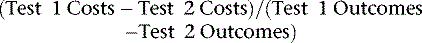Chapter 24 Cost-Effectiveness of Myocardial Perfusion Single-Photon Emission Computed Tomography
INTRODUCTION
Since peaking in the mid-1960s, reductions in mortality from coronary artery diseases have approached 50%.1 Although a number of reasons have been put forth for this marked decline in death rates, a proportion of this reduction can be attributed to early and effective diagnostic testing techniques that result in improved outcomes for at-risk patients. Along with marked improvements in outcome, procedural utilization rates have skyrocketed. The current focus on the part of public and private health care payers is to contain “out of control” health care costs. Health care payers have reacted by focusing on programs to decrease reimbursement levels and to manage utilization of major growth procedures such as myocardial perfusion single-photon emission computed tomography (SPECT).2 In fact, myocardial perfusion SPECT has been one of the largest growing procedures and is estimated to encumber approximately 2% of annual Medicare expenditures. To this end throughout the last few decades, there have been shifting foci between marked improvements in disease detection with medical advances in new technology and the reverberating excess expenditures associated with such developments. As a result, there has been a focus on developing rational approaches to containing health care costs and curbing unrestrained spending. The development of evidence-based medicine is an attempt to set guidelines for thresholds of evidence to justify the cost of a procedure. The bar has been set very high for diagnostic procedures where a test must now result in a net health improvement in clinical outcome (i.e., be effective). This is at the heart of cost-effectiveness analysis: striking a balance between spending and value. For a diagnostic test in cardiology to be accepted and receive widespread utilization, using evidence-based standards, it must have a clear and demonstrated economic and clinical incremental value when compared with other modalities. Nuclear cardiology has a clear advantage with regard to setting higher standards of evidence, owing to the large (unparalleled) body of evidence from multicenter, observational series and randomized or controlled clinical trials from statistically powered, diverse patient populations. The current chapter will provide a synopsis of available evidence of gated myocardial perfusion SPECT, as well as review the guiding principles applied in the development of cost-effectiveness evidence.
HEALTH CARE COSTS FOR CARDIOVASCULAR DISEASE
In the United States, total health care costs for cardiovascular disease approached $450 billion in 2007.3 In fact, lifetime costs for one symptomatic patient are reported at $1 million dollars.4 Given that nearly 19 million patients have chronic stable angina, these costs of care are astronomical. It is then no wonder that the Medicare Panel Expenditure Survey ranked ischemic heart disease as the most expensive condition.5 Recent estimates from the American College of Cardiology have noted that approximately 38% of all payments to cardiologists were for echocardiography and nuclear imaging.6 Estimates from the 34th Bethesda Conference on atherosclerotic imaging noted an estimated 40 million noninvasive cardiac tests were performed during each of the past few years,7 with total costs of over $1 billion in the year 2000.3 For example, every year, a total of 6 million exercise electrocardiograms are billed. In the area of imaging, approximately 8.5 million nuclear scans are performed.6 A report from the American College of Radiology reported annual growth rates for myocardial perfusion imaging exceeding 30% for cardiologists.8 The largest growth sectors have been in the outpatient setting where reimbursement strategies have been more favorable. Given our aging population, as well as the dramatic increases in diabetes and obesity, it is estimated that there will be a continuing need for diagnostic imaging services to identify patients at risk for coronary artery disease (CAD). However, the challenge is to adequately service this growing population of at-risk patients with limited financial means within our current environment.
With the recent history of marked growth in nuclear cardiology, payers consider this test a “big target” for focusing cost-reduction efforts—in many cases, encouraging shifting to lower-cost procedures such as stress echocardiography. Thus the current review hopes to frame the utilization as supported by effective evidence to guide medical decision making, as well as to consider the advantages of a more noninvasive center approach to contain large health care costs associated with invasive coronary angiography. Similar to nuclear imaging, growth rates for cardiac catheterization have increased 300%+ with, on average, a third of catheterizations being considered inappropriate or not supported by accepted clinical indications.9 From a clinical point of view, it is easy to envision that an overuse of coronary angiography can lead to higher rates and in some cases the unnecessary use of coronary revascularization procedures, defined as utilization that is not supported by evidence noting substantive improvements in outcome. Although SPECT imaging growth rates have been high, the cost savings resulting from decreased angiographic utilization, as will be illustrated later in this chapter, have been reportedly quite substantial. This is particularly true of patients with stable chest pain symptoms who are referred to diagnostic left heart catheterization.
Prior to discussing the available economic evidence, it will be helpful to define the calculation of cost-effectiveness and related standards for health policy decision making. An incremental cost-effectiveness ratio (ICER) requires not only necessary information on cost but also integrated evidence on diagnostic test accuracy and the ensuing therapeutic benefit of treatment.10 Cost-effectiveness analyses are increasingly favored within health care systems where health care resources are increasingly finite; ICER is a tool to assist decision makers to assess and devise cost containment and value within their health care system. ICER is defined as an incremental or marginal cost-effectiveness ratio because it compares more than one test, therapy, or patient management approach. In general for cardiac imaging modalities, there are a number of published cost analyses.7,11–26
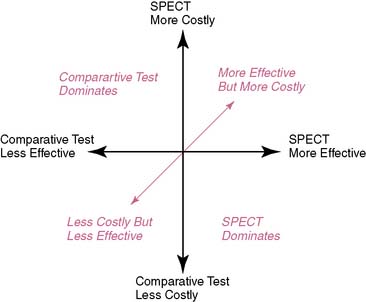
A challenge with this reasoning is that for many comparisons, nuclear cardiology is a more expensive test. However, more expensive procedures can be cost-effective if they reduce induced costs or are substantially more effective. There are several scenarios in which nuclear cardiology may result in a favorable ICER. For the first case, if nuclear cardiology is less expensive and equally effective, then the result could be a favorable ICER. A favorable ICER could also result for nuclear cardiology testing even if it is initially more expensive but results in a reduction in downstream procedures with equally effective outcomes results. And finally, nuclear cardiology testing could be substantively more costly but also have a favorable ICER should it result in decidedly better outcome results. All of the above are simplified examples of how nuclear cardiology could be cost-effective; greater details of the methods for these types of analyses will be provided in the following discussions (Fig. 24-1).
