Chapter 21 Coronary Artery Computed Tomography Angiography
COMPUTED TOMOGRAPHY CORONARY ARTERY ANGIOGRAPHY: TECHNOLOGIC CONSIDERATIONS
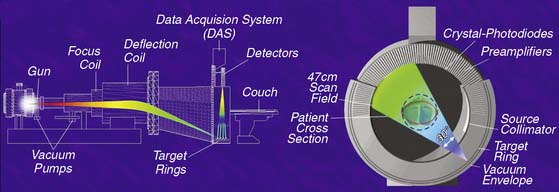
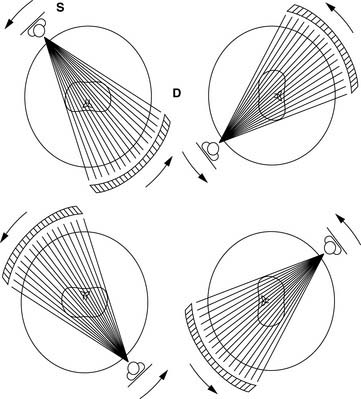
The main challenge in imaging the coronary arteries with CT is the complex and continuous motion of these small targets. Therefore, both high temporal and spatial resolution are of paramount importance to obtain diagnostic quality images. The field of noninvasive coronary angiography started with the introduction of electron beam CT (EBCT; Imatron, San Francisco, CA) that operated at a temporal resolution varying between 50 and 100 milliseconds (ms) per tomographic image. This was a fourth-generation CT scanner that utilized a beam of electrons directed toward a tungsten ring; the impact of the electrons against the tungsten created a fan of x-rays moved along an arc of 210 degrees around the gantry (Fig. 21-1). The issuance of the electron beam, followed by the generation of the x-ray fan, was timed at 60% to 80% of the R-R interval on the surface electrocardiogram (ECG), and the patient was moved in small increments of 3 mm through the stationary gantry at each heartbeat while holding his breath. This step-and-shoot prospective (i.e., ECG triggered) acquisition required a 20- to 30-second breath hold to cover the entire z-axis (cranial-caudal distance) of the heart. The benefits of the EBCT scanner were the very high temporal resolution and the relatively low radiation dose provided to the patient because of the intermittent (once per heartbeat) exposure to x-rays. However, there were also important drawbacks to the technology, such as the low spatial resolution, a significant partial volume effect due to the thick tomographic slices, and the fact that the axial acquisition of the slices with a small interslice gap often created a stair-step artifact in the reconstructed images. Finally, the prospective ECG-triggered acquisition forced patients with slow heart rates to maintain long breath holds, often inducing artifacts when the patients breathed in the middle of the test. In the early 2000s, the field witnessed an important evolution with the introduction of the first 4-slice mechanical CT. A dual-slice helical CT (Elscint, Haifa, Israel) had been in use for a few years1–3 but had not received much attention in cardiovascular circles. The development of multislice CT (also known as multidetector CT [MDCT]) was made possible by the introduction of the slip-ring technology that permitted the continuous revolution of the x-ray source-detector pair in the CT gantry, allowing a perpetual spiraling motion around the patient. With this new technology, an x-ray tube generates a beam of x-rays that is split, collimated, and detected by several rows of detectors positioned on the other side of the patient’s table (Figs. 21-2 and 21-3).
As can be deduced from the preceding discussion, the introduction of scanners with more than 4 rows (16 to 64), despite better spatial resolution, did not fully improve the temporal resolution limitation of MDCT. As a result, even when using most current MDCT technology, β-blockade is still necessary to limit motion artifacts if the heart rate is greater than 70 beats/min. Nonetheless, by increasing the distance covered in the z-axis during a single rotation of the gantry, they shortened the acquisition time. This weakness of MDCT was partially overcome with the introduction of dual-source 64-row MDCT scanners (Siemens, Forcheim, Germany).4–6 These scanners utilize two x-ray tubes and two sets of detector rows set at 90 degrees from each other that operate simultaneously, reducing the effective temporal resolution to about 83 ms per tomographic slice. Furthermore, the two tubes can issue x-rays of different energy spectra (dual-energy systems), with potential advantages for the study of tissues with different x-ray absorption characteristics, as discussed later in this chapter.
The temporal resolution of the dual-source MDCT is similar to that of EBCT, and no β-blockade appears to be necessary when imaging, reportedly even in the presence of atrial fibrillation.7,8
COMPARISON OF CORONARY COMPUTED TOMOGRAPHY ANGIOGRAPHY WITH INVASIVE ANGIOGRAPHY FOR THE DETECTION OF LUMINAL STENOSIS
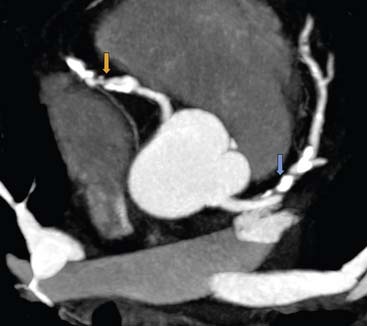
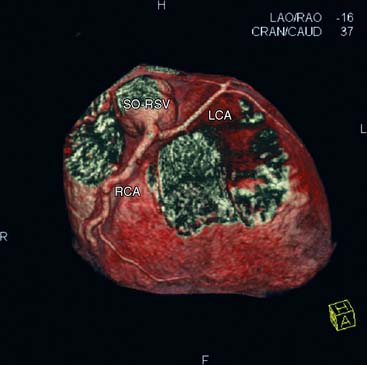
Native Coronary Arteries (Figs. 21-4 and 21-5)
The initial results of coronary CT angiography with EBCT were encouraging but also demonstrated the limitations of the technology. Achenbach et al9 reported a sensitivity and specificity of 92% and 94% for EBCT angiography compared to invasive coronary angiography for the detection of a luminal obstruction greater than 50% in 125 patients. However, 25% of the coronary segments were not evaluable, owing to technical limitations such as motion artifacts, calcium overlay, high image noise, and so forth. Similar results were obtained with 4-slice MDCT technology. Leber et al.10 reported a sensitivity and specificity of 82% and 96% for 4-slice MDCT with 25% to 30% nonevaluable coronary segments, and similar results were also shown by other investigators.11 A recent meta-analysis, including 22 studies of 4-slice MDCT versus invasive angiography, yielded a sensitivity of 87% and specificity of 87% on a per-vessel basis and 91% and 83% on a per-patient basis, respectively.12
The newer generations of MDCT scanners, starting with the 16-slice MDCTs, have shown improved accuracy compared to 4-slice MDCT. Each advance in technology with increased spatial and temporal resolution has been accompanied by improved results. Better spatial resolution leads to improved ability to assess coronary stents and calcified coronary artery segments (decreased partial volume effect) and improved ability to assess smaller arteries and side branches. As discussed, 16-slice MDCT typically has a spatial resolution of 0.7 mm and a temporal resolution of about 200 ms,13 whereas 64-slice MDCTs typically have a spatial resolution of 0.5 mm and a temporal resolution of 165 ms,14 except for the dual-source 64-slice MDCT that has a temporal resolution of 83 ms.15 These technical characteristics need to be compared to those of invasive angiography with a spatial resolution of 0.2 mm and temporal resolution of 5 to 20 ms.13
A number of relatively small studies compared 16-slice16–29 and 64-slice MDCT30–47 to invasive coronary angiography as the gold standard for detection of obstructive coronary artery disease (CAD) defined as either =50% or =70% luminal artery stenosis. These studies had limitations: they tended to be small (most enrolled less than 100 patients) single-center studies and involved mostly men. To date, there have been three multicenter trials comparing 64-slice MDCT to invasive coronary angiography.48–50 The published estimates of accuracy are somewhat variable because of the use of different scanning protocols and different hardware. Many of the earlier studies excluded unassessable coronary artery segments from statistical analysis, which resulted in artificially elevated measurements of accuracy.12 Most studies included only coronary segments over 1.5 to 2 mm in diameter, although a few evaluated all segments, including small side branches, leading to lower estimates of accuracy. Finally, some of the investigators reported results only on a coronary artery segment or vessel basis, rather than on a patient basis. A segment-by-segment analysis obviously provides more statistical power for the investigation, but a patient-based analysis tends to be more relevant to clinical practice and to the current use of CTA, which revolves around the decision as to whether a patient should be sent to the invasive catheterization laboratory or not.51
Three meta-analyses summarized several studies comparing the diagnostic accuracy of coronary MDCT angiography versus invasive angiography as the reference standard.12,51,52 Vanhoenacker et al.12 included 22 4-slice MDCT studies, 26 16-slice studies, and 6 64-slice studies. Each study in this meta-analysis assessed the ability of MDCT angiography to detect =50% coronary stenosis on invasive angiography. Analyses were performed on three levels: per patient (1474 total patients), in which the presence of any stenosis in any coronary artery was considered a positive result; per vessel (2692 vessels); and per coronary artery segment, based on the American Heart Association approved 15-segment model (30,775 segments). On a per-patient basis, the 4-slice MDCTs, 16-slice MDCTs, and 64-slice MDCTs demonstrated the following sensitivity and specificity: 91% and 83%, 97% and 81%, and 99% and 93%, respectively. Thus, with an increasing number of detectors, the diagnostic performance of MDCT for detection of obstructive CAD seems to have increased. Additionally, there were fewer unassessable segments with 16- and 64-slice MDCT in comparison to 4-slice MDCT.
A second meta-analysis51 comparing 4-, 8-, 16-, and 64-slice MDCT to invasive angiography reached similar conclusions. In this analysis, the authors used 41 single-center studies published between 1997 and 2006 and analyzed data per patient (2515) and per segment (21,821). Diagnostic accuracy improved with each newer generation of scanner. On a per-patient basis, 4- and 8-slice MDCT (combined analysis) showed a sensitivity and specificity of 97% and 81%, respectively, 16-slice MDCT had a sensitivity and specificity of 99% and 83%, and 64-slice MDCT had a sensitivity and specificity of 98% and 92%.
In the third meta-analysis,52 the authors compared 64-slice MDCT alone to invasive angiography. Twenty-seven studies including 1740 patients were reviewed, including four studies with patients who had undergone coronary artery bypass grafting (CABG) and five studies of patients with coronary stents. Of the 18,920 coronary segments, only 4% were unassessable and thus excluded from analysis. A positive study by MDCT or invasive angiography was defined as =50% coronary stenosis. For stenoses in the native coronary artery on a per-patient basis, 64-slice MDCT showed a sensitivity of 97.5%, specificity of 91%, positive predictive value (PPV) of 93%, and negative predictive value (NPV) of 96.5%, consistent with findings in the other two meta-analyses. For post-CABG patients, sensitivity was 98.5%, specificity 96%, PPV 92%, and NPV 99%. Accuracy was considerably less for detection of in-stent restenosis, with a sensitivity of 87%, specificity 96%, PPV 83.5%, and NPV 97%.52
The more recent prospective multicenter trials conducted utilizing 64-slice CT, however, did not completely confirm the very optimistic results reported in the earlier meta-analyses. Budoff et al. compared coronary CTA using 64-slice MDCT to invasive coronary angiography in 230 patients.48 As reported in the meta-analyses, this study showed a high NPV (99%) of coronary 64-slice MDCT for the detection of =50% or =70% coronary luminal stenoses, but a lower specificity and PPV (83% and 99%, respectively, for both per-patient and per-vessel analyses). The authors further noted a substantial decrease in specificity of MDCT in the presence of a coronary artery calcium (CAC) score over 400. The study by Meijboom et al. again illustrated the high NPV of 64-slice MDCT (97% and 99% for patient and segmental analyses) but also the lower than expected specificity and PPV of 64-slice MDCT (64% and 86% for patient and 90% and 47% for segmental analyses).49 In this study of 360 patients with stable or unstable angina, the authors reported a high false-positive rate for MDCT: 41 patients with only mild CAD on invasive angiography were classified by MDCT as having obstructive CAD. However, the investigators did not exclude any coronary segment from evaluation, and heavily calcified segments were considered to harbor obstructive stenoses. Finally, Miller et al.50 reported the results of a nine-center study conducted with Toshiba 64-slice scanners involving 291 patients. Patients with a CAC score over 600 and vessels with a diameter less than 1.5 mm were excluded. The overall area under the curve for detection of more than 50% stenosis by MDCT was 0.93 for patient and 0.91 for vessel analyses. Although the specificities were high (90% and 93%), the NPVs were lower than previously reported (83% and 89%) for both patient and vessel analyses.
Dual-source 64-slice MDCT (Siemens, Forcheim, Germany) has improved temporal resolution, with fewer motion artifacts,53 and the initial clinical experience has shown excellent diagnostic performance of this scanner.4–6
Finally, the new 320-slice MDCT scanner (Toshiba, Tochigi-ken, Japan) has undergone a very initial evaluation.54 The attractiveness of this technology is the potential to limit radiation exposure by allowing whole-heart coverage with a single rotation, although this cannot be obtained for high heart rates, as explained earlier in this chapter. In a study of 40 patients, only one of 1166 coronary artery segments was unassessable, and 89% of the segments showed excellent image quality. Four of the 40 patients underwent invasive angiography. There were seven coronary stenoses =50% noted on 320-row MDCT, and all were confirmed by invasive angiography.54 The average radiation dose was 6.8 ± 1.4 mSv for the single-rotation protocol, although several patients required more rotations, with a substantial increase in radiation dose.
In aggregate, the various studies conducted so far, with the exception of one outlying study showing an NPV of only 83%,50 have consistently shown a high NPV of CTA (in every other case >97% and often close to 100%). It is therefore appropriate to assume that at this stage of development, CTA is especially useful to exclude the presence of obstructive CAD, rather than conclusively demonstrating its presence. In fact, as illustrated by the most recent randomized studies,48,49,50 MDCT does appear to carry a significant false-positive risk for prediction of obstructive luminal disease on invasive angiography.
