Corticosteroids in respiratory care
After reading this chapter, the reader will be able to:
1. Define key terms that pertain to corticosteroids
2. Discuss the indications for inhaled corticosteroid use
3. List all available inhaled corticosteroids used in respiratory therapy
4. Differentiate between specific corticosteroid formulations
5. Describe the route of administration available for corticosteroids
6. Describe the mode of action for corticosteroids
7. Discuss the effect corticosteroids have on the white blood cell count
8. Discuss the effect corticosteroids have on β receptors
9. Differentiate between systemic and local side effects of corticosteroids
10. Discuss the use of corticosteroids in the treatment of asthma and chronic obstructive pulmonary disease
11. Be able to assess clinically corticosteroid use in patient care
Chapter 11 discusses the use of corticosteroids in respiratory care and provides a brief review of the physiology of endogenous corticosteroid hormones in the body. A brief description of inflammation, and specifically of airway inflammation in asthma, forms the basis for a discussion of the pharmacology of corticosteroids as antiinflammatory drugs. Aerosolized glucocorticoids and their uses and side effects are described.
Clinical indications for use of inhaled corticosteroids
• Orally inhaled agents: Maintenance, control therapy of chronic asthma, identified as requiring step 2 care or greater by the National Asthma Education and Prevention Program Expert Panel Report 3 Guidelines for the Diagnosis and Management of Asthma—Update on Selected Topics (available at http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm):1
• Step 2 asthma is defined as symptoms occurring more than 2 days/week but not daily and night awakenings occurring 3 to 4 nights/month, with forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) 80% predicted or greater.
• Inhaled agents can be used with systemic corticosteroids in severe asthma and may allow systemic dose reduction or elimination for asthma control.
• Inhaled corticosteroids are recommended by the American Thoracic Society (ATS)2 (available at: http://www.thoracic.org/sections/publications/statements/pages/respiratory-disease-adults/copdexecsum.html) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD)3 (available at: http://www.goldcopd .org/Guidelineitem.asp?l1=2&l2=1&intId=996) for chronic obstructive pulmonary disease (COPD).
• Intranasal aerosol agents: Management of seasonal and perennial allergic and nonallergic rhinitis.
Identification of aerosolized corticosteroids
Increased numbers of aerosolized corticosteroid preparations are becoming available for oral inhalation and intranasal delivery. Table 11-1 lists currently available aerosol formulations of corticosteroids for oral inhalation, and Table 11-2 lists intranasal formulations. The rationale for inhaled aerosol agents is discussed and the properties of corticosteroids required for success as topical agents are described subsequently, along with additional detail on individual agents.
TABLE 11-1
Corticosteroids Available by Aerosol for Oral Inhalation*
| DRUG | BRAND NAME | FORMULATION AND DOSAGE |
| Beclomethasone dipropionate HFA | Qvar | MDI: 40 μg/puff and 80 μg/puff |
| Adults ≥12 yr: 40-80 μg twice daily† or 40-160 μg twice daily‡ | ||
| Children ≥5 yr: 40-80 μg twice daily | ||
| Flunisolide | AeroBid | MDI: 250 μg/puff |
| Adults and children ≥6 yr: 2 puffs bid, adults no more than 4 puffs daily | ||
| Children ≤15 yr: no more than 2 puffs daily | ||
| Flunisolide hemihydrate HFA | AeroSpan | MDI: 80 μg/puff |
| Adults ≥12 yr: 2 puffs bid, adults no more than 4 puffs daily | ||
| Children 6-11 yr: 1 puff daily, no more than 2 puffs daily | ||
| Fluticasone propionate | Flovent HFA | MDI: 44 μg/puff, 110 μg/puff, and 220 μg/puff |
| Adults ≥12 yr: 88 μg bid†, 88-220 μg bid‡, or 880 μg bid§ | ||
| Children 4-11 yr: 88 μg bid¶ | ||
| Flovent Diskus | DPI: 50 μg, 100 μg, and 250 μg | |
| Adults: 100 μg bid†, 100-250 μg bid‡, 1000 μg bid§ | ||
| Children 4-11 yr: 50 μg twice daily | ||
| Budesonide | Pulmicort | DPI: 90 μg/actuation and 180 μg/actuation |
| Adults: 180-360 μg bid†, 180-360 μg bid‡, 360-720 μg bid§ | ||
| Children ≥6 yr: 180-360 μg bid | ||
| Pulmicort Respules | SVN: 0.25 mg/2 mL, 0.5 mg/2 mL, 1 mg/2 mL | |
| Children 1-8 yr: 0.5 mg total dose given once daily or twice daily in divided doses†, ‡; 1 mg given as 0.5 mg bid or once daily§ | ||
| Mometasone furoate | Asmanex Twisthaler | DPI: 110 μg/actuation and 220 μg/actuation |
| Adults and children ≥12 yr: 220-880 μg daily | ||
| Children 4-11 yr: 110 μg daily | ||
| Ciclesonide | Alvesco | MDI: 40 μg/puff and 80 μg/puff |
| Adults ≥12 yr: 80-160 μg twice daily†, or 80-320 μg twice daily‡ | ||
| Fluticasone propionate/salmeterol | Advair Diskus | DPI: 100 μg fluticasone/50 μg salmeterol, 250 μg fluticasone/50 μg salmeterol, or 500 μg fluticasone/50 μg salmeterol |
| Advair HFA | Adults and children ≥12 yr: 100 μg fluticasone/50 μg salmeterol, 1 inhalation twice daily, about 12 hr apart (starting dose if not currently taking inhaled corticosteroids) | |
| Maximal recommended dose is 500 μg fluticasone/50 μg salmeterol twice daily | ||
| Children ≥4 yr: 100 μg fluticasone/50 μg salmeterol, 1 inhalation twice daily, about 12 hr apart (for patients who are symptomatic while taking inhaled corticosteroid) | ||
| MDI: 45 μg fluticasone/21 μg salmeterol, 115 μg fluticasone/21 μg salmeterol, or 230 μg fluticasone/21 μg salmeterol | ||
| Adults and children ≥12 yr: 2 inhalations twice daily, about 12 hr apart | ||
| Budesonide/formoterol fumarate HFA | Symbicort | MDI: 80 μg budesonide/4.5 μg formoterol and 160 μg budesonide/4.5 μg formoterol |
| Adults and children ≥12 yr: 160 μg budesonide/9 μg formoterol bid, 320 μg budesonide/9 μg formoterol bid; daily maximum: 640 μg budesonide/18 μg formoterol | ||
| Mometasone furoate/formoterol fumarate HFA | Dulera | MDI: 100 μg mometasone/5 μg formoterol and 200 μg mometasone/5 μg formoterol |
| Adults and children ≥12 yr: If previously on medium dose of corticosteroids, ≤400 μg mometasone/20 μg formoterol daily; if previously on high dose of corticosteroid, ≤800 μg mometasone/20 μg formoterol daily |
*Individual agents are discussed in text. Detailed information about each agent should be obtained from the manufacturer’s drug insert.
