Chapter 8 Corneal Diseases
Introduction
Ultrasound biomicroscopy (UBM) systems are suitable for imaging of virtually all aspects of anterior segment including the cornea, anterior chamber, iris and iridocorneal angle, ciliary body and lens (Chapter 4). Ultrasound is therefore applicable to a host of corneal disorders. Although anterior segment examinations are most commonly performed with a fluid-filled scleral shell, they may also utilize a water bath or a membrane-enclosed tip applied to the eye after topical anesthetic. A gentle through-the-lid approach is most commonly used in cases of trauma, albeit at the cost of reduced sensitivity due to attenuation by the lids (Chapter 3).1,2
Pavlin et al were the first to describe UBM imaging of the cornea.2,3 Diagnostic uses in the setting of corneal pathology include congenital corneal opacification, edema,4 keratoconus,5,6 dystrophies,7 corneal scars,8,9 trauma,10 and corneal keloid.11
The earliest commercial UBM system from Zeiss-Humphrey (later Paradigm Medical Industries, Salt Lake City, UT, USA) consisted of a handheld 50 MHz probe that allowed unparalleled non-invasive imaging of anatomic areas of interest throughout the anterior segment. Later generation handheld systems from several manufacturers offer higher scan rates and more compact probes.1 An automated high frequency ultrasound system developed at Cornell University and commercialized as the Artemis-2 (ArcScan) couples a disposable water bath interface and an arcuate probe scanning pathway that approximates normality between the ultrasound beam axis and the corneal surface to facilitate corneal biometric analysis with sufficient scan width to visualize the anterior segment from sulcus to sulcus (Chapter 6). The Artemis very high frequency digital ultrasound system (bandwidth 10 to 60 MHz) has advantages over analog processing systems in its ability to consistently detect internal corneal interfaces due to the higher signal-to-noise ratio between the interface echo complex and the surrounding tissue. These sensitivity gains and a more systematic measurement approach favor excellent quantitative repeatability for small ocular structures such as corneal sublayers compared to UBM.12 Optical coherence tomography (OCT), an interferometric technique first described by Huang et al in 1991,13 has also been shown to be capable of detecting corneal sublayer interfaces such as the laser in-situ keratomileusis (LASIK) flap in the early postoperative period.14 A limitation of OCT is its inability to image the ciliary sulcus through the iris.
Cornea
Normal cornea
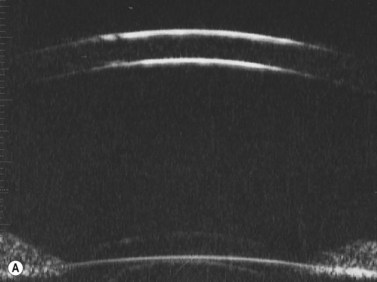
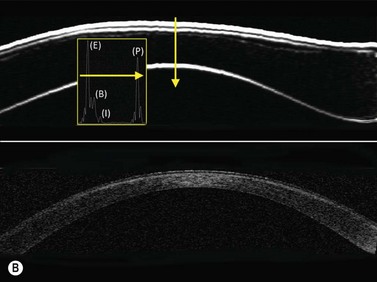
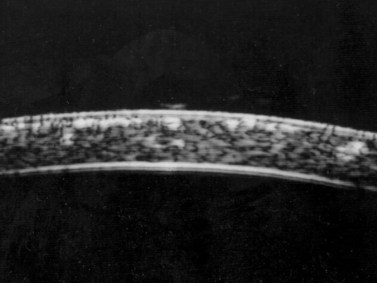
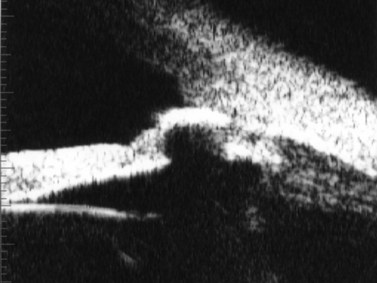
Previous studies have shown that the corneal imaging is best conducted with 80–100-MHz probes because the axial resolution is directly proportional to the emission frequency. Pavlin et al showed that even with high-frequency probes, the cornea appears to be divided into four layers rather than its true five layers.2,3
