhat poorer results seen when ocular surface disease is associated with inflammation and severe dry eye.2 Hence, the recipient ocular surface may require pretreatment to optimize the conditions for the cultured LESC graft. Most importantly, this involves assessment of the ocular adnexa and subsequent correction of any lid dysfunction, treatment of dry eye disease, as well as thorough control of ocular surface inflammation.25 To avoid complications during surgery, preoperative pachymetry is recommended in cases where corneal thickness cannot be assessed reliably at the slit lamp.2
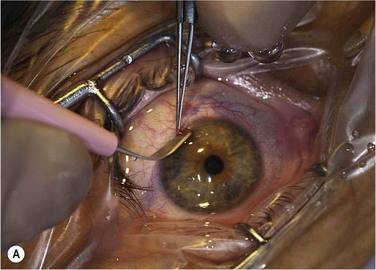
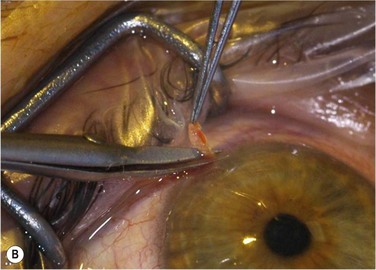
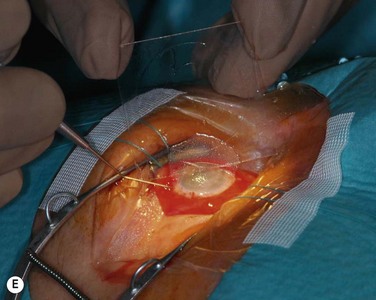
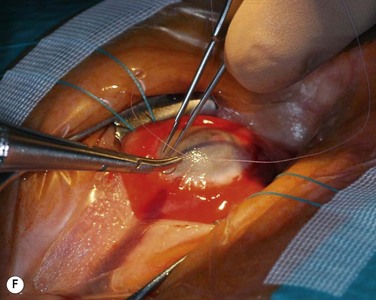
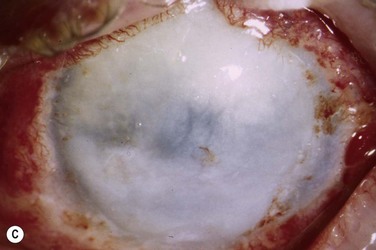
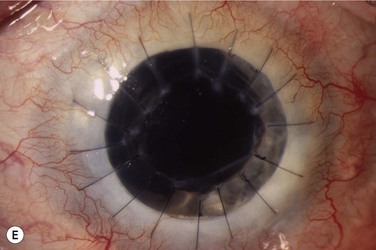
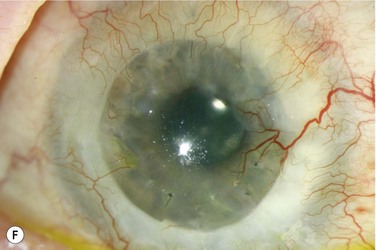
The host ocular surface is surgically prepared by performing a 360° conjunctival peritomy and removing any remaining epithelium and fibrovascular tissue which may cover the diseased cornea (see Fig. 44.6 and supplementary video 2). At this stage in some studies, mitomycin C is applied topically for a brief period.6 If significant corneal stromal thinning or scarring is present, a full-thickness or anterior lamellar corneal graft may need to be performed simultaneously or subsequent to the surface reconstruction procedure (Fig. 44.9). The cultured LESC graft is transferred to the corneal surface and sutured in place. For mechanical protection of the graft during the postoperative period, available options are therapeutic contact lenses, a secondary layer of amniotic membrane and/or temporary lid closure.