†Recommended starting dose if taking only bronchodilators.
‡Recommended starting dose if previously taking inhaled corticosteroids.
§Recommended starting dose if previously taking oral corticosteroids.
TABLE 11-2
Aerosol Corticosteroid Preparations Available for Intranasal Delivery*
| DRUG | BRAND NAME | FORMULATION AND DOSAGE |
| Beclomethasone | Beconase AQ | Spray: 42 μg/actuation |
| Adults ≥12 yr: 1 or 2 sprays each nostril twice daily | ||
| Children 6-11 yr: 1 spray each nostril twice daily, may increase to 2 sprays | ||
| Triamcinolone acetonide | Nasacort AQ | Spray: 55 μg/actuation |
| Adults and children ≥12 yr: 2 sprays each nostril once daily (starting dose) | ||
| Children 6-11 yr: 1 spray each nostril once daily (starting dose) | ||
| Flunisolide | Spray: 25 μg/actuation and 29 μg/actuation | |
| Adults and children ≥14 yr: 2 actuations each nostril bid | ||
| Children 6-14 yr: 1 actuation each nostril tid or 2 actuations each nostril bid | ||
| Budesonide | Rhinocort Aqua | Spray: 32 μg/actuation |
| Adults and children ≥6 yr: 1 spray each nostril daily (starting dose) | ||
| Fluticasone | Flonase | Spray: 50 μg/actuation |
| Adults: 2 sprays each nostril once daily (starting dose) | ||
| Children ≥4 yr: 1 spray each nostril once daily (starting dose) | ||
| Mometasone furoate | Nasonex | Spray: 50 μg/actuation |
| Adults and children ≥12 yr: 2 sprays each nostril once daily | ||
| Children 2-11 yr: 1 spray each nostril once daily | ||
| Fluticasone furoate | Veramyst | Spray: 27.5 μg/actuation |
| Adults and children ≥12 yr: 2 sprays each nostril once daily | ||
| Children 2-11 yr: 1 spray each nostril once daily | ||
| Ciclesonide | Omnaris | Spray: 50 μg/actuation |
| Adults and children ≥6 yr: 2 sprays each nostril once daily |
*Detailed information about each agent should be obtained from the manufacturer’s drug insert.
Physiology of corticosteroids
Identification and source
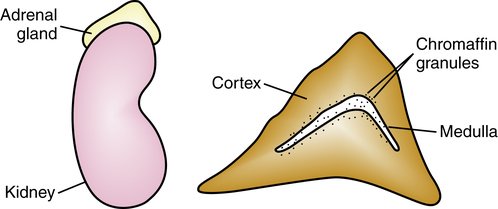
Corticosteroids are a group of chemicals secreted by the adrenal cortex and are referred to as adrenal cortical hormones. The adrenal or suprarenal gland is composed of two portions (Figure 11-1). The inner zone is the adrenal medulla and produces epinephrine. The outer zone is the cortex, which is the source of corticosteroids. Three types of corticosteroid hormones are produced by the adrenal cortex: glucocorticoids (e.g., cortisol), mineralocorticoids (e.g., aldosterone), and sex hormones (e.g., androgens and estrogens). The mineralocorticoid aldosterone regulates body water by increasing the amount of sodium reabsorption in the renal tubules. The corticosteroids used in pulmonary disease are all analogues of cortisol, or hydrocortisone as it is also termed. Glucocorticoid agents are referred to as glucocorticosteroids and by the more general term corticosteroid, or simply as steroids.
Hypothalamic-pituitary-adrenal axis
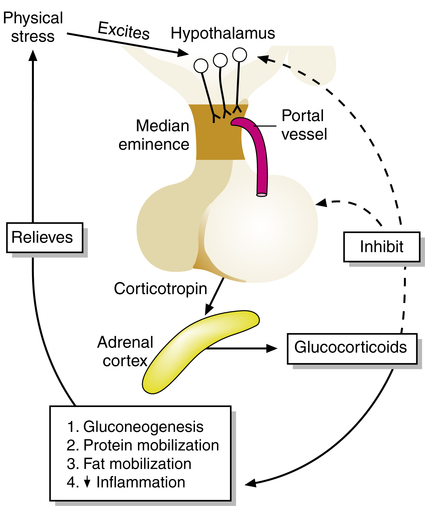
The side effects of corticosteroids and the rationale for aerosol or alternate-day therapy can be understood if the production and control of endogenous (the body’s own) corticosteroids are grasped. The pathway for release and control of corticosteroids is the hypothalamic-pituitary-adrenal (HPA) axis (Figure 11-2). Stimulation of the hypothalamus causes impulses to be sent to the area known as the median eminence, where corticotropin-releasing factor (CRF) is released. CRF circulates through the portal vessel to the anterior pituitary gland, which then releases corticotropin, or adrenocorticotropic hormone (ACTH), into the bloodstream. ACTH in turn stimulates the adrenal cortex to secrete glucocorticoids, such as cortisol. Cortisol and glucocorticoids in general regulate the metabolism of carbohydrates, fats, and proteins, generally to increase levels of glucose for body energy. This is the reason cortisol and its analogues are called glucocorticoids. They can also cause lipolysis, redistribution of fat stores, and breakdown of tissue protein stores. These actions are the basis for many of the side effects seen with glucocorticoid drugs. The breakdown of proteins for use of the amino acids (gluconeogenesis) is responsible for muscle wasting, and the effects on glucose metabolism can increase plasma glucose levels. The latter is sometimes referred to as steroid diabetes.4
Hypothalamic-pituitary-adrenal suppression with steroid use
One of the most significant side effects of treatment with glucocorticoid drugs (exogenous corticosteroids) is adrenal suppression or, more generally, HPA suppression. When the body produces endogenous glucocorticoids, there is a normal feedback mechanism within the HPA axis to limit production. As glucocorticoid levels increase, release of CRF and ACTH is inhibited, and further adrenal production of glucocorticoids is stopped. This feedback inhibition of the hypothalamus and the pituitary is shown in Figure 11-2 and is analogous to the servo mechanism by which a thermostat regulates furnace production of heat by monitoring temperature levels.
