11 Coronary Artery Disease
Ischemia, Infarction, and Complications
Wall Motion Abnormalities and Their Relation to Blood Flow
Although echocardiographic estimates using wall motion correlate well with autopsy estimates of infarction,1,2 in general, echocardiography tends to overestimate the amount of ischemic/infarcting muscle.3 Pathologic:echocardiographic correlations
Echocardiography for Wall Assessment in Acute Myocardial Infarction
In terms of 1-year mortality, a WMS <2 has high predictive value for a good prognosis and low complication rate. A WMS >7 on admission predicts Killip Class 3 or 4 with a sensitivity of 88%, specificity of 57%, positive predictive value of 35%, and negative predictive value of 95%, and may, therefore, be helpful in identifying early low-risk patients.4 The degree of systolic function of the nonischemic/infarcting myocardial segments is largely a function of the adequacy of perfusion to these other territories. The normal response of noninfarcting myocardium is hyperkinesia. The absence of hyperkinesia correlates with more extensive CAD (e.g., prior infarction, acute ischemia, stunning, or hibernation), and high risk for early mortality.4,5 Echocardiography is superior to electrocardiography in determining infarct extension.6 Echocardiographic assessment of left ventricular systolic function (presence/absence of WMAs) in patients presenting to the emergency department with cardiac-related symptoms is able to stratify patients (Table 11-1).
Echocardiographic Views to Assess Left Ventricular Wall Motion and its Relation to Coronary Arteries
Apical 5-Chamber View
 The A5CV images through the anterior septum; therefore, this is predominantly LAD territory.
The A5CV images through the anterior septum; therefore, this is predominantly LAD territory.
 The apex is usually LAD territory.
The apex is usually LAD territory.
 The lateral wall perfusion may reflect any combination of LAD–diagonal branches, left circumflex–obtuse marginal branches, and right coronary distal posterolateral branches.
The lateral wall perfusion may reflect any combination of LAD–diagonal branches, left circumflex–obtuse marginal branches, and right coronary distal posterolateral branches.
 A5CV sampling, as with all “apical” views, is prone to foreshortening.
A5CV sampling, as with all “apical” views, is prone to foreshortening.
 The A4CV images through the inferior interventricular septum.
The A4CV images through the inferior interventricular septum.
 The basal portion is typically perfused by septal branches of the posterior descending artery.
The basal portion is typically perfused by septal branches of the posterior descending artery.
 The lateral wall perfusion may reflect any combination of LAD–diagonal branches, left circumflex–obtuse marginal branches, and right coronary distal posterolateral branches.
The lateral wall perfusion may reflect any combination of LAD–diagonal branches, left circumflex–obtuse marginal branches, and right coronary distal posterolateral branches.
Coronary Care Unit Echocardiographic Studies
Scanning Issues/Required Parameters to Obtain from Scanning
 LV quantification (Simpson’s for ejection fraction, end-systolic volume)
LV quantification (Simpson’s for ejection fraction, end-systolic volume)
 Stroke volume, cardiac insufficiency, right ventricular systolic pressure
Stroke volume, cardiac insufficiency, right ventricular systolic pressure
 Weight and height for body surface area
Weight and height for body surface area
 Note on the worksheet if the patient was on inotropes (e.g., dobutamine, milrinone) or an intra-aortic balloon pump.
Note on the worksheet if the patient was on inotropes (e.g., dobutamine, milrinone) or an intra-aortic balloon pump.
 If a cardiogenic shock case, have the echocardiography attending review the case before disconnecting.
If a cardiogenic shock case, have the echocardiography attending review the case before disconnecting.
 If on an intra-aortic balloon pump, partial rolling of the patient may be acceptable in some cases, at the discretion of the attending in the cardiac care unit.
If on an intra-aortic balloon pump, partial rolling of the patient may be acceptable in some cases, at the discretion of the attending in the cardiac care unit.
Echocardiography for the Assessment of Complications of Acute Myocardial Infarction
Pump Failure
Left Ventricular Systolic Dysfunction and Failure
LV pump failure is the dominant cause (>65%) of in-hospital mortality from acute MI. It may occur with a first, and massive, infarction, or with a second or third infarction. Severe WMAs in extensive territories are expected in cardiogenic shock due to pump failure. The LV may or may not be dilated. Massive acute first MI is unlikely to have prominent dilation. It is the combination of abrupt fall in ejection fraction and lack of dilation that is responsible for the low stroke volume, and resultant low output, despite tachycardia.
