94 Critical Care Nutrition
Less than ideal NT is unfortunately provided to a significant proportion of ICU patients. A recent evaluation of nutrition practices in 158 ICUs across 20 countries by Cahill et al.1 reported significant deficits in meeting caloric and protein goals and adhering to the provision of specialized nutrition. For example, delivery of protein goals was only achieved 60.3% of the time.
 Gut Use and Differential Response to Feeding and Starvation
Gut Use and Differential Response to Feeding and Starvation
Lack of adequate food intake is a frequent problem in the ICU. Diseases are frequently associated with significant anorexia and/or inability to eat. Surgical procedures and diagnostic tests often demand an empty stomach. A nil per os (NPO) order is too easily written even in the absence of a logical reason to do it. Despite physiologic differences between starvation in a healthy individual and lack of adequate intake during illness, it is essential to study starvation as an important aspect of nutritional care in the critically ill patient. The functional and structural integrity of the GI tract is affected by whether the gut is used and the patient receives enteral feeding. Animal and human studies suggest that enteral feeding maintains mucosal mass, stimulates cellular proliferation and production of brush-border enzymes, and maintains villus height.2–4 Enteral nutrients maintain the integrity of tight junctions between intestinal epithelial cells, stimulate blood flow to the gut, and promote release of a variety of endogenous agents such as cholecystokinin, gastrin, bombesin, and bile salts—substances with trophic effects on intestinal epithelium. Bombesin, for example, can reverse all the histologic and functional deficits caused by parenteral feeding,5 and gastrin and cholecystokinin can encourage partial recovery of gut-associated lymphoid tissue after the use of parenteral nutrition (PN).6 Secretory immunoglobulin A (sIgA) and the production of bile salts help coat bacteria within the GI tract, preventing adherence. Along with the production of mucus and good GI contractility, this helps wash away bacteria in a caudad direction.3 These mechanisms, together with antimicrobial secretions such as pancreatic enzymes, proteases, and lactoferrin help keep the total number of bacteria in check. The normal predominant anaerobic flora of the gut is maintained, preventing overgrowth of more pathogenic organisms such as Enterobacteriaceae, a process referred to as colonization resistance.7
Gut disuse, with or without PN, can lead to deterioration of the functional and structural integrity of the gut. In animals, gut disuse is associated with a marked reduction in villus height, cellular proliferation, mucosal mass, and brush-border enzymes. Intestinal changes caused by starvation in humans are less pronounced than in rodents, but whereas gut disuse may result in a 40% decrease of mucosal mass in rats, the decrease in humans still appears to be about 10% to 15%.2 In humans, loss of villus height in response to pancreatitis is diminished by enteral feeding.4 Villus atrophy is perpetuated in a time-dependent fashion with parenteral feeding.3 Starvation alone may be insufficient to increase gut permeability, but injury followed by starvation increases mucosal permeability proportional to the severity of disease.3,8 Increased permeability is prevented through early feeding, and in burns inversely correlates with the amount of enteral feeding delivered.9 Increases in gut permeability are associated with systemic endotoxemia in humans.8,10 Among burn patients, infection is associated with increased gut mucosal permeability.9 Increases in gut permeability in critically ill patients correlate with the development of organ dysfunction.11
Lack of feeding in animals results in bacterial overgrowth and loss of mucosal defenses against bacterial invasion.7,12 Reduced peristalsis (ileus) can contribute to bacterial overgrowth. Reduced secretions of bile salts and sIgA promote bacterial adherence to the mucosa. Bacterial translocation, a process whereby bacteria transgress the mucosal barrier, is associated with aerobic bacterial overgrowth and decreased intestinal sIgA levels.3 Recent animal studies suggest that these gut-derived factors can reach the systemic circulation via the lymphatic system rather than via the portal bloodstream and thereby cause distant organ injury.13 Thus, animal models suggest that bacterial overgrowth in the lumen leads to bacterial translocation, potentially being a portal for development of sepsis and organ failure.
The significance of bacterial translocation in humans as a cause of systemic illness is still unclear.14,15 Translocation of bacterial products such as endotoxin may also occur. Endotoxin itself, when infused in even small doses in normal volunteers, increases gut mucosal permeability.7 The intestinal secretion of sIgA is diminished within 5 days of gut disuse, with or without PN.5,16 Respiratory tract secretion of IgA may be diminished even sooner. Reduction in the mucosal mass of gut-associated lymphoid tissue and decreased sIgA production increase susceptibility to infections normally controlled by IgA-mediated defenses in experimental animals.17 In mice, as little as 5 days of gut disuse with PN results in loss of protection against respiratory viral infection and reduces clearance of the virus.18 Refeeding with enteral nutrients restores antiviral defenses. Established antiviral mucosal immunity is lost when the GI tract is not stimulated by enteral feeding.17
Dendritic macrophages act as antigen-presenting cells that release cytokines and activate naive CD4+ helper T cells (TH0).19 Secretion of interleukin (IL)-12 stimulates the naive cells to differentiate into T helper 1 (TH1) lymphocytes, favoring a proinflammatory response and release of other proinflammatory cytokines such as IL-2, interferon gamma (IFN-γ), and tumor necrosis factor (TNF). TH1 responses are associated with increased inflammation and are essential for host defenses against infection. Uncontrolled TH1 responses, however, can result in self-injury. Production of IL-4 also stimulates differentiation of TH0 into TH2 lymphocytes,19 leading to secretion of additional IL-4, IL-6, and IL-10. The TH2 response tends to curb or check the TH1 inflammatory response. TH2 responses are essential to prevent self-injury caused by inflammation. However, excessive regulation of inflammatory responses by TH2 cytokines can lead to immune suppression.19
Gut disuse, with or without PN, alters the balance of these lymphocyte populations and the profile of associated cytokines. In animals, gut disuse with PN for 5 days decreases IL-4 and IL-10 secretion and markedly reduces sIgA levels.17 In human babies, use of PN reduces sIgA in intestinal immunocytes.20 IFN-β, IL-5, and IL-6 production by TH1 lymphocytes is not affected by gut disuse and PN.21 Thus, the absence of enteral nutrition (EN) can unbalance the ratio of proinflammatory to antiinflammatory responses.
Gut disuse affects expression of adhesion molecules required for proper homing by naive B cells to the intestinal lamina propria and gut-associated lymphoid tissue. MADCAM-1 is the primary ligand required for the proper homing of B cells, and decreased expression of this molecule interferes with the normal migration of B cells from the vascular space into the lamina propria, leading to atrophy of Peyer’s patches. In animals, there is a 60% decrease in MADCAM-1 expression within 4 days of initiating PN.17 Within 3 days of starting PN, the number of T and B cells in the lamina propria and Peyer’s patches decreases by about 50%.3 In this model, secretion of the TH1 cytokine, IFN-γ, is unchanged, but secretion of the TH2 cytokines, IL-4 and IL-10, decreases. Decreased production of IL-4 and IL-10 leads to increased expression of the adhesion molecules, ICAM-1 and E-selectin, in both the intestinal and pulmonary microvasculature. Increased E-selectin expression on endothelial cells in the pulmonary microvasculature promotes sequestration and extravasation of polymorphonuclear neutrophils.22–24 As a result, any subsequent injury (e.g., ischemia-reperfusion) can promote accumulation of polymorphonuclear neutrophils in the lungs, exacerbating organ injury and even increasing mortality.25,26 Abundant data demonstrate that gut disuse through starvation, either caused by disease or through ill-advised physician orders or neglect, is a real problem that contributes to development of systemic infections, a systemic inflammatory response, and development of multiple organ failure.
