CHAPTER 17 Cross-sectional investigations, nuclear medicine and ultrasound of the small and large bowel
Introduction
Most abdominal imaging is performed to investigate general abdominal symptoms, such as pain, weight loss, bloating or abdominal distension. Abdominal imaging involves a multitude of complex investigations to the uninitiated. Ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and radionuclide scanning are all important adjuncts to first line imaging: the abdominal radiograph. They are complementary to each other and the majority of clinical questions can be answered by cross- sectional imaging. Occasionally specialist investigations will be required e.g. indium-111 labelled white cell nuclear scintigraphy when the other imaging modalities have been negative and there are ongoing clinical symptoms or concern. Each investigative method is appropriate for certain clinical questions, when used appropriately it will help make an accurate diagnosis, preventing any treatment delays. To understand the role of each imaging modality you must understand the principle technique, including its value and limitations.
Evidence-based medicine can be defined as the integration of best available research with clinical expertise and patient values (Erturk et al., 2006). You can apply evidence-based practice to any clinical discipline using five basic principles:
Ultrasound
The basic essential component of an ultrasound probe is the piezoelectric (PZE) crystal in a shape of a rectangle or disk. When an alternating (AC) current is applied to the crystal, it expands and contracts with the same frequency: this is the ‘piezoelectric effect’. This produces sound waves or echoes. The echoes are transmitted undergoing ‘reflection’, returning at different velocities depending on the media the echoes have encountered within the body. Different tissues will have different acoustic impedance. The proportion of energy (or sound) reflected and transmitted depends on the acoustic impedances of the two materials. In physics terms, the acoustic impedance of a material is defined as the product of the density of the material and the velocity of the sound within it. The greater the acoustic impedance mismatches between two materials the greater the fraction of sound which is reflected and thus imaging is limited. The reflected/returning sound waves act on the PZE crystal in the ultrasound probe to produce an electric signal. The probe is connected to a powerful computer processor that converts the electrical signal into a cross-sectional image. The images are captured in real time and it can show the solid organs and the movement and flow through blood vessels using a special technique of Doppler ultrasound. The image is then displayed on a monitor and may be stored or printed.
The properties of ultrasound are unique. Unlike x-rays or light waves, which can travel through a vacuum, sound waves require a material medium to travel through and thus cannot penetrate air gaps. Ultrasound cannot image through bone or large collections of air. There is also a high acoustic impedance mismatch between the patient’s skin and the probe. This is overcome by the use of ultrasound scanning gel. The advantages and limitations of ultrasound as an imaging modality are outlined in Boxes 17.1 and 17.2.
Doppler ultrasound
The combination of two-dimensional ultrasound imaging with Doppler ultrasound is known as duplex ultrasound. Duplex is an important technique for the examination of arteries and veins.
Applied terminology
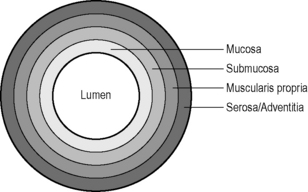
Hypoechoic: dark gray, nearly black, e.g. fat, muscularis propria of the bowel wall.
Patient preparation
Ultrasound of the kidneys
To evaluate the bladder, it must be distended fully. This is achieved by drinking one litre of water or more, an hour before the examination. If the patient is catheterized it should be clamped off, again to fill the bladder. If the bladder is only partially filled, then only comment can be made on any gross/large abnormalities. Small lesions cannot be confidently detected.
Ultrasound of the Bowel
Ultrasound of small and large bowel
Acute appendicitis in experienced hands is usually diagnosed on history, physical examination and laboratory investigations. The aim of imaging is either to confirm or refute the diagnosis; however, if the appendix is not visualized then appendicitis cannot be excluded. In a female, diagnoses which mimic appendicitis include enlarged ovarian cysts or torsion of the ovaries. Thickening of the wall of a normal appendix wall to 6 mm (3 mm or less is normal) is suggestive of appendicitis (Guillerman and Ng, 2005). Other features include non-compressibility and round in cross-section rather than oval.
Ultrasound of the bowel can be used for bowel obstruction and in Crohn’s disease. Bowel obstruction is considered present at sonography when the lumen of the fluid-filled small bowel loops were dilated more than 3 cm and the length of a segment of the dilated small bowel was over 10 cm; peristalsis of the dilated segment was increased. This is shown by rapid progression or whirling movement of bowel content (Ko et al., 1993).
Ultrasound can be used for the diagnosis of Crohn’s disease (an inflammatory bowel disease that may involve various segments of the GIT, although ileal and/or colonic involvement is most frequent); however, the specificity and sensitivity of any test depends on the prevalence of the disease in the population (i.e. the pre-test probability) (Fraquelli et al., 2005).
