CHAPTER 18 CT colonography
Introduction
Colon cancer is the third most common malignancy in men and the second most common malignancy in women with 35 599 new cases reported in the UK in 2005 (Cancer Research UK, 2008). Most colon cancers arise from pre-existing adenomatous polyps where the incidence of malignancy increases with size. There are well known risk factors to developing colon cancer, such as hereditary polyposis syndromes and chronic inflammatory bowel disease, but most cancers occur in patients with no predisposing risk factors other than advancing age (Tolan et al., 2007).
Computed tomography colonography (CTC) was first described in 1994 as dual position, helical computed tomography (CT) of a cleansed, gas distended colon (Vining et al., 1994). Since that time, the examination has steadily evolved to the point where it is not only advocated for the investigation of patients with symptoms of colon cancer but also as a screening test for asymptomatic patients (Levin et al., 2008).
Meta-analysis of published data demonstrates that CTC has high sensitivity and specificity rates (using conventional colonoscopy as the reference standard) for large and medium size polyps and a high reported diagnostic accuracy for symptomatic cancer (Pickhardt et al., 2003; National Institute for Health and Clinical Excellence, 2005). However, diagnostic accuracy falls with polyp size and, as with conventional colonoscopy, there may be low sensitivity in detecting flat colonic lesions (Hoon et al., 2003). Studies have also shown that CTC is significantly more sensitive than barium enema at polyp detection and has the additional advantage of being able to demonstrate significant extracolonic pathology in approximately 9% of patients (Yee et al., 2005; Spreng et al., 2005; Taylor et al., 2006; Tolan et al., 2007).
Research shows that the diagnostic accuracy of CTC is highly dependent on the quality of the examination and that meticulous attention must be given to both examination and interpretation techniques in order to achieve acceptable diagnostic performance (Ho Park et al., 2007; Rockey et al., 2007).
CTC technique
Patient information
Patient compliance is vital to achieve an optimal CTC examination, therefore, it is most important that high quality, accurate written information should be available so that patients are aware of the bowel preparation, the dietary restrictions and their effect on the diagnostic outcome. A description of what the procedure involves must be included so patients are forewarned about what they will undergo, especially the need to distend the colon with gas and the administration of spasmolytic drugs and contrast media. The information leaflet should clearly explain the risks, benefits and alternatives to the procedure, including the risk of perforation, contrast reaction, side effects of the spasmolytic drugs and the radiation dose. The information should also include advice for patients with diabetes on where and how to obtain specific information on glycemic control and specialist information for those taking Metformin (Tolan et al., 2007).
Bowel preparation and fecal tagging
Reliable detection of small polyps at CTC is heavily dependent on optimum bowel preparation. The presence of fecal residue and retained fluid can cover colonic mucosa, hide pathology and reduce the sensitivity and specificity of the examination (Mang et al., 2007).
There is currently no consensus as to the optimum bowel preparation regime for CTC, although it is generally recognized that a clean, dry colon is required. Picolax (sodium picosulphate and magnesium citrate) is widely used in the UK for barium enema and colonoscopy bowel preparation (Box 18.1) and there is evidence to support its efficacy for CTC (Taylor et al., 2003a). Patient safety and tolerance should also be considered in the choice and administration of bowel preparation as vulnerable groups, such as the elderly and those with renal impairment, may be at risk of dehydration and electrolyte disturbance. Patients with diabetes should be advised to contact departments so they can be scheduled first on the list and to contact a diabetic nurse specialist to obtain advice regarding glycemic control for the period of dietary restriction (Tolan et al., 2007).
