Chapter 20 Coronary Artery Calcification
Pathogenesis, Imaging, and Risk Stratification
INTRODUCTION
Assessing atherosclerotic plaque burden has long been the goal of cardiologists, considering that the initial presentation of a significant percentage of previously asymptomatic patients is with myocardial infarction (MI) or sudden cardiac death.1 Large epidemiologic studies have identified a host of “conventional” risk factors that are able to predict only about two-thirds of those patients who will eventually go on to develop coronary artery disease (CAD).2 Nearly a third of patients dying of CAD in the United States are classified as being at low risk with the Framingham Risk Index score.3
It is now well known that most of the acute coronary events result from the rupture and/or erosion of non-stenotic but vulnerable plaques.4,5 To date, invasive coronary angiography remains the gold standard for imaging the coronary arteries and intravascular ultrasonography (IVUS) is the investigation of choice to accurately assess the total atherosclerotic plaque burden and plaque morphology. With the attending risk (though small) involved with an non-invasive technique to image the coronary arteries and accurately assess the total atherosclerotic plaque burden. The exponential improvements in imaging technology in the last 2 decades, most notably the improvements in computed tomography (CT) and the rapid growth of computer technology, have made this goal a reality. There has been a plethora of articles published on the subject of detection of coronary artery calcification (CAC) using electron beam CT (EBCT) and multislice CT (MSCT) and its value in prognosis and risk stratification, particularly in asymptomatic patients or in those patients with a low to intermediate probability of developing CAD.
TYPES OF ARTERIAL CALCIFICATION
Two distinct types of arterial calcification have been described: medial arterial calcification and calcification associated with atherosclerotic plaque.6 These two types of calcification differ in terms of their pathophysiology, morphologic features, and clinical significance. In atherosclerotic plaque, calcium deposits are typically found in the intima. In contrast, medial calcification, also known as Mönckeberg’s sclerosis, occurs independently of atherosclerosis. Medial arterial calcification (MAC) occurs in the small and medium sized muscular arteries. It is typically noted in the arteries of the abdominal viscera, breast and the thyroid gland. Medial calcification of the coronary arteries, though rare, has been noted in patients with advanced diabetes and/or chronic kidney disease. MAC is not associated with luminal stenosis but causes stiffening and decreased compliance of the arteries.
PATHOGENESIS OF CORONARY ARTERY CALCIFICATION
Atherosclerosis is known to be a chronic inflammatory process where various components of the immune system are implicated.7,8 In the majority of cases, the presence of a high plasma lipid concentration fuels the process of atherosclerosis. Endothelial cells, leukocytes, and intimal smooth muscle cells, as well as other contributory factors such as smoking, hypertension, diabetes, and in some cases, high homocysteine levels, all play an important part in the process of atherosclerosis, but the exact nature of their interactions with each other is yet to be fully unraveled.
There have been different views regarding the mechanism of calcium deposition in atherosclerotic plaques.9 Some initial theories suggest that calcification results from passive adsorption of Gla-containing proteins with a high affinity for calcium phosphate and hydroxyapatite, whose only known function is to bind calcium.10,11 This seems unlikely in light of the fact that calcification occurs in only those vessels with atherosclerosis and is absent in normal arteries.12 Other evidence suggests that coronary calcification is an actively regulated process rather than passive adsorption and precipitation.13 In fact, several intriguing similarities have been noted between coronary calcification and bone formation.14
Calcium deposition within an atherosclerotic plaque lesion starts as early as the second decade of life, when the plaque lesion is no more than a fatty streak15 (type III plaque lesion). When calcification becomes widespread, it is then classified as a type IV lesion, which contains granules of calcium within the smooth muscle cells and the extracellular matrix. The process of calcification starts in the damaged intracellular organelles of smooth muscle cells that subsequently become apoptotic and act as niduses of further calcification.16 The extracellular accumulation of lipids within the plaque deep within the intima appears to be the starting point for this process.17 The small granules of calcium fuse together to form larger deposits in the form of lumps and plates. These are progressively classified as type V, VI, and VII plaque lesions, with increasing quantities of calcium. The stages in the progression of atherosclerotic plaque have been illustrated in Figure 20-1.
Clinical Relevance of Coronary Artery Calcification
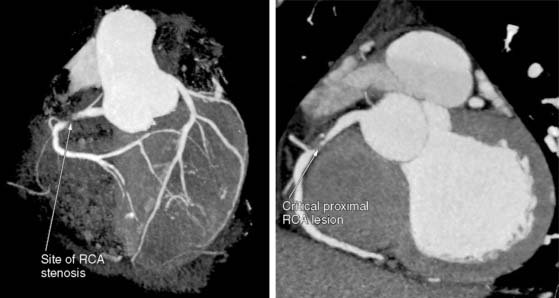
The amount of coronary calcium correlates well with the segmental atherosclerotic plaque burden18 and is clearly illustrated in Figure 20-2. Histologic study by Rumberger et al. showed that on average, calcium represents about a fifth of the total atherosclerotic burden.19 However, the amount of calcium does not correlate with the severity of angiographic luminal stenosis, and this is most likely due to the remodeling process, whereby there is an increase in size of the arteries, compensating for the atherosclerotic plaque (Fig. 20-3).20 Furthermore, presence and extent of calcification do not predict the future risk of plaque rupture. In fact, there are conflicting theories regarding the effect of calcium on plaque stability. It has been suggested that calcified plaques are much stiffer and hence less likely to rupture than cellular plaques. Culprit lesions revealed by IVUS were found to contain less calcium than stable lesions,21 lending credibility to the notion that calcification stabilizes a plaque. There have also been studies suggesting that calcification is a result of subclinical plaque rupture, a sort of protective mechanism of the plaque, to stabilize and prevent further plaque rupture.
On the contrary, Abedin et al. suggest that the vessel is rendered less vulnerable to rupture only when extensive calcification has occurred, whereas the early stages of calcification may actually enhance plaque vulnerability.22 It has been shown that calcified atherosclerotic plaque is at least 4 to 5 times stiffer than cellular plaque.23 In the initial stages of calcification, plaques are most prone to rupture at areas of interface between high- and low-density tissue.24 As the degree of calcification increases, the number of interfaces between rigid and distensible plaque initially would increase until the point at which the rigid plaques coalesce. Calcification beyond this point may be associated with decreasing risk of plaque rupture. However, further research is needed to unravel the association between calcification and plaque vulnerability.
Biochemical Factors Implicated in Coronary Calcification
Current opinion suggests that coronary artery calcification is an active process mediated by the production of ectopic bone matrix proteins by either vascular pericyte-like cells, smooth muscle cells or macrophage-derived foam cells.25,26 Fitzpatrick and colleagues have identified the mRNA of the matrix protein Osteopontin in coronary artery specimens with calcification.27 The bone matrix proteins implicated in the development of coronary artery calcification so far are shown in Table 20-1.
Table 20-1 Biochemical Factors Implicated in Coronary Artery Calcification
| Biomarker | Class | Action/Role |
|---|---|---|
| Osteopontin | Glycoprotein | Cell attachment |
| Osteonectin | Calcium-binding glycoprotein | Binds strongly to both hydroxyapatite and collagen |
| Osteocalcin | Gamma-carboxylated protein | Responsible for mineralization, only produced by osteoblasts |
| Osteoprotegerin | Cytokine of the tumor necrosis factor family (glycoprotein) | Osteoclastogenesis inhibitory factor, inhibiting the differentiation of macrophages into osteoclasts |
| Bone morphogenetic protein (BMP) 2a | Cytokine belonging to the transforming growth factor beta superfamily of proteins | Induces the pericyte-like cells to undergo osteogenic differentiation leading to production of bonelike matrix |
Osteoprotegerin (OPG) in particular appears to have garnered a fair share of attention. Anand and colleagues showed that OPG levels correlated significantly with increased CAC scores in a cohort of asymptomatic diabetics with an unadjusted odds ratio of 3.08 (95% CI: 2.42 to 3.92, P < 0.001).28 More recently, in a very high-risk population of patients with chronic kidney disease (CKD), it was shown that the levels of OPG correlated significantly with the degree of CAC as well as survival. In a multivariate logistic regression model, OPG independently predicted all-cause mortality in CKD patients.29
CORONARY ARTERY CALCIUM IMAGING USING ELECTRON BEAM COMPUTED TOMOGRAPHY/MULTISLICE COMPUTED TOMOGRAPHY
A few studies have compared the data variability between these two imaging modalities (EBCT and MSCT scanners), and they show good correlation between the scanners in terms of accuracy and reproducibility. Knez et al. showed an excellent correlation between MSCT and EBCT for quantification of coronary calcium in 99 patients (r = 0.994; P = 0.01).30 Becker et al. compared the two modalities in 100 patients and found a good correlation between the two types of scanners.31
ALGORITHMS FOR QUANTIFICATION OF CORONARY CALCIUM
Agatston Score
The calcium scoring system first described by Agatston et al.32 in 1990 is widely used even today. They described a scoring algorithm that takes into consideration the area and density of the calcified plaque. Calcified foci within the outline of epicardial coronary arteries with a threshold area of 1 mm2 and a threshold attenuation value of 130 Hounsfield units are scored. CAC score is calculated as maximal computed tomographic number (MCTN) multiplied by area of calcification in mm2. The MCTN is obtained from the maximal Hounsfield intensity within the area of interest, as shown in Table 20-2. For example, if the peak x-ray density of a calcified lesion is 400 Hounsfield units, and the total area occupied is 10 mm2, then the CAC score using this method is: 4 × 10 = 40 Agatston units (Au). The score for each lesion in a given patient is measured, and all the scores are added to give the total CAC score for the patient.
| Attenuation Density of Coronary Plaque (Hounsfield Units) | Maximum Computed Tomographic Number |
|---|---|
| 130–200 | 1 |
| 201–300 | 2 |
| 301–400 | 3 |
| >400 | 4 |
* Used in coronary artery calcium scoring using the Agatston method.
The Agatston method is susceptible to partial volume effects, and the reported variability of repeated EBCT scanning ranges from 22% to 49%.33–35 In a study by Bielak et al.,36 hyperattenuating foci less than 2 mm2 showed less than 50% reproducibility in 256 subjects who underwent two sequential electron beam CT examinations several minutes apart. The factors mainly responsible for poor reproducibility are cardiac, respiratory, and patient motion; arrhythmias; image noise; and image gaps due to discontinuous acquisition of image data. There are a few other factors that can also cause artifacts and difficulty in scan analysis, such as the presence of pacing or ICD wires and coronary stents.
Volume Score
One of the ways of improving reproducibility is to use volume-based calcium quantification, as reported by Callister et al.37 An important limitation of the Agatston scoring algorithm is that it is based on the area (rather than the volume) of the calcified plaque. This means only the length and breadth of a plaque are taken into account, and the depth is ignored. Volume-based calcium quantification uses isotropic interpolation, and thus the value obtained is the volume of calcium present and not an abstract number. The absence of a Hounsfield units–dependent factor makes this system less susceptible to the effects of partial-volume averaging. This is particularly useful with the newer spiral scanners, which use overlapping image reconstruction, since the volume score is independent of the image overlap and the slice thickness used.
Detrano et al.38 evaluated the effect of scanner type and scoring algorithm on the reproducibility of CAC measurements among the participants of the Multi-Ethnic Study of Atherosclerosis (MESA), a multicenter observational study of 6814 asymptomatic subjects. Three of the study centers used EBCT, and the other three centers used MSCT. Each participant underwent two scans 2 minutes apart. EBCT and MSCT scanners showed equivalent reproducibility for measuring coronary artery calcium. Calcium volume scores showed slightly better reproducibility compared to Agatston scores.
Mass Score
It has also been suggested that calculating absolute calcium mass is a reliable method of quantification of coronary artery calcium. If appropriate calibration is used, either with an external standard or a calibration phantom, the reliability is improved and is independent of the CT hardware or the differing scanning protocols.39 Calcium mass is calculated as a product of lesion volume, average CT density of the lesion in Hounsfield units, and a calibration factor. These newer methods of calcium quantification are not widely being used, because there is no standardized reference database for them as yet. Since the majority of the large-scale trials have used the Agatston score, this method continues to be the clinical standard used in most centers.
The age/sex percentile rank and diagrammatic representation of the coronary arteries with CAC lesions (depicted in black) in a typical CAC score report is shown in Figure 20-4.
Coronary Artery Calcium Score: What Does It Mean?