EVALUATING PROCEDURAL COST
Although much has been made of the variability in imaging costs, it is helpful to examine data on the unit operating cost of a variety of cardiac diagnostic procedures.7 There are two methods that can be applied to estimating cost: top-down and bottom-up. A top-down cost approach uses adjusted (technical and professional) charges and is calculated as the charge × the hospital-specific cost-charge ratio (set by the Center for Medicare and Medicaid Services). Recently, bottom-up costs have been employed and involve calculating both the fixed and variable labor costs (e.g., supplies, equipment, and labor costs). Using this type of calculation, procedural cost is predominately driven by laboratory volume, where high-volume laboratories have lower cost per test, achieving economies of scale with increasing volume. For SPECT imaging, cost is also influenced by noncardiac procedural volume. The use of quantitative scoring systems, such as calculating left ventricular ejection fraction, have lower labor inputs and are generally lower cost than systems utilizing greater labor components, especially those including physician labor. In large part, the use of quantitative scoring systems generally not only lowers initial costs but (because of improved reproducibility) also aids physician diagnostic confidence and the impact on induced posttest costs.27
For SPECT imaging, the cost of radioisotopes and pharmacologic stress agents is also included in the unit cost of a test. Recently, the commonly used technetium-99m sestamibi became generic, allowing for lower radioisotope costs to be realized. Factors that also influence test cost include labor and equipment necessary for laboratory standards or certification. A major component of the unit operating cost is equipment, where costs can vary widely from less than $1 million to up to $3 million for magnetic resonance, positron emission tomography, or multislice computed tomography scanners. Despite these high cost estimates, discounted prices and leasing agreements are common and reduce equipment prices substantially for most imaging modalities. The equipment space (e.g., cost at total square footage) and any necessary requirements (e.g., added concrete thickness for magnetic resonance imaging) for the equipment should be included in the equipment or fixed cost estimate. In the final step in calculating costs, estimates are then discounted and inflation-corrected to a given time period in order to establish a common metric for individual procedural estimates and for comparisons to other modalities.28,29
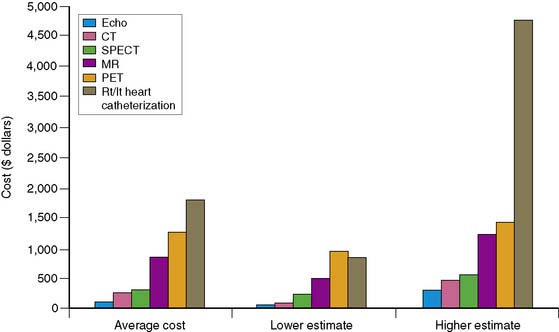
A review of current estimates for diagnostic procedures has been reported in several publications and is depicted in Figure 24-2.7 Cost estimates should be viewed for all the modalities as “guesstimates,” because there have been few attempts to calculate the true unit operating cost of each procedure. New imaging modalities in general have higher initial costs, but with greater experience and efficiency and equipment price reductions, cost estimates will decrease. Inexpensive tests include those that are “low tech” such as exercise electrocardiography. When integrating the available data, nuclear cardiology procedures appear to be in the midrange of diagnostic procedure cost estimates.
Adding Downstream Costs
Although rarely considered, the total cost of a procedure not only should include the upfront costs but should be totaled throughout the episode of care (importantly, including induced costs). Drummond and Jefferson30 have advised that any economic evaluation should consider the impact of test use on downstream resource consumption, including both additional procedure use and treatment costs. From a societal point of view, cost estimates should also consider those consumed by the patient, or indirect costs. By definition, indirect costs include travel time, out-of-pocket expenses, and days lost from work for both the patient and other caregivers. Downstream costs should also consider the induced costs of incidental findings, and this latter point will be a more critical cost component in vascular imaging. More recently, reports have been published on the radiation exposure associated with a number of cardiac imaging procedures. The associated cancer risk is also one component of cost that is rarely included but should be considered as a component of induced cost.31
Although precise delineation of induced costs may be difficult to enumerate, there is an expected proportional relationship between test results and expected costs of care.32 That is, greater costs are expected for higher-risk test results (e.g., significant left ventricular dysfunction and multivessel or severely abnormal perfusion abnormalities), particularly in higher-likelihood patients. As expected, this latter population will require higher rates of coronary angiography, as well as revascularization procedures and (because of elevated, cumulative event rates) greater hospital admissions for acute coronary syndromes and lost life years as a result of premature death. Estimates of induced costs have revealed that the costs of downstream hospital and procedural care can be approximately 10- to 100-fold higher than the initial test cost itself.32
An easy method for estimating downstream care costs is to calculate a test’s negative and positive disease rates, and this may be used to define cost waste induced by the procedure. True negative (lowest cost cohort) and positive tests result in effective resource consumption or costs as a result of their accurate identification or exclusion of disease. Of course, the economics of test misclassification can be affected by changes to the positivity threshold, the prevalence of disease in the population, and the length of follow-up for evaluating, in particular, false-negative results.33 By definition, a false-positive test result is documented when a patient with abnormal SPECT results undergoes coronary angiography revealing normal or insignificant disease (Table 24-1). Of course, the defining of an intermediate stenosis that elicited a flow limitation may be wrongly termed a false positive. Yet it may be viewed as a false positive insofar as the abnormal nuclear finding failed to define revascularizable disease and, as such, from that perspective may be termed a false positive. This latter point suggests that the defining of a false positive should be used cautiously as a measure of cost inefficiency. Despite this, when downstream testing results in a lack of confirmatory findings, then the initial results misclassified the expected disease state in that patient. Repeated, redundant, and unnecessary testing contributes to high costs of care. Although false-positive results can be difficult to assess, many studies do not allow a sufficient time period for follow-up to discern the rate of false-negative test results. A false-negative test result would be expected to occur when a patient with initially normal findings has an adverse event, or disease is confirmed at a later date. There are additional challenges with negative findings: They may change a patient’s health-seeking behaviors, resulting in delays to treatment as well as litigation costs that are rarely considered in an economic analysis.34 It is impossible to expect that any test would perfectly classify patients 100% of the time, and there are no standards for acceptability of false-negative and false-positive findings; however, a low rate of false-negative results is preferable (i.e., perhaps < 10%), because false-negative results are more costly and harmful to the patient.