EN, particularly if started early, prevents the ill effects of starvation. Normal enteral feeding stimulates proliferation of TH2 CD4+ helper T lymphocytes and the production and release of IgA-stimulating cytokines including IL-4, IL-5, IL-6, IL-10, and IL-13.27 This process is normally counterbalanced by proliferation of TH1 CD4+ helper T lymphocytes and IgA-inhibitory cytokines including IFN-β, TNF, and IL-2. IL-4 stimulates naive CD4+ helper T lymphocytes to convert to IgA-positive B cells in Peyer’s patches. IL-10, IL-5, and IL-6 stimulate the differentiation of IgA-positive B cells into sIgA-secreting plasma cells in the lamina propria.17
Approximately 1 ton of food passes through the intestinal tract of an adult human every year.20 About 1/100,000 of this intake represents intact immunologic antigen.20 Oral tolerance refers to the process whereby the immune response is down-regulated to prevent excessive responses to common antigens found in food and in the commensal bacterial flora of the GI tract. During the induction of oral tolerance, an alternative pathway for CD4+ helper T-cell activation leads to proliferation of special regulatory T cells (TH3 and Tr1) which produce the counter-regulatory cytokines, IL-10 and transforming growth factor beta (TGF-β).20 The stimulation and proliferation of TH3 cells induced by enteral feeding therefore promotes expression of a balanced TH2/TH1 profile. The large dietary and indigenous microbial antigenic load is extremely important for maintaining normal mucosal immunity.20 Antigenic constituents of food clearly exert a stimulatory effect on the intestinal B-cell system, helping to explain why enteral feeding supports a high density of IgA-secreting immunocytes within the intestinal lamina propria. Continued enteral feeding, as well as maintenance of the indigenous microbial flora in the gut, may help keep a balance between the TH1 and TH2 profile and prevent an exaggerated TH1 inflammatory response.
The importance of EN for modulating the inflammatory response was illustrated by a classic study of human volunteers challenged with a small dose of Escherichia coli lipopolysaccharide (endotoxin).28 One group of subjects was maintained for 1 week without feeding and received PN, whereas another group was fed enterally during the same period. After 7 days of either PN or EN, both groups were challenged with lipopolysaccharide. The subjects in the PN group had an exaggerated response to the proinflammatory stimulus, manifested by higher circulating levels of cortisol and TNF, among other findings. Similarly, following injury or an inflammatory disease process, early enteral feeding can blunt the hypermetabolic response.29,30 Among patients with acute pancreatitis, those fed enterally rather than parenterally had significantly lower circulating levels of C-reactive protein, less evidence of oxidative stress, faster resolution of systemic inflammatory response syndrome (SIRS), and a greater decrease in their Acute Physiology and Chronic Health Evaluation (APACHE) II scores over a week of nutritional therapy.14 In another study of patients with acute pancreatitis, there was faster resolution of the disease process among patients treated with enteral feeding compared with similar patients receiving PN.31
The traditional model of SIRS and the compensatory antiinflammatory response syndrome (CARS) described in trauma and sepsis may be influenced by the differential immunologic response between enteral feeding and starvation or gut disuse.32 In SIRS, there appears to be an up-regulated, nonspecific activation of the innate immune system, with an increase in the expression of proinflammatory cytokines such as IL-1, TNF, IL-2, and IFN-γ. This profile is similar to that of the TH1 subset response (in which IFN-γ, TNF, and IL-2 are produced). Intracellular bacteria and viruses absorbed through the intestinal epithelium may activate dendritic cells, macrophages, and natural killer cells to produce IL-2 and IFN-γ, which causes naive CD4 cells to proliferate into TH1 cells. CARS, in contrast, appears to be a pattern of macrophage deactivation, reduced antigen presentation, and T-cell anergy, which results in a shift of the T–helper cell pattern to a TH2 response.32 Gut disuse following injury or illness may promote a SIRS response through stimulation of both the innate immune system (causing a hyperinflammatory response from macrophages and natural killer cells) and the acquired immune system (resulting in a shift from a TH2 to a TH1 profile).
Compared with the metabolic response to enteral feeding, this exaggerated stress response to gut disuse with or without PN has been shown to exacerbate disease severity, increase the rate of complications, and lead to prolongation of the disease process.14,31 There is thus little justification to prolonging NPO status in critically ill patients beyond the period absolutely necessary during resuscitation, surgery, or other procedures. Process-improvement efforts aimed at minimizing starvation is an important goal of any modern ICU.
 Impact of Enteral Nutrition on Outcome
Impact of Enteral Nutrition on Outcome
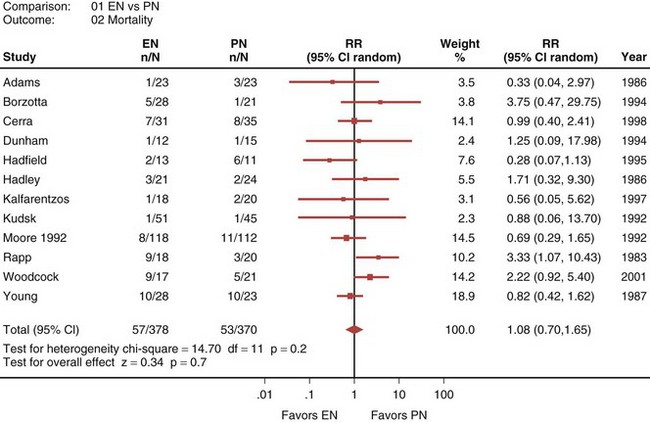
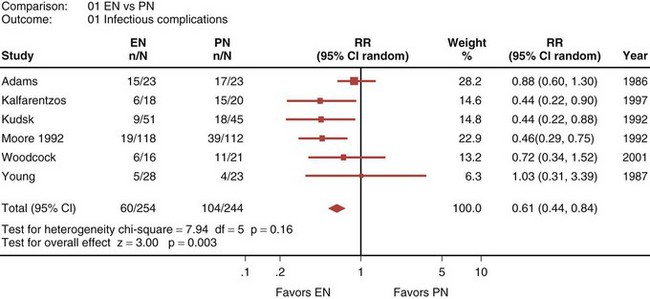
A recently published systematic analysis reviewed data from 13 randomized controlled studies comparing EN and PN in heterogeneous populations of ICU patients, including those with head trauma, abdominal trauma, sepsis, and severe acute pancreatitis, among other conditions.33 When a meta-analysis was carried out, there was no apparent difference in mortality rate between patients treated with EN and those treated with PN (relative risk [RR] 1.08; 95% confidence interval [CI], 0.70-1.65; Figure 94-1). However, compared with PN, EN was associated with a significant reduction in infectious complications (RR 0.61; 95% CI, 0.44-0.84; Figure 94-2).
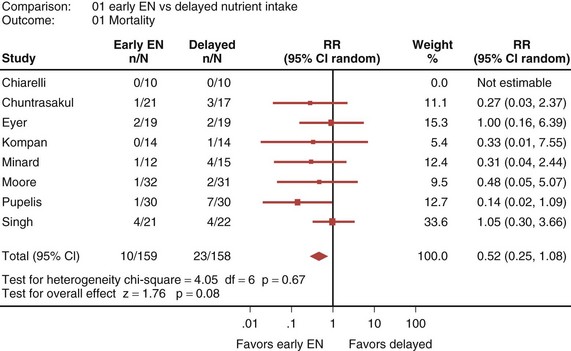
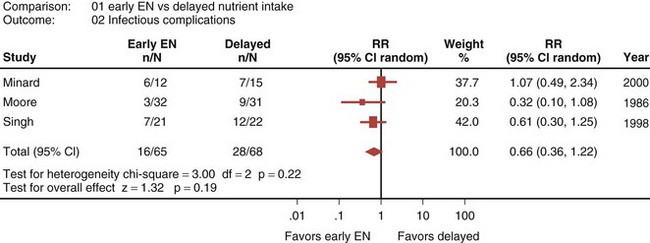
Eight randomized controlled trials that compared early EN with more delayed forms of nutrition were recently reviewed and analyzed.33 When these studies were aggregated, early EN was associated with treatment benefits that approached statistical significance. Early EN was associated with reduced mortality (RR 0.52; 95% CI, 0.25-1.08; Figure 94-3) and fewer infectious complications (RR 0.66; 95% CI, 0.36-1.22; Figure 94-4) compared with delayed nutrient intake. These differences approached but did not achieve statistical significance. No differences in length of hospital stay were observed between the groups. All seven studies that reported nutritional endpoints (e.g., nitrogen balance) showed a significant benefit for early EN. There were no differences in complications between the groups.
A number of strategies can be employed to maximize the delivery of EN while minimizing the risks of gastric colonization, gastroesophageal regurgitation, and pulmonary aspiration (Box 94-1). By delivering enteral feeds into the small bowel beyond the pylorus, the frequency of regurgitation and aspiration is decreased.34 In a recent meta-analysis, there were seven randomized trials that evaluated the effect of route of feeding on rates of ventilator-associated pneumonia.35 When these results were aggregated, there was a significant reduction in ventilator-associated pneumonia with feeding distal to the pylorus (RR 0.76; 95% CI, 0.59-0.99). These studies also demonstrated that small-bowel feeding is associated with an increase in protein and calories delivered and a shorter time to attain the target dose of nutrition.
