Chapter 4 Cranial Meninges
The brain and spinal cord are entirely enveloped by three concentric membranes, the meninges, which provide support and protection. The outermost meningeal layer is the dura mater (pachymeninx). Beneath this lies the arachnoid mater. The innermost layer is the pia mater. The dura is an opaque, tough, fibrous coat. It incompletely divides the cranial cavity into compartments and accommodates the dural venous sinuses. It is separated from the arachnoid by a narrow subdural space. The arachnoid mater and pia mater are sometimes referred to collectively as the leptomeninges, and they share many similarities. The arachnoid is much thinner than the dura and is mostly translucent. It surrounds the brain loosely, spanning depressions and concavities. Beneath the arachnoid lies the subarachnoid space, which contains cerebrospinal fluid (CSF), secreted by the choroid plexuses of the cerebroventricular system. The pia mater is a transparent, microscopically thin membrane that follows the contours of the brain and is closely adherent to its surface. The subarachnoid space varies greatly in depth, and the larger expanses are termed subarachnoid cisterns. CSF circulates within the subarachnoid space and is reabsorbed into the venous system through arachnoid villi and granulations associated with the dural venous sinuses. Cranial and spinal meninges are continuous through the foramen magnum. Only the cranial meninges are described in this chapter.
Dura Mater
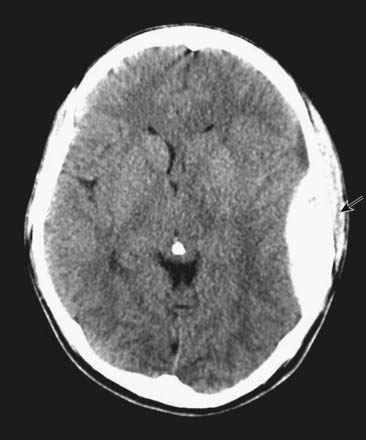
CASE 1 EPIDURAL HAEMATOMA
An EDH can readily be seen on an unenhanced head CT scan and typically has a lens-shaped appearance, as it lies in the potential space between the dura and the calvaria (Fig. 4.1). It does not cross the cranial suture lines because at those locations the dura is tightly adherent to the skull. Emergency surgery is required in most cases to relieve the pressure caused by the haematoma and, if possible, identify the source of bleeding.
Dural Partitions
Falx Cerebri
The falx cerebri is a strong, crescent-shaped sheet of dura mater lying in the sagittal plane and occupying the great longitudinal fissure between the two cerebral hemispheres (Figs. 4.2, 4.3). The crescent is narrow in front, where the falx is fixed to the crista galli, and broad behind, where it blends into the midline with the tentorium cerebelli. The anterior part of the falx is thin and may have a number of irregular perforations (see Fig. 4.2). Its convex upper margin is attached to the internal cranial surface on each side of the midline, as far back as the internal occipital protuberance. The superior sagittal sinus runs within the dura along this margin, in a cranial groove, and the falx is attached to the lips of this groove. At its lower edge, the falx is free and concave and contains the inferior sagittal sinus. The straight sinus runs along the line of attachment of the falx to the tentorium cerebelli (see Fig. 4.2).
Tentorium Cerebelli
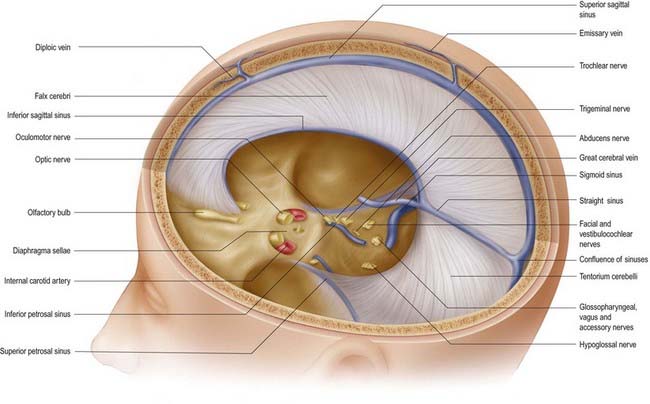
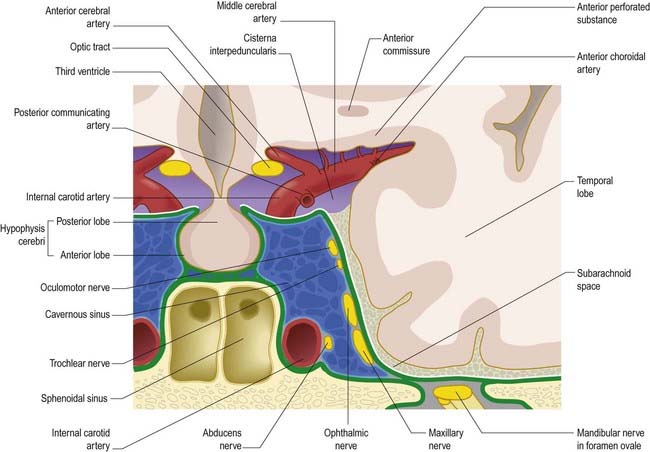
The tentorium cerebelli (Figs. 4.2–4.4) is a sheet of dura mater with a peaked configuration reminiscent of a single-poled tent, from which its name is derived. It covers the cerebellum and passes under the occipital lobes of the cerebral hemispheres. Its concave anterior edge is free; between it and the dorsum sellae of the sphenoid bone is a large curved hiatus (the tentorial incisure or notch), which is occupied by the midbrain and the anterior part of the superior aspect of the cerebellar vermis. The tentorium divides the cranial cavity into supratentorial and infratentorial compartments that contain the forebrain and hindbrain, respectively. The convex outer limit of the tentorium is attached posteriorly to the lips of the transverse sulci of the occipital bone and the posteroinferior angles of the parietal bones, where it encloses the transverse sinuses. Laterally, the tentorium is attached to the superior borders of the petrous temporal bones, where it contains the superior petrosal sinuses (see Fig. 4.3). Near the apex of the petrous temporal bone, the lower layer of the tentorium is evaginated anterolaterally under the superior petrosal sinus to form a recess between the endosteal and meningeal layers in the middle cranial fossa. This recess is the trigeminal cave (Meckel’s cave) and contains the roots and ganglion of the trigeminal nerve. The evaginated meningeal layer fuses in front with the anterior part of the trigeminal ganglion. At the apex of the petrous temporal bone, the free border and attached periphery of the tentorium cross each other (see Fig. 4.4). The anterior ends of the free border are fixed to the anterior clinoid processes, and the attached periphery is fixed to the posterior clinoid processes. The oculomotor nerve lies in the groove between them on each side.
