Chapter 13 CPAP Treatment for Moderate Diffuse Excessive Dynamic Airway Collapse Caused by Mounier-Kuhn Syndrome
Case Description
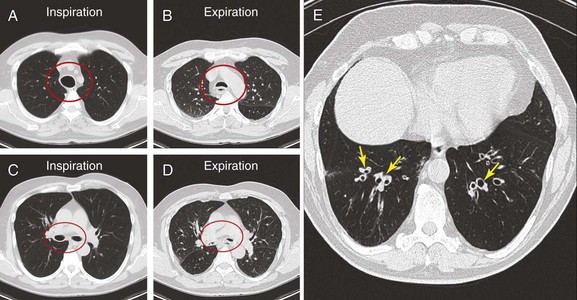
The patient was a 70-year-old man with “seal barking” cough for longer than a year. His cough was associated with congestion and was worse at night and in the supine position. He had dyspnea on exertion (World Health Organization [WHO] class II). His past medical history was significant for asthma and frequent hospitalizations for pneumonia and “bronchitis.” At the time of our evaluation, his medications included fluticasone/salmeterol 250/50 mg twice daily, tiotropium one inhalation daily, prednisone 10 mg/day, mometasone nasal spray, Mucinex, loratadine, and inhaled tobramycin for recurrent lower respiratory tract Pseudomonas aeruginosa infection. In addition, he was using omeprazole 20 mg daily for gastroesophageal reflux disease (GERD) and albuterol 2.5 mg nebulization 3 times daily, followed by application of high-frequency chest oscillation (i.e., percussion vest). He was not a smoker and had no occupational exposure to fumes and toxins. On examination, wheezing over the trachea and bilateral rhonchi could be heard early during expiration. His oxygen saturation was 92% on 4 L/min of oxygen. Pulmonary function testing (PFT) revealed the following: forced expiratory volume in 1 second (FEV1) 1.93 L (51% predicted), which increased to 2.26 L (60% predicted), resulting in 17% improvement after bronchodilators; and forced vital capacity (FVC) 2.62 L (52% predicted) increased to 3.19 L (63% predicted)—a 22% improvement after bronchodilators. Total lung capacity (TLC) was 98% predicted, residual volume (RV) 171% predicted, and diffusing capacity of the lung for carbon monoxide (DLCO) 89% predicted. A paired inspiratory-expiratory dynamic computed tomography (CT) scan of the chest showed tracheobronchomegaly, expiratory bulging of the posterior membrane inside the airway lumen, narrowing the cross-sectional area of the trachea and mainstem bronchi by 67%, and bronchiectasis (Figure 13-1). A review of his old records revealed that several sputum and bronchioloalveolar lavage (BAL) cultures showed multiple bacteria, including Stenotrophomonas, Nocardia, Escherichia coli, Pseudomonas, and Aspergillus.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
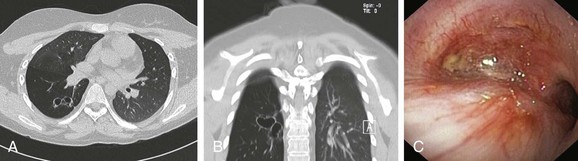
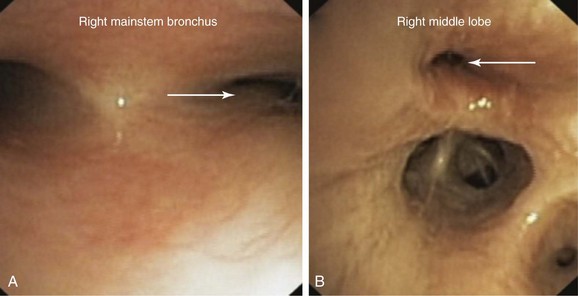
This patient’s PFTs showed moderate obstructive ventilatory impairment responsive to bronchodilators, a normal DLCO, and evidence of air trapping. These findings were consistent with his diagnosis of asthma. The CT scan, however, showed tracheobronchomegaly, moderate EDAC on expiratory images, bronchiectasis, bronchial thickening, and bronchiolitis in the lower lobes (see Figure 13-1). During the initial evaluation of a patient with bronchiectasis, the presumed cause of this patient’s recurrent episodes of bronchitis and pneumonia, the clinician must select diagnostic studies to determine the cause of the disorder. If bronchiectasis is truly focal (i.e., confined to one lobe), then it is highly unlikely that inherited or systemic causes are responsible, and investigations can be tailored appropriately,1 for example, bronchoscopy might be performed to identify possible obstruction by foreign body, tumor, or stricture (Figure 13-2).