BOX 18.1 Standard bowel preparation
The day before the procedure
| 0800 | Take one sachet of Picolax |
| Then drink as much clear fluid as you can, including clear soups, Oxo, Bovril, jelly and sweet, fizzy drinks | |
| 1600 | Take the second sachet of Picolax |
| Continue to drink as much clear fluid as you can, including clear soups, Oxo, Bovril, jelly and sweet, fizzy drinks until your examination |
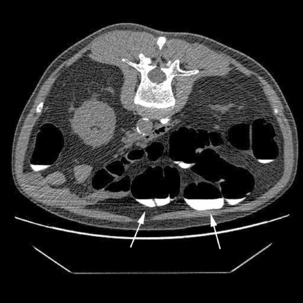
It is recognized that full bowel preparation does not always result in a completely clean colon. Techniques have been developed that allow residue and fluid to be labeled or tagged using oral contrast agents in order to avoid them being confused with pathology. There are a number of tagging protocols in use which include barium compounds, iodinated contrast media or a combination of the two (Box 18.2). It is suggested that barium is superior at tagging solid residue and iodinated contrast media is better at tagging fluid, although there is undoubtedly some overlap (Figure 18.1). The diagnostic accuracy of CTC can be further increased by using specialized computer software to perform ‘electronic cleansing’. This allows the opacified colonic fluid and barium tagged stool to be digitally removed at the post processing stage so that it does not obscure the visualization of polyps or significant pathology (Rockey et al., 2007; Mang et al., 2007).
BOX 18.2 Full purgation with stool/fluid tagging: University of Wisconsin (standard regimen)
| 24 h before | Clear liquids only |
| 18 h before | Fleet phosphosoda (45 ml) undiluted followed by 1–2 l of clear fluids |
| 15 h before | Barium 2.1% (250 ml) (plus 296 ml of magnesium citrate if bowel cleansing not commenced). Further 1–2 l of clear fluid |
| 12 h before | 60 ml gastrograffin (Bracco Diagnostics) with clear fluids |
| 8 h before | Nil by mouth until examination |
The development of fecal tagging protocols and ‘electronic cleansing’ has resulted in some centers reducing the laxative regime given to patients. This undoubtedly increases patient acceptability as the bowel preparation is often considered the most intolerable part of the examination. It also allows CTC to be performed with reduced or no laxation in those patients where it may be harmful, e.g. the elderly and those with significant comorbidity (O’Hare and Fenlon, 2006; Laudi et al., 2008).
Colonic insufflation
Good colonic distension is fundamental to obtaining a high quality examination and optimal mucosal visualization. Imaging under-distended or collapsed segments of bowel can render the examination non-diagnostic, necessitating a repeat examination or referral for colonoscopy. It may also result in pathologies being missed or lead to a false positive diagnosis. There are several strategies currently used to achieve good colonic distension which include the use of different catheters and insufflation devices, administering spasmolytics and obtaining images in the prone and supine position (Burling et al., 2006a; Mang et al., 2007).
Insufflation techniques vary between centers and a number of methods are currently used to distend the colon. These include manual insufflation of room air or carbon dioxide or the use of a commercially available automated carbon dioxide colon inflator. Inflating carbon dioxide is preferable to room air as it is more readily absorbed, resulting in less post-procedural discomfort and bloating (Burling et al., 2006a).
A small caliber (20F), soft flexible catheter is adequate to obtain optimal colonic distension and is well tolerated by the patient. Large, rigid rectal catheters of the type used for barium enema examinations have not been found to improve colonic distension and, in some cases, have led to rectal perforation and their use should be actively discouraged (Sosna et al., 2006).
The practice of using a retention balloon varies and, although the occurrence of perforation during barium enema examination is estimated to increase by a factor of 2.5 when a retention balloon is used, there are no published data to suggest this is the case during CTC. If an inflated rectal catheter balloon is used, it should be deflated during the prone series, which best visualizes the lower rectum. This, together with performing a per rectal (PR) examination, will minimize the risk of missing a low rectal tumor (Burling et al., 2006a; Tolan et al., 2007).
Manually inflating room air or carbon dioxide has the advantage of being relatively cheap and readily available. The automated colon inflator (PROTOCO2L E-Z-Em) has the advantage of using carbon dioxide as well as maintaining constant pressure and adequate insufflation of the colon throughout the study by regulating the pressure and volume of gas administered. It has also been found significantly to improve colonic distension when compared to manual insufflation. The disadvantages of the automated colon inflator are the high capital cost of the machine and the ongoing costs of the consumables (rectal catheter and tubing) (Burling et al., 2006a).
Whichever method of gas insufflation is used, there is always a risk of colonic perforation and care must be taken throughout the procedure. If gas insufflation proves difficult or the patient complains of undue discomfort, careful consideration of the causes must be made. Symptoms of bloating and mild cramping are usually good indicators that reasonable distension has been achieved. If there is any doubt a scout view should be obtained to assess the level of distension (Figure 18.2) or identify any abrupt cut off in the colonic gas pattern which may represent the presence of an obstructing stricture or mass (Tolan et al., 2007).