Unless logistic problems represent an unacceptable hurdle, we recommend routine use of small-bowel feedings. If routine use of this strategy is not feasible, small-bowel feedings should be considered for patients at high risk for intolerance to EN (e.g., patients receiving inotropic or vasoactive drugs, continuous infusion of sedatives, or paralytic agents; or those with large volumes of nasogastric drainage) or at high risk for regurgitation and aspiration (e.g., patients kept supine). Finally, if obtaining small-bowel access is not feasible (e.g., because access to fluoroscopy or endoscopy is limited and blind techniques are not reliable), small-bowel feedings should be considered for selected patients who repeatedly have large gastric residual volumes and are not tolerating adequate amounts of EN intragastrically.33 Additional strategies to maximize the benefits of EN while minimizing the risks (see Box 94-1) include caring for the patient with the head of the bed elevated 30 to 45 degrees,36 using GI promotility agents, reducing doses of opioids,37 and using nurse-directed feeding protocols that include frequent checking of gastric residual volumes.38,39
 Assessment of the Critically Ill Patient
Assessment of the Critically Ill Patient
The clinician must first evaluate the level of stress in a critically ill patient to determine the likelihood of deterioration in nutritional status and to assess the overall need for aggressive nutritional support. Standardized scoring systems such as APACHE II or APACHE III, the Injury Severity Score (ISS), and the Abdominal Trauma Index (ATI) can be helpful for determining the level of stress and the likelihood of deterioration in nutritional status.40,41 Scoring systems have also been used to assess the need for nutritional support in patients with acute pancreatitis.42 Patients with an APACHE II score greater than 10 and having more than three Ranson’s criteria require additional nutritional support.42
Assessment of the patient’s nutritional status is difficult in the ICU. Classic chemical biomarkers, such as circulating levels of albumin and prealbumin, and immunologic parameters, such as lymphocyte counts, are all affected by the inflammatory response observed in critical illness. It is also the case for other parameters such as isokinetic dynamometry. Clinicians therefore have to be diligent at obtaining an excellent history and physical examination, identifying clinical signs of malnutrition. A history of poor nutrient intake and recent weight loss should alert the clinician that gut assimilation may be a problem, that more aggressive delivery of enteral or parenteral nutrients is appropriate, and that there is a greater need to meet calorie and protein requirements sooner in the hospital course (see Figure 94-2).
If the overall level of stress and severity of illness indicate the need for nutritional support, the clinician must next evaluate the status of the GI tract. Intravascular volume status should be optimized before initiating enteral feeds. It is not safe to infuse nutrients into the gut if there is ongoing ischemia or a high risk of mesenteric hypoperfusion. Feeding standard enteral formulas to patients with hypotension, hypovolemia, or septic shock, especially when vasopressors are being used to support blood pressure, may precipitate bowel ischemia.43
When EN is started soon after the onset of critical illness (i.e., enteral nutrients have been lacking only a short time), one can presume that the integrity of the intestinal mucosa is well maintained, and a standard enteral formula can be used. If the period of gut disuse has been more prolonged, the clinician must consider the possibility that mucosal integrity is not normal. If there is evidence of malassimilation and diarrhea, enteral formulas containing oligopeptides may enhance absorption and assimilation of protein.44 In a recent review of 19 prospective randomized trials in humans, 11 studies showed evidence of clinical benefit when oligopeptide-containing formulas were used instead of standard enteral (intact protein) formulas. Although there was no impact on patient outcome, the benefits of oligopeptide-based diets included significantly improved nitrogen absorption, higher visceral protein levels, more weight gain, less frequent stooling, and reduced stool volume.44
Once EN is started, the clinician must monitor tolerance. Overall assimilation of nutrients by the enteral route is assessed clinically by checking for the presence or absence of diarrhea. Additionally, it is important to monitor circulating concentrations of glucose, triglycerides, urea nitrogen, and creatinine ratio. Risk factors for aspiration include age older than 60 years, decreased level of consciousness, bolus feeding, and supine position.45–47 Gastric residual volumes, output from the gastric port of an aspiration or feeding tube, and passage of stool and gas are valuable indices of intestinal motility.
 Practical Considerations
Practical Considerations
All critically ill patients require a nutritional evaluation, and many benefit from receiving nutritional therapy. The complexity and degree of nutritional intervention necessary is proportional to the patient’s severity of illness. In the ICU, nutritional intervention is subject to the same rules of any medical therapy demonstrating a mechanism of action, a benefit in clinical outcome, acceptable risks and side effects, and ideally a cost benefit. The form of nutritional intervention a given patient should get is not necessarily intuitive. In certain disease processes associated with high severity of illness—trauma, burns, acute pancreatitis, acute respiratory failure requiring mechanical ventilation, for example—the decision to use the parenteral rather than the enteral route for feeding can significantly affect outcome.48,49
The greater importance of EN among sicker patients was first shown by evaluating septic complications in trauma patients randomized at the time of surgery to receive either PN or EN.50 Patients were ranked for severity of disease by their ATI scores. Among patients with ATI scores higher than 24, the incidence of septic complications was greater in the PN group than in the EN group (47.6% versus 11.1%; P < 0.05). Among patients with moderate illness and ATI scores lower than 24, there was no significant difference in the incidence of septic complications between the PN and EN groups (29.2% versus 20.8%; P = NS).50
Further evidence of the importance of maintaining gut integrity in patients with more severe disease was provided by a series of prospective randomized controlled trials of EN versus PN in patients with acute pancreatitis.14,51,52 In the first trial published, feeding by the enteral route was shown to be safe, but only 19% of the patients had severe pancreatitis, and there were no differences in the rates of nosocomial infection, organ failure, or overall complications.52 In a second study, 38% of the patients had severe pancreatitis, and a significantly greater percentage of those fed enterally rather than parenterally had resolution of SIRS over the first week of therapy (81% versus 17%; P<0.05); nevertheless, there were no differences between the groups with respect to rates of nosocomial infection or complications.14 In a third study, 100% of the patients had severe pancreatitis, and septic complications were reduced from 50% in the PN group to 28% in the EN group (P<0.05); the overall rate of complications was reduced from 75% in the PN group to 44% in the EN group (P<0.05).51
For EN support, clinicians must determine caloric requirements in order to set a goal or mandatory threshold for the volume, or “dose,” of enteral feeding provided. Use of indirect calorimetry or simplistic equations (e.g., 25 kcal/kg/d) to estimate caloric requirements can help identify this threshold amount. Focusing on such a goal volume allows clinicians to determine a dose/response effect of enteral tube feeding; that is, the percentage of this goal volume required to achieve desired therapeutic endpoints (maintenance of gut integrity, containment of intestinal permeability, attenuation of the stress response, reduction of overall disease severity). In the early stages of critical illness, patients are in the throes of the hypermetabolic stress response and more prone to ileus owing to higher doses of narcotics, electrolyte abnormalities, and shifts in fluid volume. In this situation, it is difficult to provide full caloric requirements. The minimum amount or volume of feeds (as a percentage of total caloric requirements) sufficient to achieve the desired therapeutic effect is not known. Recent evidence suggests that “trophic” or “trickle” rates of feeding (usually meaning 10-30 mL/h of a nutritional formula containing ≈1 kcal/mL) are probably inadequate to provide demonstrable benefits. Data from clinical studies indicate that 50% to 65% of goal calories are needed to prevent increases in intestinal permeability in burn victims9,53 and bone marrow transplant patients (M.T. Demeo, personal communication), promote better and faster return of cognitive function in head injury victims,54 and reduce the duration of mechanical ventilation and ICU and hospital length of stay in critically ill patients.55 When higher feeding rates are not feasible, trickle feeds may have limited value and should be provided, but efforts to infuse greater volumes should be continued.