In a patient with bilateral bronchiectasis, such as that noted in our patient, a battery of immunologic and genetic tests can be tailored on the basis of clinical suspicions.1 Our patient had no evidence of autoimmune disease, immunodeficiency, or genetic disorders such as alpha 1-antitrypsin deficiency or cystic fibrosis: The autoantibody screen that comprised antinuclear antibodies (ANAs), antineutrophil cytoplasmic antibodies (ANCAs), anti-SSA, anti-SSB, and rheumatoid factor, along with cystic fibrosis (CF) testing for 97 mutations, was negative; the alpha 1-antitrypsin level and immunoglobulin (Ig)G, IgM, IgA, and IgE were normal; and the erythrocyte sedimentation rate (ESR) was slightly elevated at 25 (normal <20). Because chronic aspiration is another cause of bilateral bronchiectasis, an esophagogram and a hypopharyngogram were performed, revealing no evidence of aspiration or obstruction. However, high-grade reflux, which in fact can be seen in almost a fourth of patients with bronchiectasis, is usually asymptomatic. One study showed that only 27% of patients with bronchiectasis and coexisting GERD have typical reflux symptoms such as heartburn, regurgitation, or dyspepsia.2
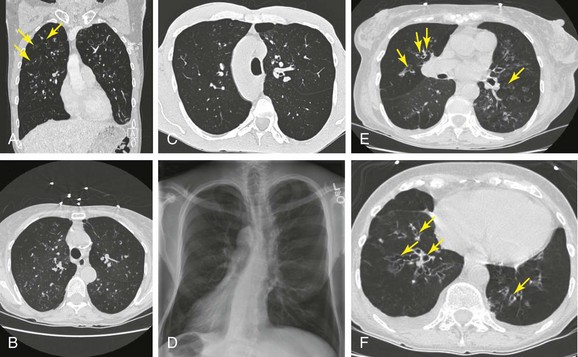
Today, chest high-resolution computed tomography (HRCT) scans are performed in almost anyone suspected of having bronchiectasis. A classic finding of bronchiectasis is the “signet ring” sign (this was present in our patient), characterized by a diameter of the bronchus greater than the adjacent artery (broncho-arterial ratio >1)* (see Figure 13-1). Bronchial wall thickening,† bronchi filled with mucus, and mosaic perfusion (air trapping on expiration) are indirect HRCT signs that assist in diagnosis (Figure 13-3; see also Figure 13-1). HRCT also narrows the differential diagnosis by revealing specific disease processes such as situs inversus in Kartagener syndrome‡ (see Figure 13-3), or by revealing a specific distribution of the abnormalities. For instance, in allergic bronchopulmonary aspergillosis (ABPA),* the distribution of bronchiectasis is usually central (see Figure 13-3), but in tuberculosis, it is usually unilateral and upper lobe predominant. In our patient, the distribution was in the lower lobes, suggesting impaired mucociliary mechanisms or postinfectious causes. In addition, HRCT, when performed with a dynamic protocol,† can detect and quantify the degree of central airway collapse.3 Very high resolution images and three-dimensional (3D) reconstruction using 320-slice CT have been reported for diagnosis of EDAC.‡4
The patient’s “seal barking” cough, nocturnal congestion in the supine position, and wheezing were suggestive of expiratory central airway collapse. The paired inspiratory-expiratory dynamic CT showed tracheobronchomegaly, mild tracheal collapse (67%), and moderate collapse in the mainstem bronchi, with near complete closure of the lumen during exhalation (90%)* (see Figure 13-1). Tracheobronchomegaly is usually secondary to bilateral upper lobe fibrosis as a sequel of pulmonary fibrosis, tuberculosis, cystic fibrosis, or sarcoidosis (Figure 13-4), but our patient had other parenchymal/interstitial lung findings on HRCT.
In the absence of alternative explanations, we believed that our patient had primary tracheobronchomegaly in association with bronchiectasis—findings known as Mounier-Kuhn syndrome. This syndrome is characterized by atrophy or absence of elastic fibers and smooth muscle cells. These structural alterations may lead to collapse of the airway during exhalation, making expectoration by cough inefficient. Although considered to be a congenital condition, 50% of patients have no symptoms until the third decade of life, when frequent lower respiratory tract infections and sputum production become manifest, probably as a result of bronchiectasis.5
Support System
The patient was married, but separated. He told us that he spent occasional holidays with his wife, but that he was alone often and occasionally felt depressed. He felt isolated and lonely, in part because of his symptoms. Jokingly he told us that no woman would want to bear a man who barked all the time. Once the suspicion for EDAC had been raised by the CT findings, we believed it was important to inquire further into this patient’s social support environment, and we took the liberty to ask about his home situation. Published evidence suggests that one’s living situation and potential resolution of marital conflicts may be targeted by interventions leading to improved CPAP adherence—one of the conservative treatments for EDAC.*6,7
Patient Preferences and Expectations
The patient was a retired physician who showed good understanding of his disease process. He was willing to try wearing a CPAP mask but was concerned that he would not tolerate it, citing examples of many of his patients who suffered from obstructive sleep apnea (OSA). Because his cough was interfering with his social life and his frequent lower respiratory tract infections required multiple hospitalizations, he was determined to try his best to wear CPAP apparatus, if indicated, provided that a notable symptomatic benefit would be derived. Although he expressed strong motivation initially, we were not certain about the long-term health benefits of wearing CPAP for this disease. We explained to him that the immediate benefit of CPAP for patients with EDAC (in terms of severity of airway collapse) could be detected at the time of bronchoscopy, and that in general, with regard to adherence to treatment, data from patients with OSA show increased adherence to CPAP with time in continuous users.8
Procedural Strategies
Indications
1. Bronchoscopic diagnosis of infection: Infections play an important role in exacerbation of bronchiectasis and baseline symptoms. Our patient’s main reason for chronic infections and exacerbations was his anatomic airway abnormality. Excessive collapse of the trachea and mainstem bronchi leads to inability to raise secretions, infections, damage to the bronchial walls, and progressive airway dilation (bronchiectasis). This further reduces one’s ability to clear secretions from the distal airways, setting up a repetitive cycle of purulent drainage and tissue damage.9 Studies using techniques that have avoided contamination by upper airway flora (such as bronchoscopic protected brush specimens and BAL) show that more than 50% of patients with bronchiectasis have potentially pathogenic bacteria in their lower airways. Although the presence of pathogenic bacteria in the lower airways in these patients has often been termed colonization, the spectrum of pathogenic bacteria isolated in patients at baseline and during exacerbations is remarkably similar.* Furthermore, improvement in baseline symptoms after long-term antibiotics suggests that bacteria may be causing inflammation.10–12 Some pathogens have a definite role in long-term prognosis. Relevant to this patient, the isolation of Pseudomonas aeruginosa correlates with severe clinical disease in bronchiectasis, occurs later in the course of disease,13 and is associated with a decline in lung function as measured by FEV1 (50 mL/yr).14
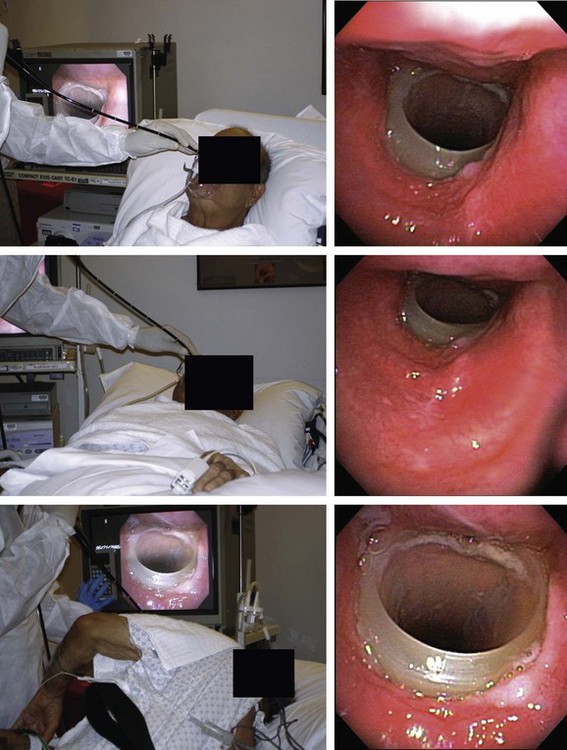
2. One indication† for dynamic bronchoscopy (aka functional bronchoscopy) is the evaluation of EDAC. This technique allows for real-time observations of changes in the airways in response to various maneuvers. It is performed using flexible bronchoscopy with minimal or moderate (conscious) sedation so that the patient can cooperate and obey commands during the procedure.15 With the flexible bronchoscope at the midline, the patient is asked to inhale and exhale deeply, hyperflex and hyperextend the neck, and cough. These maneuvers are done while the patient is placed in upright, supine, and lateral decubitus positions (Figure 13-5).