Patient positioning and technique
CTC insufflations/technique protocols will vary between centers and a typical technique protocol is outlined in Box 18.3. In order to reduce the duration of patient discomfort and to ensure an optimum examination is obtained, it is important the CTC is performed by well-trained, motivated staff with a special interest. Ideally, the primary read of the axial images should take place while the patient is in the examination room so that full staging can be performed if a colon cancer or extra colonic pathology is demonstrated (Tolan et al., 2007).
BOX 18.3 CTC technique using automated or manual insufflation
 For automated insufflation, zero the volume reading and set pressure to 25 mmHg or start manual insufflation
For automated insufflation, zero the volume reading and set pressure to 25 mmHg or start manual insufflation Wait until 2.5–4 l of CO2 and pressure has risen to 20 mmHg or a further 20 bulb puffs have been performed
Wait until 2.5–4 l of CO2 and pressure has risen to 20 mmHg or a further 20 bulb puffs have been performedAntispasmodics
Antispasmodics are used routinely in gastrointestinal imaging to improve colonic distension and reduce patient discomfort. There are currently two antispasmodics in general use in the UK – hyoscine-N-butylbromide (Buscopan) and glucagon (Glucagen).
Hyoscine-N-butylbromide (Buscopan) is widely used in barium enema practice and has also been shown significantly to improve colonic distension during CTC. It is only effective for about 15 minutes after injection so should be administered just before insufflation begins to allow enough time to acquire the prone and supine data sets with optimum distension. Due to the drug’s anticholinergic effect, caution must be used when giving it to patients with a cardiac history or a history of glaucoma (Taylor et al., 2003a).
Glucagon is often used in barium enema practice when Buscopan is contraindicated. However, it has been shown that during CTC, distension of the colon is not significantly improved after glucagon and that patients often experience nausea and vomiting (Rogalla et al., 2005).
Intravenous contrast
Image enhancement with IV contrast can be helpful in differentiating polyps from fecal residue and improving the detection of polyps in a poorly prepared colon. Currently, the use of IV contrast in the screening population is not advocated due to the increased risk involved and the uncertain cost effectiveness. However, IV contrast enhancement has been shown to be of benefit in symptomatic patients where the examination of extracolonic organs and detection of extracolonic abnormalities is of clinical benefit. The symptoms of colon cancer can be very non-specific and it has been demonstrated that significant extracolonic findings are relatively frequent in older symptomatic patients (Rockey et al., 2007; Soo Lee et al., 2007).
If IV contrast is administered, it is easier for both the patient and operator if this is done during the supine acquisition. Acquisition of images during the arterial phase (45s) may be more appropriate for the purposes of polyp detection since bowel wall enhancement is maximized and polyps are better visualized in this phase. However, in reality, acquisition of images in the portal phase (65–70s) is more appropriate to maximize extracolonic evaluation and optimize tumor staging (Spreng et al., 2005; Tolan et al., 2007).
CT acquisition technique
The ability of CT colonography to detect colorectal polyps is dependent on the CT acquisition parameters including slice thickness. The slice thickness should be at least half of the target polyp size to minimize partial volume averaging with adjacent air. Therefore, in practice, slice thickness should be no more than 2.5 mm, however, in reality much thinner slices are obtained with modern 16 and 64 slice scanners. Faster tube rotation times and increased detectors permit faster table speeds so that patients can be scanned quicker, reducing the chance of movement artefact and allowing scans to be acquired during one breath hold. It is generally accepted that the thin sections and faster scanning times provided by multidetector CT scanners are required for optimum results. The American College of Radiology practice guidelines (2006) recommend a KVp of 120 KV and a tube current of <100 mA for routine colonography examinations in adult patients; if IV contrast is given then normal dose settings should be employed. Typical protocols using a 16 and 64 detector CT are given in Box 18.4 (Taylor et al., 2003b; Tolan et al., 2007).