Age and sex of a patient play an important role in determining the prevalence and extent of CAC. In both the sexes, prevalence of CAC increases with age.40 CAC scores vary greatly, so even in subjects of similar age groups, an age/sex nomogram is required to standardize an individual patient value against that of a matched population—or in essence, to suggest a “coronary age.”41,42 Hoff et al. analyzed 35,246 self-referred, predominantly white, asymptomatic subjects between the ages of 30 and 90 years and categorized them according to percentiles.43 The total CAC scores in this study group were decidedly non-Gaussian. The age/sex nomogram generated from this study is particularly useful in the risk stratification of individuals, depending on their CAC scores. For example, a calcium score of 15 Au in a male subject younger than 40 years of age, though not alarming in itself, places him above the 90th percentile when ranked with the nomogram. From these data, it is also clear that CAC measured in men is similar to the scores measured in women who are 15 years older.
However, a recent study by Budoff and colleagues44 demonstrated that absolute CAC score groups were a better predictor of incident CHD events than age/sex and ethnicity-based percentile rank in the MESA cohort of patients. This is clearly illustrated in Figure 20-5. The Kaplan-Meier curves based on absolute CAC scores appear to have a better separation than those based on age-sex-race/ethnicity percentile ranks, indicative of better risk stratification capability in the short term. What this suggests is that patients in the lower absolute CAC score group are at low risk of CHD events irrespective of their age-sex-race/ethnicity percentile rank. This appears to be true for prediction of obstructive CAD as well in both asymptomatic and symptomatic patients.45
Severity of CAC score is arbitrarily classified as minimal, mild, moderate, severe, and extensive based on the absolute score, as shown in Table 20-3. Snapshotsof the varying degrees of severity of CAC score in a typical scan are clearly illustrated in Figure 20-6.
| CAC Score (Agatston Units) | Coronary Calcification Category |
|---|---|
| 0 | Absent |
| 1–10 | Minimal |
| 11–100 | Mild |
| 101–400 | Moderate |
| 401–1000 | Severe |
| >1000 | Extensive |
ROLE OF CAC IMAGING IN RISK STRATIFICATION
The Significance of Risk Stratification in Coronary Artery Disease
In approximately 50% of patients with newly diagnosed CAD, the first presentation is either MI or sudden cardiac death.1 Nearly half a million sudden deaths occur in the United States each year, and approximately 70% of them are due to CAD.46 Although the overall mortality of cardiovascular diseases has decreased in the United States, mortality due to SCD has remained largely unchanged.47 Conventional cardiovascular risk factors account for a large proportion of CAD burden48; early identification and timely intervention to lower risk in asymptomatic individuals could prevent or postpone a majority of CAD events.49 Risk stratification is an important initial step in the clinical management of cardiovascular risk factors. Risk stratification should start with simple evaluation of clinical risk markers, including age, sex, history of hypertension, diabetes, smoking, hypercholesterolemia, and family history of premature cardiovascular disease. Commonly used risk algorithms include the Framingham Risk Score (FRS),50 PROCAM score,51 or the European risk prediction system called SCORE52 (systemic cardiovascular risk evaluation). These risk scores predict the 10-year absolute risk of cardiovascular events and are helpful in selecting the most appropriate candidates for risk-reduction treatments. Such office-based, clinically derived risk scores, though practical, inexpensive, and easy to calculate, have significant limitations53 and are not uniformly applicable in different populations.54–57 CAC imaging, by virtue of its ability to detect and accurately quantify subclinical atherosclerosis in coronary arteries, offers promise to refine the current risk stratification strategies.
Correlation Between Coronary Artery Calcium and Conventional Cardiovascular Risk Factors
The prevalence of coronary calcification is significantly higher in subjects with traditional cardiovascular risk factors such as hypertension, diabetes, obesity, infrequent exercise, previous smoking, and hypercholesterolemia.58 There was a significant, continuous graded relationship between prevalence of coronary calcium, mean CAC scores, and the number of risk factors. The prevalence of CAC deposits was 40% in asymptomatic men aged younger than 60 years with no risk factors, as opposed to 74% in those with three or more risk factors. However, in older men (>60 years of age), the prevalence of CAC deposits was more than 80% regardless of the number of risk factors present. Multiple logistic regression analysis showed age (relative risk [RR] = 2.82 per 10 years), female gender (RR = 0.34), history of hypertension (RR = 1.53), diabetes (RR = 2.32), obesity (RR = 2.22), and hypercholesterolemia (RR = 1.63) to be significant independent predictors of the presence of coronary calcium. In a recent study, Parikh et al.59 performed CAC measurements in the second- and third-generation descendents of original Framingham cohorts and showed that parental premature cardiovascular disease was associated with a significantly high likelihood of coronary calcification in the offspring, after adjustment for other cardiovascular risk factors.
The Coronary Artery Risk Development in Young Adults (CARDIA) Study60 observed the effect of cardiovascular risk factors in young adults on subsequent development of coronary calcification. The study involved over 3000 adults, aged between 18 and 30 years, who underwent risk assessment at years 0, 3, 5, 7, 10, and 15, followed by CAC measurement in year 15. Individuals with above-average baseline risk were significantly more likely to develop coronary atherosclerosis as indicated by the presence of CAC, underscoring the importance of risk assessment in younger individuals.
Who Should Undergo CAC Screening?
The fundamental principle of preventive cardiology is that the intensity of risk-reduction measures should match the baseline risk of disease,61 so that the benefits of intervention outweigh the risks. Accordingly, the National Cholesterol Educational Programme (NCEP) Expert Panel62 recommends that individuals with multiple cardiovascular risk factors that confer a CAD risk greater than 20% in 10 years should be targeted for intensive lipid-lowering therapy, with a target LDL cholesterol level of under 100 mg/dL, similar to those with established CAD or CAD risk equivalents such as diabetes, peripheral artery disease, and symptomatic carotid artery disease. Hence, high-risk individuals should be targeted for aggressive risk-reduction measures without need for additional risk assessment tests. At the other end of the spectrum, in low-risk individuals (10-year absolute CAD risk < 10%), the likelihood of adverse cardiovascular events is too low to justify any interventions beyond simple lifestyle changes.
In individuals at intermediate risk, it is difficult to make decisions regarding the administration of risk-reduction treatments. Subclinical disease markers such as CAC imaging are most likely to be effective in this group by reclassifying them into either low- or high-risk groups, thereby influencing management decisions. In a prospective observational study,63 Greenland et al. demonstrated that asymptomatic individuals at intermediate risk according to Framingham criteria but with CAC scores above 300 had an annualized hard cardiac event rate of 2.8% (10-year absolute risk > 20%) and therefore would have to be reclassified as high-risk patients. This information would then mandate a more intensive approach to modification of risk factors, including goals for reducing LDL cholesterol. Furthermore, in low-risk (<10% over 10 years) and high-risk (>20% over 10 years) individuals, CAC measurements did not change the risk prediction substantially enough to alter their prognosis and clinical management.
As a caveat, estimation of lifetime risk of cardiovascular disease (CVD) should be considered as an adjunct to 10-year risk calculation in adults younger than 50 years of age. A significant percentage of adults younger than 50 are placed in the low-risk category for developing CVD over a 10-year period, despite a high lifetime risk of developing CVD. Estimation of lifetime risk for CVD has been elucidated by Lloyd-Jones et al. previously.64 Berry et al.65 studied 2988 subjects aged younger than 50 years at study entry into the MESA cohort and at year 15 of follow-up of the CARDIA trial, respectively, and divided them into three distinct groups. The first group was composed of subjects with low (<10%) 10-year risk of CVD and low lifetime risk (<39%) of developing CVD, the second group was composed of subjects with low 10-year risk but high lifetime risk (>39%) for CVD, and finally those with high 10-year risk or diagnosed diabetes mellitus (DM). In the cohort of subjects with low 10-year risk of CVD, those with a high lifetime risk had a significantly higher prevalence of CAC in men and women, as well as greater carotid intima media thickness. Subjects with a higher lifetime risk also showed greater CAC progression than those with lower lifetime risk. This was true for subjects in both the CARDIA and the MESA trials.
The ACC/AHA expert committee task force analysis66 of intermediate FRS patients from four studies63,67–69 reported annual CAD death/MI rates of 0.4%, 1.3%, and 2.5% for CAC score categories of less than 100, 100 to 400, and =400, respectively. This is illustrated in Figure 20-7 and reflects the discriminatory ability of CAC in this important intermediate-risk group, which represents a third of the U.S. population aged 60 years or older, according to data from the third National Health and Nutrition Examination Survey (NHANES III).70
THE PROGNOSTIC VALUE OF CAC: AVAILABLE EVIDENCE
A meta-analysis71 of 4 studies72–75 showed that CAC scores remained predictive of coronary events, even after adjustment for established cardiovascular risk factors. The event rates in individuals with even mild coronary calcification (CAC scores of 1 to 100) were twice as high compared to those with no detectable coronary calcium (RR = 2.1). CAC scores over 400 were associated with very high relative risks (RR = 4.3 to 17) after adjustment for age, sex, and other cardiovascular risk. However, the meta-analysis showed a significant heterogeneity in the quality of these studies, most of which used self-reported or historical as opposed to measured risk factor data and did not use blinded outcome adjudication, which could have overestimated the predictive accuracy of CAC measurements. Selection bias was also an issue, since most of these studies recruited self-referred or physician-referred individuals, who are not necessarily representative of the general population. Furthermore, earlier studies included “soft” endpoints, including coronary revascularization, which could have overestimated the predictive ability of CAC, since CAC results per se could influence the decision to undertake revascularization.
More recent studies have focused on the prediction of “hard” cardiac events (myocardial infarction, CAD death) and are less likely to be subjective. One such study conducted by Ostrom and colleagues76 included 2538 consecutive patients referred for CT coronary angiography. Correlation of mortality and absolute CAC score groups (1 to 9, 10 to 99, 100 to 399 and > 400) was assessed in the 1060 patients diagnosed with nonobstructive CAD. The survival rates for different CAC score groups over a follow-up period of 78 ± 12 months were 99.2%, 99%, 96.9%, and 96.1%, respectively. The risk factor adjusted RR of 5.1 (P = 0.003) and 6.2 (P = 0.001) for CAC scores of 100 to 399 and over 400, respectively, elegantly demonstrated the independent and incremental value of the CAC score.
A recent meta-analysis reported as part of the ACC/AHA 2007 Expert Consensus statement66 on CAC imaging included six studies63,67–69,77,78 published between 2003 and 2005, involving a total of 27,622 patients with 395 hard cardiac events. CAC score of zero was associated with a very low risk (0.4%; 49 events in 11,815 individuals) of CAD death or MI over 3 to 5 years of observation, and presence of any detectable coronary calcium increased the risk fourfold (P < 0.0001). The summary RR increased significantly in those with more severe coronary calcification (7.2 for CAC score of 400 to 1000, and 10.8 for CAC scores >1000, compared to zero CAC score), establishing a continuous graded relationship between CAC scores and risk of coronary events.
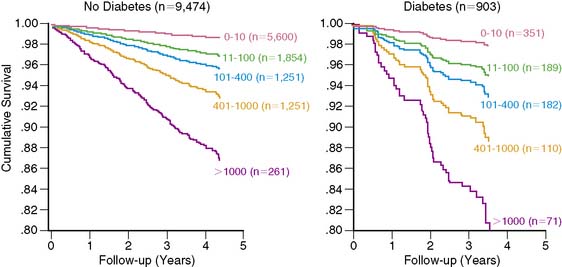
A subsequent large observational study79 published in 2007 evaluated the effect of CAC on all-cause mortality in a cohort of 25,253 patients followed for 6.8 ± 3 years. The study conclusively established the independent and incremental prognostic ability of CAC to predict all-cause mortality. The 10-year cumulative survival was 99.4% for a CAC score of zero and reduced to 87.8% for a score of over 1000 (P < 0.0001) (Fig. 20-8). ROC analysis showed a significant increase in the area under the curve when CAC score was added to risk factors and age. The improved strength and quality of available evidence were enough for the 2007 Expert Consensus Committee of the AHA/ACC to recommend CAC imaging as a reasonable choice for refining the risk stratification in asymptomatic individuals with intermediate Framingham Risk Score,66 which is a significant change from the earlier recommendation made in 2000 by the same body.80
Limitations of CAC Imaging as a Screening Tool in the General Population
For a screening program to be successful, both the disease to be screened and the screening test should satisfy certain criteria.81 The disease should be of considerable public health importance and should have a prolonged latent, preclinical stage, and early identification of preclinical patients should lead to treatments that can reduce the risk of subsequent adverse clinical events. The screening test should be practical, safe, acceptable, available, and affordable, and should accurately differentiate between high-risk and low-risk patients. CAD remains the most deadly and the also most expensive disease in most industrialized nations. Autopsy studies82 showed atherosclerotic lesions in men in their 20s and 30s, indicating a long subclinical asymptomatic stage. Studies have consistently shown that institution of aggressive risk-reduction treatments in high-risk asymptomatic individuals is associated with a significant reduction in mortality and morbidity. Although CAD appears to be an ideal disease for screening, CAC imaging as a universal screening test for CAD has certain limitations.