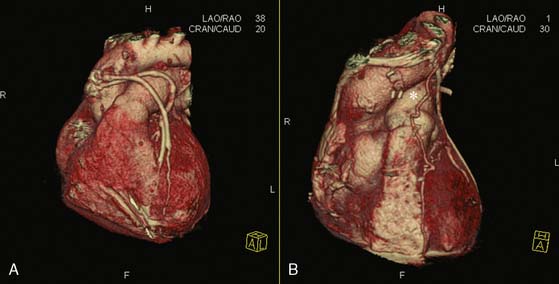
Coronary CTA for Evaluation of Bypass Grafts (Fig. 21-6)
Coronary CTA is useful for the evaluation of bypass grafts. Saphenous vein grafts are larger than coronary arteries and less mobile, making them easier to image. Arterial grafts are smaller in caliber than vein grafts but can be evaluated accurately by coronary CTA. Coronary CTA has shown excellent diagnostic performance for the evaluation of coronary grafts compared to invasive angiography, with 64-slice MDCT being more accurate than 16-slice MDCT.36,55–62 Several studies have quoted a sensitivity and specificity of 100% for evaluation of patency of the body of the grafts. Difficulty can arise, however, in evaluation of the anastomotic site and of the native coronary arteries distal to the anastomosis. A clear visibility of the anastomotic site to the native coronary artery is often hampered by the presence of surgical clips that cause significant image artifact. The distal segments of the native coronary arteries after the anastomosis are often small, diseased, and at times heavily calcified. These factors limit the ability of CTA to accurately detect for full spectrum of disease in patients submitted to CABG. Another potentially useful application of coronary CTA is planning for repeat bypass surgery, as shown in a publication by Gasparovic et al.63 The clear three-dimensional (3D) reconstruction of the chest cavity and visualization of the course of previous grafts, mammary arteries, location of an aortic aneurysm with respect to other vital organs, and so forth, provide an accurate in-space orientation and may be of great aid to the surgeon planning an often difficult and at times dangerous reoperation.
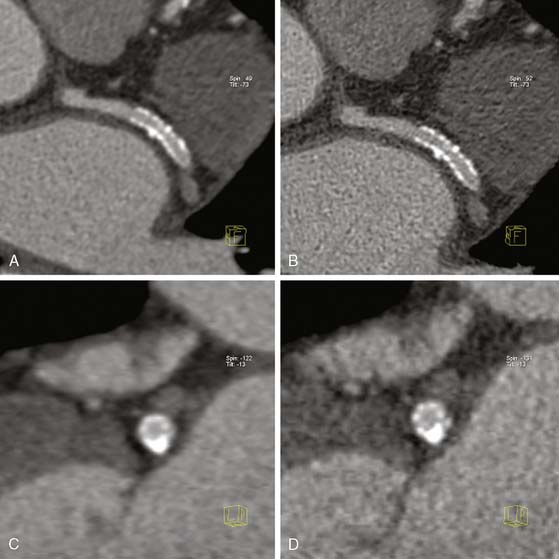
Coronary CTA for Evaluation of Coronary Stent Patency (Fig. 21-7)
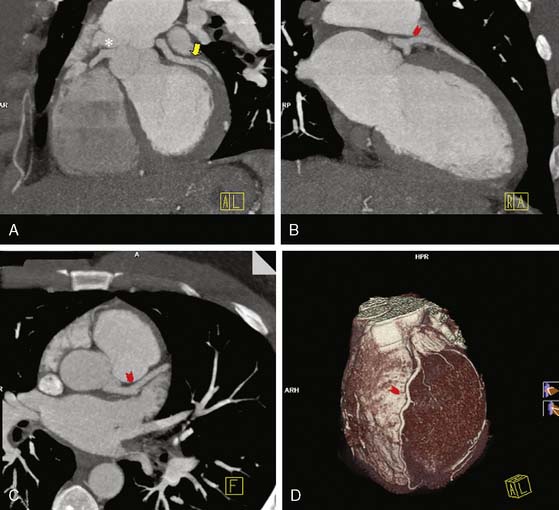
In-stent restenosis occurs at a relatively high rate, and patients with prior stent placement comprise a good proportion of patients referred for invasive coronary angiography. Therefore, the development of a noninvasive modality that can accurately detect in-stent restenosis would be very clinically useful. Coronary stent evaluation, however, continues to be limited with coronary CTA. The main problem encountered is the “blooming artifact” (see artificial overexpansion of the borders of the stent in Fig. 21-7) caused by the high-density material of the struts that makes assessment of stent lumen patency difficult. The size of the stent64 and the coexistence of calcification in the vicinity of the stent further complicate the performance of diagnostic-quality CTA imaging. Some improvement in diagnostic quality can be obtained by reconstructing angiographic images with a sharper kernel, as shown in Figure 21-7. Naturally, the evaluation of large stents, such as those in the left main coronary artery, is more accurate.65,66
In an earlier study, 64 patients with 102 stents were assessed with 64-slice MDCT, and the results were compared to those of invasive quantitative coronary angiography.67 Only 58% of the stents were assessable, and these were larger than the stents that could not be accurately viewed (average diameter 3.28 versus 3.03 mm; P = 0.0002), yielding an overall sensitivity and specificity of 50% and 57%, respectively. For the assessable stents, the sensitivity and specificity rose to 86% and 98%, respectively. The authors further reported that paclitaxel-eluting stents (Boston Scientific, Maple Grove, MN) with 0.13-mm strut thickness were more likely to be assessable than sirolimus-eluting stents (Cordis Corporation, Miami Lakes, FL) with 0.14-mm strut thickness (P < 0.001 for frequency of assessability). Two more recent publications addressed the ability of 64-slice MDCT to establish the diagnosis of in-stent restenosis.68,69 Cademartiri et al.68 evaluated 182 patients who had received 192 stents larger than 2.5 mm in diameter and reported that 7.3% of the stents could not be evaluated. For the remaining stents, 64-slice MDCT demonstrated excellent test characteristics as follows: sensitivity 95%, specificity 93%, PPV 63%, and NPV 99%. Schuijf et al.69 performed a similar analysis in 50 patients who had received 76 stents (2.25 to 4.0 mm in diameter and 8 to 33 mm in length); of these, 14% could not be evaluated, mostly due to motion artifacts (mean heart rate in patients with nonevaluable stents versus evaluable stents: 72+9 versus 55+2; P = 0.002). In the remaining 56 stents, 64-slice MDCT demonstrated both a sensitivity and specificity of 100%. The authors further reported that nonobstructive disease within the stent was clearly diagnosable in 71% of the cases. Dual-source 64-slice MDCT also been evaluated in a recent study of 35 patients with 48 stents.70 All 48 stents were assessable by dual-source MDCT that showed a diagnostic performance versus invasive angiography as follows: sensitivity of 100%, specificity of 94%, PPV 89%, and NPV 100%. However, 67% of the stents were over 3 mm in diameter.
CORONARY COMPUTED TOMOGRAPHY ANGIOGRAPHY: COMPARISON WITH NUCLEAR MYOCARDIAL PERFUSION IMAGING
In an early study, 114 patients at intermediate pretest likelihood of CAD underwent CTA (mostly by 64-slice MDCT) and MPI.71 Of the 114 patients, 58 also underwent invasive angiography, based on the results of imaging studies and clinical data, once again introducing a bias due to the presence of an abnormal test guiding the performance of the subsequent test. All patients with a normal CTA had a normal invasive angiogram, and 90% had a normal MPI, emphasizing the ability of a normal CTA to rule out obstructive CAD in an intermediate-risk population. All patients with obstructive or nonobstructive disease on CTA had CAD on invasive angiography, but only 59% had an abnormal MPI. Of the patients with obstructive CAD on CTA, 82% had obstructive disease on invasive angiography, and 50% had an abnormal MPI. Di Carli et al.,72 Hacker et al.,73 and Gaemperli et al.74 reached the same conclusion: There is often a substantial discrepancy between severity of stenosis assessed by CTA and MPI results. Lin et al.75 proposed a method for improving detection of perfusion abnormalities in patients with atherosclerotic disease on CTA. Taking advantage of the ability of CTA to detect the presence and extent of obstructive and nonobstructive atherosclerosis along the coronary arteries, the authors devised a variety of scores based not only on the severity of stenosis but also the location, extent, and composition of all visualized plaques. They utilized data from 163 patients submitted to sequential CTA and MPI, and were able to show that a score based on the sum of all stenoses, or one based on the presence of mixed plaque (calcified and noncalcified), as well as the proximity of plaques to the coronary ostia, predicted an abnormal result on MPI with high accuracy. Hence, plaque location and morphology may provide additional data that improve the physician’s ability to predict based on abnormal MPI results.
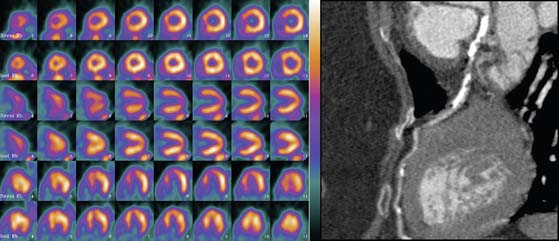
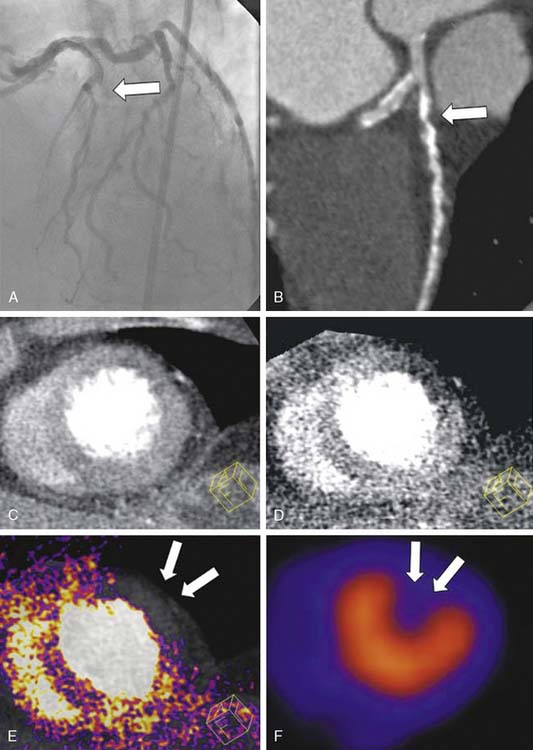
An elegant method to potentially overcome the partial limitations of both CTA and MPI and improve the diagnostic accuracy of these tools is image fusion. The existence of hybrid imaging equipment, such as PET/CT and SPECT/CT scanners, and the implementation of postprocessing software to merge data obtained from separate MPI and CTA equipment allow superimposing the coronary artery tree over perfusion maps of the left ventricle (Figs. 21-8 and 21-9).76,77 Fusion imaging appears to provide a more precise co-localization of perfusion defects in a specific area of the myocardium with coronary artery stenoses, and to improve the overall diagnostic accuracy of either tool taken separately.
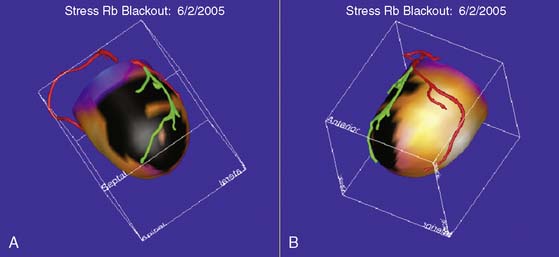
Figure 21-9 Fusion of SPECT and computed tomography angiography images shown in Fig. 21-8, in a patient with single-vessel coronary artery disease. In the fused display, the black area identifies a region of myocardial hypoperfusion during stress. The segment of the left anterior descending coronary artery rendered in green identifies the area of disease seen on CT angiography.
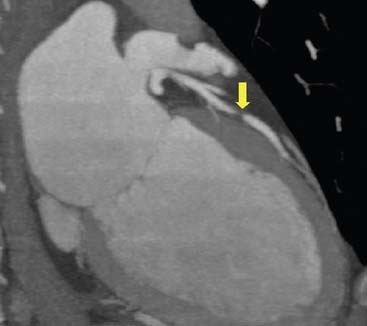
The preceding discussion clearly demonstrates that MPI and CTA provide complementary information, since a stenotic lesion on CTA or invasive angiography is not necessarily associated with a hemodynamic abnormality, and an abnormal MPI is not necessarily accompanied by a critical luminal stenosis on CTA. An abnormal CTA in the presence of a normal MPI, however, indicates the presence of subclinical atherosclerosis that should prompt more aggressive medical therapy even in the absence of hemodynamic alterations. The discrepancy between severity of stenosis on CTA and invasive angiography (described in the first section of this chapter), as well as the discrepancy between severity of stenosis on CTA and inducibility of myocardial ischemia on MPI, should not come as a complete surprise. Indeed, it is important to remember that with CTA, the clinician identifies more than the mere luminal stenosis of a vessel, as can be seen with invasive angiography. In fact, with CTA it is possible to identify the presence of outward and inward vessel wall remodeling, which increases the difficulty of clearly assessing the severity of luminal restriction. Furthermore, the presence of mere stenosis does not necessarily establish the presence of flow abnormalities. Neither invasive coronary angiography nor coronary CTA correlate well with the assessment of the hemodynamic significance of coronary stenoses by intracoronary fractional flow reserve measured by Doppler flow wires.78,79 Finally, as said in other sections of this chapter, the presence of dense calcification causing blooming artifact (see Fig. 21-5) and the occurrence of motion artifacts render the interpretation of severity of stenosis very difficult. All of these factors mitigate the probability of a perfect match between anatomic and functional imaging.
