Coronary Artery Disease in Children
Most coronary artery diseases in children are congenital.1,2 A large number of anomalous arrangements of the coronary arteries exist, but only a few have clinical significance. Among acquired diseases of the coronary arteries in children, Kawasaki disease (KD) is the most common. Other diseases include sequelae from trauma, vasculitides (see Chapter 82), radiation injury after oncologic treatment, and rare cases of familial hyperlipidemia and idiopathic infantile arterial calcification. Box 78-1 outlines the two principal manifestations of coronary artery diseases, together with the diseases in which they are commonly seen in children. In clinical practice, patients presenting with syncope, arrhythmia, or near sudden death usually are referred for coronary computed tomography (CT) or magnetic resonance imaging (MRI) to diagnose a coronary anomaly, to confirm and clarify echocardiographic findings of coronary anomalies, to map out the coronary arteries for surgical planning, and to evaluate the coronary tree for aneurysms and stenosis.
Imaging Considerations
Catheter angiography is the gold standard for the determination of luminal coronary abnormalities, such as coronary stenosis.3 During catheterization, the arterial wall can be characterized with intravascular ultrasound,4 and the hemodynamic significance of a stenotic coronary lesion on myocardial perfusion can be measured using the fractional flow reserve technique.5 In infants, transthoracic echocardiography is the first-line imaging tool for the evaluation of structural anomalies of the proximal coronary arteries. Echocardiography is usually adequate to exclude the major types of lethal anomalous coronary arteries. Echocardiography is less useful in older children, and it cannot visualize the entire coronary vasculature.
Cardiac-gated multidetector row CT angiography (CCTA) and cardiac magnetic resonance angiography (CMRA) are important noninvasive alternatives to catheter coronary angiography.6 Both techniques record three-dimensional images of the heart, with the coronary vessels shown in the context of adjacent cardiac structures. Visualization is facilitated by three-dimensional postprocessing. These capabilities have made CCTA and CMRA the preferred method for the diagnosis and characterization of anomalous coronary arteries.
The greatest concern regarding CCTA is that it uses ionizing radiation (see Chapter 66). Every measure must be taken to reduce the dose of ionizing radiation delivered to a patient. First, CCTA should be undertaken only if the diagnostic goals are achievable and the results affect management of the patient’s condition. As much as possible, scanning should be limited to a single pass over the heart only. Tube voltage and tube current should be kept to a minimum for the patient’s size and acceptable image quality. If possible, the pitch factor should be adjusted to the heart rate. Prospective ECG gating and ECG dose modulation should be used when appropriate. With these measures, dose-equivalent radiation is less than 10 mSv for retrospectively gated CCTA and 3 mSv for prospective gated or nongated CCTA.7 Noise reduction with iterative reconstruction currently is being investigated as a method to achieve diagnostic images at a very low radiation dose.8 A dose equivalent to less than 1 mSv for prospectively gated or nongated CCTA likely is achievable.
The lack of ionizing radiation makes CMRA an attractive imaging choice. On most clinically available magnetic resonance (MR) scanners, whole heart CMRA is built on a three-dimensional, cardiac-gated, navigated echo, T2-prepared, steady-state, free-precession sequence.9 Depending on coverage, spatial resolution, heart rate, and breathing pattern, imaging time typically is 10 to 20 minutes. For this amount of time, breath holding is impractical. Respiratory motion is managed by restricting data acquisition at a predetermined range of diaphragm position monitored in real time with the navigator echo technique. Contrast for the coronary vessels is based on the intrinsic long-T2 value of blood, and no gadolinium contrast administration is required, which is an important advantage in patients who have renal failure and the risk of gadolinium contrast–related nephrogenic systemic fibrosis.10 However, the T2-contrast mechanism is not selective, and all vascular channels are equally bright. Furthermore, other materials with a high T2 value, such as pericardial effusion and pleural effusion, are as bright as blood. This lack of differentiation sometimes can confound diagnostic interpretation.
Normal Coronary Artery Anatomy
Many anatomic variations of the coronary arteries exist, and the separation of normal variants from anomalous coronary arteries is arbitrary (Fig. 78-1). In persons with conventional coronary anatomy, two main coronary arteries originate from two of the three aortic sinuses of Valsalva closest to the pulmonary trunk. The three aortic sinuses are labeled according to their attached coronary arteries: the right coronary sinus giving rise to the right coronary artery (RCA), the left coronary sinus giving rise to the left main coronary artery (LMCA), and the noncoronary sinus giving rise to no coronary branch.
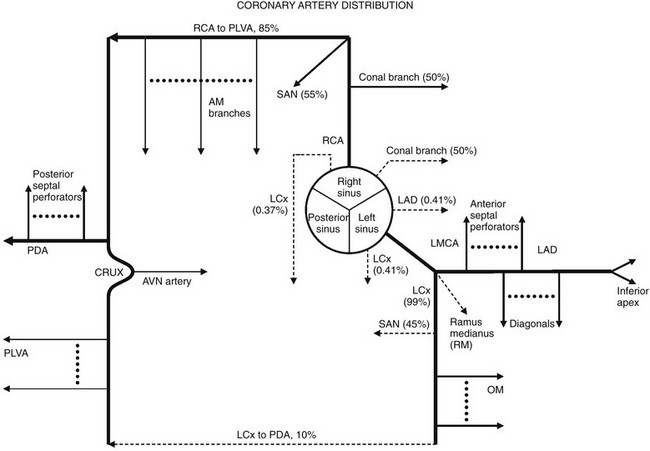
Figure 78-1 Coronary artery distribution diagram.
Solid lines represent the most common coronary artery pattern. Dashed lines represent common variants (prevalences in percentages). AM, Acute marginal; AVN, atrioventricular nodal; LAD, left anterior descending; LCx, left circumflex; LMCA, left main coronary artery; OM, obtuse marginal; PDA, posterior descending artery; PLVA, posterior left ventricular artery; RCA, right coronary artery; RM, ramus medianus; SAN, sinoatrial nodal.