 Immunonutrition
Immunonutrition
L-arginine
The amino acid, L-arginine, plays fundamental roles in protein metabolism and polyamine synthesis and is a critical substrate for nitric oxide (NO) production.56 L-Arginine stimulates the release of growth hormone, insulin growth factor, and insulin, all of which may stimulate protein synthesis and promote wound healing. The enzyme, L-arginase, metabolizes L-arginine to L-ornithine, an amino acid implicated in wound healing. NO is produced by a family of enzymes called nitric oxide synthases (NOSs) which exist in constitutive and inducible isoforms.57 Under normal conditions and in some disease states, small quantities of NO are synthesized by the constitutive forms, which have a beneficial effect on tissue oxygenation, vasodilation, and immune function.58
Omega-3 Fatty Acids
Experimentally increasing the quantity of omega-3 fatty acids (found in fish oils) in the diet reduces platelet aggregation, slows blood clotting, and limits the production of proinflammatory cytokines.69 Data from studies using animal models suggest that a diet enriched with fish and borage oils can ameliorate inflammation-induced acute lung injury.70,71 The only clinical study of fish oil (omega-3 fatty acid) supplementation pertinent to the care of critically ill patients was carried out by Gadek and colleagues.72 In a randomized multicenter double-blind clinical trial, these investigators studied the effects of a diet (Oxepa7; Ross Products, Columbus, Ohio) supplemented with fish oils (containing EPA and docosahexaenoic acid), borage oil (rich in γ-linolenic acid), and antioxidants on markers of lung inflammation and survival. Patients (n=146) with acute respiratory distress syndrome (ARDS) were randomized within 24 hours of meeting entrance criteria to either a high-fat, low-carbohydrate control diet or the experimental diet. Only 98 of the 146 patients were deemed evaluable and included in the efficacy analysis. Among the evaluable patients, those who received the experimental diet had higher plasma phospholipid fatty acid levels (i.e., dihomo-γ-linolenic acid, EPA, and EPA/arachidonic acid ratio) and fewer total cells and neutrophils recovered from bronchoalveolar lavage fluid obtained on study days 4 and 7.
In addition, PaO2/FIO2 ratios on days 4 and 7 showed greater improvement in patients receiving the experimental diet compared with control patients. There was a non-significant improvement in survival in the experimental group compared with controls (16% versus 25%; P=0.17). Patients fed the experimental diet required fewer days on supplemental oxygen (13.6 versus 17.1; P=0.078), required significantly fewer days of ventilatory support (9.6 versus 13.2; P=0.027), spent less time in the ICU (11.0 versus 14.8 days; P=0.016), and had fewer new organ failures (10% versus 25%; P=0.018). Thus, the findings from this study support the view that administration of dietary lipids rich in omega-3 fatty acids can modify the lipid profile and favorably affect clinical outcome among critically ill patients with ARDS. However, a high-fat diet may be harmful, at least in critically ill burn victims,73 so the results of the Gadek study may be confounded by use of a high-fat control formula.72 Further, because of the addition of supplements other than fish oils (e.g., antioxidants), it is not possible to definitively attribute the beneficial effects of the experimental diet to its higher content of omega-3 fatty acids.
L-glutamine
The amino acid, L-glutamine, plays a central role in nitrogen transport within the body. It is used as a fuel by rapidly dividing cells, particularly lymphocytes and gut epithelial cells,74–76 and is also a substrate for synthesis of the important endogenous antioxidant, glutathione. Although L-glutamine is not an essential amino acid under normal conditions, plasma L-glutamine concentration decreases during critical illness, and low circulating levels of L-glutamine have been associated with immune dysfunction77 and increased mortality.78 Thus, L-glutamine may be regarded as a “conditionally essential” amino acid.
The effects of L-glutamine supplementation on clinically important outcomes have been assessed in several randomized trials of surgical and critically ill patients,79 and the results from these studies have been subjected to meta-analysis.80 In the aggregate, L-glutamine supplementation is associated with a significant reduction in mortality (RR 0.78; 95% CI, 0.61-0.99; P=0.04), a trend toward a reduction in infectious complications (RR 0.89; 95% CI, 0.73-1.08; P=0.2), and no overall effect on length of stay (weighted mean difference in days, −1.30; 95% CI, −4.77 to 2.17). When route of administration (parenteral versus enteral) was assessed in a subgroup analysis, the majority of the treatment effect with respect to mortality and infectious complications was associated with parenteral administration of L-glutamine in patients receiving PN. Because the majority of L-glutamine provided enterally is metabolized in the gut and liver, it may not have a systemic effect. Only one small study in burn patients demonstrated a reduction in mortality with enteral L-glutamine.81 In a study of trauma patients, administration of an enteral formula supplemented with L-glutamine was associated with a non-significant decrease in the number of infections compared with the number of infections observed with administration of the control formula (20 of 35 [57%] versus 26 of 37 [70%]).82
Antioxidants, Vitamins, and Trace Minerals
For a variety of inflammatory, infectious, and ischemic diseases, reactive oxygen species (ROS) represent a final common pathway. These toxic mediators (e.g., superoxide anion, hydroxyl radical, hydrogen peroxide, hypochlorous acid) can cause cellular injury by numerous mechanisms including destruction of cell membranes through the peroxidation of fatty acids; disruption of organelle membranes, such as those bounding lysosomes and mitochondria; degradation of hyaluronic acid and collagen; and disruption of key proteins and enzymes such as Na+/K+-ATPase or alpha1-proteinase inhibitor. To protect tissues from ROS-induced injury, the body maintains a complex endogenous defense system including enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase. These enzymes all have metals—notably, manganese, selenium, copper, or zinc—at their active sites. When these enzymatic antioxidants are overwhelmed, ROS are free to react with susceptible target molecules and cause cellular damage. Thus, cells have a secondary means of scavenging ROS using nonenzymatic antioxidants that are either water soluble, such as glutathione and vitamin C, or lipid soluble, such as vitamin E and beta carotene.83
In critical illness, oxidative stress arises as the result of an imbalance between protective antioxidant mechanisms and generation of ROS. This imbalance may be due to excess generation of ROS, low antioxidant capacity, or both. Plasma and intracellular concentrations of the various antioxidants are abnormally low in subpopulations of critically ill patients.84–86 In critical illness, evidence of oxidative stress includes high circulating levels of byproducts of lipid per oxidation, markers of protein oxidation, nitration or nitrosylation, or increased activity of ROS-producing enzymatic systems.87
In a recent meta-analysis,88 we aggregated results from 12 randomized trials that were designed to assess the value of administering exogenous antioxidants to critically ill patients.87,89–99 Of the included studies, several examined the effects of a single nutrient with antioxidant properties.90,92–94,96 In most cases, the nutrient evaluated was selenium,90,92–94 but one study assessed the effect of zinc supplementation on outcome in ventilated patients with head trauma.96 The effects of selenium combined with other antioxidants were assessed in four studies,89–9199 and four studies focused on the effects of vitamin A, vitamin C, vitamin E, N-acetylcysteine, and glutathione.87,95,97,98 When the 12 trials were aggregated, antioxidants were associated with a significant reduction in mortality (RR 0.66; 95% CI, 0.45-0.95; P=0.03). Only five of these studies reported on infectious complications.87,90,91,95,97 When these results were aggregated, antioxidants had no effect on infectious complications (RR 0.94; 95% CI, 0.63-1.40; P = 0.8). In further subgroup analysis, the majority of the treatment effect seemed to be related to parenteral rather than enteral administration of antioxidants or antioxidant nutrients, especially selenium. Thus for critically ill patients, selenium supplementation in combination with other antioxidants (vitamin E or alpha tocopherol, vitamin C, N-acetylcysteine, zinc) may be beneficial.