Endoluminal ultrasound is excellent for local staging of esophageal cancer. It can define tumor infiltration, as well as local nodal staging. This has major prognostic and management implications, and associated morbidity of open surgery if lesions are detected early. Puncture endosonographic scopes have also been developed to enable fine needle aspiration of lymph nodes and more accurately stage nodal burden (Binmoeller, 1999).
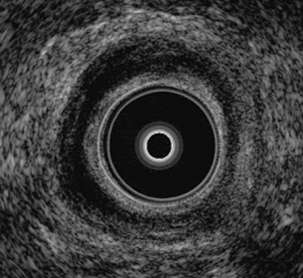
Endoanal ultrasound (Figure 17.2) essentially follows the same principle and has several specific indications. It is used to assess the anal–rectal region, in particular the sphincters. The main indication for endoanal ultrasound is in the investigation of fecal incontinence as well as anal pain (Rottenberg and Wiiliams, 2002).
Computed tomography (CT)
CT uses x-rays to produce the cross-sectional images. The x-ray tube is mounted on a gantry, which is a set of circular rotating metal rings. Directly opposite the x-ray tube is a set of detectors, which collect the information that is analyzed by a powerful computer and displayed on high resolution monitors. The gantry is positioned at the level of interest and the x-ray tube rotates 360 degrees on the gantry. The patient passes through the gantry and the image is acquired in a spiral fashion (or by multislice techniques); previously only one slice was acquired at a time. CT previously was long and cumbersome with respiratory and motion artefact causing limitation in the information acquired. When we discuss spiral or multislice imaging, imagine that the x-ray tube is mounted on an imaginary large Slinky around the patient. These data are transformed into a cross-sectional image using a mathematical technique called Fourier’s transformation. The cross-sectional data are called a data set, just like a stack of coins. The computers give an illusion of one continuous image, when in fact you are scrolling through the data set.
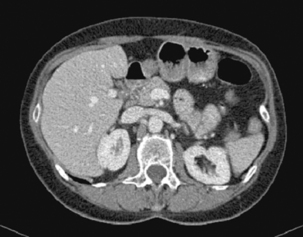
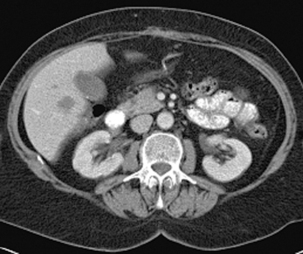
The x-ray beam passes through the patient and is detected on the corresponding side. The x-rays are attenuated to varying degree according to the different tissue densities of the body. High density components (e.g. bone, calcium, metal or contrast material) are attenuated to a greater degree than lower density tissues (fat or soft tissues) and appear white. Fat, soft tissues and lung/air allow more x-rays to pass through and appear black. The image can be manipulated using the computer by altering the ‘window’ settings. Thus you can concentrate on looking at just the lung, bone or soft tissue settings. Figures 17.3, 17.4, 17.5 and 17.6 are examples of abdominal CT anatomy.
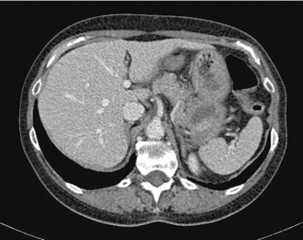
Figure 17.3 Abdominal CT with intravenous contrast (portal venous phase) at the level of the origin of the celiac axis.
Advances in modern computers, software platforms, medical physics and detector technology have all allowed greater imaging capability with improved detail and image manipulations. The data set can be reconstructed into multiplanar reconstruction (MPR) images (all three orthogonal planes; sagittal, coronal and axial) or maximum image projection (MIP) images. The advantages and limitations of CT are outlined in Boxes 17.3 and 17.4
BOX 17.3 Advantages of CT
Applied terminology
Density can be used as an alternative to attenuation.
Hounsfield unit: a unit of measurement for CT densities, named after Geoffrey Hounsfield, the inventor of CT.
Windowing: manipulation of the CT image to display differences in the gray scale
Magnetic resonance imaging (MRI)
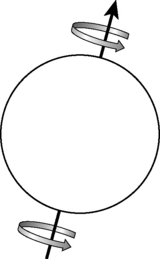
The proton has a positive electric charge and spin. Imagine the proton represents the Earth; it has a North and a South pole and a spin along an axis which is slightly tilted off the centre (Figure 17.8). This is occurring in the human body; the protons are spinning along their axis but spinning randomly. However, once placed inside the magnetic bore, the protons will align in one of two stable states. They will either align parallel (spin-up) or anti-parallel (spin-down) to the strong external magnetic field. A greater proportion will align in the parallel direction and so the net vector (the net direction) will be in the direction of the magnetic field (known as B zero).