After its takeoff from the right coronary sinus, the RCA courses anteriorly into the right atrioventricular groove. It gives rise to a right conal artery as the first branch 50% of the time. Otherwise, the right conal artery arises directly from the right coronary sinus. This conal artery supplies the myocardium of the right ventricular outflow tract (RVOT). In the majority of cases, a sinoatrial nodal artery branches from the proximal RCA (Fig. 78-2, A). Otherwise, it arises from the proximal left circumflex (LCx) artery. The middle portion of the RCA gives rise to one or more right ventricular branches (Fig. 78-2, B). These branches are called acute marginal branches, analogous to the obtuse marginal branches from the LCx artery. The distal portion of the RCA wraps around the inferior surface of the heart. In most people, the RCA gives rise to a posterior descending artery (PDA) (Fig. 78-2, C). The RCA then enters the “crux,” the intersection between the interventricular septum and the atrioventricular groove. At the crux, the RCA bends and forms an inverted U and then continues into the left atrioventricular groove at the bottom of the heart, where it gives off multiple posterior left ventricular arteries that supply the basilar inferior wall of the left ventricle. This pattern of the distal RCA is called a right-dominant coronary system and occurs in 85% of the population. An atrioventricular nodal branch often arises from the RCA near the apex of the inverted U (Fig. 78-2, D). The PDA gives rise to many inferior septal perforator branches that supply the inferior septum, analogous to the anterior septal perforator branches that originate from the left anterior descending (LAD) artery.
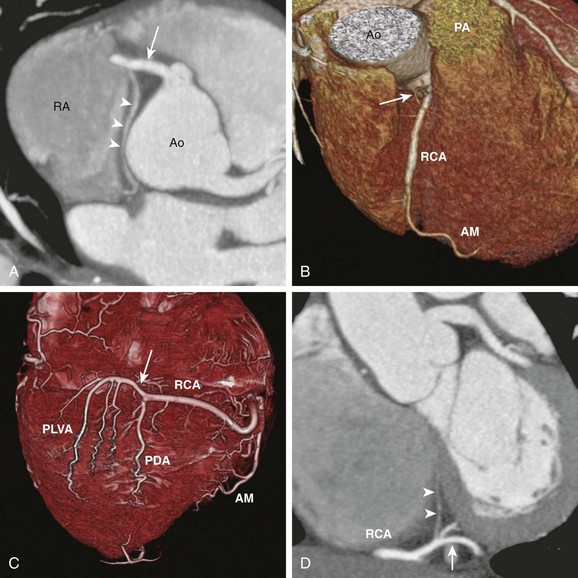
Figure 78-2 Major branches of the normal right coronary artery (RCA).
A, Axial cardiac-gated multidetector row computed tomography angiography image shows the RCA (arrow) originating from the aorta (Ao) and extending toward the right atrioventricular groove. It gives off a sinoatrial nodal branch (arrowheads) that terminates at the right atrium (RA) near the atrial septum. B, A volume-rendered image shows an acute marginal (AM) branch arising from the RCA at the right ventricular free wall. The conal branch (arrow) is the first branch of the RCA. PA, pulmonary artery. C, A volume-rendered image shows the inferior surface of a heart. The RCA gives off in succession the AM branch, the posterior descending artery (PDA), and the posterior left ventricular arteries (PLVA), which lie on the left side of the crux (arrow). D, A short-axis view shows the RCA at the crux (arrow) giving off an atrioventricular nodal branch (arrowheads).
The LMCA originates from the left coronary sinus and courses to the left for a short distance. In most people, it bifurcates into an LAD artery and an LCx artery. In other people, the LMCA trifurcates into an LAD artery, an LCx artery, and, in between, a ramus medianus branch (Fig. 78-3, A). This branch supplies the anterior left ventricular wall. The LAD artery gives rise to two sets of vessels: an epicardial set called the diagonal branches and an intramuscular set called the anterior septal perforator branches. The diagonal branches are responsible for perfusing the anterior left ventricular wall, whereas the septal perforator branches are responsible for the anterior septum. The distal LAD artery typically wraps around the cardiac apex and terminates at the inferior wall of the apex (Fig. 78-3, B). In a minority of cases, the LCx artery gives off a sinoatrial nodal branch (Fig. 78-3, C) before it enters the left atrioventricular groove. The LCx artery then gives rise to a number of obtuse marginal branches, which supply the lateral left ventricular wall (see Fig. 78-3, B). In 10% of the population, the LCx artery supplies the posterior left ventricular branches and the PDA instead of the RCA. This system is called a left-dominant coronary system. In 5% of the population, the RCA supplies the PDA while the LCx artery supplies the posterior left ventricular branches. This system is called a co-dominant coronary system.
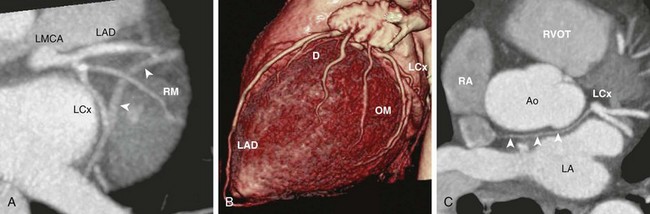
Figure 78-3 Major branches of the normal left main coronary artery (LMCA).
A, Oblique axial cardiac-gated multidetector row computed tomography angiography image shows the trifurcation of the LMCA into a left anterior descending (LAD) artery, a ramus medianus (RM) branch, and a left circumflex (LCx) artery. The crossing vessel (arrowheads) is the greater cardiac vein. B, A volume-rendered image shows the LAD and several diagonal (D) branches. The LAD wraps around the apex to reach the apical inferior surface. The LCx artery gives off several obtuse marginal (OM) branches. C, An axial image shows the LCx giving off a sinoatrial nodal branch (arrowheads) that travels behind the aorta (Ao) and terminates at the right atrium (RA) near the atrial septum. LA, left atrium; RVOT, right ventricular outflow tract.
Congenital Coronary Artery Anomalies
The true prevalence of congenital coronary artery anomalies is unknown. According to adult catheter angiographic data, 0.6% to 1.5% of patients were found to have coronary artery anomalies.11,12 The largest study of this type reviewed more than 126,000 coronary studies and revealed a prevalence of 1.3%.13 Of these anomalous cases, 80% were judged to have no clinical significance.14 Ectopic coronary origin and aberrant course is estimated to affect 1% of the population. The most common type is separate origins of the LAD and LCx arteries arising from the left sinus of Valsalva (0.41%) (Fig. 78-4, A). The next most common variation is an ectopic LCx artery arising from the right sinus or the RCA and then coursing posterior to the aorta (0.37%) (Fig. 78-4, B). Both configurations are clinically benign. Other examples of benign anomalies are an absent LCx artery with a superdominant RCA that reaches the anterior atrioventricular groove, an ectopic right or left coronary artery from the posterior sinus, and an ectopic left or right coronary origin from the ascending aorta. Clinically significant coronary artery anomalies are listed in Box 78-2.