3. CPAP (or BiPAP) assistance during flexible bronchoscopy can be used to confirm the possible effectiveness of conservative management in symptomatic patients with expiratory central airway collapse (EDAC or malacia), as well as to prevent respiratory distress and intubation in high-risk patients requiring flexible bronchoscopy.16,17 Bronchoscopy allows removal of mucus plugs and improves ventilation and oxygen exchange, but by occupying 10% to 15% of the normal tracheal lumen, the bronchoscope can decrease PaO2 (partial pressure of oxygen in arterial blood) by 10 to 20 mm Hg, which may contribute to respiratory complications and cardiac arrhythmias. Hypoxemia is worsened when local anesthetics or saline solution is instilled into the lower airways. The American Thoracic Society recommends avoiding flexible bronchoscopy and lavage in patients with hypoxemia that cannot be corrected to at least a PaO2 of 75 mm Hg or an SaO2 (saturated oxygen level in hemoglobin) greater than 90%.18,19 In patients with severe obstructive ventilatory impairment, bronchoscopy can lead to air trapping because functional residual capacity (FRC) increases when the scope is inserted nasally; and in children and in patients with OSA or obesity hypoventilation syndrome (OHS) who already have awake hypercapnia, bronchoscopy can exacerbate hypoxemia and hypercapnia because of sedation-related increased collapsibility of the central and upper airways. In these settings, bronchoscopy can be performed with CPAP or BiPAP assistance, potentially sparing patients the discomfort and risks of refractory hypoxemia, intubation, and mechanical ventilation.20,21 However, CPAP can thwart an accurate evaluation of dynamic airway obstruction by preventing airway collapse. For this reason, dynamic airway lesions within extrathoracic and intrathoracic airways (e.g., laryngomalacia, tracheobronchomalacia [TBM], EDAC) should be bronchoscopically visualized with and without CPAP to assess collapsibility.22
Contraindications
Contraindications to performing flexible bronchoscopy on CPAP include respiratory insufficiency requiring endotracheal intubation (e.g., cardiopulmonary resuscitation for respiratory arrest), severe hemodynamic instability (i.e., systolic blood pressure <80 mm Hg), encephalopathy, recent (within 1 week) myocardial infarction, respiratory failure caused by neurologic disease or status asthmaticus, and the presence of facial deformities. Another contraindication is recent oral, esophageal, or gastric surgery that prohibits the use of a face mask or increases the risk of gastric insufflation and aspiration.23 Our patient had no such contraindications.
Expected Results
CPAP can be used in patients with EDAC or TBM to ameliorate airway collapse. The exact CPAP settings that maintain airway patency in expiratory central airway collapse of various degrees of severity have not been systematically studied. If an evaluation of central airway collapse is performed, digital imaging documentation is obtained in upright and supine positions both on and off CPAP so the degree of airway narrowing and the response to positive pressure can be evaluated. The pressure requirements are individualized and determined during CPAP-assisted bronchoscopy, or, alternatively, with CPAP-assisted CT scanning.4 A recent case study reported that a pressure of 10 cm H2O abolished a patient’s symptoms and ameliorated EDAC, as evaluated by 320-slice CT scanning with 3D reconstruction.*4 In EDAC or TBM, a CPAP of 7 to 10 cm H2O usually ensures airway patency.24,25 Overall, noninvasive positive-pressure ventilation (CPAP or BiPAP) assistance helps ensure an uncomplicated bronchoscopy in hypercapnic chronic obstructive pulmonary disease (COPD) patients with pneumonia,26 compensates for the hypotonicity of the upper airway muscles in patients with OSA,27 and improves tidal volume and respiratory flow in spontaneously breathing children with easily collapsible upper airways.22
Team Experience
CPAP-assisted bronchoscopies can be performed by a bronchoscopist (i.e., intensivist, pulmonologist, thoracic or trauma surgeon) with the help of a bronchoscopy assistant and respiratory therapists or nurses who are familiar with noninvasive positive-pressure ventilation. These nonphysician health care providers are thus actively involved in preparation for and performance of this procedure, form a relationship with the patient, and are involved in or responsible for postprocedure surveillance,28 including telephone follow-up to troubleshoot and to ensure compliance.
Diagnostic Alternatives
1. CPAP-assisted computed tomography: Noninvasive methods of assessing the degree of collapse and response to CPAP can be offered by CPAP-assisted computed tomography.4 Evaluation of the lung parenchyma may be necessary during CPAP application, especially in patients with COPD. Results from one study showed that a CPAP of 5 cm H2O caused little increase in lung aeration in healthy volunteers and COPD patients, but in a couple of patients with COPD (2 out of 11), CPAP deflated some regions of the lungs. CPAP levels of 10 cm H2O and 15 cm H2O increased the emphysematous zones in all sectors of the lungs, including dorsal and apical regions, in patients with COPD, but caused little hyperaeration predominantly in the ventral areas in healthy volunteers.29 This is potentially harmful, given the already hyperinflated status of a COPD patient’s lungs. Optimal CPAP levels should counteract the increased effort of breathing while improving expiratory airflow to its maximum without causing further hyperinflation.30 Thin HRCT scan slices may better estimate CPAP-induced lung hyperinflation, provided that an appropriate radiologic density threshold is used for quantification of hyperinflated zones.31 This method is useful for EDAC assessment, but not for collecting specimens for microbiologic analysis.