The first layer encountered by an acoustic signal is the epithelium, which is approximately 50 µm thick and has a slightly irregular anterior surface. Bowman’s layer is visible under the epithelium as a highly reflective continuous line because of several interfaces within it. It should be noted that these two layers are not always clearly distinguishable with a 50-MHz probe and can be better identified by post-processing of the signal.2–4
With a more regular internal lamellar structure, the corneal stroma appears as a uniform layer with medium to low reflectivity. This is in contrast to the sclera and Bowman’s layer, which are more reflective because of the more irregular arrangement of collagen fibrils. The fourth layer is represented by Descemet’s membrane (DM) and the endothelial monolayer, which cannot be visualized as two distinct layers and therefore appear as a highly reflective continuous line behind the stroma (Figure 8.1).2–4
Congenital corneal opacity
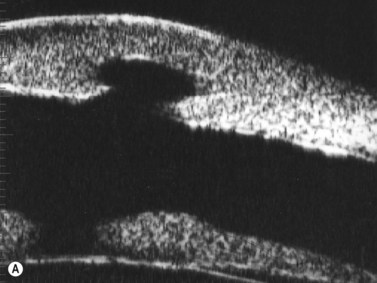
UBM is useful not only for determining the clinical diagnosis in congenital corneal opacification, but also for refining the surgical plan before keratoplasty where keratolenticular and iridocorneal adhesions and other ocular abnormalities such as aniridia and congenital aphakia are detected.15
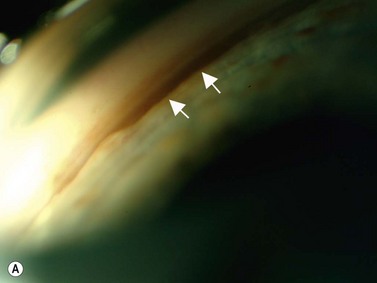
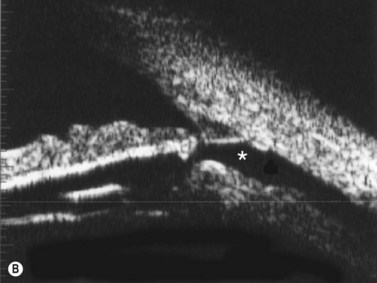
Nischal et al studied the correlation of ultrasound biomicroscopy and histological findings in cases of congenital corneal opacification.15 In a report of 22 eyes in 13 patients who were diagnosed with Peter’s anomaly, corneal dystrophy, and sclerocornea, UBM findings changed the final diagnosis in 5 of the 13 cases. They showed that the usually contiguous reflectivity of the DM/endothelium layer seen on UBM was instead irregular, a finding confirmed on histology to be due to focal absences of Descemet’s membrane with multilayering of the endothelium in cases of posterior polymorphous dystrophy (PPMD) and absence of endothelium in cases of congenital hereditary endothelial dystrophy (CHED). Similarly, the presence of a hypoechoic layer in the anterior stroma just below the hyperechoic epithelial layer may be indicative of an absent Bowman’s layer with concomitant edema.15 The ability to delineate the zone of endothelial pathology and the presence or absence of iridocorneal adhesions is particularly important given recent advances in endothelial keratoplasty and the potential application of these approaches to appropriate cases of congenital corneal opacification (Figure 8.2).
Corneal edema
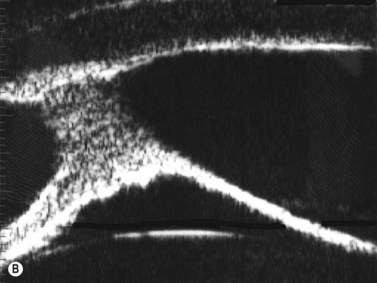
Some of the characteristic features of corneal epithelial edema – hydropic intra- and extracellular changes and intercellular vesicles – increase the quantity and intensity of echo interfaces and result in the appearance of a thicker, more reflective epithelium and Bowman’s layer. An increase in the thickness and the reflectivity of the stroma also occurs with the increased spacing of corneal lamellae and appearance of large interlamellar vesicles.2,4
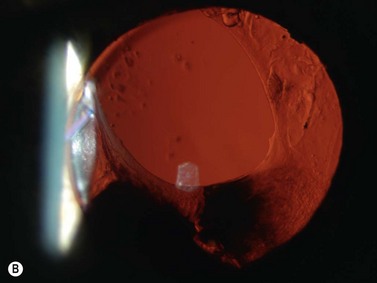
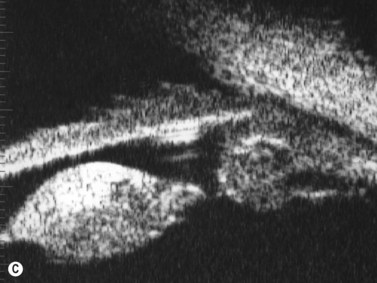
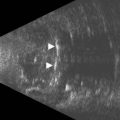
With UBM it is possible to detect the cause of corneal edema in the majority of cases and to plan the correct surgical approach (Figure 8.3).4 UBM can provide particularly useful information in cases of corneal edema related to acute hydrops in patients with keratoconus, bullous keratopathy,16 and total corneal decompensation secondary to iridocorneal touch in iridoschisis (Figure 8.4).17