Although CAC imaging is a safe, noninvasive test and generally acceptable, currently its availability is limited, especially outside the United States. The current cost of a CAC scan (approximately $500 per scan)83 makes it a relatively expensive test for screening the general population. Also, it involves exposure to radiation, which, though very low for an individual patient, might have consequences when applied in the general population.84 Perhaps the most important limitation for CAC screening is its limited ability to accurately differentiate between individuals who are likely to have events from those who will not.85 In most of the outcome analysis studies performed to date, in spite of the positive correlation between CAC scores and likelihood of events, 85% to 95% of those with high CAC scores remained event free over 2 to 7 years of follow-up, and absence of coronary calcium did not always preclude events.86 Calcium is just one of the components of the atherosclerotic plaque and tends to develop late in the natural course of an individual plaque. Thus a CAC score of zero, though it may indicate a low risk of coronary events, by no means excludes the possibility of acute coronary events, since such events can result from the rupture of plaques that have not yet calcified or accumulated enough calcium to exceed the threshold for detection by Agatston method. The study by Cheng and colleagues87 showed that 6.5% of patients with zero CAC had evidence of noncalcified coronary plaque on CT angiography, though only a small minority (0.5%) had evidence of significant (= 50%) luminal stenosis.
When individuals were stratified according to FRS, CAC results did not cause substantial change in treatment targets in low- and high-risk groups.63 Also, there are no prospective data to show that the use of CAC screening can reduce CAD-associated mortality and morbidity. Therefore, even after a cumulative experience of nearly 400,000 patient years of observation in various prospective and observational studies, CAC is not approved as a screening tool for CAD in the general population, unlike other established screening strategies for abdominal aortic aneurysm,88 colon cancer, and breast cancer.89,90
Role of CAC Imaging in the Diagnosis of CAD in Symptomatic Patients
Several studies evaluating the role of CAC imaging in the diagnosis of symptomatic patients with suspected CAD reported reasonable diagnostic accuracy, with the cutoff CAC score chosen in a particular study determining the relative sensitivity and specificity. The 2000 ACC/AHA Expert Consensus document80 on EBCT reported a meta-analysis of 16 studies32,36,91–104 published before 1999, involving a total of 3683 patients without prior CAD who underwent EBCT and diagnostic angiography. The weighted average sensitivity and specificity were 80.4% and 39.9%, respectively. For non-zero CAC scores, the summary odds of having significant obstructive CAD elevated 20-fold. However, there was heterogeneity in the included studies in terms of the use coronary calcium score thresholds, patient entry criteria, and angiographic stenosis thresholds to define significant obstructive CAD. Subsequent studies established the ability of CAC scoring to improve diagnostic discrimination over conventional risk factors in the identification of patients with angiographic coronary disease.105–110
Haberl et al. studied 1764 patients with suspected CAD from a single center.107 CAC score greater than 0 Au had very high sensitivity (99% in men and 100% in women) and negative predictive value (97% in men and 100% in women) in detecting the equivalent of 50% angiographic stenosis. However, the specificity was poor (23% in men and 40% in women), and the positive predictive value was moderate (62% in men and 66% in women). Importantly, in the absence of detectable coronary calcium, angiographically significant stenosis was a rare finding, even in the presence of symptoms (n = 5, of whom only 2 patients needed intervention). There was no evidence of coronary calcium in 11% of men and 22% of women with high pretest probability of CAD. Calcium scoring can therefore identify a subset of patients with a very low risk of significant CAD in whom invasive diagnostic procedures may be omitted; it can thus act as a potential gatekeeper before more invasive tests. In another large multicenter trial, Budoff and colleagues evaluated 1851 patients who underwent angiography for clinical indications.108 The overall sensitivity and specificity of non-zero CAC score to predict obstructive CAD were 95% and 40%, respectively. Increasing the cutoff CAC score to 20, 80, and 100 Au decreased the sensitivity to 90%, 79%, and 76%, whereas the specificity increased to 58%, 72%, and 75%, respectively. CAC scores demonstrated independent and incremental power to predict obstructive CAD when added to age and sex (area under the curve increased from 0.67 to 0.84; P < 0.001). CAC scores, when added to clinically derived pretest probabilities, dramatically changed the posttest probability of obstructive coronary disease across a wide range of patients; the greatest change was noted in patients with intermediate (20% to 70%) pretest probabilities. These earlier studies were subjected to verification bias, which could increase sensitivity and reduce specificity.
In a more recent large, angiographically correlated study109 involving 2115 symptomatic patients, Knez et al. used volumetric calcium scores for prediction of obstructive CAD. In symptomatic patients with no detectable coronary calcium, the likelihood of angiographic stenosis was very low (<1%). Similar to the previous studies, this finding reinforces the reliability of a calcium score of zero to essentially rule out obstructive disease in symptomatic subjects. The presence of any calcium was highly sensitive (99%) for diagnosis of obstructive coronary disease, but the high sensitivity was achieved at the expense of a high percentage of false-positive results and a low specificity (28%), which may lead to unnecessary additional investigations; hence, the use of calcium score percentiles was suggested. For CAC equal to 75%, the overall predictive accuracy was 80% to 82%, which is comparable to stress echocardiography and scintigraphy.
Keelan et al. followed 288 symptomatic patients who underwent EBCT and coronary angiography within 4 weeks of each other, for a mean duration of 6.9 years.110 Only 1 of the 87 patients in this cohort with a CAC score less than 20 Au experienced a hard coronary event, in spite of the presence of symptoms (the patient also had a normal coronary angiogram). In the stepwise Cox proportional hazards model that included risk factors, CAD event history, CAC scores, and angiographic measures of disease, only age and CAC scores were predictive of hard coronary events (risk ratios 1.72 and 1.88, respectively; P < 0.05). CAC scores provided more prognostic information than angiography in symptomatic patients. Although regarded as the gold-standard investigation for diagnosis of obstructive CAD, the angiogram is essentially a “lumenogram” and provides no information on plaque burden or the likelihood of vulnerable plaque.111 Budoff et al. evaluated the ability of EBCT to noninvasively differentiate between ischemic and nonischemic etiologies of cardiomyopathy.112 EBCT was performed within 3 months of coronary angiography in 125 patients with cardiomyopathy. EBCT-derived CAC measurements were 99% sensitive and 83% specific in detecting an ischemic etiology.
COMPARISON OF CAC WITH OTHER DIAGNOSTIC TESTS
To secure its place in the diagnostic armamentarium of a cardiologist, CAC imaging should prove its cost and clinical effectiveness in comparison to other diagnostic tests that are well established and readily available. Earlier studies comparing EBCT with stress electrocardiography (ECG) and scintigraphy indicated that CAC scores were at least as good if not better in predicting angiographically confirmed obstructive coronary disease.113,114 The anatomic information obtained by CAC imaging can complement the functional information from stress testing and improve the overall diagnostic accuracy for obstructive CAD.
CAC and Stress Electrocardiography
The clinical advantage of CAC imaging lies in its remarkably high negative predictive value.107–110 In the study by Lamont et al.,115 the absence of coronary calcium in symptomatic patients with abnormal stress ECG result correlated very well with the absence of obstructive CAD. Only 2 out of 112 patients with angiographic CAD had a zero CAC score. Thus the absence of coronary calcium by EBCT in symptomatic patients with a positive stress ECG test accurately identified those with a false-positive treadmill test. In a similar study by Shavelle et al.,113 when stress ECG results were combined with CAC scores, the specificity of stress ECG increased from 47% to 83% (P < 0.005). The relative risk of obstructive angiographic CAD for an abnormal test was higher for EBCT (RR = 4.53) than either stress ECG (RR = 1.72) or stress single-photon emission computed tomography (SPECT) (RR = 1.96). The study by Schmermund et al.116 showed that addition of CAC result was most beneficial in patients with equivocal stress ECG due to either inadequate exercise or nondiagnostic ECG changes. When CAC scores were added to a negative or equivocal stress ECG result, 84% of false-negative results were correctly classified as having obstructive CAD.
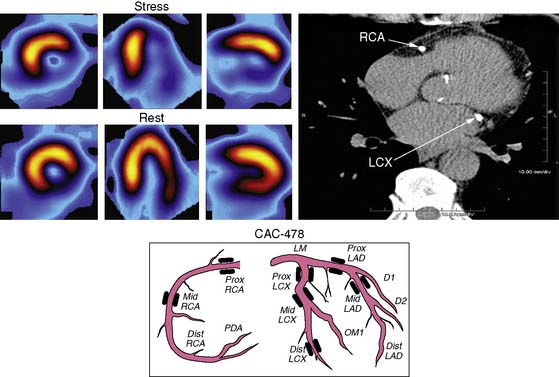
CAC and Myocardial Perfusion Scintigraphy: Role of Synergistic Imaging
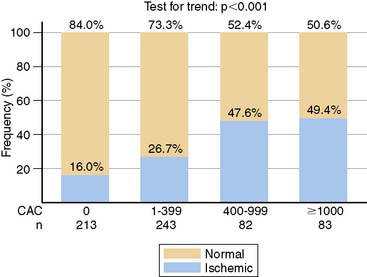
For over 3 decades, myocardial perfusion scintigraphy (MPS) by SPECT has been extensively validated for the diagnosis of patients with suspected CAD, and has played a key role in formulating management decisions regarding revascularization or medical therapy in symptomatic patients with established obstructive CAD.117–124 Attempts have been made to integrate a newer, evolving technology such as CAC imaging with SPECT in investigating patients with suspected or known CAD. Berman et al.125 observed the relationship between CAC scores and stress MPS results in 1195 individuals who underwent both these tests within 7.2 + 44.8 days of each other. While only 2% of patients with CAC scores under 100 Au had evidence of ischemia on MPS, 20% of those with CAC scores over 1000 Au had ischemic MPS, indicating significant relationship between these two clinical markers of atherosclerosis and ischemia, respectively. This relationship is elucidated in (Figure 20-9). After multivariable logistic regression analysis, CAC score was found to be the most potent predictor of ischemia. However, 56% of all normal MPS results were noted in those with significant subclinical atherosclerosis, as indicated by CAC score over 100 Au. Furthermore, even among patients with CAC score over 1000 Au, 85% of asymptomatic and 68% of symptomatic individuals had normal MPS.
In another study to assess the predictive ability of CAC score for the presence of ischemia, Schenker et al.126 looked at 621 patients who had no known CAD but were clinically referred to have a PET scan. The frequency of myocardial ischemia showed significant correlation with the severity of coronary calcification; patients with CAC over 400 Au were more likely to have an abnormal scan than those with CAC 1 to 399 Au (48.5% compared to 21.7%; P < 0.001) (Fig. 20-10). But what was interesting was that even in patients with a zero CAC score, 16% had abnormal scans. At the other end of the spectrum, in patients with a CAC score over 1000 Au, 50.6% had a normal perfusion scan. In the same study, CAC score was found to be strongly predictive of survival in patients with abnormal PET scans and also in those with normal scans. In the presence of a normal PET scan, annualized event rates in patients with a CAC score over 1000 Au and those with no CAC were 12.3% and 2.6%, respectively. Similarly, in those patients with demonstrable ischemia on PET scan, CAC scores of over 1000 Au and under 1000 Au were associated with annualized event rates of 22.1% and 8.1%, respectively.
This disparity between CAC and MPS results reflects the fundamental difference in the nature of information provided by these two tests. Coronary calcium allows detection of atherosclerotic lesions, both obstructive and nonobstructive. Secondary to expansive remodeling of the vessel wall, a considerable amount of plaque can accumulate before luminal encroachment starts. Stress scintigraphy (as with all stress imaging methods), in contrast, requires the presence of a hemodynamically significant obstructive lesion, either fixed or dynamic, before an abnormality becomes evident. The study by Ramakrishna et al.127 also showed a weak correlation between CAC scores and summed stress scores by SPECT; however, the two tests were complementary in predicting mortality. CAC and MPS appear to have different but complementary roles in risk prediction and diagnosis. While MPS is an excellent tool for predicting short-term risk, thereby guiding decisions regarding revascularization, CAC is a better predictor of long-term risk and hence more useful in the determination of the need for aggressive medical prevention measures. Absence of ischemia on MPS is associated with a low short-term risk of coronary events, even when the CAC scores are high (>1000 Au).128
The high sensitivity and negative predictive value of CAC scoring can be combined with relatively high specificity and positive predictive value of MPS to achieve high overall diagnostic accuracy. CAC scores less than 100 Au are typically associated with a low probability (> 2%) of abnormal perfusion on nuclear stress tests125 and less than 3% probability of significant obstruction on cardiac catheterization.107–108 He et al.129 evaluated the relationship between the severity of coronary calcification and stress-induced myocardial ischemia in a large cohort of asymptomatic subjects with risk factors for CAD. CAC score was the best predictor of an abnormal SPECT. Nearly half of those with scores over 400 Au had evidence of ischemia, compared to only 6.6% of those with scores under 400 Au.