 Appropriate Use of Total Parenteral Nutrition in the Intensive Care Unit
Appropriate Use of Total Parenteral Nutrition in the Intensive Care Unit
Patient Selection
In almost all critical care patient populations involving a wide range of disease processes (from surgery and pancreatitis to trauma, burns, and critically ill patients on mechanical ventilation), EN is first-line therapy and should be chosen before PN. Reduction of infections by the use of EN compared with PN is consistent regardless of whether patients have cancer or protein-energy malnutrition.100 In the critical care of an average patient with an intact GI tract, PN should never be selected ahead of EN. When studies from diverse critical care patient populations are combined, “standard therapy” in which no artificial nutritional support is provided has a more favorable impact on patient outcome than PN does. In a recent meta-analysis, Braunschweig and colleagues showed a statistically significant reduction in infections with standard therapy compared with PN (RR 0.77; 95% CI, 0.65-0.91).100 If the patients were clearly well nourished, an even greater reduction in the incidence of infections was seen with standard therapy compared with PN (RR 0.61; 95% CI, 0.50-0.76).100 There was a trend toward reduced overall complications with standard therapy, which just missed statistical significance (RR 0.87; 95% CI, 0.74-1.03).100 Hospital length of stay was reduced significantly in 8 of the 14 studies reviewed by Heyland and coworkers in which standard therapy was compared with PN.101
The presence of protein-calorie malnutrition (PCM) reverses the choice between standard therapy and PN. In general, PN has greater efficacy in patients with PCM, and the chance of a favorable impact on patient outcome is more likely with PN than with standard therapy. PCM is most commonly defined by a greater than 10% to 15% weight loss101 or a low body mass index.102 In patients with severe PCM, PN reduces infectious morbidity, overall major complications, and even mortality in comparison to standard therapy. In their meta-analysis, Heyland and coworkers showed a 48% reduction in risk of major complications with the use of total PN compared with standard therapy in malnourished surgery patients (RR 0.52; 95% CI, 0.30-0.91).101 In a diverse population of malnourished patients, giving no nutritional support and providing standard therapy are associated with a trend toward increased infection (RR 1.17; 95% CI, 0.88-1.56) and a significant threefold increase in mortality (RR 3.0; 95% CI, 1.09-8.56).100 Those patients with severe PCM, the ones most likely to benefit from PN, usually represent a very small minority of patients. The prevalence of severe PCM in some studies of ICU patients ranged from 8.3% to 12.6%.103–105
Critically ill patients with sepsis and multiple organ dysfunction respond poorly to PN. Heyland et al. showed a trend toward a 2.5-fold increase in complications (RR 2.40; 95% CI, 0.88-6.58) and a significant twofold increase in mortality (RR 0.178; 95% CI, 1.11-2.85) from the use of PN compared with standard therapy with no nutritional support.101
Lipid Content
Use of emulsified lipids (Intralipid) with PN is controversial because previous studies have shown that long-chain fats can cause immune suppression.106 Intralipid can promote dysfunction of the reticuloendothelial system, enhance formation of prostanoids and leukotrienes, increase generation of ROS, and adversely affect the composition of cell membranes.106
Several reports demonstrate that intravenous lipids can adversely affect immune status and clinical outcome.106–108 Results of a meta-analysis of PN suggest that the adverse effects of lipids may negate any beneficial effects of nonlipid PN supplementation.101 Two studies compared the use of lipids to no lipids in PN.106,109 Among trauma patients, the use of PN without lipids versus with lipids was associated with a significant reduction in pneumonia (48% versus 73%; P=0.05), catheter-related sepsis (19% versus 43%; P=0.04), length of ICU stay (18 versus 29 days; P=0.02), and length of hospital stay (27 versus 39 days; P=0.03).110 In another study, the group that received no lipids (hypocaloric group) showed a trend toward a reduction in infections compared with the group that received lipids (29% versus 53%; P=0.2).109 Combining these two studies, a meta-analysis showed a significant reduction in infections in the group that received no lipids (RR 0.63; 95% CI, 0.42-0.93) and no difference in mortality (RR 1.29; 95% CI, 0.16-10.7).101
The long-term effects of fat-free PN are unknown. However, some fat—at least 5% of total calories—has to be provided as lipid emulsion to prevent essential fatty acid deficiency, although this issue is usually not important until after the first 10 days of hospitalization.102 Therefore, lipid-free PN is probably best given to those patients requiring only short-term PN (<10 days). This recommendation cannot be extrapolated to those who have an absolute contraindication to EN and need PN for a longer duration.
Effect of Hyperglycemia
Hyperglycemia might be a key factor in the reduced efficacy and increased rate of complications associated with PN. Hyperglycemia impairs neutrophil chemotaxis and phagocytosis,100 leads to glycosylation of immunoglobulins,111 impairs wound healing,112 alters function of the complement cascade,113 and exacerbates inflammation.110
Compared with EN, PN more frequently leads to hyperglycemia. For a variety of reasons, patients receiving EN often receive fewer total calories than those receiving PN.100 Whereas PN formulas typically contain 60% to 75% carbohydrate, EN formulas usually contain 40% to 55% carbohydrate.100 The parenteral route of feeding has been shown to lead to an increased stress response compared with enteral feeding. This effect in turn may increase endogenous glucose production and decrease glucose oxidation.100
The results from a number of early studies highlight the relationship between hyperglycemia and incidence of nosocomial infection. In an early meta-analysis by Moore et al. comparing parenteral and enteral routes of feeding in trauma patients, mean blood glucose concentration was greater than 200 mg/dL in the PN group on postoperative days 7 to 9, whereas it was only 132 mg/dL during the same period in patients receiving EN (P<0.05).110 Incidence of infection was 44% in the PN group and 17% in the EN group (P<0.05).110 In a different study, Kudsk and colleagues provided further evidence that hyperglycemia increases risk of infection.111 Among trauma patients randomized to EN or PN, those with a blood glucose concentration greater than 220 mg/dL had a 53% incidence of infection, whereas those with a blood glucose concentration less than 220 mg/dL had 23% incidence of infection (P<0.03).
Van den Berghe et al. compared intensive insulin therapy (target range for blood glucose concentration, 4.4-6.1 mmol/L) and conventional treatment (target range for blood glucose concentration, 10.0-11.1 mmol/L) in critically ill patients receiving nutritional support.114 This was a large study (n=1548) of surgical ICU patients (predominantly elective cardiovascular surgery) with relatively low APACHE II scores (median 9). Study patients were started on a glucose load (200-300 g/day) and then were advanced to PN, combined PN-EN, or EN after 24 hours of admission. Intensive insulin therapy was associated with a lower incidence of sepsis (P=0.003), a trend toward a reduction in ventilator days, reduced ICU length of stay (P<0.04), and decreased hospital mortality (P=0.01) compared with conventional insulin therapy.114
From these studies, one can infer that hyperglycemia (defined as a circulating glucose concentration > 200 mg/dL) is associated with poor outcome in different critically ill patient populations including trauma, strokes, and acute coronary syndromes.115–118 Using conventional glucose monitoring systems, glucose levels below 180 mg/dL should be maintained in critically ill patients.
Caloric Provision—Permissive Underfeeding
Several studies have shown a correlation between provision of excessive amounts of calories and increased rates of insulin resistance, infectious morbidity, and mortality. Hyperglycemia (blood glucose concentration > 220 mg/dL) has been shown to occur in greater than 50% of nondiabetic patients receiving PN in excess of 35 kcal/kg actual body weight per day.119 In a retrospective study, patients who received a high dose of carbohydrates (77% of total calories and 42.4 kcal/kg/d on average) were compared with patients who received a lower dose of carbohydrates (60.6% of total calories and 34.3 kcal/kg/d on average).120 The group that received more carbohydrates had significantly more episodes of sepsis (14 episodes in 26 patients versus 4 episodes in 17 patients; P < 0.05) and significantly higher mortality (28% versus 10%; P < 0.05).120 Although the group on the higher-carbohydrate regimen received less protein than the group on the lower-carbohydrate regimen (82.5 versus 98.7 g/day), this difference did not reach statistical significance.120
In another study, children with greater than 60% total body surface area burns were randomized to either a control group that received a high-carbohydrate, normal-protein regimen of PN or a study group that received a reduced-carbohydrate, high-protein regimen.121 The control group received 87% of goal calories, whereas the study group received only 77.7% of goal calories (P<0.002). The number of bacteremic days was 11% in the control group but only 8% in the experimental group (P<0.05). Mortality was 44% in the control group and 0% in the experimental group (P<0.03).121
Two additional studies evaluated the effect of hypocaloric feeding in critically ill patients. To achieve a hypocaloric dose of PN, Choban et al.122 reduced both carbohydrates and lipids in morbidly obese critically ill patients, whereas McCowen et al.109 withheld lipids in a heterogeneous group of patients including critically ill patients. In the study by McCowen’s group, hypocaloric feeding was associated with a trend toward a reduction in infectious complications (P=0.2)109; infectious complications were not reported in the study by Choban’s group.122 There were no significant differences in mortality or length of stay between groups in either study.