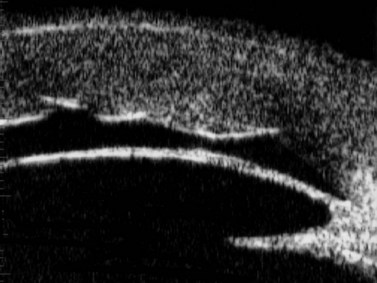
Reproduced with permission from: Heur M, Jeng BH. Anterior segment disorders. Ultrasound Clin 2008; 3(2):201–206.51
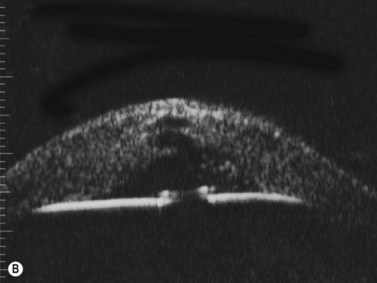
Reproduced with permission from: Nakagawa T, Maeda N, Okazaki N, et al. Ultrasound biomicroscopic examination of acute hydrops in patients with keratoconus. Am J Ophthalmol 2006; 141(6):1134–1136.16
Descemet’s membrane detachments are a common complication of intraocular surgery that can lead to corneal edema. Although spontaneous reattachment occurs in some cases, edema and visual loss can persist and diagnosis is often difficult at the slit lamp microscope in cases of diffuse edema. UBM can be a useful adjunct in visualizing the membrane for diagnosis and for confirming correct suture placement and membrane repositioning during the repair.18,19
Corneal dystrophy
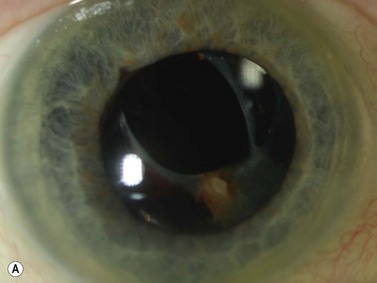
Corneal dystrophies typically are diagnosed clinically based on the specific layer of the cornea affected and characteristic appearances on slit lamp biomicroscopy. UBM of granular dystrophy shows highly reflective hyaline deposits in the superficial stroma and correlates well with OCT findings (Figure 8.5).
Although UBM may be useful to identify affected regions in the setting of corneal dystrophy, Rapuano has presented data that casts doubt upon the accuracy of central stromal lesion depth measurements with a 50 MHz UBM device.7 In 34 eyes undergoing excimer laser phototherapeutic keratectomy (PTK) for a variety of primary or recurrent anterior stromal corneal dystrophies, post-hoc determinations of lesion depth from UBM images did not correlate significantly with the PTK ablation depth.
Corneal infection
UBM can be used to assess the severity of corneal infections. In opacified corneas with bacterial, fungal or amebal keratitis, the extent of corneal thinning and risk of perforation can be assessed serially even when the view at the slit lamp microscope prohibits a view of deep corneal structures. In one case of Acanthamoeba keratitis with acute hydrops, UBM was used acutely to demonstrate Descemet’s membrane rupture and anterior chamber inflammation without corneal perforation, and serially to track resolution of the Descemet’s rupture and infectious keratitis.20
Corneal transplants
Descemet’s-stripping automated endothelial keratoplasty (DSAEK) is gaining prominence as a surgical approach to treating corneal endothelial diseases such as Fuchs’ endothelial dystrophy and pseudophakic or aphakic bullous keratopathy. Patients typically enjoy more rapid visual rehabilitation than historically achieved with penetrating keratoplasty with less profound ametropic and astigmatic effects.21,22 VHF arc-scanning ultrasound offers important advantages over uniaxial central corneal thickness measurements in this setting. UBM and VHF digital UBM can be used to visualize and quantify host and donor cornea thickness profiles after DSAEK. These modalities also provide sublayer anatomy information that ultrasound pachymetry cannot.