Nicol et al.80 addressed the question of what type of CTA results should prompt a referral to MPI. In a study of 52 symptomatic patients with low to intermediate pretest probability of disease, an abnormal MPI result was observed more often (86% versus 50%) in subjects with a CTA stenosis =70% than =50%. They concluded that CTA lesions narrowing the coronary lumen =70% should be considered “hemodynamically significant,” and patients hosting this type of lesion should be referred for invasive angiography. Since the NPV of a CTA stenosis less than 50% was 100% for an abnormal MPI, no further testing should be recommended for these patients. Further MPI testing, however, should be considered for patients with stenosis severity graded as 50% to 69%. Similarly, Sato et al.81 performed 64-slice CTA and MPI in 104 lean Japanese patients; once again the probability of an abnormal MPI was significantly greater for CTA stenosis =80% (PPV: 86%) compared to lower degrees of stenosis. It should be noted that both investigator groups (Nicol and Sato) emphasized ischemia detected by MPI as the primary target of investigation for CAD, a viewpoint that clinicians interested in the detection of preclinical coronary atherosclerosis may find arguable and oppose.
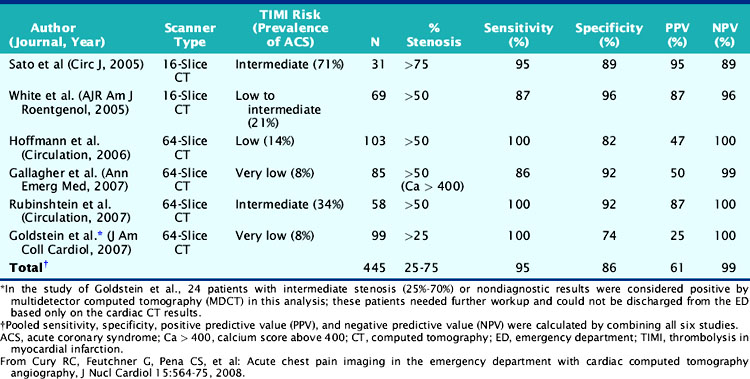
MPI and CTA have been compared in the setting of acute chest pain in the emergency room. In low-risk patients, the accuracy of CTA appears to be at least as good as MPI for the diagnosis of acute coronary syndrome (ACS).82 In one study, a low-risk population of 85 patients with chest pain, negative serial cardiac enzymes, and normal ECG were submitted to both rest/stress MPI and CTA (64-slice MDCT). Patients with a reversible defect on MPI or =50% stenosis on CTA were referred for invasive angiography. The endpoints were accurate identification of greater than 70% stenosis by invasive angiography or a major adverse cardiac event within 30 days. Seven patients met a primary endpoint (all > 70% stenosis by invasive angiography). Sensitivity and specificity of MPI for predicting an endpoint were 71% and 90%, respectively, while sensitivity and specificity of 64-slice MDCT were 86% and 92%. In a similar emergency room setting, Goldstein et al.83 randomized 99 low-risk patients to 64-slice CTA and 98 similar patients to MPI at a single medical center. The patients in whom CTA showed minimal or no disease were discharged home; if CTA showed over 70% stenosis in one or more vessels, they were sent directly to invasive angiography, and if the lesion was determined to be intermediate, they were referred for MPI. A standard protocol was followed for the patients referred to MPI first. Among the CTA patients, 68 were immediately discharged home, 8 were referred for invasive angiography, and 24 to MPI. Patients were managed equally well in the two imaging arms, and there were no untoward events at 6 months in either group. In the CTA group, however, the final diagnosis was reached in a significantly shorter time (3.4 versus 15 hours; P < 0.001), with a cost saving of about $300 per patient, and the patients required fewer recurrent evaluations for chest pain. It would appear, therefore, that in the emergency room setting, CTA may be an acceptable alternative to MPI in patients at low pretest likelihood of disease. Of interest, in a matched-cohort observational study of patients with new-onset chest pain undergoing coronary CTA or MPI, the 2-year mortality rate based on severity of CAD as assessed by coronary CTA was not significantly different from the rate based on severity of ischemia as assessed by MPI.84 This isolated outcome study suggests that the noninvasive assessment of severity of anatomic stenosis or hemodynamic impediment to blood flow may have similar prognostic implications. Obviously, such data will need to be confirmed in larger prospective studies, but they constitute interesting hypothesis-generating information. Cury et al. recently reviewed the use of coronary CTA in the emergency department setting (Table 21-1).85 This review confirmed the high NPV of CTA for the exclusion of acute coronary syndromes in patients at low to intermediate pretest probability of disease.
CORONARY COMPUTED TOMOGRAPHY ANGIOGRAPHY TO PERFORM MYOCARDIAL PERFUSION IMAGING AND ASSESS VIABILITY
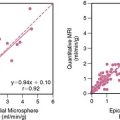
The option to utilize CTA to evaluate myocardial perfusion, infarct size, and viability has been recently explored. Although MDCT can be used for these applications, there is a substantial difference between CTA and MPI; with the former, one can obtain mostly perfusion information (i.e., presence or absence of blood flow in the coronary arteries and into the myocardium), the latter provides information on cellular and molecular function, which is, of course, dependent on coronary blood flow. During the arterial phase of CT angiography, reduced contrast enhancement may be due to severe epicardial coronary stenosis or occlusion, obstruction of the microcirculation, or myocardial scar. Chronic myocardial infarction can usually be identified as an area of reduced myocardial enhancement, but wall thickness and abnormal wall motion, differentiation of severe stenosis, acute myocardial infarction, or microvascular obstruction are problematic on resting CTA angiography. One of the major limiting factors for CTA is the rapid fading of contrast from the myocardium. MPI imaging is an “equilibrium imaging” acquired over several minutes, while CTA perfusion imaging is an instantaneous form of myocardial imaging, and the fleeting appearance of iodine contrast hampers the clear distinction of hypoperfused myocardium, normally perfused myocardium, and the blood pool. Pharmacologic stress testing prior to CTA to improve detection of myocardial ischemia with this tool has been applied in both animal86 and human experiments.87 George et al.86 created coronary artery stenoses in the LAD of eight anesthetized dogs and performed rest and adenosine stress CTA. They then compared the myocardial signal intensity (measured in Hounsfield units [HU]) of ischemic and remote nonischemic regions and measured myocardial blood flow (MBF) with infusion of microphages. They found a close correlation between MBF and myocardial signal intensity, both in ischemic and distant regions. Kurata et al.87 performed rest and stress CTA in 12 patients. All 12 patients were also submitted to MPI and 9 of them to invasive coronary angiography. Adenosine triphosphate (ATP) stress CTA and MPI showed an agreement of 83% (P < 0.05 for concordance), with only a few discordant myocardial segments between the two techniques. Hence, it appears that stress CTA may perform fairly well for detection of myocardial ischemia. However, the performance of stress CTA implies not only a double exposure to a considerable amount of radiation but also a double dose of iodine contrast, rendering this technique unattractive at the current state of development. An interesting application of CTA for myocardial perfusion was recently proposed by Ruzsics et al.53 In 35 patients with chest pain, the authors performed CTA utilizing a dual-source 64-slice MDCT (Siemens, Forcheism, Germany) with each x-ray tube operated at different x-ray energy spectra. The aim of the authors was to obtain simultaneous diagnostic information on coronary artery stenosis and myocardial perfusion from the same CT acquisition without the need to repeat two separate studies. The patients were also submitted to invasive coronary angiography and MPI for comparison. CT demonstrated an accuracy of 92% to detect both coronary artery obstructive disease and myocardial perfusion abnormalities (Fig. 21-10). Since dual-energy imaging will become available in several CT systems in the near future, these findings suggest that the presence of perfusion defects may enhance the diagnostic accuracy of CTA for coronary stenoses, often hampered by the presence of severe artifacts (as discussed elsewhere in this chapter).
Numerous publications have addressed the ability of CTA to identify areas of acute myocardial infarction as well as areas of scar, as defined by the presence of delayed hyperenhancement. In fact, it appears that gadolinium and iodine contrast demonstrate similar molecular weight and volume of distribution in areas of myocardial infarction. The best time delay to evaluate presence of infarction by CTA is not yet defined. Investigators have used a time delay varying between 5 and 15 minutes, although there is some evidence that the best results are obtained when imaging at 5 minutes after contrast infusion.88 Possible explanations for the accumulation of iodine contrast in areas of acute infarction include the accumulation of iodine in the extracellular matrix and/or the inability of necrotic myocardial cells to excrete the iodine due to sarcolemmal membrane dysfunction.89 In the chronic phase of a myocardial infarction, the iodine contrast is accumulated in the extracellular matrix of a scar. Infarcted areas with microvascular obstruction due to cellular debris blockage of intramyocardial capillaries appear as subendocardial areas of hypoenhancement adjacent to the hyperenhanced areas.89
In animal experiments, myocardial infarction has been induced with ligation of a coronary artery90 or via prolonged inflations of angioplasty balloons,89,91 and the size of the infarct measured by means of postmortem triphenyltetrazolium chloride (TTC) myocardial staining. Hoffmann et al.90 ligated the LAD and performed first-pass CTA in 5 pigs within 3 hours of inducing myocardial infarction. A distinct area of hypoenhancement, which correlated well with reduced blood flow measured by microspheres and size of infarction by TTC, was seen in all animals. Baks et al.91 performed CTA imaging in pigs 5 days after inducing myocardial infarction with prolonged balloon inflation, while Lardo et al.89 waited 8 weeks after inducing the infarction. Both groups of investigators showed a good correlation between the area of hyperenhancement detected on delayed CTA and infarct size on TTC staining. A hypoenhanced area on delayed CTA images was found to be a reliable indicator of microvascular obstruction (i.e., no-reflow phenomenon).89 Finally, there was an excellent correlation between the area of delayed hyperenhancement found on CTA and MRI.91 Very similar correlation results between CT and MRI were reported in humans by Gerber et al.88 and Nieman et al.92 In several small human studies, the extent of myocardial scarring, subendocardial to transmyocardial, was correlated with subsequent recovery of left ventricular function.93–96
ASSESSMENT OF LEFT VENTRICULAR FUNCTION AND MASS BY CARDIAC COMPUTED TOMOGRAPHY
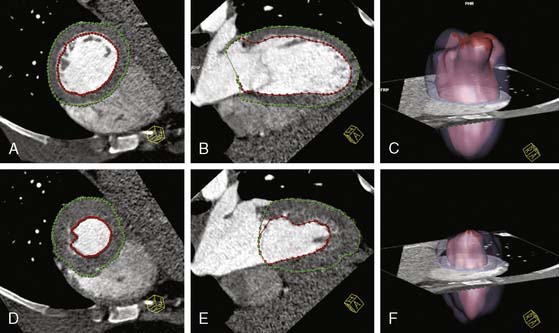
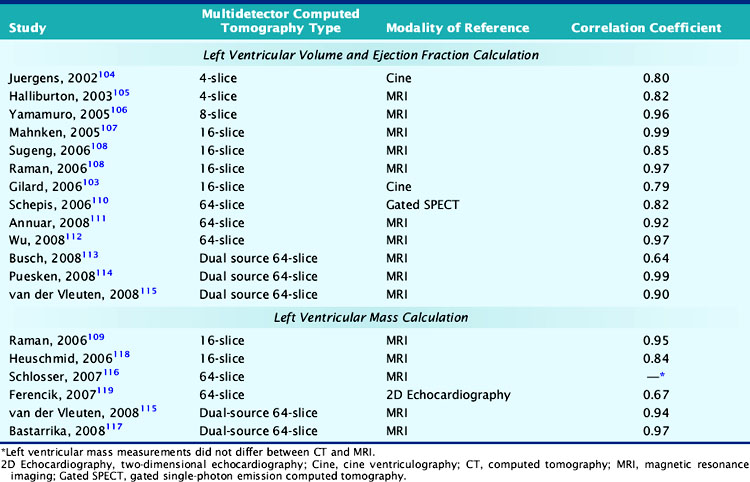
The assessment of left ventricular volumes, function, and mass was shown to be feasible and very accurate with the older EBCT technique.97–101 However, since EBCT used a step-and-shoot acquisition protocol for the coronary arteries (one image per heartbeat or every other beat), left ventricular function could only be obtained after the injection of a second dose of iodine contrast and the acquisition of a multiphase and multilevel scan; this of course increased the risk and discomfort for the patient. With MDCT scanners, the continuous helical acquisition of hundreds of slices throughout the cardiac cycle makes it possible to utilize the same scan used to assess CAD to evaluate left ventricular function. To assess left ventricular function, end-diastolic and end-systolic volumes are usually measured using the Simpson method or, more recently, using a threshold-based volumetric method that takes advantage of the high contrast-to-noise ratio between the left ventricular cavity filled with contrast and the myocardium.102 The end-diastolic frames are obtained from a late-diastolic phase at 85% to 95% of the R-R interval on the surface ECG and the end-systolic frames from an early systolic phase at 25% to 30% of the R-R interval, respectively (Fig. 21-11). Magnetic resonance imaging (MRI), currently considered the noninvasive modality of reference for the assessment of global and regional left ventricular function, has been compared to MDCT in several publications. Whereas MDCT has a better spatial resolution than EBCT and MRI, its accuracy for the determination of left ventricular ejection fraction is affected by its lower temporal resolution, which usually results in the overestimation of end-systolic volumes and an underestimation of left ventricular ejection fraction. Despite this limitation, the left ventricular ejection fraction obtained with 4- and 16-slice cardiac MDCT showed a very good correlation with that obtained with invasive contrast ventriculography103 and with MRI.104–115 In several comparative studies, the MDCT and MRI left ventricular ejection fraction showed a correlation coefficient varying from 0.82 to 0.99. Although the majority of studies demonstrated an excellent correlation of 64-slice MDCT with MRI (r = 0.92 to 0.95),111,112 Schlosser et al.116 calculated a significantly lower left ventricular ejection fraction with 64-slice cardiac MDCT than MRI. So far, dual-source MDCT, with greater temporal resolution than other currently available 64-slice MDCT scanners, has shown mixed results, with an excellent114 to modest113 correlation with MRI (Table 21-2). Finally, Schepis et al.110 compared 64-slice MDCT with gated SPECT and found a correlation coefficient of 0.82 for the measurement of left ventricular function. Mass can also be accurately assessed with MDCT, with a closer similarity to MRI than seen with measurement of left ventricular volumes and function.109,115–118 However, Ferencik et al.119 calculated a significantly lower left ventricular mass with 64-slice MDCT than echocardiography in heart transplant recipients. This systematic error between the techniques may be secondary to the very different assumptions made with 3D and 2D imaging techniques.