Supplemental Total Parenteral Nutrition
Few studies have looked at the impact of supplemental PN in patients receiving an insufficient volume of enteral feeding. In a study of 120 critically ill patients, Bauer and colleagues compared a control group receiving EN alone with a study group treated with EN supplemented with PN; both groups were fed for at least 4 to 7 days after starting nutritional support.123 Overall, there was no difference in morbidity or mortality between the two groups. Duration of stay in the ICU, duration of mechanical ventilation, incidence of respiratory infection, and mortality were equal between the two groups. Hospital length of stay was shorter in the study group receiving supplemental PN than in the control group (31.2 versus 33.7 days; P=0.002), but this effect was easily explained by a statistically significant earlier date of entry into the study (1.1 versus 1.5 days; P=0.002). The cost of nutritional support was doubled by the addition of supplemental PN.
Of greater concern was a study by Herndon and colleagues of patients with greater than 50% total body surface area burns.124 Mortality was significantly higher among the 16 study patients treated with EN and supplemental PN than it was in the 23 control patients treated with EN alone (63% versus 26%; P<0.05). Supplemental PN added to EN in the study group decreased the amount of enteral calories patients tolerated. Although both groups exhibited depressed natural killer cell activity from days 0 to 14, the group receiving supplemental PN experienced greater depression of T cell helper-suppressor ratios from days 7 to 14.
A recent meta-analysis evaluated five randomized trials that addressed the clinical benefits of supplemental PN in critically ill patients.125 The aggregated results demonstrated a trend toward increased mortality associated with the use of combination EN and PN (RR 1.27; 95% CI, 0.82-1.94; P=0.3). Supplemental PN was not associated with a difference in the incidence of infection (RR 1.14; 95% CI, 0.66-1.96; P=0.6). Supplemental PN had no effect on hospital stay (standardized mean difference −0.12 days; 95% CI, −0.45 to 0.2 days; P=0.5) or ventilator days. Thus, there appears to be no clinical evidence to support the practice of supplementing EN with PN when EN is initiated. Supplemental PN adds nothing and may actually worsen the outcome for patients already on EN.
Duration and Timing of Parenteral Nutrition
When EN is not feasible, providing standard therapy with no artificial nutritional support may be better than PN in well-nourished patients, regardless of their disease process. The timing of PN initiation is based on the underlying nutritional status of the patient. In a previously well-nourished but otherwise critically ill patient who has not resumed oral intake, it is reasonable to wait 7 to 10 days before initiating PN.100,126 Some experts recommend a longer waiting period (10 to 14 days) before initiating PN in a previously well-nourished patient who is not expected to resume oral intake soon.92,93 However, after 14 days, increased mortality is seen in most patients who are not yet eating and remain on standard therapy with no nutritional support.127 After 14 days, initiating PN is clearly associated with less mortality than providing no nutritional support.127 PN is indicated over standard therapy for the first 7 to 10 days when the enteral route is not available in malnourished patients (usually characterized by >10%-15% weight loss). PN should not be initiated unless more than 7 to 10 days of therapy is anticipated. No studies of short-term PN (<7 days) have shown it to be efficacious or to impact favorably on patient outcome.
 Future Considerations
Future Considerations
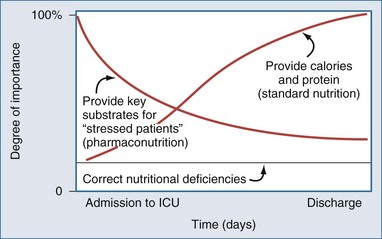
In the future, nutritional prescriptions will likely be complex recommendations that continue to consider protein and calorie requirements but, in addition, consider the key nutrients needed to modulate the stress response, maintain gut integrity, and ameliorate the pathophysiology of the underlying critical illness (Figure 94-5). It may turn out that prescription of key substrates will have a greater effect on outcome than provision of calories or protein per se. For example, consider a critically ill patient with clinical or biochemical evidence of hypoperfusion. Early in the course of the illness, current thinking would say that EN is contraindicated. However, providing L-glutamine or antioxidants enterally may be exactly what this patient needs to recover from the oxidative stress associated with critical illness. The need to meet protein and calorie requirements might occur much later in the course of the illness (see Figure 94-5). Although this example represents an extreme case, nutritional prescriptions in the future will have to be more cognizant of evolving pathophysiology and the ability of nutrients to modulate the integrity of the immune system, systemic inflammatory response, and the underlying disease in the early phases of the clinical course.
Key Points
Brandtzaeg PE. Current understanding of gastrointestinal immunoregulation and its relation to food allergy. Ann N Y Acad Sci. 2002;964:13-45.
Taylor SJ, Fettes SB, Jewkes C, Nelson RJ. Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Crit Care Med. 1999;27:2525-2531.
Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367.
Windsor AC, Kanwar S, Li AG, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431-435.
Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13:25-34.
1 Cahill NE, Dhaliwal R, Day AG, et al. Nutrition therapy in the critical care setting: what is “best achievable” practice? An international multicenter observational study. Crit Care Med. 2010;38:395-401.
2 van der Hulst RR, van Kreel BK, von Meyenfeldt MF, et al. Glutamine and the preservation of gut integrity. Lancet. 1993;341:1363-1365.
3 Kudsk KA. Importance of enteral feeding in maintaining gut integrity. Tech Gastro Endoscopy. 2001;3:2-8.
4 Groos S, Hunefeld G, Luciano L. Parenteral versus enteral nutrition: Morphological changes in human adult intestinal mucosa. J Submicrosc Cytol Pathol. 1996;28:61-74.
5 Li J, Kudsk KA, Hamidian M, Gocinski BL. Bombesin affects mucosal immunity and gut-associated lymphoid tissue in intravenously fed mice. Arch Surg. 1995;130:1164-1169.
6 Dobbins WO. Gut immunophysiology: A gastroenterologist’s view with emphasis on pathophysiology. Am J Physiol. 1982;242:G1-G8.
7 Fink MP. Why the GI tract is pivotal in trauma, sepsis, and MOF. J Crit Illness. 1991;6:253-269.
8 Ammori BJ, Leeder PC, King RF, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: Correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252-262.
9 Ziegler TR, Smith RJ, O’Dwyer ST, et al. Increased intestinal permeability associated with infection in burn patients. Arch Surg. 1988;123:1313-1319.
10 Windsor AC, Kanwar S, Li AG, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431-435.
11 Doig CJ, Sutherland LR, Sandham JD, et al. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444-451.
12 Alverdy JC, Aoys E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988;104:185-190.
13 Deitch EA. Role of the gut lymphatic system in multiple organ failure. Curr Opin Crit Care. 2001;7:92-98.
14 Moore FA, Moore EE, Poggetti R, et al. Gut bacterial translocation via the portal vein: A clinical perspective with major torso trauma. J Trauma. 1991;31:629-636. discussion 636-638
15 Kane TD, Alexander JW, Johannigman JA. The detection of microbial DNA in the blood: A sensitive method for diagnosing bacteremia and/or bacterial translocation in surgical patients. Ann Surg. 1998;227:1-9.
16 King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132:1303-1309.
17 Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002;183:390-398.
18 Renegar KB, Small PAJr. Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146-2148.
19 Elson CO. The immunology of inflammatory bowel disease. In: Kirsner JB, editor. Inflammatory Bowel Disease. Philadelphia: WB Saunders; 2000:208-239.
20 Brandtzaeg PE. Current understanding of gastrointestinal immunoregulation and its relation to food allergy. Ann N Y Acad Sci. 2002;964:13-45.
21 Wu Y, Kudsk KA, DeWitt RC, et al. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999;229:662-667.
22 Krakauer T. IL-10 inhibits the adhesion of leukocytic cells to IL-1-activated human endothelial cells. Immunol Lett. 1995;45:61-65.
23 Fukatsu K, Lundberg AH, Hanna MK, et al. Route of nutrition influences intercellular adhesion molecule-1 expression and neutrophil accumulation in intestine. Arch Surg. 1999;134:1055-1060.
24 Fukatsu K, Lundberg AH, Hanna MK, et al. Increased expression of intestinal P-selectin and pulmonary E-selectin during intravenous total parenteral nutrition. Arch Surg. 2000;135:1177-1182.
25 Fukatsu K, Zarzaur BL, Johnson CD, et al. Enteral nutrition prevents remote organ injury and death after a gut ischemic insult. Ann Surg. 2001;233:660-668.
26 Fukatsu K, Kudsk KA, Zarzaur BL, et al. Increased ICAM-1 and beta2 integrin expression in parenterally fed mice after a gut ischemic insult. Shock. 2002;18:119-124.
27 Lebman DA, Coffman RL. Cytokines in the mucosal immune system. In: Ogra PL, Lamm ME, McGhee JR, et al, editors. Handbook of Mucosal Immunology. San Diego: Academic; 1994:243-249.