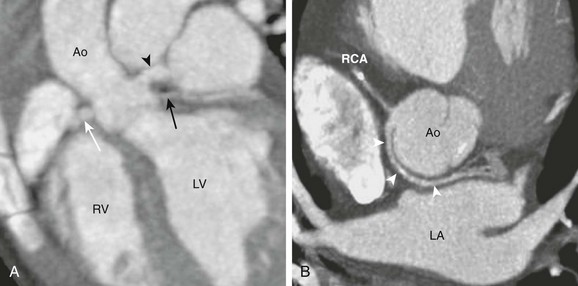
e-Figure 78-4 Common benign variants of the coronary arteries.
A, A horizontal long-axis view reconstructed from a coronary computed tomography angiogram of a 1-year-old boy shows separate origins for the left anterior descending (arrowhead) and the left circumflex (LCx) (black arrow) arteries. The right coronary artery (RCA) (white arrow) is in normal position. This variant occurs in 0.41% of the population. B, A short-axis view from a 40-year-old man shows the LCx artery and the RCA arising separately from the right sinus. The LCx artery (white arrowheads) wraps around posterior to the aorta (Ao) and then enters the left atrioventricular groove. This variant occurs in 0.37% of the population. LA, Left atrium; LV, left ventricle; RV, right ventricle.
Coronary Artery with Interarterial Course
Overview: An anomalous LMCA may originate from the right coronary sinus or from the RCA. Before bifurcating into the LAD and LCx arteries, the LMCA must travel toward the left by one of three routes: anterior to the RVOT, between the aorta and the pulmonary artery, or posterior to the aorta. Similarly, an anomalous RCA may originate from the left coronary sinus or from the LMCA. It must travel toward the right atrioventricular groove by one of these three routes. For both coronary arteries, the anterior and posterior courses are clinically benign, whereas the interarterial course—that is, between the aorta and the pulmonary artery (Fig. 78-5)—is associated with sudden death.15 An aberrant LMCA with an interarterial course is estimated to affect 0.03% to 0.05% of the population. An anomalous RCA with an interarterial course is more common, with a prevalence estimated at 0.1%. The interarterial segment of the coronary artery may be intramural within the aortic wall, intramuscular within the myocardium, or free between the aorta and the pulmonary artery.
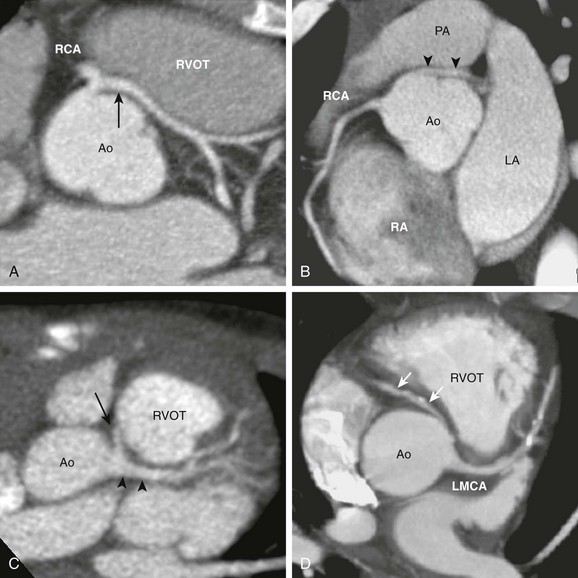
Figure 78-5 An anomalous coronary artery with an interarterial course.
A, The axial view from a cardiac computed tomography angiogram of a 10-year-old boy shows a common origin of the right coronary artery (RCA) and the left main coronary artery (LMCA) (arrow) arising from the right sinus of the aorta (Ao). The LMCA has a long interarterial course as it travels between the aorta and the right ventricular outflow tract (RVOT). B, In this 20-year-old man, an LMCA (arrowheads) originates from the right sinus separate from the RCA, then travels between the Ao and the pulmonary artery (PA). C, In this 2-year-old boy, the RCA (arrow) arises from the LMCA (arrowheads). D, In this adult with calcific coronary plaques, an RCA (arrows) originates from the left sinus separate from the LMCA. A tapered narrowing is evident at the proximal, interarterial portion of the RCA. LA, Left atrium; RA, right atrium.
The association between an anomalous coronary artery with an interarterial course and sudden death in young athletes was discovered in autopsy series.16–18 Sudden death in this otherwise healthy population is rare, with an incidence estimated to be 5 in 1 million people per year,19 with anomalous coronary arteries found in up to 20% of these cases. Most autopsy series report a greater number of anomalous LMCAs than anomalous RCAs, some by a ratio of 3 : 1. Given that the anomalous RCA is three times more common, the autopsy results suggest that anomalous LMCA is a more lethal lesion. The mortality rate of these lesions is unknown, because most patients have not been diagnosed and followed up prospectively.
In patients with lethal types of coronary anomalies, sudden death almost always occurs during exertion. Most of these patients who died had no known premonitory symptoms.20 In about 30% of the cases, syncope or chest pain occurred within 2 years of death, but resting ECG and stress ECG have been reported as normal. The pathophysiology of sudden death has not been conclusively determined. A lethal arrhythmia may be triggered by transient ischemia caused by inadequate coronary flow through either the slitlike opening of the anomalous coronary artery or the narrowed lumen compressed by the aorta and pulmonary artery.
In addition to interarterial coronary arteries, sudden death has been associated with an aberrant LMCA arising from the noncoronary sinus without an interarterial course.21 In this configuration, the alignment of the LMCA origin and the anterior interventricular groove is such that the LMCA must have a more acute takeoff angle from the noncoronary sinus (Fig. 78-6). This acute takeoff angle creates a narrowing at the ostium that is thought to be a factor leading to ischemia and sudden death.
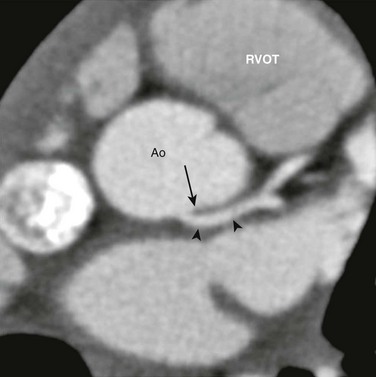
e-Figure 78-6 Computed tomography angiogram of an anomalous left main coronary artery (LMCA) from the noncoronary sinus.
The short-axis view from a coronary computed tomography angiogram of a 3-year-old girl shows the LMCA (arrowheads) arising from the noncoronary sinus just to the left of the commissure (arrow). Ao, Aorta; RVOT, right ventricular outflow tract.
Imaging: In neonates and young children, echocardiography sometimes provides sufficient visualization of the proximal coronary arteries to suggest the lethal anomalous coronary types previously discussed. These patients are referred for CMRA or CCTA to confirm the diagnosis and to map out the coronary vessels for surgical planning. In adolescents and young adults, the coronary arteries may not be adequately assessed with echocardiography. Anomalous coronary arteries are suspected on the basis of clinical symptoms, which often are nonspecific. The pretest probability of disease usually is low. To avoid exposing a large number of normal patients to radiation, CMRA should be the first test of choice. If the CMRA is not diagnostic, then CCTA can be used as a backup test. Diagnostic catheter angiography does not have much of a role for this indication.
