Chapter 11 Cranial Nerves
Cranial nerves are the means by which the brain receives information from, and controls the activities of, the head and neck and, to a lesser extent, the thoracic and abdominal viscera. Briefly, there are 12 pairs of cranial nerves that are individually named and numbered (using roman numerals) in a rostrocaudal sequence (see Table 1.1). Unlike spinal nerves, only some are mixed in function and thus carry both sensory and motor fibres. Others are purely sensory or purely motor. The first cranial nerve (I; olfactory) has an ancient lineage and is derived from the forerunner of the cerebral hemisphere. It retains this unique position through the connections of the olfactory bulb and is the only sensory cranial nerve that projects directly to the cerebral cortex rather than via the thalamus, as do all other sensory modalities. The areas of cerebral cortex involved have a primitive cellular organization and are an integral part of the limbic system, which is concerned with the emotional aspects of behaviour. The second cranial nerve (II; optic) consists of the axons of second-order visual neurones and terminates in the thalamus. The other 10 pairs of cranial nerves attach to the brain stem. Most of the component fibres originate from, or terminate in, named cranial nerve nuclei (Ch. 10).
Olfactory Nerve (I)
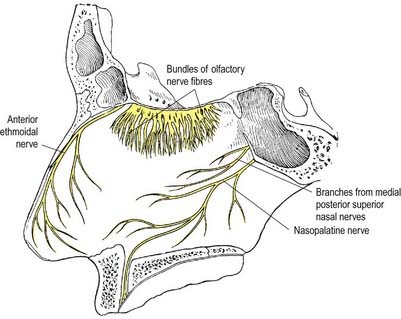
The cell of origin of the olfactory nerves serving the sense of smell is in the olfactory mucosa covering the superior nasal concha, the upper part of the vertical portion of the middle concha and the opposite part of the nasal septum. The axons, which are unmyelinated, originate as the central or deep processes of the olfactory neurones and collect in bundles that cross in various directions, forming a plexiform network in the mucosa. The bundles finally form approximately 20 branches that traverse the cribriform plate in lateral and medial groups and end in the glomeruli of the olfactory bulb. Each branch has a sheath consisting of dura mater and pia-arachnoid; the former continues into the nasal periosteum, and the latter into the connective tissue sheaths surrounding the nerve bundles (Figs 11.1, 11.2).
Olfactory pathways subserving the sense of smell are described in Chapter 12.
CASE 1 Anosmia
Discussion: This man has suffered a basal skull fracture involving the floor of the anterior fossa. The fracture line crosses the olfactory groove, resulting in laceration of the olfactory nerve and thus anosmia. Tumours and other mass lesions on the floor of the anterior fossa may similarly result in ipsilateral loss of the sense of smell; bilateral anosmia, with or without impairment of taste, may be due to the use of a variety of drugs, such as calcium channel blockers, some antibiotics, anticonvulsants and opiates, among others. (See also Ch. 9, Case 1.)
Optic Nerve (II)
The optic nerve is the second cranial nerve (Figs 11.3, 11.4). It arises from the optic chiasma on the floor of the diencephalon and enters the orbit through the optic canal, accompanied by the ophthalmic artery. It changes shape, starting out flat at the chiasma and becoming rounded as it passes through the optic canal. In the orbit it passes forward, laterally and downward and pierces the sclera at the lamina cribrosa, slightly medial to the posterior pole. It has a somewhat tortuous course within the orbit to allow for movements of the eyeball. It is surrounded by extensions of the three layers of meninges.
Oculomotor Nerve (III)
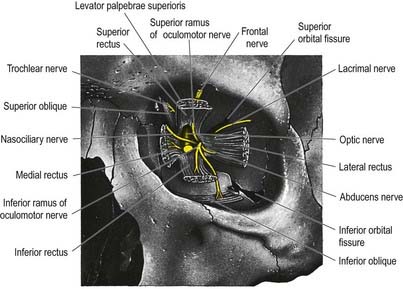
The oculomotor nerve is the third cranial nerve (Figs 11.3, 11.4, 11.8). It is the main source of innervation to the extraocular muscles and also contains parasympathetic fibres that relay in the ciliary ganglion.
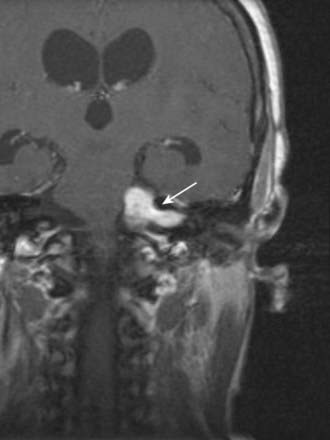
CASE 3 Thyroid-Associated Ophthalmopathy (Graves’ Disease)
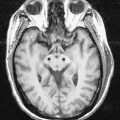
A 45-year-old woman is referred for evaluation of a mild action tremor. She has a history of a mixed seizure disorder in the first two decades of her life, completely controlled by sodium divalproate. She subsequently complains of double vision, and neuro-ophthalmological a examination shows impaired up-gaze of the right eye with resultant vertical diplopia. There is slight lid retraction. Thyroid function testing reveals an elevated thyroxine (T4) level and reduced thyroid-stimulating hormone (TSH). Orbital magnetic resonance imaging (MRI) documents thickening of the extraocular muscles, particularly involving the medial and inferior recti of the right eye (Fig. 11.9).
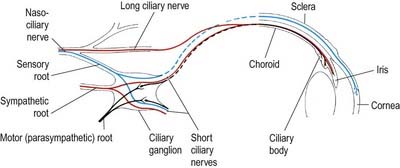
Ciliary Ganglion
The ciliary ganglion is a parasympathetic ganglion concerned functionally with the motor innervation of certain intraocular muscles (Figs 11.5, 11.6). It is a small, flat, reddish grey swelling, 1 to 2 mm in diameter, connected to the nasociliary nerve and located near the apex of the orbit in loose fat approximately 1 cm in front of the medial end of the superior orbital fissure. It lies between the optic nerve and lateral rectus, usually lateral to the ophthalmic artery. Its neurones, which are multipolar, are larger than in typical autonomic ganglia; a very small number of more typical neurones are also present.
The sympathetic root contains fibres from the plexus around the internal carotid artery within the cavernous sinus. These postganglionic fibres, derived from the superior cervical ganglion, form a fine branch that enters the orbit through the superior orbital fissure inside the common tendinous ring of recti muscles. The fibres either pass directly to the ganglion or join the nasociliary nerve and travel to the ganglion in its sensory root. Either way, they traverse the ganglion without synapsing to emerge into the short ciliary nerves. They are distributed to the blood vessels of the eyeball. Sympathetic fibres innervating dilator pupillae may sometimes travel via the short ciliary nerves (rather than the more usual route via the ophthalmic, nasociliary and long ciliary nerves).
CASE 2 Nutritional Amblyopia
Discussion: This man has nutritional amblyopia, sometimes referred to as tobacco-alcohol amblyopia. The clinical manifestations are due to bilateral involvement of the neural fibres constituting the macular projection system, resulting in a macular syndrome. The lesions predominate in the central portion of the optic nerves bilaterally, where the macular fibres are found, thus making it a disorder of the anterior conducting system (Fig. 11.7). Although tobacco use is sometimes implicated as a cause, this is in all likelihood a nutritional disorder due to deficiency of one or more B vitamins. It responds quickly to vitamin supplementation.
Trochlear Nerve (IV)
The trochlear nerve is the fourth cranial nerve and is the only one that emerges from the dorsal surface of the brainstem (see Fig. 11.35). It passes from the midbrain onto the lateral surface of the crus of the cerebral peduncle and runs through the lateral dural wall of the cavernous sinus. It then crosses the oculomotor nerve and enters the orbit through the superior orbital fissure, above the common tendinous ring of the recti muscles and levator palpebrae superioris and medial to the frontal and lacrimal nerves. The trochlear nerve travels only a short distance to enter the superior (orbital) surface of superior oblique, which is its sole target (see Fig. 11.8).