Then a second phenomenon on the spinning protons occurs due to the influence of the magnetic field. The B zero causes a secondary spin known as precession. The frequency of the precession is an inherent property of the hydrogen atom in a specific magnetic field strength and is known as the Larmor frequency. Larmor frequency will change depending on the magnetic field strength.
The net vector in the transverse plane induces a current in magnetic coils known as radiofrequency and the RF receiver coils pick up the signal, to be converted to an image. The advantages and limitations of MRI are given in Boxes 17.5 and 17.6.
Applied terminology
The accepted MRI terminology is signal characteristics.
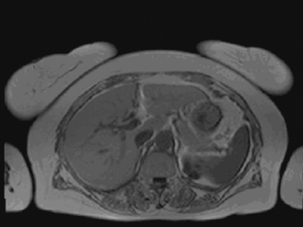
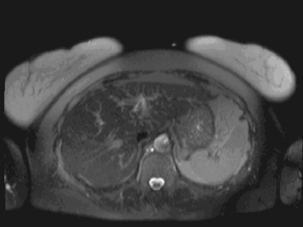
T1 weighting: This is the spin/lattice interaction, where energy is given off to surrounding tissue. It is used to indicate an image where most of the contrast between tissues is due to differences in the T1 value. A T1-weighted magnetic resonance image (Figure 17.9) is created typically by using short TE (time to echo) and TR (time to relaxation) times.
Lesions with short T1 are (bright in T1-weighted sequences):
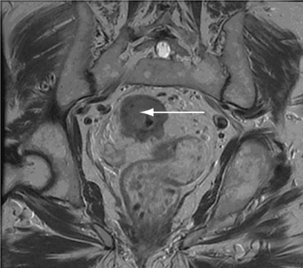
Lesions with short T2 are (dark in T2-weighted sequences) (Figure 17.10):
Lesions with long T2 are bright.
Clinical indications for magnetic resonance imaging
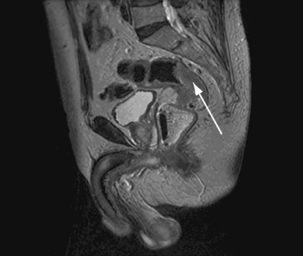
Magnetic resonance cholangiopancreaticography
The image is heavily T2-weighted so the signal from solid parenchyma is suppressed and fluid-filled structures are highlighted (Figure 17.13).
CT, MR colonography and enteroclysis
Each year in the UK, around 16 000 people die from colorectal cancer. At disease presentation, around 55% of people have advanced cancer that has spread to lymph nodes, metastasized to other organs or is so locally advanced that surgery is unlikely to be curative (Dukes’ stage C or D) (Drug Therapy Bulletin, 2006). Overall 5-year survival for colorectal cancer in the UK is around 47–51% (compared to 64% in the USA), but only 7% at most in those presenting with metastatic disease (Drug Therapy Bulletin, 2006). It is now known that colorectal carcinoma can start as an adenomatous (precancerous) polyp then develop to carcinoma in-situ and finally to frankly invasive. The risk of malignant progression increases with the increasing size of the polyp: generally adenomatous polyps 5 mm and below have 0.5% risk, greater than 10 mm have 3–10% risk and greater than 20 mm have 10–50% risk (Dahnert, 2003). Thus there is a significant advantage in detecting adenomatous polyps. Direct colonoscopy is the reference standard as simultaneous biopsy and polyp removal can be achieved. However, it is painful, requires analgesia ± sedatives, may not reach the right side of the colon and has a risk of perforation (Lee et al., 1994). (Please refer to Chapter 20 for a more detailed discussion.)
Developments such as MR and CT colonography (CTC) allow examination of the colon and rectum with the possibilities of detecting polyps and/or carcinomas (Pickhardt et al., 2003). It can be used in both symptomatic and asymptomatic patients who have a high risk of developing cancer or even those of average risks.
CTC (Figures 17.14, 17.15, see color insert and 17.16) is less invasive with no clinically significant complications (Yee et al., 2001; Pickhardt et al., 2003) unlike direct colonoscopy; though full bowel preparation is required so fecal residue is not misinterpreted. Antispasmodic prior to the scan is given and a distended colon to patient’s toleration, with full distention being the optimum situation. Distention is achieved by insufflation with air or carbon dioxide and spiral acquisition with breath hold in both supine and prone positions (National Institute for Clinical Excellence). The data obtained are used to produce a 2- and 3-dimensional image of the entire colon and rectum, the software enabling the operator to manipulate the data set in order to define lesions. CTC has high average sensitivity and specificity for large and medium polyps in the symptomatic population, but the findings in the asymptomatic population have not been established due to heterogeneity and small numbers in the cohort groups for the various studies (Halligan et al., 2005). CTC techniques are explored further in Chapter 18.