2. Polysomnography: This technique was reportedly useful in a case of EDAC due to tracheobronchomegaly; evidence of both obstructive apnea and hypopnea was found with an apnea-hypopnea index (AHI) of 11, no snoring, and associated oxygen desaturation of 75%. During a second overnight study with nasal CPAP at a pressure of 8 cm, the AHI decreased to 3 and no drop in oxygen saturation was noted.32 This raises the yet unstudied issue of a possible role for polysomnography in patients with expiratory central airway collapse.
3. Endotracheal intubation–assisted bronchoscopy: This approach should be used instead of CPAP-assisted bronchoscopy when it is not possible to maintain SaO2 above 85% despite the use of a high fraction of inspired oxygen (FiO2),33 or when a patient has copious secretions that cannot be cleared or are associated with acidosis or changes in mental status.34 Mechanical ventilation through an indwelling endotracheal tube offers the benefit of a secure airway, facilitates carbon dioxide clearance, improves gas exchange, unloads respiratory muscles, and reduces the risk for massive aspiration during bronchoscopy. It also allows repeated insertions of the bronchoscope to remove obstructing mucus plugs or blood clots. Obviously, proper assessment of airway dynamics is impaired by the indwelling endotracheal tube and the need for sedation in intubated patients.
Therapeutic Alternatives
With regard to bronchiectasis, large population-based, long-term studies of efficacy are lacking. Still, loosening of secretions combined with enhanced removal using standard pulmonary hygiene measures is probably warranted, regardless of the cause. Approaches include administration of bronchodilators, chest physiotherapy (postural drainage, hand or mechanical chest clapping, incentive spirometry, and high-frequency chest wall compression), hypertonic saline,* and mucolytics and anti-inflammatory medications (i.e., inhaled glucocorticosteroids).35
With regard to EDAC, laser application on the posterior membrane to stiffen the airway and prevent collapse, although it has been described,36 has not been systematically studied, and its safety profile is unknown. Therefore, this approach cannot be recommended as of this writing. Stent insertion and membranous tracheoplasty are indicated for severely symptomatic patients with severe and diffuse collapse of the central airways, usually caused by cartilaginous weakness in the setting of crescent-type TBM and rarely EDAC.37,38
Techniques and Results
Anesthesia and Perioperative Care
Intravenous sedation is usually necessary to ensure patient comfort, but it can compromise ventilatory status, especially in children and in patients with awake hypercapnia, OSA, or OHS who are at risk for sedation-related increased collapsibility of the upper or central airways.39 In our experience, it is helpful for one designated team member to continuously observe the patient and monitors, and to be ready to reposition the patient or suction the mouth in case of aspiration of gastric contents. Reassurance is provided continuously because patient cooperation is essential, even when sedation is being administered. The choice of sedative agent and its dose generally depend on age, underlying medical comorbidities, concomitant medications, and operator preference. Careful monitoring and adherence to sedation guidelines help to ensure a safe outcome.33 Monitoring helps prevent procedure-related morbidity and assists the clinician in applying appropriate ventilatory settings and detecting early signs of respiratory or hemodynamic compromise.40 These may occur as a result of performing bronchoscopy in an already frail patient (i.e., extensive pulmonary infiltrates, SaO2 <90%, PaO2 <75 mm Hg, FEV1 <1 L),41 but may also be caused by aspiration, which is more likely with CPAP owing to gastric distention.42 Electrocardiography and pulse oximetry are continuously monitored. If the oxygen saturation drops below 80% for longer than 1 minute, or if poor clinical tolerance is observed, the nurse or respiratory therapist should increase the FiO2 to keep the SaO2 above 90%.21
Technique and Instrumentation
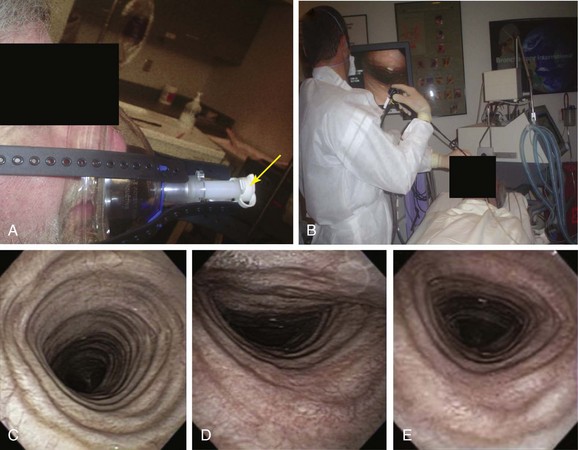
A full face mask is secured to the patient’s face with elastic straps (Figure 13-6). The patient is then connected to the ventilator with a dual-axis swivel adapter (T-adapter) attached to the face mask. CPAP (or BiPAP) is delivered at parameters that depend on clinical indications. We start at a pressure of 0 cm H2O and titrate upward according to the indications. For instance, in cases of refractory hypoxemia or hypercarbia from COPD, ventilator parameters are set at CPAP of 5 cm H2O and pressure support ventilation at 15 to 17 cm H2O. During bronchoscopy, FiO2 is kept at 1 and is decreased to prebronchoscopy levels after the procedure if the patient is able to maintain SpO2 (pulse oximeter oxygen saturation) greater than 92%. The bronchoscope is advanced to the nares through the swivel adapter and face mask (see video on ExpertConsult.com) (Video III.13.1![]() ). If necessary, as in evaluation of central airway collapse, procedures are performed in the upright and supine positions, as well as on and off CPAP, to evaluate the degree of airway narrowing and response to CPAP (see Figure 13-6). In EDAC, CPAP pressures of 7 to 10 cm H2O usually ensure airway patency, but pressures can be raised incrementally by 3 cm H2O until airway caliber during exhalation is at least 50% of that noted during inspiration* (see video on ExpertConsult.com) (Video III.13.2
). If necessary, as in evaluation of central airway collapse, procedures are performed in the upright and supine positions, as well as on and off CPAP, to evaluate the degree of airway narrowing and response to CPAP (see Figure 13-6). In EDAC, CPAP pressures of 7 to 10 cm H2O usually ensure airway patency, but pressures can be raised incrementally by 3 cm H2O until airway caliber during exhalation is at least 50% of that noted during inspiration* (see video on ExpertConsult.com) (Video III.13.2![]() ). When CPAP is used for bronchoscopy in severely hypoxemic patients, positive-pressure ventilation is maintained for at least 30 minutes after the procedure, after which CPAP is discontinued if SpO2 is greater than 92% and no evidence of respiratory insufficiency is noted.