Postoperative care routinely involves administration of topical unpreserved steroid and broad-spectrum antibiotics. In addition, corticosteroids may also be administered systemically with the objective of controlling inflammation and preventing allograft rejection. Intravenous methylprednisolone can be given postoperatively for 3 days; however, most studies used a 1-month course of betamethasone or prednisolone (1 mg/kg/day).7 Although it has not been shown specifically that systemic immunosuppression is able to reduce the risk of immune rejection following cultured LESC transplantation, it is known that allogeneic limbal epithelial cells can become the target of an immunological reaction.26 Therefore, long-term immunosuppression may be of value and should be considered following cultured LESC transplantation (see Chapter 46). However, in view of the report that donor DNA could not be detected on host ocular surfaces 9 months after transplantation, it can be argued that immunosuppression should not be continued beyond this point.8 Immunosuppression is commonly achieved using oral cyclosporine A; however, there is no consensus as to what dose should be administered and when or if treatment should be reduced or discontinued. Additional topical cyclosporine A or systemic cyclophosphamide have been used in some studies, but again it remains unclear whether this yields any further reduction of the risk of immune rejection.7
Assessment of the ocular surface integrity is widely regarded the most relevant measure to judge the success of a cultured LESC graft. There is no general consensus as to how successful restoration of corneal epithelium should be determined clinically. Most studies rely on epithelial transparency and superficial vascularization, but often the parameters used to assess LESC deficiency pre- and postoperatively remain unspecified. Rama et al. developed a scoring system to assess the severity of LESC deficiency.27 This assessment relies on clinical findings, as well as the cytokeratin expression of corneal epithelial cells obtained via impression cytology and may therefore, offer an objective measure of ocular surface improvement. Other authors have advocated the use of confocal microscopy to detect the presence of conjunctival epithelium on the corneal surface.18 This method correlated well with impression cytology findings, and does not carry the risk of damaging the reconstructed epithelium.
In contrast to many other medical or surgical procedures in ophthalmology, visual acuity is considered only a secondary end point in many studies of LESC transplantation. This stems from the fact that, frequently, considerable stromal scarring impedes immediate visual recovery following ocular surface treatment. Instead, while reconstruction of the ocular surface by cultured LESC transplantation paves the way for subsequent surgery which may restore visual function, reduction of ocular symptoms has been reported from various studies.6 It has recently been suggested that quality of life should be included as an outcome measure for LESC transplantation, as this may allow a combined and structured measure of useful vision, ocular discomfort and also cosmesis.28
The most common underlying condition in patients who received cultured LESC grafts is chemical or thermal trauma, which accounts for 75% of the patients reported.6 Upon reviewing clinical studies on cultured LESC transplantation, independent groups found a generally favorable outcome with around 75% of all published cases being classified as clinically successful.6,7 This is despite the fact that the published evidence on clinical results of cultured LESC transplantation is limited by inconsistent inclusion criteria, culture protocols, and outcome measures. Systematic meta-analysis of success rates of cultured LESC grafts is therefore, not feasible at this stage.
Adverse events have been reported from a number of studies that transplanted cultured LESCs. Among the most frequently reported adverse events were bleeding (2.8%) and perforation (1.3%).6 Out of a total of 150 cases reviewed by Shortt et al.,7 infectious keratitis occurred in five patients, all of whom had received cultured limbal allografts and were therefore, immunosuppressed. In another five eyes, epithelial rejection of allografts was observed despite systemic immunosuppression. As a result, graft failure occurred in three of the five eyes. Kaplan – Meier analyses indicate that, generally, failure occurs predominantly within the first 1–2 years of grafting.
Outlook
Since the early successes in LESC culture, a multitude of clinical trials has examined their clinical usefulness for the reconstruction of the corneal epithelium. Results are favorable across many studies, and follow-up data have been recorded for periods up to 10 years in some cases.1 Molecular characterization of LESCs and the limbal stem cell niche are likely to translate into improved culture techniques in the future. However, only in conjunction with thorough understanding of the mechanisms of action of LESC transplantation in vivo and through coordinated efforts for clinical evaluation and implementation will defined culture conditions lead to clinical success in reconstruction of the corneal epithelium. Studies to compare cultured LESC grafts to other methods of limbal stem cell transplantation (which consume fewer resources for their preparation) are missing.29 Currently, there is no standard protocol for clinical grading of LESC deficiency, and no consensus on how outcomes of cultured LESC transplantation should be reported, impeding direct comparison between studies on cultured LESC transplantation. At the time of writing, efforts are under way within the LESC research community to compile such a set of standards for clinical and research applications. Furthermore, randomized, multicenter trials are required in order to standardize and optimize the available protocols for LESC expansion and transplantation.
1. Rama, P, Matuska, S, Paganoni, G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155.
2. Shortt, AJ, Tuft, SJ, Daniels, JT. Ex vivo cultured limbal epithelial transplantation. A clinical perspective. Ocul Surf. 2010;8:80–90.
3. Ahmad, S, Osei-Bempong, C, Dana, R, et al. The culture and transplantation of human limbal stem cells. J Cell Physiol. 2010;225:15–19.
