CHAPTER 39 Critical Care Medicine
Critical illness occurs in stages. The acute illness is followed by a plateau phase, which may be prolonged and is poorly understood. The biphasic response to stress exhibited by the hypothalamic pituitary adrenal axis is also poorly understood. In critically ill patients, an initial surge in adrenocorticotropic hormone (ACTH) and cortisol is followed by a prolonged state of decreased ACTH levels and persistently elevated cortisol (Beishuizen and Thijs, 2004). Persistent hypercortisolism may benefit the patient by promoting the availability of fuel substrates and minimizing inflammation, or it may be harmful, causing hyperglycemia, delayed wound healing, immune suppression, and myopathy. The immune or inflammatory response to injury follows a similar pattern (Hotchkiss and Karl, 2003).
An increasing number of studies have focused on long-term functional outcomes, and these are likely to change critical care practice in coming years. For example, although tolerance of relative hypoxia has become the norm for treating patients with acute respiratory distress syndrome (ARDS), outcome studies show that duration of saturation less than 90%, less than 85%, and less than 80% correlated with impairment in memory, attention, and processing speed, respectively (Hopkins et al., 1999). Pediatric patients undergoing arterial switch, a procedure that should leave them with normal long-term heart function, had a higher than expected incidence of neurodevelopmental disabilities at school age (Mehta and Arnold, 2004). As more information about how to promote good long-term functional outcomes becomes available, our practice will evolve.
Respiratory system
Actual or impending respiratory failure is a common reason for admission to the PICU, and one in six patients admitted requires mechanical ventilation (Epstein, 2009). Although some patients with healthy lungs require mechanical ventilation for nonrespiratory reasons, the majority have acute respiratory failure due to lung parenchymal disease. The treatment of acute respiratory failure remains primarily supportive. The goal of treatment is to avoid further damage to the lungs and other organ systems during the period required for the lungs to heal.
Modes of Mechanical Ventilation in the PICU
Support Modes
Effort above a preset rate may or may not be supported. The most common form of support is pressure support. Pressure support has been shown to facilitate weaning, and it may also improve patient comfort. Pressure support is frequently used to recondition weakened respiratory muscles while still providing mandatory breaths to prevent atelectasis; however, data to support this approach do not exist (Turner and Arnold, 2007).
Noninvasive Positive Pressure Ventilation
Over the past few years, there has been an increase in the use of noninvasive ventilation to prevent or delay endotracheal intubation. Noninvasive positive pressure ventilation (NPPV) includes continuous positive airway pressure (CPAP), which is used to increase functional residual capacity (FRC) and to overcome inspiratory airway collapse, and biphasic positive airway pressure, which also supports respiratory effort with positive inspiratory pressure. Studies in adults have shown clear benefit to NPPV, especially when endotracheal intubation can be avoided, with no decrement when endotracheal intubation is merely delayed (Pagano and Barazzone-Argiroffo, 2007). NPPV has been demonstrated successfully in pediatric patients with neuromuscular disease, upper airway obstruction, status asthmaticus, and cystic fibrosis (Deis, 2008).
Ventilator-Induced Lung Injury
Ventilator-induced lung injury is caused by prolonged exposure to high Fio2, by alveolar stretch from excessive tidal volumes (volutrauma), and by opening and closing of alveoli (shear stress trauma). Although hyperoxic inspired gas is essential for patients with lung injury, exposure to Fio2 greater than 95% for 24 to 48 hours causes lung injury similar to ARDS and causes death within 48 to 72 hours due to oxygen toxicity (Adams et al., 2003).
Mechanical trauma from ventilation causes injury both through physical disruption of the architecture of the lung and through release of inflammatory mediators. Atelectatrauma, which is thought to occur when PEEP is set below the lower inflection point of the lung hysteresis curve, is particularly problematic in lung disease states associated with surfactant dysfunction, such as ARDS. Patients with heterogeneous lung disease are at increased risk for volutrauma. Transpulmonary pressure is not uniformly distributed to alveoli, and collapsed alveoli adjacent to inflated alveoli may be subject to extreme shearing forces. Mechanical trauma leads to release of inflammatory mediators, which may spill over to systemic circulation and play a role in the development of multiorgan failure (Plötz et al., 2004; Harris, 2005; Tremblay and Slutsky, 2006).
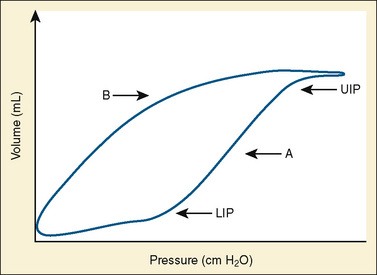
Use of Lung Hysteresis Curves
More sophisticated ventilators can calculate pressure-volume loops showing the difference in the pressure-volume relationship between the inspiratory and expiratory limbs of the respiratory cycle (Fig. 39-1). The sigmoidal shape of the hysteresis curve reflects differing compliance at different lung volumes. The lower inflection point represents the point during inspiration at which compliance improves. Below this point, the majority of alveoli are closed. The upper inflection point represents another change in compliance. Above a certain volume, more pressure is required to further distend alveoli, and the upper inflection point is thought to represent the beginning of a zone in which further pressure causes significant alveolar strain. The curve is steepest and nearly linear between these points, reflecting maximal compliance. Lung injury is thought to be minimized by keeping PEEP above the lower inflection point to maintain recruitment, and keeping tidal volumes below the upper inflection point by minimizing peak inspiratory pressure (Tremblay and Slutsky, 2006).
Alternative Modes of Ventilation
High-Frequency Oscillatory Ventilation
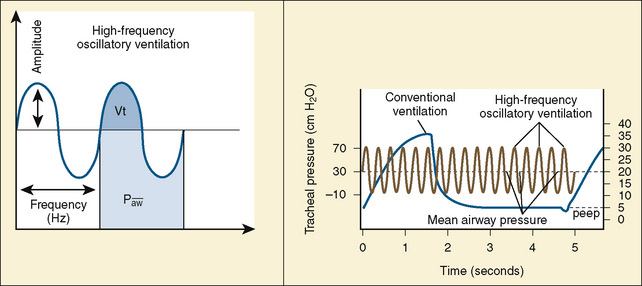
Despite conventional ventilation strategies to reduce VILI, about one third of patients with ARDS die, and many of the survivors experience a significant decrement in function. The object of high-frequency oscillatory ventilation (HFOV) is to accomplish oxygenation and ventilation with less injury to the lung. HFOV uses laminar flow at high MAPs to maintain the lung open, and it uses an oscillating piston to create pressure displacement above and below the MAP to deliver very small tidal volumes in the range of the volume of the patient’s anatomic dead space (Stawicki et al., 2009) (Fig. 39-2). The result, ideally, is ventilation only on the steep and linear part of the hysteresis curve. Compliant alveoli, which might receive an excessively large share of the delivered tidal volume in conventional ventilation, are protected from volutrauma. With this strategy, noncompliant alveoli—those with time constants long enough to preclude opening with conventional ventilation—may be recruited. Recruitment of new lung units may improve V/Q matching. A crossover trial comparing HFOV with conventional ventilation in children with severe lung disease showed that HFOV was associated with improved oxygenation and, despite higher MAPs, a reduced need for supplemental oxygen at 30 days (Arnold et al., 1994).
In HFOV, the variables controlled on the ventilator are frequency of respiration, (measured in cycles per second, or Hertz), amplitude of ventilation (power), and MAP. Frequency is measured in cycles per second. A frequency of 10 is 600 “breaths” per second, and a frequency of 3 is 180 breaths per second. A lower frequency functionally creates a longer inspiratory time and therefore a larger breath. The frequency is initially set at 10 for infants, and somewhat lower for older children. Amplitude, also referred to as power, is measured in centimeters of water. There is no standard starting pressure. The amplitude is initially set to produce a visible wiggling motion of the patient’s body to the level of the lower abdomen or groin. MAP is initially set to 5 cm H2O greater than the last MAP on conventional ventilation (Stawicki et al., 2009). A chest radiograph performed within an hour or two after initiating HFOV should confirm an appropriate MAP setting by showing approximately nine visible ribs from the top of the thorax to the diaphragm.
Ventilation can be improved by increasing the amplitude and decreasing the frequency. As the frequency of breaths decreases, a higher percentage of the set amplitude will be delivered. This is like increasing the tidal volume in a conventional ventilator; decreasing frequency is a way to increase ventilation, thereby decreasing Paco2. Frequencies less than 3 (180 cycles per minute) may increase the risk for volutrauma (Stawicki et al., 2009).
For a more complete treatment of the mechanics of HFOV, see Stawicki and colleagues (2009).
Airway Pressure-Release Ventilation
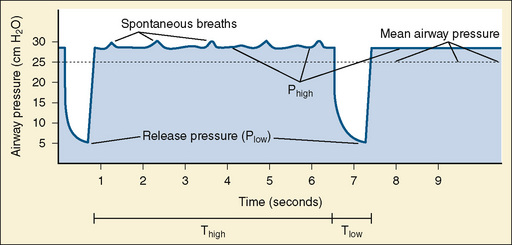
Airway pressure-release ventilation (APRV) cycles between a high- (Phigh) and a low-pressure level (Plow) in a high-flow CPAP circuit (Fig. 39-3). The continuous high flow allows spontaneous breathing throughout the ventilatory cycle, and it may reduce the need for heavy sedation and neuromuscular blockade. Spontaneous breathing is necessary to realize the benefits of this mode of ventilation (Stawicki et al., 2009).
Most of the respiratory cycle is spent at Phigh, and pressure is briefly released to Plow several times per minute. Ideally, time spent at Phigh will maximize recruitment and, by placing the lung on the steep part of the hysteresis curve, make spontaneous breathing easier. The time spent at Plow allows ventilation, but it is believed to be brief enough to prevent atelectasis of alveoli with long time constants (Stawicki et al., 2009).
Initially, Phigh is set at the plateau pressure measured from an inspiratory pause on conventional ventilation and adjusted to allow weaning of Fio2. Plow is set at zero to maximize the velocity of exhalation. The time (T) of Thigh is initially set between 4 to 6 seconds and adjusted to maintain adequate oxygenation and ventilation. Tlow is set at 0.4 to 0.5 seconds and adjusted to maintain a tidal volume of about 5 mL/kg, or such that expiration ends when the flow reaches 50% to 75% of the measured maximal expiratory flow. Tlow is the critical variable that affects bulk air flow and thus ventilation. Oxygenation is increased by increasing Fio2 or by increasing Phigh or Thigh, both of which will increase MAP. Ventilation is increased by encouraging spontaneous respiration or increasing Tlow (Stawicki et al., 2009).
Although it was described in 1987, APRV is relatively new in pediatrics and has not been thoroughly studied. Case reports of APRV in pediatric patients have shown improvement in oxygenation with the ventilation strategy (Krishnan and Morrison, 2007; Kuruma et al., 2008). With a crossover design, the one randomized controlled trial of APRV in children demonstrated comparable ventilation at lower peak inspiratory and plateau pressures (Schultz et al., 2001). The potential advantages of decreasing mechanical ventilatory pressures and allowing spontaneous breathing and a reduced need for sedation and neuromuscular blockade in patients with severe lung disease make it worth considering despite the paucity of data. APRV may be contraindicated in patients with severe obstruction to exhalation, such as status asthmaticus.
Adjuncts to Mechanical Ventilation for Patients with Severe Lung Disease
Helium-Oxygen Mixtures (Heliox)
Helium is a low-density, biologically inert gas that can be safely mixed with oxygen. When air flow through a tube is turbulent, the flow rate depends on the density of the gas. With high helium concentrations, heliox can flow more rapidly through narrowed airways than nitrogen-oxygen mixtures. Additionally, carbon dioxide diffuses through helium faster than through air. The Fio2 required by the patient may limit the use of heliox, as minimal benefit is believed to occur from heliox concentrations of less than 60%. When used with a spontaneously breathing patient, efforts should be made to prevent entrainment of room air with a well-fitting nonrebreather mask. Heliox can be delivered through the ventilator but may interfere with tidal volume measurements. This difficulty can be overcome with in-line monitors of respiratory mechanics (Myers, 2006).
Heliox may improve the delivery of nebulized medications. It improves dyspnea and peak expiratory flow in spontaneously breathing asthmatics, and it may limit peak inspiratory pressure in ventilated patients with status asthmaticus (Gupta and Cheifetz, 2005; Kissoon et al., 2008). Heliox improves respiratory distress associated with upper airway obstruction and improves the work of breathing in patients with bronchiolitis (Gupta and Cheifetz, 2005). No adverse effects of heliox have been reported. Despite a widespread belief that early application of heliox can prevent the need for intubation in select patients, there are no studies of heliox directed at patient outcomes.
Prone Positioning
In a supine patient, the dependent lung is underventilated and at risk for collapse, and the antidependent lung is aerated and at risk for overdistention. The mechanism by which prone positioning can improve oxygenation is not completely clear, but it may improve V/Q matching by improving the aeration of the dependent lung (Ryu et al., 2008). Prone positioning can also improve lung mechanics by decreasing thoracoabdominal compliance. In adult patients, a protocol of prone positioning for 7 hr/day improved oxygenation but did not alter ventilator-free days or mortality (Gattinoni et al., 2001). In pediatric patients, a protocol of prone positioning for 20 hr/day improved efficiency of oxygenation but did not improve ventilator-free days or organ failure–free days (Curley et al., 2005).
Nitric Oxide
Inhaled nitric oxide (iNO) has been a mainstay of treatment for both primary and secondary pulmonary hypertension of the newborn since its introduction. In these patients, it causes a rapid and sustained improvement in efficiency of oxygenation by preferentially vasodilating ventilated lung units (Konduri and Kim, 2009). Because it is rapidly inactivated by hemoglobin, the effect of iNO is limited to the pulmonary vasculature (Adhikari et al., 2007).
Because the pathophysiology of ARDS and acute lung injury (ALI) involves V/Q mismatching, there has been interest in using iNO for patients outside the neonatal age range. In adult studies, iNO improved oxygenation (i.e., the ratio of pulmonary arterial oxygen pressure [Pao2] to Fio2, and the oxygenation index) but did not improve mortality. There has been some concern in adult patients that iNO may be associated with an increase in the need for new renal replacement therapy (Adhikari et al., 2007). In a randomized trial in children, iNO improved oxygenation without harmful side effects from methemoglobinemia at dosages up to 20 parts per million. The study was not powered to detect a difference in mortality (Tang et al., 1998).