BOX 18.4 Parameters for 16 and 64 section MD CTC with IV contrast
Supine with contrast
| 16 section | 64 section | |
|---|---|---|
| Voltage | 120 KV | 120 KV |
| Effective current | 100 mA (current modulation to minimum dose) | |
| Acquisition | 16 × 0.75 mm | 64 × 0.6 mm |
| Collimation | 0.75 mm | 0.6 mm |
| Feed/rotation | 12 mm | 26.9 mm |
| Increment | 1 mm | 0.7 mm |
| Reconstructed section thickness | 1 mm |
Post CTC patient care
Patients who have undergone CTC require specific after care due to the combined effects of the bowel preparation, abdominal distension and intravenous injection of IV contrast and a spasmolytic. Once the rectal catheter is removed, the patient should be assisted to sit up slowly and advised to sit on the examination couch for a few minutes. The patient should be warned and reassured about abdominal pain. They should be escorted to a toilet which must be immediately available and near by; ideally, a shower should also be available. The patient should be offered refreshments, e.g. a cup of tea or coffee and a light snack which should be taken in a comfortable quiet environment. They should be advised to remain in the department for 15–30 minutes, especially if IV contrast has been administered (the cannula should be removed before they leave). Verbal and written information must be given with advice about the side effects of the spasmolytic drugs, the possible complications that can occur and symptoms to look out for as well as reassuring advice about abdominal pain caused by the gas (Tolan et al., 2007; Burling et al., 2006b).
Interpretation and reporting
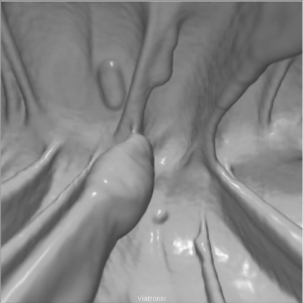
CTC images can be reviewed as two-dimensional (2D) or three-dimensional (3D) images. 2D image review refers to the sagittal images of the colon from the rectum to the cecum viewed by scrolling through the serial images in a stack mode. 3D image review refers to an optical colonoscopy-like endoluminal fly-through of a 3D reconstructed colon (Figure 18.3) and relies on specialized computer software to manipulate the data set (Figure 18.4, see color insert).
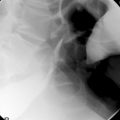
The primary 2D read should ideally occur in the CT control room while the patient is still in the examination room. This allows any significant colonic or extracolonic pathology to be identified and means that any additional imaging such as chest images or images with IV contrast may be acquired (Figure 18.5).
Formal CTC interpretation must take place at a dedicated workstation using specialist software. Optimized interpretation of CTC requires specialist reader training and robust audit practices. The method of image interpretation is the personal choice of the reporter and may consist of a primary 2D read, where the axial images are interrogated first, or a primary 3D read, where the colon is examined principally using the 3D endoluminal images. However, standard practice appears to consist of a simultaneous review of the prone and supine 2D images viewed side by side. This allows tracing of the colonic outline on each image to find small contour abnormalities which can be compared in the supine and prone view (Figures 18.6 and 18.7). 3D endoluminal fly through images can be obtained as antegrade and retrograde in both the supine and prone positions (see Figure 18.4). Taking advantage of this plethora of data can make CTC interpretation extremely time consuming and, in reality, the 3D endoluminal views are often used to problem solve in a segment of abnormal colon, to confirm the presence of a polyp (Figure 18.8) or to help distinguish between fecal residue and polyp (Rockey et al., 2007).
A recent development in CTC interpretation has been the availability of computer-aided detection (CAD). This is currently an area of active research and will undoubtedly have an impact on the future of CTC interpretation. CAD has the potential to improve the sensitivity for polyp detection and has already been incorporated into several commercial CTC reporting software systems. Although it has many advantages, CAD has the potential, when incorporated into established reading strategies, to increase reporting time and reduce specificity. Most established readers currently use CAD as a ‘second reader’, reading the data set first without using the CAD prompts, then repeating the analysis using CAD (Yoshida and Nappi, 2007).
American College of Radiology. ACR Practice guideline for the performance of computed tomography (CT) colonography in adults. 2006.
Burling D., Taylor S.A., Halligan S., et al. Automated insufflation of carbon dioxide for MDCT colonography: distension and patient experience compared with manual insufflation. Am. J. Roentgenol. 2006;186:96-103.