4. Osei-Bempong, C, Henein, C, Ahmad, S. Culture conditions for primary human limbal epithelial cells. Regen Med. 2009;4:461–470.
5. Sangwan, VS, Vemuganti, GK, Iftekhar, G, et al. Use of autologous cultured limbal and conjunctival epithelium in a patient with severe bilateral ocular surface disease induced by acid injury: a case report of unique application. Cornea. 2003;22:478–481.
6. Baylis, O, Figueiredo, F, Henein, C, et al. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem. 2011;112:993–1002.
7. Shortt, AJ, Secker, GA, Notara, MD, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52:483–502.
8. Daya, SM, Watson, A, Sharpe, JR, et al. Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology. 2005;112:470–477.
9. O’Callaghan, AR, Daniels, JT. Concise review: limbal epithelial stem cell therapy: controversies and challenges. Stem Cells. 2011;29:1923–1932.
10. Meyer-Blazejewska, EA, Kruse, FE, Bitterer, K, et al. Preservation of the limbal stem cell phenotype by appropriate culture techniques. Invest Ophthalmol Vis Sci. 2010;51:765–774.
11. Notara, M, Shortt, AJ, O’Callaghan, AR, et al. The impact of age on the physical and cellular properties of the human limbal stem cell niche. Age (Dordr). 2012. [Epub ahead of print]
12. Saghizadeh, M, Soleymani, S, Harounian, A, et al. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Mol Vis. 2011;17:2177–2190.
13. Schlötzer-Schrehardt, U, Dietrich, T, Saito, K, et al. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85:845–860.
14. Grueterich, M, Espana, EM, Tseng, SCG. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–646.
15. Koizumi, N, Rigby, H, Fullwood, NJ, et al. Comparison of intact and denuded amniotic membrane as a substrate for cell-suspension culture of human limbal epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2007;245:123–134.
16. Shortt, AJ, Secker, GA, Lomas, RJ, et al. The effect of amniotic membrane preparation method on its ability to serve as a substrate for the ex-vivo expansion of limbal epithelial cells. Biomaterials. 2009;30:1056–1065.
17. Dua, HS, Rahman, I, Miri, A, et al. Variations in amniotic membrane: relevance for clinical applications. Br J Ophthalmol. 2010;94:963–964.
18. Shortt, AJ, Secker, GA, Rajan, MS, et al. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology. 2008;115:1989–1997.
19. Nakamura, T, Yoshitani, M, Rigby, H, et al. Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004;45:93–99.
20. Yokoo, S, Yamagami, S, Usui, T, et al. Human corneal epithelial equivalents for ocular surface reconstruction in a complete serum-free culture system without unknown factors. Invest Ophthalmol Vis Sci. 2008;49:2438–2443.
21. Li, QJ, Ashraf, FM, Rana, TS, et al. Long-term survival of allogeneic donor cell-derived corneal epithelium in limbal deficient rabbits. Curr Eye Res. 2001;23:336–345.
22. Daniels, JT, Secker, GA, Shortt, AJ, et al. Stem cell therapy delivery: treading the regulatory tightrope. Regen Med. 2006;1:715–719.
23. Schwab, IR, Johnson, NT, Harkin, DG. Inherent risks associated with manufacture of bioengineered ocular surface tissue. Arch Ophthalmol. 2006;124:1734–1740.
24. Pellegrini, G, Rama, P, De Luca, M. Vision from the right stem. Trends Mol Med. 2010;17:2177–2190.
25. DeSousa, JL, Daya, S, Malhotra, R. Adnexal surgery in patients undergoing ocular surface stem cell transplantation. Ophthalmology. 2009;116:235–242.
26. Cooper, LJ, Fullwood, NJ, Koizumi, N, et al. An investigation of removed cultivated epithelial transplants in patients after allocultivated corneal epithelial transplantation. Cornea. 2004;23:235–242.
27. Rama, P, Bonini, S, Lambiase, A, et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72:1478–1485.
28. Miri, A, Mathew, M, Dua, HS. Quality of life after limbal transplants. Ophthalmology. 2010;117:638. e1–3
29. Cauchi, PA, Ang, GS, Azuara-Blanco, A, et al. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am J Ophthalmol. 2008;146:251–259.
30. Nakamura, T, Ang, LP, Rigby, H, et al. The use of autologous serum in the development of corneal and oral epithelial equivalents in patients with Stevens – Johnson syndrome. Invest Ophthalmol Vis Sci. 2006;47:909–916.