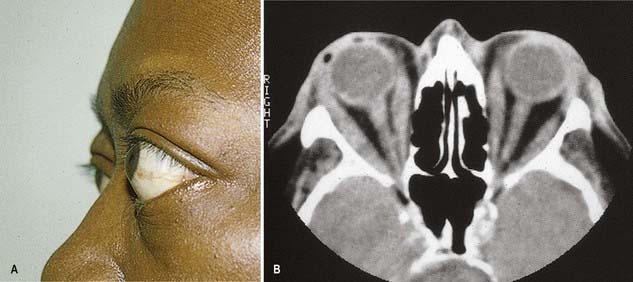
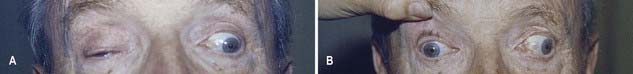
CASE 4 Diabetic Third Nerve Palsy
Two months later the patient is entirely normal except for persistent mild left ptosis.
Discussion: Vasculopathic third nerve palsies are most commonly associated with diabetes mellitus and usually come under the rubric of diabetic ophthalmoplegia. Other conditions associated with microvascular disease, such as hypertension and dyslipidemia, may also be responsible. Most cases are thought to be the result of ischaemia affecting the central portion of the oculomotor nerve, sparing the peripherally located pupillary fibre bundle; nonetheless, a small proportion of patients exhibits minor pupillary involvement. In contrast, compressive third nerve palsies, such as those associated with an intracranial aneurysm, almost always have marked pupillary involvement and usually present with more pain. See Figure 11.10.
Trigeminal Nerve (V)
Innervation of the Face and Scalp
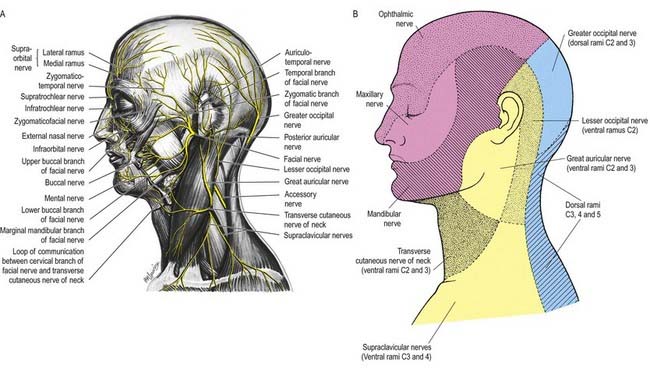
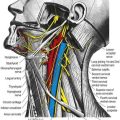
Three large areas of the face can be mapped to indicate the peripheral nerve fields associated with the three divisions of the trigeminal nerve. The fields are not horizontal but curve upward (Fig. 11.11A), apparently because the facial skin moves upward with growth of the brain and skull. Embryologically, each division of the trigeminal nerve is associated with a developing facial process that gives rise to a specific area of the face in the adult. Thus the ophthalmic nerve is associated with the frontonasal process, the maxillary nerve with the maxillary process and the mandibular nerve with the mandibular process.
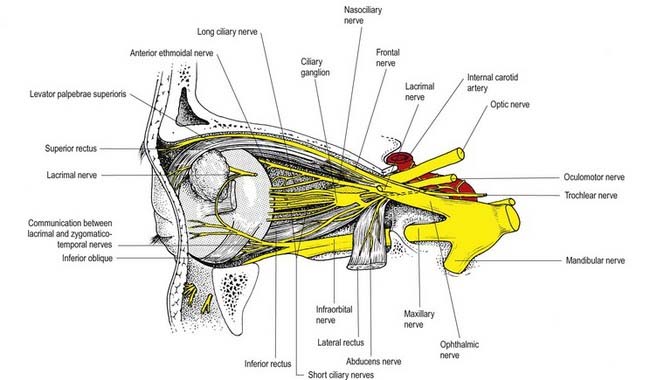
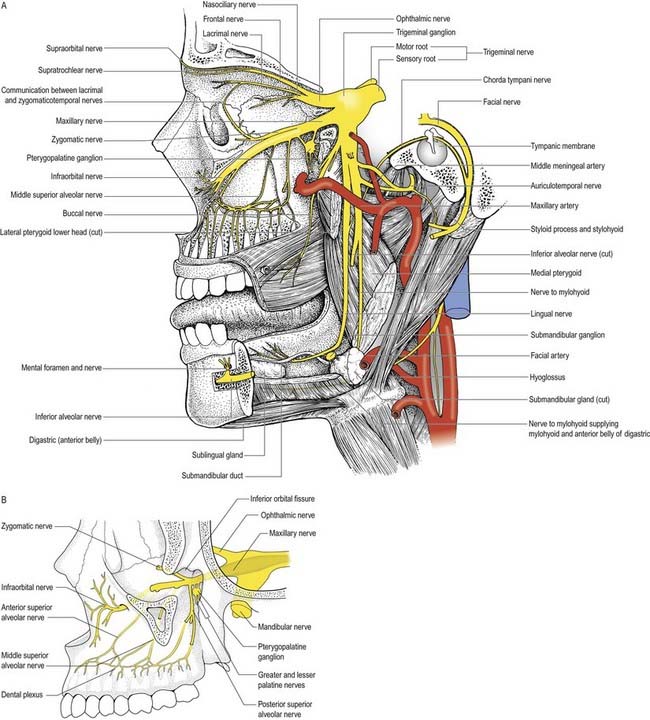
Ophthalmic Nerve
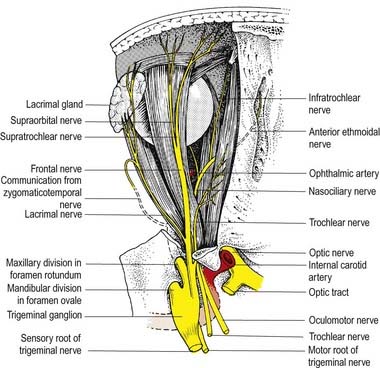
The ophthalmic nerve (Fig. 11.11B), a division of the trigeminal nerve, travels through the orbit to supply targets primarily in the upper part of the face. It arises from the trigeminal ganglion in the middle cranial fossa and passes forward along the lateral dural wall of the cavernous sinus. It gives off three main branches—the lacrimal, frontal and nasociliary nerves—just before it reaches the superior orbital fissure. The cutaneous branches of the ophthalmic nerve supply the conjunctiva, skin over the forehead, upper eyelids and much of the external surface of the nose.
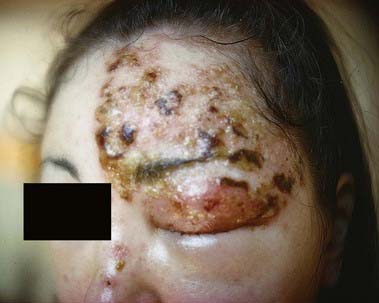
CASE 5 Herpes Zoster Ophthalmicus
She is diagnosed with herpes zoster ophthalmicus. Within several days, vesicles appear in the territory of the ophthalmic division of the right trigeminal nerve, along with keratitis, conjunctivitis and iritis of the right eye (Fig. 11.12).
Lacrimal Nerve
The lacrimal nerve enters the orbit through the superior orbital fissure, above the common tendinous ring of the recti muscles and lateral to the frontal and trochlear nerves (see Figs 11.4, 11.5, 11.8). It passes forward along the lateral wall of the orbit on the superior border of the lateral rectus and travels through the lacrimal gland and the orbital septum to supply conjunctiva and skin covering the lateral part of the upper eyelid. The lacrimal nerve communicates with the zygomatic branch of the maxillary nerve, so parasympathetic fibres associated with the pterygopalatine ganglion might be conveyed to the lacrimal gland.
Frontal Nerve
The frontal nerve is the largest branch of the ophthalmic nerve (see Figs 11.4, 11.5, 11.8). It enters the orbit through the superior orbital fissure, above the common tendinous ring of the recti muscles, and lies between the lacrimal nerve laterally and the trochlear nerve medially. It passes forward on the levator palpebrae superioris, toward the rim of the orbit; about halfway along this course it divides into the supraorbital and supratrochlear nerves.