PROGNOSIS OF PATIENTS UNDERGOING COMPUTED TOMOGRAPHY ANGIOGRAPHY TO DIAGNOSE CORONARY ARTERY DISEASE
Based on the findings of a CTA study, patients could be categorized as having (1) no disease, (2) nonobstructive disease, and (3) obstructive CAD. As expected, patients with no disease have the lowest risk for subsequent cardiovascular events or death. This has been clearly demonstrated in several studies. Gilard et al. followed 141 patients with normal CTA findings for a mean period of 14.7 months. These patients experienced 0% mortality, a 3.5% rate of subsequent conventional coronary angiography, and a 0.7% rate of myocardial infarction, which compared favorably with those following conventional coronary angiography with normal findings.120 In two other recent studies, patients without abnormal findings on CTA had no major cardiovascular events (i.e., cardiac death, nonfatal myocardial infarction, unstable angina, revascularization) after an average follow-up of 12 months.121,122 In the experience reported by Matsumoto et al.,123 the annual rate of acute coronary syndromes (nonfatal myocardial infarction, unstable angina) and cardiac death was 0.66% and 0.21%, respectively, over a 3-year follow-up period.
The high NPV of CTA makes it an attractive alternative for low-risk patients in the emergency room setting. Rubinshtein et al.124 discharged 35 patients from the emergency room after initial triage and CTA and showed that no patient died or had a myocardial infarction during a 15-month period, and only one underwent late percutaneous coronary intervention; despite this apparent mishap, the NPV of CTA was still 97%.
When the findings of CTA are indicative of atherosclerotic disease with nonobstructive lesions, this confers a moderately elevated risk for subsequent cardiovascular events. The risk of first-year major cardiovascular events (cardiac death, nonfatal myocardial infarction, unstable angina, and revascularization) in this group of patients is in the range of 3% to 8%.121,122 In a 3-year follow-up, the annual event rate for any acute coronary syndrome or cardiac death in patients with mild to moderate stenoses on CTA (25% to 75%) was about 2%, significantly higher than the event rate in patients with no stenoses.123
One of the main advantages of CTA over conventional coronary angiography is its ability to visualize not only the lumen but also the plaque itself. In more than 50% of myocardial infarctions, the culprit lesion is a nonstenotic yet vulnerable lipid-rich plaque.125 CTA is currently the only noninvasive technology capable of evaluating plaque morphology, and this specific advantage of CTA may be the source of the additional prognostic information provided by this imaging modality. Indeed, the presence of nonobstructive disease on invasive angiography has also been shown to be predictive of an adverse outcome in the Coronary Artery Surgery Study.126
Finally, patients with obstructive coronary artery disease on CTA have a very high risk for subsequent cardiovascular events and death. Depending on multiple factors beyond the anatomic findings per se, this risk may be as high as 63% for the first year.122 However, it is well known that the location and extent of CAD play an important role in determining prognosis. These findings are in line with the findings from conventional coronary angiography that have been widely used for risk stratification and management of patients with CAD. The presence of either obstructive or nonobstructive plaques in the left main (LM) coronary artery, the left anterior descending (LAD) coronary artery, and in the proximal segment of any of the three major coronary arteries confers an unfavorable prognosis. Another factor that affects prognosis is the number of segments with plaques.121
In the study of Pundziute et al.,122 multivariate analyses revealed that the presence of obstructive CAD, obstructive CAD in left main or left anterior descending coronary artery, the number of segments with plaques, the number of segments with obstructive plaques, and the number of segments with mixed plaques were independent predictors of events.
In an attempt to assess the association of all-cause mortality with the extent and severity of CAD, Min et al.127 followed 1127 patients with chest pain symptoms for 15 months after undergoing CTA. They applied several methods of estimating CAD severity and extent: (1) assessment of moderate (=50% of the vessel diameter) or severe (=70% of the vessel diameter) stenosis in the three coronary arteries and the left main trunk, (2) the Duke prognostic CAD index128 and one modification of it in patients with <50% stenosis, and (3) three different clinical plaque scores measuring plaque burden and distribution. Each one of these methods gave important prognostic information.
OTHER USES OF COMPUTED TOMOGRAPHY ANGIOGRAPHY
Noncalcified Atherosclerotic Plaque
The superior spatial resolution of MDCT allows the visualization of the noncalcified component of atherosclerotic plaques (Fig. 21-12).129–131 However, the identification of areas of low attenuation along the course of the coronary arteries is at times challenging and much more difficult than the identification of calcified foci, owing to the similar attenuation of plaque and pericoronary fat. Furthermore, the spatial resolution of MDCT is not yet sufficient to visualize the finer components of a plaque, such as the lipid core or the fibrous cap. Therefore, the precise quantification and characterization of noncalcified plaques remains an elusive goal at this time. Nonetheless, there is obviously a high degree of interest in pursuing this imaging venue because of the well-known tenet that plaques more prone to rupture (i.e., vulnerable) contain a large lipid core and are covered by a thin fibrous cap and should therefore have a lower attenuation on CT imaging than more stable and fibrotic plaques. Several attempts were made at separating lipid-rich from fibrotic plaques by assessing the mean CT attenuation in areas of apparent noncalcified atherosclerosis, and the CT findings were compared to intravascular ultrasound (IVUS) data. Achenbach et al.130 with 16-slice MDCT and Leber et al.132 with 64-slice MDCT showed that CTA underestimates plaque volume compared to IVUS and has modest (53%) to good sensitivity (83%) for the identification of noncalcified plaques compared to the invasive technique. The mean CT attenuation of hypoechogenic plaques on IVUS (i.e., lipid rich) was significantly lower than that of hyperechogenic (i.e., fibrotic) plaques, with values ranging from 14 to 58 HU for the former and 90-120 HU for the latter.129,131,133 Patients with acute coronary syndromes show less coronary calcification, larger areas and greater numbers of low attenuation plaques,134,135 and more plaques with positive remodeling136 than patients with stable angina. In Motoyama’s report,136 the simultaneous presence of positive remodeling, areas of low attenuation, and spotty calcification identified with 95% accuracy the plaque associated with the acute coronary syndrome (i.e., culprit plaque). Since the mean CT attenuation in plaques exhibiting positive remodeling is lower than in vessels without remodeling,137 and the plaques with the lowest attenuation have been associated with the presence of a necrotic core on spectral analysis of IVUS images (i.e., virtual histology),138,139 the combination of low attenuation and positive remodeling makes for a severely increased risk of unstable plaques. Attempts at assessing changes in noncalcified plaque volume with sequential CTA have been conducted in animals140 as well as in humans.141,142 There appears to be a significant change in volume over time without treatment,141 although this trend seems to be reversible with treatment.140,142 Although interesting, there are some limitations to the data presented so far: The studies were very small, and data on the prognostic value of noncalcified plaque are missing, with an isolated small exception.122 Many investigators reported a substantial overlap between CT attenuation values of lipid-rich and fibrotic plaques, hence the mere measurement of plaque attenuation may not be sufficient to define its vulnerability. Additionally, a change in image filter (technically called kernel) may substantially affect the measurement of CT attenuation within the plaque.143 To overcome some of these limitations, more work is ongoing in the field. The application of dual-energy CT imaging, to take advantage of the difference in radiation absorption of different tissues, has been proposed to improve the chance to detect and characterize noncalcified plaques.144 Although accurate, to date, this application has been limited to ex vivo experiments.144 Finally, in animal testing, iodine-containing nanoparticles selectively uptaken by macrophages have been used in a rabbit model of atherosclerosis to image inflammation within a noncalcified plaque.145 It is obvious that although attractive, this field needs to further expand before becoming a clinical reality.
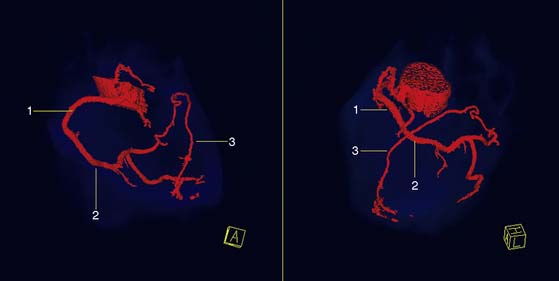
Coronary Artery Anomalies
Visualization of congenital anomalies of the coronary arteries is achievable with high accuracy with both EBCT and MDCT.146–149 With the isotropic volumetric information acquired with cardiac CT, it is possible to clearly delineate the origin and the course of the anomalous coronary in 3D-rendered images, as shown in Figs. 21-13 and 21-14. Although MDCT could be particularly helpful in children for early diagnosis of coronary anomalies, the younger the patients, the greater the risk linked with radiation exposure; therefore, MRI is often the preferred noninvasive imaging method for children. However, many anomalies are found serendipitously in scans ordered because of atypical chest pain or other unrelated reasons in unsuspecting adult individuals.150
Guidance Prior to Attempting Angioplasty of a Chronic Total Occlusion
One of the main hurdles to performing a successful percutaneous angioplasty and one of the most frequent reasons for complications and restenosis after angioplasty of a chronic total occlusion is heavy calcification at the site of occlusion. Furthermore, it is often difficult for the operator to predict the length of the occlusion and the size and course of the occluded vessel. CTA has been reported to be of aide in the preoperative evaluation of patients scheduled to undergo angioplasty of totally occluded vessels. Mollet et al.151 found two independent predictors of failure to complete the angioplasty: an occlusion length exceeding 15 mm and calcification involving more than 50% of the cross-sectional area of the coronary artery at the site of occlusion. Soon et al.152 confirmed these findings. Yokoyama et al.153 noted that the majority of calcification is usually located very proximal to the total occlusion, and the success rate (ability to cross the total occlusion with a guide wire) was 91% under MDCT guidance because of the visual guidance provided to the operator.
Presurgical Risk Stratification
Cardiac CT may be of value in excluding the presence of obstructive CAD prior to surgery in patients with a contraindication to cardiac catheterization or a very high risk for it. Gilard et al.154 performed CTA with a 16-slice MDCT in 55 patients scheduled to undergo invasive coronary angiography prior to surgery for aortic valve stenosis. In cases with a CAC score <1000, 80% of the patients would not have needed an invasive angiogram, because the CT clearly excluded the presence of critical CAD. For patients with more extensive calcium scores, this proportion dropped to 6%.154 Similar conclusions were reached by Holmström et al.,155 who showed a sensitivity of 8-slice MDCT angiography of 63% but a NPV of 98% for obstructive CAD in 22 patients with severe aortic stenosis. A different result was reported by Scheffel et al.156 in 50 patients awaiting surgery for aortic valve regurgitation. Given the much lower CAC score in these patients, CTA performed much better in comparison with invasive angiography to exclude obstructive disease with sensitivity, specificity, PPV and NPVs of 100%, 95%, 87%, and 100%, respectively. Accordingly, preoperative invasive angiography could have been avoided in 70% of the patients. Schlosser et al.157 reviewed a small case series of 11 patients scheduled to undergo repair of a thoracic aorta aneurysm or aortic dissection in whom 64-slice CTA was performed instead of invasive angiography. They reported 100% success rate in excluding or confirming the presence of CAD, but they were also able to visualize and size the thoracic pathology of interest and clearly assess the calcification and mobility status of the mitral and aortic valves.
Assessment of Etiology of Left Ventricular Dysfunction
One of the most frequent etiologies of left ventricular dysfunction in Western countries is CAD, and invasive angiography is often performed at the time of first diagnosis of congestive heart failure. Cardiac CT can assist in excluding CAD as a cause of left ventricular dysfunction. The initial demonstration of the utility of CT in this field was provided several years ago by Budoff et al.,158 who showed that absence of coronary artery calcium is associated with a very low probability of CAD in patients with recent diagnosis of left ventricular dysfunction. More recently, other investigators have shown that CTA is a valid alternative to invasive angiography for the exclusion of ischemic heart disease in patients with systolic dysfunction but otherwise no clinical suspicion of CAD.159,160
RADIATION EXPOSURE (See Chapter 10)
Despite the numerous helpful applications and the enthusiasm we witnessed in recent years, patients submitted to CTA should be carefully chosen because of the potential risks associated with the use of ionizing radiation, especially in young patients and women (added risk inherent with additional radiation to the breast and ovaries). The effective dose, expressed in millisieverts (mSv), is the dose parameter most frequently cited in the literature.161 While coronary calcium screening delivers a relatively low radiation dose (effective dose of 0.7 mSv with EBCT and 1.0 to 4.1 mSv with MDCT), coronary CT angiography delivers much higher levels of radiation (effective dose of 9 to 20 mSv).162 This should be compared to the radiation dose provided by MPI studies (effective dose range of 13 to 16 mSv), especially those conducted using thallium-201 or dual-isotope techniques (effective dose of 25 to 30 mSv)163 or invasive diagnostic coronary catheterization (effective dose of 3 to 10 mSv).164 The dose delivered depends both on the imaging protocol used and the patient’s body habitus: the heavier the patient, the greater the photon flux needed to obtain a good-quality image and the greater the radiation dose. The effective dose can be decreased by using ECG-triggered tube current modulation. With this method, the x-ray tube current is decreased during systole and increased during diastole, since images typically are reconstructed from data obtained during diastole. Tube current modulation can decrease radiation dose by about a third, depending on the heart rate (the slower the heart rate, the longer the systolic phase during which current is reduced).165–168 Prospective gating can also help reduce radiation dose while performing coronary CTA. With standard retrospective gating, the patient moves through the gantry at a steady speed and images are obtained throughout the cardiac cycle. With prospective gating, the scans are initiated at a certain point on the cardiac cycle and typically obtained during the later portion of diastole. A limitation of this method is that systolic images are not obtained, and ventricular function cannot be assessed. Earls et al.169 studied 82 patients with retrospective gating and 121 patients with prospective gating. They reported 83% reduction in effective dose with prospective gating compared to retrospective gating, with no difference in proportion of unassessable coronary segments and better image quality for prospective gating.169
Much of our knowledge on the carcinogenic effects of low doses of radiation (whole body exposures of 5 to 150 mSv) derives from follow-up data on the survivors of the atomic bombing during World War II. Although small, there appears to be an increase in incidence of cancer in subjects exposed to low doses of radiation—especially in children because of the higher radiation sensitivity and the long lifetime during which they can receive further radiation exposure and can develop cancer. There are several theories as to how risk should be estimated. The linear no-threshold theory assumes that risk increases linearly with dose. The supralinear no-threshold theory considers the risk increasing at a greater rate than the dose received. Finally, the threshold theory assumes that risk starts increasing only after a certain dose of radiation is absorbed. The linear no-threshold theory is the predominant view. Using this model and the organ-specific risk from Biological Effects of Ionizing Radiation (BEIR VII),170 the data presented by Einstein et al.171 indicate that the lifetime risk of cancer for a CTA in a 50-year-old individual is 0.4% for a man and 1.2% for a woman. Obviously these data should be interpreted in light of the overall clinical scenario for the patient under study, including the background risk of cancer incidence in the general population and any other risk factors such as diabetes mellitus, high blood pressure, or a family history of cancer or heart disease. According to statistics from the American Cancer Society, the lifetime risk of cancer at any site is 45% for men and 38% for women; the respective death rates are 23% and 20%.172 Thus, the additive cancer risk may be negligible for a 50-year-old, hypertensive, diabetic man who might benefit substantially from the information collected with CTA. Hence, an appropriate selection of subjects to be submitted to CTA, as well as any other test utilizing ionizing radiation, will guarantee that the gain derived from the examination is greater than the added risk to the patient.