Treatment: Because most deaths occur in patients between 10 and 30 years of age, teenagers and young adults who are diagnosed with an anomalous coronary artery with an interarterial course or rare types of anomalous LMCA from the noncoronary sinus should consider surgery with the goal of preventing sudden death. Preventive surgery in patients outside this age group is controversial, because their risk of sudden death is not known. The surgical approach depends on whether the interarterial segment is intramural or free. If it is intramural, then the ostium can be surgically enlarged by unroofing the common wall between the aorta and the coronary segment. Otherwise, the coronary artery must be reimplanted or bypassed. Determination of the intramural course can be difficult with CCTA and CMRA. Correlation with surgical pathology suggests that CCTA findings of a slitlike orifice, an acute angle at vessel takeoff, and an elliptical cross section are related to an intramural coronary segment (Fig. 78-7).22
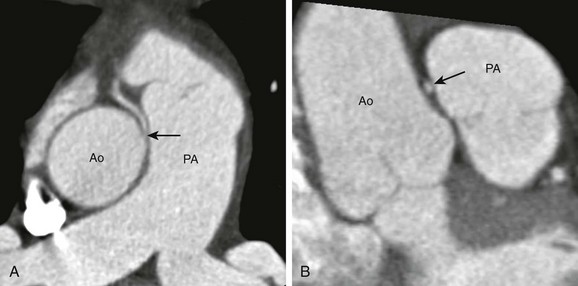
Figure 78-7 Coronary computed tomography angiogram of a surgically proven case of intramural right coronary artery (RCA) arising from the left coronary sinus in an 8-year-old girl.
A, The short-axis view shows an interarterial RCA (arrow) arising from the aorta (Ao) in an acute angle. The proximal RCA tapers to a slitlike opening at the ostium. B, A longitudinal view shows the RCA compressed (arrow) by the Ao and the pulmonary artery (PA). The cross section of the RCA is elliptical in shape.
Coronary Artery From Pulmonary Artery
Overview: Origination of a coronary artery from a pulmonary artery includes anomalous origin of the LMCA, LAD artery, LCx artery, or RCA from the pulmonary trunk or the main branch pulmonary arteries. The most common and clinically most important type is the anomalous left coronary artery from the pulmonary artery (ALCAPA) (Fig. 78-8). The incidence is approximately 1 in 300,000 live births, accounting for 0.24% to 0.5% of congenital cardiac anomalies.23 Origination of the anomalous right coronary artery from the pulmonary artery is four times less common than is ALCAPA.24 The clinical presentation depends on how much of the coronary flow to the left ventricle is compromised.
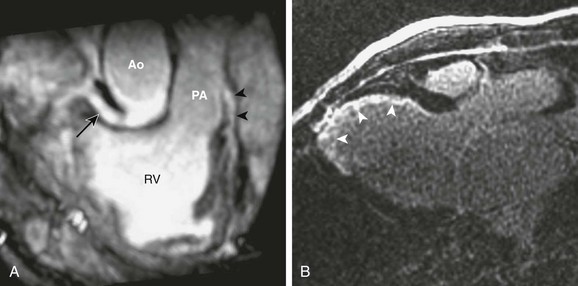
Figure 78-8 Magnetic resonance imaging of a 12-year-old boy with an anomalous left coronary artery from the pulmonary artery (PA).
A, An oblique image reformatted from a noncontrast coronary magnetic resonance angiogram shows the right coronary artery (arrow) arising from the aorta (Ao) at the normal location and the left main coronary artery (LMCA) (arrowheads) originating from the PA. B, Three-chamber view from a delayed-enhancement study shows endocardial infarction at the left ventricular anteroseptal wall and the apex (arrowheads) caused by inadequate myocardial perfusion from the LMCA. RV, Right ventricle.
Imaging: Chest radiographs for patients with the infantile form of ALCAPA usually show an enlarged cardiac silhouette and signs of pulmonary venous congestion or pulmonary edema. ECG and other laboratory tests suggest myocardial ischemia and infarction. When selective coronary angiography is performed, the catheter fails to engage the LMCA ostium. An aortogram fills a single right coronary artery in the early phase, with late opacification of the LMCA and retrograde flow of contrast material into the pulmonary artery. Echocardiography usually can detect the connection between the anomalous coronary artery and the pulmonary artery and can visualize the reversed coronary flow by color Doppler imaging. In difficult cases, the connection between the anomalous coronary artery and the pulmonary artery can be seen readily with CCTA or CMRA.25
Management: The definitive treatment is surgery. The most straightforward approach is to reimplant the anomalous coronary artery from the pulmonary artery to the aorta.26 With ALCAPA, reimplantation of the LMCA can be difficult because the LMCA may be too short to reach the centrally located aorta. This problem can be solved with the Takeuchi operation,27 in which an aortopulmonary window is created first. Then a baffled tunnel is created inside the pulmonary trunk to connect the aortopulmonary window to the ostium of the anomalous LMCA. Blood flows from the aorta, through the aortopulmonary window, into the baffled tunnel, and then into the anomalous LMCA ostium.
Coronary Artery Fistula
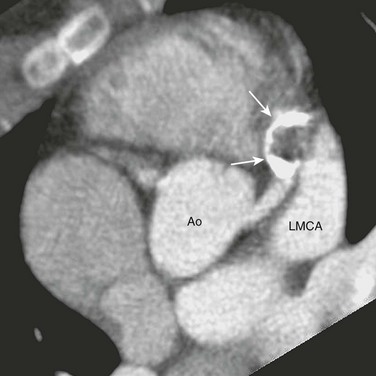
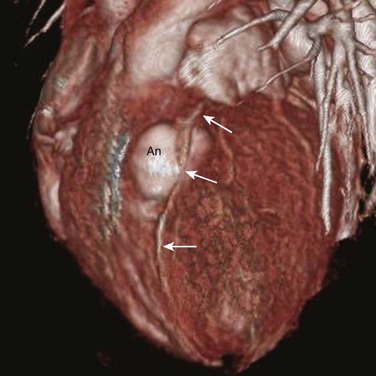
Overview: A coronary artery fistula is a common coronary abnormality seen in 0.3% to 0.8% of patients referred for cardiac catheterization.28 It is an abnormal communication between a normal coronary artery and another cardiovascular structure. Although it usually is congenital, it can form after trauma or after a surgical procedure such as a myomectomy. Anatomically, a coronary fistula most frequently originates from the RCA (55%), followed in frequency by the LAD artery (35%), both arteries (5%), and others (5%). A coronary fistula terminates in the right side of the heart far more often than in the left side of the heart. The most common site of termination is the right ventricle (41%) (Fig. 78-9), followed by the right atrium (26%), pulmonary trunk (17%) (Fig. 78-10), left ventricle (3%), and superior vena cava (1%).29
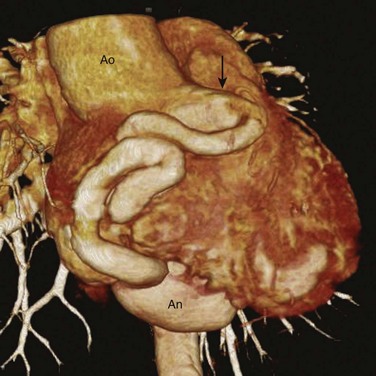
Figure 78-9 Coronary fistula to the right ventricle.