Mechanical Complications of Infarction
Free Wall Rupture
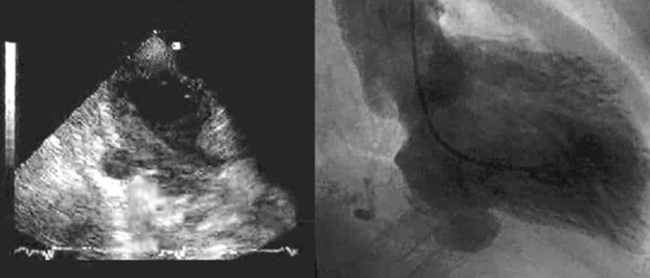
Free wall rupture accounts for approximately 10% of postinfarction sudden deaths. Rupture typically occurs through the lateral (but it may be through any) wall of the left ventricle. The right ventricle is one-seventh as likely to rupture as the LV. Rupture occurs only following a transmural infarction. The time is typically 3 to 5 days following MI, but may be earlier, especially if the patient received fibrinolytics. A subset of patients with free wall rupture will not experience immediate sudden death: this group is said to have “subacute rupture”7 with echocardiographic signs of tamponade, pericardial effusion >5 mm, and echodensities in the pericardial space that represent coagulated blood. Suspected clot in the pericardial space, which appears as an echo-dense mass in the pericardial space, may be a sign of free wall rupture8 and renders the likelihood of evacuation through a pericardiocentesis needle unlikely. Urgent surgical repair will salvage some cases. Bedside echocardiography is the most suitable test to identify free wall rupture. Ultimately, there is no perfect echocardiographic sign to distinguish severe early postinfarction tamponade from rupture. Identification of clot in the pericardial space is useful, however.
Left Ventricular (or Other) False Aneurysm
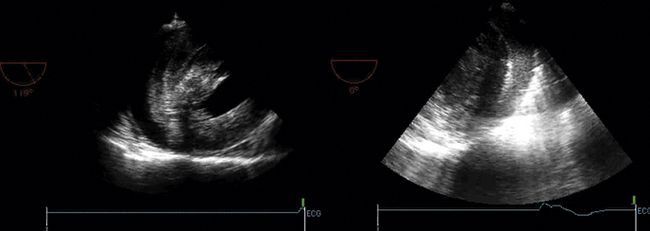
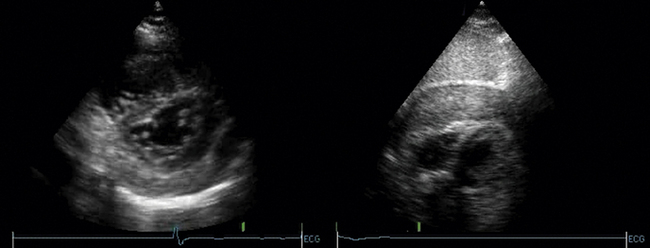
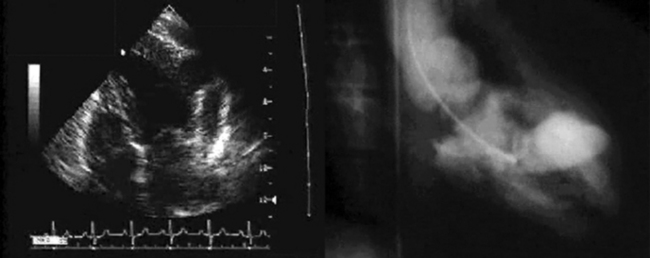
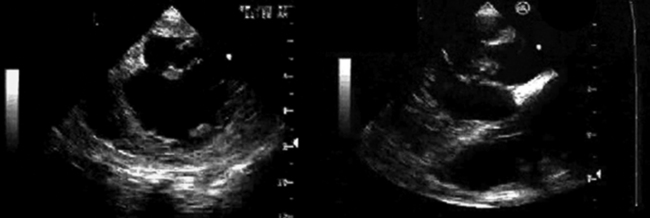
Ventricular false or “pseudo” aneurysms contain no myocardial layer; a free wall rupture is contained by adherence of overlying pericardium or by a thin layer of epicardium beneath pericardium. A false aneurysm develops because a tear into a portion of recently transmurally infarcted myocardium extended up to the epicardium or even through it, and complete rupture of the LV into the pericardial cavity was avoided because pericardial adhesions (the result of prior