Table 24-1 Cost Advantages Using Myocardial Perfusion Single-Photon Emission Computed Tomography
| High sensitivity excludes disease more accurately and avoids the need for a secondary test if a less accurate primary test is used. |
| High sensitivity leads to fewer false-negative test results and avoids the cost of future events in patients with undiagnosed disease. |
| High specificity reduces the number of false-positive test results and consequent downstream testing. |
| Additional prognostic information avoids the need for further prognostic testing and focuses high-cost interventional care on patients with advanced disease with the most to gain in terms of improvement in clinical outcome. |
DEFINING INCREMENTAL EFFECTIVENESS
In addition to delineating the costs of a diagnostic procedure, there are a variety of methods to define its effectiveness. There have been recent active discussions within health policy arenas as to what is the correct method to define the effectiveness of a diagnostic procedure. Historically, the diagnostic accuracy of a test has been the mainstay for gaining U.S. Food and Drug Administration (FDA) approval. More recently there has been an abundance of evidence on the prognostic accuracy of testing, including a large body of evidence on risk stratification with nuclear cardiology that is discussed in Chapters 15 and 16. A new standard of net improvement in health outcome has been introduced as a standard upon which to guide reimbursement. Where evidence for a given modality meets the criteria of net improvement in health outcome, then reimbursement should be favored for that given test or patient indication. In terms of defining what a net improvement in outcome means, patients must be “better off” after undergoing the diagnostic test when compared to no testing or an alternative modality. This type of examination of the effectiveness of a diagnostic test has to do with how the procedure is used to guide subsequent decisions on starting, stopping, or modifying therapies.35 Other positive improvements in health outcomes following a diagnostic test include:
DEFINING INCREMENTAL COST-EFFECTIVENESS RATIO
For many clinicians, cost-effectiveness analysis can seem a bit obtuse; however, it is simply a ratio that reflects the amount of resources needed to change a patient’s outcome. In other words, it is used to reflect the intensity of management in relation to any given outcome achieved. The aim of a cost-effectiveness analysis is to reflect or mirror clinical decision making where physicians make choices based on the information content and, generally, the invasive nature of the procedure (i.e., a surrogate for cost). A cost-effectiveness ratio (ICER) is most commonly expressed in cost per life year saved or, if adjusted by patient functional gain, in a modification as cost per quality-adjusted life year saved. For ICERs, cost per life year saved is rapidly becoming a common metric for comparisons to other medical interventions. A compendium of ICER data can be compiled in the form of a league table for comparisons to other medical and nonmedical procedures, therapies, and so forth.32–57 Such comparisons may be more relevant to the health care policy analyst, but for the clinician, the link to an ICER for any given diagnostic test is best understood by a combination of factors that integrate accuracy, and resulting treatment efficacy and management intensity and timing. That is, a diagnostic test that is effective at identifying patients whose ensuing risk may be altered by aggressive therapeutic intervention will result in an aversion to more costly, end-stage care, thus resulting in cost-effective care for similar patients.
The theoretical approaches to adding value and improved cost-effectiveness with noninvasive testing are noted in Table 24-2. Tests that are ineffective result in redundant testing with rising cost-ineffective care. Simply stated, diagnostic tests that have high rates of false-negative and false-positive test results have excessive cost waste and result in ICERs that are not economically attractive for the health and well-being of our society. High rates of false-positive tests lead to greater use of unnecessary coronary angiography, and high false-negative rates lead to higher rates of acute coronary syndromes in patients with initially negative results. This inefficiency leads to patient care that does not improve outcome and is cost inefficient.
Table 24-2 Theoretical Approaches to Adding Value and Improved Cost-Effectiveness With Noninvasive Testing
| Adding Value but Minimizing Cost Through Improved Test Accuracy Combination of Physiologic/Anatomic Assessment Adds to Cost Efficiency |
| For example, gated SPECT imaging, including evaluation of myocardial perfusion and global ventricular function |
| Containing Diagnostic Test Costs |
| For example, initial exercise electrocardiography followed by a cardiac imaging test (e.g., SPECT) in patients who have indeterminate or mildly positive ST-segment changes; lower overall evaluation costs, especially for patients with a normal resting 12-lead electrocardiogram |
| Tiered approach to testing, with a selective use of higher cost test for example, SPECT to those higher-risk patients |
| Lower Costs Tests Applied to Non-High-Risk |
| Selective coronary angiography in patients with SPECT ischemia |
| Using SPECT as a gatekeeper to conventional angiography, especially for patients with stable chest pain symptoms. The results of this strategy could be: |
| A reduction in procedural complications, hospital costs, and overall “workup” cost for a diagnostic catheterization; in-laboratory complications are ~1%. |
An incremental or marginal cost-effectiveness ratio includes a comparison of the differences in cost and effectiveness of more than one imaging modality. As previously stated, an ICER includes the calculation of upfront and downstream cost differences as well as near-term and/or long-term (i.e., life expectancy) outcome differences. Based on early work done on the evaluation of renal dialysis programs, the threshold for economic efficiency is set at less than $50,000 per life year saved (LYS), with many countries setting thresholds as low as less than $20,000 per LYS.7,32–57 It does appear that the standards for an ICER are more appropriately designed for the evaluation of therapeutic regimens and, in some cases, screening programs.7
For clinicians, previous discussions on risk stratification have particular relevance and are the critical points for affecting cost-effective care for patients. That is, when a test risk stratifies, it also is a measure of the intensity of resources required to manage a given risk cohort and provides insight into the expected costs of care. There is a directly proportional relationship between risk and cost. Each event that is estimated in the many published reports should be equated to a given “high ticket” item in health care resource consumption (a myocardial infarction costs on average $14,000; chest pain hospitalization ≅ $6000, to name a few). In Chapters 15 and 16, there are reviews of the large body of evidence on risk stratification with SPECT imaging results. This compendium of data reveals that gated myocardial perfusion imaging is highly accurate for estimating major adverse cardiac events, including cardiac death and nonfatal myocardial infarction. This accuracy results in cost-effective care by streamlining the need for additional testing, resulting in more efficient care. In a recent review of the literature by Underwood and colleagues,16 the rate of false-negative test results is minimal at around 12%, while the rate of false-positive results is around 26%. Opponents to SPECT imaging have been critical of this higher rate of false-positive results (i.e., diminished specificity). One should remember that flow limitations would be observed at subcritical lesions, and thus the calculation of diagnostic specificity using an obstructive lesion threshold of 70% or greater would be less valuable than understanding the ensuing prognosis associated with any given test abnormality. Accordingly, in many cases, diagnostic accuracy is not helpful in understanding the clinical or cost-effectiveness of a procedure. Risk stratification, however, has tremendous value in the course of everyday laboratory practice, in which the vast majority of patients undergoing SPECT imaging will have normal perfusion and function results, thus receiving posttest “low-cost” care. That is, the necessity for additional testing, in the setting of normal gated SPECT imaging, is minimal, and this information should be important to large health care payers and systems alike.
Mansley and McKenna51 illustrate how one may design an ICER using five clear-cut steps:
USE OF INTERMEDIATE OUTCOME MEASURES
A critical challenge of determining cost-effective testing is that the traditional definition includes examination of the incremental differences in cost per life year saved. As many clinicians involved in diagnostic testing understand, the use of any imaging modality does not directly impact patient survival but is used to identify risk. The ensuing risk is affected by the initiation of life-saving therapeutic intervention. An additional challenge with the use of cost-effective decision models is the lack of long-term data that may be used to estimate changes in life expectancy. Many individuals have advocated the use of intermediate outcome measures for diagnostic testing in order to emulate how clinicians utilize test content. This would include the derivation of economic models that determine the cost to identify or avert a major adverse cardiac event at 2 to 5 years after testing. As Hunink and Krestin recently described, outcome measures should reflect the physician’s decision-making process.52 In a review of the methods applied to establish cost-effectiveness of a diagnostic test, the link between diagnosis and end-stage care is often disparate or unrelated; most patients undergoing testing have nearly 2 decades of life years remaining.53 From a practical standpoint, not all test abnormalities are acted on by the overseeing physicians. For example, one-third to two-thirds of patients with SPECT perfusion abnormalities are referred to coronary angiography, so the cost-effective models would be highly responsive and influenced by the intensity of posttest management, including both aggressive antiischemic and risk-factor modification therapies.