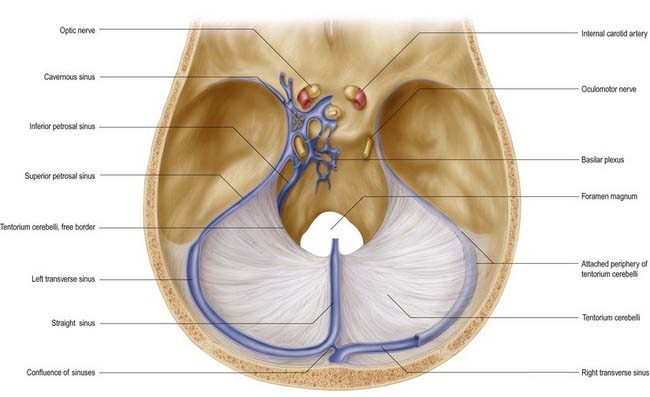
Fig. 4.4 Dura mater of the floor of the cranial cavity and the superior aspect of the tentorium cerebelli. Representations of the cavernous sinus and its venous relationships are greatly simplified and are shown on the left only. Note that the trochlear and abducens nerves are not shown (see Fig. 4.6).
Falx Cerebelli
The falx cerebelli is a small midline fold of dura mater lying below the tentorium cerebelli. It projects forward into the posterior cerebellar notch between the cerebellar hemispheres. Its base is directed upward and attached to the posterior part of the inferior surface of the tentorium cerebelli in the midline. Its posterior margin is attached to the internal occipital crest and contains the occipital sinus. The apex of the falx cerebelli frequently divides into two small folds, which disappear at the sides of the foramen magnum. Frequently the falx is double.
Diaphragma Sellae
The diaphragma sellae (see Fig. 4.2) is a small, circular, horizontal sheet of dura mater that forms a roof to the sella turcica and, in many cases, almost completely covers the pituitary gland (hypophysis). The central opening in the diaphragma allows the infundibulum and pituitary stalk to pass into the pituitary fossa. There is wide individual variation in the size of the central opening. The diaphragma sellae was an important landmark in pituitary surgery in the past—extension of a pituitary tumour above it was an indication for a subfrontal approach through a craniotomy. A transsphenoidal approach is currently preferred, irrespective of whether there is suprasellar extension.
The arrangement of the dura mater in the central part of the middle cranial fossa is complex (see Fig. 4.4). The tentorium cerebelli forms a large part of the floor of the middle cranial fossa and fills much of the gap between the ridges of the petrous temporal bones. On both sides, the rim of the tentorial incisure is attached to the apex of the petrous temporal bone and continues forward as a ridge of dura mater to attach to the anterior clinoid process. This ridge marks the junction of the roof and the lateral part of the cavernous sinus (Figs. 4.5, 4.6). The periphery of the tentorium cerebelli is attached to the superior border of the petrous temporal bone, crosses under the free border of the tentorial incisure and continues forward to the posterior clinoid processes as a rounded, indefinite ridge of the dura mater. Thus, an angular depression exists between the anterior parts of the peripheral attachment of the tentorium and the free border of the tentorial incisure (see Figs. 4.2, 4.4). This depression in the dura mater is part of the roof of the cavernous sinus and is pierced in front by the oculomotor nerve and behind by the trochlear nerve, which proceed anteroinferiorly into the lateral wall of the cavernous sinus (Fig. 4.7). In the anteromedial part of the middle cranial fossa, the dura mater ascends as the lateral wall of the cavernous sinus. It reaches the ridge produced by the anterior continuation of the free border of the tentorium and runs medially as the roof of the cavernous sinus, where it is pierced by the internal carotid artery (see Figs. 4.2, 4.4). Medially, the roof of the sinus is continuous with the upper layer of the diaphragma sellae. At or just below the opening in the diaphragma for the infundibulum and pituitary stalk, the dura, arachnoid and pia mater blend with one another and with the capsule of the pituitary gland. It is not possible to distinguish the layers of the meninges within the sella turcica, and the subarachnoid space is obliterated.