Nasociliary Nerve
The nasociliary nerve is intermediate in size between the frontal and lacrimal nerves and is more deeply placed in the orbit, which it enters through the common tendinous ring, lying between the two rami of the oculomotor nerve (see Figs 11.4, 11.5, 11.8). It crosses the optic nerve with the ophthalmic artery and runs obliquely below superior rectus and superior oblique to reach the medial orbital wall. Here, as the anterior ethmoidal nerve, it passes through the anterior ethmoidal foramen and canal and enters the cranial cavity. It runs forward in a groove on the upper surface of the cribriform plate beneath the dura mater and descends through a slit lateral to the crista galli into the nasal cavity, where it occupies a groove on the internal surface of the nasal bone and gives off two internal nasal branches. The medial internal nasal nerve supplies the anterior septal mucosa, and the lateral internal nasal nerve supplies the anterior part of the lateral nasal wall. The anterior ethmoidal nerve emerges, as the external nasal nerve, at the lower border of the nasal bone and descends under the transverse part of the nasalis to supply the skin of the nasal alae, apex and vestibule.
Two or three long ciliary nerves branch from the nasociliary nerve as it crosses the optic nerve (see Fig. 11.5). They accompany the short ciliary nerves and pierce the sclera near the attachment of the optic nerve. Running forward between the sclera and choroid, they supply the ciliary body, iris and cornea and are thought to contain postganglionic sympathetic fibres for the dilator pupillae from neurones in the superior cervical ganglion. An alternative pathway for the supply of the dilator pupillae is via the sympathetic root associated with the ciliary ganglion.
Maxillary Nerve
Zygomatic Nerve
The zygomatic nerve is located close to the base of the lateral wall of the orbit. It soon divides into two branches—the zygomaticotemporal and zygomaticofacial nerves—which run for only a short distance in the orbit before passing onto the face through the lateral wall of the orbit. Either these two branches enter separate canals within the zygomatic bone or the zygomatic nerve itself enters the bone before dividing.
The zygomaticotemporal nerve exits the zygomatic bone on its medial surface and pierces the temporal fascia to supply the skin over the temple. It also gives a branch to the lacrimal nerve, which may carry parasympathetic fibres to the lacrimal gland (Figs 11.5, 11.13).
Infraorbital Nerve
The infraorbital nerve emerges onto the face at the infraorbital foramen (see Fig. 11.5), where it lies between the levator labii superioris and levator anguli oris. It divides into three additional groups of branches. The palpebral branches ascend deep to orbicularis oculi and pierce the muscle to supply the skin of the lower eyelid and join with the facial and zygomaticofacial nerves near the lateral canthus. Nasal branches supply the skin of the side of the nose and movable part of the nasal septum and join the external nasal branch of the anterior ethmoidal nerve. Superior labial branches, large and numerous, descend behind levator labii superioris to supply the skin of the anterior part of the cheek and upper lip. They are joined by branches from the facial nerve to form the infraorbital plexus.
Mandibular Nerve
The mandibular nerve is the largest trigeminal division and is a mixed nerve (Figs 11.11, 11.13, 11.14). Its sensory branches supply the teeth and gums of the mandible; the skin in the temporal region; part of the auricle, including the external meatus and tympanic membrane, and the lower lip; the lower part of the face (see Fig. 11.11); and the mucosa of the anterior two-thirds (presulcal part) of the tongue and floor of the oral cavity. The motor branches innervate the muscles of mastication. The large sensory root emerges from the lateral part of the trigeminal ganglion and exits the cranial cavity through the foramen ovale. The small motor root passes under the ganglion and through the foramen ovale to unite with the sensory root just outside the skull. As it descends from the foramen ovale, the nerve is approximately 4 cm from the surface and a little anterior to the neck of the mandible. The mandibular nerve immediately passes between tensor veli palatini, which is medial, and lateral pterygoid, which is lateral, and gives off a meningeal branch and nerve to the medial pterygoid from its medial side. The nerve then divides into small anterior and large posterior trunks. The anterior division gives off branches to the four main muscles of mastication and a buccal branch that is sensory to the cheek. The posterior division gives off three main sensory branches—the auriculotemporal, lingual and inferior alveolar nerves—and motor fibres to supply the mylohyoid and anterior belly of the digastric (see Figs 11.13, 11.14).

Fig. 11.14 Right otic and pterygopalatine ganglia and their branches displayed from the medial side.
Anterior Trunk
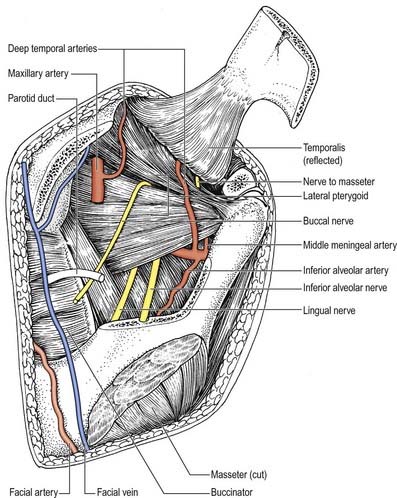
Buccal nerve
The buccal nerve (Fig. 11.15) passes between the two heads of the lateral pterygoid. It descends deep to the temporalis tendon, passes laterally in front of the masseter, and anastomoses with the buccal branches of the facial nerve. It carries the motor fibres to the lateral pterygoid, and these are given off as the buccal nerve passes through the muscle. It may also give off the anterior deep temporal nerve. The buccal nerve supplies sensation to the skin over the anterior part of the buccinator and buccal mucous membrane, together with the posterior part of the buccal gingivae adjacent to the second and third molar teeth.
Nerve to masseter
The nerve to the masseter (see Fig. 11.15) passes laterally above the lateral pterygoid and on to the skull base, anterior to the temporomandibular joint and posterior to the temporalis tendon. It crosses the posterior part of the mandibular notch with the masseteric artery and ramifies on and enters the deep surface of the masseter. It also provides articular branches that supply the temporomandibular joint.
Posterior Trunk
Auriculotemporal nerve
The auriculotemporal nerve usually has two roots that encircle the middle meningeal artery (see Figs 11.13, 11.14). It runs back under the lateral pterygoid on the surface of tensor veli palatini, passes between the sphenomandibular ligament and neck of the mandible and then runs laterally behind the temporomandibular joint related to the upper part of the parotid gland. Emerging from behind the joint, it ascends over the posterior root of the zygoma, posterior to the superficial temporal vessels, and divides into superficial temporal branches. It communicates with the facial nerve and otic ganglion. The rami to the facial nerve, usually two, pass anterolaterally behind the neck of the mandible to join the facial nerve at the posterior border of the masseter. Filaments from the otic ganglion join the roots of the auriculotemporal nerve close to their origin (Figs 11.14, 11.16). The cutaneous branches of the auriculotemporal nerve supply the tragus and part of the adjoining auricle of the ear and posterior part of the temple.
Lingual nerve
The lingual nerve (see Figs 11.13–11.15) is sensory to the mucosa of the anterior two-thirds of the tongue, the floor of the mouth and the mandibular lingual gingivae. It arises from the posterior trunk of the mandibular nerve and at first runs beneath the lateral pterygoid and superficial to tensor veli palatini, where it is joined by the chorda tympani branch of the facial nerve and often by a branch of the inferior alveolar nerve. Emerging from under cover of the lateral pterygoid, the lingual nerve then runs downward and forward on the surface of the medial pterygoid and is thus carried progressively closer to the medial surface of the mandibular ramus. It becomes intimately related to the bone a few millimetres below and behind the junction of the vertical ramus and horizontal body of the mandible. Here it lies anterior to, and slightly deeper than, the inferior alveolar (dental) nerve. It next passes below the mandibular attachment of the superior pharyngeal constrictor and pterygomandibular raphe, closely applied to the periosteum of the medial surface of the mandible, until it lies opposite the posterior root of the third molar tooth, where it is covered only by the gingival mucoperiosteum. At this point it usually lies 2 to 3 mm below the alveolar crest and 0.6 mm from the bone; however, in approximately 5% of cases, it lies above the alveolar crest. It next passes medial to the mandibular origin of mylohyoid, and this carries it progressively away from the mandible and separates it from the alveolar bone covering the mesial root of the third molar tooth.