). When CPAP is used for bronchoscopy in severely hypoxemic patients, positive-pressure ventilation is maintained for at least 30 minutes after the procedure, after which CPAP is discontinued if SpO2 is greater than 92% and no evidence of respiratory insufficiency is noted.
Anatomic Dangers and Other Risks
The flexible bronchoscope occupies 10% to 15% of a normal tracheal lumen;43 this may result in hypoventilation and hypoxemia. Bronchoscopy may induce or exacerbate bronchospasm, especially in patients with known asthma.44 The risk for respiratory insufficiency or cardiac arrhythmias45–47 is increased in hypoxemic patients.†
Topical anesthetics such as lidocaine or tetracaine, saline washes, and bronchoalveolar lavage (BAL) fluid administered during the procedure may induce bronchospasm and allergic reactions. Furthermore, gas exchange is impaired through a process of alveolar filling, especially after BAL, and even in relatively normal patients, hypoxemia may persist for up to 6 hours.40
If excessive cough is noted before the procedure, as was the case in our patient, the bronchoscopist may be tempted to use higher doses of lidocaine for local laryngeal analgesia. The major toxic side effect of lidocaine for local anesthesia is seizures (also seen with tetracaine). Concern for this is increased when plasma levels are greater than 5 mg/L. The dose of lidocaine should therefore be limited to 400 mg or 3 to 4 mg/kg.48 Vigilance is warranted in elderly patients with liver or cardiac involvement because lidocaine is metabolized mainly in the liver, and low cardiac output delays its metabolism. The bronchoscopy team should continuously monitor the patient’s mental status because seizures may present in a nonconvulsive pattern. Adverse reactions to lidocaine during bronchoscopy are, in fact, primarily related to toxicity rather than to allergy,49 but true allergic reactions to local anesthetics are rare and account for less than 1% of adverse reactions to these anesthetic preparations.* In our practice, we do not use nebulized lidocaine. Studies have shown that the addition of nebulized lidocaine for airway analgesia before the start of flexible bronchoscopy offered no benefit when flexible bronchoscopy was performed using combined topical analgesia and moderate (conscious) sedation.50 Peak lidocaine plasma levels occur 15 minutes after direct topical application to the larynx and trachea, and 5 minutes after ultrasonic or other nebulization, but the maximum duration of the anesthetic effect of lidocaine, regardless of the mode of administration, is between 20 and 30 minutes.51 These pharmacodynamic properties of lidocaine should be taken into account when patients cough excessively during bronchoscopy, because the cough can be solely related to inadequate laryngeal analgesia and often improves after the bronchoscope is removed from the patient’s airway.
Results and Procedure-Related Complications
Bronchoscopy on CPAP was performed with minimal sedation (midazolam 2 mg intravenously) and with 300 mg of 1% lidocaine for laryngeal analgesia. We evaluated central airway dynamics during upright and supine positions at CPAP pressures of 0, 5, 7, 10, and 12 cm H2O. The airway lumen was evaluated first in the upright position during tidal respiration, then during forced expiration and cough. We observed that the degree of airway compression was normal during tidal respiration but became abnormal with forced exhalation and cough (see video on ExpertConsult.com) (Video III.13.3![]() ). These findings were worse in the supine position when collapse in the mainstem bronchi was near complete. Findings were consistent with the patient’s worsened symptoms at night. The degree of collapse improved with application of CPAP of 12 cm H2O (see video on ExpertConsult.com) (Video III.13.4
). These findings were worse in the supine position when collapse in the mainstem bronchi was near complete. Findings were consistent with the patient’s worsened symptoms at night. The degree of collapse improved with application of CPAP of 12 cm H2O (see video on ExpertConsult.com) (Video III.13.4![]() ). After airway dynamics had been evaluated, BAL was performed by sequential instillation and aspiration of 3 aliquots of 30 mL of physiologic saline solution, with the tip of the bronchoscope wedged in the right lower lobe bronchus. After BAL was completed, positive-pressure ventilation could have been maintained for at least 30 minutes after the procedure and discontinued if SaO2 were greater than 92% and the patient was without evidence of respiratory insufficiency.20 In our patient, however, CPAP was discontinued immediately after removal of the bronchoscope because we used CPAP assistance to determine the optimal pressure that maintained airway patency for EDAC, not for severe refractory hypoxemia. Monitoring continued until the effects of intravenous sedation had subsided and the gag reflex had returned. Nursing staff who monitored the patient during this period were aware that respiratory distress from hypoxemia or bronchospasm can occur after the scope is removed from the airway.
). After airway dynamics had been evaluated, BAL was performed by sequential instillation and aspiration of 3 aliquots of 30 mL of physiologic saline solution, with the tip of the bronchoscope wedged in the right lower lobe bronchus. After BAL was completed, positive-pressure ventilation could have been maintained for at least 30 minutes after the procedure and discontinued if SaO2 were greater than 92% and the patient was without evidence of respiratory insufficiency.20 In our patient, however, CPAP was discontinued immediately after removal of the bronchoscope because we used CPAP assistance to determine the optimal pressure that maintained airway patency for EDAC, not for severe refractory hypoxemia. Monitoring continued until the effects of intravenous sedation had subsided and the gag reflex had returned. Nursing staff who monitored the patient during this period were aware that respiratory distress from hypoxemia or bronchospasm can occur after the scope is removed from the airway.
Long-Term Management
Outcome Assessment
The patient was discharged home after he had been monitored for 30 minutes. He continued treatment with bronchodilators and pulmonary hygiene (postural drainage and percussion vest). Based on bronchoscopy findings and in light of evidence of improved airflow, symptoms, and atelectasis with the use of CPAP,24,25 CPAP 12 cm H2O was prescribed at night and intermittently during the day as conservative management of his EDAC.
Referrals
He was referred to an infectious disease specialist to determine whether his chronic Pseudomonas aeruginosa infection should be treated. He was also referred to a sleep center to further explore and potentially resolve issues of CPAP noncompliance.* A gastroenterologist was asked to readdress his GERD.