A volume-rendered image from a coronary computed tomography angiogram of a 22-year-old woman shows a large and extremely tortuous right coronary artery arising (arrow) from the aorta (Ao). At the inferior surface of the heart, this coronary fistula connects to a giant aneurysm (An), which empties into the right ventricle (not shown).
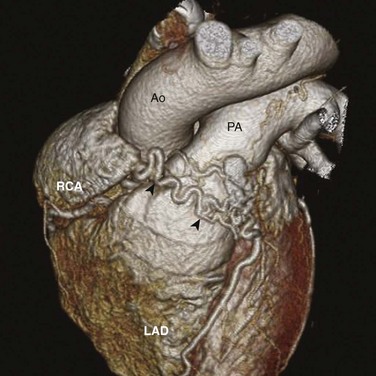
e-Figure 78-10 Coronary fistulae to the pulmonary artery.
A volume-rendered image from a coronary computed tomography angiogram of a 30-year-old man shows multiple, small, tortuous coronary fistulae (arrowheads) arising from both the right coronary artery (RCA) and the left anterior descending (LAD) artery, feeding into the pulmonary artery (PA). Ao, Aorta.
Imaging: A small coronary artery fistula may be detected incidentally when a screening echocardiography is ordered for an unexplained murmur or for unrelated symptoms. If the fistula is large, the course and connections of the fistula, as well as aneurysmal or stenotic segments, should be defined with catheter angiography, CCTA, or CMRA. In addition, MRI can quantify the shunt flow and shunt ratio with use of phase-contrast techniques.
Management: Patients with fistulae of any size should be given antibiotic prophylaxis to prevent endocarditis.30 For patients with a shunt ratio greater than 1.5, the fistula should be closed to prevent the development of pulmonary hypertension. Some authors advocate elective closure of all fistulae to prevent myocardial ischemia, endocarditis, and aneurysm formation. Fistulae with favorable connections and shapes can be closed with catheter embolization. Otherwise, they can be closed with surgical ligation.31,32 Aneurysmal segments should be surgically reduced to prevent rupture.
Anomalous Coronary Artery in Structural Heart Disease
Overview: TOF is the most common cyanotic congenital heart disease, accounting for 10% of all congenital heart defects (see Chapter 76). The standard treatment today is early total correction between 3 and 12 months of age. The surgical repair has two components: a patch closure of the VSD and a transannular patch augmentation of the RVOT and the pulmonary trunk. In 4% of patients with TOF, an anomalous LAD artery arises from the RCA and crosses anterior to the RVOT before entering the anterior interventricular septal groove (Fig. 78-11).33 This LAD artery lies in the path of the transannular incision and can be accidentally transected. If it cannot be adequately mobilized, it may prevent sufficient augmentation of the RVOT.34
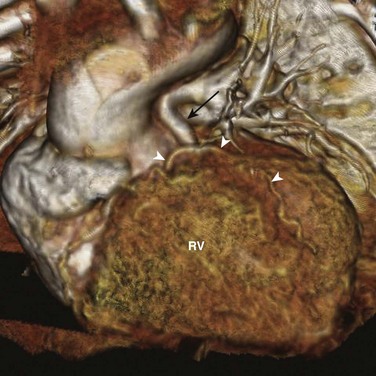
Figure 78-11 Aberrant left anterior descending (LAD) artery in a child with tetralogy of Fallot.
A volume-rendered image from a 1-year-old boy viewed from the front shows an LAD artery (arrowheads) that travels in front of the stenotic pulmonary trunk (black arrow). The position of this LAD artery may complicate the surgical repair. RV, Right ventricle.
Transposition of the Great Arteries
Overview: TGA accounts for 5% of all congenital heart defects (see Chapter 76). Most patients with TGA have normal ventricular positioning (D-looping), atrioventricular concordance, and ventriculoarterial discordance with the aorta anterior and to the right of the pulmonary trunk (Fig. 78-12).
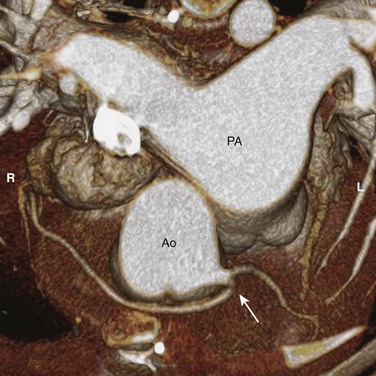
Figure 78-12 Coronary artery arrangement in d-transposition of the great arteries (d-TGA).
A volume-rendered image from a coronary computed tomography angiogram of a 12-year-old girl shows an aorta (Ao) that is situated in front and to the right of the pulmonary artery (PA), consistent with a d-TGA. A single coronary ostium is present (arrow), and the right coronary artery courses in front of the Ao. This coronary pattern is not favorable for the arterial switch operation.
Imaging and Treatment: The preferred surgical treatment for complete TGA is the arterial switch, or Jatene operation. This surgery is a definitive operation in which the aorta and the pulmonary trunk are surgically transposed, thereby returning the circulatory anatomy to a “normal” state. In this operation, the coronary arteries also must be reimplanted from the aortic sinuses to the pulmonary sinuses. In normal truncal anatomy, the left and right coronary arteries arise from the aortic sinuses closest to the pulmonary trunk. This relationship usually is preserved even when the aorta and the pulmonary trunk are congenitally transposed. The close proximity of the coronary origins from both great arteries makes surgical translocation of the coronary arteries feasible. However, frequent variations of coronary origins and course occur that make this operation difficult or even impossible.35 By mapping out the coronary anatomy, CCTA or CMRA can be very useful for surgical planning.36
Pulmonary Atresia with Intact Ventricular Septum
Overview: PAIVS is believed to be caused by the in-utero obstruction of the pulmonary valve after the formation of the pulmonary infundibulum and the central pulmonary arteries (see Chapter 74). The reduced right ventricular flow prevents the normal growth of the right ventricle and the tricuspid valve, causing hypoplasia of both structures. Without an outlet, the right ventricular systolic pressure can rise above the left ventricular systolic pressure and the coronary arterial pressure. This mechanism, in turn, disrupts the normal regression of embryologic communications between epicardial coronary arteries and the right ventricle, resulting in coronary fistulae between the two in 75% of the patients at birth.