Abducens Nerve (VI)
The abducens nerve is the sixth cranial nerve, and it emerges from the brain-stem between the pons and the medulla oblongata (see Figs 11.5, 11.35). It is related to the cavernous sinus, but unlike the oculomotor, trochlear, ophthalmic and maxillary nerves, which merely invaginate the lateral dural wall, it passes through the sinus itself, lying lateral to the internal carotid artery (see Fig. 4.6). The abducens nerve enters the orbit through the superior orbital fissure, within the common tendinous ring of the recti muscles (see Fig. 11.3), at first below and then between the two divisions of the oculomotor nerve and lateral to the nasociliary nerve. It passes forward to enter the medial (ocular) surface of the lateral rectus, which is its sole target.
CASE 8 Sixth Cranial Nerve Palsy
A 61-year-old woman with diabetes and hypertension complains of double vision while looking to the left. On examination, she has incomplete paralysis of gaze past the midline bilaterally, worse in the left eye (Fig. 11.17). Upward gaze is also slightly impaired. She complains of headache and difficulty walking. Neuroimaging reveals a posterior fossa ependymoma with hydrocephalus. She undergoes a suboccipital craniectomy for resection of the ependymoma; after several months, her vision returns to normal.
Facial Nerve (VII)
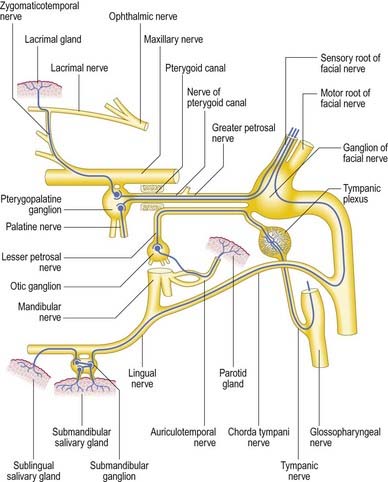
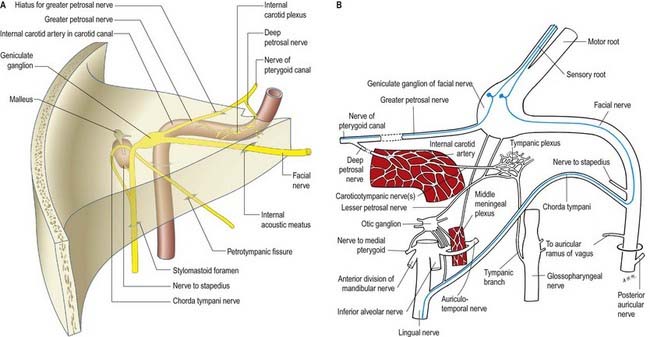
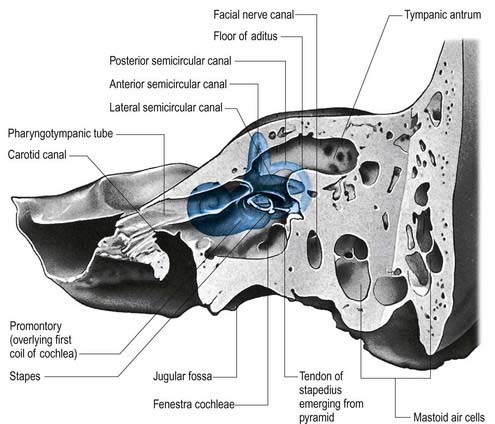
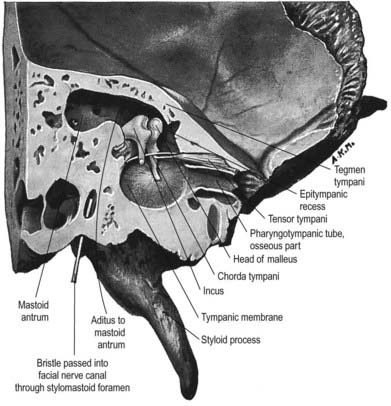
The facial nerve enters the temporal bone through the internal acoustic meatus accompanied by the vestibulocochlear nerve (Fig. 11.18; see also Fig. 10.20). At this point, the motor root, which supplies the muscles of the face, and the nervus intermedius, which contains sensory fibres concerned with the perception of taste and parasympathetic (secretomotor) fibres to various glands, are separate components. They merge within the meatus. At the end of the meatus, the facial nerve enters its own canal, the facial canal, which runs across the medial wall and down the posterior wall of the tympanic cavity to the stylomastoid foramen (Fig. 11.19). As the nerve enters the facial canal, there is a bend that contains the geniculate ganglion (Figs 11.18, 11.20). The branches that arise from the facial nerve within the temporal bone can be divided into those that come from the geniculate ganglion and those that arise within the facial canal.
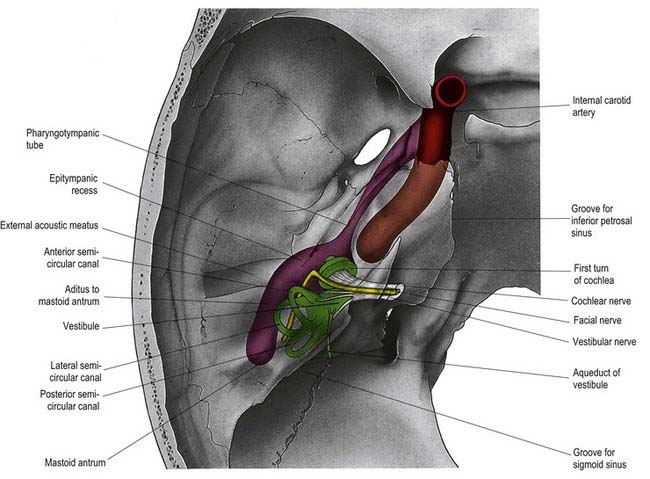
Fig. 11.20 Left auditory apparatus, as if viewed through a semitransparent temporal bone. Compare with Figure 11.19. Note the bend (genu) in the facial nerve at the site of the geniculate ganglion.
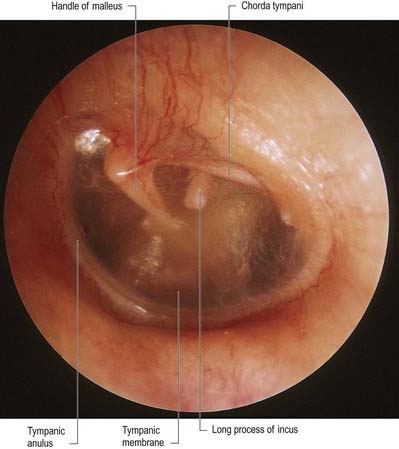
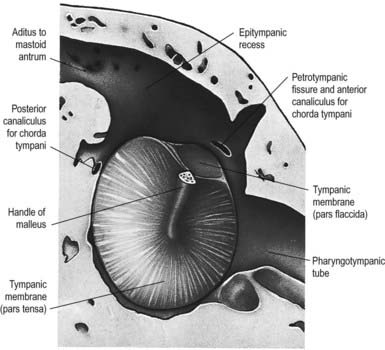
The chorda tympani (Figs 11.14, 11.21) leaves the facial nerve approximately 6 mm above the stylomastoid foramen and runs anterosuperiorly in a canal to enter the tympanic cavity via the posterior canaliculus. It then curves anteriorly in the substance of the tympanic membrane between its mucous and fibrous layers (Fig. 11.22) and crosses medial to the upper part of the handle of the malleus to the anterior wall, where it enters the anterior canaliculus (Fig. 11.23). It exits the skull at the petrotympanic fissure. It contains parasympathetic fibres that supply the submandibular and sublingual salivary glands via the submandibular ganglion (see Fig. 11.16) and taste fibres from the anterior two-thirds of the tongue.
