14 Critical care
Severity of illness scoring systems
Initial assessment: ABCDE
Guidelines for initial and daily patient assessment
Initial assessment
• Age, number of days on the unit
Daily investigations
Radiological investigations
Some radiological investigations may require the need for IV and/or oral contrast. Most IV contrast agents are nephrotoxic. The mainstay of prevention is good hydration. An additional bolus of an appropriate crystalloid is often required prior to giving the contrast agent. IV N-acetylcysteine (NAC) is used (p. 686) as a prophylactic agent against contrast-induced nephropathy. Prophylactic pre-procedural haemofiltration appears to be effective in patients with severely diminished renal function.
Discharge from the critical care environment
Dying patients and end-of-life care
Transportation of the critically ill patient
General considerations for patient care in the critical care environment
Infection control
Patient care
Gastrointestinal care
Nutritional support
Early enteral feeding
Prokinetic therapy/enteral feeding failure
Maintenance fluids — IV and enteral
Assessing the adequacy of fluid replacement
Packed red cell transfusion
Glycaemic control
Thrombo-prophylaxis
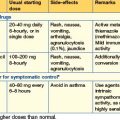
Analgesia and sedation
Guidelines
| Drug | Regime | Notes |
|---|---|---|
| Morphine | Loading 5–15 mg Maintenance 1–12 mg/hour |
Slow onset. Long-acting. Active metabolites. Accumulates in renal and hepatic impairment |
| Fentanyl | Loading 25–100 mcg Maintenance 25–250 mcg/hour |
Rapid onset. Modest duration of action. No active metabolites. Renally excreted |
| Alfentanil | Loading 15–50 mcg/kg Maintenance 30–85 mcg/kg/hour (1–6 mg/hour) |
Rapid onset. Relatively short-acting. Accumulates in hepatic failure |
| Remifentanil | Maintenance 6–12 mcg/kg/hour | Rapid onset and offset of action, with minimal if any accumulation of the weakly active metabolite. Significant incidence of problematic bradycardia. Expensive |
| Clonidine | Maintenance 1–4 mcg/kg/hour | An α2 agonist. Has marked sedative and atypical analgesic effects |
| Ketamine | Analgesia Induction 0.2 mg/kg/hour Maintenance 0.5–2.0 mg/kg 1–2 mg/kg/hour |
Atypical analgesic with hypnotic effects at higher doses. Sympathomimetic; associated with emergence phenomena when given at hypnotic doses when usually co-administered with a benzodiazepine. Contraindicated in raised intracranial pressure |
Table 14.1b Continuous infusion sedative regimens
| Drug | Regime | Notes |
|---|---|---|
| Propofol 1% | Loading 1.5–2.5 mg/kg Maintenance 0.5–4 mg/kg/hour (0–200 mg/hour) |
IV anaesthetic agent. Causes vasodilatation and hence hypotension. Extrahepatic metabolism, thus does not accumulate in hepatic failure. Has no analgesic properties |
| Midazolam | Loading 30–300 mcg/kg Maintenance 30–200 mcg/kg/hour (0–14 mg/hour) |
Short-acting benzodiazepine. Used with morphine. Active metabolites accumulate in all patients, esp in renal failure |
Respiratory failure
Respiratory support
Continuous positive airway pressure (CPAP)
Non-invasive ventilation
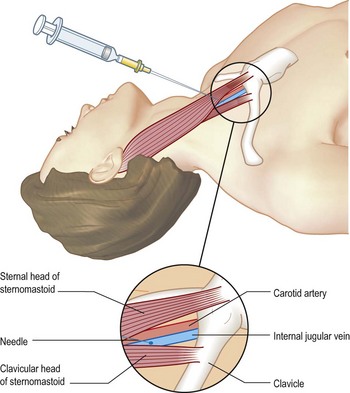
Endotracheal intubation (Box 14.1)
Box 14.1 Endotracheal intubation and tracheostomy
Technique
Mechanical ventilation
The mainstay of respiratory support is intermittent positive pressure ventilation (IPPV).
• To fix hypercapnia
• Unconventional ventilation
Complications associated with mechanical ventilation
• Respiratory complications
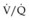 mismatch and collapse of peripheral alveoli. Traditionally, the latter was prevented by using high tidal volumes (10–12 mL/kg), but high inflation pressures, with over-distension of compliant alveoli, perhaps exacerbated by the repeated opening and closure of distal airways, can disrupt the alveolar–capillary membrane. There is an increase in microvascular permeability and release of inflammatory mediators, leading to VILI. Extreme over-distension of the lungs during mechanical ventilation with high tidal volumes and PEEP can rupture alveoli and cause air to dissect centrally along the perivascular sheaths. This ‘barotrauma’ may be complicated by pneumomediastinum, subcutaneous emphysema, pneumoperitoneum, pneumothorax and intra-abdominal air. The risk of pneumothorax is increased in those with destructive lung disease (e.g. necrotizing pneumonia, emphysema), asthma or fractured ribs.
mismatch and collapse of peripheral alveoli. Traditionally, the latter was prevented by using high tidal volumes (10–12 mL/kg), but high inflation pressures, with over-distension of compliant alveoli, perhaps exacerbated by the repeated opening and closure of distal airways, can disrupt the alveolar–capillary membrane. There is an increase in microvascular permeability and release of inflammatory mediators, leading to VILI. Extreme over-distension of the lungs during mechanical ventilation with high tidal volumes and PEEP can rupture alveoli and cause air to dissect centrally along the perivascular sheaths. This ‘barotrauma’ may be complicated by pneumomediastinum, subcutaneous emphysema, pneumoperitoneum, pneumothorax and intra-abdominal air. The risk of pneumothorax is increased in those with destructive lung disease (e.g. necrotizing pneumonia, emphysema), asthma or fractured ribs.Weaning from IPPV
The weaning process starts the moment IPPV is initiated.