Discussion Points
1. Provide two indications for bronchoscopy on CPAP in this patient.
2. List two differential diagnoses for excessive dynamic airway collapse (EDAC).
3. Provide two reasons why CPAP might help a patient with expiratory central airway collapse.
Expert Commentary
The belief that real-time bronchoscopy can determine the severity of EDAC is based on visual perspectives and perceptions of changes in size or cross-sectional area across the respiratory cycle. This is a controversial concept. In clinical terms, it could be regarded as being “good enough.” However, this type of assessment has many limitations.58 Short excerpts of the patient’s bronchoscopy display many of the issues that will be raised in the following paragraphs.
It is worthwhile remembering that the airway does lengthen and dilate with inspiration and then progressively contracts to its original position by the end of expiration. Bronchoscopic appreciation of this change is gained by holding the scope in a fixed position, but as the object of interest moves away from the tip of the scope, it actually moves to a lower magnification power; thus perceptions of change are gained through unknown but lower values of magnification, distortion effects, unknown distance traveled by the point of interest, and perspective change (Figure 13-7). In addition, effects on cross-sectional area change are due to uncontrolled changes in lung volume associated with the depth of anesthesia/sedation effects on respiratory system mechanics, resistance effects of the bronchoscope, and effects of movement of mucus on ventilation distribution and regional lung volumes (Figure 13-8). These effects are not readily perceived or appreciated despite the use of modern digital imaging techniques, television screens, or computer display panels. Memory function and the level of experience of the bronchoscopist are also likely to change these perceptions.59 Thus perceived change in airway size should be treated cautiously, or at its best should be regarded as an appreciable underestimate.
Planar quantitative or morphometric bronchoscopy, which enables assessment of airway lumen size, is available with techniques such as color histogram mode techniques,58,60,61 but precise measurement is limited to the expiratory position because the peak inspiratory position may be difficult to define in complex lesions (Figure 13-9). The expiratory position is chosen because it can be touched by the bronchoscope (the zero position), and because airway collapse may be present across both inspiration and expiration (see video on ExpertConsult.com) (Video III.13.2![]() ), and it may be available over long distances. Thus, the bronchoscope can be entrapped by collapsed tissue, or perspective may be lost by angulation changes (see video on ExpertConsult.com) (Video III.13.3
), and it may be available over long distances. Thus, the bronchoscope can be entrapped by collapsed tissue, or perspective may be lost by angulation changes (see video on ExpertConsult.com) (Video III.13.3![]() ). Change in airway size at the point of interest cannot be measured without knowledge of the distance traveled during the respiratory cycle and without placement of calibration objects in the airways.*In addition, this method does require quantification of magnification characteristics and power of the particular bronchoscope selected. Post hoc analyses are necessary to attain quantitative measurements with strong intraobserver and interobserver agreement.60,61 Three-dimensional (3D) bronchoscopic reconstruction of the airways and morphometry across both phases of respiration represent an advance in determining precise relative change, but accurate distance measurements are still needed to allow quantitative measurements. Computing power will have to be substantial to enable real-time in vivo measurements.
). Change in airway size at the point of interest cannot be measured without knowledge of the distance traveled during the respiratory cycle and without placement of calibration objects in the airways.*In addition, this method does require quantification of magnification characteristics and power of the particular bronchoscope selected. Post hoc analyses are necessary to attain quantitative measurements with strong intraobserver and interobserver agreement.60,61 Three-dimensional (3D) bronchoscopic reconstruction of the airways and morphometry across both phases of respiration represent an advance in determining precise relative change, but accurate distance measurements are still needed to allow quantitative measurements. Computing power will have to be substantial to enable real-time in vivo measurements.
Reference points are required for comparative quantitative bronchoscopy assessments. Because the cricoid is a complete ring and a relatively rigid structure, reproducibility of measurement is high. Measurements in this region have strong relationships to lower airway measurements. However, the weak point of this technique is that intersubject variability is relatively high.62 This potentially makes grading of severity relatively weak, but it does provide a quantifiable comparative approach.
1. Bilton D. Update on non-cystic fibrosis bronchiectasis. Curr Opin Pulm Med. 2008;14:595-599.
2. Koh WJ, Lee JH, Kwon YS, et al. Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest. 2007;131:1825-1830.
3. Boiselle PM, Feller-Kopman D, Ashiku S, et al. Tracheobronchomalacia: evolving role of dynamic multislice helical CT. Radiol Clin North Am. 2003;41:627-636.
4. Joosten S, Macdonald M, Lau KK, et al. Excessive dynamic airway collapse co-morbid with COPD diagnosed using 320-slice dynamic CT scanning technology. Thorax. 2010. Jul 7. [Epub ahead of print]
5. Bateson EM, Woo-Ming M. Tracheo-bronchomegaly. Clin Radiol. 1973;24:345-358.
6. Baron KG, Smith TW, Czajkowski LA, et al. Relationship quality and CPAP adherence in patients with obstructive sleep apnea. Behav Sleep Med. 2009;7:22-36.
7. Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology. 2006;11:388-406.
8. Sucena M, Liistro G, Aubert G, et al. Continuous positive airway pressure treatment for sleep apnoea: compliance increases with time in continuing users. Eur Respir J. 2006;27:761-766.
9. Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383-1393.
10. Pang JA, Cheng A, Chan HS, et al. The bacteriology of bronchiectasis in Hong Kong investigated by protected catheter brush and bronchoalveolar lavage. Am Rev Respir Dis. 1989;139:14-17.
11. Tsang KW, Ho PI, Chan KN, et al. A pilot study of low-dose erythromycin in bronchiectasis. Eur Respir J. 1999;13:361-364.
12. Angrill J, Agustí C, de Celis R, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2002;57:15-19.
13. Ho PL, Chan KN, Ip MS, et al. The effect of Pseudomonas aeruginosa infection on clinical parameters in steady-state bronchiectasis. Chest. 1998;114:1594-1598.
14. Martínez-García MA, Soler-Cataluña JJ, Perpiñá-Tordera M, et al. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest. 2007;132:1565-1572.
15. Cortese DA, Prakash UBS, Stubbs SE. Technical solutions to common problems in bronchoscopy. In: Prakash UBS, editor. Bronchoscopy. New York: Raven Press; 1994:111-133.