One of the major drawbacks for using an intermediate outcome measure is the lack of standardization or accepted metric (such as with cost per life year saved) upon which to derive comparisons across other health care choices. Nevertheless, there are several approaches that can be employed to discern whether ICER results are economically attractive societal health care choices. That is, the cost to avert a death or myocardial infarction should be less than the actual cost of these events, or in the range of $20,000 to $50,000 per patient. Another approach would be to examine the population benefit of this type of ICER calculation for a given cohort of 1000 patients. The resulting ranges would appear to be in the range of $250,000 to $500,000 (or under) to detect cardiac death or myocardial infarction.32
High-Risk Cost-Effectiveness Models
The concept of a high-risk cost-effectiveness model was introduced a number of years ago, and its utility appears to be highly relevant to the body of prognostic evidence with SPECT imaging.53,54 A high-risk cost-effectiveness model is defined using the concept of proportional risk reduction as it is directly related to the underlying hazard in the population. That is, higher-risk patients receive a greater proportional therapeutic benefit from intervention. Using this reasoning, the more clinically beneficial the treatment, the more cost-effective care patterns will ensue. Or, in terms of imaging modalities, clinical effectiveness and cost-effectiveness generally parallel one another. For the clinician who struggles to find relevance in an ICER, this type of reasoning can be very helpful. Especially for nuclear cardiology procedures, the wealth and depth of evidence on risk assessment can become a template for understanding ICERs for SPECT. When SPECT is highly effective in any given patient cohort (intermediate-risk patients, stable chest pain patients, to name a few), one would also expect it to be cost-effective, since these two measures generally parallel one another. Clinicians should remember that clinical events are “big ticket” items that have direct cost implications, and it may be helpful to learn general ballpark estimates of major adverse events such as myocardial infarction (average cost = $14,000) or cardiac death (average societal cost = $10,000 per lost year of life).
ICERs have been evaluated using other imaging modalities, and the results reveal that economically attractive ratios can be achieved when identifying an optimal patient cohort, particularly where disease is prevalent48 or for relevant age subsets.55,56 For SPECT imaging, these results would yield attractive ICERs in intermediate high-risk patients (as compared to low-risk individuals) or in elderly (as compared with middle-aged) patients. This latter exercise also illustrates further how we can extrapolate ICERs from our existing data on risk stratification. That is, for every prognostic series illustrating effective risk stratification, we can envision that this subset also derives a cost-effective benefit from testing. In a recent review of the prognostic value of SPECT imaging,58 we noted that the differences in risk between low- and high-risk test results ranged from approximately 0.5% to 1.6% to 3% to 12% for suspected coronary disease to multivessel coronary disease patient subsets. ICERs would be favorable in patients with high-risk, multivessel disease cohorts (i.e., < $20,000 per life year saved [LYS]). These results would also be applicable to high-risk-disease equivalent patients such as diabetics or those with known cardiovascular disease (see Chapter 22). By comparison, in suspected disease subsets, cost-effective subsets (i.e., cohorts with an ICER < $50,000/LYS) of this population may include only those patients older than age 65 years or those with typical angina, to name a few.
ECONOMIC IMPACT OF NUCLEAR CARDIOLOGY AS A GATEKEEPER TO THE DIAGNOSTIC CATHETERIZATION LABORATORY
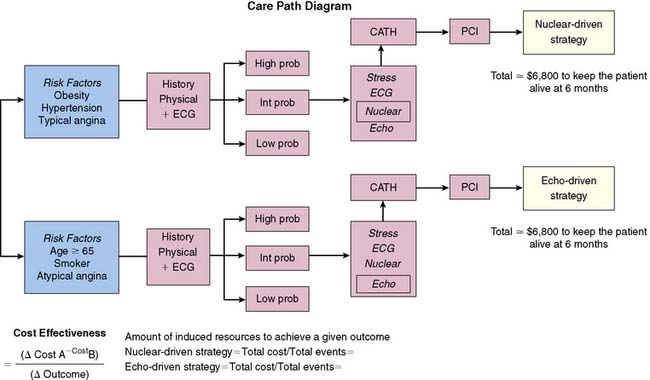
This section of the chapter will deal specifically with the available evidence on ICERs, as well as the simplistic comparison of marginal cost savings with SPECT imaging. Generally, the available economic data with SPECT imaging as compared with other diagnostic testing techniques—including exercise electrocardiography, echocardiography, and coronary angiography—evaluate costs as a result of a given management strategy resulting from the initial procedure. An example of how such an evaluation would be constructed is illustrated in Figure 24-3 and compares an echocardiographic versus SPECT imaging management approach. When evaluating the economic literature, one can see that there has been a growing use of more sophisticated ICER models over time. Older reports examined simple differences in test cost, but newer strategies employ models examining near-term and long-term ICERs.
Cost Minimization or Savings
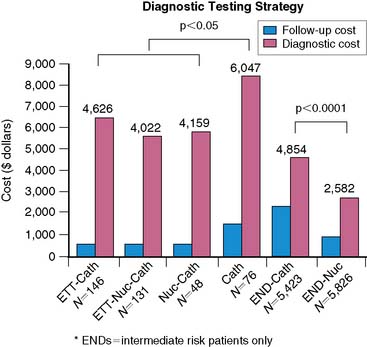
One form of an ICER is a cost-savings model. When examining the calculation of an ICER, if the denominator is equivalent, then the economic advantages are leveraged based solely on cost differences (i.e., savings). Of course, this point is critical to most cost-savings models that consider resource consumption alone and fail to account for differences in patient outcome. Thus, a true cost-savings analysis should compare equivalent test choices that achieve similar outcomes. An example of this type of analysis was published from the Economics of Noninvasive Diagnosis (END) study group, in which 3-year rates of cardiac death or nonfatal myocardial infarction were statistically similar (Fig. 24-4).15 This type of economic analysis is perhaps the most intuitive, with the greatest potential for ease of understanding and assimilation of evidence into clinical practice. As a result, it is also one of the most commonly misapplied. That is, often the comparisons are based on cost only, without considering whether the two choices are equivalent or have similar diagnostic or prognostic accuracy. The term cost savings is also called cost minimization or cost efficiency because of the goal of achieving a streamlined pattern of care. An additional component to this type of analysis is that it also considers not only the upfront cost of a procedure but also the downstream use induced by the procedure itself.