In the study by Anand et al.,130 CAC imaging and MPS were synergistic for the prediction of short-term cardiovascular events in 510 asymptomatic diabetic subjects without prior cardiovascular disease. Those with CAC scores over 100 Au (n = 127) and a random sample of the remaining participants with a CAC score of 100 Au (n = 53) underwent MPS and were followed up. In the multivariable model, CAC score was the only predictor of myocardial perfusion abnormality (P < 0.001); CAC score and extent of myocardial ischemia were the only independent predictors of outcome. Twenty cardiovascular events were noted over a median follow-up of 2.2 years, of which 15 were in those with CAC score over 400 Au, and no events were noted in those with scores less than 10 Au. The CAC score predicted events more accurately than the UKPDS (UK Prospective Diabetes Study) and Framingham Risk Scores (area under the curves were 0.92, 0.74, and 0.60 for CAC, UKPDS, and FRS, respectively; P < 0.0001). In a similar study by Wong et al.,131 CAC scores below 100 Au were associated with a very low risk (<3%) of ischemic MPS, even in patients with metabolic syndrome and diabetes. The strong and independent relationship between CAC scores and ischemic MPS persisted, even in patients receiving comprehensive medical therapy with adequate control of risk factors.132
In view of this association between the presence of CAC, especially above a threshold value of 400 Au, and abnormal myocardial perfusion study in a wide variety of populations, a sequential screening approach with initial CAC scoring followed by MPS in those with high CAC scores was suggested for maximizing the yield of MPS and improving cost-effectiveness.133 Using CAC imaging prior to MPS seems logical from a pathophysiologic point of view as well, since the development of coronary atherosclerosis almost always precedes the onset of myocardial ischemia in the ischemic cascade. The incremental value of an integrated approach is clearly shown in Figure 20-11.
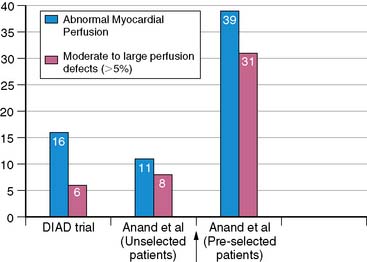
Figure 20-11 Preselection of asymptomatic diabetic subjects by electron beam computed tomography improves the yield of perfusion abnormalities detected by myocardial perfusion imaging. Left, Myocardial perfusion imaging was performed in asymptomatic diabetic subjects (n = 522) in the DIAD study. In the study of Anand et al,130 myocardial perfusion imaging was performed in asymptomatic diabetic subjects with coronary artery calcium scores of greater than 100: unselected patients (middle) and patients selected based on electron beam computed tomography (coronary calcium scores >100 Agatston units) (right).
(Reproduced from Wackers FJ, Young LH, Inzucchi SE, et al: Detection of silent myocardial ischemia in asymptomatic diabetic subjects: The DIAD Study, Diabetes Care 27:1954-1961, 2004; and Lim E, Lahiri A: The importance of the link between coronary artery calcification and myocardial ischemia: A developing argument, J Nucl Cardiol 14:272-274, 2007.)
CAC and Other Stress Tests
CAC scores also showed a weak but statistically significant correlation with wall-motion abnormalities on stress echocardiography.134 The proportion of patients with abnormal exercise Wall Motion Score Index (WMSI) was higher with increasing CAC scores; but even in patients with severe coronary calcification, the majority of patients had a normal WMSI. Symptoms, age, and CAC score were independently associated with abnormal exercise WMSI. More recently, Wang et al. explored the correlation between CAC scores and myocardial perfusion reserve in a subgroup of MESA cohorts using magnetic resonance imaging (MRI) at rest and during adenosine-induced hyperemia.135 Higher CAC scores were associated with low myocardial perfusion reserve independent of the coronary risk factors. The study suggests that subclinical coronary atherosclerosis can impair coronary vasoreactivity and myocardial perfusion reserve in the absence of clinical CAD. Another study using CAC measurements and tagged MRI showed a positive correlation between subclinical atherosclerosis, as indicated by CAC score in a particular coronary artery, and subclinical regional ventricular dysfunction, as indicated by myocardial strain and strain rate in the corresponding perfusion territory.136
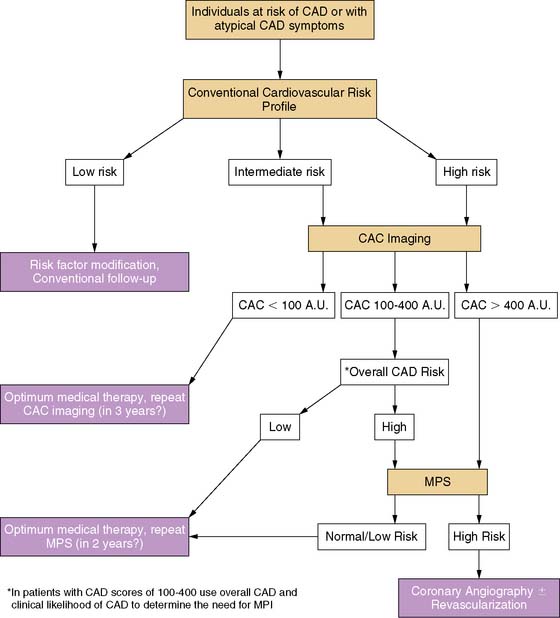
PROPOSED ALGORITHM FOR SEQUENTIAL SCREENING
Based on the available evidence, a diagnostic algorithm was proposed for assessing patients at risk of CAD and those with atypical symptoms (Fig. 20-12).133 CAC scoring can be used in those subjects assessed to be at intermediate or high risk after an initial clinical evaluation using established risk-assessment systems such as FRS. In individuals with CAC scores above 400 Au, the frequency of ischemia is usually substantial, and MPS is thus likely to be cost-effective. This threshold could be lowered perhaps to 200 Au in diabetic subjects, but more data are required before an exact cutoff value can be agreed upon. In individuals with CAC scores in the intermediate range of 100 to 400 Au, the need for MPS should be determined on the basis of overall risk profile and the likelihood of CAD. The severity of perfusion abnormality on MPS determines the need for angiography. When less than 10% of the myocardium is involved, optimum medical therapy was shown to be better than revascularization; hence, coronary angiography can be avoided in these patients. Patients with more extensive ischemia (>10% myocardial involvement) could be referred for angiography and possible revascularization. Finally, those individuals with severe coronary calcification and normal MPS were shown to have a low frequency of hard coronary events (<1% per year) over 3 years of follow-up128; therefore, these patients can be targeted for aggressive medical treatments and spared from invasive interventions.
CAC IMAGING IN PATIENTS WITH DIABETES MELLITUS AND CARDIOMETABOLIC SYNDROME
CAD accounts for 70% of the deaths among patients with DM.137 Diabetic patients face a two- to fourfold higher risk of cardiac events than their nondiabetic counterparts, and in fact, diabetes is now treated as CAD equivalent138 in risk stratifying patients. CAD is often asymptomatic and is associated with worse prognosis in diabetic patients, thus posing a major dilemma in identifying those at increased risk.139 CAC imaging in diabetic patients promises to be an effective test for early detection of silent CAD.
Mielke et al.140 showed that the median CAC score was higher in diabetic subjects compared to their nondiabetic counterparts. In a large study comparing the CAC scores in self-referred diabetic (n = 1075) and nondiabetic subjects (n = 29,829) with no known previous CAD, Hoff et al.141 reported that diabetes was the strongest predictor for having a CAC score in the highest quartile, irrespective of sex.
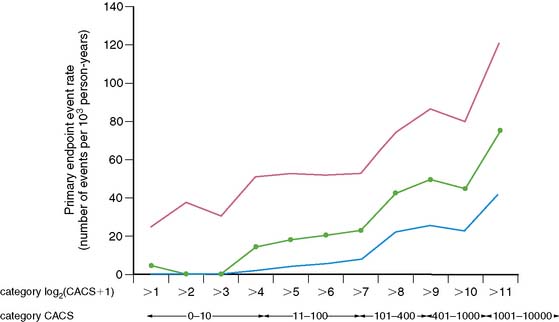
The PREDICT trial142 researchers prospectively studied 589 asymptomatic diabetic patients who were followed up for a median of 4 years with first-incident stroke or CAD events as primary endpoints. Similar to previous large observational studies, CAC score was an independent predictor of primary endpoint events, inclusion of which increased the ROC area under the curve from 0.63 to 0.73, using Framingham CAD risk, and from 0.67 to 0.75 for UKPDS CAD risk prediction. What was interesting was that the doubling of CAC score was associated with a 32% increased risk for primary endpoint events. The authors were able to demonstrate this by using a base 2 logarithm (CAC score + 1), as seen in Figure 20-13.
Diabetic patients with coronary calcification have worse prognosis than nondiabetic subjects with the same extent of coronary calcification (Fig. 20-14).143 Furthermore, in the presence of low CAC scores, the risk of coronary events is low even in diabetic patients. The study by Raggi et al.143 showed that diabetic patients with no detectable coronary calcium had excellent 5-year survival that was not significantly different from nondiabetic subjects (survival 98.8% versus 99.4%, respectively, P = 0.49). The South Bay Heart Watch Study,144 in which 1312 diabetic and nondiabetic patients were screened with CAC scoring using an EBCT scanner and followed up for 6.3 ± 1.4 years, with the endpoints of coronary death, nonfatal MI, coronary revascularization, or stroke, demonstrated a nearly fourfold increase in event rates in diabetics with a higher CAC than in the nondiabetics with a lower CAC.
In patients with diabetes, the presence of metabolic abnormalities increases the likelihood of an abnormal myocardial perfusion scan.145 Wong et al.146 examined 4468 asymptomatic individuals who underwent CAC imaging. The prevalence of CAC ranged from 51% in men and 35% in women with neither DM nor cardiometabolic syndrome (CMS) to 75% in men and 58% in women with both DM and CMS. These data concur with the results of the NHANES study147 that reported the highest prevalence of CAD in patients with both DM and CMS in the United States. The same study also reported higher prevalence of CAD in women with CMS without DM compared to those with DM without CMS (13.9% and 7.5%, respectively).
So far, there are no data to suggest that patients with CMS but no diabetes benefit from aggressive risk-factor modification, as do patients with overt diabetes.148 This makes a good case for CAC imaging screening of asymptomatic diabetic patients, as well as those with CMS but without overt diabetes, to better guide their clinical management. Metabolic syndrome alone is not a robust marker for 10-year cardiovascular risk, but the presence of metabolic syndrome even in the absence of DM should prompt a reassessment of cardiovascular risk, ideally with imaging for the presence of subclinical atherosclerosis.
EFFECT OF ETHNICITY ON PREVALENCE AND PROGNOSTIC SIGNIFICANCE OF CORONARY CALCIUM
CVD-associated morbidity and mortality varies widely across different ethnic groups, and it is likely that these differences are related to genetic as well as environmental factors. Most of the studies that investigated the prevalence and prognostic significance of coronary calcium were based on a predominantly white population. Earlier studies based on necropsy reports149,150 and cine-fluoroscopy,151 and more recent studies using EBCT-derived CAC measurements,152–154 found higher prevalence of coronary calcium in Caucasians compared to African Americans. Using EBCT and coronary angiography, Budoff et al. reported a higher prevalence of coronary atherosclerosis in Caucasians compared to African Americans or Hispanics.155 On the contrary, the CARDIA study156 showed no significant difference in the prevalence of coronary calcification in 443 young black and white adults aged between 28 and 40 years. However, the prevalence of CAC was low in this young cohort. Also, the threshold area for CAC detection was 2.06 mm2, twice the threshold area used in most of the other studies. In another smaller study of 128 black and 733 white asymptomatic postmenopausal women (mean age, 63 ± 8 years), a similar distribution of CAC scores was found in both the ethnic groups.157
The Dallas Heart Study evaluated the prevalence and severity of CAC in a multiethnic population-based sample in which blacks were systematically oversampled to make the final sample 50% black.158 The prevalence of subclinical atherosclerosis was similar in the black and white participants, as were the mean FRSs.159 However, this study used a score of greater than 10 Au (as opposed to > 0 Au) as the minimum score to define CAC prevalence. Also, greater obesity in black women could have resulted in increased noise, which could be misinterpreted as calcium. In a more recent larger study160 involving over 16,000 asymptomatic individuals, Budoff and colleagues reported that African American men were least likely to have any coronary calcium, after adjusting for age and risk factors. African American women, on the other hand, had significantly higher odds of having non-zero CAC scores. Caucasians and Hispanics have a significantly higher prevalence of CAC when compared to African and Asian men. Similarly, in the MESA cohort, white men had the highest prevalence of coronary calcification (70.4%), followed by Chinese (59.2%), Hispanic (55.6%), and black men (52.1%). The weight of evidence points to higher prevalence and severity of CAC in Caucasians compared to ethnic minorities, but it is not yet clear if this is due to differences in the plaque burden or due to differing degrees of plaque calcification among different ethnicities.