Burling D., Halligan S., Slater A., et al. Potentially serious adverse events at CT colonography in symptomatic patients: national survey of the United Kingdom. Radiology. 2006;239:464-471.
Cancer Research UK. UK Bowel Cancer Incidence Statistics, Available: Accessed 28/09/2008 http://www.info.cancerresearchuk.org/cancerstats/tyoes/bowel/incidence/
Ho Park S., Yee J., Hyung Kim S., et al. Fundamental elements for successful performance of CT colonography. Korean J. Radiol.. 2007;8(4):264-275.
Hoon J., Rolnick J.A., Haker S., et al. Multislice CT colonography: current status and limitations. Eur. J. Radiol.. 2003;47(2):123-134.
Laudi C., Campanella G., Galatola G., et al. PA.246 virtual colonoscopy (VC) using same day faecal tagging and limited bowel preparation. Dig. Liver Dis.. 2008;40(1):S165.
Levin B., Lieberman D.A., McFarland B., et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps. A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer and the American College of Radiology. Cancer J. Clin.. 2008;58:130-160. and for the American Cancer Society Colorectal Cancer Advisory Group, the US Multi-Society Task Force and the American College of Radiology Colon Cancer Committee
Mang T., Graser A., Schima W., et al. CT colonography: techniques, indications, findings. Eur. J. Radiol.. 2007;61(3):388-399.
National Institute for Health and Clinical Excellence. Computed tomographic colonography. (virtual colonoscopy). 2005. Interventional procedure guidance 129. Available http://www.nice.org.uk/Guidance/IPG129 Accessed 29/09/08
O Hare A., Fenlon H. Virtual colonoscopy in the detection of colonic polyps and neoplasms. Best Pract. Res. Clin. Gastroenterol.. 2006;20(1):79-92.
Pickhardt P.J., Choi R.J., Hwang I., et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N. Engl. J. Med.. 2003;349(23):2191-2200.
Rockey D., Barish M., Brill J.V., et al. CT colonography standards. Standards for gastroenterologists for performing and interpreting diagnostic computed tomographic colonography. Gastroenterology. 2007;133:1005-1024.
Rogalla P., Lemboke A., Ruckert J.C., et al. Spasmolysis at CT colonography: butyl scopolamine versus glucagon. Radiology. 2005;236:184-188.
Soo Lee S., Ho Park S., Choi E.K., et al. Colorectal polyps on portal phase contrast-enhanced CT colonography: lesion attenuation and distinction from tagged feces. Am. J. Roentgenol. 2007;189:35-40.
Sosna J., Blachar A., Amitai M., et al. Colonic perforation at CT colonography: assessment of risk in a multicenter large cohort. Radiology. 2006;239:457-463.
Spreng A., Netzer P., Mattich J., et al. Importance of extracolonic findings at IV contrast medium-enhanced CT colonography versus those at non enhanced CT colonography. Eur. Radiol.. 2005;15:2088-2095.
Taylor S.A., Halligan S., Goh V., et al. Optimizing colonic distension for multi-detector row CT colonography: effect of hyocine butylbromide and rectal balloon catheter. Radiology. 2003;229:99-108.
Taylor S.A., Halligan S., Bartram C.I., et al. Multi-detector row CT colonography: effect of collimation, pitch, and orientation on polyp detection in a human colectomy specimen. Radiology. 2003;229:109-118.
Taylor S.A., Halligan S., Slater A., et al. Comparison of radiologists’ confidence in excluding significant colorectal neoplasia with multidetector-row CT colonography compared with double contrast barium enema. Br. J. Radiol.. 2006;79:208-214.
Tolan D.J.M., Armstrong E.M., Burling D., et al. Optimization of CT colonography technique: a practical guide. Clin. Radiol.. 2007;62(9):819-827.
Vining D.J., Gelfand R.E., Bechtold R.E., et al. Technical feasibility of colon imaging with helical CT and virtual reality. Am. J. Roentgenol. 1994;162(Suppl.):104.
Yee J., Kumar N.N., Godara S., et al. Extracolonic abnormalities discovered incidentally at CT colonography in a male population. Radiology. 2005;236:519-526.
Yoshida H., Näppi J. CAD in CT colonography without and with oral contrast agents: progress and challenges. Comput. Med. Imag. Graph.. 2007;31(4–5):267-284.