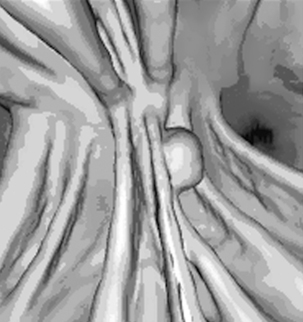
Figure 17.16 Reconstructed CT colonographic image of the colonic polyp as seen in the original dataset of Figure 17.14.
MR colonography on the other hand has no radiation exposure but still requires antispasmodics to minimize bowel peristalsis and spasm. The colon is distended with several litres of tap water with the patient in the prone position. Once complete filling is achieved with adequate colonic distention, intravenous gadolinium is given. The examination is only performed in the prone position and generally it is well tolerated (Lauenstein et al., 2002).
MR enteroclysis imaging is a technique for evaluation of the small bowel, in particular patients with Crohn’s disease. The technique involves administration of 1.5–2 litres of isosmotic water solution through a nasojejunal catheter, which ensures distention of the bowel and facilitates identification of wall abnormalities. This is similar to the traditional enteroclysis technique, but an antiperistaltic agent is essential to acquire images free of motion artefacts. The patient is then imaged with special MRI sequences: FISP (fast imaging with steady state precession) and HASTE (half Fourier acquisition single shot turbo spin echo) (Prassopoulos et al., 2001). The major advantage is the lack of ionizing radiation especially in patients who are diagnosed at a young age with inflammatory bowel disease and will have to have repeated examinations for follow up, disease status or its complications. It provides excellent soft tissue contrast with 3-dimensional images and is able to provide extramural information. However, the spatial resolution is not yet sufficient to delineate superficial abnormalities. It is emerging, but it is not the initial investigation of choice as its clinical utility has not been fully established.
Nuclear medicine
Nuclear medicine uses radioactive labeled pharmaceuticals, which are injected into the patient, and the image is captured by a gamma camera. The radionuclide emits gamma rays (majority of cases); the most commonly used radionuclide is technetium. The radionuclide compound used depends on the area to be examined. The gamma camera detects the gamma rays and converts the absorbed energy into an electrical signal; this is analysed by a computer and then displayed as an image.
Nuclear scintigraphic examinations are highly sensitive to pathology and can detect abnormalities early before changes are apparent on any other cross-sectional imaging. Anatomically detail is not specific but broad anatomical regions can be read. Its other strength is that it provides functional imaging, i.e. how an organ is working or not. The advantages and limitations of scintigraphy are given in Boxes 17.7 and 17. 8.
BOX 17.8 Limitations of scintigraphy
Applied terminology
Hot: an area of increased radionuclide activity.
Photopenic/cold: an area of decreased radionuclide activity.
Nuclear medicine in abdominal imaging
The role of nuclear medicine in relation to the detection of GI bleeding has specific indications. GI bleeding is typically intermittent and requires active bleeding to be localized. When other investigations have been unable to detect the exact site, it is imperative to ‘stop the tap’ in patients where the bleeding is massive or recurrent. This will determine who may benefit from aggressive treatment and who would be appropriate for medical management. The full scintigraphy protocol has been set by the Society of Nuclear Medicine, which can be downloaded from the website (www.snm.org). It must be emphasized that only hemodynamically stable patients should undergo nuclear scintigraphic scan and the unstable patients should immediately undergo angiography (Hastings, 2000). A high level of certainty in localizing the site of bleeding is possible when located in the stomach or the colon. Localizing the bleeding site is less sensitive in the small bowel, especially from the jejunum and ileum (Holder, 2000).
There are several scintigraphic factors which affect the differences in detecting bleeding sites: they include dynamic imaging, delayed imaging and the extra large field of view needed to visualize the upper and lower gastrointestinal tract simultaneously.
Physiological factors affecting detection are: the bleeding flow rate (rapid, moderate, slow, intermittent, minimal, antegrade and retrograde bleeding); the specific bleeding site; and any previous bowel surgery. An indication of the clinical application for scintigraphic assessment in abdominal imaging is given in Table 17.1.