Two recent studies evaluated the influence of ethnicity on the predictive value of CAC. Nasir et al. reported the prevalence and the prognostic value of CAC in predicting all-cause mortality in a large, ethnically diverse population of over 14,000 asymptomatic individuals with no prior history of CHD.161 After a mean follow-up of 6.8 years, CAC score was the best predictor of time to death among all ethnic groups. Compared to non-Hispanic whites, ethnic minorities have lower odds of having any, as well as increasing, CAC burden (P < 0.0001). Higher CAC scores were associated with greater mortality among ethnic minorities. However, in the MESA study, no interaction was noted between ethnicity and the risk associated with increasing calcium score.162 Following a median follow-up of 3.9 years, CAC scores were independently predictive of the risk of major coronary events in all ethnic groups; no major differences in the predictive value of calcium scores were detected among different ethnic groups.
The recently concluded London Life Sciences Population (LOLIPOP) study163 evaluated the ethnic differences in the prevalence of subclinical atherosclerosis among 2398 asymptomatic participants with no known previous CAD, recruited from the primary care practices in west London, UK. In comparison to the Europeans (n = 1031), Indian Asians (n = 1367) had approximately twofold higher prevalence of hypertension and type 2 diabetes, higher waist-hip ratio and triglycerides, and lower HDL cholesterol. However, there was no difference in CAC prevalence or mean CAC scores between Indian Asians and Europeans either before or after adjustment for the measured cardiovascular risk factors. In the same study, 262 patients with CAC over 100 Au underwent myocardial perfusion scan. There was no difference in prevalence of silent myocardial ischemia between the two ethnic groups, following adjustment for conventional risk factors. These results contrast with an almost twofold higher risk of MI and CAD mortality in Asians compared to Europeans, and indicate the need for more studies to conclusively establish the relationship between ethnicity and CAD.
PROGRESSION OF CORONARY CALCIUM—CAUSES AND CONSEQUENCES
There is a wealth of published data highlighting the prognostic significance of CAC scoring, but there have been very few trials studying the factors responsible for the progression of CAC scores. Yoon et al.164 showed that the baseline CAC score was the most important determinant of rate of calcium progression. This study showed that neither age nor sex significantly correlated with progression of CAC. But from more robust data from the MESA cohort,165 annual CAC progression had a linear correlation with age irrespective of race and showed a similar trend in both sexes. Among the 2948 participants (51.2%) without detectable CAC at baseline, 475 (16.1%) went on to develop incident CAC over an average of 2.4 years of follow-up. Thus, the estimated incidence rate was 6.6% per year. The incidence was less than 5% per year at younger ages and more than 12% per year at the highest ages. It is important to note that this study did not control for baseline CAC score, since CAC progression is assumed to be a continuous spectrum of change from the initial focus of calcification in the atherosclerotic plaque lesion; therefore, to control for baseline CAC score would be to ignore the effect of causative factors from initiation untill the baseline scan. The study showed that the age differences were similar across gender and race/ethnicity subgroups. Black women had the highest yearly incident rates at 6.3%. Hispanic, white, and Chinese women had yearly rates of 6%, 5.6%, and 4.7%, respectively. These ethnic differences in yearly incident rates were non significant when adjusted for age. With the exception of Chinese participants, men had higher incidence rates than women (P < 0.001). The yearly incidence rates in decreasing order are 10.3% for white men, 8.2% for black men, 7.2% for Hispanic men, and 4.4% for Chinese men. In the same cohort, after adjusting for age, gender, race, and smoking, systolic blood pressure (SBP) and serum glucose were found to be strong predictors of progression of CAC. Serum glucose level was strongly associated with progression even in patients with no diabetes.
Anand et al.166 reported a similar finding in a cohort of 398 asymptomatic diabetic subjects. The study revealed that whereas traditional risk factors except smoking and hyperlipidemia were all univariate predictors of CAC progression, suboptimal glycemic control was one of the strongest predictors of CAC progression. Anand et al. also demonstrated that patients with minimal CAC scores did not progress significantly, but those who already had evidence of increased CAC earlier showed considerable CAC progression despite treatment. Though presence of cardiometabolic syndrome with or without diabetes was associated with a higher prevalence of CAC scores, in the recent Rancho Bernardo study,167 only hypertension and fasting plasma glucose levels significantly predicted the progression of CAC, whereas metabolic syndrome did not.
Yoon et al. also showed that hypertension and diabetes were independent predictors of progression of CAC.164 In a few studies it has been shown that family history of CAD is a strong predictor for the progression of CAC scores, pointing to hitherto undiscovered genetic factors at play in the progression of CAC scores.168,169 Surprisingly, most of the studies looking at progression did not find any significant correlation between levels of LDL and progression of CAC.
It has been shown that increased rate of progression of CAC is associated with increased cardiac event rates, as demonstrated by Shemesh et al.170 in a cohort of high-risk hypertensive patients. The percentage change in CAC score in the three groups of their study population of asymptomatic patients, patients with stable CAD, and patients who suffered a cardiovascular event during the 3 years of follow-up were 118%, 124%, and 180% (P < 0.05). Similarly, Raggi et al.171 also showed that in asymptomatic patients, higher progression rate of calcium volume score was significantly associated with increased risk of coronary events. In a study population of 1310 subjects undergoing sequential EBCT scanning with at least a 1-year interval between scans and average follow up of 2.2 to 2.7 years, 49 subjects suffered from an MI; 90% of the diabetics (n = 9) and 71% of nondiabetics (n = 24) who suffered from an MI had greater than 15% annualized change in their calcium volume score.
It is important to know how frequently a patient should be imaged in order to monitor the progression of CAC, especially when the score is zero. In an interesting study by Gopal et al.,172 710 physician-referred patients with an initial CAC of zero were followed up differentially: 35% for 1 to 3 years, 36% for 3 to 5 years, and 29% for over 5 years. A CAC change/year of zero was found in 61% of the study population; in fact 97% of the subjects had a change in CAC/year of less than 10 Au. Only 1% had a CAC change/year of more than 50 Au, and 2% were in the intermediate group of 10 to 50 Au. They concluded that with a baseline score of zero, there was no evidence to suggest repeating the scan before 5 years. These data are supported by an earlier study by Budoff et al., who reported that only 2% of their study population with an initial score of zero had a CAC score of greater than 10 Au at repeat scanning after 1 to 6 years.173
EFFECT OF TREATMENT ON PROGRESSION OF CORONARY CALCIUM
The evidence for the importance of CAC imaging as a prognostic indicator and as a method of risk stratification has already been elucidated. Logically, identification of high-risk patients based on CAC imaging would lead to the institution of more intense risk-reduction measures. Some trials have suggested targets for LDL as low as 70 mg/dL for additional benefit in selected populations.174–176 However, the evidence for the effect of aggressive HMG-CoA reductase inhibitor therapy on the rate of progression of CAC is conflicting. Earlier studies177,178 that showed slowing of progression of CAC score with HMG-CoA reductase inhibitor therapy are not supported by the data from more recent, larger prospective trials.179–181 Houslay et al.181 showed that despite aggressive therapy with atorvastatin 80 mg compared with placebo, leading to significant reduction in both LDL (more than half of baseline) and CRP, there was no significant correlation with the progression of CAC. The annualized change in CAC in the atorvastatin group was 26%/year, and in the placebo group it was 18%/year (P = NS). Owing to progression in CAC despite aggressive LDL lowering, they also concluded that there was no significant correlation between LDL levels and progression of CAC. In the special case of familial hypercholesterolemia, it has been shown in a very small study group of 8 patients (7 male and 1 female), that LDL apheresis with an extracorporeal device on a weekly or twice-weekly basis in addition to 80 mg of atorvastatin resulted in a significant reduction in coronary calcium scores (26 ± 14%, P < 0.01) during a 29-month follow-up period.182 It is postulated that the reduction in cholesterol levels reduced the exposure of macrophages to the toxic effects of oxidized LDL, thus reducing cell death and denying a focus for calcium deposition.
In a larger observational study, Hecht et al.183 studied a group of 182 asymptomatic patients who were treated with an HMG-CoA reductase inhibitor, either alone or in combination with niacin. The study population was divided into those who had achieved an LDL of lower than 80 mg/dL and those who had an LDL level of higher than 80 mg/dL. Repeat scan after 1.2 years did not show any significant difference in the annualized progression rates of the calcium score between the two groups (9.3% in the group with LDL < 80 mg/dL and 9.1% in the group with LDL > 80 mg/dL). Similar annual progression was noted in the group of patients given an HMG-CoA reductase inhibitor alone and the group given a combination of HMG-CoA reductase inhibitor and niacin (12.2% and 12.1%, respectively). Similarly, in the St. Francis Heart Study,179 treatment with 20 mg of atorvastatin, 1g of vitamin C, and 1000 units of vitamin E (alpha tocopherol) induced a significant reduction in LDL but no significant reduction in CAC progression, compared to treatment with placebo. Interestingly, in subjects with a CAC score of over 400, treatment reduced the incidence of atherosclerotic cardiovascular disease events by 42% (P = 0.046). This discrepancy between the effect of HMG-CoA reductase inhibitors on cardiovascular event rates and CAC progression could be due to the fact that they stabilize the plaque by reducing the free lipid core and inducing a fibrovascular transformation of the thin fibrous cap.184 HMG-CoA reductase inhibitor therapy also reduces the number of macrophages in the plaque,185 therefore attenuating the inflammatory impetus rendering the plaque vulnerable,186 consequently reducing the plaque vulnerability and cardiovascular mortality in general. This effect of HMG-CoA reductase inhibitor therapy on stabilization of the plaque has very little effect on the calcium component of the atherosclerotic plaque lesion or in some cases, an increase in the proportion of calcification in the plaque even as the total volume of the lesion regresses.187
COST-EFFECTIVENESS OF CAC IMAGING
In the current era of inflation beating health care costs and increasing budgetary constraints, imaging services are under growing scrutiny to prove their cost and clinical effectiveness before they can be accepted as part of routine clinical care. Cardiovascular disease already imposes a huge economic burden on the society, costing in excess of $329 billion per year in the United States,188 and can increase substantially in the future as a result of an aging population and growing prevalence of risk factors. Approximately 300,000 EBCT CAC are scans are performed annually in 79 centers in the United States. In the last 2 decades, the number of cardiac imaging procedures has grown markedly while prevalence of CAD has remained steady.189 The American College of Cardiology and the American Heart Association now recommend CAC imaging as a reasonable choice in intermediate Framingham risk patients.66 The 34th Bethesda Conference190 on Atherosclerosis Imaging estimated that applying CAC screening to the 34 million intermediate-risk individuals in the United States could result in direct imaging costs of approximately $1 billion, with additional downstream costs from the ensuing tests and treatments. It is estimated that using CAC screening with a cutoff value of above 75% of age- and gender-adjusted percentile, 25% of intermediate-risk individuals could be reclassified into the high-risk group,191 which will have a significant impact on the extra cost of additional aggressive risk-reduction treatments.
Most of the evidence for cost effectiveness of CAC imaging comes from analysis based on decision modeling rather than actual trial data. The cost-effectiveness of CAC scoring depends largely on the population being screened. Cost-effectiveness analysis based on a decision model192 estimated that the cost to identify a CAD event was $73,070 for low-risk and $37,260 for intermediate-risk individuals (P < 0.00001). When the costs and benefits of resulting treatment were taken into account, the cost was $500,000 per life year saved in the low-risk group, whereas in intermediate-risk individuals, cost-effectiveness ratios were much more favorable, ranging between $30,000 and $42,000 per life year saved. Similarly, when CAC imaging is used as a diagnostic test in symptomatic patients, cost-effectiveness depends largely on the disease prevalence and the cutoff CAC score. In a decision-tree model,83 CAC scan with a cutoff score of 168 Au represented the most cost-effective initial test in population groups with low/intermediate disease prevalence (<70%), compared to other investigations such as stress ECG, stress echo, stress scintigraphy, or direct angiography. CAC scan with a cutoff score of greater than 0 proved to be the least cost-effective noninvasive strategy in the same group. In high-prevalence groups, initial angiography with no prior noninvasive testing proved to be the most cost-effective diagnostic strategy. However, an inherent problem with these decision models is that they either are too simplistic in their assumptions or make too many assumptions. Thus, there is a need for prospective studies evaluating the cost performance and economic implications of CAC scoring in the screening and diagnosis of CAD.