Nitric oxide is commonly used at a level of 20 ppm. Very high concentrations of NO may paradoxically worsen V/Q matching by diffusing into nonventilated areas and improving the perfusion there. High concentrations also carry a significantly increased risk for methemoglobinemia. Methemoglobin should be monitored daily while therapy with iNO is used. If methemoglobin becomes elevated, it is usually sufficient to decrease the concentration of iNO. For very high methemoglobin levels, a scavenger, such as methylene blue, may be used. Exposure to NO for even a brief period can sensitize the pulmonary vasculature to its effects. Rapid weaning to low levels (5 ppm) is usually well tolerated, but thereafter, iNO should be weaned slowly to prevent rebound vasoconstriction (Adhikari et al., 2007).
Surfactant
Surfactant deficiency has been described in ARDS and ALI, particularly in the acute phase of the illness. The best-studied application of surfactant is in meconium aspiration syndrome, where significant clinical improvement is reported after surfactant instillation (Willson et al., 2008). Surfactant instillation in infants mechanically ventilated for respiratory syncytial virus disease leads to improvement in oxygenation and decrease in duration of mechanical ventilation (Tibby et al., 2000; Luchetti et al., 2002).
Trials of endotracheal instillation or aerosolization of surfactant in adults have shown little or no benefit. In children, treatment with calfactant, a natural preparation relatively rich in surfactant specific protein B, has been associated with improved oxygenation and mortality, with particular benefit seen in patients younger than 12 months (Willson et al., 2005). Although this trial generated significant interest in the use of calfactant for pediatric ARDS and ALI, an independent analysis of the data noted that the placebo arm of the trial had more immunocompromised patients, and that statistical significance was lost when controlling for immune status (Czaja, 2007). Surfactant therapy for ARDS/ALI in patients outside of the neonatal age range remains controversial.
Surfactant is administered by direct endotracheal instillation through an endotracheal tube or bronchoscope at a dosage of 100 mg/kg body weight. The total volume of the drug preparation is significant. Complications of surfactant instillation include volume-responsive hypotension and transient hypoxia. Most authors cite pulmonary hemorrhage as a relative contraindication (Willson et al., 2008). Surfactant is probably best used early in the course of mechanical ventilation. Administration of surfactant in patients with severe hypoxia can provoke severe desaturation. In patients with severe hypoxia, surfactant may be safely given while the patient is supported with extracorporeal therapies.
Weaning and Extubation
Not all patients require gradual weaning. An alternative approach is to perform a daily test of extubation readiness, such as a spontaneous breathing trial, and give higher amounts of support to provide periods of rest for the respiratory muscles between these tests. A third approach is to provide periods of rest interspersed with periods of lower support designed to exercise or train the muscles of respiration. Several adult studies have shown that protocolized weaning results in earlier extubation without an increase in complications (Newth et al., 2009). Studies of weaning protocols in children are ongoing. Pediatric studies have corroborated adult data showing that spontaneous breathing trials with a T-piece (i.e., a tracheotomy tube collar) or pressure support can predict successful extubation (Newth et al., 2009). A negative inspiratory force of –30 correlates with extubation success in adults but has not been validated in children.
Despite weaning and careful testing, 8% to 20% of patients fail extubation. Extubation failure is independently associated with a fivefold increased risk for mortality in children, and upper airway obstruction accounts for or contributes to 25% to 40% of extubation failures (Newth et al., 2009). Testing for a leak around the endotracheal tube may predict post-extubation stridor, particularly in patients with uncuffed endotracheal tubes and those whose intubation has been prolonged. The absence of a leak at 30 cm H2O does not accurately predict extubation failure, but it is commonly a trigger for the use of steroids. Dexamethasone given 24 hours in advance of extubation may reduce the inflammation associated with airway injury from the endotracheal tube (Randolph, 2009).
Specific Disease Processes Requiring Mechanical Ventilation
Asthma
NPPV, if applied early in patients with status asthmaticus, can take advantage of patient’s respiratory efforts and prevent the muscle fatigue that leads to the need for intubation (Stather and Stewart, 2005). There is evidence that NPPV prevents hospitalization in patients presenting to the emergency department with status asthmaticus. Mental status changes, anxiety, exhaustion, or refractory hypoxemia preclude the use of NPPV, and it is these clinical changes rather than arterial blood gas analysis results that should guide the decision to intubate.
Once the patient is intubated, the primary strategy is to limit gas trapping, which causes intrinsic PEEP and forces the lung to operate on the less compliant portion of the hysteresis curve. Gas trapping is evident in flow-versus-time graphs, where inspiration is seen to start before flow returns to zero, or in graphs of end-tidal CO2 versus time, where CO2 fails to reach a plateau before the next inspiration begins (Stather and Stewart, 2005).
Significant controversy exists as to the best method for ventilating asthmatics. There are advocates for both pressure- and volume-control ventilation. Because of the high air-flow rates required to achieve targeted pressure in pressure-control ventilation, this mode may cause more turbulence in the airways of ventilated asthmatics. Volume-control modes may require higher set pressures to deliver adequate tidal volumes; however, because much of the pressure is lost in overcoming proximal airway resistance, alveolar hyperinflation may not occur. Alveolar pressure can be measured with an inspiratory pause pressure; complications are rare when this pressure does not exceed 30 cm H2O (Stather and Stewart, 2005).
Intrinsic PEEP can be measured with an expiratory pause pressure in a muscle-relaxed patient. Because complete exhalation is the goal, some authors recommend minimizing PEEP with an initial setting from 0 to 5 cm H2O. Others, acting on the theory that PEEP will stent airways open and ameliorate gas trapping, recommend setting PEEP at or slightly higher than the measured intrinsic PEEP. If a higher-PEEP strategy is used, intrinsic PEEP should be measured frequently, and the ventilator settings should be adjusted accordingly (Stather and Stewart, 2005).
There is general agreement that exhalation time should be as long as possible. Low respiratory rates and short inspiratory times give maximum exhalation time, but often at the expense of minute ventilation. Permissive hypercapnia (see Acute Respiratory Distress Syndrome and Acute Lung Injury, next) has been successfully used in the ventilation of asthmatics (Stather and Stewart, 2005). With permissive hypercapnia, patient ventilatory dyssynchrony is common. Neuromuscular blockade with adequate sedation is often necessary until severe bronchospasm resolves, usually within 24 hours. Weaning and extubation should be prompt, because spontaneous respiration is favorable for asthmatics. There are no generally agreed on criteria for extubating asthmatics, except that it should be performed as soon as possible. Negative inspiratory pressure with active exhalation is more effective than positive pressure in obstructive lung disease. Blood gas and ventilatory parameters that suggest the patient with asthma is improving include a normalizing partial pressure of CO2 and pH, and a peak inspiratory pressure that begins to approach the inspiratory pause pressure. The difference between peak inspiratory pressure and inspiratory pause pressure correlates with airway resistance.
Acute Respiratory Distress Syndrome and Acute Lung Injury
The clinicopathologic picture of ARDS and ALI is diffuse alveolar damage, and four clinical parameters are involved: acute onset, severe arterial hypoxemia resistant to supplemental oxygen, bilateral infiltrates, and absence of left atrial hypertension. A Pao2/Fio2 ratio of less than 200 mm Hg defines ARDS, and a ratio of less than 300 mm Hg defines ALI (Randolph et al., 2003).
The most common cause of ARDS and ALI is lower respiratory tract infection, but it can be precipitated by a variety of systemic inflammatory conditions, including infection outside the respiratory tract, pancreatitis, and tissue necrosis. Overall mortality for children ranges between 8% and 15%, which is lower than that for adults (Randolph et al., 2003). Patients with bone marrow transplantation may have a significantly higher mortality. Because of the low mortality in children, duration of mechanical ventilation or number of ventilator-free days are both considered acceptable endpoints for studies of ARDS and ALI.
Although not a predictor of mortality in adults with ARDS, multiple single-center and multicenter studies of the epidemiology of ARDS and ALI in pediatric patients confirm that the ratio of the alveolar oxygen pressure (Pao2) to Fio2 at the onset of ARDS or ALI is an independent predictor of mortality (Flori et al., 2005; Erickson et al., 2007). Mortality rates for children with ARDS or ALI from studies in the past 10 years range from 8% to 35% (Willson et al., 2008; Randolph et al., 2003). A recent prospective multicenter observational study of 320 children with ARDS or ALI found that mortality in patients with a Pao2/Fio2 ratio of less than 200 is nearly twice the mortality in those with a ratio between 200 to 300 (26% versus 13%) (Erickson, 2007). The Pao2/Fio2 ratio also predicted the number of ventilator-free days (Flori et al., 2005). The severity of hypoxia is also sometimes measured by the oxygenation index, defined as (MAP × Fio2)/Pao2 (Randolph, 2009).
Treatment of ARDS and ALI
Control of a persistent trigger of the inflammatory cascade is critical to eliminating the stimulus for ongoing lung injury. Because inflammation with capillary leak is part of the pathophysiology of ARDS, patients may benefit from a restrictive fluid strategy (Wiedemann et al., 2006). Fluid restriction should be initiated only after a patient with shock has been adequately resuscitated. Treatment of hypoproteinemia with albumin and furosemide may also be beneficial (Randolph, 2009).
Intensive care unit (ICU) stays for patients with ARDS or ALI may be prolonged, so is important to be attentive to other organ systems and to the prevention of iatrogenic injury. Strategies to avoid ICU-related pneumonia, critical care myopathy, central line infection, deep venous thrombosis, and gastritis, will be discussed later (see Prophylaxis).
Mechanical ventilation strategies for ARDS and ALI are designed to minimize VILI while protecting the patient from profound hypoxia. A Pao2 target of 55 to 80 mm Hg (i.e., an arterial oxygen saturation as measured by pulse oximetry [SpO2] of 88% to 95%) is commonly recommended for adult patients but may be too low for children, as the effect of prolonged marginal oxygenation on the developing brain is unknown. Most authors recommend an SpO2 target of greater than 90% for children (Randolph, 2009).
Alveolar collapse is possible with a low tidal volume strategy. Recruitment maneuvers with such a strategy include application of a sustained increase in airway pressure (i.e., a pressure of 40 mm Hg for 40 seconds) with a goal of opening the collapsed lung. After recruitment, PEEP is optimized to maintain the open lung (Randolph, 2009).
Permissive Hypercapnia
If the mode of ventilation that maintains ideal oxygenation and minimizes VILI is not capable of adequate CO2 exchange, a lower pH and a higher Pco2 may be tolerated. Patients in whom hypercapnia is being tolerated to minimize lung stretch will exhibit increased air hunger and anxiety and will require increased sedation. Hypercapnia is accompanied by acidosis in the acute phase, until renal compensation can buffer the pH. Hypercapnic acidosis (HCA) may benefit patients with ARDS or ALI apart from its association with ventilator strategies designed to decrease lung stretch. HCA increases cardiac output and organ blood flow (Rogovik and Goldman, 2008). It has antiinflammatory effects and attenuates free radical production. It may thus function to attenuate further lung injury from mechanical ventilation and from evolving infection.
HCA may have deleterious effects in select populations. Decreases in neutrophil chemotaxis and in the bactericidal activity of neutrophils and macrophages seen with HCA may be harmful, particularly in patients with sepsis (Curley et al., 2010). HCA increases pulmonary vascular resistance, and therefore it may not be appropriate for patients who are anticipated to have reactive pulmonary vasculature, particularly those in the neonatal age range (Curley et al., 2010). The increase in organ blood flow with HCA can cause increased intracranial pressure (ICP) in at-risk patients. Higher levels of CO2 are not believed to damage the brain, but rigorous long-term outcome studies in children have not been done (Curley et al., 2010).
Extracorporeal Membrane Oxygenation
Despite all the innovative therapies, deterioration of lung function in patients with acute respiratory failure may lead to a requirement for 100% oxygen and high ventilatory pressures. In some of these patients, extracorporeal membrane oxygenation (ECMO) can ensure adequate oxygenation and ventilation without high Fio2 and without potentially injurious airway pressures, allowing time for the lungs to heal. ECMO has been a particularly effective treatment for neonates with persistent pulmonary hypertension of the newborn: the use of ECMO reduced mortality in this group from between 80% and 85% to about 20% (Roy et al., 2000). Although older trials had suggested that ECMO for acute respiratory failure in adults was futile, the Extracorporeal Life Support Organization registry reports a survival rate of 53% for the small number of adult patients treated with ECMO for respiratory failure (Zapol et al., 1979; Conrad et al., 2005). A recent randomized trial of ECMO for adults with acute respiratory failure found the therapy effective in terms of improving survival without disability, and in terms of cost effectiveness (Peek et al., 2009). The use of ECMO was associated with an improved survival in pediatric patients with respiratory failure (Green et al., 1996). In pediatric patients, the mortality reported by the Extracorporeal Life Support Organization registry was 56% (Conrad et al., 2005). A randomized controlled trial of ECMO in pediatric patients was attempted but failed because of an overall decrease in mortality, probably resulting from lung-protective ventilation strategies.
The general indication for ECMO is reversible lung disease that prevents adequate oxygenation with conventional therapy, or shock refractory to medical treatment in a patient who is believed to have reversible disease (see ECMO for Circulatory Support of the Patient with Shock, later).
It is difficult to precisely identify the indications for ECMO for the patient with respiratory failure. The issue is clearer in neonates, who may be expected to have a relatively rapid resolution of pulmonary hypertension and a good outcome with extracorporeal therapy. Before the advent of high-frequency oscillating ventilation and nitric oxide, an oxygenation index of 40 was the criterion for considering ECMO. A recent review of 174 newborns with respiratory failure suggested that using a lower oxygenation index (the authors suggested 33.2) as the criterion for ECMO resulted in a lower mortality rate and a lower rate of chronic lung disease (Bayrakci et al., 2007). As mechanical ventilation becomes more sophisticated, it becomes more difficult to identify its moment of failure. With continuous innovation, it is also difficult to collect historical data for criteria that would identify patients in whom mortality with conventional ventilation exceeds the expected mortality with ECMO.
ECMO should be considered in a patient with high airway pressures and high Fio2 who may be expected to require a long course of mechanical ventilation, or when the clinician believes that the probability of chronic lung disease induced by mechanical ventilation outweighs the risk of ECMO. The best outcomes are seen in patients with single-organ dysfunction and viral pneumonia. Mortality is highest in patients with trauma, immune suppression, or multiple-organ dysfunction syndrome (MODS) at the time of ECMO initiation. Most authors consider prolonged ventilatory course (>10 to 14 days) with high pressures and high Fio2 to contraindicate ECMO, because significant VILI has already occurred (Frenckner and Radell, 2008). Survival to hospital discharge is less likely in patients who have been ventilated longer than 10 days (Nehra et al., 2009). Absolute contraindications to ECMO include conditions that make cannulation impossible, including severe prematurity or small size (e.g., infants <34 weeks old and weighing <2 kg), any condition that would preclude systemic heparinization (e.g., major coagulopathy, active hemorrhage, or recent intracranial hemorrhage), or conditions amenable to surgical repair (e.g., total anomalous pulmonary venous return and lethal anomalies).