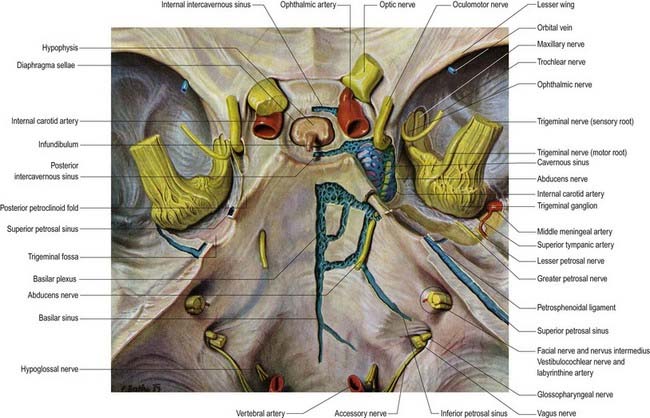
Fig. 4.6 Middle cranial fossa, viewed from above to show the cavernous and related sinuses. These have been exposed by partial removal of the dura matter. The trigeminal, trochlear and oculomotor nerves have been reflected forward on both sides.
Dural Venous Sinuses
Dural venous sinuses (see Fig. 6.16) are a complex of venous channels that lie between the two layers of dura mater, draining blood from the brain and cranial bones. They are lined by endothelium and have no valves; their walls are devoid of muscular tissue. Developmentally, the venous sinuses emerge as venous plexuses, and most sinuses preserve a plexiform arrangement to a variable degree rather than being simple vessels with a single lumen. Browder and Kaplan (1976) examined human venous sinuses in hundreds of corrosion casts and observed vascular plexuses adjoining the superior and inferior sagittal and straight sinuses and, with a lower incidence, the transverse sinuses. There was much individual variation, and departures from ‘average’ patterns were frequent in early life; for example, in infancy, the falx cerebelli may contain large plexiform channels and venous lacunae, augmenting the occipital sinus. These variations cannot be detailed in a general text. They must be established on an individual basis by angiography when the clinical necessity arises. However, it is important to emphasize the wide variation possible in the structure of cranial venous sinuses, together with their plexiform nature and wide connections with cerebral and cerebellar veins. Another kind of connection has been shown experimentally. Parts of sinuses (and even diploic veins) can be filled by forcible internal carotid injection, suggesting the existence of arteriovenous shunts (Browder and Kaplan 1976). A connection between the middle meningeal arteries and the superior sagittal sinus has been demonstrated in this way, although the sites of communication are unknown.
Superior Sagittal Sinus
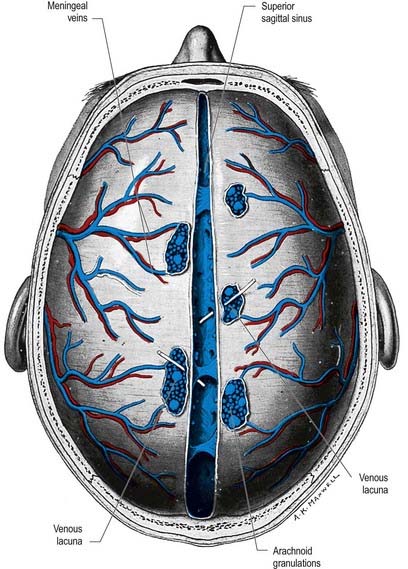
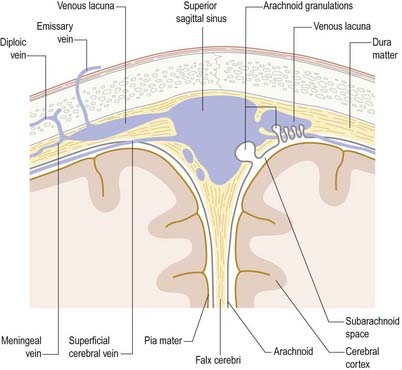
The superior sagittal sinus runs in the attached, convex margin of the falx cerebri; it grooves the internal surface of the frontal bone, the adjacent margins of the two parietal bones and the squamous part of the occipital bone (Fig. 4.8; see also Figs. 4.2, 6.16). It begins near the crista galli, a few millimetres posterior to the foramen caecum, and receives primary tributaries from cortical veins of the frontal lobes, the ascending frontal veins. Narrow anteriorly, the sinus runs backward, gradually widening to approximately 1 cm. Near the internal occipital protuberance it deviates, usually to the right, and continues as a transverse sinus. Triangular in cross-section, the interior of the superior sagittal sinus possesses the openings of superior cerebral veins and projecting arachnoid granulations. It is traversed by many fibrous bands. It also communicates by small orifices with irregular venous lacunae, situated in the dura mater near the sinus. There are usually two or three of these on each side—a small frontal, a large parietal and an intermediate-sized occipital. In the elderly, the lacunae tend to become confluent, so there is one elongated lacuna on each side. Fine fibrous bands cross them, and numerous arachnoid granulations project into them. The superior sagittal sinus receives the superior cerebral veins and, near the posterior end of the sagittal suture, veins from the pericranium, which pass through the parietal foramina. The lacunae also drain the diploic veins and meningeal veins.