Images obtained with VHF digital UBM have been used to study the etiology of a tendency toward hyperopic shift after DSAEK that, although mild compared with refractive instability after penetrating keratoplasty, remains an important consideration especially in combined or staged management of endothelial disease and cataract. Dupps et al used arc-scanning US to analyze donor and recipient corneal thicknesses after DSAEK and test the hypothesis that multiple elements of the in situ donor lenticle shape contribute to DSAEK-induced refractive shift.23 Donor lenticles produced with microkeratomes favored non-uniform thickness profiles that tended to be thinner centrally than peripherally. UBM has also been used to demonstrate iridocorneal adhesion with localized graft edema during an acute rejection episode in a DSAEK patient.
Photoablative corneal surgery
The applications of arc-scanning VHF digital UBM have perhaps been best defined in the area of refractive surgery. Several studies have demonstrated the utility of VHF digital UBM for obtaining reproducible measurements of flap thickness,24 measuring residual stromal thickness (RST) for assessing suitability for LASIK enhancement,25 detecting irregularities of the epithelial and stromal thickness profiles in candidates for phototherapeutic keratectomy,26,27 guiding repositioning of a dislocated flap (“free cap”) after LASIK,28 and characterizing changes in the corneal epithelial thickness profile induced by myopic LASIK and PRK to assess sources of regression or residual refractive error in patients being considered for enhancement procedures (Chapter 6).29
VHF digital UBM has been used as a tool to guide transepithelial PTK together with a wavefront-guided treatment to reduce stromal surface irregularities and higher-order aberrations.27
A notable advance in keratoconus diagnostics first enabled by VHF digital UBM is the ability to separate the contributions of epithelial and stromal thickness to corneal surface topography. Reinstein et al have published their finding that the normal corneal epithelium has a typical non-uniform thickness distribution30 and that this distribution is altered in a characteristic way in keratoconus.31 Epithelial thinning over the steepest point of the stromal cone masks the cone from the perspective of common anterior surface curvature and elevation mapping tools and can confound the identification of early cases of keratoconus.
Corneal biomechanical imaging
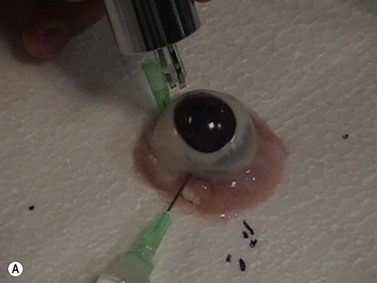
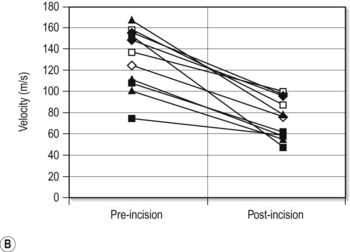
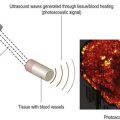
A variety of acoustic imaging techniques are being explored as methods of more directly characterizing corneal biomechanical properties and ultimately, ectasia risk (Chapter 19). In 1991, Ophir first described the use of ultrasound speckle tracking during tissue compression for inferring elastic properties – specifically, the elastic modulus – in a non-destructive manner.32 By measuring the displacement of tissue during application of a known deforming force, absolute and relative estimates of local tissue stiffness can be obtained. Hollman et al subsequently extended the concept to porcine cornea (Figure 8.6).33 More recently, sonic wave velocity measurements34,35 and supersonic shear imaging36 have been used to demonstrate the stiffening effects of collagen crosslinking, an emerging treatment for keratoconus (Figure 8.7). Advances in this area are likely to lead to more sensitive and specific diagnostic tools for detecting keratoconus, assessing the biomechanical impact of keratorefractive surgery, and monitoring the effects of treatments for ectasia.