FUTURE DIRECTIONS: CAN CT CORONARY ANGIOGRAPHY REPLACE CAC IMAGING?
CAC imaging is already being used extensively in the United States and some European countries, mainly for reclassifying patients who have a 10-year intermediate cardiovascular risk based on conventional risk factors. Despite the extensive research and the wide availability of CT scanners, there is still a reluctance to integrate CAC imaging into the burgeoning investigative arsenal at the disposal of cardiologists today. With the introduction of successive generations of MSCT scanners and gradual improvements in temporal and spatial resolution, it is now possible to make a more comprehensive assessment of coronary atherosclerotic plaques, both calcified and noncalcified. It is well known that although CAC correlates well with the overall atherosclerotic burden, it does not represent the atherosclerotic plaque in its entirety. More important, CAC imaging provides no information regarding the degree of luminal stenosis caused by an individual plaque. CT coronary angiography can provide important information regarding the location,193 extent, and morphology of the plaque,194 vessel wall remodelling,195,196 and the degree of luminal compromise caused by an individual lesion (Fig. 20-15).197 Although there is not enough evidence currently to advocate the use of CTA as a screening tool in asymptomatic patients, aggressive marketing strategies and mass media have, on occasions, prompted self-referral from the public, posing additional dilemmas to the physician. A recent study by Choi et al.198 evaluated the use of CTA as a screening tool in 1000 middle-aged asymptomatic subjects (age 50 ± 9 years; 63% men). Interestingly, 40 participants (4%) had exclusively noncalcified plaque; 10 of these subjects had significant stenosis on CTA (defined as > 50%), and 5 of them had severe stenosis (defined as > 70%). Midterm follow-up of the study population revealed 15 events; however, only one of these events was ACS, and the rest were revascularizations, mostly prompted by the results of the scan. The study showed that the prevalence of significant noncalcified atherosclerotic lesions in asymptomatic individuals is low (1%) but non-negligible. With the additional costs and risks involved with CTA, there is (1) a need for more robust data to prove that CTA can provide prognostic information that is independent of and incremental to CAC imaging and (2) a need to lower the radiation burden significantly before it can replace the latter as a tool for CAD screening and risk stratification.
There is active ongoing research in the field of atherosclerosis imaging, with an intense pursuit for imaging modalities that can assess not only the extent and severity of the plaque but also its composition and vulnerability.199 Newer imaging modalities are in various stages of development and mostly rely on the use of specific antibodies and ligands with high affinity for various components of atherosclerotic plaque. Since inflammation plays an important role in all stages of atherosclerosis, macrophages have been an attractive target for most of these modalities. MRI studies using targeted immuno-micelles showed strong correlation with macrophage content in atherosclerotic plaques200; however, these studies were mostly limited to extracardiac vessels. Also, positron emission tomography (PET) studies using fluorodeoxyglucose (FDG) demonstrated increased FDG uptake in atherosclerotic plaques, and there was a significant correlation between the PET signal from carotid plaques and the macrophage staining from corresponding histologic sections (r = 0.70; P < 0.0001).201 Similarly, in rabbits, specific uptake of an iodine-containing contrast agent by macrophages allows atherosclerotic lesions to be detected using CT.202 Pending further clinical trials, this contrast agent may become an important adjunct to the clinical evaluation of coronary arteries with CT. These evolving techniques, when successfully validated for use in humans, can potentially transform cardiovascular imaging from a method of diagnosis in symptomatic patients to a tool for noninvasive detection of early subclinical abnormalities. Until such time, CAC imaging, by virtue of its established efficacy and ease of use, remains the method of choice for noninvasive detection of coronary atherosclerosis.
1. Myerburg R.J., Kessler K.M., Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187-1197.
2. Kannel W.B., Neaton J.D., Wentworth D., et al. Overall and coronary heart disease mortality rates in relation to major risk factors in 325,348 men screened for the MRFIT: Multiple Risk Factor Intervention Trial. Am Heart J. 1986;112:825-836.
3. Taylor A.J., Burke A.P., O’Malley P.G., et al. A comparison of Framingham Risk Index, Coronary Artery Calcification and culprit plaque morphology in sudden cardiac death. Circulation. 2000;101:1243-1248.
4. Little W.C., Constantinescu M., Applegate R.J., et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157-1166.
5. Giroud D., Li J.M., Urban P., et al. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol. 1993;71:257-258.
6. Proudfoot D., Shanahan C.M. Biology of calcification in vascular cells: intima versus media. Herz. 2001;26:245-251.
7. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801-809.
8. Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868-874.
9. Doherty T.M., Fitzpatrick L.A., Inoue D., et al. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629-672.
10. Vermeer C. Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem J. 1990;266:625-636.
11. Price P.A. Gla-containing proteins of bone. Connect Tissue Res. 1989;21:51-60.
12. Simons D.B., Schwartz R.S., Edwards W.D., et al. Noninvasive definition of anatomic coronary artery disease by ultrafast computed tomographic scanning: a quantitative pathologic comparison study. J Am Coll Cardiol. 1992;20:1118-1126.
13. Doherty T.M., Detrano R.C. Coronary arterial calcification as an active process: a new perspective on an old problem. Calcif Tissue Int. 1994;54:224-230.
14. Doherty T.M., Asotra K., Fitzpatrick L.A., et al. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci U S A. 2003;100:11201-11206.
15. Stary H.C. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur Heart J. 1990;11(Suppl E):3-19.
16. Proudfoot D., Skepper J.N., Hegyi L., et al. The role of apoptosis in the initiation of vascular calcification. Z Kardiol. 2001;90(Suppl 3):43-46.
17. Proudfoot D., Davies J.D., Skepper J.N., et al. Acetylated low-density lipoprotein stimulates human vascular smooth muscle cell calcification by promoting osteoblastic differentiation and inhibiting phagocytosis. Circulation. 2002;106:3044-3050.
18. Sangiorgi G., Rumberger J.A., Severson A., et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126-133.
19. Rumberger J.A., Simons D.B., Fitzpatrick L.A., et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157-2162.
20. Clarkson T.B., Prichard R.W., Morgan T.M., et al. Remodelling of coronary arteries in human and non-human primates. JAMA. 1994;279:289-294.
21. Ehara S., Kobayashi Y., Yoshiyama M., et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424-3429.
22. Abedin M., Tintut Y., Demer L.L. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161-1170.
23. Lee R.T., Grodzinsky A.J., Frank E.H., et al. Structure-dependent dynamic mechanical behavior of fibrous caps from human atherosclerotic plaques. Circulation. 1993;83:1764-1770.
24. Richardson P.D., Davies M.J., Born G.V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989;2:941-944.
25. Johnson R.C., Leopold J.A., Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044-1059.
26. Doherty T.M., Fitzpatrick L.A., Inoue D., et al. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629-672.
27. Fitzpatrick L.A., Severson A., Edwards W.D., Ingram R.T. Diffuse calcification in human coronary arteries: association of osteopontin with atherosclerosis. J Clin Invest. 1994;94:1597-1604.
28. Anand D.V., Lahiri A., Lim E., et al. The relationship between osteoprotegerin levels and coronary artery calcification in uncomplicated type 2 diabetic subjects. J Am Coll Cardiol. 2006;47(9):1850-1857.
29. Mesquita M., Demulder A., Damry N., et al. Plasma osteoprotegerin is an independent risk factor for mortality and an early bio-marker of coronary vascular calcification in chronic kidney disease. Clin Chem and Lab Med. 2009. (Epub ahead of print)
30. Knez A., Becker C., Becker A., et al. Determination of coronary calcium with multi-slice spiral computed tomography: a comparative study with electron-beam CT. Int J Cardiovasc Imaging. 2002;18:295-303.
31. Becker C.R., Kleffel T., Crispin A. Coronary artery calcium measurement: agreement of multirow detector and electron beam CT. AJR Am J Roentgenol. 2001;176:1295-1298.
32. Agatston A.S., Janowitz W.R., Hildner F.J. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827-832.
33. Becker C.R., Jakobs T.F., Aydemir S. Helical and single slice conventional CT versus EBCT for the quantitation of coronary artery calcification. AJR Am J Roentgenol. 2000;174:543-547.
34. Bielak L.F., Sheedy P.F.2nd, Peyser P.A. Coronary artery calcification measured at EBCT: agreement in dual scan runs and change over time. Radiology. 2001;218:224-229.
35. Yoon H.C., Greaser L.E.3rd, Mather R. Coronary artery calcium: alternate methods for accurate and reproducible quantitation. Acad Radiol. 1997;4:666-673.
36. Bielak L.F., Kaufmann R.B., Moll P.P. Small lesions identified in the heart by EBCT: calcification or noise? Radiology. 1994;192:631-636.
37. Callister T.Q., Cooil B., Raya S.P., et al. Coronary artery disease: Improved reproducibility of calcium scoring with an EBCT volumetric method. Radiology. 1998;208:807-814.
38. Detrano R.C., Anderson M., Nelson J., et al. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility—MESA study. Radiology. 2005;236:477-484.
39. Hong C., Becker C.R., Schoepf U.J., et al. Coronary artery calcium: absolute quantification in non-enhanced and contrast enhanced multi-detector row CT studies. Radiology. 2002;223:474-480.
40. Shisen J., Leung D.Y., Juergens C.P. Gender and age differences in the prevalence of coronary artery calcification in 953 Chinese subjects. Heart Lung Circ. 2005;14:69-73.
41. Shaw L.J., Raggi P., Berman D.S., Callister T.Q. Coronary artery calcium as a measure of biologic age. Atherosclerosis. 2006;188:112-119.
42. Anand D.V., Lipkin D., Lahiri A. Finding the age of the patient’s heart. BMJ. 2003;326:1045-1046.
43. Hoff J.A., Chomka E.V., Krainik A.J., et al. Age and gender distributions of coronary artery calcium detected by EBCT in 35246 adults. Am J Cardiol. 2001;87:1335-1339.
44. Budoff M.J., Nasir K., McClelland R.L., et al. Coronary artery calcium predicts coronary events better with absolute calcium scores than age-sex-race/ethnicity percentiles: (MESA) Multi Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2009;53:345-352.
45. Akram K., Voros S. Absolute coronary artery calcium scores are superior to MESA percentile rank in predicting obstructive coronary artery disease. Int J Cardiovasc Imaging. 2008;24:743-749.
46. Centers for Disease Control and Prevention (CDC). State-specific mortality from sudden cardiac death–United States, 1999. MMWR Morb Mortal Wkly Rep. 2002;51:123-126.
47. Goraya T.Y., Jacobsen S.J., Kottke T.E., et al. Coronary heart disease death and sudden cardiac death: a 20-year population-based study. Am J Epidemiol. 2003;157:763-770.
48. EUROASPIRE II Study Group. Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries; principal results from EUROASPIRE II Euro Heart Survey Programme. Eur Heart J. 2001;22:554-572.
49. Stampfer M.J., Hu F.B., Manson J.E., et al. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16-22.
50. Truett J., Cornfield J., Kannel W. A multivariate analysis of the risk of coronary heart disease in Framingham. J Chronic Dis. 1967;20:511-524.
51. Assmann G., Cullen P., Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation. 2002;105:310-315.
52. Conroy R.M., Pyorala K., Fitzgerald A.P., et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987-1003.
53. Hemann B.A., Bimson W.F., Taylor A.J. The Framingham Risk Score: an appraisal of its benefits and limitations. Am Heart Hosp J. 2007;5:91-96.
54. Coleman R.L., Stevens R.J., Retnakaran R., Holman R. Framingham, SCORE, and DECODE risk equations do not provide reliable cardiovascular risk estimates in type 2 diabetes. Diabetes Care. 2007;30:1292-1293.
55. Brindle P., Emberson J., Lampe F., et al. Predictive accuracy of the Framingham coronary risk score in British men: prospective cohort study. BMJ. 2003;327:1267.
56. Eichler K., Puhan M.A., Steurer J., Bachmann L.M. Prediction of first coronary events with the Framingham score: a systematic review. Am Heart J. 2007;153:722-731.
57. Empana J.P., Ducimetière P., Arveiler D., et al. PRIME Study Group. Are the Framingham and PROCAM coronary heart disease risk functions applicable to different European populations? The PRIME Study. Eur Heart J. 2003;24:1903-1911.
58. Wong N.D., Kouwabunpat D., Vo A.N., et al. Coronary calcium and atherosclerosis by ultrafast computed tomography in asymptomatic men and women: relation to age and risk factors. Am Heart J. 1994;127:422-430.