Diurnal steroid cycle
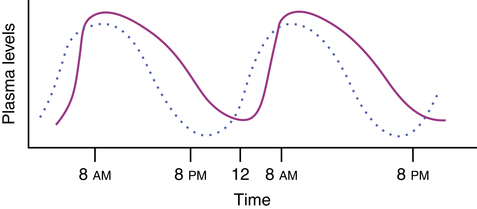
The production of the body’s own glucocorticoids also follows a rhythmic cycle, termed a diurnal or circadian rhythm. This daily rise and fall of glucocorticoid levels in the body are shown in Figure 11-3. On a daily schedule of daytime work and nighttime sleep, cortisol levels are highest in the morning around 8 am. These high plasma levels inhibit further production and release of glucocorticoids and ACTH by the HPA axis because of the feedback mechanism previously described. During the day, plasma levels of ACTH (see Figure 11-3, dotted line) and cortisol (see Figure 11-3, solid line) gradually decrease. As the glucocorticoid level decreases, the anterior pituitary is reactivated to begin releasing ACTH, which stimulates production of cortisol by the adrenal cortex. This lag between increased ACTH and cortisol levels is illustrated in Figure 11-3. One of the reasons for jet lag and the delay in adjusting to night shift from day shift is that this diurnal and regular rhythm of corticosteroid levels becomes out of synchronization with the time zone and the work time. Although a worker needs to sleep at 8 am. after working all night, the body is wide awake, with energy stores being released.
Nature of inflammatory response
Redness: Local dilation of blood vessels, occurring in seconds
Flare: Reddish color several centimeters from the site, occurring 15 to 30 seconds after injury
Increased vascular permeability: An exudate is formed in the surrounding tissues.
Leukocytic infiltration: White blood cells emigrate through capillary walls (diapedesis) in response to attractant chemicals (chemotaxis).
Phagocytosis: White blood cells and macrophages (in the lungs) ingest and process foreign material such as bacteria.
Mediator cascade: Histamine and chemoattractant factors are released at the site of injury, and various inflammatory mediators such as complement and arachidonic acid products are generated.
Inflammation in the airway
Because glucocorticoids are a mainstay for treating asthma, the multiple pathways and mediators for the genesis of airway inflammation seen in asthma are briefly described. Asthma is currently understood as a disease in which there is chronic inflammation of the airway wall, causing airflow limitation and a hyperresponsiveness to various stimuli (Box 11-1).4,5 The airway inflammation is mediated by inflammatory cells, such as mast cells, eosinophils, T lymphocytes, and macrophages. The mast cell and the eosinophil are considered to be the major effector cells of the inflammatory response, regardless of whether the asthma is allergic or nonallergic.6 T lymphocytes may be pivotal in coordinating the inflammatory response by release of numerous proinflammatory cytokines (proteins that regulate immune/inflammatory responses), which act on basophils, epithelial cells, and endothelial cells in the airway to further the inflammatory process. The potent mediators released during an asthmatic reaction cause airway smooth muscle contraction (bronchospasm), increased microvascular leakage and airway wall swelling, mucus secretion, and remodeling of the airway wall over the longer term. In an acute state, people with asthma exhibit wheezing, breathlessness, chest tightness, and cough, especially at night or early morning. The acute symptoms produced by the airway inflammation are at least partly reversible either spontaneously or with pharmacologic treatment. Treatment with antiinflammatory agents such as glucocorticoids is important to reduce the basal level of airway inflammation and reduce airway hyperresponsiveness and the predisposition to acute episodes of obstruction.
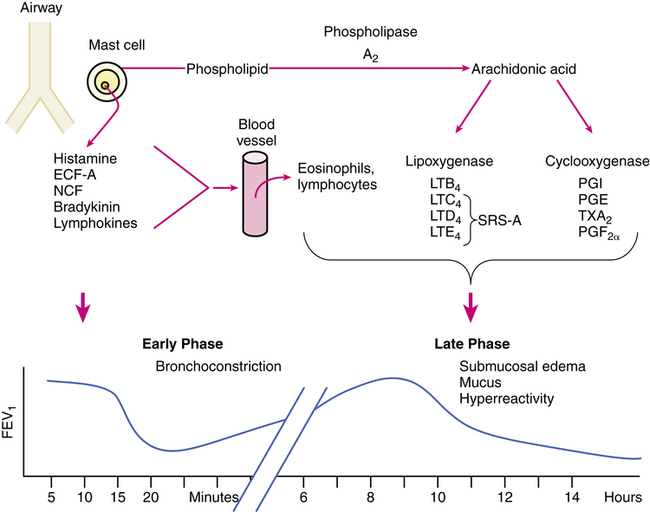
Asthmatic reactions are biphasic, including an early phase and a late phase. Figure 11-4 shows a conceptual representation of the overall process. After an insult to the asthmatic airway by an allergen, cold air, viral infection, or noxious gas, there is evidence that the early asthmatic response is caused by immunoglobulin E (IgE)–dependent activation of airway mast cells, which can release inflammatory mediators such as histamine, prostaglandin D2 (PGD2), and leukotriene C4.5 The immediate response of the airway to chemicals such as histamine is bronchospasm. This response peaks at about 15 minutes and then declines over the next hour; this produces the early phase decrease in expiratory flow rates illustrated in Figure 11-4.
Although the early bronchoconstriction of smooth muscle may self-limit or respond to β agonists, the progression of cellular events can continue. Mast cell mediators and the release of cytokines recruit other inflammatory cells (eosinophils, basophils, monocytes/macrophages, and lymphocytes) by activating epithelial cells and endothelial cells to release adhesion molecules (e.g., intercellular adhesion molecule [ICAM]) and other cytokines to cause the late phase reaction. During the late phase response, mast cells and recruited eosinophils, lymphocytes, or macrophages that have infiltrated the airway release a range of inflammatory mediators. Neutrophils are not generally associated with asthma and allergic reactions in the absence of infection. The late asthmatic response occurs 6 to 8 hours after a challenge, and it may last for 24 hours. The late-phase reaction is thought to be reflective of the chronic inflammation characterizing asthma between acute episodes.6
Phospholipids in the cell membrane of mast cells and other cells are converted by phospholipase A2 to arachidonic acid and then to various bronchoactive and vasoactive substances by the two metabolic paths shown in Figure 11-4: the cyclooxygenase and lipoxygenase pathways. The term eicosanoid is used to refer to the products of the two pathways. The migration of eosinophils and lymphocytes and further development of inflammation-producing chemicals such as the arachidonic acid metabolites and cytokines all contribute to build an inflammatory response in the lung.7 In addition to smooth muscle spasm, mucus secretion occurs, along with mucosal swelling resulting from increased vascular permeability. Shedding of airway cells (desquamation) and goblet cell hyperplasia are seen. The result is mucous plugging of the airway, complicated by the cellular debris in the bronchial lumen. The pathology of bronchial asthma has been described as “chronic desquamating eosinophilic bronchitis.”8 These airway changes lead to further bronchial hyperreactivity seen in asthma. There is evidence of airway remodeling, with increased tenascin (an extracellular matrix protein for cell development), collagens, and fibronectin, all of which can cause thickening of the basement membrane in the airway wall. This airway remodeling deregulates communication between cells, promoting epithelial damage and enhancing the inflammatory response.9
Aerosolized corticosteroids
Aerosolized corticosteroid agents
Several aerosol steroid agents, all of which are glucocorticoids, are available for inhalational use in the United States at the time of this edition. These are identified in Table 11-1, which summarizes steroids used for oral inhalation, and Table 11-2, which lists agents for intranasal delivery, giving strengths and recommended doses. Originally, in the United States, all of the orally inhaled corticosteroids were available as metered dose inhaler (MDI) formulations. Three dry powder inhalers (DPIs), Diskus (fluticasone), Pulmicort Flexhaler (budesonide), and Asmanex Twisthaler (mometasone furoate), have also been introduced, along with the only approved small volume nebulizer (SVN) formulation (budesonide [Pulmicort Respules]). At the time of this edition, all of the MDI formulations are available with hydrofluoroalkane (HFA) propellant (beclomethasone, flunisolide, fluticasone, and ciclesonide) with the exception of triamcinolone. Azmacort (triamcinolone) inhaler was discontinued as of December 31, 2010.