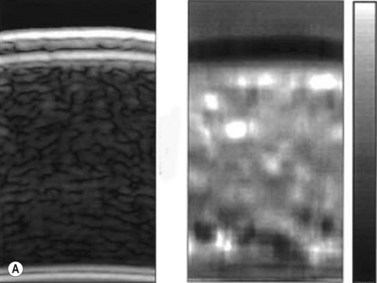
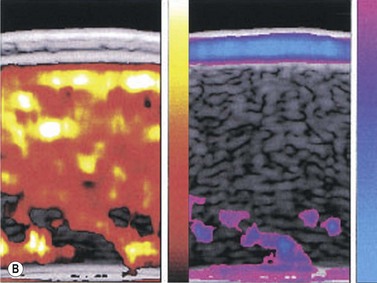
Reproduced with permission from: Hollman KW, Emelianov SY, Neiss JH, et al. Strain imaging of corneal tissue with an ultrasound elasticity microscope. Cornea 2002; 21(1):68–73.33
Intraocular lens implantation
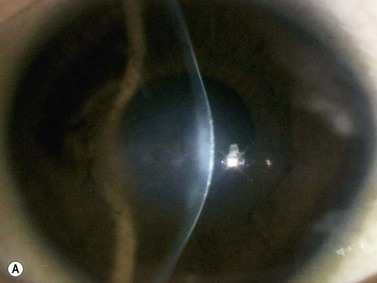
Intraocular lens (IOL) implantation is the optimal method of correcting aphakia after cataract extraction (Chapter 7). Ideally, the IOL is placed in the normal anatomic lens position, behind the iris plane, with support from the capsular bag. There are some instances, however, when the lens cannot be placed in the bag or the haptics are inadvertently malpositioned (Figure 8.8). UBM has been used to evaluate the position of the IOL optic and haptics after cataract surgery and is particularly helpful in cases of posterior capsule rupture, zonular instability, and determining the position of scleral-fixated IOL haptics relative to the ciliary sulcus (Figure 8.9). UBM is therefore an important tool for planning appropriate intervention and minimizing complications such as hyphema (Figure 8.10), uveitis, corneal decompensation, IOP elevation, lens remnants (Figure 8.11), retinal detachment, lens malposition, and a poor visual outcome.37–43
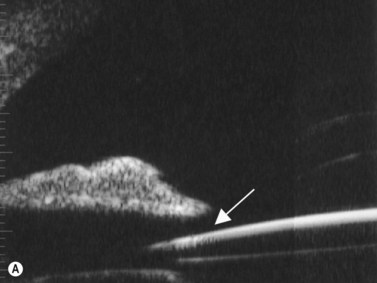
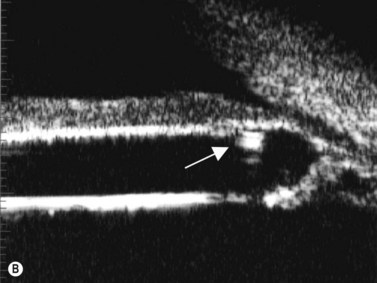
Reproduced with permission from: Heur M, Jeng BH. Anterior segment disorders. Ultrasound Clin 2008; 3(2):201–206.51
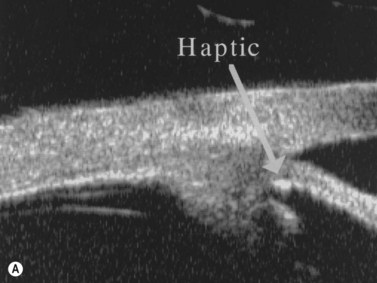
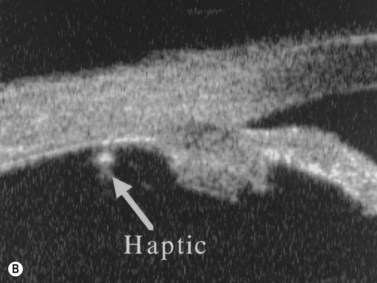
Figure 8.9 Haptic sutured at the sulcus region (A, arrow) and sutured posterior to the pars plicata (B, arrow).
Reproduced with permission from: Manabe S, Oh H, Amino K, Hata N, Yamakawa R. Ultrasound biomicroscopic analysis of posterior chamber intraocular lenses with transscleral sulcus suture. Ophthalmology 2000; 107(12):2172–2178.42
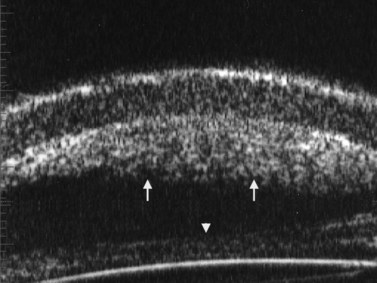
Figure 8.10 Layered hyphema on the posterior corneal surface (arrows) and on the posterior chamber IOL (arrowhead).