There are further limitations to the wide implementation of CTA that have been discussed in part in this chapter. Calcified lesions (and stents) cause partial volume and beam-hardening artifacts (see Fig. 21-5). A given voxel (volume unit) in the image can contain more than one tissue type. If the voxel contains calcium and another tissue type, the dense calcium signal will overwhelm the other tissue type, and the entire voxel will appear bright, making a calcified lesion appear larger than the actual size (see Fig. 21-5). This partial-volume or blooming artifact can induce a false-positive reading. A beam-hardening artifact typically causes a “shadow” adjacent to a densely calcified lesion that can be confused with a noncalcified plaque. Additionally, CTA requires use of iodinated contrast (50 to 120 mL per study) with risk of both nephrotoxicity and anaphylactic reactions. In patients with impaired renal function, the use of coronary CTA is limited, especially since an abnormal result may lead to further testing by MPI and/or invasive coronary angiography with additional radiation exposure and contrast use. Small vessels (<1.5 to 2 mm), including branches of main vessels, the distal segments of main vessels, or the native vessel distal to a graft anastomosis, are difficult to visualize. As explained in the introductory remarks, at the present state of technologic development, the heart rate should be less than 60 to 65 beats/min to obtain diagnostic-quality images with most scanners. Administration of β-blockers is therefore commonly required to lower the heart rate prior to CTA with a 16- or 64-slice MDCT, and this may limit the application of CTA to those patients who can tolerate β-blockade. Additionally, patients with atrial fibrillation or frequent ectopic beats may have decreased-quality studies.
WHEN SHOULD CORONARY COMPUTED TOMOGRAPHY BE UTILIZED?
Both the American College of Cardiology173 and the European Society of Cardiology174 in association with several other professional societies summarized the current appropriate indications for CTA. Table 21-3 summarizes the American recommendations, which for the most part are similar to those issued by the European Writing Group. Asymptomatic patients should not be exposed to ionizing radiation unnecessarily, so there is no current indication for CTA in these patients. Patients with unclear functional stress test results, patients experiencing chest discomfort with normal ECG and no cardiac enzyme elevation, and cases of suspected coronary anomaly or exclusion of CAD in patients with new-onset congestive heart failure constitute the main indications. Owing to the consistently high NPV, coronary CTA is better suited to ruling out CAD than establishing a firm diagnosis of obstructive disease. This could be especially useful in the emergency department, where a quick and accurate diagnosis may be desirable for a rapid and cost-effective disposition of the patient.175 Finally, to improve overall quality as well as reduce inappropriate uses of the technique, both the European174 and American173 professional associations called for specific training prior to pursuing CT credentials.
Table 21-3 Appropriate Indications for Computed Tomography Angiography
| Asymptomatic patients | No indications |
| Symptomatic patients | Indeterminate pretest probability of coronary artery disease with uninterpretable ECG or patient unable to exercise Intermediate pretest probability of coronary artery disease with normal ECG and cardiac enzymes Uninterpretable or equivocal stress test Suspected coronary anomaly Evaluation of coronary artery disease in new-onset congestive heart failure |
Summarized from Hendel RC et al.167
ACKNOWLEDGEMENTS
Nikolaos Alexopoulos, MD was supported by a scholarship from the Hellenic Cardiology Society.
1. Shemesh J., Apter S., Rozenman J., Lusky A., Rath S., Itzchak Y., Motro M. Calcification of coronary arteries: detection and quantification with double-helix CT. Radiology. 1995;197(3):779-783.
2. Shemesh J., Stroh C.I., Tenenbaum A., Hod H., Boyko V., Fisman E.Z., Motro M. Comparison of coronary calcium in stable angina pectoris and in first acute myocardial infarction utilizing double helical computerized tomography. Am J Cardiol. 1998;81(3):271-275.
3. Broderick L.S., Shemesh J., Wilensky R.L., Eckert G.J., Zhou X., Torres W.E., Balk M.A., Rogers W.J., Conces D.J.Jr, Kopecky K.K. Measurement of coronary artery calcium with dual-slice helical CT compared with coronary angiography: evaluation of CT scoring methods, interobserver variations, and reproducibility. AJR Am J Roentgenol. 1996;167(2):439-444.
4. Scheffel H., Alkadhi H., Plass A., Vachenauer R., Desbiolles L., Gaemperli O., Schepis T., Frauenfelder T., Schertler T., Husmann L., Grunenfelder J., Genoni M., Kaufmann P.A., Marincek B., Leschka S. Accuracy of dual-source CT coronary angiography: First experience in a high pre-test probability population without heart rate control. Eur Radiol. 2006;16(12):2739-2747.
5. Johnson T.R., Nikolaou K., Wintersperger B.J., Leber A.W., von Ziegler F., Rist C., Buhmann S., Knez A., Reiser M.F., Becker C.R. Dual-source CT cardiac imaging: initial experience. Eur Radiol. 2006;16(7):1409-1415.
6. Achenbach S., Ropers D., Kuettner A., Flohr T., Ohnesorge B., Bruder H., Theessen H., Karakaya M., Daniel W.G., Bautz W., Kalender W.A., Anders K. Contrast-enhanced coronary artery visualization by dual-source computed tomography—initial experience. Eur J Radiol. 2006;57(3):331-335.
7. Oncel D., Oncel G., Tastan A. Effectiveness of dual-source CT coronary angiography for the evaluation of coronary artery disease in patients with atrial fibrillation: initial experience. Radiology. 2007;245(3):703-711.
8. Strub W.M., Vagal A., Meyer C. Optimizing coronary artery imaging in patients with atrial fibrillation with ECG-gated 64-MDCT. AJR Am J Roentgenol. 2007;189(1):W50-W51.
9. Achenbach S., Moshage W., Ropers D., Nossen J., Daniel W.G. Value of electron-beam computed tomography for the noninvasive detection of high-grade coronary-artery stenoses and occlusions. N Engl J Med. 1998;339(27):1964-1971.
10. Leber A.W., Knez A., Becker C., Becker A., White C., Thilo C., Reiser M., Haberl R., Steinbeck G. Non-invasive intravenous coronary angiography using electron beam tomography and multislice computed tomography. Heart. 2003;89(6):633-639.
11. Achenbach S., Ulzheimer S., Baum U., Kachelriess M., Ropers D., Giesler T., Bautz W., Daniel W.G., Kalender W.A., Moshage W. Noninvasive coronary angiography by retrospectively ECG-gated multislice spiral CT. Circulation. 2000;102(23):2823-2828.
12. Vanhoenacker P.K., Heijenbrok-Kal M.H., Van Heste R., Decramer I., Van Hoe L.R., Wijns W., Hunink M.G. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology. 2007;244(2):419-428.
13. Roberts W.T., Bax J.J., Davies L.C. Cardiac CT and CT coronary angiography: technology and application. Heart. 2008;94(6):781-792.
14. Nikolaou K., Becker C.R., Muders M., Babaryka G., Scheidler J., Flohr T., Loehrs U., Reiser M.F., Fayad Z.A. Multidetector-row computed tomography and magnetic resonance imaging of atherosclerotic lesions in human ex vivo coronary arteries. Atherosclerosis. 2004;174(2):243-252.
15. Flohr T.G., McCollough C.H., Bruder H., Petersilka M., Gruber K., Suss C., Grasruck M., Stierstorfer K., Krauss B., Raupach R., Primak A.N., Kuttner A., Achenbach S., Becker C., Kopp A., Ohnesorge B.M. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol. 2006;16(2):256-268.
16. Achenbach S., Ropers D., Pohle F.K., Raaz D., von Erffa J., Yilmaz A., Muschiol G., Daniel W.G. Detection of coronary artery stenoses using multi-detector CT with 16 x 0.75 collimation and 375 ms rotation. Eur Heart J. 2005;26(19):1978-1986.
17. Aviram G., Finkelstein A., Herz I., Lessick J., Miller H., Graif M., Keren G. Clinical value of 16-slice multi-detector CT compared to invasive coronary angiography. Int J Cardiovasc Intervent. 2005;7(1):21-28.
18. Dewey M., Laule M., Krug L., Schnapauff D., Rogalla P., Rutsch W., Hamm B., Lembcke A. Multisegment and halfscan reconstruction of 16-slice computed tomography for detection of coronary artery stenoses. Invest Radiol. 2004;39(4):223-229.
19. Fine J.J., Hopkins C.B., Hall P.A., Delphia R.E., Attebery T.W., Newton F.C. Noninvasive coronary angiography: agreement of multi-slice spiral computed tomography and selective catheter angiography. Int J Cardiovasc Imaging. 2004;20(6):549-552.
20. Garcia M.J., Lessick J., Hoffmann M.H. Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296(4):403-411.
21. Hoffmann M.H., Shi H., Schmitz B.L., Schmid F.T., Lieberknecht M., Schulze R., Ludwig B., Kroschel U., Jahnke N., Haerer W., Brambs H.J., Aschoff A.J. Noninvasive coronary angiography with multislice computed tomography. JAMA. 2005;293(20):2471-2478.
22. Kaiser C., Bremerich J., Haller S., Brunner-La Rocca H.P., Bongartz G., Pfisterer M., Buser P. Limited diagnostic yield of non-invasive coronary angiography by 16-slice multi-detector spiral computed tomography in routine patients referred for evaluation of coronary artery disease. Eur Heart J. 2005;26(19):1987-1992.
23. Kuettner A., Trabold T., Schroeder S., Feyer A., Beck T., Brueckner A., Heuschmid M., Burgstahler C., Kopp A.F., Claussen C.D. Noninvasive detection of coronary lesions using 16-detector multislice spiral computed tomography technology: initial clinical results. J Am Coll Cardiol. 2004;44(6):1230-1237.
24. Martuscelli E., Romagnoli A., D’Eliseo A., Razzini C., Tomassini M., Sperandio M., Simonetti G., Romeo F. Accuracy of thin-slice computed tomography in the detection of coronary stenoses. Eur Heart J. 2004;25(12):1043-1048.
25. Mollet N.R., Cademartiri F., Krestin G.P., McFadden E.P., Arampatzis C.A., Serruys P.W., de Feyter P.J. Improved diagnostic accuracy with 16-row multi-slice computed tomography coronary angiography. J Am Coll Cardiol. 2005;45(1):128-132.
26. Morgan-Hughes G.J., Roobottom C.A., Owens P.E., Marshall A.J. Highly accurate coronary angiography with submillimetre, 16 slice computed tomography. Heart. 2005;91(3):308-313.
27. Nieman K., Cademartiri F., Lemos P.A., Raaijmakers R., Pattynama P.M., de Feyter P.J. Reliable noninvasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation. 2002;106(16):2051-2054.
28. Ropers D., Baum U., Pohle K., Anders K., Ulzheimer S., Ohnesorge B., Schlundt C., Bautz W., Daniel W.G., Achenbach S. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation. 2003;107(5):664-666.
29. Schuijf J.D., Bax J.J., Salm L.P., Jukema J.W., Lamb H.J., van der Wall E.E., de Roos A. Noninvasive coronary imaging and assessment of left ventricular function using 16-slice computed tomography. Am J Cardiol. 2005;95(5):571-574.
30. Cademartiri F., Maffei E., Palumbo A., Malago R., Alberghina F., Aldrovandi A., Brambilla V., Runza G., La Grutta L., Menozzi A., Vignali L., Casolo G., Midiri M., Mollet N.R. Diagnostic accuracy of 64-slice computed tomography coronary angiography in patients with low-to-intermediate risk. Radiol Med (Torino). 2007;112(7):969-981.
31. Ehara M., Surmely J.F., Kawai M., Katoh O., Matsubara T., Terashima M., Tsuchikane E., Kinoshita Y., Suzuki T., Ito T., Takeda Y., Nasu K., Tanaka N., Murata A., Suzuki Y., Sato K. Diagnostic accuracy of 64-slice computed tomography for detecting angiographically significant coronary artery stenosis in an unselected consecutive patient population: comparison with conventional invasive angiography. Circ J. 2006;70(5):564-571.