16. Benditt JO. Novel uses of noninvasive ventilation. Respir Care. 2009;54:212-219.
17. Murgu SD, Colt HG. Treatment of adult tracheobronchomalacia and excessive dynamic airway collapse: an update. Treat Respir Med. 2006;5:103-115.
18. Goldstein RA, Rohatgi PK, Bergofsky EH, et al. Clinical role of bronchoalveolar lavage in adults with pulmonary disease. Am Rev Respir Dis. 1990;142:481-486.
19. Dellinger RP. Fiberoptic bronchoscopy in adult airway management. Crit Care Med. 1990;18:882-887.
20. Antonelli M, Conti G, Rocco M, et al. Noninvasive positive-pressure ventilation versus conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest. 2002;121:1149-1154.
21. Maitre B, Jaber S, Maggiore SM, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients: a randomized double-blind study using a new device. Am J Respir Crit Care Med. 2000;162:1063-1067.
22. Trachsel D, Erb TO, Frei FJ, et al. Use of continuous positive airway pressure during flexible bronchoscopy in young children. Eur Respir J. 2005;26:773-777.
23. Antonelli M, Pennisi M, Conti G, et al. Fiberoptic bronchoscopy during noninvasive positive pressure ventilation delivered by helmet. Intensive Care Med. 2003;29:126-129.
24. Wiseman NE, Duncan PG, Cameron CB. Management of tracheobronchomalacia with continuous positive airway pressure. J Pediatr Surg. 1985;20:489-493.
25. Adliff M, Ngato D, Keshavjee S, et al. Treatment of diffuse tracheomalacia secondary to relapsing polychondritis with continuous positive airway pressure. Chest. 1997;112:1701-1704.
26. Da Conceicao M, Genco G, Favier JC, et al. Fiberoptic bronchoscopy during non-invasive positive pressure ventilation in patients with chronic obstructive lung disease with hypoxemia and hypercapnia. Ann Fr Anesth Reanim. 2000;19:231-233.
27. Borowiecki B, Pollack CP, Weitzman ED, et al. Fibro-optic study of pharyngeal airway during sleep in patients with hypersomnia obstructive sleep-apnea syndrome. Laryngoscope. 1978;88:1310-1313.
28. Murgu SD, Pecson J, Colt HG. Bronchoscopy on noninvasive positive pressure ventilation: indications and technique. Respir Care. 2010;55:595-600.
29. Holanda MA, Fortaleza SC, Alves-de-Almeida M, et al. Continuous positive airway pressure effects on regional lung aeration in patients with COPD: a high-resolution CT scan study. Chest. 2010;138:305-314.
30. Khirani S, Biot L, Eberhard A, et al. Positive end expiratory pressure and expiratory flow limitation: a model study. Acta Biotheor. 2001;49:277-290.
31. Parr DG, Stoel BC, Stolk J, et al. Influence of calibration on densitometric studies of emphysema progression using computed tomography. Am J Respir Crit Care Med. 2004;170:883-890.
32. Sundaram P, Joshi JM. Tracheobronchomegaly associated tracheomalacia: analysis by sleep study. Indian J Chest Dis Allied Sci. 2004;46:47-49.
33. Martin J. Preparing and supporting patients undergoing a bronchoscopy. Nurse Times. 2003;99:52-55.
34. Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452-2460.
35. McCool FD, Rosen MJ. Nonpharmacologic airway clearance therapies: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:250S.
36. Dutau H, Maldonado F, Breen DP, et al. Endoscopic successful management of tracheobronchomalacia with laser: apropos of a Mounier-Kuhn syndrome. Eur J Cardiothorac Surg. 2011. Mar 5. [Epub ahead of print]
37. Murgu SD, Colt HG. Complications of silicone stent insertion in patients with expiratory central airway collapse. Ann Thorac Surg. 2007;84:1870-1877.
38. Wright CD, Grillo HC, Hammoud ZT, et al. Tracheoplasty for expiratory collapse of central airways. Ann Thorac Surg. 2005;80:259-267.
39. Chhajed PN, Aboyoun C, Malouf MA, et al. Management of acute hypoxemia during flexible bronchoscopy with insertion of a nasopharyngeal tube in lung transplant recipients. Chest. 2002;121:1350-1354.
40. Matsushima Y, Jones RL, King EG, et al. Alterations in pulmonary mechanics and gas exchange during routine fiberoptic bronchoscopy. Chest. 1984;86:184-188.
41. Klech J, Pohl W. Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Report of the European Society of Pneumology Task Group. Eur Respir J. 1989;2:561-585.
42. Aoyama K, Yasunaga E, Takenaka I, et al. Positive pressure ventilation during fibreoptic intubation: comparison of the laryngeal mask airway, intubating laryngeal mask and endoscopy mask techniques. Br J Anaesth. 2002;88:246-254.
43. Lindholm CE, Ollman B, Snyder GV, et al. Cardiorespiratory effects of flexible fiberoptic bronchoscopy in critically ill patients. Chest. 1978;74:362-368.
44. Pue CA, Pacht ER. Complications of fiberoptic bronchoscopy at a university hospital. Chest. 1995;107:430-432.
45. Payne CBJr, Goyal PC, Gupta SC. Effects of transoral and transnasal fiberoptic bronchoscopy on oxygenation and cardiac rhythm. Endoscopy. 1986;18:1-3.
46. Katz AS, Michelson EL, Stawicki J, et al. Cardiac arrhythmias, frequency during fiberoptic bronchoscopy and correlation with hypoxemia. Arch Intern Med. 1981;141:603-606.
47. Albertini R, Harrel JH, Moser KM. Hypoxemia during fiberoptic bronchoscopy. Chest. 1974;65:117-118.
48. Langmarc EL. Serum lidocaine concentration in asthmatics undergoing research bronchoscopy. Chest. 2000;117:1055-1060.
49. Gall H, Kaufman CM. Adverse reactions to local anesthetics: analysis 7 of 179 cases. J Allergy Clin Immunol. 1996;97:933-937.