In anterior chamber IOL (ACIOL) implantation for aphakia or phakic correction of ametropia, ultrasound techniques are useful for preoperative determination of anterior chamber depth and angle-to-angle diameter to facilitate proper lens sizing and minimize the risk of corneal endothelial decompensation. Postoperative imaging is useful for visualizing the location of the haptics in relation to the iris, iridocorneal angle and ciliary body in cases of suspected malposition44 and for directly assessing the distance between the corneal endothelium and ACIOL.45 Corneal decompensation secondary to malpositioned ACIOL haptics can progress to the point of requiring a corneal transplant, and in such cases, UBM can be used to determine the extent of adhesions over implant haptics and predict the ease of IOL explantation.46
UBM and VHF digital UBM also provide key anatomic dimensions for more optimal sizing of posterior chamber phakic IOL, which rest in the pre-crystalline lens space and are supported by haptics in the ciliary sulcus (Chapter 6).47 The postoperative vault between the phakic IOL and the crystalline lens can be evaluated and is thought to be a predictor of anterior cataract formation and pupillary block glaucoma, the two most important complications of such lenses.48
Anterior segment trauma
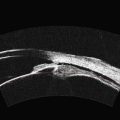
UBM can be used to visualize traumatic lens changes such as focal cataract and zonular dehiscence (Figure 8.12). Iridocorneal angle structures and the ciliary body can also be assessed by UBM in detail allowing for differentiation between iridodialysis (Figure 8.13), angle recession, and cyclodialysis in cases of trauma (Figure 8.14). UBM is of particular importance for diagnosing and evaluating cyclodialysis clefts, even in the presence of a closed angle, allowing for determination of the extent of dialysis and refinement of the surgical plan (Chapter 16).49,50
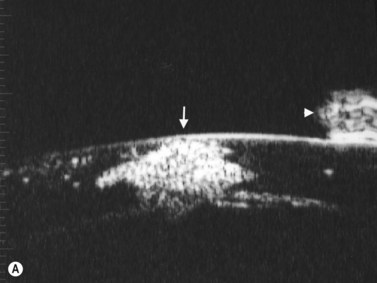
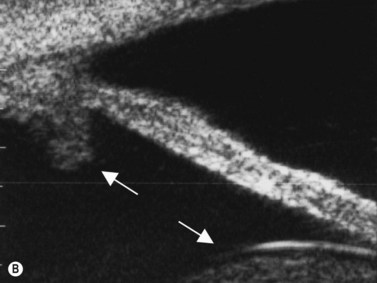
(Image courtesy of Charles J. Pavlin MD, Toronto, Canada.) Reproduced with permission from: Heur M, Jeng BH. Anterior segment disorders. Ultrasound Clin 2008; 3(2):201–206.51
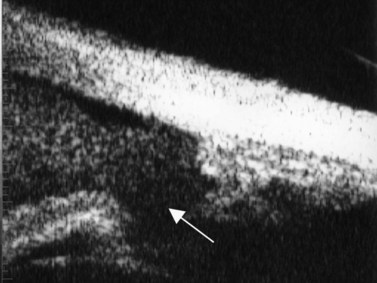
Figure 8.13 Iridodialysis. Radial scan shows complete separation of the iris from the root (arrow).
Reproduced with permission from: Heur M, Jeng BH. Anterior segment disorders. Ultrasound Clin 2008; 3(2):201–206.51
Anterior segment foreign body
Foreign bodies are localized readily by UBM, and they generate characteristic acoustic artifacts on ultrasonography based on their composition that allow detection of even small foreign bodies through media opacities or in concealed locations such as the iridocorneal angle or behind the iris (Figure 8.15).50 Foreign bodies containing pockets of air, such as wood and concrete, create shadowing because of the dampening of the sound waves by air; foreign bodies that are more dense, such as metal and glass, create reflective tails because of internal reflection of the sound waves.
1 Silverman RH. High-resolution ultrasound imaging of the eye – a review. Clin Exp Ophthalmol. 2009;37(1):54-67.
2 Pavlin CJ, Sherar MD, Foster FS. Subsurface ultrasound microscopic imaging of the intact eye. Ophthalmology. 1990;97(2):244-250.
3 Pavlin CJ, Harasiewicz K, Sherar MD, et al. Clinical use of ultrasound biomicroscopy. Ophthalmology. 1991;98(3):287-295.
4 Avitabile T, Russo V, Ghirlanda R, et al. Corneal oedemas: diagnosis and surgical planning with ultrasound biomicroscopy. Ophthalmologica. 1998;212(Suppl 1)):13-16.
5 Avitabile T, Marano F, Uva MG, et al. Evaluation of central and peripheral corneal thickness with ultrasound biomicroscopy in normal and keratoconic eyes. Cornea. 1997;16(6):639-644.
6 Avitabile T, Franco L, Ortisi E, et al. Keratoconus staging: a computer-assisted ultrabiomicroscopic method compared with videokeratographic analysis. Cornea. 2004;23(7):655-660.
7 Rapuano CJ. Excimer laser phototherapeutic keratectomy in eyes with anterior corneal dystrophies: short-term clinical outcomes with and without an antihyperopia treatment and poor effectiveness of ultrasound biomicroscopic evaluation. Cornea. 2005;24(1):20-31.