The dilated posterior end of the superior sagittal sinus is referred to as the confluence of the sinuses (see Fig. 4.4). This is situated to one side (usually the right) of the internal occipital protuberance, where the superior sagittal sinus turns to become a transverse sinus. It also connects with the occipital and contralateral transverse sinus. The size and degree of communication of the channels meeting at the confluence are highly variable. In more than half of subjects, all venous channels that converge toward the occiput interconnect, including the straight and occipital sinuses. In many instances, however, communication is absent or tenuous. Any sinus involved may be duplicated, narrowed or widened near the confluence.
Inferior Sagittal Sinus
The inferior sagittal sinus is located in the posterior half or two-thirds of the free margin of the falx cerebri (see Fig. 4.2). It increases in size posteriorly and ends in the straight sinus. It receives veins from the falx and sometimes from the medial surfaces of the cerebral hemispheres.
Straight Sinus
The straight sinus lies in the junction of the falx cerebri and tentorium cerebelli (see Figs. 4.2, 4.4). It runs posteroinferiorly as a continuation of the inferior sagittal sinus into the transverse sinus. It is not continuous (or only tenuously so) with the superior sagittal sinus. Its tributaries include the great cerebral vein (see Figs. 4.2, 6.16) and some superior cerebellar veins. Internally, the straight sinus is triangular in cross-section.
Transverse Sinus
The transverse sinuses begin at the internal occipital protuberance (see Figs. 4.4, 4.5). One of them, usually the right, is directly continuous with the superior sagittal sinus; the other is continuous with the straight sinus. On both sides the sinuses run in the attached margin of the tentorium cerebelli, first on the squama of the occipital bone, then on the mastoid angle of the parietal bone. Each follows a gentle anterolateral curve, increasing in size as it does so, to the posterolateral part of the petrous temporal bone. There it turns down as a sigmoid sinus, which ultimately becomes continuous with the internal jugular vein. Transverse sinuses are triangular in section and usually unequal in size; the one draining the superior sagittal sinus is larger. They receive the inferior cerebral, inferior cerebellar, diploic and inferior anastomotic veins and are joined by the superior petrosal sinuses, where they continue as sigmoid sinuses.
Sigmoid Sinus
The sigmoid sinuses are continuations of the transverse sinuses, beginning where these leave the tentorium cerebelli (Figs. 4.5, 4.10). Each sigmoid sinus curves inferomedially in a groove on the mastoid process of the temporal bone, crosses the jugular process of the occipital bone and turns forward to the superior jugular bulb, lying posterior in the jugular foramen. Anteriorly, a thin plate of bone separates its upper part from the mastoid antrum and air cells. It connects with pericranial veins via mastoid and condylar emissary veins.
Occipital Sinus
The occipital sinus is the smallest of the sinuses. It lies in the attached margin of the falx cerebelli (see Fig. 4.5) and is occasionally paired. It commences near the foramen magnum in several small channels, one joining the end of the sigmoid sinus, and connects with the internal vertebral plexuses. It ends in the confluence of the sinuses.
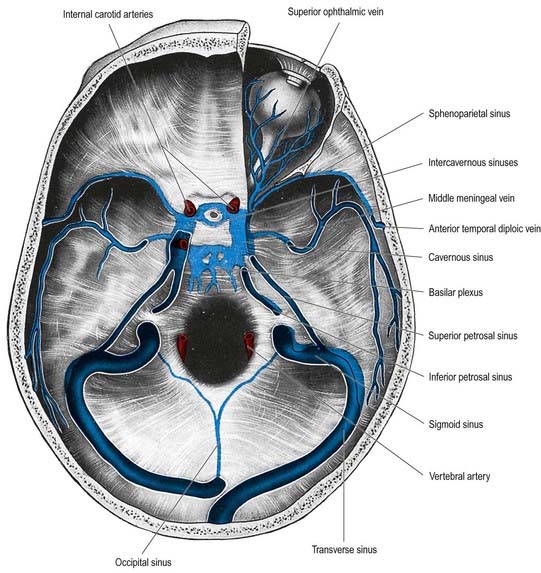
Cavernous Sinus
The cavernous sinus is a large venous plexus that lies on both sides of the body of the sphenoid bone (see Figs. 4.5–4.7). The sinus extends from the superior orbital fissure to the apex of the petrous temporal bone, with an average length of 2 cm and a width of 1 cm. The sphenoidal air sinus and pituitary gland are medial to the cavernous sinus. The trigeminal cave is near the inferoposterior part of its lateral wall and extends posteriorly beyond it to enclose the trigeminal ganglion. The uncus of the temporal lobe is also lateral to the sinus.
The internal carotid artery, and its associated sympathetic plexus, passes forward through the sinus together with the abducens nerve, which lies lateral to the artery. The oculomotor and trochlear nerves and the ophthalmic and maxillary divisions of the trigeminal nerve all lie in the lateral wall of the sinus (see Fig. 4.7). Their diameters are such that they project into the lumen and are usually covered medially by little more than endothelium. Propulsion of blood in the cavernous sinus is partly due to pulsation of the internal carotid artery, but it is also influenced by gravity and hence by the position of the head.