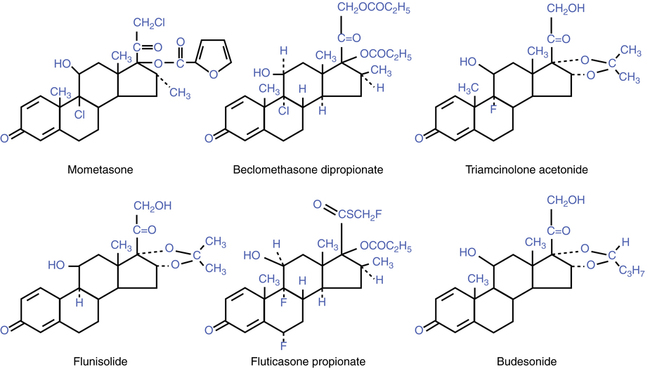
These agents possess a high topical-to-systemic potency ratio, which makes them suitable for control of asthma or COPD with minimal systemic side effects. The chemical structures of the aerosol agents available for oral and nasal inhalation are shown in Figure 11-5. Each of the aerosol agents is briefly described next.
Beclomethasone dipropionate (Qvar)
Beclomethasone dipropionate (Qvar) has been known by several names, including Vanceril and Beclovent; however, with the transition from chlorofluorocarbon (CFC)-propelled MDI formulations, beclomethasone dipropionate has been reformulated with an HFA propellant in a 40-μg and 80-μg MDI strength as Qvar (see Table 11-1). Along with the change in propellant, many components of the MDI system were reengineered, significantly increasing the efficiency of this drug delivery system. Lung deposition with Qvar has been measured at 50% to 60% of the emitted dose (see discussion of MDIs in Chapter 3). The usual starting dose of Qvar is 40 to 80 μg twice daily (see Table 11-1).
Absorption of the drug across the pulmonary epithelium is good, but rapid inactivation prevents systemic accumulation. After inhalation of a 2-mg dose, plasma levels of beclomethasone dipropionate are very low, but the active metabolite, beclomethasone monopropionate (often designated 17-BMP), reaches significant plasma levels of 1.8 to 2.5 ng/mL.10
Flunisolide (aerobid, aerospan)
Flunisolide shows a peak plasma level after inhalation at between 2 minutes and 60 minutes, indicating good absorption from the lungs, as with beclomethasone. The half-life in plasma with inhalation is approximately 1.8 hours and similar to that with oral or intravenous dosing, indicating a rapid first-pass metabolism.12
Fluticasone propionate (flovent hfa, flovent diskus)
Fluticasone propionate (Flovent HFA, Flovent Diskus) is a synthetic, trifluorinated glucocorticoid with high topical antiinflammatory potency and is available in MDI and DPI forms. The MDI is available in three different strengths: 44 μg, 110 μg, and 220 μg. The DPI is also available in three different strengths: 50 μg, 100 μg, and 250 μg. The drug is a further analogue of previous agents with high topical potency, synthesized in an attempt to avoid systemic side effects. Fluticasone is derived from the 17β-carbothioate series of androstane analogues, a group that has very weak HPA inhibitory activity but high antiinflammatory effect.13 Using fluocinolone acetonide as a reference standard, fluticasone propionate was found to have an antiinflammatory potency of 91 in mice, with an HPA-inhibitory activity of only 1, giving a therapeutic index (antiinflammatory potency/HPA potency) of 91. By comparison, beclomethasone dipropionate has an antiinflammatory potency of 21, with an HPA-inhibitory potency of 49 in mice.15 If given by subcutaneous injection to mice, fluticasone propionate exhibits HPA inhibition; however, the oral route gives only weak HPA suppression; this is useful with inhaled aerosols because a portion of the aerosol may be swallowed and contribute to systemic activity of a drug. An explanation for the weak HPA suppression when given orally may be its high first-pass effect, resulting in less than 1% of active drug in the circulation because fluticasone is rapidly metabolized in the liver to the inactive product, 17β-carboxylic acid.14
Budesonide (pulmicort, pulmicort respules)
Budesonide (Pulmicort, Pulmicort Respules) is available as a DPI (Pulmicort) or as an inhalation solution (Pulmicort Respules). The DPI delivers two strengths, 90 μg/metered dose and 180 μg/metered dose, whereas Pulmicort Respules is available in doses of 0.25 mg, 0.5 mg, and 1 mg. The benefit of using Respules is that it can be mixed with other agents such as bronchodilators (e.g., albuterol, levalbuterol, and ipratropium). Numerous studies have shown that mixing the agents had no effect on the drugs mixed.15
Budesonide is a topically active inhaled corticosteroid with a potency greater than beclomethasone dipropionate, triamcinolone, or flunisolide, but it is less potent than fluticasone, as estimated by skin vasoconstriction assay. With oral administration, only 10% of budesonide enters the systemic circulation because of high (approximately 89%) first-pass metabolism in the liver. After inhalation with a spacer device, peak plasma concentrations occur between 15 minutes and 45 minutes. The plasma half-life is 2 hours. There appears to be minimal metabolism in the lung, with approximately 70% of the inhaled dose reaching the circulation.16 Budesonide was found to exhibit about half the adrenal suppression of fluticasone, on a microgram equivalent basis in asthmatic patients.17
Mometasone furoate (asmanex twisthaler)
Mometasone furoate (Asmanex Twisthaler) is available as a DPI in two strengths, 110 μg/actuation and 220 μg/actuation dosing. McCormack and Plosker18 reviewed its use in asthma. Asmanex can be given once or twice daily. Single-day dosing may be beneficial to increase consistency in usage of an inhaled corticosteroid. Karpel and others19 found that pulmonary function results increased in patients receiving once-daily Asmanex compared with placebo in patients previously using twice-daily doses of inhaled corticosteroids. Asmanex is approved for patients as young as 4 years of age.