8 Reinstein DZ, Aslanides IM, Silverman RH, et al. High-frequency ultrasound corneal pachymetry in the assessment of corneal scars for therapeutic planning. CLAO J. 1994;20(3):198-203.
9 Allemann N, Chamon W, Silverman RH, et al. High-frequency ultrasound quantitative analyses of corneal scarring following excimer laser keratectomy. Arch Ophthalmol. 1993;111(7):968-973.
10 Pavlin CJ, Foster FS. Ultrasound biomicroscopy. High-frequency ultrasound imaging of the eye at microscopic resolution. Radiol Clin North Am. 1998;36(6):1047-1058.
11 Chawla B, Agarwal A, Kashyap S, et al. Diagnosis and management of corneal keloid. Clin Exp Ophthalmol. 2007;35(9):855-857.
12 Reinstein DZ, Archer TJ, Gobbe M, et al. Accuracy and reproducibility of artemis central flap thickness and visual outcomes of LASIK with the Carl Zeiss Meditec VisuMax femtosecond laser and MEL 80 excimer laser platforms. J Refract Surg. 2010;26(2):107-119.
13 Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178-1181.
14 Li Y, Netto MV, Shekhar R, et al. A longitudinal study of LASIK flap and stromal thickness with high-speed optical coherence tomography. Ophthalmology. 2007;114(6):1124-1132.
15 Nischal KK, Naor J, Jay V, et al. Clinicopathological correlation of congenital corneal opacification using ultrasound biomicroscopy. Br J Ophthalmol. 2002;86(1):62-69.
16 Nakagawa T, Maeda N, Okazaki N, et al. Ultrasound biomicroscopic examination of acute hydrops in patients with keratoconus. Am J Ophthalmol. 2006;141(6):1134-1136.
17 Srinivasan S, Batterbury M, Hiscott P. Bullous keratopathy and corneal decompensation secondary to iridoschisis: a clinicopathological report. Cornea. 2005;24(7):867-869.
18 Morinelli EN, Najac RD, Speaker MG, et al. Repair of Descemet’s membrane detachment with the assistance of intraoperative ultrasound biomicroscopy. Am J Ophthalmol. 1996;121(6):718-720.
19 Jeng BH, Meisler DM. A combined technique for surgical repair of Descemet’s membrane detachments. Ophthal Surg Lasers Imaging. 2006;37(4):291-297.
20 Guerriero S, La Tegola MG, Monno R, et al. A case of Descemet’s membrane rupture in a patient affected by Acanthamoeba keratitis. Eye Contact Lens. 2009;35(6):338-340.
21 Price FWJr, Price MO. Descemet’s stripping with endothelial keratoplasty in 50 eyes: a refractive neutral corneal transplant. J Refract Surg. 2005;21(4):339-345.
22 Koenig SB, Covert DJ. Early results of small-incision Descemet’s stripping and automated endothelial keratoplasty. Ophthalmology. 2007;114(2):221-226.
23 Dupps WJJr, Qian Y, Meisler DM. Multivariate model of refractive shift in Descemet-stripping automated endothelial keratoplasty. J Cataract Refract Surg. 2008;34(4):578-584.
24 Reinstein DZ, Sutton HF, Srivannaboon S, et al. Evaluating microkeratome efficacy by 3D corneal lamellar flap thickness accuracy and reproducibility using Artemis VHF digital ultrasound arc-scanning. J Refract Surg. 2006;22(5):431-440.
25 Reinstein DZ, Couch DG, Archer T. Direct residual stromal thickness measurement for assessing suitability for LASIK enhancement by Artemis 3D very high-frequency digital ultrasound arc scanning. J Cataract Refract Surg. 2006;32(11):1884-1888.
26 Reinstein DZ, Silverman RH, Raevsky T, et al. Arc-scanning very high-frequency digital ultrasound for 3D pachymetric mapping of the corneal epithelium and stroma in laser in situ keratomileusis. J Refract Surg. 2000;16(4):414-430.
27 Reinstein DZ, Archer T. Combined Artemis very high-frequency digital ultrasound-assisted transepithelial phototherapeutic keratectomy and wavefront-guided treatment following multiple corneal refractive procedures. J Cataract Refract Surg. 2006;32(11):1870-1876.
28 Reinstein DZ, Rothman RC, Couch DG, et al. Artemis very high-frequency digital ultrasound-guided repositioning of a free cap after laser in situ keratomileusis. J Cataract Refract Surg. 2006;32(11):1877-1883.