pericarditis) contained the rupture. Thus, a false aneurysm is to be considered an intermediate form of rupture, and reparative surgery is urgently indicated. The contained rupture typically extends away from the neck to appear as an echo-free space with the shape of a mushroom cap. The neck usually is less than half the width of the body of the false aneurysm. This is in contradistinction to the wide neck of a (true) aneurysm, which usually is as wide as the body of the aneurysm itself (the dimension of the neck/the dimension of the body is 0.9 to 1.0).9 It is the width of the neck, more than any other detail, that renders the outpouching of the LV a false aneurysm rather than a true aneurysm. In systole, the false aneurysm typically bulges out as it receives part of the LV stroke volume. The flow in and out (or “to-and-fro”) is evident by color and spectral Doppler.10 Two-dimensional echocardiography is the procedure of choice to establish the presence of a pseudoaneurysm. Cineangiography and MRI also are excellent means to image false aneurysms. Confusion occurs about aneurysms of the base of the LV between the body of the posteromedial papillary muscle and the mitral annulus. Often the traction of the papillary muscle keeps the neck narrow, replicating the sign of a false aneurysm. Thus the “neck” sign is best applied away from the trouble spot on the posterolateral wall, where both aneurysms and false aneurysms occur, but where they cannot be easily distinguished by neck morphology alone.
Septal Rupture
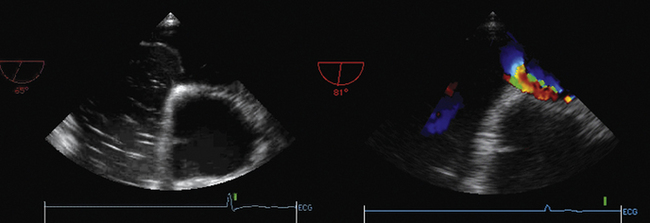
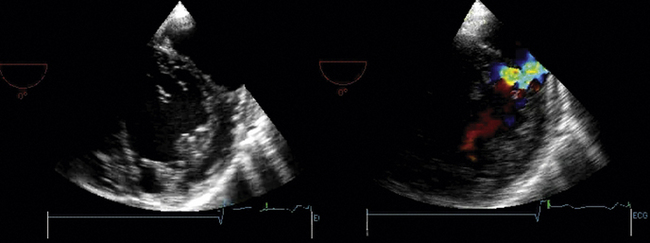
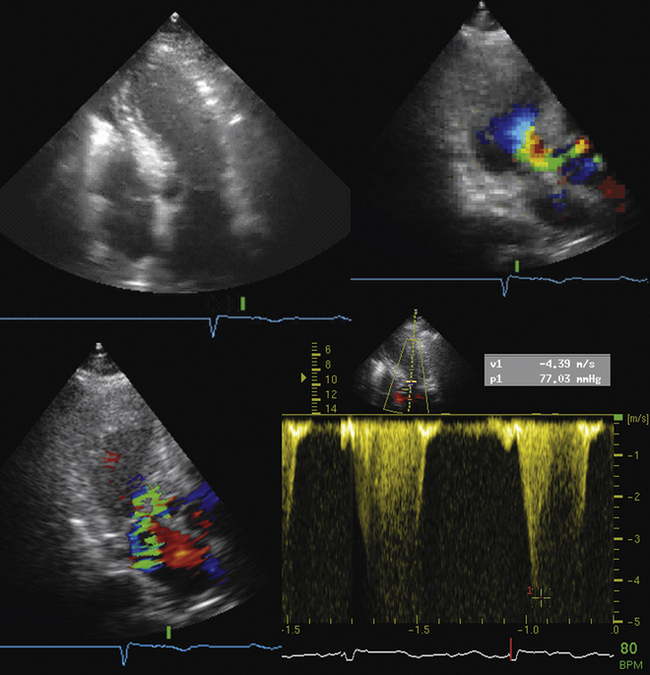
Associated RV MI is common, which indicates a poor prognosis.11 Two-dimensional echocardiography identifies the defect in more than half of cases, but color Doppler will find nearly 100% of defects.12–14 Agitated saline contrast studies are not needed. Urgent surgical repair is indicated. Patients with large concurrent RV infarctions do very poorly with or without surgery.