Table 17.1 Clinical application for scintigraphic assessment in abdominal imaging
| Organ | Radionuclide | Clinical application |
|---|---|---|
| Liver/spleen | 99 mTc | Liver/splenic masses |
| Bile ducts/gallbladder | 99 mTc-IDA | Acute cholecystitis, biliary obstruction, biliary atresia, post liver transplant |
| Adrenal medulla | 131I-MIBG | Localization of pheochromocytoma, staging of neuroblastoma (pediatric population in particular) |
| Gastrointestinal bleeding | 99 mTc-labeled red blood cells | Acute gastrointestinal bleeding |
| Occult infections | 67gallium, 111 indium | Patients with abdominal abscess or pyrexia of unknown origin |
Positron emission tomography CT (PET-CT)
PET-CT is a relatively new imaging technique. PET requires radionuclides that decay by positron emission. A cyclotron produces positron-emitting isotopes. The radionuclide is attached to a biological compound to form a radiopharmaceutical. This is injected into the patient and a PET camera detects the emission. PET is similar to the other scintigraphic examinations: it provides functional information, but similar to other nuclear medicine investigations, it is not specific anatomically (Figures 17.17, 17.18, see color insert).
Acknowledgment
I would like to acknowledge Emma Hornsby and Dinesh Ranatunga for their help in proof reading.
Binmoeller K.F. Endoluminal ultrasound for oesophageal cancer. Dig. Endosc. 1999;11(1):3-6.
Dahnert W. Radiology review manual, fifth ed. Lippincott Williams & Wilkins, 2003. ISBN 0–7817–4822–4
Drug Therapy, Bulletin. Population screening for colorectal cancer. Drug Ther. Bull.. 2006;44(9):65-68.
Erturk S.M., Ondategui-Parra S., Otero H., Ros P.R. Evidence-based radiology. J. Am. Coll. Radiol. 2006;3(7):513-519.
Fraquelli M., Colli A., Paggi S., et al. Role of ultrasound in detection of Crohn’s disease: Meta-analysis. Radiology. 2005;236:95-101.
Guillerman R.P., Ng C.S. Ultrasound of appendicitis. Ultrasound. 2005;13(2):78-85.
Halligan S., Altman D.G., Taylor S.A., et al. Evidence-based practice. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology. 2005;237:893-904.
Hastings G.S. Angiographic localization and transcatheter treatment of gastrointestinal bleeding. Radiographics. 2000;20:1160-1168.
Holder L.E. Radionuclide imaging in the evaluation of acute gastrointestinal bleeding. Radiographics. 2000;20(4):1153-1159.
Ko Y.T., Lim J.H., Lee D.H., Lee H.W., Lim J.W. Small bowel obstruction: sonographic evaluation. Radiology. 1993;188:649-653.
Lauenstein T.C., Goehde S.C., Ruehm S.G., Holtmann G., Debatin J.F. MR colonography with barium-based faecal tagging: initial clinical experience. Radiology. 2002;223:248.
Ledermann H.P., Borner N., Strunk H., Bongartz G., Zollikofer C., Struckmann G. Review: bowel wall thickening on transabdominal sonography. Am. J. Radiol. 2000;174:107-117.
Lee P.Y., Fletcher W.S., Sullivan E.S., Vetto J.T. Colorectal cancer in young patients: characteristics and outcome. Am. Surg. 1994;60:607-612.
National Institute for Clinical Excellence, Interventional procedures programme. Interventional procedures overview of computed tomography colonography (virtual colonoscopy). www.nice.com
Pickhardt P.J., Choi J.R., Hwang I., et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N. Eng. J. Med.. 2003;349:2191-2199.
Prassopoulos P., Papanikolaou N., et al. MR enteroclysis imaging of Crohn disease. Radiographics. 2001;21:S161-S172.
Rottenberg G.T., Williams A.B. Pictorial review: Endoanal ultrasound. Br. J. Radiol. 2002;75:482-488.
Society of Nuclear Medicine procedure guidelines for gastrointestinal bleeding and Meckel’s diverticulum scintigraphy. 1999 www.snm.org Version 1.0
Strassburg J. The MERCURY experience of MRI. Tech. Coloproctology. 2004;8:S16-S18.
Yee J., Akerkar G.A., Hung R.K., et al. Colorectal neoplasia: performance characteristics of CT colonography for detection in 300 patients. Gastrointest. Imag. 2001;219:685-692.
Farr R.F., Allisy-Roberts P.J. Physics for medical imaging. W.B. Saunders, 2002.
McRobbie D.W., Moore E.A., Graves M.J., Prince M.R. MRI from picture to proton. Cambridge University Press, 2002.
Wheater P.R., Burkitt H.G., Daniels V.G. Functional histology. A text and colour atlas, second ed., Churchill Livingstone; 1996:201-224.