The pulmonary hypertension that leads to neonates requiring ECMO resolves in a few days, but the lung damage that leads to pediatric patients requiring ECMO is much slower to heal. For pediatric patients, the mean time on ECMO is almost 2 weeks (Frenckner and Radell, 2008; Nehra et al., 2009). A recently published 8-year experience with ECMO from Massachusetts General Hospital described an average duration of ECMO for respiratory failure of 274 hours (11.4 days), with a range of 1 to 1154 hours (48 days) (Nehra et al., 2009). The duration of a course of ECMO should be considered on a case-by-case basis. In general, it is reasonable to continue ECMO support for a pediatric patient with respiratory failure until there is clear evidence that pulmonary disease is irreversible, or until complications preclude survival.
Every day on ECMO increases the risk for complications. Bleeding occurs in up to 16% of pediatric patients on ECMO, and it is most commonly seen at surgical sites, at cannula sites, and in the GI tract. Complications related to the ECMO circuit (e.g., oxygenator failure, cannula dysfunction, tubing rupture, pump malfunction) occur for 14% of pediatric patients (Frenckner and Radell, 2008). During prolonged ECMO runs, attention to the support and preservation of extrapulmonary organ systems becomes paramount. Patients who may require ECMO should be sent to a center with expertise in the procedure before the severity of lung disease prevents safe transport.
For a thorough review of management of the patient on ECMO, see Frenckner and Radell (2008). For the most complete resource on ECMO management, see Van Meurs and colleagues (2005).
Cardiovascular system
Treatment of shock is the mainstay of emergent support of the cardiovascular system in the pediatric ICU. It is difficult to determine what percentage of shock seen in pediatric patients presenting to the PICU is septic shock, because nearly three fourths of children admitted to the PICU meet the criteria for systemic inflammatory response syndrome (SIRS) (Carvalho et al., 2005; Wessel and Laussen, 2006). Therefore, recommendations for treatment of shock are skewed toward SIRS, probably the most common cause of shock in the PICU. Despite their disparate causes, the various types of shock share a common pathophysiology and treatment, and classifying shock by etiology is not particularly useful in children. Pediatric patients with septic shock present with elements of hypovolemic, cardiogenic, and distributive shock (Zanotti-Cavazzoni and Dellinger, 2006).
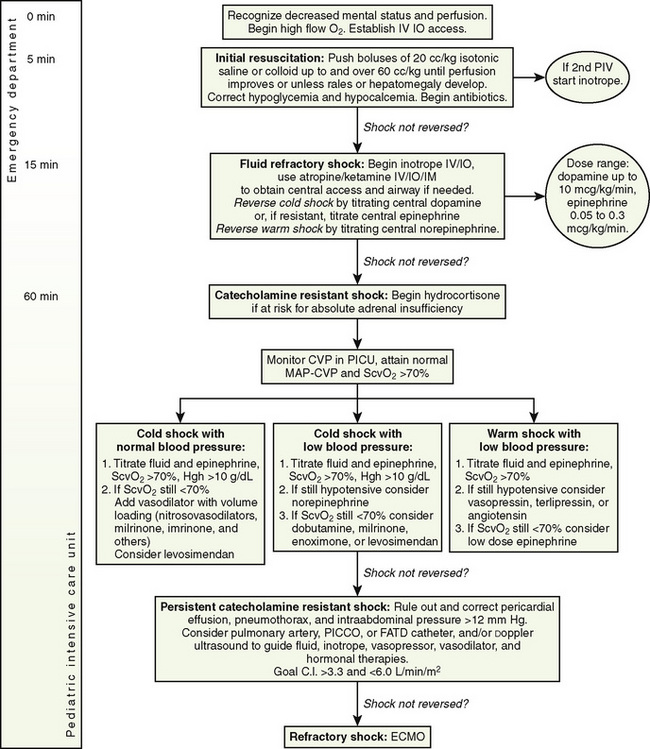
Rapid resuscitation and management of shock are critical to the treatment of children with shock, and diagnosing the type of shock is secondary to initiating resuscitation (Brierley et al., 2009). Aggressive fluid resuscitation and initiation of inotropes and antibiotics should be accomplished within the first hour after presentation. In adults, an early and aggressive protocol for septic shock has proved vastly more effective than any pharmacologic intervention (Rivers et al., 2001). Pediatric shock resuscitation protocols call for symptomatic treatment of shock using clinical signs as endpoints. The guidelines are divided into specific tasks to be accomplished in the first 5 minutes, the first 15 minutes, and the first hour (Fig. 39-4). The guidelines from the American College of Critical Care Medicine (ACCM) have resulted in improved outcomes for children with septic shock (Han et al., 2003; de Oliveira et al., 2008).
Recognition of Shock and Initial Resuscitation
Outcomes are improved if shock is recognized by its early signs, before the onset of arterial hypotension (Han et al., 2003). Because of children’s capacity to increase systemic vascular resistance, they may be normotensive in shock. Tachycardia is a sensitive but nonspecific indicator. Prolonged capillary refill in a central location, particularly when combined with tachycardia, is a more specific indicator of shock. Once the diagnosis of shock has been made, resolution of tachycardia is a useful indicator of successful treatment. Severe tachycardia (>180 beats/min in a child younger than 1 year, or >140 bpm in a younger child) in a severely ill child may be rapidly followed by a precipitous decline in blood pressure (Dabrowski et al., 2000). Treating shock in a timely manner, before hypotension develops, may prevent circulatory collapse.
The two distinct clinical presentations of shock are warm shock (characterized by full, bounding pulses, warm skin, and hypotension) and cold shock (characterized by hypotension and delayed capillary refill). Warm shock is more common in adults, but it is present in only 20% of children who present with septic shock (Zanotti-Cavazzoni and Hollenberg, 2009). Particularly in patients with cold shock, tissue hypoperfusion begins to occur long before compensatory mechanisms fail and hypotension develops (Dabrowski et al., 2000).
Shock resuscitation should begin with assessment of the airway, breathing, and circulation. All children with suspected shock should receive supplemental oxygen. Pulse oximetry is useful, not only to monitor adequacy of oxygenation but also because a poor signal indicative of poor perfusion may prompt a more aggressive resuscitation. Children with shock and severely increased work of breathing, or a Glasgow Coma Scale of less than 8, are at high risk for respiratory arrest, and the airway should be secured. Although etomidate as an induction agent favors hemodynamic stability, it impairs steroidogenesis and has been associated with increased mortality in patients with septic shock (Hildreth et al., 2008; Cuthbertson et al., 2009). Ketamine, because of its favorable effects on hemodynamics and its antiinflammatory properties, may be the drug of choice for intubating patients in shock (Lois and De Kock, 2008).
Vascular access should be established as quickly as possible. Clinicians should have a low threshold for using the intraosseous route. Central venous access is recommended for fluid refractory shock, but resuscitation should begin via intravenous or intraosseous access (Brierley et al., 2009). Anything that can be given through an intravenous line can be given via the intraosseous route. For a child with impaired perfusion, the time to central circulation for drugs administered via the intraosseous route may be shorter than for drugs given though a peripheral intravenous line. A bedside glucose test is appropriate for any acutely ill child, as high metabolic rates and limited glycogen stores predispose infants and small children with sepsis to hypoglycemia. Hypocalcemia is an easily reversible contributor to myocardial dysfunction. An ionized calcium level should be obtained, and hypocalcemia should be treated in the initial phase of resuscitation (Brierley et al., 2009).
Fluid resuscitation should be accomplished as quickly as possible. No consensus exists with regard to the use of crystalloid versus colloid (Brierley et al., 2009). The most common initial resuscitation fluid is normal saline. Crystalloid resuscitation should be delivered in 20 mL/kg aliquots to most patients in 15 minutes or less (Brierley et al., 2009). Because it is impossible to administer large volumes rapidly via a standard intravenous pump, resuscitation fluids need to be pushed by hand in children larger than 10 to 15 kg. Children with shock may require large volumes of fluid resuscitation, often in excess of 100 mL/kg, and increased fluid requirements may persist for several days (Carcillo and Fields, 2002). Children can develop severe hypovolemia before presenting with obvious shock. They may also have significant ongoing losses from the intravascular space as a result of capillary leak. Outcomes are improved when at least 60 mL/kg are given within in the first hour of resuscitation (Carcillo et al., 1991). Large volumes of fluid given to pediatric patients with shock have not been shown to increase the rate of ARDS (Carcillo et al., 1991). Fluid resuscitation should continue until there is clinical improvement, with normalization of heart rate, perfusion, and mental status, or evidence of a hypervolemic state. Smaller fluid boluses (5 to 10 mL/kg) should be considered in some patients, particularly neonates and patients with suspected heart disease. The relatively thick right ventricle of the newborn may be less compliant and therefore less able to tolerate larger fluid boluses.
Fluid Refractory Shock
Vasoactive agents are an important adjunct to the stabilization of children in shock, but they should not be considered a replacement for adequate fluid resuscitation. Current ACCM guidelines recommend beginning with an inotrope (dopamine, up to 10 mcg/kg per minute, or lower-dosage epinephrine, beginning at 0.05 mcg/kg per minute). Mortality increases with delay in initiation of inotropes. Inotropes may be given through peripheral venous access (with close monitoring of the access site) or through an intraosseous line (Carcillo et al., 1991). In patients who require vasoactive medications, central venous access should be established. Warm shock should be treated with norepinephrine delivered via a central line (Carcillo et al., 1991).
Catecholamine-Resistant Shock
When shock is not reversed by the addition of centrally delivered inotropes or vasopressors, adrenal insufficiency should be considered (Carcillo et al., 1991). The diagnosis of relative adrenal insufficiency in patients with septic shock is controversial. Hypothalamic pituitary adrenal axis suppression can occur in patients with sepsis by a variety of mechanisms, as a result of dysfunction at any point along the hypothalamic pituitary adrenal axis. A landmark study by Annane and colleagues (2002) found an improvement in mortality rates when septic patients with relative adrenal insufficiency were treated with steroid replacement. This study found a high incidence of relative adrenal insufficiency, which was probably related to a high rate of etomidate exposure in these patients, leading the author to recommend abandoning the use of etomidate (Annane, 2005). A subsequent multicenter randomized controlled trial failed to find a mortality benefit, although corticosteroid replacement did hasten reversal of shock in patients when shock could be reversed (Sprung et al., 2008). Exogenous corticosteroids have been shown to improve the vascular response to exogenous catecholamines in patients with shock (Oppert et al., 2005). The definition of an adequate circulating cortisol level is a subject of debate and probably depends on the severity of the illness. The most widely accepted definition in adults is a serum cortisol level of less than 15 mcg/dL, or an increase of less than 9 mcg/dL after ACTH stimulation (Annane et al., 2000). Because the dosage used in the ACTH stimulation test is supraphysiologic, some question its usefulness in identifying patients who could benefit from steroid replacement. Current recommendations for adult sepsis recommend use of low-dosage steroids (<300 mg/day of hydrocortisone) for shock that is resistant to fluid resuscitation and catecholamines without use of ACTH stimulation test (Dellinger et al., 2008; Marik et al., 2008). Because the P450 cytochrome system, which is responsible for steroidogenesis, matures to an adult level of functioning by about 1 year of age, infants and young children are at higher risk for adrenal insufficiency (Bartelink et al., 2006; Masumoto et al., 2008; Fernandez and Watterberg, 2009).
Adrenal insufficiency should be suspected in patients with fluid- and catecholamine-resistant shock who have risk factors for adrenal insufficiency, including a history of systemic steroid use, with a rapidly evolving purpuric rash, a history of panhypopituitarism, or exposure to etomidate (Brierley et al., 2009). The ACCM guidelines recommend hydrocortisone replacement for children with risk factors for adrenal insufficiency and catecholamine-resistant shock and for those with shock and inadequate response to the corticotropin stimulation test (peak cortisol, <18 mcg/dL after stimulation) (Brierley et al., 2009). A cortisol level should be drawn before the first dose of hydrocortisone. It is not always possible to wait for results of a stimulation test before initiating steroid replacement therapy. The recommended dosage for hydrocortisone ranges from 2 mg/kg per day (stress dosage) to 50 mg/kg per day (shock dosage) (Brierley et al., 2009). The dosage may be titrated to resolution of shock. Results of serum cortisol, when available, may lead to discontinuation of replacement hydrocortisone. The patient should be weaned from steroid therapy as soon as hemodynamics allow, because the impact of exogenous stress-dosage steroids on sensitive elements of the immune system is not fully understood. Prospective randomized controlled trials in children have not been done.
Increasing Oxygen Delivery and Normalizing Perfusion
The 2007 clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock from the ACCM recommend use of clinical goals to direct shock resuscitation in the first hour. Shock resuscitation should be continued until therapeutic endpoints are reached (Box 39-1). Adult studies have shown improved outcomes when therapies for shock are titrated to maximize oxygen delivery, using venous oxyhemoglobin saturation (Svo2) as a surrogate (Rivers et al., 2001). After the first hour of resuscitation in children, shock management should be directed to generating an Svo2 of greater than 70% and, when pulmonary artery (PA) catheters are used, a cardiac index of 3.3 to 6.0 L/min per square meter (Brierley et al., 2009). A low Svo2 may be treated with increasing inotropes or with blood transfusion, aiming for a hemoglobin level of about 10 mg/dL.
Box 39-1 Therapeutic Endpoints for the Resuscitation of Shock
Adapted from Brierley J, Carcillo JA, Choong K, et al: Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine, Crit Care Med 37:666, 2009.
In adults, the use of PA catheters has not been shown to improve mortality, but comparable studies have not been done in children. PA catheters have been shown to identify incorrect assessment of hemodynamic status made on the basis of clinical parameters in children (Ceneviva et al., 1998). PA catheters may be useful in children when therapy directed by central venous pressure and Svo2 is inadequate to reverse shock (Carcillo and Fields, 2002).
Vasopressin has been used in catecholamine refractory shock, particularly in the setting of decreased vascular tone. Children with severe shock often have high levels of endogenous catecholamines and some degree of catecholamine resistance. This, coupled with the fact that catecholamines have some unfavorable effects on immune function and catabolism, have led investigators to consider other biochemical pathways to reverse shock. Vasopressin is a powerful vasoconstrictor, but at low dosages it has organ-specific vasodilatory effects (Holmes et al., 2003). Vasopressin deficiency has been described in vasodilatory shock states in both adults and children (Landry et al., 1997; Lodha et al., 2006). Studies in adults have shown that vasopressin improves blood pressure and urine output and allows lowering of the dosage of norepinephrine; however, a recent large randomized controlled trial of low-dosage vasopressin infusion found a good safety profile but no impact on mortality when compared with norepinephrine alone (Russell et al., 2008).