Papillary Muscle Rupture
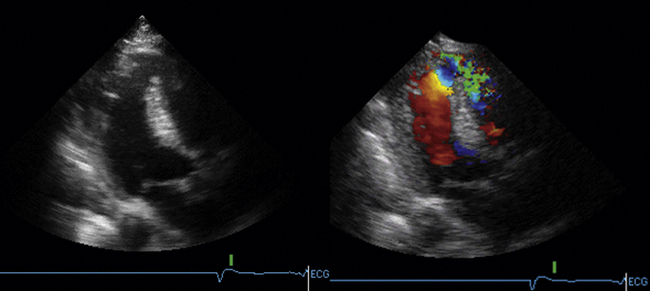
Papillary muscle rupture causes severe disruption of the mitral apparatus and leads to severe mitral insufficiency and marked reduction in forward cardiac output. Clinically, papillary muscle rupture is a severe event, progressive, and usually fatal if not successfully operated upon (80% mortality within 1 week). In the setting of inferior infarction, the posteromedial papillary muscle usually is the affected one. In the setting of anterolateral infarction, the anterolateral papillary muscle usually is the affected one. Overall, the posteromedial papillary muscle, which usually is supplied by the posterior descending artery alone, is 5 to 10 times more likely to rupture than the anterolateral one, which usually is supplied by both the posterior descending artery and the left circumflex coronary artery.15 In a short-axis view, the anterolateral papillary muscle is seen at 3 o’clock, and the posteromedial papillary muscle is seen at 7 o’clock. Of note, even tricuspid papillary muscles can rupture in the case of RV MI. Rupture may be complete or partial. Partial rupture results in severe MR, not cardiogenic shock. In a posterior long-axis view, the posteromedial papillary muscle or muscles are seen. On an A4CV, the anterolateral papillary muscle is seen. On TEE transgastric short-axis views, as with transthoracic short-axis views, both papillary muscle sets are seen. On a TEE long-axis view, both papillary muscles can be seen, although often with slight angulations to optimize the imaging of each. Imaging of papillary muscles requires specific views to assess their integrity. Zoom views are helpful. The echocardiographic signs of papillary muscle rupture depend on whether the rupture is complete or partial.
 Complete papillary muscle rupture
Complete papillary muscle rupture
Left Ventricular Aneurysm
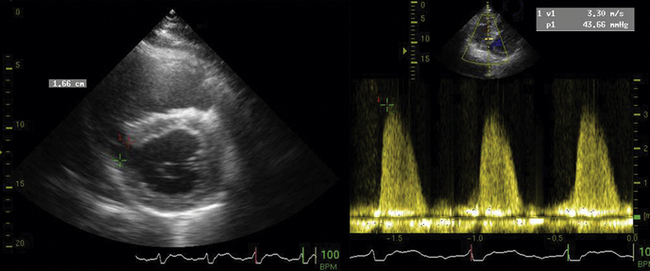
Two-dimensional echocardiography is the procedure of choice to establish the presence and size of ventricular aneurysms (sensitivity 93%, specificity 94%).16 Aneurysms may occur anywhere, but most commonly occur over the anterior or apical regions. Typically, a recently formed aneurysm is dyskinetic. Chronically, as they scar and often calcify, they become less dyskinetic. Early formation of aneurysm (<5 days) is associated with high mortality (80% at 1 year).17,18 True aneurysms of the LV only rarely rupture. They commonly are associated with intracavitary clot, as the flow within them is stagnant, and permissive of thrombus formation.19–22 The size of the aneurysm may be described in several ways, including the ratio of the circumferential length of the aneurysm to that of the overall LV, and area of the aneurysm to the overall LV area. Patients with circumference ratios >0.4 or area ratios >0.3 have significantly more congestive heart failure and mortality.18,22 The location of the aneurysm and the systolic function of the remainder of the LV are considered when surgical aneurysmectomy is entertained. Surgical aneurysmectomy is not a primary indication for surgery, but may be entertained when coronary bypass or valve surgery is the primary indication. If the aneurysm involves the papillary muscles, then surgical results are less good, as the mitral apparatus will be affected by the surgery. Similarly, if the condition of the nonaneurysmal LV (what will be left after the surgery) is poor, then the outcome will be poor.19,20
Other Complications
Intracavitary Thrombus
“Functional” Mitral Regurgiation
Transthoracic Echocardiography
ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography23
Myocardial Ischemia/Infarction with TTE
 Acute chest pain with suspected MI and nondiagnostic ECG when a resting ECG can be performed during pain