Fluticasone propionate/salmeterol (advair)
Fluticasone propionate/salmeterol (Advair) is a combination product of the corticosteroid fluticasone with the long-acting β2-agonist bronchodilator salmeterol. Advair is available as a DPI and HFA MDI in three different strengths (fluticasone/salmeterol): DPI, 100 μg/50 μg, 250 μg/50 μg, and 500 μg/50 μg; HFA MDI, 45 μg/21 μg, 115 μg/21 μg, and 230 μg/21 μg. The combination of inhaled steroid and long-acting β2 agonist in a convenient dose package is useful in patients with asthma requiring step 3 care or higher, needing both types of drug. In a large multicenter clinical study by Shapiro and colleagues,20 patients with asthma who were taking medium doses of inhaled corticosteroids were treated with 250 μg of fluticasone in combination with 50 μg of salmeterol from the DPI Diskus device for 12 weeks. Patients in the treatment group had significantly better FEV1 profiles over 12 hours, a significantly greater probability of remaining in the study and not withdrawing because of worsening symptoms, a significantly increased morning PEF, reduced asthma symptom scores, reduced rescue albuterol use, and significantly fewer nights with no awakenings compared with patients taking salmeterol or fluticasone alone or placebo.
Mometasone furoate/formoterol (dulera)
Mometasone furoate/formoterol (Dulera) is a combination product of the corticosteroid mometasone with the long-acting β2-agonist bronchodilator formoterol (see Chapter 6 for a discussion of formoterol). Dulera is available as an MDI in two strengths (mometasone/formoterol): 100 μg/5 μg and 200 μg/5 μg, respectively). In two randomized, double-blind, placebo-controlled studies involving more than 1500 patients, Dulera was able to increase lung function as a combination drug better than formoterol alone or placebo. Fewer patients reported a deterioration on Dulera than formoterol alone (manufacturer’s literature).
Budesonide/formoterol (symbicort)
Budesonide/formoterol (Symbicort) is a combination product of the corticosteroid budesonide with the long-acting β2-agonist bronchodilator formoterol (see Chapter 6 for a discussion of formoterol). Symbicort is available as an MDI in two strengths (budesonide/formoterol): 80 μg/4.5 μg and 160 μg/4.5 μg, respectively). In two large, double-blind, placebo-controlled studies involving more than 100 patients, Symbicort was able to increase lung function as a combination drug better than either drug separately or placebo, and it was found that the combination therapy was able to increase lung function 15 minutes after administration (manufacturer’s literature).
Although combination products have the disadvantage of not allowing changes in dose for each drug separately and have been discouraged, there is evidence of a beneficial, complementary interaction between glucocorticoids and β-adrenergic agonists. The addition of long-acting bronchodilators to inhaled corticosteroids has no negative effect and shows improvements in lung function and symptom control, as shown in the clinical trial by Shapiro and associates,20 by Chung21 for Advair, and by Jenkins and colleagues22 for Symbicort.
• Steroids increase β2-adrenergic receptor transcription (upregulation of β receptors).21,23
• Inhaled corticosteroid therapy can provide partial protection against the development of tolerance to β2-adrenergic agonists.21
• Salmeterol has been shown to promote binding of the glucocorticoid receptor to the response element of the cell’s nuclear DNA, without the glucocorticoid present, in vascular cells, initiating the antiinflammatory effect at least partially.23
Intranasal corticosteroids
All of the steroids available as orally inhaled agents are also available in an intranasal formulation. Exact indications for the intranasal preparations vary by specific agent, but intranasal steroids generally are used to treat allergic or inflammatory nasal conditions and seasonal or perennial allergic or nonallergic rhinitis and to prevent recurrence of nasal polyps. Available preparations are listed in Table 11-2, with strengths and recommended doses. Other agents that are used to treat seasonal allergic rhinitis include H1-receptor antagonists (e.g., loratadine), cromolyn sodium (see Chapter 12), topical vasoconstrictors such as oxymetazoline or ephedrine, and anticholinergics such as ipratropium bromide.
Pharmacology of corticosteroids
Mode of action
Glucocorticoids are highly lipophilic and enter airway cells to bind to intracellular receptors.24 This mechanism of drug signaling action was described briefly in Chapter 2 in the discussion of the pharmacodynamics of lipid-soluble drugs that interact with intracellular receptors. Originally, investigators thought that corticosteroids or, more simply, steroids exerted antiinflammatory activity by stabilizing lysosomes within neutrophils; this prevented degranulation and an inflammatory response. In the mid-1960s, steroid receptors were discovered, and it was realized that steroids modify the inflammatory response by inducing gene expression within the cell. By the 1980s, it was shown that glucocorticoids induce gene expression for the antiinflammatory protein lipocortin, which inhibits the enzyme phospholipase A2 (PLA2), preventing the arachidonic acid cascade, which leads to prostaglandin synthesis and lipoxygenase products. However, now it is understood that PLA2 inhibition is only one of multiple mechanisms by which steroids attenuate the inflammatory response.6,25–27
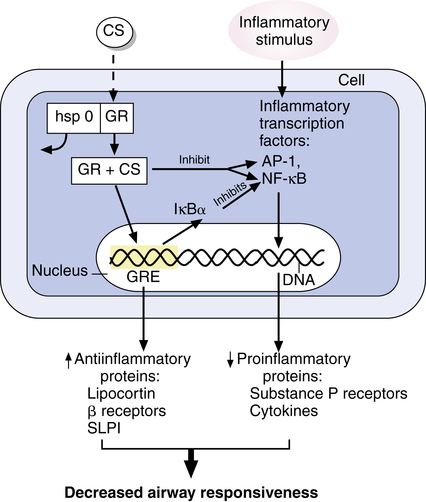
Steroids suppress a local or systemic inflammatory response by at least three general actions; these actions are illustrated in Figure 11-6. Generally, steroids diffuse into the cell and bind to a glucocorticoid receptor. Before binding by a steroid, the glucocorticoid receptor is in an inactive state and is bound to a protein complex termed heat shock protein 90 (hsp90), which prevents the unoccupied receptor from translocating to the nucleus of the cell. When the steroid binds to the receptor, hsp90 dissociates, and the steroid-receptor complex translocates to the cell nucleus. One general action (not always the first temporally) of a glucocorticoid is to upregulate the transcription of antiinflammatory genes for substances such as lipocortin, previously described.25–27 In the nucleus, the steroid produces this part of its effect on the cell by binding to portions of the nuclear DNA termed glucocorticoid response elements. Binding of the drug-receptor complex to glucocorticoid response elements upregulates, or induces, transcription of antiinflammatory substances such as lipocortin, neutral endopeptidase, secretory leukocyte protease inhibitor (SLPI), or inhibitors of plasminogen activator.6 These are all antiinflammatory substances. A second general action of glucocorticoids is the suppression of factors such as activator protein-1 (AP-1) and nuclear factor-κB (NF-κB), which cause transcription of genes involved in inflammation. This suppression may