Vasopressin and its synthetic analogue, terlipressin, have been used in children in two ways: as a rescue therapy for severe shock, and as a catecholamine-sparing hormone replacement. Case series of the use of vasopressin in patients with refractory shock have described the use of dosages in the range of 0.00001 to 0.08 units/kg per minute (Matok et al., 2005; Choong and Kissoon, 2008; Yildizdas et al., 2008). When vasopressin is used as a hormone replacement, it is not titrated to clinical effect but used as a low-dosage continuous infusion. The only pediatric randomized controlled trial so far used a dosage of 0.0005 units/kg per minute, as in adult trials, and found an increase in MAP but no differences in time to hemodynamic stability or other clinical outcomes (Choong et al., 2009).
Reassessment of Fluid Balance and Hemodynamic Profile
Serial reassessments of patients admitted with shock are critical to good outcomes, and require continuous and meticulous attention to the maintenance of optimal preload, contractility, and afterload. Shock in neonates and children is more variable than in adults. Children who present in warm shock with an increased cardiac output and low systemic vascular resistance often progress to cold shock with myocardial dysfunction within 24 hours. Shock that is refractory to fluid resuscitation and vasoactive agents should prompt review of volume status. A high percentage of patients who have persistent shock after volume resuscitation have an underfilled ventricle, seen by echocardiography (Jardin et al., 1999). It should also raise the possibility of alternative diagnoses, including acquired cardiac disease, obstructive etiologies, toxins, occult blood loss, and inborn errors of metabolism.
Other Causes of Shock
Causes of shock in the neonatal age range include congenital heart disease, particularly ductal-dependent lesions. An infusion of prostaglandin E2 should be started immediately in neonates with shock until a ductal-dependent congenital heart lesion can be ruled out. Prostaglandin may cause apnea at higher dosages. Very young infants still transitioning from fetal to neonatal circulation are also at high risk for pulmonary hypertension and right-sided heart failure. Acidosis from shock and hypoxia can trigger this pathophysiology. Therapies that reduce pulmonary arterial pressure, including supplemental oxygen, sedation, maintenance of an alkalotic pH, and nitric oxide, are commonly needed for neonates with fluid-refractory shock and may be started empirically if necessary (Carcillo and Fields, 2002). Particular attention should be paid to maintenance of adequate temperature and glucose level in neonates with shock.
Early antibiotic administration is a high priority in patients presenting with septic shock. In adults with septic shock, a delay in antibiotic therapy is associated with worse survival, with mortality rates increasing by 7% for every 30 minutes that passes without delivery of appropriate antibiotic therapy (Kumar et al., 2006). Cultures are extremely important, but antibiotics should not be delayed if it is difficult to secure appropriate samples. In particular, lumbar puncture should be deferred until the patient is more stable and an evaluation of the coagulation system can be performed.
ECMO for Circulatory Support of the Patient with Shock
Shock that is refractory to medical interventions may be treated with extracorporeal support. ECMO for pediatric circulatory support was used in 29% of all cases in 2004, but most of these were cardiac patients requiring perioperative support (Conrad et al., 2005). Shock related to acute fulminant myocarditis has been successfully treated with extracorporeal support, with survival rates approaching 80% for patients on ECMO (Duncan et al., 2001). ECMO may be a bridge to myocardial recovery or to heart transplantation in these patients. It may be more effective in the support of shock characterized by inadequate cardiac output than of shock with a prominent component of vasoplegia or failure of oxygen extraction.
The use of ECMO for other causes of shock is uncommon and poorly studied. Historically, sepsis was considered a contraindication to ECMO because of concerns that the circuit would be come irreversibly contaminated, but today, sepsis causing pulmonary hypertension is a standard indication for ECMO in neonates. Randomized controlled trials of ECMO for refractory septic shock are not possible, because the condition is rare and the options for treatment are few. A recent single-center report of 45 pediatric patients treated with ECMO for refractory circulatory failure due to sepsis, collected over 18 years, is the largest study published to date. These authors reported a 47% survival to hospital discharge despite the fact that 91% of the patients had three or more organ failures (Maclaren et al., 2007). Estimates of mortality without ECMO in case series of patients in which ECMO was used for this indication support the idea that ECMO as a treatment for septic shock significantly improves mortality rates (Bartlett, 2007). The reported average durations of ECMO for sepsis-related circulatory failure are 137 hours and 76 hours in case series of 9 and 12 patients, respectively (Beca and Butt, 1994; Goldman et al., 1997).
Cardiac Arrest in Children
Management of the Patient after Cardiac Arrest
Protocols for the management of pediatric cardiac arrest have been developed by the American Heart Association and are covered in Chapter 38, Cardiopulmonary Resuscitation. The focus here is on the care of children after the return of spontaneous circulation.
The most important treatment for pediatric cardiac arrest is prevention. Because pediatric cardiac arrest is not usually sudden in onset, a window of opportunity exists in the period before it occurs. Medical emergency teams designed to respond to patients in danger of decompensation have been shown to decrease hospital mortality rates and the frequency of more serious codes (Topjian et al., 2009). In addition to early recognition of impending cardiac arrest, extracorporeal cardiopulmonary resuscitation (ECPR) has been used in an attempt to improve these outcomes. Intact survival from isolated respiratory arrest is the rule, but outcomes from in-hospital pediatric cardiac arrest remain poor. Return of spontaneous circulation is established in one half to two thirds of cases of in-hospital arrest, but less than 18% of patients survive with good neurologic outcome (Nadkarni et al., 2006). Survival rates and neurologic outcomes for out-of-hospital cardiac arrest are much poorer (Young et al., 2004). Cold-water submersion for cardiac arrest, even for relatively long duration, may be associated with a much higher rate of intact survival.
Extracorporeal Cardiopulmonary Resuscitation
Multiple physicians have examined the application of extracorporeal therapy as a rescue therapy during cardiac arrest before the return of spontaneous circulation. Survival to hospital discharge in these case series is surprisingly high—35%—despite a relatively long median duration of cardiopulmonary resuscitation (CPR) before the establishment of flow on ECMO (mean duration of 50 minutes) (Morris et al., 2004). With ECPR, survival to hospital discharge does not correlate with duration of CPR before ECMO. Neurologic outcomes have been encouraging: good neurologic outcomes are reported in greater than 75% of patients (Huang et al., 2008; Prodhan et al., 2009). It is important to select patients who have a reversible disease process for use of ECPR.
Management of Hypoxic-Ischemic Brain Injury after Resuscitation
Controlled hypothermia is associated with improved mortality rates and improved functional outcome after cardiac arrest in adults and in neonates with birth asphyxia (Bernard et al., 2002; Hypothermia after Cardiac Arrest Study Group, 2002; Gluckman et al., 2005; Shankaran et al., 2005). It is difficult to extrapolate these findings directly to children, because the circumstances surrounding cardiac arrest in children are different from those in adults. Ventricular fibrillation is the most common cause of cardiac arrest in adults, but cardiac arrest in children is predominately caused by asphyxia. Asphyxial arrest occurs after a period of hypoxia, and the total duration of cerebral insult (hypoxia plus anoxia) may be very long. Ischemic injury may be seen in brain cells after relatively short periods of interrupted circulation if they are preceded by a period of hypoxia (Vaagenes et al., 1997).
Clinical trials of therapeutic hypothermia after cardiac arrest in children are ongoing. Although there are not yet randomized controlled data supporting controlled hypothermia for pediatric patients after cardiac arrest, it is being used in the United States “consistently” by 9% of the pediatric intensivists surveyed and “sometimes” by 38% (Haque et al., 2006). Target temperatures vary widely, with most using temperatures of 32° to 35° for 12 to 24 hours (Haque et al., 2006). It is relatively labor intensive to rapidly achieve and maintain hypothermia. Neuromuscular blockade may be necessary to control shivering. Slow and controlled rewarming (0.5° C every 2 hours) is an important part of this therapy. Vigilance during the rewarming phase is necessary to avoid hyperthermia, which is known to exacerbate ischemic brain injury, but also to allow careful monitoring of the blood pressure and electrolyte disturbances that are common with rewarming (Wang et al., 2009; Fink et al., 2010a, 2010b). In a retrospective analysis of children after cardiac arrest, there was no increase in the number of hypothermia-associated adverse events in patients who received this therapy (Doherty et al., 2009).
Hypoglycemia has a synergistic, deleterious effect when coupled with neonatal asphyxia (Lubchenco and Bard, 1971). Hyperglycemia has been shown to be detrimental after near-drowning and traumatic brain injury, and, in general, glucose-containing solutions are not used in the first 48 hours after brain injury in older children (Ashwal et al., 1990; Chiaretti et al., 2002). Both hypotension and hypertension during reperfusion have been shown to have deleterious effects in animal models (Miller and Myers, 1972; Bleyaert et al., 1980).
Oxygen toxicity is posited to play a role in postischemic reperfusion injury. The brain is susceptible to oxygen free-radical damage (Siesjö et al., 1989). On the basis of this, there has been some interest in using room air to resuscitate newborns, particularly those with preserved circulation (Ten and Matsiukevich, 2009). In older patients, avoidance of hyperoxia may be beneficial.
Seizure activity increases cerebral metabolic rate and may worsen ischemic injury. Subclinical seizure activity is common after cardiac arrest, particularly in patients treated with therapeutic hypothermia, because of the requirement for neuromuscular blockade. Prophylactic use of antiepileptic drugs in patients who require paralysis is reasonable (Abend et al., 2009).
Prediction of Outcome after Cardiac Arrest
Attempts to use variables such as duration of cardiac arrest, initial pH, or early neurologic examination to predict neurologic outcome after cardiac arrest in children have been misleading. Electroencephalography can provide some prognostic information. The burst suppression pattern seen on the electroencephalogram after cardiac arrest is sensitive and specific for poor outcome (Wijdicks et al., 2006). No single test has been validated to predict outcome with a high sensitivity and specificity. High-quality CPR, and well-established ventilation and oxygenation in the period before the return of spontaneous circulation or the establishment of ECMO flow, may be the best predictors of outcome (Topjian et al., 2009).
Renal system
Fluid Balance and Renal Replacement
Fluid overload and renal insufficiency are common in critically ill patients because of requirements for fluid resuscitation, medications, and blood products. Fluid overload is associated with increased incidence of multiorgan failure in critically ill adults and children (Schuller et al., 1991; Rosenberg, 2003; Goldstein et al., 2005). Decreased fluid overload is associated with improved outcomes in neonates on ECMO, adults with ARDS, and children requiring continuous hemofiltration (Kelly et al., 1991; Foland et al., 2004; National Heart, Lung and Blood Institute, 2006). Diuretics and fluid restriction are routinely used to ameliorate the fluid overload accumulated with initial resuscitation. Fluid restriction may be necessary when fluid excretion is limited by kidney injury, but over time it may impair caloric intake.
Acute Kidney Injury
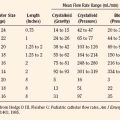
Acute kidney injury is fairly common in pediatric ICU patients and is independently associated with mortality (Plötz et al., 2008). The definition of acute renal failure is not mutually agreed on: it is described as a change from baseline in serum creatinine, or an elevation in blood urea nitrogen level, or urine output inadequate to match required intake (Plötz et al., 2008). A uniform definition of renal injury is important for research, but it does not yet inform clinical decision making. The RIFLE criteria (risk, injury, failure, loss, end-stage renal disease) have been proposed to classify renal injury in adult patients (Kellum et al., 2002). A pediatric modification of the adult RIFLE criteria has been proposed and independently evaluated (Table 39-1) (Akcan-Arikan et al., 2007). Among children requiring mechanical ventilation for less than 4 days, 58% met pediatric RIFLE criteria for acute kidney injury, most on the first day of admission. Patients with acute kidney injury according to the pediatric RIFLE scoring system had a mortality rate that was five times higher than patients without acute kidney injury (Akcan-Arikan et al., 2007; Plötz et al., 2008).
| Estimated Creatinine Clearance Index | Urine Output | |
| Risk | eCCI decreased by 25% | <0.5 mL/kg per hr for 8 hr |
| Injury | eCCI decreased by 50% | <0.5 mL/kg per hr for 16 hr |
| Failure | eCCI decreased by 75% or eCCI <35 mL/min per 1.73 square meter | <0.3 mL/kg per hr for 24 hr, or anuric for 12 hr |
| Loss | Persistent failure > 4 wk | — |
| End-stage renal disease | End-stage renal disease (persistent failure > 3 mo) | — |
eCCI, Estimated creatinine clearance index; RIFLE, risk, injury, failure, loss, and end-stage renal disease.
From Akcan-Arikan A, Zappitelli M, Loftis LL, et al: Modified RIFLE criteria in critically ill children with acute kidney injury, Kidney Int 71:1028, 2007.
Renal Replacement Therapy
Criteria for Initiating Renal Replacement Therapy
There are no nationally published guidelines for the initiation of RRT, and there is wide variation among practitioners as to its timing of initiation and discontinuation. Absolute indications for renal replacement therapies include life-threatening electrolyte abnormalities and anuria. However, RRT is more commonly used for the relative indication of fluid overload with inadequate urine output (Gibney et al., 2008). The guidelines used at the Children’s Hospital of Pittsburgh (University of Pittsburgh Medical Center) are shown in Box 39-2. There is increasing evidence that fluid overload contributes to mortality, particularly in children. Observational studies suggest that increasing severity of fluid overload before or at initiation of RRT is an independent predictor of mortality after controlling for severity of illness. RRT should be considered when accumulated fluid overload reaches 10% (Ricci and Ronco, 2008; Sutherland et al., 2010).
Box 39-2 Indications for Continuous Renal Replacement Therapy
Practical Aspects of Renal Replacement Therapy
Continuous renal replacement therapy (CRRT) is the preferred mode of renal replacement therapy in ICU patients with hemodynamic instability. The advantages of CRRT over intermittent hemodialysis are that it allows correction of metabolic acidosis, provides temperature control, and provides constant fluid hemostasis with better avoidance of intracerebral fluid shifts. Its main drawback is the need for anticoagulation. Although CRRT shows no survival benefit over intermittent therapy, it may predict renal recovery in survivors (Uchino et al., 2007).