Acute chest pain with suspected MI and nondiagnostic ECG when a resting ECG can be performed during pain
 Evaluation of a patient without chest pain but with other features of an ischemic equivalent or laboratory markers indicative of ongoing MI
Evaluation of a patient without chest pain but with other features of an ischemic equivalent or laboratory markers indicative of ongoing MI
 Suspected complication of MI, including, but not limited to, acute mitral regurgitation, ventricular septal defect, free-wall rupture/tamponade, shock, RV involvement, HF, or thrombus
Suspected complication of MI, including, but not limited to, acute mitral regurgitation, ventricular septal defect, free-wall rupture/tamponade, shock, RV involvement, HF, or thrombus
ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography24
Recommendations for Echocardiography in the Diagnosis of Acute MI Syndromes
Recommendations for Echocardiography in Risk Assessment, Prognosis, and Assessment of Therapy in Acute MI Syndromes
Transesophageal Echocardiography
ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography23
TEE as Initial or Supplemental Test—General Uses
Cardiac Computed Tomography
ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac CT25
Cardiac Magnetic Resonance
ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 Appropriateness Criteria for Cardiac Computed Tomography and Cardiac Magnetic Resonance Imaging26
Evaluation of Ventricular and Valvular Function
 Procedures may include LV/RV mass and volumes, MR angiography, quantification of valvular disease, and delayed contrast enhancement
Procedures may include LV/RV mass and volumes, MR angiography, quantification of valvular disease, and delayed contrast enhancement
 Evaluation of LV function following MI or in heart failure patients
Evaluation of LV function following MI or in heart failure patients
 Evaluation of LV function following MI or in heart failure patients
Evaluation of LV function following MI or in heart failure patients
Patients with technically limited images from echocardiogram
Discordant information that is clinically significant from prior tests
Nuclear
ACCF/ASNC/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging27
Evaluation of LV Function
 Assessment of LV function with radionuclide angiography (ERNA or FP RNA)
Assessment of LV function with radionuclide angiography (ERNA or FP RNA)
In absence of recent reliable diagnostic information regarding ventricular function obtained with another imaging modality
 Routine† use of rest/stress ECG-gating with SPECT or PET MPI
Routine† use of rest/stress ECG-gating with SPECT or PET MPI
Appropriateness criteria: A, appropriate; I, inappropriate; U, uncertain.
1. Wilkins G.T., Southern J.F., Choong C.Y., et al. Correlation between echocardiographic endocardial surface mapping of abnormal wall motion and pathologic infarct size in autopsied hearts. Circulation. 1988;77:978-987.
2. Parisi A.F. The case for echocardiography in acute myocardial infarction. J Am Soc Echocardiogr. 1988;1:173-178.
3. Force T., Kemper A., Perkins L., et al. Overestimation of infarct size by quantitative two-dimensional echocardiography: the role of tethering and of analytic procedures. Circulation. 1986;73:1360-1368.
4. Jaarsma W., Visser C.A., Eenige van M.J., et al. Predictive value of two-dimensional echocardiographic and hemodynamic measurements on admission with acute myocardial infarction. J Am Soc Echocardiogr. 1988;1:187-193.
5. Jaarsma W., Visser C.A., Eenige van M.J., et al. Prognostic implications of regional hyperkinesia and remote asynergy of noninfarcted myocardium. Am J Cardiol. 1986;58:394-398.
6. Isaacsohn J.L., Earle M.G., Kemper A.J., et al. Postmyocardial infarction pain and infarct extension in the coronary care unit: role of two-dimensional echocardiography. J Am Coll Cardiol. 1988;1988(11):246-251.