32. Fine J.J., Hopkins C.B., Ruff N., Newton F.C. Comparison of accuracy of 64-slice cardiovascular computed tomography with coronary angiography in patients with suspected coronary artery disease. Am J Cardiol. 2006;97(2):173-174.
33. Herzog C., Zwerner P.L., Doll J.R., Nielsen C.D., Nguyen S.A., Savino G., Vogl T.J., Costello P., Schoepf U.J. Significant coronary artery stenosis: comparison on per-patient and per-vessel or per-segment basis at 64-section CT angiography. Radiology. 2007;244(1):112-120.
34. Leber A.W., Knez A., von Ziegler F., Becker A., Nikolaou K., Paul S., Wintersperger B., Reiser M., Becker C.R., Steinbeck G., Boekstegers P. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46(1):147-154.
35. Leschka S., Alkadhi H., Plass A., Desbiolles L., Grunenfelder J., Marincek B., Wildermuth S. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J. 2005;26(15):1482-1487.
36. Malagutti P., Nieman K., Meijboom W.B., van Mieghem C.A., Pugliese F., Cademartiri F., Mollet N.R., Boersma E., de Jaegere P.P., de Feyter P.J. Use of 64-slice CT in symptomatic patients after coronary bypass surgery: evaluation of grafts and coronary arteries. Eur Heart J. 2007;28(15):1879-1885.
37. Meijboom W.B., Mollet N.R., Van Mieghem C.A., Weustink A.C., Pugliese F., van Pelt N., Cademartiri F., Vourvouri E., de Jaegere P., Krestin G.P., de Feyter P.J. 64-Slice CT coronary angiography in patients with non-ST elevation acute coronary syndrome. Heart. 2007;93(11):1386-1392.
38. Mollet N.R., Cademartiri F., van Mieghem C.A., Runza G., McFadden E.P., Baks T., Serruys P.W., Krestin G.P., de Feyter P.J. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112(15):2318-2323.
39. Muhlenbruch G., Seyfarth T., Soo C.S., Pregalathan N., Mahnken A.H. Diagnostic value of 64-slice multi-detector row cardiac CTA in symptomatic patients. Eur Radiol. 2007;17(3):603-609.
40. Oncel D., Oncel G., Tastan A., Tamci B. Detection of significant coronary artery stenosis with 64-section MDCT angiography. Eur J Radiol. 2007;62(3):394-405.
41. Plass A., Grunenfelder J., Leschka S., Alkadhi H., Eberli F.R., Wildermuth S., Zund G., Genoni M. Coronary artery imaging with 64-slice computed tomography from cardiac surgical perspective. Eur J Cardiothorac Surg. 2006;30(1):109-116.
42. Pugliese F., Mollet N.R., Runza G., van Mieghem C., Meijboom W.B., Malagutti P., Baks T., Krestin G.P., deFeyter P.J., Cademartiri F. Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006;16(3):575-582.
43. Raff G.L., Gallagher M.J., O’Neill W.W., Goldstein J.A. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46(3):552-557.
44. Ropers D., Rixe J., Anders K., Kuttner A., Baum U., Bautz W., Daniel W.G., Achenbach S. Usefulness of multidetector row spiral computed tomography with 64- × 0.6-mm collimation and 330-ms rotation for the noninvasive detection of significant coronary artery stenoses. Am J Cardiol. 2006;97(3):343-348.
45. Schlosser T., Mohrs O.K., Magedanz A., Nowak B., Voigtlander T., Barkhausen J., Schmermund A. Noninvasive coronary angiography using 64-detector-row computed tomography in patients with a low to moderate pretest probability of significant coronary artery disease. Acta Radiol. 2007;48(3):300-307.
46. Schuijf J.D., Pundziute G., Jukema J.W., Lamb H.J., van der Hoeven B.L., de Roos A., van der Wall E.E., Bax J.J. Diagnostic accuracy of 64-slice multislice computed tomography in the noninvasive evaluation of significant coronary artery disease. Am J Cardiol. 2006;98(2):145-148.
47. Shabestari A.A., Abdi S., Akhlaghpoor S., Azadi M., Baharjoo H., Pajouh M.D., Emami Z., Esfahani F., Firouzi I., Hashemian M., Kouhi M., Mozafari M., Nazeri I., Roshani M., Salevatipour B., Tavalla H., Tehrai M., Zarrabi A. Diagnostic performance of 64-channel multislice computed tomography in assessment of significant coronary artery disease in symptomatic subjects. Am J Cardiol. 2007;99(12):1656-1661.
48. Budoff M.J., Dowe D., Jollis J.G., Gitter M., Sutherland J., Halamert E., Scherer M., Bellinger R., Martin A., Benton R., Delago A., Min J.K. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724-1732.
49. Meijboom W.B., Meijs M.F., Schuijf J.D., Cramer M.J., Mollet N.R., van Mieghem C.A., Nieman K., van Werkhoven J.M., Pundziute G., Weustink A.C., de Vos A.M., Pugliese F., Rensing B., Jukema J.W., Bax J.J., Prokop M., Doevendans P.A., Hunink M.G., Krestin G.P., de Feyter P.J. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135-2144.
50. Miller J.M., Rochitte C.E., Dewey M., Arbab-Zadeh A., Niinuma H., Gottlieb I., Paul N., Clouse M.E., Shapiro E.P., Hoe J., Lardo A.C., Bush D.E., de Roos A., Cox C., Brinker J., Lima J.A. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359(22):2324-2336.
51. Janne d’Othee B., Siebert U., Cury R., Jadvar H., Dunn E.J., Hoffmann U. A systematic review on diagnostic accuracy of CT-based detection of significant coronary artery disease. Eur J Radiol. 2008;65(3):449-461.
52. Abdulla J., Abildstrom S.Z., Gotzsche O., Christensen E., Kober L. Torp-Pedersen C. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur Heart J. 2007;28(24):3042-3050.
53. Ruzsics B., Lee H., Zwerner P.L., Gebregziabher M., Costello P., Schoepf U.J. Dual-energy CT of the heart for diagnosing coronary artery stenosis and myocardial ischemia-initial experience. Eur Radiol. 2008.
54. Rybicki F.J., Otero H.J., Steigner M.L., Vorobiof G., Nallamshetty L., Mitsouras D., Ersoy H., Mather R.T., Judy P.F., Cai T., Coyner K., Schultz K., Whitmore A.G., Di Carli M.F. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24(5):535-546.
55. Anders K., Baum U., Schmid M., Ropers D., Schmid A., Pohle K., Daniel W.G., Bautz W., Achenbach S. Coronary artery bypass graft (CABG) patency: assessment with high-resolution submillimeter 16-slice multidetector-row computed tomography (MDCT) versus coronary angiography. Eur J Radiol. 2006;57(3):336-344.
56. Burgstahler C., Beck T., Kuettner A., Drosch T., Kopp A.F., Heuschmid M., Claussen C.D., Schroeder S. Non-invasive evaluation of coronary artery bypass grafts using 16-row multi-slice computed tomography with 188 ms temporal resolution. Int J Cardiol. 2006;106(2):244-249.
57. Jabara R., Chronos N., Klein L., Eisenberg S., Allen R., Bradford S., Frohwein S. Comparison of multidetector 64-slice computed tomographic angiography to coronary angiography to assess the patency of coronary artery bypass grafts. Am J Cardiol. 2007;99(11):1529-1534.
58. Meyer T.S., Martinoff S., Hadamitzky M., Will A., Kastrati A., Schomig A., Hausleiter J. Improved noninvasive assessment of coronary artery bypass grafts with 64-slice computed tomographic angiography in an unselected patient population. J Am Coll Cardiol. 2007;49(9):946-950.
59. Pache G., Saueressig U., Frydrychowicz A., Foell D., Ghanem N., Kotter E., Geibel-Zehender A., Bode C., Langer M., Bley T. Initial experience with 64-slice cardiac CT: non-invasive visualization of coronary artery bypass grafts. Eur Heart J. 2006;27(8):976-980.
60. Ropers D., Pohle F.K., Kuettner A., Pflederer T., Anders K., Daniel W.G., Bautz W., Baum U., Achenbach S. Diagnostic accuracy of noninvasive coronary angiography in patients after bypass surgery using 64-slice spiral computed tomography with 330-ms gantry rotation. Circulation. 2006;114(22):2334-2341. quiz 2334
61. Schlosser T., Konorza T., Hunold P., Kuhl H., Schmermund A., Barkhausen J. Noninvasive visualization of coronary artery bypass grafts using 16-detector row computed tomography. J Am Coll Cardiol. 2004;44(6):1224-1229.
62. Stauder N.I., Kuttner A., Schroder S., Drosch T., Beck T., Stauder H., Blumenstock G., Claussen C.D., Kopp A.F. Coronary artery bypass grafts: assessment of graft patency and native coronary artery lesions using 16-slice MDCT. Eur Radiol. 2006;16(11):2512-2520.
63. Gasparovic H., Rybicki F.J., Millstine J., Unic D., Byrne J.G., Yucel K., Mihaljevic T. Three dimensional computed tomographic imaging in planning the surgical approach for redo cardiac surgery after coronary revascularization. Eur J Cardiothorac Surg. 2005;28(2):244-249.
64. Maintz D., Seifarth H., Raupach R., Flohr T., Rink M., Sommer T., Ozgun M., Heindel W. Fischbach R. 64-slice multidetector coronary CT angiography: in vitro evaluation of 68 different stents. Eur Radiol. 2006;16(4):818-826.
65. Gilard M., Cornily J.C., Rioufol G., Finet G., Pennec P.Y., Mansourati J., Blanc J.J., Boschat J. Noninvasive assessment of left main coronary stent patency with 16-slice computed tomography. Am J Cardiol. 2005;95(1):110-112.
66. Van Mieghem C.A., Cademartiri F., Mollet N.R., Malagutti P., Valgimigli M., Meijboom W.B., Pugliese F., McFadden E.P., Ligthart J., Runza G., Bruining N., Smits P.C., Regar E., van der Giessen W.J., Sianos G., van Domburg R., de Jaegere P., Krestin G.P., Serruys P.W., de Feyter P.J. Multislice spiral computed tomography for the evaluation of stent patency after left main coronary artery stenting: a comparison with conventional coronary angiography and intravascular ultrasound. Circulation. 2006;114(7):645-653.
67. Rixe J., Achenbach S., Ropers D., Baum U., Kuettner A., Ropers U., Bautz W., Daniel W.G., Anders K. Assessment of coronary artery stent restenosis by 64-slice multi-detector computed tomography. Eur Heart J. 2006;27(21):2567-2572.
68. Cademartiri F., Schuijf J.D., Pugliese F., Mollet N.R., Jukema J.W., Maffei E., Kroft L.J., Palumbo A., Ardissino D., Serruys P.W., Krestin G.P., Van der Wall E.E., de Feyter P.J., Bax J.J. Usefulness of 64-slice multislice computed tomography coronary angiography to assess in-stent restenosis. J Am Coll Cardiol. 2007;49(22):2204-2210.
69. Schuijf J.D., Pundziute G., Jukema J.W., Lamb H.J., Tuinenburg J.C., van der Hoeven B.L., de Roos A., Reiber J.H., van der Wall E.E., Schalij M.J., Bax J.J. Evaluation of patients with previous coronary stent implantation with 64-section CT. Radiology. 2007;245(2):416-423.
70. Oncel D., Oncel G., Tastan A., Tamci B. Evaluation of coronary stent patency and in-stent restenosis with dual-source CT coronary angiography without heart rate control. AJR Am J Roentgenol. 2008;191(1):56-63.
71. Schuijf J.D., Wijns W., Jukema J.W., Atsma D.E., de Roos A., Lamb H.J., Stokkel M.P., Dibbets-Schneider P., Decramer I., De Bondt P., van der Wall E.E., Vanhoenacker P.K., Bax J.J. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006;48(12):2508-2514.
72. Di Carli M.F., Dorbala S., Curillova Z., Kwong R.J., Goldhaber S.Z., Rybicki F.J., Hachamovitch R. Relationship between CT coronary angiography and stress perfusion imaging in patients with suspected ischemic heart disease assessed by integrated PET-CT imaging. J Nucl Cardiol. 2007;14(6):799-809.
73. Hacker M., Jakobs T., Hack N., Nikolaou K., Becker C., von Ziegler F., Knez A., Konig A., Klauss V., Reiser M., Hahn K., Tiling R. Sixty-four slice spiral CT angiography does not predict the functional relevance of coronary artery stenoses in patients with stable angina. Eur J Nucl Med Mol Imaging. 2007;34(1):4-10.
74. Gaemperli O., Schepis T., Koepfli P., Valenta I., Soyka J., Leschka S., Desbiolles L., Husmann L., Alkadhi H., Kaufmann P.A. Accuracy of 64-slice CT angiography for the detection of functionally relevant coronary stenoses as assessed with myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging. 2007;34(8):1162-1171.
75. Lin F., Shaw L.J., Berman D.S., Callister T.Q., Weinsaft J.W., Wong F.J., Szulc M., Tandon V., Okin P.M., Devereux R.B., Min J.K. Multidetector computed tomography coronary artery plaque predictors of stress-induced myocardial ischemia by SPECT. Atherosclerosis. 2008;197(2):700-709.
76. Rispler S., Keidar Z., Ghersin E., Roguin A., Soil A., Dragu R., Litmanovich D., Frenkel A., Aronson D., Engel A., Beyar R., Israel O. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007;49(10):1059-1067.
77. Gaemperli O., Schepis T., Valenta I., Husmann L., Scheffel H., Duerst V., Eberli F.R., Luscher T.F., Alkadhi H., Kaufmann P.A. Cardiac image fusion from stand-alone SPECT and CT: clinical experience. J Nucl Med. 2007;48(5):696-703.
78. Christou M.A., Siontis G.C., Katritsis D.G., Ioannidis J.P. Meta-analysis of fractional flow reserve versus quantitative coronary angiography and noninvasive imaging for evaluation of myocardial ischemia. Am J Cardiol. 2007;99(4):450-456.