28 Fong YM, Marano MA, Barber A, et al. Ann Surg. 1989;210:449-456.
29 Chiarelli A, Enzi G, Casadei A, et al. Very early nutrition supplementation in burned patients. Am J Clin Nutr. 1990;51:1035-1039.
30 Poret HA, Kudsk KA, Croce MA, et al. The effect of enteral feeding on catecholamine response following trauma. Surg Forum. 1991;42:11.
31 Abou-Assi S, Craig K, O’Keefe SJ. Hypocaloric jejunal feeding is better than total parenteral nutrition in acute pancreatitis: Results of a randomized comparative study. Am J Gastroenterol. 2002;97:2255-2262.
32 Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: Understanding the role of innate and acquired immunity. Shock. 2001;16:83-96.
33 Heyland DK, Dhaliwal R, Drover JW, et al. Canadian clinical practice guidelines for nutrition support in the adult critically ill patient. JPEN J Parenter Enteral Nutr. 2003;27:355-373.
34 Heyland DK, Drover JW, MacDonald S, et al. Effect of postpyloric feeding on gastroesophageal regurgitation and pulmonary microaspiration: Results of a randomized controlled trial. Crit Care Med. 2001;29:1495-1501.
35 Heyland DK, Drover JD, Dhaliwal R, Greenwood J. Optimizing the benefits and minimizing the risks of enteral nutrition in the critically ill: Role of small bowel feeding. JPEN J Parenter Enteral Nutr. 2002;26:S51-S57.
36 Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: A randomised trial. Lancet. 1999;354:1851-1858.
37 Booth CM, Heyland DK, Paterson WG. Gastrointestinal promotility drugs in the critical care setting: A systematic review of the evidence. Crit Care Med. 2002;30:1429-1435.
38 Spain DA, McClave SA, Sexton LK, et al. Infusion protocol improves delivery of enteral tube feeding in the critical care unit. JPEN J Parenter Enteral Nutr. 1999;23:288-292.
39 Pinilla JC, Samphire J, Arnold C, et al. Comparison of gastrointestinal tolerance to two feeding protocols in critically ill patients: A prospective, randomized controlled trial. JPEN J Parenter Enteral Nutr. 2001;25:81-86.
40 Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Apache II: A severity of disease classification system. Crit Care Med. 1985;13:818-829.
41 Greenspan L, McLellan BA, Greig H. Abbreviated injury scale and injury severity score: A scoring chart. J Trauma. 1985;25:60-64.
42 McClave SA, Spain DA, Snider HL. Nutritional management in acute and chronic pancreatitis. Gastroenterol Clin North Am. 1998;27:421-434.
43 McClave SA, Chang WK. Feeding the hypotensive patient: Does enteral feeding precipitate or protect against ischemic bowel? Nutr Clin Pract. 2003;18:279-284.
44 McClave SA. Do peptide-based enteral formulas provide any benefit over intact protein diets? Nutrition. 1995;11:395-397.
45 Metheny N. Minimizing respiratory complications of nasoenteric tube feedings: State of the science. Heart Lung. 1993;22:213-223.
46 Metheny N. Preventing pulmonary complications during enteral feeding in the critically ill. In: Program Manual, ASPEN 18th Clinical Congress. San Antonio: Tex; Jan 30-Feb 2, 1994:318-322.
47 Mullan H, Roubenoff RA, Roubenoff R. Risk of pulmonary aspiration among patients receiving enteral nutrition support. JPEN J Parenter Enteral Nutr. 1992;16:160-164.
48 Heyland DK. Nutritional support in the critically ill patient: A critical review of the evidence. Crit Care Clin. 1998;14:423-440.
49 McClave SA, Snider HL, Spain DA. Preoperative issues in clinical nutrition. Chest. 1999;115:64S-70S.
50 Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding—effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503-511.
51 Kalfarentzos F, Kehagias J, Mead N, et al. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: Results of a randomized prospective trial. Br J Surg. 1997;84:1665-1669.
52 McClave SA, Greene LM, Snider HL, et al. Comparison of the safety of early enteral vs parenteral nutrition in mild acute pancreatitis. JPEN J Parenter Enteral Nutr. 1997;21:14-20.
53 Peng YZ, Yuan ZQ, Xiao GX. Effects of early enteral feeding on the prevention of enterogenic infection in severely burned patients. Burns. 2001;27:145-149.
54 Taylor SJ, Fettes SB, Jewkes C, Nelson RJ. Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Crit Care Med. 1999;27:2525-2531.
55 Atkinson S, Sieffert E, Bihari D. A prospective, randomized, double-blind, controlled clinical trial of enteral immunonutrition in the critically ill. Crit Care Med. 1998;26:1164-1172.
56 Evoy D, Lieberman MD, Fahey TH, Daly JM. Immunonutrition: The role of arginine. Nutrition. 1998;14:611-617.
57 Salzman AL. Nitric oxide in the gut. New Horiz. 1995;3:33-45.
58 Muscara MN, Wallace JL. Nitric oxide: Therapeutic potential of nitric oxide donors and inhibitors. Am J Physiol. 1999;276:G1313-G1316.
59 Marik PE, Zaloga GP. Immunonutrition in high-risk surgical patients: a systematic review and analysis of the literature. JPEN J Parenter Enteral Nutr. 2010;34:378-386.
60 Waitzberg DL, Saito H, Plank LD, et al. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006;30:1592-1604.
61 Braga M, Gianotti L. Preoperative immunonutrition: cost-benefit analysis. JPEN J Parenter Enteral Nutr. 2005;29:S57-S61.
62 McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2009;33:277-316.
63 Martindale RG, McClave SA, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009;37:1757-1761.
64 Moore FA, Moore EE, Kudsk KA, et al. Clinical benefits of an immune-enhancing diet for early postinjury enteral feeding. J Trauma. 1994;37:607-615.
65 Heyland DK, Dhaliwal R, Drover JW, et al. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355-373.
66 Ligthart-Melis GC, van de Poll MC, Vermeulen MA, et al. Enteral administration of alanyl-[2-(15)N]glutamine contributes more to the de novo synthesis of arginine than does intravenous infusion of the dipeptide in humans. Am J Clin Nutr. 2009;90:95-105.
67 Heyland DK, Novak F, Drover JW, et al. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944-953.
68 Galban C, Montejo JC, Mesejo A, et al. An immune-enhancing enteral diet reduces mortality rate and episodes of bacteremia in septic intensive care unit patients. Crit Care Med. 2000;28:643-648.
69 Grimm H, Mayer K, Mayser P, Eigenbrodt E. Regulatory potential of W3 fatty acids in immunological and inflammatory processes. Br J Nutr. 2002;87:S59-S67.
70 Mancuso P, Whelan J, DeMichele SJ, et al. Dietary fish oil and fish and borage oil suppress intrapulmonary proinflammatory eicosanoid biosynthesis and attenuate pulmonary neutrophil accumulation in endotoxic rats. Crit Care Med. 1997;25:1198-1206.
71 Mancuso P, Whelan J, DeMichele SJ, et al. Effects of eicosapentaenoic and linolenic acid on lung permeability and alveolar macrophage eicosanoid synthesis in endotoxic rats. Crit Care Med. 1997;25:523-532.
72 Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Crit Care Med. 1999;27:1409-1420.
73 Garrel D, Razi M, Lariviere F, et al. Improved clinical status and length of care with low-fat nutrition support in burn patients. JPEN J Parenter Enteral Nutr. 1995;19:482-491.
74 Ogle CK, Ogle JD, Mao JX, et al. Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. JPEN J Parenter Enteral Nutr. 1994;18:128-133.
75 O’Riordain MG, De Beaux A, Fearon KC. Effect of glutamine on immune function in the surgical patient. Nutrition. 1996;12:S82-S84.
76 Aosasa S, Mochizuki H, Yamamoto T, et al. A clinical study of the effectiveness of oral glutamine supplementation during total parenteral nutrition: Influence on mesenteric mononuclear cells. JPEN J Parenter Enteral Nutr. 1999;23:S41-S44.
77 Oehler R, Pusch E, Dungel P, et al. Glutamine depletion impairs cellular stress response in human leucocytes. Br J Nutr. 2002;87:S17-S21.