29 Reinstein DZ, Srivannaboon S, Gobbe M, et al. Epithelial thickness profile changes induced by myopic LASIK as measured by Artemis very high-frequency digital ultrasound. J Refract Surg. 2009;25(5):444-450.
30 Reinstein DZ, Archer TJ, Gobbe M, et al. Epithelial thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2008;24(6):571-581.
31 Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009;25(7):604-610.
32 Ophir J, Cespedes I, Ponnekanti H, et al. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13(2):111-134.
33 Hollman KW, Emelianov SY, Neiss JH, et al. Strain imaging of corneal tissue with an ultrasound elasticity microscope. Cornea. 2002;21(1):68-73.
34 Dupps WJJr, Netto MV, Herekar S, et al. Surface wave elastometry of the cornea in porcine and human donor eyes. J Refract Surg. 2007;23(1):66-75.
35 Thornton IL, Dupps WJ, Roy AS, et al. Biomechanical effects of intraocular pressure elevation on optic nerve/lamina cribrosa before and after peripapillary scleral collagen cross-linking. Invest Ophthalmol Vis Sci. 2009;50(3):1227-1233.
36 Tanter M, Touboul D, Gennisson JL, et al. High-resolution quantitative imaging of cornea elasticity using supersonic shear imaging. IEEE Trans Med Imaging. 2009;28(12):1881-1893.
37 Landau IM, Laurell CG. Ultrasound biomicroscopy examination of intraocular lens haptic position after phacoemulsification with continuous curvilinear capsulorhexis and extracapsular cataract extraction with linear capsulotomy. Acta Ophthalmol Scand. 1999;77(4):394-396.
38 Loya N, Lichter H, Barash D, et al. Posterior chamber intraocular lens implantation after capsular tear: ultrasound biomicroscopy evaluation. J Cataract Refract Surg. 2001;27(9):1423-1427.
39 Ozdal PC, Mansour M, Deschenes J. Ultrasound biomicroscopy of pseudophakic eyes with chronic postoperative inflammation. J Cataract Refract Surg. 2003;29(6):1185-1191.
40 LeBoyer RM, Werner L, Snyder ME, et al. Acute haptic-induced ciliary sulcus irritation associated with single-piece AcrySof intraocular lenses. J Cataract Refract Surg. 2005;31(7):1421-1427.
41 Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopic analysis of haptic position in late-onset, recurrent hyphema after posterior chamber lens implantation. J Cataract Refract Surg. 1994;20(2):182-185.
42 Manabe S, Oh H, Amino K, et al. Ultrasound biomicroscopic analysis of posterior chamber intraocular lenses with transscleral sulcus suture. Ophthalmology. 2000;107(12):2172-2178.
43 Sewelam A, Ismail AM, El Serogy H. Ultrasound biomicroscopy of haptic position after transscleral fixation of posterior chamber intraocular lenses. J Cataract Refract Surg. 2001;27(9):1418-1422.
44 Anton A, Weinreb RN. Recurrent hyphema secondary to anterior chamber lens implant. Surv Ophthalmol. 1997;41(5):414-416.
45 Jimenez-Alfaro I, Garcia-Feijoo J, Perez-Santonja JJ, et al. Ultrasound biomicroscopy of ZSAL-4 anterior chamber phakic intraocular lens for high myopia. J Cataract Refract Surg. 2001;27(10):1567-1573.
46 Rutnin SS, Pavlin CJ, Slomovic AR, et al. Preoperative ultrasound biomicroscopy to assess ease of haptic removal before penetrating keratoplasty combined with lens exchange. J Cataract Refract Surg. 1997;23(2):239-243.
47 Dougherty PJ, Rivera RP, Schneider D, et al. Improving accuracy of phakic intraocular lens sizing using high-frequency ultrasound biomicroscopy. J Cataract Refract Surg. 2011;37(1):13-18.
48 Garcia-Feijoo J, Alfaro IJ, Cuina-Sardina R, et al. Ultrasound biomicroscopy examination of posterior chamber phakic intraocular lens position. Ophthalmology. 2003;110(1):163-172.
49 Gentile RC, Pavlin CJ, Liebmann JM, et al. Diagnosis of traumatic cyclodialysis by ultrasound biomicroscopy. Ophthalmic Surg Lasers. 1996;27(2):97-105.
50 Berinstein DM, Gentile RC, Sidoti PA, et al. Ultrasound biomicroscopy in anterior ocular trauma. Ophthalmic Surg Lasers. 1997;28(3):201-207.
51 Heur M, Jeng BH. Anterior segment disorders. Ultrasound Clin. 2008;3(2):201-206.