After birth, the right ventricular pressure remains high and blood flows from the right ventricle into the coronary system. If the right ventricle is decompressed by surgically relieving the pulmonary obstruction, blood flow in the coronary fistulae abruptly reverses, shunting blood away from the myocardium into the right ventricle and leading to myocardial ischemia and infarction. Normal coronary circulation, then, depends on a pressurized right ventricle. A pressurized right ventricle, in turn, precludes surgery that restores the right ventricle to a functioning pump for the pulmonary circulation. This physiology is called a “right ventricle–dependent coronary circulation.”37
Imaging and Management: Catheter coronary angiography is the standard imaging study to detect PAIVS-related coronary fistulae. Classic findings on coronary angiography include systolic retrograde filling of the RCA and the LAD artery during right ventriculography (Fig. 78-13) and end-diastolic filling of the right ventricle during coronary angiography. This type of coronary fistula can be seen readily with CCTA, although its role in the surgical decision of PAIVS is not defined.
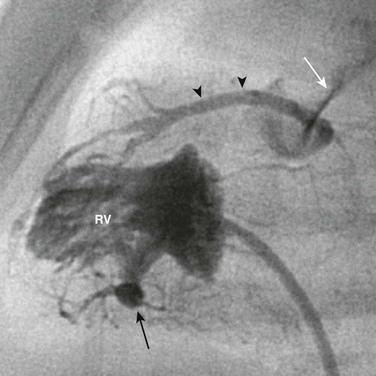
Figure 78-13 Catheter angiography of the right ventricle of a 1-year-old boy with pulmonary atresia and an intact ventricular septum.
Contrast material injected into the right ventricle (RV) flows through the sinusoids (black arrow) into the coronary arterial system (arrowheads) and then flows retrograde out of the pulmonary artery (white arrow). This coronary circulation is judged right-ventricular dependent.
Coronary Artery Hypoplasia
Overview: Coronary artery hypoplasia (CAH) is a rare condition characterized by a diffusely narrowed, underdeveloped major coronary segment or branch.38 The prevalence of CAH is estimated to be 0.03% based on adult coronary angiography, and it represents 6% of all coronary anomalies. Most reported cases involve the left coronary system (Fig. 78-14). The pathogenesis of this lesion is unknown, but an in-utero embolic event affecting a coronary artery may be a cause. The clinical significance of CAH also is unknown. In a few cases it was related to sudden death, but other persons with this anomaly led normal lives, with the diagnosis being made at autopsy after they died from unrelated causes.
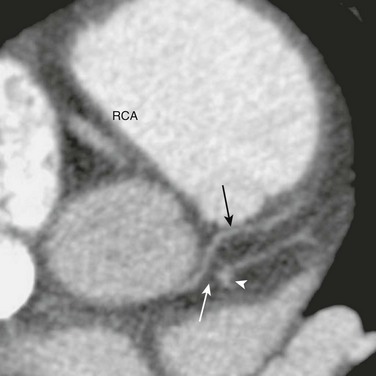
Figure 78-14 Coronary artery hypoplasia in a 15-year-old girl with exertional angina.
An axial view from a coronary computed tomography angiogram shows diffusely small left coronary arteries compared with the right coronary artery (RCA). The left circumflex artery (arrowhead) is occluded (white arrow) from the left main coronary artery (black arrow).
Imaging and Management: CCTA can be used to visualize the threadlike epicardial coronary artery. However, catheter angiography is required to delineate all collateral vessels. The optimal treatment has not been determined, but in patients who have CAH along with documented arrhythmia or syncope, implantation of a defibrillator would be a prudent choice.
Acquired Coronary Artery Diseases
KD, or mucocutaneous lymph node syndrome, was first described by Tomisaku Kawasaki in 1967. It is an acute, febrile, multisystem vasculitis of unknown etiology that affects primarily children younger than 5 years of age, with a slight male predominance. It is most common in Japan, although the number of cases in the United States is increasing. The number of children hospitalized for KD is estimated to be 3000 per year in the United States. KD is the most common acquired coronary artery disease in children. Diagnosis is made by clinical characteristics of prolonged high fever, conjunctivitis, red, cracked lips, a red oral mucous membrane, a strawberry tongue, a multiform rash, cervical lymphadenopathy, erythema of the palms and the soles, swollen hands and feet, and desquamation around fingers and toes 1 to 2 weeks after the onset of symptoms.39 The disease usually is self-limiting, although recurrence has been seen in 3% of patients.
Imaging: The most important complication of KD is the development of coronary artery aneurysms. Coronary artery aneurysms develop in 15% to 30% of patients who do not receive gamma globulin therapy, usually 1 to 4 weeks after disease onset. Thromboembolism of coronary artery aneurysms (Fig. 78-15) and coronary artery stenosis are the causes of myocardial ischemia, infarction, heart failure, and sudden death in these patients. Expanding aneurysms may rupture. Echocardiography is the primary imaging tool in persons with KD. In young children, echocardiography is quite effective in detecting coronary aneurysms. Giant aneurysms (>8 mm in diameter) are associated with increased risk of myocardial infarction and sudden death. Echocardiography is less effective in older children and young adults, and serial angiography is needed to monitor progression of aneurysms and the development of stenosis. Both CCTA and CMRA are useful, noninvasive alternatives for this purpose.40,41 In addition, MRI is useful in the assessment of myocardial ischemia, infarction, and ventricular function.
Treatment: In multiple studies, it has been shown that administration of intravenous gamma globulin therapy lowers the risk of aneurysm formation from more than 15% to 5%. High-dose aspirin is used as adjunctive therapy during the acute phase to lower coagulation activation. In 1970 the mortality rate of KD was 2%, mostly as a result of cardiac complications, but with improvements in treatment, it has dropped to 0.1%.