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
Diagnostic criteria
Pathophysiology
Changes include alveolar flooding with proteinaceous fluid (exacerbated by reduced clearance); decreased surfactant production, resulting in atelectasis and decreased compliance; and hyaline membrane formation and interstitial oedema, leading to impaired gaseous diffusion. There is increased intrapulmonary shunt and loss of mechanical defence barrier; hence the high risk of sepsis.
Management
Cardiovascular failure
Terminology and normal values
Methods of continuous haemodynamic monitoring
Invasive arterial and venous pressure monitoring
Pulmonary artery catheterization and thermodilution
This is still the gold standard technique for measurement of haemodynamic status (see below).
Oesophageal Doppler
Cardiovascular supportive therapies
Rate and rhythm control
IV fluid resuscitation, the dynamic fluid challenge or pre-load optimization
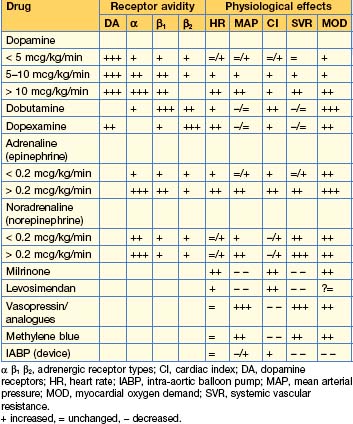
Inotropes, vasopressors and other vasoactive drugs
Table 14.6 Dosage regimens of commonly used vasoactive drugs
| Drug | Dosage range | Notes |
|---|---|---|
| Dopamine | 1–20 mcg/kg/min | Dominant effect dependent on dose. Some evidence of worse outcome compared to other drugs, therefore out of favour |
| Dobutamine | 5–20 mcg/kg/min | Inodilator (positive inotropic and causes systemic vasodilatation) |
| Dopexamine | 0.25–2.0 mcg/kg/min | Positive chronotrope/inodilator/anti-inflammatory? |
| Adrenaline (epinephrine) | 0.01–1 mcg/kg/min | Inoconstrictor (positive inotropic causing systemic vasoconstriction) |
| Noradrenaline (norepinephrine) | 0.01–1 mcg/kg/min | Vasoconstrictor (some inotropic activity), first-line vasopressor |
| Milrinone (phosphodiesterase inhibitor) | 150–750 ng/kg/min | Inodilator. Significantly longer onset and elimination half-life than dobutamine. Accumulates in renal failure. Do not give loading dose |
| Levosimendan (Ca sensitizer and K channel opener) | 0.05–0.2 mcg/kg/min | Inodilator. Active metabolite with long elimination half-life, hence 24-hour infusion will have measurable effects for up to 7 days. Do not give loading dose |
| Vasopressin | 0.01–0.05 U/min | Vasoconstrictor. Second-line vasopressor in noradrenaline-resistant shock (see also functional hypoadrenalism) |
| Terlipressin (vasopressin analogue) | 0.25–2 mg bolus | Vasoconstrictor. Duration of action 4–6 hours. Alternative to vasopressin |
| Methylene blue (nitric oxide antagonist) | 2 mg/kg loading 0.25–2 mg/kg/hour |
Vasoconstrictor. 2nd/3rd line vasopressor in norepinephrine resistant shock (see also functional hypoadrenalism) |
Systemic Inflammatory Response Syndrome (SIRS), Sepsis, Severe Sepsis and Septic Shock
Definitions
Sepsis is defined as SIRS with a documented or suspected infection. Severe sepsis is sepsis with evidence of organ dysfunction (as defined by SOFA or equivalent, p. 31). Septic shock is defined as severe sepsis with hypotension despite adequate fluid resuscitation. Severe sepsis and septic shock are the commonest conditions requiring critical care. Their incidence is high and increasing. They are associated with a very high morbidity and an overall mortality of 30–60%.
The Surviving Sepsis campaign’s recommendations (2004) may be found at www.survivingsepsis.org.
Initial resuscitation: goals during the first 6 hours
Management goals within the first 24 hours (see Emergencies in Medicine, p. 718)
Brain injury
Brainstem death
The process of diagnosing brain death is divided into three parts: pre-conditions, exclusions and tests.
Pre-conditions
Brainstem death tests
Organ and tissue donation
Individual countries have their own processes. In the UK, organ and tissue donation are governed by the Human Tissue Act 2004 (see http://body.orpheusweb.co.uk/HTA2004/20040030.htm). Always consider donation in dying patients, especially in those with brainstem death and, in particular, if the brain injury is the sole organ failure. In the UK, to discuss any aspect of donation, contact your local transplant coordinator.
acute kidney injury
Acute kidney injury (p. 348) is a common complication of critical illness. Kidney injury is associated with increased morbidity and mortality regardless of the primary pathology, with a direct correlation between the severity of renal injury and poor outcome.
Medical management of acute oliguric/anuric renal failure
Renal replacement therapy (p. 357)
Adhikari NKJ, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. 2010;376:1339-1346.
Randall CurtisJ, Vincent J-L. Ethics and end-of-life care for adults in the intensive care unit. Lancet. 2010;376:1347-1353.
Vincent J-L, Singer M. Critical care: advances and future perspectives. Lancet. 2010;376:1354-1361.