59. Parikh N.I., Hwang S.J., Larson M.G., et al. Parental occurrence of premature cardiovascular disease predicts increased coronary artery and abdominal aortic calcification in the Framingham offspring and third-generation cohorts. Circulation. 2007;116:1473-1481.
60. Loria C.M., Liu K., Lewis C.E., et al. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007;49:2013-2020.
61. 27th Bethesda Conference. Matching the Intensity of Risk Factor Management with the Hazard for Coronary Disease Events. September 14 to 15, 1995. J Am Coll Cardiol. 1996;27:957-1047.
62. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
63. Greenland P., LaBree L., Azen S.P., et al. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210-215.
64. Lloyd-Jones D., Leip E.P., Larson M.G., et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;117:791-798.
65. Berry J.D., Liu K., Folsom A.R., et al. Prevalence and progression of sub-clinical atherosclerosis in younger adults with low short term but high lifetime estimated risk for cardiovascular disease: The Coronary Artery Risk Development in Young Adults Study and Multi-Ethnic Study of Atherosclerosis. Circulation. 2009;119:382-389.
66. Greenland P., Bonow R.O., Brundage B.H., et al. ACCF/AHA 2007 Clinical Expert Consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force. J Am Coll Cardiol. 2007;49:378-402.
67. Arad Y., Goodman K.J., Roth M., et al. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158-165.
68. Vliegenthart R., Oudkerk M., Hofman A., et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572-577.
69. LaMonte M.J., FitzGerald S.J., Church T.S., et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162:421-429.
70. Ford E.S., Giles W.H., Mokdad A.H. The distribution of 10-year risk for coronary heart disease among U.S. adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol. 2004;43:1791-1796.
71. Pletcher M.J., Tice J.A., Pignone M., Browner W.S. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med. 2004;164:1285-1292.
72. Yang T., Doherty T.M., Wong N.D., Detrano R.C. Alcohol consumption, coronary calcium, and coronary heart disease events. Am J Cardiol. 1999;84:802-806.
73. Arad Y., Spadaro L.A., Goodman K., et al. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol. 2000;36:1253-1260.
74. Wong N.D., Hsu J.C., Detrano R.C., et al. Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol. 2000;86:495-498.
75. Raggi P., Cooil B., Callister T.Q. Use of electron beam tomography data to develop models for prediction of hard coronary events. Am Heart J. 2001;141:375-382.
76. Ostrom M.P., Gopal A., Ahmadi N., et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol. 2008;52:1335-1343.
77. Taylor A.J., Bindeman J., Feuerstein I., et al. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) Project. J Am Coll Cardiol. 2005;46:807-814.
78. Kondos G.T., Hoff J.A., Sevrukov A., et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571-2576.
79. Budoff M.J., Shaw L.J., Liu S.T., et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860-1870.
80. O’Rourke R.A., Brundage B.H., Froelicher V.F., et al. American College of Cardiology/American Heart Association Expert Consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation. 2000;102:126-140.
81. Criteria for appraising the viability, effectiveness and appropriateness of a screening programme. http://www.nsc.nhs.uk/uk_nsc/uk_nsc_ind.htm. Accessed 23 April 2008
82. Virmani R., Robinowitz M., McAllister H.A.Jr. Coronary heart disease in 48 autopsy patients 30 years old and younger. Arch Pathol Lab Med. 1983;107:535-540.
83. Rumberger J.A., Behrenbeck T., Breen J.F., Sheedy PFII. Coronary calcification by electron beam computed tomography and obstructive coronary artery disease: a model for costs and effectiveness of diagnosis as compared with conventional cardiac testing methods. J Am Coll Cardiol. 1999;33:453-462.
84. Nickoloff E.L., Alderson P.O. Radiation exposures to patients from CT: reality, public perception, and policy. AJR Am J Roentgenol. 2001;177:285-287.
85. Mozaffarian D. Electron-beam computed tomography for coronary calcium: a useful test to screen for coronary heart disease? JAMA. 2005;294:2897-12890.
86. Doherty T.M., Wong N.D., Shavelle R.M., et al. Coronary heart disease deaths and infarctions in people with little or no coronary calcium. Lancet. 1999;353:41-42.
87. Cheng V.Y., Lepor N.E., Madyoon H., et al. Presence and severity of noncalcified coronary plaque on 64-slice computed tomographic coronary angiography in patients with zero and low coronary artery calcium. Am J Cardiol. 2007;99:1183-1186.
88. Fleming C., Whitlock E.P., Beil T.L., Lederle F.A. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142:203-211.
89. Pignone M., Rich M., Teutsch S.M., et al. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:132-141.
90. Humphrey L.L., Helfand M., Chan B.K., Woolf S.H. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:347-360.
91. Tanenbaum S.R., Kondos G.T., Veselik K.E., et al. Detection of calcific deposits in coronary arteries by ultrafast computed tomography and correlation with angiography. Am J Cardiol. 1989;63:870-872.
92. Detrano R., Hsiai T., Wang S., et al. Prognostic value of coronary calcification and angiographic stenoses in patients undergoing coronary angiography. J Am Coll Cardiol. 1996;27:285-290.
93. Schmermund A., Baumgart D., Adamzik M., et al. Comparison of electron-beam computed tomography and intracoronary ultrasound in detecting calcified and noncalcified plaques in patients with acute coronary syndromes and no or minimal to moderate angiographic coronary artery disease. Am J Cardiol. 1998;81:141-146.
94. Breen J.F., Sheedy P.F., Schwartz R.S., et al. Coronary artery calcification detected with ultrafast CT as an indication of coronary artery disease. Radiology. 1992;185:435-439.
95. Kaufmann R.B., Sheedy P.F., Maher J.E., et al. Quantity of coronary artery calcium detected by electron beam computed tomography in asymptomatic subjects and angiographically studied patients. Mayo Clin Proc. 1995;70:223-232.
96. Devries S., Wolfkiel C., Shah V., Chomka E., Rich S. Reproducibility of the measurement of coronary calcium with ultrafast computed tomography. Am J Cardiol. 1995;75:973-975.
97. Kajinami K., Seki H., Takekoshi N., Mabuchi H. Quantification of coronary artery calcification using ultrafast computed tomography: reproducibility of measurements. Coron Artery Dis. 1993;4:1103-1108.
98. Rumberger J.A., Sheedy P.F., Breen J.F., Schwartz R.S. Coronary calcium, as determined by electron beam computed tomography, and coronary disease on arteriogram: effect of patient’s sex on diagnosis. Circulation. 1995;91:1363-1367.
99. Braun J., Oldendorf M., Moshage W., et al. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis. 1996;27:394-401.
100. Budoff M.J., Georgiou D., Brody A., et al. Ultrafast computed tomography as a diagnostic modality in the detection of coronary artery disease: a multicenter study. Circulation. 1996;93:898-904.
101. Fallavollita J.A., Brody A.S., Bunnell I.L., et al. Fast computed tomography detection of coronary calcification in the diagnosis of coronary artery disease: comparison with angiography in patients, 50 years old. Circulation. 1994;89:285-290.
102. Baumgart D., Schmermund A., Goerge G., et al. Comparison of electron beam computed tomography with intracoronary ultrasound and coronary angiography for detection of coronary atherosclerosis. J Am Coll Cardiol. 1997;30:57-64.
103. Schmermund A., Baumgart D., Goörge G., et al. Coronary artery calcium in acute coronary syndromes: a comparative study of electron-beam computed tomography, coronary angiography, and intracoronary ultrasound in survivors of acute myocardial infarction and unstable angina. Circulation. 1997;96:1461-1469.
104. Kennedy J., Shavelle R., Wang S., et al. Coronary calcium and standard risk factors in symptomatic patients referred for coronary angiography. Am Heart J. 1998;135:696-702.
105. Schmermund A., Bailey K.R., Rumberger J.A., et al. An algorithm for noninvasive identification of angiographic three-vessel and/or left main coronary artery disease in symptomatic patients on the basis of cardiac risk and electron-beam computed tomographic calcium scores. J Am Coll Cardiol. 1999;33:444-452.
106. Guerci A.D., Spadaro L.A., Goodman K.J., et al. Comparison of electron beam computed tomography scanning and conventional risk factor assessment for the prediction of angiographic coronary artery disease. J Am Coll Cardiol. 1998;32:673-679.
107. Haberl R., Becker A., Leber A., et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: results of 1,764 patients. J Am Coll Cardiol. 2001;37:451-457.
108. Budoff M.J., Diamond G.A., Raggi P., et al. Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography. Circulation. 2002;105:1791-1796.
109. Knez A., Becker A., Leber A., et al. Relation of coronary calcium scores by electron beam tomography to obstructive disease in 2,115 symptomatic patients. Am J Cardiol. 2004;93:1150-1152.
110. Keelan P.C., Bielak L.F., Ashai K., et al. Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation. 2001;104:412-417.
111. Topol E.J., Nissen S.E. Our preoccupation with coronary luminology: the dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333-2342.
112. Budoff M.J., Shavelle D.M., Lamont D.H., et al. Usefulness of electron beam computed tomography scanning for distinguishing ischemic from nonischemic cardiomyopathy. J Am Coll Cardiol. 1998;32:1173-1178.
113. Shavelle D.M., Budoff M.J., LaMont D.H., et al. Exercise testing and electron beam computed tomography in the evaluation of coronary artery disease. J Am Coll Cardiol. 2000;36:32-38.
114. Kajinami K., Seki H., Takekoshi N., Mabuchi H. Noninvasive prediction of coronary atherosclerosis by quantification of coronary artery calcification using electron beam computed tomography: comparison with electrocardiographic and thallium exercise stress test results. J Am Coll Cardiol. 1995;26:1209-1221.
115. Lamont D.H., Budoff M.J., Shavelle D.M., et al. Coronary calcium scanning adds incremental value to patients with positive stress tests. Am Heart J. 2002;143:861-867.
116. Schmermund A., Baumgart D., Sack S., et al. Assessment of coronary calcification by electron-beam computed tomography in symptomatic patients with normal, abnormal or equivocal exercise stress test. Eur Heart J. 2000;21:1674-1682.
117. Ladenheim M.L., Pollock B.H., Rozanski A., et al. Extent and severity of myocardial hypoperfusion as predictors of prognosis in patients with suspected coronary artery disease. J Am Coll Cardiol. 1986;7:464-471.
118. Staniloff H.M., Forrester J.S., Berman D.S., Swan H.J. Prediction of death, myocardial infarction, and worsening chest pain using thallium scintigraphy and exercise electrocardiography. J Nucl Med. 1986;27:1842-1848.
119. Brown K.A. Prognostic value of thallium-201 myocardial perfusion imaging. A diagnostic tool comes of age. Circulation. 1991;83:363-381.
120. Iskandrian A.S., Chae S.C., Heo J., et al. Independent and incremental prognostic value of exercise single photon emission computed tomographic (SPECT) thallium imaging in coronary artery disease. J Am Coll Cardiol. 1993;22:665-670.
121. Heller G.V., Herman S.D., Travin M.I., et al. Independent prognostic value of intravenous dipyridamole with technetium-99m sestamibi tomographic imaging in predicting cardiac events and cardiac-related hospital admissions. J Am Coll Cardiol. 1995;26:1202-1208.
122. Hachamovitch R., Berman D.S., Kiat H., et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93:905-914.
123. Hachamovitch R., Berman D.S., Shaw L.J., et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535-543.
124. Hachamovitch R., Hayes S.W., Friedman J.D., et al. Stress myocardial perfusion SPECT is clinically effective and cost-effective in risk-stratification of patients with a high likelihood of CAD but no known CAD. J Am Coll Cardiol. 2004;43:200-208.
125. Berman D.S., Wong N.D., Gransar H., et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923-930.
126. Schenker M.P., Dorbala S., Hong E.C.T., et al. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: A combined positron emission tomography/computed tomography study. Circulation. 2008;117:1693-1700.
127. Ramakrishna G., Miller T.D., Breen J.F., et al. Relationship and prognostic value of coronary artery calcification by electron beam computed tomography to stress-induced ischemia by single photon emission computed tomography. Am Heart J. 2007;153:807-814.
128. Rozanski A., Gransar H., Wong N.D., et al. Clinical outcomes after both coronary calcium scanning and exercise myocardial perfusion scintigraphy. J Am Coll Cardiol. 2007;49:1352-1361.
129. He Z.X., Hedrick T.D., Pratt C.M., et al. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101:244-251.
130. Anand D.V., Lim E., Hopkins D., et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27:713-721.
131. Wong N.D., Rozanski A., Gransar H., et al. Metabolic syndrome and diabetes are associated with an increased likelihood of inducible myocardial ischemia among patients with subclinical atherosclerosis. Diabetes Care. 2005;28:1445-1450.