Carotid cavernous fistula and cavernous sinus thrombosis
The unique location of the internal carotid artery within a venous structure occasionally gives rise to direct communication between the two structures by means of a carotid cavernous fistula (CCF), which is established as a result of either severe head trauma or degenerative or aneurysmal vessel disease (see Chapter 6, Case 3). A CCF causes proptosis, which may be pulsatile, together with vascular dilatation in the tissues of the orbit and globe and combinations of third, fourth and sixth cranial nerve palsies. These changes can cause permanent blindness. CCFs are most commonly treated by passing a catheter up the carotid into the fistula and then occluding it with dilatable balloons or flexible metal coils. Any spreading infection involving the upper nasal cavities, paranasal sinuses, cheek (especially near the medial canthus), upper lip, anterior nares or even upper incisor or canine tooth may rarely lead to septic thrombosis of the cavernous sinuses as infected thrombi pass from the facial vein or pterygoid venous complex into the sinus (via either ophthalmic veins or emissary veins that enter the cranial cavity through the foramen ovale). This is a critical medical emergency with a high risk of disseminated cerebritis and cerebral venous thrombosis (see Case 2).
Intercavernous Sinus
The two cavernous sinuses are connected by anterior and posterior intercavernous sinuses (see Fig. 4.5) and the basilar plexus (see Fig. 4.6). The intercavernous sinuses lie in the anterior and posterior attached borders of the diaphragma sellae, thus forming a complete circular venous sinus (see Fig. 4.6). All connections are valveless, and the direction of flow is reversible. Small irregular sinuses inferior to the pituitary gland drain into the intercavernous sinuses. These inferior intercavernous sinuses are plexiform in nature and are important in a transnasal surgical approach to the pituitary.
Superior Petrosal Sinus
This small, narrow sinus drains the cavernous sinus into the transverse sinus on either side (see Figs. 4.4–4.6). It leaves the posterosuperior part of the cavernous sinus, runs posterolaterally in the attached margin of the tentorium cerebelli and crosses above the trigeminal nerve to lie in a groove on the superior border of the petrous part of the temporal bone. It ends by joining a transverse sinus where this curves down to become the sigmoid. It receives cerebellar, inferior cerebral and tympanic veins and connects with the inferior petrosal sinus and the basilar plexus.
Inferior Petrosal Sinus
The inferior petrosal sinus drains the cavernous sinus into the internal jugular vein (see Figs. 4.5, 6.16). It begins at the posteroinferior aspect of the cavernous sinus and runs back in a groove between the petrous temporal and basilar occipital bones. It traverses the anterior part of the jugular foramen and ends in the superior jugular bulb. It receives labyrinthine veins via the cochlear canaliculus and the vestibular aqueduct and tributaries from the medulla oblongata, pons and inferior cerebellar surface. The sinus is often a plexus and sometimes drains by a vein in the hypoglossal canal to the suboccipital vertebral plexus.
Sphenoparietal Sinus
The sphenoparietal sinus is located below the periosteum of the lesser wing of the sphenoid bone, near its posterior edge (see Fig. 4.5). It curves medially to open into the anterior part of the cavernous sinus. It receives small veins from the adjacent dura mater and sometimes the frontal ramus of the middle meningeal vein. It may also receive connecting rami, in its middle course, from the superficial middle cerebral vein and veins from the temporal lobe and the anterior temporal diploic vein. When these connections are well developed, the sphenoparietal sinus is a large channel.
Basilar Sinus and Plexus
The basilar sinus and plexus consist of interconnecting channels between layers of dura mater on the clivus (see Fig. 4.6). The basilar venous plexus interconnects the inferior petrosal sinuses and joins with the internal vertebral venous plexus. It also usually connects with the cavernous and superior petrosal sinuses at its anterior end. When veins around the foramen magnum (so-called marginal sinuses) are large, they communicate anteriorly with the plexus, and this produces an almost complete circular venous channel around the foramen magnum, connecting the basilar plexus intracranially to the inferior petrosal, sigmoid and occipital sinuses and extracranially to variable vertebral plexuses in the suboccipital region.
Middle Meningeal Vein (Sinus)
Tributaries of the middle meningeal vein communicate with the superior sagittal sinus through its venous lacunae. Below, they converge and unite as frontal and parietal trunks, which accompany branches of the middle meningeal arteries in grooves on the internal parietal surfaces. The veins lie closer to the bone than the arteries do, and they may occupy separate grooves. This situation makes them particularly liable to tear in cranial fractures. Their termination is variable. The parietal trunk may traverse the foramen spinosum to the pterygoid venous plexus. The frontal trunk may also reach this plexus via the foramen ovale, or it may end in the sphenoparietal or cavernous sinus (see Fig. 4.5). The middle meningeal vein receives meningeal tributaries and small inferior cerebral veins, and it connects with the diploic and superficial middle cerebral veins. It frequently bears arachnoid granulations.