be by means of a direct interaction with these transcription factors, by which the transcription factor is inactivated before it induces gene expression in the nucleus.25 Direct inactivation of AP-1 and NF-κB leads to downregulation of gene expression for proinflammatory mediators such as cytokines. NF-κB regulates genes that have increased expression in asthma, including genes for cytokines such as interleukins (e.g., interleukin-1 [IL-1], interleukin-3 [IL-3]); chemokines; tumor necrosis factor-α (TNF-α); nitric oxide synthase, which produces nitric oxide in the airway; and adhesion molecules, which promote recruitment and attachment of leukocytes (eosinophils and basophils) from the circulation to the airway endothelium.14,25,26 A direct inactivation of inflammatory transcription factors such as AP-1 or NF-κB may account for the rapidity with which some cellular effects of steroids are seen and that are not well explained by the time needed for modification of gene expression within a cell. A third action of glucocorticoids is to upregulate the expression of inhibitors of NF-κB, such as the inhibitor protein IκBα. This inhibitor of NF-κB further suppresses gene expression for proinflammatory proteins, such as cytokines.26–28
The general result of these actions is to induce gene expression for antiinflammatory proteins and receptors and to suppress gene expression for proinflammatory proteins. Overall, glucocorticoids inhibit the cytokine production responsible for recruitment and migration of inflammatory cells such as eosinophils and lymphocytes into the airway. Examples of cytokines that are suppressed through gene suppression activity of steroids are listed in Box 11-2.
Glucocorticoids inhibit many of the cells involved in airway inflammation, including macrophages, T lymphocytes, eosinophils, and mast cells in the bronchial airway epithelium and submucosa, and reverse the shedding of epithelial cells and goblet cell hyperplasia seen in asthma.25,30 By decreasing cytokine-mediated survival of eosinophils, apoptosis of eosinophils occurs, reducing the number of eosinophils in the circulation and in the airway of subjects with asthma. Glucocorticoids also reduce the number of mast cells within the airways; mast cells are sources of histamine and other mediators of inflammation and inhibit plasma exudation and mucus secretion in inflamed airways.24,29
Effect on β receptors
β-adrenergic agents are among the most potent inhibitors of mast cell release, yet an individual with asthma in an acute episode may be unresponsive to these drugs. A beneficial effect of glucocorticoids is their ability to restore responsiveness to β-adrenergic stimulation.23,30 This effect can be seen within 1 to 4 hours after intravenous administration of glucocorticoids and is the rationale for administering a bolus of steroid in status asthmaticus as part of acute treatment. Although steroid action is slow, the sooner steroids are given, the sooner the asthmatic begins to respond to β-adrenergic drugs, and supported ventilation may be avoided. Glucocorticoids enhance β-receptor stimulation by increasing the number and availability of β receptors on the cell surfaces and by increasing affinity of the receptor for β agonists. There is also evidence that glucocorticoids prolong endogenous circulatory catecholamine action by inhibiting the uptake-2 mechanism. The mechanisms for a positive interaction between β2 agonists and corticosteroids are described in the discussion at the end of the section, Aerosolized Corticosteroid Agents.
Hazards and side effects of steroids
Systemic administration of steroids
The complicating side effects of systemic steroid treatment are well known and provide the motivation to switch to aerosolized, inhaled steroids when possible. These complications arise from the physiologic effects of steroids on the body. These physiologic effects are often exaggerated with systemic drug therapy because potency and plasma levels are higher than with the body’s own steroids. Complications of systemic therapy are reviewed by Truhan and Ahmed.31 These complications are summarized in Box 11-3 and are briefly described here:
• Suppression of the HPA axis by exogenous steroids may occur, causing inhibition of ACTH release and cortisol secretion from the adrenal gland. The length of time to recover from this suppression varies with patient, dose, and duration of treatment.
• With sufficient dose and duration, immunosuppression can be caused by systemic use of steroids; this can lead to increased susceptibility to infection by bacterial, viral, or fungal agents.
• Psychiatric reactions can occur, including insomnia, mood changes, and bipolar or schizophrenic psychoses.
• Cataract formation has been noted, and, rarely, intraocular pressure may increase with systemic steroid therapy.
• Myopathy of striated skeletal muscle can occur.
• Steroid-induced osteoporosis is debated, but is thought to be a limitation of extended steroid therapy. Aseptic necrosis of the bone is also caused by steroid therapy.
• Peptic ulcer is thought to be a complication of steroid therapy, but evidence for this is debated. Patients may often be receiving other ulcerogenic medications such as aspirin or nonsteroidal antiinflammatory drugs (NSAIDs).
• Fluid retention can occur as a result of the sodium-sparing effects of glucocorticoids, giving a puffy appearance.
• Hypertension may accompany the fluid retention or be aggravated by it.
• Corticosteroids given systemically can increase the white blood cell count, with an increase in neutrophils and a decrease in lymphocytes and eosinophils.
• Dermatologic changes can occur with steroid therapy, including a redistribution of subcutaneous fat causing the cushingoid appearance of central obesity, humpback, and moon face.
• Growth of children can be slowed by prolonged systemic therapy because corticosteroids retard bone growth and epiphyseal maturation.
• Corticosteroids lead to gluconeogenesis and antagonize glucose uptake, causing hyperglycemia. This can lead to reversible steroid-induced diabetes.
Systemic side effects with aerosol administration
The rationale for the introduction of inhaled aerosol steroids was to eliminate or reduce the side effects seen with systemic therapy. Although aerosol steroids are administered in low doses because of their high topical activity, local side effects may occur, and certain systemic side effects, listed in Box 11-4, are also a concern. Some side effects may occur with transfer from oral therapy to the inhaled route. Three systemic effects of concern with inhaled steroids are HPA suppression, loss of bone density, and growth restriction in children. Possible systemic side effects with inhaled steroids are the following:
• Adrenal insufficiency may occur after transfer from systemic to inhaled aerosol steroids. Weaning from systemic steroids to allow recovery of adrenal cortex and HPA function and careful monitoring of pulmonary function can help control this problem.