Clinical Changes When Initiating CRRT
Clinicians should be alert to the risk for hypotension when CRRT is initiated. Hypotension may occur as a result of inadequate intravascular volume, hypocalcemia, or bradykinin release syndrome, particularly when patients are acidotic at the time of CRRT initiation. Once CRRT is begun, therapies aimed at preventing fluid overload should be discontinued. Diuretics should be decreased or stopped. Nutrition should be increased if it had been limited by fluid restriction. The amount of protein in parenteral nutrition should be increased to 1.5 to 4 mg/kg per day, because hemofiltration results in significant amino acid loss across the membrane (Maxvold et al., 2000). A low blood urea nitrogen level in a patient on RRT reflects inadequate nitrogen balance. A patient on CRRT requires additional potassium and phosphorus to replace that lost to the circuit. When CRRT is discontinued or interrupted, diuretics, fluid rate, TPN prescription, and electrolyte infusions should be returned to normal. The removal of medications across the CRRT membrane is difficult to predict. Consultation with a pharmacist is recommended with initiation of CRRT.
High-Clearance CRRT for Treatment of Sepsis
It is conventional to speak of the dosage of CRRT, meaning the quantity of blood purified per unit time. Higher-clearance CRRT (at least 35 mL/kg per hour versus 20 mL/kg per hour) is associated with improved survival in adults, with the greatest improvement seen in septic patients (Ronco et al., 2000). There has been some interest in using extremely high clearance CRRT to remove cytokines and modulate inflammation. This approach has shown some promise in patients with ARDS after bone marrow transplantation (DiCarlo et al., 2003). The pore size in most filters used for hemofiltration and dialysis admit passage of proteins whose size is in the range of 30 to 40 kDa, which is more than adequate for removal of interleukins 1, 6, 8, and 10, and tumor necrosis factor. Studies of high-clearance CRRT in septic patients have not shown significant and sustained decreases in cytokine levels, and high-clearance CRRT has not been conclusively shown to improve outcome (Joannidis, 2009).
Contraindications to CRRT
There are no absolute contraindications for the use of CRRT. Patients at risk for life-threatening bleeding with systemic heparinization may be put on CRRT with a citrate anticoagulation protocol. Citrate is added to the CRRT circuit, generating a level of hypocalcemia in the circuit sufficient to prevent clotting. Calcium must be infused centrally to replace the calcium lost in the circuit. Most of the citrate is removed in the circuit, and the liver metabolizes what remains. When citrate is used for anticoagulation, patients with liver failure who are unable to metabolize citrate are at risk for citrate lock, manifested by an increased ratio of total to ionized calcium (Morgera et al., 2004). Risks and benefits should be weighed carefully when considering the use of citrate CRRT in patients with liver failure.
Nutritional Support
Premorbid, protein-energy malnutrition is associated with poor immune function and wound healing, multiorgan failure, longer hospital stays, and poor outcomes, and the nutritional status of children deteriorates while they are in the PICU (Pollack et al., 1982; Pollack et al., 1985; Hulst et al., 2004). It can be difficult to assess the nutritional needs of PICU patients. Adults have a hypermetabolic response to critical illness. Calculations of resting energy expenditures and correction factors for a variety of critical illness states in children have been proposed, but, with the exception of burn injury, for which the hypermetabolic state is well documented, these have not been validated. There are factors that both increase and decrease metabolic needs during critical illness. Resting energy expenditure may be higher than normal, but the energy used by muscle activity is much lower for healthy children. Metabolic calculations in critically ill children demonstrated that energy expenditure was lower than predicted by common formulas and was lowest in patients with multiple-organ dysfunction syndrome (Turi et al., 2001). Both overfeeding and underfeeding have deleterious consequences in critically ill children (Skillman and Wischmeyer, 2008). Optimal energy intake during the acute phase and the recovery phase of critical illness remains uncertain.
The recommended daily calorie requirements for healthy children are a reasonable starting target for critically ill children. A general estimate of calorie requirements by age is presented in Table 39-2 (Agus and Jaksic, 2002).
TABLE 39-2 Estimated Calorie and Protein Requirements of Children During Critical Illness
| Age | Calories | Protein (g/kg per day) |
| Premature infant | 120 | 3.5 |
| Term infant | 100 | 2.5-3 |
| Child, 1 to 12 years | 70 | 1.5-2 |
| Child, >12 years | 70 | 1.5 |
Enteral Feeding
Enteral feeding is usually withheld until cardiovascular stability is established, because generalized hypoperfusion and vasoactive agents limit intestinal blood flow. However, early enteral feeding may preserve gut mucosal integrity, may decrease septic episodes, and may decrease the incidence of ventilator-associated pneumonia (Jeejeebhoy, 2001; Kompan et al., 2004). Aspiration is thought to be a risk factor for ventilator-associated pneumonia (VAP), but enteral nutrition decreased the risk for VAP in adults. Similar studies have not been done in children. The frequent interruptions to enteral feeding (for many reasons, including perceived intolerance, procedures, diagnostic tests, medication administration) may result in inadequate nutrition delivery (de Neef et al., 2008). Attention should be given to calories delivered, not just calories prescribed. Feeding protocols may limit the impact of these factors on the delivery of adequate nutrition (Meyer et al., 2009). When complete enteral nutrition is impossible, low-dosage enteral nutrition in combination with parenteral nutrition is probably preferable to parenteral nutrition alone (Heidegger et al., 2008).
Transpyloric feeding may help to avoid unnecessary feeding interruptions, particularly in patients with gastroparesis or those who are at increased risk for aspiration, but it does not prevent aspiration of gastric contents (Meert et al., 2004). Because infants and children are at higher risk for malplacement of enteral feeding tubes, tube placement should be confirmed radiographically before use. Enteral formulas exist for special situations such as renal failure, general malabsorption, fat malabsorption, and pancreatitis. Although it is generally accepted that enteral nutrition is superior to parenteral nutrition, studies in pediatric patients are lacking.
Parenteral Nutrition
Total parenteral nutrition (TPN) is indicated for patients with contraindications to enteral feeding. TPN is associated with a number of complications, including intestinal mucosal atrophy with increased bacterial translocation, metabolic abnormalities including hyperglycema and hypertriglyceridemia, and immune dysfunction and infection. With long-term therapy, hepatic steatosis, cholestasis, and irreversible liver failure can occur. When there is risk for liver injury, TPN is often cycled to mimic the postabsorptive state (Kelly, 2006). Timing of initiation of parenteral nutrition is controversial. A recent review suggested starting it when enteral nutrition could not be used for 3 to 5 days (Skillman and Wischmeyer, 2008). In patients at high risk for malnutrition, it may be appropriate to initiate parenteral nutrition sooner.
The glucose infusion is typically started at 5 to 6 mg/kg per minute and increased by 0.5 to 1 mg/kg per minute per day for preterm infants, or 2 to 4 mg/kg per minute per day for older children. For patients with sepsis, the ACCM guidelines recommend at least 8 mg/kg per minute for neonates, 5 mg/kg per minute in children, and 2 mg/kg per minute in adolescents (Brierley et al., 2009). Glucose control may be important in critically ill patients (see later). Some of the negative effects of parenteral nutrition seen in studies done in the past two decades may be related to carbohydrate overfeeding and poor glycemic control.
Provision of adequate protein intake prevents negative nitrogen balance and skeletal muscle wasting and supports growth and wound healing. To prevent protein from being used as a calorie source and to preserve its availability for growth and repair, adequate nonprotein calories must be provided (see Table 39-2). In renal failure, it may be necessary to decrease the amount of protein delivered. When CRRT is initiated, protein delivery should increase to compensate for protein lost across the membrane.
Intensive Insulin Therapy in Critically Ill Patients
Hyperglycemia is common in critically ill patients. In children, hyperglycemia is associated with organ dysfunction and poor outcomes (Kyle et al., 2010). Maintenance of normal fasting blood glucose values (80 to 100 mg/dL) conferred a survival advantage over maintenance blood glucose values (180 to 200 mg/dL) in critically ill adult surgical patients and was associated with a shorter ICU and hospital stay, a shorter period of mechanical ventilation, and less renal injury in adult medical patients (van den Berghe et al., 2001, 2006). In a recently published trial of tight glucose control using age-stratified glucose targets in critically ill children, tight control was associated with improvement in morbidity and mortality over a glucose target of 180 to 214 mg/dL, despite episodes of hypoglycemia occurring in 25% of the treatment group. More than two thirds of the children in this study were postoperative cardiac surgery patients; this may hamper the study’s generalizability to medical patients (Rouette et al., 2010).
Neurologic systems
Traumatic Brain Injury
See Adelson and colleagues (2003) for a thorough review of traumatic brain injury in infants, children, and adolescents. See Chapter 22, Anesthesia for Neurosurgery, for perioperative treatment of the pediatric trauma patient. Here, the focus is on the critical care management of traumatic brain injury (TBI).
Physiology of Traumatic Brain Injury
In the injured brain, there is an area of primary injury that cannot be ameliorated, a penumbra of brain tissue that is at risk for further injury, and normal brain distant from the site of initial injury. The goal of critical care after TBI is to keep that penumbra as small as possible and to protect the normal brain from further injury. Only part of the injury sustained in TBI occurs with the initial injury. The most important causes of secondary injury include hypoxia, hypotension, hyperglycemia and hyperthermia, seizures, and intracranial hypertension. The mechanisms for secondary brain injury, including excitotoxicity, have been reviewed by Kochanek and coworkers (2008).
Initial Management of the Brain-Injured Patient
Early management of airway, breathing, and circulation should take into account the devastating effect of even transient hypoxia and the impact of hypercarbia on ICP. Prevention of hypoxia by intubation is strongly associated with improved outcomes (Franschman et al., 2009; Zebrack et al., 2009). The initial management of TBI is presented in Box 39-3. The examiner should always be sensitive to signs of intracranial hypertension or impending herniation, including pupillary dilation, Cushing’s triad, and extensor posturing. The Glasgow Coma Scale has been adapted for use in children and should be calculated for all patients with TBI on arrival and in subsequent examinations (Table 39-3). Brain injuries evolve over time, and the initial computed tomography scan might not reflect the current state of brain edema or intracranial hemorrhage. A sustained change in ICP or in the neurologic examination should prompt repeat imaging.
Box 39-3 Initial Management of Traumatic Brain Injury
Modified from Franschman G, Peerdeman SM, Greuters S, et al: ALARM-TBI investigators: Prehospital endotracheal intubation in patients with severe traumatic brain injury: guidelines versus reality, Resuscitation 80(10):147, 2009; Zebrack M, Dandoy C, Hansen K, et al: Early resuscitation of children with moderate to severe traumatic brain injury, Pediatrics 124(1):56, 2009.
| Infants and Young Children | Older Children and Adults | |
| Eye Opening | ||
| 4 | Spontaneous | Spontaneous |
| 3 | To voice | To voice |
| 2 | To pain | To pain |
| 1 | None | None |
| Verbal | ||
| 5 | Coos, babbles | Oriented |
| 4 | Irritable | Confused |
| 3 | Cries to pain | Inappropriate words |
| 2 | Moans to pain | Incomprehensible sounds |
| 1 | None | None |
| Motor | ||
| 6 | Normal spontaneous movements | Obeys commands |
| 5 | Withdraws to touch | Localizes pain |
| 4 | Withdraws to pain | Withdraws to pain |
| 3 | Abnormal flexion | Abnormal flexion |
| 2 | Abnormal extension | Abnormal extension |
| 1 | Flaccid | None |
Minimizing Secondary Injury
Once the initial assessment and resuscitation are complete, further management is aimed at preventing and treating causes of secondary brain injury. The goals are to preserve delivery of fuel substrates, to prevent increases in cerebral metabolic needs, and to minimize ICP. The management for minimizing secondary injury is presented in Box 39-4 (Chiaretti et al., 2002; Adelson et al., 2003; Davis, 2008). Therapeutic hypothermia is under investigation for children with TBI, and although there are no data to support broad application of this therapy, it might be considered for severe refractory intracranial hypertension.
Box 39-4 Minimizing Secondary Injury
Modified from Chiaretti A, Piastra M, Pulitanò S, et al: Prognostic factors and outcome of children with severe head injury: an 8-year experience, Childs Nerv Syst 18(3–4):129, 2002; Adelson PD, Bratton SL, Carney NA, et al; American Association for Surgery of Trauma; Child Neurology Society; International Society for Pediatric Neurosurgery; International Trauma Anesthesia and Critical Care Society; Society of Critical Care Medicine; World Federation of Pediatric Intensive and Critical Care Societies: Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 5. Indications for intracranial pressure monitoring in pediatric patients with severe traumatic brain injury, Pediatr Crit Care Med 4(3 Suppl):S1, 2003; Davis DP: Early ventilation in traumatic brain injury, Resuscitation 76(3):333, 2008.
Management of Intracranial Hypertension
Intracranial hypertension may impair cerebral blood flow and is associated with poor outcome. It results from osmolar shifts, cellular edema, vasogenic edema (blood-brain barrier breakdown), mass lesions, and intracranial hemorrhage. Protocols exist for the management of intracranial hypertension, but they should not be seen as a surrogate for cooperative management with a neurosurgical team (Adelson et al., 2003). Therapies aimed at controlling intracranial pressure are applied in a stepwise fashion, beginning with the least invasive measures (Box 39-5).
Box 39-5 Management of Intracranial Hypertension
Adapted from Davis DP: Early ventilation in traumatic brain injury, Resuscitation 76(3):333, 2008; Adelson PD, Bratton SL, Carney NA, et al; American Association for Surgery of Trauma; Child Neurology Society International Society for Pediatric Neurosurgery; International Trauma Anesthesia and Critical Care Society; Society of Critical Care Medicine; World Federation of Pediatric Intensive and Critical Care Societies: Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 5. Indications for intracranial pressure monitoring in pediatric patients with severe traumatic brain injury, Pediatr Crit Care Med 4(Suppl 3):S1, 2003.
Hematology and immunology
Red Blood Cell Transfusion in the PICU
Critically ill patients are prone to anemia as a result of hemodilution, phlebotomy, and suppression of hematopoiesis by, for example, inflammation and malnutrition. Red blood cell transfusions are associated with significant risks related to immune suppression, transfusion reactions, transfusion associated lung injury, infection. Changes in the red blood cell that occur during storage may decrease oxygen delivery, negating the benefit expected from transfusion (Tinmouth et al., 2006). Adult studies have shown that setting a conservative threshold for transfusion (i.e., a hemoglobin level of 7 mg/dL) is safer than a threshold of 10 mg/dL. A conservative threshold reduced the number of transfusions and decreased the incidence of organ failure, cardiac complications, and mortality (Hébert et al., 1999).
Pediatric studies support that it is safer to use a conservative (7 mg/dL) than a liberal (9.5 mg/dL) threshold for stable critically ill children and postsurgical pediatric patients. This restrictive strategy reduces the number of transfusions per patient and dramatically reduces the number of patients who receive transfusions without worsening organ dysfunction (Lacroix et al., 2007; Rouette et al., 2010). The optimal transfusion threshold for unstable patients is unknown. It is probably appropriate to target the normal range in children with profound hypoxemia and shock (Carcillo and Fields, 2002; Randolph, 2009).