Figure 24-2 explores the amount of resource consumption based on nuclear- and echocardiographic-driven diagnostic strategies. Using this example, patients with abnormal scans are referred for coronary angiography directly as a result of the SPECT results. Thus, including the cost of cardiac catheterization is a major component of calculating the costs of care. Although the added cost of coronary angiography was effective in identifying a patient with coronary disease in this illustration, false-positive costs, defined as an abnormal test but with normal coronaries, are considered cost waste in this diagnostic algorithm. Tests that are highly accurate also minimize cost waste, including a reduced false-positive rate. Therefore, the clinician evaluating not only cost evaluations but also diagnostic accuracy data may make inferences about cost waste by knowing the diagnostic specificity (= true negative/[true negative + false positive]) of a procedure. Since specificity gives us a false-positive rate, any laboratory can focus its daily laboratory practice of “cath correlations” on minimizing false positives, thus creating laboratory cost efficiency.
Furthermore, false-negative results are also a cost waste within this diagnostic construct. False-negative tests may be defined by calculating diagnostic sensitivity. However, a false negative is associated with a substantial economic burden to society, since it usually occurs when a patient presents downstream for additional testing or an acute coronary syndrome or death. In the latter case, average costs of this type of event are in the tens of thousands of dollars, with a great economic burden to society. To understand a false-negative rate, one must look beyond the near term and evaluate an adequate length of follow-up (e.g., 2 to 3 years). Although this is time-consuming for most laboratories, recent evidence from a community evaluation of prognosis with SPECT revealed that the overall false-negative rate is exceedingly low at less than 1%.59 Using this type of analysis, most laboratories in the course of their quality evaluations may guesstimate their false-positive and false-negative or induced cost waste as well as their effective costs. The goal of this type of analysis would then be to optimize effective costs and minimize cost waste. This can be effective evidence to guide health care payer contracting.
One can then synthesize the published evidence about the cost savings of nuclear imaging based on several key publications from Cedars-Sinai Medical Center and from the Economics of Noninvasive Diagnosis multicenter study group.15,60 One strategy that has been effective at realizing approximately 30% cost savings is that of limiting diagnostic catheterization to only those with inducible ischemia (i.e., conservative management or ischemia-guided care). This type of care is based on large bodies of evidence noting that the extent and severity of ischemia may be used to guide the proportional therapeutic benefit of risk reductions. For stable angina populations commonly referred to SPECT imaging, those indications for percutaneous coronary interventions noted in the recent stable angina guidelines include measures of the amount and severity of perfusion defects. Thus, by limiting referral to catheterization to only those with ischemia, a clinically effective paradigm is followed by a cost-efficient pattern of care. Cost savings are further realized for those patients with normal perfusion studies, inasmuch as intervention in this latter cohort is not associated with changes in outcome. Of course, this type of care is based on generalities that may not fit a given patient; other considerations such as exercise capacity and past medical history should also be considered in the management of stable chest-pain patients. However, this type of cost-efficient pathway can be used to guide a health care system that is both effective and cost efficient.
In more recent examples of cost savings, additional cost savings have been reported for patients who had an indeterminate exercise electrocardiogram. Due to effective risk stratification with SPECT imaging, cost efficiency can also be noted for patients with a normal resting 12-lead electrocardiogram. There are two large controlled clinical studies that have examined the cost implications of SPECT imaging when compared with other diagnostic approaches.15,16 The smaller of the two studies, the Economics of Myocardial Perfusion Imaging in Europe (EMPIRE) study, compared stable chest-pain patients undergoing exercise electrocardiography, SPECT imaging, and direct coronary angiography (see Fig. 24-3). This study has value to extending prior results noted in the United States by the inclusion of four European countries with diverse health care and economic structures. The results of this study revealed that a noninvasive approach was decidedly cost efficient when compared with an invasive approach to care, at least for this cohort of patients with stable chest pain symptoms.
In the United States, the END study included a geographically diverse representation of multiple hospitals from within the United States and included 11,372 patients with Canadian Cardiovascular Society class II angina.15 Patients enrolled in END were referred to direct coronary angiography or to initial SPECT imaging followed by selective catheterization in the setting of provocative ischemia. Figure 24-3 illustrates the diagnostic and follow-up costs of care for aggressively versus conservatively managed patients. These results revealed that over 3 years, there was an approximate 30% to 40% cost savings with initial SPECT imaging and a strategy of selective coronary angiography.
Both of these two large study results are consistent with prior modeling and observational datasets that support an economic advantage with the use of SPECT imaging in patients with stable chest-pain symptoms. If applied to a larger cohort of patients evaluated within a health care system, cost savings could be dramatic and approach several million dollars for every 1000 patients tested. The economic benefit of SPECT is based on the low catheterization rate required for patients with a normal perfusion scan. A number of reports have noted that only 1% to 3% of patients with normal perfusion results are referred to coronary angiography. Thus, the confirmation of normal SPECT findings can result in a marked reduction in costs of care and a reduced intensity of management. In the United Kingdom, the routine application of SPECT in this setting has been estimated to reduce costs by approximately £65,000 per year.16
At the heart of this research lies the concept of SPECT imaging as a gatekeeper to invasive coronary angiography. The above-mentioned research notes that gatekeeping can elicit substantive cost savings while maintaining equivalent patient outcomes. Recently, a review from the United Kingdom’s National Institute of Clinical Excellence reported that when coronary angiography is used selectively following SPECT imaging, the resulting revascularization rates can be reduced by up to 50%, without a negative impact on outcomes.61
A recent report utilized this analytic method of identifying cost savings of SPECT compared with computed tomographic angiography (CTA).62 This report compared 2313 patients who underwent CTA with 9252 patients referred to SPECT imaging. At 9 months of follow-up, the rates of worsening chest pain, myocardial infarction, or for any coronary disease hospitalization were similar for SPECT and CTA. However, dramatic cost differences were noted for both tests. That is, for patients with a history of coronary artery disease, CTA resulted in approximately $2500 higher costs of care. This makes sense because of the importance of provocative ischemia in guiding therapeutic decision making in patients with a high likelihood for CAD and more prevalent revascularizable disease states. Conversely, in the low-risk patient with more atypical symptoms, the use of CTA resulted in cost savings of $603, largely driven by its high negative predictive accuracy. That is, the likelihood of significant CAD is very low when the CTA results do not reveal even minimal luminal irregularities. This report highlights the importance of pretest risk as a covariate in cost analysis. As shown in other reports, the extent and severity of regional myocardial perfusion provides important information that most prominently affects patients at higher risk, including those with known CAD.