The diploic veins constitute a hypothetical fourth venous tier. However, because they drain into dural veins, they are grouped here with them, following Browder and Kaplan (1976). It should be noted that intracranial veins communicate at many points with extracranial vessels via emissary and other veins.
Arterial Supply and Venous Drainage of the Cranial Dura Mater
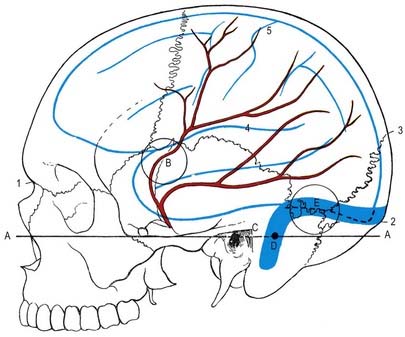
The middle meningeal is the largest of the meningeal arteries. It passes between the roots of the auriculotemporal nerve and may lie lateral to the tensor veli palatini before entering the cranial cavity through the foramen spinosum. It then runs in an anterolateral groove on the squamous part of the temporal bone, dividing into frontal and parietal branches. The larger frontal (anterior) branch crosses the greater wing of the sphenoid, reaches a groove or canal in the sphenoidal angle of the parietal bone and divides into branches between the dura mater and cranium, with some ascending to the vertex and others to the occipital region. One ascending branch grooves the parietal bone approximately 15 mm behind the coronal suture, corresponding approximately to the precentral sulcus (see Fig. 4.10). The parietal (posterior) branch curves back on the squamous temporal bone, reaching the lower border of the parietal bone anterior to its mastoid angle and dividing to supply the posterior parts of the dura mater and cranium. These branches anastomose with their fellows and with the anterior and posterior meningeal arteries.
Arachnoid Mater
The arachnoid and pia are separated by the subarachnoid space and joined by trabeculae (Fig. 4.11). The anatomical relationships of the arachnoid and pia differ to some extent in the cerebral and spinal regions.
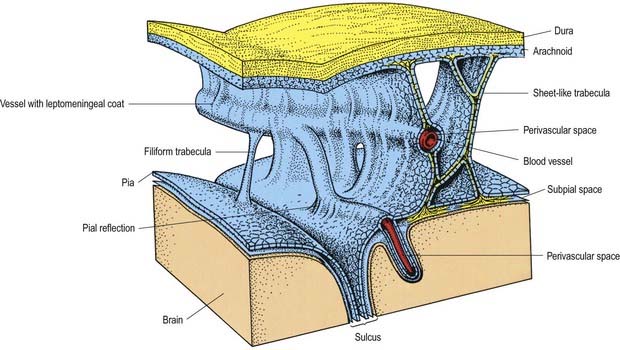
Fig. 4.11 Relationships of the pia and arachnoid mater to the dura, brain and vessels.
(Modified from Alcolado et al, 1988, according to Zhang, E.T., Inman, C.B.E., Weller, R.O. 1990. Interrelationships of the pia mater and the perivascular (Virchow–Robin) spaces in the human cerebrum. J. Anat. 170, 111–123, by permission from Blackwell Science.)
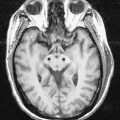
CASE 3 SUBDURAL HAEMATOMA
SDH can be seen on head CT or MRI; the latter is more sensitive for small SDHs (Fig. 4.12). The accumulated subdural blood typically has a crescent shape, as it can flow freely in the subdural space. Larger SDHs, or those causing neurological dysfunction, require surgical drainage; smaller, asymptomatic SDHs can sometimes be followed expectantly.
Subarachnoid Space
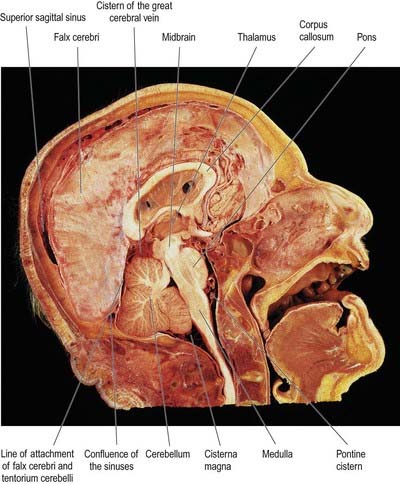
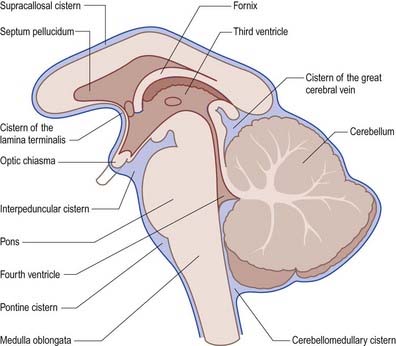
The subarachnoid space lies between the arachnoid and the pia mater. It contains CSF and the larger arteries and veins that traverse the surface of the brain. Arachnoid and pia mater are in close apposition over the convexities of the brain, such as the cortical gyri, whereas concavities are followed by the pia but spanned by the arachnoid. This arrangement produces a subarachnoid space of greatly variable depth that is location dependent. The more expansive spaces are identified as subarachnoid cisterns (Fig. 4.13). Cisterns are continuous with each other through the general subarachnoid space, of which there are dilatations.