Sepsis and Multiple Organ Dysfunction Syndrome
No treatment improves outcomes in pediatric patients with sepsis besides providing appropriate antibiotic therapy and rapidly and aggressively treating shock (for discussions on the latter, see Cardiovascular System, p. 1258). Administration of appropriate antimicrobial therapy should be considered urgent, as a delay of even 30 minutes has been shown to increase mortality in adults. It is appropriate to start with broad coverage, using local antimicrobial resistance patterns to guide antibiotic choice. The most common bacterial causes of sepsis vary by age and immunization status, with group B Streptococcus and Escherichia coli predominating in newborns, and Streptococcus pneumonia, Staphylococcus aureus, and Neisseria meningitides predominating in older children. Viruses and fungi can also cause sepsis, particularly in immunocompromised hosts. The pathophysiology of sepsis is complex and incompletely understood (but see Hotchkiss and Karl, 2003).
Activated protein C, which held some promise in adult patients with sepsis, is not recommended in children. In a randomized controlled trial of activated protein C in pediatric patients with sepsis, no difference in organ failure or mortality was found between groups, but there was an increase in central nervous system bleeding in the treatment group (Nadel et al., 2007).
Multiple organ dysfunction syndrome represents a continuum of physiologic abnormalities rather than a dichotomous state of organ function or failure. MODS develops quickly in children, with a maximum number of organ failures seen at 72 hours in most children (Proulx et al., 1994). Diagnostic criteria for organ failures are useful for comparing patients in research studies and evaluating outcome in clinical trials (Wilkinson et al., 1987; Leteurtre et al., 2003). Because overall mortality is lower in critically ill children than in adults, organ failure scores and time to resolution of organ failure are considered appropriate endpoints for clinical trials in pediatric intensive care. Children with MODS have a better chance of a good outcome than adults. Six months after discharge from the PICU, 60% of survivors of MODS have a normal quality of life, and 32% have ongoing health, emotional, or cognitive problems (Ambuehl et al., 2002). The number and severity of organ failures are associated with mortality, but it is rarely the case that failure of a specific organ can be implicated as a cause of death. Children with severe MODS usually die when the decision to withdraw support is made in response to an increasingly grim prognosis.
Shock states may cause relative hypoperfusion to abdominal organs, as sympathetic stimulation preferentially diverts blood flow from the periphery and the splanchnic circulation and toward the myocardium and the brain. This is particularly true in children, for whom the most common hemodynamic profile is cold shock. Elevated levels of both proinflammatory and antiinflammatory cytokines are associated with severity of MODS (Doughty et al., 1998; Whalen et al., 2000). Breakdown of the barrier function of intestinal mucosa has been postulated to contribute to MODS by initiating or exacerbating the inflammatory cascade (Proulx et al., 2009). Increased prothrombotic and antifibrinolytic activity in plasma is associated with severity of MODS. Dysregulation of normal clotting and fibrinolytic mechanisms may lead to microvascular thrombosis, impairing organ perfusion locally and perpetuating the inflammatory state (Proulx et al., 2009). Nitric oxide, which is present in increased amounts in the blood during shock states, may combine with superoxides to produce peroxynitrites. Peroxynitrites can damage lipid membranes and DNA and alter mitochondrial function (Wong et al., 1995; Burney et al., 1999).
Mechanical ventilation causing excessive stretch of poorly compliant lungs leads to the release of inflammatory mediators and has been implicated in the development of multiorgan failure in ARDS patients (Tremblay and Slutsky, 2006). The abnormalities of metabolism and neuroendocrine response noted in patients with multiorgan failure may represent other organ failures or may contribute to the syndrome (Proulx et al., 2009). Impairments in the immune response have also been noted in these patients. Failure of antigen presentation (i.e., immunoparalysis) and depletion of lymphoid elements predispose patients with MODS to secondary infection and may contribute to mortality (Peters et al., 1999; Felmet et al., 2005). Finally, mitochondrial dysfunction has been posited to be a part of the final common pathway of MODS and may some day be treatable (Singer, 2007).
The treatment of MODS is supportive. Treatments discussed earlier and later in this chapter are intended to minimize the development and severity of organ dysfunction. Treatments that have been associated with a decrease in the incidence or severity of MODS in adults include use of ventilator strategies that avoid of excessive stretch or atelectrauma, aggressive shock resuscitation, control of source of infection or inflammation, early enteric feeding to prevent intestinal mucosal atrophy, tight glycemic control, and a conservative transfusion threshold (Proulx et al., 2009).
Prophylaxis
Gastritis
The association between severe stress and GI ulceration is well established. The incidence of clinically important GI bleeding in both adult and pediatric patients with critical illness is low (Quenot et al., 2009; Reveiz et al., 2010). The mechanism of stress ulcer formation is incompletely understood, but it includes impairment of blood flow to the intestinal mucosa and circulation of proinflammatory cytokines (Quenot et al., 2009). Direct damage to the epithelium by hydrogen ions is preventable to some degree, but this is only one of many factors. Options for prophylaxis include antacids, sucralfate, histamine type 2 (H2) receptor antagonists (H2RAs), and proton pump inhibitors (PPIs).
Sucralfate works by improving the neutralization capacity of the mucous layer of the gastric lining. It may decrease the absorption of concomitantly administered medications. Because it is not systemically absorbed, it may not be administered via duodenal or jejunostomy tubes. H2RAs decrease gastric acid secretion through competitive inhibition of histamine-stimulated acid secretion. PPIs irreversibly inhibit the final step in acid production (the transport of hydrogen), providing long-lasting acid suppression. PPIs inhibit both vagally mediated and histamine-induced acid secretion. Both PPIs and H2RAs are superior to placebo in suppressing clinically significant stress-ulcer–related bleeding (Reveiz et al., 2010).
Stress-ulcer prophylaxis is not routinely indicated for ICU patients but should be reserved for patients at risk for stress ulcer–related bleeding. These include patients with sepsis, coagulopathy, or a history of ulcer or GI bleeding within the past year, and patients requiring mechanical ventilation for longer than 48 hours, a prolonged ICU stay, or high-dosage corticosteroids (Reveiz et al., 2010). A recent meta-analysis failed to find convincing evidence that PPIs are superior to H2RAs in terms of preventing stress ulcer–related bleeding (Lin et al., 2010). Many clinicians discontinue stress ulcer prophylaxis when the patient begins an oral diet, begins a complete enteral diet, or discontinues steroid therapy.
Deep Venous Thrombosis
Among adult trauma patients, the risk for deep venous thrombosis (DVT) is greater than 50% without venous thromboembolism (VTE) prophylaxis. Prophylaxis reduces the incidence of new DVT in the adult medical and surgical ICU to between 10% and 33% (Cook et al., 2005). The incidence in pediatrics is much lower, but comprehensive screening studies have not been done. The reported incidence of DVT among pediatric trauma patients is 0.2 to 3.3 per 1000 trauma discharges. Older adolescents and young adults may be at higher risk than younger pediatric patients (O’Brien et al., 2008). For children who are critically ill after trauma, the incidence of symptomatic DVT is reported to be much higher (6.2%). The presence of central venous lines, poor perfusion and immobility, and a family history of thrombosis are risk factors. Studies screening for asymptomatic VTE found central venous line–associated VTE rates in critically ill children ranging from 8% to 35% (Hanson et al., 2010).
An algorithm for the use of VTE prophylaxis in pediatric trauma patients has been proposed (Fig. 39-5) (O’Brien et al., 2008). In critically ill pediatric patients without trauma, mechanical prophylaxis and vigilance for signs of DVT may be appropriate. Pulmonary embolus is rare in children, with about 40 per year occurring in the United States. The nonspecific symptoms of pulmonary embolus may be responsible for its underrecognition in critically ill patients. Massive pulmonary embolus in children is most often described at autopsy (Baird et al., 2010).
Ventilator-Associated Pneumonia
The frequency of VAP in pediatric patients is 2.9 episodes per 1000 ventilator days (Principi and Esposito, 2007). The presence of an endotracheal tube is the most important risk factor, and shortening the duration of ventilation is the most important preventative. Organisms responsible are typically S. aureus and gram-negative bacteria (Grohskopf et al., 2002). Early VAP may involve organisms commonly associated with community-acquired pneumonia. Specific measures for VAP prevention recommended in adult patients include raising the head of the bed by 30 to 45 degrees, instituting a daily sedation holiday with an extubation readiness trial, stress-ulcer prophylaxis, and DVT prophylaxis (Grohskopf et al., 2002). It is essential to identify the causative agent. Because of antimicrobial resistance in hospital-acquired organisms, initial therapy should be broad and should be tailored once sensitivities are known. Compared with placebo, sucralfate and H2 blockers do not alter the incidence of VAP or upper airway colonization in children (Lopriore et al., 2002).
Heparin-Induced Thrombocytopenia
Heparin-induced thrombocytopenia (HIT) is an immune response to platelet factor–heparin complexes that results in platelet activation and thrombocytopenia. These signs appear 4 to 5 days after initial heparin exposure, or more abruptly after reexposure. The primary complications are mostly thrombotic, although bleeding may occur, often as a postthrombotic complication. The diagnosis of HIT depends on demonstration of antibodies to platelet factor 4 (PF4)-heparin complexes, and on the appearance of the syndrome’s clinical features, including a 50% fall in platelet count with appropriate timing and absence of a more likely cause of thrombocytopenia. Demonstration of PF4 heparin antibodies is necessary but not sufficient for diagnosis, as only a small fraction of patients with the antibody have the syndrome (Hassell, 2008).
Treatment consists of removal of all forms of heparin and using of alternative anticoagulants. Low-molecular-weight heparin has been used when platelet aggregation is not demonstrated. Warfarin and aspirin have been used in pediatric patients. Direct thrombin inhibitors such as argatroban, and anti-Xa agents such as fondaparanux have been used in adult patients. These drugs have been used in pediatric patients, but the efficacy and safety have not been well established (Rayapudi et al., 2008; Dhillon, 2009). Simple discontinuation of heparin without alternative anticoagulation is associated with severe complications (Hassell, 2008).
Trials in adults have reported an incidence of HIT that varies between 1% and 30%. The diagnosis can be challenging in severely ill patients, because heparin use is a ubiquitous part of intravenous and central line flushes in the pediatric ICU, and because most of these patients have a variety of potential reasons for thrombocytopenia. Venous clots around indwelling catheters may be more common in children because the diameter of central lines is larger than the size of the vessel (DeAngelis et al., 1996).
Critical Care Myopathy
Survivors of critical illness are at risk for polyneuropathy and myopathy syndromes causing weakness ranging in severity from brief and reversible skeletal muscle weakness to inability to wean from mechanical ventilation. There is an enormous deficit in knowledge about the incidence, causes, treatment, and prognosis of these syndromes, particularly in children (Williams et al., 2007). Pediatric patients with sepsis and SIRS are at particular risk, and in adult patients with sepsis and multiorgan failure the incidence of these syndromes approaches 100% (Berek et al., 1996; Latronico et al., 1996). Although data linking the combination of neuromuscular blockade and systemic steroids with ICU-acquired polyneuropathy and myopathy are limited, most clinicians agree that the combination should be avoided whenever possible. Hyperglycemia is a modifiable independent risk factor for critical care myopathy (Herridge et al., 2008). Tight glucose control may decrease its incidence in adults, but this preventive strategy not been investigated in children (van den Berghe et al., 2001).
A high index of suspicion is required for diagnosis. Identification of critical illness myopathy and polyneuropathy helps to predict respiratory failure after extubation and can help in planning rehabilitation. Neuropathy and myopathies are frequently lumped together because they are indistinguishable without relatively invasive tests, and because treatment for both syndromes is supportive. In both syndromes, weakness is usually symmetrical, and sensory symptoms are less common. Laboratory tests are usually normal and are of limited use. Nerve conduction studies and electromyography can help to differentiate between axonal neuropathy and muscle atrophy or necrosis. Slow recovery over weeks to months is the norm, although many adults show diminished exercise capacity and quality of life years after critical illness (Herridge, 2009).
ICU-Acquired Infections
The National Nosocomial Infections Surveillance system found that nosocomial infections occur in ICUs at a rate of 13 per 1000 patient days. About 23% of nosocomial infections are urinary tract infections, and 97% of these are associated with urinary catheters. Central line–associated bloodstream infection (CLABSI) occurs in 1.0 to 5.6 per 1000 catheter days. Bloodstream infections associated with catheters are the most commonly reported nosocomial infection in pediatric intensive care. Duration of catheter use, TPN, and immunosuppression are correlated with CLABSI (Barsanti and Woeltje, 2009). The majority of pathogens causing CLABSI are found on the skin. A multidisciplinary strategy, involving, for example, maximum barrier precautions for catheter placement, antibiotic-coated catheters, handwashing campaigns, and use of chlorhexidine for dressing changes, significantly decreased rates of infection (Bhutta et al., 2007). The most important preventative for CLABSI is early removal of central venous catheters.
Clostridium difficile is increasingly recognized as a preventable cause of significant morbidity in the ICU. More virulent strains are emerging and have been implicated in ICU mortality. C. difficile spores can remain in the environment for a prolonged period of time and are spread by health care workers (Barsanti and Woeltje, 2009). Affected patients should be isolated, and general infection prevention measures should be in place for all patients. Use of broad-spectrum antibiotics increases the risk for C. difficile infection and should be carefully considered.
Summary: the role of the intensivist
As our support of individual organ systems becomes more complex, it is ever more tempting to refer to the organ-specific subspecialist to manage that aspect of care, but there is evidence that the continuous presence of a fellowship-trained intensivist improves outcomes and lowers cost (Pollack et al., 1988; Pronovost et al., 2002). Consultation with the specialist is invaluable, but each consultant focuses on the specialty’s organ system. It is the job of the intensivist to consider the specialist’s recommendations, and then negotiate the barriers of ego, control, and reimbursement to do what best serves the patient’s long-term interests.
Abend N.S., Topjian A., Ichord R., et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72(22):1931.
Adams A.B., Simonson D.A., Dries D.J., et al. Ventilator-induced lung injury. Respir Care Clin N Am. 2003;9(3):343.
Adelson P.D., Bratton S.L., Carney N.A., American Association for Surgery of Trauma; Child Neurology SocietyInternational Society for Pediatric Neurosurgery; International Trauma Anesthesia and Critical Care Society; Society of Critical Care Medicine; World Federation of Pediatric Intensive and Critical Care Societies. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 5. Indications for intracranial pressure monitoring in pediatric patients with severe traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S1.