79. Meijboom W.B., Van Mieghem C.A., van Pelt N., Weustink A., Pugliese F., Mollet N.R., Boersma E., Regar E., van Geuns R.J., de Jaegere P.J., Serruys P.W., Krestin G.P., de Feyter P.J. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008;52(8):636-643.
80. Nicol E.D., Stirrup J., Reyes E., Roughton M., Padley S.P., Rubens M.B., Underwood S.R. Sixty-four-slice computed tomography coronary angiography compared with myocardial perfusion scintigraphy for the diagnosis of functionally significant coronary stenoses in patients with a low to intermediate likelihood of coronary artery disease. J Nucl Cardiol. 2008;15(3):311-318.
81. Sato A., Hiroe M., Tamura M., Ohigashi H., Nozato T., Hikita H., Takahashi A., Aonuma K., Isobe M. Quantitative measures of coronary stenosis severity by 64-Slice CT angiography and relation to physiologic significance of perfusion in nonobese patients: comparison with stress myocardial perfusion imaging. J Nucl Med. 2008;49(4):564-572.
82. Gallagher M.J., Ross M.A., Raff G.L., Goldstein J.A., O’Neill W.W., O’Neil B. The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med. 2007;49(2):125-136.
83. Goldstein J.A., Gallagher M.J., O’Neill W.W., Ross M.A., O’Neil B.J., Raff G.L. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49(8):863-871.
84. Shaw L.J., Berman D.S., Hendel R.C., Borges Neto S., Min J.K., Callister T.Q. Prognosis by coronary computed tomographic angiography: matched comparison with myocardial perfusion single-photon emission computed tomography. J Cardiovasc Comput Tomogr. 2008;2(2):93-101. Epub 2008 Jan 12
85. Cury R.C., Feutchner G., Pena C.S., Janowitz W.R., Katzen B.T., Ziffer J.A. Acute chest pain imaging in the emergency department with cardiac computed tomography angiography. J Nucl Cardiol. 2008;15(4):564-575.
86. George R.T., Silva C., Cordeiro M.A., DiPaula A., Thompson D.R., McCarthy W.F., Ichihara T., Lima J.A., Lardo A.C. Multidetector computed tomography myocardial perfusion imaging during adenosine stress. J Am Coll Cardiol. 2006;48(1):153-160.
87. Kurata A., Mochizuki T., Koyama Y., Haraikawa T., Suzuki J., Shigematsu Y., Higaki J. Myocardial perfusion imaging using adenosine triphosphate stress multi-slice spiral computed tomography: alternative to stress myocardial perfusion scintigraphy. Circ J. 2005;69(5):550-557.
88. Gerber B.L., Belge B., Legros G.J., Lim P., Poncelet A., Pasquet A., Gisellu G., Coche E., Vanoverschelde J.L. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113(6):823-833.
89. Lardo A.C., Cordeiro M.A., Silva C., Amado L.C., George R.T., Saliaris A.P., Schuleri K.H., Fernandes V.R., Zviman M., Nazarian S., Halperin H.R., Wu K.C., Hare J.M., Lima J.A. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006;113(3):394-404.
90. Hoffmann U., Millea R., Enzweiler C., Ferencik M., Gulick S., Titus J., Achenbach S., Kwait D., Sosnovik D., Brady T.J. Acute myocardial infarction: contrast-enhanced multi-detector row CT in a porcine model. Radiology. 2004;231(3):697-701.
91. Baks T., Cademartiri F., Moelker A.D., Weustink A.C., van Geuns R.J., Mollet N.R., Krestin G.P., Duncker D.J., de Feyter P.J. Multislice computed tomography and magnetic resonance imaging for the assessment of reperfused acute myocardial infarction. J Am Coll Cardiol. 2006;48(1):144-152.
92. Nieman K., Shapiro M.D., Ferencik M., Nomura C.H., Abbara S., Hoffmann U., Gold H.K., Jang I.K., Brady T.J., Cury R.C. Reperfused myocardial infarction: contrast-enhanced 64-section CT in comparison to MR imaging. Radiology. 2008;247(1):49-56.
93. Wada H., Kobayashi Y., Yasu T., Tsukamoto Y., Kobayashi N., Ishida T., Kubo N., Kawakami M., Saito M. Multi-detector computed tomography for imaging of subendocardial infarction: prediction of wall motion recovery after reperfused anterior myocardial infarction. Circ J. 2004;68(5):512-514.
94. Koyama Y., Matsuoka H., Mochizuki T., Higashino H., Kawakami H., Nakata S., Aono J., Ito T., Naka M., Ohashi Y., Higaki J. Assessment of reperfused acute myocardial infarction with two-phase contrast-enhanced helical CT: prediction of left ventricular function and wall thickness. Radiology. 2005;235(3):804-811.
95. Lessick J., Dragu R., Mutlak D., Rispler S., Beyar R., Litmanovich D., Engel A., Agmon Y., Kapeliovich M., Hammerman H., Ghersin E. Is functional improvement after myocardial infarction predicted with myocardial enhancement patterns at multidetector CT? Radiology. 2007;244(3):736-744.
96. Sato A., Hiroe M., Nozato T., Hikita H., Ito Y., Ohigashi H., Tamura M., Takahashi A., Isobe M., Aonuma K. Early validation study of 64-slice multidetector computed tomography for the assessment of myocardial viability and the prediction of left ventricular remodelling after acute myocardial infarction. Eur Heart J. 2008;29(4):490-498.
97. Reiter S.J., Rumberger J.A., Feiring A.J., Stanford W., Marcus M.L. Precision of measurements of right and left ventricular volume by cine computed tomography. Circulation. 1986;74(4):890-900.
98. Rich S., Chomka E.V., Stagl R., Shanes J.G., Kondos G.T., Brundage B.H. Determination of left ventricular ejection fraction using ultrafast computed tomography. Am Heart J. 1986;112(2):392-396.
99. Roig E., Georgiou D., Chomka E.V., Wolfkiel C., LoGalbo-Zak C., Rich S., Brundage B.H. Reproducibility of left ventricular myocardial volume and mass measurements by ultrafast computed tomography. J Am Coll Cardiol. 1991;18(4):990-996.
100. Feiring A.J., Rumberger J.A., Reiter S.J., Skorton D.J., Collins S.M., Lipton M.J., Higgins C.B., Ell S., Marcus M.L. Determination of left ventricular mass in dogs with rapid-acquisition cardiac computed tomographic scanning. Circulation. 1985;72(6):1355-1364.
101. Mao S., Takasu J., Child J., Carson S., Oudiz R., Budoff M.J. Comparison of LV mass and volume measurements derived from electron beam tomography using cine imaging and angiographic imaging. Int J Cardiovasc Imaging. 2003;19(5):439-445.
102. Juergens K.U., Seifarth H., Maintz D., Grude M., Ozgun M., Wichter T., Heindel W., Fischbach R. MDCT determination of volume and function of the left ventricle: are short-axis image reformations necessary? AJR Am J Roentgenol. 2006;186(6 Suppl 2):S371-378.
103. Gilard M., Pennec P.Y., Cornily J.C., Vinsonneau U., Le Gal G., Nonent M., Mansourati J., Boschat J. Multi-slice computer tomography of left ventricular function with automated analysis software in comparison with conventional ventriculography. Eur J Radiol. 2006;59(2):270-275.
104. Juergens K.U., Grude M., Fallenberg E.M., Opitz C., Wichter T., Heindel W., Fischbach R. Using ECG-gated multidetector CT to evaluate global left ventricular myocardial function in patients with coronary artery disease. AJR Am J Roentgenol. 2002;179(6):1545-1550.
105. Halliburton S.S., Petersilka M., Schvartzman P.R., Obuchowski N., White R.D. Evaluation of left ventricular dysfunction using multiphasic reconstructions of coronary multi-slice computed tomography data in patients with chronic ischemic heart disease: validation against cine magnetic resonance imaging. Int J Cardiovasc Imaging. 2003;19(1):73-83.
106. Yamamuro M., Tadamura E., Kubo S., Toyoda H., Nishina T., Ohba M., Hosokawa R., Kimura T., Tamaki N., Komeda M., Kita T., Konishi J. Cardiac functional analysis with multi-detector row CT and segmental reconstruction algorithm: comparison with echocardiography, SPECT, and MR imaging. Radiology. 2005;234(2):381-390.
107. Mahnken A.H., Koos R., Katoh M., Spuentrup E., Busch P., Wildberger J.E., Kuhl H.P., Gunther R.W. Sixteen-slice spiral CT versus MR imaging for the assessment of left ventricular function in acute myocardial infarction. Eur Radiol. 2005;15(4):714-720.
108. Sugeng L., Mor-Avi V., Weinert L., Niel J., Ebner C., Steringer-Mascherbauer R., Schmidt F., Galuschky C., Schummers G., Lang R.M., Nesser H.J. Quantitative assessment of left ventricular size and function: side-by-side comparison of real-time three-dimensional echocardiography and computed tomography with magnetic resonance reference. Circulation. 2006;114(7):654-661.
109. Raman S.V., Shah M., McCarthy B., Garcia A., Ferketich A.K. Multi-detector row cardiac computed tomography accurately quantifies right and left ventricular size and function compared with cardiac magnetic resonance. Am Heart J. 2006;151(3):736-744.
110. Schepis T., Gaemperli O., Koepfli P., Valenta I., Strobel K., Brunner A., Leschka S., Desbiolles L., Husmann L., Alkadhi H., Kaufmann P.A. Comparison of 64-slice CT with gated SPECT for evaluation of left ventricular function. J Nucl Med. 2006;47(8):1288-1294.
111. Annuar B.R., Liew C.K., Chin S.P., Ong T.K., Seyfarth M.T., Chan W.L., Fong Y.Y., Ang C.K., Lin N., Liew H.B., Sim K.H. Assessment of global and regional left ventricular function using 64-slice multislice computed tomography and 2D echocardiography: a comparison with cardiac magnetic resonance. Eur J Radiol. 2008;65(1):112-119.
112. Wu Y.W., Tadamura E., Yamamuro M., Kanao S., Okayama S., Ozasa N., Toma M., Kimura T., Komeda M., Togashi K. Estimation of global and regional cardiac function using 64-slice computed tomography: a comparison study with echocardiography, gated-SPECT and cardiovascular magnetic resonance. Int J Cardiol. 2008;128(1):69-76.
113. Busch S., Johnson T.R., Wintersperger B.J., Minaifar N., Bhargava A., Rist C., Reiser M.F., Becker C., Nikolaou K. Quantitative assessment of left ventricular function with dual-source CT in comparison to cardiac magnetic resonance imaging: initial findings. Eur Radiol. 2008;18(3):570-575.
114. Puesken M., Fischbach R., Wenker M., Seifarth H., Maintz D., Heindel W., Juergens K.U. Global left-ventricular function assessment using dual-source multidetector CT: effect of improved temporal resolution on ventricular volume measurement. Eur Radiol. 2008;18:2087-2094.
115. van der Vleuten P.A., de Jonge G.J., Lubbers D.D., Tio R.A., Willems T.P., Oudkerk M., Zijlstra F. Evaluation of global left ventricular function assessment by dual-source computed tomography compared with MRI. Eur Radiol. 2009;19:271-277.
116. Schlosser T., Mohrs O.K., Magedanz A., Voigtlander T., Schmermund A., Barkhausen J. Assessment of left ventricular function and mass in patients undergoing computed tomography (CT) coronary angiography using 64-detector-row CT: comparison to magnetic resonance imaging. Acta Radiol. 2007;48(1):30-35.
117. Bastarrika G., Arraiza M., De Cecco C.N., Mastrobuoni S., Ubilla M., Rabago G. Quantification of left ventricular function and mass in heart transplant recipients using dual-source CT and MRI: initial clinical experience. Eur Radiol. 2008;18(9):1784-1790.
118. Heuschmid M., Rothfuss J.K., Schroeder S., Fenchel M., Stauder N., Burgstahler C., Franow A., Kuzo R.S., Kuettner A., Miller S., Claussen C.D., Kopp A.F. Assessment of left ventricular myocardial function using 16-slice multidetector-row computed tomography: comparison with magnetic resonance imaging and echocardiography. Eur Radiol. 2006;16(3):551-559.
119. Ferencik M., Gregory S.A., Butler J., Achenbach S., Yeh R.W., Hoffmann U., Inglessis I., Cury R.C., Nieman K., McNulty I.A., Healy J.A., Brady T.J., Semigran M.J., Jang I.K. Analysis of cardiac dimensions, mass and function in heart transplant recipients using 64-slice multi-detector computed tomography. J Heart Lung Transplant. 2007;26(5):478-484.
120. Gilard M., Le Gal G., Cornily J.C., Vinsonneau U., Joret C., Pennec P.Y., Mansourati J., Boschat J. Midterm prognosis of patients with suspected coronary artery disease and normal multislice computed tomographic findings: a prospective management outcome study. Arch Intern Med. 2007;167(15):1686-1689.
121. Gaemperli O., Valenta I., Schepis T., Husmann L., Scheffel H., Desbiolles L., Leschka S., Alkadhi H., Kaufmann P.A. Coronary 64-slice CT angiography predicts outcome in patients with known or suspected coronary artery disease. Eur Radiol. 2008;18(6):1162-1173.
122. Pundziute G., Schuijf J.D., Jukema J.W., Boersma E., de Roos A., van der Wall E.E., Bax J.J. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;49(1):62-70.
123. Matsumoto N., Sato Y., Yoda S., Nakano Y., Kunimasa T., Matsuo S., Komatsu S., Saito S., Hirayama A. Prognostic value of non-obstructive CT low-dense coronary artery plaques detected by multislice computed tomography. Circ J. 2007;71(12):1898-1903.
124. Rubinshtein R., Halon D.A., Gaspar T., Jaffe R., Karkabi B., Flugelman M.Y., Kogan A., Shapira R., Peled N., Lewis B.S. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation. 2007;115(13):1762-1768.