There are also a number of reports focusing solely on the cost savings of SPECT imaging.63 One area where the data are particularly strong for the utility of SPECT imaging is in the acute evaluation of chest pain.63 In this setting, SPECT imaging has a high negative predictive value exceeding 95% and supported in the recent ACC/AHA guidelines by a class IA indication for patients with a nondiagnostic ECG and initially normal serum markers and enzymes.64,65 Although SPECT imaging has been proposed as one of many strategies employed as a gatekeeper to hospitalization, the value of some form of testing lies in the nearly 3 million unnecessary hospitalizations accounting for some $5 to $8 billion dollars, as well as the approximately 5% of patients with acute coronary syndromes who are inappropriately sent home from the emergency department each year.66–68 A review of this evidence was recently synthesized within the statement on cost-effectiveness published by the American Society of Nuclear Cardiology (ASNC),63 in which the authors posit a nearly $800 cost savings when SPECT imaging is applied in the acute evaluation of chest pain in patients with indeterminate biomarkers. When compared to hospitalized patients, the use of SPECT imaging results in a lower rate of cardiac catheterization and shorter lengths of stay, both of which contribute to a positive yield in cost minimization. Moreover, the use of SPECT imaging to guide admissions results in a decrease in the rate of unnecessary hospitalizations by nearly 30% and a 6% reduction in inappropriate discharges. Perhaps the strongest piece of evidence was published from the ERASE trial noting that the use of SPECT imaging resulted in a 32% lower rate of admissions when compared to usual care.69
Several reports on the cost savings associated with predischarge use of SPECT imaging in patients with acute coronary syndromes have been published recently. The largest of these is from the Adenosine Sestamibi SPECT Postinfarction Evaluation (INSPIRE) Trial, where risk stratification based on quantitative estimates of ischemia resulted in substantial differences in cost (Table 24-3).70 This report enrolled over 700 patients and noted that low-risk patients had average hospitalization costs of $5609 compared to high-risk patients, where the costs of care averaged $13,269 dollars (P < 0.0001). Much of the difference in cost had to do with a reduced length of stay for the low-risk patient with minimal ischemia. In fact, the average length of stay for low-risk patients was 5.5 days versus 13.9 days for high-risk patients (P < 0.0001), with high-risk patients remaining in the coronary care unit (CCU) an average of 2 additional days. The take-home message from the INSPIRE study was that low-risk patients with minimal ischemia can be safely discharged as soon as they are clinically stable. In fact, many of the low-risk patients spent little to no time in the CCU and were safely discharged within 3 days of admission. The majority of the aforementioned studies support the potential for significant cost savings as a result of using myocardial perfusion SPECT, with growing support of its utility postinfarction as an effective means of risk stratification and for identification of patients ready for early discharge.
Table 24-3 Average In-Hospital Cost and Length of Stay in Postinfarction Patients Undergoing Myocardial Perfusion SPECT*
| Estimated Hospital Costs (Not Charges) | |
Cost-Effectiveness of Myocardial Perfusion SPECT Compared to Other Diagnostic Procedures
There are a number of reports that have compared the marginal cost-effectiveness of stress myocardial perfusion SPECT with other modalities, notably stress echocardiography.17–20 The majority of these reports have been decision models that have culled together pieces of data from related research, including test diagnostic accuracy and the effectiveness of revascularization in patients with CAD. The decision model is then formulated by including snippets of information from many diverse research reports. The problem with this type of analysis is that there are many assumptions about data inputs, and the selection of data is critical to devising the model and can heavily influence its results. It is for this reason that physicians should take care to scrutinize decision models, paying particular attention to the model inputs to evaluate their clinical relevance.
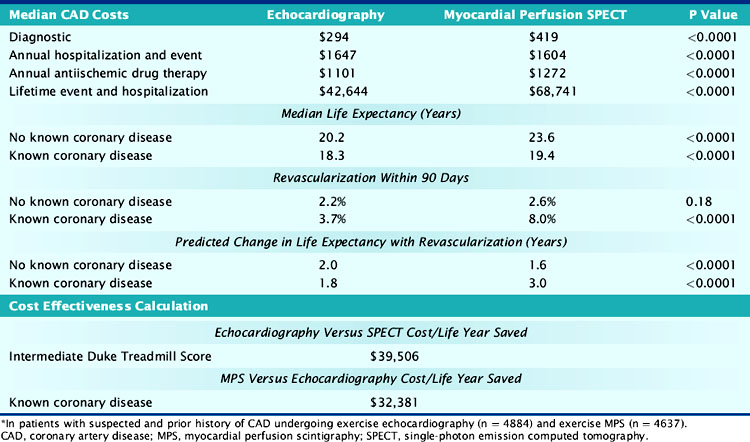
One recent report, however, evaluated a total of 4884 and 4637 patients undergoing exercise echocardiography and myocardial perfusion SPECT, respectively (Table 24-4).71 The results reveal that for patients with a high CAD likelihood, SPECT was decidedly more cost-effective than echocardiography. A number of patient subsets were included in this high-CAD-likelihood patient subset, notably the elderly, diabetics, patients with peripheral arterial disease, or those who are functionally impaired. The major driver for this cost-effectiveness advantage was the rapid referral of patients with abnormal myocardial perfusion results to coronary revascularization, with the result being a total of 3 additional years of life expectancy. Based on this, the resulting ICER was $32,381 for exercise SPECT versus echocardiography for the evaluation of stable chest pain. However, exercise echocardiography revealed a favorable ICER for lower-risk patients with a prior intermediate Duke Treadmill Score or indeterminate test results. In fact, the ICER was less than $20,000 for echocardiography compared to SPECT imaging when the estimated risk of cardiac death or nonfatal myocardial infarction was less than 2% per year (and largely included lower-risk patient cohorts). Once again, note the importance of pretest risk as guiding cost-effective test selection.
American Society of Nuclear Cardiology Statement on Cost-Effectiveness of Nuclear Cardiology
The ASNC published a consensus statement on cost-effectiveness that may be helpful for readers to provide to local payers and as a tool for administrators to structure pathways of care that create efficiency and cost savings when using myocardial perfusion SPECT.63 This ASNC statement highlighted several critical factors as strongly influencing downstream costs of care, including the greater accuracy of myocardial perfusion SPECT. A pattern of highly accurate testing can create cost efficiency or minimization of resources by reducing the need to layer testing; this pattern of cost efficiency has been reported for women and men with stable chest pain in diabetics, in the evaluation of acute chest pain in the emergency department, and for the predischarge evaluation following acute coronary syndrome. Each of these areas has been discussed in greater detail in prior parts of this chapter. For the interested laboratory, the creation of programs to ensure high-quality imaging will allow for pathways of care that create diagnostic efficiency. These programs should contain active quality assessment programs to assess near-term diagnostic or prognostic accuracy, including the assessment of referral patterns to coronary angiography for both low- and high-risk patients. The development of such programs can be important to payers and patients, ensuring that high-quality imaging is ensured within a given laboratory.
1. Benjamin E.J., Smith S.C.Jr, Cooper R.S., et al. Task force #1: magnitude of the prevention problem: opportunities and challenges. 33rd Bethesda Conference. J Am Coll Cardiol. 2002;40:588-603.