The geniculate ganglion also communicates with the lesser petrosal nerve.
The facial nerve emerges from the base of the skull at the stylomastoid foramen. At this point, the facial nerve lies approximately 9 mm from the posterior belly of the digastric muscle and 11 mm from the bony external acoustic meatus. It gains access to the face by passing through the substance of the parotid gland. Although mainly motor, there are some cutaneous fibres from the facial nerve that accompany the auricular branch of the vagus and probably innervate the skin on both auricular aspects, in the conchal depression and over its eminence.
The temporal branches are generally multiple and pass across the zygomatic arch to the temple to supply intrinsic muscles on the lateral surface of the auricle and the anterior and superior auricular muscles. They join with the zygomaticotemporal branch of the maxillary nerve and the auriculotemporal branch of the mandibular nerve. The more anterior branches supply the frontal belly of occipitofrontalis, orbicularis oculi and corrugator and join the supraorbital and lacrimal branches of the ophthalmic nerve.
The peripheral branches of the facial nerve just described are joined by anastomotic arcades between adjacent branches to form the parotid plexus of nerves, which shows considerable variation. In surgical terms, these anastomoses are important and presumably explain why accidental or essential division of a small branch often fails to result in the expected facial nerve weakness. Six distinctive anastomotic patterns were originally classified by Davis and colleagues (1956) and are illustrated in Figure 11.24. These observations have been confirmed by others, although some variation in the frequency has been reported.
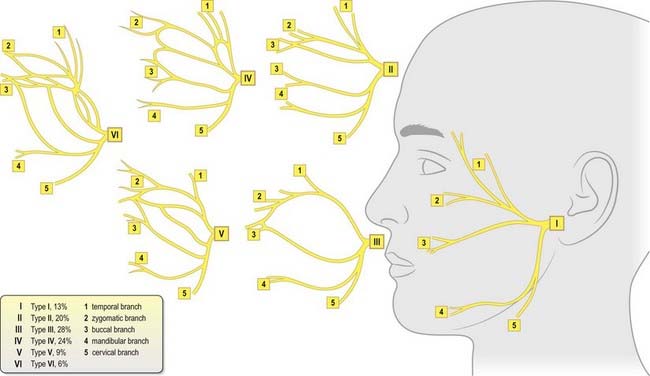
Fig. 11.24 Patterns of branching of the facial nerve.
(Modified with permission from Berkovitz, B.K.B., Moxham, B.J., Head and Neck Anatomy. 2002. © 2002 Informa Healthcare and from Davis, R.A., Anson, B.J., Budinger, J.M., Kurth, I.E., 1956. Surgical anatomy of the facial nerve and parotid gland based upon a study of 350 cervicofacial halves. Surg. Gynecol. Obstet. 102, 385–412, with permission from the American College of Surgeons.)
Facial Nerve Lesions
Facial nerve paralysis may be due to an upper motor neurone lesion (when frontalis is partially spared due to bilateral innervation of the muscle of the upper part of the face) or a lower motor neurone lesion (when all branches may be involved). Bell’s palsy and acoustic neuromas can produce a complete lower motor neurone facial paralysis as a result of compression of the facial nerve trunk as it passes through the middle ear. More commonly, cheek lacerations or malignant parotid tumours result in weakness in part of the face, depending on which branch of the nerve is involved. Unfortunately, the presence of facial paralysis is not a reliable diagnostic sign of a malignant tumour. It is not uncommon for a facial nerve infiltrated by a malignant tumour to continue to function normally. However, when paralysis does accompany a parotid mass, it is certainly malignant.
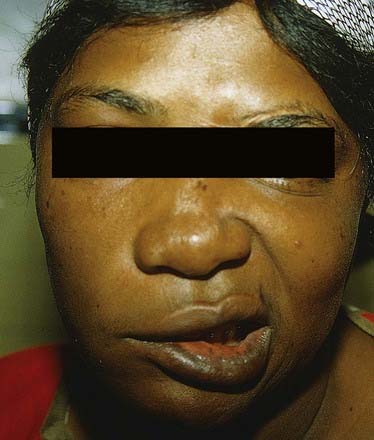
CASE 9 Bell’s Palsy
Discussion: This woman has typical Bell’s palsy, or idiopathic peripheral facial palsy (Fig. 11.25). The seventh cranial (facial) nerve has a complex anatomy and subserves multiple functions. Idiopathic facial palsy is most often the result of a lesion (probably inflammatory) within the confines of the fallopian canal. This often impacts the greater superficial petrosal nerve (decreased tearing), nerve to the stapedius (hyperacusis) and chorda tympani (dysgeusia, with impaired taste on the ipsilateral anterior two-thirds of the tongue), in addition to the main branch of the facial nerve (ipsilateral facial paralysis and subtle sensory disturbance in the region of the ipsilateral ear).
Vestibulocochlear Nerve (VIII)
The vestibulocochlear nerve emerges from the pontocerebellar angle (Fig. 11.35). It courses through the posterior cranial fossa to enter the petrous temporal bone via the internal acoustic meatus, where it divides into an anterior trunk, the cochlear nerve, and a posterior trunk, the vestibular nerve. Both contain the centrally directed axons of bipolar neurones, the cell bodies of which are situated close to their peripheral terminals, together with a smaller number of efferent fibres that arise from brain stem neurones and terminate on cochlear and vestibular sensory cells (Figs. 11.20, 11.29).
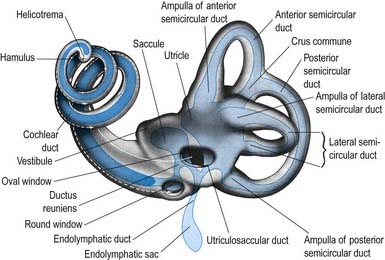
Intratemporal Vestibular Nerve
The maculae and crests are innervated by dendrites of bipolar neurones in the vestibular (Scarpa’s) ganglion situated in the trunk of the nerve within the lateral end of the internal auditory meatus (Fig. 11.27).
The peripheral processes of the ganglion cells are aggregated into definable nerves, each with a specific distribution (Fig. 11.28). The main nerve divides at and within the ganglion into superior and inferior divisions, which are connected by an isthmus. The superior division, the larger of the two, passes through the small holes in the superior vestibular area to supply the ampullary crests of the lateral and anterior semicircular canals via the lateral and anterior ampullary nerves, respectively. A secondary branch of the lateral ampullary nerve supplies the macula of the utricle; however, the greater part of the utricular macula is innervated by the utricular nerve, which is a separate branch of the superior division. Another branch of the superior division, Voit’s nerve, supplies part of the saccule.
Afferent and efferent cochlear fibres are also present in the inferior division of the vestibular nerve, but they leave at the anastomosis of Oort to join the main cochlear nerve (see review by Warr 1992). Another anastomosis, the vestibulofacial anastomosis, is situated more centrally between the facial and vestibular nerves and is the point at which fibres originating in the intermediate nerve pass from the vestibular nerve to the main trunk of the facial nerve.
The cell bodies of the bipolar neurones that contribute to the vestibular nerve vary considerably in size: their circumferences range from 45 to 160 µm (Felix et al 1987). No topographically ordered distribution relating to size has been found. The cell bodies are notable for their abundant granular endoplasmic reticulum, which forms Nissl bodies in places, and their prominent Golgi complexes (see Fig. 11.27). They are covered by a thin layer of satellite cells and are often arranged in pairs, closely abutting each other so that only a thin layer of endoneurium separates the adjacent coverings of satellite cells. This arrangement has led to speculation that ganglion cells may affect one another directly by electrotonic spread (ephaptic transmission).