132. Ho J., FitzGerald S., Stolfus L., et al. Severe coronary artery calcifications are associated with ischemia in patients undergoing medical therapy. J Nucl Cardiol. 2007;14:341-346.
133. Yerramasu A., Maggae S.V., Lahiri A., Anand D.V. Cardiac computed tomography and myocardial perfusion imaging for risk stratification in asymptomatic diabetic patients: a critical review. J Nucl Cardiol. 2008;15:13-22.
134. Ramakrishna G., Breen J.F., Mulvagh S.L., et al. Relationship between coronary artery calcification detected by electron-beam computed tomography and abnormal stress echocardiography: association and prognostic implications. J Am Coll Cardiol. 2006;48:2125-2131.
135. Wang L., Jerosch-Herold M., Jacobs D.R., et al. Coronary artery calcification and myocardial perfusion in asymptomatic adults: The MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2006;48:1018-1026.
136. Edvardsen T., Detrano R., Rosen B.D., et al. Coronary artery atherosclerosis is related to reduced regional left ventricular function in individuals without history of clinical cardiovascular disease: the Multiethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:206-211.
137. Gu K., Cowie C.C., Harris M.I. Mortality in adults with and without diabetes in a national cohort of the US population, 1971–1993. Diabetes Care. 1998;21:1138-1145.
138. Haffner S.M., Lehto S., Rönemaa T., et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234.
139. Donnan P.T., Boyle D.I., Broomhall J., Hunter K., et al. Prognosis following first acute myocardial infarction in type 2 diabetes: A comparative population study. Diabet Med. 2002;19:448-455.
140. Mielke C.H., Shields J.P., Broemeling L.D. Coronary artery calcium, coronary artery disease, and diabetes. Diabetes Res Clin Pract. 2001;53:55-61.
141. Hoff J.A., Quinn L., Sevrukov A., et al. The prevalence of CAD among diabetic individuals without known CAD. J Am Coll Cardiol. 2003;41:1008-1012.
142. Elkeles R.S., Godsland I.F., Feher M.D., et al. Coronary artery calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J. 2008;29:2244-2251.
143. Raggi P., Shaw L.J., Berman D.S., Callister T.Q. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663-1669.
144. Qu W., Le T.T., Azen S.P., et al. Value of coronary artery calcium scanning by computed tomography for predicting coronary heart disease in diabetic subjects. Diabetes Care. 2003;26:905-910.
145. Wong N.D., Rozanski A., Gransar H., et al. Metabolic syndrome and diabetes are associated with an increased likelihood of inducible myocardial ischaemia among patients with sub-clinical atherosclerosis. Diabetes Care. 2005;28:1445-1450.
146. Wong N.D., Gransar H., Shaw L.J., et al. Comparison of atherosclerotic calcification burden in persons with cardiometabolic syndrome and diabetes. J Cardiometab Syndr. 2006;1:90-94.
147. Alexander C.M., Landsman P.B., Teutsch S.M., et al. Third National Health and Nutritional Survey (NHANES III); National Cholesterol Education Programme (NCEP). NCEP defined metabolic syndrome, diabetes and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;26:3160-3167.
148. Budoff M.J., Yu D., Nasir K., et al. Diabetes and the progression of coronary calcium under the influence of statin therapy. Am Heart J. 2005;149:695-700.
149. Strong J.P., Oalmann M.C., Newman W.P., et al. Coronary heart disease in young black and white males in New Orleans: community pathology study. Am Heart J. 1984;108:747-759.
150. Eggen D.A., Stong J.P., McGill H.C. Coronary calcification: relationship to clinically significant coronary lesions and race, sex and topographic distribution. Circulation. 1965;32:948-955.
151. Tang W., Detrano R.C., Brezden O.S., et al. Racial differences in coronary calcium prevalence among high-risk adults. Am J Cardiol. 1995;75:1088-1091.
152. Lee T.C., O’Malley P.G., Feuerstein I., Taylor A.J. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41:39-44.
153. Newman A.B., Naydeck B.L., Whittle J., et al. Racial differences in coronary artery calcification in older adults. Arterioscler Thromb Vasc Biol. 2002;122:424-430.
154. Doherty T.M., Tang W., Detrano R.C. Racial differences in the significance of coronary calcium in asymptomatic black and white subjects with coronary risk factors. J Am Coll Cardiol. 1999;34:787-794.
155. Budoff M.J., Yang T.P., Shavelle R.M., et al. Ethnic differences in coronary atherosclerosis. J Am Coll Cardiol. 2002;39:408-441.
156. Bild D.E., Folsom A.R., Lowe L.P., et al. Prevalence and correlates of coronary calcification in black and white young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arterioscler Thromb Vasc Biol. 2001;21:852-857.
157. Khurana C., Rosenbaum C.G., Howard B.V., et al. Coronary artery calcification in black women and white women. Am Heart J. 2003;145:724-729.
158. Victor R.G., Haley R.W., Willett D., et al. A population-based probability sample for the multidisciplinary study of ethnic disparities in cardiovascular disease: recruitment and validation in the Dallas Heart study. Am J Cardiol. 2004;93:1473-1480.
159. Jain T., Peshock R., McGuire D.K., et al. Dallas Heart Study Investigators. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011-1017.
160. Budoff M.J., Nasir K., Mao S., et al. Ethnic differences of the presence and severity of coronary atherosclerosis. Atherosclerosis. 2006;187:343-350.
161. Nasir K., Shaw L.J., Liu S.T., et al. Ethnic differences in the prognostic value of coronary artery calcification for all-cause mortality. J Am Coll Cardiol. 2007;50:953-960.
162. Detrano R., Guerci A.D., Carr J.J., et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336.
163. Jain P., Chambers J.C., Elliott P., et al. Coronary artery calcification as a predictor of increased coronary heart disease risk in UK Indian Asians [abstract]. Heart. 2008;94(Suppl II):A77.
164. Yoon H.C., Emerick A.M., Hill J.A., et al. Calcium begets calcium: progression of coronary artery calcification in asymptomatic subjects. Radiology. 2002;224:236-241.
165. Kronmal R.A., McClelland R.L., Detrano R., et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115:2722-2730.
166. Anand D.V., Lim E., Darko D., et al. Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol. 2007;50:2218-2225.
167. Kramer C.K., von Muhlen D., Gross J.L., et al. Blood pressure and fasting plasma glucose rather than metabolic syndrome predict progression of coronary artery calcium: Rancho Bernardo study. Diabetes Care. 2009;32:141-146.
168. Peyser P.A., Bielak L.F., Chu J.S., et al. Heritability of coronary artery calcium quantity measured by electron beam computed tomography in asymptomatic adults. Circulation. 2002;106:304-308.
169. Cassidy-Bushrow A.E., Bielak L.F., Sheedy P.F.2nd, et al. Coronary artery calcification progression is heritable. Circulation. 2007;116:25-31.
170. Shemesh J., Apter S., Stolero D., et al. Annual progression of coronary artery calcium by spiral computed tomography in hypertensive patients without myocardial ischemia but with prominent atherosclerotic risk factors, in patients with previous angina pectoris or healed acute myocardial infarction, and in patients with coronary events during follow-up. Am J Cardiol. 2001;87:1395-1397.
171. Raggi P., Cooil B., Ratti C., et al. Progression of coronary artery calcium and occurrence of myocardial infarction in patients with and without diabetes mellitus. Hypertension. 2005;46:238-243.
172. Gopal A., Nasir K., Liu S.T., et al. Coronary calcium progression rates with a zero initial score by electron beam tomography. Int J Cardiol. 2007;117:227-231.
173. Budoff M.J., Lane K.L., Bakhsheshi H., et al. Rates of progression of coronary calcium by electron beam tomography. Am J Cardiol. 2000;86:8-11.
174. Nissen S.E., Tuzcu E.M., Schoenhagen P., et al. Effect of intensive compared with moderate lipid lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071-1080.
175. O’keefe J.H.Jr, Cordain L., Harris W.H., et al. Optimal LDL is 50–70 mgs/dl: lower is better and physiologically normal. J Am Coll Cardiol. 2004;43:2142-2146.
176. Larosa J.C., Grundy S.M., Waters D.D., et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425-1435.
177. Callister T.Q., Raggi P., Cooil B., et al. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339:1972-1978.
178. Achenbach S., Ropers D., Pohle K., et al. Influence of lipid-lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation. 2002;106:1077-1082.
179. Arad Y., Spadaro L.A., Roth M., et al. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005;46:166-172.
180. Raggi P., Davidson M., Callister T.Q., et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation. 2005;112:563-571.
181. Houslay E.S., Cowell S.J., Prescott R.J., et al. Progressive coronary calcification despite intensive lipid lowering treatment. Heart. 2006;92:1207-1212.
182. Hoffmann U., Derfler K., Haas M., et al. Effects of combined LDL apheresis and aggressive statin therapy on coronary calcified plaque as measured by computed tomography. Am J Cardiol. 2003;91:461-463.
183. Hecht H.S., Harman S.M. Relation of aggressiveness of lipid-lowering treatment to changes in calcified plaque burden by EBCT. Am J Cardiol. 2003;92:334-336.
184. Crisby M., Nordin-Fredriksson G., Shah P.K., et al. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103:926-933.
185. Aikawa M., Rabkin E., Sugiyama S., et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases, and tissue factor in vivo, and in vitro. Circulation. 2001;103:276-283.
186. Kwak B.R., Mulhaupt F., Mach F. Atherosclerosis: anti-inflammatory and immunomodulatory activities of statins. Autoimmun Rev. 2003;2:332-338.
187. Trion A., Schutte-Bart C., Bax W.H. Modulation of calcification of vascular smooth muscle cells in culture by calcium antagonists, statins, and their combination. Mol Cell Biochem. 2008;308:25-33.
188. Weinstein M.C., Stason W.B. Cost-effectiveness of interventions to prevent or treat coronary heart disease. Annu Rev Public Health. 1985;6:41-63.
189. Lucas F.L., DeLorenzo M.A., Siewers A.E., Wennberg D.E. Temporal trends in the utilization of diagnostic testing and treatments for cardiovascular disease in the United States, 1993–2001. Circulation. 2006;113:374-379.
190. Mark D.B., Shaw L.J., Lauer M.S., O’Malley P.G., Heidenreich P. 34th Bethesda Conference: Task Force #5—Is atherosclerosis imaging cost effective. J Am Coll Cardiol. 2003;4:1906-1917.
191. Lakoski S.G., Cushman M., Blumenthal R.S., et al. Implications of C-reactive protein or coronary artery calcium score as an adjunct to global risk assessment for primary prevention of CHD. Atherosclerosis. 2007;193:401-407.
192. Shaw L.J., Raggi P., Berman D.S., Callister T.Q. Cost effectiveness of screening for cardiovascular disease with measures of coronary calcium. Prog Cardiovasc Dis. 2003;46:171-184.
193. Sheth T., Amlani S., Ellins M.L., et al. Computed tomographic coronary angiographic assessment of high-risk coronary anatomy in patients with suspected coronary artery disease and intermediate pretest probability. Am Heart J. 2008;155:918-923.
194. Carrascosa P.M., Capuñay C.M., Garcia-Merletti P., et al. Characterization of coronary atherosclerotic plaques by multidetector computed tomography. Am J Cardiol. 2006;97:598-602.
195. Achenbach S., Ropers D., Hoffmann U., et al. Assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J Am Coll Cardiol. 2004;43:842-847.
196. Schmid M., Pflederer T., Jang I.K., et al. Relationship between degree of remodeling and CT attenuation of plaque in coronary atherosclerotic lesions: an in-vivo analysis by multi-detector computed tomography. Atherosclerosis. 2008;197:457-464.
197. Leber A.W., Johnson T., Becker A., et al. Diagnostic accuracy of dual-source multi-slice CT-coronary angiography in patients with an intermediate pretest likelihood for coronary artery disease. Eur Heart J. 2007;28:2354-2360.
198. Choi E.K., Choi S.I., Rivera J.J., et al. Coronary computed tomography angiography as a screening tool for the detection of occult coronary artery disease in asymptomatic individuals. J Am Coll Cardiol. 2008;52:357-365.
199. Rudd J.H., Fayad Z.A. Imaging atherosclerotic plaque inflammation. Nat Clin Pract Cardiovasc Med. 2008;5(Suppl 2):S11-S17.
200. Amirbekian V., Lipinski M.J., Briley-Saebo K.C., et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A. 2007;104:961-966.
201. Tawakol A., Migrino R.Q., Bashian G.G., et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818-1824.
202. Hyafil F., et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nature Med. 2007;13:636-641.