Adhikari N.K., Burns K.E., Friedrich J.O., et al. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334(7597):779.
Agus M.S., Jaksic T. Nutritional support of the critically ill child. Curr Opin Pediatr. 2002;14(4):470.
Akcan-Arikan A., Zappitelli M., Loftis L.L., et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028.
Altemeier W.A., et al. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care. 2007;13:73.
Ambuehl J., Karrer A., Meer A., et al. Quality of life of survivors of pediatric intensive care. Pediatr Crit Care Med. 2002;3:1.
Annane D. ICU physicians should abandon the use of etomidate!. Intensive Care Med. 2005;31(3):325.
Annane D., Sébille V., Troché G., et al. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283(8):1038.
Annane D., Sébille V., Charpentier C., et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862.
Arnold J.H., Hanson J.H., Toto-Figuero L.O., et al. Prospective randomized comparison of high frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Crit Care Med. 1994;22:1530.
Ashwal S., Schneider S., Tomasi L., et al. Prognostic implications of hyperglycemia and reduced cerebral blood flow in childhood near-drowning. Neurology. 1990;40(5):820.
Baird J.S., Killinger J.S., Kalkbrenner K.J., et al. Massive pulmonary embolism in children. J Pediatr. 2010;156(1):148.
Barsanti M.C., Woeltje K.F. Infection prevention in the intensive care unit. Infect Dis Clin North Am. 2009;23(3):703.
Bartelink I.H., Rademaker C.M., Schobben A.F., et al. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet. 2006;45(11):1077.
Bartlett R.H. Extracorporeal support for septic shock. Pediatr Crit Care Med. 2007;8(5):498.
Bayrakci B., Josephson C., Fackler J., et al. Oxygenation index for extracorporeal membrane oxygenation: is there predictive significance? J Artif Organs. 2007;10(1):6.
Beca J., Butt W. Extracorporeal membrane oxygenation for refractory septic shock in children. Pediatrics. 1994;93(5):726.
Beishuizen A., Thijs L.G. The immunoneuroendocrine axis in critical illness: beneficial adaptation or neuroendocrine exhaustion? Curr Opin Crit Care. 2004;10(6):461.
Berek K., Margreiter J., Willeit J., et al. Polyneuropathies in critically ill patients: a prospective evaluation. Intensive Care Med. 1996;22(9):849.
Bernard S.A., Gray T.W., Buist M.D., et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557.
Bhutta A., Gilliam C., Honeycutt M., et al. Reduction of bloodstream infections associated with catheters in paediatric intensive care unit: stepwise approach. BMJ. 2007;334(7589):362.
Bleyaert A.L., Sands P.A., Safar P., et al. Augmentation of postischemic brain damage by severe intermittent hypertension. Crit Care Med. 1980;8(1):41.
Brierley J., Carcillo J.A., Choong K., et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37(2):666.
Burney S., Caulfield J.L., Niles J.C., et al. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res. 1999;424:37.
Carcillo J.A., Fields A.I., American College of Critical Care Medicine Task Force Committee Members. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30(6):1365.
Carcillo J.A., Davis A.L., Zaritsky A., et al. Role of early fluid resuscitation in pediatric septic shock. JAMA. 1991;2266:1242.
Carvalho P.R., Feldens L., Seitz E.E., et al. Prevalence of systemic inflammatory syndromes at a tertiary pediatric intensive care unit. J Pediatr (Rio J). 2005;81:143.
Ceneviva G., Paschall J.A., Maffei F., et al. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics. 1998;102(2):e19.
Chiaretti A., Piastra M., Pulitanò S., et al. Prognostic factors and outcome of children with severe head injury: an 8-year experience. Childs Nerv Syst. 2002;18(3–4):129.
Choong K., Kissoon N. Vasopressin in pediatric shock and cardiac arrest. Pediatr Crit Care Med. 2008;9(4):372.
Choong K., Bohn D., Fraser D.D., Canadian Critical Care Trials Group. Vasopressin in pediatric vasodilatory shock: a multicenter randomized controlled trial. Am J Respir Crit Care Med. 2009;180(7):632.
Conrad S.A., Rycus P.T., Dalton H., et al. Extracorporeal Life Support Registry Report 2004. ASAIO J. 2005;51(1):4.
Cook D., Crowther M., Meade M., et al. Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med. 2005;33(7):1565.
Curley G., Contreras M.M., Nichol A.D., et al. Hypercapnia and acidosis in sepsis: a double-edged sword? Anesthesiology. 2010;112(2):462.
Curley M.A., Hibberd P.L., Fineman L.D., et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294(2):229.
Cuthbertson B.H., Sprung C.L., Annane D., et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Med. 2009;35(11):1868.
Czaja A.S. A critical appraisal of a randomized controlled trial: Willson et al: Effect of exogenous surfactant (calfactant) in pediatric acute lung injury. JAMA. 2005;293:470-476. Pediatr Crit Care Med. 2007;8(1):50.
Dabrowski G.P., Steinberg S.M., Ferrara J.J., et al. A critical assessment of endpoints of shock resuscitation. Surg Clin North Am. 2000;80(3):825.
Davis D.P. Early ventilation in traumatic brain injury. Resuscitation. 2008;76(3):333.
DeAngelis G.A., McIlhenny J., Willson D.F., et al. Prevalence of deep venous thrombosis in the lower extremities of children in the intensive care unit. Pediatr Radiol. 1996;26(11):821.
Deis J.N., Abramo T.J., Crawley L., et al. Noninvasive respiratory support. Pediatr Emerg Care. 2008;24(5):331-338.
Dellinger R.P., Levy M.M., Carlet J.M., International Surviving Sepsis Campaign Guidelines CommitteeAmerican Association of Critical-Care Nurses; American College of Chest Physicians; American College of Emergency Physicians; Canadian Critical Care Society; European Society of Clinical Microbiology and Infectious Diseases; European Society of Intensive Care Medicine; European Respiratory Society; International Sepsis Forum; Japanese Association for Acute Medicine; Japanese Society of Intensive Care Medicine; Society of Critical Care Medicine; Society of Hospital Medicine; Surgical Infection Society; World Federation of Societies of Intensive and Critical Care Medicine. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296.
de Neef M., Geukers V.G., Dral A., et al. Nutritional goals, prescription and delivery in a pediatric intensive care unit. Clin Nutr. 2008;27(1):65.
de Oliveira C.F., de Oliveira D.S., Gottschald A.F., et al. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 2008;34(6):1065.
Dhillon S. Argatroban: a review of its use in the management of heparin-induced thrombocytopenia. Am J Cardiovasc Drugs. 2009;9(4):261.
DiCarlo J.V., Alexander S.R., Agarwal R., et al. Continuous veno-venous hemofiltration may improve survival from acute respiratory distress syndrome after bone marrow transplantation or chemotherapy. J Pediatr Hematol Oncol. 2003;25(10):801.
Doherty D.R., Parshuram C.S., Gaboury I., Canadian Critical Care Trials Group. Hypothermia therapy after pediatric cardiac arrest. Circulation. 2009;119(11):1492.
Doughty L., Carcillo J.A., Kaplan S., et al. The compensatory anti-inflammatory cytokine interleukin 10 response in pediatric sepsis-induced multiple organ failure. Chest. 1998;113:1625-17163.
Duncan B.W., Bohn D.J., Atz A.M., et al. Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg. 2001;122(3):440.
Epstein S.K. Weaning from ventilatory support. Curr Opin Crit Care. 2009;15(1):36.
Erickson S., Schibler A., Numa A., Paediatric Study Group. Australian and New Zealand Intensive Care Society Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8(4):317-323.
Felmet K.A., Hall M.W., Clark R.S., et al. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174:3765.
Fernandez E.F., Watterberg K.L. Relative adrenal insufficiency in the preterm and term infant. J Perinatol. 2009;29(Suppl 2):S44.
Fink E.L., Clark R.S., Kochanek P.M., et al. A tertiary care center’s experience with therapeutic hypothermia after pediatric cardiac arrest. Pediatr Crit Care Med. 2010;11(1):66.
Fink E.L., Kochanek P.M., Clark R.S., et al. How I cool children in neurocritical care. Neurocrit Care. 2010. Feb 10 [Epub ahead of print]
Flori H.R., Glidden D.V., Rutherford G.W., et al. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171(9):995-1001.
Foland J.A., Fortenberry J.D., Warshaw B.L., et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32(8):1771.
Franschman G., Peerdeman S.M., Greuters S., ALARM-TBI investigators. Prehospital endotracheal intubation in patients with severe traumatic brain injury: guidelines versus reality. Resuscitation. 2009;80(10):1147.
Frenckner B., Radell P. Respiratory failure and extracorporeal membrane oxygenation. Semin Pediatr Surg. 2008;17(1):34.
Gattinoni L., Tognoni G., Pesenti A., Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345(8):568.
Gibney N., Hoste E., Burdmann E.A., et al. Timing of initiation and discontinuation of renal replacement therapy in AKI: unanswered key questions. Clin J Am Soc Nephrol. 2008;3(3):876.
Gluckman P.D., Wyatt J.S., Azzopardi D., et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663.
Goldman A.P., Kerr S.J., Butt W., et al. Extracorporeal support for intractable cardiorespiratory failure due to meningococcal disease. Lancet. 1997;349(9050):466.
Goldstein S.L., Somers M.J., Baum M.A., et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(2):653.
Green T.P., Timmons O.D., Fackler J.C., et al. The impact of extracorporeal membrane oxygenation on survival in pediatric patients with acute respiratory failure. Pediatric Critical Care Study Group. Crit Care Med. 1996;24(2):323.
Grohskopf L.A., Sinkowitz-Cochran R.L., Prevention Network. A national point-prevalence survey of pediatric intensive care unit-acquired infections in the United States. J Pediatr. 2002;140(4):432.
Gupta V.K., Cheifetz I.M. Heliox administration in the pediatric intensive care unit: an evidence-based review. Pediatr Crit Care Med. 2005;6(2):204.
Hanson S.J., Punzalan R.C., Greenup R.A., et al. Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Trauma. 2010;68(1):52.
Han Y.Y., Carcillo J.A., Dragotta M.A., et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112(4):793.
Haque I.U., Latour M.C., Zaritsky A.L., et al. Pediatric critical care community survey of knowledge and attitudes toward therapeutic hypothermia in comatose children after cardiac arrest. Pediatr Crit Care Med. 2006;7(1):7.
Harris R.S. Pressure-volume curves of the respiratory system. Respir Care. 2005;50(1):78.
Hassell K. Heparin-induced thrombocytopenia: diagnosis and management. Thromb Res. 2008;123(Suppl 1):S16.
Hébert P.C., Wells G., Blajchman M.A., et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409.
Heidegger C.P., Darmon P., Pichard C., et al. Enteral vs. parenteral nutrition for the critically ill patient: a combined support should be preferred. Curr Opin Crit Care. 2008;14(4):408.
Herridge M.S. Legacy of intensive care unit-acquired weakness. Crit Care Med. 2009;37(Suppl 10):S457.
Herridge M.S., Batt J., Hopkins R.O., et al. The pathophysiology of long-term neuromuscular and cognitive outcomes following critical illness. Crit Care Clin. 2008;24(1):179.
Hildreth N., Mejia V.A., Maxwell R.A., et al. Adrenal suppression following a single dose of etomidate for rapid sequence induction: a prospective randomized study. J Trauma. 2008;65:573.
Holmes C.L., Landry D.W., Granton J.T., et al. Science review: vasopressin and the cardiovascular system part 1—receptor physiology. Crit Care. 2003;7(6):427.
Hopkins R.O., Weaver L.K., Pope D., et al. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50.
Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138.
Huang S.C., Wu E.T., Chen Y.S., et al. Extracorporeal membrane oxygenation rescue for cardiopulmonary resuscitation in pediatric patients. Crit Care Med. 2008;36(5):1607.
Hulst J., Joosten K., Zimmermann L., et al. Malnutrition in critically ill children: from admission to 6 months after discharge. Clin Nutr. 2004;23:223.
Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549.
Jardin F., Fourme T., Page B., et al. Persistent preload defect in severe sepsis despite fluid loading: a longitudinal echocardiographic study in patients with septic shock. Chest. 1999;116(5):1354.
Jeejeebhoy K.N. Total parenteral nutrition: potion or poison? Am J Clin Nutr. 2001;74(2):160.
Joannidis M. Continuous renal replacement therapy in sepsis and multisystem organ failure. Semin Dial. 2009;22(2):160.
Kellum J.A., Levin N., Bouman C., et al. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care. 2002;8(6):509.
Kelly D.A. Intestinal failure-associated liver disease: what do we know today? Gastroenterology. 2006;130(2 Suppl 1):S70.
Kelly R.E.Jr, Phillips J.D., Foglia R.P., et al. Pulmonary edema and fluid mobilization as determinants of the duration of ECMO support. J Pediatr Surg. 1991;26(9):1016.
Kissoon N., Rimensberger P.C, Bohn D., et al. Ventilation strategies and adjunctive therapy in severe lung disease. Pediatr Clin North Am. 2008;55(3):709.
Kochanek P.M., Bayir H., Jenkins L.W., et al. Molecular Biology of Brain Injury. In: Nichols D., editor. Rogers textbook of pediatric intensive care. ed 4. Pennsylvania: Lippincott Williams & Wilkins; 2008:26-45.
Kompan L., Vidmar G., Spindler-Vesel A., et al. Is early enteral nutrition a risk factor for gastric intolerance and pneumonia? Clin Nutr. 2004;23(4):527.
Konduri G.G., Kim U.O. Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr Clin North Am. 2009;56(3):579.
Krishnan J., Morrison W. Airway pressure release ventilation: a pediatric case series. Pediatr Pulmonol. 2007;42(1):83.
Kumar A., Roberts D., Wood K.E., et al. Duration of hypotension prior to initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589.
Kuruma Y., Hirasaki Y., Taniguchi Y., et al. Airway pressure release ventilation for respiratory management in a pediatric case after adult-size kidney transplantation. Paediatr Anaesth. 2008;18(12):1271.
Kyle U.G., Coss Bu J.A., Kennedy C.E., et al. Organ dysfunction is associated with hyperglycemia in critically ill children. Intensive Care Med. 2010;36(2):312.
Lacroix J., Herbert P.C., Hurchison J.S., et al. Transfusion strategies for patients in the pediatric intensive care units. N Engl J Med. 2007;356:1609.
Landry D.W., Levin H.R., Gallant E.M., et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95(5):1122.
Latronico N., Fenzi F., Recupero D., et al. Critical illness myopathy and neuropathy. Lancet. 1996;347(9015):1579.