7. Lopez-Sendon J., Gonzalez A., Lopez D.S., et al. Diagnosis of subacute ventricular wall rupture after acute myocardial infarction: sensitivity and specificity of clinical, hemodynamic and echocardiographic criteria. J Am Coll Cardiol. 1992;19:1145-1153.
8. Knopf W.D., Talley J.D., Murphy D.A. An echo-dense mass in the pericardial space as a sign of left ventricular free wall rupture during acute myocardial infarction. Am J Cardiol. 1987;59:1202.
9. Gatewood R.P.Jr., Nanda N.C. Differentiation of left ventricular pseudoaneurysm from true aneurysm with two dimensional echocardiography. Am J Cardiol. 1980;46:869-878.
10. Roelandt J.R., Sutherland G.R., Yoshida K., et al. Improved diagnosis and characterization of left ventricular pseudoaneurysm by Doppler color flow imaging. J Am Coll Cardiol. 1988;12:807-811.
11. Moore C.A., Nygaard T.W., Kaiser D.L., et al. Postinfarction ventricular septal rupture: the importance of location of infarction and right ventricular function in determining survival. Circulation. 1986;74:45-55.
12. Maurer G., Czer L.S., Shah P.K., et al. Assessment by Doppler color flow mapping of ventricular septal defect after acute myocardial infarction. Am J Cardiol. 1989;64:668-671.
13. Harrison M.R., MacPhail B., Gurley J.C., et al. Usefulness of color Doppler flow imaging to distinguish ventricular septal defect from acute mitral regurgitation complicating acute myocardial infarction. Am J Cardiol. 1989;64:697-701.
14. Helmcke F., Mahan E.F.III, Nanda N.C., et al. Two-dimensional echocardiography and Doppler color flow mapping in the diagnosis and prognosis of ventricular septal rupture. Circulation. 1990;81:1775-1783.
15. Barbour D.J., Roberts W.C. Rupture of a left ventricular papillary muscle during acute myocardial infarction: analysis of 22 necropsy patients. J Am Coll Cardiol. 1986;8:558-565.
16. Visser C.A., Kan G., David G.K., et al. Echocardiographic-cineangiographic correlation in detecting left ventricular aneurysm: a prospective study of 422 patients. Am J Cardiol. 1982;50:337-341.
17. Visser C.A., Kan G., Meltzer R.S., et al. Incidence, timing and prognostic value of left ventricular aneurysm formation after myocardial infarction: a prospective, serial echocardiographic study of 158 patients. Am J Cardiol. 1986;57:729-732.
18. Visser C.A., Kan G., Meltzer R.S., et al. Assessment of left ventricular aneurysm resectability by two-dimensional echocardiography. Am J Cardiol. 1985;1985(56):857-860.
19. Edwards B.S., Edwards W.D., Edwards J.E. Ventricular septal rupture complicating acute myocardial infarction: identification of simple and complex types in 53 autopsied hearts. Am J Cardiol. 1984;54:1201-1205.
20. Mann J.M., Roberts W.C. Rupture of the left ventricular free wall during acute myocardial infarction: analysis of 138 necropsy patients and comparison with 50 necropsy patients with acute myocardial infarction without rupture. Am J Cardiol. 1988;62:847-859.
21. Cabin H.S., Roberts W.C. True left ventricular aneurysm and healed myocardial infarction. Clinical and necropsy observations including quantification of degrees of coronary arterial narrowing. Am J Cardiol. 1980;46:754-763.
22. Matsumoto M., Watanabe F., Goto A., et al. Left ventricular aneurysm and the prediction of left ventricular enlargement studied by two-dimensional echocardiography: quantitative assessment of aneurysm size in relation to clinical course. Circulation. 1985;72:280-286.
23. Douglas P.S., Garcia M.J., Haines D.E., et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57(9):1126-1166.
24. Cheitlin M.D., Armstrong W.F., Aurigemma G.P., et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography—summary article. J Am Coll Cardiol. 2003;42(5):954-970.
25. Taylor A.J., Cerqueira M., Hodgson J.M., et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. J Am Coll Cardiol. 2010;56(22):1864-1894.
26. Hendel R.C., Patel M.R., Kramer C.M., et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Cardiol. 2006;48(7):1475-1497.
27. Hendel R.C., Berman D.S., Di Carli M.F., et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging. J Am Coll Cardiol. 2009;53(23):2201-2229.