2. http://www.rwjf.org/files/research/022008ib118final.pdf Accessed March 15, 2008
3. http://www.americanheart.org/presenter.jhtml?identifier=3018163 Accessed September 1, 2008
4. Shaw L.J., Bairey Merz C.N., Pepine C.J., Reis S.E., Bittner V., Kip K., Kelsey S.F., Olson M., Johnson B.D., Mankad S., Sharaf B.L., Rogers W.J., Pohost G.M., Sopko G. for the WISE Investigators: The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health–National Heart, Lung, and Blood Institute–Sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114:894-904.
5. http://www.meps.ahrq.gov/mepsweb/ Accessed March 15, 2008
6. American College of Cardiology, Available at www.acc.org/advocacy/advoc_issues/impactchart.htm Accessed May 11, 2004
7. Mark D.B., Shaw L.J., Lauer M.S., et al. Task force #5: is atherosclerotic imaging cost effective? From the 34th Bethesda Conference on Atherosclerotic Imaging. J Am Coll Cardiol. 2003;41:1906-1917.
8. Levin D.C., Parker L., Intenzo C.M., et al. Recent rapid increase in utilization of radionuclide myocardial perfusion imaging and related procedures: 1996–1998 practice patterns. Radiology. 2002;222:144-148.
9. Scanlon P.J., Faxon D.P., Audet A.M., et al. ACC/AHA Guidelines for coronary angiography: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 1999;33:1756-1824.
10. Goldman L., Garber A.M., Grover S.A., et al. 27th Bethesda Conference: matching the intensity of risk factor management with the hazard for CAD events. Task Force 6. Cost effectiveness of assessment and management of risk factors. J Am Coll Cardiol. 1996;27:1020-1030.
11. Underwood S.R., Anagnostopoulos C., Cerqueira M., et al. Myocardial perfusion scintigraphy: the evidence. A consensus conference organised by the British Cardiac Society, the British Nuclear Cardiology Society and the British Nuclear Medicine Society, endorsed by the Royal College of Physicians of London and the Royal College of Radiologists. Eur J Nuc Med Mol Imaging. 2003;31:261-291.
12. Berry E., Kelly S., Hutton J., et al. A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease. Health Tech Assess. 1999;3:1-118.
13. Berry E., Kelly S., Westwood M.E., et al. The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review. Health Tech Assess. 2002;6:1-155.
14. Rumberger J.A., Behrenbeck T., Breen J.F., et al. Coronary calcification by electron beam computed Tomography and obstructive coronary artery disease: a model for costs and effectiveness of diagnosis as compared with conventional cardiac testing methods. J Am Coll Cardiol. 1999;33:453-462.
15. Shaw L.J., Hachamovitch R., Berman D.S., et al. The economic consequences of available diagnostic and prognostic strategies for the evaluation of stable angina patients: an observational assessment of the value of precatheterization ischemia. Economics of Noninvasive Diagnosis (END) Multicenter Study Group. J Am Coll Cardiol. 1999;33:661-669.
16. Underwood S.R., Godman B., Salyani S., et al. Economics of myocardial perfusion imaging in Europe: the EMPIRE study. Eur Heart J. 1999;20:157-166.
17. Garber A.M., Solomon N.A. Cost-effectiveness of alternative test strategies for the diagnosis of coronary artery disease. Ann Intern Med. 1999;130:719-728.
18. Kuntz K.M. Cost-effectiveness of diagnostic strategies for patients with chest pain. Ann Intern Med. 1999;130:709-718.
19. Marwick T.H., Shaw L.J., Case C., et al. Clinical and economic impact of exercise electrocardiography and exercise echocardiography in clinical practice. Eur Heart J. 2003;24:1153-1163.
20. Lee D.S., Jang M.J., Cheon G.J., et al. Comparison of the cost-effectiveness of stress myocardial perfusion SPECT and stress echocardiography in suspected coronary artery disease considering the prognostic value of false-negative results. J Nucl Cardiol. 2002;9:515-522.
21. Shaw L.J., Hachamovitch R., Eisenstein E., et al. Cost implications for implementing a selective preoperative risk screening approach for peripheral vascular surgery patients. Am J Managed Care. 1997;3:1817-1827.
22. Shaw L.J., Miller D.D., Berman D.S., et al. Clinical and economic outcomes assessment in nuclear cardiology. Q J Nucl Med. 2000;44:138-152.
23. Maddahi J., Ghambir S.S. Cost-effective selection of patients for coronary angiography. J Nucl Cardiol. 1997;4:S141-S151.
24. Patterson R.E., Eng C., Horowitz S.F., et al. Bayesian comparison of cost-effectiveness of different clinical approaches to coronary artery disease. J Am Coll Cardiol. 1984;4:278-289.
25. Hunink M.G., Kuntz K.M., Fleischmann K.E., et al. Noninvasive imaging for the diagnosis of coronary artery disease: focusing the development of new diagnostic technology. Ann Intern Med. 1999;131:673-680.
26. Sculpher M., Drummond M., Buxton M. The iterative use of economic evaluation as part of the process of health technology assessment. J Health Serv Res. 1997;2:26-30.
27. Callister T.Q., Cooil B., Raya S.P., et al. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208:807-814.
28. Smith D.H., Hugh Gravelle H. The practice of discounting in economic evaluations of healthcare interventions. Int J Tech Assess Health Care. 2001;17:236-243.
29. Sheldon T.A. Discounting in Health care decision-making: time for a change? J Pub Health Med. 1992;14:250-256.
30. Drummond M.F., Jefferson T.O. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313:275-283.
31. Einstein A.J., Henzlova M.J., Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298(3):317-323. (Jul 18)
32. Shaw L.J., Raggi P., Berman D.S., et al. Cost effectiveness of screening for cardiovascular disease with measures of coronary calcium. Prog Cardiov Dis. 2003;46:171-184.
33. Bell R., Petticrew M., Luengo S., et al. Screening for ovarian cancer: a systematic review. Health Tech Assess. 1998;2:1-84.
34. Petticrew M.P., Sowden A.J., Lister-Sharp D., et al. False-negative results in screening programmes: systematic review of impact and implications. Health Tech Assess. 2000;4:1-60.
35. Mol. Characteristics of good diagnostic studies. Semin Reprod Med. 2003;21(1):17-25.
36. Shaw L.J., Eisenstein E.L., Hachamovitch R., et al. A primer of biostatistic and economic methods for diagnostic and prognostic modeling in nuclear cardiology: Part II. J Nucl Cardiol. 1997;4(1 Pt 1):52-60.
37. Shaw L.J., Hachamovitch R., Papatheofanis F.J. Outcomes and technology assessment in nuclear medicine. Reston, VA: Society of Nuclear Medicine Press, 1999.
38. Mark D. Medical economics and health policy issues for interventional cardiology. In Topol E., editor: Textbook of Interventional Cardiology, ed 2, Philadelphia: WB Saunders, 1993.