The largest cistern, the cisterna magna or cerebellomedullary cistern, is formed where the arachnoid bridges the interval between the medulla oblongata and the inferior surface of the cerebellum. The cistern is continuous above with the lumen of the fourth ventricle through its median aperture—the foramen of Magendie—and below with the subarachnoid space of the spinal cord. The pontine cistern is an extensive space ventral to the pons, continuous below with the spinal subarachnoid space, behind with the cisterna magna and, rostral to the pons, with the interpeduncular cistern. The basilar artery runs through the pontine cistern into the interpeduncular cistern. As the arachnoid mater spans between the two temporal lobes, it is separated from the cerebral peduncles and structures within the interpeduncular fossa by the interpeduncular cistern, which contains the circulus arteriosus (circle of Willis). Anteriorly, the interpeduncular cistern extends to the optic chiasma. The cistern of the lateral fossa is formed by the arachnoid as it bridges the lateral sulcus between the frontal, parietal and temporal opercula, and it contains the middle cerebral artery. The cistern of the great cerebral vein (cisterna ambiens or superior cistern) lies posterior to the brain stem and third ventricle and occupies the interval between the splenium of the corpus callosum and the superior cerebellar surface. The great cerebral vein traverses this cistern, and the pineal gland protrudes into it.
The cerebral subarachnoid space is connected with the fourth ventricle of the brain by three openings, through which CSF flows. The median aperture, or foramen of Magendie, lies in the median plane in the inferior part of the roof of the fourth ventricle and provides communication with the cisterna magna. The paired lateral apertures, or foramina of Luschka, are located at the ends of the lateral recesses of the fourth ventricle and open into the subarachnoid space at the cerebellopontine angle, behind the upper roots of the glossopharyngeal nerves (see Fig. 5.10). Occlusion of these foramina in cases of chronic meningitis, such as tuberculous meningitis, may lead to interruption of CSF outflow from the ventricular system, resulting in hydrocephalus.
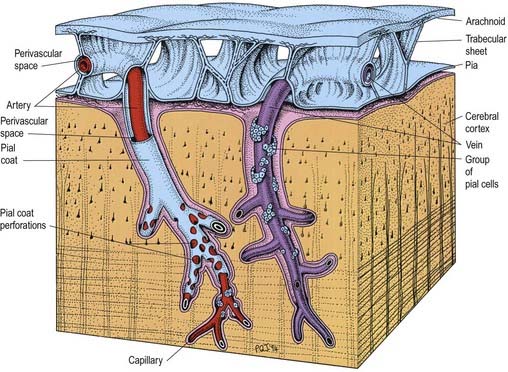
Trabeculae, in the form of sheets or fine filiform structures, traverse the subarachnoid space from the deep layers of the arachnoid mater to the pia mater and are also attached to large blood vessels within the subarachnoid space (Figs. 4.11, 4.14). Each trabecula has a core of collagen and is coated by leptomeningeal cells. Subarachnoid trabeculae are long and filamentous and cross the subarachnoid cisterns. The topography of trabeculae that cross the subarachnoid space may, in effect, form compartments, particularly in the perivascular regions, enabling directional flow of CSF through the subarachnoid space.
Arteries and veins in the subarachnoid space are coated by a thin layer of leptomeninges, often only one cell thick. The pia mater, the blood vessels and the arachnoid mater are connected by collagenous trabeculae and sheets, which are also coated by leptomeningeal cells (see Fig. 4.11). Cranial and spinal nerves that traverse the subarachnoid space to pass out of cranial or intervertebral foramina are coated by a thin layer of leptomeninges, which fuses with the arachnoid at the exit foramina.
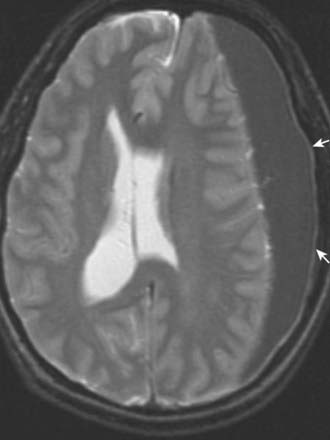
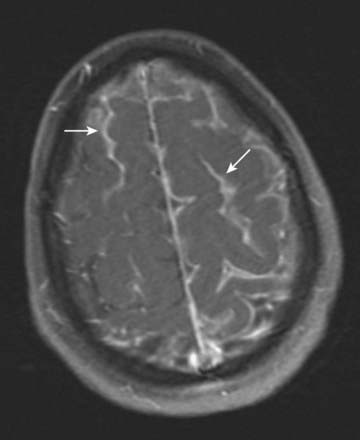
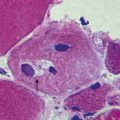
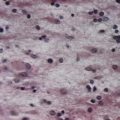
CASE 4 MENINGITIS
Discussion: This patient has the classic signs of meningitis—fever, altered mental status and a stiff neck. Inflammation of the meninges may be caused by a variety of agents, including infectious (viral, bacterial, fungal) or non-infectious (medications, vasculitis, subarachnoid haemorrhage). Diagnosis of meningitis is by spinal fluid analysis and culture; when CSF cultures are negative, the disease is called aseptic meningitis. Treatment of meningitis is directed by the underlying cause. See Figure 4.15.