• There may be a recurrence of allergic inflammation in other organs, such as nasal polyps or atopic dermatitis, after cessation of systemic steroids.
• Acute severe episodes of asthma may occur after withdrawal from oral steroids and transfer to inhaled forms. Aerosolized steroids may be inadequate to control asthma, especially during periods of stress, and short courses of oral drug may be necessary (“burst” therapy).
• Suppression of HPA function is nonexistent or low at small doses of inhaled aerosol steroids and increases with higher doses. Clinically significant suppression is rare at inhaled doses less than 800 μg/day in adults and less than 400 μg/day in children.32 Goldberg and associates33 investigated MDI beclomethasone administration in children with and without a reservoir. They found that 7 of 15 subjects using the MDI alone (average dose 474 ± 220 μg/day) showed adrenal suppression as measured by 24-hour urinary free cortisol excretion. Only 2 of 24 subjects using an MDI-reservoir system (average dose 563 ± 249 μg/day) showed such suppression. These results indicated that inhalation of low to moderate doses can cause some adrenal suppression and that use of a reservoir can reduce this, probably by reducing the amount of drug swallowed. Although higher inhaled doses of steroid have a greater risk of adrenal suppression, the dose at which the risk for toxicity outweighs the beneficial effect of an inhaled steroid is unknown.32
• Questions have been raised about the effect of inhaled steroids on growth when used in prepubertal children. A study by Wolthers and Pedersen34 found a reduction in rate of lower leg growth with inhaled budesonide compared with placebo. Growth restriction was seen in some studies with beclomethasone dipropionate, but other studies have found no effect on growth with the same drug by inhalation. Results may be confounded by the moderate growth restriction and delay in puberty seen as a result of asthma.3 The authors cite a meta-analysis that found no association between growth impairment and inhaled beclomethasone dipropionate, even at higher doses.35 Sharek and Bergman36 found in a meta-analysis that the use of beclomethasone and fluticasone resulted in a decrease in growth. The benefits of inhaled corticosteroids in the treatment of asthma outweigh the possible consequence of growth reduction, however.
• No data have shown clearly the effect of inhaled glucocorticoids in asthma on bone density and osteoporosis. However, Israel and others37 discovered that the higher the dose of inhaled corticosteroid, the greater the effect on bone density seen in premenopausal, asthmatic women.
It is logical that the risks of steroid-induced adverse effects are lower with the relatively low doses of inhaled steroids compared with systemic administration. However, the threshold doses by inhalation causing adrenal suppression or other effects are unknown. These effects generally are rare with doses of 800 μg/day or less in adults and 400 μg/day or less in children. Absorption of inhaled steroid leads to systemic bioavailability, from both the swallowed portion and the inhaled portion reaching the lung. Table 11-3 summarizes data on the bioavailability of four agents. When administered orally, bioavailability ranges from less than 1% to more than 20% of the total dose; this is due to the high first-pass metabolism of the swallowed drug. In contrast, all of the inhaled dose reaching the lung is absorbed and enters the systemic circulation, where it is ultimately metabolized in the liver or extrapulmonary tissues.11 The efficiency of the delivery device in depositing drug in the lungs determines the amount of drug entering the systemic circulation from the airway. Thorsson and colleagues38 reported that an MDI of budesonide delivered 18% of the dose to the lungs, whereas a DPI preparation (Turbuhaler) delivered nearly twice as much (32%). Leach and colleagues39 found that HFA formulations gave more than CFC formulations, with 53% of HFA beclomethasone deposited in the lung.
TABLE 11-3
Bioavailability of Oral and Inhaled Corticosteroid Agents
| ORAL (%)* | INHALED (%)† | |
| Beclomethasone dipropionate | <20 | <20 |
| Triamcinolone acetonide | 22.5 | 21.5 |
| Budesonide | 11 | 25.0 |
| Fluticasone propionate | <1 | 20.0 |
| Des-ciclesonide | <1 | 31.0 |
| Mometasone furoate | <1 | 19.0 |
*Figures represent percentages of a 100% oral dose.
†Figures represent the 20% of an inhaled dose that reaches the lungs and indicate complete absorption of that fraction.
Modified from Winkler J, Hochhaus G, Derendorf H: How the lung handles drugs: pharmacokinetics and pharmacodynamics of inhaled corticosteroids, Proc Am Thorac Soc 1:356-363, 2004.
Topical (local) side effects with aerosol administration
Oropharyngeal fungal infections
Infections with C. albicans or Aspergillus niger may occur in the mouth, pharynx, or larynx with aerosolized steroid treatment. Some form of this may be seen in one-third of patients taking the aerosol formulations; however, these infections respond to topical antifungal agents and seem to diminish with continued aerosol steroid use.8 Occurrence and severity are dose-related and are more likely in patients who are also taking oral steroids. The use of a spacer device and gargling after treatment can reduce oropharyngeal deposition of the steroid and the incidence or severity of oropharyngeal fungal infections.
Dysphonia
Hoarseness and changes in voice quality also may occur with inhaled steroids in one-third of patients. This dysphonia can be minimized by use of a spacer and by gargling. The effect is caused primarily by adductor vocal cord paresis, which is thought to be a local steroid-induced myopathy.40
Other complications or precautions
• Cough and bronchoconstriction: Occasionally, cough or bronchoconstriction may occur after inhalation of an aerosol steroid.8
• Incorrect use: Incorrect use of the MDI delivery vehicle represents a possible risk factor because inadequate amounts of the topical inhaled steroid are delivered.
With inhaled steroids, the following can minimize the risk of local and systemic adverse effects:
Clinical application of aerosol steroids
Use in asthma
The 2009 Global Initiative for Asthma (GINA)4 and the 2007 National Asthma Education and Prevention Program Expert Panel Report 3 (NAEPP EPR-3), Guidelines for the Diagnosis and Management of Asthma,1 identify corticosteroids as long-term control agents rather than quick-relief agents. Corticosteroids traditionally have been used in asthma by the oral route for maintenance therapy of severe asthma, by the oral or intravenous route for treatment of status asthmaticus, and by inhalation for maintenance of asthma control. However, increased emphasis on asthma as a disease of inflammation leading to bronchial hyperresponsiveness has shifted the use of inhaled aerosol steroids from second-line or third-line therapy to first-line, primary therapy. The use of corticosteroids can control asthma and improve asthma symptoms by reducing exacerbations and improving lung function.1
• Bronchial hyperresponsiveness is characteristic of asthma and is related to the degree of airway inflammation.