Intratemporal Cochlear Nerve
The cochlear nerve connects the organ of Corti to the cochlear and related nuclei of the brain stem. The cochlear nerve lies inferior to the facial nerve throughout the internal acoustic meatus. It becomes intimately associated with the superior and inferior divisions of the vestibular nerve, which are situated in the posterior compartment of the canal, and leaves the internal acoustic meatus in a common fascicle (Fig. 11.29).
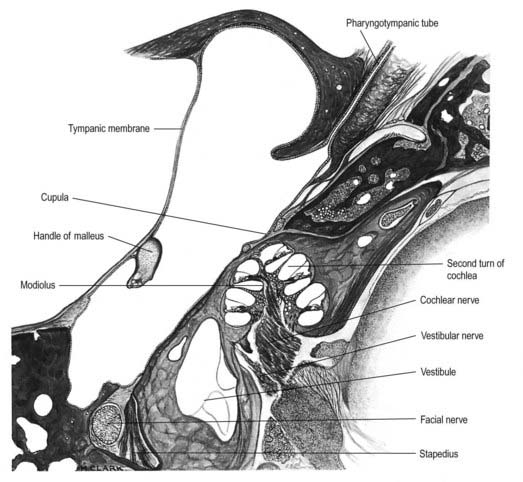
Fig. 11.29 Horizontal section through the left temporal bone.
(Drawn from a section prepared at the Ferens Institute and lent by the late J. Kirk.)
There are approximately 30,000 to 40,000 nerve fibres in the human cochlear nerve (for review, see Nadol 1988). Their fibre diameter distribution is unimodal and ranges from 1 to 11 µm, with a peak at 4 to 5 µm. Functionally, the nerve contains both afferent and efferent somatic fibres, together with adrenergic postganglionic sympathetic fibres from the cervical sympathetic system.
Afferent Cochlear Innervation
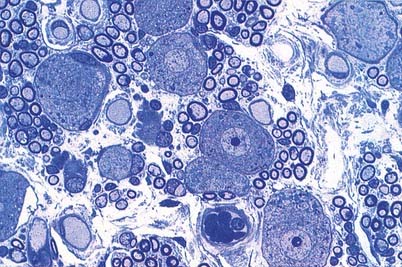
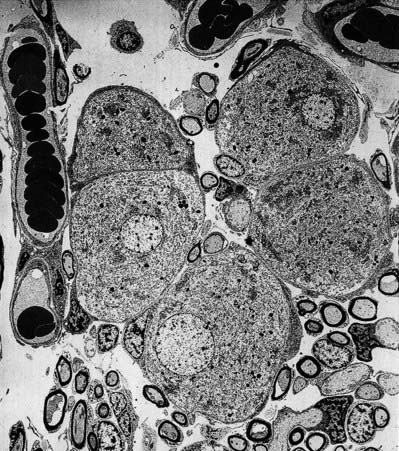
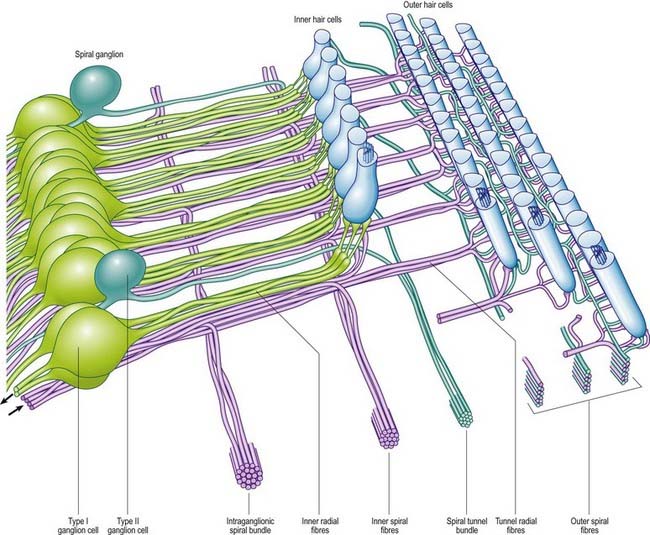
The afferent fibres are myelinated axons with bipolar cell bodies that lie in the spiral ganglion in the modiolus (Fig. 11.30). There are two types of ganglion cell: most (90% to 95%) are large Type I cells, and the remainder are smaller Type II cells (see reviews by Nadol 1988, Eybalin 1993). Type I cells contain a prominent spherical nucleus, abundant ribosomes and many mitochondria; in many mammals (although possibly not in humans) they are surrounded by myelin sheaths. In contrast, Type II cells are smaller, are always unmyelinated and have a lobulated nucleus. The cytoplasm of Type II cells is enriched with neurofilaments but has fewer mitochondria and ribosomes than Type I cells.
Three distinct groupings of afferent fibres have been identified: inner radial, basilar and outer spiral fibres (Fig. 11.31).
Efferent Cochlear Fibres
The efferent nerve fibres in the cochlear nerve are derived from the olivocochlear system (see reviews by Warr 1992, Guinan 1996). Within the modiolus, the efferent fibres form the intraganglionic spiral bundle, which may be one or more discrete groups of fibres situated at the periphery of the spiral ganglion (see Fig. 11.31). There are two main groups of olivocochlear efferents: lateral and medial. The lateral efferents come from small neurones in and near the lateral superior olivary nucleus and arise mainly, but not exclusively, ipsilaterally. They are organized into inner spiral fibres that run in the inner spiral bundle before terminating on the afferent axons that supply the inner hair cells. The medial efferents originate from larger neurones in the vicinity of the medial superior olivary nucleus, and the majority arise contralaterally. They are myelinated and cross the tunnel of Corti to synapse with the outer hair cells mainly by direct contact with their bases, although a few synapse with the afferent terminals. The efferent innervation of the outer hair cells decreases along the organ of Corti from cochlear base to apex, and from the first (inner) row to the third. The efferents use acetylcholine, γ-aminobutyric acid (GABA) or both as their neurotransmitter. They may also contain other neurotransmitters and neuromodulators.
Activity of the medial efferents inhibits cochlear responses to sound; the strength of the activity grows slowly with increasing sound levels. They are believed to modulate the micromechanics of the cochlea by altering the mechanical responses of the outer hair cells, thus changing their contribution to frequency sensitivity and selectivity. The lateral efferents related to the inner hair cells also respond to sound. They appear to modify transmission through their postsynaptic action on inner hair cell afferents. The cholinergic fibres may excite the radial fibres, whereas those containing GABA may inhibit them, although their role is less well understood than that of the medial efferents (see review by Guinan 1996).
Autonomic Cochlear Innervation
CASE 10 Acoustic Neuroma
Discussion: This patient exhibits an indolent clinical course typical of an acoustic neuroma, with early symptoms attributed to eighth nerve dysfunction reflecting the site of the tumour, which is virtually always within the internal auditory meatus (Fig. 11.32). Although the tumour is immediately adjacent to the facial nerve in this location, the first clinical manifestations beyond the eighth nerve itself are generally attributed to the trigeminal nerve. As in this patient, loss of the corneal reflex is a relatively early sign. As the tumour grows, other cranial nerves are affected. Eventually, the enlarging mass impinges, directly or indirectly, on the fourth ventricle, with resultant hydrocephalus; increased intracranial pressure is unusual under these circumstances, in light of contemporary diagnostic procedures.