Arachnoid Villi and Granulations
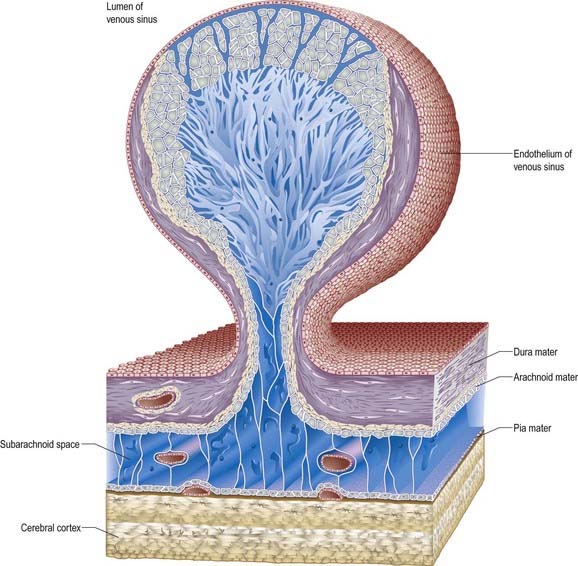
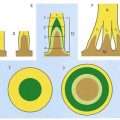
Arachnoid villi and the larger arachnoid granulations represent extensions of the arachnoid mater and subarachnoid space through the wall of the dural venous sinuses. As such, they present an exchange surface to the sinus endothelium (Fig. 4.16), which constitutes the major pathway for the passage of CSF from the subarachnoid space into the blood. They are, therefore, an essential step in the normal circulation and reabsorption of CSF.
These structures are most prominent along the margins of the great longitudinal fissure, where they project into the superior sagittal sinus (see Fig. 4.8). Arachnoid villi are also found in association with other cerebral venous sinuses, such as the transverse sinus. Microscopic villi are present in the superior sagittal sinus of the fetus and newborn infant. These hypertrophy to form granulations that are visible by age 18 months in the parieto-occipital region of the superior sagittal sinus and by 3 years in the laterally located sinuses of the posterior fossa. The arachnoid granulations become more lobulated and complex with increasing age. They may become calcified, when they are known as Pacchionian bodies.
At the base of each arachnoid granulation, a thin neck of arachnoid mater projects through an aperture in the dural lining of the venous sinus and expands to form a core of collagenous trabeculae and interwoven channels (Fig. 4.17). The core is surmounted by an apical cap of arachnoid cells, some 150 µm thick. Channels extend through the cap to reach the subendothelial regions of the granulation. The cap region of each granulation is attached to the endothelium of the sinus over an area some 300 µm in diameter, whereas the rest of the granulation core is separated from the endothelium by a fibrous dural cupola.
Pia Mater
The pia mater is a delicate membrane that closely invests the surface of the brain, from which it is separated by a microscopic subpial space (see Fig. 4.11). It follows the contours of the brain into concavities and into the depths of fissures and sulci. During development, it becomes apposed to the ependyma in the roof of the telencephalon and fourth ventricle to form the stroma of the choroid plexus.
Pia mater is formed from a layer of leptomeningeal cells that is often only one to two cells thick. The cells are joined by desmosomes and gap junctions but few if any tight junctions, and they are continuous with the coating of the subarachnoid trabeculae. They are separated from the basal lamina of the glia limitans by collagen bundles, fibroblast-like cells and arteries and veins lying in the subpial space (see Fig. 4.14).
Despite its delicate and thin nature, the pia mater appears to form a regulatory interface between the subarachnoid space and the brain. In addition to separating the subarachnoid space from the subpial and perivascular spaces, cells of the pia mater exhibit pinocytotic activity and ingest particles up to 1 µm in diameter. They contain enzymes such as catechol-O-methyltransferase and glutamine synthetase, which degrade neurotransmitters. Further evidence of the pia’s effectiveness as a barrier is seen in subarachnoid haemorrhages and in subpial haemorrhages in infants—in neither of these situations do red blood cells penetrate the pia mater.
It was long thought that the subarachnoid space connected directly with the perivascular spaces (Virchow–Robin spaces) surrounding blood vessels in the brain. However, it is now recognized that the pia mater is reflected from the surface of the brain onto the surface of blood vessels in the subarachnoid space. Thus, the subarachnoid space is separated by a layer of pia from the subpial and perivascular spaces of the brain (see Figs. 4.11, 4.14).
Browder, Kaplan, 1976Browder J. Kaplan H.A. Cerebral Dural Sinuses and Their Tributaries. 1976. Thomas. Springfield, Illinois.
Describes variations in the superior sagittal and other venous sinuses.
Kida, Weller, 1994Kida S. Weller R.O. Morphology of CSF drainage pathways in man. Raimondi A. Principles of Pediatric Neurosurgery. vol 4. 1994. Berlin. Springer.
Klintworth G.K. The ontogeny and growth of the human tentorium cerebelli. Anat. Rec. 1967;158:433-442.
Zhang E.T., Inman C.B.E., Weller R.O. Interrelationships of the pia mater and the perivascular (Virchow–Robin) spaces in the human cerebrum. J. Anat. 1990;170:111-123.