Leteurtre S., Martinot A., Duhamel A., et al. Validation of the paediatric logistic organ dysfunction score: prospective, observational, multicentre study. Lancet. 2003;362:192.
Lin P.C., Chang C.H., Hsu P.I., et al. The efficacy and safety of proton pump inhibitors vs histamine-2 receptor antagonists for stress ulcer bleeding prophylaxis among critical care patients: a meta-analysis. Crit Care Med. 2010. Feb 18 [Epub ahead of print]
Lodha R., Vivekanandhan S., Sarthi M., et al. Serial circulating vasopressin levels in children with septic shock. Pediatr Crit Care Med. 2006;7(3):220.
Lois F., De Kock M. Something new about ketamine for pediatric anesthesia? Curr Opin Anaesthesiol. 2008;21(3):340.
Lopriore E., Markhorst D.G., Gemke R.J., et al. Ventilator-associated pneumonia and upper airway colonisation with Gram negative bacilli: the role of stress ulcer prophylaxis in children. Intensive Care Med. 2002;28(6):763.
Lubchenco L.O., Bard H.. Incidence of hypoglycemia in newborn infants classified by birth weight and gestational age. Pediatrics. 1971(5):831.
Luchetti M., Ferrero F., Gallini C., et al. Multicenter, randomized, controlled study of porcine surfactant in severe respiratory syncytial virus-induced respiratory failure. Pediatr Crit Care Med. 2002;3(3):261.
Maclaren G., Butt W., Best D., et al. Extracorporeal membrane oxygenation for refractory septic shock in children: one institution’s experience. Pediatr Crit Care Med. 2007;8(5):447.
Marik P.E., Pastores S.M., Annane D., American College of Critical Care Medicine. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36(6):1937.
Masumoto K., Kusuda S., Aoyagi H., et al. Comparison of serum cortisol concentrations in preterm infants with or without late-onset circulatory collapse due to adrenal insufficiency of prematurity. Pediatr Res. 2008;63(6):686.
Matok I., Vard A., Efrati O., et al. Terlipressin as rescue therapy for intractable hypotension due to septic shock in children. Shock. 2005;23(4):305.
Maxvold N.J., Smoyer W.E., Custer J.R., et al. Amino acid loss and nitrogen balance in critically ill children with acute renal failure: a prospective comparison between classic hemofiltration and hemofiltration with dialysis. Crit Care Med. 2000;28(4):1161.
Meert K.L., Daphtary K.M., Metheny N.A., et al. Gastric vs small-bowel feeding in critically ill children receiving mechanical ventilation: a randomized controlled trial. Chest. 2004;126:872.
Mehta N.M., Arnold J.H. Mechanical ventilation in children with acute respiratory failure. Curr Opin Crit Care. 2004;10(1):7.
Meyer R., Harrison S., Sargent S., et al. The impact of enteral feeding protocols on nutritional support in critically ill children. J Hum Nutr Diet. 2009;22(5):428.
Miller J.R., Myers R.E. Neuropathology of systemic circulatory arrest in adult monkeys. Neurology. 1972;22(9):888.
Morgera S., Scholle C., Voss G., et al. Metabolic complications during regional citrate anticoagulation in continuous venovenous hemodialysis: single-center experience. Nephron Clin Pract. 2004;97(4):c131.
Morris M.C, Wernovsky G., Nadkarni V.M., et al. Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2004;5(5):440.
Myers T.R. Use of heliox in children. Respir Care. 2006;51(6):619.
Nadel S., Goldstein B., Williams M.D., et al. Researching severe sepsis and organ dysfunction in children: a global perspective (RESOLVE) study group. Drotrecogin alfa in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369:836.
Nadkarni V.M., Larkin G.L., Peberdy M.A., National Registry of Cardiopulmonary Resuscitation Investigators. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50.
National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network,Wiedemann H.P., Wheeler A.P., Bernard G.R., et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564.
Wiedemann H.P., Wheeler A.P., Bernard G.R., et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564.
Nehra D., Goldstein A.M., Doody D.P., et al. Extracorporeal membrane oxygenation for nonneonatal acute respiratory failure: the Massachusetts General Hospital experience from 1990 to 2008. Arch Surg. 2009;144(5):427.
Newth C.J., Venkataraman S., Willson D.F., Eunice Shriver Kennedy National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med. 2009;10(1):1.
O’Brien S.H, Haley K., Kelleher K.J., et al. Variation in DVT prophylaxis for adolescent trauma patients: a survey of the Society of Trauma Nurses. J Trauma Nurs. 2008(2):53.
Oppert M., Schindler R., Husung C., et al. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005;33(11):2457.
Pagano A., Barazzone-Argiroffo C. Alveolar cell death in hyperoxia-induced lung injury. Ann N Y Acad Sci. 2003;1010(1):405-416.
Peek G.J., Mugford M., Tiruvoipati R., CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351.
Peters M., Petros A., Dixon G., et al. Acquired immunoparalysis in paediatric intensive care: prospective observational study. BMJ. 1999;319:609.
Plötz F.B., Slutsky A.S., van Vught A.J., et al. Ventilator-induced lung injury and multiple system organ failure: a critical review of facts and hypotheses. Intensive Care Med. 2004;30(10):1865.
Plötz F.B., Bouma A.B., van Wijk J.A., et al. Pediatric acute kidney injury in the ICU: an independent evaluation of RIFLE criteria. Intensive Care Med. 2008;34(9):1713.
Pollack M.M., Wiley J.S., Kanter R., et al. Malnutrition in critically ill infants and children. JPEN J Parenter Enteral Nutr. 1982;6:20-24.
Pollack M.M., Ruttimann U.E., Wiley J.S., et al. Nutritional depletions in critically ill children: associations with physiologic instability and increased quantity of care. JPEN J Parenter Enteral Nutr. 1985;9:309-313.
Pollack M.M., Katz R.W., Ruttimann U.E., et al. Improving the outcome and efficiency of intensive care: the impact of an intensivist. Crit Care Med. 1988;16(1):11-17.
Principi N., Esposito S. Ventilator-associated pneumonia (VAP) in pediatric intensive care units. Pediatr Infect Dis J. 2007;26(9):841.
Prodhan P., Fiser R.T., Dyamenahalli U., et al. Outcomes after extracorporeal cardiopulmonary resuscitation (ECPR) following refractory pediatric cardiac arrest in the intensive care unit. Resuscitation. 2009;80(10):1124.
Pronovost P.J., Angus D.C., Dorman T., et al. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288(17):2151.
Proulx F., Gauthier M., Nadeau D., et al. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994;22:1025.
Proulx F., Joyal J.S., Mariscalco M.M., et al. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;10(1):12.
Quenot J.P., Thiery N., Barbar S., et al. When should stress ulcer prophylaxis be used in the ICU? Curr Opin Crit Care. 2009;15(2):139.
Randolph A.G. Management of acute lung injury and acute respiratory distress syndrome in children. Crit Care Med. 2009;37(8):2448.
Randolph A.G., Meert K.L., O’Neil M.E., et al. The feasibility of conducting clinical trials in infants and children with acute respiratory failure. Am J Respir Crit Care Med. 2003;167:1334.
Rayapudi S., Torres A.Jr, Deshpande G.G., et al. Bivalirudin for anticoagulation in children. Pediatr Blood Cancer. 2008;51(6):798.
Reveiz L., Guerrero-Lozano R., Camacho A., et al. Stress ulcer, gastritis, and gastrointestinal bleeding prophylaxis in critically ill pediatric patients: a systematic review. Pediatr Crit Care Med. 2010;11(1):124.
Ricci Z., Ronco C. Dose and efficiency of renal replacement therapy: continuous renal replacement therapy versus intermittent hemodialysis versus slow extended daily dialysis. Crit Care Med. 2008;36(Suppl 4):S229.
Rivers E., Nguyen B., Havstad S., Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368.
Rogovik A., Goldman R. Permissive hypercapnia. Emerg Med Clin North Am. 2008;26(4):941.
Ronco C., Bellomo R., Homel P., et al. Effects of different doses in continuous venovenous haemofiltration on outcomes of acute renal failure: a prospective randomized trial. Lancet. 2000;356:26.
Rosenberg A.L. Fluid management in patients with acute respiratory distress syndrome. Respir Care Clin N Am. 2003;9(4):481.
Rouette J., Trottier H., Ducruet T., for the Canadian Critical Care Trials Group: the PALISI Network. Red Blood Cell Transfusion Threshold in Postsurgical Pediatric Intensive Care Patients: A Randomized Clinical Trial. Ann Surg. 2010;251(3):421.
Roy B.J., Rycus P., Conrad S.A., et al. The changing demographics of neonatal extracorporeal membrane oxygenation patients reported to the Extracorporeal Life Support Organization (ELSO) Registry. Pediatrics. 2000;106(6):1334.
Russell J.A., Walley K.R., Singer J., VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877.
Ryu H.G., Bahk J.H., Lee H.J., et al. Effect of recruitment and body positioning on lung volume and oxygenation in acute lung injury model. Anaesth Intensive Care. 2008;36(6):792.
Schuller D., Mitchell J.P., Calandrino F.S., et al. Fluid balance during pulmonary edema. Is fluid gain a marker or a cause of poor outcome? Chest. 1991;100(4):1068.
Schultz T.R., Costarino A.T., Durning S.M., et al. Airway pressure release ventilation in pediatrics. Pediatr Crit Care Med. 2001;2(3):243.
Shankaran S., Laptook A.R., Ehrenkranz R.A., National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574.
Siesjö B.K., Agardh C.D., Bengtsson F., et al. Free radicals and brain damage. Cerebrovasc Brain Metab Rev. 1989;1(3):165.
Singer M. Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit Care Med. 2007;35:S441.
Skillman H.E., Wischmeyer P.E. Nutrition therapy in critically ill infants and children. JPEN J Parenter Enteral Nutr. 2008;32(5):520.
Sprung C.L., Annane D., Keh D., CORTICUS Study Group. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111.
Stather D.R., Stewart T.E. Clinical review: mechanical ventilation in severe asthma. Crit Care. 2005;9(6):581.
Stawicki S.P., Goyal M., Sarani B. Analytic reviews: high-frequency oscillatory ventilation (HFOV) and airway pressure release ventilation (APRV) a practical guide. J Intensive Care Med. 2009;24:215.
Sutherland S.M., Zappitelli M., Alexander S.R., et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316.
Tang S.F., Sherwood M.C., Miller O.I., et al. Randomised trial of three doses of inhaled nitric oxide in acute respiratory distress syndrome. Arch Dis Child. 1998;79(5):415.
Ten V.S., Matsiukevich D. Room air or 100% oxygen for resuscitation of infants with perinatal depression. Curr Opin Pediatr. 2009;21(2):188.
The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome,. N Engl J Med. 2000;342(18):1301-1308.
Tibby S.M., Hatherill M., Wright S.M., et al. Exogenous surfactant supplementation in infants with respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1251.
Tinmouth A., Fergusson D., Yee I.C, ABLE InvestigatorsCanadian Critical Care Trials Group. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014.
Topjian A.A., Nadkarni V.M., Berg R.A., et al. Cardiopulmonary resuscitation in children. Curr Opin Crit Care. 2009;15(3):203.
Tremblay L.N., Slutsky A.S. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med. 2006;32(1):24.
Turi R.A., Petros A.J., Eaton S., et al. Energy metabolism of infants and children with systemic inflammatory response syndrome and sepsis. Ann Surg. 2001;233:581.
Turner D.A., Arnold J.H. Insights in pediatric ventilation: timing of intubation, ventilatory strategies, and weaning. Curr Opin Crit Care. 2007;13(1):57.
Uchino S., Bellomo R., Kellum J.A, Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) Investigators Writing Committee. Patient and kidney survival by dialysis modality in critically ill patients with acute kidney injury. Int J Artif Organs. 2007;30(4):281.
Vaagenes P., Safar P., Moossy J., et al. Asphyxiation versus ventricular fibrillation cardiac arrest in dogs. Differences in cerebral resuscitation effects: a preliminary study. Resuscitation. 1997;35(1):41.
van den Berghe G., Wouters P., Weekers F., et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359.
van den Berghe G., Wilmer A., Hermans G., et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449.
Van Meurs K., Lally K.P., Peek G., et al. ECMO Extracorporeal cardiopulmonary support in critical care, ed 3. Ann Arbor: Extracorporeal life support organization, 2005.
Wang C.X., Stroink A., Casto J.M., et al. Hyperthermia exacerbates ischaemic brain injury. Int J Stroke. 2009;4(4):274.
Wessel D., Laussen P. Cardiac Intensive Care. In: Fuhrman B.P., Zimmerman J., editors. Pediatric critical care. ed 3,. Philadelphia: Mosby; 2006:419-474.
Whalen M.J., Doughty L.A., Carlos T.M., et al. Intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 are increased in the plasma of children with sepsis-induced multiple organ failure. Crit Care Med. 2000;28:2600.
Wijdicks E.F., Hijdra A., Young G.B., Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203.
Wilkinson J.D., Pollack M.M., Glass N.L., et al. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr. 1987;111:324.
Williams S., Horrocks I.A., Ouvrier R.A., et al. Critical illness polyneuropathy and myopathy in pediatric intensive care: a review. Pediatr Crit Care Med. 2007;8(1):18.
Willson D.F., Chess P.R., Notter R.H., et al. Surfactant for pediatric acute lung injury. Pediatr Clin North Am. 2008;55(3):545.
Wilson D.F., Thomas N.J., Markovitz B.P., et al. Effect of exogenous surfactant (Calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293(4):470.
Wong H.R., Carcillo J.A., Burckart G., et al. Increased serum nitrite and nitrate concentrations in children with the sepsis syndrome. Crit Care Med. 1995;23:835.
Yildizdas D., Yapicioglu H., Celik U., et al. Terlipressin as a rescue therapy for catecholamine-resistant septic shock in children. Intensive Care Med. 2008;34(3):511.
Young K.D., Gausche-Hill M., McClung C.D., et al. A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardiopulmonary arrest. Pediatrics. 2004;114(1):157.
Zanotti Cavazzoni S.L., Dellinger R.P. Hemodynamic optimization of sepsis-induced tissue hypoperfusion. Crit Care. 2006;10(Suppl 3):S2.
Zanotti-Cavazzoni S.L., Hollenberg S.M. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009;15(5):392.
Zapol W.M., Snider M.T., Hill J.D., et al. Extracorporeal membrane oxygenation in severe acute respiratory failure: a randomized prospective study. JAMA. 1979;242(20):2193.
Zebrack M., Dandoy C., Hansen K., et al. Early resuscitation of children with moderate-to-severe traumatic brain injury. Pediatrics. 2009;124(1):56.