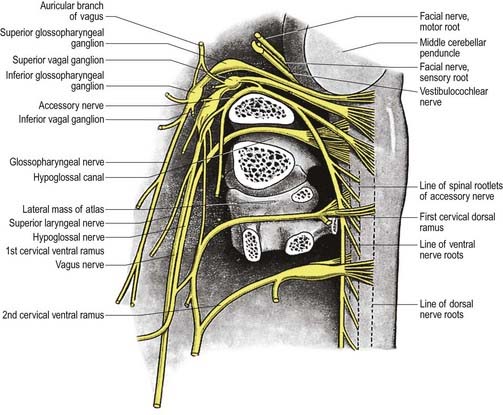
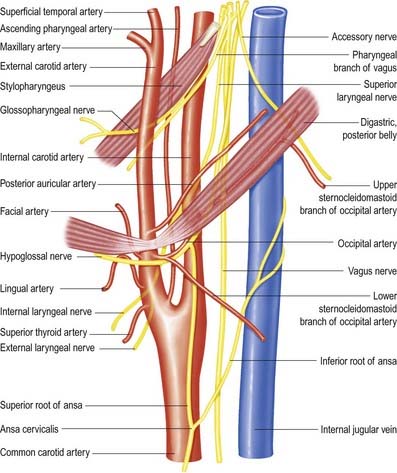
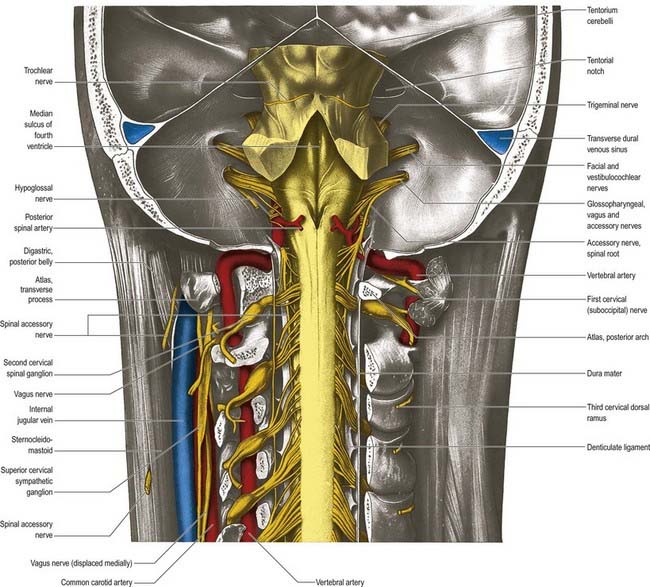
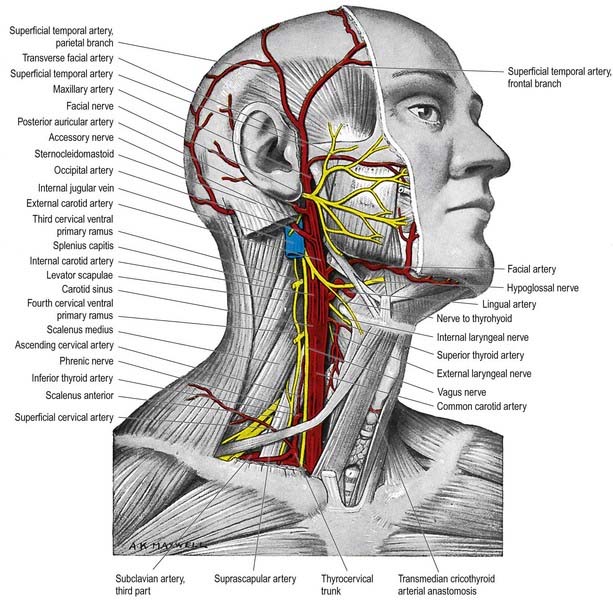
Glossopharyngeal Nerve (IX)
The glossopharyngeal nerve (Figs 11.33–11.35) supplies motor fibres to stylopharyngeus; parasympathetic secretomotor fibres to the parotid gland (derived from the inferior salivatory nucleus); sensory fibres to the tympanic cavity, pharyngotympanic tube, fauces, tonsils, nasopharynx, uvula and posterior (postsulcal) third of the tongue; and gustatory fibres to the postsulcal part of the tongue.
The nerve leaves the skull through the anteromedial part of the jugular foramen, anterior to the vagus and accessory nerves, and in a separate dural sheath. In the foramen it is lodged in a deep groove leading from the cochlear aqueductal depression and is separated by the inferior petrosal sinus from the vagus and accessory nerves. The groove is bridged by fibrous tissue, which is calcified in approximately 25% of skulls. After leaving the foramen, the nerve passes forward between the internal jugular vein and internal carotid artery and then descends anterior to the latter, deep to the styloid process and its attached muscles, to reach the posterior border of stylopharyngeus. It curves forward on the stylopharyngeus and either pierces the lower fibres of the superior pharyngeal constrictor or passes between it and the middle constrictor to be distributed to the tonsil, mucosa of the pharynx and postsulcal part of the tongue, vallate papillae and oral mucous glands.
Two ganglia, superior and inferior, are situated on the glossopharyngeal nerve as it traverses the jugular foramen (see Fig. 11.33). The superior ganglion is in the upper part of the groove occupied by the nerve in the jugular foramen. It is small, has no branches and is usually regarded as a detached part of the inferior ganglion. The inferior ganglion is larger and lies in a notch in the lower border of the petrous part of the temporal bone. Its cells are typical unipolar neurones, and their peripheral branches convey gustatory and tactile signals from the mucosa of the tongue (posterior third, including the sulcus terminalis and vallate papillae) and general sensation from the oropharynx, where it is responsible for initiating the gag reflex.
Branches of Distribution
The branches of distribution are the tympanic, carotid, pharyngeal, muscular, tonsillar and lingual.
Lesions of the Glossopharyngeal Nerve
Damage to the glossopharyngeal nerve rarely occurs without involvement of other lower cranial nerves. Transient or sustained hypertension may follow surgical section of the nerve, reflecting involvement of the carotid branch. Isolated lesions of the glossopharyngeal nerve lead to loss of sensation over the ipsilateral soft palate, fauces, pharynx and posterior third of the tongue, although this is difficult to assess clinically and requires galvanic stimulation. The palatal and pharyngeal (gag) reflexes are reduced or absent, and salivary secretion from the parotid gland may also be reduced. Stylopharyngeus weakness cannot be tested individually. Glossopharyngeal neuralgia consists of brief episodes of severe pain, often precipitated by swallowing, experienced in the throat, behind the angle of the jaw and within the ear. Superior jugular bulb thromboses (e.g. in otitis media) and jugular foramen syndrome (associated with nasopharyngeal carcinoma and glomus tumour) may cause lesions of the adjacent glossopharyngeal, vagus and accessory nerves, with associated weakness in the muscles supplied (in the pharynx and larynx).
Vagus Nerve (X)
The vagus is a large mixed nerve. It has a more extensive course and distribution than any other cranial nerve and traverses the neck, thorax and abdomen (Figs 11.33, 11.34, 11.36; see also Figs 10.3, 10.8). Its central connections are described in Chapter 10.
After emerging from the jugular foramen, the vagus bears two marked enlargements: a small, round superior ganglion and a larger inferior ganglion (see Fig. 11.33).
Branches in the Neck
Auricular Branch
The auricular branch arises from the superior vagal ganglion and is joined by a branch from the inferior ganglion of the glossopharyngeal nerve. It passes behind the internal jugular vein and enters the mastoid canaliculus on the lateral wall of the jugular fossa. Traversing the temporal bone, it crosses the facial canal approximately 4 mm above the stylomastoid foramen and there supplies an ascending branch to the facial nerve. Fibres of the nervus intermedius may pass to the auricular branch at this point, which may explain the cutaneous vesiculation in the auricle that sometimes accompanies geniculate herpes. The auricular branch then traverses the tympanomastoid fissure and divides into two rami. One ramus joins the posterior auricular nerve, and the other is distributed to the skin of part of the ear and to the external acoustic meatus.
Superior Laryngeal Nerve
The external laryngeal nerve, smaller than the internal, descends behind the sternohyoid with the superior thyroid artery, but on a deeper plane. It lies first on the inferior pharyngeal constrictor, then pierces it to curve around the inferior thyroid tubercle and reach the cricothyroid, which it supplies. The nerve also gives branches to the pharyngeal plexus and inferior constrictor. Behind the common carotid artery, the external laryngeal nerve communicates with the superior cardiac nerve and superior cervical sympathetic ganglion.
Accessory Nerve (XI)
The accessory nerve is conventionally described as a single entity (see Figs 11.33, 11.35, 11.37), even though its two components—the cranial root and spinal root, which join for a relatively short part of its course—are of separate origin.