• The basic pathology of asthma, previously emphasizing bronchospasm, is now understood to be a chronic inflammatory disorder of the airways resulting from a complex interaction among inflammatory cells, mediators, and airway tissue.1 The phrase “chronic desquamating eosinophilic bronchitis” has been used to describe asthma.8
• Inhaled corticosteroids are considered to be the most effective long-term therapy for mild, moderate, or severe persistent asthma, and they are well tolerated and safe at recommended dosages.1,4 Several points are related to the use of inhaled steroids:
• Barnes41 suggested starting inhaled corticosteroids at a high enough dose to be effective and then reducing the dose. Alternatively, a short course of systemic corticosteroids can be used to gain control of symptoms followed by a step-down in therapy.1 Loss of patient confidence and compliance with prescribed use of inhaled corticosteroids may be avoided in this way, especially because steroids do not give an immediate effect as a bronchodilator does. Any reduction in pharmacologic management should be monitored by symptoms, concomitant need for β2 agonists, and peak flow rates.
• An increase in dose of inhaled corticosteroids, such as doubling of the current dose, if peak expiratory flow rates decline 25% to 30% may avoid the need for oral steroids. However, controlled studies are needed to confirm the effectiveness of such practice.41
• If asthma is not controlled by inhaled corticosteroids and other types of drug therapy, a short burst of oral steroids may be required to regain control of the asthma and to help clear the airways.1,9
Early use of corticosteroids in asthma
There is evidence that the addition of an inhaled corticosteroid to first-line β-agonist maintenance treatment of asthma reduces morbidity and airway hyperresponsiveness.1,42 Haahtela and associates43 showed that subjects with mild asthma maintained on inhaled budesonide (1200 μg/day for 2 years and then 400 μg/day) had decreased bronchial response to histamine challenge compared with subjects taking inhaled terbutaline (375 μg twice daily) over a 2-year period. Perhaps the most significant finding was that the later addition of inhaled budesonide after use of a β2 agonist was unable to give as high a level of bronchoprotection as achieved by subjects who had started with and continued taking the inhaled steroid. This finding suggests that irreversible changes had occurred during the 2 years of β2-agonist therapy and supports earlier use of inhaled steroids.
Pauwels and colleagues44 found that early treatment in mild persistent asthma with low-dose corticosteroids decreased exacerbations, increased symptom-free days, improved FEV1, and decreased the need for systemic corticosteroids. In a meta-analysis, Masoli and colleagues45 reported that inhaled corticosteroids are best in treating asthma when kept to a therapeutic range of 400 μg/day. Although inhaled corticosteroids are first-line antiinflammatory agents and acceptable for primary therapy of moderate asthma in children, the antiasthmatic prophylactic agents cromolyn sodium, nedocromil sodium, and leukotriene modifiers may be used as an initial choice for long-term control therapy of mild persistent asthma (step 2 therapy) in children because these medications have excellent safety profiles.1
Inhaled corticosteroids for acute severe asthma
Inhaled corticosteroids have not been considered useful for treatment of acute, severe asthma episodes, and drug labeling contraindicates this use because there is no bronchodilator effect. In addition, the dose of inhaled steroids is low compared with oral administration. A study by Rodrigo and Rodrigo46 examined the addition of high, cumulative doses of inhaled flunisolide added to albuterol in emergency department treatment of acute adult asthma. Both drugs were given by MDI with a spacer. Flunisolide was given as 4 puffs (250 μg/actuation) every 10 minutes. Their protocol allowed 3 hours of this treatment, with a cumulative dose of 6 mg of flunisolide each hour, and equally aggressive albuterol dosing. The use of flunisolide resulted in better lung function at 90 minutes and afterward compared with the use of albuterol alone. Although preliminary, these results suggest that the contraindication to the use of inhaled corticosteroids for treating acute severe asthma may need to be reconsidered.
Clinical use of inhaled corticosteroids
Other considerations in the clinical application of inhaled corticosteroids are as follows:
• High-dose inhaled steroids can be tried in cases of severe, persistent asthma to replace or reduce oral corticosteroid dependence. High doses of inhaled steroids are two to four times the usual recommended dose. Oral steroid therapy can be reduced slowly while monitoring the patient’s pulmonary function.1 (Note that relative inhaled doses considered low, medium, and high are given in the NAEPP guidelines.)
• Although more control may be achieved with high doses of inhaled steroids, side effects, including systemic effects, are also likely to increase with inhaled doses greater than 1 mg/day. However, if oral steroids can be replaced or even reduced, this can be an overall improvement in the risk-to-benefit ratio.
• MDI-formulated corticosteroids should be administered for oral inhalation using a reservoir device (preferably a holding chamber rather than a spacer), and all formulations should include mouth rinsing to reduce the risk of oropharyngeal candidiasis or other fungal infections and to reduce systemic absorption from swallowed drug.
• Use of a long-acting β2 agonist such as salmeterol in subjects with inadequate symptom control, who are already receiving low to moderate doses of inhaled corticosteroids, may prevent the need to increase the inhaled corticosteroid dose.1
• The use of long-term β-agonist therapy with corticosteroid can improve lung function.1
• Compliance with prescribed steroid therapy by inhalation seems to be poor and can be a complicating factor in the management of asthma and COPD. The ability to reduce agents or move to once-a-day dosing may be helpful.
Use in chronic obstructive pulmonary disease
The use of steroids in COPD is recognized as having potential action in relieving symptoms and exacerbations, but steroid use has little to no effect on FEV1. The use of corticosteroids is described in the 2004 ATS guidelines2 and in the 2009 GOLD guidelines.3 A review and update on COPD have been provided by Fabbri and colleagues.47
COPD is characterized by a different pattern of inflammatory cells than is seen in asthma.48,49 Eosinophils predominate in asthma, whereas neutrophils predominate in COPD. Oral and inhaled corticosteroids do not influence the inflammatory changes driven by neutrophils.26,48,49
Available studies show that corticosteroid use in COPD reduces exacerbations, symptoms, and mortality50 but has little effect on pulmonary function results.51–54 Other studies have found that inhaled corticosteroids may slow the decline of FEV1.53,55
In acute exacerbations of COPD, oral or parenteral steroids are often given. Short-term corticosteroid therapy has shown benefit in hospitalized patients.3,4 Maltais and associates56 found that 2 mg of liquid, nebulized budesonide improved FEV1 compared with placebo and had similar results to 30 mg of oral prednisolone. Use of inhaled corticosteroids is much safer than oral steroid use. Patients with stable COPD should not be given systemic corticosteroids.2