Trauma
Overview: Trauma to the chest is relatively common in children; however, few reported injuries are specific to the coronary arteries. The most common cause of pediatric trauma is blunt trauma from motor vehicle accidents, although in urban areas, penetrating injuries from assaults and other violence occur not infrequently. Blunt injury to the coronary artery can cause an intimal tear, dissection, and acute thrombosis, leading to myocardial infarction and arrhythmia.42,43 Both blunt and penetrating injury can cause rupture of the coronary arteries, resulting in hemorrhage, tamponade, and myocardial infarction. Commotio cordis, or concussion of the heart, has led to dramatic sudden death during play in otherwise healthy children.44 The pathophysiology is not known, but postulated mechanisms include ventricular fibrillation brought on by mechanical shock to the myocardium during its vulnerable repolarization period, or by myocardial ischemia resulting from coronary vasospasm or dissection. Over time, the weakened or ruptured coronary vessel wall can form a pseudoaneurysm. Traumatic coronary artery fistulae to the ventricles have been reported. The infarcted or contused myocardium can become aneurysmal (Fig. 78-16), and if the injury to myocardium is extensive, heart failure can result.
Imaging and Management: The initial management of chest trauma focuses on more common lethal injuries such as airway compromise, pneumothorax, aortic rupture, and tamponade. Conventional computed tomographic angiography is routinely performed to evaluate for these findings. If signs of cardiac injury such as an abnormal ECG, hemodynamic instability, and elevated myocardial enzymes are found, echocardiography is performed to evaluate wall motion abnormality, abnormal valvular function, myocardial hematoma, and pericardial effusion or blood. To assess for acute coronary injury, catheter angiography is the imaging modality of choice. Less acutely, CCTA can be used to assess structural damages in the heart, such as traumatic VSD and myocardial aneurysm. MRI is useful for the evaluation of myocardial infarction and ventricular functions.
Metabolic Diseases Affecting the Coronary Artery
Familial hypercholesterolemia (FH) is an autosomal dominant disorder that causes a severe elevation in total cholesterol and low-density lipoprotein (LDL) levels. It is caused by mutations in the LDL receptor gene in chromosome 19. The prevalence of the heterozygous form of FH is 1 in 500 people, and the prevalence of homozygous FH is 1 in 1 million people.45 Children with the heterozygous form usually are asymptomatic. Children with the rare but clinically much more severe homozygous form present with early atherosclerosis of the coronary arteries, leading to acute myocardial infarction and sudden death at as young as 1 to 2 years of age. These patients also experience peripheral vascular disease, cerebrovascular disease, aortic stenosis, cutaneous xanthomas, corneal arcus, and an LDL level of greater than 600 mg/dL. The role of imaging, except for echocardiography screening for aortic stenosis, has not been defined for this rare disease. The mainstay of treatment is aggressive cholesterol-lowering medical therapy and treatments of atherosclerosis-related complications. Because FH is a disease of a defective LDL receptor in the liver, liver transplantation has been attempted as a curative procedure.
Coronary Calcinosis
Although coronary calcification is rare in children, it can occur in children with chronic vasculitis, which is seen with KD, radiation, end-stage renal disease, and type I diabetes mellitus. Coronary calcification can be seen as part of idiopathic infantile arterial calcification, a very rare disease characterized by extensive depositions of hydroxyapatite in the elastic layer of the large and medium-size muscular arteries, including the coronary arteries.46 Coronary stenosis is caused by intimal proliferation, and patients with idiopathic infantile arterial calcification experience myocardial infarction in infancy. Similarly, renal artery stenosis causes hypertension, and carotid stenosis causes cerebral infarction. Patients rarely survive beyond infancy, and many die prenatally. Diagnosis is made by radiologic demonstration of diffuse arterial calcification, arterial biopsy, or prenatal ultrasound demonstrating dilated cardiac ventricles, hydrops fetalis, and hyperechogenic large vessels. Patients are treated with etidronate, which inhibits calcific mineralization in addition to inhibiting bone resorption. Regression of arterial calcification has been shown after treatment, but vascular stenoses may not resolve.
Basso, C, Maron, BJ, Corrado, D, et al. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501.
Frommelt, PC, Frommelt, MA. Congenital coronary artery anomalies. Pediatr Clin North Am. 2004;51:1273–1288.
Hellinger, JC, Pena, A, Poon, M, et al. Pediatric computed tomographic angiography: imaging the cardiovascular system gently. Radiol Clin North Am. 2010;48:439–467.
Stuber, M, Weiss, RG. Coronary magnetic resonance angiography. J Magn Reson Imaging. 2007;26:219–234.
Tacke, CE, Kuipers, IM, Groenink, M, et al. Cardiac magnetic resonance imaging for noninvasive assessment of cardiovascular disease during the follow-up of patients with Kawasaki disease. Circ Cardiovasc Imaging. 2011;4:712–720.
References
1. De WD, Vercruysse, T, Suys, B, et al. Major coronary anomalies in childhood. Eur J Pediatr. 2002;161:637–642.
2. Frommelt, PC, Frommelt, MA. Congenital coronary artery anomalies. Pediatr Clin North Am. 2004;51:1273–1288.
3. Levin, DC, Fellows, KE, Abrams, HL. Hemodynamically significant primary anomalies of the coronary arteries: angiographic aspects. Circulation. 1978;58:25–34.
4. Sugimura, T, Kato, H, Inoue, O, et al. Intravascular ultrasound of coronary arteries in children. Assessment of the wall morphology and the lumen after Kawasaki disease. Circulation. 1994;89:258–265.
5. De Bruyne, B, Sarma, J. Fractional flow reserve: a review. Heart. 2008;94:949–959.
6. Chan, FP. MR and CT imaging of the pediatric patient with structural heart disease. Semin Thorac Cardiovasc Surg. 2008;20:393–399.
7. Hellinger, JC, Pena, A, Poon, M, et al. Pediatric computed tomographic angiography: imaging the cardiovascular system gently. Radiol Clin North Am. 2010;48:439–467.
8. Moscariello, A, Takx, RA, Schoepf, UJ, et al. Coronary CT angiography: image quality, diagnostic accuracy, and potential for radiation dose reduction using a novel iterative image reconstruction technique-comparison with traditional filtered back projection. Eur Radiol. 2011;21:2130–2138.
9. Stuber, M, Weiss, RG. Coronary magnetic resonance angiography. J Magn Reson Imaging. 2007;26:219–234.
10. Hellman, RN. Gadolinium-induced nephrogenic systemic fibrosis. Semin Nephrol. 2011;31:310–316.
11. Rigatelli, G, Docali, G, Rossi, P, et al. Congenital coronary artery anomalies: angiographic classification revisited. Int J Cardiovasc Imaging. 2003;19:361–366.
12. Engel, HJ, Torres, C, Page, HL. Major variations in anatomical origin of the coronary arteries: angiographic observations in 4,250 patients without associated congenital heart disease. Cathet Cardiovasc Diagn. 1975;1:157.