125. Falk E., Shah P.K., Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657-671.
126. Mock M.B., Ringqvist I., Fisher L.D., Davis K.B., Chaitman B.R., Kouchoukos N.T., Kaiser G.C., Alderman E., Ryan T.J., Russell R.O.Jr., Mullin S., Fray D., Killip T.3rd. Survival of medically treated patients in the coronary artery surgery study (CASS) registry. Circulation. 1982;66(3):562-568.
127. Min J.K., Shaw L.J., Devereux R.B., Okin P.M., Weinsaft J.W., Russo D.J., Lippolis N.J., Berman D.S., Callister T.Q. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161-1170.
128. Mark D.B., Nelson C.L., Califf R.M., Harrell F.E.Jr., Lee K.L., Jones R.H., Fortin D.F., Stack R.S., Glower D.D., Smith L.R., et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89(5):2015-2025.
129. Schroeder S., Kopp A.F., Baumbach A., Meisner C., Kuettner A., Georg C., Ohnesorge B., Herdeg C., Claussen C.D., Karsch K.R. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol. 2001;37(5):1430-1435.
130. Achenbach S., Moselewski F., Ropers D., Ferencik M., Hoffmann U., MacNeill B., Pohle K., Baum U., Anders K., Jang I.K., Daniel W.G., Brady T.J. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109(1):14-17.
131. Leber A.W., Knez A., Becker A., Becker C., von Ziegler F., Nikolaou K., Rist C., Reiser M., White C., Steinbeck G., Boekstegers P. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol. 2004;43(7):1241-1247.
132. Leber A.W., Becker A., Knez A., von Ziegler F., Sirol M., Nikolaou K., Ohnesorge B., Fayad Z.A., Becker C.R., Reiser M., Steinbeck G., Boekstegers P. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47(3):672-677.
133. Pohle K., Achenbach S., Macneill B., Ropers D., Ferencik M., Moselewski F., Hoffmann U., Brady T.J., Jang I.K., Daniel W.G. Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: comparison to IVUS. Atherosclerosis. 2007;190(1):174-180.
134. Leber A.W., Knez A., White C.W., Becker A., von Ziegler F., Muehling O., Becker C., Reiser M., Steinbeck G., Boekstegers P. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast-enhanced multislice computed tomography. Am J Cardiol. 2003;91(6):714-718.
135. Schuijf J.D., Beck T., Burgstahler C., Jukema J.W., Dirksen M.S., de Roos A., van der Wall E.E., Schroeder S., Wijns W., Bax J.J. Differences in plaque composition and distribution in stable coronary artery disease versus acute coronary syndromes; non-invasive evaluation with multi-slice computed tomography. Acute Card Care. 2007;9(1):48-53.
136. Motoyama S., Kondo T., Sarai M., Sugiura A., Harigaya H., Sato T., Inoue K., Okumura M., Ishii J., Anno H., Virmani R., Ozaki Y., Hishida H., Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50(4):319-326.
137. Schmid M., Pflederer T., Jang I.K., Ropers D., Sei K., Daniel W.G., Achenbach S. Relationship between degree of remodeling and CT attenuation of plaque in coronary atherosclerotic lesions: an in-vivo analysis by multi-detector computed tomography. Atherosclerosis. 2008;197(1):457-464.
138. Pundziute G., Schuijf J.D., Jukema J.W., Decramer I., Sarno G., Vanhoenacker P.K., Boersma E., Reiber J.H., Schalij M.J., Wijns W., Bax J.J. Evaluation of plaque characteristics in acute coronary syndromes: non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. Eur Heart J. 2008;29:2373-2381.
139. Choi B.-J., Kang D.-K., Tahk S.-J., Choi S.-Y., Yoon M.-H., Lim H.-S., Kang S.-J., Yang H.-M., Park J.-S., Zheng M., Hwang G.-S., Shin J.-H. Comparison of 64-slice multidetector computed tomography with spectral analysis of intravascular ultrasound backscatter signals for characterizations of noncalcified coronary arterial plaques. Am J Cardiol. 2008;102:988-993.
140. Ibanez B., Cimmino G., Benezet-Mazuecos J., Gallego C.G., Pinero A., Prat-Gonzalez S., Speidl W.S., Fuster V., Garcia M.J., Sanz J., Badimon J.J. Quantification of serial changes in plaque burden using multi-detector computed tomography in experimental atherosclerosis. Atherosclerosis. 2009;202:185-191.
141. Schmid M., Achenbach S., Ropers D., Komatsu S., Ropers U., Daniel W.G., Pflederer T. Assessment of changes in non-calcified atherosclerotic plaque volume in the left main and left anterior descending coronary arteries over time by 64-slice computed tomography. Am J Cardiol. 2008;101(5):579-584.
142. Burgstahler C., Reimann A., Beck T., Kuettner A., Baumann D., Heuschmid M., Brodoefel H., Claussen C.D., Kopp A.F., Schroeder S. Influence of a lipid-lowering therapy on calcified and noncalcified coronary plaques monitored by multislice detector computed tomography: results of the New Age II Pilot Study. Invest Radiol. 2007;42(3):189-195.
143. Cademartiri F., La Grutta L., Runza G., Palumbo A., Maffei E., Mollet N.R., Bartolotta T.V., Somers P., Knaapen M., Verheye S., Midiri M., Hamers R., Bruining N. Influence of convolution filtering on coronary plaque attenuation values: observations in an ex vivo model of multislice computed tomography coronary angiography. Eur Radiol. 2007;17(7):1842-1849.
144. Boll D.T., Hoffmann M.H., Huber N., Bossert A.S., Aschoff A.J., Fleiter T.R. Spectral coronary multidetector computed tomography angiography: dual benefit by facilitating plaque characterization and enhancing lumen depiction. J Comput Assist Tomogr. 2006;30(5):804-811.
145. Hyafil F., Cornily J.C., Feig J.E., Gordon R., Vucic E., Amirbekian V., Fisher E.A., Fuster V., Feldman L.J., Fayad Z.A. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13(5):636-641.
146. Ropers D., Moshage W., Daniel W.G., Jessl J., Gottwik M., Achenbach S. Visualization of coronary artery anomalies and their anatomic course by contrast-enhanced electron beam tomography and three-dimensional reconstruction. Am J Cardiol. 2001;87(2):193-197.
147. Schmitt R., Froehner S., Brunn J., Wagner M., Brunner H., Cherevatyy O., Gietzen F., Christopoulos G., Kerber S., Fellner F. Congenital anomalies of the coronary arteries: imaging with contrast-enhanced, multidetector computed tomography. Eur Radiol. 2005;15(6):1110-1121.
148. Schmid M., Achenbach S., Ludwig J., Baum U., Anders K., Pohle K., Daniel W.G., Ropers D. Visualization of coronary artery anomalies by contrast-enhanced multi-detector row spiral computed tomography. Int J Cardiol. 2006;111(3):430-435.
149. Shi H., Aschoff A.J., Brambs H.J., Hoffmann M.H. Multislice CT imaging of anomalous coronary arteries. Eur Radiol. 2004;14(12):2172-2181.
150. Cademartiri F., La Grutta L., Malago R., Alberghina F., Meijboom W.B., Pugliese F., Maffei E., Palumbo A.A., Aldrovandi A., Fusaro M., Brambilla V., Coruzzi P., Midiri M., Mollet N.R., Krestin G.P. Prevalence of anatomical variants and coronary anomalies in 543 consecutive patients studied with 64-slice CT coronary angiography. Eur Radiol. 2008;18(4):781-791.
151. Mollet N.R., Hoye A., Lemos P.A., Cademartiri F., Sianos G., McFadden E.P., Krestin G.P., Serruys P.W., de Feyter P.J. Value of preprocedure multislice computed tomographic coronary angiography to predict the outcome of percutaneous recanalization of chronic total occlusions. Am J Cardiol. 2005;95(2):240-243.
152. Soon K.H., Cox N., Wong A., Chaitowitz I., Macgregor L., Santos P.T., Selvanayagam J.B., Farouque H.M., Rametta S., Bell K.W., Lim Y.L. CT coronary angiography predicts the outcome of percutaneous coronary intervention of chronic total occlusion. J Interv Cardiol. 2007;20(5):359-366.
153. Yokoyama N., Yamamoto Y., Suzuki S., Suzuki M., Konno K., Kozuma K., Kaminaga T., Isshiki T. Impact of 16-slice computed tomography in percutaneous coronary intervention of chronic total occlusions. Catheter Cardiovasc Interv. 2006;68(1):1-7.
154. Gilard M., Cornily J.C., Pennec P.Y., Joret C., Le Gal G., Mansourati J., Blanc J.J., Boschat J. Accuracy of multislice computed tomography in the preoperative assessment of coronary disease in patients with aortic valve stenosis. J Am Coll Cardiol. 2006;47(10):2020-2024.
155. Holmström M., Sillanpaa M.A., Kupari M., Kivisto S., Lauerma K. Eight-row multidetector computed tomography coronary angiography evaluation of significant coronary artery disease in patients with severe aortic valve stenosis. Int J Cardiovasc Imaging. 2006;22(5):703-710.
156. Scheffel H., Leschka S., Plass A., Vachenauer R., Gaemperli O., Garzoli E., Genoni M., Marincek B., Kaufmann P., Alkadhi H. Accuracy of 64-slice computed tomography for the preoperative detection of coronary artery disease in patients with chronic aortic regurgitation. Am J Cardiol. 2007;100(4):701-706.
157. Schlosser F.J., Mojibian H.R., Dardik A., Verhagen H.J., Moll F.L., Muhs B.E. Simultaneous sizing and preoperative risk stratification for thoracic endovascular aneurysm repair: Role of gated computed tomography. J Vasc Surg. 2008.
158. Budoff M.J., Shavelle D.M., Lamont D.H., Kim H.T., Akinwale P., Kennedy J.M., Brundage B.H. Usefulness of electron beam computed tomography scanning for distinguishing ischemic from nonischemic cardiomyopathy. J Am Coll Cardiol. 1998;32(5):1173-1178.
159. Andreini D., Pontone G., Pepi M., Ballerini G., Bartorelli A.L., Magini A., Quaglia C., Nobili E., Agostoni P. Diagnostic accuracy of multidetector computed tomography coronary angiography in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2007;49(20):2044-2050.
160. Ghostine S., Caussin C., Habis M., Habib Y., Clement C., Sigal-Cinqualbre A., Angel C.Y., Lancelin B., Capderou A., Paul J.F. Non-invasive diagnosis of ischaemic heart failure using 64-slice computed tomography. Eur Heart J. 2008.
161. Hunold P., Vogt F.M., Schmermund A., Debatin J.F., Kerkhoff G., Budde T., Erbel R., Ewen K., Barkhausen J. Radiation exposure during cardiac CT: effective doses at multi-detector row CT and electron-beam CT. Radiology. 2003;226(1):145-152.
162. Hausleiter J., Meyer T., Hadamitzky M., Huber E., Zankl M., Martinoff S., Kastrati A., Schomig A. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation. 2006;113(10):1305-1310.
163. Thompson R.C., Cullom S.J. Issues regarding radiation dosage of cardiac nuclear and radiography procedures. J Nucl Cardiol. 2006;13(1):19-23.
164. Einstein A.J., Moser K.W., Thompson R.C., Cerqueira M.D., Henzlova M.J. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116(11):1290-1305.
165. Gerber T.C., Stratmann B.P., Kuzo R.S., Kantor B., Morin R.L. Effect of acquisition technique on radiation dose and image quality in multidetector row computed tomography coronary angiography with submillimeter collimation. Invest Radiol. 2005;40(8):556-563.
166. Jakobs T.F., Becker C.R., Ohnesorge B., Flohr T., Suess C., Schoepf U.J., Reiser M.F. Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol. 2002;12(5):1081-1086.
167. Sanz J., Rius T., Kuschnir P., Fuster V., Goldberg J., Ye X.Y., Wisdom P., Poon M. The importance of end-systole for optimal reconstruction protocol of coronary angiography with 16-slice multidetector computed tomography. Invest Radiol. 2005;40(3):155-163.
168. Trabold T., Buchgeister M., Kuttner A., Heuschmid M., Kopp A.F., Schroder S., Claussen C.D. Estimation of radiation exposure in 16-detector row computed tomography of the heart with retrospective ECG-gating. Rofo. 2003;175(8):1051-1055.
169. Earls J.P., Berman E.L., Urban B.A., Curry C.A., Lane J.L., Jennings R.S., McCulloch C.C., Hsieh J., Londt J.H. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology. 2008;246(3):742-753.
170. National Research Council (U.S.). Committee to Assess Health Risks from Exposure to Low Level of Ionizing Radiation. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington, DC: National Academies Press, 2006.
171. Einstein A.J., Henzlova M.J., Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298(3):317-323.
172. Jemal A., Siegel R., Ward E., Murray T., Xu J., Thun M.J. Cancer statistics, 2007. CA: Cancer J Clin. 2007;57(1):43-66.
173. Hendel R.C., Patel M.R., Kramer C.M., Poon M., Carr J.C., Gerstad N.A., Gillam L.D., Hodgson J.M., Kim R.J., Lesser J.R., Martin E.T., Messer J.V., Redberg R.F., Rubin G.D., Rumsfeld J.S., Taylor A.J., Weigold W.G., Woodard P.K., Brindis R.G., Douglas P.S., Peterson E.D., Wolk M.J., Allen J.M. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48(7):1475-1497.
174. Schroeder S., Achenbach S., Bengel F., Burgstahler C., Cademartiri F., de Feyter P., George R., Kaufmann P., Kopp A.F., Knuuti J., Ropers D., Schuijf J., Tops L.F., Bax J.J. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2008;29(4):531-556.
175. Raff G.L., Goldstein J.A. Coronary angiography by computed tomography: coronary imaging evolves. J Am Coll Cardiol. 2007;49(18):1830-1833.