39. Finkler S. Cost accounting for health care organizations: concepts and applications. Gaithersburg, MD: Aspen Publishers, 1994.
40. Laupacis A., Feeny D., Detsky A.S., et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473-481.
41. Gold M. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press, 1996.
42. Office of Technology Assessment. The implications of cost-effectiveness analysis of medical technology, Chapters 1–4. Washington, DC: U.S. Government Printing Office, 1980.
43. Doubilet P., Weinstein M.C., McNeil B.J. Use and misuse of the term “cost effective” in medicine. N Engl J Med. 1986;314:253-256.
44. Garber A.M., Phelps C.E. Economic foundations of cost-effectiveness analysis. J Health Econ. 1997;16:1-31.
45. Siegel J.E., Weinstein M.C., Russell L.B., et al. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339-1341.
46. Weinstein M.C., Siegel J.E., Gold M.R., et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253-1258.
47. Russell L.B., Gold M.R., Siegel J.E., et al. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172-1177.
48. Shaw L.J., Culler S.D., Becker N.R. Current evidence on cost effectiveness of noninvasive cardiac testing. Subsection E: analytic approaches to cost effectiveness and outcomes measurement in cardiovascular imaging. In: Pohost G., O’Rourke R., Shah P., Berman D., editors. Imaging in Cardiovascular Disease. Philadelphia: Lippincott Williams & Wilkins; 2000:479-500.
49. Petitti D.B. Meta-analysis, decision analysis, and cost-effectiveness analysis: Methods for quantitative synthesis in medicine. New York: Oxford University Press, 1994.
50. Krumholz H.M., Weintraub W.S., Bradford W.D., et al. The cost of prevention: can we afford it? Can we afford not to do it? J Am Coll Cardiol. 2002;40:603-605.
51. Mansley E.C., McKenna M.T. Importance of perspective in economic analyses of cancer screening decisions. Lancet. 2001;358:1169-1173.
52. Hunink M.G., Krestin G.P. Study design for concurrent development, assessment, and implementation of new diagnostic imaging technology. Radiology. 2002;222:604-614.
53. Mushlin A.I., Ruchlin H.S., Callahan M.A. Cost effectiveness of diagnostic tests. Lancet. 2001;358:1353-1355.
54. Goldman L., Garber A.M., Grover S.A., et al. 27th Bethesda Conference: matching the intensity of risk factor management with the hazard for CAD events. Task Force 6. Cost effectiveness of assessment and management of risk factors. J Am Coll Cardiol. 1996;27:1020-1030.
55. Weinstein M.C., Stason W.B. Cost-effectiveness of interventions to prevent or treat coronary heart disease. Annu Rev Public Health. 1985;6:41-63.
56. Sonnenberg A., Delco F. Cost-effectiveness of a single colonoscopy in screening for colorectal cancer. Arch Intern Med. 2002;162:163-168.
57. Johnstone P.A., Moore E.M., Carrillo R., et al. Yield of mammography in selected patients age ≤30 years. Cancer. 2001;91:1075-1078.
58. Shaw L.J., Iskandrian A.E. Prognostic value of stress gated SPECT in patients with known or suspected coronary artery disease. J Nucl Cardiol. 2004;11:171-185.
59. Thomas G.S., Miyamoto M.I., Morello A.P.III, et al. Technetium-99m based myocardial perfusion imaging predicts clinical outcome in the community outpatient setting: The Nuclear Utility in the Community (“NUC”) Study. J Am Coll Cardiol. 2004;43:213-223.
60. Hachamovitch R., Berman D.S., Shaw L.J., Kiat H., Cohen I., Cabico J.A., Friedman J., Diamond G.A. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535-543.
61. Mowatt G., Brazzelli M., Gemmell H., Hillis G.S., Metcalfe M., Vale L. Aberdeen Technology Assessment Group. Systematic review of the prognostic effectiveness of SPECT myocardial perfusion scintigraphy in patients with suspected or known coronary artery disease and following myocardial infarction. Nucl Med Commun. 2005;26:217-229.
62. Min J.K., Shaw L.J., Berman D.S. Cost-effective applications of cardiac computed tomography in coronary artery disease. Expert Rev Cardiovasc Ther. 2008;6(1):43-55.
63. DesPrez R.D., Gillespie R.L., Jaber W.A., Noble G.L., Soman P., Wolinsky D.G., Williams K.A., Shaw L.J. American Society of Nuclear Cardiology Information Statement on the Cost Effectiveness of Myocardial Perfusion Imaging. J Nucl Cardiol. 2005;12(6):750-759.
64. Heller G.V., Stowers S.A., Hendel R.C., Herman S.D., Daher E., Ahlberg A.W., Baron J.M., Mendes de Leon C.F., Rizzo J.A., Wackers F.J. Clinical value of acute rest technetium-99m tetrofosmin tomographic myocardial perfusion imaging in patients with acute chest pain and nondiagnostic electrocardiograms. J Am Coll Cardiol. 1998;31:1011-1017.
65. Klocke F.J., et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging: executive summary. Circulation. 2003;108:1404-1418.
66. Fineberg H.V., Scadden D., Goldman L. Care of patients with a low probability of acute myocardial infarction. Cost effectiveness of alternatives to coronary-care-unit admission. N Engl J Med. 1984;310:1301-1307.
67. Lee T.H., Rouan G.W., Weisberg M.C., Brand D.A., Acampora D., Stasiulewicz C., Walshon J., Terranova G., Gottlieb L., Goldstein-Wayne B., et al. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60(4):219-224.
68. Pope J.H., Aufderheide T.P., Ruthazer R., Woolard R.H., Feldman J.A., Beshansky J.R., Griffith J.L., Selker H.P. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163-1170.
69. Udelson J.E., Beshansky J.R., Ballin D.S., Feldman J.A., Griffith J.L., Handler J., Heller G.V., Hendel R.C., Pope J.H., Ruthazer R., Spiegler E.J., Woolard R.H., Selker H.P. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: a randomized controlled trial. JAMA.. 2002;288(21):2693-2700.
70. Mahmarian J.J., Shaw L.J., Filipchuk N.G., Dakik H.A., Iskander S.S., Ruddy T.D., Henzlova M.J., Keng F., Allam A., Moye L.A., Pratt C.M. INSPIRE Investigators. A multinational study to establish the value of early adenosine technetium-99m sestamibi myocardial perfusion imaging in identifying a low-risk group for early hospital discharge after acute myocardial infarction. J Am Coll Cardiol. 2006;48(12):2448-2457. Epub 2006 Nov 28.2006 Dec 19
71. Shaw L.J., Marwick T.H., Berman D.S., Sawada S., Heller G.V., Vasey C., Miller D.D. Incremental cost effectiveness of exercise echocardiography versus SPECT imaging for the evaluation of stable chest pain. Eur Heart J. 2006;27(20):2448-2458. Epub 2006, Sep 26, 2006 Oct