13. Yamanaka, O, Hobbs, RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21:28–40.
14. Donaldson, RM, Raphael, MJ, Yacoub, MH, et al. Hemodynamically significant anomalies of the coronary arteries: surgical aspects. Thorac Cardiovasc Surg. 1982;30:7–13.
15. Frommelt, PC, Frommelt, MA, Tweddell, JS, et al. Prospective echocardiographic diagnosis and surgical repair of anomalous origin of a coronary artery from the opposite sinus with an interarterial course. J Am Coll Cardiol. 2003;42:148–154.
16. Kragel, AH, Roberts, WC. Anomalous origin of either the right or left main coronary artery from the aorta with subsequent coursing between aorta and pulmonary trunk: analysis of 32 necropsy cases. Am J Cardiol. 1988;62:771–777.
17. Frescura, C, Basso, C, Thiene, G, et al. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol. 1998;29:689–695.
18. Roberts, WC, Siegel, RJ, Zipes, DP. Origin of the right coronary artery from the left sinus of Valsalva and its functional consequences: analysis of 10 necropsy patients. Am J Cardiol. 1982;49:863–868.
19. Basso, C, Maron, BJ, Corrado, D, et al. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501.
20. Liberthson, RR. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334:1039–1044.
21. Hamamichi, Y, Okada, E, Ichida, F. Anomalous origin of the main stem of the left coronary artery from the non-facing sinus of Valsalva associated with sudden death in a young athlete. Cardiol Young. 2000;10:147–149.
22. Miller, JA, Anavekar, NS, El Yaman, MM, et al. Computed tomographic angiography identification of intramural segments in anomalous coronary arteries with interarterial course [published online ahead of print September 3, 2011]. Int J Cardiovasc Imaging. 2013;30(2):225–233.
23. Lee, AC, Foster, E, Yeghiazarians, Y. Anomalous origin of the left coronary artery from the pulmonary artery: a case series and brief review. Congenit Heart Dis. 2006;1:111–115.
24. William, IA, Gersony, WM, Hellenbrand, WE. Anomalous right coronary artery arising from the pulmonary artery: a report of 7 cases and a review of the literature. Am Heart J. 2006;152:1004.e9–1004.e17.
25. Ten Kate, GJ, Weustink, AC, de Feyter, PJ. Coronary artery anomalies detected by MSCT-coronary angiography in the adult. Neth Heart J. 2008;16:369–375.
26. Alexi-Meskishvili, V, Nasseri, BA, Nordmeyer, S, et al. Repair of anomalous origin of the left coronary artery from the pulmonary artery in infants and children. J Thorac Cardiovasc Surg. 2011;142:868–874.
27. Ginde, S, Earing, MG, Bartz, PJ, et al. Late complications after Takeuchi repair of anomalous left coronary artery from the pulmonary artery: case series and review of literature. Pediatr Cardiol. 2012;33(7):1115–1123.
28. Vavuranakis, M, Bush, CA, Boudoulas, H. Coronary artery fistulas in adults: incidence, angiographic characteristics, natural history. Cathet Cardiovasc Diagn. 1995;35:116–120.
29. Schumacher, G, Roithmaier, A, Lorenz, HP, et al. Congenital coronary artery fistula in infancy and childhood: diagnostic and therapeutic aspects. Thorac Cardiovasc Surg. 1997;45:287–294.
30. Gowda, RM, Vasavada, BC, Khan, IA. Coronary artery fistulas: clinical and therapeutic considerations. Int J Cardiol. 2006;107:7–10.
31. Carrel, T, Tkebuchava, T, Jenni, R, et al. Congenital coronary fistulas in children and adults: diagnosis, surgical technique and results. Cardiology. 1996;87:325–330.
32. Said, SA, Landman, GH. Coronary-pulmonary fistula: long-term follow-up in operated and non-operated patients. Int J Cardiol. 1990;27:203–210.
33. Dabizzi, RP, Caprioli, G, Aiazzi, L, et al. Distribution and anomalies of coronary arteries in tetralogy of Fallot. Circulation. 1980;61:95–102.
34. Ruzmetov, M, Jimenez, MA, Pruitt, A, et al. Repair of tetralogy of Fallot with anomalous coronary arteries coursing across the obstructed right ventricular outflow tract. Pediatr Cardiol. 2005;26:537–542.
35. Sim, EK, van Son, JA, Edwards, WD, et al. Coronary artery anatomy in complete transposition of the great arteries. Ann Thorac Surg. 1994;57:890–894.
36. McMahon, CJ, el Said, HG, Feltes, TF, et al. Preoperative identification of coronary arterial anatomy in complete transposition, and outcome after the arterial switch operation. Cardiol Young. 2002;12:240–247.
37. Giglia, TM, Mandell, VS, Connor, AR, et al. Diagnosis and management of right ventricle-dependent coronary circulation in pulmonary atresia with intact ventricular septum. Circulation. 1992;86:1516–1528.
38. Amabile, N, Fraisse, A, Quilici, J. Hypoplastic coronary artery disease: report of one case. Heart. 2005;91:e12.
39. Freeman, AF, Shulman, ST. Kawasaki disease: summary of the American Heart Association guidelines. Am Fam Physician. 2006;74:1141–1148.
40. Peng, Y, Zeng, J, Du, Z, et al. Usefulness of 64-slice MDCT for follow-up of young children with coronary artery aneurysm due to Kawasaki disease: initial experience. Eur J Radiol. 2009;69:500–509.
41. Tacke, CE, Kuipers, IM, Groenink, M, et al. Cardiac magnetic resonance imaging for noninvasive assessment of cardiovascular disease during the follow-up of patients with Kawasaki disease. Circ Cardiovasc Imaging. 2011;4:712–720.
42. Cizmarova, E, Simkovic, I, Zelenay, J, et al. Post-traumatic coronary occlusion and its consequences in a young child. Pediatr Cardiol. 1988;9:117–120.
43. Harada, H, Honma, Y, Hachiro, Y, et al. Traumatic coronary artery dissection. Ann Thorac Surg. 2002;74:236–237.
44. Wang, JN, Tsai, YC, Chen, SL, et al. Dangerous impact: commotio cordis. Cardiology. 2000;93:124–126.
45. Marais, AD, Firth, JC, Blom, DJ. Homozygous familial hypercholesterolemia and its management. Semin Vasc Med. 2004;4:43–50.
46. van de Sluis, I, Boot, AM, Vernooij, M, et al. Idiopathic infantile arterial calcification: clinical presentation, therapy and long-term follow-up. Eur J Pediatr. 2006;165:590–593.