78 Roth E, Funovics J, Muhlbacher F, et al. Metabolic disorders in severe abdominal sepsis: Glutamine deficiency in skeletal muscle. Clin Nutr. 1982;1:25-41.
79 Novak F, Heyland DK, Avenell A, et al. Glutamine supplementation in serious illness: A systematic review of the evidence. Crit Care Med. 2002;30:2022-2029.
80 Heyland DK, Suchner U. Immunonutrition. In Rolandelli Bankhead, Boullata Compher, editors: Clinical nutrition: enteral and tube feeding, 4th ed, Philadelphia: Saunders, 2004.
81 Garrel D, Nedelec B, Samson L, et al. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplementation. Crit Care Med. 2003;31:2444-2449.
82 Houdijk AP, Rijnsburger ER, Jansen J, et al. Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet. 1998;352:772-776.
83 Tanswell AK, Freeman BA. Antioxidant therapy in critical care medicine. New Horiz. 1995;3:330-341.
84 Goode HF, Cowley HC, Walker BE, et al. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med. 1995;23:646-651.
85 Cowley HC, Bacon PJ, Goode HF, et al. Plasma antioxidant potential in severe sepsis: A comparison of survivors and non-survivors. Crit Care Med. 1996;24:1179-1183.
86 Forceville X, Vitoux D, Guazit R, et al. Selenium, systemic immune response syndrome, sepsis and outcomes in critically ill patients. Crit Care Med. 1998;26:1536-1544.
87 Preiser JC, Van Gossum A, Berré J, et al. Enteral feeding with a solution enriched with antioxidant vitamins A, C, E enhances the resistance to oxidative stress. Crit Care Med. 2000;28:3828-3832.
88 Suchner U, Heyland DK, Dhaliwal R. The effect of antioxidant replacement strategies in critical illness: the results of a meta-analysis (submitted for publication).
89 Berger MM, Spertini F, Shenkin A, et al. Trace element supplementation modulates pulmonary infection rates after major burns: A double-blind, placebo-controlled trial. Am J Clin Nutr. 1998;68:365-371.
90 Berger MM, Recmond MJ, Shenkin A, et al. Influence of selenium supplements on the post-traumatic alterations of the thyroid axis: A placebo-controlled trial. Intensive Care Med. 2001;27:91-100.
91 Porter JM, Ivatury RR, Azimuddin K, Swami. Antioxidant therapy in the prevention of organ dysfunction syndrome and infectious complications after trauma: Early results of a prospective randomized study. Am Surg. 1999;65:478-483.
92 Kuklinski B, Buchner M, Schweder R, Nagel R. Akute Pancreatitisine “free radical disease”: Letalitatssenkung durch Natriumselenit (Na2SeO3)-Therapie. Z Gestame. Inn Med. 1991;46:S145-S149.
93 Zimmermann T, Albrecht S, Kühne H, et al. Selensubstitution bei Sepsispatienten. Med Klin. 1997;92(Suppl 5):3-4.
94 Angstwurm MW, Schottdorf J, Schopohl J, Gaertner R. Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit Care Med. 1999;27:1807-1813.
95 Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814-822.
96 Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13:25-34.
97 Maderazo EG, Woronick CL, Hickingbotham N, et al. A randomized trial of replacement antioxidant vitamin therapy for neutrophil locomotory dysfunction in blunt trauma. J Trauma. 1991;31:1142-1150.
98 Ortolani O, Conti A, Raffaele De Gaudio A, et al. The effect of glutathione and N-acetylcysteine on lipoperoxidative damage in patients with early septic shock. Am J Respir Crit Care Med. 2000;161:1907-1911.
99 Berger MM, Baines M, Wardle CA, et al. Trace element supplements modulate tissue levels, antioxidant status and clinical course after major burns—preliminary results. Clin Nutr. 2002;21(Suppl):66.
100 Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: A meta-analysis. Am J Clin Nutr. 2001;74:534-542.
101 Heyland DK, MacDonald S, Keefe L, Drover JW. Total parenteral nutrition in the critically ill patient: A meta-analysis. JAMA. 1998;280:2013-2019.
102 Koretz RL, Lipman TO, Klein S. AGA technical review on parenteral nutrition. Gastroenterology. 2001;121:970-1001.
103 Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325:525-532.
104 Detsky AS, Baker JP, O’Rourke K, Goel V. Perioperative parenteral nutrition: A meta-analysis. Ann Intern Med. 1987;107:195-203.
105 Muller JM, Keller HW, Brenner U, et al. Indications and effects of preoperative parenteral nutrition. World J Surg. 1986;10:53-63.
106 Basttistella FD, Widergren JT, Anderson JT, et al. A prospective, randomized trial of intravenous fat emulsion administration in trauma victims requiring total parenteral nutrition. J Trauma. 1997;43:52-58. discussion 58-60
107 Seidener DL, Mascioli EA, Istfan NW, et al. Effects of long-chain triglyceride emulsions on reticuloendothelial system function in humans. JPEN J Parenter Enteral Nutr. 1989;13:614-619.
108 Freeman J, Goldmann DA, Smith NE, et al. Association of intravenous lipid emulsion and coagulase-negative staphylococcal bacteremia in neonatal intensive care units. N Engl J Med. 1990;323:301-308.
109 McCowen KC, Friel C, Sternberg J, et al. Hypocaloric total parenteral nutrition: Effectiveness in prevention of hyperglycemia and infectious complications. A randomized clinical trial. Crit Care Med. 2000;28:3606-3611.
110 Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications: The results of a meta-analysis. Ann Surg. 1992;216:172-183.
111 Kudsk KA, Laulederkind A, Hanna MK. Most infectious complications in parenterally fed trauma patients are not due to elevated blood glucose levels. JPEN J Parenter Enteral Nutr. 2001;25:174-179.
112 Bistrian BR. Hyperglycemia and infection: Which is the chicken and which is the egg? JPEN J Parenter Enteral Nutr. 2001;25:180-181.
113 Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22:1408-1414.
114 Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367.
115 Eakins J. Blood glucose control in the trauma patient. J Diabetes Sci Technol. 2009;3:1373-1376.
116 Kosiborod M, Deedwania P. An overview of glycemic control in the coronary care unit with recommendations for clinical management. J Diabetes Sci Technol. 2009;3:1342-1351.
117 Kovalaske MA, Gandhi GY. Glycemic control in the medical intensive care unit. J Diabetes Sci Technol. 2009;3:1330-1341.
118 Quinn TJ, Lees KR. Hyperglycaemia in acute stroke–to treat or not to treat. Cerebrovasc Dis. 2009;27(Suppl 1)):148-155.
119 Rosmarin DK, Wardlaw GM, Mirtallo J. Hyperglycemia associated with high, continuous infusion rates of total parenteral nutrition dextrose. Nutr Clin Pract. 1996;11:151-156.
120 Vo NM, Waycaster M, Acuff RV, Lefemine AA. Effects of postoperative carbohydrate overfeeding. Am Surg. 1987;53:632-635.
121 Alexander JW, MacMillan BG, Stinnett JD, et al. Beneficial effects of aggressive protein feeding in severely burned children. Ann Surg. 1980;192:505-517.
122 Choban PS, Burge JC, Scales D, et al. Hypoenergetic nutrition support in hospitalized obese patients: A simplified method for clinical application. Am J Clin Nutr. 1997;66:546-550.
123 Bauer P, Charpentier C, Bouchet C, et al. Parenteral with enteral nutrition in the critically ill. Intensive Care Med. 2000;26:893-900.
124 Herndon DN, Barrow RE, Stein M, et al. Increased mortality with intravenous supplemental feeding in severely burned patients. J Burn Care Rehabil. 1989;10:309-313.
125 Dhaliwal R, Jurewitsch B, Harrietta D, Heyland DK. Combination enteral nutrition and parenteral nutrition in critically ill patients: Harmful or beneficial? A review of the evidence. Intensive Care Med. 2004;30:1666-1671.
126 Klein S, Kinney J, Jeejeebhoy K, et al. Nutrition support in clinical practice: Review of published data and recommendations for future research directions. National Institutes of Health, American Society for Parenteral and Enteral Nutrition, and American Society for Clinical Nutrition. JPEN J Parenter Enteral Nutr. 1997;21:133-156.
127 Sandstrom R, Drott C, Hyltander A, et al. The effect of postoperative intravenous feeding (TPN) on outcome following major surgery evaluated in a randomized study. Ann Surg. 1993;217:185-195.