Spinal Root
The spinal root arises from an elongated nucleus of motor cells situated in the lateral aspect of the ventral horn that extends from the junction of the spinal cord and medulla to the sixth cervical segment (see Fig. 11.35). Some rootlets emerge directly; others turn cranially before exiting. Their line of exit is irregular rather than linear, and the spinal root usually passes through the first cervical dorsal root ganglion. The rootlets form a trunk that ascends between the ligamentum denticulatum and the dorsal roots of the spinal nerves and enters the skull via the foramen magnum, behind the vertebral artery. It then turns upward and passes laterally to reach the jugular foramen, which it traverses in a common dural sheath with the vagus, but separated from that nerve by a fold of arachnoid mater. As the spinal root exits the jugular foramen, it runs posterolaterally and passes either medial or lateral to the internal jugular vein. Occasionally, it passes through the vein. The nerve then crosses the transverse process of the atlas and is itself crossed by the occipital artery. It descends obliquely, medial to the styloid process, stylohyoid and posterior belly of the digastric. Running with the superior sternocleidomastoid branch of the occipital artery, it reaches the upper part of the sternocleidomastoid and enters its deep surface, to form an anastomosis with fibres from C2 alone, C3 alone, or C2 and C3, the ansa of Maubrac. The nerve occasionally terminates in the muscle. More commonly, it emerges a little above the midpoint of the posterior border of the sternocleidomastoid, generally above the emergence of the great auricular nerve (usually within 2 cm of it) and between 4 and 6 cm from the tip of the mastoid process. However, the point of emergence is very variable. It crosses the posterior triangle on the levator scapulae (see Fig. 11.37), separated from it by the prevertebral layer of deep cervical fascia and adipose tissue. There the nerve is relatively superficial and related to the superficial cervical lymph nodes. About 3 to 5 cm above the clavicle, it passes behind the anterior border of the trapezius, often dividing to form a plexus on its deep surface that receives contributions from C3 and C4 or from C4 alone. It then enters the deep surface of the muscle.
Sensory ganglia have been described along the course of the spinal root.
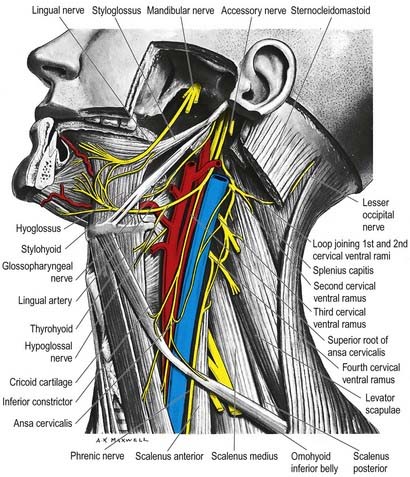
Hypoglossal Nerve (XII)
The hypoglossal nerve is motor to all the muscles of the tongue, except the palatoglossus (see Figs 11.34, 11.36–11.38). The hypoglossal rootlets run laterally behind the vertebral artery, collected into two bundles that perforate the dura mater separately opposite the hypoglossal canal in the occipital bone and then unite after traversing it. The canal is sometimes divided by a spicule of bone. The nerve emerges from the canal in a plane medial to the internal jugular vein, internal carotid artery and ninth, tenth and eleventh cranial nerves and passes inferolaterally behind the internal carotid artery and glossopharyngeal and vagus nerves to the interval between the artery and the internal jugular vein. There it makes a half-spiral turn around the inferior vagal ganglion and is united with it by connective tissue. It then descends almost vertically between the vessels and anterior to the vagus to a point level with the angle of the mandible, becoming superficial below the posterior belly of the digastric and emerging between the internal jugular vein and internal carotid artery. It loops around the inferior sternocleidomastoid branch of the occipital artery and crosses lateral to both the internal and external carotid arteries and the loop of the lingual artery a little above the tip of the greater cornu of the hyoid; it is crossed itself by the facial vein.
Communications
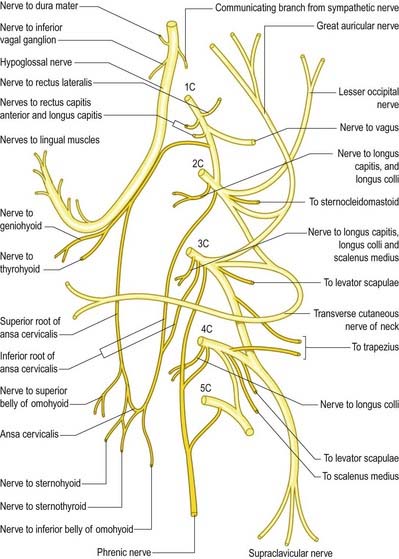
The hypoglossal nerve communicates with the sympathetic trunk, vagus, first and second cervical nerves and lingual nerve. Near the atlas it is joined by branches from the superior cervical sympathetic ganglion and by a filament from the loop between the first and second cervical nerves, which leaves the hypoglossal as the upper root of the ansa cervicalis (see Fig. 11.38). The vagal connections occur close to the skull, and numerous filaments pass between the hypoglossal nerve and the inferior vagal ganglion in the connective tissue uniting them. As the hypoglossal nerve curves around the occipital artery, it receives the ramus lingualis vagi from the pharyngeal plexus. Near the anterior border of the hyoglossus, it is connected with the lingual nerve by many filaments that ascend on the muscle.
Branches of Distribution
Descending Branch
The descending branch (descendens hypoglossi) contains fibres from the first cervical spinal nerve. It leaves the hypoglossal nerve when it curves around the occipital artery and runs down on the carotid sheath. It provides a branch to the superior belly of the omohyoid before joining with the descendens cervicalis to form the ansa cervicalis (see Fig. 11.38).
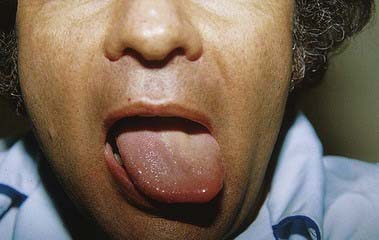
Lesions of the Hypoglossal Nerve
Brodal A. Neurological Anatomy in Relation to Clinical Medicine, third ed. Oxford: Oxford University Press; 1981. Unconventional but highly readable text of neuroanatomy with an emphasis on clinical relevance. Particularly good account of motor pathways
Crossman A.R., Neary D. Neuroanatomy: An Illustrated Colour Text, second ed. Edinburgh: Churchill Livingstone; 2000.
Davis R.A., Anson B.J., Budinger J.M., Kurth L.E. Surgical anatomy of the facial nerve and parotid gland based upon a study of 350 cervicofacial halves. Surg. Gynecol. Obstet.. 1956;102:385-412.
England M.A., Wakely J. A Colour Atlas of the Brain and Spinal Cord. London: Wolfe Publishing; 1991.
Eybalin M. Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol. Rev.. 1993;73:309-373.
Felix H., Hoffman V., Wright A., Gleeson M.J. Ultrastructural findings on human Scarpa’s ganglion. Acta. Otolaryngol. Suppl.. 1987;436:85-92.
Guinan J.Jr. Physiology of olivocochlear efferents. In: Dallos P., Popper A.N., Fay R.R., editors. The Cochlea. New York: Springer Verlag; 1996:435-502. Comprehensive description of the efferent innervation of the cochlea and its function
Haines D.E. Neuroanatomy: An Atlas of Structures, Sections, and Systems, fifth ed. Philadelphia: Lippincott Williams and Wilkins; 2000.
Lee H., et al. Sudden deafness and anterior inferior cerebellar artery infarction. Stroke. 2002;33:2807.
Nadol J.B. Comparative anatomy of the cochlea and auditory nerve in mammals. Hear Res.. 1988;34:253-266.
Warr W.B. Organization of olivocochlear efferent systems in mammals. In: Webster D.B., Popper A.N., Fay R.R., editors. Mammalian Auditory Pathway: Neuroanatomy. New York: Springer Verlag; 1992:410-448.