50. Stolz D, Chhajed P, Leuppi J, et al. Nebulized lidocaine for flexible bronchoscopy. Chest. 2005;128:1756-1760.
51. Reed A. Preparation of the patient for awake flexible fiberoptic bronchoscopy. Chest. 1992;101:244.
52. Baram D, Smaldone G. Tracheal collapse versus tracheobronchomalacia: normal function versus disease. Am J Respir Crit Care Med. 2006;174:724.
53. Guerin C, LeMasson S, de Varax R, et al. Small airway closure and positive end-expiratory pressure in mechanically ventilated patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155:1949-1956.
54. Schwab RJ, Pack AI, Gupta KB, et al. Upper airway and soft tissue structural changes induced by CPAP in normal subjects. Am J Respir Crit Care Med. 1996;154(4 Pt 1):1106-1116.
55. Dellacà RL, Rotger M, Aliverti A, et al. Noninvasive detection of expiratory flow limitation in COPD patients during nasal CPAP. Eur Respir J. 2006;27:983-991.
56. Lourens MS, van den Berg B, Verbraak AFM, et al. Effect of series of resistance levels on flow limitation in mechanically ventilated COPD patients. Respir Physiol. 2001;127:39-52.
57. Davis S, Jones M, Kisling J, et al. Effect of continuous positive airway pressure on forced expiratory flows in infants with tracheomalacia. Am J Respir Crit Care Med. 1998;158:148-152.
58. Murgu S, Colt HG. Morphometric bronchoscopy in adults with central airway obstruction: case illustrations and review of the literature. Laryngoscope. 2009;119:1318-1324.
59. Masters IB, Chang AB. Tracheobronchomalacia in children. Expert Rev Respir Med. 2009;3:425-439.
60. Masters IB, Eastburn MM, Francis PW, et al. Quantification of the magnification and distortion effects of a pediatric flexible video-bronchoscope. Respir Res. 2005;6:16.
61. Masters IB, Eastburn MM, Wootton R, et al. A new method for objective identification and measurement of airway lumen in paediatric flexible videobronchoscopy. Thorax. 2005;60:652-658.
62. Masters IB, Ware RS, Zimmerman PV, et al. Airway sizes and proportions in children quantified by a video-bronchoscopic technique. BMC Pulm Med. 2006;6:5.
* Other direct signs of bronchiectasis include tram tracks, string of pearls, lack of tapering, and visualization of the airway within 1 cm of coastal/adjacent mediastinal pleura.
† Bronchial wall thickening is defined as thickness of the bronchial wall that is >0.5 times the diameter of the adjacent vertical pulmonary artery.
‡ Usually an autosomal recessive disorder characterized by mirror image organ arrangement, bronchiectasis, and sinusitis due to congenital reduction or absence of ciliary function.
* ABPA is characterized by a history of asthma, immediate skin test reactivity to Aspergillus antigens, precipitating serum antibodies to Aspergillus fumigatus, serum total IgE concentration greater than 1000 ng/mL, peripheral blood eosinophilia >500/mm3, lung infiltrates on chest x-ray or chest HRCT, central bronchiectasis on chest CT, and elevated specific serum IgE and IgG to A. fumigatus.
† Images are initially obtained using a standard radiation dose technique at end inspiration (170 mAs, 120 kVp, 2.5-mm collimation, high-speed mode, and pitch equivalent of 1.5). This is followed by a low-dose examination (40 mAs, 120 kVp, 2.5-mm collimation, high-speed mode, and pitch equivalent of 1.5) during the dynamic expiratory phase of respiration.
‡ Images obtained during this study were conducted at a dose of 7.2 mSv, which is comparable with the dose received during a standard CT chest.
* The severity of airway narrowing in expiratory central airway collapse describes the degree of airway collapse during expiration, as assessed by bronchoscopy or radiographic studies, and can be classified as follows: 1, Normal: expiratory collapse of less than 50%; 2, Mild: expiratory airway collapse of 50% to 75%; 3, Moderate: expiratory airway collapse of 75% to 100%; and 4, Severe: expiratory airway collapse of 100% (the airway walls make contact).
* Of note, this was studied in patients suffering from obstructive sleep apnea (OSA), not from EDAC.
* In non–cystic fibrosis bronchiectasis, Haemophilus influenzae has been the most common pathogen isolated, followed by Streptococcus pneumoniae and Pseudomonas aeruginosa. Less common pathogens include Enterobacter, Nocardia, Staphylococcus, and nontuberculous mycobacteria.
† This bronchoscopic technique can also be used to explore tracheoesophageal or bronchoesophageal fistulas, as well as tracheobronchomalacia.
* Clinicians, when performing these types of studies, should take into account the potential effects of radiation exposure in a given individual.
* The mechanism of action probably is the result of improved mucus flow, increased ciliary motility, and enhanced cough.
* This is usually a subjective assessment during bronchoscopy; a more accurate analysis of the still images obtained during bronchoscopy and quantification of the degree of airway narrowing (aka morphometric bronchoscopy) can be performed post procedure with the use of image processing software.
† Procedure-related hypoxemia occurs after insertion of the bronchoscope through the glottis, which causes partial tracheal obstruction and ventilation-perfusion mismatch. It is also caused by excessive suctioning, which reduces end-expiratory volume and promotes alveolar closure.
* With the exception of the gastrointestinal system, side effects are primarily dose related. Most lidocaine-related side effects involve the central nervous system—seizures, tremors, dysarthria, ataxia, hallucinations, nystagmus, and memory impairment; the cardiovascular system—sinus slowing, asystole, hypotension, and shock; and the gastrointestinal tract—nausea, vomiting, and anorexia. If an allergy to a local anesthetic belonging to the ester class is found, then generally speaking, an anesthetic from the amide class may be used, and vice versa.
* Reasons for CPAP noncompliance may include true anatomic reasons (beards, mustaches, or facial irregularities), as well as poor mask fit, leaks, humidifier problems, high flow impeding exhalation, and nasal congestion.
* It should be noted, however, that oxygen supplementation alone is not the standard of practice in severe respiratory conditions with severe hypoxemia. This population might be better served by invasive or noninvasive positive-pressure ventilation (NPPV), regardless of their need for bronchoscopy. To our knowledge, no head-to-head trials have compared NPPV versus endotracheal intubation for severely hypoxemic patients requiring bronchoscopy.
* This is possible if no associated increased work of breathing is seen to result from decreased pulmonary compliance at increased lung volumes.